Abstract
BACKGROUND
In the United States, more than 30,000 cases of mpox (formerly known as monkeypox) had occurred as of March 1, 2023, in an outbreak disproportionately affecting transgender persons and gay, bisexual, and other men who have sex with men. In 2019, the JYNNEOS vaccine was approved for subcutaneous administration (0.5 ml per dose) to prevent mpox infection. On August 9, 2022, an emergency use authorization was issued for intradermal administration (0.1 ml per dose); however, real-world effectiveness data are limited for either route.
METHODS
We conducted a case–control study based on data from Cosmos, a nationwide Epic electronic health record (EHR) database, to assess the effectiveness of JYNNEOS vaccination in preventing medically attended mpox disease among adults. Case patients had an mpox diagnosis code or positive orthopoxvirus or mpox virus laboratory result, and control patients had an incident diagnosis of human immunodeficiency virus (HIV) infection or a new or refill order for preexposure prophylaxis against HIV infection between August 15, 2022, and November 19, 2022. Odds ratios and 95% confidence intervals were estimated from conditional logistic-regression models, adjusted for confounders; vaccine effectiveness was calculated as (1 – odds ratio for vaccination in case patients vs. controls) × 100.
RESULTS
Among 2193 case patients and 8319 control patients, 25 case patients and 335 control patients received two doses (full vaccination), among whom the estimated adjusted vaccine effectiveness was 66.0% (95% confidence interval [CI], 47.4 to 78.1), and 146 case patients and 1000 control patients received one dose (partial vaccination), among whom the estimated adjusted vaccine effectiveness was 35.8% (95% CI, 22.1 to 47.1).
CONCLUSIONS
In this study using nationwide EHR data, patients with mpox were less likely to have received one or two doses of JYNNEOS vaccine than control patients. The findings suggest that JYNNEOS vaccine was effective in preventing mpox disease, and a two-dose series appeared to provide better protection. (Funded by the Centers for Disease Control and Prevention and Epic Research.)
IN THE UNITED STATES, MORE THAN 30,000 confirmed and probable cases of mpox (formerly known as monkeypox) were reported as of March 1, 2023, in an outbreak disproportionately affecting transgender persons and gay, bisexual, and other men who have sex with men.1,2 Among persons with mpox disease, a large proportion are persons with human immunodeficiency virus (HIV) infection, those who have had a recent sexually transmitted infection (STI), or those who are receiving preexposure prophylaxis (PrEP) against HIV infection.3 However, mpox infection can be acquired by anyone, regardless of sex, gender identity, or sexual orientation, through close, sustained physical contact.4
In 2019, the Food and Drug Administration (FDA) approved JYNNEOS vaccine (modified vaccinia Ankara [MVA] vaccine, Bavarian Nordic) administered subcutaneously in a two-dose series (0.5 ml per dose, 4 weeks apart) for prevention of smallpox and mpox.5 On August 9, 2022, in order to increase the supply of vaccine available for use, an emergency use authorization (EUA) was issued for intradermal administration of the vaccine in a two-dose series (0.1 ml per dose, 4 weeks apart).6,7 Vaccination is available for persons with known or presumed exposure to a person with mpox (offered as postexposure prophylaxis) or with factors potentially increasing the likelihood of having been exposed (previously referred to as expanded postexposure prophylaxis).8,9 Vaccination may also be offered to persons at high risk for exposure to mpox virus (MPXV) or who might benefit from vaccination (PrEP for mpox), including gay, bisexual, or other men who have sex with men; transgender persons; nonbinary or gender nonconforming persons; persons with HIV; persons eligible for PrEP against HIV; or persons with a recent (past 6 months) diagnosis of HIV or other nationally reportable STI.8,9
The effectiveness of JYNNEOS vaccine against mpox disease has been inferred from animal and immunogenicity studies for both the subcutaneous10 and intradermal7 administration routes; however, real-world effectiveness data about mpox PrEP are limited.11,12 We analyzed a large, nationwide electronic health record (EHR) database in the United States to estimate the effectiveness of partial (one dose) and full (two doses) vaccination with JYNNEOS as PrEP against mpox disease overall, among select subpopulations, and according to route of administration.
METHODS
STUDY DESIGN
We designed a case–control study using data from the Epic Cosmos platform, an integrated EHR database with information on more than 173 million patients (as of the study period) from all 50 states in the United States (see the Supplementary Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org).13 We defined our study period as August 15, 2022, through November 19, 2022, to capture the period of increased vaccine availability after the EUA for intradermal administration was issued. The study was designed by staff from the Centers for Disease Control and Prevention (CDC) and Epic Research; staff from Epic Research gathered and analyzed the data, and staff from the CDC and Epic Research vouch for the accuracy and completeness of the data and for the fidelity of the study to the protocol, available at NEJM.org. Both CDC and Epic Research staff interpreted the results, drafted the manuscript, and decided to submit the manuscript for publication. All the authors contributed to developing the study design, interpreting the results, and critically reviewing and revising the manuscript. This activity was reviewed by the CDC, and the conduct of the study was consistent with applicable federal law and CDC policy.21
CASE PATIENT AND CONTROL PATIENT SELECTION
Case patients were persons of any gender identity and age with either an initial mpox diagnosis code or a positive orthopoxvirus or MPXV laboratory test result during the study period (Table S1 in the Supplementary Appendix). We selected control patients on the basis of vaccine distribution guidance from the Administration for Strategic Preparedness and Response8 and population characteristics of other studies examining vaccine performance.11 Control patients included those with an incident clinical HIV diagnosis or a new positive HIV antibody or antigen–antibody test, or a new or refill order for HIV PrEP who also had an in-person clinic visit during the study period. Patients with a previous mpox diagnosis or positive orthopoxvirus or MPXV laboratory test result (from August 15, 2021, through August 14, 2022) were excluded. For both case and control patients, we considered the date of diagnosis, laboratory test result, or prescription date as the date of the index event.
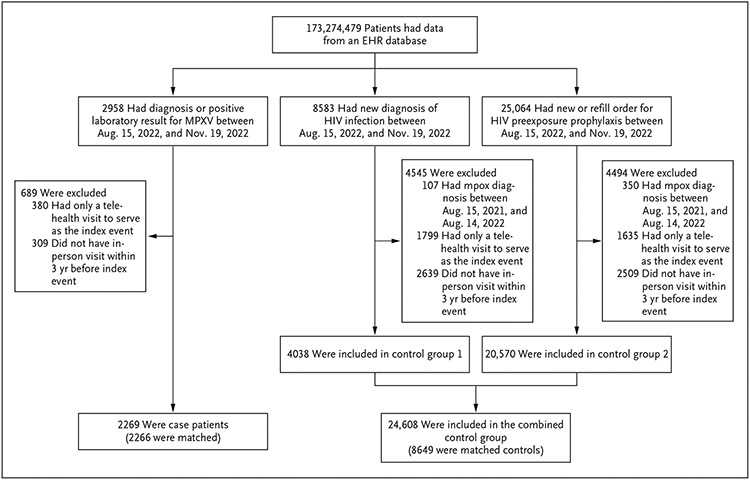
To achieve comparability in routine health care–seeking behaviors between case patients and control patients, we excluded patients who had no in-person medical encounters during the 3 years before their index event or who had only a telehealth visit to serve as the index event during the study period. We also excluded control patients who had a documented prescription for tecovirimat (brand name, TPOXX, a smallpox and mpox antiviral agent) in the period from 14 days before their index event through the end of the study period (Fig. 1).
Figure 1. Assessment of Eligibility for Case Patients and Control Patients.
Among 350 of 4494 patients with a new or refill order for human immunodeficiency virus (HIV) preexposure prophylaxis who were excluded because of an mpox diagnosis, 2 patients had a prescription for tecovirimat from 14 days before the index event through November 19, 2022. EHR denotes electronic health record, and MPXV mpox virus.
Each case patient was matched with up to four control patients without replacement, according to the week of index event, Department of Health and Human Services census region, and gender identity (as categorized in Table 1). Case patients and control patients were randomly matched when more than four control patients were identified.
Table 1.
Characteristics of Case Patients and Control Patients, among Persons seeking Health Care.*
| Characteristic | Case Patients (N = 2266) |
Control Patients (N = 8649) |
|---|---|---|
| Demographic and clinical characteristics | ||
| Age at index event — no. (%) | ||
| 18–35 yr | 1252 (55.3) | 4221 (48.8) |
| 36–49 yr | 707 (31.2) | 2434 (28.1) |
| 50–64 yr | 273 (12.0) | 1619 (18.7) |
| ≥65 yr | 34 (1.5) | 375 (4.3) |
| Legal sex — no. (%) | ||
| Male | 2059 (90.9) | 7821 (90.4) |
| Female | 204 (9.0) | 815 (9.4) |
| Other | 1 (<0.1) | 5 (0.1) |
| Unknown | 2 (0.1) | 8 (0.1) |
| Gender identity — no. (%) | ||
| Male | 2022 (89.2) | 7717 (89.2) |
| Female | 199 (8.8) | 790 (9.1) |
| Transgender male | 2 (0.1) | 4 (0.1) |
| Transgender female | 23 (1.0) | 75 (0.9) |
| Other | 19 (0.8) | 62 (0.7) |
| Chose not to disclose | 1 (<0.1) | 1 (<0.1) |
| Race or ethnic group — no. (%)† | ||
| Non-Hispanic White | 792 (35.0) | 4800 (55.5) |
| Non-Hispanic Black | 816 (36.0) | 1545 (17.9) |
| Hispanic | 500 (22.1) | 1519 (17.6) |
| Non-Hispanic Asian | 71 (3.1) | 413 (4.8) |
| Non-Hispanic Alaska Native or American Indian | 8 (0.4) | 24 (0.3) |
| Other, non-Hispanic | 79 (3.5) | 348 (4.0) |
| Social Vulnerability Index quartile — no. (%)‡ | ||
| 0–0.24 | 268 (11.8) | 1423 (16.5) |
| 0.25–0.49 | 374 (16.5) | 2030 (23.5) |
| 0.50–0.74 | 501 (22.1) | 2209 (25.5) |
| 0.75–1.00 | 1106 (48.8) | 2954 (34.2) |
| Unknown | 17 (0.8) | 33 (0.4) |
| U.S. Census region — no. (%) | ||
| South | 879 (38.8) | 3235 (37.4) |
| Northeast | 322 (14.2) | 1273 (14.7) |
| West | 708 (31.2) | 2693 (31.1) |
| Midwest | 357 (15.8) | 1448 (16.7) |
| Immunocompromising condition — no. (%)§ | ||
| No | 1304 (57.5) | 6924 (80.1) |
| Yes | 962 (42.5) | 1725 (19.9) |
| HIV infection — no. (%) | ||
| No | 1407 (62.1) | 7241 (83.7) |
| Yes | 859 (37.9) | 1408 (16.3) |
| HIV infection and CD4 cell count <200/mm3 in previous 6 mo — no. (%) | ||
| No | 2052 (90.6) | 8394 (97.1) |
| Yes | 214 (9.4) | 255 (2.9) |
| New diagnosis ofHIV infection — no./total no. (%)¶ | ||
| No | 1407/1526 (92.2) | 7241/8649 (83.7) |
| Yes | 119/1526 (7.8) | 1408/8649 (16.3) |
| Active HIV PrEP prescription among persons without HIV infection — no./total no. (%) | ||
| No | 1217/1407 (86.5) | 0 |
| Yes | 190/1407 (13.5) | 7241/7241 (100.0) |
| Vaccinated with ACAM2000 before 2022 — no. (%) | ||
| No | 2258 (99.6) | 8562 (99.0) |
| Yes | 8 (0.4) | 87 (1.0) |
| Vaccination status | ||
| Fully vaccinated‖ | ||
| Overall — no./total no. (%) | 25/2193 (1.1) | 335/8319 (4.0) |
| Administration timing relative to index event — no./total no. (%) | ||
| Second dose, 24–28-day interval and ≥14 days before index event | 11/25 (44.0) | 133/335 (39.7) |
| Second dose, 29–45-day interval and ≥14 days before index event | 11/25 (44.0) | 164/335 (49.0) |
| Second dose, ≥46-day interval and ≥14 days before index event | 3/25 (12.0) | 38/335 (11.3) |
| Administration route for both doses — no./total no. (%) | ||
| Subcutaneous | 6/25 (24.0) | 63/335 (18.8) |
| Intradermal | 5/25 (20.0) | 42/335 (12.5) |
| Heterologous | 8/25 (32.0) | 150/335 (44.8) |
| Other or missing | 6/25 (24.0) | 80/335 (23.9) |
| Partially vaccinated** | ||
| Overall — no./total no. (%) | 146/2193 (6.7) | 1000/8319 (12.0) |
| Administration timing relative to index event — no./total no. (%) | ||
| First dose, ≥14 days before index event | 80/146 (54.8) | 366/1000 (36.6) |
| First dose, ≥24-day interval and <14 days before index event | 53/146 (36.3) | 269/1000 (26.9) |
| Second dose after index event | 13/146 (8.9) | 365/1000 (36.5) |
| Administration route — no./total no. (%) | ||
| Subcutaneous | 106/146 (72.6) | 704/1000 (70.4) |
| Intradermal | 27/146 (18.5) | 186/1000 (18.6) |
| Other or missing | 13/146 (8.9) | 110/1000 (11.0) |
| Unvaccinated†† | ||
| Overall — no./total no. (%) | 2022/2193 (92.2) | 6984/8319 (84.0) |
| Administration timing relative to index event — no./total no. (%) | ||
| No documented doses | 1922/2022 (95.1) | 6406/6984 (91.7) |
| First dose on same day as index event | 70/2022 (3.5) | 171/6984 (2.4) |
| First dose after index event | 30/2022 (1.5) | 407/6984 (5.8) |
Case patients had an mpox diagnosis code or a positive orthopoxvirus or mpox virus laboratory result, and control patients had an incident diagnosis of human immunodeficiency virus (HIV) infection or a new or refill order for preexposure prophylaxis (PrEP) against HIV infection between August 15, 2022, and November 19, 2022. Percentages may not sum to 100 because of rounding.
Race or ethnic group was reported by the patients.
Scores on the Social Vulnerability Index, an indicator of community-level vulnerability, range from 0 to 1.0, with higher scores indicating greater vulnerability.
Immunocompromising conditions include selected conditions identified on the basis of electronic health record documentation of International Classification of Diseases, 10th revision, diagnostic codes or prescriptions for immunosuppressive medications in the previous 6 months (Table S2).
Totals exclude patients who were not at risk for a new HIV diagnosis (owing to an existing HIV diagnosis).
Patients were considered to be fully vaccinated if they had received two doses of JYNNEOS vaccine at least 24 days apart and the second dose was at least 14 days before the index event. Patients were excluded if they had received two doses less than 24 days apart (no case patients and 7 control patients).
Patients were considered to be partially vaccinated if they had received one dose at least 14 days before the index event, or if the second dose was less than 14 days before the index event; patients who had received one dose less than 14 days before the index event were excluded because vaccination might represent postexposure prophylaxis (73 case patients and 323 control patients).
Patients were considered to be unvaccinated if there were no documented doses before the index event.
DEFINITIONS
Cosmos obtains vaccination information from state registries, health systems that contribute to Cosmos, other health systems through clinical record exchanges, and patient-reported histories verified by clinic staff; information is transferred within approximately 14 days after a health care encounter. Patients were considered to be fully vaccinated if they had received two doses of JYNNEOS vaccine at least 24 days apart (to account for a 4-day grace period),9,11,14 with the second dose at least 14 days before the index event. Patients were considered to be partially vaccinated if they had received one dose at least 14 days before the index event or if they had received a second dose less than 14 days before the index event; patients who had received two doses less than 24 days apart were excluded from vaccine-effectiveness estimates. Patients were considered to be unvaccinated if there were no documented doses before the index event; patients who had received one dose less than 14 days before the index event were excluded from vaccine-effectiveness estimates because vaccination might represent postexposure prophylaxis against mpox.
We categorized the route of administration as subcutaneous, intradermal, other (i.e., improbable administration routes, including intramuscular, oral, or sublingual), or missing. For vaccinations before August 9, 2022, we recategorized the route of administration from “other” or “missing” to “subcutaneous” because JYNNEOS was authorized only for subcutaneous administration during this time. For vaccinations on or after August 9, 2022, we used dosage information to recategorize the route of administration: vaccinations with a route documented as “other” or “missing” and a dose of 0.5 ml were recategorized as “subcutaneous,” and those with a dose of 0.1 ml were recategorized as “intradermal.” Vaccines administered on or after August 9, 2022, with a route of “missing” or “other” and a dose other than 0.1 ml or 0.5 ml were not recategorized.
We examined several covariates, including demographic characteristics (e.g., score on the Social Vulnerability Index [SVI], an indicator of community-level vulnerability),15,16 patient medical conditions (e.g., immunocompromising conditions, including HIV, identified on the basis of EHR documentation of International Classification of Diseases, 10th revision, diagnostic codes or prescriptions for immunosuppressive medications in the previous 6 months17,18 [Table S2]), characteristics of health care use (e.g., the number of in-person encounters during the year before the index event), and characteristics associated with the index event (e.g., hospitalization).
STATISTICAL ANALYSIS
We used conditional logistic regression to account for matching and to estimate crude odds ratios and adjusted odds ratios evaluating the association between vaccination status and case patient or control patient status; adjusted odds ratios accounted for potential confounders (age, race or ethnic group, SVI score, and the presence or absence of immunocompromising conditions) identified a priori through causal diagrams. Vaccine effectiveness and adjusted vaccine effectiveness were calculated as (1 – odds ratio or adjusted odds ratio, respectively) × 100. Associations between age, race or ethnic group, SVI score, and the presence or absence of immunocompromising conditions and case patient or control patient status were also estimated (Table S3). Confidence intervals were not adjusted for multiplicity and should not be used in place of hypothesis tests. Analyses were conducted with the use of R software, version 4.1.2 (R Foundation for Statistical Computing).
In secondary analyses, we sought to examine a priori vaccine effectiveness among six subpopulations of interest: persons who identified as female, persons who identified as male, persons who identified as male who were 18 to 49 years of age and had not had ACAM2000 vaccination (to account for documented or potential smallpox vaccination among persons ≥50 years of age, because routine smallpox vaccination ended in 1972),19 persons without immunocompromising conditions, persons with immunocompromising conditions, and persons with HIV infection. However, limited sample sizes precluded us from examining vaccine effectiveness among persons who identified as female (none of the patients with female gender identity, as compared with 2 female patients according to legal sex, were fully vaccinated [Table S4]), persons with immunocompromising conditions (34 of 2633 patients with immunocompromising conditions [1.3%] were fully vaccinated), or persons with HIV (22 of 2222 patients with HIV [1.0%] were fully vaccinated). Subpopulation analyses were adjusted for age, race or ethnic group, and SVI scores; analyses for men only and for men who were 18 to 49 years of age who had not received ACAM2000 vaccination were also adjusted for the presence or absence of immunocompromising conditions.
In addition, we a priori sought to examine vaccine effectiveness according to route of vaccine administration among persons who were fully vaccinated. Limited sample sizes precluded the examination of vaccine effectiveness among persons who were fully vaccinated subcutaneously (69 patients) or intradermally (47 patients). Finally, to assess the robustness of our findings against unmeasured confounders, E values were calculated overall and according to subpopulations of interest.20
RESULTS
PATIENTS
Between August 15, 2022, and November 19, 2022, a total of 2958 case patients and 33,647 control patients were identified (Fig. 1). After exclusions, 2269 case patients and 24,608 controls remained, and 2266 case patients (99.9%) were matched to 8649 controls (35.1%).
Overall, 89.2% of the patients identified as men, 9.1% as women, 1.0% as transgender women or men, and 0.7% as another gender identity (Table 1). Case patients were younger than control patients, and more case patients were non-Hispanic Black or Hispanic; more case patients were in the highest quartile of SVI scores than control patients. More case patients than control patients had an immunocompromising condition (42.5% vs. 19.9%). During the study period, more case patients than control patients had HIV and had had a CD4 cell count of less than 200 per cubic millimeter in the previous 6 months (9.4% vs. 2.9%), but fewer case patients than control patients had a new HIV diagnosis (7.8% vs. 16.3%). A total of 8 case patients and 87 control patients had documentation of previous ACAM2000 vaccination. Case patients and control patients had a similar number of underlying conditions and in-person health care encounters in the previous year (Table S5).
VACCINE EFFECTIVENESS
Among 2193 case patients included in vaccine-effectiveness analyses, 2022 were unvaccinated, 146 were partially vaccinated, and 25 were fully vaccinated (Table 2). Among 8319 control patients included in vaccine-effectiveness analyses, 6984 were unvaccinated, 1000 were partially vaccinated, and 335 were fully vaccinated. Unadjusted vaccine effectiveness was 52.0% (95% confidence interval [CI], 42.3 to 60.1) for partial vaccination and 77.2% (95% CI, 65.0 to 85.1) for full vaccination. After adjustment for age, race or ethnic group, SVI score, and the presence or absence of immunocompromising conditions, vaccine effectiveness was 35.8% (95% CI, 22.1 to 47.1) for partial vaccination and 66.0% (95% CI, 47.4 to 78.1) for full vaccination (Table 2). Among men 18 to 49 years of age who had not received ACAM2000 vaccination, adjusted JYNNEOS vaccine effectiveness was 35.5% (95% CI, 19.1 to 48.6) for partial vaccination and 58.7% (95% CI, 33.9 to 74.3) for full vaccination (Table 3). In patients without an immunocompromising condition, adjusted vaccine effectiveness was 40.8% (95% CI, 24.8 to 53.4) with partial vaccination and 76.3% (95% CI, 57.7 to 86.8) with full vaccination.
Table 2.
Estimated Vaccine Effectiveness against Diagnosed Mpox among Persons Seeking Health Care, August 15 through November 19, 2022.*
| Persons Seeking Health Care | Case Patients |
Control Patients |
Vaccine Effectiveness (95% CI) |
|
|---|---|---|---|---|
| Unadjusted | Adjusted† | |||
| number | percent | |||
| Unvaccinated, reference population | 2022 | 6984 | ||
| Partially vaccinated, 1 dose | 146 | 1000 | 52.0 (42.3–60.1) | 35.8 (22.1–47.1) |
| Fully vaccinated, 2 doses | 25 | 335 | 77.2 (65.0–85.1) | 66.0 (47.4–78.1) |
CI denotes confidence interval.
Adjustment was for age group (18 to 35, 36 to 49, and ≥50 years), race or ethnic group (non-Hispanic White, non-Hispanic Black, and other non-Hispanic), Social Vulnerability Index quartile (quartile 1 to 4, or unknown), and the presence or absence of an immunocompromising condition.
Table 3.
Estimated Vaccine Effectiveness against Diagnosed Mpox among Persons Seeking Health Care, According to Subpopulations of Interest, August 15 through November 19, 2022.
| Subpopulation | Case Patients |
Control Patients |
Vaccine Effectiveness (95% CI) |
|
|---|---|---|---|---|
| Unadjusted | Adjusted* | |||
| number | percent | |||
| Men only † | ||||
| Unvaccinated, reference group | 1792 | 6075 | ||
| Partially vaccinated | 136 | 983 | 54.5 (45.0–62.5) | 35.9 (21.6–47.6) |
| Fully vaccinated | 25 | 335 | 77.3 (65.3–85.2) | 64.8 (45.2–77.3) |
| Men only, 18–49 yr of age and without ACAM2000 vaccination † | ||||
| Unvaccinated, reference group | 1561 | 4632 | ||
| Partially vaccinated | 119 | 787 | 56.9 (46.7–65.2) | 35.5 (19.1–48.6) |
| Fully vaccinated | 23 | 247 | 73.4 (58.3–83.0) | 58.7 (33.9–74.3) |
| Not immunocompromised | ||||
| Unvaccinated, reference group | 1151 | 5368 | ||
| Partially vaccinated | 102 | 932 | 47.0 (33.2–58.0) | 40.8 (24.8–53.4) |
| Fully vaccinated | 14 | 312 | 80.6 (65.5–89.1) | 76.3 (57.7–86.8) |
Adjustment was for age group (18 to 35, 36 to 49, and ≥50 years), race or ethnic group (non-Hispanic White, non-Hispanic Black, and other non-Hispanic), Social Vulnerability Index quartile (quartile 1 to 4, or unknown), and the presence or absence of an immunocompromising condition.
This category includes patients whose gender identity was male.
We examined vaccine effectiveness according to route of administration among fully vaccinated persons for whom information regarding the route of administration was available. A total of 32.0% of case patients and 44.8% of control patients received vaccine with a heterologous regimen (i.e., one dose subcutaneously and one dose intradermally) (Table 1); unadjusted vaccine effectiveness for heterologous administration was 84.1% (95% CI, 67.1 to 92.3), and adjusted vaccine effectiveness was 75.2% (95% CI, 48.0 to 88.2).
E values suggested that point estimates for associations of interest would be completely explained by unmeasured confounders that were moderately associated (i.e., relative risk ≥2.5) with vaccination status and case patient or control patient status (Table S6).
DISCUSSION
In this study using nationwide EHR data, patients with mpox were less likely to be vaccinated with JYNNEOS vaccine than control patients; the estimated adjusted vaccine effectiveness was 66.0% (95% CI, 47.4 to 78.1) in fully vaccinated persons and 35.8% (95% CI, 22.1 to 47.1) in partially vaccinated persons. These results suggest that both one and two doses of the vaccine provide protection against mpox disease.
Among men 18 to 49 years of age without a history of ACAM2000 vaccination, adjusted vaccine effectiveness was 58.7% (95% CI, 33.9 to 74.3) in fully vaccinated persons and 35.5% (95% CI, 19.1 to 48.6) in those who were partially vaccinated. Persons 50 years of age or older, who were excluded from this subpopulation analysis, might have had a previous smallpox vaccination19 or might have been at lower risk for mpox disease than younger men22; however, the point estimates for this subpopulation were similar to the overall point estimates.
Among persons without immunocompromising conditions, vaccine effectiveness was 76.3% (95% CI, 57.7 to 86.8) in those who were fully vaccinated and 40.8% (95% CI, 24.8 to 53.4) in those who were partially vaccinated; these percentages were higher than the overall point estimate, although confidence intervals overlapped. Persons with immunocompromising conditions might mount a less effective immune response after vaccinations,23 such that vaccine effectiveness is lower in this group than among persons without such conditions. Persons with immunocompromising conditions compose a large proportion of those with mpox and may be more likely to have severe outcomes.3,4,22,24 We could not estimate vaccine effectiveness among the immunocompromised population because vaccine coverage was low (1.3% were fully vaccinated). Additional research is needed to evaluate vaccine effectiveness among persons with immunocompromising conditions, and these persons may choose to take additional precautions to reduce the risk of or prevent mpox infection.25
Our findings are consistent with those of the few studies examining the effectiveness of JYNNEOS (MVA) vaccine against mpox disease. A study that examined vaccine performance with the use of surveillance data from 43 U.S. jurisdictions showed that the incidence of mpox disease was 7.4 times and 9.6 times as high among unvaccinated men who were 18 to 49 years of age and who were eligible for JYNNEOS vaccination as among those who received a first or second vaccine dose at least 14 days earlier, respectively; it is notable that this study relied on data from separate surveillance systems, which required several assumptions, and was unable to account for potential confounding factors.11 A cohort study from Israel showed that MVA vaccine was associated with an 86% decrease in the incidence of mpox disease among men receiving HIV PrEP or men with HIV who had a diagnosis of one or more STIs; however, the study examined only a single, subcutaneously administered dose, and the cohort included only 21 persons with mpox, factors that resulted in imprecise estimates.12
Our findings have implications for public health and clinical practice. Evidence from immunogenicity studies provided the basis for implementing intradermal vaccination as a strategy to increase vaccine supply, because intradermal administration requires one fifth the dosage of subcutaneous administration.7,26 We found that two doses of vaccine, including those administered subcutaneously, intradermally, or heterologously, provided the highest protection against mpox disease.26 Furthermore, our finding that vaccine effectiveness was higher among persons who received two doses, rather than one dose, highlights the importance of following the approved dosing schedule. Recent data suggest that only 57.6% of first-dose vaccine recipients who were eligible to receive a second dose did so.27
Our study has several limitations. This was an observational study, and therefore it does not provide definitive evidence of causality. In addition, although Cosmos data reflect the age and race or ethnic group distribution of the U.S. population, the data represent only persons who sought health care during the previous 3 years. Because information regarding sexual orientation and behavior are not consistently documented in the Cosmos database, we relied on clinical encounter and diagnostic information to select control patients with characteristics that were consistent with characteristics outlined in the Mpox National Vaccine Strategy,8 which included patients receiving HIV PrEP or with a new HIV diagnosis. However, we might have inadvertently missed or included persons who were eligible or ineligible, respectively, for vaccination. In addition, case patients and control patients differed slightly according to HIV status; eligible case patients had either an existing or a new HIV diagnosis, whereas eligible control patients had only a new HIV diagnosis. Underascertainment of vaccination status, which would occur for patients vaccinated at non-Epic sites outside their state of residence, would underestimate vaccine effectiveness if vaccinated control patients were misclassified as unvaccinated. For vaccinations before the EUA was issued on August 9, 2022, we recategorized “other” routes of administration as “subcutaneous”; however, it is possible that we erroneously recategorized vaccinations administered incorrectly. Because we lacked information on patients’ exposures to persons with mpox, we could not differentiate those vaccinated less than 14 days after their index event from those receiving mpox postexposure prophylaxis or expanded postexposure prophylaxis, and as a result we excluded those persons from analyses. Limited sample sizes resulted in imprecise estimates and precluded us from examining vaccine effectiveness among all subpopulations of interest, or according to all routes of administration. Although unmeasured confounding might influence vaccine effectiveness, E values suggested that our findings were robust to unmeasured confounding.
This study, which used data from a national EHR data platform, suggested that JYNNEOS vaccine was effective at reducing the risk of mpox disease, with effectiveness that appeared to be greater among persons who completed the two-dose series. In addition to vaccination, persons might benefit from strategies to prevent or reduce the risk of acquiring or transmitting mpox.25,28 Further research is needed to understand vaccine effectiveness according to route of administration and among subpopulations at risk for severe outcomes.
Supplementary Material
Acknowledgments
Supported by the Centers for Disease Control and Prevention and Epic Research.
We thank Caleb Cox for data management and analytic support.
Footnotes
The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Contributor Information
Nicholas P. Deputy, Mpox Emergency Response Team, Centers for Disease Control and Prevention, Atlanta; Public Health Service Commissioned Corps, Rockville, MD
Joseph Deckert, Epic Research, Epic Systems, Verona, WI
Anna N. Chard, Mpox Emergency Response Team, Centers for Disease Control and Prevention, Atlanta; Public Health Service Commissioned Corps, Rockville, MD
Neil Sandberg, Epic Research, Epic Systems, Verona, WI
Danielle L. Moulia, Mpox Emergency Response Team, Centers for Disease Control and Prevention, Atlanta
Eric Barkley, Epic Research, Epic Systems, Verona, WI
Alexandra F. Dalton, Mpox Emergency Response Team, Centers for Disease Control and Prevention, Atlanta
Cory Sweet, Epic Research, Epic Systems, Verona, WI
Amanda C. Cohn, Mpox Emergency Response Team, Centers for Disease Control and Prevention, Atlanta; Public Health Service Commissioned Corps, Rockville, MD
David R. Little, Epic Research, Epic Systems, Verona, WI
Adam L. Cohen, Mpox Emergency Response Team, Centers for Disease Control and Prevention, Atlanta; Public Health Service Commissioned Corps, Rockville, MD
Danessa Sandmann, Epic Research, Epic Systems, Verona, WI
Daniel C. Payne, Mpox Emergency Response Team, Centers for Disease Control and Prevention, Atlanta
Jacqueline L. Gerhart, Epic Research, Epic Systems, Verona, WI
Leora R. Feldstein, Mpox Emergency Response Team, Centers for Disease Control and Prevention, Atlanta; Public Health Service Commissioned Corps, Rockville, MD
REFERENCES
- 1.Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak — nine states, May 2022. MMWR Morb Mortal Wkly Rep 2022;71:764–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Mpox: 2022 outbreak cases and data. April 12, 2023. (https://www.cdc.gov/poxvirus/monkeypox/response/2022/index.html).
- 3.Curran KG, Eberly K, Russell OO, et al. HIV and sexually transmitted infections among persons with monkeypox — eight U.S. jurisdictions, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of Monkeypox cases — United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1018–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Food and Drug Administration. JYNNEOS. March 2023. (package insert) (https://www.fda.gov/media/131078/download).
- 6.Food and Drug Administration. Fact sheet for healthcare providers administering vaccine: emergency use authorization of JYNNEOS (smallpox and monkeypox vaccine, live, non-replicating) for prevention of monkeypox disease in individuals determined to be at high risk for monkeypox infection. August 2022. (https://www.fda.gov/media/160774/download).
- 7.Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine 2015;33:5225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Administration for Strategic Preparedness and Response. JYNNEOS vaccine distribution by jurisdiction. Washington, DC: Department of Health and Human Services; (https://aspr.hhs.gov/SNS/Pages/JYNNEOS-Distribution.aspx). [Google Scholar]
- 9.Centers for Disease Control and Prevention. JYNNEOS vaccine. December 22, 2022. (https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/jynneos-vaccine.html).
- 10.Overton ET, Stapleton J, Frank I, et al. Safety and immunogenicity of modified vaccinia Ankara-Bavarian Nordic smallpox vaccine in vaccinia-naive and experienced human immunodeficiency virus-infected individuals: an open-label, controlled clinical phase II trial. Open Forum Infect Dis 2015;2(2):ofv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payne AB, Ray LC, Cole MM, et al. Reduced risk for mpox after receipt of 1 or 2 doses of JYNNEOS vaccine compared with risk among unvaccinated persons — 43 U.S. jurisdictions, July 31–October 1, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff Sagy Y, Zucker R, Hammerman A, et al. Real-world effectiveness of a single dose of mpox vaccine in males. Nat Med 2023;29:748–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epic Systems. Epic Cosmos https://cosmos.epic.com/).
- 14.Kroger A, Bahta L, Hunter P. General best practice guidelines for immunization. Atlanta: Centers for Disease Control and Prevention; (https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/downloads/general-recs.pdf). [Google Scholar]
- 15.Agency for Toxic Substances and Disease Registry. CDC/ATSDR Social Vulnerability Index. Atlanta: Centers for Disease Control and Prevention. November 16, 2022. (https://www.atsdr.cdc.gov/placeandhealth/svi/index.html). [Google Scholar]
- 16.Flanagan BE, Hallisey EJ, Adams E, Lavery A. Measuring community vulnerability to natural and anthropogenic hazards: the Centers for Disease Control and Prevention’s Social Vulnerability Index. J Environ Health 2018;80:34–6. [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg JA, Hohmann SF, Hall JB, Kress JP, David MZ. Validation of a method to identify immunocompromised patients with severe sepsis in administrative databases. Ann Am Thorac Soc 2016;13:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes K, Middleton DB, Nowalk MP, et al. Effectiveness of influenza vaccine for preventing laboratory-confirmed influenza hospitalizations in immunocompromised adults. Clin Infect Dis 2021;73(11):e4353–e4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. Smallpox vaccine basics. August 8, 2022. (https://www.cdc.gov/smallpox/vaccine-basics/index.html).
- 20.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- 21.Office of the Federal Registrar. Electronic code of federal regulations. Title 45: part 46 — protection of human subjects. National Archives, March 30, 2023. (https://www.ecfr.gov/cgi-bin/text-idx?SID=fc043bd2812f0775fa80066558a6bbcf&mc=true&node=pt45.1.46&rgn=div5#se45.1.46_1102). [Google Scholar]
- 22.Kava CM, Rohraff DM, Wallace B, et al. Epidemiologic features of the monkeypox outbreak and the public health response — United States, May 17–October 6, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1449–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Clinician FAQs: people who are immunocompromised. February 2, 2023. (https://www.cdc.gov/poxvirus/monkeypox/clinicians/faq.html#People-who-are-Immunocompromised).
- 24.Miller MJ, Cash-Goldwasser S, Marx GE, et al. Severe monkeypox in hospitalized pat ients — United States, August 10–October 10, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. How to protect yourself — mpox prevention steps. October 31, 2022. https://www.cdc.gov/poxvirus/mpox/prevention/protect-yourself.html).
- 26.Brooks JT, Marks P, Goldstein RH, Walensky RP. Intradermal vaccination for monkeypox — benefits for individual and public health. N Engl J Med 2022;387:1151–3. [DOI] [PubMed] [Google Scholar]
- 27.Kriss JL, Boersma PM, Martin E, et al. Receipt of first and second doses of JYNNEOS vaccine for prevention of monkeypox — United States, May 22–October 10, 2022. MMWR Morb Mortal Wkly Rep 2022;71:1374–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delaney KP, Sanchez T, Hannah M, et al. Strategies adopted by gay, bisexual, and other men who have sex with men to prevent monkeypox virus transmission — United States, August 2022. MMWR Morb Mortal Wkly Rep 2022;71:1126–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.