Significance
Here, we use a globally derived camera trapping dataset to evaluate theorized drivers of group formation in a species complex, where all species are assumed to be obligately solitary. We find empirical support consistent with the resource dispersion hypothesis, and a combination of physiological and environmental factors underpinning tendencies toward aggregation. We demonstrate that the probability of group formation in these “solitary” species varies by over an order of magnitude, highlighting how the use of “social” vs. “solitary” categorizations of animals limits our understanding of both individual species and their ecologies, and the causes and consequences of group formation.
Keywords: sociality, social organisation, group-living, camera trap, resource dispersion hypothesis
Abstract
The social system of animals involves a complex interplay between physiology, natural history, and the environment. Long relied upon discrete categorizations of “social” and “solitary” inhibit our capacity to understand species and their interactions with the world around them. Here, we use a globally distributed camera trapping dataset to test the drivers of aggregating into groups in a species complex (martens and relatives, family Mustelidae, Order Carnivora) assumed to be obligately solitary. We use a simple quantification, the probability of being detected in a group, that was applied across our globally derived camera trap dataset. Using a series of binomial generalized mixed-effects models applied to a dataset of 16,483 independent detections across 17 countries on four continents we test explicit hypotheses about potential drivers of group formation. We observe a wide range of probabilities of being detected in groups within the solitary model system, with the probability of aggregating in groups varying by more than an order of magnitude. We demonstrate that a species’ context-dependent proclivity toward aggregating in groups is underpinned by a range of resource-related factors, primarily the distribution of resources, with increasing patchiness of resources facilitating group formation, as well as interactions between environmental conditions (resource constancy/winter severity) and physiology (energy storage capabilities). The wide variation in propensities to aggregate with conspecifics observed here highlights how continued failure to recognize complexities in the social behaviors of apparently solitary species limits our understanding not only of the individual species but also the causes and consequences of group formation.
Understanding the evolutionary, physiological, and proximal drivers of animals’ social organizations and structures is foundational to understanding their ecology and, in turn, assessing organisms’ ecological niches and propensities for adaptation. As a prerequisite, we must understand what drives conspecifics to congregate, with the potential to establish groups. Most carnivorans are described as being solitary (80 to 95%, 1, 2), with direct social interactions restricted to mating and young rearing, suggesting that they have limited potential for cooperative behaviors (3). Yet, there is growing evidence that social organizations and structures are complex across even the most socially restricted species including sibling coalitions, male–male alliances, and spatial group formations (4–6). Despite growing recognition that the social structure and organization of species is likely dynamic, with “group-living” and “solitary” representing extremes of a spectrum (7), our current schema of discrete categorizations remains clouded by anthropocentrism that limits our understanding of countless species.
At all trophic levels, life imposes energetic requirements. Nonetheless, constraints imposed by body–mass ratios, spatial heterogeneity, and temporal environmental stochasticity of resources may be mitigated through cooperative behaviours that alter what, how, and when resources are obtained (8). The propensity of members of a species to form groups (typically of related individuals and breeding pairs) is thought to be influenced by benefits gained from cooperative hunting, alloparental care, social learning, and defense against predators and conspecific territorial intruders, offset by the disadvantages of high parasite burdens, high infanticide risk, and intraspecific competition (9–11). To avoid ambiguity and confusion caused by irregular use and a lack of consensus regarding terminology in the social systems literature, here, for clarity we define critical terms used, adapting the framework and definitions presented and discussed in refs. 12 and 13; see Table 1.
Table 1.
Definition of critical terms used in our research with key references for each term
| Term | Definition | References |
|---|---|---|
| Social complexity | Social complexity refers to a continuous spectrum in which complex social systems are those where individuals frequently interact in many different contexts with many different individuals and often repeatedly interact with many of the same individuals over time. | (14) |
| Social structure | Social structure is defined by the content, quality, and patterning of social relationships emerging from repeated interactions between pairs of individuals belonging to the same social unit. | (13) |
| Social organization | Social organization refers to the size and composition of a social unit. | (13) |
| Group/Aggregation | Group, grouping, or aggregation (used interchangeably) refers to when animals are located together in time and space. The smallest group size equals 2; the largest animal groups can include millions of individuals. For our research, we define a group or aggregation to be ≥2 animals detected in the same image together. | (13, 15) |
| Association | Associations are non-solitary social units defined as “a set of animals that interact regularly and more so with each other than with members of other such groups.” | (13, 16) |
A general model of social organization, the resource variance-life history model (17), which has the resource dispersion hypothesis as a special case (10, 18), predicts that patterns of resource availability, in both space and time influence tolerance of conspecifics and thus group formation. This model considers productivity and variation of resources in space (patchiness) and time (predictability) to be key for explaining mating and dispersal strategies of mammals (17). It predicts that groups will develop in landscapes where resources are dispersed spatially in resource-rich patches with high temporal constancy whereby the smallest economically defensible territory for a single individual can sustain additional animals. Empirical tests of these predictions generally support resource availability patterns being central to social organization and complexity (19).
Examining the variation in the ecology and life history of closely related species can clarify important evolutionary processes (20). Mustelids are the most species-rich superfamily amongst the carnivorans (21). Most mustelids display some form of intra-sexual territoriality, a simple social structure amongst solitary carnivorans where individuals exclude conspecifics of the same sex. Females are thought to try to maximize resources within their range, while males try to maximize access to females and, therefore, impose territory overlap on females (22, 23). Group formation in mustelids is thought to be constrained by a combination of factors. Mustelids have retained the slender morphology of their ancestral viverravids and miacids (24, 25) providing access to small semi-arboreal, fossorial, and subnivean prey. Small vertebrate prey are diverse and have populations on nearly every landscape on the planet. Generally, small vertebrate prey are theorized to be distributed homogenously within their preferred habitats (21), making the establishment of territories and exclusion of conspecifics, a beneficial strategy to ensure sufficient resources for survival (10, 26; 14 presented the theoretical derivation). Mustelids’ asocial tendencies are thought to be exacerbated by the pleiotropic biochemical costs of delayed implantation. Oxytocin, the “affection hormone” (27), is inhibited for delayed implantation to the apparent detriment of affectionate dispositions in mustelids (21, 28). Amongst this generally asocial taxon, martens and their close relatives (sometimes called the “Martes complex,” hereafter, martens) are exemplars of animals thought to be restricted in terms of tolerance on conspecifics and that have been described as “obligately solitary” (22, 29–31). Semi-arboreal and fossorial life histories are underpinned physiologically in mustelids by an inability to produce substantial fat deposits (32). Harlow hypothesized that martens store excess energy as muscle instead of fat (33). Thus, as with other mammals, large individuals experience higher survival rates during periods of resource scarcity because they have longer fasting endurance due to metabolizing somatic stores at a lower weight-specific rate (34). Nevertheless, the elongated and thin body shape of small mustelids increases vulnerability to starvation (32), thereby reducing food security during severe winters. This is thought to preclude the tolerance of conspecifics, which is the fundamental prerequisite to the formation of groups (35).
Recent evidence challenges the established dogma of obligate solitariness in martens. For example, cooperative foraging has been observed in tropical yellow-throated martens (Martes flavigula, 36). Sibling coalitions and paternal philopatry have been documented for wolverines (Gulo gulo), stone martens (Martes foina), and pine martens (Martes martes, 37, 38). Whilst scattered, these observations of cooperative and social behaviors suggest greater social complexity than previously envisaged in this animal group. The contrast between these recent observations and the expected solitary life histories makes martens an ideal model system for testing hypotheses related to the drivers of group formation.
We assembled a global collation of camera trapping data to test the drivers of group formation across seven species within the Martes complex that vary in weight over an order of magnitude (1 kg to 20 kg): Four species in the genus Martes [Martes americana (including Martes caurina), M. flavigula, M. foina, M. martes], the wolverine, the tayra (Eira barbara), and the fisher (Pekania pennanti). The resource variance-life history models present hypotheses relating to productivity, patchiness, and predictability of resources (17, 19). Additionally, recent hypotheses have emerged regarding energy storage capabilities as a facilitator of aggregation (35). Thus, based on these lines of evidence, we hypothesize that a) individuals of a species will be more likely to aggregate on low productivity landscapes; b) animals that use more patchily distributed food resources (e.g., fruit, insect nests, and large prey) will have a greater probability of aggregating with conspecifics, c) individuals of a species will be more likely to aggregate with conspecifics on landscapes which do not undergo prolonged periods of resource scarcity resultant from severe winter conditions, and d) larger species will be more likely to aggregate with conspecifics than smaller ones due to energy stores buffering against periods of food scarcity.
Methods
Collection and Preparation of Global Camera Trapping Data.
We conducted a literature review of camera trap research in regions across the globe within the expected ranges of any member of the Martes complex during 2000 to 2020. We used search terms related to specific species names as well as generic terms such as “marten,” “camera trap,” “survey,” and “study.” We used these to create a database of correspondence authors from whom we requested data. In addition, we contacted experts and reviewed the activities of major international non-governmental organizations. We conducted snowball sampling, obtaining additional datasets from colleagues recommended by previous contacts. Data gathered included longitude and latitude of camera stations, date, time, species names, number of individuals in each image, and other associated information (e.g., use of lure or bait). Whilst camera deployment methods varied across the collated studies (see SI Appendix: Camera Trapping Studies for full details of each locality), the general method involved deploying camera traps either without bait/lure or facing a bait station (bait varied with the focal species but was most commonly a commercial scent lure, but also included peanuts, eggs, beaver meat, and so forth). Cameras were set to take photos in bursts of 1 to 10 images or videos of 10 s–1 min with short interval times (1 to 20 s). Camera makes and models are listed in SI Appendix: Camera Trapping Studies.
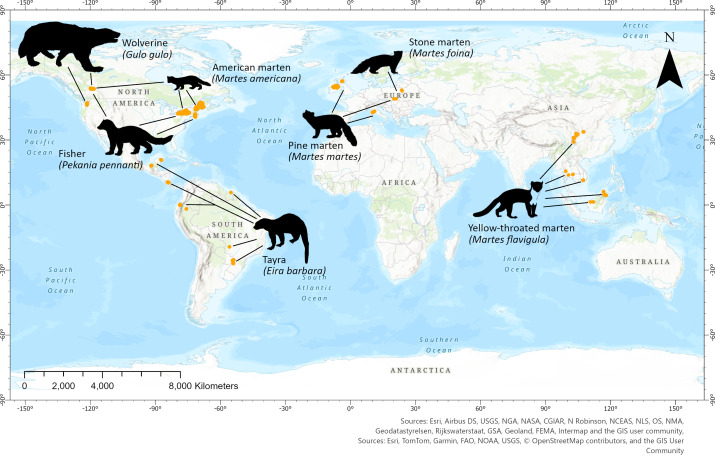
Our total collated dataset contained 33,996 detections of members of seven focal species from 29 study sites across 17 countries and four continents (Fig. 1). We considered a maximum of 1 detection per day at any site to ensure independence between detections. Over the course of the twenty-year period between 2000 and 2020; there were 16,483 independent detections (maximum of 1 per 24-h period), which were composed of 7,657 fisher detections, 3,578 American marten detections, 2,130 pine marten detections, 911 tayra detections, 771 stone marten detections, 730 yellow-throated marten detections, and 706 wolverine detections.
Fig. 1.
Map showing the location of the 29 camera trapping studies with detections of Martes complex species collated from 17 countries across four continents during 2000 to 2020. Site locations are orange dots.
Statistical Analysis.
We initially examined interspecific differences in the Martes complex by examining how probability of being detected in a group varied among species. A group detection was defined as a single image that contained two or more individuals of the same species (to remove uncertainty two or more individuals must be detected in the same individual image to be classified as being in a group, a sequence of images containing single animals would all be classified as individual detections). This conservative approach to individual vs. group assignment removes the possibility of false positives (assuming animals were in a group because they were in a sequence of photos over some arbitrary timeframe) and minimizes the chance of considering antagonistic interactions (i.e., one animal chasing another as in a territorial dispute) as individuals aggregating in groups. While there is a degree of ambiguity with images, no obvious evidence of hostile encounters (e.g., fighting; snarling; aggressive body positioning) was found using this individual image-based group classification approach. To test our hypotheses, we used a series of binomial generalized mixed effect models with the binary response variable of detections of individuals (0) or groups (1). Modeling was conducted in R version 4.3.1 using the function glmer in package lme4 (39, 40). The covariates we tested were average body weight of each species (kg, log-transformed), resource productivity (gross primary productivity: GPP), average annual temperature change as a proxy for winter severity (average annual temperature difference, °C, power-transformed, λ = 2), mean SD of daily temperature differences from annual averages as a quantification of resource constancy (°C), and a metric for patchiness of resources used by the species (see below). We quantified resource productivity of each site (camera trap station) using MODIS Land Satellite data (41) and summarized gross primary productivity over 8-d periods at each site. From MODIS Land Surface Temperature estimates (42), we quantified winter severity by calculating the mean annual temperature difference between monthly averages for January and July for each year for each site. We produced a proxy for resource constancy by 1) using daily estimates of temperature at each independent detection over the 20-y focal period to estimate annual averages, 2) calculating daily differences from annual averages at each independent detection, and then 3) calculating mean SD of daily temperature differences from average annual temperatures. We calculated a resource dispersion metric to quantify the patchiness of the distribution of resources for each species in each focal area as:
where HDR is the frequency of occurrence of homogenously distributed resources (HDR) in the diet, and PDR is the frequency of occurrence of patchily distributed resources (PDR) in the diet. HDR are all small vertebrate prey (e.g., small mammals, birds, and reptiles), and patchily distributed resources are comprised of invertebrates, fruit, and carrion (21). For each species, we conducted literature searches for dietary studies at each camera site using search terms that included the species name, the country, and generic terms such as “diet,” “predation,” “food resources.” Where specific locality data were not available, we adopted results from the nearest location. See SI Appendix, Table S1 for details on each species and locality. To account for the effects of variation in bait and scent-lure use in camera trapping methods, and how these might affect behavior and aggregation of individuals, we considered four alternate parameterizations of bait status, H1—All different [3 level factor, 1 = food reward, 2 = scent lure, 3 = none]; H2—Attractant [2 level factor, 1 = food/scent lure, 2 = none]; H3—Food different [2 level factor, 1 = food reward, 2 = scent lure and none]; H4—Lure different [2 level factor, 1 = lure only, 2 = food reward and none] and used AIC-based model selection to identify to the most parsimonious parametrization of bait/scent-lure usage (SI Appendix, Table S2 and Bait Covariate Parameterization). The two-level attractant parameterization (food/scent lure vs. unbaited) was the most supported and was included as a fixed effect in all models. To account for natural annual and interannual temporal variation in group formation in species expected due to breeding we include ordinal day, both the linear and quadratic terms, as fixed effects, and year as a random effect in all models. We attempted to fit a date and species interaction, but models failed to converge. To examine how resource constancy, and severe winter or summer conditions, interact with physiological factors (e.g., energy stores as fat or muscle) to drive propensity to associate with conspecifics, we added an interaction between log transformed weight (kg) and mean annual temperature difference. To account for potential unaccounted for landscape effects that may introduce non-independence within study regions, we also included study region as a random effect on all models. We excluded data that were based on monitoring of denning sites and breeding sites so as not to introduce bias through saturation of detections of multiple individuals where such monitoring was conducted. It was not possible to assign relationship identifiers to photographs, so each independent detection was scored binomially (0—single individual, 1—two or more individuals). We attempted to include phylogenetic contrasts (43) to account for taxonomic relatedness in the analysis. Phylogenetic contrasts require consideration of species trait values averaged at shared ancestral nodes (43, 44). Given we only consider seven species, four of which are in the same genus, and thus share the same ancestral node, such an approach was not viable. Nonetheless, qualitative examination of phylogenetic positioning against the species trait of interest (e.g., probability of aggregating in groups) did not provide evidence of correlation (SI Appendix, Fig. S1).
All continuous covariates were scaled and standardized to have unit variance and a mean of zero. Based on variance inflation factors, there was evidence of strong collinearity between our metrics for winter severity and resource constancy (VIF = 7.59, see SI Appendix, Fig. S2). Thus, resource constancy was dropped from the global model and we assume that our winter severity metric (average annual temperature difference) provides a proxy for both winter severity and resource constancy. There was no evidence of collinearity between any covariates after this removal (45, VIF < 3). We compared all combinations of covariates using AIC-based ranking methods to assess the most parsimonious model combination for grouping within martens (46). This resulted in 20 candidate models which were compared using the package “MuMin” (47, see SI Appendix, Table S3 for full model list). Redundancy of parameters was evaluated following Arnold (48) such that the parameters that were included but resulted in less than −2 AIC units from the next best model were considered uninformative and removed. To visualize the effects of important covariates, we estimate the marginal probability of an animal being detected in a group across the entire gradient of parameter space. All analyses were conducted in R version 4.2 (40).
Results
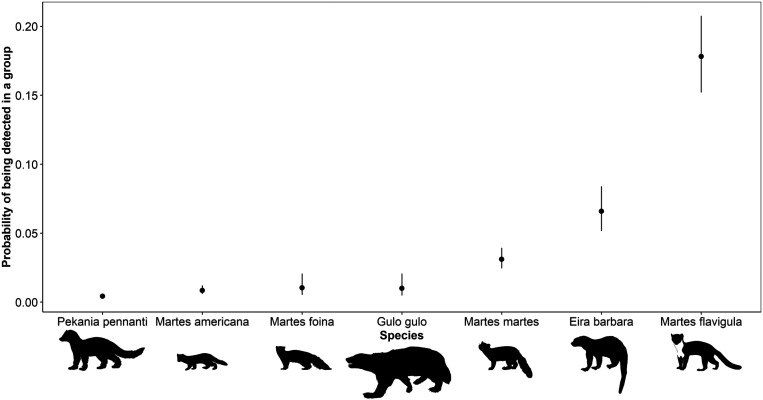
The initial exploratory species model showed clear differences among species in their probability of being detected in groups (Fig. 2). The two species whose ranges are partly tropical, the yellow-throated marten (0.18, CI 95% = 0.15 to 0.21) and the tayra (0.08, CI 95% = 0.05 to 0.09), displayed the highest probabilities of being detected in groups. This was followed by the pine marten (0.03, CI 95% = 0.02 to 0.04), the wolverine (0.01, CI 95% = 0.01 to 0.02), then by the stone marten (0.01, CI 95% = 0.01 to 0.02), and the American marten (0.01, CI 95% = 0.01 to 0.01). Finally, the least likely to be detected in groups was the fisher (0.00, CI 95% = 0 to 0.01).
Fig. 2.
The probability of each member of the Martes complex (martens and close relatives) being detected in groups (two or more individuals) based on generalized linear mixed effects models of a globally derived camera trapping dataset collected between 2000 and 2020.
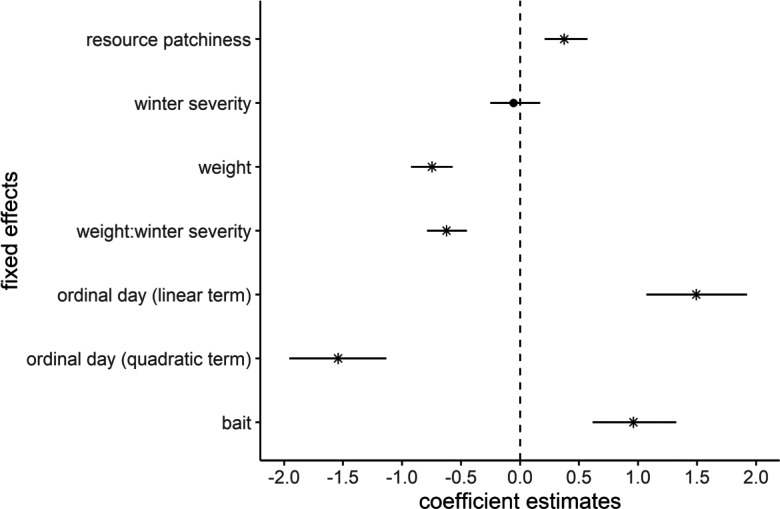
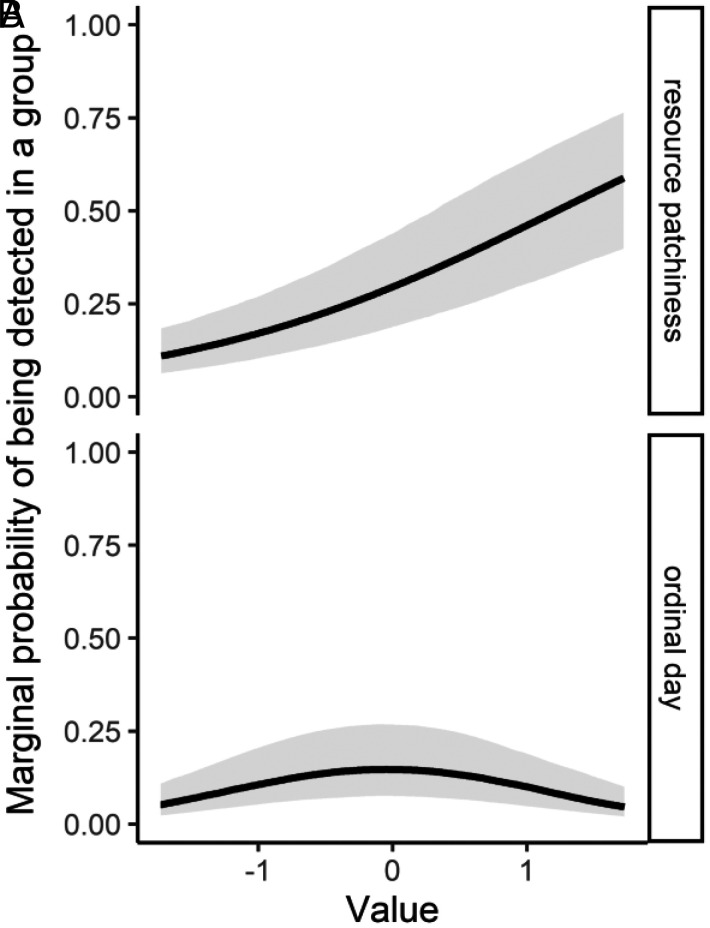
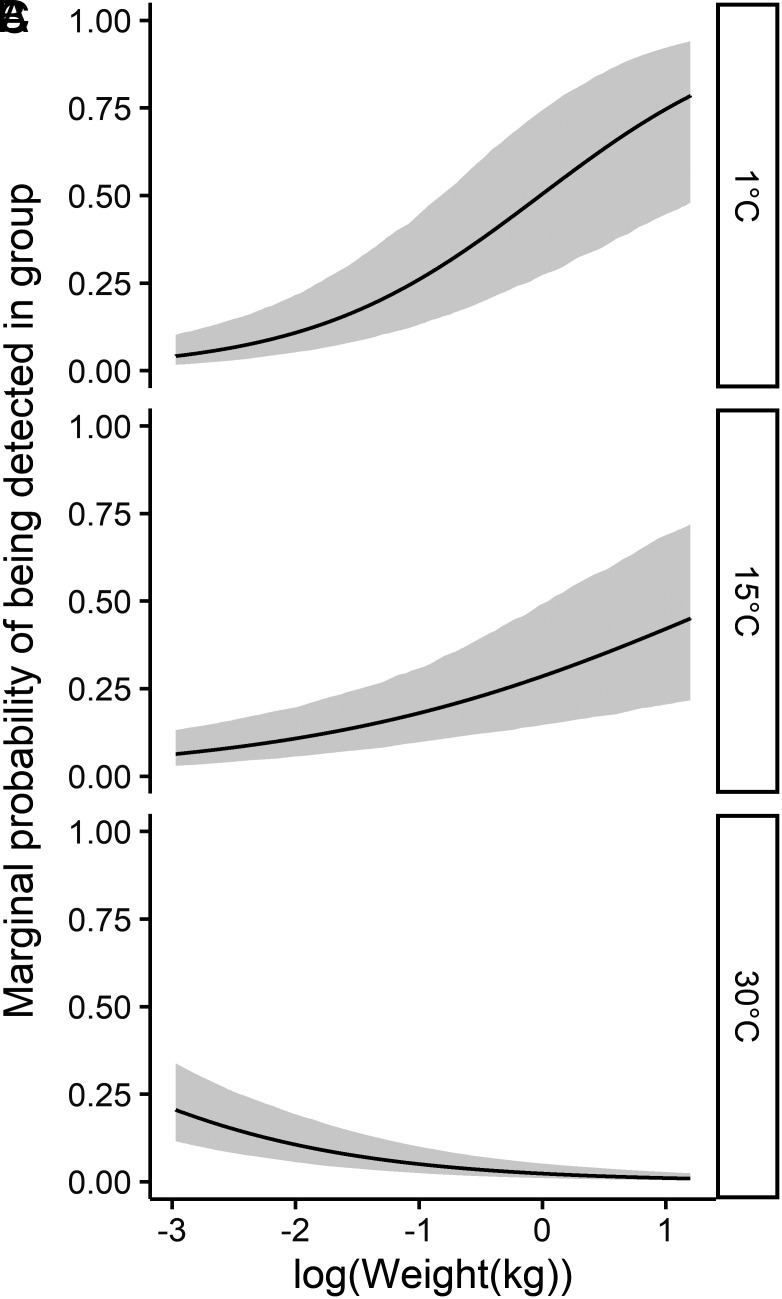
Using AIC to rank the 20 models that included all covariate combinations, we found clear support for a single top model (SI Appendix, Table S4), providing evidence that resource-related and physiological factors (i.e., energy storing capabilities) interact to jointly explain the variation in tendencies toward aggregation amongst species (Fig. 3). Specifically, the top model showed that higher reliance on patchy resources (e.g., fruit, invertebrates, and large prey) was associated with increases in grouping of individuals (βpatchiness = 0.37 ± 0.14, Figs. 3 and 4A). The use of attractants at camera sites had a strong effect, with use of bait/scent-lures at sites increasing probability of detecting aggregations of individuals (βbait = 0.96 ± 0.18, Fig. 3). Evidence existed of pronounced annual variation in group formation as expected due to the requirements of breeding, with the quadratic term showing a peak mid-year which aligns with the kit rearing period for most of the species (βday(quadratic) = −1.54 ± 0.21, Fig. 3). The impacts of temporal variation on overall interspecific differences observed were, however, comparatively minor, as evidenced by limited variation in marginal probabilities of grouping throughout the year (Fig. 4B). The interaction between body weight (as a proxy for energy storage capabilities) and mean annual temperature difference (our proxy for winter severity/resource constancy) was also important (βweight:resource constancy = −0.62 ± 0.08, Fig. 3), whereby in environments which do not undergo resource scarcity induced by severe winter conditions (e.g., those with lower mean annual temperature differences) higher body weights were positively associated with tendencies to aggregate with conspecifics (Fig. 5 A and B). Nonetheless, in systems displaying harsh winter conditions (i.e., ≥30 °C mean annual temperature difference), this positive association between weight and being detected in groups was absent (Fig. 5C). Resource productivity was a redundant parameter and did not explain variance in probability of being detected in groups (SI Appendix, Table S4).
Fig. 3.
Model coefficient estimates with 95% CIs showing associations between the probability of martens and close relatives (Martes complex) being detected in groups and fixed effects in the top AIC-ranked generalized mixed effect model from a globally derived camera trapping dataset between 2000 and 2020. An asterisk represents coefficients with strong relationships with this quantitative metric for group formation (i.e., CIs do not overlap 0).
Fig. 4.
“Marginal” probability of being detected in a group from the seven species of the Martes complex as a function of (A) resource patchiness and (B) ordinal day based on the top AIC-ranked generalized mixed effect model of a global camera trapping dataset collected between 2000 and 2020. Marginal probability estimates are made along the whole sequence of values sampled in parameter space for the covariate of interest while keeping all other covariates at their mean value (0 as all covariates were centered to have a mean and unit variance of 0).
Fig. 5.
“Marginal” probability of being detected in a group from the seven species of the Martes complex as a function of the interaction between body weight (kg) and mean annual temperature difference (°C) of (A) 1 °C mean annual temperature difference, (B) 15 °C mean annual temperature difference, and (C) 30 °C mean annual temperature difference based on the top AIC-ranked generalized mixed effect model of a global camera trapping dataset collected between 2000 and 2020. Marginal probability estimates are made along the whole sequence of values sampled in parameter space for the covariate of interest while keeping all other covariates at their mean value (0 as all covariates were centered to have a mean and unit variance of 0).
Discussion
In a closely related species complex where all species were previously assumed to be obligately solitary, we observe a wide range of tendencies toward group formation, with the probability of aggregating in groups varying by over an order of magnitude between species. All of the theorized resource-focused predictors except resource productivity contributed toward variation in tendency toward aggregating into groups, including winter severity/resource constancy, energy storing capabilities, and the distribution of resources. Species in environments with greater seasonal constancy, which exploit resources that are widely dispersed in resource-rich patches, had the highest tendencies towards grouping. On the contrary, animals that must tolerate extreme winter severity with diets dominated by relatively homogeneously distributed small vertebrate prey show almost no evidence of aggregating with conspecifics. We, therefore, provide empirical evidence to support the resource dispersion hypothesis as one of the key drivers of aggregation and highlight that our correlational evidence suggests that it is the critical interactions between physiology (e.g., energy storage capabilities to buffer against periods of resource scarcity) and resource constancy and dispersion in the environment, that underpin tendency of animals to aggregate and form groups. We make the case that while species may be predisposed to a certain level of solitariness, we should be cautious in the use of discrete generalizations of solitary or social, with a dynamic spectrum of environment-dependent likelihood of group formation possibly exhibited within any single species.
Here, we focused on how both physiological factors related to energy storage (body weight) and resource availability influence grouping behavior. Following predictions of the resource-variance life history model and the resource dispersion hypothesis, we observe that the dispersion of resources, measured here as the species-specific ratio of homogenously vs. patchily dispersed resources in the diet, was the best predictor of probability of being detected in groups. In our examination of species that are all considered to be intra-sexually territorial and solitary (17), we observed that species that relied primarily on homogenously dispersed prey (e.g., fisher) were less likely to associate with conspecifics. In contrast, the two species with the highest probability of being observed in groups (yellow-throated marten and tayra) use patchily distributed resources most extensively and in most of their sampled range live in environments that do not undergo prolonged periods of winter-induced resource scarcity.
For these latter two species, natural history notes have recently emerged describing observations of truly social behaviors, specifically, cooperative hunting of prey significantly larger than themselves. Yellow-throated martens have been reported to prey upon macaques (Macaca fascicularis) and muntjac (Muntiacus sp.) in pairs and threes (36); whilst tayra have been observed to hunt large species such as armadillo (Dasypus sp.) in groups (49). Under certain circumstances, a key benefit resulting from aggregation with conspecifics appears to be the development of social behaviors facilitating access to new resources in the form of significantly larger prey. Thus, while the resource dispersion hypothesis describes how groups can form in the absence of any functional advantage to any individual from the presence of others, we highlight that consideration of resource-based factors and the benefits and costs of spatial aggregations are not dichotomous, or alternative theories, but rather, the use of rich and patchily distributed resources are a prerequisite for tolerance of conspecifics, and thus necessary for, but not a certain predictor of more complex associations and behaviors.
Our results also provide mixed evidence regarding the hypothesis that low energy-storing capabilities constrains martens in terms of group formation (35). The interaction term with mean annual temperature difference highlights that physiological factors must be considered alongside winter severity/resource constancy to be meaningful. We see that in less extreme environments that do not undergo annual periods of resource scarcity induced by severe winter conditions, probability of being observed in groups scales with body weight; however, this is not the case in environments where annual temperature change is ≥30 °C. If energy-storing capabilities were sufficient to predict group formation, the wolverine, which is threefold-to-fourfold heavier than the next largest species would be the most frequently detected in groups. It is important to highlight that due to the conservative nature of our grouping metric, even species with very low probability of being detected in groups are not necessarily precluded from displaying social associations and complexity (see below). The nature and magnitude of the benefits of grouping are expected to vary across species and habitats (50); as seen here [and in other species, such as Chinese ferret badgers (Melogale moschata), which form groups in sub-tropical environments (51)], proclivity toward aggregation in groups is dynamic, with specific local conditions being critical.
Whilst tolerance of conspecifics and group formation may facilitate strategies to better exploit available resources as with the cooperative hunting observed in the yellow-throated marten and tayra, other potential benefits exist (52). For example, cooperation in the rearing of offspring can increase survival rates (53, 54). Such systems that include mated pair familiarity, extended tolerance of sub-adults, and male parental association with participation in young rearing have recently been observed in two of the “solitary” species we examine here, notably, these two species had the next highest probabilities of aggregating into groups as determined by our global camera trapping analysis, e.g., the wolverine (38) and the pine marten (55). The low predicted probabilities of grouping by our metric for these species, despite observations of such behaviors may have been due to our exclusion of data targeting dens, where such behaviors would have been observable. We hypothesize that the development of cooperative behaviors is facilitated by extended natal philopatry, occurring only where resource-related factors permit it. Where absent (e.g., individuals using more HDR in low resource constancy environments), juveniles would be expected to disperse early, thus limiting the potential for the development of such behaviors.
Our approach is observational. We have collated a large globally derived dataset and applied a simple quantification of group formation, a binary coding of detections as individual or in groups. Whilst we recognize this is a coarse metric, lacking information on relationships of groupings, many are plausible [e.g., parent-offspring (mature or dependant), sibling coalitions, breeding pairs, unrelated same-sex groupings] or the nature or purpose of relationships (e.g., tolerance only without cooperation, predator avoidance, thermoregulatory, cooperative foraging). We nonetheless provide proof of concept for how simple quantification of an otherwise difficult to measure and assess life history trait such as group formation can open opportunities for empirical examination and testing of general ecological theories. We are witnessing an exponential growth in the availability of such datasets (sensu 56) as camera trapping has become mainstream and is employed as a critical and everyday tool by conservationists globally (57, 58). A simple metric such as this facilitates the use of a globally distributed dataset to empirically examine questions that are otherwise difficult to address.
Nonetheless, we must be cautious in our inference, with various probabilistic factors that could impact the likelihood of individuals being photographed together. For example, the variable territory sizes and densities observed in the species complex [ranging from 1 to 2.2 km2 in pine martens (59, 60), to up to approximately 796 km2 in wolverines (61, 62)] results in notable variation in the probability that animals are co-located by chance alone. Despite this potential for variation resultant from interspecific heterogeneity in space use, this did not appear to be an important factor within this analysis (territory size was colinear with weight, which was included in the models). This is likely underpinned by the fact that all species in the complex are intra-sexually territorial and exclude same-sex conspecifics.
From a methodological perspective, we observed a strong positive effect of scent/bait at a camera station associated with grouping. This could tentatively be used to provide additional support for the influence of patchy resources promoting group tolerance in the sense that some of the baits used were food-based and thus represent valuable and patchily distributed resources in the environment that may act to relax agonistic behaviors and promote aggregation at the cameras [as observed by Pulliainen et al. (63) for pine martens at deer carcasses, a behavior termed “martelism”]. Yet, our examination of potential different responses to food rewards vs. scent lures vs. no bait showed no difference in response to two attractant types (SI Appendix: Bait Covariate Parameterization). Thus, it appears probable that the use of scents/baits simply decreases the probability of false negatives by causing animals to pause and investigate the scent or food resource. The use of scents/baits may increase the likelihood of detecting when animals are in groups compared to non-baited cameras where fast-traveling animals and small image viewsheds result in expected low probabilities of detecting animals in a group, even when they are in a group. Thus, the effect of scents/baits we see may be resultant from changes in detection probability, not the true state of the social unit (solitary vs. in a group). An additional source of potential heterogeneity in detection probability stems from variation in the detection cone of cameras, which may differ between individual camera deployments and across study regions, with the field of views of individual cameras impacting likelihood of detecting animals together. In these considerations, we see that the approach has limitations. The nature and structure of these data, which lack replicates at the sample level (i.e., image), makes it challenging to distinguish between methodological impacts on detection probability and the true latent grouping. Even with this limitation, we argue that the simple quantification of aggregation used here is an effective empirical tool when used conservatively on an appropriate model system to test the prevailing theories that underpin our understanding of group formation and its drivers. We find a wealth of variation and evidence of grouping across an animal group that has long been used as an exemplar of species that are constrained to a solitary lifestyle (35).
In conclusion, we find in the Martes complex model system that it is a species’ environment and the constancy and distribution of resources that underpin an individual’s propensity to aggregate with conspecifics, a prerequisite for associations and more complex social behaviors. We find evidence to support the role of interactions between climate and energy-storing capabilities in facilitating the tendency toward grouping displayed by species. Inescapably, we observe where resources are homogeneously distributed, and individuals undergo prolonged resource scarcity, groups cannot form. We hypothesize that these results are likely to be widely generalizable outside the taxonomic unit of the model system. The wide variety of propensities to aggregate here support the need to recognize underlying complexities in the social organization and behaviors of apparently “solitary” species. Failure to do so limits our understanding not only of the individual species but also the causes and consequences of group formation and ultimately sociality.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We would like to thank Megan Whatton, Belden Giman, Hongliang Bu, Dajun Wang, Fang Wang, and Roland Kays for their roles in contributing data that was used in this analysis. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the US Government.
Author contributions
J.P.T., A.Z., M.V.C., and R.A.P. designed research; J.P.T. performed research; J.P.T. analyzed data; C.S. provided supervision, mentorship, and extensive discussion and was critical in reviewing and revising the manuscript; A.Z. data contributor, provided comments and revisions to paper; M.V.C., O.R.W., J.H., A.W., E.M., P.B., A.M., B.E., B.D.G., T.J.M., L.S.G., J.M., A.E.M., I.W., J.L., J.A., D.D., W.M., S.M., L.P., A.J.B., S.L., R.B.R., R.S., Á.J.V.T., C.L.-G., N.E.L.-D., O.C., C.N.W., J. Bamber, F.S., J.F., A.K.F., K.A.P., and R.A.P. contributed data and to manuscript revisions; J. Birks contributed to ideas and manuscript revisions; and J.P.T., C.S., J. Birks, O.R.W., J.H., A.W., E.M., P.B., A.M., B.E., B.D.G., T.J.M., L.S.G., J.M., A.E.M., I.W., J.L., J.A., D.D., W.M., S.M., L.P., A.J.B., S.L., R.B.R., R.S., Á.J.V.T., C.L.-G., N.E.L.-D., O.C., C.N.W., J. Bamber, F.S., J.F., A.K.F., K.A.P., and R.A.P. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Although PNAS asks authors to adhere to United Nations naming conventions for maps (https://www.un.org/geospatial/mapsgeo), our policy is to publish maps as provided by the authors.
Data, Materials, and Software Availability
CSV data have been deposited in Dryad (https://doi.org/10.5061/dryad.sn02v6x8c) (64).
Supporting Information
References
- 1.Bekoff M., Daniels T. J., Gittleman J. L., Life history patterns and the comparative social ecology of carnivores. Ann. Rev. Ecol. Syst. 15, 191–232 (1984). [Google Scholar]
- 2.Gittleman J. L., Carnivore life history patterns: Allometric, phylogenetic, and ecological associations. Am. Nat. 127, 744–771 (1986). [Google Scholar]
- 3.Sandell M., “The mating tactics and spacing patterns of solitary carnivores in Carnivore Behaviour, Ecology, and Evolution, Gittleman J. L., Ed. (Comstock Publishing Associates, Cornell University Press, Ithaca, NY, 1989), pp. 164–182. [Google Scholar]
- 4.Caro T. M., Collins D. A., Male cheetah social organization and territoriality. Ethology 74, 52–64 (1987). [Google Scholar]
- 5.Waser P. M., Keane B., Creel S. R., Elliott L. F., Minchella D. J., Possible male coalitions in a solitary mongoose. Anim. Behav. 47, 289–294 (1994). [Google Scholar]
- 6.Lührs M. L., Dammhahn M., Kappeler P. M., Strength in numbers: Males in a carnivore grow bigger when they associate and hunt cooperatively. Behav. Ecol. 24, 21–28 (2013). [Google Scholar]
- 7.Graw B., Kranstauber B., Manser M. B., Social organization of a solitary carnivore: Spatial behaviour, interactions and relatedness in the slender mongoose. R. Soc. Open Sci. 6, 182160 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoener T. W., Resource portioning in ecology communities. Science 185, 27–39 (1974). [DOI] [PubMed] [Google Scholar]
- 9.Alexander R. D., The evolution of social behavior. Ann. Rev. Ecol. Syst. 5, 325–383 (1974). [Google Scholar]
- 10.Johnson D. D. P., Kays R., Blackwell P. G., Macdonald D. W., Does the resource dispersion hypothesis explain group living? Trends Ecol. Evol. 17, 563–570 (2002). [Google Scholar]
- 11.Albery G. F., et al. , Negative density-dependent parasitism in a group-living carnivore. Proc. R. Soc. B. 287, 20202655 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kappeler P. M., van Schaik C. P., Evolution of primate social systems. Int. J. Primatol. 23, 707–740 (2002). [Google Scholar]
- 13.Kappeler P. M., A framework for studying social complexity. Behav. Ecol. Sociobiol. 73, 13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeberg T. M., Dunbar R. I. M., Ord T. J., Social complexity as a proximate and ultimate factor in communicative complexity. Philos. Trans. R. Soc. B 367, 1785–1801 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrish J. K., Edelstein-Keshet L., Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 284, 99–101 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Struhsaker T. T., Correlates of ecology and social organization among African cercopithecines. Folia Primatol. 11, 80–118 (1969). [DOI] [PubMed] [Google Scholar]
- 17.Powell R. A., “Effects of resource productivity, patchiness and predictability on mating and dispersal strategies” in Comparative Socioecology of Mammals and Humans, Standen V., Foley R. A., Eds. (British Ecological Society Symposium, Blackwell, Oxford, 1989), pp. 101–123. [Google Scholar]
- 18.Carr G. M., Macdonald D. W., The sociality of solitary foragers: A model based on resource dispersion. Anim. Behav. 34, 1540–1549 (1986). [Google Scholar]
- 19.Macdonald D. W., Johnson D. D. P., Patchwork planet: The resource dispersion hypothesis, society and the ecology of life. J. Zool. 295, 75–107 (2015). [Google Scholar]
- 20.Standen V., Foley R. A., Comparative Socioecology: The Behavioural Ecology of Humans and Other Mammals (Blackwell Scientific Publications, Oxford, UK, 1989), p. 519. [Google Scholar]
- 21.Macdonald D. W., Newman C., “Mustelid sociality: The grassroots of society” in Biology and conservation of Musteloids, Macdonald D. W., Newman C., Harrington L. A., Eds. (Oxford University Press, UK, 2017), pp. 167–188. [Google Scholar]
- 22.Powell R. A., Mustelid spacing patterns: Variations on a theme by Mustela. Z. Tierpsychol. 50, 153–165 (1979). [Google Scholar]
- 23.Facka A. N., Powell R. A., Intraspecific competition, habitat quality, niche partitioning, and causes of intrasexual territoriality in a reintroduced carnivoran. Front. Ecol. Evol. 9, 734155 (2021). [Google Scholar]
- 24.Koepfli K. P., Dragoo J. W., Wang X., “The evolutionary history and molecular systematics of the Musteloidea” in Biology and Conservation of Musteloids, Macdonald D. W., Newman C., Harrington L. A., Eds. (Oxford Univeristy Press, Oxford, 2017), pp. 75–91. [Google Scholar]
- 25.Spaulding M., Flynn J. J., Phylogeny of the Carnivormorpha: The impact postcranial characters. J. Syst. Paleontol. 10, 654–677 (2012). [Google Scholar]
- 26.Johnson D. D. P., Macdonald D. W., Newman C., Morecroft M. D., Group size versus territory size in group-living badgers: A large-sample field test of the resource dispersion hypothesis. Oikos 95, 265–274 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magon N., Kalra S., The orgasmic history of oxytocin: Love, lust, and labor. Indian J. Endocrinol. Metab. 15, 156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caldwell H. K., Stephens S. L., Young W. S., Oxytocin as a natural antipsychotic: A study using oxytocin knockout mice. Mol. Psychiatry 14, 190–196 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Buskirk S. W., Harestad A. S., Raphael M. G., Powell R., Martens, Sables, and Fishers: Biology and Conservation (Cornell University Press, Ithaca, NY, 1994). [Google Scholar]
- 30.Zalewski A., Jędrzejewski W., Spatial organisation and dynamics of the pine marten Martes martes population in Białowieza Forest (E Poland) compared with other European woodlands. Ecography 29, 31–43 (2006). [Google Scholar]
- 31.O’Mahony D. T., Socio-spatial ecology of pine marten (Martes martes) in conifer forests, Ireland. Acta Theriol. 59, 251–256 (2014). [Google Scholar]
- 32.Brown J. H., Lasiewski R. C., Metabolism of weasels: The cost of being long and thin. Ecology 53, 939–943 (1972). [Google Scholar]
- 33.Harlow H. J., “Trade-offs associated with the size and shape of American martens in Martens, Sables and Fishers: Biology and Conservation, Buskirk S. W., Harestad A. S., Raphael M. G., Powell R. A., Eds. (Cornell University Press, Ithaca, NY, 1994), pp. 391–403. [Google Scholar]
- 34.Lindstedt S. L., Boyce M. S., Seasonality, fasting endurance, and body size in mammals. Am. Nat. 125, 873–878 (1985). [Google Scholar]
- 35.Newman C., Zhou Y. B., Buesching C. D., Kaneko Y., Macdonald D. W., Contrasting sociality in two widespread, generalist, mustelid genera, Meles and Martes. Mamm. Study 36, 169–188 (2011). [Google Scholar]
- 36.Twining J. P., Mills C., Cooperative hunting in the yellow-throated marten (Martes flavigula): Evidence for the not-so-solitary marten? Ecosphere 12, e03398 (2021). [Google Scholar]
- 37.Genovesi P., Sinibaldi I., Boitani L., Spacing patterns and territoriality of the stone marten. Can. J. Zool. 75, 1996–1971 (1997). [Google Scholar]
- 38.Copeland J. P., et al. , “Social ethology of the wolverine” in Biology and Conservation of Musteloids, Macdonald D. W., Newman C., Eds. (Harrington Oxford University Press, Oxford, UK, 2017), pp. 388–398. [Google Scholar]
- 39.Bates D., Mächler M., Bolker B., Walker S., Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015), 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 40.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna, 2022), https://www.R-project.org. [Google Scholar]
- 41.Running S., Mu Q., Zhao M., MODIS/Terra gross primary productivity 8-day L4 global 500m SIN Grid V061 (2021), distributed by NASA EOSDIS Land Processes DAAC, 10.5067/MODIS/MOD17A2H.06. [DOI]
- 42.Wan Z., Hook S., Hulley G., MODIS/Terra land surface temperature/emissivity monthly L3 global 0.05Deg CMG V061 (2021), distributed by NASA EOSDIS Land Processes DAAC, 10.5067/MODIS/MOD11C3.061. [DOI]
- 43.Felsenstein J., Phylogenies and the comparative method. Am. Nat. 125, 1–15 (1995). [DOI] [PubMed] [Google Scholar]
- 44.Garland T. Jr., Adolph S. C., Why not to do two-species comparative studies: Limitations on inferring adaptation. Physiol. Zool. 67, 797–828 (1994). [Google Scholar]
- 45.Zuur A. F., Leno E. N., Walker N. J., Saveliev A. A., Smith G. M., Mixed Effects Models and Extensions in Ecology with R (Springer, New York, NY, 2009). [Google Scholar]
- 46.Burnham K. P., Anderson D. R., Model Selection and Multimodel Inference: A Practical Information-Theoretical Approach (Springer-Verlag, New York, ed. 2, 2002). [Google Scholar]
- 47.Bartoń K., Multi-model inference ( Version 1.46.0, R Package, The Comprehensive R Archive Network, 2022).
- 48.Arnold T. W., Uninformative parameters and model selection using Akaike’s information criterion. J. Wildlife Manag. 74, 1175–1178 (2010). [Google Scholar]
- 49.Grotta-Neto F., et al. , The role of tayra (Eira barbara) as predator of medium and large-sized mammals. Austral Ecol. 46, 329–333 (2020). [Google Scholar]
- 50.Silk J. B., The adaptive value of sociality in mammalian groups. Philos. Trans. R. Soc. B 362, 539–559 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L., et al. , Ranging and activity patterns of the group-living ferret badger Melogale moschata in central China. J. Mammal. 91, 101–108 (2010). [Google Scholar]
- 52.Creel S., Macdonald D. W., Sociality, group size, and reproductive suppression among Carnivores. Adv. Study Behav. 24, 203–257 (1995). [Google Scholar]
- 53.Gittleman J. L., “Carnivore group living: Comparative trends” in Carnivore Behaviour, Ecology, and Evolution, Gittleman J., Ed. (Springer, Boston, 1989), pp. 183–207, p. 620. [Google Scholar]
- 54.Gubernick D. J., Teferi T., Adaptive significance of male parental care in a monogamous mammal. Proc. R. Soc. B 267, 20000979 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zalewski A., Alternative strategies in the acquisition of home ranges by male pine martens in a high-density population. Acta Theriol. 57, 371–375 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cove M. V., et al. , SNAPSHOT USA 2019: A coordinated national camera trap survey of the United States. Ecology 102, e03353 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Burton A. C., et al. , Wildlife camera trapping: A review and recommendations for linking surveys to ecological processes. J. Appl. Ecol. 52, 675–685 (2015). [Google Scholar]
- 58.Wearn O., Glover-Kapfer P., Snap happy: Camera traps are an effective sampling tool when compared with alternative methods. R. Soc. Open Sci. 6, 181748 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zalewski A., Jędrzejewski W., Jędrzejewska B., Mobility and home range use by pine martens (Martes martes) in a Polish primeval forest. Ecoscience 11, 113–122 (2004). [Google Scholar]
- 60.Twining J. P., et al. , All forests are not equal: Population demographics and denning behaviour of a recovering small carnivore in human modified landscapes. Wildlife Biol. 2020, wlb.0076 (2020). [Google Scholar]
- 61.Persson J., Wedholm P., Segerstrom P., Space use and territoriality of wolverines (Gulo gulo) in northern Scandinavia. Eur. J. Wildlife Res. 56, 49–57 (2009). [Google Scholar]
- 62.Dawson N. F., Magoun A. J., Bowman J., Ray J. C., Wolverine, Gulo gulo, home range size and denning habitat in lowland boreal forest in Ontario. Can. Field Nat. 124, 2010 (2010). [Google Scholar]
- 63.Pulliainen E., Use of the home range by pine martens (Martes martes L.). Acta Zool. Fenn. 171, 271–274 (1984). [Google Scholar]
- 64.Twining J., et al. , Using global remote camera data of a “solitary” species complex to evaluate the drivers of group formation [Dataset]. Dryad. 10.5061/dryad.sn02v6x8c. Deposited 16 February 2024. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
CSV data have been deposited in Dryad (https://doi.org/10.5061/dryad.sn02v6x8c) (64).