Abstract
There exists no cure for acute, recurrent acute or chronic pancreatitis and treatments to date have been mainly focused on managing symptoms. A recent workshop held by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) focused on interventions that might disrupt or perhaps even reverse the natural course of this heterogenous disease, aiming to identify knowledge gaps and research opportunities that might inform possible future funding initiatives for NIDDK. The breadth and variety of identified active or planned clinical trials traverses the spectrum of the disease and was conceptually grouped for the workshop into behavioral, nutritional, pharmacologic and biologic, and mechanical interventions. Cognitive and other behavioral therapies are proven interventions for pain and addiction, but access and other barriers have limited their use. Whilst a disease specific instrument quantifying pain is now validated, an equivalent is lacking for nutrition - and both face challenges in ease and frequency of administration. Digital technologies have great potential to overcome such barriers and could also remedy access to behavioral therapy. Pancreatic enzyme replacement therapy, repurposing of anti-fibrosis drugs, and stem-cell-based interventions all hold promise. Ongoing development of Patient Reported Outcome (PRO) measurements can serve to satisfy Investigative New Drug (IND) regulatory assessments for these and all of the above-mentioned interventions. Finally, despite multiple randomized clinical trials demonstrating benefit, great uncertainty remains regarding patient selection, timing of intervention, and type of mechanical intervention (endoscopic versus surgery). Access to an appropriately trained surgeon is a significant barrier in this space. Challenges and opportunities to establish beneficial interventions for patients were identified.
Keywords: chronic pancreatitis, recurrent acute pancreatitis, clinical trial, intervention, patient-reported outcomes, health outcomes
INTRODUCTION
There currently exists no cure or drug therapy for acute (AP), recurrent acute (RAP) or chronic pancreatitis (CP) and to date treatments are focused on management of symptoms alone. A recent workshop held by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) focused on interventions that have the potential to disrupt progression or even reverse the natural course of this heterogenous disease, aiming to identify knowledge gaps and research opportunities that might inform possible future funding initiatives for NIDDK. The breadth and variety of identified active or planned clinical trials traverses the spectrum of the disease and was conceptually grouped for the workshop into behavioral, nutritional, pharmacologic and biologic, and mechanical (endoscopic, surgical, and bioengineering) interventions.
BEHAVIORAL INTERVENTIONS
Behavioral, social, psychological, and environmental factors contribute significantly to the disease burden associated with RAP and CP, and furthermore health outcomes in these individuals. Specific health behaviors such as smoking and alcohol use place individuals at risk for progression of RAP to end-stage CP with higher associated risks of diabetes and exocrine insufficiency. More constant and more severe pain is also often seen in individuals with ongoing alcohol and smoking. Pain is the most common symptom experienced in RAP and CP, is the main driver of disease morbidity,1 and is the highest priority symptom identified by patients.2 Pain is associated with comorbidities affecting quality of life (QOL) such as low physical activity, sleep problems, and symptoms of anxiety and depression, all of which are targets for treatment.3 Many of thecomorbid symptoms are modifiable with behavioral interventions; however, these have been sparsely used to date and access to adequate behavioral health treatment has remained a common issue in this patient population. Both baseline assessment of disease symptoms – most commonly pain and related comorbid conditions – and the impact of interventions have remained a challenge to quantify and characterize in the absence of disease-specific tools that acknowledge both the complexity and the specific needs of this patient population. Disease-specific pain assessment with a dedicated instrument may offer more precision in measuring both pain in CP and response to interventions. Widely disseminated systematic approaches are needed to screen RAP and CP patients for a range of comorbidities (e.g., nicotine, alcohol use, depression), and to deliver interventions that can be accessed equitably by all individuals with RAP and CP.
Whole-person Approach: Connections across Biological, Behavioral, Social and Environmental Domains
For many patients, RAP and CP exist in a disease continuum where they may progress in a single direction toward the latter with repeated episodes of acute inflammation, or persistent toxic or metabolic factors that cause progressive fibrosis or scarring of the pancreas.4 In the absence of a cure for either disease, cessation of risky health behaviors (alcohol or tobacco use), correction of metabolic factors (triglyceride or calcium levels) or removal of an impetus for the disease (gallstones) represent the lone interventions that can be performed to stop this progression. A whole-person health approach involves considering multiple factors that promote either health or disease across interconnected biological, behavioral, social, and environmental domains. This approach may be especially useful given that for many patients RAP and CP may result from multiple factors, creating opportunities to intervene in multiple domains. Using the concept of whole-person health, the interventions pursued for each individual will be different according to their needs, but allow for the acknowledgement of areas not commonly addressed in conventional interactions surrounding pancreatic health. In addition to this, whole-person health allows for the acknowledgement of multiple consequences of the disease, and the treatment of those resultant comorbidities. Some of these additional domains were addressed at length, including mental health, addiction behaviors, and racial factors that may influence outcomes.
Of specific relevance to CP, multi-component cognitive behavioral therapies (CBT) are available for chronic pain management and have a strong evidence base for reducing pain, disability, and distress in other chronic pain conditions. Behavioral interventions are underutilized in patients with pancreatitis, but early studies show promise.5, 6 Barriers in implementation of CBT include shortage of interdisciplinary clinics that address pain and GI disorders, geographic distance to trained specialists, insurance limitations, and stigma. A pilot feasibility study of Internet CBT for painful chronic pancreatitis7 demonstrated that a series of skills-building lessons, including thought challenging, relaxation, and activity pacing, substantially reduced pain and pain interference in patients with RAP and CP compared to a control condition. CBT approaches are also used to treat other comorbidities. Insomnia is an important yet underappreciated comorbidity affecting over 50% of people with chronic pain, is related to worse pain and disability, and potentially reduces effectiveness of pain interventions. Interventions that collectively address insomnia and pain are being evaluated in ongoing trials.8
It is unclear how the effectiveness of interventions for CP differs across groups that vary by their cultural and social experiences (e.g., poverty, access to care) and this area requires more study. Disparities in mortality rates have been documented for CP in Black males compared to White males,9 and disparities in pain management and quality of pain care have been documented across racial minority groups.10, 11 Racial minorities are underrepresented in pain intervention RCTs. In fact, race distribution is under-reported in clinical trials of pain, making the disparity difficult to enumerate. Inclusive practices for the conduct of clinical trials are critical to developing equitable interventions in CP.
In the future, the implementation of screening tools to identify patients who would benefit from whole-person interventions and implementing multi-component behavioral interventions are likely to improve the management and QOL in CP.12 Longitudinal data collection in prospective studies using multiple biomarkers such as brain imaging, quantitative sensory testing, assessment of psychosocial factors and blood markers may predict progression of CP and aid in identifying patients suitable for whole person interventions. Specific research methodologies and clinical trial designs to study interconnected systems (e.g., machine learning methods, latent class/profile analysis, adaptive interventions) may be particularly pertinent in CP clinical trials.
Alcohol Use Disorder (AUD) in Patients with RAP and CP: Context and Treatment Strategies
Alcohol is identified as an etiology in 25% of patients with acute pancreatitis (AP) and at least 30% of CP patients, and is associated with more than 200 health conditions.13 Alcohol is the 3rd leading cause of cancer and causes 1 in 10 deaths among adults 20-64 years in the U.S.14 High recidivism to drinking even after disease occurrence warrants intervention beyond mere recommendation, though this is rarely pursued, often due to reluctance on the part of patient or provider, or lack of knowledge or resources regarding how to proceed.
Problematic alcohol use, however, is easily identifiable and measurable. Criteria for Alcohol Use Disorder (AUD) include cravings, loss of control, interruption in life activities due to alcohol, tolerance and withdrawal symptoms.15 The Alcohol Use Disorder Identification Test – C (AUDIT-C) is a 3-question version of a longer questionnaire specifically developed by the World Health Organization to identify problematic alcohol use, and can be implemented as a screening tool in clinical settings.16 Heavy drinking, another measure of degree of use, is defined by the National Institute on Alcohol Abuse and Alcoholism as ≥4 drinks/day or ≥14 drinks/week for men, and ≥3 drinks/day or ≥7 drinks/week for women.17
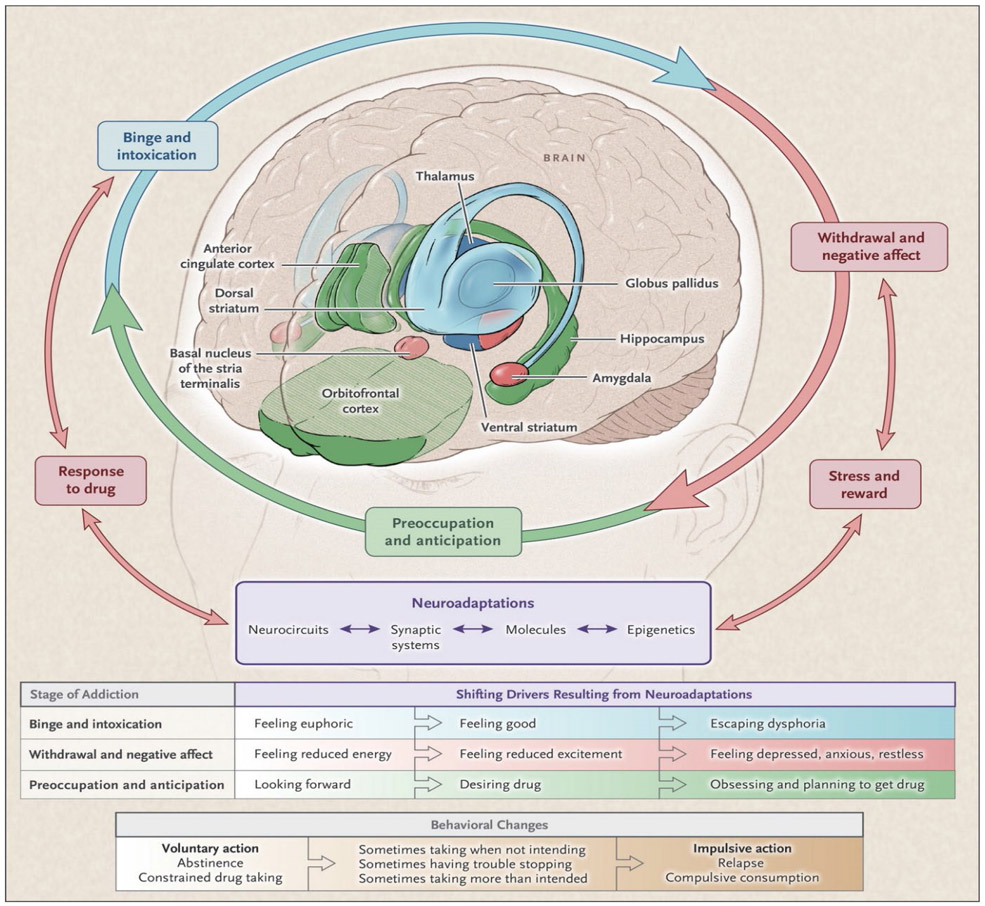
Appreciation for the neurobiology of AUD may help to tailor treatments for alcohol-associated pancreatitis. More than half of AUD is heritable, and patients who tout tolerance are at higher risk for AUD.18 The euphoric rewards of alcohol diminishes with continued use, and with prolonged history of dependence, a state of ‘relief craving’ sets in, when low mood, elevated anxiety and increased sensitivity to stress become dominant.19 Recognizing the stages of addiction will also enhance and inform treatment strategies. (Figure 1)
Figure 1: Cycle of Addiction.
Stages of the Addiction Cycle.
During intoxication, drug-induced activation of the brain’s reward regions (blue) is enhanced by conditioned cues in areas of increased sensitization (green). During withdrawal, the activation of brain regions involved in emotions (pink) results in negative mood and enhanced sensitivity to stress. During preoccupation, the decreased function of the prefrontal cortex leads to an inability to balance the strong desire for the drug with the will to abstain, which triggers relapse and reinitiates the cycle of addiction. The compromised neuro-circuitry reflects the disruption of the dopamine and glutamate systems and the stress-control systems of the brain, which are affected by corticotropin-releasing factor and dynorphin. The behaviors during the three stages of addiction change as a person transitions from drug experimentation to addiction as a function of the progressive neuroadaptations that occur in the brain.
(Adapted From) and reproduced with permission NEJM 2016 Jan 28;374(4):363-71. doi: 10.1056/NEJMra1511480.
Both pharmacological and non-pharmacological treatments are available to address AUD, including CBT in individual or group settings.20 Among pharmacological options, naltrexone and acamprosate have demonstrated decreases in number of drinking days and frequency of heavy drinking.21 Family, community, and vocational interventions may also play an important role.
Disease-Specific Assessment of Pain in CP
Pain assessment instruments used in clinical trials and studies of CP to date have frequently lacked a multidimensional approach limiting their use and interpretation, especially in areas such as total duration of pain and impact of food intake on pain, both disease-specific features important in CP.22 Because pain is such a salient feature of CP (>80% over course of disease, ~60% in past week, 50% managed with chronic opioids), a standardized disease-specific pain assessment tool is needed to accurately identify pain profiles and also measure effectiveness of treatments.3 While pain remains a predominant feature of CP among all age groups, the pattern and impact on daily activities may differ.23, 24 An ideal pain assessment tool would would have developmentally relevant versions applicable to children and adults in order to maintain consistency throughout trials across the lifespan.
As described in a previous workshop held by the NIDDK,24 the Comprehensive Pain Assessment Tool for Chronic Pancreatitis (COMPAT), a self-administered research instrument specific to CP consisting of six pain dimensions,25 has been made more useful through distillation into a short-form (COMPAT-SF) that can be completed in under 10 minutes at bedside. The COMPAT-SF includes 6 questions total that evaluate a) pain severity, b) pain pattern, c) factors provoking pain, d) widespread pain, and e) qualitative pain descriptors.26 Criterion validity has been demonstrated by the association of higher COMPAT-SF scores with more frequent hospitalizations, and with higher levels of pain as measured by the Izbicki and Brief Pain Inventory. In patients with CP disease, COMPAT-SF also demonstrated high test-retest reliability. The COMPAT-SF is an important patient-reported outcome (PRO) measurement that holds promise for wide dissemination and use in interventional studies in order to chart patient progress. Ongoing efforts to translate the current version into multiple languages for use in centers around the world will open pathways to assess CP symptoms across multiple populations (potentially helping to identify disparities), make it affordable for patients and clinicians, and accessible on mobile platforms.
NUTRITIONAL INTERVENTIONS
Overview of Nutrition Research Priorities in Pancreatitis
The effect of nutritional disorders experienced by patients with AP, RAP, and CP is broad considering the dual endocrine and exocrine functions of the pancreas. The spectrum of complications include malnutrition, micronutrient deficiencies, sarcopenia, diabetes, and metabolic bone disease.27-29 Input from patients and patient advocates has indicated that nutrition, diet, and the ability to eat are important issues.2 In fact, these concerns rank second only to abdominal pain which, in turn, has a central role in feeding and nutrition. Unfortunately, data on directly recorded dietary patterns and nutrition-focused interventions in AP, RAP, and CP remain sparse.30 More of both are needed to inform better management of pancreatitis.
Several important considerations must be accounted for when approaching study design for nutrition studies in pancreatitis. As with all clinical trials in pancreatitis, the development and implementation of a core outcome set is desirable to reduce heterogeneity and produce higher quality evidence.31 In the absence of such guidance, investigators are encouraged to adopt standard definitions from nutrition societies. For example, the Global Leadership Initiative on Malnutrition (GLIM) criteria was developed by an international consortium and represents a consensus for the diagnosis of malnutrition.32 This model can be followed in the future by other groups to develop consensus agreements as a foundation for future studies.
Considering the multifactorial contributions to nutritional compromise, multimodal interventions represent the preferred approach for successful application of findings to clinical practice. Types of interventions include dietary modifications, micronutrient supplementation, pancreatic enzyme replacement therapy (PERT), prescriptive physical activity, and nutrition support (i.e., oral nutrient supplementation, enteral nutrition, or parenteral nutrition). As an example from other pancreatic diseases, a group has recently completed a feasibility study in pancreatic cancer with an intervention comprised of a fish oil-based supplement (eicosapentaenoic acid-enriched), PERT, a prescriptive exercise regime, and registered dietician-led dietary counseling.33
Dietary Patterns
The role of medical nutrition therapy for prevention and treatment of AP, RAP, and CP remain in their infancy. Prior to identifying candidate approaches to investigate in nutrition-based clinical trials, it is necessary to understand differences in baseline dietary patterns in patients with pancreatitis compared to control populations and define the degree of variability within groups. In addition to potential heterogeneity of dietary intake other modifiable (e.g., gut microbiota, adiposity, smoking, and alcohol status) and nonmodifiable factors (e.g., age, race, sex) should be considered in the design of nutrition-based trials. Due to these complexities, careful assessment of dietary intake prior to and during the intervention is necessary. Additionally, disease-related factors, such as the etiology of pancreatitis may be associated with differences in dietary patterns and should be accounted for in the trial design and analysis plan.27 Also, food security remains an important consideration in all nutrition research, and given suspected disparities within the population of patients across the pancreatitis disease spectrum, this needs to be taken into account.34
Quantitative estimation of dietary intake for the purpose of nutrition research can be performed in several ways and there is no universal standard. Dietary records, often identified as the gold standard dietary assessment tool, require high participant literacy and can be resource intensive. Larger population studies often rely on food frequency questionnaires which provide a less costly approach to categorizing habitual dietary intake but lack the precision of dietary records. Ultimately, the selection of the most appropriate dietary assessment tool and the frequency of assessment should be determined based on the scientific question being addressed.
Qualitative assessments of dietary patterns - measurements of dietary patterns and behaviors for optimal health – have recently evolved with the introduction and dissemination of dietary inflammatory scores. Previously, the Healthy Eating Index (HEI) or Mediterranean diet scores were used by nutrition scientists to estimate the quality of the diet of a group compared to normative population values. In recent years, dietary inflammatory scores have been introduced that study dietary patterns associated with pro-inflammatory biomarkers.35 This approach has been used in the investigation of other gastrointestinal disorders: higher dietary inflammatory scores have even previously been associated with higher risk of developing Crohn’s disease.36 The empirical dietary inflammatory index (EDII), empirical dietary inflammatory pattern (EDIP), and the dietary inflammation score and lifestyle inflammation score (DIS/LIS) have been of interest in chronic inflammatory conditions while other tools, like polyphenol or phytochemical assessments, are rarely reported in AP or CP, but may also be reflective of the inflammatory potential of the diet. Recent pilot studies have shown more inflammatory diet characteristics in CP patients compared to controls on the HEI, alternative Mediterranean diet score, EDIP and polyphenol intake, findings that merit further study moving forward.34, 37
Enteral Nutrition in Pancreatitis
In pancreatitis it has long been recognized that when both are feasible, enteral nutrition remains the preferred route over parenteral route for caloric delivery as well as the non-caloric benefits of maintaining gut integrity, modulating disease burden, and enhancing the immune system in severe medical illnesses. Enteral nutrition supplementation as a gold standard is supported by data from randomized controlled trials, observational studies, expert consensus, and society recommendations.38-41 Additional studies are needed to better ascertain discernible outcome differences between proximal and distal feeding in AP and RAP, as well as clear benefits of distal feeding to provide ‘pancreatic rest’ in these disorders.
There is growing interest in the pancreatitis field to use nutrition as a therapy in order to provide nutrients to promote enhanced recovery, decrease inflammation, restore immunity, combat catabolism, maintain gut function, and ultimately improve patient outcomes.42 Borrowing from concepts and tools used in critical care and pancreatic cancer nutrition research, adaptive nutrition for AP, RAP and CP also include immune enhancing formulas, novel nutraceuticals, and disease specific treatment. Nutritional candidates with emerging preliminary data that warrant further investigation include omega 3 fatty acids, arginine, leucine, and specialized pro-resolving mediators.43-45 These supplements may be considered in combination with other behavioral and/or nutritional interventions.
Pancreatic Enzyme Replacement Therapy
The role of exogenous PERT for the management of exocrine pancreatic insufficiency (EPI) is a cornerstone in the nutritional management of patients with chronic pancreatitis.46 EPI is also an increasingly recognized phenomenon in AP: it is estimated that approximately two-thirds of AP patients may be affected with EPI during their AP admission and one-third of AP patients have EPI during followup.47 There is a physiological basis to hypothesize that PERT may be useful to improve other clinical outcomes in pancreatitis by reducing nutrient stimulated secretion of cholecystokinin. Studies to date investigating the use of PERT for management of abdominal pain have been challenging to interpret due to heterogeneity in study designs, including the dose and formulation of PERT.48 Of note, there are observational retrospective data from a pediatric pancreatitis study population demonstrating that use of PERT was associated with a reduction in the incidence rate of recurrent AP (though no change in pain pattern) following PERT initiation (unpublished data). These preliminary data also warrant careful design and conduct of a clinical trial to understand the potential role of PERT for preventing AP in high-risk populations. Lastly, there is an ongoing need for additional research to identify effective alternatives to provide exogenous pancreas enzyme supplementation for patients who have either allergies to active or inactive ingredients of current PERT formulations or personal objections to consumption of animal-derived products.
PHARMACOLOGIC AND BIOLOGIC INTERVENTIONS
There exists no current disease-modifying drug or biologic therapy for treatment of AP, RAP, or CP. Approved pharmacologic or biologic therapies are currently lacking and few agents are in development.49 Ongoing experimental efforts focus on several known agents that are being repurposed to target pathologic processes in both AP and CP, such as inflammation and fibrosis. Consensus is lacking at present on optimal targets for drug development, or acceptable outcome measures to assess efficacy. Several ongoing trials were highlighted to serve as examples for discussion in how to best move forward the field of pharmacologic or biologic interventions in pancreatic disease.
Anti-fibrotic agents
Pirfenidone, an anti-fibrosis agent approved by the Food and Drug Administration for treatment of idiopathic pulmonary fibrosis50 has been repurposed in an effort to test its use as a disease-modifying therapy for predicted moderately severe and severe AP.50 Preclinical mouse models of AP demonstrate that pirfenidone reduces the severity of inflammation through an increase in levels of the anti-inflammatory cytokine IL-10.51 Pirfenidone has previously been used safely in humans (as treatment for idiopathic pulmonary fibrosis)52 but has never been used in acutely ill patients with AP. A clinical trial has been initiated to test the safety and tolerability in patients with predicted moderately severe and severe AP (NCT05350371). This trial will also measure multiple clinical endpoints in order to assess for a signal of efficacy. Future studies on prevention of chronic pancreatitis are also being considered.
Another anti-fibrotic agent, TLY012, a soluble trimeric form of human tumor necrosis factor-related apoptosis inducing ligand (TRAIL) has been developed with the help of animal models in which it has shown potent anti-fibrotic activity in CP and models of other fibrotic diseases such as scleroderma and NASH.53-55 TLY012 acts on activated myofibroblast and pancreatic stellate cells (PSC), but not quiescent PSCs, driving apoptosis and resulting in rapid reductions in fibrosis, reducing pancreatic injury in animal models of CP compared to controls. It is also thought to limit fibrosis-associated pain: in rat models of CP, TLY012 treatment reduced somatic-referred hyperalgesia and direct visceral pain sensitivity in the pancreas. While the concept of an anti-fibrotic agent is well established, development of a stable trimer with a long half-life and good solubility provides the first opportunity to evaluate potential efficacy in humans. The FDA has granted orphan drug designation for this agent in both CP and scleroderma and authorized initiation of a Phase 1 trial in healthy volunteers. This safety study will open the door for proof-of-concept trials in patients with painful CP and other fibrotic diseases.
Neuroleptic agents
Lacosamide, an anti-epileptic agent that dampens voltage-gated sodium (NaV1.7) ion channel excitability, has previously been observed in pre-clinical studies in combination with opioid analgesics to diminish sodium channel activity and improve pain control.56 Previous human studies with lacosamide for chronic pain conditions including fibromyalgia and diabetic peripheral neuropathy have failed to demonstrate significant pain reduction, but patients on opioids were excluded.57 The working hypothesis of the present study (extrapolated from an understanding of the mechanism for opioid-induced hyperalgesia) is that opioids activate the non-classical opioid receptor, toll-like receptor 4 (TLR4), and elicit greater pain in individuals due to the opening of the voltage-gated sodium channel NaV1.7. Lacosamide dampens NaV1.7 ion channel excitability, and therefore would allow for an improvement in pain in response to opioids at potentially lower doses. Given the key role of opioid analgesics in this mechanism, previous work excluding patients on opioids eliminated participants most likely to respond to treatment.57 The aim of the ongoing phase 1 study (NCT05603702) is to evaluate the effect of adding lacosamide to opioid therapy on the safety and tolerability in treating CP pain. The results of this pilot study may support a subsequent phase 2 trial assessing the efficacy of lacosamide added to opioid therapy to alleviate abdominal pain in CP.
Human Autologous Stem Cell Therapies
The use of human autologous stem cells represents a potential non-drug biologic therapy that may serve as a disease-modifying intervention to reduce inflammation AP and has previously been evaluated in pre-clinical studies on necrotizing disease. In severe AP, inflammation is exacerbated by resident macrophage activation potentiating the immune response leading to acinar cell death that further amplifies the early inflammatory cascade locally and systemically. Previous clinical attempts to target specific inflammatory modulators to ameliorate the inflammatory response in AP have failed. In contrast, systemic delivery of mesenchymal stem/stromal cells (MSC) has reproducibly shown marked anti-inflammatory effects in rat models of severe AP, even after inflammation was well established. 58, 59 While autologous bone marrow MSC require prolonged processing, adipose-derived MSC, adipose stem/stromal cells (ASC), are readily available by liposuction and require no ex vivo expansion. Pre-clinical studies with human ASC have confirmed that systemic provision of human ASC, like MSC, significantly reduces local tissue injury and systemic inflammation in a murine model of AP.60 The breadth of evidence of the ability of ASC to ameliorate inflammation, in conjunction with their safety profile in several clinical studies, provides a strong rationale to consider a clinical trial of ASC in severe AP.61
ENDOSCOPIC, SURGICAL, and BIOENGINEERING APPROACHES
In the early stages of CP, medical management of exocrine and endocrine functions and mild to moderate pain can be effective in many cases.62, 63 With progression of disease, long-term pain relief can often only be achieved using additional invasive interventions such as endoscopy and surgery in conjunction with adjunctive therapies for comorbid conditions.64, 65 While technical aspects of endoscopic and surgical interventions have changed little in recent years, the debate is ongoing about optimal patient selection, timing, and modality of treatment.66 A multi-modal and multi-disciplinary approach is often required to determine the best treatment plan, frequently multifaceted to manage all treatable aspects of painful CP.
Advances in Pancreatic Endoscopy
Imaging
Endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS) are the two endoscopic modalities that are commonly used in the management of patients with pancreatitis. ERCP is no longer used in the diagnostic setting, but rather only in settings of a therapeutic intervention. EUS, a commonly used imaging modality for the pancreas, can visualize the entire pancreas and offers the potential for simultaneous tissue sampling when appropriate. It has excellent sensitivity and specificity for features of late disease (main pancreatic dilation and irregularity or calcifications), but current criteria for diagnosing early CP remains suboptimal due to lack of specificity, poor inter-observer reliability and inconsistent histopathologic correlation.67-69 There is a critical need to leverage ongoing innovation in artificial intelligence to address these diagnostic limitations, likely in conjunction with imaging enhancements such as strain or shear wave elastography. Importantly, there are no tests to confirm the presence of pancreatic hypertension within a dilated (“obstructed”) pancreatic duct.
Therapy for Pain
Since endoscopic therapy is predicated on decompression of a dilated pancreatic duct, a biomarker for pressure-mediated pancreatic pain may be useful in predicting which patients will derive the greatest benefit from endoscopic drainage. Pancreatic duct dilation is considered a phenotypic manifestation of increased pancreatic duct pressure, which is hypothesized to represent one mechanism for pain in chronic pancreatitis.70, 71 However, small studies suggest poor correlation between findings at ERCP, pain scores, and intraductal pressure.72, 73 It is widely recognized that the etiology of pain is multifactorial, with pancreatic duct obstruction representing one of several mechanisms that include pancreatic ischemia, intrapancreatic nerve damage (manifested by inflammation, enlargement, fibrosis, or some combination), and impaired central nervous system modulation.74 Given the complex pathogenesis of chronic pancreatitis pain and variability in pain phenotypes,75 treatment of pancreatic duct obstruction (and thus, pancreatic duct hypertension) is logical but not unequivocally beneficial—randomized trials comparing endoscopy with surgery or extracorporeal shock wave lithotripsy reported favorable outcomes in < 50% of the endoscopy population.76-79
Approaches to endoscopic transpapillary pancreatic duct drainage have continued to focus on the basic principles: (1) traverse the segment of dominant obstruction using a guidewire; (2) remove main duct calculi, perhaps first by debulking them via lithotripsy before or after; (3) dilating the obstruction using graduated catheters or hydrostatic balloon catheters; and (4) maintain duct patency through placement of one or more plastic pancreatic duct stents in parallel. A complete treatment course usually requires at least 3-4 procedures over approximately 1 year. There are two approaches to pancreatic lithotripsy: intraductal (electrohydraulic or laser mediated) and through extracorporeal shock waves. Preliminary studies suggest extracorporeal shock wave lithotripsy alone may have comparable efficacy to the same technique accompanied by ERCP with stone fragment extraction and stenting. It is also possible that neither are superior to medical therapy and the placebo effect; there are two ongoing sham-controlled trials (SCHOKE,12 NCT03966781, and PERCePT NCT04232670) and several other longitudinal cohort or clinical trials comparing endoscopic techniques.80 Pancreatic stents have not changed in decades. Recent development of a removal, self-expandable metallic stent has been halted by a study which failed to meet its pain endpoint.81 Advances in intraductal pancreatoscopy allow for a more sophisticated—and probably more efficient—approach to removing main duct calculi during the first endoscopic session when the anatomy is favorable. Innovation in interventional EUS is expected to expand the role of endoscopy in treating pancreatic pain, ideally with more objective imaging and prognostic tests to optimize patient selection.
Therapy for local complications of acute pancreatitis
Endoscopy now represents the first-line modality for treating most local complications of pancreatitis—leaks, cysts, and necrotic collections.82 Natural orifice transluminal endoscopic surgery (NOTES) was merely a concept until the endoscopic treatment of walled off pancreatic necrosis evolved to become the preferred strategy for debriding necrotic collections with favorable anatomic boundaries. Initially, these drainage and debridement procedures were performed using tools developed for tissue sampling and biliary drainage. The advent of lumen apposing metal stents preloaded onto an electrocautery enhanced cystotome (Axios™, Boston Scientific Corp., Marlborough, Massachusetts) reduces procedure times, technical complexity to gain access, and facilitates repeated intubations of the necrotic cavity. Internal drainage procedures spare the patient the consequences of external drains and eliminate the likelihood of cutaneous fistulae. However, endoscopic treatment of pancreatic necrosis remains imperfect. Each debridement requires anesthesia, is time consuming, and not without risk of hemorrhage, perforation, and infection. Cases that require 3-4 debridements—each 60-90 minutes—are resource intense and inconvenient for patients who may have to travel from a distance.
Surgery in Pancreatitis
Endoscopy Versus Surgery
There lacks consensus agreement in the field regarding the optimal timing and order of invasive interventions to treat painful CP.83, 84 Despite well-conducted randomized studies showing superiority of pain outcomes with surgical drainage compared to endoscopic drainage and early surgery to be favored over later surgery, many clinicians persist with multiple endoscopic procedures before surgical referral. The reasons for this are many and include patient preference and reluctance to undergo surgical intervention, regional differences in practice patterns, lack of adequate surgical experts to perform these interventions as training for these surgeries has declined, and lack of consensus definition on identification of an early stage of CP during which intervention is theoretically optimized in order to prevent progression to end-stage disease.85, 86
A nuanced surgical approach is necessary to adequately address the individual pathological pattern of each candidate for intervention. Several randomized controlled trials have compared classical pancreato-duodenectomies with Beger-type procedures, but have not adequately addressed the fact that more advanced cases with venous compression are able to undergo a Beger-type procedure but not a classical resection of the head of the pancreas. What has additionally not been addressed in the literature is the type of patient with advanced symptoms and total parenchymal and vascular involvement with a non-dilated main pancreatic duct. Although a formal total pancreatectomy is often not possible in this situation, a subtotal in situ resection often achieves good clinical outcomes.87 Contrary to widely held perceptions, both the Frey procedure and the Beger procedure can be applied in patients who have a small main pancreatic duct; previous work has established the success of drainage procedures in these patients despite small duct disease.88 There has been a recent rise in the application and ongoing study of outcomes related to total pancreatectomy and islet auto transplantation (TPIAT).89 In patients with small duct disease or patients with hereditary pancreatitis, this can result in significant improvements in QOL for patients who typically require frequent hospitalizations for pain, and theoretically eliminates the risk of malignant degeneration in those patients with a genetic basis for disease.
Although a conceptual mechanism for progression to end-stage CP has been published and widely discussed, a universally-accepted definition for each of the individual stages of CP remains a barrier to forward progress.4 Wider agreement on this would provide a foundation on which to build future randomized clinical trials of appropriate interventions at each stage.
Chemical Pancreatectomy: An Experimental Non-Operative Treatment for Chronic Pancreatitis
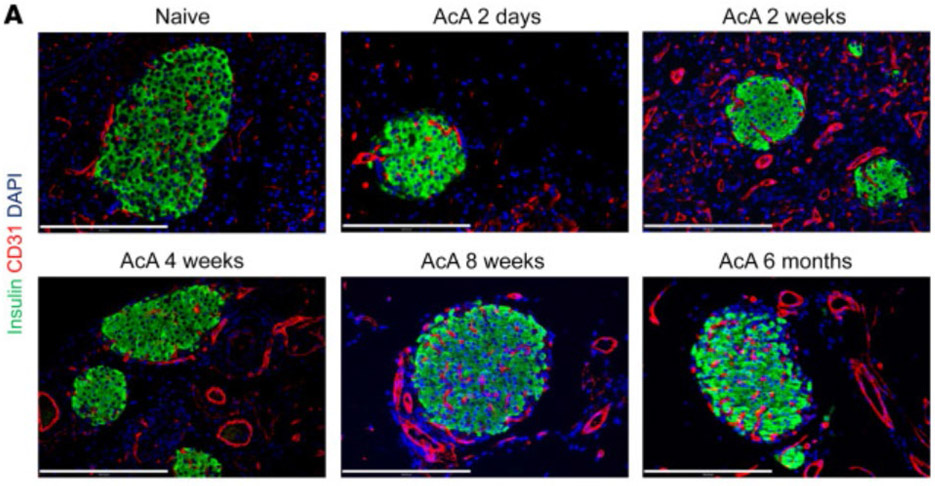
A serendipitous observation in mice that an infusion of 100% ethanol into the pancreatic duct led to total ablation of the ducts and acini, with complete preservation of the endocrine islets may lead to the development of a non-operative approach for RAP and CP, having potential to serve as a non-operative alternative to TPIAT. Subsequent investigation in this area found that a simple dilute 1% solution of acetic acid achieved the same effects with much less toxicity, and a simultaneous improvement in glucose tolerance.90 Subsequently, a cerulein and diet-induced murine model of CP showed improved pain signaling and avoidance of pancreatitis-induced dysglycemia. It was found that the islet innervation and vascularization were maintained.(Figure 2) Fenestrations adjacent to islet endocrine cells seen in normal islet cell architecture that facilitate glucose sensing and hormone release were also preserved on electron microscopy. Evaluation in non-human primates (NHP) found that 2% acetic acid was more effective than 1%, but supra-normal glucose tolerance and enhanced insulin release were again observed.91 Follow-up in some NHPs for up to 20 months revealed no evidence of exocrine regeneration. Currently, there is ongoing work with the FDA to begin a human trial.
Figure 2. Preservation of islets following acetic acid-induced pancreatectomy.
Morphological and histological changes in mouse pancreas specimens following chemical pancreatectomy. (A) After AcA infusion, gross morphology of the pancreas was abnormally white and edematous at 2 days, translucent with visible islets (arrow) at 2 weeks, and replaced by fatty tissue with visible islets (arrow) at 8 weeks. (B and C) Normal pancreas histology is seen at 2 days after saline infusion (B). Two days after AcA infusion, exocrine tissue necrosis (asterisks) is seen with intact islets (i). Magnification (inset) of the apparently preserved acinar cells revealed cell swelling and cytoplasmic vacuolization (arrows) (C). (D) Histology of the pancreas after AcA infusion. Intact islets are denoted by i. Arrows denote fat cells at 4 weeks. (E) Immunostaining after AcA infusion showed negative amylase staining (arrows denote amylase remnants at 2 days), with normal insulin and glucagon staining. Illustrative histology results from 5 animals per time point are shown. Scale bars: 200 μm.
Reproduced (adapted) with permission J Clin Invest. 2021 Feb 1; 131(3): e143301. Published online 2021 Feb 1. doi: 10.1172/JCI143301
CONCLUSIONS
In summary, there remain many challenges to establishing a set of beneficial interventions that will achieve meaningful improvement in clinical outcomes for patients with AP, RAP, and CP. Opportunities for advancement in the field were identified during the course of workshop discussions and outlined to target future developments that will specifically benefit patients (Table 1). This summary report represents the opinions and expertise of the invited speakers, and does not include a systematic review of all available treatments for RAP or CP nor does exclusion of a treatment suggest that it has less value than those discussed here. The items discussed in Table 1 include many efforts that will require cooperation of large consortia and consensus agreements in the field in order to establish foundational definitions on which future advancements can be built. Through these efforts we anticipate iterative progress toward the ultimate goal of addressing the unmet needs of patients with AP, RAP, and CP, and improvement in clinical care and overall outcomes.
Table 1.
Selected Gaps and Opportunities for the Advancement of Development of Interventions for Acute, Recurrent Acute, and Chronic Pancreatitis
| Behavioral Interventions | -Understand and overcome access limitations to behavioral health resources (i.e. shortage of interdisciplinary GI and pain clinics, geographic distance to trained specialists, insurance limitations, stigma) -Development of study designs to evaluate integrated multimodal interventions -Assessment of and intervention to reduce healthcare disparities across the spectrum of pancreatic disease -Further development of psychological interventions including CBT for pain and behavioral contributors to pancreatic disease -Development of novel treatment strategies for alcohol and tobacco addiction in patients with RAP and CP -Expansion of the use of digital technologies for data measurement and delivery of interventions including disease-specific tools |
| Nutritional Interventions | -Consensus development for key definitions of nutritional problems (i.e. malnutrition, sarcopenia, dynamic frailty) in patients with AP, RAP, and CP -Development of a disease-specific standardized PRO tool for nutritional endpoints in AP, RAP, and CP -Clinical trial assessment of frequently used or promising dietary supplements in the treatment of AP, RAP, and CP -Development of collaborative intervention trials assessing the efficacy of multimodal nutrition-centered interventions on QOL and PROs for CP -Further assess the role and effects of PERT in AP, RAP, and CP |
| Pharmacologic And Biologic Interventions | -Development of an FDA-approved PRO tool that can be used in standardized fashion to measure efficacy -Collaboration between basic scientists and clinical trial specialists to harmonize the experimental pipeline: animal models to resemble the human experience of disease (i.e. etiology, disease course, fibrosis) -Further study of existing pharmacologic agents that can be repurposed, and experimental therapies such as stem cell therapy for intervention in AP, RAP, and CP -Development of large-scale collaborative networks for the execution of clinical trials of pharmacologic/biologic agents |
| Bioengineering, Surgical, and Endoscopic Interventions | -Consensus definition for the understanding of stages of RAP and CP (to include multi-modal clinical metrics, etiologic contributors, and additional endpoints) -Guideline creation by consensus agreement on the interventional management of painful CP (incorporating known data on prediction of success, timing of procedure, and modality of procedure) -Further exploration of safety and efficacy of experimental techniques for painful CP (i.e. chemical pancreatectomy and others) |
CBT: Cognitive Behavioral Therapy, RAP: Recurrent Acute Pancreatitis, CP: Chronic Pancreatitis, AP: Acute Pancreatitis, FDA: Food and Drug Administration, PRO: Patient-Reported Outcome, QOL: Quality of Life, PERT: Pancreatic Enzyme Replacement Therapy
Acknowledgements:
The authors gratefully acknowledge the support of the National Pancreas Foundation (NPF) for the workshop, including the assistance of NPF staff Ms. Sokphal Tun and Ms. Trish O'Neill. The logistical and organizational support of Ms. Danielle Johnikin and Mr. John Hare of Scientific Consulting Group Inc is also gratefully acknowledged.
Research Support:
Research reported in this publication was supported by the National Cancer Institute, Department of Defense, and National Institute of Diabetes and Digestive and Kidney U01DK127392 (S.H., C.F.), U01DK108320 (S.H., C.F.), W81XWH-21-1-0665 (V.D.), R01DK 111834 (V.D.), U01DK108323 (E.F.), U01DK127382 (E.F.), U01DK116743 (E.F.), R21AG068607 (G.G.), R01 DK112836 (G.G.), R01DK120698 (G.G.), R01DK120377 (G.G.), R21DA052419, U01DK108327 (P.H.), U01DK127388 (P.H.), R01DK118752 (T.P.), U01DK108314 (S.P.), U01 DK127377 (D.Y.), U01DK108306 (D.Y., R.B.), U01116743 (D.Y.), DoDPR182623 (D.Y.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: The following authors disclose potential conflicts of interest: A.E.P. (Board Member, National Pancreas Foundation), R.B. (Board Member, National Pancreas Foundation), G.C. (Consultant, Olympus America; Consultant, Interpace Diagnostics; Scientific Advisory Board, Genprex), S.D. (co-recipient of unrestricted industry research funding from Mylan Healthcare, expert panel for Mylan Healthcare), A.F. (Consultant work for Takeda and Abbvie), C.F. (Board Member, National Pancreas Foundation; Research support Abbvie), M.R. (honoraria from Fresenius Kabi as speaker); V.S. (Board Member, National Panreas Foundation; Consultant to Abbvie, Ariel Precision Medicine, Organon, Nestle Health Sciences, Panafina and Horizon Therapeutics; Scientific Advisory Board Member and Equity Holder in Kyttaro and Origin Endoscopy), D.W. (Consultant to Abbvie, Ariel Precision Medicine, Nestle, Organon, Regeneron. Co-Founder and Chief Scientific Officer, Ariel Precision Medicine.), D.Y. (Consultant - Pfizer Inc.).
REFERENCES
- 1.Singh VK, Yadav D, Garg PK. Diagnosis and Management of Chronic Pancreatitis: A Review. JAMA 2019;322:2422–2434. [DOI] [PubMed] [Google Scholar]
- 2.Cure M. Pancreatitis patient survey: final report. Available at: https://www.mission-cure.org/patient-resources/patient-survey-report., 2018.
- 3.Yadav D, Askew RL, Palermo T, et al. Association of Chronic Pancreatitis Pain Features With Physical, Mental, and Social Health. Clin Gastroenterol Hepatol 2023;21:1781–1791 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitcomb DC, Frulloni L, Garg P, et al. Chronic pancreatitis: An international draft consensus proposal for a new mechanistic definition. Pancreatology 2016;16:218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rich KL, Abu-El-Haija M, Nathan JD, et al. The Role of Psychology in the Care of Children With Pancreatitis. Pancreas 2020;49:887–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrik ML, Freeman ML, Trikudanathan G. Multidisciplinary Care for Adults With Chronic Pancreatitis: Incorporating Psychological Therapies to Optimize Outcomes. Pancreas 2022;51:4–12. [DOI] [PubMed] [Google Scholar]
- 7.Palermo TM, Law EF, Topazian MD, et al. Internet Cognitive-Behavioral Therapy for Painful Chronic Pancreatitis: A Pilot Feasibility Randomized Controlled Trial. Clin Transl Gastroenterol 2021;12:e00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palermo TM, Beals-Erickson S, Bromberg M, et al. A Single Arm Pilot Trial of Brief Cognitive Behavioral Therapy for Insomnia in Adolescents with Physical and Psychiatric Comorbidities. J Clin Sleep Med 2017;13:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polednak AP. Temporal trend in the U.S. black-white disparity in mortality rates from selected alcohol-related chronic diseases. J Ethn Subst Abuse 2008;7:154–64. [DOI] [PubMed] [Google Scholar]
- 10.McHenry N, Ahmed A, Shah I, et al. Racial and Ethnic Disparities in Opioid Prescriptions in Benign and Malignant Pancreatic Disease in the United States. Pancreas 2022;51:1359–1364. [DOI] [PubMed] [Google Scholar]
- 11.McHenry N, Shah I, Ahmed A, et al. Racial Variations in Pain Management and Outcomes in Hospitalized Patients With Acute Pancreatitis. Pancreas 2022;51:1248–1250. [DOI] [PubMed] [Google Scholar]
- 12.Ocskay K, Juhasz MF, Farkas N, et al. Recurrent acute pancreatitis prevention by the elimination of alcohol and cigarette smoking (REAPPEAR): protocol of a randomised controlled trial and a cohort study. BMJ Open 2022;12:e050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irving HM, Samokhvalov AV, Rehm J. Alcohol as a risk factor for pancreatitis. A systematic review and meta-analysis. JOP 2009;10:387–92. [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute on Alcohol Abuse and Alcoholism. Alcohol Facts and Statistics: National Institute on Alcohol Abuse and Alcoholism, 2023. [Google Scholar]
- 15.American Psychiatric Association., American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders : DSM-5. Washington, D.C.: American Psychiatric Association, 2013. [Google Scholar]
- 16.Bradley KA, DeBenedetti AF, Volk RJ, et al. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 2007;31:1208–17. [DOI] [PubMed] [Google Scholar]
- 17.National Institute on Alcohol Abuse and Alcoholism. Rethinking Alcohol, Alcohol & your health. Volume 2023: National Intstitute on Alcohol Abuse and Alcoholism, 2023. [Google Scholar]
- 18.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet 2005;6:521–32. [DOI] [PubMed] [Google Scholar]
- 19.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 2001;24:97–129. [DOI] [PubMed] [Google Scholar]
- 20.Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. Am J Psychiatry 2018;175:86–90. [DOI] [PubMed] [Google Scholar]
- 21.Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 2014;311:1889–900. [DOI] [PubMed] [Google Scholar]
- 22.Teo K, Johnson MH, Truter S, et al. Pain assessment in chronic pancreatitis: A comparative review of methods. Pancreatology 2016;16:931–939. [DOI] [PubMed] [Google Scholar]
- 23.Perito ER, Pohl JF, Bakker C, et al. Outpatient Pain Management in Children With Chronic Pancreatitis: A Scoping Systematic Review. Pancreas 2022;51:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uc A, Andersen DK, Apkarian AV, et al. Pancreatic Pain-Knowledge Gaps and Research Opportunities in Children and Adults: Summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Pancreas 2021;50:906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teo K, Johnson MH, Drewes AM, et al. A comprehensive pain assessment tool (COMPAT) for chronic pancreatitis: Development, face validation and pilot evaluation. Pancreatology 2017;17:706–719. [DOI] [PubMed] [Google Scholar]
- 26.Kuhlmann L, Teo K, Olesen SS, et al. Development of the Comprehensive Pain Assessment Tool Short Form for Chronic Pancreatitis: Validity and Reliability Testing. Clin Gastroenterol Hepatol 2022;20:e770–e783. [DOI] [PubMed] [Google Scholar]
- 27.Ul Ain Q, Bashir Y, Kelleher L, et al. Dietary intake in patients with chronic pancreatitis: A systematic review and meta-analysis. World J Gastroenterol 2021;27:5775–5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaghan B, Monaghan A, Mockler D, et al. Physical activity for chronic pancreatitis: a systematic review. HPB (Oxford) 2022;24:1217–1222. [DOI] [PubMed] [Google Scholar]
- 29.Ramai D, Facciorusso A, Maida M, et al. Prevalence of Osteopathy In Chronic Pancreatitis: A Systematic Review and Meta-Analysis. Clin Transl Gastroenterol 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiese M, Gartner S, Doller J, et al. Nutritional management of chronic pancreatitis: A systematic review and meta-analysis of randomized controlled trials. J Gastroenterol Hepatol 2021;36:588–600. [DOI] [PubMed] [Google Scholar]
- 31.Hart PA, Andersen DK, Lyons E, et al. Clinical Trials in Pancreatitis: Opportunities and Challenges in the Design and Conduct of Patient-Focused Clinical Trials in Recurrent Acute and Chronic Pancreatitis: Summary of a National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Pancreas 2022;51:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cederholm T, Jensen GL, Correia M, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1–9. [DOI] [PubMed] [Google Scholar]
- 33.Griffin OM DS, Fennelly D, McDermott R, Geoghegan J, Conlon K Exploring the feasibility of a supportive care intervention for patients undergoing neo-adjuvant chemotherapy for pancreatic cancer: The FEED study (a fish oil supplement, pancreatic enzyme supplement, exercise advice and individualised dietary counselling). HPB (Oxford) 2020:S239–S330. [Google Scholar]
- 34.Roberts KM, Golian P, Nahikian-Nelms M, et al. Does the Healthy Eating Index and Mediterranean Diet Score Identify the Nutritional Adequacy of Dietary Patterns in Chronic Pancreatitis? Dig Dis Sci 2019;64:2318–2326. [DOI] [PubMed] [Google Scholar]
- 35.Bahr LS, Franz K, Mahler A. Assessing the (anti)-inflammatory potential of diets. Curr Opin Clin Nutr Metab Care 2021;24:402–410. [DOI] [PubMed] [Google Scholar]
- 36.Lo CH, Lochhead P, Khalili H, et al. Dietary Inflammatory Potential and Risk of Crohn's Disease and Ulcerative Colitis. Gastroenterology 2020;159:873–883 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siprelle KA HE, Nahikian-Nelsm M, et al. The Assessment of Polyphenols in Chronic Pancreatitis Using a Web-Based Food Frequency Questionnaire and the Phenol Explorer Database. Journal of the Academy of Nutrition and Dietetics. 2021;121:PA129. [Google Scholar]
- 38.Bakker OJ, van Brunschot S, van Santvoort HC, et al. Early versus on-demand nasoenteric tube feeding in acute pancreatitis. N Engl J Med 2014;371:1983–93. [DOI] [PubMed] [Google Scholar]
- 39.Chang YS, Fu HQ, Xiao YM, et al. Nasogastric or nasojejunal feeding in predicted severe acute pancreatitis: a meta-analysis. Crit Care 2013;17:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arvanitakis M, Ockenga J, Bezmarevic M, et al. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis. Clin Nutr 2020;39:612–631. [DOI] [PubMed] [Google Scholar]
- 41.Machicado JD, Gougol A, Paragomi P, et al. Practice Patterns and Utilization of Tube Feedings in Acute Pancreatitis Patients at a Large US Referral Center. Pancreas 2018;47:1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr 2016;40:159–211. [DOI] [PubMed] [Google Scholar]
- 43.Pradelli L, Klek S, Mayer K, et al. Omega-3 fatty acid-containing parenteral nutrition in ICU patients: systematic review with meta-analysis and cost-effectiveness analysis. Crit Care 2020;24:634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenthal MD, Patel J, Staton K, et al. Can Specialized Pro-resolving Mediators Deliver Benefit Originally Expected from Fish Oil? Curr Gastroenterol Rep 2018;20:40. [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Go VL, Sarkar FH. The Role of Nutraceuticals in Pancreatic Cancer Prevention and Therapy: Targeting Cellular Signaling, MicroRNAs, and Epigenome. Pancreas 2015;44:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de la Iglesia-Garcia D, Huang W, Szatmary P, et al. Efficacy of pancreatic enzyme replacement therapy in chronic pancreatitis: systematic review and meta-analysis. Gut 2017;66:1354–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang W, de la Iglesia-Garcia D, Baston-Rey I, et al. Exocrine Pancreatic Insufficiency Following Acute Pancreatitis: Systematic Review and Meta-Analysis. Dig Dis Sci 2019;64:1985–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winstead NS, Wilcox CM. Clinical trials of pancreatic enzyme replacement for painful chronic pancreatitis--a review. Pancreatology 2009;9:344–50. [DOI] [PubMed] [Google Scholar]
- 49.Abu-El-Haija M, Gukovskaya AS, Andersen DK, et al. Accelerating the Drug Delivery Pipeline for Acute and Chronic Pancreatitis: Summary of the Working Group on Drug Development and Trials in Acute Pancreatitis at the National Institute of Diabetes and Digestive and Kidney Diseases Workshop. Pancreas 2018;47:1185–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nathan SD, Costabel U, Glaspole I, et al. Efficacy of Pirfenidone in the Context of Multiple Disease Progression Events in Patients With Idiopathic Pulmonary Fibrosis. Chest 2019;155:712–719. [DOI] [PubMed] [Google Scholar]
- 51.Palathingal Bava E, George J, Tarique M, et al. Pirfenidone increases IL-10 and improves acute pancreatitis in multiple clinically relevant murine models. JCI Insight 2022;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.King TE Jr., Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. [DOI] [PubMed] [Google Scholar]
- 53.Guicciardi ME, Gores GJ. Paving the TRAIL to anti-fibrotic therapy. Hepatology 2016;64:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh Y, Park O, Swierczewska M, et al. Systemic PEGylated TRAIL treatment ameliorates liver cirrhosis in rats by eliminating activated hepatic stellate cells. Hepatology 2016;64:209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park JS, Oh Y, Park YJ, et al. Targeting of dermal myofibroblasts through death receptor 5 arrests fibrosis in mouse models of scleroderma. Nat Commun 2019;10:1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim SA, Albany Z, Albany C. Significant response to lacosamide in a patient with severe chemotherapy-induced peripheral neuropathy. J Community Support Oncol 2015;13:202–4. [DOI] [PubMed] [Google Scholar]
- 57.Hearn L, Derry S, Moore RA. Lacosamide for neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev 2012;2012:CD009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung KH, Song SU, Yi T, et al. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology 2011;140:998–1008. [DOI] [PubMed] [Google Scholar]
- 59.Tu XH, Song JX, Xue XJ, et al. Role of bone marrow-derived mesenchymal stem cells in a rat model of severe acute pancreatitis. World J Gastroenterol 2012;18:2270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roch AM, Maatman TK, Cook TG, et al. Therapeutic Use of Adipose-Derived Stromal Cells in a Murine Model of Acute Pancreatitis. J Gastrointest Surg 2020;24:67–75. [DOI] [PubMed] [Google Scholar]
- 61.Bourin P, Bunnell BA, Casteilla L, et al. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013;15:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rustagi T, Njei B. Antioxidant therapy for pain reduction in patients with chronic pancreatitis: a systematic review and meta-analysis. Pancreas 2015;44:812–8. [DOI] [PubMed] [Google Scholar]
- 63.Olesen SS, Bouwense SA, Wilder-Smith OH, et al. Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology 2011;141:536–43. [DOI] [PubMed] [Google Scholar]
- 64.Nusrat S, Yadav D, Bielefeldt K. Pain and opioid use in chronic pancreatitis. Pancreas 2012;41:264–70. [DOI] [PubMed] [Google Scholar]
- 65.Glass LM, Whitcomb DC, Yadav D, et al. Spectrum of use and effectiveness of endoscopic and surgical therapies for chronic pancreatitis in the United States. Pancreas 2014;43:539–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drewes AM, Kempeneers MA, Andersen DK, et al. Controversies on the endoscopic and surgical management of pain in patients with chronic pancreatitis: pros and cons! Gut 2019;68:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sheel ARG, Baron RD, Sarantitis I, et al. The diagnostic value of Rosemont and Japanese diagnostic criteria for 'indeterminate', 'suggestive', 'possible' and 'early' chronic pancreatitis. Pancreatology 2018;18:774–784. [DOI] [PubMed] [Google Scholar]
- 68.Rana SS, Vilmann P. Endoscopic ultrasound features of chronic pancreatitis: A pictorial review. Endosc Ultrasound 2015;4:10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallace MB, Hawes RH, Durkalski V, et al. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointest Endosc 2001;53:294–9. [DOI] [PubMed] [Google Scholar]
- 70.Ebbehoj N, Borly L, Bulow J, et al. Pancreatic tissue fluid pressure in chronic pancreatitis. Relation to pain, morphology, and function. Scand J Gastroenterol 1990;25:1046–51. [DOI] [PubMed] [Google Scholar]
- 71.Bradley EL 3rd. Pancreatic duct pressure in chronic pancreatitis. Am J Surg 1982;144:313–6. [DOI] [PubMed] [Google Scholar]
- 72.Ebbehoj N, Borly L, Madsen P, et al. Pancreatic tissue fluid pressure during drainage operations for chronic pancreatitis. Scand J Gastroenterol 1990;25:1041–5. [DOI] [PubMed] [Google Scholar]
- 73.Manes G, Buchler M, Pieramico O, et al. Is increased pancreatic pressure related to pain in chronic pancreatitis? Int J Pancreatol 1994;15:113–7. [DOI] [PubMed] [Google Scholar]
- 74.Drewes AM, Bouwense SAW, Campbell CM, et al. Guidelines for the understanding and management of pain in chronic pancreatitis. Pancreatology 2017;17:720–731. [DOI] [PubMed] [Google Scholar]
- 75.Mullady DK, Yadav D, Amann ST, et al. Type of pain, pain-associated complications, quality of life, disability and resource utilisation in chronic pancreatitis: a prospective cohort study. Gut 2011;60:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med 2007;356:676–84. [DOI] [PubMed] [Google Scholar]
- 77.Dite P, Ruzicka M, Zboril V, et al. A prospective, randomized trial comparing endoscopic and surgical therapy for chronic pancreatitis. Endoscopy 2003;35:553–8. [DOI] [PubMed] [Google Scholar]
- 78.Dumonceau JM, Costamagna G, Tringali A, et al. Treatment for painful calcified chronic pancreatitis: extracorporeal shock wave lithotripsy versus endoscopic treatment: a randomised controlled trial. Gut 2007;56:545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Issa Y, Kempeneers MA, Bruno MJ, et al. Effect of Early Surgery vs Endoscopy-First Approach on Pain in Patients With Chronic Pancreatitis: The ESCAPE Randomized Clinical Trial. JAMA 2020;323:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olesen SS, Drewes AM, Gaud R, et al. Combined extracorporeal shock wave lithotripsy and endoscopic treatment for pain in chronic pancreatitis (SCHOKE trial): study protocol for a randomized, sham-controlled trial. Trials 2020;21:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sherman S, Kozarek RA, Costamagna G, et al. Soft self-expandable metal stent to treat painful pancreatic duct strictures secondary to chronic pancreatitis: a prospective multicenter trial. Gastrointest Endosc 2023;97:472–481 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roug S, Novovic S, Hansen EF, et al. Short- and Long-Term Outcomes After Multimodal Treatment of Pancreatic Duct Leakage in Patients With Chronic Pancreatitis. Pancreas 2022;51:1315–1319. [DOI] [PubMed] [Google Scholar]
- 83.Yang CJ, Bliss LA, Freedman SD, et al. Surgery for chronic pancreatitis: the role of early surgery in pain management. Pancreas 2015;44:819–23. [DOI] [PubMed] [Google Scholar]
- 84.Guo JY, Qian YY, Sun H, et al. Optimal Timing of Endoscopic Intervention After Extracorporeal Shock-Wave Lithotripsy in the Treatment of Chronic Calcified Pancreatitis. Pancreas 2021;50:633–638. [DOI] [PubMed] [Google Scholar]
- 85.Frokjaer JB, Akisik F, Farooq A, et al. Guidelines for the Diagnostic Cross Sectional Imaging and Severity Scoring of Chronic Pancreatitis. Pancreatology 2018;18:764–773. [DOI] [PubMed] [Google Scholar]
- 86.Whitcomb DC, Shimosegawa T, Chari ST, et al. International consensus statements on early chronic Pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with The International Association of Pancreatology, American Pancreatic Association, Japan Pancreas Society, PancreasFest Working Group and European Pancreatic Club. Pancreatology 2018;18:516–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baron RD, Sheel ARG, Farooq A, et al. The in situ near-total pancreatectomy (LIVOCADO procedure) for end-staged chronic pancreatitis. Langenbecks Arch Surg 2021;406:2657–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yekebas EF, Bogoevski D, Honarpisheh H, et al. Long-term follow-up in small duct chronic pancreatitis: A plea for extended drainage by "V-shaped excision" of the anterior aspect of the pancreas. Ann Surg 2006;244:940–6; discussion 946-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bellin MD, Ramanathan K, Chinnakotla S. Total Pancreatectomy with Islet Auto-Transplantation: Surgical Procedure, Outcomes, and Quality of Life. Adv Surg 2023;57:15–30. [DOI] [PubMed] [Google Scholar]
- 90.Saleh M, Sharma K, Kalsi R, et al. Chemical pancreatectomy treats chronic pancreatitis while preserving endocrine function in preclinical models. J Clin Invest 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kalsi RS, Kreger AM, Saleh M, et al. Chemical pancreatectomy in non-human primates ablates the acini and ducts and enhances beta-cell function. Sci Rep 2023;13:9113. [DOI] [PMC free article] [PubMed] [Google Scholar]