Abstract
Background and Objectives
Bisphosphonates are routinely used to treat osteoporosis in patients with Duchenne muscular dystrophy (DMD), a rare, severely debilitating neuromuscular disease. We sought to synthesize and grade benefits and harms evidence of bisphosphonates in glucocorticoid-treated patients with DMD.
Methods
In this systematic review (PROSPERO identifier: CRD42020157606), we searched MEDLINE, CINAHL, Embase, PsycINFO, Web of Science, and CENTRAL for articles published from inception up to and including March 31, 2023, reporting results in any language from any study type. Quality of evidence was assessed using the Grading of Recommendations, Assessment, Development, and Evaluations framework.
Results
We identified 19 publications involving 1,010 children and adults from 12 countries across all inhabited continents except South America. We found high-quality evidence that bisphosphonates significantly increase the areal lumbar spine bone mineral density (BMD) Z score in glucocorticoid-treated patients with DMD. The greatest improvements were recorded in controlled settings among patients treated with intravenous zoledronate. Evidence of benefits to fracture risks was inconclusive and/or of low quality, primarily due to lack of controlled data and small samples. Bisphosphonates were generally well-tolerated, although adverse events related to the first infusion (i.e., “acute phase reaction”) were frequently reported.
Discussion
There is high-quality evidence supporting the use of bisphosphonates to increase the areal lumbar spine BMD Z score in patients with DMD and glucocorticoid-induced osteoporosis. Our synthesis and grading affirm current recommendations put forward in the 2018 DMD Clinical Care Considerations and should be helpful in raising awareness about anticipated benefits of bisphosphonates, prevailing unmet needs, and potential safety issues in their use.
Introduction
Duchenne muscular dystrophy (DMD) is a rare, X-linked recessive, severely debilitating disease characterized by progressive muscle degeneration.1 Due to the underlying myopathy and associated risk factors (e.g., inadequate intake of calcium and vitamin D due to nutritional problems and reduced sunlight exposure), patients with DMD are predisposed to loss of bone strength (i.e., osteoporosis) resulting in vertebral and nonvertebral fragility fractures.2 In addition, prolonged exposure to glucocorticoids—the cornerstone of the current pharmacologic management of DMD, initiated at a young age—causes further loss of bone strength due to direct and indirect osteotoxicity, resulting in even greater susceptibility to fracture.3 Indeed, the risk of long bone fractures in boys with DMD more than doubles with glucocorticoid therapy,4 and vertebral fractures occur in most patients,5,6 with no reports of growth-mediated spontaneous (i.e., without bone-active medications) vertebral body reshaping in the glucocorticoid-treated DMD setting.7
Given the high incidence of fractures in patients with DMD, treatment of secondary osteoporosis constitutes an important clinical component of the multidisciplinary management of the disease. Per the most recent clinical care considerations published in 2018,2 indications for treatment with intravenous bisphosphonate in DMD include a single low-trauma vertebral fracture or single long bone fracture (i.e., multiple fractures are not required). In doing so, the main clinical aims were to identify and treat the earliest signs of bone fragility to better preserve the heights of the vertebral bodies, minimize back pain, prevent new fractures, the extremity fracture/refracture cycle, and loss of ambulation due to fractures.
We presently lack a comprehensive and up-to-date understanding of the current body of evidence, including observational studies, non-RCT or quasi-RCTs, and RCTs addressing the combined effects of bisphosphonates and glucocorticoid therapies in DMD. To bridge this data gap, the objective of our study was to perform a systematic review and grading of the benefits and harms evidence of bisphosphonates among glucocorticoid-treated children and adults with DMD.
Methods
This systematic review was registered with the International Prospective Registry of Systematic Reviews (PROSPERO) (identifier: CRD42020157606) and conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.8
Search Strategy
On April 1, 2023, we searched MEDLINE (through Ovid), the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase (through Ovid), PsycINFO, Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL) for studies published from inception up until March 31, 2023, reporting results of benefits and harms of bisphosphonates in glucocorticoid-treated children and adults with DMD. The search string contained a combination of the following Medical Subject Heading terms, title/abstract, and topic/all-field tags: “Duchenne muscular dystrophy,” “bisphosphonate,” “diphosphonate,” “alendronate,” “alendronic acid,” “etidronate,” “etidronic acid,” “risedronate,” “risedronic acid,” “zoledronate,” “zoledronic acid,” “ibandronate,” “ibandronic acid,” “olpadronate,” “olpadronic acid,” “pamidronate,” “pamidronic acid,” “tiludronate,” and “tiludronic acid” (full search strings are provided in eTable 1 (links.lww.com/WNL/D296) in the online supplemental material).
Selection Criteria
Based on eligibility criteria derived from the Population, Intervention, Comparison, Outcomes, and Study design framework, we included all publications that reported benefits and/or harms of bisphosphonates in glucocorticoid-treated human children and adults with DMD from any study type in any language. Review articles were not included but screened for additional references, and we did not consider conference abstracts (because these contain too few details for meaningful synthesis and grading). No further criteria were imposed for study eligibility.
Screening, Data Extraction, and Synthesis
Two investigators (E.L. and F.Z.) independently screened article titles and abstracts for eligibility and subsequently reviewed full-text versions of selected records. Reasons for article exclusion were recorded and disagreements were resolved by the involvement of a third investigator (T.S.). Details of extracted data elements are summarized in eTable 2 (links.lww.com/WNL/D296) in the online supplemental material. Evidence regarding benefits of bisphosphonates in glucocorticoid-treated DMD was synthesized and reported for the following predefined outcome categories: long bone fractures, vertebral fractures, vertebral body reshaping, bone mineral density (BMD), serum biomarkers of bone turnover, and transiliac bone formation rates. In addition, based on reported follow-up durations, we calculated the mean annual change in areal lumbar spine (LS) BMD Z score. Reported fracture rates were also annualized (where possible) to facilitate comparison. Adverse event/safety data were summarized as reported in the included publications. For meaningful synthesis, we did not consider evidence of effectiveness from included case studies/series (i.e., for which results are reported at the case level).
Rating of the Quality of the Evidence
We assessed the quality of the identified evidence regarding effects of bisphosphonates in glucocorticoid-treated patients with DMD using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework9 (details provided in eTable 3 (links.lww.com/WNL/D296) in the online supplemental material).
Standard Protocol Approvals, Registrations, and Patient Consents
Not applicable (literature review of previously published data).
Data Availability
Data analyzed as part of this literature review are included in the article or its supplemental material (online).
Results
The database searches resulted in the identification of 163 unique publications (Figure 1). Of these, 18 full-text articles4,10-26 and 1 editorial letter (reporting previously unpublished case series data)27 were included for extraction, synthesis, and grading. Summary data of the included publications are listed in Table 1.
Figure 1. PRISMA Diagram of the Selection Process of the Included Publications.
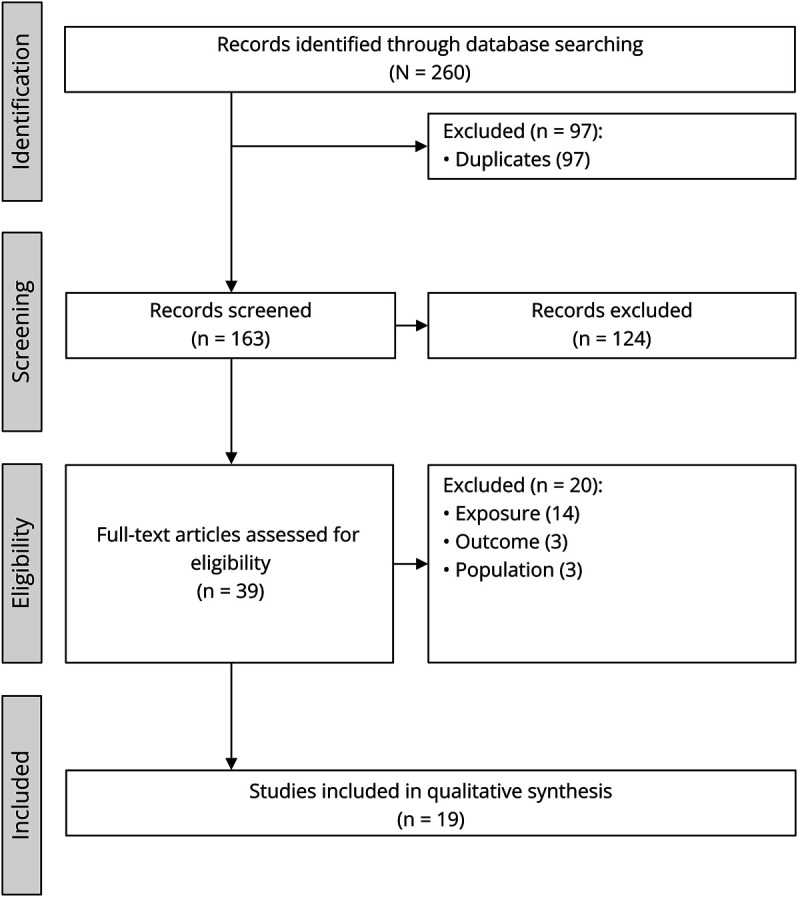
DMD = Duchenne muscular dystrophy.
Table 1.
Characteristics of Included Studies
| Glucocorticoid exposure | Bisphosphonate exposure | |||||||||
| Author [country] | Study design | Sample, n (age)a | Pharmacologic agent(s) | Dose, mean | Duration, mean | n (%) exposed | Pharmacologic agent(s) | Administration route | Dose, mean | Duration, mean |
| Case et al. [UK]10 | Case study | Case: 11 y | DFZ and PRED | • DFZ: 0.7 mg/kg/d • PRED: 0.8 mg/kg/d |
6.3 y | 1 (100) | ZLN | IV | 0.05 mg/kg | <6 mo (1 IV infusion) |
| Gordon et al. [CAN]11 | Retrospective cohort study | 44 (median age: 13 y, range: 7–23 y) | DFZ and/or PDN | NR | >1 y | 16 (36) | ALN, CLO, and/or PAM | NR | NR | 6 yc |
| Hawker et al. [CAN]12 | Nonrandomized, uncontrolled before-and-after trial | 16 (mean age: 11 y, range: 7–16 y) | DFZ | 0.69 mg/kg/d | 2.6 y | 16 (100) | ALN | Oral | 0.08 mg/kg/d | 2 y |
| Houston et al. [USA]13 | Retrospective cohort study | 19 (age NR)d | DFZ and/or PDN | NR | NR | 19 (100)e | ALN | Oral | 35 mg and/or 70 mg weekly | 3.4 y |
| Ivanyuk et al. [CH]14 | Case series | • Case 1: 14 y • Case 2: 13 y |
NR | NR | Case 1: 5 y Case 2: 9 y |
2 (100) | ZLN | IV | • Case 1: 0.04 mg/kg • Case 2: 0.02 mg/kg |
• Case 1: <12 mo (2 IV infusions) • Case 2: <6 mo (1 IV infusion) |
| Joseph et al. [UK]4 | Retrospective cohort study | 520 (age NR)f | DFZ and/or PRED | NR | 4.0 y | NRf | NR | Oral and IV | NR | NR |
| Lemon et al. [UK]15 | Case series | • Case 1: 11 y • Case 2: 15 y |
DFZ | 18 mg/d | NR | 2 (100) | ZLN | IV | • Case 1: 0.025 mg/kg • Case 2: 0.05 mg/kg |
<6 mo (1 IV infusion) |
| Lim et al. [AUS]27 | Case series | 9 (mean age: 13 y, range: 9–15 y) (see article for case-specific details) | DFZ or PRED | • DFZ: 0.9 mg/kg/dg • PRED: 0.75 mg/kg/dg |
6.8 y (see article for case-specific details) | 9 (100) | ZLN | IV | 0.12 mg/kg/y (see article for case-specific details) | 2.3 y (see article for case-specific details) |
| Misof et al. [CAN]16 | Prospective cohort study | 9 (mean age: 11 y, range: 8–14 y)h | DFZ or PDN | • DFZ: 0.6 mg/kg/d • PDN: 0.3 mg/kg/d |
3.4 y | 9 (100) | PAM or ZLN | IV | • PAM: 9 mg/kg/y • ZLN: 0.05–0.10 mg/kg/y |
2.4 y |
| Moretti et al. [IT]17 | Retrospective cohort study | 8 (mean age: 19 y, range NR) | DFZ | 15 mg/d | 11.5 yc | 8 (100) | NER | IM injection | 25 mg/mo | 12 mo |
| Nasomyont et al. [USA]18 | Retrospective cohort study | 52 (median age: 12 y, range: 8–20 y) | DFZ or PDN | • DFZ: 0.9 mg/kg/dg • PRED: 0.75 mg/kg/dg |
4.7 y | 52 (100) | ALN | Oral | 17.5 mg/wk (age <7 y) and 35 mg/wk (age ≥8 y)g | ≤5 y |
| Palomo Atance et al. [ES]19 | Case series | • Case 1: 8 y • Case 2: 16 y |
DFZ | • Case 1: 0.7 mg/kg/d • Case 2: 1.0 mg/kg/d |
NR | 2 (100) | ALN | Oral | 10 mg/d | • Case 1: 2.1 y • Case 2: 1.2 y |
| Ronsley et al. [CAN]20 | Retrospective cohort study | 68 (mean age: 13 y, range: NR) | DFZ or PDN | • DFZ: 0.9 mg/kg/d (maximum 36 mg) • PDN: 0.7 mg/kg/d |
8.5 y | 32 (47) | PAM and/or ZLN | IV | • PAM: 9 mg/kg/y • ZLN: 0.10 mg/kg/y |
4.2 y |
| Sbrocchi et al. [CAN]21 | Retrospective cohort study | 5 (mean age: 10 y, range: 9–12 y) | DFZ | 0.9 mg/kg/d | 2.8 y | 5 (100) | PAM or ZLN | IV | • PAM: 9 mg/kg/y • ZLN: 0.10 mg/kg/y |
2 y |
| Srinivasan et al. [UK]22 | Nonrandomized case-control trial | 51 (mean age: 9 y, range: NR) | DFZ or PRED | NR | 3.3 yc | 36 (71)i | RIS | Oral | 35 mg/wkj | 3.6 y |
| Tian et al. [USA]23 | Retrospective cohort study | 54 (median age: 11 y, range: 6–17 y) | NR | NR | 4.0 yc | 54 (100)k | ALN | Oral | 17.5 mg/wk (age <7 y) and 35 mg/wk (age ≥8 y)g | 6.0 yc |
| Ward et al. [multinationalb]24 | Randomized controlled trial | 34 (mean age: 13 y, range: 9–17 y)l | NR | NR | ≥1 dose within 1 y of trial | 18 (53)m | ZLN | IV | 0.05 mg/kg (maximum 5 mg) once every 6 mo | 1 y |
| Zacharin et al. [AUS/NZ]25 | Randomized controlled trial | 62 (mean age: 10 y, range: 6–16 y)n | DFZ or PRED | NR | ≥3 mo | 31 (50)n | ZLN | IV | • Dose 1 and 2 at 0.025 mg/kg (at 0 and 3 mo) • Dose 3, 4, and 5 at 0.05 mg/kg (at 6, 12, and 18 mo) |
2 y |
| Zheng et al. [CN]26 | Prospective cohort study | 52 (mean age: 10 y, range: 5–16 y) | PRED | 0.66 mg/kg/d | 21 mo | 17 (33) | ALN | Oral | 70 mg/wk | 24 mo |
| 18 (35) | ZLN | IV | 5 mg/y | 24 mo | ||||||
Abbreviations: ALN = Alendronate; AUS = Australia; CAN = Canada; CH = Switzerland; CLO = Clodronate; CN = China; DFZ = Deflazacort; DMD = Duchenne muscular dystrophy; ES = Spain; IM = Intramuscular; IT = Italy; IV = Intravenous; NER = Neridronate; NR = Not reported; NZ = New Zealand; PAM = Pamidronate; PDN = Prednisone; PRED = Prednisolone; RIS = Risedronate; SQ = Subcutaneous; UK = United Kingdom; USA = United States of America; ZLN = Zoledronate.
Number of patients treated with glucocorticoids (age at study baseline or bisphosphonate initiation).
Canada, Australia, the United Kingdom, South Africa, Russia, and Hungary.
Median.
A proportion of patients receiving bisphosphonates were not treated with glucocorticoids.
Nineteen of 29 and 13 of 29 patients treated with bisphosphonates had areal bone mineral density measurements at the total hip and lumbar spine, respectively, at both baseline and the follow-up visit.
The proportion of patients treated with bisphosphonates (n = 47) also treated with glucocorticoids is not reported.
Starting dose.
One patient was not treated with glucocorticoids.
Bone mineral density measurements were only available for a subset of the total sample (see article for details).
One mg/kg/wk for patients weighting <20 kg.
Measures of areal bone mineral density at the lumbar spine and whole body were only available for 51 and 49 patients, respectively.
Thirteen patients diagnosed with DMD (38% of the total cohort).
Six patients diagnosed with DMD (33% of all exposed to bisphosphonates).
Fourteen patients withdrew before study completion (5 treated with bisphosphonates and 9 controls). In total, 56 patients completed the 24-mo follow-up (27 treated with zoledronate).
Summary of the Evidence
Identified studies encompassed 1,010 patients from 12 countries: Australia, Canada, China, Hungary, Italy, New Zealand, South Africa, Spain, Switzerland, Russia, the UK, and the United States. Individual study samples comprised between 1 and 520 cases, and the age of patients ranged between 5 and 23 years (5 studies did not report minimum and maximum ages).4,13,17,20,22 Two (11%) studies reported results from randomized controlled trials,24,25 8 (42%) from retrospective cohort studies,4,11,13,17,18,20,21,23 5 (26%) from case studies/series,10,14,15,19,27 2 (11%) from prospective cohort studies,16,26 1 (5%) from a case-control trial,22 and 1 (5%) from an uncontrolled before-and-after trial.12
In total, 10 (53%) publications10,14-16,20,21,24-27 reported evidence from studies of intravenous (IV) bisphosphonates and 6 (32%) of orally administered bisphosphonates12,13,18,19,22,23 (2 studies reported oral and IV administration,4,26 and 1 did not disclose the bisphosphonate administration route11). Details of the reported methods for the quantification of osteoporosis clinical outcomes are summarized in Table 2.
Table 2.
Methods for Quantification of Osteoporosis Clinical Outcomes
| Author [country] | VF evaluation criteria | VF analysis method | Vertebral body reshaping following VF | Non-VF evaluation criteria | Non-VF analysis method | DXA assessment method | DXA analysis method |
| Gordon et al. [CAN]11 | NA | NA | NA | NA | NA | NA | NA |
| Hawker et al. [CAN]12 | NA | NA | NA | NA | NA | Single Lunar DPX-L DXA | Areal LS and TB BMD Z scores |
| Houston et al. [USA]13 | NA | NA | NA | NA | NA | Single Hologic QDR Delphi-4500A DXA | Areal LS and TH BMD Z scores |
| Joseph et al. [UK]4 | NR | NR | NA | NR | NR | NA | NA |
| Misof et al. [CAN]16 | Genant semiquantitative method | NA | NA | NA | NA | NR | Areal and volumetric LS BMD Z scores |
| Moretti et al. [IT]17 | NR | NR | NA | NR | NR | GE Lunar i-DXA | Areal LS BMD Z score |
| Nasomyont et al. [USA]18 | Genant semiquantitative method | Six-point vertebral morphometry on radiographs to document change in VF prevalence and severity | Decrease in Genant grade | NA | NA | NA | NA |
| Ronsley et al. [CAN]20 | NR | Fracture yes/no from clinical reports | NA | NR | NR | Hologic Discovery A | Areal LS, LH, and TB BMD Z scores |
| Sbrocchi et al. [CAN]21 | Genant semiquantitative method | Triple read by 3 paediatric radiologists (2 independent reads, followed by third party discrepancy resolution) | Change in vertebral height ratio which exceeds the least significant change on 6-point vertebral morphometry | NR | NR | Lunar Prodigy (General Electric; Madison, WI, USA) | Areal and volumetric LS BMD Z scores |
| Srinivasan et al. [UK]22 | NR | Plain radiographs | NA | NR | Plain radiographs | HOLOGIC equipment (Delphi W and Discovery A) | Areal LS BMD Z score and TB (excl. head) BMC |
| Tian et al. [USA]23 | NR | Determined by radiologist and verified by inspection of radiographs by the treating and study physician | Increase in vertebral height and improvement of preexisting VF on radiographs (no detailed morphometry analyses performed) | NA | NA | Hologic Discovery A | Areal LS, TB, and LDF BMD Z scores |
| Ward et al. [multinationala]24 | Genant semiquantitative method | Triple read by 3 pediatric radiologists (2 independent reads, followed by third-party discrepancy resolution) | NR | Any low-trauma fracture (excluding those of the face, skull, and digits of the hands and feet) that was diagnosed following presentation to medical attention with signs or symptoms of a fracture | Central reading at BioClinica, Inc | Lunar (Prodigy, GE Lunar Corp, DPX-NT, iDXA software) or Hologic machines (fan beam scanners) | Areal LS BMD Z score, height-adjusted areal LS BMD Z score, areal LDF BMD, and LS and TB BMC |
| Zacharin et al. [AUS/NZ]25 | Genant semiquantitative method | Single-read lateral thoracolumbar spine radiographs by blinded radiologist; spinal deformity index calculated (sum of Genant grades from T4 to L4) | NR | NR | NR | Hologic QDR 4500 initially then Horizon (RCH) or GE-Lunar Prodigy (CHW, PMH and The Liggins Institute) | Areal LS BMD Z score, areal heigh-adjusted LS BMD Z score, and LS and TB BMC |
| Zheng et al. [CN]26 | NA | NA | NA | NA | NA | GE-Lunar Prodigy Advance (Madison, WI) | Areal FN, LS, and TH BMD Z scores |
Abbreviations: AUS = Australia; BMC = Bone mineral content; BMD = Bone mineral density; CAN = Canada; CN = China; DMD = Duchenne muscular dystrophy; FN = Femoral neck; IT = Italy; LDF = Lateral distal femur; LH = Left hip; LS = Lumbar spine; NA = Not applicable; NR = Not reported; NZ = New Zealand; TB = Total body; TH = Total hip; UK = United Kingdom; USA = United States of America; VF = Vertebral fracture.
Canada, Australia, the United Kingdom, South Africa, Russia, and Hungary.
Rating of the Quality of the Evidence
We initially assigned included RCTs to a high rating, observational studies and nonrandomized trials to a low rating, and case reports/series to a very low rating (per the GRADE manual, Step 1). Next, in Step 2, called “Outcome grade modification,” we downgraded the rating for the study conducted by Gordon et al.11 due to serious limitations (risk of bias) from immortal time bias, those conducted by Joseph et al.4 and Ronsley et al.20 due to serious limitations (risk of bias) pertaining to lack of information regarding the bisphosphonate exposure, and those conducted by Hawker et al.,12 Misof et al.,16 and Sbrocchi et al.21 due to small sample sizes. Finally, in Step 3, we provided an overall rating of the quality of the evidence of each publication. The results of these 3 steps to rate the quality of evidence are summarized in eTable 4 (links.lww.com/WNL/D296) in the online supplemental material.
Benefits of Bisphosphonates in Glucocorticoid-Treated Patients With DMD
Long Bone Fractures
None of the identified studies were specifically designed and sufficiently powered to detect an effect of bisphosphonates on long bone fracture rates in glucocorticoid-treated patients with DMD. However, 2 RCTs24,25 and 2 uncontrolled observational studies17,21 recorded incident long bone fractures during the bisphosphonate treatment period. In the RCT by Ward et al.,24 involving an international cohort of 34 children with bone fragility and chronic disorders (of whom 38% had DMD and all of whom had with at least 1 dose of glucocorticoid therapy for the treatment of their underlying disease in the 12 months preceding enrollment), the incidence rate per person-year was estimated at 0.17 in patients treated with IV zoledronate and 0.25 in those without IV zoledronate therapy. In the RCT by Zacharin et al.,25 encompassing 62 patients from Australia/New Zealand with DMD followed up for 24 months, the mean long bone fracture incidence rate was 0.02 per person-year in both treatment groups (IV zoledronic plus nutritional support [calcium and vitamin D supplementation] vs nutritional support alone). In the nonrandomized case-control trial by Srinivasan et al.,22 long bone fractures were systematically recorded only in the treated cohort (but rates were not reported).
Vertebral Fractures
We found 1 study22 that was designed to evaluate the effects of bisphosphonates on vertebral fractures in glucocorticoid-treated patients with DMD and 2 RCTs24,25 and 4 uncontrolled observational studies17,18,21,23 recording incident vertebral fractures during bisphosphonate treatment. Specifically, Srinivasan et al.22 conducted a nonrandomized case-control trial of vertebral fracture risk in 51 glucocorticoid-treated UK patients with DMD, of whom 36 received oral risedronate. The mean vertebral fracture incidence rate per person-year was estimated at 0.02 with oral risedronate therapy and 0.06 without oral risedronate therapy (p = 0.045). In the 12-month RCT by Ward et al.,24 the mean low-trauma vertebral fracture incidence rate per person-year in patients treated with IV zoledronate was 0 (i.e., no low-trauma vertebral fractures sustained during follow-up) and 0.13 in those who received IV placebo. In the 24-month RCT by Zacharin et al.,25 15% (4 of 27 patients) sustained new vertebral fractures on zoledronic acid compared with 24% (7 of 29 patients) in the control arm (randomized to nutritional support alone with calcium and vitamin D supplements), with each group having the same mean vertebral fracture incidence rate per person-year of 0.28. Zacharin et al.25 also found no evidence of a difference in spinal deformity index (sum of Genant grade from T4 to L4) at 24 months between patients having received IV zoledronic acid and those who received nutritional support alone.
Looking into descriptive results from included observational cohort studies, Nasomyont et al.18 observed a similar prevalence of thoracic and lumbar vertebral fractures at baseline and each year of follow-up (ranging up to 5 years) in 52 US patients treated with oral alendronate; Sbrocchi et al.21 documented 3 incident vertebral fractures in 2 patients across the 2-year follow-up period (corresponding to a mean incidence rate per person-year of 0.30) in 5 Canadian patients treated with IV pamidronate or zoledronate; and Moretti et al.17 recorded no new fractures during follow-up.
Vertebral Body Reshaping
We found 3 retrospective cohort studies18,21,23 reporting evidence of the effects of bisphosphonates on vertebral body reshaping in glucocorticoid-treated patients with DMD. Specifically, Nasomyont et al.18 found that 46% (24 of 52) of patients with at least 1 vertebral fracture treated with weekly oral alendronate showed improvement (i.e., a decrease) in the Genant grade after up to 5 years of follow-up; Tian et al.23 recorded an increase in vertebral height and improvement of preexisting vertebral fractures (described as a decrease in the Genant grade by at least 1) in 9% (5 of 54) of US patients treated with oral alendronate across a median of 6 years; and Sbrocchi et al.21 documented reshaping of the vertebral bodies (quantified by changes that exceeded the least significant change on a 6-point vertebral morphometry) after 24 months in 40% (2 of 5) of Canadian patients treated with IV pamidronate or zoledronate.
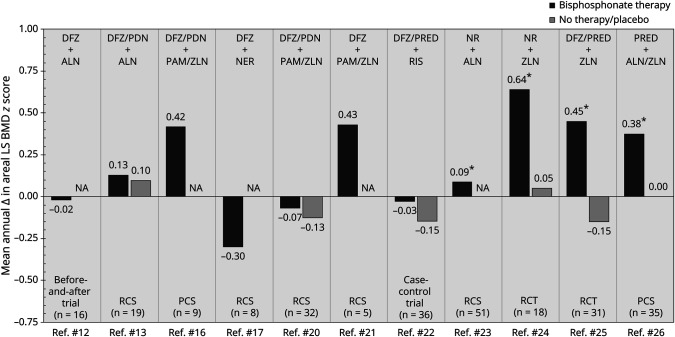
Bone Mineral Density
We found 11 studies12,13,16,17,20-26 reporting evidence of the effects of bisphosphonates on BMD in glucocorticoid-treated patients with DMD. Identified estimates of the mean change in areal LS BMD Z score, annualized based on study-specific follow-up durations, are presented in Figure 2. In the RCT by Ward et al.,24 the mean change in height Z score–adjusted areal LS BMD Z score (IV zoledronate therapy vs IV placebo) was estimated at 0.75 after 1 year of treatment (p = 0.040). The corresponding estimated increase in areal LS BMD Z score across 24 months in the RCT by Zacharin et al.,25 including 62 Australian/New Zealander patients with DMD, was 1.30 (p < 0.001).
Figure 2. Mean Annual Change in Areal LS BMD Z Score in Glucocorticoid-Exposed Patients With DMD Treated With Bisphosphonates.
ALN = Alendronate; BMD = Bone mineral density; DFZ = Deflazacort; DMD = Duchenne muscular dystrophy; LS = Lumbar spine; NA = Not applicable; NER = Neridronate; NR = Not reported; PAM = Pamidronate; PCS = Prospective cohort study; PDN = Prednisone; PRED = Prednisolone; RCS = Retrospective cohort study; RCT = Randomized controlled trial; RIS = Risedronate; ZLN = Zoledronate. * Statistically significant change.
Looking into the evidence of volumetric BMD, Misof et al.16 estimated the mean change in volumetric LS BMD Z score across an average of 2.4 years at 1.30 in a prospective cohort study of 9 patients treated with IV pamidronate or zoledronate. A numerically larger change in volumetric LS BMD Z score (1.64) was reported by Sbrocchi et al.21 in their retrospective cohort study of 5 Canadian patients treated with IV pamidronate or zoledronate across 24 months. Finally, in the RCT by Zacharin et al.,25 the estimated mean change in 4% volumetric radius trabecular BMD Z score by peripheral quantitative CT after 2 years of zoledronate therapy was reported at 2.8 (p = 0.007).
In a nonrandomized, uncontrolled before-and-after trial, Hawker et al.12 studied changes in areal total body (TB) BMD Z score in 16 Canadian patients with DMD treated with oral alendronate. The mean change in areal TB BMD Z score after 2 years of treatment was estimated at 0.04 (99% CI 0.62–0.81), corresponding to an annual increase of 0.02. By contrast, Ronsley et al.20 estimated the mean change in areal TB BMD Z score at −0.62 (p = 0.028) across an average of 4.2 years in a retrospective cohort study of 68 glucocorticoid-treated Canadian patients with DMD receiving IV pamidronate or zoledronate. A similar estimate (−0.95) was reported by Tian et al.23 after an average of 3 years of treatment with oral alendronate among 54 US patients.
Houston et al.13 investigated changes in areal total hip (TH) BMD Z score in 19 US patients with DMD treated with oral alendronate. The mean change in areal TH BMD Z score after an average of 3.4 years of bisphosphonate therapy was estimated at 0.21 (p = 0.38).
Finally, in a retrospective cohort study, Tian et al.23 investigated changes in areal lateral distal femur BMD in 54 US patients with DMD treated with oral alendronate. The mean change in areal lateral distal femur BMD Z score after an average of 3.0 years was −0.30 (R1), −1.41 (R2), and −1.42 (R3), corresponding to annual changes of −0.10, −0.47, and −0.47, respectively.
Serum Bone Turnover Markers and Bone Formation Rates on Transiliac Bone Biopsies
None of the identified studies were specifically designed and sufficiently powered to detect an effect of bisphosphonates on serum bone turnover markers or bone formation rates on transiliac bone biopsies in glucocorticoid-treated patients with DMD. However, 2 RCTs24,25 and 2 uncontrolled observational studies16,21 recorded these outcomes during the bisphosphonate treatment period. Specifically, in the RCT by Ward et al.,24 serum cross-linked N-terminal telopeptide of type I collagen (NTx), bone-specific alkaline phosphatase, and procollagen type I aminoterminal propeptide (PINP) declined significantly on IV zoledronic acid compared with that on IV placebo. In the RCT by Zacharin et al.,25 there were no difference in serum alkaline phosphatase level at baseline and 24 months between patients on zoledronic acid and those on nutritional support with calcium supplements and vitamin D alone. Misof et al.16 reported significant decrease in static and dynamic indices of bone formation on transiliac bone biopsy with IV bisphosphonate treatment. Bone resorption–related parameters (osteoclast surface per bone surface) did not change with IV bisphosphonate therapy, whereas eroded surface increased. Misof et al.16 found no correlation between histomorphometric bone turnover indices and serum c-telopeptide and alkaline phosphatase levels after IV bisphosphonates. Finally, Sbrocchi et al.21 did not observe significant trends in longitudinal changes of serum bone-specific alkaline phosphatase and c-telopeptide Z scores after IV bisphosphonates, whereas transiliac histomorphometry results showed decline in bone turnover indices after 2 years of IV bisphosphonates.
Nonmusculoskeletal Outcomes
Gordon et al.11 investigated life expectancy in 44 US patients with DMD treated with prednisone and/or deflazacort, of which 16 patients also received alendronate, pamidronate, and/or clodronate. The median survival of patients treated with bisphosphonates was 27 years, compared with 21 years for those not treated with bisphosphonates (p = 0.005). None of the other studies were sufficiently powered to assess nonmusculoskeletal outcomes.
Harms of Bisphosphonates in Glucocorticoid-Treated Patients With DMD
A summary of reported adverse events of bisphosphonates in glucocorticoid-treated patients with DMD is detailed in Table 3. Reported events were largely due to the known “acute phase reaction” associated with the first dose; however, additional less common side effects were also reported, including rhabdomyolysis with myoglobinuria (IV zoledronate),14,15 intracardiac thrombosis (IV zoledronate),10 and memory loss (oral alendronate).12
Table 3.
Adverse Events of Bisphosphonates in Glucocorticoid-Treated Patients With DMD
| Adverse event category | ||||||||
| Author [country] | Interventions | Dizziness/vomiting | Fever | Fatigue | Headache | Gastrointestinala | Muscle/bone pain | Other |
| Case et al. [UK]10 | DFZ/PRED + ZLN | 1 (100%) | 1 (100%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | • Chest pain: 1 (100%) • Tachycardia: 1 (100%) • Tachypnea: 1 (100%) • Hypoglycemia: 1 (100%) • Hypocalcemia: 1 (100%) • Hypophosphatemia: 1 (100%) • Hyponatremia: 1 (100%) • Hypokalemia: 1 (100%) |
| Gordon et al. [CAN]11 | DFZ/PDN + ALN/CLO/PAM | NR | NR | NR | NR | NR | NR | NR |
| Hawker et al. [CAN]12 | DFZ + ALN | 2 (13%) | 0 (0%) | 0 (0%) | 4 (25%) | 8 (50%) | 4 (25%) | • Memory loss: 1 (6%) • Rash: 1 (6%) |
| Houston et al. [USA]13 | DFZ/PDN + ALN | NR | NR | NR | NR | NR | NR | NR |
| Ivanyuk et al. [CH]14 | NR + ZLN | NR | NR | NR | NR | NR | NR | Rhabdomyolysis with myoglobinuriac |
| Joseph et al. [UK]4 | DFZ/PRED + NR | NR | NR | NR | NR | NR | NR | NR |
| Lemon et al. [UK]15 | DFZ + ZLN | NR | NR | NR | NR | NR | NR | Rhabdomyolysis with myoglobinuriac |
| Lim et al. [AUS]27 | DFZ/PRED + ZLN | 0 (0%)d | 0 (0%)d | 0 (0%)d | 0 (0%)d | 0 (0%)d | 0 (0%)d | 0 (0%)d |
| Misof et al. [CAN]16 | DFZ/PDN + PAM/ZLN | NR | NR | NR | NR | NR | NR | NR |
| Moretti et al. [IT]17 | DFZ + NER | NR | NR | NR | NR | NR | NR | NR |
| Nasomyont et al. [USA]18 | DFZ/PDN + ALN | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Palomo Atance et al. [ES]19 | DFZ + ALN | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ronsley et al. [CAN]20 | DFZ/PDN + PAM/ZLN | NR | NR | NR | NR | NR | 22 (69%) | • Chills: 22 (69%) • Muscle cramps/spasms: 19 (59%) • Numbness/tingling: 21 (66%) |
| Sbrocchi et al. [CAN]21 | DFZ + PAM/ZLN | 0 (0%) | 4 (57%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | • Asymptomatic ionized hypocalcemia: 2 (29%) |
| Srinivasan et al. [UK]22 | DFZ/PRED + RIS | Yesc | Yesc | 0 (0%) | 0 (0%) | Yesc | Yesc | 0 (0%) |
| Tian et al. [USA]23 | NR + ALN | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Ward et al. [multinationalb]24 | NR + ZLN | 4 (22%) | 4 (22%) | 1 (6%) | 4 (22%) | 4 (22%) | 6 (33%) | • Hypocalcemia: 2 (11%) • Tachycardia: 3 (17%) • Adrenal insufficiency: 3 (17%) • Nausea: 3 (17%) |
| Zacharin et al. [AUS/NZ]25 | DFZ/PRED + ZLN | NR | NR | NR | NR | NR | NR | • Asymptomatic hypocalcemia: 10 (37%) after 48 h and 4 (16%) after 72 h • Asymptomatic hypophosphatemia: 1 (4%) after 48 h and 3 (13%) after 72 h |
| Zheng et al. [CN]26 | PRED + ALN | 0 (0%) | 0 (0%) | 0 (0%) | 1 (6%) | 5 (28%) | 1 (6%) | 0 (0%) |
| PRED + ZLN | 0 (0%) | 12 (71%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
Abbreviations: ALN = Alendronate; AUS = Australia; CAN = Canada; CH = Switzerland; CLO = Clodronate; CN = China; DFZ = Deflazacort; DMD = Duchenne muscular dystrophy; ES = Spain; IT = Italy; NER = Neridronate; NR = Not reported; NZ = New Zealand; PAM = Pamidronate; PDN = Prednisone; PRED = Prednisolone; RIS = Risedronate; UK = United Kingdom; USA = United States of America; ZLN = Zoledronate.
Data reported as n (proportion %) if not otherwise stated.
Diarrhea, constipation, nausea, heartburn, or abdominal pain.
Canada, Australia, the United Kingdom, South Africa, Russia, and Hungary.
Number of affected patients not reported.
Serious adverse events.
Discussion
The objective of our study was to perform a systematic review and grading of evidence for benefits and harms of bisphosphonates in glucocorticoid-treated patients with DMD. We found a total of 19 articles that met the review inclusion criteria, of which only 2 had high-quality evidence arising from randomized controlled trial designs. The paucity of randomized controlled data in DMD likely reflects a number of factors, including that DMD is a rare disease (with limited numbers of patients available to enroll in clinical trials), that patients may prioritize enrollment in myopathy-targeted trials rather than those which target secondary clinical outcomes, and that bisphosphonates are already used routinely in clinical practice, rendering the conduct of clinical trials with a nonintervention control group challenging to obsolete in contemporary times. This is, combined with the fact that regulated clinical trials are logistically demanding, which may damper time and/or enthusiasm for their conduct. Nevertheless, the 2 RCTs24,25 that have been published assessing the impact of bisphosphonate therapy on skeletal health in glucocorticoid-treated DMD, combined with the data arising from uncontrolled observational studies, provide evidence for an overall increase in mean annualized areal LS BMD Z scores (with trial durations of 12 and 24 months, respectively). Of importance, the greatest increases in mean annualized areal LS BMD Z scores in the controlled settings were observed on IV bisphosphonate therapy, an observation that is not surprising given the greater oral bioavailability of IV compared with oral bisphosphonate agents in children with osteogenesis imperfecta and glucocorticoid-treated conditions.28,29
None of the controlled studies in this systematic review were sufficiently powered to assess differences in clinical outcomes beyond the primary outcome, namely changes in areal LS BMD Z scores.13,20,22,24,25 It is interesting to note, however, that descriptively, there were fewer low-trauma vertebral fractures in patients treated with IV zoledronate compared with those treated with placebo in the RCT by Ward et al.24 (mean incidence rate per person-year: 0.00 vs 0.13 [38% of whom had glucocorticoid-treated DMD]). This differs from the findings from Zacharin et al.,25 in which the incidence rate was identical between trial arms. Nevertheless, even IV zoledronic acid, the most potent IV bisphosphonate currently available, was not sufficient to completely prevent incident vertebral fractures in the glucocorticoid-treated DMD setting, an observation that speaks to the aggressivity of the underlying osteoporosis. Currently, the 2018 Centers for Disease Control Clinical Care Considerations for osteoporosis management in DMD is to initiate (preferably IV) bisphosphonate therapy at early signs of bone fragility (i.e., a single low-trauma vertebral or long bone fracture)2; whether even earlier initiation of bone protection therapy will be even more effective in preventing incident vertebral fractures remains unknown, an important clinical question going forward. The spirit behind this question is whether prevention of even subtle vertebral collapse can reign in the vertebral fracture cascade, a phenomenon whereby vertebral fractures at a given time point are linked to further vertebral collapse in the future among those with persistent risk factors. On the contrary, long bone fractures, while infrequent, were similar between groups in these 2 high-quality controlled studies.24,25 This was also expected, given that long bones are less amenable to the BMD-modifying effect of antiresorptive therapy due to more limited surface area of compact bone (in contrast to the greater surface area of porous trabecular bone that is characteristic of vertebral bodies).
Vertebral body reshaping following vertebral fractures is a key clinical outcome in pediatric osteoporosis studies, one that is sometimes overlooked because the phenomenon is not part of the standard adult osteoporosis battery of drug trial outcomes. The reason for this is that adults lack the ability (postepiphyseal fusion) to undergo growth-mediated restoration of normal vertebral dimensions following fractures. Vertebral body reshaping following vertebral fractures is indeed a growth-mediated phenomenon, one that arises from the synergistic effect of antiresorptive therapy and endochondral ossification on bone modeling (the process by which bones get taller [in the case of vertebral bodies] and longer [in the case of long bones]). In this systematic review, we found that 3 studies reported partial or complete vertebral body reshaping on bisphosphonate therapy, whereas the phenomenon was not observed (or not reported) in the absence of bisphosphonate treatment in any of the studies.18,21,23 Given the aggressivity of the osteoporosis in glucocorticoid-treated DMD, combined with the virtually universal growth failure in this glucocorticoid-treated setting, lack of vertebral body reshaping in the absence of bisphosphonate therapy is expected. This is in contrast to the prototypical osteoporotic condition of childhood that is characterized by significant potential for vertebral body reshaping following vertebral fractures—pediatric leukemia.30 The frequent vertebral body reshaping in this setting is attributed to the young age diagnosis (and significant residual growth potential), the intermittency of the glucocorticoid prescriptions, and the fact that the disease is fortunately transient for most patients (given the high cure rates).
The fact that degrees of vertebral body reshaping were observed on bisphosphonate therapy in this systematic review is encouraging, given adult studies that have shown improved pulmonary function testing in women without vertebral collapse compared with those with vertebral fractures.31 In a condition such as DMD where cardiorespiratory failure signals end of life, it seems logical to maintain the integrity of the vertebral bodies as far as possible, to maximize lung volumes by preserving vertebral heights (theoretically). Of interest, Gordon et al.11 showed that bisphosphonate use was associated with greater longevity in DMD, an observation that has been postulated, but never proven, to result from preservation of vertebral heights and possibly prevention of the “fat embolism syndrome.” Taken together, preservation of vertebral heights and prevention of incident vertebral (and nonvertebral) fractures are important clinical goals for patients with DMD.
The low bone turnover on trabecular surfaces (measured on transiliac histomorphometry) observed in the studies conducted by Sbrocchi et al.21 and Misof et al.,16 prebisphosphonate and postbisphosphonate therapy provides the most robust evidence that osteoporosis in DMD is a low bone turnover state. Serum bone turnover markers largely reflect growth velocity, rendering their use challenging in clinical settings marked by poor growth such as glucocorticoid-treated DMD. The fact that markers of bone resorption (and formation, which is coupled to resorption on trabecular surfaces) declined in the RCT by Ward et al.24 is in line with the expected mechanism of action of bisphosphonate therapy; this was the only study in our review where bone-specific serum turnover markers were measured, highlighting the typical, profound drop in bone turnover with bisphosphonate therapy.
Given the multiplicity of factors that contribute to bone fragility in DMD and the potential for adverse effects of bisphosphonates to mimic common flu-like symptoms, the importance of controlled study designs cannot be underestimated in this setting. It was not surprising, based on drug potency and clinical experience, that IV bisphosphonates showed consistently greater efficacy in increasing areal LS BMD Z scores compared with the comparator arms in the controlled trials24,25 and compared with oral agents; it was also not surprising that most patients on IV zoledronic acid experienced 1 or more adverse effects of bisphosphonate therapy. However, it was unexpected that 25% of patients on intravenous placebo in the Ward et al.24 RCT had adverse events that occurred within the first 10 days of the first infusion. This study included not only patients with DMD but also children with inflammatory disorders, a fact that may have predisposed the patients to systemic side effects on IV placebo. Nevertheless, the unexpected frequency of adverse effects on intravenous zoledronic acid serves as a reminder to clinicians that not all symptoms reported in the first 10 days following the first infusion are necessarily bisphosphonate related and that symptoms arising from the underlying disease should also be considered in the face of first-infusion reactions. Of importance, bisphosphonates have been known in DMD to precipitate adrenal insufficiency, an observation that was documented in this systematic review.24 As such, the clinical significance of glucocorticoid stress dosing around the time of first (and potentially subsequent) bisphosphonate infusions cannot be underscored enough, given the potential for adrenal insufficiency to be life-threatening. Patients presenting with cardiac compromise after bisphosphonate infusion should also receive prompt treatment for adrenal insufficiency. Although rare, intracardiac thrombosis has also been reported and could potentially be precipitated by adrenal insufficiency, along with inflammation from the acute phase reaction and electrolyte disturbances. As such, precautions to provide adequate glucocorticoid stress dosing and to maintain hydration plus normal ions of bone and mineral metabolism are important. Unquestionably, a comprehensive cardiac workup should be undertaken in patients presenting with cardiac compromise postbisphosphonate. Rhabdomyolysis is another rare but, nonetheless, serious consequence of intravenous bisphosphonate therapy. Although never proven unequivocally, it is logical to encourage adequate hydration pre-, during- and post-intravenous bisphosphonate infusions in efforts to mitigate the potential for this rare complication.
While the 2018 Clinical Care Considerations2 recommended intravenous bisphosphonate therapy due to more extensive evidence for treatment efficacy compared with oral bisphosphonates (both within DMD and across osteoporosis disease categories such as osteogenesis imperfecta), it is recognized that significant adverse events of intravenous bisphosphonates may trigger some clinicians to recommend oral bisphosphonate therapy to achieve more attenuated side effects (which our data suggest will come at a cost to treatment benefits). Ultimately, the decision to treat with an oral or intravenous bisphosphonate should be made collaboratively with the patient and family, in view of the existing benefits and harms evidence highlighted in this systematic review. Furthermore, the administration of bisphosphonate therapy should be undertaken by clinicians with expertise in their prescription, including supportive care management of potential adverse events postinfusion.
A strength of our systematic review was the unrestricted search strategy, designed to identify any record with data concerning bisphosphonates in glucocorticoid-treated DMD and our in-depth assessment of study quality. A limitation to our work concerns the fact that we did not include gray literature or conference abstracts. As a result, some evidence of benefits and/or harms of bisphosphonates in glucocorticoid-treated patients with DMD might not have been fully synthesized (although the quality of such formally unpublished data would be expected to be low). In addition, for evidence from observational studies, it is important to interpret conclusions regarding causality with some caution.
In conclusion, we show that there is high-quality evidence supporting the use of bisphosphonates to increase the areal lumbar spine BMD Z score in patients with DMD and glucocorticoid-induced osteoporosis, with the greatest improvements recorded in controlled settings among patients treated with intravenous zoledronate. Yet, evidence of benefits to fracture risks was inconclusive and/or of low quality, primarily due to lack of controlled data and small samples. We also found that bisphosphonates were generally well-tolerated, although adverse events related to the first infusion (i.e., “acute phase reaction”) were frequently reported. Our synthesis and grading affirm current recommendations put forward in the 2018 DMD Clinical Care Considerations2 and should be helpful in raising awareness about anticipated benefits of bisphosphonates, prevailing unmet needs, and potential safety issues in their use.
Acknowledgment
Professor Ward has been supported by a Tier 1 Research Chair in Pediatric Bone Disorders from the University of Ottawa and the Children's Hospital of Eastern Ontario Research Institute. Professor Ward has also received educational grants from Parent Project Muscular Dystrophy and Defeat Duchenne Canada.
Glossary
- BMD
bone mineral density
- DMD
Duchenne muscular dystrophy
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluations
- LS
lumbar spine
- TB
total body
Appendix. Authors
| Name | Location | Contribution |
| Erik Landfeldt, PhD | IQVIA, Stockholm, Sweden | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Kim Phuong, MD | Children's Hospital of Eastern Ontario, Ottawa, Canada | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Farasat Zaman, PhD | Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Eva Åström, MD, PhD | Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Sophia Abner, MPH | IQVIA, London, United Kingdom | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Hanns Lochmüller, MD | Children's Hospital of Eastern Ontario Research Institute; Division of Neurology, Department of Medicine, the Ottawa Hospital, Canada | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Thomas Sejersen, MD, PhD | Department of Women's and Children's Health, Karolinska Institutet, Stockholm, Sweden | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
| Leanne M. Ward, MD | Division of Endocrinology, Department of Pediatrics, University of Ottawa, Children's Hospital of Eastern Ontario, Canada | Drafting/revision of the article for content, including medical writing for content; study concept or design; and analysis or interpretation of data |
Study Funding
The authors report no targeted funding.
Disclosure
E. Landfeldt, K. Phung, F. Zaman, E. Åström, S. Abner, H. Lochmüller, and T. Sejersen report no conflicts of interest. Unrelated to this study, L.M. Ward has been a consultant to and participated in clinical trials with Amgen, Santhera, Catabasis, Edgewise, and ReveraGen (with funds to Professor Ward's institution). Go to Neurology.org/N for full disclosures.
References
- 1.Emery AE. The muscular dystrophies. Lancet. 2002;359(9307):687-695. doi: 10.1016/S0140-6736(02)07815-7 [DOI] [PubMed] [Google Scholar]
- 2.Birnkrant DJ, Bushby K, Bann CM, et al. . Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347-361. doi: 10.1016/S1474-4422(18)30025-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King WM, Ruttencutter R, Nagaraja HN, et al. . Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68(19):1607-1613. doi: 10.1212/01.wnl.0000260974.41514.83 [DOI] [PubMed] [Google Scholar]
- 4.Joseph S, Wang C, Bushby K, et al. . Fractures and linear growth in a nationwide cohort of boys with Duchenne muscular dystrophy with and without glucocorticoid treatment: results from the UK NorthStar database. JAMA Neurol. 2019;76(6):701-709. doi: 10.1001/jamaneurol.2019.0242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh A, Schaeffer EK, Reilly CW. Vertebral fractures in Duchenne muscular dystrophy patients managed with deflazacort. J Pediatr Orthop. 2018;38(6):320-324. doi: 10.1097/BPO.0000000000000817 [DOI] [PubMed] [Google Scholar]
- 6.Bothwell JE, Gordon KE, Dooley JM, MacSween J, Cummings EA, Salisbury S. Vertebral fractures in boys with Duchenne muscular dystrophy. Clin Pediatr (Phila). 2003;42(4):353-356. doi: 10.1177/000992280304200408 [DOI] [PubMed] [Google Scholar]
- 7.Ma J, McMillan HJ, Karagüzel G, et al. . The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporos Int. 2017;28(2):597-608. doi: 10.1007/s00198-016-3774-5 [DOI] [PubMed] [Google Scholar]
- 8.Page MJ, McKenzie JE, Bossuyt PM, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380-382. doi: 10.1016/j.jclinepi.2010.09.011 [DOI] [PubMed] [Google Scholar]
- 10.Case SJ, Moon RJ, Bharucha T, Davies JH. Intracardiac thrombosis following intravenous zoledronate treatment in a child with steroid-induced osteoporosis. J Pediatr Endocrinol Metab. 2023;36(3):327-330. doi: 10.1515/jpem-2022-0475 [DOI] [PubMed] [Google Scholar]
- 11.Gordon KE, Dooley JM, Sheppard KM, MacSween J, Esser MJ. Impact of bisphosphonates on survival for patients with Duchenne muscular dystrophy. Pediatrics. 2011;127(2):e353-e358. doi: 10.1542/peds.2010-1666 [DOI] [PubMed] [Google Scholar]
- 12.Hawker GA, Ridout R, Harris VA, Chase CC, Fielding LJ, Biggar WD. Alendronate in the treatment of low bone mass in steroid-treated boys with Duchenne's muscular dystrophy. Arch Phys Med Rehabil. 2005;86(2):284-288. doi: 10.1016/j.apmr.2004.04.021 [DOI] [PubMed] [Google Scholar]
- 13.Houston C, Mathews K, Shibli-Rahhal A. Bone density and alendronate effects in Duchenne muscular dystrophy patients. Muscle Nerve 2014;49(4):506-511. doi: 10.1002/mus.23948 [DOI] [PubMed] [Google Scholar]
- 14.Ivanyuk A, García Segarra N, Buclin T, et al. . Myoglobinuria in two patients with Duchenne muscular dystrophy after treatment with zoledronate: a case-report and call for caution. Neuromuscul Disord. 2018;28(10):865-867. doi: 10.1016/j.nmd.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 15.Lemon J, Turner L, Dharmaraj P, Spinty S. Rhabdomyolysis and myoglobinuria following bisphosphonate infusion in patients with Duchenne muscular dystrophy. Neuromuscul Disord. 2019;29(7):567-568. doi: 10.1016/j.nmd.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 16.Misof BM, Roschger P, McMillan HJ, et al. . Histomorphometry and bone matrix mineralization before and after bisphosphonate treatment in boys with duchenne muscular dystrophy: a paired transiliac biopsy study. J Bone Miner Res. 2016;31(5):1060-1069. doi: 10.1002/jbmr.2756 [DOI] [PubMed] [Google Scholar]
- 17.Moretti A, Liguori S, Paoletta M, Gimigliano F, Iolascon G. Effectiveness of neridronate in the management of bone loss in patients with duchenne muscular dystrophy: results from a pilot study. Adv Ther. 2022;39(7):3308-3315. doi: 10.1007/s12325-022-02179-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasomyont N, Tian C, Hornung L, et al. . The effect of oral bisphosphonate therapy on vertebral morphometry and fractures in patients with Duchenne muscular dystrophy and glucocorticoid-induced osteoporosis. Muscle Nerve. 2021;64(6):710-716. doi: 10.1002/mus.27416 [DOI] [PubMed] [Google Scholar]
- 19.Palomo Atance E, Ballester Herrera MJ, Márquez de La Plata M, Medina Cano E, Carmona Vilchez RM. Alendronate treatment of osteoporosis secondary to Duchenne muscular dystrophy. Anales de Pediatría. 2011;74(2):122-125. doi: 10.1016/j.anpedi.2010.10.002 [DOI] [PubMed] [Google Scholar]
- 20.Ronsley R, Islam N, Kang M, et al. . Effects of bisphosphonate therapy on bone mineral density in boys with Duchenne muscular dystrophy. Clin Med Insights Endocrinol Diabetes. 2020;13:1179551420972400. doi: 10.1177/1179551420972400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sbrocchi AM, Rauch F, Jacob P, et al. . The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int. 2012;23(11):2703-2711. doi: 10.1007/s00198-012-1911-3 [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan R, Rawlings D, Wood CL, et al. . Prophylactic oral bisphosphonate therapy in Duchenne muscular dystrophy. Muscle Nerve. 2016;54(1):79-85. doi: 10.1002/mus.24991 [DOI] [PubMed] [Google Scholar]
- 23.Tian C, Wong BL, Hornung L, et al. . Oral bisphosphonate treatment in patients with Duchenne muscular dystrophy on long term glucocorticoid therapy. Neuromuscul Disord. 2020;30(7):599-610. doi: 10.1016/j.nmd.2020.06.005 [DOI] [PubMed] [Google Scholar]
- 24.Ward LM, Choudhury A, Alos N, et al. . Zoledronic acid vs placebo in pediatric glucocorticoid-induced osteoporosis: a randomized, double-blind, phase 3 trial. J Clin Endocrinol Metab. 2021;106(12):E5222-E5235. doi: 10.1210/clinem/dgab458 [DOI] [PubMed] [Google Scholar]
- 25.Zacharin M, Lim A, Gryllakis J, et al. . Randomized controlled trial evaluating the use of zoledronic acid in Duchenne muscular dystrophy. J Clin Endocrinol Metab. 2021;106(8):2328-2342. doi: 10.1210/clinem/dgab302 [DOI] [PubMed] [Google Scholar]
- 26.Zheng WB, Dai Y, Hu J, et al. . Effects of bisphosphonates on osteoporosis induced by Duchenne muscular dystrophy: a prospective study. Endocr Pract. 2020;26(12):1477-1485. doi: 10.4158/EP-2020-0073 [DOI] [PubMed] [Google Scholar]
- 27.Lim A, Zacharin M, Pitkin J, de Valle K, Ryan MM, Simm PJ. Therapeutic options to improve bone health outcomes in Duchenne muscular dystrophy: zoledronic acid and pubertal induction. J Paediatr Child Health. 2017;53(12):1247-1248. doi: 10.1111/jpc.13692 [DOI] [PubMed] [Google Scholar]
- 28.Nakhla M, Denker AE, Connor JD, et al. . Bioavailability and short-term tolerability of alendronate in glucocorticoid-treated children. Clin Ther. 2011;33(10):1516-1523. doi: 10.1016/j.clinthera.2011.09.001 [DOI] [PubMed] [Google Scholar]
- 29.Ward LM, Denker AE, Porras A, et al. . Single-dose pharmacokinetics and tolerability of alendronate 35- and 70-milligram tablets in children and adolescents with osteogenesis imperfecta type I. J Clin Endocrinol Metab. 2005;90(7):4051-4056. doi: 10.1210/jc.2004-2054 [DOI] [PubMed] [Google Scholar]
- 30.Ward LM, Ma J, Lang B, et al. . Bone morbidity and recovery in children with acute lymphoblastic leukemia: results of a six-year prospective cohort study. J Bone Miner Res. 2018;33(8):1435-1443. doi: 10.1002/jbmr.3447 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe R, Shiraki M, Saito M, Okazaki R, Inoue D. Restrictive pulmonary dysfunction is associated with vertebral fractures and bone loss in elderly postmenopausal women. Osteoporos Int. 2018;29(3):625-633. doi: 10.1007/s00198-017-4337-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data analyzed as part of this literature review are included in the article or its supplemental material (online).