Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a common respiratory disorder in pulmonology. Chuanbeimu (CBM) is a traditional Chinese medicinal herb for treating COPD and has been widely utilized in clinical practice. However, the mechanism of CBM in the treatment of COPD remains incompletely understood. This study aims to investigate the underlying therapeutic mechanism of CBM for COPD using network pharmacology and experimental approaches.
Methods
Active ingredients and their targets were obtained from the Traditional Chinese Medicine Systems Pharmacology database. COPD-associated targets were retrieved from the GeneCards database. The common targets for CBM and COPD were identified through Venn diagram analysis. Protein-protein interaction (PPI) networks and disease-herb-ingredient-target networks were constructed. Subsequently, the results of the network pharmacology were validated by molecular docking and in vitro experiments.
Results
Seven active ingredients and 32 potential targets for CBM were identified as closely associated with COPD. The results of the disease-herb-ingredient-target network and PPI network showed that peimisine emerged as the core ingredient, and SRC, ADRB2, MMP2, and NOS3 were the potential targets for CBM in treating COPD. Molecular docking analysis confirmed that peimisine exhibited high binding affinity with SRC, ADRB2, MMP2, and NOS3. In vitro experiments demonstrated that peimisine significantly upregulated the expression of ADRB2 and NOS3 and downregulated the expression of SRC and MMP2.
Conclusion
These findings indicate that CBM may modulate the expression of SRC, ADRB2, MMP2, and NOS3, thereby exerting a protective effect against COPD.
Keywords: chuanbeimu, molecular docking, network pharmacology, experiment verification, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a prevalent respiratory condition within pulmonary disease. In clinical practice, it is essential to consider respiratory difficulties, long-term chronic cough or expectoration, and recurrent lower respiratory tract infections when accompanied by long-term exposure to risk factors such as smoking, exposure to fumes, as well as air pollution. According to the 2022 revision of Global Initiative for Chronic Obstructive Lung Disease (GOLD), the initial assessment and diagnosis categorizes COPD into two categories: stable COPD and Acute exacerbation of COPD (AECOPD), based on evaluation results and characteristics.1 The prevalence and mortality of COPD in China are increasing annually due to an aging population, an increasing number of smokers, and rising levels environmental pollution. According to World Health Organization (WHO) statistics, it is estimated that approximately 5.4 million individuals will succumb to COPD and associated diseases by 2060.2 The pathogenesis of COPD remains elusive, however, studies reveal that lung parenchymal damage, inflammatory responses, and structural alterations are the most characteristic pathological changes in the occurrence and development of COPD.3 Pulmonary function tests are currently objective indicator for the diagnosis and evaluation of COPD. When combined with the COPD Assessment Test (CAT), it can markedly improve the sensitivity of COPD diagnosis.4
Currently, some drugs such as glucocorticoids, bronchodilators, antibiotics, anti-inflammatory drugs, and immunosuppressants, are commonly used in the treatment of COPD.5,6 While these medications have demonstrated certain efficacy in treating COPD, they also trigger various adverse reactions such as increased drug resistance and hormone-related side effects.7 Thus, alternative therapies such as traditional Chinese medicine (TCM) may be a treatment option for COPD with fewer side effects and lower cost. TCM, a system with a long history, has demonstrated unique therapeutic advantages and significant research potential in treating COPD. TCM emphasizes holistic conditioning, individualized treatment, and herbal medicine, offering significant advantages in alleviating symptoms, reducing the frequency of acute exacerbation, and improving the quality of life in COPD.8,9 Unlike western medicine, which often targets specific symptoms, TCM aims to harmonize the balance of Yin and Yang in the human body, thereby improving the quality of life of COPD patients through a comprehensive treatment approach.10 Recent studies have provided further evidence supporting the effectiveness and elucidating the mechanisms of TCM in treating COPD,11,12 suggesting that TCM offers a broader range of treatment options and substantial potential for ongoing research in this field.
Chuanbeimu (CBM) is a valued and time-honored Chinese medicinal material with a rich medicinal history. Over the past few decades, several chemical components of CBM have been identified, which primarily include steroid alkaloids, saponins, and terpenoids.13 In recent years, numerous studies have shown that CBM exhibits a multiple biological activities, including expectorant, antitussive, antiasthmatic, anti-inflammatory, anti-tumor, and anti-oxidant effects.14–16 Historically, CBM has been used both in China and abroad for the treatment of lung diseases.17 Its therapeutic effects are primarily mediated by biological processes, notably inflammation, to confer therapeutic benefits in lung diseases.18 Currently, over 200 CBM-related products are available on the market. Examples include Nin Jiom Pei Pa Koa, Chuanbei Zhike Lu, and Chuanbei Pipa Capsules. These products are widely used in clinical practice for treating respiratory difficulties, bronchitis, COPD, and other pulmonary conditions.19–22 Wang et al found that imperialine, a steroidal alkaloid derived from CBM, may alleviate both lung function and structural integrity in a rat model of COPD, potentially delaying disease progression.22 Investigating the mechanism of CBM in the treatment of COPD holds significant importance. COPD, a debilitating respiratory disease, profoundly affects patients’ quality of life. Among the pharmacologically active components of CBM, total alkaloids have demonstrated potential therapeutic effects on COPD symptoms in animal studies. These components may exert their mechanism of action through the inhibition of TGF-β and NF-κB signaling pathways, both of which play crucial roles in the inflammatory response and pulmonary fibrosis associated with COPD.23 However, the research on CBM for COPD treatment encounters challenges primarily stemming from the intricate nature of its chemical composition. Therefore, further investigation is necessary to identify effective components and elucidate their mechanisms of action.
Network pharmacology is a widely employed approach for investigating the biological mechanisms of TCM in complex diseases at the molecular level. This method utilizes the principles of multi-directional pharmacology, systems biology, network analysis, and computational biology to systematically elucidate the potential targets and mechanisms of TCM.24 Moreover, it facilitates the establishment of a relationship network encompassing the disease-herb-compound-target pathway, thus uncovering the molecular basis and predicting the pharmacological mechanism.25 Network pharmacology is gaining popularity as a method for investigating the mechanisms through which TCM treats a wide range of diseases.
Molecular docking plays a crucial role in contemporary drug design and discovery. It facilitates the comprehension of drug mechanisms, prediction of efficacy, and assessment of side effects by simulating the interactions between drugs and their target receptors.26 Molecular docking enables precise demonstration of the interactions between drugs and their receptors. It facilitates the determination of the drug’s position within the binding pocket and the forces involved with amino acids, both of which are essential for understanding the drug’s affinity and specificity. For instance, Hamza et al determined the binding mode of Pyrazolo-pyridazinone derivatives as FGFR1 inhibitors through docking analysis.27 Molecular docking analysis assists in optimizing drug structures, thereby enhancing their activity and selectivity. Through simulating interactions between various structures and the receptor, this approach enables the design of more effective drugs. For instance, previous studies have guided the design of pyrrolo-pyrimidine analogs as FGFR1 inhibitors through docking analysis.28 In summary, molecular docking plays a pivotal role in drug development, offering efficient computational tools for design and the elucidation of mechanisms.
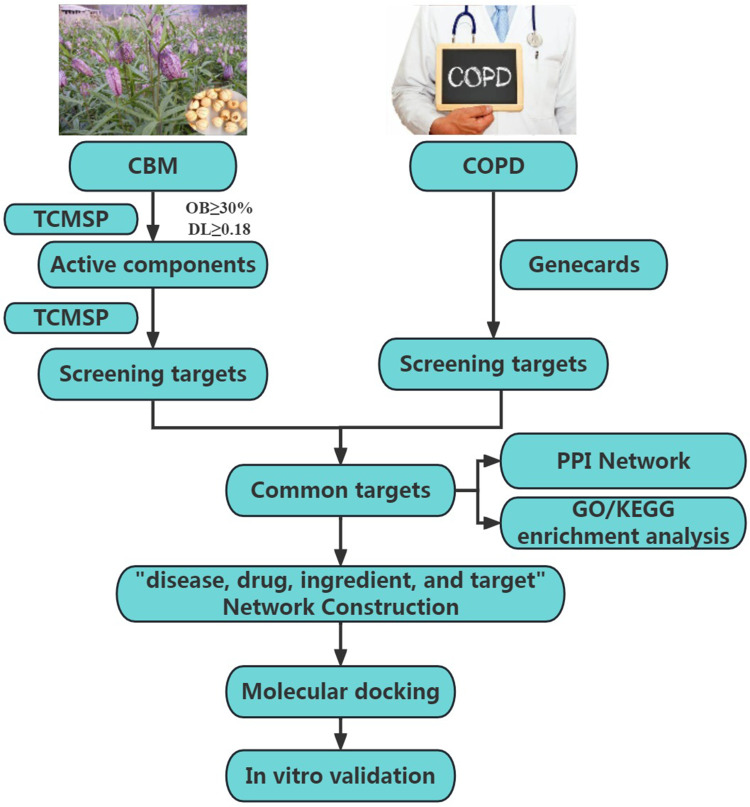
In this study, we screened the key active ingredients and targets for CBM against COPD and investigated the potential mechanism of CBM in the treatment of COPD through network pharmacology, molecular docking, and in vitro experiments. The flowchart of our research is illustrated in Figure 1.
Figure 1.
The overall flow chart of this work.
Abbreviations: CMB, chuanbeimu; COPD, chronic obstructive pulmonary disease; TCMSP, Traditional Chinese Medicine Systems Pharmacology; OB, oral bioavailability; DL, drug-likeness; PPI, protein-protein interaction; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Materials and Methods
Screening of Active Ingredients and Targets of CBM
The chemical components of CBM were screened using the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database (https://old.tcmsp-e. com/index.php).29 From these, we selected active components based on the criteria of oral bioavailability (OB) ≥ 30% and drug-likeness (DL) ≥ 0.18.30 Subsequently, TCMSP was employed to predict targets of the active components. Furthermore, the UniProt database (https://www.uniprot.org/) was utilized to standardize the targets.
Prediction of COPD Targets
The search for targets associated with COPD was conducted in the GeneCards database (https://www.genecards.org/).31 The keyword used for the search was “chronic obstructive pulmonary disease”. The targets associated with COPD were identified based on a relevance score greater than 1.
Screening for CBM-COPD Common Targets and Construction of a Protein-Protein Interaction (PPI) Network
The overlapping targets between CBM and COPD were identified using the Venn diagram tool 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/index.html). The potential targets of CBM for the treatment of COPD were then entered into the STRING database (http://string-db.org/)32 for PPI analysis. The biological species “Homo sapiens” was selected as the study organism, the isolated protein was masked, and the TSV file was exported. Cytoscape 3.10.0 was utilized to generate the PPI network diagram and conduct topological analysis to identify the central protein.
GO and KEGG Enrichment Analysis
The Database for Annotation, Visualization, and Integrated Discovery (DAVID) (http://david.abcc.ncifcrf.gov)33 and KEGG Orthology Based Annotation System (KOBAS) (https://www.biostars.org/p/200126/) databases were utilized to search for the relevant gene functions. Gene ontology (GO) functional enrichment analysis and KEGG pathway enrichment analysis were performed using R package version 4.1.1. The variables for plotting were selected based on the p-values.
Construction of Disease-Herb- Ingredient-Target Network
Cytoscape 3.10.0 (https://cytoscape.org/)34 was used to generate a network diagram illustrating the interactions among disease, herb, active ingredients, and targets in CBM for the treatment of COPD. Nodes represent diseases, drugs, active ingredients, and targets. Edges represent the connections between nodes.
Molecular Docking
To gain a more comprehensive understanding of the correlation between key active ingredients and their targets, we assessed the strength and pattern of their interactions through molecular docking. The 3D molecular structure of the active ingredient was retrieved from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The crystal structure of the target proteins were obtained from the RCSB Protein Data Bank (PDB, http://www.rcsb.org/).35 Subsequently, molecular docking between the ligands and receptors was performed using Autodock 4.2 software.36 The results of molecular docking were analyzed using Discovery Studio Visualizer software and PyMOL.
Experimental Verification
Preparation of Cigarette Smoke Extract (CSE)
Septwolves cigarettes (tobacco type of tar: 10 mg, nicotine content: 0.8 mg, and carbon monoxide fumes: 12 mg) were purchased from China Tobacco Fujian Industry Limited Liability Company (Xiamen, China) and used to prepare CSE through a puffing mechanism mimicking a standardized human smoking pattern (volume 30 mL/puff, duration 2 s/puff, and frequency 1 puff/min) as described elsewhere with minor modifications.37 A total of 300 mL cigarette smoke was collected by a mechanical vacuum pump and bubbled in 10 mL DMEM medium without serum. The crude CSE was adjusted pH to 7 and filtered through a 0.22 μm filter, and this solution was defined as 100% CSE. Working concentration was prepared by dilution the 100% CSE with culture medium.
Establishing a COPD Cell Model
The human bronchial epithelial cell line (BEAS-2B) was purchased from the Procell Life Science and Technology Co.,Ltd. (Wuhan, China) and cultured in DMEM medium (Procell, Wuhan, China) in a humidified incubator under 5% CO2 at 37 °C. The cells were exposed to varying concentrations of CSE (0%, 1%, 2.5%, 5%, 7.5%, 10%, 25%) for different durations (24 h and 48 h). Following the replacement of the CCK-8 mixture (Beyotime, Hangzhou, China), the cells were incubated for 4 hours. Subsequently, the absorbance values of each well were measured using a full-spectrum microplate reader (Molecular Devices, Sunnyvale, CA, USA) at a wavelength of 450 nm. Specific concentrations (low, medium, and high) were then selected, and a singular treatment time was chosen for subsequent model validation experiments. For the cell apoptosis assessment, these cells were stained with Muse Annexin V & Dead Cell kit (Muse, Beijing, China), and then was detected and analyzed by the flow cytometry. RT-qPCR was conducted to evaluate the levels of inflammatory factors IL-1β and TNF-α. Integrating the results from the above experiments allows for a comprehensive evaluation of the successful construction of the model and aids in identifying the most suitable modeling concentration.
Drug Treatment of BEAS-2B
Peimisine (purity ≥ 98%) was purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). Cell viability was assessed using the CCK-8 proliferation assay (Beyotime, Hangzhou, China). BEAS-2B cells were seeded in a 96-well plate at a density of 3 × 105, and after culturing for 24 h, different concentrations of peimisine (o μM, 2.5 μM, 5 μM, 10 μM, 20 μM, 40 μM, 80 μM, 160 μM, and 320 μM) were added, respectively. Following 24 h and 48 h of incubation, CCK8 was added to each well and the cells were cultured at 37 °C for 4 h. The OD value (450 nm) of each well was measured using a full-spectrum microplate reader (Molecular Devices, Sunnyvale, CA, USA) to determine the safe concentration range of peimisine.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
Total RNA was extracted from cells using Trizol reagent (Agbio, Hunan, China) following the manufacturer’s protocol. A NanoDrop 2000 (Thermo Fisher Scientific, USA) was used to determine the RNA purity and concentration. A reverse transcription kit (TaKaRa, Clontech) was employed to transcribe RNA into cDNA. After reverse transcription, qRT-PCR was conducted using the ABI 7500 Real-Time PCR System (Applied Biosystems, USA). The primer sequences are provided in Table S1, and the expression of IL-1β, TNF-α, SRC, ADRB2, and MMP2 were assessed. The qRT-PCR reaction system had a total volume of 20 μL, comprising 10 μL 2 × TB Green Premix Ex Taq II, 0.8 μL of 10 μM forward and reverse primers each, 0.4 μL 50 × ROX Reference Dye II, 2 μL cDNA, supplemented with double-distilled water. The qRT-PCR conditions were as follows: 40 cycles of denaturation at 95 °C for 30s, followed by denaturation at 95 °C for 5s, and annealing at 60 °C for 34s. GAPDH served as the internal control, and data analysis utilized the 2−ΔΔCt method. The experiment was repeated three times.
Statistical Analysis
The data were analyzed using GraphPad Prism 8 software for statistical analysis. All experimental data obtained from the study were collected from three biological replicates. Measurement data were presented as mean ± standard deviation (SD). Inter-group comparisons were conducted using one-way analysis of variance (ANOVA) or Student’s t-test. The results were statistically significant, with a p-value of less than 0.05. * p < 0.05, ** p < 0.01, and *** p < 0.001.
Results
Identification of Bioactive Ingredients and Targets in CBM
The OB and DL indices are critical tools for the screening of active ingredients in TCM. OB indicates the degree of absorption of compounds within TCM formulations, where substances with an OB ≥ 30% are regarded as having enhanced pharmaceutical potential. DL reflects the potential for pharmaceutical development of compounds, with those exhibiting a DL ≥ 0.18 chosen as markers of substantial drug delivery potential. Integrating these two indices can improve the precision of predicting TCM efficacy and optimize the in vivo absorption and utilization. In this study, thirteen active ingredients of CBM were identified based on the screening criteria (OB ≥ 30% and DL ≥ 0.18). After excluding active ingredients lacking corresponding targets and removing duplicates, seven active ingredients, as listed in Table 1, along with 175 corresponding targets, were obtained from the TCMSP database for further analysis.
Table 1.
Information on the Candidate Active Components of CBM
| Mol ID | Molecule Name | Molecular Formula | Molecular Weight | OB (%) | DL |
|---|---|---|---|---|---|
| MOL001749 | ZINC03860434 | C24H38O4 | 390.62 | 43.59 | 0.35 |
| MOL000358 | Beta-sitosterol | C29H50O | 414.79 | 36.91 | 0.75 |
| MOL004440 | Peimisine | C27H41NO3 | 427.69 | 57.40 | 0.81 |
| MOL009027 | Cyclopamine | C27H41NO2 | 411.69 | 55.42 | 0.82 |
| MOL009589 | Korseverinine | C2H6 | 413.71 | 53.51 | 0.71 |
| MOL009593 | Verticinone | C27H45NO3 | 429.71 | 60.07 | 0.67 |
| MOL009596 | Sinpemine A | C27H43NO2 | 413.71 | 46.96 | 0.71 |
Abbreviations: CBM, Chuanbeimu; OB, oral bioavailability; DL, drug-likeness; ID, Identity document.
Targets Identification of CBM in the Treatment of COPD and the PPI Network
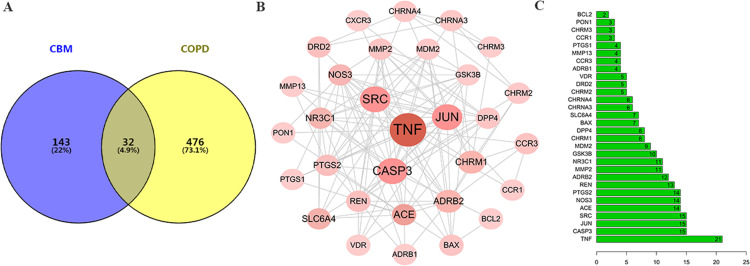
A total of 508 targets associated with COPD were retrieved from the GeneCards database with a relevance score greater than 1. All targets associated with CBM and COPD were cataloged as two distinct sets. Figure 2A illustrates that 32 common targets between CBM and COPD were identified as potential therapeutic targets for CBM in treating COPD. Subsequently, the PPI network was constructed using the STRING database and visualized with Cytoscape 3.7.2. As depicted in Figure 2B, the PPI network comprises 32 nodes. The nodes with the highest degree values included TNF (degree = 21), CASP3 (degree = 15), JUN (degree = 15), SRC (degree = 15), ACE (degree = 14), NOS3 (degree = 14), PTGS2 (degree = 14), REN (degree= 13), ADRB2 (degree = 12), and MMP2 (degree = 11), as indicated in Figure 2C.
Figure 2.
Screening the targets of CBM and COPD. (A) Venn diagram showing the common targets of CBM in the treatment of COPD; (B) PPI network of the common targets; (C) Barplot of the common targets.
Abbreviations: CMB, chuanbeimu; COPD, chronic obstructive pulmonary disease; PPI, protein-protein interaction.
GO and KEGG Enrichment Analyses
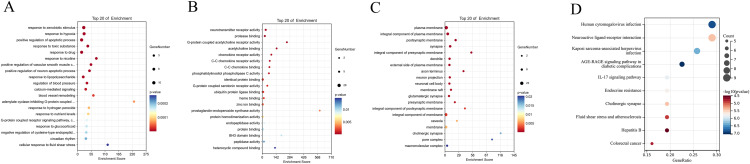
To delve deeper into the functions of the 32 identified targets, GO enrichment analysis was performed. The top 20 enriched terms within the categories of biological process (BP), molecular function (MF), and cellular component (CC) terms are illustrated using bubble charts in Figures 3A–C. Among these terms, the BP category showed predominant enrichment in processes such as response to xenobiotic stimulus, hypoxia, and positive regulation of the apoptotic process. In the MF category, enrichment was primarily observed in functions including neurotransmitter receptor activity, protease binding, and G-protein-coupled acetylcholine receptor activity. The CC category was primarily enriched in components such as the plasma membrane, integral components of the plasma membrane, and postsynaptic membrane. The top 10 enriched KEGG terms are displayed in the bubble chart depicted in Figure 3D.
Figure 3.
Functional enrichment analysis of the common targets. (A) Bubble chart of the biological process category terms; (B) Bubble chart of the molecular function category terms; (C) Bubble chart of the cellular component category terms; (D) Bubble chart showing the top 10 enriched KEGG terms.
Abbreviation: KEGG, Kyoto Encyclopedia of Genes and Genomes.
Construction and Analysis of the Disease-Herb-Ingredient-Target Network
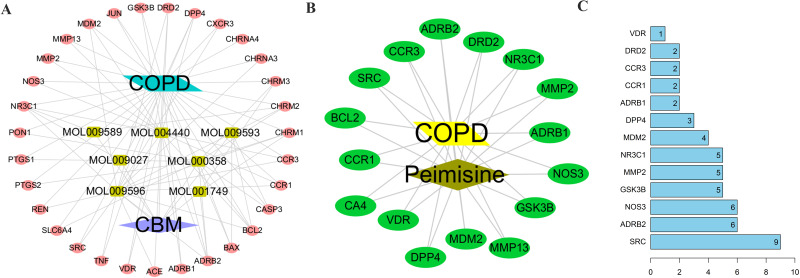
We constructed a disease-herb-ingredient-target network using Cytoscape 3.10.0 (Figure 4A), comprising 41 nodes:7 representing active ingredients, 32 for targets, one for disease, and one for herb). Table 2 illustrated that certain ingredients, such as peimisine, sinpemine, and beta-sitosterol, exhibited higher degree values. Notably, peimisine, with the highest degree value of 16, may play a pivotal role in COPD treatment. Additionally, we constructed an ingredient-target network centered on peimisine and its associated targets (Figure 4B). The target with the highest node degree were SRC, ADRB2, NOS3, GSK3B, and MMP2, as depicted in Figure 4C. Integrating this with the PPI network of the 32 common targets, where SRC, ADRB2, NOS3, and MMP2 exhibited the highest degree values, we hypothesize that peimisine may regulate the expression of SRC, ADRB2, NOS3, and MMP2, contributing to its therapeutic potential in COPD treatment.
Figure 4.
Network construction of the herb, ingredient, and targets. (A) Network of the disease-herb-ingredient-target for the treatment of COPD using the CBM; (B) Peimisine and disease-target network; (C) Barplot of the 16 targets of peimisine.
Abbreviations: CBM, chuanbeimu; COPD, chronic obstructive pulmonary disease.
Table 2.
CBM Active Ingredients Correspond to Targets
| Mol ID | Molecule Name | Targets Number | Targets |
|---|---|---|---|
| MOL004440 | Peimisine | 16 | NR3C1 |
| MOL004440 | Peimisine | NOS3 | |
| MOL004440 | Peimisine | DPP4 | |
| MOL004440 | Peimisine | CCR3 | |
| MOL004440 | Peimisine | DRD2 | |
| MOL004440 | Peimisine | CCR1 | |
| MOL004440 | Peimisine | VDR | |
| MOL004440 | Peimisine | MDM2 | |
| MOL004440 | Peimisine | SRC | |
| MOL004440 | Peimisine | MMP13 | |
| MOL004440 | Peimisine | MMP2 | |
| MOL004440 | Peimisine | GSK3B | |
| MOL004440 | Peimisine | BCL2 | |
| MOL004440 | Peimisine | ADRB2 | |
| MOL004440 | Peimisine | ADRB1 | |
| MOL004440 | Peimisine | CA4 | |
| MOL009596 | Sinpemine | 15 | CHRM2 |
| MOL009596 | Sinpemine | CHRM1 | |
| MOL009596 | Sinpemine | REN | |
| MOL009596 | Sinpemine | MDM2 | |
| MOL009596 | Sinpemine | DPP4 | |
| MOL009596 | Sinpemine | ADRB2 | |
| MOL009596 | Sinpemine | CCR1 | |
| MOL009596 | Sinpemine | CCR3 | |
| MOL009596 | Sinpemine | NR3C1 | |
| MOL009596 | Sinpemine | CHRNA3 | |
| MOL009596 | Sinpemine | CXCR3 | |
| MOL009596 | Sinpemine | DRD2 | |
| MOL009596 | Sinpemine | BCL2 | |
| MOL009596 | Sinpemine | TNF | |
| MOL009596 | Sinpemine | ACE | |
| MOL000358 | Beta-sitosterol | 12 | PTGS1 |
| MOL000358 | Beta-sitosterol | PTGS2 | |
| MOL000358 | Beta-sitosterol | CHRM3 | |
| MOL000358 | Beta-sitosterol | CHRM1 | |
| MOL000358 | Beta-sitosterol | CHRM2 | |
| MOL000358 | Beta-sitosterol | ADRB2 | |
| MOL000358 | Beta-sitosterol | SLC6A4 | |
| MOL000358 | Beta-sitosterol | BCL2 | |
| MOL000358 | Beta-sitosterol | BAX | |
| MOL000358 | Beta-sitosterol | JUN | |
| MOL000358 | Beta-sitosterol | CASP3 | |
| MOL000358 | Beta-sitosterol | PON1 | |
| MOL001749 | ZINC03860434 | 3 | CHRM3 |
| MOL001749 | ZINC03860434 | ADRB2 | |
| MOL001749 | ZINC03860434 | CHRM1 | |
| MOL009593 | Verticinone | 3 | BCL2 |
| MOL009593 | Verticinone | BAX | |
| MOL009593 | Verticinone | CASP3 | |
| MOL009589 | Korseverinine | 2 | CHRM2 |
| MOL009589 | Korseverinine | NR3C1 | |
| MOL009027 | Cyclopamine | 1 | NR3C1 |
Abbreviations: CBM, Chuanbeimu; ID, Identity document.
Molecular Docking results
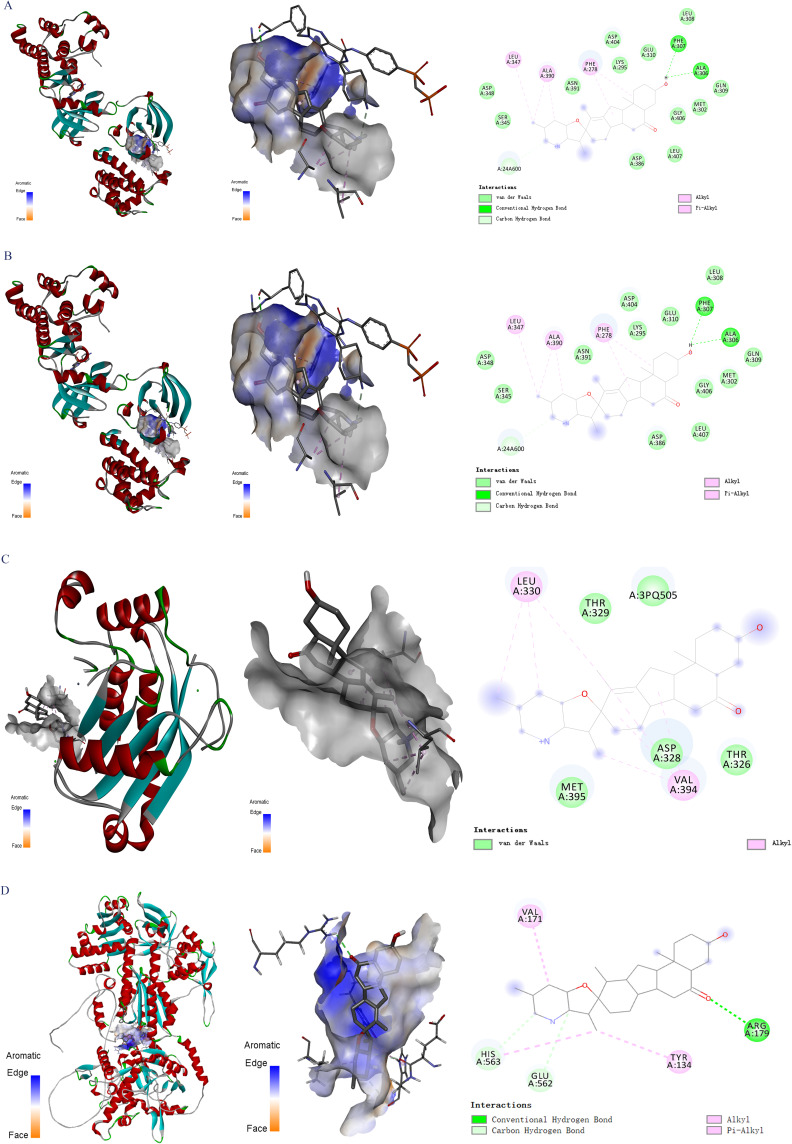
To assess the validity of molecule-target interactions and ascertain the exact binding patterns, we identified peimisine as the principal molecule, with SRC, ADRB2, MMP2, and NOS3 according to the preceding results. Lower binding energy correlates with increased stability of the ligand-receptor binding conformation. A binding energy of −5 kcal/mol or lower signifies robust binding affinity between molecules and proteins.
As depicted in Figure 5 and Table 3, our findings indicate that peimisine exhibits strong interactions with SRC, ADRB2, MMP2, and NOS3, characterized by binding energies of −9.1 kcal/mol, −9.2 kcal/mol, −8.0 kcal/mol, and −10 kcal/mol, respectively. This affirms the stability of the binding activity between the core active ingredient and the key target proteins.
Figure 5.
Molecular docking analysis between peimisine and the targets. (A) SRC, (B) ADRB2, (C) MMP2, and (D) NOS3.
Table 3.
Molecular Docking Information of Peimisine and Targets
| Compound | Target | PDB ID | Affinity (kcal/mol) |
|---|---|---|---|
| Peimisine | NOS3 | P29474 | −10.0 |
| Peimisine | SRC | 2BDF | −9.1 |
| Peimisine | ADRB2 | 6PS2 | −9.2 |
| Peimisine | MMP2 | 4WK7 | −8.0 |
Abbreviations: PDB, Protein data bank; ID, Identity document.
Establishment and Evaluation of a Cell Model for COPD
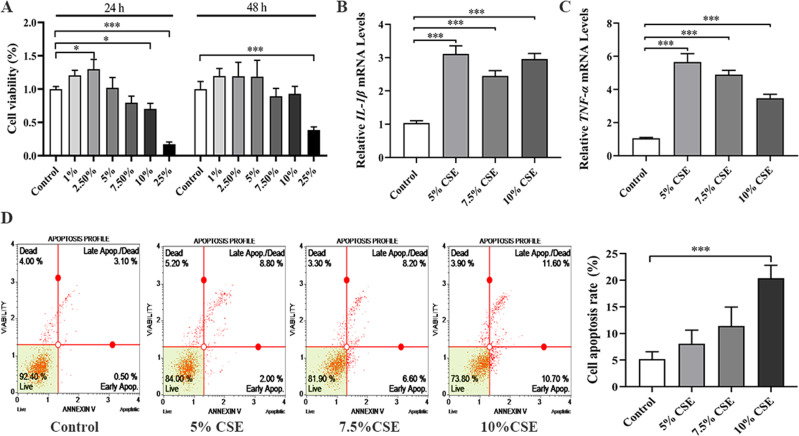
To assess the successful establishment of the COPD model, we evaluated cell proliferation with CCK-8 and quantified inflammatory factor levels via PCR. CCK-8 assays (Figure 6A) demonstrated cytotoxicity to the cells at a 25% CSE concentration. Consequently, we selected CSE concentrations of 5%, 7.5%, and 10% for the subsequent 24-hour experiments. PCR analyses showed that various CSE concentrations significantly upregulated the expression of inflammatory markers IL-1β and TNF-α, as depicted in Figure 6B and C. Furthermore, apoptosis assays indicated that disparate CSE concentrations induced apoptosis, evidenced by Figure 6D. Taken together with the apoptosis data, these findings suggest a successful construction of our COPD cell model. In light of these findings, we selected a 10% CSE concentration as optimal for further experimentation.
Figure 6.
Establishment and evaluation of COPD cell model. (A) Cell proliferation was detected by CCK8 assay at 24 h and 48 h; (B and C) Detection of inflammatory factors; (D) Detection of cell apoptosis. All in vitro experiments were repeated three times independently. *p < 0.05 and ***p < 0.001.
Abbreviations: COPD, chronic obstructive pulmonary disease; CCK-8, Cell Counting Kit-8.
Effect of Peimisine on Cell Viability in a Cell Model of COPD
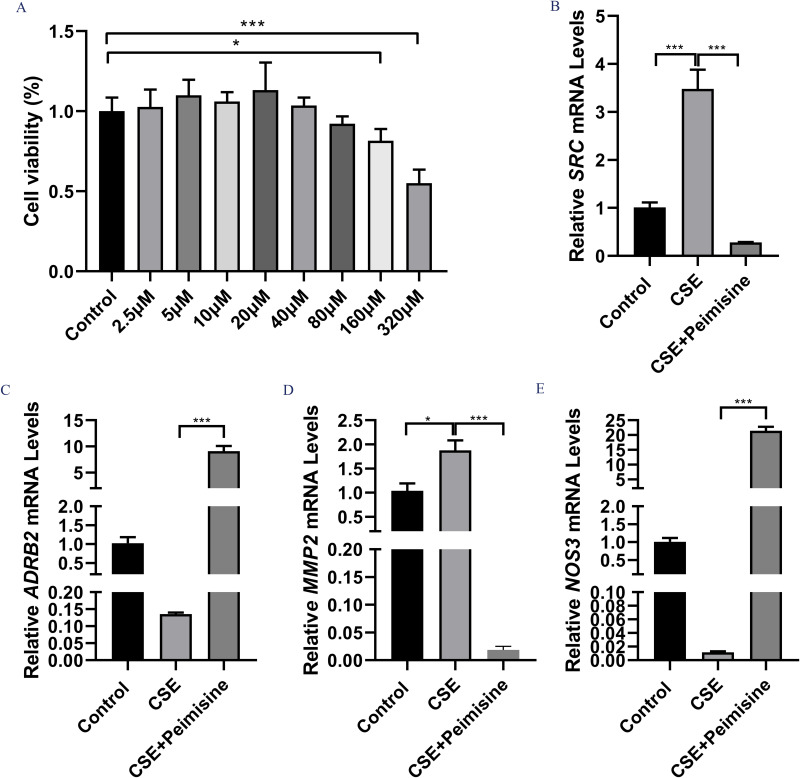
In this study, we determined the safe concentration range of peimisine for BEAS-2B cells to accurately assess its therapeutic potential for COPD. The results indicated that peimisine addition led to a reduction in cell proliferation (Figure 7A). Following the experimental results, we selected a concentration of 160 μM peimisine for a 24-hour exposure as the optimal concentration for subsequent experiments.
Figure 7.
To clarify the effect of Peimisine on target gene expression in COPD. Effect of Peimisine on cell viability at 24h (A). RT-qPCR was used to detect the mRNA expression levels of SRC (B), ADRB2 (C), MMP2 (D), and NOS3 (E). All experiments were repeated three times independently. *p < 0.05 and and ***p < 0.001.
Abbreviation: COPD, chronic obstructive pulmonary disease.
The Influence of CBM on the Expression Levels of SRC, ADRB2, MMP2, and NOS3
To examine the effects of peimisine on the expression of target genes in COPD, we quantified the mRNA expression levels of SRC, ADRB2, MMP2, and NOS3 using qRT-qPCR. The results demonstrated that peimisine significantly increased ADRB2 and NOS3 mRNA expression levels (p < 0.001), while notably reducing SRC and MMP2 mRNA expression levels of (p < 0.001), as shown in Figure 7B–E.
Discussion
TCM exerts a beneficial therapeutic effect on COPD. CBM represents a TCM therapeutic strategy for COPD, with prior research illustrating the significant role of CBM and its components in the treatment of COPD.22,38 However, the complex composition of TCM poses challenges to exploring the potential therapeutic mechanisms underlying CBM. Consequently, this study validated the mechanism of action of CBM for treating COPD through network pharmacology and in vitro experimentation.
Within the framework of TCM, compounds that lack appropriate pharmacokinetic profiles are unable to effectively reach the target organs to manifest their biological activity. Consequently, we screened active compounds of CBM based on OB ≥ 30% and DL ≥ 0.18, revealing that the selected compounds exhibited pharmacokinetic properties, which suggests their potential for effective absorption and metabolism in humans.29 Significantly, research has indicated that ingredient with a high degree value may exert therapeutic benefits for COPD. In our study, peimisine might be the primary active ingredient of CBM in the treatment of COPD with a high degree value. Peimisine, an alkaloid found in CBM, has expectorant and cough-relieving effects.39 A study has shown that peimisine, a steroid alkaloid from CBM, reduces the expression of p-AKT and phosphorylated glycogen synthase kinase 3β (p-GSK3β), while increasing the expression of phosphorylated myosin light chain 2 (p-MLC2) to prevent the progression of COPD.38 Meanwhile, peimisine can alleviate inflammation, acute lung injury, and oxidative stress.40 Chen et al discovered that peimisine was mainly distributed in tissues such as the lungs, spleen, and kidneys in rats.41 Jin et al discovered that peimisine (10 mg/kg) significantly decreased the levels of TNF-α and IL-1β, and mitigated the extent of lung tissue damage.42 TNF-α and IL-1β are the primary inflammatory factors in human COPD.43 Therefore, peimisine may have certain anti-inflammatory effects by reducing the levels of inflammatory factors. As a result, it has potential in the treatment of COPD. Furthermore, the PPI analysis and disease-herb-ingredient-target network revealed that SRC, ADRB2, MMP2, and NOS3 were the potential targets for CBM in treating COPD. The study has shown that SRC is activated in the lungs of patients with COPD.44 The Beta 2-adrenergic receptor (ADRB2) plays a crucial role in regulating airway smooth muscle tension in COPD.45 In COPD, mutations that impair ADRB2 function may increase the risk of the disease or reduce the response to naturally occurring and inhaled adrenergic agonists.45 Chi et al found elevated MMP2 expression in the lung tissue of rats with COPD.46 Smoking can increase MMP2 activity in human lung fibroblasts and mouse bronchoalveolar lavage fluid.47 NOS3 has a substantial role in the development of COPD is substantial. For example, Ben Nasr et al demonstrated that NOS3 polymorphism is associated with an increased susceptibility to COPD, which strongly associated with NO levels and the degree of airflow limitation.48 In addition, Barreiro et al observed that in the quadriceps femoris of patients with COPD, NOS3 activity is elevated and protein oxidation levels are higher.49 Ito et al further highlighted the pivotal role of NOS3 in the pathophysiology of COPD, particularly in maintaining and repairing alveolar structures.50 Furthermore, Csoma et al reported that dysregulation of the endothelial nitric oxide pathway is linked to airway inflammation in patients with COPD.51 These findings suggest that SRC, ADRB2, MMP2, and NOS3 have an important role in COPD for treatment with CBM.
Molecular docking represents a pivotal computational strategy employed to simulate the interactions between pharmaceutical agents and protein targets, aiding researchers in predicting the binding modes and affinity of pharmaceutical compounds to proteins. In the field of traditional Chinese medicine research, this technology is indispensable for elucidating the interactions of complex herbal constituents with biomolecules and their pharmacological impacts.52 For instance, the research conducted by Liu et al identified the principal constituents of Huanglian-Jiedu decoction and their potential targets using molecular docking, which furnished novel insights into the treatment of sepsis.8 Furthermore, Jiao et al utilized molecular docking and network pharmacology approaches to comprehensively predict the biological activities of TCM constituents and elucidate their mechanisms of action.53 In our research, we performed molecular docking to assess the validity of molecule-target interactions, peimisine demonstrates a strong binding affinity with SRC, ADRB2, MMP2, and NOS3.
Furthermore, we substantiated our findings with additional validation through in vitro experiments. First, we established a cell model for COPD by preparing CSE. CS is a primary causes of COPD,54 as it contains high concentrations of reactive oxygen species that can induce chemokines and lead to neutrophil accumulation in the lungs.55 CSE comprises numerous harmful substances, including tar, nitric oxide, nicotine, and oxides. Upon inhaling these harmful substances through smoke, ciliary motility of tracheal mucosal epithelial cells is inhibited, secretion is stimulated, and macrophage phagocytosis is impaired, ultimately resulting in inflammation.56 Consequently, we validated the COPD cell model by identifying cellular inflammatory factors. Yuan et al demonstrated that the severity of lung injury can be evaluated based on the release of various inflammatory mediators, including macrophages, neutrophils, and cytokines.57 Inflammation plays a crucial role in COPD. In the early stages of COPD development, macrophages and neutrophils infiltrate the airways and alveoli. These inflammatory cells subsequently elevate the levels of a complex cascade of inflammatory mediators, such as TNF-α, IL-1β, and MMPs,58 which aligns with our findings. Finally, the results indicated that peimisine significantly elevated the mRNA expression levels of ADRB2 and NOS3 and notably decreased the mRNA expression levels of SRC and MMP2. Collectively, our findings suggest that CBM may treat COPD by modulating the expression of SRC, ADRB2, MMP2, and NOS3.
It is noteworthy that network pharmacology and in vitro experiments may offer preliminary insights into the mechanisms underlying CBM in treatmenting COPD. However, further investigation, encompassing both in vivo studies and clinical trials, is essential to confirm the therapeutic efficacy of CBM for COPD management.
Conclusion
In conclusion, our study is the pioneering investigation into the core ingredient, key targets, and underlying mechanisms of CBM for treating COPD. The findings indicate that peimisine, the core ingredient of CBM, may exert an anti-COPD effect by modulating the expression of SRC, ADRB2, MMP2, and NOS3. This study provides a novel outlook on the development of drugs for COPD by elucidating the underlying mechanisms responsible for the therapeutic effects of CBM. Subsequent research will entail animal experiments to further validate the safety and efficacy of CBM in the treatment of COPD.
Funding Statement
This study was supported by the specific research fund of The Innovation Platform for Academicians of Hainan Province (No.YSPTZX202312), Hainan Provincial Natural Science Foundation of China (No. 823RC559), and Hainan Province Science and Technology Special Fund (No. ZDYF2024SHFZ094).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Hainan Medical College, and the data used in this study obtained the informed consent of the Ethics Committee of Hainan Medical College.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Halpin DMG, Criner GJ, Papi A., et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD Science Committee Report on COVID-19 and Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2021;203:24–36. doi: 10.1164/rccm.202009-3533SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh D, D’Urzo AD, Chuecos F, et al. Reduction in clinically important deterioration in chronic obstructive pulmonary disease with Aclidinium/formoterol. Respir Res. 2017;18:106. doi: 10.1186/s12931-017-0583-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jolobe OMP. Pulmonary thromboembolism, blood gas status and left ventricular function in COPD. Int J Cardiol. 2019;292:211. doi: 10.1016/j.ijcard.2019.05.062 [DOI] [PubMed] [Google Scholar]
- 4.Kart L, Akkoyunlu ME, Bayram M, et al. COPD: an underdiagnosed disease at hospital environment. Wiener klinische Wochenschrift. 2014;126:73–78. doi: 10.1007/s00508-013-0458-4 [DOI] [PubMed] [Google Scholar]
- 5.Terry PD, Dhand R. Inhalation Therapy for Stable COPD: 20 Years of GOLD Reports. Adv Ther. 2020;37:1812–1828. doi: 10.1007/s12325-020-01289-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uwagboe I, Adcock IM, Lo Bello F, et al. New drugs under development for COPD. Minerva Med. 2022;113:471–496. doi: 10.23736/s0026-4806.22.08024-7 [DOI] [PubMed] [Google Scholar]
- 7.Vogelmeier CF, Román-Rodríguez M, Singh D, et al. Goals of COPD treatment: focus on symptoms and exacerbations. Respir Med. 2020;166:105938. doi: 10.1016/j.rmed.2020.105938 [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Liu J, Tong X, et al. Network pharmacology prediction and molecular docking-based strategy to discover the potential pharmacological mechanism of huai hua san against ulcerative colitis. Drug Des Devel Ther. 2021;15:3255–3276. doi: 10.2147/dddt.S319786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JS. International clinical practice guideline of Chinese medicine: chronic obstructive pulmonary disease. World J Traditional Chin Med. 2020;6:39. [Google Scholar]
- 10.Cao X, Wang Y, Chen Y, et al. Advances in traditional Chinese medicine for the treatment of chronic obstructive pulmonary disease. J Ethnopharmacol. 2023;307:116229. doi: 10.1016/j.jep.2023.116229 [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Hou Y, Ma X, et al. Multi-omics analysis reveals the mechanisms of action and therapeutic regimens of traditional Chinese medicine, Bufei Jianpi granules: implication for COPD drug discovery. Phytomedicine. 2022;98:153963. doi: 10.1016/j.phymed.2022.153963 [DOI] [PubMed] [Google Scholar]
- 12.Jia Y, He T, Wu D, et al. The treatment of Qibai Pingfei Capsule on chronic obstructive pulmonary disease may be mediated by Th17/Treg balance and gut-lung axis microbiota. J Transl Med. 2022;20:281. doi: 10.1186/s12967-022-03481-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D, Chen X, Atanasov AG, et al. Plant resource availability of medicinal fritillaria species in traditional producing regions in Qinghai-Tibet plateau. Front Pharmacol. 2017;8:502. doi: 10.3389/fphar.2017.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li HJ, Jiang Y, Li P. Chemistry, bioactivity and geographical diversity of steroidal alkaloids from the Liliaceae family. Nat Product Rep. 2006;23:735–752. doi: 10.1039/b609306j [DOI] [PubMed] [Google Scholar]
- 15.Fan B, Li T, Xu S, et al. Efficient, accurate and comprehensive evaluation of polysaccharides from Fritillaria and their inhibitory responses to mouse inflammation. Food Funct. 2019;10:7913–7925. doi: 10.1039/c9fo02209k [DOI] [PubMed] [Google Scholar]
- 16.Lin Q, Qu M, Zhou B, et al. Exosome-like nanoplatform modified with targeting ligand improves anti-cancer and anti-inflammation effects of imperialine. J Control Release. 2019;311–312:104–116. doi: 10.1016/j.jconrel.2019.08.037 [DOI] [PubMed] [Google Scholar]
- 17.Wang DD, Feng Y, Li Z, et al. In vitro and in vivo antitumor activity of bulbus fritillariae cirrhosae and preliminary investigation of its mechanism. Nutr Cancer. 2014;66:441–452. doi: 10.1080/01635581.2013.878737 [DOI] [PubMed] [Google Scholar]
- 18.Quan Y, Li L, Yin Z, et al. Bulbus fritillariae cirrhosae as a respiratory medicine: is there a potential drug in the treatment of COVID-19? Front Pharmacol. 2021;12:784335. doi: 10.3389/fphar.2021.784335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham AB, Brinckmann JA, Pei SJ, et al. High altitude species, high profits: can the trade in wild harvested Fritillaria cirrhosa (Liliaceae) be sustained? J Ethnopharmacol. 2018;223:142–151. doi: 10.1016/j.jep.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 20.Guo F, Tang X, Zhang W, et al. Exploration of the mechanism of traditional Chinese medicine by AI approach using unsupervised machine learning for cellular functional similarity of compounds in heterogeneous networks, XiaoErFuPi granules as an example. Pharmacol Res. 2020;160:105077. doi: 10.1016/j.phrs.2020.105077 [DOI] [PubMed] [Google Scholar]
- 21.Zhang JL, Wang H, Pi HF, et al. Structural analysis and antitussive evaluation of five novel esters of verticinone and bile acids. Steroids. 2009;74:424–434. doi: 10.1016/j.steroids.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Du Q, Li H, et al. The isosteroid alkaloid imperialine from bulbs of fritillaria cirrhosa mitigates pulmonary functional and structural impairment and suppresses inflammatory response in a COPD-like rat model. Mediators Inflammation. 2016;2016:4192483. doi: 10.1155/2016/4192483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai M, Er-Bu A, Wu Y, et al. Total alkaloids of bulbus of Fritillaria cirrhosa alleviate bleomycin-induced inflammation and pulmonary fibrosis in rats by inhibiting TGF-β and NF-κB signaling pathway. Food Nut Res. 2023:67. doi: 10.29219/fnr.v67.10292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li S, Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chinese J Nat Med. 2013;11:110–120. doi: 10.1016/s1875-5364(13)60037-0 [DOI] [PubMed] [Google Scholar]
- 25.Zhao P, Li J, Yang L, et al. Integration of transcriptomics, proteomics, metabolomics and systems pharmacology data to reveal the therapeutic mechanism underlying Chinese herbal Bufei Yishen formula for the treatment of chronic obstructive pulmonary disease. Mol Med Rep. 2018;17:5247–5257. doi: 10.3892/mmr.2018.8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li T, Guo R, Zong Q, et al. Application of molecular docking in elaborating molecular mechanisms and interactions of supramolecular cyclodextrin. Carbohydr Polym. 2022;276:118644. doi: 10.1016/j.carbpol.2021.118644 [DOI] [PubMed] [Google Scholar]
- 27.Hamza S, Abid A, Khanum A, et al. 3D-QSAR, docking and molecular dynamics simulations of novel Pyrazolo-pyridazinone derivatives as covalent inhibitors of FGFR1: a scientific approach for possible anticancer agents. J Biomol Struct Dyn. 2023:1–15. doi: 10.1080/07391102.2023.2212306 [DOI] [PubMed] [Google Scholar]
- 28.Raza A, Chohan TA, Sarfraz M, et al. Molecular modeling of pyrrolo-pyrimidine based analogs as potential FGFR1 inhibitors: a scientific approach for therapeutic drugs. J Biomol Struct Dyn. 2023;41:14358–14371. doi: 10.1080/07391102.2023.2187638 [DOI] [PubMed] [Google Scholar]
- 29.Ru J, Li P, Wang J, et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminf. 2014;6:13. doi: 10.1186/1758-2946-6-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu X, Zhang W, Huang C, et al. A novel chemometric method for the prediction of human oral bioavailability. Int J Mol Sci. 2012;13:6964–6982. doi: 10.3390/ijms13066964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebhan M, Chalifa-Caspi V, Prilusky J, et al. GeneCards: integrating information about genes, proteins and diseases. Trends Genet. 1997;13:163. doi: 10.1016/s0168-9525(97)01103-7 [DOI] [PubMed] [Google Scholar]
- 32.von Mering C, Huynen M, Jaeggi D, et al. STRING: a database of predicted functional associations between proteins. Nucleic Acids Res. 2003;(31):258–261. doi: 10.1093/nar/gkg034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dennis G, Sherman BT, Hosack DA, et al. DAVID: database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:3. [PubMed] [Google Scholar]
- 34.Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berman HM, Westbrook J, Feng Z, et al. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–461. doi: 10.1002/jcc.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang M, Tang J, Shan H, et al. p66Shc mediates mitochondrial dysfunction dependent on PKC activation in airway epithelial cells induced by cigarette smoke. Oxid Med Cell Longev. 2018;2018:5837123. doi: 10.1155/2018/5837123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen T, Zhong F, Yao C, et al. A systematic review on traditional uses, sources, phytochemistry, pharmacology, pharmacokinetics, and toxicity of fritillariae cirrhosae bulbus. Evid Based Complement Alternat Med. 2020;2020:1536534. doi: 10.1155/2020/1536534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D, Zhu J, Wang S, et al. Antitussive, expectorant and anti-inflammatory alkaloids from bulbus fritillariae cirrhosae. Fitoterapia. 2011;82:1290–1294. doi: 10.1016/j.fitote.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Yang T, Ming TW, et al. Isosteroid alkaloids from Fritillaria cirrhosa bulbus as inhibitors of cigarette smoke-induced oxidative stress. Fitoterapia. 2020;140:104434. doi: 10.1016/j.fitote.2019.104434 [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Li D, Zhang G, et al. Pharmacokinetics, tissue distribution and excretion of peimisine in rats assessed by liquid chromatography-tandem mass spectrometry. Arch Pharmacal Res. 2015;38:1138–1146. doi: 10.1007/s12272-014-0434-1 [DOI] [PubMed] [Google Scholar]
- 42.Jin X, Gao X, Lan M, et al. Study the mechanism of peimisine derivatives on NF-κB inflammation pathway on mice with acute lung injury induced by lipopolysaccharide. Chem Biol Drug Des. 2022;99:717–726. doi: 10.1111/cbdd.14013 [DOI] [PubMed] [Google Scholar]
- 43.Simpson JL, Phipps S, Gibson PG. Inflammatory mechanisms and treatment of obstructive airway diseases with neutrophilic bronchitis. Pharmacol Ther. 2009;124:86–95. doi: 10.1016/j.pharmthera.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 44.Chung S, Vu S, Filosto S, et al. Src regulates cigarette smoke-induced ceramide generation via neutral sphingomyelinase 2 in the airway epithelium. Am J Respir Cell Mol Biol. 2015;52:738–748. doi: 10.1165/rcmb.2014-0122OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen AO, Jensen CS, Arredouani MS, et al. Variants of the ADRB2 gene in COPD: systematic review and meta-analyses of disease risk and treatment response. Copd. 2017;14:451–460. doi: 10.1080/15412555.2017.1320370 [DOI] [PubMed] [Google Scholar]
- 46.Chi Y, Di Q, Han G, et al. Mir-29b mediates the regulation of Nrf2 on airway epithelial remodeling and Th1/Th2 differentiation in COPD rats. Saudi J Biol Sci. 2019;26:1915–1921. doi: 10.1016/j.sjbs.2019.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ning W, Dong Y, Sun J, et al. Cigarette smoke stimulates matrix metalloproteinase-2 activity via EGR-1 in human lung fibroblasts. Am. J. Respir. Cell Mol. Biol. 2007;36:480–490. doi: 10.1165/rcmb.2006-0106OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben Nasr H, Bchir S, Ben Anes A, et al. The −786 T/C polymorphism of NOS3 gene is a susceptibility marker of COPD among Tunisians that correlates with nitric oxide levels and airflow obstruction. Cytokine. 2017;93:66–73. doi: 10.1016/j.cyto.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 49.Barreiro E, Gea J, Corominas JM, et al. Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2003;29:771–778. doi: 10.1165/rcmb.2003-0138OC [DOI] [PubMed] [Google Scholar]
- 50.Ito H, Matsushita S, Ishikawa S, et al. Significant correlation between endothelial nitric oxide synthase (eNOS) expression and alveolar repair in elastase-induced rat pulmonary emphysema. Surg Today. 2013;43:293–299. doi: 10.1007/s00595-012-0293-7 [DOI] [PubMed] [Google Scholar]
- 51.Csoma B, Bikov A, Nagy L, et al. Dysregulation of the endothelial nitric oxide pathway is associated with airway inflammation in COPD. Respir Res. 2019;20:156. doi: 10.1186/s12931-019-1133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Wei S, Niu S, et al. Network pharmacology prediction and molecular docking-based strategy to explore the potential mechanism of Huanglian Jiedu Decoction against sepsis. Comput Biol Med. 2022;144:105389. doi: 10.1016/j.compbiomed.2022.105389 [DOI] [PubMed] [Google Scholar]
- 53.Jiao X, Jin X, Ma Y, et al. A comprehensive application: molecular docking and network pharmacology for the prediction of bioactive constituents and elucidation of mechanisms of action in component-based Chinese medicine. Comput Biol Chem. 2021;90:107402. doi: 10.1016/j.compbiolchem.2020.107402 [DOI] [PubMed] [Google Scholar]
- 54.Nie YC, Wu H, Li PB, et al. Characteristic comparison of three rat models induced by cigarette smoke or combined with LPS: to establish a suitable model for study of airway mucus hypersecretion in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2012;25:349–356. doi: 10.1016/j.pupt.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 55.Lixuan Z, Jingcheng D, Wenqin Y, et al. Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models. Pulm Pharmacol Ther. 2010;23:411–419. doi: 10.1016/j.pupt.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 56.Lin H, Wang C, Yu H, et al. Protective effect of total Saponins from American ginseng against cigarette smoke-induced COPD in mice based on integrated metabolomics and network pharmacology. Biomed Pharmacother. 2022;149:112823. doi: 10.1016/j.biopha.2022.112823 [DOI] [PubMed] [Google Scholar]
- 57.Yuan Q, Jiang YW, Fang QH. Improving effect of Sivelestat on lipopolysaccharide-induced lung injury in rats. APMIS. 2014;122:810–817. doi: 10.1111/apm.12222 [DOI] [PubMed] [Google Scholar]
- 58.Barnes PJ. Chronic obstructive pulmonary disease * 12: new treatments for COPD. Thorax. 2003;58:803–808. doi: 10.1136/thorax.58.9.803 [DOI] [PMC free article] [PubMed] [Google Scholar]