Abstract
Objective
To assess the relationships of prenatal and childhood smoke exposure with specific neurodevelopmental and behavioral problems during early childhood.
Study design
A subsample (n = 386) of mother–child dyads from the Newborn Epigenetic Study (NEST) prebirth cohort participated in the study. Cotinine concentrations were used to objectively measure prenatal and childhood smoke exposure when youth were aged 3–13 years. Multivariable regression models were used to estimate associations of prenatal and childhood cotinine concentrations with performance on the National Institutes of Health (NIH) Toolbox and attention-deficit/hyperactivity disorder and behavioral symptoms, measured using the Behavior Assessment System for Children, 2nd edition (BASC-2).
Results
After adjusting for confounders, childhood cotinine concentrations were associated with poorer cognitive performance on tasks measuring cognitive flexibility (B = 1.29; P = .03), episodic memory (B = 0.97; P = .02), receptive language development (B = 0.58; P = .01), and inhibitory control and attention (B = 1.59; P = .006). Although childhood cotinine concentration was associated with higher levels of attention problems (B = 0.83; P = .004) on the BASC-2, after adjustment for confounders, the association is nonsignificant. Although associations for maternal cotinine concentrations were null, an interaction was detected between prenatal and childhood cotinine concentrations on the NIH Toolbox Picture Vocabulary Task (P = .02).
Conclusions
Our findings suggest that childhood tobacco smoke exposure may lead to poorer attention regulation and language acquisition, complex visual processing ability, and attention problems.
INTRODUCTION
Attention-deficit/hyperactivity disorder (ADHD) is associated with social, emotional, cognitive, and academic impairments and negative outcomes that can persist into adulthood.1–3 Children with language impairments also have difficulties establishing social relationships and maintaining employment in adulthood.4 Additionally, children’s cognitive ability in preschool and early childhood is associated with adult educational attainment.5
Tobacco smoke is a well-known teratogen, and studies have found significant associations between prenatal and childhood secondhand smoke (SHS) exposure and cognitive and behavioral outcomes in children.6–16 However, not all studies have found a clear link between prenatal maternal smoking and childhood SHS exposure and cognitive and behavioral outcomes.17–20 Some evidence suggests that compared with prenatal smoke exposure, childhood SHS exposure may lead to poorer cognitive and behavioral outcomes, such as impaired nonverbal reasoning, receptive vocabulary deficits, and elevated behavioral difficulties.17,21 Trasti et al found that once maternal education was adjusted for in multivariable models, children of mothers who smoked during pregnancy did not have worse cognitive functioning compared with children of mothers who did not smoke during pregnancy.19 However, others have found that neither prenatal nor early childhood SHS exposure were associated with behavioral problems, yet significant associations were found between prenatal and early childhood SHS exposure and deficits in executive functioning.22
The aims of the current study were to examine independent associations between prenatal smoke exposure, measured via maternal plasma cotinine concentration collected during pregnancy, and childhood SHS exposure, measured via child saliva cotinine concentration, on children’s neurodevelopment and behavioral functioning. We hypothesized that both maternal prenatal cotinine and childhood cotinine concentration would be related to poorer cognitive outcomes in children—especially those linked to the executive functions of inhibitory control, attention, and cognitive flexibility. We also hypothesized that both maternal prenatal cotinine and childhood cotinine concentration would be related to higher levels of parent-rated behavioral problems—especially ADHD symptoms.
METHODS
Participants were part of the Newborn Epigenetic Study (NEST). NEST is a prebirth cohort study based in the southeastern US that was initiated in 2005. The Duke University Health Institutional Review Board approved the studies involving these participants, and written informed consent was obtained from all mothers. Participant identification and enrollment procedures have been described in greater detail previously.23,24 In brief, 2595 pregnant women were recruited from prenatal clinics serving Duke University Hospital and Durham Regional Hospital Obstetrics facilities between April 2005 and June 2011. Eligibility criteria were age3 18 years, pregnant, and intention to use 1 of the 2 obstetrics facilities for the index pregnancy, to enable access to labor and birth outcome data. At enrollment, maternal blood specimens were collected, along with survey data on health, nutrition, stress, and lifestyle behaviors.
Women were recontacted to participate in a follow-up study between 2013 and 2019. To be eligible for the follow up study, women had to speak English and children had to be at least 3 years old to allow for cognitive testing with the National Institutes of Health (NIH) Toolbox. The current study includes data from 386 mother–child pairs who participated in the follow-up study. There were more Hispanic children, more mothers with a high school education and fewer mothers with a college degree, more underweight births, and more early births among the original cohort compared with the mother–child pairs included in the current study. There were no between-group differences in prenatal cotinine concentration and infant sex.
Eligible mothers were initially contacted via letters mailed from the study team and/or were recruited during a well child clinic visit. Mothers who agreed to participate were scheduled for a laboratory visit that included completing survey measures on their child’s health and behaviors, an IQ test, and assessment of executive functions. During the visit, children provided saliva samples for cotinine analyses, and like the mothers, completed an IQ assessment and a test of executive functions. Trained staff members administered the IQ tests and test of executive functions.
MEASURES
Tobacco Smoke Exposure
Tobacco smoke exposure was estimated objectively using cotinine, a metabolite of nicotine. Prenatal smoke exposure was estimated using maternal plasma blood samples that had been collected during pregnancy (mean, 20.7 weeks of gestation; SD, 12.9 weeks). Early childhood smoke exposure was estimated using children’s saliva samples that had been collected during their clinic visits. Children were asked to spit into a vial until approximately 5 mL of saliva was collected. Children who had difficulty producing saliva were asked to chew a parafilm to aid saliva production. In children who had difficulty spitting, an absorbent sponge tip was used to aid saliva collection.
Concentrations of cotinine were measured in plasma and saliva samples by liquid chromatography–tandem mass spectrometry. The system consists of a TSQ Quantum Access MAX triple-stage quadrupole mass spectrometer, coupled with Accela 1250 pump and Accela Open Autosampler. For plasma analysis, we spiked d3-cotinine into 200-mL samples and then added 1 mL of methanol and vortexed the mixture thoroughly. After centrifugation at 16 000 g for 10 minutes, the supernatant was transferred to a centrifuge tube and evaporated to dryness under ultrapure nitrogen gas. The residual was reconstituted with 200 mL of acetonitrile/water (15:85) for instrumental analysis. For saliva analysis, we spiked d3-cotinine into 100-mL samples and then added 50 mL of acetonitrile/methanol (80:20). After a 10-minute vortex and 30-minute centrifugation, the supernatant was used for instrumental analysis. We used a Phenomenex Luna 3m C18 (50 2 mm) column using an isocratic mobile phase elution at a flow rate of 200 mL/minute for chromatographic separation. The ion pairs of 177/98 m/z and 180/80 m/z were used to monitor cotinine and d3-cotinine concentrations, respectively. The detection limit of cotinine is 0.075 ng/mL for plasma and 0.024 ng/mL for saliva.
Cognitive and Executive Functioning
The NIH Toolbox Cognition Battery was used to assess child cognitive functioning.25 The Toolbox was administered in the laboratory by trained study staff. The Cognition Composite score as well as the following subscales were included: Flanker Inhibitory Control and Attention Test (FICA), Dimensional Change Card Sort Test (DCCS), Picture Sequence Memory Test (PSM), and Picture Vocabulary Test (PV). The FICA, DCCS, PSM, and PV subscales assess inhibitory control and attention, cognitive flexibility episodic memory, and receptive vocabulary (a proxy for language development), respectively. In addition to producing scores for each cognitive domain, a composite score was calculated from these subtest scores. The instrument was validated in a diverse sample and found to demonstrate good test–retest and convergent validity.25 Of note, the battery was administered via a laptop from December 2013 to January 2016 (n = 246) and then on an iPad from February 2016 to February 2020 (n = 138) once NIH Toolbox transitioned away from the web-based platform. Revised formulas provided by NIH Toolbox for comparison across platforms were used to calculate domain scores. According to guidelines provided by NIH Toolbox, raw scores were used in all comparisons as standardized scores were not available because we used both laptop and computer administration.
Maternal Intelligence
Maternal intelligence was assessed via the Wechsler Abbreviated Scale of Intelligence (WASI-II).26 The WASI-II provides a composite IQ score derived from 4 subtests: Block Design, Vocabulary, Matrix Reasoning, and Similarities. The WASI II was administered by study staff trained and supervised by a PhD-level clinical psychologist.
ADHD Symptoms and Self-Regulation
The Behavior Assessment System for Children, 2nd edition (BASC-2), Parent Rating Scales was used to assess ADHD related symptoms.27 There are several clinically relevant sub scales as well as composite scores, and the measure has been shown to discriminate well between children with ADHD and healthy controls.28 The current analyses included the BASC-2 Hyperactivity and Attention Problems subscales, reported as standardized T-scores (mean, 50; SD, 10).
Other Measures
Trained personnel abstracted parturition data from medical records after delivery, including birth weight, gestation period (in weeks), and infant sex. Other characteristics, such as maternal race, highest education, and prepregnancy body mass index were obtained from maternal self-report on a survey that was completed at enrollment during pregnancy. For both mothers and children, age was calculated by subtracting their birth date from the date of their follow-up visit. Given the heritability of ADHD, we also controlled for mothers’ ADHD symptoms by including mothers’ scores on the ADHD Symptoms Total subscale from the Conners Adult ADHD Rating Scales (CAARS).29
Statistical Analyses
To be included in the current analyses, participants had to have had cotinine assayed from prenatal maternal blood samples and childhood saliva samples, outcome data from the NIH Toolbox, ratings from the BASC-2 assessment, and data on covariates potentially related to the outcomes. Because cotinine concentrations were right-skewed, a natural log transformation was used to normalize the data prior to conducting analyses; for cotinine values equal to 0, 0.0001 was added only to those values prior to transformation.
Three multivariable linear regression models were conducted for each outcome variable, which included the NIH Toolbox Cognitive Composite score and the 4 subdomains (FICA, DCCS, PSM and PV), and each of the BASC-2 subscales (Attention Problems and Hyperactivity). Child’s age and sex and mother’s race were included to account for the unstandardized NIH Toolbox scores. The first model included log-transformed prenatal and childhood cotinine values (un adjusted model). The second model included log-transformed prenatal cotinine and childhood cotinine values and covariates (fully adjusted model). The covariates were selected by constructing a directed acyclic graph, which uses graphics to determine which variables to include in models to minimize bias (Figure 1).30 The selected covariates from the directed acyclic graph included mother’s race, age, education, IQ, CAARS ADHD symptoms, and child’s sex, age, gestation period, and birth weight. A third model tested for an interaction between log-transformed prenatal and childhood cotinine values, including the covariates (fully adjusted model plus interaction term). Cotinine concentration was analyzed as a continuous variable in all models. Listwise deletion was used for all regression models. All analyses were conducted using R version 4.0.2 (R Foundation for Statistical Computing).
Figure 1.
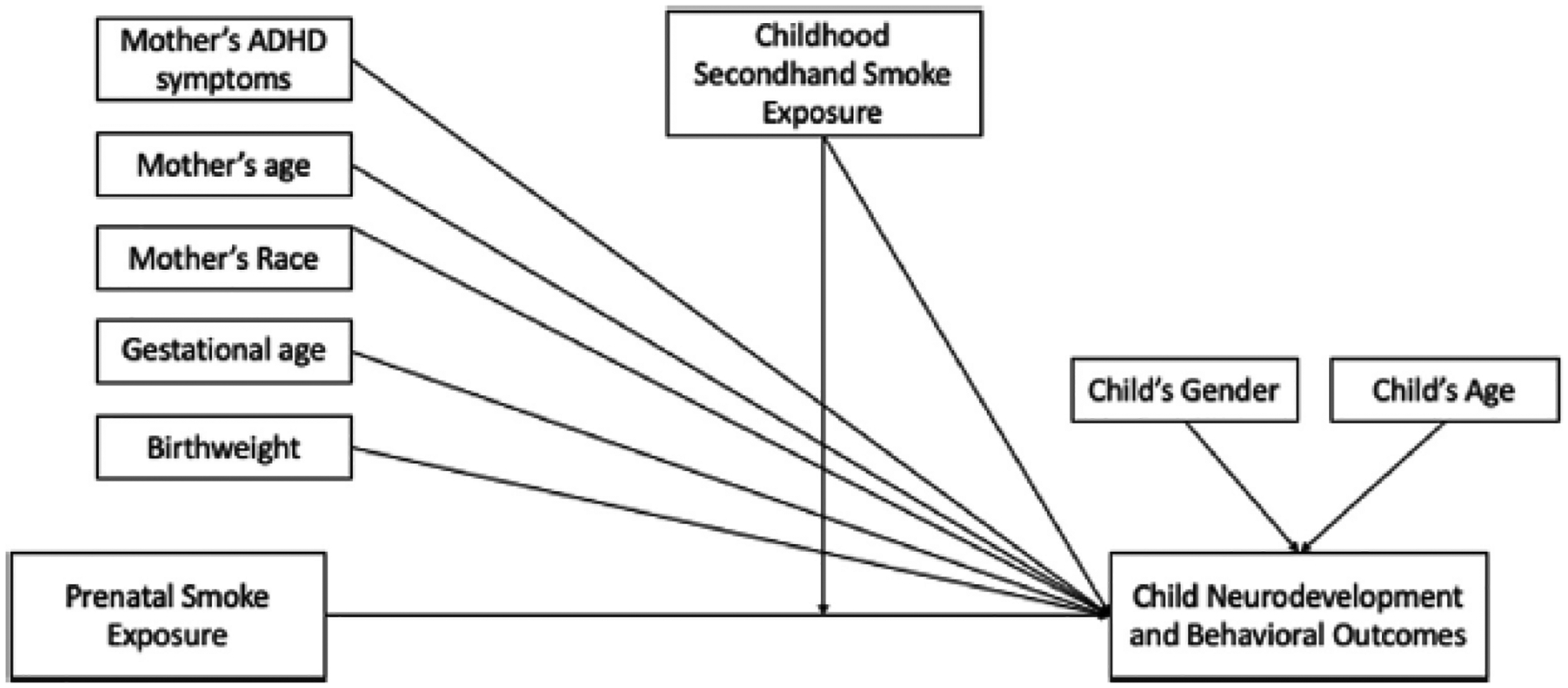
Directed acyclic graph (DAG) of the associations between prenatal and childhood smoke exposure and neurodevelopmental and behavioral outcomes. DAG with covariates included in the multivariable regression models.
RESULTS
Characteristics of the study participants are presented in Table I, along with cotinine concentration cutoff scores recommended by Benowitz et al.31 The geometric means for prenatal cotinine and childhood cotinine concentration were 0.96 and 0.24, respectively. The average maternal age at delivery was 28.3 years (SD, 5.7). The age range of children was 3 to 13, with an average age of 6.0 years (SD, 2.4). A majority of the sample self-identified as Black/African American (59.8%), and 33.4%, 2.8%, and 3.9% self-reported as White, Hispanic, and “other,” respectively. At the time of study enrollment, 39% of the mothers had a college degree, and the average family income reported by mothers was $63 259.47. Table II displays means and SDs of children’s scores on the NIH Toolbox task and BASC-2 assessment.
Table I.
Characteristics of the sample (N = 386)
| Characteristics | Values |
|---|---|
| Categorical variables, n (%) | |
| Maternal race/ethnicity | |
| White | 129 (33.4) |
| Black/African American | 231 (59.8) |
| Hispanic | 11 (2.8) |
| Other | 15 (3.9) |
| Maternal education | |
| Less than high school | 55 (14.6) |
| High school diploma/GED | 94 (24.9) |
| Some college | 81 (21.5) |
| College graduate | 147 (39.0) |
| Child sex | |
| Male | 186 (48.2) |
| Female | 200 (51.8) |
| Prenatal cotinine concentration (ng/mL) | |
| Nonsmoker (<1) | 213 (61.6) |
| SHS exposure (1–2.99) | 65 (18.8) |
| Active smoker/high SHS exposure (≥3) | 68 (19.7) |
| Childhood cotinine concentration (ng/mL) | |
| Nonsmoker (<1) | 261 (73.7) |
| SHS exposure (≥1) | 93 (26.3) |
| Continuous variables, mean ± SD | |
| Maternal age at delivery, y | 28.3 ± 5.7 |
| Maternal IQ | 95.68 ± 17.7 |
| Mother CAARS ADHD symptoms total score | 10.88 ± 8.32 |
| Gestation period, wk | 39.03 ± 1.8 |
| Birth weight, g | 3229.60 ± 542.9 |
| Child age at follow-up study, y | 6.0 ± 2.4 |
| Prenatal cotinine concentration, ng/mL | 14.90 ± 48.31 |
| Childhood cotinine concentration, ng/mL | 1.03 ± 2.01 |
GED, General Educational Development.
Table II.
Mean values and SDs for children’s cognitive performance on the NIH toolbox and BASC-2 Scores
| Variables | Construct | N | Mean ± SD | Range |
|---|---|---|---|---|
| NIH Toolbox | ||||
| Cognitive Composite | Overall cognitive capacity | 364 | 79.08 ± 28.70 | 24–190.58 |
| Flanker | Attention/inhibitory control | 379 | 60.02 ± 26.30 | 21.05–117 |
| Dimensional Card Sort | Flexibility | 377 | 63.93 ± 22.80 | 32.37–119 |
| Picture Sequence Memory | Episodic memory | 369 | 80.84 ± 14.91 | 49.61–125.19 |
| Picture Vocabulary | Receptive vocabulary | 380 | 67.43 ± 12.16 | 33.77–112 |
| BASC-2 (PRS) Scales | ||||
| BASC AP T-score (composite) | Attention problems | 369 | 51.98 ± 10.39 | 31–85 |
| BASC HY T-score (composite) | Hyperactivity | 367 | 49.29 ± 8.32 | 31–101 |
We also examined how prenatal and childhood cotinine concentration varied by the different demographic variables included in the models. Conducting t tests, ANOVA, and correlation analyses as appropriate, several between-group differences emerged. Children with Black/African American mothers had higher prenatal and childhood cotinine concentrations. Children whose mothers had college degrees had lower prenatal and childhood cotinine concentrations compared with children whose mothers had less education. Children with older mothers had lower prenatal and childhood cotinine concentrations compared with children with younger mothers. Children whose mothers scored higher on the WASI-II had lower prenatal and childhood cotinine concentrations compared with children whose mothers scored lower on the WASI-II.
Table III presents results from the 5 sets of regression models for the NIH Toolbox subscale scores. Prenatal cotinine concentration was associated with better inhibitory control (B = 1.61; P = .02) and attention and cognitive ability (B = 1.37; P = .02) in the unadjusted models, but when the covariates were included in the fully adjusted models, prenatal cotinine concentration was not associated with any of the NIH Toolbox scores. Childhood cotinine concentration was associated with poorer cognitive performance overall (B = 2.34; P < .001) and on tasks measuring inhibitory control and attention (B = 1.59; P = .006), cognitive flexibility (B = 1.29; P = .03), episodic memory (B = 0.97; P = .02), and receptive language development (B = 0.58; P = .01).
Table III.
Regression analyses examining cotinine on NIH Toolbox performance with both prenatal and childhood cotinine
| Parameters | B | 95% CI | P value |
|---|---|---|---|
| Cognitive composite | |||
| Unadjusted model | |||
| Prenatal cotinine | 1.22 | −0.10 to 2.55 | .07 |
| Childhood cotinine | −2.92*** | −4.29 to −1.55 | <.001 |
| Fully adjusted model | |||
| Prenatal cotinine | 0.71 | −0.57 to 1.99 | .27 |
| Childhood cotinine | −2.34*** | −3.69 to −0.98 | <.001 |
| Fully adjusted model plus interaction term | |||
| Prenatal cotinine and childhood cotinine interaction | −0.40 | −1.14 to 0.34 | .29 |
| Flanker (inhibitory control and attention) | |||
| Unadjusted model | |||
| Prenatal cotinine | 1.61* | 0.25–2.97 | .02 |
| Childhood cotinine | −2.56*** | −3.96 to −1.17 | <.001 |
| Fully adjusted model | |||
| Prenatal cotinine | 0.30 | −0.76 to 1.36 | .58 |
| Childhood cotinine | −1.59** | −2.71 to −0.47 | .006 |
| Fully adjusted model plus interaction term | |||
| Prenatal cotinine and childhood cotinine interaction | −0.30 | −0.91 to 0.31 | .33 |
| Dimensional Card Sort (cognitive flexibility) | |||
| Unadjusted model | |||
| Prenatal cotinine | 1.37* | 0.20–2.54 | .02 |
| Childhood cotinine | −1.87** | −3.08 to −0.67 | .002 |
| Fully adjusted model | |||
| Prenatal cotinine | 0.77 | −0.32 to 1.85 | .16 |
| Childhood cotinine | −1.29* | −2.43 to −0.14 | .03 |
| Fully adjusted model plus interaction term | |||
| Prenatal cotinine and childhood cotinine interaction | 0.06 | −0.54 to 0.65 | .85 |
| Picture Sequence Memory (episodic memory) | |||
| Unadjusted model | |||
| Prenatal cotinine | 0.15 | −0.64 to 0.94 | .70 |
| Childhood cotinine | −1.97*** | −2.79 to −1.16 | <.001 |
| Fully adjusted model | |||
| Prenatal cotinine | 0.09 | −0.66 to 0.85 | .81 |
| Childhood cotinine | −0.97* | −1.77 to −0.17 | .02 |
| Fully adjusted model plus interaction term | |||
| Prenatal cotinine and childhood cotinine interaction | −0.27 | −0.71 to 0.17 | .22 |
| Picture Vocabulary (language development) | |||
| Unadjusted model | |||
| Prenatal cotinine | 0.20 | −0.36 to 0.76 | .49 |
| Childhood cotinine | −1.37** | −1.95 to −0.80 | <.001 |
| Fully adjusted model | |||
| Prenatal cotinine | −0.06 | −0.50 to 0.38 | .79 |
| Childhood cotinine | 0.58* | −1.05 to −0.11 | .01 |
| Fully adjusted model plus interaction term | |||
| Prenatal cotinine and childhood cotinine interaction | −0.29* | −0.54 to −0.04 | .02 |
Fully adjusted models control for mother race, mother age, mother education, mother IQ, and mother CAARS ADHD Symptoms Total score and child sex, age, birth weight, and gestation period.
P < .05;
P < .01;
P < .001.
In the third model, there was a significant interaction between prenatal cotinine and childhood cotinine (P = .02) for the task measuring language development (PV task). SAS software (SAS Institute) was used to graphically interpret the interaction, and simple slope analyses were performed using the R package interactions. As can be seen in Figure 2, children with the highest concentrations of prenatal and childhood cotinine performed the worst on the PV task. Simple slope analyses were conducted by holding childhood cotinine concentration at the mean, 1 SD below the mean, and 1 SD above the mean. None of the slopes were statistically significant: 1 SD below the mean, B = 0.90, P = .06; at the mean, B = 0.29, P = .29; 1 SD above the mean, B = 0.33, P = .19.
Figure 2.
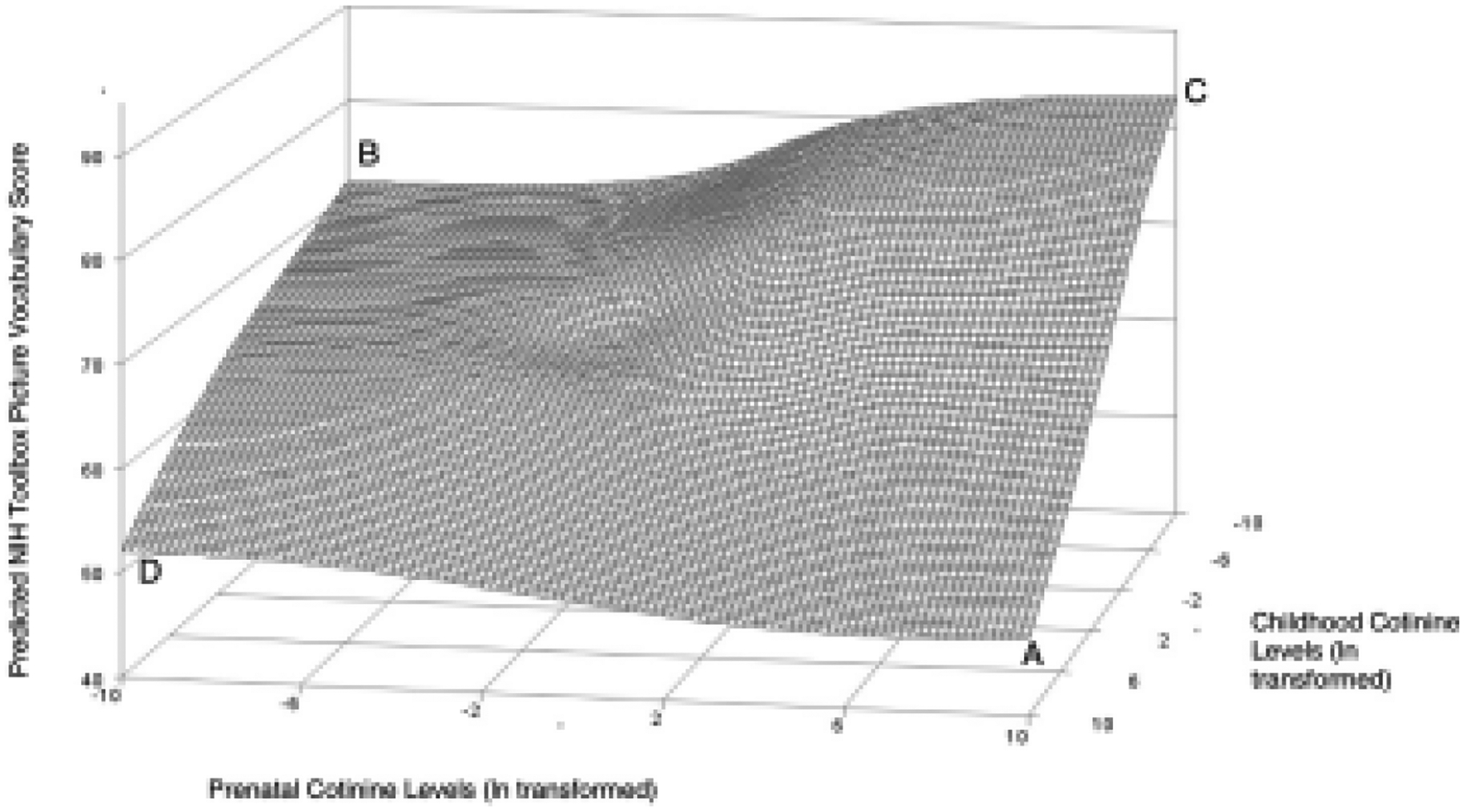
Surface plot displaying interaction between prenatal and childhood cotinine concentration (with PV as the outcome). Children with the highest prenatal and childhood cotinine concentrations performed worst on the PV task (A). Children with no prenatal exposure but the highest childhood exposure (D) and no prenatal and childhood exposure (B) performed better but children with the highest prenatal exposure and no childhood exposure performed best (C).
Table IV presents results from the 2 sets of regression models for the BASC-2 assessment scores. Prenatal cotinine concentration was not associated with any behavioral problems in any models. Greater childhood cotinine concentration was related to higher levels of attention problems (B = 0.83; P = .004) in the unadjusted model but not in the fully adjusted models. Childhood cotinine concentration was not associated with hyperactivity in any of the models.
Table IV.
Regression analyses examining cotinine on BASC-2 assessment with both prenatal and childhood cotinine
| Parameters | B | 95% Cl | P value |
|---|---|---|---|
| Attention problems | |||
| Unadjusted model | |||
| Prenatal cotinine | 0.21 | −0.34 to 0.76 | .46 |
| Childhood cotinine | 0.83* | 0.27–1.40 | .004 |
| Fully adjusted model | |||
| Prenatal cotinine | 0.22 | −0.35 to 0.80 | .45 |
| Childhood cotinine | 0.49 | −0.12 to 1.11 | .11 |
| Fully adjusted model plus interaction term | |||
| Prenatal cotinine and childhood cotinine interaction | −0.20 | −0.53 to 0.13 | .24 |
| Hyperactivity | |||
| Unadjusted model | |||
| Prenatal cotinine | 0.08 | −0.48 to 0.64 | .78 |
| Childhood cotinine | 0.56 | −0.01 to 1.13 | .06 |
| Fully adjusted model | |||
| Prenatal cotinine | 0.10 | −0.49 to 0.69 | .73 |
| Childhood cotinine | 0.49 | −0.13 to 1.12 | .12 |
| Fully adjusted model plus interaction term | |||
| Prenatal cotinine and childhood cotinine interaction | −0.07 | −0.41 to 0.26 | .67 |
Fully adjusted models control for mother’s race, age, education, IQ, and CAARS ADHD symptoms total score and child’s sex, age, birth weight and gestation period.
P < .01.
DISCUSSION
The findings suggest that childhood SHS exposure, but not prenatal maternal smoking, contributes to children’s task performance on measures of cognitive and executive functioning and maternal report of attention problems. Domains of functioning that were significantly related to SHS exposure during childhood included impaired abilities related to inhibitory control and attention, episodic memory, language development, and cognitive flexibility. Child attention problems were associated with higher levels of childhood cotinine concentrations, but this finding became nonsignificant once adjusted for covariates.
Prenatal smoke exposure was not significantly associated with any of the neurodevelopmental or behavioral outcomes once the models are adjusted for covariates. In both cases in which prenatal smoke exposure was associated with the neurodevelopmental outcomes, the associations were in the opposite hypothesized direction. Why prenatal cotinine concentrations were associated with better FICA and DCCS task performance in the unadjusted models is unclear; however, the associations become nonsignificant once the covariates were included. There was a significant interaction between prenatal and SHS smoke exposure predicting performance on a task assessing receptive vocabulary. Results suggested that children exposed to smoke prenatally and after birth could be at heightened risk for language-related impairments. However, given that all the simple slopes were nonsignificant, the interaction should be interpreted with caution.
The strength of the associations between prenatal and childhood smoke exposure and the neurodevelopmental and behavioral outcomes was also considered. To interpret the log-transformed cotinine values, the b coefficient can by multiplied by log(1.x), with x being the percent increase in the independent variable. For example, a 10% increase in cotinine concentration is associated with a 0.22 decrease in cognitive composite score based on the current findings. Although a 0.22 decrease in the NIH Toolbox cognitive composite score might not significantly impact a child’s overall cognitive ability, higher percent increases in cotinine concentration may lead to impairment in cognition.
In the epidemiologic literature, the relationship of smoke exposure with child neurodevelopment and behavioral difficulties is difficult to parse out. The data are complicated by various factors, including when smoke exposure is assessed (prenatally, early childhood, or both), how exposure is assessed (self-report vs measured in a biological sample), the time during development when assessments are conducted (infancy, early childhood vs later childhood), and the type of assessments performed (fine/gross motor, cognitive performance, behavioral ratings). A strength of this study is its inclusion of biological assessments of both prenatal and childhood SHS exposure, along with measured assessments of executive functioning domains and behavior. The findings are consistent with a study in which investigators found that prenatal smoke exposure was not as strong a predictor of children’s verbal abilities and behavioral difficulties (mean age, 5 years) as childhood SHS exposure.17 In that study, prenatal cotinine assays were used to confirm maternal reports of smoking, and SHS during childhood was based on the mother’s report of her own smoking at the time. Moore et al found similar results; prenatal smoke exposure (as assessed by prenatal urinary cotinine) was related to poorer performance by children on the NIH Toolbox Flanker sub scale measuring inattention and inhibitory control, but this effect was null after adjusting for postnatal smoke exposure (assessed by maternal self-report).32 To our knowledge, the current study is unique in its assessment of both prenatal and early childhood SHS exposure using biological assays at both time points.
Smoking during pregnancy is a well-known risk factor for an array of negative health outcomes for pregnant women and offspring33; ongoing efforts to support limiting smoke exposure during pregnancy are vitally important. However, the present results consistently indicate that early childhood smoke exposure may be a more specific risk factor for poorer neurodevelopmental and behavioral outcomes. It may be that SHS has a more significant impact on brain development during early childhood, as the brain experiences its most rapid growth between birth and age 1–2 years and continues to grow rapidly up to age 6 years.34–36 SHS exposure in early childhood also may have a greater effect on cognitive and attentional abilities compared with other aspects of neurodevelopment that may be more greatly impacted by prenatal exposure, such as early infant motor and temperament out comes. For example, Moore et al found associations between prenatal cotinine with both motor and inhibitory control in 4- to 6-year-old children, but the association between prenatal cotinine and inhibitory control became nonsignificant when postnatal exposure was included in their model.32 Furthermore, pregnant women have been found to clear cotinine at an accelerated pace compared with nonpregnant women,37,38 which also might have impacted prenatal cotinine concentrations in the current study. Nevertheless, these findings add to the literature by highlighting childhood SHS exposure as a specific risk factor for negative neurodevelopmental outcomes, underscoring that the detrimental effects of smoking on child development do not stop at birth.
This study has several strengths. We used cotinine concentration as the main method for assessing both prenatal and childhood smoke exposure. Using this objective, continuous measurement helps reduce any bias associated with self-reports.39,40 The cotinine test used has high sensitivity, allowing for the detection of even low cotinine concentrations. This study also used assessments providing information on a range of neurodevelopmental abilities. Many studies have relied solely on maternal report of their child’s neurodevelopment functioning and behavior. The use of standardized assessments helps overcome this potential bias. This study used the NIH Toolbox to assess child functioning in relation to prenatal and childhood exposure to tobacco smoke. Thus, this instrument may be a useful add-on to studies examining prenatal exposures to toxins and later childhood cognitive capacities. Our analyses also accounted for a number of potential confounding variables, including maternal cognitive functioning and ADHD symptoms.
Our findings should be interpreted in the context of some important limitations. The half-life of cotinine in both saliva and plasma is estimated to be 14–15 hours.41 Cotinine is metabolized faster in pregnant individuals (approximately 8 hours), although it is still dangerous to the developing fetus.37,38 Thus, prenatal cotinine samples may underestimate of the true level of smoke exposure experienced by women during pregnancy in the current sample. The lack of standardized timing of collection of blood and saliva samples during pregnancy and childhood is also a potential weakness of the cotinine measurements. The sample participating in the follow-up was not fully representative of the sample source, because the original cohort included a greater representation of children of Hispanic origin, more mothers with only a high school degree, and fewer mothers with a college degree. The study team faced some challenges with recruiting for the follow-up study from the sample of women in the larger cohort, owing in part to the lengthy laboratory visits required for the follow-up study. However, maternal smoking and cotinine concentration during pregnancy were unrelated to participation in the follow-up study, suggesting that there likely is minimal selection bias with respect to the level of exposure. Furthermore, although we included a broad set of variables to minimize confounding, the potential for residual confounding remains. Although we controlled for age to account for variability, there still could be age related effects that warrant further research. Developmentally, children can vary considerably in this age range, and the negative impacts of tobacco exposure may vary as a function of age, especially as children approach adolescence. Additionally, there are potential confounding variables associated with child smoke exposure that were not accounted for, such as marijuana smoke exposure, poverty,42 and neighborhood characteristics.43
Our present findings contribute to the growing evidence that childhood smoke exposure can have deleterious effects on children’s cognitive development, specifically as it relates to inhibitory control, attention, and early language development. Many women in the US are aware of the importance of stopping smoking in pregnancy; however, it is estimated that 50%-80% of women will resume smoking within the first year after birth.44,45 These findings underscore that limiting smoke exposure even after the prenatal period is essential for optimal neurodevelopmental functioning. Continued studies to help identify possible mechanisms and methods for determining the developmental timing and level of exposure more precisely are needed. For example, assays of deciduous teeth may be helpful in clarifying the level and totality of exposure over development.46 These more precise measurements may be needed to determine to what degree of smoke exposure over what period of time correlates to neurodevelopmental and behavioral outcomes.
Funding/support:
Supported by the National Institute of Environmental Health Sciences (R01 ES016772, R21 ES014947, P01 ES022831, and R24 ES028531), the US Environmental Protection Agency (RD-83543701), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD084487), the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK085173), and the Duke Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. Further, the US Environmental Protection Agency does not endorse the purchase of any commercial products or services mentioned in this publication. In addition, this research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences. The authors declare no conflicts of interest.
Abbreviations:
- ADHD
Attention-deficit/hyperactivity disorder
- BASC-2
Behavior Assessment System for Children, 2nd edition
- CAARS
Conners Adult ADHD Rating Scales
- DCCS
Dimensional Change Card Sort Test
- FICA
Flanker Inhibitory Control and Attention Test
- NEST
Newborn Epigenetic Study
- NIH
National Institutes of Health
- PSM
Picture Sequence Memory Test
- PV
Picture Vocabulary Test
- SHS
Secondhand smoke
- WASI-II
Wechsler Abbreviated Scale of Intelligence
REFERENCES
- 1.Thapar A, Cooper M, Rutter M. Neurodevelopmental disorders. Lancet Psychiatry 2017;4:339–46. 10.1016/S2215-0366(16)30376-5 [DOI] [PubMed] [Google Scholar]
- 2.Wehmeier PM, Schacht A, Barkley RA. Social and emotional impair ment in children and adolescents with ADHD and the impact on quality of life. J Adolesc Health 2010;46:209–17. 10.1016/j.jadohealth.2009.09.009 [DOI] [PubMed] [Google Scholar]
- 3.Barbaresi WJ, Colligan RC, Weaver AL, Voigt RG, Killian JM, Katusic SK. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics 2013;131:637–44. 10.1542/peds.2012-2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clegg J, Hollis C, Mawhood L, Rutter M. Developmental language dis orders–a follow-up in later adult life. Cognitive, language and psychosocial outcomes. J Child Psychol Psychiatry 2005;46:128–49. 10.1111/j.1469-7610.2004.00342.x [DOI] [PubMed] [Google Scholar]
- 5.Ahmed SF, Kuhfeld M,Watts TW, Davis-Kean PE, Vandell DL. Preschool executive function and adult outcomes: a developmental cascade model. Dev Psychol 2021;57:2234–49. 10.1037/dev0001270 [DOI] [PubMed] [Google Scholar]
- 6.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environ mental tobacco smoke exposure and children’s health. Pediatrics 2004;113:1007–15. [PubMed] [Google Scholar]
- 7.Fried PA, Watkinson B. 36- and 48-month neurobehavioral follow-up of children prenatally exposed to marijuana, cigarettes, and alcohol. J Dev Behav Pediatr 1990;11:49–58. [PubMed] [Google Scholar]
- 8.Ghassabian A, Sundaram R, Chahal N, McLain AC, Bell E, Lawrence DA, et al. Determinants of neonatal brain-derived neurotrophic factor and association with child development. Dev Psychopathol 2017;29:1499–511. 10.1017/S0954579417000414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keyes KM, Davey Smith G, Susser E. Associations of prenatal maternal smoking with offspring hyperactivity: causal or confounded? Psychol Med 2014;44:857–67. 10.1017/S0033291713000986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiechl-Kohlendorfer U, Merkle U, Deufert D, Neubauer V, Peglow UP, Griesmaier E. Effect of developmental care for very premature infants on neurodevelopmental outcome at 2 years of age. Infant Behav Dev 2015;39:166–72. 10.1016/j.infbeh.2015.02.006 [DOI] [PubMed] [Google Scholar]
- 11.Abdel Hamed NA, Hammad EEM, Salama RH, Yassa HA, Awaga MM. Secondhand smoke as a risk factor for attention deficit hyperactivity dis order in children. Inhal Toxicol 2019;31:420–7. 10.1080/08958378.2019.1705440 [DOI] [PubMed] [Google Scholar]
- 12.Kovess V, Keyes KM, Hamilton A, Pez O, Bitfoi A, Koc C, et al. Maternal smoking and offspring inattention and hyperactivity: results from a cross-national European survey. Eur Child Adolesc Psychiatry 2015;24: 919–29. 10.1007/s00787-014-0641-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joo H, Lim MH, Ha M, Kwon HJ, Yoo SJ, Choi KH, et al. Secondhand smoke exposure and low blood lead levels in association with attention deficit hyperactivity disorder and its symptom domain in children: a community-based case-control study. Nicotine Tob Res 2017;19:94–101. 10.1093/ntr/ntw152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margolis AE, Pagliaccio D, Ramphal B, Banker S, Thomas L, Robinson M, et al. Prenatal environmental tobacco smoke exposure al ters children’s cognitive control circuitry: a preliminary study. Environ Int 2021;155:106516. 10.1016/j.envint.2021.106516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellis-Suriani Z, Norsa’adah B, Othman A, Siti-Azrin AH. Association be tween secondhand smoke exposure at home and cognitive performance among rural primary school children in Malaysia. Tob Induc Dis 2021;19:27. 10.18332/tid/133638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Max W, Sung HY, Shi Y. Childhood secondhand smoke exposure and ADHD-attributable costs to the health and education system. J Sch Health 2014;84:683–6. 10.1111/josh.12191 [DOI] [PubMed] [Google Scholar]
- 17.Eskenazi B, Trupin LS. Passive and active maternal smoking during preg nancy, as measured by serum cotinine, and postnatal smoke exposure. II: effects on neurodevelopment at age 5 years. Am J Epidemiol 1995;142: S19–29. 10.1093/aje/142.Supplement_9.S19 [DOI] [PubMed] [Google Scholar]
- 18.Knopik VS. Maternal smoking during pregnancy and child outcomes: real or spurious effect? Dev Neuropsychol 2009;34:1–36. 10.1080/87565640802564366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trasti N, Vik T, Jacobsen G, Bakketeig LS. Smoking in pregnancy and children’s mental and motor development at age 1 and 5 years. Early Hum Dev 1999;55:37–147. 10.1016/S0378-3782(99)00017-1 [DOI] [PubMed] [Google Scholar]
- 20.Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R. Exposure to environmental tobacco smoke and cognitive abilities among US children and adolescents. Environ Health Perspect 2005;113:98–103. 10.1289/ehp.7210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang S, Decker A, Kramer MS. Exposure to parental smoking and child growth and development: a cohort study. BMC Pediatr 2013;13:104. 10.1186/1471-2431-13-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh K, Xu Y, Terrizzi BF, Lanphear B, Chen A, Kalkbrenner AE, et al. As sociations between early low-level tobacco smoke exposure and execu tive function at age 8 years. J Pediatr 2020;221:174–80.e1. 10.1016/j.jpeds.2019.11.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoyo C, Murtha AP, Schildkraut JM, Forman MR, Calingaert B, Demark-Wahnefried W, et al. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health 2011;11:46. 10.1186/1471-2458-11-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics 2012;7:735–46. 10.4161/epi.20734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their con tributions to complex “frontal lobe” tasks: a latent variable analysis. Cog nit Psychol 2000;41:49–100. 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- 26.Wechsler D. Wechsler abbreviated Scale of intelligence. 2nd ed. London: Pearson; 2011. [Google Scholar]
- 27.Bender AH, Auciello D, Morrison CE, MacAllister WS, Zaroff CM. Comparing the convergent validity and clinical utility of the behavior assessment system for children-parent rating Scales and child behavior checklist in children with epilepsy. Epilepsy Behav 2008;13:237–42. 10.1016/j.yebeh.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 28.Harrison JR, Vannest KJ, Reynolds CR. Behaviors that discriminate ADHD in children and adolescents: primary symptoms, symptoms of comorbid conditions, or indicators of functional impairment? J Atten Disord 2011;15:147–60. 10.1177/1087054709356170 [DOI] [PubMed] [Google Scholar]
- 29.Conners CK, Erhardt D, Epstein JN, Parker JDA, Sitarenios G, Sparrow E. Self-ratings of ADHD symptoms in adults I: factor structure and normative data. J Atten Disord 1999;3:141–51. 10.1177/108705479900300303 [DOI] [Google Scholar]
- 30.Textor J, Hardt J, Knuppel S. DAGitty: A graphical tool for analyzing € causal diagrams. Epidemiology 2011;22:745. 10.1097/EDE.0b013e318225c2be [DOI] [PubMed] [Google Scholar]
- 31.Benowitz NL, Dains KM, Dempsey D, Wilson M, Jacob P. Racial differ ences in the relationship between number of cigarettes smoked and nico tine and carcinogen exposure. Nicotine Tob Res 2011;13:772–83. 10.1093/ntr/ntr072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore BF, Shapiro AL, Wilkening G, Magzamen S, Starling AP, Allshouse WB, et al. Prenatal exposure to tobacco and offspring neuro cognitive development in the Healthy Start study. J Pediatr 2020;218:28–34.e2. 10.1016/j.jpeds.2019.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smok ing—50 years of progress: a report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention; 2014. 10.1037/e510072014-001 [DOI] [PubMed] [Google Scholar]
- 34.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev 2003;27:3–18. 10.1016/S0149-7634(03)00005-8 [DOI] [PubMed] [Google Scholar]
- 35.Deoni SCL. Neuroimaging of the developing brain and impact of nutri tion. Nestle Nutr Inst Workshop Ser 2018;89:155–74. 10.1159/000486500 [DOI] [PubMed] [Google Scholar]
- 36.Tregellas JR, Legget KT. Rapid early brain development highlights a crit ical period and possible intervention window. Biol Psychiatry Cogn Neurosci Neuroimaging 2020;5:937–8. 10.1016/j.bpsc.2020.08.007 [DOI] [PubMed] [Google Scholar]
- 37.Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 2009;192:29–60. 10.1007/978-3-540-69248-5_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dempsey D, Jacob P 3rd, Benowitz NL. Accelerated metabolism of nico tine and cotinine in pregnant smokers. J Pharmacol Exp Ther 2002;301: 594–8. 10.1124/jpet.301.2.594 [DOI] [PubMed] [Google Scholar]
- 39.Jedrychowski W, Perera F,Mroz E, Edwards S, Flak E, Bernert JT, et al. Fetal exposure to secondhand tobacco smoke assessed by maternal self-reports and cord blood cotinine: prospective cohort study in Krakow. Matern Child Health J 2009;13:415–23. 10.1007/s10995-008-0350-4 [DOI] [PubMed] [Google Scholar]
- 40.Pichini S, Basagana XB, Pacifici R, Garcia O, Puig C, Vall O, et al. Cord ~ serum cotinine as a biomarker of fetal exposure to cigarette smoke at the end of pregnancy. Environ Health Perspect 2000;108:1079–83. 10.1289/ehp.001081079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zevin S, Jacob P, Geppetti P, Benowitz NL. Clinical pharmacology of oral cotinine. Drug Alcohol Depend 2000;60:13–8. 10.1016/S0376-8716(00)80003-4 [DOI] [PubMed] [Google Scholar]
- 42.Hajizadeh M, Nandi A. The socioeconomic gradient of secondhand smoke exposure in children: evidence from 26 low-income and middle-income countries. Tob Control 2016;25:e146–55. 10.1136/tobaccocontrol-2015-052828 [DOI] [PubMed] [Google Scholar]
- 43.Galvez MP, McGovern K, Teitelbaum SL, Windham G, Wolff MS. Neighborhood factors and urinary metabolites of nicotine, phthalates, and dichlorobenzene. Pediatrics 2018;141:S87–95. 10.1542/peds.2017-1026L [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. Am J Public Health 1990;80:541–4. 10.2105/AJPH.80.5.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kahn RS, Certain L, Whitaker RC. A reexamination of smoking before, during, and after pregnancy. Am J Public Health 2002;92:1801–8. 10.2105/AJPH.92.11.1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andra SS, Austin C, Wright RO, Arora M. Reconstructing pre-natal and early childhood exposure to multi-class organic chemicals using teeth: towards a retrospective temporal exposome. Environ Int 2015;83:137–45. 10.1016/j.envint.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]


