Abstract
Background
The Gambia, located in West Africa, is one of 7 country sites conducting the Enterics for Global Health (EFGH) Shigella Surveillance Study to establish incidence and consequence of Shigella-associated medically attended diarrhea among children 6–35 months old.
Methods
Here we describe the study site and research experience, sociodemographic characteristics of the study catchment area, facilities of recruitment for diarrhea case surveillance, and known care-seeking behavior for diarrheal illness. We also describe The Gambia's healthcare system and financing, current vaccine schedule and Shigella vaccine adaptation, local diarrhea management guidelines and challenges, and antibiotic resistance patterns in the region.
Conclusions
The EFGH study in The Gambia will contribute to the multisite network of Shigella surveillance study and prepare the site for future vaccine trials. In addition, the data produced will inform policy makers about prevention strategies and upcoming Shigella vaccine studies among children in this setting.
Keywords: diarrhea, Gambia, healthcare, prevalence, Shigella
This article describes The Gambia EFGH study site, research capacities, study catchment area, and recruitment facilities. This paper also highlights sociodemographic status, healthcare-seeking behaviors for diarrhea, diarrheal case management, antibiotic resistance patterns, and new vaccine adaptation in children.
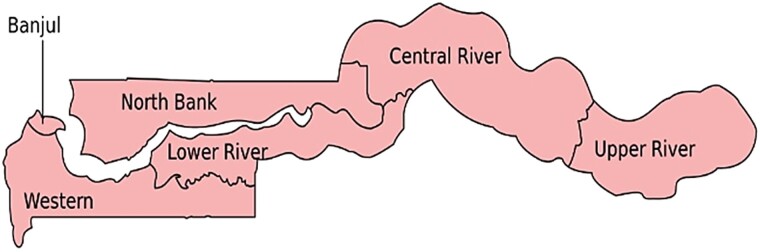
The Gambia is a small country in West Africa affectionately known as “the smiling coast of Africa,” covering approximately 11 000 km2, with 6 administrative regions (Figure 1) and a population of approximately 2.5 million people [1]. The median age is 17 years [2], life expectancy at birth is 62 years (World Bank, 2021, https://data.worldbank.org/country/GM), and infant mortality rate is 34.7 per 1000 live births [1]. The mean income per capita is US$771 per annum, less than half the sub-Saharan Africa average of US$1632, and 17.2% of the population live in poverty [1].
Figure 1.
Administrative regions in The Gambia.
MEDICAL RESEARCH COUNCIL UNIT THE GAMBIA AT THE LONDON SCHOOL OF HYGIENE AND TROPICAL MEDICINE
The Medical Research Council Unit The Gambia (MRCG), established in 1947 by the Medical Research Council United Kingdom (MRC UK), is one of the largest scientific research centers in sub-Saharan Africa. The MRCG became part of the London School of Hygiene and Tropical Medicine (LSHTM) in February 2018, according to the general strategy by the MRC UK to transfer its units to universities. The MRCG maintains its scientific independence within the LSHTM, and MRCG is considered an African-based academic research institution.
The MRCG at LSHTM has a large research portfolio of successful epidemiologic studies and clinical trials organized under 3 themes: disease control and elimination; vaccines and immunity; and nutrition and planetary health. These themes have been targeted to meet the health needs of low-income countries, reinforce regional and international collaborations, and address current priorities of the Sustainable Development Goals. MRCG at LSHTM has established strategic partnerships within the region through an alliance called the West African Global Health Alliance.
Over the past 75 years, MRCG at LSHTM has developed and maintained programs, infrastructure, and resources that support a wide spectrum of research activities, from basic science to the evaluation of interventions for the control of diseases of public health importance in sub-Saharan Africa. The unit's investigator-led research portfolio is strengthened by the availability of excellent laboratory facilities and easy access to the field, with a population willing to participate in health research. The unit provides excellent clinical services within its Clinical Services Department, maintains rigorous ethical procedures, and delivers Good Clinical Practice (GCP)–compliant clinical trials.
MRCG participated into 2 large diarrhea burden and etiology studies carried out between 2008 and 2018, namely, the Global Enterics Multicenter Study (GEMS) [3, 4] and the Vaccine Impact on Diarrhea in Africa (VIDA) study [5, 6]. Other research achievements include a large trial on the efficacy of Haemophilus influenzae type B vaccine [7] and the subsequent near-elimination of the disease, pioneering studies on the impact of insecticide-treated bed nets against malaria that supported their worldwide use [8, 9], studies demonstrating the impact of conjugate pneumococcal vaccines on pneumonia [10, 11] and child mortality, and the reduction of Hepatitis B carriage in The Gambia following vaccination [12]. A randomized controlled trial of enterotoxigenic Escherichia coli vaccine among children aged 6–18 months is ongoing to assess safety and efficacy on E coli–associated diarrhea, and has successfully completed enrollment of 4936 study participants [13].
MRCG LABORATORY FACILITIES
The MRCG laboratory facility serves as a center of excellence for training in laboratory science within the West African region. Activities span from basic research in immunology, microbiology, virology, and molecular biology to large epidemiological studies and intervention trials as well as routine clinical diagnostics. Microbiology, clinical services, molecular biology, genomic, serology, and immunology are located at the main MRCG at LSHTM campus in Fajara. The Unit also supports 2 laboratories in its field stations in rural Gambia, in Basse and Keneba. Basse field station was established in 1982; has capacity for microbiology, molecular biology, and immunology; and supports all MRCG at LSHTM research activities in this region. The Basse laboratory was responsible for conventional pathogen detection, including Shigella culture, serotyping, and antimicrobial resistance, testing nearly 8700 stool samples collected from diarrhea cases and their matched controls in the GEMS and VIDA studies [3–5, 14].
EFGH STUDY CATCHMENT AREA
The EFGH study site in The Gambia is based in Upper River Region (URR), in eastern Gambia (Figure 1), with a population of 230 000, and 1 major town called Basse. In URR, the temperature varies between 18°C and 44°C. There are distinctive dry (November–May) and wet (June–October) seasons, and the mean annual rainfall is 876 mm. The economy is primarily dependent on small-scale agriculture [15], and the main ethnic groups are the Mandinka, Fula, and Sarahulleh.
Malaria is endemic in URR, with the incidence of clinical malaria estimated between 0.08 and 1.89 per person-year during transmission season (July–December) [16]. Human immunodeficiency virus prevalence is low (<2%) [17]. Immunization coverage of all 3 doses of diphtheria, pertussis, and tetanus (DPT) was 81.7% [18]. The major drinking water sources in this region are boreholes, deep tube wells, and open wells, and some villages have a public water supply system piped to a communal tap that is shared by multiple households. Pit latrines are the most common type of toilet [19]. Only 10.2% of primary caregivers of children <5 years of age have attended primary and postprimary school [20], and the main mode of transport in rural villages are donkey carts and bicycles [15]. However, the use of commercial vehicles and motorbikes is increasing.
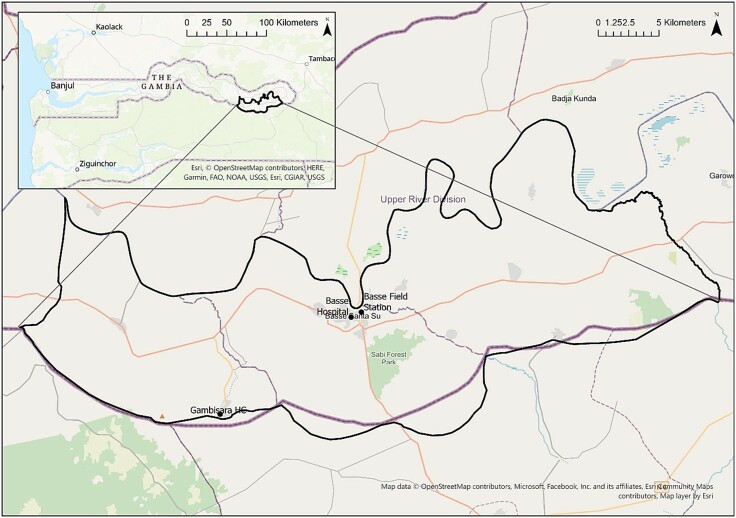
The EFGH catchment area constitutes a subset of the population of the Basse Health and Demographic Surveillance System (BHDSS), which was established in 2007 and covers the south bank of the URR (Figure 2). The BHDSS includes 224 villages across 2 districts, with a population of 213 587, of whom 30 691 (14.4%) are children <5 years old (December 2022 MRCG Census data). The population is enumerated through household visits every 4 months, with records of births, deaths, vaccination, marriage, and migration in and out of the surveillance area. The Basse Field Station, which houses the microbiology laboratory and field office, is within the BHDSS area.
Figure 2.
Enterics for Global Health Study catchment area map, The Gambia site.
The EFGH catchment consists of 134 villages with a population of approximately 138 000. Within the EFGH catchment area there are 6 public health facilities, 1 district hospital (Basse), and 5 health centers/posts (Sabi, Demba Kunda, Gambisara, Sotuma, and Bakadagy). EFGH is recruiting from 2 of these facilities, Basse District Hospital and Gambisara Health Center, which were chosen because of the high enrollment in previous diarrhea studies, the large proportion of the total population served (65%), and proximity to MRCG Basse Field Station. Basse District Hospital is a referral hospital with an outpatient department, a general adult inpatient ward, pediatric ward, labor ward, minor theater, and a small laboratory. The hospital serves about 196 000 people in URR. Gambisara is a small rural health center that served as a GEMS and VIDA health facility and refers severe cases to Basse District Hospital. Both health facilities are open 24 hours a day and provide emergency services, adult medicine, and reproductive and child health services including free healthcare for children <5 years old.
IMPLEMENTATION OF THE EFGH STUDY WITHIN THE GOVERNMENT HEALTH FACILITIES
The EFGH study staff integrate with the existing health facility staff to ensure acutely ill children are managed in accordance with The Gambian Ministry of Health guidelines [21] and the World Health Organization (WHO) Integrated Management of Childhood Illness guidelines [22]. The Ministry of Health guidelines are adapted from WHO guidelines. These guidelines are summarized in Table 1. In situations where guideline-recommended treatments are out of stock, the EFGH study supports treatment for study participants.
Table 1.
Summary Table of Treatment Guidelines of Ministry of Health and Social Welfare, Republic of The Gambia
| Dehydration Management | ||
|---|---|---|
| Children without severe acute malnutrition | ||
| Dehydration | Country guidelines (the same as WHO-indicated treatment) | |
| Severe (Plan C—facility) | Start IV fluid immediately (preferably Ringer’s lactate solution, 100 mL/kg). Reassess every 15–30 min. Give ORS (15 mL/kg/h) as soon as the child can drink. Reclassify dehydration after 6 h in infant and 3 h in child and continue with A, B, C plan. |
|
| Some (Plan B—facility) | Give recommended ORS in clinic over 4 hours. Reclassify dehydration after 4 h and continue with A, B, C plan. |
|
| None (Plan A—home) | Increase food and fluid intake to prevent dehydration | |
| Children with severe acute malnutrition and no shock | ||
| Dehydration | Country guidelines (same as WHO-indicated treatment) | |
| Severe (Plan C—facility) | Give ReSoMal (or half-strength standard low ORS with added potassium and glucose) 5 mL/kg every 30 min for the first 2 h, and 5–10 mL/kg/h for the next 4–10 h on alternate hours with F75. If rehydration still required at 10 h, give starter F75 instead of ReSoMal, at the same times. | |
| Some (Plan B—facility) | Same as above | |
| None (Plan A—home) | Not applicable (because child admitted in health facility) | |
| Therapeutic zinc | ||
| Population | Country guidelines (the same as WHO-indicated treatment) | |
| All children | Zinc supplementation for 10–14 d (age ≤6 mo: 10 mg/d; age >6 mo: 20 mg/d | |
| Antibiotics | ||
| Population | Country guidelines (the same as WHO-indicated treatment) | |
| Dysentery | Ciprofloxacin (15 mg/kg) twice daily for 3 d OR based on local sensitivity. IV/IM ceftriaxone at 50–80 mg/kg/d for 3 d (if child is severely ill or as second-line treatment) | |
| Suspected cholera (age ≥2 y + severe dehydration + cholera present in area) | Erythromycin (12 mg/kg) 4 times a day for 3 d Ciprofloxacin 10–20 mg/kg twice a day for 5 d Cotrimoxazole: 4 mg/kg trimethoprim and 20 mg/kg sulfamethoxazole twice a day |
|
HEALTHCARE SYSTEMS AND FINANCING
The Gambia operates a 3-tier health system, namely primary, secondary, and tertiary healthcare levels. The primary level serves as the first point of contact for accessing healthcare in the community and provides health promotion, preventive, curative, and rehabilitative services. Nurses (community health, state-enrolled, and state-registered nurses) and public health officers are the main care providers in the primary level in community clinics, health posts, and minor health centers. The secondary healthcare level is composed of major health centers and regional hospitals, which receive referrals from the primary level. The tertiary healthcare level serves as the referral level for secondary facilities and provides specialized consultative healthcare for inpatients, advanced medical investigation, and patient management at teaching, general, and specialized hospitals [23]. Although there is no functional health insurance policy in place for the public healthcare system, healthcare service is free for children <5 years old and pregnant women (antenatal, childbirth, and postpartum period).
CHALLENGES AFFECTING IMPLEMENTATION OF CLINICAL GUIDELINES AT HEALTH FACILITIES
Although The Gambia Ministry of Health has adopted the WHO diarrhea management guidelines, some practical implementation challenges are encountered. Health staff are not consistent in following the national and WHO guidelines, and antibiotics are frequently prescribed without the required indication [24]. The scarcity of recommended antibiotics, zinc supplements, and other drugs also hinders following recommended guidelines, and the available antibiotics are prescribed instead, even if not ideal. Low literacy and minimal formal health knowledge among caretakers of sick children can result in home instructions not being well-followed either [15].
VACCINE SCHEDULE AND VACCINE ADAPTATION
The Gambian Expanded Programme on Immunization schedule for children under 5 (Table 2) currently follows the WHO recommendations and guidelines (Table 2). The Gambia has consistently documented complete DPT vaccination coverage >90%, but there has been lower coverage among poor and marginalized groups [25]. The government of The Gambia is supported by multilateral partners and in-country nongovernmental organizations to ensure that all children receive their basic vaccinations.
Table 2.
Gambian Expanded Programme on Immunization Vaccination Schedule
| Schedule | Vaccination | |||
|---|---|---|---|---|
| At birth or soon after | BCG injection | Oral polio 0 | Hepatitis B | … |
| 2 mo | Pentavalenta 1 | Oral polio 1 | PCV 1 | Rota 1 |
| 3 mo | Pentavalent 2 | Oral polio 2 | PCV 2 | Rota 2 |
| 4 mo | Pentavalent 3 | Oral polio 3 | PCV 3 | … |
| 9 mo | Measles/rubella | Yellow fever | Oral polio 4 | … |
| 12 mo | Meningitis A | DPT booster | … | … |
| 18 mo | Measles/rubella | Oral polio 5 | … | … |
Abbreviations: DPT, diphtheria, pertussis, and tetanus; PCV, pneumococcal conjugate vaccine; Rota, rotavirus.
aPentavalent, Diphtheria, pertussis, tetanus, hepatitis B, and Hemophilus influenzae b.
SHIGELLA INCIDENCE, PREVALENCE, AND ANTIMICROBIAL RESISTANCE
In The Gambia, Shigella was the second leading cause of diarrhea identified in the earlier GEMS study, was one of the 5 most common pathogens responsible for diarrhea, and had highest incidence in older children in The Gambia compared to other GEMS sites in Africa and Asia, except Bangladesh [3, 4, 26]. In both GEMS and VIDA studies, the positivity of shigellosis was also higher in The Gambia compared to Mali and Kenya (Table 3, Table 4) [3, 4, 14, 26], and 30.8% of diarrhea cases were attributable to Shigella infection in The Gambia (Table 5) compared to 9.3% in Mali and 18.7% in Kenya [14]. In The Gambia, the incidence of Shigella is greatest during the rainy season, between June and September [14]. In the GEMS and VIDA studies, 10.6% and 12.9%, respectively, of moderate-to-severe diarrhea (MSD) cases were positive for Shigella by stool culture (Table 5). Shigella flexneri was the most common serogroup, followed by Shigella boydii, Shigella sonnei, and Shigella dysenteriae in both GEMS and VIDA studies (Table 6) [14].
Table 3.
Attributable Fraction and Attributable Incidence of Shigella by Age Group in The Gambia Site for the Global Enterics Multicenter Study and Vaccine Impact on Diarrhea in Africa Study Based on Detection by Quantitative Polymerase Chain Reaction (TaqMan)
| Age Group | VIDA (2015–2018) | GEMS (2008–2012) | ||
|---|---|---|---|---|
| AF (95% CI) | AI (95% CI) | AF (95% CI) | AI (95% CI) | |
| 0–11 mo | 14.02 (9.3–17.1) | 3.37 (2.3–4.1) | 7.5 (3.9–12.9) | 1.0 (.5–1.8) |
| 12–23 mo | 39.96 (36.5–46.2 | 8.83 (8.1–10.2) | 32.7 (27.0–42.0) | 6.5 (5.2–8.3) |
| 24–59 mo | 34.73 (28.9–40.2) | 2.1 (1.7–2.4) | 25.9 (18.5–36.1) | 0.7 (.5–1.1) |
Table 4.
Proportion of Vaccine Impact on Diarrhea in Africa Study Participants From The Gambia in Each Age and Diarrhea Group With Shigella Detection Based on Detection by TaqMan Array Card (Cycle Threshold <35)
| The Gambia | VIDA (2015–2018) | |||
|---|---|---|---|---|
| 0–5 mo | 6–11 mo | 12–23 mo | 24–59 mo | |
| MSD | ||||
| Dysentery | 6/20 (30.0%) | 48/98 (49.0%) | 170/205 (82.9%) | 125/160 (78.1%) |
| Nondysentery | 6/66 (9.1%) | 79/355 (22.3%) | 181/414 (43.7%) | 155/360 (43.1%) |
| Controls | 4/101 (4.0%) | 78/595 (13.1%) | 235/748 (31.4%) | 190/694 (27.4%) |
Source: Kasumba et al [14].
Proportion of Shigella/enteroinvasive Escherichia coli positive, where positive is defined as a cycle threshold <35.
Abbreviations: MSD, moderate-to-severe diarrhea that was medically attended; VIDA, Vaccine Impact on Diarrhea in Africa.
Table 5.
Positivity of Shigella in Cases and Controls by Both Culture and Quantitative Polymerase Chain Reaction in the Vaccine Impact on Diarrhea in Africa Study, The Gambia (2015–2018)
| Test | Cases, % | Controls, % |
|---|---|---|
| Stool culture | 12.9 | 2.2 |
| Positive (Ct <35) | 45.9 | 30.2 |
| Attributable to Shigella | 30.8 | |
Source: Kasumba et al [14].
Abbreviation: Ct, cycle threshold.
Table 6.
Distribution of Shigella Serogroup Among Shigella Culture-Positive Moderate-to-Severe Diarrhea Cases in the Global Enterics Multicenter Study and Vaccine Impact on Diarrhea in Africa Study, The Gambia
| Shigella Serogroup | VIDA Study (2015–2018) | GEMS Study (2008–2012) |
|---|---|---|
| Cases (n = 207), No. (%) | Cases (n = 116), No. (%) | |
| S flexneri | 146 (70.5) | 80 (69.0) |
| S boydii | 30 (14.5) | 7 (6.0) |
| S sonnei | 24 (11.6) | 24 (20.7) |
| S dysenteriae | 6 (2.9) | 5 (4.3) |
Source: Kasumba et al [14].
Abbreviations: GEMS, Global Enterics Multicenter Study; VIDA, Vaccine Impact on Diarrhea in Africa.
Shigella-attributable diarrhea appears to be increasing. Between 2008 and 2012, incidence in the GEMS study was estimated at 1.0 per 100 child-years in 0- to 11-month-olds, and 6.5 per 100 child-years in 12- to 23-month-olds [26]. Between 2015 and 2018, the incidence of Shigella-attributable MSD in the VIDA study was estimated at 3.6 (95% confidence interval [CI], 2.6–4.8) per 100-child-years in 0- to 11-month-olds and 9.8 (95% CI, 9.4–12.0) per 100 child-years in 12- to 23-month-olds (Kotloff et al, unpublished data).
In The Gambia, Shigella isolates were resistant to commonly used antibiotics. Almost all (95.0%) Shigella isolates were resistant to cotrimoxazole and half (48.4%) of them were resistant to ampicillin [14]. Resistance to the guideline-indicated first-line (ciprofloxacin), second-line (azithromycin), and third-line (ceftriaxone) antibiotics for Shigella was none or rare in 0.0%, 0.3%, and 0.3% of isolates, respectively [14]; these drugs are not commonly available in health facilities. The most commonly used antibiotics in The Gambia for MSD cases were ciprofloxacin (47.0%), metronidazole (29.3%), and co-trimoxazole (18.0%), and among dysenteric cases were ciprofloxacin (85.3%) and metronidazole (6.3%) [24].
HEALTHCARE-SEEKING FOR DIARRHEA
Serial healthcare utilization surveys were conducted both in GEMS (2008–2012) and VIDA (2015–2018) studies in The Gambia. In GEMS, caretakers reported that 23.3% of the children experienced diarrhea within the previous 2 weeks. About 81%–85% of primary caregivers sought care outside the home for reported diarrhea in children <5 years old and 15% did not seek any care [15, 20]. Approximately half of the children with caregiver-reported diarrhea sought care in health centers [15, 20], while others sought care with private licensed practitioners (14.0%) or unlicensed practitioners (13.9%), bought remedies at the market (9.1%) or over the counter at a pharmacy (8.3%), or sought out traditional healers (4.8%) [15]. Reasons for not seeking formal healthcare included high cost of treatment, travel cost, lack of transport, distance to the health facility, more time needed to travel to health facility, and insufficient knowledge of danger signs among primary caretakers [15, 20].
TRAINING AND CAPACITY BUILDING
The MRCG has diverse groups of researchers and staff from sub-Saharan Africa, including The Gambia and other West African countries, and from abroad (eg, Europe, Australia). MRCG at LSHTM maintains a strict portfolio of equity during employment and equal opportunities for all employees. Team leadership promotes a culture that values and respects equality, diversity, and inclusiveness where everyone feels comfortable sharing their perspective and ideas. Team members have equal access to the same resources and opportunities for career and professional development, including mentorship programs, networking opportunities, and funding for research projects.
The EFGH Gambia team comprises many young early career researchers, and actively promotes their independent research and career development. The EFGH junior investigators have participated in short courses offered by the University of Washington. Several junior investigators have also participated in EFGH supplement manuscript writing and submitted applications for the Rising Star Awards, a small fund which allows for nested studies within EFGH led by junior investigators from EFGH country sites. The EFGH study also facilitates distance learning for junior investigators to facilitate their career development.
ETHICAL REVIEW
The EFGH study was approved by The Gambia government/Medical Research Council Unit, The Gambia Joint Ethics Committee, and the Institutional Review Board of the University of Maryland, Baltimore. Informed written consent was obtained from parents or guardians of study participants.
CONCLUSIONS
The MRCG at LSHTM has excellent laboratory facilities at Fajara, Basse, and Keneba field stations, rigorous ethical procedures, and the ability to deliver GCP-compliant clinical trials. The MRCG at LSHTM has access to well-defined populations that are highly supportive of and willing to participate in its studies. The unit has excellent experience and willingness to participate in multicountry studies and to work in collaboration with other international research institutes. In the past, MRCG participated in 2 of the largest diarrhea burden and diarrhea etiology studies, GEMS and VIDA, which have provided an excellent data and infrastructure foundation, in addition to important historical data, for the current EFGH study. The EFGH Gambia site has a strong infrastructure, resources, and excellent trained staff to conduct diarrheal disease research. The Shigella burden is high and the EFGH study site can contribute substantially to future Shigella prevention studies and vaccine trials.
Contributor Information
Bakary Conteh, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Henry Badji, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Abdoulie F Jallow, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Mehrab Karim, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Alhagie Manneh, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Belali Keita, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Golam Sarwar, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Bubacarr E Ceesay, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Sheikh Jarju, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Abdoulie M J Jabang, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Ebrima Baldeh, Regional Health Directorate Upper River Region, Ministry of Health and Social Welfare, Basse, The Gambia.
Usman N Ikumapayi, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Ousman Secka, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Martin Antonio, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Anna Roca, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Umberto D’Alessandro, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Karen L Kotloff, Center for Vaccine Development and Global Health, University of Maryland School of Medicine, Baltimore, Maryland, USA; Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland, USA.
M Jahangir Hossain, Medical Research Council Unit The Gambia at the London School of Hygiene and Tropical Medicine, Fajara, The Gambia.
Notes
Acknowledgments. We express our deep gratitude to the families of the study participants in the EFGH studies; the clinical, data, field, and laboratory staff for their exceptional hard work and dedication; and the physicians, administration, and health officials at EFGH study sites who generously provided facilities and support for the conduct of the study. We are grateful to the EFGH Coordination team for their continuous extensive support to the study team whenever it is needed, including training and coordination of activities. Our special thanks to the GEMS and VIDA teams.
Financial support. This research was supported by the Bill & Melinda Gates Foundation (grant numbers INV-016650, INV-031791, INV-028721, INV-041730) and by the UK Research and Innovation Medical Research Council (program number MC_UU_00031/1—Disease Control and Elimination).
Supplement sponsorship. This article appears as part of the supplement “Enterics for Global Health (EFGH) Shigella Surveillance Study-Rationale and Methods,” sponsored by the Bill & Melinda Gates Foundation.
References
- 1.The World Bank. The World Bank in The Gambia. 2023. Available at: https://www.worldbank.org/en/country/gambia. Accessed on 19 May 2023.
- 2.World Economics. Gambia's economics. Available at: https://www.worldeconomics.com/Country-Data/Gambia.aspx. Accessed 29 June 2023.
- 3. Kotloff KL, Nasrin D, Blackwelder WC, et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: a 12-month case-control study as a follow-on to the Global Enteric Multicenter Study (GEMS). Lancet Glob Health 2019; 7:e568–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 5. Powell H, Liang Y, Neuzil KM, et al. A description of the statistical methods for the Vaccine Impact on Diarrhea in Africa (VIDA) study. Clin Infect Dis 2023; 76:S5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hossain MJ, Powell H, Sow SO, et al. Clinical and epidemiologic features of Cryptosporidium-associated diarrheal disease among young children living in sub-Saharan Africa: the Vaccine Impact on Diarrhea in Africa (VIDA) study. Clin Infect Dis 2023; 76:S97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mulholland K, Hilton S, Adegbola R, et al. Randomised trial of Haemophilus influenzae type-b tetanus protein conjugate vaccine [corrected] for prevention of pneumonia and meningitis in Gambian infants. Lancet 1997; 349:1191–7. [DOI] [PubMed] [Google Scholar]
- 8. Alonso PL, Lindsay SW, Armstrong Schellenberg JR, et al. A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. 6. The impact of the interventions on mortality and morbidity from malaria. Trans R Soc Trop Med Hyg 1993; 87:37–44. [DOI] [PubMed] [Google Scholar]
- 9. D’Alessandro U, Olaleye B, Langerock P, et al. The Gambian national impregnated bed net programme: evaluation of effectiveness by means of case-control studies. Trans R Soc Trop Med Hyg 1997; 91:638–42. [DOI] [PubMed] [Google Scholar]
- 10. Cutts FT, Zaman SM, Enwere G, et al. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 2005; 365:1139–46. [DOI] [PubMed] [Google Scholar]
- 11. Mackenzie GA, Hill PC, Sahito SM, et al. Impact of the introduction of pneumococcal conjugate vaccination on pneumonia in The Gambia: population-based surveillance and case-control studies. Lancet Infect Dis 2017; 17:965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fortuin M, Chotard J, Jack AD, et al. Efficacy of hepatitis B vaccine in the Gambian expanded programme on immunisation. Lancet 1993; 341:1129–32. [DOI] [PubMed] [Google Scholar]
- 13.Pan African Clinical Trial Registry. PACTR202010819218562. https://pactr.samrc.ac. Accessed 22 June 2023.
- 14. Kasumba IN, Badji H, Powell H, et al. Shigella in Africa: new insights from the Vaccine Impact on Diarrhea in Africa (VIDA) study. Clin Infect Dis 2023; 76:S66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nasrin D, Wu Y, Blackwelder WC, et al. Health care seeking for childhood diarrhea in developing countries: evidence from seven sites in Africa and Asia. Am J Trop Med Hyg 2013; 89:3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mwesigwa J, Achan J, Di Tanna GL, et al. Residual malaria transmission dynamics varies across The Gambia despite high coverage of control interventions. PLoS One 2017; 12:e0187059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.. Republic of The Gambia. The Gambia Global AIDS response progress report. 2015. Available at: http://www.unaids.org/sites/default/files/country/documents/GMB_narrative_report_2015.pdf. Accessed 1 May 2017.
- 18. Scott S, Odutola A, Mackenzie G, et al. Coverage and timing of children's vaccination: an evaluation of the expanded programme on immunisation in The Gambia. PLoS One 2014; 9:e107280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hossain MJ, Saha D, Antonio M, et al. Cryptosporidium infection in rural Gambian children: epidemiology and risk factors. PLoS Negl Trop Dis 2019; 13:e0007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saha D, Akinsola A, Sharples K, et al. Health care utilization and attitudes survey: understanding diarrheal disease in rural Gambia. Am J Trop Med Hyg 2013; 89:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ministry of Health and Social Welfare, Republic of The Gambia; World Health Organization; United Nations Children’s Fund. Integrated management of neonatal and childhood illness. Ministry of Health and Social Welfare, Republic of The Gambia, WHO and UNICEF; 2021.
- 22.World Health Organization. Integrated Management of Childhood Illness (IMCI) chart booklet. 2014. Available at: https://cdn.who.int/media/docs/default-source/mca-documents/child/imci-integrated-management-of-childhood-illness/imci-in-service-training/imci-chart-booklet.pdf?sfvrsn=f63af425_1. Accessed 28 March 2019.
- 23.Ministry of Health, Republic of The Gambia. National health policy 2021–2030. 2022. Available at: https://www.afro.who.int/sites/default/files/2022-07/FINAL-HEALTH-POLICY-2021–2030_18.01.22.pdf. Accessed 28 April 2023.
- 24. Awuor AO, Ogwel B, Powell H, et al. Antibiotic-prescribing practices for management of childhood diarrhea in 3 sub-Saharan African countries: findings from the Vaccine Impact on Diarrhea in Africa (VIDA) study, 2015–2018. Clin Infect Dis 2023; 76:S32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNICEF Gambia. Immunization. Available at: https://www.unicef.org/gambia/immunization. Accessed 2 July 2023.
- 26. Liu J, Platts-Mills JA, Juma J, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 2016; 388:1291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]