Abstract
INTRODUCTION:
Adenoma detection rate (ADR) is an accepted benchmark for screening colonoscopy. Factors driving ADR and its relationship with sessile serrated lesions detection rate (SSLDR) over time remain unclear. We aim to explore patient, physician, and procedural influences on ADR and SSLDR trends.
METHODS:
Using a large healthcare system in northern California from January 2010 to December 2020, a total of 146,818 screening colonoscopies performed by 33 endoscopists were included. ADR and SSLDR were calculated over time using natural language processing. Logistic regression was used to calculate the odd ratios of patient demographics, physician attributes, and procedural details over time.
RESULTS:
Between 2010 and 2020, ADR rose from 19.4% to 44.4%, whereas SSLDR increased from 1.6% to 11.6%. ADR increased by 2.7% per year (95% confidence interval 1.9%–3.4%), and SSLDR increased by 1.0% per year (95% confidence interval 0.8%–1.2%). Higher ADR was associated with older age, male sex, higher body mass index, current smoker, higher comorbidities, and high-risk colonoscopy. By contrast, SSLDR was associated with younger age, female sex, white race, and fewer comorbidities. Patient and procedure characteristics did not significantly change over time (P-interaction >0.05). Longer years in practice and male physician were associated with lower ADR and SSLDR in 2010, but significantly attenuated over time (P-interaction <0.05).
DISCUSSION:
Both ADR and SSLDR have increased over time. Patient and procedure factors did not significantly change over time. Male endoscopist and longer years in practice had lower initial ADR and SSLDR, but significantly lessened over time.
KEYWORDS: adenoma detection rate, sessile serrated lesions, colonoscopy
INTRODUCTION
Roughly 150,000 new cases of colorectal cancer are diagnosed each year in the United States primarily through colonoscopies, considered to be the gold standard for colon cancer screening. Several quality benchmarks for colonoscopy have been proposed and widely adopted in the healthcare system. In particular, adenoma detection rate (ADR) is an important quality measure for screening colonoscopy (1). Higher ADR is associated with reduction in interval colon cancer and cancer-related mortality (2,3). Recently, overall ADR is reported to increase over time (4,5); however, the factors contributing to this increase remain unclear. Some studies showed that ADR variation is affected by patient-level characteristics such as age and sex (6–8), whereas other studies have reported physician-level factors can also influence ADR, such as sex of physician and years of training (9,10). There are limited data on the relative effect of patient, physician, and procedure factors on changes in ADR over time.
Although multiple professional societies have recommended using ADR thresholds to assess the quality of colonoscopies, there are questions regarding use of ADR as the best quality metric for prevention of postcolonoscopy colorectal cancer (1,11). Sessile serrated lesions (SSLs) are recognized precursors to 30% of colon cancer (12), but a performance threshold has yet to be established. Although sessile serrated lesions detection rate (SSLDR) has been correlated with ADR, data regarding trends of SSLDR over time are limited, making an SSLDR target difficult to gauge (1,13). In this study, we aim to (i) examine the trends in ADR and SSLDR over time and (ii) explore associations of patient-level, physician-level, and procedure-level factors with ADR and SSLDR and whether the effects of these factors change over time.
METHODS
Study setting
We conducted a retrospective cohort study using data from a large integrated healthcare system in northern California, Sutter Health-Palo Alto Medical Foundation (PAMF). PAMF serves a diverse population of approximately 1 million active patients annually across 4 counties in the San Francisco Bay area. The healthcare system provides primary care, specialty care, ambulatory surgery, and laboratory, imaging, and inpatient physician services. Most patient care is recorded in an unified electronic health record system.
Study sample
Our starting sample included 192,673 total colonoscopies performed for 144,122 adult patients at the PAMF ambulatory surgical centers between January 2010 and December 2020. We excluded those younger than 18 years (n = 131), with a history of colon cancer (n = 1,212) or history of inflammatory bowel disease (n = 5,859), with diagnostic colonoscopies (n = 37,413), or with procedure performed at a non-PAMF facility (n = 8,131). This yielded a final sample of 146,818 screening colonoscopies performed by 33 endoscopists with 88,091 pathology reports (Figure 1).
Figure 1.
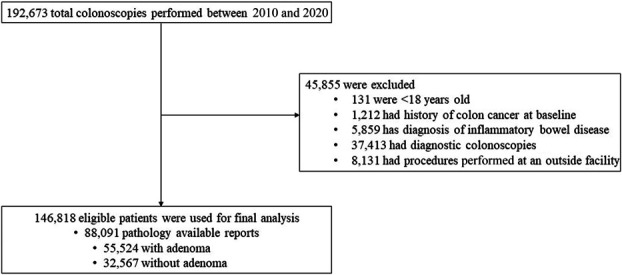
Colonoscopy study sample.
Polyp outcomes
We developed a natural language processing (NLP) algorithm to extract colon polyp data (presence of tubular, tubulovillous, villous, sessile serrated, and traditional adenoma) from available free-text pathology reports. The algorithm was validated against a random sample of 1,000 manual chart reviews with more than 98% agreement rate among 2 reviewers (authors E.S.H. and S.-Y.L.) and a Cohen's kappa statistics of 0.959. We subsequently applied this NLP algorithm to the final analytic sample (see Supplementary Appendix 1, Supplementary Digital Content 1, http://links.lww.com/CTG/B84).
For each colonoscopy, we used the NLP-derived adenoma information to classify whether at least 1 adenoma (either tubular, tubulovillous, or villous) was detected. Similarly, we used the NLP-derived information to classify whether sessile serrated lesions (either sessile serrated or traditional serrated adenoma) were detected as a secondary outcome variable. The interpretation of sessile serrated lesions was captured using histologic criteria at the time of collection (14). We defined ADR as the number of colonoscopies with adenomatous polyps over total number of screening colonoscopies and SSLDR as number of colonoscopies with sessile serrated lesion over total number of screening colonoscopies.
Patient, procedure, and physician characteristics
Patient and physician data were obtained from structured electronic health record fields. Patient characteristics were collected as follows: age, sex, race, body mass index (BMI), smoking history, Charlson comorbidity index (CCI, using International Classification of Disease, 9th revision and 10th revision), and colonoscopy indication (average risk and high risk). Average risk was defined as those without any personal history of colon polyp, family history of colon cancer, and family history of colon polyps, whereas high risk was defined as having 1 or more of these characteristics. Colonoscopy elements were collected including use of mucosal assist device, use of high-definition colonoscope, cecal withdrawal time, quality of preparation, and type of sedation. Physician-level data included sex and years in practice. At the time of data extraction, approximately 23% of procedures did not have information on preparation quality and sedation type, thus missingness was imputed by random sampling of values proportionally to retain the existing distribution.
Statistical analysis
We computed the yearly ADR and SSLDR between 2010 and 2020. Linear regressions were used to examine the trends over time. χ2 tests were used to assess bivariate associations of patient factors: age (<50, 50–75, or >75 years), sex (male or female), BMI (<25, 25–30, >30 kg/m2, or unknown), race (White, Asian American, African American, other, or unknown), smoking status (never, current, former, or unknown), CCI (0, 1, or ≥2), colonoscopy type (average risk vs high risk), procedure characteristics: use of mucosal assist device (yes or no), use of high-definition colonoscope (yes, no, or missing), cecal withdrawal time (<6, 6–9, >9 minutes, or unknown), quality of preparation (adequate or inadequate), type of sedation (moderate sedation or other), and physician factors: sex (male or female) and years in practice (<11, 11–25, or >25 years), with the study outcomes. Generalized linear models were used to estimate the odds ratios (ORs) and 95% confidence interval (CI), adjusting for covariates and account for clustering of patients cared for by the same endoscopist. To explore whether risk factors have different effects on the detection of adenoma and sessile serrated lesions over time, we conducted stratified analyses focusing on the first year (2010) and the last year (2020) of the study period. We estimated the generalized linear model using the pooled 2010 and 2020 data and adding the interaction terms between the year and risk factors. Testing the effects are the same between 2010 and 2020 was performed using Wald tests to assess whether coefficients of interaction terms being zero. We discuss results as statistically significant when P < 0.05. All P values were 2-sided. Analyses were conducted using R (version 4.2.2; The R Project) and Stata (version 16.1; StataCorp LLC, College Station, TX). The study was reviewed and approved by the Sutter Health Institutional Review Board.
RESULTS
Sample characteristics
Of 146,818 screening colonoscopies, the overall mean age of patients was 58.7 years (SD 9.1) and 50% were women. The mean BMI was 26.9 (SD 5.0). Approximately 55.5% were White, 26.6% Asian, 1.9% African American, 4.1% other, and 11.9% unknown. Nineteen-point-six percent were former smokers, whereas 3.5% were current smokers. The mean CCI was 1.33 (SD 1.79), and 55.8% of colonoscopies were average risk. Approximately 15.4% and 55.2% of colonoscopies used a mucosal assist device and high-definition colonoscopes, respectively. The median cecal withdrawal time was 13 minutes (interquartile range 10–17 minutes). Nearly all (98.9%) colonoscopies had adequate preparation quality, and 94.7% used moderate sedation. Seventy-five percent of procedures were performed by male physicians. The mean years of training was 15.4 (SD 7.7) (Table 1).
Table 1.
Baseline characteristics
| Characteristics | Overall (n = 146,818) |
| Patient-related | |
| Age, yr, mean ± SD | 58.7 ± 9.1 |
| Sex, n (%) | |
| Male | 73,645 (50.2) |
| Female | 73,173 (49.8) |
| BMI, kg/m2, mean ± SD | 26.9 ± 5.0 |
| Race, n (%) | |
| White | 81,473 (55.5) |
| Asian | 39,059 (26.6) |
| African American | 2,832 (1.9) |
| Other | 5,980 (4.1) |
| Unknown | 17,474 (11.9) |
| Smoking status, n (%) | |
| Never smoker | 101,419 (69.1) |
| Current smoker | 5,126 (3.5) |
| Former smoker | 28,785 (19.6) |
| Unknown | 11,488 (7.8) |
| Charlson comorbidity, mean ± SD | 1.3 ± 1.8 |
| Colonoscopy indication, n (%) | |
| Average risk | 81,893 (55.8) |
| High risk | 64,925 (44.2) |
| Procedure-related | |
| Mucosal assist device, n (%) | |
| No | 124,195 (84.6) |
| Yes | 22,623 (15.4) |
| High-definition colonoscope | |
| No | 30,289 (20.6) |
| Yes | 81,021 (55.2) |
| Unknown | 35,508 (24.2) |
| Withdrawal time, min, median (IQR) | 13 (10–17) |
| Preparation quality, n (%) | |
| Adequate (excellent, good, or fair) | 145,241 (98.9) |
| Inadequate (poor) | 1,577 (1.1) |
| Sedation type, n (%) | |
| Moderate sedation | 139,056 (94.7) |
| Othera | 7,762 (5.3) |
| Physician-related | |
| Procedure performed by male physician | |
| Male physician | 110,018 (74.9) |
| Female physician | 36,800 (25.1) |
| Years in practice, yr, mean ± SD | 15.4 ± 7.7 |
BMI, body mass index; IQR, interquartile range.
Other includes no sedation or general anesthesia.
Of the 88,091 pathology reports, 63.0% had adenoma, and 13.8% had sessile serrated lesion, whereas 6.2% of the pathology contained both adenoma and sessile serrated lesion (see Supplementary Table 1, Supplementary Digital Content 1, http://links.lww.com/CTG/B84).
ADR and SSLDR: trend over time
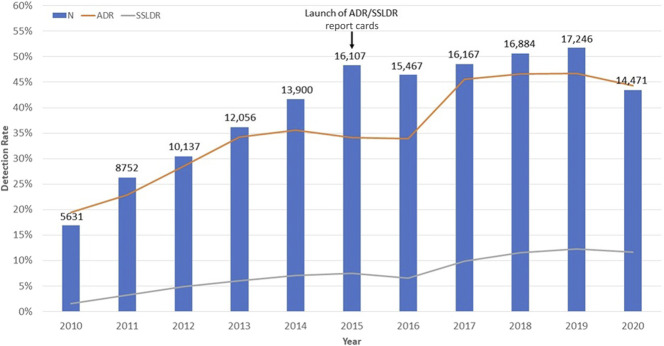
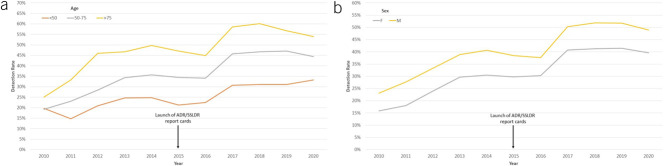
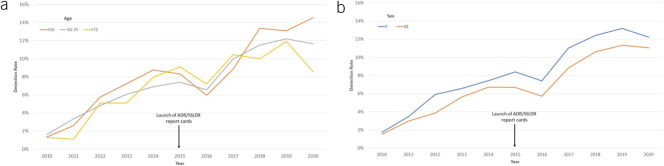
Figure 2 describes trends of ADR and SSLDR over time. The volume of colonoscopies increased between 2010 and 2019, but dropped in 2020 during the COVID-19 pandemic. The overall ADR ranged 19.4%–22.9% in 2010–2011, 28.5%–34.0% in 2012–2016, and 45.6%–44.4% in 2017–2020. ADR increased by a mean of 2.67% each year (95% CI 1.93%–3.42%) during this decade. In comparison, the overall SSLDR increased by a mean of 1.0% per year (95% CI 0.82%–1.23%) from 1.6% in 2010 to 11.6% in 2020 (Figure 2). The increase in SSLDR was dramatically higher than ADR (612% SSLDR vs 128% ADR) over the same decade. Similar trends of ADR and SSLDR were observed when colonoscopies were stratified by patient age and sex (Figures 3 and 4).
Figure 2.
Adenoma detection rate, sessile serrated lesions detection rate, volume of colonoscopy from 2010–2020. ADR, adenoma detection rate; SSLDR, sessile serrated lesions detection rate.
Figure 3.
Adenoma detection rate from 2010–2020 based on patient age (a) and sex (b). ADR, adenoma detection rate.
Figure 4.
Sessile serrated lesions detection rate from 2010–2020 based on patient age (a) and sex (b). SSLDR, sessile serrated lesions detection rate.
Patient characteristics
Overall, we found older patients were associated with a higher ADR (26.2% <50 vs 51.5% >75, P < 0.001) and male patients had a higher ADR than female patients (42.5% male vs 33.1% female, P < 0.001). Patients with a BMI over 30 had ADR of 40.7%, whereas those with a BMI under 25 had ADR of 30.5% (P < 0.001). Lower ADR was observed in Asian patients compared with other races (35.7% vs 38.9% White; 37.8% African American; 36.2% other; and 38.0% unknown, P < 0.001). Current smokers had higher ADR compared with never smokers (46.5% vs 36.9%, P < 0.001). Patients with ≥2 CCI were associated with higher ADR (40.9% vs 37% and 39.4% for those with 1 and 0 CCI, respectively, P < 0.001). High-risk colonoscopies were associated with higher ADR (41.9%) compared with 34.6% for average-risk colonoscopy (P < 0.001) (Table 2).
Table 2.
ADR and SSADR according to patient, procedure, and physician characteristics
| Characteristics | ADR | SSADR | ||
| n/N (%) | P value | n/N (%) | P value | |
| Patient-related | ||||
| Age | ||||
| <50 yr | 2,408/9,208 (26.2) | <0.001 | 847/9,208 (9.2) | 0.004 |
| 50–75 yr | 49,283/130,162 (37.9) | 10,697/130,162 (8.2) | ||
| >75 yr | 3,833/7,448 (51.5) | 633/7,448 (8.5) | ||
| Sex | ||||
| Male | 31,311/73,645 (42.5) | <0.001 | 5,583/73,645 (7.6) | <0.001 |
| Female | 24,213/73,173 (33.1) | 6,594/73,173 (9.0) | ||
| Body mass index | ||||
| <25 kg/m2 | 12,965/42,506 (30.5) | <0.001 | 3,168/42,506 (7.5) | <0.001 |
| 25–30 kg/m2 | 14,990/40,833 (36.7) | 3,076/40,833 (7.5) | ||
| >30 kg/m2 | 9,889/24,276 (40.7) | 1,828/24,276 (7.5) | ||
| Unknown | 17,680/39,153 (45.2) | 4,105/39,153 (10.5) | ||
| Race | ||||
| White | 31,694/81,473 (38.9) | <0.001 | 7,936/81,473 (9.7) | <0.001 |
| Asian | 13,944/39,059 (35.7) | 2,391/39,059 (6.1) | ||
| African American | 1,071/2,832 (37.8) | 135/2,832 (4.8) | ||
| Other | 2,168/5,980 (36.3) | 349/5,980 (5.8) | ||
| Unknown | 6,647/17,474 (38.0) | 1,366/17,474 (7.8) | ||
| Smoking status | ||||
| Never smoker | 37,419/101,419 (36.9) | <0.001 | 8,595/101,419 (8.5) | <0.001 |
| Current smoker | 2,381/5,126 (46.5) | 430/5,126 (8.4) | ||
| Former smoker | 12,122/28,785 (42.1) | 2,388/28,785 (8.3) | ||
| Unknown | 3,602/11,488 (31.4) | 764/11,488 (6.7) | ||
| Charlson comorbidity | ||||
| 0 | 24,014/67,046 (35.8) | <0.001 | 5,946/67,046 (8.9) | <0.001 |
| 1 | 10,647/28,801 (37.0) | 2,388/28,801 (8.3) | ||
| ≥2 | 20,863/50,971 (40.9) | 3,843/50,971 (7.5) | ||
| Colonoscopy indication | ||||
| Average risk | 28,342/81,893 (34.6) | <0.001 | 6,282/81,893 (7.7) | <0.001 |
| High riska | 27,182/64,925 (41.9) | 5,895/64,925 (9.1) | ||
| Procedure-related | ||||
| Mucosal assist device | ||||
| No | 44,932/124,195 (36.2) | <0.001 | 9,523/124,195 (7.7) | <0.001 |
| Yes | 10,592/22,623 (46.8) | 2,654/22,623 (11.7) | ||
| High-definition colonoscope | ||||
| No | 102,38/30,289 (33.8) | <0.001 | 1,384/30,289 (4.6) | <0.001 |
| Yes | 34,962/81,021 (43.2) | 7,972/81,021 (9.8) | ||
| Unknown | 10,324/35,508 (29.1) | 2,821/35,508 (7.9) | ||
| Withdrawal time | ||||
| <6 min | 122/903 (13.5) | <0.001 | 14/903 (1.6) | <0.001 |
| 6–9 min | 6,662/25,608 (26.0) | 802/25,608 (3.1) | ||
| >9 min | 38,519/84,644 (45.5) | 8,658/84,644 (10.2) | ||
| Missing | 10,221/35,663 (28.7) | 2,703/35,663 (7.7) | ||
| Preparation quality | ||||
| Adequate (excellent, good, or fair) | 55,063/145,241 (37.9) | <0.001 | 12,078/145,241 (8.3) | 0.004 |
| Inadequate (poor) | 461/1,577 (29.2) | 99/1,577 (6.3) | ||
| Sedation type | ||||
| Moderate sedation | 52,123/139,056 (37.5) | <0.001 | 11,426/139,056 (8.2) | <0.001 |
| Other | 3,401/7,762 (43.8) | 751/7,762 (9.7) | ||
| Physician-related | ||||
| Endoscopist sex | ||||
| Male physician | 41,072/110,018 (37.3) | <0.001 | 8,306/110,018 (7.5) | <0.001 |
| Female physician | 14,452/36,800 (39.3) | 3,871/36,800 (10.5) | ||
| Years in practice | ||||
| <11 yr | 18,701/44,765 (41.8) | <0.001 | 4,876/44,765 (10.9) | <0.001 |
| 11–25 yr | 29,574/77,299 (38.3) | 6,435/77,299 (8.3) | ||
| >25 yr | 7,249/24,754 (29.3) | 866/24,754 (3.5) | ||
ADR, adenoma detection rate; SSADR, sessile serrated adenoma detection rate.
High risk includes those with personal history of colon polyps, family history of colon cancer, and family history of colon polyps.
Younger patients were associated with a higher SSLDR (9.2% <50 vs 8.5% >75, P < 0.001). SSLDRs were higher among female patients compared with male patients (9.0% vs 7.6%, P < 0.001). Patients with unknown BMI had a higher SSLDR (10.5% vs 7.5% known BMI, P < 0.001), but SSLDRs were comparable among the known BMI groups (P = 0.91). Higher SSLDRs were seen in White patients compared with other races (9.7% vs 6.1% Asian; 4.8% African American; 5.8% other; and 7.8% unknown, P < 0.001) and 1 CCI (8.3% vs 7.6%, 7.5% for those with 0 and ≥2 CCI, respectively, P < 0.001). Lower SSLDR was observed among those with unknown smoking status (6.7% vs current smoker 8.4%; former smoker 8.3%; and never smoker 8.5%, P < 0.001). High-risk colonoscopies also had higher SSLDR compared with average-risk colonoscopies (9.1% vs 7.7%, P < 0.001) (Table 2).
Procedure characteristics
ADR and SSLDR varied by procedure characteristics. Longer cecal withdrawal time was positively associated with ADR and SSLDR (45.5% for >9 minutes vs 26.0% for 6–9 minutes, P < 0.001 for ADR; 10.2% for >9 minutes vs 3.1% for 6–9 minutes, P < 0.001 for SSLDR). The use of mucosal assist device and high-definition colonoscope had higher rates of ADR (46.8% vs 36.2%, P < 0.001; 43.2% vs 33.8%, P < 0.001, respectively) and SSLDR (11.7% vs 7.7%, P < 0.001; 9.8% vs 4.6%, P < 0.001, respectively). Similarly, both ADR and SSLDR were higher in those with adequate preparation vs inadequate preparation (37.9% vs 29.2%, P < 0.001 for ADR; 8.3% vs 6.3%, P < 0.001 for SSLDR). In addition, moderate sedation was associated with lower ADR and SSLDR compared with other types of sedation (37.5% vs 43.8%, P < 0.001 for ADR; 8.2% vs 9.7%, P < 0.001 SSLDR) (Table 2).
Physician characteristics
We found ADR and SSLDR did differ based on physician sex and the years in practice. Female physicians have higher rates of ADR and SSLDR compared with their male counterparts (39.3% vs 37.3%, P < 0.001 for ADR; 10.5% vs 7.5%, P < 0.001 for SSLDR). Furthermore, physicians who have been in practice for a shorter time tend to have higher ADR and SSLDR compared with those who have been in practice for longer (41.8% for <11 years in practice vs 29.3% >25 years in practice, P < 0.001 for ADR; 10.9% for <11 years in practice vs 3.5% >25 years in practice, P < 0.001 for SSLDR) (Table 2).
Multivariate analysis
Multivariate regressions had similar findings (Tables 3 and 4). ADRs were higher among older (>75: OR 2.67, 95% CI 2.44–2.92 compared with <50 years old), higher BMI (>30: OR 1.40, 95% CI 1.33–1.47 compared with BMI <25), smokers (current: OR 1.39, 95% CI 1.29–1.50; former: OR 1.09, 95% CI 1.06–1.13, compared with never smoker), ≥2 CCI (OR 1.14, 95% CI 1.10–1.17 compared with CCI of 0), were high-risk colonoscopy (OR 1.07, 95% CI 1.01–1.13), use of mucosal device (OR 1.18, 95% CI 1.01–1.39), or longer cecal withdrawal time (>9 minutes: OR 4.23, 95% CI 2.84–6.30; compared with <6 minutes). ADRs were lower among females (OR 0.68, 95% CI 0.66–0.70) or inadequate preparation (OR 0.72, 95% CI 0.63–0.83) (Table 3).
Table 3.
ORs of ADR according to patient, procedure, and physician characteristics by all years, 2010 and 2020
| Characteristics | All yearsa | 2010b | 2020c | P-interactiond |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Patient-related | ||||
| Age | ||||
| <50 yr | Reference | Reference | Reference | 0.28 |
| 50–75 yr | 1.75 (1.62–1.88) | 1.17 (0.73–1.88) | 1.63 (1.40–1.90) | |
| >75 yr | 2.67 (2.44–2.92) | 2.11 (1.16–3.82) | 2.25 (1.77–2.86) | |
| Sex | ||||
| Male | Reference | Reference | Reference | 0.44 |
| Female | 0.68 (0.66–0.70) | 0.62 (0.49–0.78) | 0.69 (0.63–0.75) | |
| BMI | ||||
| <25 kg/m2 | Reference | Reference | Reference | 0.33 |
| 25–30 kg/m2 | 1.18 (1.15–1.22) | 1.03 (0.86–1.22) | 1.14 (1.03–1.26) | |
| >30 kg/m2 | 1.40 (1.33–1.47) | 1.16 (0.83–1.63) | 1.48 (1.31–1.66) | |
| Unknown | 1.67 (1.57–1.78) | 2.14 (1.67–2.75) | 1.10 (0.99–1.23) | |
| Race | ||||
| White | Reference | Reference | Reference | 0.08 |
| Asian | 0.98 (0.90–1.05) | 0.83 (0.62–1.09) | 0.96 (0.86–1.08) | |
| African American | 0.92 (0.85–1.00) | 1.25 (0.78–2.01) | 0.91 (0.74–1.12) | |
| Other | 0.95 (0.89–1.02) | 0.82 (0.55–1.22) | 1.13 (0.91–1.39) | |
| Unknown | 0.98 (0.92–1.04) | 0.95 (0.74–1.22) | 0.91 (0.82–1.01) | |
| Smoking status | ||||
| Never smoker | Reference | Reference | Reference | 0.08 |
| Current smoker | 1.39 (1.29–1.50) | 1.32 (0.75–2.29) | 1.33 (1.07–1.65) | |
| Former smoker | 1.09 (1.06–1.13) | 1.27 (0.99–1.63) | 0.98 (0.89–1.08) | |
| Unknown | 0.94 (0.88–0.99) | 0.67 (0.49–0.92) | 0.99 (0.86–1.14) | |
| Charlson comorbidity | ||||
| 0 | Reference | Reference | Reference | 0.08 |
| 1 | 1.02 (0.99–1.06) | 1.24 (1.07–1.43) | 1.03 (0.95–1.11) | |
| ≥2 | 1.14 (1.10–1.17) | 1.29 (1.04–1.59) | 1.15 (1.05–1.26) | |
| Colonoscopy indication | ||||
| Average risk | Reference | Reference | Reference | 0.73 |
| High risk | 1.07 (1.01–1.13) | 1.33 (0.91–1.95) | 1.24 (1.11–1.39) | |
| Procedure-related | ||||
| Mucosal assist device | ||||
| No | Reference | Reference | Reference | NAe |
| Yes | 1.18 (1.01–1.39) | NAe | 0.92 (0.76–1.11) | |
| High-definition colonoscope | ||||
| No | Reference | Reference | Reference | |
| Yes | 1.08 (0.97–1.19) | 0.76 (0.52–1.11) | 0.97 (0.82–1.15) | 0.26 |
| Unknown | 1.04 (0.77–1.40) | 0.86 (0.24–3.07) | 1.08 (0.84–1.38) | |
| Withdrawal time | ||||
| <6 min | Reference | Reference | Reference | 0.10 |
| 6–9 min | 1.85 (1.29–2.65) | 2.54 (1.31–4.92) | 1.27 (0.75–2.16) | |
| >9 min | 4.23 (2.84–6.30) | 6.42 (2.73–15.12) | 2.46 (1.59–3.82) | |
| Missing | 2.66 (1.83–3.86) | 4.55 (2.17–9.54) | 2.20 (1.30–3.72) | |
| Preparation quality | ||||
| Adequate (excellent, good, or fair) | Reference | Reference | Reference | 0.01 |
| Inadequate (poor) | 0.72 (0.63–0.83) | 1.30 (0.71–2.40) | 0.50 (0.30–0.86) | |
| Sedation type | ||||
| Moderate sedation | Reference | Reference | Reference | 0.14 |
| Other | 1.05 (0.98–1.13) | 0.84 (0.67–1.05) | 1.02 (0.86–1.20) | |
| Physician-related | ||||
| Endoscopist sex | ||||
| Male physician | Reference | Reference | Reference | 0.03 |
| Female physician | 1.08 (0.87–1.35) | 2.80 (1.13–6.91) | 0.93 (0.67–1.29) | |
| Years in practice | ||||
| <11 yr | Reference | Reference | Reference | <0.001 |
| 11–25 yr | 0.97 (0.77–1.23) | 7.82 (2.13–28.78) | 0.86 (0.65–1.13) | |
| >25 yr | 0.80 (0.46–1.37) | 4.61 (0.88–24.15) | 0.72 (0.56–0.93) |
BMI, body mass index; CI, confidence interval; OR, odds ratio; ADR, adenoma detection rate.
Multivariate model included variables for all patient-related, procedure-related, physician-related characteristics, and fixed effects of each year from 2010 to 2020.
Multivariate model in variables for all patient-related, procedure-related, and physician-related characteristics, except for mucosal assist device.
Multivariate model in variables for all patient-related, procedure-related, and physician-related characteristics.
P-interaction was calculated using cross product of year and with respective risk factor. Missing and unknown categories were omitted in the model to improve interpretability.
No mucosal assist device was used in 2010, so interaction was not calculated.
Table 4.
ORs of SSADR according to patient, procedure, and physician characteristics by all years, 2010 and 2020
| Characteristics | All yearsa | 2010b | 2020c | P-interactiond |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Patient-related | ||||
| Age | ||||
| <50 yr | Reference | Reference | Reference | 0.30 |
| 50–75 yr | 0.82 (0.77–0.88) | 1.69 (0.58–4.93) | 0.75 (0.64–0.86) | |
| >75 yr | 0.80 (0.73–0.88) | 1.84 (0.22–15.40) | 0.54 (0.44–0.66) | |
| Sex | ||||
| Male | Reference | Reference | Reference | 0.41 |
| Female | 1.21 (1.17–1.26) | 0.99 (0.68–1.44) | 1.16 (1.04–1.29) | |
| BMI | ||||
| <25 kg/m2 | Reference | Reference | Reference | 0.98 |
| 25–30 kg/m2 | 1.04 (0.98–1.10) | 0.99 (0.42–2.35) | 0.92 (0.80–1.05) | |
| >30 kg/m2 | 0.99 (0.91–1.08) | 0.97 (0.43–2.19) | 0.93 (0.77–1.11) | |
| Unknown | 1.49 (1.33–1.66) | 2.41 (1.39–4.17) | 0.93 (0.78–1.11) | |
| Race | ||||
| White | Reference | Reference | Reference | 0.88 |
| Asian | 0.59 (0.54–0.65) | 0.63 (0.39–1.00) | 0.57 (0.48–0.68) | |
| African American | 0.43 (0.36–0.51) | 0.41 (0.06–3.06) | 0.38 (0.22–0.65) | |
| Other | 0.57 (0.49–0.66) | 0.42 (0.09–1.90) | 0.62 (0.52–0.75) | |
| Unknown | 0.74 (0.67–0.81) | 0.47 (0.15–1.46) | 0.74 (0.59–0.92) | |
| Smoking status | ||||
| Never smoker | Reference | Reference | Reference | 0.40 |
| Current smoker | 1.06 (0.97–1.15) | 0.58 (0.11–3.10) | 1.19 (0.92–1.54) | |
| Former smoker | 0.95 (0.89–1.00) | 1.02 (0.52–2.01) | 0.92 (0.80–1.05) | |
| Unknown | 0.94 (0.87–1.01) | 0.40 (0.27–0.58) | 1.10 (0.95–1.28) | |
| Charlson comorbidity | ||||
| 0 | Reference | Reference | Reference | 0.13 |
| 1 | 0.92 (0.88–0.95) | 1.34 (0.74–2.40) | 0.76 (0.67–0.87) | |
| ≥2 | 0.85 (0.82–0.88) | 1.26 (0.70–2.26) | 0.80 (0.70–0.91) | |
| Colonoscopy indication | ||||
| Average risk | Reference | Reference | Reference | 0.18 |
| High risk | 0.90 (0.85–0.96) | 1.63 (0.75–3.51) | 0.96 (0.88–1.05) | |
| Procedure-related | ||||
| Mucosal assist device | ||||
| No | Reference | Reference | Reference | NAe |
| Yes | 1.11 (0.95–1.29) | NAe | 1.07 (0.88–1.29) | |
| High-definition colonoscope | ||||
| No | Reference | Reference | Reference | NAf |
| Yes | 1.33 (1.17–1.50) | NAf | 1.13 (0.79–1.61) | |
| Unknown | 2.01 (1.68–2.41) | 0.87 (0.23–3.21) | 1.00 (0.58–1.73) | |
| Withdrawal time | ||||
| <6 min | Reference | Reference | Reference | 0.55 |
| 6–9 min | 1.38 (0.83–2.32) | 0.31 (0.05–1.83) | 1.57 (0.54–4.53) | |
| >9 min | 4.56 (2.57–8.12) | 1.11 (0.48–2.54) | 4.43 (1.26–15.59) | |
| Missing | 3.80 (2.11–6.85) | NA | 2.45 (0.66–9.13) | |
| Preparation quality | ||||
| Adequate (excellent, good, or fair) | Reference | Reference | Reference | 0.03 |
| Inadequate (poor) | 0.79 (0.53–1.18) | 3.16 (0.92–10.80) | 0.40 (0.14–1.19) | |
| Sedation type | ||||
| Moderate sedation | Reference | Reference | Reference | 0.75 |
| Other | 0.90 (0.82–0.99) | 0.83 (0.37–1.84) | 0.95 (0.81–1.12) | |
| Physician-related | ||||
| Endoscopist sex | ||||
| Male physician | Reference | Reference | Reference | 0.01 |
| Female physician | 1.11 (0.89–1.39) | 2.54 (1.21–5.33) | 0.86 (0.64–1.16) | |
| Years in practice | ||||
| <11 yr | Reference | Reference | Reference | 0.002 |
| 11–25 yr | 0.89 (0.71–1.13) | 4.36 (1.37–13.89) | 0.99 (0.77–1.27) | |
| >25 yr | 0.57 (0.30–1.08) | 1.34 (0.31–5.86) | 0.99 (0.82–1.20) |
BMI, body mass index; CI, confidence interval; OR, odds ratio; SSADR, sessile serrated adenoma detection rate.
Multivariate model included variables for all patient-related, procedure-related, and physician-related characteristics and fixed effects of each year from 2010 to 2020.
Multivariate model in variables for all patient-related, procedure-related, and physician-related characteristics, except for mucosal assist device and high-definition colonoscope.
Multivariate model in variables for all patient-related, procedure-related, and physician-related characteristics.
P-interaction was calculated using cross product of year and with respective risk factor. Missing and unknown categories were omitted in the model to improve interpretability.
No mucosal assist device was used in 2010, so interaction was not calculated.
Interaction term was not calculated because OR did not converge for high-definition colonoscope in 2010.
For the multivariate regressions, SSLDR was higher among female (OR 1.21, 95% CI 1.17–1.26), use of high-definition colonoscope (OR 1.33, 95% CI 1.17–1.50), or longer cecal withdrawal time (>9 minutes: OR 4.56, 95% CI 2.57–8.12, compared with <6 minutes). SSLDR was lower among older patients (>75: OR 0.80, 95% CI 0.73–0.88, compared with <50 years old), non-White (Asian: OR 0.59, 95% CI 0.54–0.65; African American: OR 0.43, 95% CI 0.36–0.51; and other race: OR 0.57, 95% CI 0.49–0.66), higher CCI (≥2 CCI: OR 0.85, 95% CI 0.82–0.88, compared with CCI of 0), or other sedation (other: OR 0.90, 95% CI 0.82–0.99) (Table 4).
Changes in predictors of ADR and SSLDR between 2010 and 2020
Most predictors had similar effects on ADR and SSLDR between 2010 and 2020 (Tables 3 and 4). For ADR, age (50–75 years), BMI (20–30 and >30), current smokers, and high-risk colonoscopy were insignificant predictors in 2010, but significant in 2020. CCI of 1 and withdrawal time (6–9 minutes) were significant predictors in 2010 that became nonsignificant in 2020. However, when we performed the joint tests (e.g., all age groups together vs 1 specific age group), all these changes over time were nonsignificant (P of testing the interactions between year and risk factors [P-interaction] >0.05). For SSLDR, age (50–75 and >75 years), female sex, race (Asian, African American, and other), CCI (1 and ≥2), and withdrawal time (>9 minutes) were nonsignificant predictors in 2010, but significant in 2020. These changes, when testing all categories within the same risk factors as a whole, were also all nonsignificant (P-interaction >0.05)
Significant changes over time were endoscopist sex and years in practice for both ADR and SSLDR. Male endoscopists and endoscopists with >25 years in practice had significantly lower ADR and SSLDR in 2010, but became insignificant in 2020 (endoscopist sex: P-interaction = 0.03, years in practice: P-interaction <0.001 for ADR; endoscopist sex: P-interaction = 0.01, years in practice: P-interaction = 0.002 for SSLDR).
DISCUSSION
In this study, we aimed to examine the trends in ADR and SSLDR over time. We further examined the associations of patient, physician, and procedure factors that may affect ADR and SSLDR. Our findings shed light on the changes in ADR and SSLDR over the past decade and the factors that might influence these trends.
The overall ADR of our study population increased from 19.4% in 2010 to 44.4% in 2020. Previous studies have similarly documented a steady increase in ADR (4,5). The observed ADR in our study closely matches these previous studies and demonstrates a consistent, increasing trend. Notably, we observe more than sevenfold increase in the overall SSLDR over a decade in our study population from 1.6% in 2010 to 11.6% in 2020, similar to another study over the same timeframe (13). We believe this may be related to increasing recognition of malignancy potential of sessile serrated lesions and improvements in technology such as use of high-definition colonoscopes (15) and use of mucosal assist device (16). Moreover, our medical group has instituted adenoma detection reporting for individual physicians in 2015, which also has been shown to be an effective method of increasing detection rates (17).
Our study found several patient factors that affect ADR and SSLDR. ADR was positively associated with older age, male patients, smokers, higher BMI, and high-risk colonoscopy, which is consistent with other studies (4,6,18,19). By contrast, we found SSLDR was higher among younger age and female patients, similar to previous studies (20,21). Those with fewer medical problems and average-risk colonoscopy also had higher SSLDR. However, after stratifying these patient factors between 2010 and 2020, we did not see a significant change over time. This suggests the increase in ADR and SSLDR may not be due to patient factors.
Similarly, we also observe that ADR and SSLDR vary by procedure characteristics. We found both ADR and SSLDR are positively associated with longer withdrawal time, similar to a recent meta-analysis suggesting an increased ADR associated with >9-minute withdrawal time (22). Moreover, use of high-definition colonoscope was associated with higher SSLDR, which also found in other studies (23). In addition, higher ADR was associated with use of mucosal device and adequate bowel preparation. These associations align with existing literature (24–27). Yet, when we evaluated these differences between 2010 and 2020, there was also no noticeable change over time with exception of preparation quality. Because preparation quality comprised only 1.1% of all colonoscopies, its effect on the increase in ADR and SSLDR is likely limited.
Physician characteristics, such as years in practice and physician sex, influenced ADR and SSLDR early in the decade. Male endoscopists had a lower ADR compared with female endoscopists. Existing literature also suggested that female physicians have higher ADR (9,28). Although the precise mechanism for this remains unclear, female physicians have been shown to be more compliant with guidelines, which can affect quality of colonoscopy and polyp detection (29,30). Physicians with fewer years of experience had higher ADR and SSLDR compared with their more seasoned counterparts. This observation might be attributed partly to differences in training and education over time. However, in both cases, the effects of these differences significantly attenuated over the decade. So, it is plausible that the increase ADR and SSLDR over time could be explained by improvements among older, male endoscopists in their ADR and SSLDR.
The strengths of our study include a large community-based population, diverse patient population, and wide assessment of patient, physician, and procedural factors. However, our study has some limitations. As a retrospective analysis, the data are subject to inherent biases and confounding variables. The use of a single healthcare system in northern California might limit the generalizability of our findings to other populations, but our population does have a very diverse patient population representative of the underlying catchment area. In addition, despite the use of NLP algorithms for pathology data extraction, there is still a possibility of misclassification. In our validation study, we did find high accuracy with use of our NLP. Nevertheless, the application of NLP may require further refinement because of variation of pathology formats and terminology. Further studies should evaluate the use of more sophisticated NLP algorithms such as large language models to broaden uses among various healthcare system. Although we used the best available data to define ADR/SSLDR and control for procedure characteristics, our study does not have information on proximal hyperplastic polyps and sessile serrated lesion with dysplasia and whether virtual chromoendoscopy was used. In addition, histologic criteria has changed over time for sessile serrated lesions, which has led to increased recognition of this new entity in both pathologists and endoscopists. Therefore, increased SSLDR over time may be partly contributed by pathologists (31). We were not able to account for variable experience of individual pathologists in interpreting sessile serrated lesions, which may introduce potential bias in our SSLDR calculation. However, we did not find any major variabilities in pathology reading between various sites, which suggest that this potential bias is likely small.
In conclusion, our study provides valuable insights into the trends of ADR and SSLDR over time and their associations with patient, physician, and procedure factors. We found both ADR and SSLDR increased over time. However, factors leading the increase over time may not be related to any patient- or procedure-related factors; however, physician factors such as sex and years in practice have changed over time and may play a potential role in the increase in ADR and SSLDR. Further research can help validate these findings and inform best practices for colonoscopy quality measure and surveillance strategies.
CONFLICTS OF INTEREST
Guarantor of the article: Su-Ying Liang, PhD, and Edward S. Huang, MD, MPH.
Specific author contributions: All authors made substantial contributions to the intellectual content of the paper and approved the final version of the manuscript. S.-Y.L. and E.S.H.: conception and design. S.-Y.L., E.S.H., and P.K.: acquisition of data. S.-Y.L., B.O., P.K., S.X.Y., S.M., M.C.M., and E.S.H.: analysis and interpretation of data. S.-Y.L. and E.S.H.: drafting of the manuscript. S.-Y.L., B.O., P.K., S.X.Y., S.M., M.C.M., and E.S.H.: critical revision of manuscript. S.-Y.L., B.O., S.X.Y., S.M., and E.S.H.: statistical analysis.
Financial support: Palo Alto Medical Foundation Innovation Grant.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Adenoma detection rate (ADR) is a quality metric for screening colonoscopy
✓ ADR relationship with sessile serrated lesion detection rate (SSLDR) is uncertain.
✓ The effect of patient-related, procedure-related, and physician-related factors on ADR and SSLDR over time remains unknown.
WHAT IS NEW HERE
✓ ADR and SSLDR have both increased over time.
✓ Patient- and procedure-related factors have not changed significantly over time.
✓ Male endoscopist and longer years in practice had lower initial rates of ADR and SSLDR, but these differences significantly reduced over time.
Supplementary Material
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/B84.
Contributor Information
Su-Ying Liang, Email: Su-Ying.Liang@sutterhealth.org.
Brandon Oscarson, Email: Brandon.Oscarson@sutterhealth.org.
Pragati Kenkare, Email: Pragati.Kenkare@sutterhealth.org.
Sherry X. Yan, Email: Sherry.Yan@sutterhealth.org.
Satish Mudiganti, Email: Satish.Mudiganti@sutterhealth.org.
Meghan C. Martinez, Email: Meghan.Martinez@sutterhealth.org.
REFERENCES
- 1.Shaukat A, Kahi CJ, Burke CA, et al. ACG clinical guidelines: Colorectal cancer screening 2021. Am J Gastroenterol 2021;116(3):458–79. [DOI] [PubMed] [Google Scholar]
- 2.Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014;370(26):2541. [DOI] [PubMed] [Google Scholar]
- 3.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010;362(19):1795–803. [DOI] [PubMed] [Google Scholar]
- 4.Brenner H, Altenhofen L, Kretschmann J, et al. Trends in adenoma detection rates during the first 10 years of the German Screening Colonoscopy Program. Gastroenterology 2015;149(2):356–66.e1. [DOI] [PubMed] [Google Scholar]
- 5.Pike IM, Eisen G, Greenwald DA, et al. 153 increasing adenoma detection rate over time in a national benchmarking registry. Gastrointest Endosc 2017;85(5 Suppl):AB56–7. [Google Scholar]
- 6.Diamond SJ, Enestvedt BK, Jiang Z, et al. Adenoma detection rate increases with each decade of life after 50 years of age. Gastrointest Endosc 2011;74(1):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCashland TM, Brand R, Lyden E, et al. Gender differences in colorectal polyps and tumors. Am J Gastroenterol 2001;96(3):882–6. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen SP, Bent S, Chen YH, et al. Gender as a risk factor for advanced neoplasia and colorectal cancer: A systematic review and meta-analysis. Clin Gastroenterol Hepatol 2009;7(6):676–81.e1–3. [DOI] [PubMed] [Google Scholar]
- 9.Mehrotra A, Morris M, Gourevitch RA, et al. Physician characteristics associated with higher adenoma detection rate. Gastrointest Endosc 2018;87(3):778–86.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarvepalli S, Garber A, Rothberg MB, et al. Association of adenoma and proximal sessile serrated polyp detection rates with endoscopist characteristics. JAMA Surg 2019;154(7):627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komanduri S, Dominitz JA, Rabeneck L, et al. AGA white paper: Challenges and gaps in innovation for the performance of colonoscopy for screening and surveillance of colorectal cancer. Clin Gastroenterol Hepatol 2022;20(10):2198–209.e3. [DOI] [PubMed] [Google Scholar]
- 12.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am J Gastroenterol 2012;107(9):1315–29; quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwardson N, Adsul P, Gonzalez Z, et al. Sessile serrated lesion detection rates continue to increase: 2008-2020. Endosc Int Open 2023;11(1):E107–E116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020;76(2):182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sehgal A, Aggarwal S, Mandaliya R, et al. Improving sessile serrated adenoma detection rates with high definition colonoscopy: A retrospective study. World J Gastrointest Endosc 2022;14(4):226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek MD, Jackson CS, Lunn J, et al. Endocuff assisted colonoscopy significantly increases sessile serrated adenoma detection in veterans. J Gastrointest Oncol 2017;8(4):636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser AG, Rose T, Wong P, et al. Improved detection of adenomas and sessile serrated polyps is maintained with continuous audit of colonoscopy. BMJ Open Gastroenterol 2020;7(1):e000425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson JC, Hisey W, Mackenzie TA, et al. Clinically significant serrated polyp detection rates and risk for postcolonoscopy colorectal cancer: Data from the New Hampshire Colonoscopy Registry. Gastrointest Endosc 2022;96(2):310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JC, Weiss JE, Robinson CM, et al. Adenoma detection rates for screening colonoscopies in smokers and obese adults: Data from the New Hampshire Colonoscopy Registry. J Clin Gastroenterol 2017;51(10):e95–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: Prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol 2010;63(8):681–6. [DOI] [PubMed] [Google Scholar]
- 21.Kim HY, Kim SM, Seo JH, et al. Age-specific prevalence of serrated lesions and their subtypes by screening colonoscopy: A retrospective study. BMC Gastroenterol 2014;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aziz M, Haghbin H, Gangwani MK, et al. 9-Minute withdrawal time improves adenoma detection rate compared with 6-minute withdrawal time during colonoscopy: A meta-analysis of randomized controlled trials. J Clin Gastroenterol 2023;57(9):863–70. [DOI] [PubMed] [Google Scholar]
- 23.Tziatzios G, Gkolfakis P, Lazaridis LD, et al. High-definition colonoscopy for improving adenoma detection: A systematic review and meta-analysis of randomized controlled studies. Gastrointest Endosc 2020;91(5):1027–36.e9. [DOI] [PubMed] [Google Scholar]
- 24.Williet N, Tournier Q, Vernet C, et al. Effect of Endocuff-assisted colonoscopy on adenoma detection rate: meta-analysis of randomized controlled trials. Endoscopy 2018;50(9):846–60. [DOI] [PubMed] [Google Scholar]
- 25.Shao PP, Shao CR, Romero T, et al. Sessile serrated adenoma/polyp detection rate of water exchange, Endocuff, and cap colonoscopy: A network meta-analysis. J Gastroenterol Hepatol 2021;36(12):3268–77. [DOI] [PubMed] [Google Scholar]
- 26.Aziz M, Desai M, Hassan S, et al. Improving serrated adenoma detection rate in the colon by electronic chromoendoscopy and distal attachment: Systematic review and meta-analysis. Gastrointest Endosc 2019;90(5):721–31.e1. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Yang X, Wang S, et al. Impact of 9-minute withdrawal time on the adenoma detection rate: A multicenter randomized controlled trial. Clin Gastroenterol Hepatol 2022;20(2):e168–e181. [DOI] [PubMed] [Google Scholar]
- 28.Araujo JL, Jaiswal P, Ragunathan K, et al. Impact of fellow participation during colonoscopy on adenoma detection rates. Dig Dis Sci 2022;67(1):85–92. [DOI] [PubMed] [Google Scholar]
- 29.Kim C, McEwen LN, Gerzoff RB, et al. Is physician gender associated with the quality of diabetes care?. Diabetes Care 2005;28(7):1594–8. [DOI] [PubMed] [Google Scholar]
- 30.Berthold HK, Gouni-Berthold I, Bestehorn KP, et al. Physician gender is associated with the quality of type 2 diabetes care. J Intern Med 2008;264(4):340–50. [DOI] [PubMed] [Google Scholar]
- 31.Carragher R, Ings GR, Baker G, et al. Trends in pathology diagnoses during 10 years of a colorectal cancer screening programme. Histopathology 2023;83(5):756–70. [DOI] [PubMed] [Google Scholar]