Abstract
Introduction.
Until now, there has been limited evidence, primarily from US cohorts, focusing on frailty as a patient-oriented outcome after liver transplantation (LT). Our study aimed to explore the relationship between pre- and post-LT frailty in a multicenter European cohort of outpatients with cirrhosis undergoing LT.
Methods.
We conducted a prospective analysis of data from 180 LT recipients recruited between 2018 and 2020 from 5 Spanish centers. Participants underwent objective and subjective frailty assessments using the Liver Frailty Index (LFI) and the Subjective Clinician Assessment (SCA) pretransplant and at 3- and/or 6-mo posttransplant.
Results.
The median pretransplant LFI was 3.9, showing minimal change at 3 mo (3.8; P = 0.331) and improvement at 6-mo post-LT (3.6; P = 0.001). Conversely, the SCA significantly improved early post-LT: at 3 mo, poor SCA decreased from 11% to 1%, and good SCA increased from 54% to 89% (P < 0.001), remaining stable between 3- and 6-mo post-LT. Multivariable analysis revealed that each 0.1 increase in pretransplant LFI correlated with a reduced probability of being robust at 3-mo (odds ratio [OR] = 0.75; P < 0.001) and 6-mo post-LT (OR = 0.74; P < 0.001). There was poor concordance between SCA and LFI, with SCA underestimating frailty both pre- and post-LT (Kappa < 0.20).
Conclusion.
In our European cohort, incomplete improvement of physical frailty was observed, with <20% achieving robust physical condition within 6-mo post-LT. The pretransplant LFI strongly predicted posttransplant frailty. As the SCA tends to overestimate physical function, we recommend using both subjective and objective tools for frailty assessment in LT candidates and recipients.
End-stage liver disease is at the root of the emergence of physical frailty, a complex phenomenon encompassing more than just functional impairment. It includes factors such as sarcopenia, malnutrition, reduced physical and mental fitness, muscle strength, balance, and walking speediness.1 In the field of hepatology, the evaluation of physical frailty often relies on subjective or objective tools.2,3 The Subjective Clinician Assessment (SCA), involving the clinician’s overall judgment of the patient’s health, is commonly used in transplant centers during the decision-making process for listing patients for liver transplantation (LT). Despite its subjective nature and high variability, it has been demonstrated to independently predict waiting list mortality in patients with cirrhosis.4 Recognizing the necessity for an objective assessment of physical frailty in this patient population, the American Societies of Transplantation and Liver Diseases recently recommended the integration of survey-based tools like Karnofsky Performance Status (KPS) or Activities of Daily Living (ADL) for inpatients and performance-based assessments such as the Liver Frailty Index (LFI) or the 6-min walk distance for outpatients into routine clinical practice,2,3.
The KPS and the LFI stand as the sole frailty assessment tools used to track longitudinal changes before and after LT in patients with cirrhosis.5-7 Extensive North American prospective cohort studies have revealed that physical frailty tends to worsen between listing for transplantation and the actual transplant procedure, showing only modest improvement posttransplant.5,6 The Functional Assessment in Liver Transplantation (FrAILT) study, conducted across multiple centers in the United States, involving 1093 outpatients with cirrhosis, indicated that 51% experienced a significant deterioration in frailty (measured by the LFI) during a median follow-up time of 10.6 mo on the waitlist.6 Among the 214 recipients enrolled in the FrAILT study, a majority demonstrated a gradual improvement in physical frailty compared with their pretransplant state. Median LFI scores worsened at 3-mo posttransplant but showed an overall improvement at 12 mo.7 Additionally, the pretransplant frailty status, categorized as “Frail,” “prefrail,” and “robust” with LFI values ≥4.5, 3.2–4.4, and <3.2, respectively, significantly predicted posttransplant frailty status. Less than 40% of liver transplant recipients achieved a “robust” status within 1 y after transplantation.7 Similarly, findings from a large dataset of 47 793 adults listed for LT within the United Network for Organ Sharing indicated a decline in KPS scores from listing to transplant in most candidates, with the decline worsening with longer waitlist durations. Posttransplant data for 42 339 recipients at 1 y and 30 291 at 2 y revealed that KPS scores improved in at least 90% of patients after transplantation, with a median amelioration of 20% by 1-y posttransplant and no significant change thereafter.5
As of now, to the best of our knowledge, there have been no published studies examining alterations in physical frailty after LT within European cohorts. In a previous multicenter Spanish study, we demonstrated an association between pretransplant frailty and a higher complication rate, as well as an extended posttransplant length of stay.8 Furthermore, analyses from LT registries in Europe and the United States highlight variations in the baseline characteristics of cirrhosis patients undergoing LT in these regions. For instance, the predominant indications for LT in Europe from 2007 to 2016 were viral infections (22%) and alcohol-related liver disease (19%)9 whereas in the United States, “other” diagnoses (36%), primarily including candidates with metabolically associated steatotic liver disease (MASLD), and alcohol-related liver disease (31%) were more prevalent.10 The acuity at transplant also differs, with higher Model for End-Stage Liver Disease (MELD) scores reported in US studies. In 2019, the proportion of transplanted recipients with a MELD score ≥30 in Europe was only 4%,11 compared with 39% in the United States.10 Furthermores, waiting times vary; while the median time on the LT waitlist was 5.6 mo in the United States, >50% of candidates in Europe awaited LT for <6 mo.10 These disparities underscore the importance of delving into frailty research in the European context.
In this current study, our aim was to explore the correlation between pretransplant and posttransplant frailty, as assessed by the LFI and the SCA, within the Spanish LT setting. We hypothesized that, akin to US studies, pretransplant frailty would improve after LT. However, given the typically shorter waiting times and lower MELD scores in the European setting, we anticipated that this improvement would occur sooner and at a higher rate.
MATERIALS AND METHODS
Study Design
We performed a prospective, longitudinal cohort study with both pretransplant and posttransplant frailty assessments performed in the ambulatory setting using the objective LFI and the SCA about patient’s overall health of patients from 5 transplant centers in Spain.
Our primary predictor was pretransplant frailty, as assessed by the objective LFI and the SCA in outpatients with cirrhosis waiting for LT.
Our primary outcome was frailty changes within 6 mo after LT defined as (1) changes in posttransplant LFI measurements, (2) changes in posttransplant frailty categories as measured by the LFI (robust, prefrail, and frail), and (3) changes in posttransplant frailty categories as assessed by the SCA (good, fair, poor), compared with pretransplant.
Secondary outcomes were (1) posttransplant mortality, for death in the first 6 mo after LT and (2) posttransplant morbidity which encompassed (1) length of transplant hospitalisation, defined as the number of days between transplant date and discharge date, (2) length of intensive care unit (ICU) stay, defined as the number of days in the ICU post-LT surgery, (3) early posttransplant complications, considered as adverse events occurring within 30 d post-LT, defined and graded based on the Clavien-Dindo classification (mild: <grade IIIA; severe: ≥ grade IIIA),12 (4) late posttransplant complications for adverse events needing hospital readmission between 30 and 90 d post-LT, (5) posttransplant cardiovascular events defined as ischaemic/haemorrhagic stroke, acute coronary syndrome, peripheral artery disease, heart failure, arrhythmias (ie, atrial fibrillation/flutter, barring if appearing in hemodynamic context) occurring within 6-mo post-LT, and (6) retransplant need within 6-mo post-LT.
Study Population
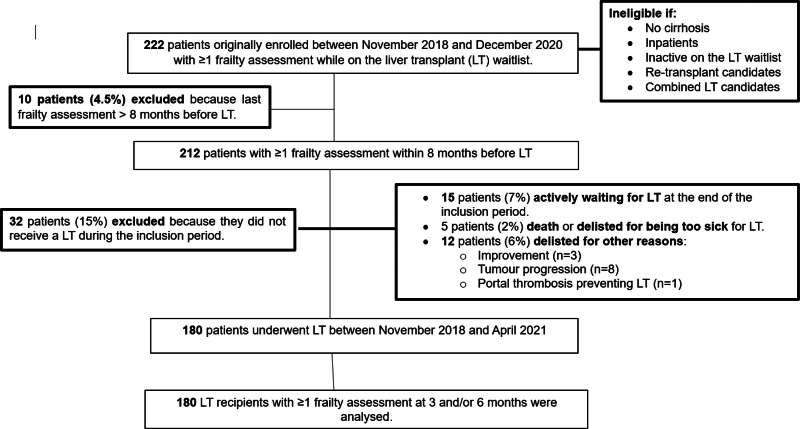
Data were acquired from a Spanish multicenter prospective cohort study that enrolled adult patients whether (1) they had an underlying cirrhosis, (2) they were actively listed for LT, and (3) they had an objective and subjective frailty assessment before LT performed in the ambulatory setting (given that the LFI was designed for outpatients) at the following institutions (1) La Fe University Hospital of Valencia, (2) Clinic University Hospital of Barcelona, (3) Reina Sofía University Hospital of Córdoba, (4) Lozano Blesa University Hospital of Zaragoza, and (5) Gregorio Marañón University General Hospital of Madrid. Patients were ineligible for enrollment if they lacked cirrhosis, were candidates for retransplantation or combined LT, or experienced any acute decompensation of their liver condition during the ambulatory visit, necessitating immediate hospitalization. To ensure the relevance of frailty assessments, participants whose most recent frailty evaluations were conducted >8 mo before LT were excluded. This criterion was established considering the potential for significant changes in frailty status within a short period among patients with cirrhosis.
For the present study, we scrutinized data from individuals who underwent LT at participating sites between November 9, 2018, and April 25, 2021. Inclusion criteria mandated that these patients had undergone at least 1 objective and subjective frailty assessment at 3-mo and/or 6-mo posttransplant, coinciding with routine clinic visits.
The institutional review committee from each site approved the study. Written informed consent was procured from each patient before participation in the study.
Frailty Assessments
All patients underwent objective and subjective measurements of frailty during an ambulatory visit using the LFI and the SCA.
The LFI, a continuous index specifically developed in the field of hepatology, was obtained from the scores of 3 easy performance-based tests:
(1) Grip strength: the average of 3 trials, measured in the subject’s dominant hand, using a hand dynamometer, in kilograms.
(2) Timed chair stands: the number of seconds required for performing 5 chair stands with the subject’s arms folded across the chest.
(3) Balance testing: the number of seconds the subject can maintain 3 positions of balance (feet side to side, semi-tandem, and tandem) for a maximum of 10 s each.
These tests were carried out by trained study collaborators. The LFI was then calculated using an online calculator available at http://liverfrailtyindex.ucsf.edu. Patients were classified as “robust,” “prefrail,” and “frail” by optimal cut points of LFI <3.2, between 3.2 and 4.4, and ≥4.5, respectively.3
The SCA was assessed by asking the hepatologist or LT surgeon who supplied ambulatory care to the patient to subjectively rate the subject’s overall health on the same day as the outpatient visit using the following question: “What is your impression about your patient’s overall health today, as compared with other patients with underlying liver disease or liver transplant recipients: Good, Fair or Poor.” The providers were blinded to the LFI measurements at the time of answering this question.
Timing of Frailty Assessments
Outpatients underwent frailty assessments both before and after LT coinciding with clinic visits previously scheduled by their regular hepatologists or LT surgeons:
(1) Pretransplant: Objective measurements using the LFI and the SCA were repeated every 6 mo (±2 mo) while active on the LT waitlist until transplant, death, or delisting. In the present study, the frailty assessments closest to the date of LT were selected as the “pretransplant frailty assessments” for our subsequent analyses.
(2) Posttransplant: After LT, frailty was evaluated for up to 6 mo. Objective and subjective assessments at 3 and 6 mo after the transplant date (±2 mo) were considered as the “posttransplant frailty assessments” for our analyses.
Additional Data Collection
Data regarding each variable of interest: (1) demographics: sex, age, height, weight, and body mass index (BMI); (2) baseline liver disease: etiology of cirrhosis (chronic hepatitis C [CHC] infection, chronic hepatitis B [CHB] infection, alcohol, MASLD, cholestasis, other), laboratory and clinical data to calculate the MELD, MELD score with the addition of sodium serum concentration (MELD-Na), MELD 3.0, and Child-Pugh scores including personal history of ascites, hepatic encephalopathy and/or presence of hepatocellular carcinoma (HCC); and (3) cardiovascular risk factors: history of dyslipidemia, diabetes (type 1 or type 2), arterial hypertension, and/or cardiovascular disease were obtained by study personnel from the clinic visit note closest to the objective LFI measurement. History of ascites or hepatic encephalopathy was recorded if it was reported in the electronic health record. Ascites were graded as “none” if never reported, “mild–moderate” if clinically controlled with both diuretic medication or sporadic paracentesis, and “severe” if large-volume paracentesis was needed on a regular schedule. Patients were considered to have a medical background of dyslipidemia, hypertension, or diabetes if the diagnosis was recorded in their medical history, or if they were prescribed treatment(s) to manage these diseases. History of cardiovascular disease was defined as a past report of coronary artery disease, peripheral artery disease, stroke (ischemic or hemorrhagic), arrhythmias, and heart failure. Donor variables (age, donation after circulatory death [DCD]) were also acquired from the electronic health report. Posttransplant outcomes regarding morbidity and mortality of LT recipients in the first 6 mo after LT were also acquired from the electronic health report.
Sample Size
To achieve an 80% statistical power for detecting changes in LFI from pretransplant up to 6-mo posttransplant with a small effect size (f = 0.1) and a 95% confidence interval (CI), we estimated a minimum of n = 172 patients to be included. Thus, the obtained sample size of n = 180 subjects is optimal to test our study hypothesis.
Statistical Analysis
Comparison of baseline characteristics according to pretransplant frailty status as assessed by the LFI (robust, prefrail, and frail) and the SCA (good, fair, and poor) were carried out using the Chi-square test for categorical variables and Kruskal-Wallis test for continuous variables. We considered that the 3 categories of both classifications were equivalents (robust/good, prefrail/fair, and frail/poor). Discrete variables were reported as frequencies (percentages) and continuous distributions were reported as medians (interquartile range [IQR]).
Kappa coefficient was used to assess concordance between the objective and the subjective frailty classifications. Because the concordance we found was poor, a McNemar test was performed to investigate the symmetry of discordant cases.
Changes over time in LFI measurements and frailty categories (according to both the LFI and the SCA) were determined with general lineal model of analyses of variance’s repeated measures and Wilcoxon test, respectively. Multiple comparisons problem was counteracted by Bonferroni correction.
Logistic regression models investigated the association between pretransplant LFI and the probability of being “robust” at 3- and 6-mo posttransplant in multivariable analysis adjusted for confounders predicting posttransplant robustness in univariable analyses (P < 0.1) such as diagnosis of HCC and HIV infection before LT, BMI at baseline, and MELD and Child-Pugh scores at transplant. Similarly, Cox, linear, and logistic regressions assessed associations between pretransplant frailty and posttransplant outcomes in multivariable analyses adjusted for covariables found to be predictive of pretransplant frailty on univariable analyses (P < 0.1) such as MELD-Na and Child-Pugh scores at transplant, recipient age, female sex, and cardiovascular risk factors.
A cut point of P < 0.05 was used to assess statistical significance. SPSS, 15.0 (Chicago, IL), was used for the statistical analyses.
The present article embraces the Strengthening The Reporting of Observational Studies in Epidemiology statement: guidelines for reporting observational studies.13
RESULTS
Participants
Of the 212 LT candidates initially enrolled in our multicenter study, we analyzed data from 180 patients who finally underwent LT. Thirty-two patients (15%) were excluded because they either died, were delisted or still waiting for LT at the end of the inclusion period (Figure 1).
FIGURE 1.
Flowchart. LT, liver transplantation.
Clinicians’ Characteristics
A total of 27 hepatologists and LT surgeons participated in this study providing a subjective assessment of their patients’ overall health status on the same day as the outpatient visit. Pretransplant and posttransplant characteristics of the providers are presented in Table 1.
TABLE 1.
Characteristics of the clinicians participating in the study providing the subjective assessment of their patients’ overall health status
| Clinicians’ characteristicsa | Pretransplant | 3-Mo posttransplant | 6-Mo posttransplant |
|---|---|---|---|
| Number of clinicians | 25 | 17 | 15 |
| % of female gender | 52 | 47 | 53 |
| % of LT surgeons | 20 | 0 | 0 |
| Median time of expertise in an LT unit, y | 11 (7–13) | 12 (8–13) | 12 (8–13) |
aMedian (interquartile range) or N (%).
LT, liver transplantation.
Of the 25 clinicians assessing LT candidates, 52% were women, 80% were hepatologists, 20% were LT surgeons and their median (IQR) time in an LT specialized unit was 11 y (7–13).
For SCA performed at 3- and 6-mo posttransplant, 17 and 15 clinicians participated in the study, respectively. They were all hepatologists. About half were women and their median time of expertise was 12 y (8–13).
Patients Characteristics
The baseline characteristics of the 180 LT recipients included in the study are listed in Table 2, column A. Median (IQR) age was 60 y (55–65) and 17% were female. With respect to baseline liver disease, the main causes of cirrhosis were CHC infection in 37%, alcohol in 35%, and MASLD in 12%. Median (IQR) laboratory MELD-Na/MELD 3.0 and Child-Pugh scores at transplant were 12 (9–19) and 7 (5–10), respectively. HCC was present in 59%. The primary indication for LT for patients with cirrhosis with concomitant HCC was the liver tumor (71% were CHILD-A, and 77% had a MELD-Na score <12 at transplant), whereas for patients without HCC, decompensated cirrhosis was the main reason to indicate LT (median [IQR] MELD-Na/MELD 3.0 score: 20 [17–25]). Regarding transplant characteristics, all patients underwent LT from a deceased donor (24% DCD), with a median (IQR) age of 59 y (50–72). Prevalence of arterial hypertension, diabetes, and dyslipidemia were 34%, 33%, and 23%, respectively, and a history of cardiovascular disease was present in 9% of the LT recipients. Median (IQR) time from pretransplant LFI measurement to LT was 41 d (14–99) for the entire cohort.
TABLE 2.
Baseline patient characteristics of the entire cohort and by frailty categories using the Liver Frailty Index or the Subjective Clinician Assessment
| Pretransplant characteristicsb | (A) All (n = 180) | Liver Frailty Indexa | Subjective Clinician Assessment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A) | (B) Robust (n = 23, 13%) | (C) Prefrail (n = 123, 68%) | (D) Frail (n = 34, 19%) | P c | (E) Good (n = 100, 55%) | (F) Fair (n = 61, 34%) | (G) Poor (n = 19, 11%) | P c | |
| Demographics | |||||||||
| Hospitals | |||||||||
| La Fe | 37% | 65% | 33% | 32% | 0.024 | 40% | 34% | 32% | 0.226 |
| Clínic | 38% | 26% | 40% | 38% | 32% | 44% | 47% | ||
| Reina Sofia | 11% | 4% | 14% | 3% | 16% | 5% | 0 | ||
| Lozano Blesa | 8% | 4% | 6% | 18% | 6% | 10% | 11% | ||
| Gregorio Marañón | 7% | 0% | 7% | 9% | 6% | 7% | 11% | ||
| Age, y | 60 (55–65) | 59 (55–62) | 59 (55–64) | 64 (60–69) | 0.005 | 58 (55–64) | 60 (54–65) | 62 (60–66) | 0.086 |
| Female sex | 30 (17%) | 4% | 20% | 15% | 0.190 | 13% | 21% | 21% | 0.336 |
| Body Mass Index | 28 (25–31) | 28 (26–30) | 28 (25–31) | 27 (24–31) | 0.545 | 27 (25–30) | 28 (25–33) | 27 (24–29) | 0.371 |
| Baseline liver disease | |||||||||
| Etiology of liver disease | |||||||||
| Chronic hepatitis C | 37% | 65% | 36% | 21% | 0.022 | 50% | 23% | 11% | 0.003 |
| Alcohol | 35% | 22% | 33% | 53% | 27% | 44% | 47% | ||
| MASH | 12% | 0% | 15% | 9% | 12% | 11% | 16% | ||
| Chronic hepatitis B | 7% | 13% | 7% | 6% | 8% | 4% | 5% | ||
| Cholestatic | 1% | 0% | 2% | 0% | 0% | 2% | 5% | ||
| Other | 8% | 0 | 8% | 12% | 3% | 13% | 16% | ||
| HIV infection | 4% | 9% | 5% | 0 | 0.273 | 6% | 3% | 0 | 0.441 |
| Hepatocellular carcinoma | 59% | 96% | 59% | 32% | <0.001 | 87% | 28% | 11% | <0.001 |
| Laboratory tests at LT | |||||||||
| MELD | 12 (9–18) | 8 (7–10) | 12 (9–17) | 16 (12–23) | <0.001 | 9 (7–12) | 17 (13–21) | 18 (14–25) | <0.001 |
| MELD score with the addition of sodium serum concentration | 12 (9–19) | 8 (7–10) | 12 (9–19) | 19 (12–27) | <0.001 | 9 (7–12) | 19 (13–24) | 21 (17–27) | <0.001 |
| MELD 3.0 | 13 (9–19) | 7 (6–11) | 13 (9–19) | 18 (13–25) | <0.001 | 9 (7–13) | 19 (14–24) | 20 (17–28) | <0.001 |
| Albumin, g/dL | 3.7 (3.2–4.2) | 4.5 (4.1–4.8) | 3.7 (3.2–4.2) | 3.2 (3.0–3.9) | <0.001 | 4.0 (3.6–4.5) | 3.3 (2.9–3.9) | 3.0 (2.7–3.9) | <0.001 |
| Ascites | |||||||||
| Absent | 41% | 78% | 42% | 12% | <0.001 | 66% | 11% | 5% | <0.001 |
| Mild–moderate | 40% | 22% | 39% | 56% | 27% | 58% | 37% | ||
| Severe | 19% | 0% | 19% | 32% | 7% | 31% | 58% | ||
| Hepatic encephalopathy | 34% | 9% | 29% | 68% | <0.001 | 13% | 56% | 74% | <0.001 |
| Child-Pugh score | 7 (5–10) | 5 (5–6) | 7 (5–9) | 10 (7–11) | <0.001 | 5 (5–7) | 9 (8–11) | 10 (8–12) | <0.001 |
| Cardiovascular Risk Factors | |||||||||
| Arterial hypertension | 34% | 22% | 35% | 38% | 0.394 | 30% | 43% | 26% | 0.198 |
| Dyslipidemia | 23% | 17% | 24% | 26% | 0.724 | 21% | 26% | 26% | 0.710 |
| Diabetes (type 1 or type 2) | 33% | 17% | 37% | 29% | 0.151 | 35% | 26% | 47% | 0.202 |
| Cardiovascular disease | 9% | 9% | 9% | 12% | 0.876 | 5% | 18% | 5% | 0.019 |
| Donor characteristics | |||||||||
| Age, y | 59 (50–72) | 62 (51–74) | 58 (49–69) | 64 (50–72) | 0.624 | 59 (50–73) | 57 (48–71) | 67 (53–74) | 0.239 |
| Donation after circulatory death LT | 24% | 13% | 28% | 12% | 0.073 | 25% | 21% | 16% | 0.643 |
| Pretransplant Frailty Assessments | |||||||||
| Liver Frailty Index | 3.9 (3.5–4.3) | 2.8 (2.6–3.0) | 3.7 (3.5–3.9) | 4.8 (4.6–5.1) | <0.001 | 3.6 (3.3–4.1) | 4.1 (3.8–4.5) | 4.9 (4.2–5.2) | <0.001 |
| Days between frailty assessment and LT date | 41 (14–99) | 50 (20–129) | 46 (16–99) | 22 (6–65) | 0.199 | 47 (19–106) | 36 (10–97) | 33 (7–84) | 0.422 |
| Posttransplant Frailty Assessments | |||||||||
| Frailty assessments at 3-mo posttransplant | 66% | 61% | 67% | 65% | 0.860 | 67% | 64% | 63% | 0.900 |
| Frailty assessments at 6-mo posttransplant | 78% | 83% | 80% | 65% | 0.123 | 81% | 75% | 68% | 0.415 |
Bold values indicate significant results.
aDefined by the Liver Frailty Index as “Robust” if the score <3.2, “Prefrail” if the score between 3.2 and 4.4 and “Frail” if the score ≥4.5
bMedian (interquartile range) or N (%).
cResults of the Chi-square or Kruskal-Wallis tests.
LT, liver transplantation; MASH, Metabolic dysfunction-associated steatohepatitis; MELD, Model for End-Stage Liver Disease.
In Table 2, columns B–D display baseline patient characteristics according to their pretransplant frailty category based on the LFI. Thirty-four patients (19%) met the criteria for “frail,” 123 (68%) for “prefrail,” and 23 (13%) for “robust” as defined by an LFI ≥4.5, between 3.2 and 4.4 and <3.2, respectively. Median (IQR) LFI among patients who were robust was 2.8 (2.6–3.0), 3.7 (3.5–3.9) among those prefrail, and 4.8 (4.6–5.1) among those frail. Frail patients (compared with prefrail and robust) were older (64 versus 59 y; P = 0.005), had less probability to have CHC infection (21% versus 36% and 65%; P = 0.022) and HCC (32% versus 59% and 96%; P < 0.001) but higher probability to have alcohol-related cirrhosis (53% versus 33% and 22%; P = 0.022). Standard metrics of liver disease severity at transplant (MELD-Na, MELD 3.0, Child-Pugh scores) including rates of ascites and history of hepatic encephalopathy were also higher in frail patients than in prefrail or robust patients (P < 0.001). The 3 categories were similar with respect to sex, BMI, cardiovascular comorbidities, donor characteristics, and time from pretransplant LFI measurement to LT (P > 0.05).
In Table 2, columns E–G list baseline patient characteristics according to their pretransplant frailty category based on the SCA. Before the transplant, 100 LT candidates (56%) were found to be in “good” condition by the attending physician, whereas the impression was “fair” in 61 (34%) and “poor” in 19 candidates (11%). To facilitate comparison of our data, we assumed the 3 categories of both classifications were equivalents (robust/good, prefrail/fair, and frail/poor). Median (IQR) LFI among patients with a pretransplant “good” SCA was 3.6 (3.3–4.1); it was 4.1 (3.8–4.5) among those with a “fair” SCA and 4.9 (4.2–5.2) among those with a “poor” SCA. Similar to frail patients, subjects with a “poor” SCA before LT (compared with those with a “fair” and “good” SCA) were less likely to have CHC infection (11% versus 23% and 50%; P = 0.003) and HCC (11% versus 28% and 87%, P < 0.001) and more likely to have alcohol-related cirrhosis (47% versus 44% and 27%; P = 0.003). Rates of severe ascites, hepatic encephalopathy, and laboratory Child-Pugh and MELD scores (in all its versions) were also higher in patients with a pretransplant-poor SCA than in the other 2 groups (P < 0.001). Unlike the pretransplant frail category, the “poor” SCA category (compared with “fair” and “good” SCA categories) was associated with increased proportion of metabolic dysfunction-associated steatohepatitis cirrhosis (16% versus 11% and 12%; P = 0.003) but not with older LT recipients (P = 0.086). Similarly to the objective classification, the 3 groups of the SCA classification were similar in terms of sex, BMI, and donor characteristics (P > 0.05).
Changes in Posttransplant Assessments Compared With Pretransplant
Posttransplant frailty assessments both by the LFI and by the SCA were available for 118 LT recipients (66%) at 3-mo and for 140 LT recipients (78%) at 6-mo posttransplant. There were no statistically significant differences in the number of posttransplant assessments by pretransplant frailty category based on the LFI (P = 0.860 and P = 0.123 at 3- and 6-mo posttransplant) or the SCA (P = 0.900 and P = 0.415 at 3- and 6-mo posttransplant).
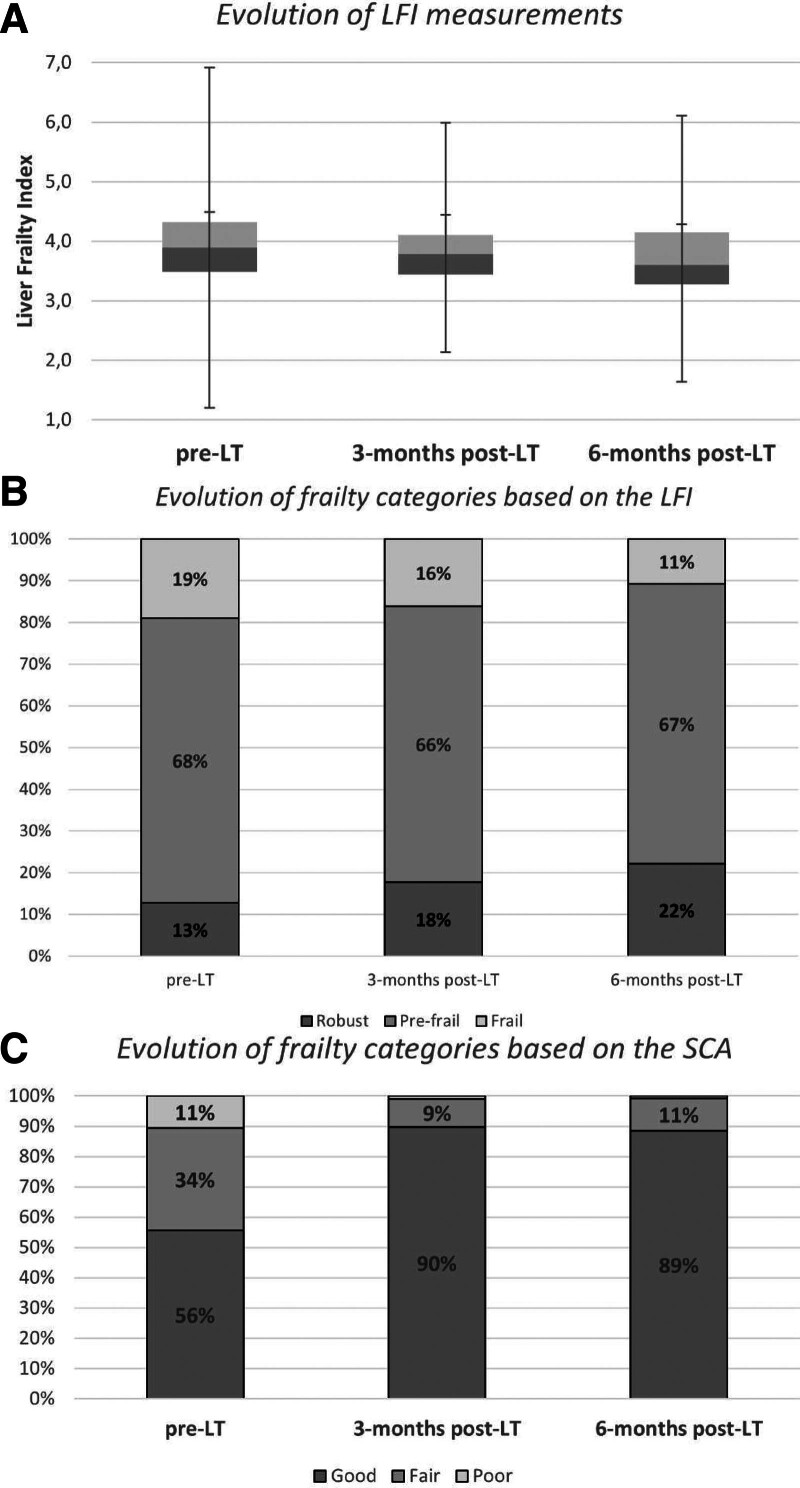
Median (IQR) pretransplant LFI was 3.9 (3.5–4.3). The scores remained similar at 3 mo (3.8; P = 0.331) and significantly improved at 6-mo posttransplant (3.6; P = 0.001) (Figure 2A and Table 3). The percentage robust pretransplant and 3-, 6-mo posttransplant was 13%, 18%, and 22%, respectively; the percentage frail was 19%, 16%, and 11% (Figure 2B). Conversely, the SCA significantly improved early posttransplant with respect to pretransplant: at 3-mo posttransplant, poor SCA decreased from 11% to 1% and good SCA increased from 56% to 90% (P < 0.001) and then remained stable between 3 and 6 mo after LT (not significant by P < 0.05) (Figure 2C and Table 3). Frailty categories of the LFI and the SCA at each time point among patients with and without HCC are shown in Figure S1 (SDC, http://links.lww.com/TXD/A630).
FIGURE 2.
Evolution of frailty assessments based on the Liver Frailty Index (LFI) and the Subjective Clinician Assessment (SCA) after liver transplantation (LT).
TABLE 3.
Frailty changes after LT compared with pretransplant and between 3- and 6-mo posttransplant
| Comparisons of frailty assessments | Liver Frailty Index | Subjective Clinician Assessment |
|---|---|---|
| P a | P b | |
| Pre-LT vs 3-mo post-LT | 0.331 | <0.001 |
| 3-mo vs 6-mo post-LT | 0.039 | 1.000 |
| Pre-LT vs 6-mo post-LT | 0.001 | <0.001 |
Bold values indicate significant results.
aMultiple comparisons result from the analyses of variance model with Bonferroni correction.
bMultiple comparisons result of the Wilcoxon test with Bonferroni correction.
LT, liver transplantation.
Pretransplant and posttransplant frailty categories among the 118 and 140 patients with a frailty assessment at 3- and 6-mo after LT are present in Table 4 (based on the LFI) and Table 5 (based on the SCA). Among the 23 patients who were robust pretransplant, 6 (26%) and 5 (22%) worsened to prefrail at 3- and 6-mo posttransplant. Among the 34 patients who were frail pretransplant, 8 (24%) improved to become prefrail at 3-mo and 12 (35%) at 6-mo posttransplant but only 1 (3%) improved to robust at 3-mo and none at 6-mo posttransplant (Table 4). Among the 100 patients with a good SCA before LT, 4 (4%) and 5 (5%) worsened to a “fair” impression at 3- and 6-mo posttransplant, and only 1 patient (1%) worsened to a “poor” impression at 6 mo after LT. Of the 19 patients with a poor pretransplant SCA, all improved to a better category after LT; 9 patients (47%) made a good impression at 3- and 6-mo, whereas 3 patients (16%) and 4 patients (11%) made a fair impression at 3- and 6-mo posttransplant, respectively (Table 5). Overall, compared with pretransplant, 60%–70% of LT recipients remained in the same frailty category based on both classifications (Table S1, SDC, http://links.lww.com/TXD/A630).
TABLE 4.
Pretransplant and posttransplant frailty categories among the 118 and the 140 LT recipients with a frailty assessment by the LFI at 3- and 6-mo after LT, respectivelya
| Pretransplant LFI-based categories (n = 180) | 3-Mo posttransplantb (n = 118) | 6-Mo posttransplantb (n = 140) | Total pre-LT | ||||
|---|---|---|---|---|---|---|---|
| Robust | Prefrail | Frail | Robust | Prefrail | Frail | ||
| Robust | 8 (35%) | 6 (26%) | 0 | 14 (61%) | 5 (22%) | 0 | 23 (13%) |
| Prefrail | 12 (10%) | 64 (52%) | 6 (5%) | 17 (14%) | 77 (63%) | 5 (4%) | 123 (68%) |
| Frail | 1 (3%) | 8 (24%) | 13 (38%) | 0 | 12 (35%) | 10 (29%) | 34 (19%) |
| Total post-LT | 21 (18%) | 78 (66%) | 19 (16%) | 31 (22%) | 94 (67%) | 15 (11%) | |
The subjects in the white cells did not experience any change in their posttransplant frailty category based on the LFI. The subjects in the dark, grey-shaded cells experienced an improvement in their frailty category at 3 and/or 6 mo after LT. The subjects in the light, grey-shaded cells experienced worsening in their frailty category at 3 and/or 6 mo after LT.
aAdapted from Lai et al.7
bN (%).
LT, liver transplantation; LFI, Liver Frailty Index.
TABLE 5.
Pretransplant and posttransplant frailty categories among the 118 and the 140 LT recipients with a frailty assessment by the SCA at 3- and 6-mo after LT, respectivelya
| Pretransplant SCA-based categoriesa (n = 180) | 3-Mo posttransplantb (n = 118) | 6-Mo posttransplantb (n = 140) | Total pre-LT | ||||
|---|---|---|---|---|---|---|---|
| Good | Fair | Poor | Good | Fair | Poor | ||
| Good | 63 (63%) | 4 (4%) | 0 | 76 (76%) | 5 (5%) | 1 (1%) | 100 (56%) |
| Fair | 34 (56%) | 4 (7%) | 1 (2%) | 39 (64%) | 6 (10%) | 0 | 61 (34%) |
| Poor | 9 (47%) | 3 (16%) | 0 | 9 (47%) | 4 (11%) | 0 | 19 (11%) |
| Total post-LT | 106 (90%) | 11 (9%) | 1 (1%) | 124 (89%) | 15 (11%) | 1(1%) | |
The subjects in the white cells did not experience any change in their posttransplant frailty category based on the SCA. The subjects in the dark, grey-shaded cells experienced improvement in their frailty category at 3 and/or 6 mo after LT. The subjects in the light, grey-shaded cells experienced worsening in their frailty category at 3 and/or 6 mo after LT.
aAdapted from Lai et al.7
bN (%).
LT, liver transplantation; SCA, Subjective Clinician Assessment.
In univariable analysis, each LFI increase of 0.1 pretransplant was associated with a decrease in the probability of being robust by approximately 25% both at 3-mo posttransplant (odds ratio [OR] = 0.75; P < 0.001) and 6-mo posttransplant (OR = 0.74; P < 0.001) which did not change substantially with multivariable adjustment for covariables that might be associated with pretransplant frailty such as MELD-Na at transplant, female sex, recipient age, and diabetes.
Concordance Between Frailty Objective and Subjective Classifications
Table 6 displays concordance between frailty classification with the LFI and the SCA both before and after LT. As previously stated, we assumed the 3 categories of both classifications were equivalents (good/robust, fair/prefrail, poor/frail). Pretransplant, the percentage of agreement was 31% using 180 frailty assessments. At 3- and 6-mo posttransplant the percentage of agreement was 23% and 26%, respectively. Kappa concordance coefficient between objective and subjective classifications was 0.19 pretransplant, 0.05 at 3-mo, and 0.00 at 6-mo posttransplant indicating that the strength of agreement between both classifications was poor (Kappa < 0.20). Before LT, 60% of prefrail and 18% of frail candidates received a “good” SCA. After LT, at 3- and 6-mo posttransplant, 93% of prefrail recipients and 72% and 76% of frail recipients were classified in the “good” SCA category, respectively. Results from the McNemar test confirmed that SCA overestimated the patients’ physical function pretransplant and posttransplant considering the LFI as the gold standard for frailty assessment (P < 0.001).
TABLE 6.
Concordance between frailty classifications with the Liver Frailty Index and the Subjective Clinician Assessment, pretransplant, and at 3- and 6-mo posttransplant
| Frailty classification | Liver Frailty Index | Concordance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Robust | Prefrail | Frail | Kappa a | P b | |||||
| N | % | N | % | N | % | N | % | |||
| Subjective Clinician Assessment | ||||||||||
| Pretransplant | ||||||||||
| Total | 180 | 100 | 23 | 13 | 123 | 68 | 34 | 19 | 0.19 | <0.001 |
| Good | 100 | 56 | 20 | 20 | 74 | 74 | 6 | 6 | ||
| Fair | 61 | 34 | 3 | 5 | 16 | 69 | 16 | 26 | ||
| Poor | 19 | 11 | 0 | 0 | 3 | 37 | 19 | 63 | ||
| 3-Mo posttransplant | ||||||||||
| Total | 118 | 100 | 21 | 18 | 68 | 58 | 29 | 25 | 0.05 | <0.001 |
| Good | 106 | 90 | 21 | 20 | 63 | 59 | 22 | 21 | ||
| Fair | 11 | 9 | 0 | 0 | 5 | 45 | 6 | 55 | ||
| Poor | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 100 | ||
| 6-Mo posttransplant | ||||||||||
| Total | 140 | 100 | 31 | 22 | 73 | 53 | 36 | 25 | 0.00 | <0.001 |
| Good | 124 | 89 | 30 | 24 | 68 | 55 | 26 | 21 | ||
| Fair | 15 | 11 | 1 | 7 | 5 | 33 | 9 | 60 | ||
| Poor | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | ||
aKappa coefficient.
bResults from McNemar test to assess the symmetry of discordant cases.
Posttransplant Outcomes
For the entire cohort, median (IQR) length of stay in the ICU was 3 d (2–5), and median time for hospitalization after the transplant surgery was 9 d (7–14). Early posttransplant (<30 d) complications occurred in 59% of LT recipients and 43% of them were severe (≥ grade 3 of the Clavien-Dindo classification).14 Median rate of late posttransplant complications (between 30 and 90 d) was 26%. Within 6 mo after LT, prevalence of cardiovascular events, retransplants and deaths were 4%, 6%, and 8%, respectively.
In multivariable analysis, after adjustment for covariables associated with frailty in univariable analysis, most results of the SCA differed from those performed for the LFI: while each 0.1 increase in the pretransplant LFI was significantly associated with higher ICU and transplant hospitalization length of stay (ẞ = 0.85, P = 0.005 and ẞ = 1.69, P = 0.007; respectively), no association was found between a “poor” pretransplant SCA and these outcomes (P > 0.005) (Table S2, SDC, http://links.lww.com/TXD/A630). In contrast, both pretransplant LFI and “poor” SCA were associated with an increased rate of early posttransplant complications (OR = 2.05; P = 0.002 and OR = 3.32; P = 0.046, respectively). None of the frailty instruments predicted the severity of postsurgical complications nor the risk of cardiovascular events, retransplant, or death in the first 6 mo after LT (P > 0.05) (Table S2, SDC, http://links.lww.com/TXD/A630).
DISCUSSION
In recent times, physical frailty has emerged as a robust predictor of adverse outcomes both before and after LT.2,3 However, there is a scarcity of studies, outside US cohorts, that have specifically delved into posttransplant frailty as a patient-centered outcome. Considering that, in individuals with cirrhosis, physical frailty is negatively associated with quality of life but not with the severity of liver disease, understanding the timing and extent of physical recovery after LT becomes crucial for patients and their caregivers in establishing realistic expectations before the transplant.15 Notably, findings from extensive prospective studies conducted in North America have indicated that while LT generally results in a swift recovery of hepatic synthetic and metabolic functions, the improvement in physical frailty is frequently delayed and incomplete.5,7,16 Nevertheless, there is a notable absence of literature addressing physical frailty after LT in contexts beyond the United States.
In our investigation involving 180 patients with cirrhosis who underwent LT across 5 Spanish centers and were monitored for up to 6-mo post-LT, we observed the following outcomes: (1) physical frailty, assessed through both the LFI and the SCA during outpatient visits, demonstrated some level of improvement in the short to intermediate term after LT compared with the pretransplant period. (2) However, physical recovery was only partial, with just 1 out of every 5 patients achieving a robust physical condition within the first 6-mo post-LT. (3) The pretransplant LFI score emerged as a strong predictor of robust physical function posttransplant. (4) The SCA tended to overestimate patients’ physical function both before and after LT. (5) Notably, the pretransplant LFI proved to be a more effective predictor of posttransplant morbidity and healthcare utilization compared with the SCA.
It is important to highlight that significant improvement of the SCA took place early at 3-mo posttransplant reaching a plateau at 6 mo; yet, changes in the objective LFI were modest and only took place at 6 mo after LT. In our Spanish cohort, median pretransplant LFI scores were higher than in the FRAILT cohort (3.9 versus 3.7) which included 214 LT recipients from 1 US center with at least 1 LFI measurement at 3-, 6-, or 12-mo posttransplant.7 Furthermore, compared with the US cohort, improvement in posttransplant LFI scores was faster (at 6-mo versus at 12-mo post-LT) and was not preceded by worsening in the early posttransplant period.7 These differences could be explained by the fact that in our study, patients were younger (60 versus 62 y), had lower MELD-Na scores at transplant (12 versus 15), and greater proportion of HCC (59% versus 45%) suggesting less complex LT surgeries and thus, easier, and faster postsurgical recovery. On the other hand, both studies concluded that achievement of a robust physical function (defined by a LFI < 3.2) after LT is incomplete, at least in the short and intermediate terms. The proportion of patients who achieved robustness at the end of follow-up was 20% in our study (up to 6-mo post-LT) compared with 40% in the FrAILT cohort (up to 1-y post-LT).7 Pretransplant frailty category was also associated with posttransplant frailty category. In the US study, of the 40 LT recipients who were robust at 12-mo posttransplant, 2 of 3 were already robust and 1 of 3 were prefrail (defined by a LFI between 3.2 and 4.4) pretransplant7; in our Spanish study, of 31 LT recipients who were robust at 6-mo posttransplant, around 1 of 2 were already robust or prefrail before LT (Table 4). In univariable and multivariable analyses, pretransplant LFI was a strong predictor of posttransplant robust physical function; in both studies, each LFI increase of 0.1 pretransplant was associated with a decrease in the probability of being robust by around 25% at 3- and 6-mo posttransplant (P ≤ 0.001).7
Although the results we obtained with the SCA went in the same direction than those obtained with the LFI, concordance between these 2 frailty classifications was poor (Kappa < 0.20). Assuming the 3 categories of both classifications were equivalents (robust/good, prefrail/fair, and frail/poor), the percentage of agreements at pretransplant, 3- and 6-mo posttransplant was only of 31%, 23%, and 26%, respectively (Table 6). Before LT, almost 3 of 5 of prefrail and 1 of 5 of frail candidates were classified in the “good” SCA category. After LT, the rate of misclassification in the “good” SCA group of frail/prefrail recipients was higher than 70%–90%. Considering the objective LFI as the gold standard for frailty assessment, we concluded that the SCA overestimated the patients’ physical function both before and after LT. Moreover, we showed that pretransplant LFI was more accurate in predicting clinically important posttransplant outcomes than the SCA. Although the pretransplant “poor” SCA was only associated with a higher risk of postsurgical complications, each 0.1 increase in pretransplant LFI predicted longer ICU and transplant hospitalization stays and higher risk of early and late posttransplant complications in multivariable analysis. These results highlight the relevance of performing objective measurements of frailty at least before LT.
We acknowledge the following limitations: first, the following time was only 6 mo, which limits possible conclusions compared with other US studies with follow-up of at least 1-y posttransplant.5,7 Second, we cannot exclude some degree of selection bias as patients who died after LT were omitted from the analysis. One would assume these to be mostly frail patients. Yet, of the 12 LT recipients who did not reach either the 3- or 6-mo timepoints because of death, only 2 were frail before LT and the median (IQR) time between transplant date and death was only 13 d (2–59), suggesting that even if present, the impact of a potential selection bias was small. Third, for ease of comparison, we deliberately assumed that the 3 categories of the objective and subjective frailty classifications were equivalent (robust/good, prefrail/fair, and frail/poor). In 2018, Lai et al17 already compared the LFI and the SCA in the FrAILT cohort of 529 patients awaiting LT at a single US center; in that study, both frailty tools were analyzed as quantitative not qualitative variables. Although in our multicentre Spanish study, the health assessment question comprised 3 possible answers (good, fair, poor), the FrAILT study included 5 response options—excellent (0), very good (1), good (2), fair (3), poor (4), or very poor (5)—derived from the National Health Interview Study published in 1999.18 Although the 3 SCA categories selected in our study differ from existing literature, our data show that they can also identify LT candidates at high risk for waitlist mortality (Table S3, SDC, http://links.lww.com/TXD/A630) thereby supporting its use in this study. Fourth, statistically significant differences between hospitals were observed by frailty categories based on the LFI (P = 0.024) but not based on the SCA (P = 0.226). This discrepancy might suggest interrater variability when administering the objective tool. The LFI has shown excellent reliability when performed by trained personnel with an intraclass correlation coefficient of 0.93 (95% CI, 0.91-0.95)19; and in our study, even though specific training was not required, instructions to evenly carry out LFI measurements in every center were shared through an explanatory video. Differences by centers in patient`s characteristics associated with frailty may explain the gap between hospitals regarding the objective but not subjective (less reliable) classification: prevalence of HCC ranged from 74% in the Reina Sofía Hospital to 36% in the Lozano Blesa Hospital; MELD-Na score at frailty assessment oscillated from 10 in La Fe Hospital to 18 in the Clinic hospital. Fifth, due to the impact of coronavirus disease 2019 with increased use of telemedicine for postoperative recipient management at participating sites, not all LT recipients had a subjective and objective frailty assessment at all timepoints (during an outpatient clinic visit); yet >60% underwent both of them at 3- and 6-mo posttransplant (66% versus 78%, respectively) and no significant differences were found between the number of posttransplant assessments by pretransplant frailty category based on the LFI (P = 0.860 and P = 0.123 at 3- and 6-mo posttransplant) or the SCA (P = 0.900 and P = 0.415 at 3- and 6-mo posttransplant) reducing the possibility of bias. Sixth, the timing of the pretransplant frailty assessment varied from 1 patient to another due to the unpredictability of LT. However, median (IQR) time between frailty assessment and LT was only 41 d (14–99), and no significant differences were found in the median time between transplant date and frailty measurement by frailty category (P = 0.199 for the LFI; P = 0.422 for the SCA), suggesting that these outpatient evaluations truly reflected the patients’ physical function on the day of transplant. Lastly, of the 212 LT candidates initially enrolled in our multicenter study, we only analyzed data from the 180 who finally underwent an LT at the end of the inclusion period. Among the 32 patients (15%) excluded, 15 patients (7%) were actively waiting for LT, 5 patients (2%) had died or been delisted for being too sick and 12 patients (6%) had been delisted for other reasons (Figure 1). Baseline characteristics of excluded patients slightly differed from the entire cohort: the percentage of frail patients and LFI measurements before LT were higher (34% versus 19% and 4.1 versus 3.9, respectively), patients were older (61 versus 60 y), there was a greater proportion of women (31% versus 17%) and a lower proportion of HCC (47% versus 59%). The number of missing patients is small but given that the frailty status seems worse than that of the entire cohort, our conclusions regarding changes in frailty after LT might be underestimated and thus, clinical implications for our patient’s care would be even more relevant.
Despite these limitations, our large multicentre study is the first to investigate physical frailty after LT through outpatient assessments of the LFI and the SCA in the European LT setting and has important clinical implications. First, our results offer the chance to improve patient care by providing accurate information about posttransplant frailty recovery. Frail LT candidates should be aware that the probability of achieving a robust physical condition within 6 mo after LT is scarce, but they should expect some degree of improvement. Prefrail patients are the ones that might improve most, as close to 20% reach robustness 6-mo posttransplant. Robust LT candidates should be informed that although most of them maintain their optimal physical function posttransplant, around 1 of 4 experience a worsening in their physical condition within 6 mo after the surgery. Therefore, regardless of pretransplant frailty status, our findings should encourage practitioners to remain vigilant of the physical condition after LT, to assess this condition through objective tools, and to consider the need for nutritional interventions or exercise. Whether strategies aimed at pre- and/or rehabilitation might be of benefit needs to be confirmed in prospective studies.
ACKNOWLEDGMENTS
We thank Ana Ibañez Escribano, PhD, in Business Administration, and Juan Luis Gómez, bachelor’s degree in mathematics, both from the University of Valencia, for their statistical input.
Supplementary Material
Footnotes
L.P. was involved in the study concept and design, acquisition of data, analysis and interpretation of data, study coordination and supervision, drafting of the article, and critical revision of the article for important intellectual content. Final approval of the version to be published and agreement to be accountable for all aspects of the work. J.H. was involved in the interpretation of data and critical revision of the article for important intellectual content. Final approval of the version to be published and agreement to be accountable for all aspects of the work. E.R., G.C., M.R.-P., L.C., T.S., and A.F.-Y. were involved in the acquisition of data and critical revision of the article for important intellectual content. Final approval of the version to be published and agreement to be accountable for all aspects of the work. E.M. was involved in the interpretation of data and critical revision of the article for important intellectual content. Final approval of the version to be published and agreement to be accountable for all aspects of the work. M.B. was involved in the study concept and design; analyses and interpretation of data, study supervision, and drafting of the article; and critical revision of the article for important intellectual content. Final approval of the version to be published and agreement to be accountable for all aspects of the work.
This research was funded by the Instituto de Salud Carlos III and co-funded by European Regional Development Fund “A Way to Make Europe” (grants number CM17/00006—L.P., PI19/01360—M.B., and INT20/00061—M.B.), by the Generalitat Valenciana (grant AICO/2021/035—M.B.), and by the CIBER-Consorcio Centro de Investigación Biomédica en Red (CB06/04/0065), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea—European Regional Development Fund. No sponsor had a role in the study design, the data collection, the analysis and interpretation of data, the writing of the article, or the decision to submit the article for publication. The other authors declare no conflicts of interest.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
Contributor Information
Julia Herreras, Email: herreras.julia8@gmail.com.
Maria Àngels Cebrià i Iranzo, Email: Angeles.Cebria@uv.es.
Érick Reyes, Email: erick.reyesc@gmail.com.
Gonzalo Crespo, Email: GCRESPO@clinic.cat.
Manuel Rodríguez-Perálvarez, Email: ropeml@hotmail.com.
Luis Cortés, Email: lucorga@hotmail.com.
Trinidad Serrano, Email: tserrano.aullo@gmail.com.
Ainhoa Fernández-Yunquera, Email: afyunquera@gmail.com.
Eva Montalvá, Email: montalva.oron@gmail.com.
Marina Berenguer, Email: marina.berenguer@uv.es.
REFERENCES
- 1.Laube R, Wang H, Park L, et al. Frailty in advanced liver disease. Liver Int. 2018;38:2117–2128. [DOI] [PubMed] [Google Scholar]
- 2.Lai JC, Tandon P, Bernal W, et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74:1611–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lai JC, Sonnenday CJ, Tapper EB, et al. Frailty in liver transplantation: an expert opinion statement from the American Society of Transplantation Liver and Intestinal Community of Practice. Am J Transplant. 2019;19:1896–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai JC, Covinsky KE, Hayssen H, et al. Clinician assessments of health status predict mortality in patients with end-stage liver disease awaiting liver transplantation. Liver Int. 2015;35:2167–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thuluvath PJ, Thuluvath AJ, Savva Y. Karnofsky Performance Status before and after liver transplantation predicts graft and patient survival. J Hepatol. 2018;69:818–825. [DOI] [PubMed] [Google Scholar]
- 6.Lai JC, Dodge JL, Kappus MR, et al. HHS public access. J Hepatol. 2020;73:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai JC, Segev DL, McCulloch CE, et al. Physical frailty after liver transplantation. Am J Transplant. 2018;18:1986–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puchades L, Herreras J, Ibañez A, et al. Waiting time dictates impact of frailty: a Spanish Multicentre Prospective Study. JHEP Reports. 2023;5:100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam R, Karam V, Cailliez V, et al. ; all the other 126 contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). 2018 Annual Report of the European Liver Transplant Registry (ELTR)—50-year evolution of liver transplantation. Transpl Int. 2018;31:1293–1317. [DOI] [PubMed] [Google Scholar]
- 10.Kwong AJ, Kim WR, Lake JR, et al. OPTN/SRTR 2019 Annual Data Report: liver. Am J Transplant. 2021;21:208–315. [DOI] [PubMed] [Google Scholar]
- 11.Eurotransplant. Statistics Report Library. Eurotransplant – statistics. Available at https://statistics.eurotransplant.org/index.php?search_type=&search_organ=liver&search_region=&search_period=2019&search_characteristic=MELD+score&search_text=&search_collection. Accessed February 27, 2024.
- 12.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo Classification of Surgical Complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, et al. ; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495–1499. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien P. Classification of surgical complications. Ann Surg. 2004;240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derck JE, Thelen AE, Cron DC, et al. Quality of life in liver transplant candidates: frailty is a better indicator than severity of liver disease. Transplantation. 2015;99:340–344. [DOI] [PubMed] [Google Scholar]
- 16.Dunn MA, Rogal SS, Duarte-Rojo A, et al. Physical function, physical activity, and quality of life after liver transplantation. Liver Transpl. 2020;26:702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai JC, Covinsky KE, McCulloch CE, et al. The Liver Frailty Index improves mortality prediction of the Subjective Clinician Assessment in patients with cirrhosis. Am J Gastroenterol. 2018;113:235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGee DL, Liao Y, Cao G, et al. Self-reported health status and mortality in a multiethnic US cohort. Am J Epidemiol. 1999;149:41–46. [DOI] [PubMed] [Google Scholar]
- 19.Wang CW, Lebsack A, Chau S, et al. The range and reproducibility of the Liver Frailty Index. Liver Transpl 2019;25:841–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.