Abstract
Background and Objectives
To evaluate in a phase 2 study the safety and efficacy of IV nipocalimab, a fully human, antineonatal Fc receptor monoclonal antibody, in patients with generalized myasthenia gravis (gMG).
Methods
Patients with gMG with inadequate response to stable standard-of-care (SOC) therapy were randomized 1:1:1:1:1 to receive either IV placebo every 2 weeks (Q2W) or one of 4 IV nipocalimab treatments: 5 mg/kg once every 4 weeks (Q4W), 30 mg/kg Q4W, 60 mg/kg Q2W each for 8 weeks, or a 60 mg/kg single dose, in addition to their background SOC therapy. Infusions (placebo or nipocalimab) were Q2W in all groups to maintain blinding. The primary safety endpoint was incidence of treatment-emergent adverse events (TEAEs), including serious adverse events and adverse events of special interest. The primary efficacy endpoint was change from baseline to day 57 in Myasthenia Gravis-Activities of Daily Living (MG-ADL) total scores. Dose response of change at day 57 was analyzed with a linear trend test over the placebo, nipocalimab 5 mg/kg Q4W, nipocalimab 30 mg/kg Q4W, and nipocalimab 60 mg/kg Q2W groups.
Results
Sixty-eight patients (nipocalimab: n = 54; placebo, n = 14) were randomized; 64 patients (94.1%) were positive for antiacetylcholine receptor autoantibodies, and 4 patients (6%) were positive for antimuscle-specific tyrosine kinase autoantibodies. Fifty-seven patients (83.8%) completed treatment through day 57. The combined nipocalimab group compared with the placebo group demonstrated similar incidences of TEAEs (83.3% vs 78.6%, respectively) and infections (33.3% vs 21.4%, respectively). No deaths or discontinuations due to TEAEs and no TEAEs of special interest (grade ≥3 infection or hypoalbuminemia) were observed with nipocalimab treatment. A statistically significant dose response was observed for change from baseline in MG-ADL at day 57 (p = 0.031, test of linear trend).
Discussion
Nipocalimab was generally safe, well-tolerated, and showed evidence of dose-dependent reduction in MG-ADL scores at day 57 in this phase 2 study. These results support further evaluation of nipocalimab for the treatment of gMG.
Trial Registration Information
Clinical Trials Registration: NCT03772587; first submitted December 10, 2018; EudraCT Number: 2018-002247-28; first submitted November 30, 2018; date of first patient dosed April 10, 2019.
Classification of Evidence
This study provides Class I evidence that for patients with gMG, nipocalimab was well-tolerated, and it did not significantly improve MG-ADL at any individual dose but demonstrated a significant dose response for improved MG-ADL across doses.
Introduction
Myasthenia gravis (MG) is an immunoglobulin G (IgG) autoantibody-mediated disease, characterized by muscle weakness due to pathogenic IgG-mediated disruption of cholinergic transmission at the neuromuscular junction.1 Available therapies for generalized MG (gMG) include cholinesterase inhibitors that ameliorate the neuromuscular transmission defect and/or immunomodulators and immunosuppressants. However, symptom persistence, exacerbations, side effects that limit tolerability, impaired quality of life, and disease morbidity limit long-term use of current therapies and create an unmet need for safer options with increased efficacy.2-4
Neonatal fragment crystallizable (Fc) receptor (FcRn) interacts with the Fc portion of IgG and extends IgG half-life by preventing its lysosomal degradation; inhibition of FcRn is an emerging treatment strategy for IgG autoantibody-mediated diseases such as MG.5-7 Recent approvals of anti-FcRn agents in gMG, including efgartigimod8 and rozanolixizumab,9 support this treatment strategy in gMG. Nipocalimab (M281) is a fully human monoclonal antibody that binds to FcRn with high affinity and blocks binding of endogenous IgG to FcRn throughout the recycling pathway.2,5 Nipocalimab is engineered to remove immune effector functions including Fc gamma receptor and complement binding due to an aglycosylated Fc. In the first-in-human phase 1 study, nipocalimab demonstrated rapid, dose-dependent reduction of IgG up to 85% following single and weekly dosing, with mild or moderate adverse events, no serious adverse events or deaths, and low incidence of infection-related adverse events that were comparable with placebo treatment.5
This phase 2 study was designed to assess the safety and efficacy of nipocalimab and evaluate its pharmacokinetics (PK) and pharmacodynamics (PD) across a range of nipocalimab doses in patients with gMG who had an inadequate response to ongoing stable standard-of-care (SOC) therapy.
Methods
Study Design and Patients
This was a multicenter, randomized, double-blind, placebo-controlled, phase 2 study, comprising a 4-week screening period followed by an 8-week double-blind treatment period and an 8-week posttreatment follow-up. Patients were enrolled at 38 sites in 8 countries (United States, Canada, Germany, Italy, Poland, Spain, Belgium, and United Kingdom). Eligible patients were adults (aged 18 years or older) with a history and clinical symptoms of gMG. Diagnosis of gMG was confirmed by a positive serologic test for gMG-related autoantibody (antiacetylcholine receptor [AChR] or antimuscle-specific tyrosine kinase [MuSK] autoantibodies). Patients were required to have insufficient control of symptoms (defined as a quantitative myasthenia gravis [QMG] score of ≥12 and Myasthenia Gravis-specific Activities of Daily Living [MG-ADL] score of ≥4) despite ongoing stable MG SOC therapy, and Myasthenia Gravis Foundation of America (MGFA) Clinical Classification Class II, III, or IVa. Women of childbearing potential were required to have a negative serum pregnancy test at screening, to have a negative urine pregnancy test at baseline, to remain abstinent, or to use an effective method of contraception during the study to 30 days after last study treatment. Exclusion criteria included patients receiving a systemic biologic antibody for any concurrent disease, rituximab or eculizumab use within 12 months before screening, plasmapheresis, immunoadsorption therapy, or intravenous immunoglobulin (IVIG) within 6 weeks before randomization, clinically significant acute or chronic infection, an unresected thymoma or history of malignant thymoma, thymectomy within 12 months before screening, and if serum IgG (unless attributed to immunomodulators), albumin or calcium levels were outside the normal range.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by an institutional review board/ethics committee at each participating center. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines, as defined by the International Conference on Harmonization. All patients provided written informed consent.
Procedures
Eligible patients were randomized 1:1:1:1:1 to one of the following 5 groups: placebo (5% dextrose in water) once every 2 weeks (Q2W); 5 mg/kg nipocalimab once every 4 weeks (Q4W); 30 mg/kg nipocalimab Q4W; 60 mg/kg nipocalimab single dose; and 60 mg/kg nipocalimab Q2W (eFigure 1, links.lww.com/WNL/D272). The 60 mg/kg nipocalimab single-dose arm was chosen to measure the durability of drug effect and magnitude of IgG reduction. Patients received infusions (placebo or nipocalimab) Q2W in all groups to maintain the blind, for a total of 5 infusions over the 8-week treatment period. Infusions were administered at the study facility on designated infusion days (days 1, 15, 29, 43, and 57). A permuted block randomization was used, stratified first by autoantibody type (anti-AChR vs anti-MuSK) and then by baseline MG-ADL score (≤10, >10) for anti-AChR–positive patients. Infusions were prepared by an unblinded site pharmacist according to an electronic password-protected randomization schedule. Patients, investigators, sponsor, and all other site personnel were blinded to treatment assignments for the study duration. Nipocalimab was supplied as a sterile solution for IV infusion in 20-mL glass vials containing 30 mg/mL (600 mg) of nipocalimab protein in solution.
Patients were required to continue stable dosing of allowed SOC therapy including acetylcholinesterase inhibitors, glucocorticoids, or nonsteroidal immunosuppressants throughout the study duration unless safety issues mandated a change. Stable doses and regimens of herbal, naturopathic, traditional remedies and nutritional supplements were allowed if their use was acceptable to the investigator. Acetylcholinesterase inhibitors were to be withheld for at least 10 hours at each study visit before conducting the efficacy assessments of MG.
Blood and serum samples were collected immediately before starting infusion of the study drug/placebo on study visits, and postinfusion on days 1 and 57, and analyzed for nipocalimab concentrations using an ELISA method. Serum total IgG levels were assessed by Roche Cobas 8000. Serum IgG subclasses (IgG1, IgG2, IgG3, and IgG4) and IgA, IgM, and IgE levels were measured by a validated immunonephelometry platform (Siemens) on the Behring Nephelometer II. Serum levels of anti-AChR and anti-MuSK autoantibodies were analyzed by ARUP Laboratories, UT, and The Doctors Laboratory, London, UK, respectively. Antidrug antibodies (ADA) and neutralizing antibodies (NAb) to nipocalimab were assessed using an electrochemiluminescence immunoassay method by Charles River Laboratories, Inc., USA, and a cell-based Fluorescence Activated Cell Sorting method based on detection by Flow Cytometry by PRA Health Sciences in Assen, Netherlands, respectively.
Endpoints
The main results were the safety and primary efficacy endpoints; secondary and exploratory endpoints were used for hypothesis generation. All efficacy measures are defined in eTable 1 (links.lww.com/WNL/D273). The primary efficacy endpoint was change from baseline to day 57 in the total MG-ADL score. Safety was evaluated based on treatment-emergent adverse events (TEAEs), with severity determined by study investigators using the National Cancer Institute's Common Terminology Criteria for Adverse Events version 5.0 criteria. Grade 3 or higher TEAEs of infection (severe or medically significant but not immediately life-threatening; IV antibiotic, antifungal, or antiviral intervention indicated; invasive intervention indicated) and grade 3 or higher hypoalbuminemia (albumin <2 g/dL) were considered adverse events of special interest (AESI). Other assessments included clinical laboratory tests, vital signs, physical examination, electrocardiograms, and Columbia-Suicide Severity Rating Scale (C-SSRS).
Secondary endpoints included the correlation between change in total MG-ADL score and total serum IgG reduction from baseline. Other secondary efficacy endpoints included change from baseline to day 57 in QMG, Revised Myasthenia Gravis Quality of Life −15 (MG-QoL 15) scores and shift in MGFA classification based on posttreatment status from baseline to day 57. Exploratory endpoints included percentage of patients with durable response (≥4 consecutive weeks with clinically meaningful improvement of ≥2 on MG-ADL),10 the PD activity of nipocalimab as measured by changes in concentrations of total IgG, IgG subclasses, IgA, IgM, and IgE, and autoantibodies (anti-AChR and anti-MuSK), and the incidence of ADA and NAb to nipocalimab.
Statistical Analysis
This study was designed to have ≥80% power with a 1-sided type 1 error of 5% to detect a dose response in change from baseline in MG-ADL total score at day 57 using a linear trend test over the placebo, nipocalimab 5 mg/kg Q4W, nipocalimab 30 mg/kg Q4W, and nipocalimab 60 mg/kg Q2W dose groups. Change from baseline in MG-ADL total score at day 57 was assumed to be −2 and −6 in the placebo and nipocalimab 60 mg/kg Q2W groups with a common standard deviation of 3. Based on these considerations, a sample size of 60 was planned with 12 patients in each of the 5 arms, assuming 10 evaluable patients (allowing 15% attrition) in each arm. Dose-response analyses included 1-sided tests of linear trend and rank-based association. Change from baseline in MG-ADL total score was also analyzed with a mixed-effects model for repeated measures (MMRM) using data from days 15, 29, 43, and 57. The MMRM included variables for baseline MG-ADL score, treatment, study week, treatment-by-study week interaction, and autoantibody type, with variance-covariance structure assumed as compound symmetry. MMRM analyses were conducted on observed data; no imputation was performed for missing observations. Similar analyses were performed for secondary endpoints. Durable response was defined as ≥4 consecutive weeks with ≥2 points improvement in MG-ADL total score. Comparisons of the proportion of patients with durable response were analyzed with the Fisher exact test. A post hoc analysis of durable response with onset by day 17 was also performed. The distribution of categorical shifts in MGFA classification from baseline to day 57 was analyzed with a Cochran-Mantel-Haenzel test. The efficacy analysis was performed using the intent-to-treat population, which included all randomized patients. Safety was summarized descriptively for the safety population, which included all patients who received any dose of nipocalimab or placebo. Serum nipocalimab concentrations were summarized by treatment groups and nominal time points using descriptive statistics. The PK population included all randomized patients in the safety population with ≥1 evaluable serum concentration of nipocalimab. The study protocol (eSAP 1, links.lww.com/WNL/D270) and statistical analysis plan (eSAP 2, links.lww.com/WNL/D271) are available for reference.
Data Availability
The clinical trial transparency policy of Janssen Pharmaceutical Companies of Johnson & Johnson is publicly available.11 Requests for access to study data can be submitted through the Yale Open Data Access (YODA) Project.12
Results
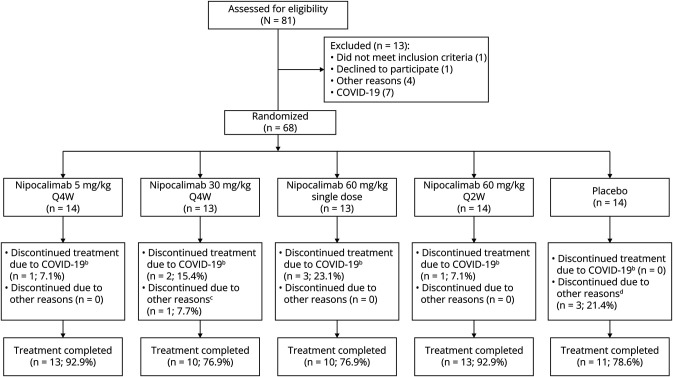
The study was initiated on December 18, 2018, the first patient was dosed on April 10, 2019, enrollment ended by early 2020, and the last patient's last visit occurred on June 25, 2020. A total of 68 patients (nipocalimab: n = 54; placebo: n = 14) were randomized, and 57 patients (83.8%; nipocalimab: n = 46, placebo: n = 11) completed the study treatment through day 57. However, study drug administration was suspended on April 17, 2020, because of the coronavirus disease 2019 (COVID-19) pandemic, and while data collection continued either remotely or in-person, 7 nipocalimab-treated patients discontinued early from the study and 14 patients missed or delayed their day 29, 43, 57, 85, and/or 113 visits. 14 patients had remote MG-ADL assessments; QMG assessments were missed during these visits because QMG cannot be performed remotely. The reasons for treatment discontinuation in the combined nipocalimab group were study disruption due to the COVID-19 pandemic (n = 7) and violation of exclusion criteria (n = 1). The reasons for discontinuation in the placebo group were adverse events (AEs) (n = 2) and withdrawal of consent (n = 1) (Figure 1). The study was completed on June 25, 2020 (date of last follow-up).
Figure 1. CONSORT Diagram: Patient Disposition.
bTreatment discontinuation was due to site not being able to conduct studies or patients not being able to travel; cOne ineligible patient discontinued treatment after 1 infusion; dTwo patients discontinued because of exacerbation of MG, and 1 patient withdrew consent. COVID-19 = coronavirus disease 2019; Q2W = every 2 weeks; Q4W = every 4 weeks.
Demographics and baseline characteristics were similar across all treatment groups (Table 1); 64 patients (94.1%) were positive for anti-AChR autoantibodies and 4 patients (6%) were positive for anti-MuSK autoantibodies. At baseline, most of the patients had previously received medications for treatment of gMG (combined nipocalimab group: 74.1%; placebo: 85.7%). All patients were on concomitant medications for treatment of gMG during this study. The most commonly prescribed medications (combined nipocalimab group vs placebo) were acetylcholinesterase inhibitors (90.7% vs 85.7%), glucocorticoids (68.5% vs 78.6%), and nonsteroidal immunosuppressants (40.7% vs 57.1%) (eTable 2, links.lww.com/WNL/D273). Fifty-four patients (5 mg/kg Q4W: 14; 30 mg/kg Q4W: 13; 60 mg/kg single-dose: 13; 60 mg/kg Q2W: 14) were treated with nipocalimab for a mean (SD) duration of 53.8 (11.80) days, while 14 patients received placebo Q2W with a mean (SD) duration of 48.1 (16.88) days.
Table 1.
Patient Demographics and Baseline Characteristics (ITT Population)
| Placebo Q2W (n = 14) |
Nipocalimab | |||||
| 5 mg/kg Q4W (n = 14) | 30 mg/kg Q4W (n = 13) | 60 mg/kg single dose (n = 13) | 60 mg/kg Q2W (n = 14) | Combined (n = 54) | ||
| Age in years, median (range) | 60.5 (25–83) | 53.0 (29–81) | 44.0 (24–74) | 47.0 (24–74) | 63.0 (27–76) | 57.5 (24–83) |
| Sex, n (%) | ||||||
| Women | 8 (57.1) | 6 (42.9) | 9 (69.2) | 9 (69.2) | 5 (35.7) | 29 (53.7) |
| Race | ||||||
| White | 12 (85.7) | 12 (85.7) | 12 (92.3) | 13 (100) | 14 (100) | 51 (94.4) |
| Black or African American | 0 | 2 (14.3) | 0 | 0 | 0 | 2 (3.7) |
| Other | 2 (14.3) | 0 | 0 | 0 | 0 | 0 |
| American Indian or Alaska Native | 0 | 0 | 1 (7.7) | 0 | 0 | 1 (1.9) |
| Ethnicity, n (%) | ||||||
| Not Hispanic or Latino | 10 (71.4) | 13 (92.9) | 10 (76.9) | 11 (84.6) | 14 (100) | 48 (88.9) |
| Hispanic or Latino | 4 (28.6) | 1 (7.1) | 3 (23.1) | 1 (7.7) | 0 | 5 (9.3) |
| Not reported | 0 | 0 | 0 | 1 (7.7) | 0 | 1 (1.9) |
| Time since symptom onset, mean (SD), y | 13.2 (9.81) | 8.0 (8.61) | 8.4 (7.15) | 7.0 (7.90) | 6.0 (5.84) | 7.3 (7.31) |
| Baseline MG-ADL total score, mean (SD) | 7.3 (2.79) | 8.0 (2.75) | 8.0 (2.61) | 7.9 (2.78) | 8.1 (3.25) | 8.0 (2.78) |
| Baseline QMG total score, mean (SD) | 17.6 (4.20) | 15.9 (2.93) | 17.1 (4.23) | 16.1 (4.07) | 16.9 (2.79) | 16.5 (3.48) |
| MGFA class, n (%) | ||||||
| IIa | 2 (14.3) | 2 (14.3) | 2 (15.4) | 3 (23.1) | 2 (14.3) | 9 (16.7) |
| IIb | 3 (21.4) | 4 (28.6) | 1 (7.7) | 2 (15.4) | 4 (28.6) | 11 (20.4) |
| IIIa | 5 (35.7) | 5 (35.7) | 6 (46.2) | 5 (38.5) | 5 (35.7) | 21 (38.9) |
| IIIb | 3 (21.4) | 3 (21.4) | 2 (15.4) | 3 (23.1) | 3 (21.4) | 11 (20.4) |
| IVa | 1 (7.1) | 0 | 2 (15.4) | 0 | 0 | 2 (3.7) |
| Anti-AChR positive | 13 (92.9) | 13 (92.9) | 12 (92.3) | 13 (100) | 13 (92.9) | 51 (94.4) |
| Anti-MuSK positive | 1 (7.1) | 1 (7.1) | 1 (7.7) | 0 | 1 (7.1) | 3 (5.6) |
Abbreviations: AChR = acetylcholine receptor; ITT = intent-to-treat; MG-ADL = Myasthenia Gravis-Activities of Daily Living Total Score; MGFA = Myasthenia Gravis Foundation of America; MuSK = muscle-specific kinase; Q2W = every 2 wk; Q4W = every 4 wk; QMG = Quantitative Myasthenia Gravis.
The overall incidence of TEAEs was similar between the combined nipocalimab group (83.3%) and placebo group (78.6%; Table 2). No relationship was observed in the overall incidence of TEAEs across the 4 nipocalimab dose regimens or for any individually reported preferred term. No TEAEs leading to death were reported during the study. Serious TEAEs were reported in 1 (1.9%) nipocalimab-treated patient (grade 1 musculoskeletal pain, worsening of shoulder pain from prestudy rotator cuff surgery) in the 30 mg/kg Q4W group and in 2 (14.3%) placebo-treated patients (grade 3 ischemic stroke and grade 3 MG worsening); all 3 events were deemed unrelated to study drug by the investigator.
Table 2.
Summary of TEAEs (Safety Population)
| Placebo Q2W (n = 14) |
Nipocalimab | |||||
| 5 mg/kg Q4W (n = 14) | 30 mg/kg Q4W (n = 13) | 60 mg/kg Single dose (n = 13) | 60 mg/kg Q2W (n = 14) | Combined (n = 54) | ||
| Any TEAEs | 11 (78.6) | 12 (85.7) | 9 (69.2) | 12 (92.3) | 12 (85.7) | 45 (83.3) |
| Any TEAEs related to study agent | 1 (7.1) | 5 (35.7) | 3 (23.1) | 6 (46.2) | 7 (50.0) | 21 (38.9) |
| Any TEAE with CTCAE grade ≥3 | 4 (28.6) | 0 | 0 | 0 | 0 | 0 |
| Any TEAE leading to treatment discontinuation | 2 (14.3) | 0 | 0 | 0 | 0 | 0 |
| Any TEAEs leading to death | 0 | 0 | 0 | 0 | 0 | 0 |
| Any serious TEAEs | 2 (14.3) | 0 | 1 (7.7) | 0 | 0 | 1 (1.9) |
| Most common TEAEs (≥5% in combined group) | ||||||
| Diarrhea | 1 (7.1) | 1 (7.1) | 1 (7.7) | 2 (15.4) | 2 (14.3) | 6 (11.1) |
| Headache | 1 (7.1) | 2 (14.3) | 1 (7.7) | 2 (15.4) | 1 (7.1) | 6 (11.1) |
| Nasopharyngitis | 0 | 1 (7.1) | 1 (7.7) | 2 (15.4) | 2 (14.3) | 6 (11.1) |
| Rash | 0 | 1 (7.1) | 0 | 0 | 3 (21.4) | 4 (7.4) |
| Back pain | 0 | 1 (7.1) | 0 | 1 (7.7) | 1 (7.1) | 3 (5.6) |
| Dizziness | 0 | 0 | 1 (7.7) | 2 (15.4) | 0 | 3 (5.6) |
| Hypertension | 0 | 2 (14.3) | 1 (7.7) | 0 | 0 | 3 (5.6) |
| Musculoskeletal pain | 0 | 0 | 1 (7.7) | 2 (15.4) | 0 | 3 (5.6) |
| Edema peripheral | 0 | 0 | 0 | 1 (7.7) | 2 (14.3) | 3 (5.6) |
| TEAEs of infections and infestations | ||||||
| Grade 1 | 2 (14.3) | 2 (14.3) | 2 (15.4) | 3 (23.1) | 3 (21.4) | 10 (18.5) |
| Grade 2 | 1 (7.1) | 3 (21.4) | 1 (7.7) | 2 (15.4) | 2 (14.3) | 8 (14.8) |
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade 4 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: CTCAE = Common Toxicity Criteria for Adverse Events; Q2W = every 2 wk; Q4W = every 4 wk; TEAE = treatment-emergent adverse event.
In the placebo group, 2 (14.3%) discontinued treatment because of TEAEs (nonserious TEAE of grade 3 myasthenia exacerbation and serious TEAE of grade 3 MG worsening; both required rescue treatment). No patient in the nipocalimab groups discontinued treatment because of TEAEs; MG worsening/exacerbation TEAE occurred in 1 patient in the 5 mg/kg Q4W group (7.1%), who did not require rescue treatment and did not discontinue treatment. All TEAEs experienced by patients in the nipocalimab group were mild or moderate (grade 1 or 2). The most frequent TEAEs in the combined nipocalimab group (n = 54) were diarrhea, headache, and nasopharyngitis (11.1% each). No grade 3 AESI of infection was reported. The percentage of infections in the combined nipocalimab group (18/54 or 33.3%) was comparable with those in the placebo group (3/14 or 21.4%) with the most common infection being nasopharyngitis.
Mild dose-dependent decreases from baseline in the mean serum albumin were observed in the nipocalimab groups. At the highest dose group of 60 mg/kg Q2W, the mean albumin reduction at day 57 was −0.83 g/dL (baseline mean 4.34 g/dL, day 57 mean 3.5 g/dL); the albumin levels returned to baseline by the end of the posttreatment follow-up (4.8 g/dL at day 113). Overall, mean serum albumin concentrations stayed within the normal limits of 3.5–5.5 g/dL throughout the treatment and follow-up periods for all doses tested (eFigure 2, links.lww.com/WNL/D272). No grade 3 AESI of hypoalbuminemia was reported.
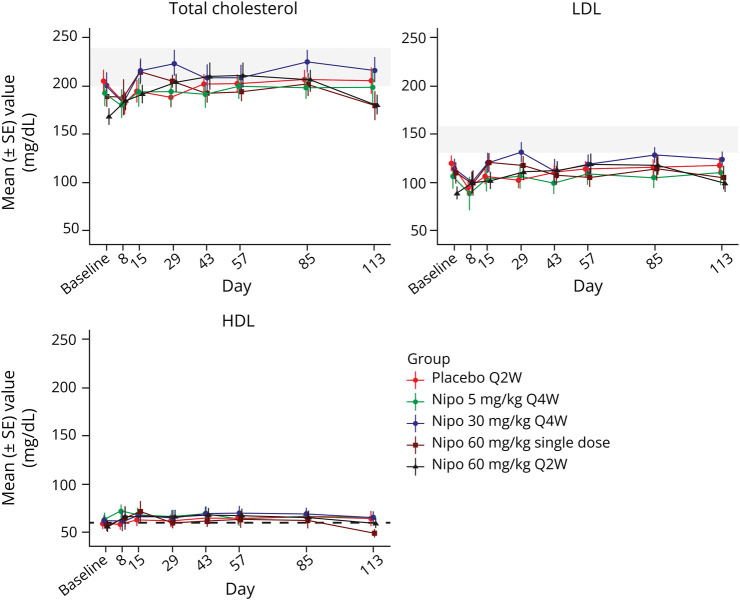
Nonfasting lipid levels were measured in this study (eTable 3, links.lww.com/WNL/D273). Mild dose-dependent and reversible elevations of cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were observed. Baseline and postbaseline (average value over all postbaseline visits from day 8 to day 113 for that patient) values in the highest dose group of 60 mg/kg Q2W for mean total cholesterol levels were 168 (range 98–210) and 198 mg/dL (range 114–268), respectively, and mean LDL levels were 90 (range 37–131) and 108 mg/dL (range 40–170), respectively. Increases in HDL levels were also seen in the highest dose group of 60 mg/kg Q2W; the baseline and postbaseline mean HDL levels were 56 (range 23–83) and 64 mg/dL (range 25–94), respectively. Due to the increase in HDL level, the maximum mean percentage increases in the cholesterol-to-HDL ratio were <5% across all nipocalimab dose groups. The average values for all dosing arms did not exceed the National Cholesterol Education Program's reference safety guidelines (Figure 2).13 No cholesterol change was reported as clinically significant in the study. Similarly, there were no clinically significant changes reported in other clinical laboratory evaluations, nor in vital signs, physical examinations, ECGs, and C-SSRS.
Figure 2. Mean (±SE) Total Cholesterol, LDL, and HDL Over Time (Safety Population).
HDL = high-density lipoprotein; LDL = low-density lipoprotein; Nipo = nipocalimab; Q2W = every 2 weeks; Q4W = every 4 weeks; SE = standard error.
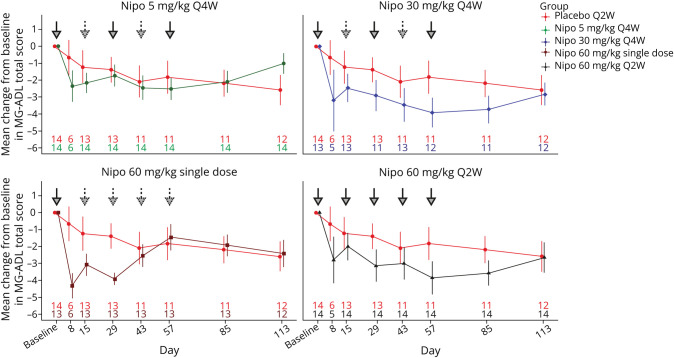
The mean change in MG-ADL total score over time is shown in Figure 3. For the primary efficacy endpoint, a statistically significant linear trend over the placebo, 5 mg/kg Q4W, 30 mg/kg Q4W, and 60 mg/kg Q2W groups, with a positive dose-response trend, was observed for the change from baseline at day 57 in the MG-ADL score (p = 0.03). A similar analysis based on the rank of the change from baseline at day 57 was also statistically significant (p = 0.004). Mean changes (LS mean (SE)) from baseline in MG-ADL total score at day 57, as estimated by the MMRM analysis, were −3.7 (0.9) for both the nipocalimab 30 mg/kg Q4W and 60 mg/kg Q2W groups but were not statistically significant compared with those for the placebo (−2.4 (0.9); p ≥ 0.22), with LS mean differences (95% CIs) with placebo of −1.3 (−3.5, 0.9) for the 30 mg/kg Q4W group and −1.3 (−3.4, 0.8) for the 60 mg/kg Q2W group.
Figure 3. Mean (±SE) Change From Baseline in MG-ADL Total Score Over Time (ITT Population).
ITT = intent-to-treat; Nipo = nipocalimab; MG-ADL = Myasthenia Gravis-Activities of Daily Living; Q2W = every 2 weeks; Q4W = every 4 weeks; SE = standard error. Bold downward arrows denote the doses of nipocalimab administered at different time points. Dotted downward arrows denote placebo administered.
For the secondary endpoints, patients with a greater magnitude of IgG lowering tended to have greater MG-ADL score reductions (eFigure 3, links.lww.com/WNL/D272). Single-dose administration of 60 mg/kg of nipocalimab assessing durability of effect with maximal IgG reduction showed large reductions in MG-ADL total scores through day 29 (mean [SD] change from baseline: −3.9 [1.32]); the magnitude of reduction decreased thereafter (mean [SD] change from baseline at day 57: −1.5 [2.82]). Reductions in QMG (eFigure 4) and MG-QoL-15r scores (eFigure 5) were observed over time in all treatment groups and were not significant compared with those in the placebo group. There were 14 patients with ≥1 missed QMG assessment due to study disruption by the COVID-19 pandemic. On day 57, mean (SD) reductions from baseline in total QMG scores were −4.1 (3.45) in the nipocalimab 30 mg/kg Q4W and −5.9 (5.30) 60 mg/kg Q2W treatment groups, although the differences between these groups and the placebo group (−3.7 [2.94]) at day 57 were not significant (p ≥ 0.23) with LS mean differences with placebo of −0.5 (−3.6 to 2.6) for the 30 mg/kg Q4W group and −1.8 (−4.8 to 1.2) for the 60 mg/kg Q2W group (Table 3). Similarly, on day 57, the mean (SD) reductions from baseline in the mean total MG-QoL-15r scores were −6.8 (5.73) in the nipocalimab 30 mg/kg Q4W group and −3.7 (5.37) in the 60 mg/kg Q2W treatment group, with a statistically significant difference between the 30 mg/kg Q4W and placebo groups at day 57 (LS mean difference (95% CI): −5.1 (−8.6 to −1.5); p = 0.005) (Table 3).
Table 3.
Changes in Efficacy Measures (ITT Population)
| Placebo Q2W (n = 14) |
Nipocalimab | ||||
| 5 mg/kg Q4W (n = 14) | 30 mg/kg Q4W (n = 13) | 60 mg/kg single dose (n = 13) | 60 mg/kg Q2W (n = 14) | ||
| MG-ADL scores | |||||
| Baseline, mean (SD) | 7.3 (2.79) | 8.0 (2.75) | 8.0 (2.61) | 7.9 (2.78) | 8.1 (3.25) |
| Day 57, mean (SD) | 5.2 (3.09) | 5.5 (3.32) | 4.0 (2.63) | 6.5 (3.84) | 4.3 (2.95) |
| Change from baseline, mean (SD) | −1.8 (3.22) | −2.5 (2.41) | −3.9 (3.00) | −1.5 (2.82) | −3.9 (3.66) |
| LS mean values (SE)a | −2.4 (0.9) | −2.4 (0.9) | −3.7 (0.9) | −1.4 (1.0) | −3.7 (0.9) |
| Difference in LS mean values (nipocalimab vs placebo)a | −0.0 (1.1) | −1.3 (1.1) | 1.0 (1.1) | −1.3 (1.1) | |
| 95% CI | −2.1 to 2.1 | −3.5 to 0.9 | −1.2 to 3.1 | −3.4 to 0.8 | |
| p Value | 0.99 | 0.24 | 0.36 | 0.22 | |
| p Value of linear trend test of change from baselineb | 0.03 | ||||
| p Value of linear trend test based on the rank of change from baselinec | 0.004 | ||||
| QMG scores | |||||
| Baseline, mean (SD) | 17.6 (4.20) | 15.9 (2.93) | 17.1 (4.23) | 16.1 (4.07) | 16.9 (2.79) |
| Day 57, mean (SD) | 13.2 (4.92) | 12.2 (4.62) | 13.1 (2.64) | 14.0 (4.56) | 11.3 (4.40) |
| Change from baseline, mean (SD) | −3.7 (2.94) | −3.5 (4.10) | −4.1 (3.45) | −1.5 (2.54) | −5.9 (5.30) |
| LS mean values (SE)a | −3.4 (1.2) | −3.5 (1.1) | −3.9 (1.2) | −1.3 (1.2) | −5.2 (1.1) |
| Difference in LS mean values (nipocalimab vs placebo)a | −0.1 (1.5) | −0.5 (1.6) | 2.1 (1.6) | −1.8 (1.5) | |
| 95% CI | −3.1 to 2.9 | −3.6 to 2.6 | −1.0 to 5.2 | −4.8 to 1.2 | |
| p Value | 0.93 | 0.73 | 0.18 | 0.23 | |
| MG-QoL-15r total scores | |||||
| Baseline, mean (SD) | 17.4 (5.24) | 15.4 (6.26) | 15.6 (7.83) | 17.8 (5.87) | 15.7 (6.82) |
| Day 57, mean (SD) | 15.6 (7.03) | 13.6 (7.49) | 9.1 (7.88) | 16.7 (5.54) | 12.0 (8.53) |
| Change from baseline, mean (SD) | −2.0 (4.58) | −1.7 (4.16) | −6.8 (5.73) | −1.2 (1.91) | −3.7 (5.37) |
| LS mean values (SE)a | −1.9 (1.4) | −2.1 (1.3) | −6.9 (1.4) | −1.3 (1.4) | −4.0 (1.3) |
| Difference in LS mean values (nipocalimab vs placebo)a | −0.2 (1.7) | −5.1 (1.8) | 0.6 (1.8) | −2.2 (1.7) | |
| 95% CI | −3.7 to 3.2 | −8.6 to −1.5 | −3.0 to 4.1 | −5.6 to 1.3 | |
| p Value | 0.90 | 0.005 | 0.754 | 0.21 | |
Abbreviations: ITT = intent-to-treat; LS mean = least squares mean; MG-ADL = Myasthenia Gravis - Activities of Daily Living Total Score; MG-QoL-15r = Revised Myasthenia Gravis Quality of Life -15; QMG = Quantitative Myasthenia Gravis; Q2W = every 2 wk; Q4W = every 4 wk; SE = standard error.
LS mean values, CIs, and p values are from a Mixed-effect Model Repeated Measures (MMRM) at day 57 with treatment group, visit, treatment group by visit interaction, and autoantibody type as fixed effects and the baseline score as a covariate. Compound symmetry covariance structure is used.
Linear trend test is based on the change from baseline at day 57. The 60 mg/kg nipocalimab single-dose treatment group was not included in this analysis, and the coefficients for testing linear trend used were −3, −1, 1, 3 for the placebo, 5 mg/kg nipocalimab Q4W, 30 mg/kg nipocalimab Q4W, and 60 mg/kg nipocalimab Q2W groups, respectively.
Linear trend test is based on the rank of the change from baseline at day 57. Patients without a day 57 result were assigned the largest rank based on the rest of the patients' results. The 60 mg/kg nipocalimab single-dose treatment group was not included in this analysis, and the coefficients for testing linear trend used were −3, −1, 1, 3 for the placebo, 5 mg/kg nipocalimab Q4W, 30 mg/kg nipocalimab Q4W, and 60 mg/kg nipocalimab Q2W groups, respectively.
MGFA classification status at day 57 compared with baseline either shifted to a lower class, for example, Class III to Class II, (21.4%–53.8% across all nipocalimab groups and 42.9% in the placebo group) or was unchanged (23.1%–64.3% across all nipocalimab groups and 42.9% in the placebo group) in most of the patients; 2 patients (n = 1 each in the 30 mg/kg Q4W and 60 mg/kg Q2W treatment groups) had worsened (shifted to a higher class). The distribution of patients across these categories (shifted lower, unchanged, shifted higher) was not statistically significantly different in any individual nipocalimab group compared with that in the placebo group (p ≥ 0.22; eTable 4, links.lww.com/WNL/D273).
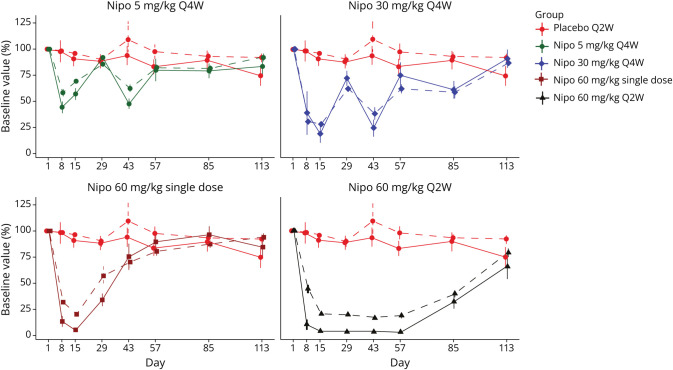
Exploratory endpoints showed that the proportion of patients with a durable response through day 57 ranged from 42.9% to 64.3% in the nipocalimab groups compared with 14.3% in the placebo group (eFigure 6, links.lww.com/WNL/D272). Comparison with the placebo group was statistically significant for the 60 mg/kg single-dose (53.9%; p = 0.046) and the 60 mg/kg Q2W dose (64.3%; p = 0.02) groups but not for the 5 mg/kg Q4W group (42.9%; p = 0.209) or 30 mg/kg Q4W group (46.2%; p = 0.103). The percentage of patients with a rapid onset of durable response within 17 days ranged from 42.9% to 46.2% in the nipocalimab groups vs 14.3% in the placebo group (eFigure 7); however, comparison with the placebo group did not reach statistical significance for any nipocalimab group (p ≥ 0.103). Treatment with nipocalimab resulted in substantial, rapid, and dose-dependent reduction in serum total IgG levels (eFigure 8). Maximal IgG reductions observed were 42% (5 mg/kg Q4W), 72% (30 mg/kg Q4W), 80% (60 mg/kg single-dose), and 83% (60 mg/kg Q2W). Mean total IgG reductions from baseline were noted as soon as 1 week after the first nipocalimab infusion, ranging from 42% at 5 mg/kg to 69% at 30 mg/kg or higher doses. The Q2W dosing regimen provided a sustained reduction in mean total IgG, and the Q4W dosing regimen produced variable changes in mean total IgG levels across the dosing interval, with a nadir approximately 2 weeks after nipocalimab administration and a peak approximately 4 weeks after administration. Corresponding to the reductions in total IgG, dose-dependent reductions in anti-AChR autoantibodies were also observed across nipocalimab treatment groups (Figure 4). Similar reductions were seen with all IgG subclasses (eFigure 9), with no changes in total IgM, IgA, and IgE across the nipocalimab treatment groups. The sample size of anti-MuSK–positive patients was insufficient to draw any conclusion regarding anti-MuSK (an IgG4 subclass autoantibody) reduction; however, the nipocalimab treatment group showed a dose-dependent reduction in IgG4 (eFigure 9).
Figure 4. Mean (±SE) Percentage of Baseline IgG and AChR-Binding Antibody by Dosing Arm Over Time (Safety Population).
AChR = acetylcholine receptor; IgG = immunoglobulin G; Nipo = nipocalimab; Q2W = every 2 weeks; Q4W = every 4 weeks; SE = standard error.
Median serum nipocalimab concentrations at the end of infusion (Ceoi) on day 1 (0 week) and day 57 (8 weeks) were approximately dose proportional across the 5 mg/kg Q4W, 30 mg/kg Q4W, and 60 mg/kg Q2W groups (eFigure 10, links.lww.com/WNL/D272). However, mean or median preinfusion serum nipocalimab concentrations on day 15 (i.e., 2 weeks after the infusion on day 1) were not dose proportional, similar to the nonlinear, dose-dependent PK of nipocalimab reported in the phase 1 study.5 No accumulations in serum nipocalimab concentrations over time were observed based on the mean or median Ctrough and Ceoi values observed across the 5 mg/kg Q4W, 30 mg/kg Q4W, and 60 mg/kg Q2W treatment groups.
A total of 54 patients receiving nipocalimab had posttreatment serum samples that were evaluable for ADA. The incidence of ADA through day 113 (16 weeks) was 40.7% (n = 22) in the combined nipocalimab group, compared with 7.1% (n = 1) in the placebo group. Of note, 35.8% samples from the combined nipocalimab group tested positive for ADA on day 15, but the ADA-positive rates dropped substantially at subsequent visits (from 12.2% on day 29 to 8.7% on day 113). The ADA-positive rates at the later time points (day 29 and beyond) in the nipocalimab-treated patients were similar to the sample ADA-positive rates in the placebo group (from 7.7% on day 29 to 10.0% on day 113). Titers of ADA were generally low (≤1:480). Eight (14.8%) of the 54 nipocalimab-treated patients were positive for NAb. The presence of ADA did not alter the PK or IgG lowering of nipocalimab based on comparisons of median serum nipocalimab concentrations or median serum IgG concentrations over time between ADA-positive and ADA-negative patients. In addition, development of ADA was not associated with reduced clinical efficacy (MG-ADL change from baseline over time) of nipocalimab or adverse events. The incidence of NAb was too low to assess its impact on PK or PD.
This study provides Class I evidence that for patients with gMG, nipocalimab was well-tolerated, and it did not significantly improve MG-ADL at any individual dose but demonstrated a significant dose response for improved MG-ADL across doses.
Discussion
This phase 2 dose ranging study evaluated the safety, efficacy, and PK/PD of nipocalimab in patients with gMG who had an inadequate response to ongoing stable SOC therapy. Nipocalimab was generally safe and well tolerated. There were no deaths, discontinuations due to TEAEs, or clinically significant grade 3 TEAEs of infection or hypoalbuminemia. Incidence of infections in the nipocalimab groups was low and comparable with those in the placebo group. Elevations in total cholesterol and LDL have been reported with drugs in the same pharmacologic class of FcRn antagonists.14 Modest elevations in nonfasting mean total cholesterol, LDL, and HDL were also observed with nipocalimab in the higher dose groups, especially in the 60 mg/kg Q2W group. These elevations were dose dependent and reversible, and there was no significant change in the lower dose groups. Furthermore, due to the concomitant elevation in HDL levels, the maximum mean percentage increases in the cholesterol-to-HDL ratio was low (<5%); low total cholesterol-to-HDL ratios are potentially suggestive of limited impact on cardiovascular risk status.15 The elevation in total, LDL, and HDL cholesterol levels may be secondary to decreases in serum albumin. Albumin has been reported as a regulator of cholesterol transport and acts as a shuttle to facilitate flux of cholesterol between plasma lipoproteins and red blood cells and transport of cholesterol to hepatocytes.16 Reduction of albumin may result in elevation of blood lipids, as has been reported with primary hypoalbuminemia.17
A positive dose response on change from baseline in MG-ADL total score at day 57 over the placebo, nipocalimab 5 mg/kg Q4W, nipocalimab 30 mg/kg Q4W, and nipocalimab 60 mg/kg Q2W groups was observed. Pairwise comparisons of each nipocalimab group with placebo were not statistically significant; however, the study was not powered for these comparisons.
The hypothesis-generating secondary and exploratory endpoints had some interesting results. Single-dose 60 mg/kg of nipocalimab showed the greatest reduction in MG-ADL through day 29 and declined thereafter, indicating the clinical effect of a single dose may not extend beyond 1 month. Duration of MG-ADL reduction was consistent with the duration of PK exposure and IgG lowering, which were dose dependent and dose-frequency dependent. The percentage of patients achieving a durable response on the MG-ADL through day 57 was also greater than that in the placebo group in all nipocalimab groups, with significant differences between placebo and 60 mg/kg (single-dose or Q2W). Overall, the results for MG-ADL indicate some clinical benefit with nipocalimab therapy. The results for QMG and MG-QoL15r did not show significance over placebo; however, missed QMG assessments due to study disruption related to the COVID-19 pandemic affected the analysis of this endpoint.
Nipocalimab is designed to bind, saturate, and block the IgG binding site on the FcRn with high affinity, thus inhibiting IgG recycling and lowering all IgG, including autoantibodies.5,18 Consistent with this mechanism of action, marked dose-dependent reductions in total serum IgG were observed across all nipocalimab treatment groups, with a maximal reduction (83%) observed in the 60 mg/kg Q2W dose group. The highest dose of 60 mg Q2W was expected to saturate the FcRn target, based on results from the phase 1 study.5 Reductions in anti-AChR autoantibodies were also observed in parallel with the reduction in total IgG and to a similar magnitude. Nipocalimab treatment was also associated with reductions in all 4 IgG subtypes. Patients with greater IgG reduction tended to have greater reduction in the MG-ADL total score, supporting the hypothesis that IgG lowering is the primary driver of efficacy for nipocalimab and supporting the causality of autoantibodies in gMG. This observation also suggests that IgG lowering could be a potential biomarker of efficacy in gMG.
ADA were developed in 40.7% of the 54 nipocalimab-treated patients, with the highest titer generally on day 15 or day 29 (19 of 22 ADA-positive patients) and diminished thereafter. Low titers of ADA (≤1:60) were seen in only 3 patients on day 113 when serum nipocalimab concentrations were no longer detected, suggesting that the ADA were developed transiently and could be diminished with continued nipocalimab treatment. Regardless, the development of ADA or NAb did not affect the PK, PD (IgG lowering), efficacy (MG-ADL reduction), and/or safety profiles of nipocalimab in patients with gMG.
Limitations to this study include the small sample size in each treatment group and the COVID-19–related study activity disruption. Although multiple doses were evaluated, the duration of treatment was short (8 weeks); further clinical benefit may be possible with prolonged treatment. Although QMG assessments were affected by pandemic-related study disruptions, MG-ADL could be assessed remotely during the pandemic. The limited sampling may potentially have affected the ability to detect a treatment effect in QMG scores.
In conclusion, the Vivacity phase 2 study in gMG showed dose-dependent reduction in MG-ADL scores at day 57 and were associated with total IgG and anti-AChR autoantibody lowering. All dose groups demonstrated acceptable safety and tolerability profile, with no clinically significant safety signals identified. Based on the analysis of safety, tolerability, and clinical endpoints from this phase 2 study, a phase 3 confirmatory study for nipocalimab in the treatment of gMG is ongoing.
Acknowledgment
The authors thank the patients, their caregivers, and Vivacity-MG trial investigators and their teams for their participation in the study. The authors also thank Andrzej Szczudlik, MD, PhD, and Brooke Hegarty, MSHS, for their contributions to the study. Priya Ganpathy, MPharm, CMPP and Sonali Satam, PhD (SIRO Clinpharm Pvt. Ltd., India) provided writing assistance, and Robert Achenbach (Janssen Global Services, LLC) provided additional editorial support.
Glossary
- AChR
acetylcholine receptor
- ADA
antidrug antibodies
- AESIs
adverse events of special interest
- COVID-19
coronavirus disease 2019
- C-SSRS
Columbia-Suicide Severity Rating Scale
- FcRn
neonatal Fc receptor
- gMG
generalized myasthenia gravis
- HDL
high-density lipoprotein
- IgG
immunoglobulin G
- LDL
low-density lipoprotein
- MG-ADL
Myasthenia Gravis-Activities of Daily Living
- MGFA
Myasthenia Gravis Foundation of America
- MMRM
mixed-effects model for repeated measures
- NAb
neutralizing antibodies
- PD
pharmacodynamics
- PK
pharmacokinetics
- Q2W
every 2 weeks
- Q4W
every 4 weeks
- QMG
quantitative myasthenia gravis
- SOC
standard-of-care
- TEAEs
treatment-emergent adverse events
Appendix 1. Authors
| Name | Location | Contribution |
| Carlo Antozzi, MD | IRCCS Neurological Institute Foundation C. Besta, Milan, Italy | Drafted and revised the article content, including analysis and interpretation of data |
| Jeffrey Guptill, MD, MA | Duke University School of Medicine, Durham, NC; Argenx US Inc., Boston, MA | Drafted and revised the article content, including analysis and interpretation of data |
| Vera Bril, MD | University of Toronto Division of Neurology, Toronto, Ontario, Canada | Drafted and revised the article content, including analysis and interpretation of data |
| Josep Gamez, MD, PhD | Department of Neurology, GMA Clinic; European Reference Network on Rare Neuromuscular Diseases (ERN EURO-NMD), Universitat Autonoma de Barcelona, Barcelona, Spain | Drafted and revised the article content, including analysis and interpretation of data |
| Sven G. Meuth, MD, PhD | Medical Faculty, Heinrich-Heine-University, Düsseldorf, Germany | Drafted and revised the article content, including analysis and interpretation of data |
| Richard J. Nowak, MD, MS | Department of Neurology, Yale University School of Medicine, New Haven, CT | Drafted and revised the article content, including analysis and interpretation of data |
| Dianna Quan, MD | University of Colorado School of Medicine, Aurora | Drafted and revised the article content, including analysis and interpretation of data |
| Teresa Sevilla, MD | Hospital Universitari i Politècnic La Fe/ IISLAFE, Universitat de Valencia, CIBERER (ERN EURO-NMD), Valencia | Drafted and revised the article content, including analysis and interpretation of data |
| Marie-Helene Jouvin, MD | Pharvaris, Inc., Boston, MA | Designed and conceptualized studies; reviewed the data; drafted and revised the manuscript content, including analysis and interpretation of data |
| Jim Jin, MS | Janssen Research & Development, LLC, Titusville, NJ | Designed and conceptualized studies; reviewed the data; drafted and revised the manuscript content, including analysis and interpretation of data |
| Keith Karcher, MS | Janssen Research & Development, LLC, Titusville, NJ | Reviewed the data, drafted and revised the article content, including analysis and interpretation of data |
| Sindhu Ramchandren, MD, MS | Janssen Research & Development, LLC, Titusville, NJ | Reviewed the data; drafted and revised the manuscript content, including analysis and interpretation of data |
| Hong Sun, MD, PhD | Janssen Research & Development, LLC, Titusville, NJ | Reviewed the data; drafted and revised the manuscript content, including analysis and interpretation of data |
| Leona Ling, PhD | Janssen Research & Development, LLC, Cambridge, MA | Designed and conceptualized studies; reviewed the data; and drafted and revised the manuscript content, including analysis and interpretation of data |
| Yaowei Zhu, PhD | Janssen Research & Development, LLC, Titusville, NJ | Reviewed the data; drafted and revised the manuscript content, including analysis and interpretation of data |
| Santiago Arroyo, MD, PhD | Marinus Pharmaceuticals, Inc. Radnor, PA; Fulcrum Therapeutics, Cambridge, MA | Designed and conceptualized studies; reviewed the data; and drafted and revised the manuscript content, including analysis and interpretation of data |
Appendix 2. Coinvestigators
| Name | Location | Role | Contribution |
| Kristl Claeys, MD PhD | UZ Leuven, Leuven, Vlaams Brabant, Belgium | Site investigator | Recruitment of patients |
| Dr. Rudy Mercelis | Belgium, University Hospital Antwerp, Antwerp, Belgium | Site investigator | Recruitment of patients |
| Gauthier Remiche, MD | Hôpital Erasme, Bruxelles, Belgium | Site investigator | Recruitment of patients |
| Annie Dionne, MD | Hopital de L'enfant Jesus, Canada | Site investigator | Recruitment of patients |
| Michael Nicolle, MD | London Health Sciences Centre, Canada | Site investigator | Recruitment of patients |
| Zaeem Siddiqi, MD PhD | University of Alberta Hospital, Canada | Site investigator | Recruitment of patients |
| Franz Blaes, MD | Kreiskrankenhaus Gummersbach, Gummersbach, Germany | Site investigator | Recruitment of patients |
| Sebastian Jander | Universitatsklinikum Dusseldorf, Düsseldorf, Germany | Site investigator | Recruitment of patients |
| Jens Schmidt, MD | Universität Georg August, Göttingen, Germany | Site investigator | Recruitment of patients |
| Rita Frangiamore, MD | IRCCS Neurological Institute Foundation C. Besta, Milan, Italy | Site investigator | Recruitment of patients |
| Fiammetta Vanoli, MD | IRCCS Neurological Institute Foundation C. Besta, Milan, Italy | Site investigator | Recruitment of patients |
| Riccardo Giossi, MD | IRCCS Neurological Institute Foundation C. Besta, Milan, Italy | Site investigator | Recruitment of patients |
| Silvia Bonanno, MD | IRCCS Neurological Institute Foundation C. Besta, Milan, Italy | Site investigator | Recruitment of patients |
| Lorenzo Maggi, MD | IRCCS Neurological Institute Foundation C. Besta, Milan, Italy | Site investigator | Recruitment of patients |
| Luigi Grimaldi, MD | Fondazione Istituto G. Giglio di Cefalù, Cefalù, Italy | Site investigator | Recruitment of patients |
| Carmelo Rodolico, MD | Az Ospedaliera Universitaria Policlinico G Martino, Messina, Italy | Site investigator | Recruitment of patients |
| Mariusz Grudniak | Centrum Medyczne NeuroProtect, Warsaw, Poland | Site investigator | Recruitment of patients |
| Krzysztof Selmaj, MD PhD | Centrum Neurologii Krzysztof Selmaj, Lodz, Poland | Site investigator | Recruitment of patients |
| Andrzej Szczudlik, MD PhD | Centrum Neurologii Klinicznej, Krakowska Akademia Neurologii, Kraków, Poland | Site investigator | Recruitment of patients |
| Malgorzata Zajda, MD | Centrum Medyczne Warszawa - PRATIA – PPDS, Warsaw, Poland | Site investigator | Recruitment of patients |
| Jose Luis Muñoz Blanco, MD | Hospital General Universitario Gregorio Marañón Servicio de Neurología, Madrid, Spain | Site investigator | Recruitment of patients |
| Carlos Casasnovas Pons, MD | Hospital Universitario de Bellvitge, L'hospitalet De Llobregat, Spain | Site investigator | Recruitment of patients |
| Antonio Guerrero Sola, MD | Hospital Clinico San Carlos, Madrid, Spain | Site investigator | Recruitment of patients |
| Isabel Illa Sendra, MD | Hospital de La Santa Creu i Sant Pau, Barcelona, Spain | Site investigator | Recruitment of patients |
| Adolfo Lopez de Munain, MD | Hospital Universitario de Donostia, San Sebastian, Spain | Site investigator | Recruitment of patients |
| Jose Luis Muñoz Blanco, MD | Hospital General Universitario Gregorio Marañon, Madrid, Spain | Site investigator | Recruitment of patients |
| Carmen Paradas, MD PhD | Hospital Universitario Virgen del Rocio – PPDS, Sevilla, Spain | Site investigator | Recruitment of patients |
| Albert Saiz, PhD | Hospital Clinic de Barcelona, Badalona, Spain | Site investigator | Recruitment of patients |
| Channa Hewamadduma | University of Sheffield, Sheffield, UK | Site investigator | Recruitment of patients |
| Saiju Jacob | Queen Elizabeth Hospital, Birmingham, UK | Site investigator | Recruitment of patients |
| Ashwin Pinto | Southampton University Hospitals NHS Trust, Southampton, UK | Site investigator | Recruitment of patients |
| Anthony Amato, MD | Brigham and Womens Hospital, Boston, USA | Site investigator | Recruitment of patients |
| Tulio Bertorini, MD | Wesley Neurology Clinic, PC, Cordova, USA | Site investigator | Recruitment of patients |
| Shan Chen, MD PhD | Robert Wood Johnson University Hospital, New Brunswick, USA | Site investigator | Recruitment of patients |
| Urvi Desai, MD | Carolinas HealthCare System Neurosciences Institute-Neurology, Charlotte, USA | Site investigator | Recruitment of patients |
| Constantine Farmakidis, MD | University of Kansas Medical Center, Fairway, USA | Site investigator | Recruitment of patients |
| Marc Feinberg, MD | South Florida Neurology Associates, Boca Raton, USA | Site investigator | Recruitment of patients |
| Miriam Freimer, MD | Ohio State University Medical Center, Columbus, USA | Site investigator | Recruitment of patients |
| Andrew Gordon, MD | Northwest Neurology Ltd. - BTC – PPDS, Lake Barrington, USA | Site investigator | Recruitment of patients |
| Raghav Govindarajan, MD | University of Missouri Health Care System, Columbia, USA | Site investigator | Recruitment of patients |
| Kavita Grover, MD | Henry Ford Health System, Detroit, USA | Site investigator | Recruitment of patients |
| Amanda Guidon, ANP MD | Massachusetts General Hospital, Boston, USA | Site investigator | Recruitment of patients |
| Shruti M. Raja, MD | Duke University School of Medicine, Durham, NC, USA | Site investigator | Recruitment of patients |
| Natalia Gonzalez, MD | Duke University School of Medicine, Durham, NC, USA | Site investigator | Recruitment of patients |
| Vern C. Juel, MD | Duke University School of Medicine, Durham, NC, USA | Site investigator | Recruitment of patients |
| Adam Horvit, MD | Central Texas Neurology Consultants PA, Round Rock, USA | Site investigator | Recruitment of patients |
| Yessar Hussain, MD | Austin Neuromuscular Center, Austin, USA | Site investigator | Recruitment of patients |
| Arnaldo Isa, MD | Neurology Associates PA, Maitland, USA | Site investigator | Recruitment of patients |
| David Konanc, MD | Raleigh Neurology Associates PA, Raleigh, USA | Site investigator | Recruitment of patients |
| Hani Kushlaf, MD | University of Cincinnati, Cincinnati, USA | Site investigator | Recruitment of patients |
| Dale Lange, MD | Hospital For Special Surgery, New York, USA | Site investigator | Recruitment of patients |
| Richard Lewis, | Cedars Sinai Medical Center, Los Angeles, USA | Site investigator | Recruitment of patients |
| George Li, MD | Medsol Clinical Research Center Inc, Port Charlotte, USA | Site investigator | Recruitment of patients |
| Tahseen Mozaffar, MD | UC Irvine Health, Orange County, USA | Site investigator | Recruitment of patients |
| Suraj Muley, MD | Barrow Neurological Institute, Phoenix, USA | Site investigator | Recruitment of patients |
| Pushpa Narayanaswami, MD | Beth Israel Deaconess Medical Center, Boston, USA | Site investigator | Recruitment of patients |
| Richard Nowak, MD | Yale School of Medicine, New Haven, USA | Site investigator | Recruitment of patients |
| Heidi Orme, MD | Les Bois Neurology, Meridian, USA | Site investigator | Recruitment of patients |
| Dianna Quan, MD | University of Colorado Anschutz Medical Campus, Aurora, USA | Site investigator | Recruitment of patients |
| Michael Rivner, MD | Augusta University, Augusta, USA | Site investigator | Recruitment of patients |
| Amit Sachdev, MD | Michigan State University, East Lansing, USA | Site investigator | Recruitment of patients |
| Katalin Scherer, MD | The University of Arizona Medical Center, Tucson, USA | Site investigator | Recruitment of patients |
| Yuen So, MD PhD | Stanford Neuromuscular Research, Palo Alto, USA | Site investigator | Recruitment of patients |
| Alberto Vasquez, MD | Suncoast Neuroscience Associates Inc, Saint Petersburg, USA | Site investigator | Recruitment of patients |
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
The study was funded by Momenta Pharmaceuticals, Inc., which has been acquired and is now part of the Janssen Pharmaceutical Companies of Johnson & Johnson.
Disclosure
C. Antozzi has received a travel grant from Biogen; J. T. Guptill was a consultant for Momenta Pharmaceuticals and is currently a clinical specialist at Argenx US Inc., USA, full disclosures available at dcri.org/about-us/conflict-of-interest/; V. Bril has been a consultant for Akcea, Alexion Pharmaceuticals, Alnylam, Argenx, CSL, Grifols, Ionis, Octapharma, Powell-Mansfield, Sanofi, Takeda (Baxalta, Shire), and UCB and has received research support from Akcea, Alexion Pharmaceuticals, Argenx, CSL, Grifols, Ionis, Octapharma, Powell-Mansfield, Takeda (Baxalta, Shire), and UCB; J. Gamez reports no disclosures relevant to the manuscript; S.G. Meuth has received honoraria for lecturing and travel expenses for attending meetings from Almirall, Amicus Therapeutics Germany, Bayer Health Care, Biogen, Celgene, Diamed, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi-Aventis, Chugai Pharma, QuintilesIMS, and Teva and has conducted research funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, German Academic Exchange Service, Hertie Foundation, Interdisciplinary Center for Clinical Studies (IZKF) Muenster, German Foundation Neurology, and by Almirall, Amicus Therapeutics Germany, Biogen, Diamed, Fresenius Medical Care, Genzyme, Merck Serono, Novartis, ONO Pharma, Roche, and Teva; R.J. Nowak has received research support from Alexion Pharmaceuticals, Genentech, Grifols, Immunovant, Momenta Pharmaceuticals, Myasthenia Gravis Foundation of America, NIH, and Ra Pharmaceuticals and has served as consultant/advisor for Alexion Pharmaceuticals, Argenx, CSL Behring, Grifols, Immunovant, Momenta Pharmaceuticals, and Ra Pharmaceuticals; D. Quan has received research funding from Alnylam, Argenx, Ionis, Momenta Pharmaceuticals, and Pfizer; T. Sevilla reports no disclosures relevant to the manuscript; M.H. Jouvin was an employee of Momenta Pharmaceuticals, Inc, part of the Janssen Pharmaceutical Companies of Johnson & Johnson; J. Jin was an employee of Momenta Pharmaceuticals, Inc, part of the Janssen Pharmaceutical Companies of Johnson & Johnson; K. Karcher is an employee of Janssen Research & Development LLC and may hold company stock or stock options; S. Ramchandren is an employee of Janssen Research & Development LLC and may hold company stock or stock options; H. Sun is an employee of Janssen Research & Development LLC and may hold company stock or stock options; L. Ling was an employee of Momenta Pharmaceuticals, Inc, part of the Janssen Pharmaceutical Companies of Johnson & Johnson; Y. Zhu is an employee of Janssen Research & Development LLC and may hold company stock or stock options; S. Arroyo is on the Board of Directors of Marinus and Lundbeck and was the Chief Medical officer of Momenta Pharmaceuticals, Inc. Go to Neurology.org/N for full disclosures.
References
- 1.Gilhus NE, Verschuuren JJ. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 2015;14(10):1023-1036. doi: 10.1016/S1474-4422(15)00145-3 [DOI] [PubMed] [Google Scholar]
- 2.Gable KL, Guptill JT. Antagonism of the neonatal Fc receptor as an emerging treatment for myasthenia gravis. Front Immunol. 2019;10:3052. doi: 10.3389/fimmu.2019.03052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen-Cao TM, Gelinas D, Griffin R, Mondou E. Myasthenia gravis: historical achievements and the "golden age" of clinical trials. J Neurol Sci. 2019;406:116428. doi: 10.1016/j.jns.2019.116428 [DOI] [PubMed] [Google Scholar]
- 4.Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419-425. doi: 10.1212/WNL.0000000000002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ling LE, Hillson JL, Tiessen RG, et al. M281, an anti-FcRn antibody: pharmacodynamics, pharmacokinetics, and safety across the full range of IgG reduction in a first-in-human study. Clin Pharmacol Ther. 2019;105(4):1031-1039. doi: 10.1002/cpt.1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Garcia AM, Santoro H, et al. Amelioration of experimental autoimmune myasthenia gravis in rats by neonatal FcR blockade. J Immunol. 2007;178(8):5390-5398. doi: 10.4049/jimmunol.178.8.5390 [DOI] [PubMed] [Google Scholar]
- 7.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715-725. doi: 10.1038/nri2155 [DOI] [PubMed] [Google Scholar]
- 8.Howard JF Jr., Bril V, Vu T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526-536. doi: 10.1016/S1474-4422(21)00159-9 [DOI] [PubMed] [Google Scholar]
- 9.Bril V, Drużdż A, Grosskreutz J, et al. Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 2023;22(5):383-394. doi: 10.1016/S1474-4422(23)00077-7 [DOI] [PubMed] [Google Scholar]
- 10.Muppidi S, Wolfe GI, Conaway M, Burns TM, MG COMPOSITE AND MG-QOL15 STUDY GROUP. MG-ADL: still a relevant outcome measure. Muscle Nerve. 2011;44(5):727-731. doi: 10.1002/mus.22140 [DOI] [PubMed] [Google Scholar]
- 11.Clinical Trial Data Transparency Policy of Janssen Pharmaceutical Companies of Johnson & Johnson. 2023. Accessed 23 July 2023. janssen.com/clinical-trials/transparency. [Google Scholar]
- 12.The Yale Open Data Access (YODA) Project. Accessed 23 July 2023. yoda.yale.edu/. [Google Scholar]
- 13.High blood cholesterol summary - National Institute of Health. Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults; 2001. Accessed November 23, 2023. nhlbi.nih.gov/files/docs/guidelines/atp3xsum.pdf. [Google Scholar]
- 14.Ward ES, Gelinas D, Dreesen E, et al. Clinical significance of serum albumin and implications of FcRn inhibitor treatment in IgG-mediated autoimmune disorders. Front Immunol. 2022;13:892534. doi: 10.3389/fimmu.2022.892534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mortensen MB, Nordestgaard BG. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70-100 years: a contemporary primary prevention cohort. Lancet. 2020;396(10263):1644-1652. doi: 10.1016/S0140-6736(20)32233-9 [DOI] [PubMed] [Google Scholar]
- 16.Sankaranarayanan S, de la Llera-Moya M, Drazul-Schrader D, Phillips MC, Kellner-Weibel G, Rothblat GH. Serum albumin acts as a shuttle to enhance cholesterol efflux from cells. J Lipid Res. 2013;54(3):671-676. doi: 10.1194/jlr.M031336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Ben M, Angelico F, Loffredo L, Violi F. Treatment of a patient with congenital analbuminemia with atorvastatin and albumin infusion. World J Clin Cases. 2013;1(1):44-48. doi: 10.12998/wjcc.v1.i1.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy S, Nanovskaya T, Patrikeeva S, et al. M281, an anti-FcRn antibody, inhibits IgG transfer in a human ex vivo placental perfusion model. Am J Obstet Gynecol. 2019;220(5):498.e1-498.e9. doi: 10.1016/j.ajog.2019.02.058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The clinical trial transparency policy of Janssen Pharmaceutical Companies of Johnson & Johnson is publicly available.11 Requests for access to study data can be submitted through the Yale Open Data Access (YODA) Project.12