Significance
Important advances in human genetics and medicine occurred in parallel since the late 20th century. I tell here a personal historical perspective of the search for discovery and translation of AIDS Restriction Genes (ARGs), human loci with polymorphic variants that influence the outcome of HIV-1 exposure, infection, AIDS progression, or treatment. We employed the principles of population genetics to describe some 36 AIDS restriction genes of interest including CCR5-∆32, a common genetic variant that prevents HIV-1 infection in homozygous carriers. The translation for AIDS and other important diseases created a harbinger for the developing discipline of genetic epidemiology. As a research director at NIH, I began a search for human genes that influence prognoses of patients exposed or infected with HIV.
Keywords: CCR5, AIDS, genetic epidemiology, natural histories
Abstract
The discovery of the 32-bp deletion allele of the chemokine receptor gene CCR5 showed that homozygous carriers display near-complete resistance to HIV infection, irrespective of exposure. Algorithms of molecular evolutionary theory suggested that the CCR5-∆32 mutation occurred but once in the last millennium and rose by strong selective pressure relatively recently to a ~10% allele frequency in Europeans. Several lines of evidence support the hypothesis that CCR5-∆32 was selected due to its protective influence to resist Yersinia pestis, the agent of the Black Death/bubonic plague of the 14th century. Powerful anti-AIDS entry inhibitors targeting CCR5 were developed as a treatment for HIV patients, particularly those whose systems had developed resistance to powerful anti-retroviral therapies. Homozygous CCR5-∆32/∆32 stem cell transplant donors were used to produce HIV-cleared AIDS patients in at least five “cures” of HIV infection. CCR5 has also been implicated in regulating infection with Staphylococcus aureus, in recovery from stroke, and in ablation of the fatal graft versus host disease (GVHD) in cancer transplant patients. While homozygous CCR5-∆32/32 carriers block HIV infection, alternatively they display an increased risk for encephalomyelitis and death when infected with the West Nile virus.
Background and Discovery of CCR5-∆32
In the five decades since I first started my career in genetic medicine, there were many important advances and changes that would influence the research I encountered. Three happenings stood out to me. First, the field of human genetics matured from simple gene mapping to full human genome sequence analyses. Human genomics became a major influence in medicine and in epidemiology. Second, the emergence of HIV-AIDS profoundly changed medical science and human society. Third, science reporting in news media went from occasional coverage of medical discoveries to a steady almost daily coverage of medical science advances. These three influences comprise the background for the review I present here. Because there were many contributions, innovative and replicating (or not) reports, please understand the narrative I present is my story, a personal recollection of the scientific advances surrounding a “magic gene” and its allele, CCR5-∆32. This is a story that features the beauty and adventure of scientific puzzle solving around a fatal disease. Let me start with the beginning days of AIDS.
Forty-two years ago (June 1981), an article appeared in a widely read CDC publication “Morbidity and Mortality Weekly Report” (MMWR) describing five homosexual men suffering from an uncommon pneumonia caused by a ubiquitous Protozoan, Pneumocystis carinii, that rarely caused disease (1). These patients also had unusual mouth sores caused by a fungus, Candida albicans. A month later MMWR reported 26 new gay men in New York, San Francisco, and Los Angeles also with the same syndrome plus a very rare purple blotch skin cancer, Kaposi’s sarcoma (2). All were nonresponsive to conventional therapy. The cluster suggested that the immune system of these men was somehow disabled and that their disease appeared to be transmitted by an infectious agent. The age of AIDS had begun.
By 1984, the cause of AIDS was shown to be an RNA “lentivirus” (a slow-growing retrovirus related to scrapie, a neurological disease-causing virus originally discovered in sheep and horses) called human immunodeficiency virus (HIV-1). That discovery was first announced by Luc Montagnier and Françoise Barré-Sinoussi of Institute Pasteur in Paris (3). In a series of four detailed reports shortly thereafter in Science, Robert Gallo’s group of the NIH established the causal role of HIV-1 for AIDS. They also developed a blood test for HIV that was licensed in 1984, an event that rapidly led to the cleansing of western blood supplies from the deadly virus (4–8). In the coming years, HIV rapidly spread across the world through sexual transmission, contaminated blood transfusions, and clotting factors supplied to hemophiliacs. Since the beginning of the epidemic, some 85 million people have become infected with HIV and ~40 million people have died of HIV (9). This puts HIV and AIDS at a mortality and cost comparable to the Spanish flu and bubonic plague.
By the mid-1980s, our research laboratory at NIH had developed a reputation for the new fields of Comparative Genomics (using gene maps of mammal species to interpret the evolution of human genome organization) and Conservation Genetics (using genetic patterns in nontraditional endangered species as an approach to imputing their natural histories of population migration, adaptation, contraction, and survival) (10–13). In part because of the increased visibility of our charismatic creatures on the covers of leading science journals, I decided that I should develop a focused medical research program to solidify my NIH budget. It occurred to me that the blossoming field of population genetics might be joined with public health to search for what we termed “Restriction Genes” that could regulate the onset and progression of disease epidemics. We needed a common deadly epidemic and the choice was obvious: HIV- AIDS, a malady with no cure, no vaccine, no treatment, and > 90% mortality within ~10 years (9).
Our quest would not be an easy one. First, there was no proven precedent for finding disease-resistant genes in humans; second, human genetics was in its infancy. In the early 1980s, fewer than 1,000 genes (of the 22,000 in our genome) had been mapped or even described. Third, the vast majority of hereditary disease genes were mapped in family pedigrees, an impossible approach for AIDS restriction genes. Last, even as AIDS had become a full-blown epidemic, there was no direct evidence that human genes played a role in the disease. Indeed, more frequently than I care to recall, I encountered harsh skepticism over my hopeful and very pricey scheme. Some critics dubbed our project a scientific “fishing expedition”.
When I explained my audacious idea to an NCI colleague, she encouraged me to apply for a grant from a US Army Fund dedicated to AIDS research, which had just opened. When I forwarded my draft proposal to my boss, NCI Division Director Richard Adamson, he reacted strenuously:
… “Why do you want to ask the army for money? NCI has plenty of resources for this novel and important project?”
By 1985, he deposited over $1,000,000 into my laboratory budget the first year and in subsequent years for a hopeful project with no real discoveries or proof of concept, something I doubt would happen so easily today. Yet, since NIH had a near–billion-dollar research budget dedicated to AIDS vaccines, treatment, prophylaxis, and remedy, genetic factors seemed a worthy idea.
We began by recruiting a cadre of epidemiologists involved in developing longitudinal AIDS “cohorts” of patients at risk for HIV infection. These were actively gay men, hemophiliacs, and blood donor recipients who had received HIV-contaminated clotting factor, IV drug users who shared HIV-contaminated needles, and infants of HIV-infected mothers. I asked them to provide me with a single blood sample from the patients they enrolled and to record the longitudinal clinical data on each (13). We recruited Cheryl Winkler and Mary Thompson, talented cell biologists who oversaw the cell transformation lab, and Michael Dean, a crack molecular biologist who would develop DNA-based assays for candidate gene variants that were reported to play important roles in AIDS pathogenesis. I turned all the blood samples over to Thompson and Winkler who made immortal B lymphocyte cell lines using Epstein–Barr virus (EBV), known to transform B cells in vitro to establish lymphoblastoid cell lines, an immortal source of DNA from each patient. EBV was also well known to cause teenage mononucleosis, and in rare cases, nasopharyngeal carcinoma and Burkitt’s lymphoma.
Dean extracted the cell line DNA from thousands of study participants and screened candidate genes for mutational variants in the cohort. Employing the principles of population genetic equilibria, we were searching for distortions in allele or genotype frequencies by comparing the AIDS disease cases versus controls. We further searched for association signals in Kaplan–Meier survival plots tracking AIDS progression since the patients’ infection in longitudinal cohorts.
For years, we continued to add more patients, more genes, more genetic variants, and improved computer algorithms to search for altered allele or genotype distributions. By the mid-1990s, we had screened thousands of patients, hundreds of candidate genes, plus DNA variants just outside coding genes. Occasionally, we thought we spotted a genetic difference between case and control patients, but they all disappeared under closer inspection. Meanwhile, we would search the AIDS literature, for new genes connected to AIDS pathology, Finally, eleven years after we had begun what was becoming a tedious, expensive, and thus far disappointing search, there appeared a glimmer of hope. Two dramatic advances would occur rapidly. The year was 1996.
The first advance was the announcement from the Vancouver International AIDS Congress in January that the “Highly Active Anti-Retroviral Treatment-HAART” for AIDS patients using three different anti-HIV drugs targeting viral genes: (inhibitors of NRTI and NNRTI reverse transcriptase and HIV protease) could reduce HIV to levels undetectable, <40 particles/mL in serum of AIDS patients (14, 15). Widespread use of the HAART drug treatment over the next several years would slow AIDS progression and cut AIDS mortality in western countries dramatically. The treatment led to a 60 percent drop in the U.S. death rate, from 50,610 AIDS mortalities in 1995 to 16,273 in 1999 (9).
The second advance was a series of revelations that led to the appreciation of CCR5-∆32 as the first verifiable AIDS Restriction Gene (ARG) allele. In December 1995, Paul Lusso, Robert Gallo, and their collaborators demonstrated that a series of cellular chemokine ligands for the CCR5 receptor (CCL3,4 and 5) blocked HIV from entering CD4 T-lymphocytes (16) Chemokine ligands are designed to ablate inflammation in joints and bruises by attracting chemokine receptors protruding from the cell surface membranes of CD4 T-lymphocytes. In the week of July 1, 1996, five separate groups published near simultaneously in Cell, Nature, and Science, the identification of three requisite co-receptors by which HIV entered lymphoid cells: CD4, and chemokine receptors CCR5 and CXCR5 (17–20).
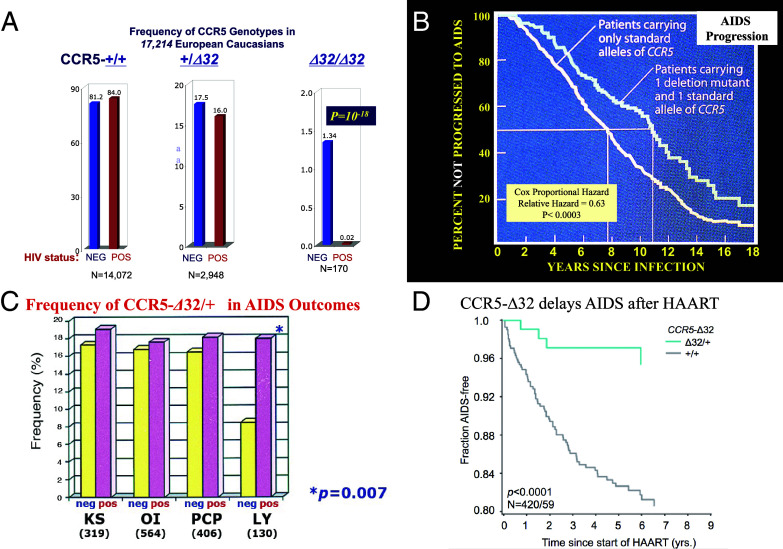
These reports invigorated my research team to rapidly sequence the three HIV receptor genes in our AIDS-cohort patients (21). We immediately identified a 32-bp deletion in the CCR5 receptor gene (Fig. 1). The CCR5-∆32 variant was common among our patients, reaching a 10% allele frequency in predominantly European American individuals. At first, we were disappointed to see little difference in the CCR5-∆32 allele frequency in HIV-infected versus uninfected individuals. The big surprise was the distribution of CCR5 genotypes in the same two categories. Although CCR5 genotypes in uninfected people conformed to Hardy–Weinberg equilibrium (HWE) (a paradigm that predicts the genotype frequencies in a population will distribute as a binomial distribution of allele frequencies), we were stunned to see zero CCR5-∆32/∆32 homozygous people among HIV-infected patients. HWE expectation predicted we would see 16 homozygotes in our 1,343 HIV-positive study participants (Fig. 2A and ref. 21). In subsequent studies of nearly 20,000 infected people, we saw virtually no infected CCR5-∆32/∆32 homozygotes producing an extraordinary statistical P-value of 10−18 for departure from HWE (Fig. 2A and refs. 21–25).
Fig. 1.
(A) CCR5 is a 7-transmembrane-spanning molecule sitting on T lymphocytes. CCR5 is a co-receptor along with CD4 for HIV-1 entry as a prelude to producing over one billion copies in HIV-1 particles circulating in AIDS patients (17–20). (B) The CCR5-∆32 variant present in European Caucasians interrupting the reading frame, leading to missense encoded protein and a premature chain-terminating nonsense codon (21–25)
Fig. 2.
Influence of CCR-∆32 on different stages of HIV AIDS. (A) HIV transmission/acquisition. The frequency (percentage) of CCR +/+. +/∆32 and ∆32/∆32 genotypes among HIV infected versus HIV uninfected people in cumulative AIDS Cohorts [Total N = 17,214 study participants (21–25)]. (B) AIDS progression in HIV-infected people. Kaplan–Meier survival curve comparing the rate of AIDS onset in CCR +/+ versus CCR5-+/∆32 (21, 22, 25). (C) Relative frequency of CCR5-∆32 allele among AIDS patients with different AIDS-defining conditions: Kaposi’s sarcoma, Pneumocystis carinii pneumonia, opportunistic infections, and B cell lymphoma (26). (D) Failure of HARRT is ablated by individuals carrying the CCR-+/∆32 genotype (27).
Because the ∆32 deletion was not in the DNA code reading frame (i.e., divisible by 3 bp), it would lead to missense and stop codons downstream from the deletion (Fig. 1B). The deletion allele produces a truncated protein that is detected as a missense peptide and digested by a cell’s endoplasmic reticulum security detail, resulting in people without any CCR5 receptors on their CD4-bearing T-lymphocytes. The CCR5-∆32/∆32 homozygosity shut the door for HIV infection; the cell entry portal was missing.
Later, the CCR5-Δ32 identification encouraged us and other AIDS researchers to search further through HIV-positive patient collections for rare CCR5-Δ32/32 patients. Over the next few years, a small handful of homozygous CCR5-Δ32/32 patients were found to be HIV infected (28–32). However, when the virus in these unfortunate exceptions was examined, they were infected with CXCR4-utilizing HIV strains. These CXCR4-utilizing HIV strains appear de novo in late-stage AIDS patients when their CD4 levels fall below 200 CD4 cells per ml of blood.
Our report of CCR5-Δ32 mediating HIV resistance appeared in Science in September 1996 (21). But we were not the only ones to discover the mutation (23, 24, 33); William Paxton, Richard Koup, and Ned Landou also discovered the same mutation in two gay men who had mysteriously avoided infection despite their admission of multiple episodes of risky sex with HIV-infected partners (24). Paxton was unable to infect their blood cells with HIV in his laboratory, prompting him to reason in their August paper that CCR5-Δ32 was a genetic shield for the two men. Our report, standing on nearly 2,000 AIDS patients in September, proved it beyond any doubt (21).
People who were heterozygous for CCR5-+/ Δ32 do get infected with HIV; however, the onset of AIDS-defining conditions is postponed for approximately 2 years on average (Fig. 2B and refs. 21 and 33). That effect seems to reflect a decrease in B-cell lymphoma, an AIDS-defining condition, which is cut in half in CCR5-+/ Δ32 heterozygotes (Fig. 2C and ref. 26). In addition, AIDS patients treated by HAART live longer when they are CCR5-+/ Δ32 heterozygotes due to faster viral load suppression and slower progression to AIDS than CCR5-+/ + patients on HAART (Fig. 2D and ref. 27). In genetic terms, CCR5- Δ32 exerts a recessive protection from HIV infection, and a dominant restriction on AIDS progression, AIDS-defining condition-B cell lymphoma, and AIDS failure upon HAART therapy.
Research on CCR5 and CCR-∆32 has exploded in the decades since the initial reports. The number of Google search hits for CCR5 and CCR5-∆32 (4,660,000 and 32,700 respectively) is evidence of their wide research interest and derivative applications. CCR5 is not the only AIDS restriction gene reported. Our group used similar techniques including genome-wide association studies to report some 40 additional markers that show distortions in population genetic equilibria when tested in AIDS cohorts, numbering some 20,000 study participants (25, 34, 35). Influences of important human regulatory loci (HLA, KIR, CCR2, SDF, TRIM5, IL10, APOBEC, PROX1, PARD3B, and many others) have been reported and replicated by ourselves and by other groups to influence the dynamic stages of HIV-AIDS (34, 35). Most of these ARGs were revealed in European American cohorts, but there were also several additional ARG effects identified in African study cohorts (viz. CCR2, AP3B1, PTPRA, NEO1, and HCG22; refs. 36–38).
A critical question involved an estimation of the fraction of the patients in each stage of HIV-AIDS progression who were actually affected by their genotypes at CCR5 or at the other AIDS Restriction Genes. George Nelson developed a mathematical approximation to estimate the “Explained Fraction” (EF) for this question based on mutual information theory (35, 39). EF quantifies the relative influence of multiple associated factors (genetic, environmental, behavioral, even stochastic) for disease progression and is additive up to a maximum of 100%. In an analysis of the kinetics of AIDS progression after HIV infection in our cohorts, the EF of CCR5-∆32 was small (0.1%) and the composite EF of fourteen ARGs considered together in our cohorts was 9.5%. This means that >90% of the epidemiological variance for AIDS pathogenesis is not explained by the sum of known ARG variants. Although the 9.5% EF for AIDS progression may seem modest, the fraction is comparable to the EF for smoking influence on lung cancer (9.7 to 11.5%), a well-known epidemiological influence (35).
Translation of CCR5-∆32 to Anti-AIDS Therapies
People homozygous for CCR5-∆32 seemed relatively healthy for long periods indicating that CCR5 function itself may be dispensable in the human genome (40). This possibility gained support from the genomic redundancy of distinct human chemokine receptors that also interact with specific chemokine ligands of CCR5 (CCL3 MCP-1å, CCL4-MCP-1ß, and CCL5-RANTES); (41, 42). The chemokine ligands can bind and simulate at least six chemokine receptors, explaining why the CCR-∆32 genetic deletion might be innocuous to carriers.
The knowledge that CCR5 is required for HIV-1 infection of lymphoid cells plus the suspicion that CCR5 function is dispensable for good health stimulated pharmaceutical development of a dozen new anti-AIDS treatments termed “HIV entry inhibitors”. Entry inhibitors were designed to interfere with HIV-CCR5 cell receptor binding as a block of HIV pathogenesis (43–46). Two powerful HIV entry inhibitors, enfuvirtide- T20 and maraviroc, were approved for AIDS patient treatment by the FDA in 2003 and 2007 respectively. These compounds had disadvantages in that they were expensive and needed to be delivered by injection. Nonetheless, they proved valuable for individuals who had developed resistance to the anti-HIV drugs, as alternative treatments. The rapid development of this avenue for AIDS therapy was clearly encouraged by the characterization of HIV resistance described in CCR5-∆32/∆32 homozygotes (Fig. 2).
Origins of CCR5-∆32
There are certain interesting details of the CCR5-∆32 mutational variant that were remarkable. First, the neighboring DNA variants closely adjacent to the CCR5-∆32 allele on chromosome 3 are the same “haplotype” (the constellation of adjacent non-CCR5 DNA allelic variants) in all CCR5-∆32 bearing chromosomes, which indicates that the mutation that caused it occurred only once. Also, CCR5-∆32 is non-existent in pure Asian or African population groups (Fig. 3A and ref. 47). This could only mean that the original ∆32 mutation had occurred sometime after the “Out of Africa” migration of human ancestors that proceeded the founding of modern human populations in Europe and Asia ~ 80,000 BP.
Fig. 3.

(A) Allele frequency cline for the CCR5-∆32 allele today in Europe, the Middle East, and Africa (47–49). (B) Amino acid substitutions encoded by alleles within the CCR5 gene as detected in a survey of 1,400 individuals across the world (49, 50). (C) Spread of bubonic plague in Europe from 1348 to 1353 (48, 51–54).
Since we know that the European population since that time grew to 1 to 2 million people, how did a spontaneous new mutation in a newborn baby go from a frequency of ~1/2,000,000 to a frequency of 10% in modern Europe? The simplest explanation seemed to be that some undiscovered large historic natural selection pressure had favored the rise of CCR5-∆32 in Europe.
Perhaps relevant, Mary Carrington and Mike Dean on our team decided to sequence CCR5 in ~1,400 people unrelated to AIDS across the globe to discover additional single-base substitutions (49, 50). They identified 22 new variants other than CCR5-∆32 in the CCR5 gene (Fig. 3B). It seemed very strange that 18 of the 22 discovered CCR5 variants (82%) were codon altering in the gene sequence or as geneticists say “non-synonymous” substitutions (i.e., altering the encoded amino acid). This was unusual because the majority of new spontaneous substitutions screened in hundreds of human genes examined to date were synonymous, because most non-synonymous variants are deleterious or harmful to a gene’s function so they are eliminated quickly by natural selection. Overall, nearly all human genes surveyed have <25% of their genetic substitutions as nonsynonymous. Not so for CCR5 (Fig. 3B)!
One important exception involves the antigen recognition site region of the HLA Class 1 (A, B, and C) molecules (55). The Antigen Recognition Site (ARS) comprises the amino acid residues in the HLA Class 1 protein that bind to incoming foreign molecules from viruses and other infectious agents to mediate their removal. The reason for elevated non-synonymous variants in the HLA-ARS has to do with historic selection pressure on HLA recognition structure inflicted by emerging infectious agents that challenge the immune system so frequently in history. New mutational variants in HLA alter immune capacity, allowing recognition and clearance of novel pathogens. Widespread outbreaks elevate new non-synonymous variant frequencies as a direct consequence of the pathogen’s exposure.
As HLA is clearly the object of strong selection for adaptive variation, then by comparison, so must CCR5. It now is known that several other infectious agents co-opt the CCR5 molecule as a step to infect human lymphocytes (56–58). This situation persuaded us all that CCR5-∆32 and probably CCR5 itself have been a continued object of strong natural selection in their history, likely by different infectious agents that like HIV try to co-opt CCR5 molecule to enter cells and wreak havoc.
Another observation that weighed into this selective idea was the phenomenon of a gene frequency gradient (evolutionary biologists call this a “cline”) for CCR5-∆32 across Europe (Fig. 3A). The allele frequency of CCR5-∆32 is highest in northern Europe: 13 to 15% in Scotland and Scandinavia; 9 to 11% in Germany/France; 4 to 6% in Italy, Greece and Turkey; 0.0% in Saudi Arabia and Africa (47–50). A cline is an indication of strong selective pressure to high frequency in one spot that got diminished to lower allele frequencies by subsequent migration. Again, the CCR5-∆32 cline supported the inference of a historic selective pressure. Most observers across the evolutionary biology discipline agreed that CCR5-∆32 must have been the object of extraordinary selective pressure (47–50)
But what was the selective pressure? Where was it? And when was it? The pressure would have favored even augmented the survival of CCR5-∆32 carriers as it does protect from HIV-AIDS. The selective agent was probably not AIDS because AIDS has not been afflicting human populations for enough time. HIV emerged to humankind from the bushmeat trade in African chimpanzees in the early 20th century (59), much too recent to cause CCR5-∆32 allele elevation in a few generations. There are plenty of other deadly epidemics that afflicted humankind in recent millennia including smallpox, tuberculosis, typhus, anthrax, cholera, typhoid, plague, and others. We needed more data.
To get at this, I wondered whether we could compute the age of the CCR5-∆32 mutation or more precisely the last time that selective elevation of CCR5-∆32 occurred. We knew that the chromosomal region adjacent to the CCR5 gene had scores of adjacent Single Nucleotide Polymorphisms (SNPs) and microsatellite loci that were the same precise constellation of alleles, termed a haplotype, surrounding the CCR5-∆32 variant. On the day a new mutation occurs on a chromosome, that entire chromosome has a certain SNP haplotype, but the length of the original SNP haplotype gets shortened by recombination each generation thereafter. The actual length of non-random haplotype stretches on either side of CCR5-∆32 would then be inversely proportionate to the time elapsed since the original ∆32 mutation event or more accurately the most recent strong selective pressure favoring the CCR5-∆32 allele.
I asked David Reich and Clay Stephens to develop an algebraic formula that could approximate the time elapsed since the last selective pressure that favored CCR5-∆32 based upon today’s haplotype length. Their equation related four measurable parameters: the chromosomal length today of the haplotype bearing CCR5-∆32, the haplotype frequency, the recombination rate in the region, and human generation time. Their equation predicted the number of generations and an approximate date of the last CCR5-∆32 allele elevation at 682 B.P. (47). That period (mid-14th century) in Europe coincided precisely with the Black Death or bubonic plague (1347–1352). Immediately the medieval plague rose to the top of the possible disease epidemics to consider for the breathtaking selective pressure that mediated the rise of CCR5-∆32 from an initial 1/2,000,000 to a frequency of 10% in modern Europe.
The Black Death posed an amazing scourge throughout Europe taking an estimated 30 to 40 million lives within a 5-y period (51–54, 60). Hundreds of books and narratives fill our libraries about the bubonic plague. A few features bear repeating here. First, plague victims first develop egg-sized swellings in the lymph nodes of their armpits pits and groin, the “buboes” from which bubonic plague got its name. Within days of the appearance of the buboes, sufferers acquired high fever, delirium, and hemorrhagic dark splotches, the mark of the plague. Plague is caused by a bacterium, Yersinia pestis, first described 350 y later in 1894 by Alexandre Yersin and Shibasaburo Kitasato.
Yersinia pestis emerged from a less lethal form that was endemic to rodents somewhere in Asia (12, 51–54, 60). An avirulent form thrives in the blood of over a dozen rodent species and had adapted itself to transport by fleas. The 14th-century plague likely started in Mongolian steppes, in Himalayan valleys or in Burmese ghettos. Consequent Asian mortalities were immense. The Chinese population would be cut in half by plague and subsequent famine, falling from 123 million in 1200 AD to 60 million by 1352. The first mortalities in Asia were recorded in the Gobi Desert where marmots carried the Yersinia. Trappers collected marmot pelts and sold them to dealers for transport west over the Silk Road to Kaffa, a Genoese seaport on the north coast of the Black Sea (Fig. 3C). Infected rats, pelts, and plague victims reached the teeming Mediterranean port city of Messina, Sicily in October 1347. Dying sailors, corpses, rats, and the plague were offloaded before the ships were dispatched from the ports, too late to stop the invasion of plague into Europe.
In the next five years, the Black Death devastated European cities and countryside. By 1352, the plague had marched north from Italy through France, Germany, England, and Spain, north to Scandinavia, and then it spread east to Poland, Lithuania to return to within a few hundred miles of where it began near Kaffa, today a city in Crimea called Feodosia (Fig. 3C). Half the population of Italy and England succumbed; In Venice, three quarters of the population, 100,000 people, died. Eighty percent of the Genoese populace was lost. From London, reports of mortality as high as 90 percent were recorded. The Islands of Cyprus and Iceland were said to have been completely depopulated by plague (51–54, 60).
Our hypothesis that the plague interacted with CCR5-∆32 to raise its frequency was met with some skepticism by the scientific community. Some argued that the plague agent was a bacterium, not a virus like HIV. Others noted that there was not sufficient mortality in the bubonic plague to cause such a dramatic elevation in CCR5-∆32 allele frequency (48, 61). Others suggested that smallpox was a more likely selective pressure due to its greater mortality (62). One group suggested that bubonic plague was not a Yersinia disease, but rather an Ebola-like hemorrhagic fever virus (63). Very few argued that CCR5-∆32 was not elevated by selection by a deadly infectious disease; they mostly differed on what the disease agent actually was. Yet our reports suggesting the bubonic plague as a selective agent had notable features that lent additional support to that notion.
The Black Death in the 15th Century was neither the first nor the last wave of plague throughout European history (51–54, 60). There were plenty of others. The horrific disease reappeared 10 y after 1,352 with almost equal intensity. Periodic regional outbreaks would occur with alarming frequency and intensity once every generation for the next three hundred years. In the centuries following the Black Death, European population declined by 60 to 75 percent. The Great Plague that swept the British Isles in 1,665 claimed 70,000 lives. The last plague outbreak in Marseilles in 1772 devoured half of the city’s populace.
There were several regular plague scourges dating back to the 6th century reign of the Roman emperor, Justinian (64, 65). The Justinian plague first began in 541 AD, subsided, but reappeared in multiple waves until 750 AD. The ferocity of the Justinian plague was huge, killing 10,000 people a day in Constantinople, and decimating the Roman Empire. Estimates of the death toll were on the order of 40 million. These mortality estimates are crude approximations, but if they anywhere approach the actual numbers, the selective pressure on a plague resistance allele would have been massive.
In 2004, a study appeared in Nature that seemed to lend functional data to the plague’s influence on CCR5-∆32 (66). The study described a Yersinia pestis infection assay in mouse strains that had a CCR5 gene Knock-Out-KO (equivalent to CCR5-∆32/-∆32 homozygotes) compared to strains with wild-type or intact CCR5. The results were dramatic. Yersinia infected normal mouse macrophages (immune defense cells which are the initial cellular target of Yersinia in humans and mice), but Yersinia infection was reduced in macrophage entry 30-fold in the CCR5−/− gene knockout strains compared to in the CCR5 +/+ normal mouse macrophages in six replicate attempts (Fig. 4A). Thus, Yersinia pestis requires an intact CCR5 gene product to enter mouse macrophages, a functional albeit indirect genetic connection of CCR5 to plague infectivity.
Fig. 4.
(A) Uptake of Yersinia pestis in mouse macrophages is reduced 30× when CCR5 -knockout mice are exposed to the bacterium; six replicate experiments (66). (B) Infection of human monocytes by yVARV-HARPER strain of the variola-smallpox virus is not affected by CCR5-∆32 genotypes. Blood samples were obtained via venipuncture from persons of known CCR5 genotypes after informed consent. Monocytes were isolated by centrifuging whole blood over a histopaque gradient. Virulent orthopoxviruses monkeypox (MPXV) and smallpox variola virus (VARV) readily enter human monocytes with three genotypes (CCR5-+/+; CCR5-+/∆32 and CCR5-∆32/∆32). Monocytes with alternative CCR5 genotypes were each infected readily by variola/smallpox virus with equivalent kinetics in vitro, a result that would not support a role for smallpox in the historic rise of CCR5-∆32 in European populations Uptake results reflect an average of four replicate experiments.
When we first raised the idea of plague and CCR5-∆32, historic smallpox epidemics were suggested as an alternative selective agent (48, 62), particularly since rabbit myxoma, a pox virus, utilizes CCR5 to enter cells (67). At that time, it was impossible to test the smallpox/variola virus because the virus had been eradicated worldwide except for a few vials stored and planned for destruction at the CDC and in Russia. However, when the 9/11 attacks occurred in New York City, quickly thereafter, a series of anthrax-laden letters arrived from an unknown terrorist to TV studios, U.S. senators’ offices and a post office in Washington D.C. leading to five fatalities (68, 69). This bio-terror incident would cause a hesitancy on the part of health officials to destroy the remaining variola/smallpox stores at CDC for anticipated bio-defense concerns.
A few years later, the CDC decided to consider properly reviewed and regulated experiments with variola-smallpox to preserve biodefense for bioterrorism. I asked smallpox virologists, Robert Fisher and Peter Jahrling, at Ft. Detrick Biohazard Laboratories of USAMIRD in Frederick, Maryland, to apply with me for permission to perform a variola experiment at the CDC Biohazard laboratories to test human monocyte cells from three CCR5 genotypes (CCR5-+/+; CCR5-+/-∆32 and CCR5-∆32/∆32) for the kinetics of variola infection. Would the CCR5 genotypes exert a differential influence on variola infection? The results of four replicate uptake experiments indicated that variola/smallpox virus infected human monocytes of each CCR5 genotype with equivalent success (Fig. 4B) Hence, we conclude that the variola/smallpox virus does not require CCR5 product to infect human cells.
In 2002, I visited Eyam, a rural village near Manchester UK well known for its suffering during the English “Great Plague” of the seventeenth century (70, 71). The death toll in the tiny village of ~900 people was considerable. Of 80 households, 70 had at least one victim, most had more. The burials would reach 280 by the end of 1666, over 50 percent of the populace of Eyam. A hundred miles to the south in London tens of thousands succumbed to the massive scourge. In the spring of 1666, the great fire of London probably played a role in ending the dying.
My trip to Eyam was prompted by Jennifer Beamish, an inquisitive television producer for London’s Channel 4. She told me that the modern township of Eyam were predominantly descendants of the survivors of the Great Plague. Beamish wondered if many survivors might have been spared by their CCR5-∆32 bearing genotype. While making a documentary for Channel 4-UK and PBS in the United States (72), we invited over 90 Eyam citizens with direct relatives from the original Eyam plague survivors to volunteer a cheek swab to assess their CCR5 genotypes. We had expected that CCR5-∆32 might be elevated among the plague survivors and their modern descendants. The survey showed a modest increase of CCR5-Δ32 allele frequency in Eyam, to 15 percent, slightly higher than the 10 percent seen in neighboring English villages (21, 22, 25, 72). Further, there were twice as many homozygous CCR5-Δ32/Δ32 people in Eyam than we found elsewhere. The results were supportive, but not conclusive, as measured numbers were too low to be statistically “significant.” Nonetheless, the trend in the right direction was consistent with what we had predicted.
The cumulative data I summarize here lean toward the Black Death and other historic plagues as principal disease agents favoring the selective rise of CCR5-Δ32 allele frequency in Europe. However, one cannot conclude that the issue is truly settled. Different diseases, genetic drift, undiscovered metabolic influences, or the combination of these are possible mediators of the remarkable elevation of the CCR5-Δ32 allele originally discovered as a factor in HIV exposure. Future approaches to this conundrum are certainly welcome.
CCR5 and Diseases Unrelated to AIDS
The discovery of the strong influence of CCR5 on HIV-AIDS and inferences on other infectious agents prompted the scientific community to design new association studies and experiments (similar to Fig. 4A) to test whether CCR5 might influence the onset of other diseases (56). Among the notable results was the discovery that CCR5 expression explodes around neural legions in stroke victims increasing inflammation and delaying recovery (73). The CCR5 antagonist maraviroc, diminishes the effect, thereby increasing rapid recovery in mice as well as in a small cohort of 68 human stroke victims who were CCR5-+/∆32 carriers (73).
CCR5-∆32 genotype also reduces the progression and pathogenesis due to hepatitis B infection (74). Further, the CCR5 receptor has been demonstrated to serve as the cell surface determinant or mediator for cytotoxic targeting of subsets of myeloid cells and T lymphocytes by the Staphylococcus aureus leukotoxin ED (LukED) (75) Administered maraviroc, the CCR5 antagonist, ablates LukED-mediated cell killing. CCR5 knock-out mice are fully resistant to the heretofore lethal S. aureus infection, suggesting a new role for CCR5-based drug targets in S. aureus pathogenesis.
CCR5-∆32 action is not always beneficial (76). One of the more dramatic examples of CCR5-∆32 vulnerability arose from the experimental studies involving the West Nile virus (WNV) (57, 58). The West Nile virus is a deadly flavivirus carried by African mosquitos that first emerged in New York City in 1999. Some 16,577 American cases have been reported of which 80% were subclinical. Approximately ~20% proceed to encephalitis with 678 of these fatal so far. To date, there is no effective vaccine or treatment for WNV.
In 2006, William Glass and Philip Murphy of NIH reported that mouse strains can develop WNV pathology, so they tested the influence of the CCR5 KO gene in WNV disease progression of mice (57). Surprisingly, CCR5 KO mice progressed to fatal encephalitis rapidly within 12 d while 60% of the CCR5 +/+ mice survived for >20 d (Fig. 5A and ref. 57). This seemed to suggest that functional CCR5 was a principal determinant in host defense of WNV disease. Their follow-up study among large human cohorts (n = 1,318) infected with WNV confirmed the effect (58). They observed a 25% incidence of CCR5-Δ32/Δ32 among WNV mortalities versus 8% CCR5-Δ32/Δ32 among non-pathogenetic infected people (OR = 8.5 to 13.2 in two population cohorts; Fig. 5B and ref. 58). This means that CCR5-∆32/∆32 poses a powerful risk factor for deadly pathology upon WNV infection, likely because functional CCR5 signaling confers a strong deterrent to WNV pathogenesis in people.
Fig. 5.
The CCR5 genotype mediates the progression of mice and people to deadly encephalomyelitis induced by West Nile virus infection. (A) Mice with CCR5 Knock-Out genotypes progress to fatal encephalitis within 12 d of challenge while 60% of CCCR5-+/+ wild-type mice did not develop pathology for over 20 d (57). (B) Frequency of CCR-∆32/∆32 homozygotes in fatal vs. overall cases infected with the West Nile virus (with permission; refs. 57 and 58).
The Berlin Patient-A Cure for AIDS
Timothy Ray Brown, a 29-y-old openly gay man living in Berlin had received an AIDS diagnosis in 1995, 1 y before the powerful HAART triple drug therapy became available (14, 15). Brown struggled with the therapy toxicity, and in 2005, he developed Acute Myelogenous Leukemia (AML), a virtual death sentence. He was initially treated with chemotherapy and radiation, but when his AML recurred in 2006, his physician Gero Hutter, a clinical hematologist, recommended a bone marrow stem cell transplant but with a twist (77). Hutter recalled the powerful resistance to HIV-1 by the CCR5-Δ32/ Δ32 genotype. He searched for and identified a potential bone marrow donor in the European Bone Marrow Transplant Registry who was HLA compatible to Brown and also homozygous for CCR5-Δ32/ Δ32. Hutter reasoned that the transplanted cells would be protected from anticipated residual HIV release from the multiple cellular reservoirs known to harbor HIV in AIDS patients.
Hutter first irradiated Brown’s lymphoid system prior to the transplant, which proceeded successfully. Because the HAART drugs Brown was taking would impede the growth of the donor cells, Hutter made the decision to suspend anti-retroviral therapy initially, but to test plasma for rebound HIV in his bloodstream. Brown was negative for circulating HIV in the first weeks following the transplant. He continued to be HIV-negative for months, then for 600 d before the case was announced by Hutter at the 2008 Conference on Retroviruses and Opportunistic Infections in Boston and in a 2009 article in the New England Journal of Medicine (77). Brown’s identity was concealed and he was referred to as the “Berlin Patient.” Three years later he revealed his identity as he continued to test negative for circulating HIV (78).
Timothy Ray Brown became a celebrity to the AIDS community and among AIDS researchers appearing at several AIDS research conferences in Europe and in America. Although he continued to struggle with debilitating non-AIDS diseases his HIV viral load remained “undetectable.” He volunteered to have biopsies tested from plasma, PBMCs, cerebral spinal fluid, lymph nodes, brain, gut, and colon (78, 79). Replicating virus was never detected and recovered. Trace-level RNA detection was interpreted to be either false positives or defective relict provirus shed from latent reservoir tissues. Despite initial skepticism from the majority of AIDS researchers, Brown’s dramatic case was pronounced the first proven cure of HIV-AIDS infection after 80 million infections worldwide. Sadly he would perish with a cancer diagnosis in 2020 at age 54.
Since the original Berlin patient, there were four additional cases of CCR5-Δ32/ Δ32 bearing marrow stem cell transplant to AIDS patients with the same result. These were named Dusseldorf Patient-2018 (80); London Patient -2019 (81); New York patient –2021 (82) a woman receiving cord blood combined with adult stem cell from a relative; and the City of Hope patient -2021 (83). All five recipients had an initial AML diagnosis. All suspended HAART therapy and each has remained clear of detectable HIV. One possible explanation, the principal source of virus rebound in patients who suspend HAART therapy is their CD4/CCR5 bearing lymphocytes, because perhaps the other reservoir tissues actually restrict productive HIV replication. I am not sure.
Bone marrow and stem cell transplants are not for everyone. They are expensive, (circa $250,000) and not so safe, primarily because, even HLA-matched donors are immunologically allogeneic (non-self). The immune system of the patient must be destroyed before cell transplant. Then, a deadly Graft Versus Host Disease (GVHD) reaction against the host is common (~20 to 30% post-transplant incidence), and the dangerous transplantation therapy must be followed by a lifelong regimen of anti-rejection therapies. A promising alternative to this may be immunologically syngeneic (self) grafts treated to knock out CCR5 of HIV-infected patients. Carl June and Bruce Levine at the University of Pennsylvania developed such a protocol using zinc finger targeted knockout of normal CCR5 genes in HIV-infected patients (84). Twelve such patients were infused with their own-engineered CCR5 −/− lymphocytes. At first, results were promising with donor cell reconstitution observed. Little safety issues were encountered, and one patient remained plasma HIV negative for the first three months post-transplant infusion (84). The remaining patients reactivated HIV within the first year, indicating that further treatment perhaps using more focused gene knockout in syngeneic cells ( e.g., by CRISPR-Cas9) should be attempted.
A Role for CCR5 and Cancer Therapy
A potential therapy for hematologic malignancies is allogeneic hematopoietic cell transplantation (AlloHCT). AlloHCT following myeloablative or non-myeloablative conditioning can be curative. However, successful AlloHCT transplantation is limited in part by GVHD. GVHD occurs in 30 to 50% of transplant patients in which donor T-cells recognize the host as non-self and mount vigorous attacks of host tissues inducing morbidity or death. The cancer transplant group at the University of Pennsylvania was intrigued by the CCR5-32 -HIV transplant successes and the zinc finger knockdown of CCR5. They had noticed that GVHD was seldom observed in several dozen such transplant recipients with CCR5-32 or with engineered inactivated CCR5 and wondered why? They reasoned that the functional CCR5 action perhaps played a powerful role in the onset of GVHD after alloHCT in leukemia patients.
But how to test that notion? They knew that the HIV entry inhibitor maraviroc acted by blocking HIV binding to CCR5, and it also blocked chemokine-ligand interaction with CCR5 (44, 45). Might treatment of AlloHCT recipients with maraviroc actually reduce the occurrence of GVHD? The FDA had approved maraviroc as a salvage pathway treatment for HIV patients ( and judged safe in HIV patents) so the Penn team promptly sought approval to try it on the alloHCT for leukemia patients.
In 2012, Ran Reshef and David Porter of the Abraham Cancer Center at Penn reported results from 38 leukemia patients who received standard GVHD prophylaxis with the addition of maraviroc for the first month after AlloHCT. They reported a 75% reduction in GVHD in these patients affirming the action of CCR5 in triggering GVHD (85) The findings were not absolute and patient numbers were too low for robust statistical significance. Subsequent Phase I and Phase II clinical trials confirmed a reduction in GVHD supporting a role of CCR5 and the protective influence of maraviroc on GVHD (86). But recently, in a multi-center randomized Phase II trial to prevent GVHD in the setting on non-myeloablative HCT, the addition of maraviroc to standard prophylaxis in one arm of the trial did not show an advantage compared to more standard regiments (87). In light of these apparently contradictory findings, future trials designed to ablate GVHD by targeting CCR5 antagonists, but without intensive immune suppressive therapy, could offer a major advance for the recipients of hematopoietic stem cell transplant therapy. Successful trials may also extend the importance of CCR5 and CCR5-∆32 in a cancer treatment context.
Germ Line Embryo Editing—An Ethical Quandary
The development of the CRISPR-cas9 gene editing technology first introduced in 2012 by Jennifer Doudna and Emmanuelle Charpentier allows one to seek out and edit by deletion or replacement of structural genes in any species genome (88). The promise of gene therapy for using CRISPR-cas9 was approved by the FDA for sickle cell disease in December 2023 and is now being tested for scores of other human diseases including, cancer, diabetes, heart disease, and AIDS (89). The CRISPR-Cas9 editing breakthrough was awarded the Nobel Prize in 2018 to Doudna and Charpentier (90). Everyone in genetics knew about it.
He Jiankui (a.k.a. “JK”) a 34-y-old Chinese scientist returned to China after his PhD training at Rice and a postdoctoral appointment at Stanford. JK accepted an appointment at the Southern University of Science and Technology in Shenzhen (termed the Chinese Caltech) in 2012. where he set up a laboratory dedicated to gene therapy for human diseases. At that time, AIDS was a scourge in China and HIV-infected people were forbidden from having children. JK reasoned that he would knock out CCR5 in a fertilized egg at risk for HIV exposure to ensure that the baby would be resistant to HIV infection throughout the baby’s life (91).
JK chose to employ CRISP-Cas9 editing to knock out two CCR5 genes on single-cell embryos, and implant them in the mother. He sought permission and approval for his audacious intervention. Some reviewers gave support, but many others condemned the germ line gene editing as unethical and in the stark violation of accepted medical ethics norms. JK recruited eight pregnant couples who volunteered to have their embryos modified by CRISPR. One couple had an HIV-infected father, who JK suspected would infect his offspring someday. The couple became pregnant with twin girls Lulu and Nana (pseudonyms to protect their privacy) (91). When JK appeared to present some preliminary animal results at an international gene-editing congress in Hong Kong in late 2018, the news of his human experiment leaked. The leak was not so surprising since he had posted videos on YouTube describing his intent and study design prior to the conference. JK was asked to present the human results.
Once JK admitted to the two twin pregnancies and a third CRISPR-edited baby named Amy, the horrified reaction of the conference and across the world was near unanimous (92, 93). Somatic editing for hereditary disease was allowed, but germ line editing which transmitted permanent gene alteration to human babies was strictly forbidden. JK was condemned in science, in the media, and shortly in Chinese courts. The consensus was that germline editing of any source or reason was simply too dangerous because of uncertainties of potentially damaging side effects or consequences of the gene meddling in an embryo. Vocal scientists and ethicists argued that JK’s editing of the twins’ DNA was neither life-saving nor preventing disease as there were effective drugs to prevent HIV transmission available. It was also reprehensible.
Further investigation showed that in one twin the CCR5 gene was disrupted by a 13-bp deletion of questionable consequence. The second twin had one deleted CCR5 but a second unaffected normal CCR5-+ allele. Chinese authorities turned on He Jiankui. They suspended him from his university, charged and convicted him of “Illegal Medical Practices,” sentenced him to three years of prison, and fined him $500,000 (91). Upon his release in 2021, JK relocated to Wuhan where he was appointed the Director of the Institute of Genomic Medicine at Wuchang University of Technology. JK never apologized for his transgressions, rather he reasoned that his mistake was simply one of moving too quickly. Today there seems consensus that germ-line gene editing should be forbidden until more of the adverse consequences are discovered and addressed. Still, most researchers believe that one day in the future germline editing will become feasible and help destroy or ablate genetic defects that affect some 75 million people worldwide.
COVID-19 and CCR5
One recent vignette relates to the recent COVID 19 scourge that emerged as a novel coronavirus in December 2019 in Wuhan China, spread throughout the world infecting over 365 million people and causing 5.6 million deaths. Genetic epidemiology association studies have pinpointed strong association signals around two human gene regions: ABO blood group antigen locus on Chromosome 9q34 and a second site on chromosome 3p21.31 near CCR5 (94, 95). The chromosome 3 resistant allele is risk factor for COVID-19-induced severe respiratory failure and the genomic region has been implicated to have been inherited from a Neanderthal ancestral genome region carried by European Caucasians (96). The COVID-19 risk allele is tightly linked (within 500k) of the CCR5 locus. The COVID-19 allele and CCR5 alleles are in strong linkage disequilibrium (LD) in our AIDS cohorts. The rare protective allele for COVID-19 is in LD with the normal CCR5-+ allele, but the LD association is likely important since there are a group of chemokine receptor genes within this interval. It is possible that the COVID-19 resistance allele may be tracking an adjacent chemokine receptor allele by LD. With little functional data connected to the important COVID 19 association study, follow-up inspection of adjacent gene alleles and functional gene demonstrations should come quickly.
Prospects for HIV-AIDS Epidemic
My initial interest in CCR5-∆32 arose from a search for genetic determinants that influenced HIV infection, AIDS progression, and therapy. The introduction of the triple drug therapy in 1996 produced stronger and safer deterrents to AIDS. These powerful drugs have led to the development of HIV pre-exposure prophylaxis (PrEP) (97, 98). PrEP prophylaxis coordinated preventive anti-viral cocktails are today recommended for high-risk uninfected individuals that include actively gay men, surgeons and nurses who treat HIV-infected patients, injection IV drug users, and other high-risk individuals.
Twenty years ago, President George W. Bush Introduced to U.S. government a program PEPFAR—the President’s Emergency Plan for AIDS Relief. Because of the proven success of the powerful anti-AIDS drugs, PEPFAR was designed to provide wide-spread anti-retroviral therapy to at-risk people of the developing world against HIV transmission and AIDS progression. Bush led a bipartisan congressional appropriation of $15 billion in 2003 and then another $48 billion in 2008. PEPFAR had been re-authorized every five years since and is credited with saving 25 million lives and preventing millions of new infections. PEPFAR was scheduled to be reauthorized before September 2023, but congressional chaos has delayed to new appropriation at this writing, As Bush noted this year in his plea to renew the PEPFAR program and its 5-y appropriation, “We are on the verge of ending the AIDS epidemic” (99). The PEPFAR program is widely successful in slowing the spread of HIV across the globe and stands as a humanitarian success of the United States people as well as cementing an important legacy of President Bush.
Conclusions
The legacy of CCR5-∆32 has been exhilarating to my team, to my collaborators, to the thousands of researchers who have contributed to the importance and translation of these findings to bedside and to society. The horrific AIDS epidemic is approaching control across the globe with the advent of HARRT, PreP, and PEPFAR. The human genome project has contributed to countless clinical advances particularly the discovery of genes that influence human hereditary maladies. I have detailed in this review the amazing role of CCR5-32 in these developments.
Science matters in many ways and for those who play a part, the times and opportunities of today are indeed breathtaking. The timing of CRISPR-cas9 based gene therapy is promising for countless biomedical applications. Witness the thousands of biotech company ideas that are dotting the scientific landscape today. A promise of personal satisfaction and intellectual luxury should be apparent to those who are trying to choose a career in medical science today.
A powerful role for science ethics has emerged over the previous decades in nearly all areas of science in human genetics. The process of discovery of many genes has involved large population cohort studies and collaborative meta-analyses. A ticklish issue has arisen around efforts toward the open release all primary data relating to medical genetic discoveries (100). But this push, albeit admirable and useful, raises the constraint posed by the informed consent agreements signed by patient volunteers. Open access to patients’ clinical data and genotypes is precluded unless prior agreement for open release is obtained. Most genome-wide association studies do not have this consent, so the primary data are unavailable for wide scientific use.
In our 20,000-patient AIDS cohorts, this constraint is real and must be respected. For our AIDS cohorts, we kept the personal genetic and clinical data shielded, although I recognized a policy that protected our study participants patient’s privacy, while still sharing detailed association test results. We developed a computer suite we call GWATCH which allowed us to release openly all of our Genome Wide Association Studies (GWAS) test results across cohorts, disease stages, and different disease association studies (101). Patients’ personal data are protected behind a GWATCH firewall, but the computed population-based results for over 100 different AIDS association tests for a million genome SNPs are openly available for review, for interpretation, for replication, and for coordinated metadata analyses (https://genomewatch.org/) (101). The data I reviewed here that led to the identification of CCR5-∆32 and additional ARGs are publicly available by permission online to all my colleagues, without any red tape or bureaucratic blocks. I invite genetic epidemiologists who have performed GWAS for any complex disease to inspect these and to release their independent gene association test results as well by adopting the GWATCH precedent.
Finally, a few thoughts for the new young scientist launching a career: Be glad when you discover or play a role in an important and relevant advance. Not everyone gets there, but many do. I believe it is critical to aim high in your research questions and plans; this will pay off. Resist the temptation to obsess over authorship priority. You will promptly forget the conflict a few years later, and that is not why you chose a science career. The tears and laughter of science adventures will soon become part of your career profile. The rewards can be frequent and promising for certain as it has been for myself and for so many successful colleagues (13).
Acknowledgments
I am deeply grateful for the very important seminal research contributions that I have reviewed here from collaborators, colleagues, and dear friends, notably: Michael Dean*, Mary Carrington*, Max Essex*, Taras Oleksyk*, James Hoxie*, David Porter*, Philip Murphy*, Mary Thompson, Cheryl Winkler, George Nelson, David Reich, Clay Stephens, Robert Gallo, Robert Fisher, Anton Sviton, Nikolay Chekasov, James Goedert, John Phair, Edward Gomperts, Sharyn Donfield, David Vlahov, Susan Buchbinder, and many others cited in the reference list. I also appreciate that early drafts of this review were read and edited constructively by scientists with an asterisk (“*”) listed above and by Bruce Grant, David Allison, William Murphy, Jose Lopez, and Laurie Goodman. Generous funding across the years was organized and administered by the National Cancer Institute, and the National Institute of Allergy and Infectious Disease under the leadership of Richard Adamson, George VandeWoude, Richard Klaussner and Anthony Fauci for which I am surely indebted.
Author contributions
S.J.O. designed research, performed research, reviewed the important advances, and wrote the paper.
Competing interests
The author declares no competing interest.
Footnotes
Reviewers: G.D., Florida International University; and M.Y., Hood College.
Data, Materials, and Software Availability
There are no data underlying this work. Data cannot be shared (human patient clinical data are not consented for release). Previously published data were used for this work (16, 21, 27, 34–38, 48, 74, 101).
References
- 1.Centers for Disease Control (CDC), Pneumocystis pneumonia—Los Angeles. MMWR. Morb. Mortal. Wkly Rep. 30, 250–252 (1981). [PubMed] [Google Scholar]
- 2.Centers for Disease Control (CDC), Kaposi’s sarcoma and pneumocystis pneumonia among homosexual men—New York City and California. MMWR Morb. Mortal. Wkly Rep. 30, 305–308 (1981). [PubMed] [Google Scholar]
- 3.Barré-Sinoussi F., et al. , Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 220, 868–871 (1983). [DOI] [PubMed] [Google Scholar]
- 4.Popovic M., et al. , Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 224, 497–500 (1984). [DOI] [PubMed] [Google Scholar]
- 5.Gallo R. C., et al. , Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 224, 500–503 (1984). [DOI] [PubMed] [Google Scholar]
- 6.Schüpbach J., et al. , “Serological analysis of a subgroup of human T-lymphotropic retroviruses (HTLV-III) associated with AIDS. Science 224, 503–505 (1984). [DOI] [PubMed] [Google Scholar]
- 7.Sarngadharan M. G., et al. , Antibodies reactive with human T-lymphotropic retroviruses (HTLV-III) in the serum of patients with AIDS. Science 224, 506–508 (1984). [DOI] [PubMed] [Google Scholar]
- 8.Vahlne A., A historical reflection on the discovery of human retroviruses. Retrovirology 40, 6–40 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO, Heath Topics: Human immunodeficiency virus (HIV). (2023). https://www.who.int/health-topics/hiv-aids#tab=tab_1 (Accessed 30 November 2023).
- 10.O’Brien S. J., Genetic Maps: Locus Maps of Complex Genomes (Cold Spring Harbor Laboratory Press, New York, 1980–1993), 6 vols. [Google Scholar]
- 11.O’Brien S. J., et al. , The promise of comparative genomics in mammals. Science 286, 458–481 (1999). [DOI] [PubMed] [Google Scholar]
- 12.O’Brien S. J., Tears of the Cheetah and Other Tales from the Genetic Frontier (St. Martin’s Press, New York, 2003). [Google Scholar]
- 13.O’Brien J. S., A beautiful life: High risk-high payoff in genetic science. Annu. Rev. An. Biosci. 8, 1–24 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Berger P. B., Hope and caution: Report from the XI International Conference on AIDS. CMAJ 155, 717–721 (1996). [PMC free article] [PubMed] [Google Scholar]
- 15.Pirisi A., Julio Montaner: King of HAART. Lancet 368, 1863 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Cocchi F., et al. , Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270, 1811–1815 (1995). [DOI] [PubMed] [Google Scholar]
- 17.Choe H., et al. , The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85, 1135–1148 (1996). [DOI] [PubMed] [Google Scholar]
- 18.Doranz B. J., et al. , A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell 85, 1149–1158 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Deng H., et al. , Identification of a major co-receptor for primary isolates of HIV-1. Nature 381, 661–666 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Alkhatib G., et al. , CC CKR5: A RANTES, MIP-lα, MIP-lβ receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272, 1955–1958 (1996). [DOI] [PubMed] [Google Scholar]
- 21.Dean M., et al. , Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 273, 1856–1862 (1996). [DOI] [PubMed] [Google Scholar]
- 22.O’Brien S. J., Moore J., The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol. Rev. 177, 99–111 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Samson M., et al. , Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 382, 722–725 (1996). [DOI] [PubMed] [Google Scholar]
- 24.Liu R., et al. , Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86, 367–377 (1996). [DOI] [PubMed] [Google Scholar]
- 25.O'Brien S. J., et al. , Polygenic and multifactorial disease gene association in man: Lessons from AIDS. Ann. Rev. Genet. 34, 563–591 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Dean M., et al. , Reduced risk of AIDS lymphoma in individuals heterozygous for the CCR5 delta32 mutation. Cancer Res. 59, 3561–3564 (1999). [PubMed] [Google Scholar]
- 27.Hendrickson S. L., et al. , Host genetic influences on highly active antiretroviral therapy efficacy and AIDS-free survival. J. Acquir. Immune. Defic. Syndr. 48, 263–271 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien T. R., et al. , HIV-1 infection in a man homozygous for CCR5 delta 32. Lancet 349, 1219 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Theodorou I., et al. , HIV-1 infection in an individual homozygous for CCR5 delta 32. Lancet 349, 1219–1220 (1997). [PubMed] [Google Scholar]
- 30.Balotta C., et al. , Homozygous delta 32 deletion of the CCR-5 chemokine receptor gene in an HIV-1-infected patient. AIDS 11, F67–F71 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Michael N. L., et al. , Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 delta32. J. Virol. 72, 6040–6047 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biti R., et al. , HIV-1 infection in an individual homozygous for the CCR5 deletion allele. Nat. Med. 3, 252–253 (1997). [DOI] [PubMed] [Google Scholar]
- 33.Zimmerman P. A., et al. , Inherited resistance to HIV-1 conferred by an inactivating mutation in CC chemokine receptor 5: Studies in populations with contrasting clinical phenotypes, defined racial background, and quantified risk. Mol. Med. 3, 23–36 (1997). [PMC free article] [PubMed] [Google Scholar]
- 34.O’Brien S. J., Hendrickson S. L., Host genomic influences on HIV/AIDS. Genome Biol. 14, 201 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien S. J., Nelson G. W., Human genes that limit AIDS. Nat. Genet. 36, 565–574 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Anzala A. O., et al. , CCR2-64I allele and genotype association with delayed AIDS progression in African women. Lancet 351, 1632–1633 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Xie W., et al. , Genome-wide analyses reveal gene influence on HIV disease progression and HIV-1C acquisition in Southern Africa. AIDS Res. Hum. Retroviruses 33, 597–609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shevchenko A. K., et al. , Genome-wide association study reveals genetic variants associated with HIV-1C infection in a Botswana study population. Proc. Natl. Acad. Sci. U.S.A. 118, e2107830118 (2021), 10.1073/pnas.2107830118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson G. W., O’Brien S. J., Using mutual information to measure the impact of multiple genetic factors on AIDS. J. Acquir. Immune Defic. Syndr. 42, 347–354 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Nguyêñ G. T., et al. , Phenotypic expressions of CCR5-delta32/delta32 homozygosity. J. Acquir. Immune Defic. Syndr. 22, 75–82 (1999). [DOI] [PubMed] [Google Scholar]
- 41.Murphy P. M., International Union of Pharmacology. XXX. Update on chemokine receptor nomenclature. Pharmacol. Rev. 54, 227–229 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Hughes C. E., Nibbs R. J., A guide to chemokines and their receptors. FEBS J. 285, 2944–2971 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao T., Cai Y., Chen B., HIV-1 entry and membrane fusion inhibitors. Viruses 13, 735 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorr P., et al. , Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49, 4721–4732 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abel S., et al. , Assessment of the absorption, metabolism and absolute bioavailability of maraviroc in healthy male subjects. Br. J. Clin. Pharmacol. 65, 60–67 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poveda E., Briz V., Soriano V., Enfuvirtide, the first fusion inhibitor to treat HIV infection. AIDS Rev. 7, 139–147 (2005). [PubMed] [Google Scholar]
- 47.Stephens J. C., et al. , Dating the origin of the CCR5-Delta32 AIDS-resistance allele by the coalescence of haplotypes. Am. J. Hum. Genet. 62, 1507–1515 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Novembre J., Galvani A. P., Slatkin M., The geographic spread of the CCR5 Δ32 HIV-resistance allele. PLoS Biol. 3, e339 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrington M., et al. , Novel alleles of the chemokine-receptor gene CCR5. Am. J. Hum. Genet. 61, 1261–1267 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dean M., Carrington M., O’Brien S. J., Balanced polymorphism selected by genetic versus infectious human disease. Ann. Rev. Genomics Hum. Genet. 3, 263–292 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Cantor N., In the Wake of the Plague: The Black Death and the World it Made (The Free Press, Simon and Schuster, New York, 2001). [Google Scholar]
- 52.Ziegler P., The Black Death (Harper Collins, New York, 2000). [Google Scholar]
- 53.Defoe D., A Journal of the Plague Year (Penguin Books, 1966). [Google Scholar]
- 54.Marriott E., Plague: A Story of Science, Rivalry, and the Scourge that Won’t Go Away (Macmillan, New York, 2004). [Google Scholar]
- 55.Hughes A. L., Nei M., Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature 335, 167–170 (1988). [DOI] [PubMed] [Google Scholar]
- 56.Ellwanger J. H., et al. , Beyond HIV infection: Neglected and varied impacts of CCR5 and CCR5Δ32 on viral diseases. Virus Res. 286, 198040 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glass W. G., et al. , Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J. Exp. Med. 202, 1087–1098 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glass W. G., et al. , CCR5 deficiency increases risk of symptomatic West Nile virus infection. J. Exp. Med. 203, 35–40 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao F., et al. , Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature 397, 436–441 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Wheelis M., Biological warfare at the 1346 siege of Caffa. Emerg. Infect. Dis. 8, 971–975 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galvani A. P., Novembre J., The evolutionary history of the CCR5-Δ32 HIV-resistance mutation. Microb. Infect. 7, 302–309 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Galvani A. P., Slatkin M., Evaluating plague and smallpox as historical selective pressures for the CCR5-Δ32 HIV-resistance allele. Proc. Natl. Acad. Sci. U.S.A. 100, 15276–15279 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scott S., Duncan C. J., Biology of Plagues: Evidence from Historical Populations (Cambridge University Press, 2005). [Google Scholar]
- 64.Horgan J., Justinian‘s Plague (541-542 CE). World History Encyclopedia (2014). https://www.worldhistory.org/article/782/justinians-plague-541-542-ce/.
- 65.Glatter K. A., Finkelmanm P., History of the plague: An ancient pandemic for the age of COVID-19. Am. J. Med. 134, 176–181 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Elvin S. J., et al. , Evolutionary genetics: Ambiguous role of CCR5 in Y. pestis infection. Nature 430, 417 (2004). [DOI] [PubMed] [Google Scholar]
- 67.Lalani A. S., et al. , Use of chemokine receptors by poxviruses. Science 286, 1968–1971 (1999). [DOI] [PubMed] [Google Scholar]
- 68.Willman D., The Mirage Man (Bantam Books, Random House New York, 2011). [Google Scholar]
- 69.Guillemin J., American Anthrax (Henry Holt and Co., New York, 2011). [Google Scholar]
- 70.Clifford J., Eyam Plague: 1665–1666 Paperback (Plague Village, Eyam, 26 May 2003). [Google Scholar]
- 71.Paul D. Eyam Plague Village (Amberley Publishing, UK, 2012). [Google Scholar]
- 72.ShaylaShaw, “Secrets of the dead - Mystery of the black death” (video recording, 2011). https://www.youtube.com/watch?v=hpXJNpymNts. Accessed 22 February 2024.
- 73.Joy M. T., et al. , CCR5 is a therapeutic target for recovery after stroke and traumatic brain injury. Cell 176, 1143–1157.e13 (2019), 10.1016/j.cell.2019.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thio C. L., et al. , Genetic protection against hepatitis B virus conferred by CCR5Delta32: Evidence that CCR5 contributes to viral persistence. J. Virol. 81, 441–445 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alonzo F. III, et al. , CCR5 is a receptor for Staphylococcus aureus leukotoxin ED. Nature 493, 51–55 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei X., Nielsen R., CCR5-d32 is deleterious in the homozygous state in humans. Nat Med. 25, 1796 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hütter G., et al. , Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl. J. Med. 360, 692–698 (2009). [DOI] [PubMed] [Google Scholar]
- 78.Brown T. R., I am the Berlin patient: A personal reflection. AIDS Res. Hum. Retroviruses. 31, 2–3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yukl S. A., et al. , Challenges in detecting HIV persistence during potentially curative interventions: A study of the Berlin patient. PLoS Pathog. 9, e1003347 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jensen B.-E. O., et al. , In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat. Med. 29, 583–587 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gupta R. K., et al. , Evidence for HIV-1 cure after CCR5Δ32/Δ32 allogeneic hemopoietic stem-cell transplantation 30 months post analytical treatment interruption: a case report. Lancet HIV. 7, e340–e347 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hsu J., et al. , International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) P1107 Team. HIV-1 remission and possible cure in a woman after haplo-cord blood transplant. Cell 186, 1115–1126 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson M., Longtime HIV patient is effectively cured after stem cell transplant. Washington Post July 27, 2022.
- 84.June C., Levine B., Blocking HIV’s attack. Sci. Am. 306, 54–59 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Reshef R., et al. , Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N. Engl. J. Med. 367, 135–145 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reshef R., et al. , Extended CCR5 blockade for graft-versus-host disease prophylaxis improves outcomes of reduced-intensity unrelated donor hematopoietic cell transplantation: A phase II clinical trial. Biol. Blood Marrow Transplant. 25, 515–521 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bolaños-Meade J., et al. , Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: A randomized phase 2 trial with a non-randomized contemporaneous control group (BMT CTN 1203). Lancet Haematol. 6, e132–e143 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Doudna J. A., Sternberg S. H., A Crack In Creation: Gene Editing and the Unthinkable Power to Control Evolution (Mariner Books, 2018). [Google Scholar]
- 89.Ran F. A., et al. , Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cohen J., CRISPR, the revolutionary genetic “scissors”, honored by Chemistry Nobel. Science (2020), 10.1126/science.abf0540. [DOI] [Google Scholar]
- 91.Goodyear D., Dangerous Designs. The New Yorker, 11 September 2023. https://www.magzter.com/stories/Culture/The-New-Yorker/Dangerous-Designs. Accessed 22 October 2023.
- 92.Normile D., Shock greets claim of CRISPR-edited babies. Science 362, 978–979 (2018). [DOI] [PubMed] [Google Scholar]
- 93.Greely H. T., CRISPR’d babies: Human germline genome editing in the “He Jiankui affair”. J. Law Biosci. 6, 111–183 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ellinghaus D., et al. , Severe Covid-19 GWAS Group, Genomewide association study of severe Covid-19 with respiratory failure. N. Engl. J. Med. 383, 1522–1534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zeberg H., The major genetic risk factor for severe COVID-19 is associated with protection against HIV. Proc. Natl. Acad. Sci. U.S.A. 119, e2116435119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeberg H., Pääbo S., The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature 587, 610–612 (2020), 10.1038/s41586-020-2818-3. [DOI] [PubMed] [Google Scholar]
- 97.Riddell J. IV, et al. , HIV preexposure prophylaxis: A review. JAMA 319, 1261–1268 (2018). [DOI] [PubMed] [Google Scholar]
- 98.Hojilla J. C., et al. , Characterization of HIV preexposure prophylaxis use behaviors and HIV incidence among US adults in an integrated health care system. JAMA Netw. Open 4, e2122692 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bush G. W., George W. Bush: Michael Gerson’s words make the case for saving PEPFAR. The Washington Post, 13 September 2023. https://www.washingtonpost.com/opinions/2023/09/13/george-bush-pepfar-michael-gerson-words/. Accessed 22 October 2023.
- 100.O’Brien S. J., et al. , Stewardship of human biospecimens, DNA, genotype, and clinical data in the GWAS era. Annu. Rev. Genomics Hum. Genet. 10, 193–209 (2009). [DOI] [PubMed] [Google Scholar]
- 101.Svitin A., et al. , GWATCH: A web platform for automated gene association discovery analysis. Gigascience 3, 2047-217X-3-18 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data underlying this work. Data cannot be shared (human patient clinical data are not consented for release). Previously published data were used for this work (16, 21, 27, 34–38, 48, 74, 101).