Abstract
INTRODUCTION:
Isolated case reports and case series have linked the use of sevelamer to severe gastrointestinal (GI) inflammation and perforation among patients with end-stage renal disease.
METHODS:
In this study, we identified 12 cases of biopsy-proven sevelamer-induced gastrointestinal disease from a large urban community hospital over the course of 5 years. We described baseline characteristics, sites and types of injury, histological findings, timing and dosing of sevelamer initiation compared with symptom onset, and in a smaller subset, endoscopic resolution post drug cessation. We also reviewed preexisting conditions to identify trends in populations at risk.
RESULTS:
Several of the patients reviewed had preexisting conditions of decreased motility and/or impaired mucosal integrity. The presentation of disease was broad and included both upper-GI and lower-GI pathologies and in varying severity.
DISCUSSION:
There is a broad phenotypic range of sevelamer-induced gastrointestinal disease. As this becomes a more frequently recognized pathology, clinicians should be aware of how it may present and which populations may be more susceptible.
KEYWORDS: sevelamer, gastrointestinal bleed, gastritis, colitis, bowel perforation
INTRODUCTION
Drug-induced gastrointestinal (GI) disease is a frequent cause of patient hospitalization and mortality, and the list of culprit medications is lengthy and growing (1). Some of the classic gastrointestinal-toxic medications include nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, immunosuppressives, chemotherapy agents, and bisphosphonates, but as newer drug classes become widely prescribed, their adverse effects are revealed. One such medication is sevelamer, a crystalline phosphate binder used among patients with end-stage renal disease (ESRD) with hyperphosphatemia. Whereas it has shown benefit in decreasing serum phosphorus versus placebo, meta-analyses have also shown increased GI adverse effects such as constipation (risk ratio 6.92, 95% confidence interval 2.24–21.38) (2). Isolated case reports and case series have also linked the use of sevelamer to severe intestinal inflammation and perforation (3–14). Sevelamer-induced gastrointestinal disease (SIGD) is becoming a more recognized cause of morbidity in patients with end-stage renal disease.
Attributing the cause of severe GI events to sevelamer is challenging because there needs to be an established temporal relationship between the initiation of medication and onset of symptoms and healing after discontinuation. Of the several existing case reports, few have reported evidence of postdiscontinuation healing as observed by repeat endoscopy (5,6), rather most make inferences based on improvement of symptoms or do not address resolution of symptoms at all. Other case series demonstrate GI damage in the setting of sevelamer use and positive histology findings but do not comment on length of sevelamer use before diagnosis or resolution following discontinuation (7,8). A large randomized trial is not reasonable to perform because of the risks that would be imposed on the study groups; thus the existing pool of data is restricted to anecdotal evidence and observational studies. One such observational study from 2013 (n = 1,060,132) attempted to make a correlation by comparing the incidence of intestinal perforation in US patients with dialysis before and after the introduction of sevelamer in 1999 and found no such statistically significant difference through formal testing in the level or slope of incidence (15). One major limitation of this study is that there was no individual patient-level data on sevelamer use, so exposure was only inferred by a calendar date as a proxy. The current data on the temporal relationship between sevelamer use and GI disease remains limited.
Twelve cases of sevelamer crystal–induced GI disease from a large urban community hospital over the course of 5 years were identified and reviewed for this case series. We hope to use this case series to better describe the breadth of clinical presentations of SIGD to inform clinicians on complications of this common drug. We also aim to contribute toward understanding the temporal relationship between sevelamer initiation and symptom onset and identify preexisting risk factors among affected patients to better understand this increasingly prevalent pathology.
METHODS
We identified 12 cases of sevelamer-induced GI events from 2016 to 2020 in a large community hospital. Patients were identified by search of a pathology database for sevelamer crystal inclusions in tissue samples reviewed by 2 expert GI pathologists. Institutional review board approval was received for this study (IRB # 02-22-14E). Diagnosis of SIGD was made based on GI symptomatology and histologically proven disease with inflammation and sevelamer crystals deposited within the mucosal wall, as assessed by a pathologist at our institution through immunohistochemical staining on formalin-fixed paraffin-embedded tissue sections. Four samples were from specimens following surgical resection and 8 from endoscopically retrieved biopsies. Patients were deidentified before storing patient-specific data in a password-protected Excel file located in an internal hospital drive accessible only to members of the study team. We collected the baseline characteristics including preexisting comorbid conditions, date of sevelamer initiation, date of symptom(s) onset, characterization of symptom(s), other possible offending medications, endoscopic findings, histological findings, and time to resolution from medication cessation. The patient charts were then reviewed for any pertinent follow-up details such as resolution of symptoms or further diagnostics that contributed to the initial diagnosis. Cases were not included in the series if they were identified to have another plausible explanation for their findings such as NSAID use, Helicobacter pylori infection, Clostridioides difficile positivity, or other confounding conditions.
RESULTS
The median patient age was 55 years with a range of 27–81 years, 8 female and 4 male patients (Table 1). Of the 12 patients, 8 had a documented history of at least 1 comorbid condition associated with GI dysmotility such as chronic constipation (n = 4), diabetes (n = 4), chronic opioid use (n = 2), and hypothyroidism (n = 1). Six patients had at least 1 comorbid condition associated with hypoperfusion and/or decreased mucosal integrity, including diabetes (n = 4), congestive heart failure (n = 2), hepatic cirrhosis (n = 1), chronic hypotension (n = 1), and Crohn's disease (n = 1). Four patients had at least 1 condition associated with GI dysmotility in addition to another condition associated with hypoperfusion or impaired mucosal integrity.
Table 1.
Baseline characteristics and clinical presentation
| Patient no. | Age and sex | Sevelamer dose (mg/d) | Days on medication | Presentation | Endoscopic or surgical findings | Comorbid conditions | Follow-up |
| 1 | 55 M | 4,800 | 36 | Diarrhea, nausea/vomiting | Ulcerated stenosis of sigmoid colon | Opioid-dependent chronic pain, constipation, HTN | Persistent stricture on colonoscopy, bleeding resolved |
| 2 | 69 F | 9,600 | 1,183 | Diarrhea, melena | Gastric ulcer, duodenitis, ulcerated proctitis | HTN, secondary hyperparathyroidism, hypothyroidism, diabetes | No further bleeding on long-term PPI |
| 3 | 52 F | 12,000 | 1,344 | Melena, hematochezia | Gastritis, gastric ulcer, normal colonic mucosa biopsied showing sevelamer crystals | CHF, orthotopic heart transplant with graft failure, chronic constipation | Repeat EGD with nonbleeding ulcer |
| 4 | 32 F | 2,400 | 60 | Abdominal pain, diarrhea, jejunitis on CT | Severe jejunitis with confluent ulcerations | HIV (CD4 = 292), secondary hyperparathyroidism, HTN, chronic constipation | No further bleeding |
| 5 | 27 M | 4,800 | 117 | Abdominal pain, hematochezia, fever | Ulcerated sigmoid colitis and proctitis | HIV (CD4 = 346), HTN, diabetes | No further bleeding |
| 6 | 54 F | 1,600 | 471 | Abdominal pain, diarrhea, perforated appendix | Perforated appendix, surgically excised | Hepatic cirrhosis, CHF, HTN, diabetes | No surgical complications or related follow-up |
| 7 | 55 F | 2,400 | 371 | Melena | Esophagitis, gastric ulcer | Dementia, HCV | H. pylori stain negative, no further follow-up |
| 8 | 74 F | 12,000 | 1,244 | Abdominal pain, melena | Gastritis, gastric ulcer | HTN, PAF, calciphylaxis, opioid-dependent chronic pain, constipation | Colonoscopy next day within normal limits |
| 9 | 63 M | 2,400 | 3,249 | Abdominal pain, diarrhea, diverticulitis on CT | Sigmoid perforation, surgically excised | Gout, HTN, renal transplant, prediabetes | No further bleed, on alternative phosphorus binder |
| 10 | 42 F | 2,400 | 980 | Nausea/vomiting, diarrhea | Diffuse pseudomembranous enterocolitis | Crohn disease, HPV, hypertrophic cardiomyopathy, diabetes | No further bleeding on PPI, C. difficile antigen and toxin negative |
| 11 | 78 F | 2,400 | 29 | Anemia | Transmural cecal necrosis, surgically excised | HTN, diverticulosis, DVT, gout | Passed away shortly after surgery |
| 12 | 81 M | 2,400 | 2,198 | Abdominal pain | Exploratory laparoscopy with dilated bowel, partially excised | Chronic hypotension, multiple myeloma | No further bleeding |
CHF, congestive heart failure; DVT, deep vein thrombosis; HCV, hepatitis C virus; HPV, human papilloma virus; HTN, hypertension; PAF, paroxysmal atrial fibrillation; PPI, proton pump inhibitor.
The daily total dosage per day of sevelamer use was on average 4,900 mg (range = 1,600 mg to 12,000 mg). The range from initiation of sevelamer to sampling of affected tissue through endoscopy or surgery was from 29 days to 3,249 days with a median of 471 days. Five patients were diagnosed with SGID within 1 year, 5 within 1–4 years, and 2 at 6 years or greater within starting sevelamer. The most common presentation was diarrhea (n = 6), abdominal pain (n = 6), melena (n = 4), and hematochezia (n = 2). Other symptoms included nausea and vomiting (n = 2), abnormal imaging findings (n = 2), anemia (n = 1), and fever (n = 1).
A broad range of endoscopic or surgical findings including ulcerations (n = 7), mucosal inflammation (n = 8), C. difficile–negative pseudomembranous colitis (n = 2), transmural necrosis (n = 3), and perforation (n = 2). These were reported throughout the GI tract including the esophagus (n = 1), stomach (n = 4), duodenum (n = 1), jejunum (n = 1), proximal colon including appendix (n = 3), distal colon (n = 4), and rectum (n = 2). Histologically, tissue biopsies had inclusions with the characteristic “fish-scale” appearance (Figures 1–3), which has been attributed to sevelamer crystals (13).
Figure 1.
Patient no. 3. (Left image) Colonic mucosa with chronic inflammation, reactive changes, and luminal crystalloid structures (darker pigmented material) consistent with sevelamer crystals. (Right image) Magnified view of sevelamer crystals.
Figure 3.
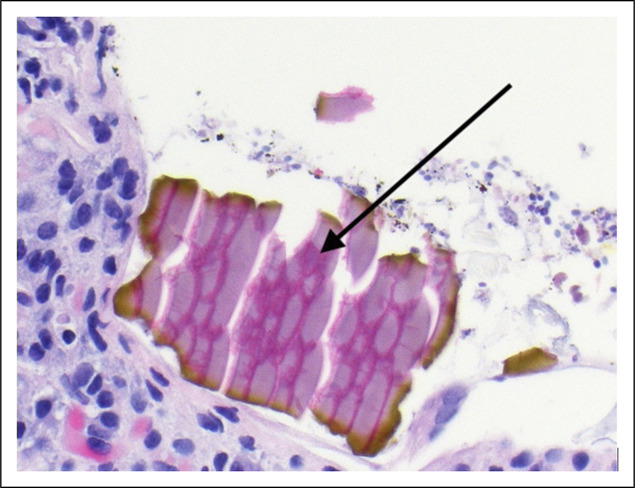
Patient no. 7. Gastric ulcer biopsy demonstrating two-toned “fish-scale” appearance of sevelamer crystals (arrow).
Figure 2.
Patient no. 4. (Left image) Small bowel mucosa demonstrating severe acute inflammation with extensive areas of ulceration and focal vascular thrombi. Sevelamer crystals are identified in the ulcer exudate (darker pigmented material). (Right image) Magnified view of sevelamer crystals.
Two patients had follow-up endoscopies after their initial endoscopic procedure performed for GI symptom evaluation demonstrating sevelamer crystal inclusions despite cessation of sevelamer (Table 2). Patient number 1 had a repeat colonoscopy 1 year later with resolution of ulcerations and no further sevelamer crystals on biopsy but did have persistence of sigmoid stenosis. Patient number 3 was started on daily proton pump inhibitor (PPI) therapy due to the presence of gastric ulcerations and underwent a repeat upper endoscopy 48 days after the initial endoscopy revealing persistent gastric ulceration without repeat biopsies undertaken. Notably, specimens were negative for H. pylori.
Table 2.
Patients with repeat endoscopy post sevelamer-induced gastrointestinal disease diagnosis
| Patient no. | Initial procedure findings | Interim between procedures | Interventions taken | Follow-up procedure findings |
| 1 | Ulcerated stenosis of sigmoid colon, pathology with sevelamer crystals | 366 d | Discontinued sevelamer | Sigmoid colon stricture, nonulcerated, unable to be traversed. No sevelamer on biopsies. |
| 3 | Gastritis, gastric ulcer, pathology with sevelamer crystals | 48 d | Discontinued sevelamer, started PPI | Nonbleeding gastric ulcer with pigmented material. No biopsies taken. |
PPI, proton pump inhibitor.
DISCUSSION
Identifying risk factors of the development of sevelamer-induced mucosal injury assists clinicians to risk stratify before initiation and may guide diagnostic workup in those who develop various GI symptoms. In our case series, there were twice as many women as men who had an adverse event related to sevelamer use, and most affected patients were in their sixth decade of life or older. According to the Centers for Disease Control and Prevention, chronic kidney disease is slightly more common in women (14%) than in men (12%) and most common among people aged 65 years or older followed by people aged 45–64 years (19). Thus, the baseline characteristics in our case series are in line with the general underlying population characteristics of patients with chronic kidney disease and does not necessarily show that female gender or older age is associated with higher risk of SGID.
In this study, we have highlighted the time course from initiation of sevelamer use to presentation and diagnosis of SGID and showed that it varies considerably, and thus exposure time does not seem to a be strong risk factor. Daily dosage of sevelamer also varied and did not seem to correlate to severity of SGID outcome. Sevelamer-induced perforation did occur at 1,600 mg (the lowest dose in our study).
Only 2 patients in this study had follow-up endoscopic evaluations to assess for resolution of initial SGID. Colonic mucosal healing with ongoing stricture was seen in a patient who underwent repeat endoscopy 1 year after initial diagnosis and cessation of sevelamer. Gastric ulceration persisted at least 48 days post sevelamer cessation and PPI initiation on repeat endoscopy in another patient. A major limitation to this case series is that there are few endoscopic evaluations post discontinuation of sevelamer. However, by symptom criteria alone, 11 of 12 of patients did improve clinically with the additional 1 patient passing away shortly after diagnosis. None of the patients reviewed have developed any further GI bleeding to this point (Table 1). More studies would be helpful in guiding physicians on the appropriate timing to examine for resolution in SGID.
Two mechanisms seen in the literature that may increase risk of adverse events are preexisting loss of mucosal integrity and increased length of exposure (7,8,10,11,17). Chronic conditions contributing to intestinal hypoperfusion such as diabetes, chronic liver disease, and heart failure can also adversely affect mucosal integrity over time (17). Active inflammatory bowel disease, enteric infections, and mesenteric ischemia disrupt the epithelial barrier, thus allowing nondegradable particles such as sevelamer crystals to infiltrate into the submucosa where the inflammatory reaction begins. These processes then cause a slowing of gut motility and further prolong mucosal exposure to these crystalline particles and may increase the risk and severity of SIGD. Currently, there are a lack of data on preexisting conditions that can predict the development of GI mucosal injury secondary to sevelamer exposure. Patients who do not have these risk factors are still at risk of developing SIGD, as seen in patients 7 and 11 (Table 1).
Recent discoveries in the mechanism of damage from crystalline drugs have been made since the disease process was first described (16). In vitro, sevelamer has been found to disrupt intestinal epithelium and induce the formation of neutrophil extracellular traps and macrophage extracellular traps, amplifying local inflammation and necrosis. This effect is posited to be exacerbated in the setting of preexisting intestinal epithelial damage such as inflammatory bowel disease, enteric infections, or mesenteric ischemia. There may also be risk of pressure necrosis due to stercoral ulceration from sevelamer-induced constipation, one of the more well-described adverse reactions (2,17).
Our study identified chronic conditions supported by available literature that may raise the risk of SGID. Specifically, conditions contributing to decreased gut motility including chronic opioid use, constipation, and hypothyroidism should give the clinician pause. Because sevelamer has the known side effect of constipation in some patients (2), consideration should be given to drug cessation if constipation develops. Our data showed that diarrhea was in fact a more common presentation leading to endoscopic evaluation. A metanalysis of adverse events associated with sevelamer indicated that patients are at increased risk of developing constipation (2). A distinction to make on our results is that these patients required endoscopic or surgical intervention. Based on these findings, SIGD may be more likely to result in an invasive diagnostic procedure in the setting of diarrhea or gastrointestinal bleed, whereas constipation may just be treated clinically.
As highlighted in our study, conditions that lead to loss of mucosal integrity may also increase the risk of developing severe gastrointestinal disease. These conditions are more common in immunocompromised states such as organ transplant recipients, liver disease, chronic heart failure, HIV-infected persons, patients with inflammatory bowel disease, and those with underlying gastroenteric infections. Diabetes can cause neuropathy in the GI tract and has effects on mucosal integrity by the accumulation of proinflammatory advanced glycation end-products (19) and alteration of the natural gut flora. NSAIDs are also commonly implicated in mucosal disruption; however, they are also contraindicated in ESRD, so are less frequently used in this population. None of the patients in this case series had documented NSAID use within 1 month of presentation.
As SIGD becomes a more recognized pathology, questions arise regarding vulnerable populations, range of presenting symptoms, and the time frame of the syndrome to initiation of drug. This case series serves to demonstrate that SIGD is quite variable, with a wide range of dosages, time to initial symptoms, and presenting symptoms. On the contrary, some aspects among cases were similar such as resolution of symptoms after discontinuation of sevelamer. A limitation of this report is the small sample size of 12 and the prospective nature of study including lack of postcessation endoscopic evaluations. Given these limitations, we cannot make population-wide extrapolations. However, our study highlights the finding that SIGD can occur anywhere in the GI tract and the GI symptoms associated and endoscopic findings vary; therefore, clinicians should be made aware of the heterogeneity in presentation of patients with SIGD.
We recommend clinicians prescribing sevelamer undertake a careful review of the patient’s preexisting conditions before sevelamer initiation. If underlying risk factors for gut dysmotility and/or conditions leading to loss of GI mucosal integrity are present, it may be prudent to consider an alternative phosphate binder such as calcium salts, which is as effective as sevelamer in reducing hyperphosphatemia but associated with decreased incidence of all-cause gastrointestinal adverse events (2,18). Therefore, any patient on sevelamer with new concerning gastrointestinal symptoms should be considered for a manifestation of SIGD.
CONFLICTS OF INTEREST
Guarantor of the article: James Todd, DO.
Specific author contributions: J.T.: conceptualization, investigation, formal analysis, writing. S.S.: supervision, writing, review and editing. J.Z.: conceptualization, investigation. W.A.: investigation, supervision, review and editing. C.J.: investigation, supervision, review and editing. B.M.: conceptualization, supervision, review and editing. All authors had access to the study data and reviewed and approved the final manuscript.
Financial support: None to report.
Potential competing interests: None to report.
Study Highlights.
WHAT IS KNOWN
✓ Sevelamer is a common medication used for treatment of hyperphosphatemia, particularly in patients with end-stage renal disease.
✓ Sevelamer-induced gastrointestinal disease (SIGD) has been previously described in isolated case studies and case series.
WHAT IS NEW HERE
✓ Preexisting decreased gastrointestinal motility and impaired mucosal integrity have been frequently seen in patients that develop SIGD.
✓ The time course between initiation of sevelamer and presentation of disease varies widely.
✓ The severity of disease does not seem to be strongly linked to dosage or length of exposure to sevelamer.
Contributor Information
Shadab Saboori, Email: shadab.saboori@atriumhealth.org.
Joseph Zeidan, Email: joseph.zeidan@atriumhealth.org.
William Ahrens, Email: william.ahrens@atriumhealth.org.
Carl Jacobs, Email: carl.jacobs@atriumhealth.org.
Baha Moshiree, Email: baha.moshiree@atriumhealth.org.
REFERENCES
- 1.Philpott HL, Nandurkar S, Lubel J, et al. Drug-induced gastrointestinal disorders. Frontline Gastroenterol 2014;5(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Navaneethan SD, Palmer SC, Vecchio M, et al. Phosphate binders for preventing and treating bone disease in chronic kidney disease patients. Cochrane Database Syst Rev 2011(2):CD006023. [DOI] [PubMed] [Google Scholar]
- 3.Amer S, Nguyen C, DePetris G. Images of the month: Gastric pneumatosis due to sevelamer-mediated necrosis. Am J Gastroenterol 2015;110(6):799. [DOI] [PubMed] [Google Scholar]
- 4.Tieu C, Moreira RK, Song LMWK, et al. A case report of sevelamer-associated recto-sigmoid ulcers. BMC Gastroenterol 2016;16(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magee J, Robles M, Dunaway P. Sevelamer-induced gastrointestinal injury presenting as gastroenteritis. Case Rep Gastroenterol 2018;12(1):41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai T, Frugoli A, Barrows B, et al. Sevelamer carbonate crystal-induced colitis. Case Rep Gastrointest Med 2020;2020:4646732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arriola AG, Martin ND, Tondon R. Crystals in perforated bowel: Culprits or innocent bystanders. Int J Surg Pathol 2018;26(3):238–9. [DOI] [PubMed] [Google Scholar]
- 8.George SA, Francis I. Pathology of resin-induced gastrointestinal damage: Report of 15 cases and review of literature. Turkish J Pathol 2019;35(3):221–7. [DOI] [PubMed] [Google Scholar]
- 9.Modi RM, Swanson B, Duggirala V. Long-standing diarrhea associated with sevelamer crystalopathy in colonic mucosa. Clin Gastroenterol Hepatol 2017;15(2):A23-7. [DOI] [PubMed] [Google Scholar]
- 10.Nambiar S, Pillai UK, Devasahayam J, et al. Colonic mucosal ulceration and gastrointestinal bleeding associated with sevelamer crystal deposition in a patient with end stage renal disease. Case Rep Nephrol 2018;2018:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okwara CJ, Gulati R, Rustagi T, et al. Upper gastrointestinal bleeding of unusual causation. Dig Dis Sci 2018;63(10):2541–6. [DOI] [PubMed] [Google Scholar]
- 12.Keri KC, Veitla V, Samji NS. Ischemic colitis in association with sevelamer crystals. Indian J Nephrol 2019;29(3):191–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai M, Reiprich A, Khov N, et al. Crystal-associated colitis with ulceration leading to hematochezia and abdominal pain. Case Rep Gastroenterol 2016;10(2):332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okwara C, Choi C, Park JY. Sevelamer-induced colitis presenting as a pseudotumor. Clin Gastroenterol Hepatol 2015;13(7):A39–A40. [DOI] [PubMed] [Google Scholar]
- 15.Yang JY, Lee TC, Montez-Rath ME, et al. Trends in the incidence of intestinal perforation in US dialysis patients (1992–2005). J Nephrol 2013;26(2):281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim T, de Oliveira Silva Lautenschlager S, Ma Q, et al. Drug crystal-related gastrointestinal complications involve crystal-induced release of neutrophil and monocyte extracellular traps. Cells 2020;9(11):2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madan P, Bhayana S, Chandra P, et al. Lower gastrointestinal bleeding: Association with sevelamer use. World J Gastroenterol 2008;14(16):2615–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qunibi WY, Hootkins RE, McDowell LL, et al. Treatment of hyperphosphatemia in hemodialysis patients: The calcium acetate renagel evaluation (care study). Kidney Int 2004;65(5):1914–26. [DOI] [PubMed] [Google Scholar]
- 19.Phuong-Nguyen K, McNeill BA, Aston-Mourney K, et al. Advanced glycation end-products and their effects on gut health. Nutrients 2023;15(2):405. [DOI] [PMC free article] [PubMed] [Google Scholar]





