Summary
CRISPR/Cas9-based gene-drive systems possess the inherent capacity to spread progressively throughout target populations. Here we describe two self-copying (or active) guide-RNA-only genetic elements, called e-CHACRs and ERACRs. These elements employ Cas9 produced in-trans by a gene-drive either to inactivate the Cas9 transgene (e-CHACRs) or to delete and replace the gene-drive (ERACRs). e-CHACRs can be inserted at various genomic locations and carry two or more gRNAs: the first copying the e-CHACR, and the second mutating and inactivating the Cas9 transgene. Alternatively, ERACRs are inserted at the same genomic location as a gene-drive, carry two gRNAs that cut on either side of the gene-drive to excise it. e-CHACRs efficiently inactivate Cas9, and can drive to completion in cage experiments. Similarly, ERACRs, particularly those carrying a recoded cDNA restoring endogenous gene activity, can drive reliably to fully replace a gene-drive. We compare the strengths of these two systems.
Keywords: Active genetics, gene-drive, MCR, e-CHACR, ERACR, CRISPR, Cas9, drive-neutralizing, Drosophila, modeling, risk management
Graphical Abstract
eToc blurb
Xu et al. describes two genetic systems for neutralizing an active gene-drive that efficiently attenuate drive frequency in both pair crosses and cage population experiments. These neutralization systems either delete the gene-drive from the genome (ERACRs) or inactivate the Cas9 protein (e-CHACRs) to halt the gene-drive.
Introduction
Harnessing natural or synthetic gene-drives to bias inheritance of beneficial traits in populations was proposed over 60 years ago (Curtis, 1968), and variations on efficient “low-threshold” systems, such as homing endonucleases (Chevalier and Stoddard, 2001; Macreadie et al., 1985), have been modeled extensively over the past two decades (Burt, 2003; Deredec et al., 2008; Eckhoff et al., 2017). Recently, CRISPR/Cas9 genome editing tools have enabled development of several highly efficient gene-drive (or active genetic) systems in insects (Gantz and Bier, 2015; Gantz et al., 2015; Hammond et al., 2016; Kyrou et al., 2018; Li et al., 2019), yeast (DiCarlo et al., 2015), and bacteria (Valderrama et al., 2019). A mammalian gRNA-only “split-drive” prototype has also shown significant promise in the mouse (Grunwald et al., 2019).
CRISPR/Cas9-based gene-drives propagate by creating double-stranded DNA breaks at the precise site on the homologous chromosome where they are inserted into the genome. In the germline, the homology directed repair (HDR) pathway copies the gene-drive element into the break on the homologous chromosome, resulting in super-Mendelian transmission of that element to progeny. Mathematical modeling predicts that such efficient gene-drive systems should spread rapidly throughout a population following logistic growth dynamics, even when released at low seeding levels (Burt, 2003; Eckhoff et al., 2017; Gantz and Bier, 2016).
Discussion in the scientific literature, workshops, and the media have raised several potential concerns including scenarios in which a low-threshold system spreads beyond its intended zone of application (Adelman et al., 2017; James et al., 2018; Organisms, 2016; Warmbrod et al., 2020). One mitigation strategy for limiting gene-drive systems to a chosen region is to develop neutralizing genetic systems that eliminate or prevent further dissemination of the gene-drive.
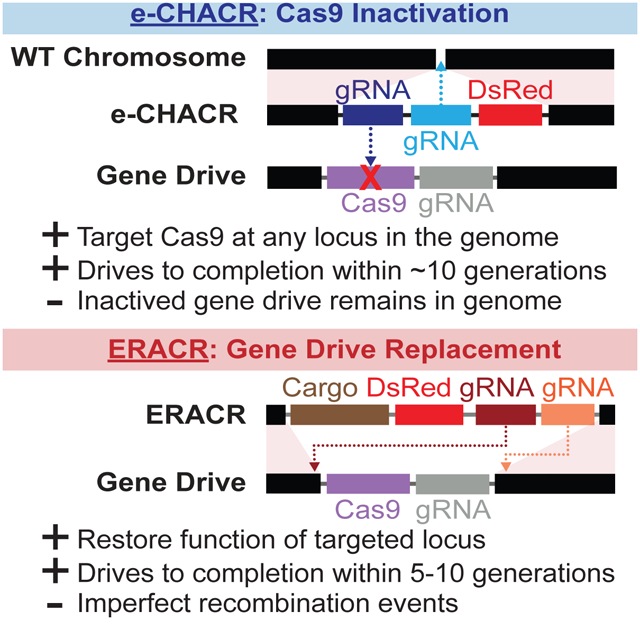
We previously proposed two designs for self-copying (or “active”) neutralizing genetic elements that either inactivate Cas9 carried by a gene-drive (e-CHACR; [erasing] CHACR = Construct Hichhiking on the Autocatalytic Chain Reaction), or delete and replace the gene-drive (ERACR = Element Reversing the Autocatalytic Chain Reaction) (Gantz and Bier, 2016) (Fig. 1A). A key design feature of both elements is that they encode guide RNAs (gRNAs) but not Cas9. e-CHACRs can be inserted into the genome at any desired location and encode two or more gRNAs. One gRNA cuts at the genomic site of e-CHACR insertion, enabling self-copying in the presence of a transacting source of Cas9. The additional gRNA(s) target cleavage and inactivation of the Cas9 transgene component of a gene-drive element. ERACRs are inserted at the same genomic site as a gene-drive and encode two gRNAs that combine with Cas9 produced by the drive, to cut on either side of the drive element to delete and replace it.
Figure 1: Gene-drive and neutralizing drive elements.
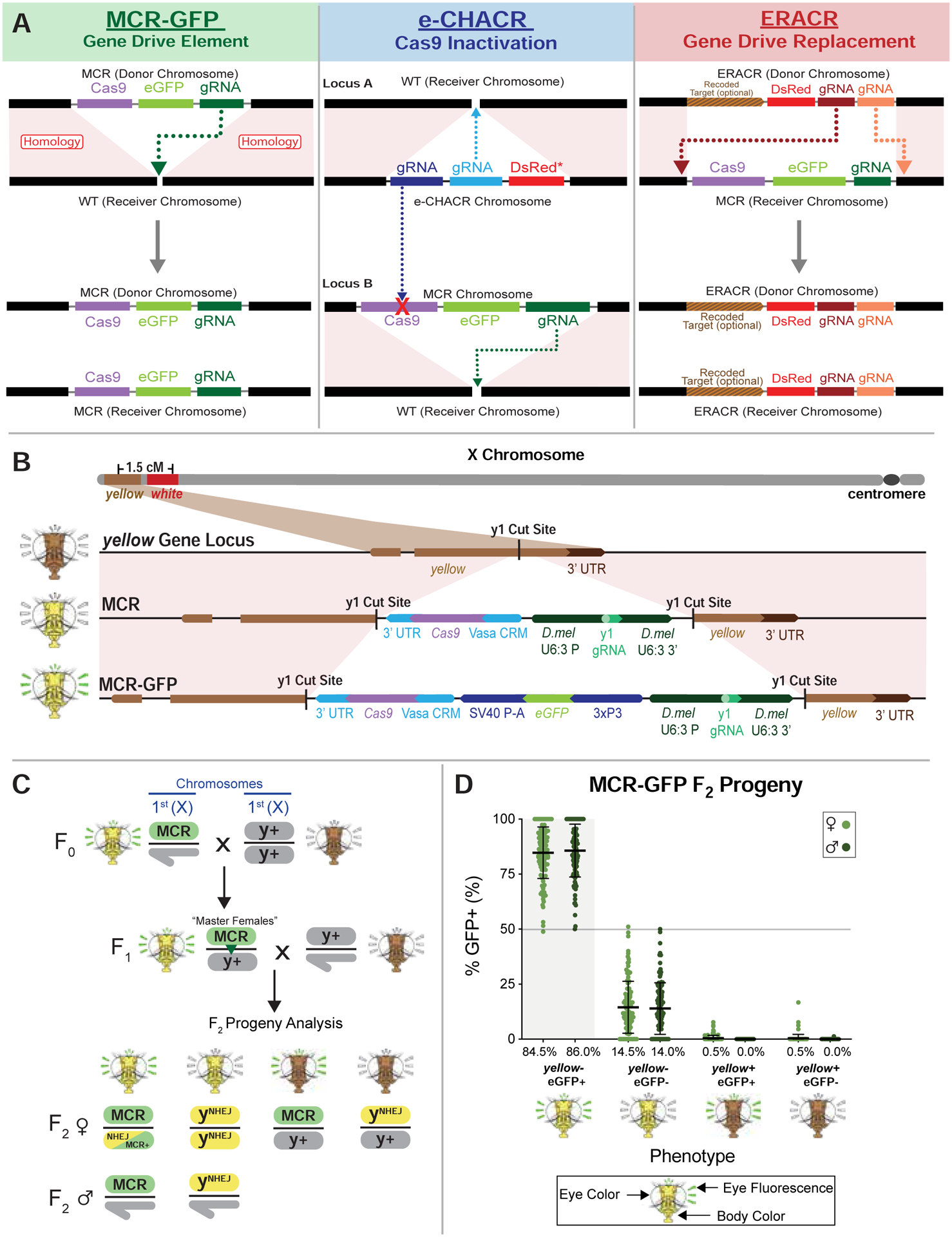
A) Scheme depicting gene-drives and neutralizing elements. Left: MCR (mutagenic chain reaction) gene-drive element carrying Cas9, an eGFP fluorescent marker, and a gRNA- for copying. Center: eCHACR carrying two or more gRNAs: one gRNA (blue) for copying at its genomic insertion site, a second gRNA (purple) targeting Cas9, and a DsRed (or eGFP) fluorescent eye marker. e-CHACRs are typically inserted at a different chromosomal site (locus A) than the gene-drive (locus B). Right: ERACR carrying gRNAs that target sequences flanking the MCR-GFP element and a DsRed marker. B) Two MCR elements inserted at the same site in the y locus: 1) MCR lacking a fluorescent marker (third row), and 2) MCR-GFP, an eGFP-marked version (bottom row) carrying the same core components (vasa-Cas9 and gRNA-y1). C) Cross scheme for generating MCR-GFP F1 “master females” and their F2 progeny. Phenotypes of F0, F1, and F2 progeny are depicted schematically. D) Percentage of GFP+ F2 progeny (carrying MCR-GFP element) recovered per cross. Fly heads depict eye phenotypes determined by the white (w) locus: wild-type = w+ (red eyes), recessive w− (white eyes), eye fluorescence markers (GFP = radiating green lines), and body color (y+ = brown, y− = yellow).
When seeded into a gene-drive population, such gRNA-only driven neutralizing systems should follow the same logistic growth trajectory as a gene-drive released into a native population (Gantz and Bier, 2016). In the absence of a Cas9 source, however, these neutralizing elements are inherited in a standard Mendelian fashion, as when they either inactivate (e-CHACR) or eliminate (ERACR) a gene-drive element.
In this study, we test and analyze the activities of several e-CHACR and ERACR elements in Drosophila melanogaster (D. mel.). We find that while the e-CHACRs vary in their copying efficiency, all mutate and inactivate Cas9 efficiently, and the one tested drives to completion in population cages. Similarly, ERACRs often copy and delete a gene-drive element as intended, but can damage target chromosomes and generate various rare recombinant outcomes. Despite these imperfections, ERACRs, particularly those carrying functional recoded sequences that restore endogenous gene activity, can fully replace a gene-drive element in cage experiments. We discuss these results with regard to the potential utility of e-CHACRs to halt the spread of gene-drives.
Results
Active genetic elements such as gene-drives and gRNA-only neutralizing elements (Fig. 1A) bypass standard rules of inheritance imposed by independent chromosome assortment and linkage of nearby loci. Tracking such fluidly copying elements requires careful genetic bookkeeping by following each genetic element with a different fluorescent transgene and using genetic markers tightly linked to these elements to distinguish donor (chromosome of origin for a given active genetic element) versus receiver (target chromosome to which a drive element copies) chromosome homologs. For example, in this study, we used an eGFP-marked gene-drive (MCR-GFP) element inserted in the yellow (y) locus and DsRed-marked neutralization elements (e-CHACRs and ERACRs). We also marked donor versus receiver X chromosomes either with different alleles of white (e.g., white apricot = wa; phenotype: orange eyes), located 1.5 centimorgans centromere proximal to y (Fig. 1B, 3A), or a viable allele of the achaete-scute locus (ac4; phenotype: missing innervated thoracic bristles) located immediately nearby (~9 kb centromere proximal to y: Fig. 2A).
Figure 3: ERACR construct designs and crossing schemes.
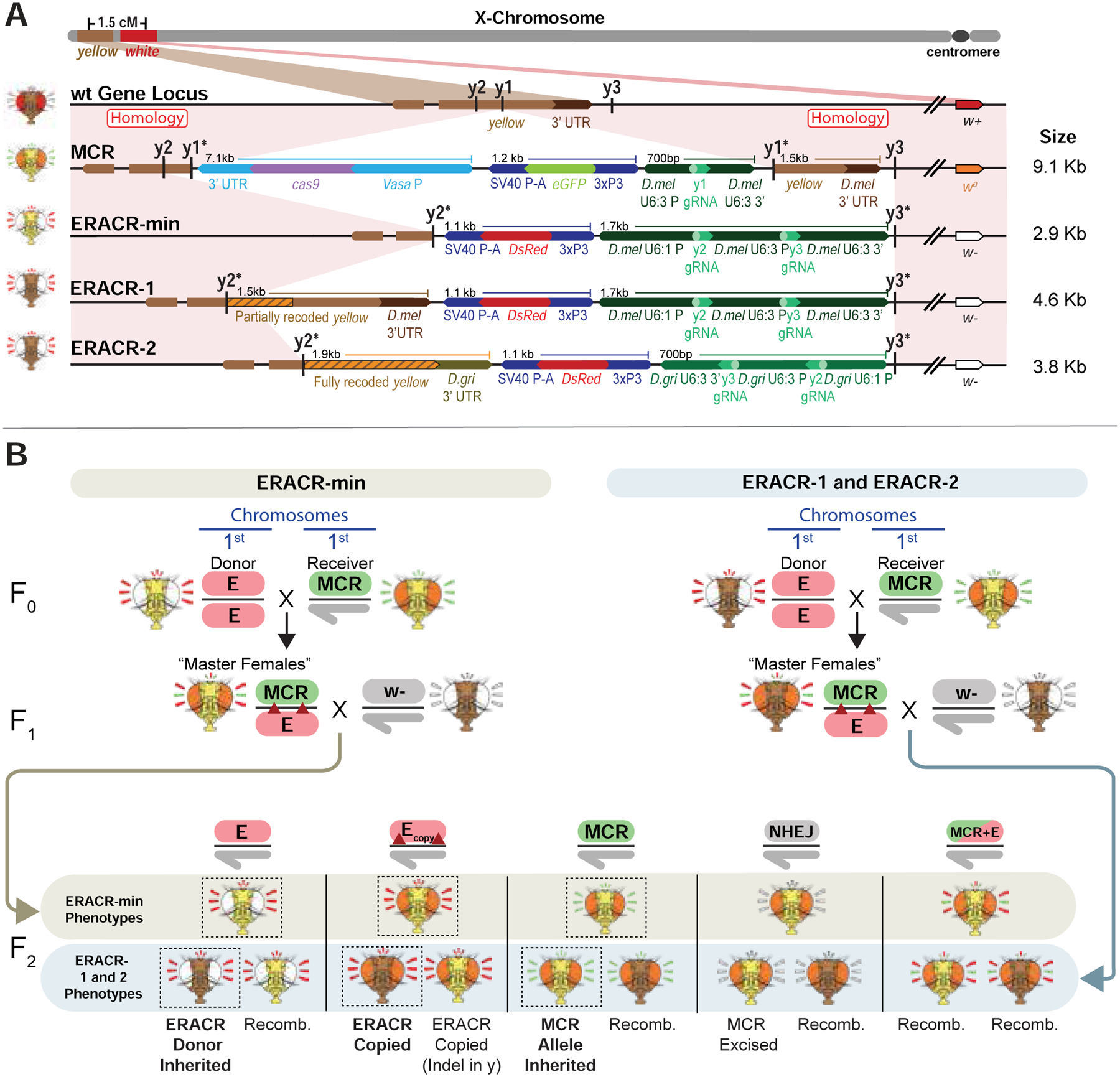
A) Diagrams of MCR-GFP and ERACR elements. Fly heads on the left indicate the phenotype of each strain. Schematic not drawn to scale. B) Cross schemes to generate F1 “master females” with ERACRs in-trans to the MCR-GFP element and their F2 progeny. Crossing schemes for y− ERACR-min (left) and for y+ ERACR-1 and y+ ERACR-2 (right). Fly heads depict eye color (wild-type = w+ (red), w− (white), or wa (orange), eye fluorescence (radial emanating lines of eGFP (green), DsRed (red), both eGFP and DsRed (alternating green and red), or neither fluorescence (white), and body color: y+ (brown) or y− (yellow). Expected F2 phenotypes bolded and outlined with black boxes.
Figure 2: e-CHACR-wR versus the MCR-GFP element.
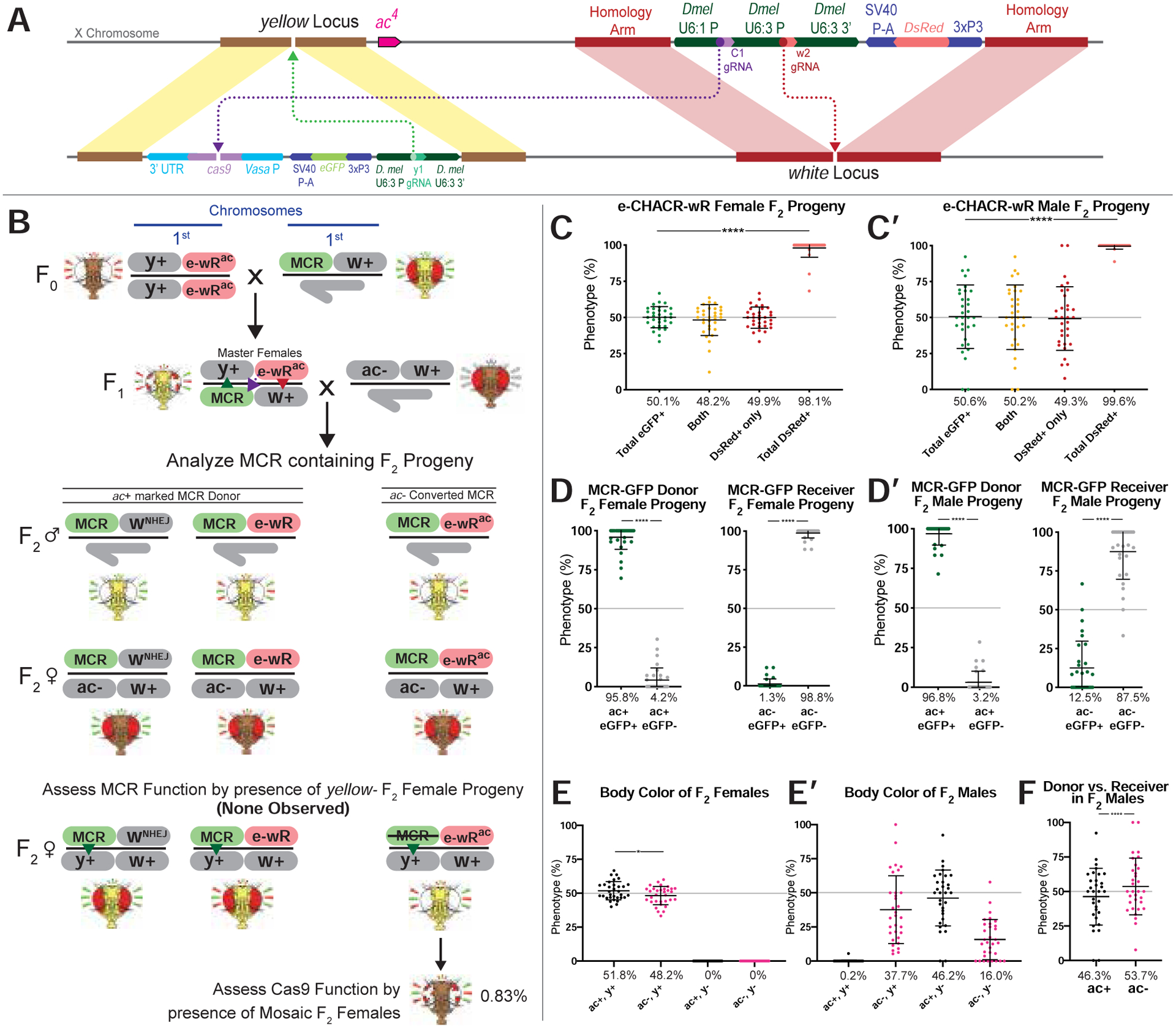
A) Schematic of DsRed+ e-CHACR-wR element linked to the ac4 allele (e-wRac) and the y+ ac+ marked MCR-GFP allele. B) Crossing scheme for testing the e-CHACR-wR (e-wR) against the MCR-GFP element. e-CHACR-wR females were mated to MCR-eGFP males to generate F1 master females that were then pair-mated to ac4 (ac−) males. F2 progeny were screened and analyzed for MCR-GFP presence and presence (DsRed+) or absence (wNHEJ) of e-CHACR-wR on ac+ or ac4 marked chromosomes. C,C’) Percentage of fluorescence phenotypes in total female (C) or male (C’) F2 progeny per cross. D,D’) Prevalence of GFP+ and GFP− alleles in MCR-GFP donor (left) and receiver F2 D) females or D’) males (right). E,E’) Prevalence of body color in total F2 E) females or E’) males. F) Percentage of MCR-GFP donor (ac+, black dots) and receiver (ac−, pink dots) alleles in F2 males.
Super-Mendelian transmission of the MCR-GFP gene-drive element
As a first step in our analysis we generated an eGFP-marked MCR-GFP gene-drive element carrying a Cas9 transgene expressed under control of the vasa promoter (vasaCas9) and a gRNA (gRNA-y1) directing its copying at the y locus (Gantz and Bier, 2015) using a genetic “tagging” method (Materials and Methods) (Fig. 1B). We assessed the drive performance of the MCR-GFP element in numerous parallel single-pair mating crosses in which so-called F1 “master females” (females carrying both gRNA and Cas9 transgenes) were crossed to y+ w− males (Fig. 1C). A substantial fraction (~20–35%) of crosses resulted in 100% transmission of the MCR-GFP element to all F2 progeny (Fig. 1D). Overall, the MCR-GFP element exhibited an average super-Mendelian transmission rate of ~85%. Similar results were obtained in crosses generated from master females that inherited the drive paternally (Fig. 1D) or maternally (Figs. S1A,B), albeit with somewhat reduced efficiency in maternal crosses. A noteworthy feature of these experiments, revealed most obviously in histogram summaries of many individual pair matings (Fig. S1C–D”), was that the frequencies of F2 progeny inheriting the gene-drive element were distributed in a non-Gaussian fashion. Potential explanations for this Poisson-like distribution of gene-drive transmission are considered in Data S1. Also, nearly all F2 progeny displayed a y− mutant phenotype whether not they carried the MCR-GFP drive, consistent with both zygotically and maternally provided Cas9/gRNA complexes acting on and mutating the wild-type paternal y+ allele, a phenomenon that we and others have previously documented (Champer et al., 2017; Gantz and Bier, 2015; Gantz et al., 2015; Guichard et al., 2019; Hammond et al., 2016; Li et al., 2019; Lin and Potter, 2016).
Experiments with wa marked receiver chromosomes (Fig. S2A) revealed that the two chromosome homologs were inherited following standard Mendelian segregation (Fig. S2B). We conclude that the great majority of the super-Mendelian inheritance displayed by the MCR-GFP element in such crosses can be attributed to gene-drive copying events rather than to biased transmission of donor versus target receiver chromosomes.
e-CHACRs efficiently inactivate Cas9, but copy with a range of frequencies
We inserted e-CHACRs into loci on different chromosomes: the X-linked white locus (e-CHACR-wG: eGFP marked; e-CHACR-wR: DsRed marked), and the third chromosome ebony (e-CHACR-e) and knirps (e-CHACR-k) loci (see online Data S1 file for further details on e-CHACR analysis). Strains carrying these e-CHACRs were crossed to those harboring a standard “static” (non-copying) vasaCas9 source (Figs. S3–5,S10) or the active MCR-GFP gene-drive inserted at y (Fig. 2; Figs. S7–9,S11) to generate F1-generation master females bearing both elements. The phenotypes of F2 progeny from many parallel pair-mating crosses between F1 master females and males of informative genotypes (i.e., y±, w±, ac±) were tabulated, as discussed below. We evaluated two key e-CHACR performance parameters: 1) the efficiency of anti-Cas9 gRNAs (gRNA-C1, carried by the w and kni e-CHACRs; or gRNA-C3, carried by the ebony e-CHACR) in mutating and inactivating Cas9, and 2) the rate of e-CHACR copying to the target receiver chromosome.
Cas9 inactivation by e-CHACRs was assessed in several ways. For e-CHACRs inserted at w (Fig. 2; Figs. S3,S4,S7), we monitored a highly penetrant Cas9-dependent somatic mosaic eye phenotype (eyes consist of red and white sectors). By this mosaic eye metric, we estimate that Cas9 was inactivated in ~99% of F2 progeny by either gRNA-C1 or gRNA-C3, both of which target sites in the Cas9 transgene encoding catalytically essential amino acids. Non-mosaic F2 progeny carrying e-CHACR-wG and the presumably mutated Cas9 source transmitted the GFP+ element to F3 progeny at standard Mendelian ratios (Fig. S3E), in contrast to the high Super-Mendelian frequencies observed in F2 progeny (Figs. S3C,C’). In experiments involving the MCR-GFP drive (Fig. 2; Figs. S7;S9;S11) we also assessed the full-body y− pigmentation phenotype, which as mentioned above, is fully penetrant in F2 progeny of master females carrying an active MCR-GFP element. 100% of F2 progeny had y+ phenotypes, revealing that Cas9 activity was eliminated by all e-CHACRs. We also sequenced genomic DNA isolated from individual F2 progeny, confirming that the gRNA target sites had been mutated in all samples analyzed from crosses with e-CHACR-wg (carrying gRNA-C1) and e-CHACR-e (carrying gRNA-C3) (Fig. S6).
We observed a range of e-CHACR transmission frequencies. Copying was highly efficient (95–99% transmission to F2 progeny) for both GFP and DsRed-marked e-CHACRs inserted at w (e-CHACR-wG and e-CHACR-wR, respectively)(Fig. 2; Figs. S3–5). e-CHACR-k, inserted in the knirps (kni) locus, exhibited intermediate levels of copying (87–90% transmission; Fig. S11), while e-CHACR-e, inserted at ebony (e), was transmitted at Mendelian frequencies (Fig. S9). The absence of drive for e-CHACR-e was puzzling since previous experiments with other split-drive elements indicated that the same gRNA-e1 carried by e-CHACR-e sustained modest super-Mendelian copying (López Del Amo et al., 2019). We speculated that efficient mutagenesis of the Cas9 transgene by gRNA-C3 might prevent e-CHACR-e from copying. Indeed a cleavage-resistant form of Cas9 (Cas9*) that could not be targeted by gRNA-C3 sustained modest copying of e-CHACR-e (~60% transmission) (Fig. S10), similar to that previously reported for the split system driven by the same gRNA-e1 (López Del Amo et al., 2019). We conclude that all three e-CHACRs are highly efficient at eliminating Cas9 activity with either of two Cas9 targeting gRNAs, and that their differing ability to copy most likely reflects the relative activities of the gRNAs that sustain their copying (see Data S1 and Discussion for further analysis).
e-CHACRs efficiently inactivate the MCR-GFP drive
We tested the ability of the three different e-CHACRs to inactivate the MCR-GFP full-drive element, and all functioned efficiently to mutate Cas9 as judged by the virtual absence of F2 individuals displaying mosaic eye (>99%) or full-body y− (100%) phenotypes (Fig. 2E,E’; Fig. S9E,E’; Fig. S11E,E’). All three e-CHACRs also markedly reduced the frequency of MCR-GFP copying, albeit to varying extents. e-CHACR-wR reduced MCR-GFP copying by 5–10 fold (i.e., from ~70%: Fig. 1D to 7–13%: Fig. 2D,D’; Fig. S7D,D’), e-CHACR-e by ~ 5-fold (Fig. S9D,D’), and e-CHACR-k by ~2-fold (Fig. S11D,D’). We conclude that e-CHACRs efficiently inactivate Cas9, copy themselves in the presence of a full-drive element or static sources of Cas9, and significantly reduce copying of the MCR-GFP gene-drive element (see Discussion). These various reinforcing activities likely contribute to the efficient performance of e-CHACR-wR in population cage experiments described below.
e-CHACRS can preferentially bias inheritance of donor chromosomes
Visible markers (e.g., wa, ac4) closely linked to the e-CHACR and MCR-GFP elements, permitted us to determine whether the donor and receiver chromosome homologs segregated in a biased fashion. We observed two types of significant bias in chromosomal inheritance. First, when the X-linked e-CHACR-wG targets a static Cas9 source inserted at the closely linked y locus, we observed a nearly 2:1 bias in the inheritance of the donor e-CHACR chromosome over the receiver chromosome in male (Fig. S3C’) but not female (Fig. S3C) F2 progeny, which was accompanied by a corresponding excess of females to males (Fig. S8B,D,F). In contrast, when autosomal e-CHACR-e targeted a nearby Cas9-GFP source at w, the single-cut target chromosome was inherited at Mendelian frequencies in F2 progeny of both sexes (Fig. S10A–C). Also, e-CHACR-wR driven by an autosomal source of Cas9 did not bias the sex of recovered F2 progeny (Fig. S5). The handicap against receiver chromosomes in the former crosses, however, did not permanently damage target chromosomes since F2 females carrying mutated Cas9 chromosomes transmitted them to ~50% of their F3 progeny (Fig. S3E).
The second type of biased chromosome inheritance occurred when any of the three e-CHACRs targeted the MCR-GFP element. In these scenarios, the same locus on both chromosome homologs is targeted for cleavage (the donor MCR-GFP allele with anti-Cas9 gRNAs, and the receiver target allele with gRNA-y1 expressed by the MCR-GFP element). We observed excess transmission of y+ac4 receiver chromosomes in F2 males, but not females (Fig. 2F; Figs. S7F;S9F;S11F). We also recovered an unexpected class of GFP− donor chromosomes (Fig. 2D,D’; Figs. S7D,D’;S9D,D’). Of 17 such isogenized GFP− target chromosomes tested, 10 (59%) carried male-lethal alleles, and all could be rescued by a duplication covering the y locus (Fig. S12A; Table S1). As analyzed in greater detail in Data S1, we conclude that at least two different types of chromosome bias can be induced by e-CHACR elements: one, seen when cutting chromosomes carrying a static Cas9 source twice, does not irreparably damage the receiver chromosome. The second, associated with e-CHACRs targeting the MCR-GFP drive element, can induce local damage to the donor chromosome resulting in male lethality (or homozygous lethality in females).
ERACR versus MCR-GFP crossing schemes
We tested three DsRed-marked ERACR constructs designed to delete and replace the MCR-GFP element inserted at y (see online Data S2 for in-depth analysis of ERACRs). All three ERACRs carry two gRNAs (gRNA-y2 and gRNA-y3) that direct Cas9 cleavage to either side of the MCR-GFP element (Fig. 3A). ERACR-min carries just the aforementioned minimal elements (DsRed and two gRNAs) while ERACR-1 and ERACR-2 also carry recoded y cDNA coding sequences at their 5’ junction that seamlessly restore function of the y locus. ERACR-2 shares fewer homologous sequences with the MCR-GFP element than ERACR-1. y cDNA sequences are fully recoded for ERACR-2, whereas only the 5’ junction is recoded in ERACR-1. Also, U6-promoter sequences driving expression of gRNAs carried by ERACR-2 derive from a distantly related Drosophilid (D. grimshawi), which have little sequence homology to those carried by the MCR-GFP element, and are oriented in the opposite direction to those carried on the gene-drive to minimize potential spurious recombination events between the two elements.
Fly strains carrying the different DsRed+ ERACR constructs were crossed to a wa marked MCR-GFP strain (Fig. 3B; see also Fig. S13 and Data S2 for additional crossing schemes and analysis). F1 master females carrying the wa MCR-GFP chromosome and w− ERACR elements were crossed to w− males and their F2 progeny scored for fluorescence, eye color, and whole-body pigmentation phenotypes. This scheme permits w− ERACR-bearing donor chromosomes to be distinguished from wa MCR-GFP target chromosomes.
ERACRs frequently delete and often replace a gene-drive element
Three prominent outcomes were observed among progeny inheriting the wa receiver chromosome: 1) deletion and replacement of the MCR-GFP element with the ERACR (phenotype: DsRed+,GFP−, Fig. 4A–C); 2) retention of the MCR-GFP element (phenotype: DsRed−,GFP+, Fig. 4D,E) with NHEJ induced indels at both the gRNA-y2 and gRNA-y3 cut sites (i.e., both ERACR gRNAs direct efficient target cleavage); and 3) deletion of the MCR-GFP element without copying the ERACR (phenotype: DsRed−,GFP−, Fig. 5A–E), a category only abundantly observed in females. Comprehensive analysis of the various ERACR versus MCR-GFP outcomes is presented in Fig. 4 and Fig. 5 and Figs. S14–17A and in-depth analysis of these events is presented in Data S2).
Figure 4: ERACRs delete and replace the MCR-GFP drive.
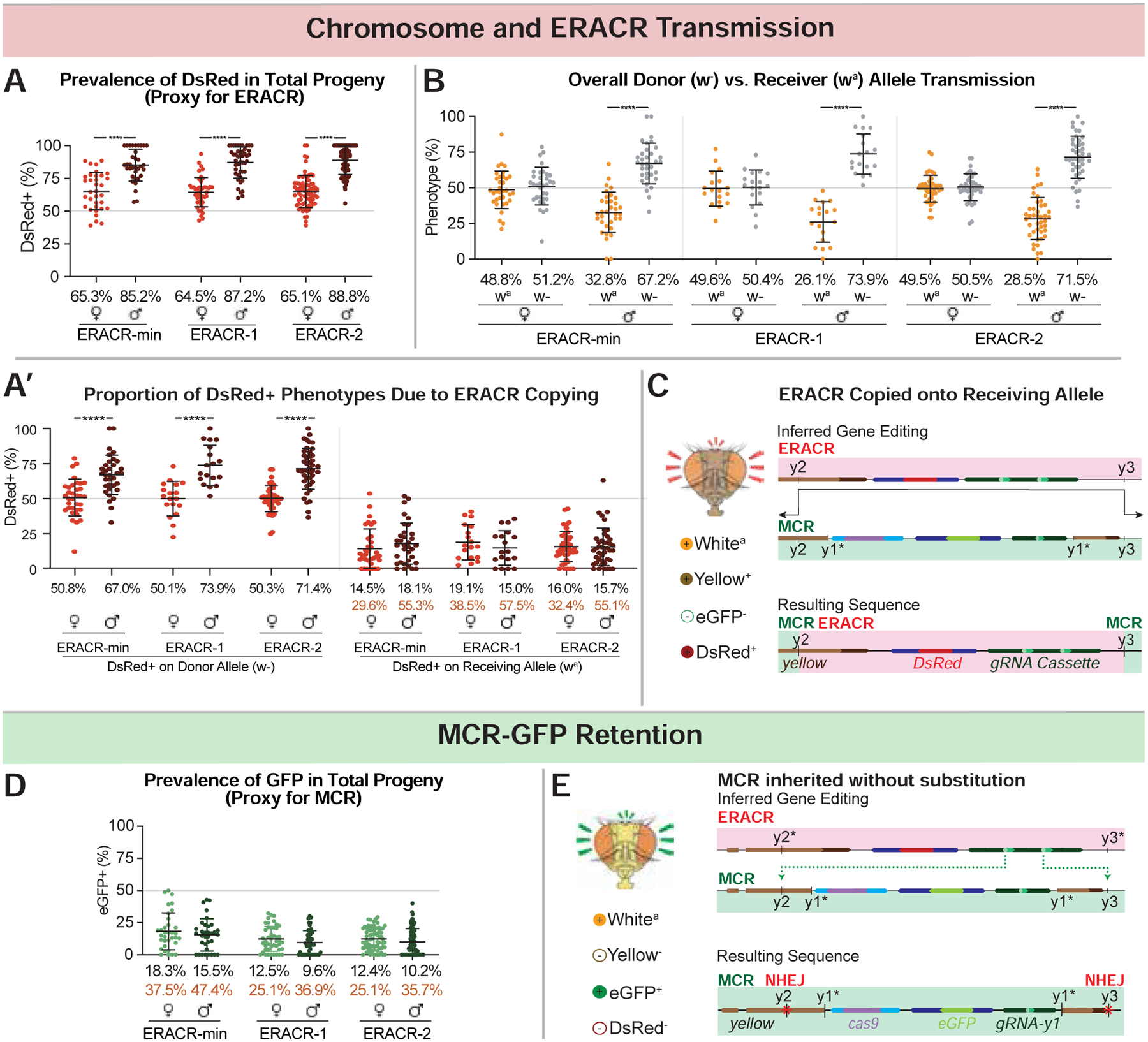
Phenotypic frequencies and deduced gene conversion events in F2 progeny from crosses depicted in Fig. 3A. (additional crossing schemes in Figs. S13A–E). Black type = mean of percentages across all vials and orange type = estimated receiver conversion frequencies (e.g., DsRed wa males/total wa males) (see Data S3 for details). A) DsRed+ inheritance is a proxy for scoring ERACR prevalence (DsRed+, GFP− and DsRed+, GFP+ progeny included). A’) The subset of data plotted in panel 4A with traceable donor and receiver chromosomes re-plotted by donor (w−; left graph) versus receiver (wa; right graph) chromosomes. B) Proportion of F2 males or females inheriting the donor (w−) versus receiver (wa) chromosome. C,E) Schematics illustrating predicted gene conversion events responsible for specific phenotypes: sequences of relevant junctions shown in (Fig. S14). D) GFP inheritance is a proxy for MCR-GFP prevalence (DsRed+,GFP+ plus DsRed−,GFP+ F2 progeny). E) MCR-GFP alleles, although intact, have NHEJ-induced indels at the y2 and y3 cut sites.
Figure 5: Alternative ERACR versus MCR-GFP outcomes.
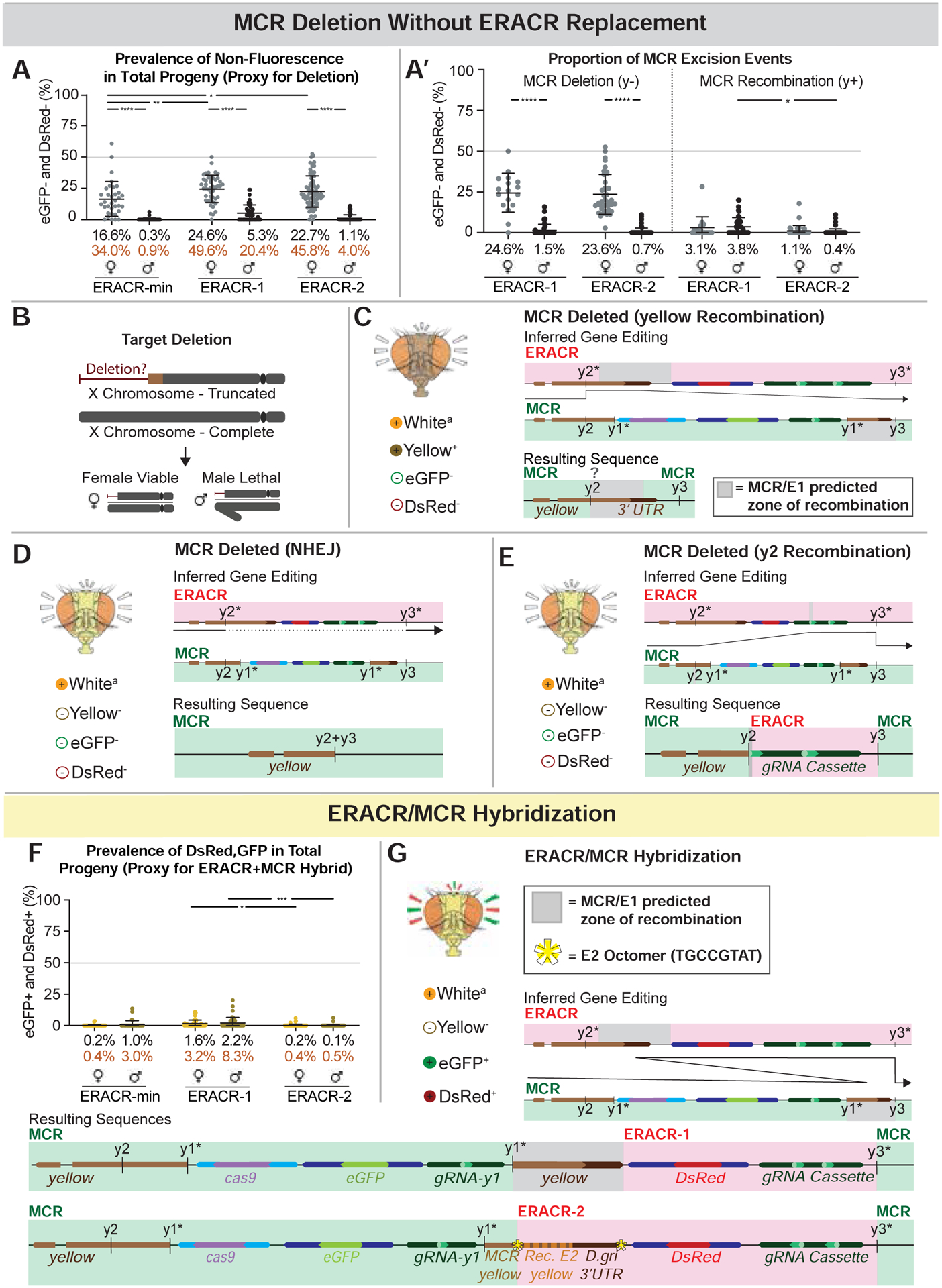
Partial copying or fusion of sequences on the receiver chromosome. A) Non-fluorescent DsRed−,GFP− F2 progeny as a proxy for MCR-GFP deletion events. A’) Data in panel A re-plotted by inheritance of y− (lethal deletion) versus y+ (recombination) alleles. Body color permits inference of the type of excision event that occurred, as depicted in C-E. B) Loss of essential sequences distal to the ERACR gRNA cut sites result in male-lethal alleles. Viability of several such alleles can be rescued in males by duplications covering the tip of the X chromosome (Fig. S12B). C-E) The MCR-GFP element is deleted, but not replaced by ERACR, producing three distinct observed outcomes. C) Hypothesized repair mediated by partial pairing of un-recoded y sequences carried by ERACR-1 and endogenous y sequences 3’ to the MCR-GFP element result in expression of the recoded y cassette and a wild-type body color. D) NHEJ events joining adjacent sequences at the gRNA-y2 and gRNA-y3 cut sites. E) Likely pairing between 17 bp of the gRNA-y2 genomic target sequences 5’ to the MCR-GFP with correspondingly oriented sequences in the gRNA-y2 transgene carried by either ERACR-min or ERACR-1. F) Prevalence of DsRed+, GFP+ F2 progeny in which MCR-GFP and ERACR sequences are both present on the receiver chromosome. G) Two examples of MCR-GFP/ERACR fusion events (see Fig. S14).
Several salient features emerged from these experiments. First, the three ERACRs performed similarly overall (Figs. 4,5; Fig. S15), deleting and replacing the MCR-GFP element (~31%), leaving the MCR-GFP in place (~24%), and, in females, deleting the MCR-GFP without copying (~43%). There were also various rare outcomes (~1%) based on illegitimate recombination events, which differed based on ERACR design (discussed further in Data S2). Second, the DsRed−, GFP− phenotype was recovered >10-fold more often in females than males (Fig. 5A,A’). The great majority of such alleles were male lethal, resulting in a 2:1 excess of female over male F2 progeny (Fig. S17A). In all tested cases, lethality of these DsRed−,GFP− alleles could be rescued by a duplication of the tip of the X chromosome covering the y locus (Fig. S12B). These findings indicate that ERACRs often damaged the target chromosome, disrupting essential functions in the vicinity of the target locus. Finally, although illegitimate recombinant events were rare, they were recovered (Fig. 5C,E,F,G). A few events lead to the generation of DsRed+,GFP+ mosaic elements (Fig. 5F,G) that retained some capacity to copy all (Fig. S20A–C’) or portions (Fig. S20B–D’) of the elements. Also noteworthy, the frequency of such outlier events was significantly reduced for ERACR-2 relative to ERACR-1 (Fig. 5A,A’,F; Figs. S14,S15), indicating that elimination of homologous sequences between ERACR and gene-drive targets is a favorable design strategy.
One-sided homology mismatch underlies ERACR-induced chromosome damage
Like e-CHACRs targeting receiver chromosomes for dual cleavage, ERACRs favor inheritance of donor chromosomes, suggesting that ERACR-induced chromosome damage might result from cutting the receiver chromosome twice at neighboring sites. We tested this hypothesis by constructing single-cut versions of ERACR-2, which should not generate damage according to the double-cut model. Single-cut ERACRs carried either only gRNA-y2 (ERACR2-y2: cutting 5’ and centromere distal to the MCR-GFP element), or only gRNA-y3 (ERACR2-y3, cutting 3’ and centromere proximal to the MCR-GFP element) (Fig. 6A). We tested these elements for super-Mendelian transmission as well as induction of damage to the target chromosome.
Figure 6: Drive performance of single-cut ERACRs versus MCR-GFP drive.
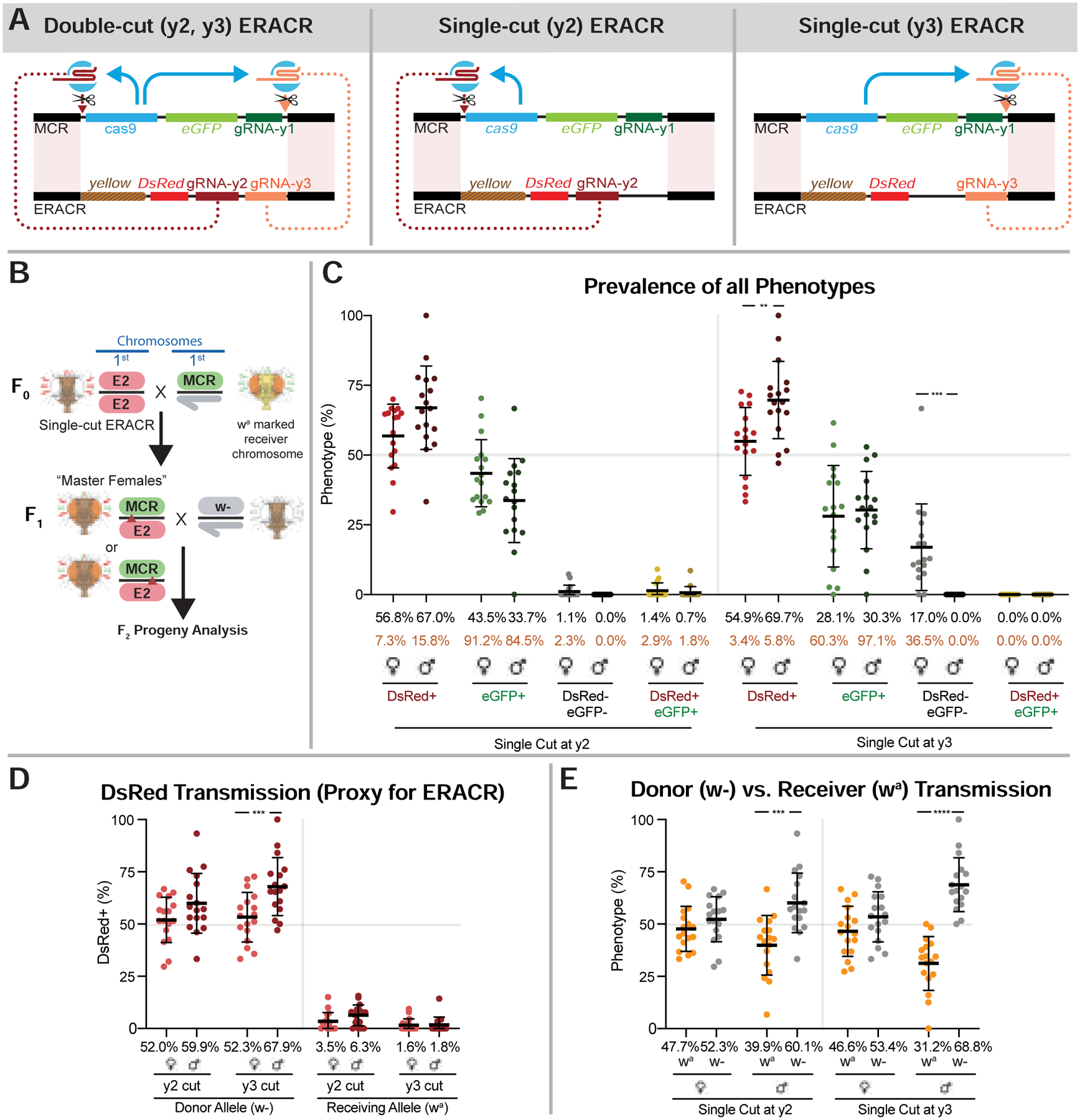
A) Schemes illustrating single-cut and double-cut ERACR designs. B) Crossing scheme for generating and testing transmission by single-cut wa ERACR/MCR-GFP F1 master females. C-E) Single-cut versions of ERACR-2 are placed in-trans to the MCR-GFP element where the gRNA-y2 and gRNA-y3 are separated by a distance of 11.3 kb (see also Fig. S19 for Copy-Cat analysis). C) Fluorescent phenotypes for single-cut DsRed+ ERACR2-y2 and ERACR2-y3 and MCR-GFP. D) Percent DsRed+ females or males inheriting either the donor (w−) ERACR-2 single-cut chromosome or the receiver (wa) chromosome. E) Donor versus receiver chromosome transmission for single-cut ERACR2-y2 and ERACR2-y3.
Copying of single-cut ERACRs was assessed by pair-mating them to wa MCR-GFP individuals according to the scheme depicted in Fig. 6B, which is analogous to that used for the double-cut ERACRs (Fig. 3B). Analysis of fluorescence associated with wa receiver chromosomes in F2 progeny (Fig. 6C,D) revealed that the frequency of deleting and replacing the MCR-GFP element was significantly reduced for both ERACR2-y2 (avg. = 4.9%, Fig. 6D) and ERACR2-y3 (avg. = 1.7%, Fig. 6D) relative to the dual-cutting ERACR-2 (avg. = 16%, Fig. 4A’, right panels), even when combined.
Crosses of ERACR2-y3 to the MCR-GFP element also reveal whether these single-cut elements might damage the target chromosome. We observed a prominent category (17%) of DsRed− GFP− F2 female progeny that was absent in male siblings (Fig. 6C), which is indicative of deleting the MCR-GFP element without copying the ERACR. This rate of DsRed−,GFP− progeny is similar to that observed for the double-cut ERACR-2 (22.7% in F2 females, versus 1.1% in males: Fig. 5A), suggesting that single-cut ERACR2-y3 damages the target chromosome at a frequency comparable to that of the double-cut elements. Although crosses of ERACR2-y2 to the MCR-GFP element did not produce a prominent class of DsRed−,GFP− F2 female progeny, we did observe a similar ~10% disparity in the fraction of GFP+ female versus male progeny. This finding could be explained by chromosome damage being induced distal to the gRNA-y2 cut site, leaving the centromere-proximal GFP transgene intact. Both single-cut-ERACRs also lead to an excess of females over males F2 progeny inheriting the receiver chromosome (Fig. S17B) as did all double-cut ERACRs (Fig. S17A). These results suggest that both single-cut ERACRs damage the target chromosome at appreciable frequencies and that damage is localized centromere-distal to the gRNA-directed cleavage sites.
We also compared the frequencies with which single- versus double-cut ERACRs copied and induced chromosome damage when confronting a wild-type y+ allele in a so-called “copy-cat” configuration with a static source of Cas9 provided in-trans (Figs. S18;S19). Here, the single-cut ERACR2-y2 (Fig. S19B) copied nearly as well as the full double-cut ERACR-2 (Fig. S19A) as judged by the proportion of DsRed+,GFP+ progeny (~20%). ERACR2-y3 copied less well, albeit notably better (~6%) than when challenged with the MCR-GFP (~1.5%). Both single- and double-cut ERACRs again induced comparable reductions in transmission of the GFP-marked target chromosome in F2 males, but not females, consistent with damage to receiver chromosomes. We conclude that the likely basis for the majority of ERACR-induced chromosomal damage is lack of DNA homology on one side of the ERACR (the side missing the second gRNA), rather than cutting the target chromosome twice. We also note that single-cut ERACRs copy better with less distance between flanking homology sequences (e.g., 2.2 kb in a copy-cat versus 11.3 kb MCR-GFP mode).
ERACR and e-CHACRs do not sustain shadow drive
We previously documented a maternal “shadow-drive” phenomenon in individuals descended from Cas9-bearing mothers that inherit a gRNA-based drive element but not the Cas9 transgene (Guichard et al., 2019). Since both e-CHACRs and ERACRs eliminate Cas9 activity, we wondered whether this early action might preclude accumulation of sufficient Cas9/gRNA stores to sustain shadow-drive in the subsequent generation. We examined the drive potential of e-CHACR or ERACR elements in F2 progeny of F1 master females lacking the Cas9 transgene and thus carrying only maternal stores of the endonuclease provided by their F1 mothers (Fig. S21A,C). We tested e-CHACR or ERACR transmission to F3 progeny from such F2 females and observed Mendelian frequencies of GFP transmission (Fig S21,B,D–F), revealing that neither neutralization element sustained significant shadow-drive.
e-CHACR-wR inactivates the MCR-GFP gene-drive in population cages
Results from pair-mating crosses revealed that e-CHACR-wR efficiently eliminated Cas9 activity and copied efficiently when combined with either static (Figs. S3;S4;S5) or gene-drive (Figs. 2; Fig. S7) borne sources of Cas9. We generated mathematical models based on these single-generation data (Data S3) and fitted parameters based on observed time-series data from the cage trials (Fig. S22, Fig. 7A - solid lines). These initial values were then used to run simulations, shown as arrays of pale-colored lines, matching the schemes for the different displayed phenotypes (Fig. 7B). Simulations with the set of fitted parameters (Data S3, Table Smod4) were largely consistent with experimental values for the frequencies of different phenotypic classes observed in the pair-mating crosses. We were also able to infer parameters revealing informative deviations from assumptions of random mating between all genotypes. In particular, the modeling indicated a relatively high degree of assortative mating within groups having shared eye pigmentation (i.e., w+ females preferentially mating with w+ males over w− males).
Figure 7: Cage experiments: MCR-GFP versus e-CHACR-wR and ERACRs.
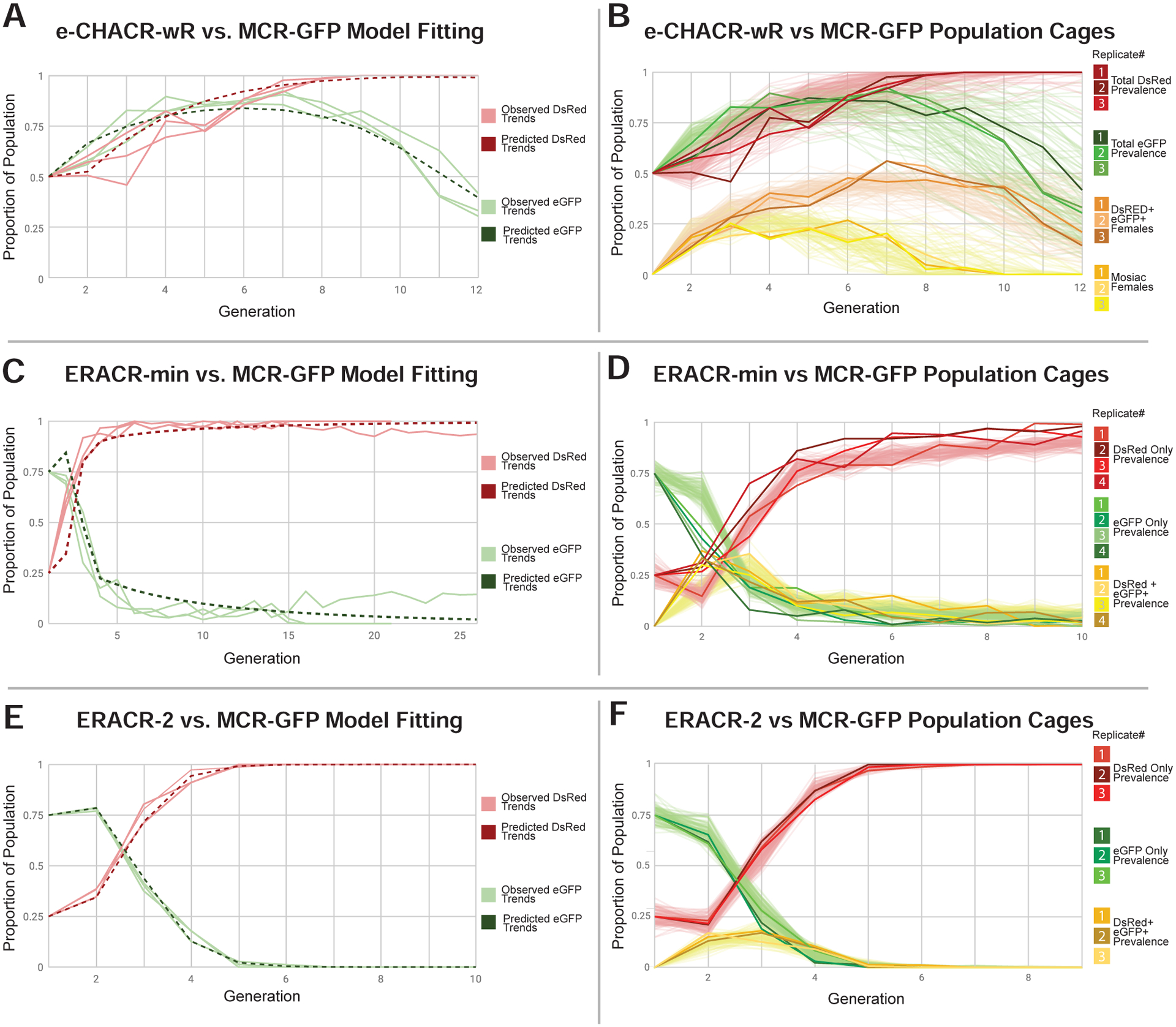
A,C,E) Modeling of MCR-GFP versus e-CHACR-wR (A) and ERACR (C,D) dynamics (solid lines: same as B,D,E) and model fits (dotted lines) plotted for the frequencies of MCR-GFP (green) and e-CHACR-wR or ERACRs (red). B) Plot of fraction of individuals with different phenotypes over 12 generations. Green = MCR-GFP and Red = e-CHACR-wR prevalence in the total population (e.g., both males and females). Orange = females carrying both elements. Yellow = females with mosaic eyes indicative of Cas9 activity. Dark traces represent separate cage replicates and pale lines = model simulations (also in panels D,F). C,E) Modeling of MCR-GFP versus ERACR dynamics over 26 generations (C) and ERACR-2 (E). D,F) ERACR-min (D) and ERACR-2 (F) versus MCR-GFP cage experiments. Red = DsRed+ ERACRs. Green = MCR-GFP+. Yellow = both markers.
In conjunction with the modeling above, we competed e-CHACR-wR against the MCR-GFP element in population cages. We seeded triplicate cages with equal numbers of homozygous e-CHACR-wR and MCR-GFP individuals. At each generation (n) we scored half of the individuals per cage (approximately 150 random flies) for their fluorescent and y± phenotypes, while the other half was used to seed the next generation (n+1) of cages. In all three cages, e-CHACR (red curves) drove to completion in 9 generations (Fig. 7A,B). In addition, we observed a transient appearance and then disappearance (after 8 generations) of a population of DsRed+, GFP+ females that exhibited mosaic eye phenotypes (yellow curves in Fig. 7B), which reveals Cas9 activity when the two elements first encounter each other (e.g., as in master females). Outcrosses of sampled DsRed+, GFP+ males and females from generations 9 and 10 revealed that all tested MCR-GFP alleles had lost Cas9 activity (judged by the absence of female progeny with mosaic eyes - Fig. S22B,B’). In addition, 100% of progeny from crosses of DsRed+,GFP+ females from generations 9 and 10 to wild-type males were DsRed+,GFP− indicated that the e-CHACR-wR had achieved 100% homozygous introgression (Fig. S22C,C’). Overall, simulations derived from these assumptions matched well with the observed efficient experimental performance of the e-CHACR (Fig. 7B).
Another multi-generational trend was that following an initial expected increase in the MCR-GFP frequency, which peaked at generation 7, prevalence of this element declined precipitously (green curve in Fig. 7B). Although a similar trend of initial surge followed by waning was observed in control crosses of the MCR-GFP element to y− mutants (which harbor an intact gRNA-y1 cleavage site) (Fig. S22A), the peak was earlier (generation 5), and the decline more gradual. These later observations suggest that the MCR-GFP element may carry a fitness cost (hence its slow loss in control experiments). The e-CHACR may accelerate clearance of the MCR-GFP element, potentially by inaccurate repair of the targeted Cas9 transgene.
ERACRs replace the MCR-GFP gene-drive in population cages
Mathematical modeling based on single generation pair-mating ERACR data (Fig. S15) suggested that these neutralizing elements should behave as designed to eliminate the gene-drive element over multiple generations (Fig. 7C,E; Fig. S23F,F’,G,G’). In these simulations we made the following two simplified assumptions: 1) MCR-GFP elements retained after confrontation with an ERACR are immune to further action by ERACRs (due to generation of cleavage-resistant NHEJ mutations at both cut sites); and 2) double-negative (DsRed−,GFP−) alleles generated by ERACRs deleting, but not replacing, the MCR-GFP element, are fully viable in heterozygous females, but lethal in homozygous or hemizygous conditions. In addition, consistent with prior classic studies (Barker, 1962; Bastock, 1956; Diederich, 1941; Merrell, 1949; Sturtevant, 1915), we modeled a range of potential fitness costs for y− versus ERACR-2 (y+) males based on y+ females preferring to mate with y+ males, as well as an apparent fitness cost associated with the MCR-GFP gene-drive. We confirmed a potent assortative mating preference of y+ females in single generation control crosses with our own stocks (Fig. S23A–D), and in competitive multi-generational cage experiments (Fig. S23E). Sampling over a broad range of parameters suggested that ERACR-min (y−) and, particularly ERACR-2 (y+), should produce sufficient drive in mixed populations carrying the MCR-GFP and ERACR (but no wild-type alleles) to replace and ultimately eliminate the gene-drive over several generations (Fig. 7C,E).
We conducted 3–4 separate cage replicates per ERACR composed of 25% homozygous ERACR and 75% homozygous MCR-GFP virgin male and female individuals in each cage. The frequency of the DsRed+ ERACR cassette for the ERACR-min experiment tripled in ~4 generations and then gradually approached fixation over the next 5 generations, with a modest degree of variation (~10–15%) exhibited between the four cages tested (Fig. 7D). Reciprocally, the frequency of the MCR-GFP marker decreased until it was nearly eliminated by generation 9. Consistent with random mating among y− ERACR-min and MCR-GFP individuals, 30–35% of progeny from the first generation were trans-heterozygotes carrying both fluorescent markers. This double-positive (GFP+,DsRed+) population peaked in generation 3 and then steadily diminished. In contrast, the proportion of DsRed individuals in the ERACR-2 cages (Fig. 7F) remained approximately constant for one generation and then increased steeply and in tight synchrony, reaching fixation in 5–6 generations, with concomitant reduction and then complete elimination of the MCR-GFP element. Notably, ERACR-2 replicats displayed little variation in their drive trajectories. These experimental results match well with simulations (pale lines in Figs. 7D,F) based on parameters identified by model fitting (dotted lines in Figs. 7C, E), capturing key features of the overall kinetics and inter-trial variations.
Although there was an excellent overall fit between the observed data and modeling (Fig. 7C,E; Fig. S23F–G’), two ERACR-min replicates (#1 and #4) pursued moderately different trajectories from the other two (#2,#3), with the latter two matching the predicted modeling (Fig. 7C,E). Since no such events were observed in any of the y+ ERACR-2 replicates, these outlier trajectories may result from formation of y− resistance alleles that would have been selected against in the ERACR-2 replicates due to assortative mating, but that could persist and potentially spread in the y− ERACR-min replicates where they were on even footing. In addition, modeling and cage studies (Fig. S22) suggest that a fitness cost is associated with the MCR-GFP element, which might be aggravated by cryptic ERACR-induced damage by gRNA-y2.
We also competed the y+ ERACR-2 against a y− NHEJ-induced point allele generated at the gRNA-y1 cleavage site (i.e., the same gRNA driving the MCR-GFP element) in the absence of Cas9 to isolate the effect of assortative mating (Fig. S23E). Over ~10 generations, chromosomes carrying the y+ ERACR-2 successfully overtook the y− NHEJ population with typical inter-cage variation (Fig. S23E). These observations confirm that the y+ genotype has an appreciable competitive advantage over y− in a cage setting. Also consistent with y+ ERACR-2 females mating preferentially with y+ ERACR-2 males, the frequency of DsRed+,GFP+ (ERACR-2 + MCR-GFP) flies in first generation progeny (Fig. 7F, yellow curves) was considerably lower (15–20%) than that predicted by random mating (37.5%) or observed for ERACR-min (Fig. 7D). We conclude that the modeling predictions conform closely to the experimental outcomes and that ERACRs, particularly those carrying a recoded transgene restoring function of a functionally important gene, have the potential to eliminate and replace a gene-drive element even once it has attained fixation in a population.
Discussion
Overall, both e-CHACRs (Fig. 8A) and (ERACRs) (Fig. 8B). offer promise for fulfilling their purpose of countering a gene-drive system (Fig. 8C–D), however, it is also important to take into account some of their unintended actions.
Figure 8: Summary Diagrams.
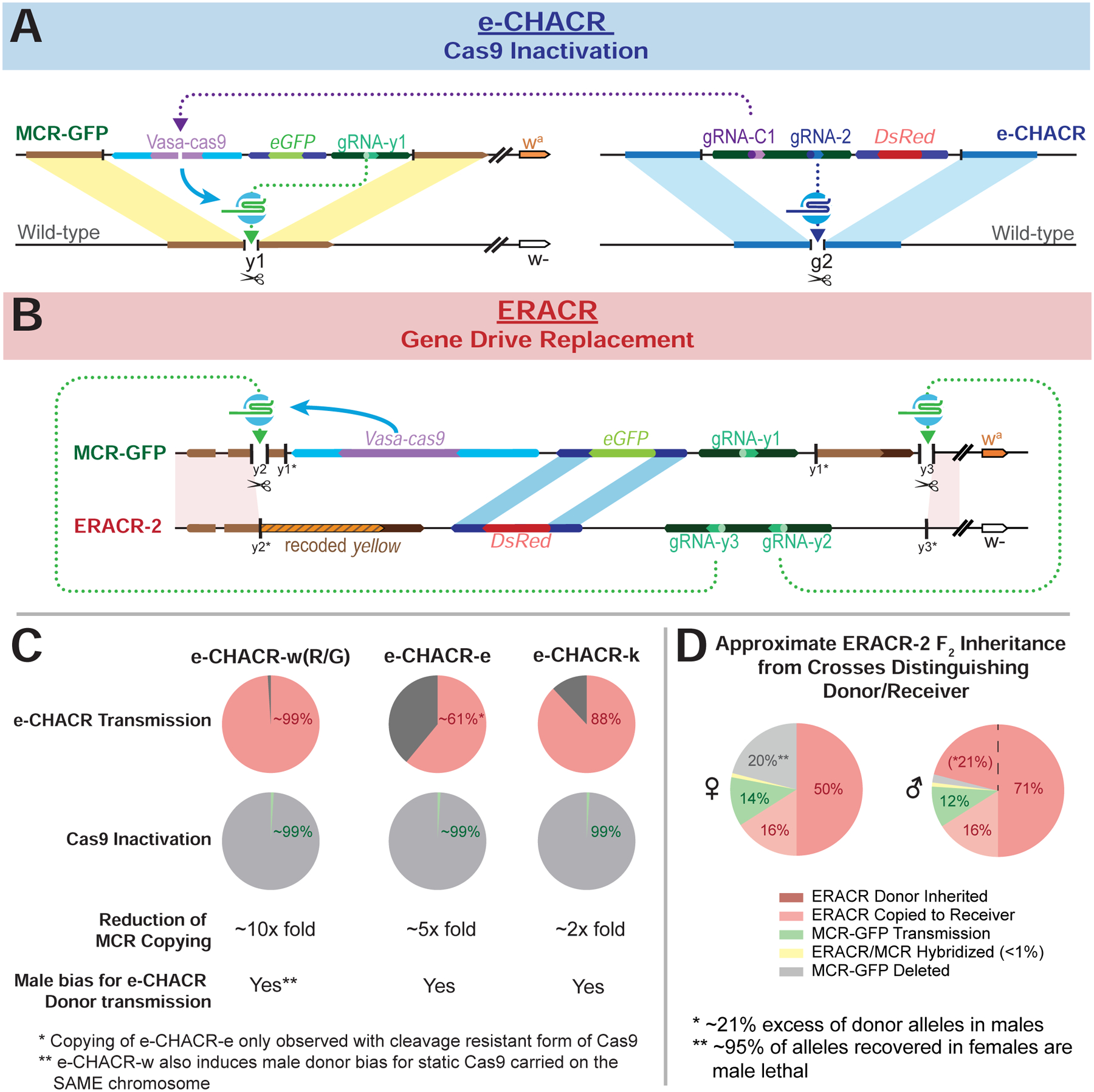
A) Diagram illustrating the generic action of the e-CHACRs against the MCR-GFP gene-drive. B) Diagram outlining the structures and homologous sequences serving as sites for potential SDSA-mediated partial copying between ERACR-2 and the MCR-GFP constructs (lightly shaded parallelograms). y1*, y2* and y3* indicate the loss of the gRNA-y1, gRNA-y2 and gRNA-y3 cut sites accompanying genomic insertion of the MCR-GFP element (gRNA-y1) or ERACRs (gRNA-y2 and gRNA-y3). C) Pie charts summarizing key e-CHACR performance parameters. D) Pie chart summary of F2 male and female progeny outcomes derived from F1 ERACR-2/MCR-GFP master females separated by inferred donor (w− = red shaded sectors) versus receiver (wa: peach = ERACR copied, green = MCR-GFP retention; gray =MCR-GFP deleted) chromosomes.
Drive performance of drive and neutralization systems
Several factors contribute to levels of drive we observed, which ranged in transmission frequencies from >95% (e-CHACR-wG/R inserted into the w locus) to only ~50% Mendelian inheritance (e-CHACR-e, but see below). e-CHACR-k and the MCR-GFP drive element copied at intermediate rates (~85%), which for the MCR-GFP is somewhat lower than estimated in prior experiments where transmission of an unmarked MCR element was estimated based on full yellow body phenotypes (Gantz and Bier, 2015). Since a significant fraction of crosses using the marked MCR-GFP element transmitted it to 100% of progeny, this may also have been the case in those original experiments, which were based on only a small number of crosses. Alternatively, it is possible that we overestimated gene conversion (e.g., the mosaic group might not have inherited the MCR). The size of the drive cassette could also impact copying efficiency since we have noted higher inheritance rates for a smaller drive element using the same gRNA-y1 (e.g., (López Del Amo et al., 2019), which was in the 90% range.
In pair-mating crosses, all three ERACRs deleted the target MCR-GFP gene-drive element from 74% of target chromosomes and copied themselves in its place as intended in 31% of those cases. The combined effect of these two outcomes is pronounced super-Mendelian inheritance of ERACR alleles in F2 males (85–90%). In females, only copying events contributed to biased inheritance of the ERACR (e.g., ~65%), since male-lethal MCR-deleted chromosomes can survive in the heterozygous state (~20% of total females). In multi-generational contexts, however, damaged alleles carried by females are rapidly purged from the population (see below).
As noted above, e-CHACRs copied at different rates, which most likely reflects relative gRNA efficiencies. Two cases are informative in this regard. First, e-CHACR-e did not copy when combined with Cas9, although in a split-drive configuration, without the Cas9-targeting gRNA, it exhibited modest drive (~60%) (López Del Amo et al., 2019). When combined with a cleavage resistant Cas9* form, however, e-CHACR-e drove at levels similar to those in the split-drive experiments. We hypothesize that a “weak” gRNA, such as that carried by e-CHACR-e, can fail entirely if the source of Cas9 is eliminated prematurely. Similarly, the intermediate level of MCR-GFP drive (~85%) was reduced 2–10 fold when combined with various e-CHACRs, again suggesting that early elimination of Cas9 activity can reduce the performance of suboptimal gRNAs. In contrast, when the e-CHACR-wG/Rs carrying an efficient gRNA were combined with static or MCR-GFP sources of Cas9, they copied at rates equal to those observed in split-drive experiments (López Del Amo et al., 2019). Thus, choosing an efficient gRNA seems to be an important design feature for e-CHACR elements.
ERACRs carry two gRNAs targeting the receiver chromosome at two neighboring sites. The two gRNAs increase the rate of ERACR copying relative to single-cut ERACRs that carry only one or the other gRNA - suggesting some form of synergy even though each gRNA was expected to act independently. This disparity was significantly more pronounced when the two gRNA cut sites were spaced further apart (e.g., when the MCR-GFP element is inserted between them).
Neutralizing elements can bias chromosomal inheritance
Marking donor versus receiver chromosomes permitted us to discern three different types of biased inheritance of these chromosome homologues. First, e-CHACRs cutting the same chromosome twice generated a 2:1 bias in favor of transmitting the donor X chromosome in males, but not females. This bias did not lead to any obvious permanent damage, however, since females segregated both donor and receiver chromosomes to their progeny with equal frequency (Fig. S3E). Second, e-CHACRs generated a class of donor MCR-GFP alleles lacking the internal GFP marker, over half of which were male lethal. In this scenario, gRNAs from e-CHACRs (anti-Cas9 gRNAs) and MCR-GFP (driving gRNA) elements target both chromosome homologs in almost exactly same location, a situation most often favoring the receiver allele, which, unlike the Cas9 transgene, is flanked by perfect homology on both sides. Finally, ERACRs with two gRNAs targeting neighboring sites on the same chromosome homolog generated an abundant class of damaged chromosomes. This effect was not the result of dual cutting per-se, since single-cut ERACRs generated comparable frequencies of damage. Thus, one-sided homology mismatch rather than dual cutting of the target most likely underlies ERACR-induced chromosome damage.
e-CHACRs and ERACRs perform effectively as designed in cage experiments
Despite imperfections revealed in pair-matings, e-CHACRs and ERACRs performed largely as intended in multi-generational cage studies. In these experiments, e-CHACR-wR completely eliminated Cas9 activity and drove itself to 100% homozygous introgression in 9–10 generations. Similarly, ERACR-2 entirely replaced the MCR-GFP element over 6 generations. Several factors contributed to these successful outcomes (Fig. 8B): 1) efficient Cas9 mutagenesis by the targeting gRNA, 2) efficient e-CHACR-wR copying, and 3) absence of somatic mosaicism or shadow-drive caused by accumulated Cas9/gRNA complexes (this factor, which greatly reduces formation of drive-resistant NHEJ alleles, likewise pertains to ERACR performance). Also, a fitness cost may be associated with the MCR-GFP element, inferred from modeling and competition between the MCR-GFP element and a y− allele in cages (Fig. S22A). In addition, single generation crosses revealed that e-CHACRs can damage MCR-GFP chromosomes, generating a class of GFP− male-lethal alleles. These alleles would be invisible in the cage experiments since they do not survive either in males or homozygous females but would deplete the MCR-GFP allelic pool.
Three notable features distinguished the drive trajectories of the y− ERACR-min and y+ ERACR-2, all of which derive from their y− versus y+ phenotypes. First, ERACR-2 rapidly drove to 100% replacement, while ERACR-min plateaued and then increased more slowly without entirely eliminating the MCR-GFP element. Second, inter-cage variation was much reduced for the ERACR-2 trials than for ERACR-min, where the observed variation was more typical (~5–10%). Finally, ERACR-2 experienced a one generation lag in producing progeny carrying both the DsRed+ ERACR and MCR-GFP elements (yellow curves in Fig. 7D versus Fig. 7F).
The significant assortative mating advantage conferred upon y+ ERACR-2 males relative to y− MCR-GFP males is well-documented (Barker, 1962; Bastock, 1956; Diederich, 1941; Merrell, 1949; Sturtevant, 1915), and was confirmed in our own experiments (Fig. S23A–E). Indeed, the y+ ERACR-2 allele overtook a point-mutant y− allele in competitive cage experiments (Fig. S23E), albeit more gradually than when driving in a Cas9-dependent fashion against the MCR-GFP element (Fig. 7F). The more rapid and complete drive of ERACR-2 compared to ERACR-min can be attributed to dual (Cas9-mediated + assortative mating) versus single (Cas9-mediated) drive mechanisms being operative. Similarly, the initial delay in generating ERACR-2/MCR-GFP heterozygotes is expected if y+ ERACR-2 females preferentially mated with their own kind. The much-reduced inter-cage variation observed for the ERACR-2 trajectories may reflect two independently acting processes: drive produced by Cas9 delivered in-trans and a selective pressure resulting from assortative mating favoring y+ males. These dual factors provide fewer opportunities for stochastic variation to accumulate.
Strengths of ERACR and e-CHACR neutralizing elements:
These studies provide encouraging support for neutralizing a gene-drive with e-CHACRs or ERACRs. The major strength of e-CHACRs is that they act generically and can neutralize SpCas9 gene-drives inserted at various locations in the genome. e-CHACRs can efficiently inactivate Cas9 and copy themselves (e-CHACR-wG/R elements). The primary advantage of ERACRs is that they can delete and replace the gene-drive, thereby eliminating any undesired effect associated with that element. ERACRs perform as intended less often, replacing the gene-drive as designed only about a third of the time (Fig. 4A–B). Nonetheless, ERACRs, particularly ERACR-2 carrying recoded sequences to restore target locus function, performed very effectively in population cages.
In the future, these two neutralizing strategies could be combined to exploit the strengths of each system. These systems also could be implemented with other proposed mitigation measures such as elements carrying anti-Cas9 proteins or inundative releases of strains carrying functional cleavage-resistant alleles of the targeted locus. Such passive elements could spread rapidly if insertion of a gene-drive disrupted locus function. Also a CATCHA construct has been described that is inserted at the site of the gene-drive (similar to ERACRs), and is designed to copy into the Cas9 transgene (Wu et al., 2016). While CATCHA elements could target Cas9 sources located elsewhere in the genome, they would not copy in those contexts. Also, because the cassette must be flanked with Cas9 homology arms, it does not readily deliver an in-frame recoded target gene.
Another potential strategy is to incorporate a “weakened” gRNA* targeting the same essential Cas9 amino acid residues as gRNA-C1 or gRNA-C3 into the drive element itself. gRNA*s carrying one or two base-pair mismatches to the target sequence should reduce Cas9 cleavage by 1–2 logs. While not expected to appreciably interfere with the spread of a gene-drive carrying such a gRNA*, the Cas9 transgene should be mutated at rates ~105 greater than by spontaneous mutation once the drive attains full introgression into a population, thereby accelerating elimination of Cas9 from the population if it imposed a fitness cost.
Implications for the potential implementations of neutralizing systems:
While e-CHACR and ERACR elements behave largely as expected to curtail the spread of gene-drives, they also produce unexpected outcomes (e.g., generation of drive-competent chimeric ERACR/MCR-GFP elements) and can falter in copying (e.g., e-CHACRs with non-optimal gRNAs). Thus, while these experiments provide optimism for strategies to retard wayward gene-drive systems if necessary, we agree with the recommendations of the National Academy of Sciences Report on Gene-Drives (Organisms, 2016) regarding the cautious use of such neutralization systems. We concur with their appraisal that the decision to go forward with potential releases of gene-drive systems should not be predicated on constructing neutralizing elements, and that such systems should be developed only for precautionary purposes. If one has concerns about a potential gene-drive system somehow going awry, then surely such concerns would only be amplified by release of a second element which could generate yet more complex genetic outcomes. Nonetheless, the current in-depth studies provide encouraging support for the use of these types of mitigating strategies should there be an unanticipated need for them.
STAR Methods
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Ethan Bier (ebier@ucsd.edu).
Materials Availability
All unique plasmids or Drosophila lines generated by this study are available from the Lead Contact with a completed Materials Transfer Agreement.
Data and Code Availability
Raw counting data from Drosophila melanogaster fly crosses, construct maps, and all sequencing data/chromatograms have been deposited on Mendeley Data (doi: 10.17632/hjcpd6j8rn.1).
Experimental Model and Subject Details
Drosophila rearing and Genetic Experiments
All genetic experiments were conducted in a high-security Arthropod Containment Level 2 (ACL2) barrier facility, in accordance with protocols approved by the Institutional Biosafety Committee from University of California San Diego. All materials and waste are frozen for 48 hours prior to removal from the facility followed by autoclaving. Flies were kept triple-contained in shatter-proof polypropylene plastic vials. Stocks were maintained at 18°C while crosses were carried out at 25°C on a 12 hour day/night cycle on cornmeal media.
Multi-Generational Population cage studies
All population cage experiments were conducted at 25°C with a 12 hour day-night cycle, in 250 ml bottles containing standard cornmeal medium. Seeding populations for all drive experiments included equal numbers of unmated females and males for each genotype. 5–7 days following introduction into the cage and successful mating, all flies (generation n) are removed. Subsequent progeny (generation n+1) were collected, then randomly separated into two pools, one group is further analyzed and screened while the other pool is used for further seeding of the next cage (generation n+2).
Method Details
Plasmid Construction
All Constructs were cloned using standard recombinant DNA techniques, including PCR amplification with Q5 Hotstart master mix (NEB #M0494S) and Gibson assembly with NEBuilder HiFi DNA Assembly Master Mix (New England Biolabs, Cat. # E2621) . Following successful cloning, the plasmids were transformed into NEB 5-alpha Electrocompetent Competent E. coli (New England Biolabs, Cat. # C2989). We list starting plasmids, oligos, and restriction enzymes used to generate each construct. Refer to supplementary materials (Table S2) for full sequences of plasmids and oligos.
MCR-GFP construct by “tagging”
The MCR-GPF construct used for these studies was generated by genetic “tagging” of the unmarked prototype yMCR construct (Gantz and Bier, 2015). Briefly, a plasmid construct carrying the eGFP gene expressed under control of the 3XP3 eye promoter, followed by 3’ SV40 polyadenylation sequences, flanked by homology sequences present in the unmarked yMCR construct (Cas9 transgene and U6 gRNA homology arms), and carrying a construct expressing a gRNA directing cleavage between the Cas9 transgene and U6-gRNA of the yMCR was inserted into the genome at the same site as the yMCR using gRNA-y1. Transgenic fly strains carrying this tagging construct (eGFP-Tagger) were recovered and crossed to the yMCR line to generate MCR/eGFP-Tagger females. Single crosses were performed by mating with Basc balancer males and the daughters in the resulting offspring were screened for a y- body color, indicative of Cas9 activity. Vials with Cas9 activity indicated a successful tagging of the yMCR construct and individual males were crossed with cut resistant gRNA-y1 site females (contain static Cas9 at gRNAy1 site) to generate isogenic homozygous stocks for use in this study.
Microinjection of ERACR and e-CHACR Constructs
Plasmids were purified using the Qiagen Plasmid Midi kit (#12191). ERACR and e-CHACR constructs were co-injected with a transient source of pAct-Cas9 (pAct-Cas9 was a gift from Fillip Port (Addgene plasmid # 62209) by Best Gene Inc. Injection mixes were assembled with either ERACR or e-CHACR donor plasmids (final concentration: 700 ng/μl) and pAct-Cas9 (final concentration: 300 ng/μl) in a volume of 50 μl. ERACR mixes were injected into a w1118 stock (BDSC #5905) while e-CHACR mixes were injected into an Oregon-R stock (BDSC #2376). All constructs were fully sequenced prior to injection and subsequently generated transgenic stocks were confirmed through sanger sequencing as well.
Recovery of Transformants and Genomic DNA Isolation
Injected flies were crossed the w1118 stock and progeny were screened for DsRed or GFP positive individuals, which were then subsequently crossed to an FM7 (first chromosome) or TM3 (third chromosome) balancer. Homozygous lines were established based on the absence of balancer alleles. For analysis of individuals containing gene-drive elements, flies were frozen in an ACL-2 facility for 48 hours prior to removal from the facility and DNA isolation. Genomic DNA was prepared from individual male flies according to protocols by (Gloor et al., 1993).
Drosophila PCR and Sequence Analysis
Sequencing PCR reactions were assembled with Q5 Hotstart master mix (NEB #M0494S), KOD Xtreme Hot Start DNA Polymerase (Millipore #71975), Phusion High-Fidelity PCR Master Mix with HF Buffer (Thermo-Fisher #F531S), or Platinum SuperFi DNA Polymerase (ThermoFisher #12351010). Primers used to amplify PCR products are listed in Table S4. PCR samples were purified using the QIAquick PCR purification kit (Qiagen #28104) prior to sanger sequencing.
Quantification and Statistical Analysis
Mathematical Modeling
Model fitting was carried out for the ERACR-min and ERACR-2 cage experiments using Markov chain Monte Carlo (MCMC) methods in which genotype-specific fitness parameters were estimated, including 95% credible intervals (CrIs). We considered discrete generations and Mendelian inheritance rules at the gene drive locus, with the exception that, for females heterozygous for the ERACR (E) and homing (H) allele, a proportion of the homing alleles are cleaved, while a proportion, , remain H alleles (Fig. Smod1). Of those that are cleaved, a proportion are subject to accurate homology-directed repair (HDR) and become E alleles, while a proportion become resistant alleles. Of those that become resistant alleles, a proportion become in-frame, cost-free resistant (R) alleles, while the remainder become out-of-frame or otherwise costly resistant (broken, B) alleles. Parameter values were fixed for and based on the experimental results depicted in Fig. S12. These considerations allowed us to calculate the expected genotype frequencies in the next generation, and to explore the fitness and assortative mating parameters that maximize the likelihood of the experimental data. Estimated parameters include the fitness cost associated with males having the homing allele females homozygous for the homing allele and having one copy of the homing or broken allele in females or respectively. BB females and BY males were known to be unviable. Populations were assumed to be randomly mixing, with the exception of the ERACR-2 experiment, in which females having the y+ phenotype preferentially mated with males having the y+ phenotype. For ERACR-2, we estimated an additional parameter representing the fraction by which y+ females reduce their mating with y- males. The modeling framework is described in full in the Supporting Information.
Statistical Methods
Statistical analysis was performed using GraphPad Prism. One-way independent measures ANOVA followed by Sidak’s Multiple Comparisons test were performed for graphs in Figure 1D and Figure 6 (all panels). All other graphs use one-way independent measures ANOVA followed by Tukey’s post-hoc test. Significance was determined as follows: * denotes p<0.05, ** denotes p<0.01, *** denotes p<0.001, and **** denotes p<0.0001.
Supplementary Material
Table S4: PCR primers for sequence analysis for Figures S6 and S14, related to STAR methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| NEB 5-alpha Electrocompetent Competent E. coli | NEB | C2989 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Q5 Hotstart High-Fidelity 2X Master Mix | NEB | M0494S |
| Phusion High-Fidelity PCR Master Mix with HF Buffer | Thermo-Fisher | F531S |
| Platinum SuperFi DNA Polymerase | Thermo-Fisher | 12351010 |
| KOD Xtreme Hot Start DNA Polymerase | Millipore | 71975 |
| Critical Commercial Assays | ||
| Qiagen Plasmid Midi Kit | Qiagen | 12191 |
| QIAquick PCR purification kit | Qiagen | 28104 |
| NEBuilder HiFi DNA Assembly Master Mix | NEB | E2621 |
| Deposited Data | ||
| Raw data (counts from fly experiments, construct maps, sequencing chromatograms) | Generated by this study | doi: 10.17632/hjcpd6j8rn.1 |
| Experimental Models: Organisms/Strains | ||
| Oregon-R | Bloomington Drosophila Stock Center | BDSC #5905 |
| W1118 | Bloomington Drosophila Stock Center | BDSC #2376 |
| FM7a | Bloomington Drosophila Stock Center | BDSC #785 |
| Basc | Bloomington Drosophila Stock Center | BDSC #806 |
| w[a] | Bloomington Drosophila Stock Center | BDSC #148 |
| ac[4] w[a] | Bloomington Drosophila Stock Center | BDSC #8 |
| w[*]; TM3, Sb[1] Ser[1]/TM6B, Tb[1] | Bloomington Drosophila Stock Center | BDSC#2537 |
| y-; w- (used in MCR cage trial) | Gift from the Gantz Lab | N/A |
| y-; w- (F5 line used in ERACR cage trial) | Gantz et al., 2015 | N/A |
| Vasa Cas9 in yellow (y1) w[1118A] | Gift from the Gantz Lab | N/A |
| Vasa Cas9 in yellow (y1) w+ | Gift from the Gantz Lab | N/A |
| Vasa Cas9 in white (w2) | Gift from the Gantz Lab | N/A |
| w- MCR GFP | This study | N/A |
| w[a] MCR GFP | This study | N/A |
| w+ MCR GFP | This study | N/A |
| e-CHACR-AG; see Table S3 | This study | N/A |
| e-CHACR-AR; see Table S3 | This study | N/A |
| eCHACR-e; see Table S3 | This study | N/A |
| e-CHACR-k; see Table S3 | This study | N/A |
| vCas9-R; see Table S3 | This study | N/A |
| ERACR-min; see Table S3 | This study | N/A |
| ERACR-1; see Table S3 | This study | N/A |
| ERACR-2; see Table S3 | This study | N/A |
| ERACR-2 y2 Single-cut; see Table S3 | This study | N/A |
| ERACR-2 y3 Single-cut; see Table S3 | This study | N/A |
| X-duplication lines; see Table S4 | Bloomington Drosophila Stock Center | Various; See Table S5 |
| Oligonucleotides | ||
| Cloning/construct design primers; see Table S2 | N/A | N/A |
| PCR primers; see Table S4 | N/A | N/A |
| Recombinant DNA | ||
| ERACR-min | This study | N/A |
| ERACR-1 | This study | N/A |
| ERACR-2 | This study | N/A |
| ERACR-2 y2 single cut | This study | N/A |
| ERACR-2 y3 single cut | This study | N/A |
| e-CHACR-AG | This study | N/A |
| e-CHACR-AR | This study | N/A |
| e-CHACR-e | This study | N/A |
| e-CHACR-k | This study | N/A |
| Vasa Cas9 resistant to e-CHACR-e | This study | N/A |
| pAct-Cas9 | Gift from Fillip Port | Addgene # 66209 |
| Software and Algorithms | ||
| GraphPad Prism | GraphPad | https://www.graphpad.com/scientificsoftware/prism/ |
| Snapgene | Snapgene | https://www.snapgene.com |
| Microsoft Excel | Microsoft | N/A |
Highlights.
e-CHACRs can efficiently copy and inactivate Cas9 activity (~99%).
e-CHACRs spread to 100% prevalence in cage trials and eliminate Cas9 activity.
ERACRs often copy, but can also damage the target chromosome.
ERACRs can efficiently delete and replace a gene-drive in population cages
Acknowledgements:
We thank Anthony James, Bill McGinnis, Kim Cooper, Sara Werner, and members of the Bier laboratory for comments and suggestions on the manuscript. We thank James Haber for suggesting the idea of testing single-cut versions of the ERACR and Gerard Terradas for help counting crosses during the campus Covid-19 lockdown. These studies were supported by NIH grant R01 GM117321, a Paul G. Allen Frontiers Group Distinguished Investigator Award to E. Bier (E.B.), funding from DARPA’s Safe Genes program (Contract # HR0011-17-2- 0047) to O.S.A., J.M.M., and E.B. (the views, opinions and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the U.S. Government), NIH grant DP5OD023098, which supported V.M.G., and a gift from the Tata Trusts in India to TIGS-UCSD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: E. Bier, V. Gantz, and O.S. Akbari have equity interest in two companies: Synbal Inc. (E.B. and V.G) and Agragene, Inc. (E.B., V.G, and O.S.A). These companies may potentially benefit from the research results. E.B. and V.G also serve on Synbal Inc.’s Board of Directors and Scientific Advisory Board, and E.B., V.G, and O.S.A serve on Agragene Inc.’s Scientific Advisory Board. The terms of these arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. All other authors declare no competing interests.
References
- Adelman Z, Akbari O, Bauer J, Bier E, Bloss C, Carter SR, Callender C, Denis AC, Cowhey P, Dass B, et al. (2017). Rules of the road for insect gene drive research and testing. Nat Biotechnol 35, 716–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker JS (1962). Studies of Selective Mating Using the Yellow Mutant of Drosophila Melanogaster. Genetics 47, 623–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock M (1956). A gene mutation which changes a behaviour pattern. Evolution 10, 421–439. [Google Scholar]
- Bozas A, Beumer KJ, Trautman JK, and Carroll D (2009). Genetic analysis of zinc-finger nuclease-induced gene targeting in Drosophila. Genetics 182, 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E, Yagi R, Debrunner M, Beck-Schneider D, Burger A, Escher E, Mosimann C, Hausmann G, and Basler K (2019). CRISPR-induced double-strand breaks trigger recombination between homologous chromosome arms. Life Sci Alliance 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt A (2003). Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc Biol Sci 270, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champer J, Reeves R, Oh SY, Liu C, Liu J, Clark AG, and Messer PW (2017). Novel CRISPR/Cas9 gene drive constructs reveal insights into mechanisms of resistance allele formation and drive efficiency in genetically diverse populations. PLoS Genet 13, e1006796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier BS, and Stoddard BL (2001). Homing endonucleases: structural and functional insight into the catalysts of intron/intein mobility. Nucleic Acids Res 29, 3757–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis CF (1968). Possible use of translocations to fix desirable genes in insect pest populations. Nature 218, 368–369. [DOI] [PubMed] [Google Scholar]
- Delattre M, Anxolabehere D, and Coen D (1995). Prevalence of localized rearrangements vs. transpositions among events induced by Drosophila P element transposase on a P transgene. Genetics 141, 1407–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deredec A, Burt A, and Godfray HC (2008). The population genetics of using homing endonuclease genes in vector and pest management. Genetics 179, 2013–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo JE, Chavez A, Dietz SL, Esvelt KM, and Church GM (2015). Safeguarding CRISPR-Cas9 gene drives in yeast. Nat Biotechnol 33, 1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich GW (1941). Non-random mating between yellow-white and wild type Drosophila melanogaster. Genetics 26, 148. [Google Scholar]
- Do AT, Brooks JT, Le Neveu MK, and LaRocque JR (2014). Double-strand break repair assays determine pathway choice and structure of gene conversion events in Drosophila melanogaster. G3 (Bethesda) 4, 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury DW, Dapper AL, Siniard DJ, Zentner GE, and Wade MJ (2017). CRISPR/Cas9 gene drives in genetically variable and nonrandomly mating wild populations. Sci Adv 3, e1601910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhoff PA, Wenger EA, Godfray HC, and Burt A (2017). Impact of mosquito gene drive on malaria elimination in a computational model with explicit spatial and temporal dynamics. Proc Natl Acad Sci U S A 114, E255–E264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz VM, and Bier E (2015). The mutagenic chain reaction: a method for converting heterozygous to homozygous mutations. Science 348, 442–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz VM, and Bier E (2016). The dawn of active genetics. Bioessays 38, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz VM, Jasinskiene N, Tatarenkova O, Fazekas A, Macias VM, Bier E, and James AA (2015). Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc Natl Acad Sci U S A 112, E6736–6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald HA, Gantz VM, Poplawski G, Xu XS, Bier E, and Cooper KL (2019). Super-Mendelian inheritance mediated by CRISPR-Cas9 in the female mouse germline. Nature 566, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A, Haque T, Bobik M, Xu XS, Klanseck C, Kushwah RBS, Berni M, Kaduskar B, Gantz VM, and Bier E (2019). Efficient allelic-drive in Drosophila. Nat Commun 10, 1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond A, Galizi R, Kyrou K, Simoni A, Siniscalchi C, Katsanos D, Gribble M, Baker D, Marois E, Russell S, et al. (2016). A CRISPR-Cas9 gene drive system targeting female reproduction in the malaria mosquito vector Anopheles gambiae. Nat Biotechnol 34, 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S, Collins FH, Welkhoff PA, Emerson C, Godfray HCJ, Gottlieb M, Greenwood B, Lindsay SW, Mbogo CM, Okumu FO, et al. (2018). Pathway to Deployment of Gene Drive Mosquitoes as a Potential Biocontrol Tool for Elimination of Malaria in Sub-Saharan Africa: Recommendations of a Scientific Working Group(dagger). Am J Trop Med Hyg 98, 1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce EF, Paul A, Chen KE, Tanneti N, and McKim KS (2012). Multiple barriers to nonhomologous DNA end joining during meiosis in Drosophila. Genetics 191, 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrou K, Hammond AM, Galizi R, Kranjc N, Burt A, Beaghton AK, Nolan T, and Crisanti A (2018). A CRISPR-Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat Biotechnol 36, 1062–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Yang T, Kandul NP, Bui M, Gamez S, Raban R, Bennett J, Sánchez C,HM, Lanzaro GC, Schmidt H, et al. (2019). Development of a Confinable Gene-Drive System in the Human Disease Vector, Aedes aegypti. bioRxiv, 645440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, and Potter CJ (2016). Non-Mendelian Dominant Maternal Effects Caused by CRISPR/Cas9 Transgenic Components in Drosophila melanogaster. G3 (Bethesda). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DL, Hardy RW, Ripoll P, and Lindsley D (2016). Gonadal Mosaicism Induced by Chemical Treatment of Sperm in Drosophila melanogaster. Genetics 202, 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez Del Amo V, Bishop AL, Sanchez CH, Bennett JB, Feng X, Marshall JM, Bier E, and Gantz VM (2020). A transcomplementing gene drive provides a flexible platform for laboratory investigation and potential field deployment. Nat Commun 11, 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Del Amo V, Leger BS, Cox KJ, Gill S, Bishop AL, Scanlon GD, Walker JA, Gantz VM, and Choudhary A (2019). Small-molecule control of super-Mendelian inheritance in gene drives. bioRxiv, 665620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macreadie IG, Scott RM, Zinn AR, and Butow RA (1985). Transposition of an intron in yeast mitochondria requires a protein encoded by that intron. Cell 41, 395–402. [DOI] [PubMed] [Google Scholar]
- Merrell DJ (1949). Selective Mating in Drosophila Melanogaster. Genetics 34, 370–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisms, C.o.G.D.R.i.N.-H. (2016). Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values (THE NATIONAL ACADEMIES PRESS, Washington D.C.). [PubMed] [Google Scholar]
- Pham TB, Phong CH, Bennett JB, Hwang K, Jasinskiene N, Parker K, Stillinger D, Marshall JM, Carballar-Lejarazu R, and James AA (2019). Experimental population modification of the malaria vector mosquito, Anopheles stephensi. PLoS Genet 15, e1008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant AH (1915). Experiments on sex recognition and the problem of sexual selection in Drosophila. J Animal Behavior 5, 351–366. [Google Scholar]
- Valderrama JA, Kulkarni SS, Nizet V, and Bier E (2019). A bacterial gene-drive system efficiently edits and inactivates a high copy number antibiotic resistance locus. Nature Communications 10, 5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella MR, Gunning CE, Lloyd AL, and Gould F (2017). Evaluating strategies for reversing CRISPR-Cas9 gene drives. Scientific Reports 7, 11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmbrod KL, Kobokovich K, West R, Ray G, Trotochaud M, and Montague M (2020). Gene Drives: Pursuing Opportunities, Minimizing Risk - A Johns Hopkins University Report on Responsible Governance (Johns Hopkins University: Johns Hopkins Bloomberg School of Public Health, Center for Health Security; ). [Google Scholar]
- Wei DS, and Rong YS (2007). A genetic screen for DNA double-strand break repair mutations in Drosophila. Genetics 177, 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Luo L, and Gao XJ (2016). Cas9-triggered chain ablation of cas9 as a gene drive brake. Nat Biotechnol 34, 137–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XS, Gantz VM, Siomava N, and Bier E (2017). CRISPR/Cas9 and active genetics-based trans-species replacement of the endogenous Drosophila kni-L2 CRM reveals unexpected complexity. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S4: PCR primers for sequence analysis for Figures S6 and S14, related to STAR methods
Data Availability Statement
Raw counting data from Drosophila melanogaster fly crosses, construct maps, and all sequencing data/chromatograms have been deposited on Mendeley Data (doi: 10.17632/hjcpd6j8rn.1).


