Abstract
Hepatopulmonary syndrome (HPS) is a serious vascular complication of liver disease that occurs in 5–32% of patients with cirrhosis. The presence of HPS markedly increases mortality. No effective medical therapies are currently available and liver transplantation is the only established treatment option for HPS. The definition and diagnosis of HPS are established by the presence of a triad of liver disease with intrapulmonary vascular dilation that causes abnormal arterial gas exchange. Experimental biliary cirrhosis induced by common bile duct ligation in the rat reproduces the pulmonary vascular and gas exchange abnormalities of human HPS and serves as a pertinent animal model. Pulmonary microvascular dilation and angiogenesis are two central pathogenic features that drive abnormal pulmonary gas exchange in experimental HPS, and thus might underlie HPS in humans. Defining the mechanisms involved in the microvascular alterations of HPS has the potential to lead to effective medical therapies. This Review focuses on the current understanding of the pathogenesis, clinical features and management of HPS.
Introduction
Cirrhosis and portal hypertension result in alterations in the vasculature in a number of organ systems, which affects function in these organs and increases mortality. Pulmonary vascular involvement in liver disease includes two unique entities: hepatopulmonary syndrome (HPS) and portopulmonary hypertension. HPS occurs in 5–32% of patients with cirrhosis and occurs when pulmonary microvascular dilatation causes impaired oxygenation in the absence of marked intrinsic cardiopulmonary disease.1–3 The presence of HPS markedly increases mortality in affected patients. Currently, no effective nonsurgical treatments are available for HPS—liver transplantation is the only treatment option.4–8 Unlike HPS, portopulmonary hypertension occurs in only 5–8% of patients with cirrhosis when increased pulmonary arterial pressure (pulmonary arterial hypertension) develops in the setting of portal hypertension.9–16 Medical therapy with vasoactive agents improves pulmonary arterial pressure and symptoms in portopulmonary hypertension.17–21 In the past few years, the coexistence of HPS and portopulmonary hypertension has been reported, implying that these two disorders might share pathogenic mechanisms. Moreover, the coexistence of HPS and portopulmonary hypertension could mask the findings of raised pulmonary arterial pressure in portopulmonary hypertension.22–27 The purpose of this Review is to provide an update on HPS, focusing on the pathophysiology, clinical features and recommendations for diagnosis and management.
Definition
HPS is defined by the presence of liver disease and/or portal hypertension and intrapulmonary vascular dilation that causes an abnormal age-corrected alveolar–arterial oxygen gradient.16 HPS can coexist with other cardiopulmonary disorders and contributes substantially to gas exchange abnormalities in this setting.28,29 In general, HPS is reversible with liver transplantation.
HPS is fairly common in patients with cirrhosis being evaluated for liver transplantation and can occur across the spectrum of severity of cirrhosis.2,30,31 Although some studies find HPS to be more common in more advanced liver disease and in more severe portal hypertension, it clearly occurs in both well compensated and decompensated liver disease, and in situations in which portal hypertension is present in the absence of cirrhosis. HPS has been described in portal hypertension without cirrhosis (prehepatic portal hypertension, nodular regenerative hyperplasia, congenital hepatic fibrosis and hepatic venous outflow obstruction)32–34 and hepatic dysfunction in the absence of established portal hypertension (acute and chronic hepatitis).35–37 In a prospective, multicentre US study, no difference in severity of liver disease in patients with or without HPS was observed.6 Intrapulmonary shunting and hypoxaemia have also been reported in patients with metastatic carcinoid in the absence of portal hypertension38 and in those with vascular abnormalities that result in limited portal flow to the liver (Abernethy malformations)39–42 or that have reduced hepatic venous drainage to the pulmonary arterial bed (Glenn or cavopulmonary shunt).43–46 These observations indicate that factors normally produced or metabolized in the liver could influence the lung microvasculature in susceptible individuals when hepatic function or blood flow are altered.
The pulmonary gas exchange abnormalities of HPS are characterized by hyperventilation and arterial deoxygenation that can be mild (partial pressure of oxygen [PaO2] <80 mmHg), moderate (PaO2 <70 mmHg) or severe (PaO2 <60 mmHg).16,31,47,48 There is an increased alveolar–arterial oxygen gradient (AaPO2) whilst breathing room air (>15 mmHg, or >20 mmHg in patients >64 years of age) with or without hypoxaemia. The prevalence of HPS (range 5–32%) varies depending on whether abnormalities in arterial gas exchange are defined by an abnormal AaPO2 or arterial hypoxaemia (in PaO2).2,30,31,49–52 From a practical perspective, identifying patients with PaO2 <70 mmHg (detected in the sitting position to avoid effects of positional changes on PaO2) is useful for recognizing those with clinically important HPS.53 Calculation of AaPO2 is one of the most sensitive approaches for the detection of early arterial deoxygenation,47 as AaPO2 can increase before arterial oxygen tension (PaO2) itself becomes abnormally low. However, AaPO2 can vary markedly in healthy adults and usually increases with age.54,55 At sea level and whilst breathing room air, a resting AaPO2 of >15 mmHg is abnormal, and an AaPO2 of >20 mmHg is considered abnormal for an individual who is >64 years of age.2,53 Therefore, targeting values above the 95% confidence interval for the age-corrected AaPO2 is appropriate to avoid overdiagnosis of HPS.56
Evidence that gas exchange abnormalities are attributable to intrapulmonary shunting (impaired oxygenation of blood in abnormal pulmonary capillaries) must also be present to confirm the existence of HPS.31 Shunting can result from microvascular dilatations, direct arteriovenous connections or angiogenesis in more severe cases.16 The vascular component characteristically includes diffuse dilated pulmonary capillaries near gas exchange units or localized dilation of larger capillaries and, less commonly, pleural and pulmonary arteriovenous communications.57–60 The diameter of the pulmonary capillaries in healthy individuals at rest can reach about 15 μm.61 Intrapulmonary vascular dilatation is considered to exist when pulmonary capillary diameter increases (15–60 μm) and is the major structural derangement in HPS.62 In some cases, diameters can reach as much as 500 μm, predominately in the lung bases, where increased blood flow exists as a result of gravity.63
Diagnosis
The diagnosis of HPS requires a high degree of clinical suspicion and rests on evidence of the presence of arterial gas exchange abnormalities resulting from intrapulmonary vascular dilatation in the appropriate clinical setting. The threshold for pursuing the diagnosis is influenced by the presence of specific signs and symptoms of HPS, risk factors for intrinsic cardiopulmonary disease and whether liver transplantation is being considered. In patients with risk factors for intrinsic cardiopulmonary disease (smoking and other cardiovascular risk factors, occupational exposure to asbestos, silica or coal dust, liver diseases associated with intrinsic lung disease), these factors, rather than HPS, are appropriate initial considerations. In patients with clubbing (proliferation of soft tissue under the nail bed resulting in abnormal curvature of the nail) or dyspnoea in the absence of risk of intrinsic cardiopulmonary disease and in those being considered for liver transplantation, screening for HPS is appropriate and cost-effective.64 In the latter group, it is particularly important to diagnose and differentiate HPS and portopulmonary hypertension, given that the presence of these disorders can influence treatment and candidacy and priority for liver transplantation.
Gas exchange abnormalities
Gas exchange abnormalities are detected by arterial blood gas measurements and quantified by calculating the AaPO2 (>15–20 mmHg abnormal based on age) and assessing for hypoxaemia (PaO2 <80 mmHg).16 Including mild gas exchange abnormalities (increased AaPO2, PaO2 >80 mmHg) in the diagnostic criteria for HPS seems to be important on the basis of findings that mortality is increased in this subset of patients with these abnormalities compared with patients with cirrhosis without HPS.2,6 Obtaining arterial blood gases in the sitting position— to minimize increases in PaO2 sometimes seen in the supine position (orthodeoxia)—could enhance the detection of arterial deoxygenation in HPS.57 Pulse oximetry is an established screening modality for detecting hypoxaemia and HPS in patients being evaluated for liver transplantation.5 Using a threshold SpO2 (arterial oxygen saturation with pulse oximetry) value of ≤96% provides a sensitivity of 100% and specificity of 88% for detecting patients with HPS who have a PaO2 of <70 mmHg.5,52 This technique can target the use of tests for HPS to those with a higher risk of disease. On the basis of the utility of pulse oximetry for detecting hypoxaemia in a wide range of disorders, this technique could also be useful for screening all populations with cirrhosis.
Intrapulmonary vascular dilatation
In adults, contrast-enhanced echocardiography using a transthoracic approach is the most sensitive and commonly used screening technique for detecting intrapulmonary vascular dilation. Lung perfusion scanning, pulmonary angiography and high-resolution CT scanning are additional studies that can be useful as adjunctive tests in selected individuals. Contrast-enhanced echocardiography and perfusion lung scanning using technetium-99m-labelled macroaggregated albumin (99mTcMAA) are the two most well-accepted approaches for assessing intrapulmonary vascular dilatation.48,63,65–67 Typically, agitated saline is used to generate microbubbles during echocardiography. Intrapulmonary vascular dilatation is diagnosed when microbubbles are observed in the left cardiac chambers three cardiac cycles after intravenous injection.30,68,69 Immediate visualization of injected contrast (microbubbles) in the left side of the heart indicates intracardiac shunting. Transoesophageal contrast echocardiography can increase the sensitivity of detecting intrapulmonary vascular dilatation compared with transthoracic echocardiography, but is invasive and more expensive.30,68–70 Echocardiography also assesses cardiac function and estimates pulmonary arterial systolic pressure, and is useful for screening for cardiac dysfunction and portopulmonary hypertension. As many as 40–60% of patients with cirrhosis and normal levels of arterial blood gases can have a positive contrast echocardiogram, suggesting that mild intrapulmonary vascular dilatation insufficient to alter gas exchange is common.2,30,31 Also, a positive result on contrast echocardiography in a patient who has hypoxaemia with concomitant pulmonary dysfunction (pleural effusion and chronic obstructive pulmonary disease) does not establish HPS as a cause of gas exchange abnormalities, because either intrapulmonary vascular dilatation or the underlying pulmonary process could be responsible. In these patients, additional testing with radionuclide lung perfusion scanning is useful for further diagnosis.
Radionuclide lung perfusion scanning (99mTcMAA scan) can be used to quantify intrapulmonary shunting in HPS. Normally, most particles are trapped in the lung microvasculature, but in HPS, some particles escape through abnormal capillaries and lodge downstream.28 Quantitative imaging of the lung and brain using a standardized methodology has been validated as a means to calculate the fraction of particles that escape the lung and reach the brain.1,28,30 Using this methodology, a positive 99mTcMAA scan (shunting >6%) is found only in patients with HPS who have a PaO2 <60 mmHg and not in those with intrinsic lung disease alone.28,30 A positive finding from a 99mTcMAA scan supports the presence of advanced HPS even in the setting of coexistent intrinsic lung disease. However, as a screening test in adults, 99mTcMAA scanning is less sensitive than contrast echocardiography in detecting intrapulmonary vascular dilatation, and cannot evaluate cardiac function, intracardiac shunting or pulmonary artery pressures.
Pulmonary angiography is an invasive and insensitive diagnostic modality for detecting intrapulmonary vasodilatation in HPS and is not useful as a screening test. Two types of angiographic findings have been reported: type 1, a diffuse ‘spongiform’ appearance of pulmonary vessels during the arterial phase; and type 2, small discrete arteriovenous communication.16,57–60 The great majority of patients with HPS have either normal angiograms or type 1 findings even when hypoxaemia is severe. Therefore, angiography has a very limited diagnostic and therapeutic role in HPS.71,72 High-resolution chest CT is a less invasive radiological method to detect discrete arteriovenous communications than angiography in HPS.38,73,74 The degree of dilatation observed on CT correlates with the severity of gas exchange abnormalities in several studies, suggesting that CT might be useful in assessing the presence and severity of HPS.
Pathophysiology and pathogenesis
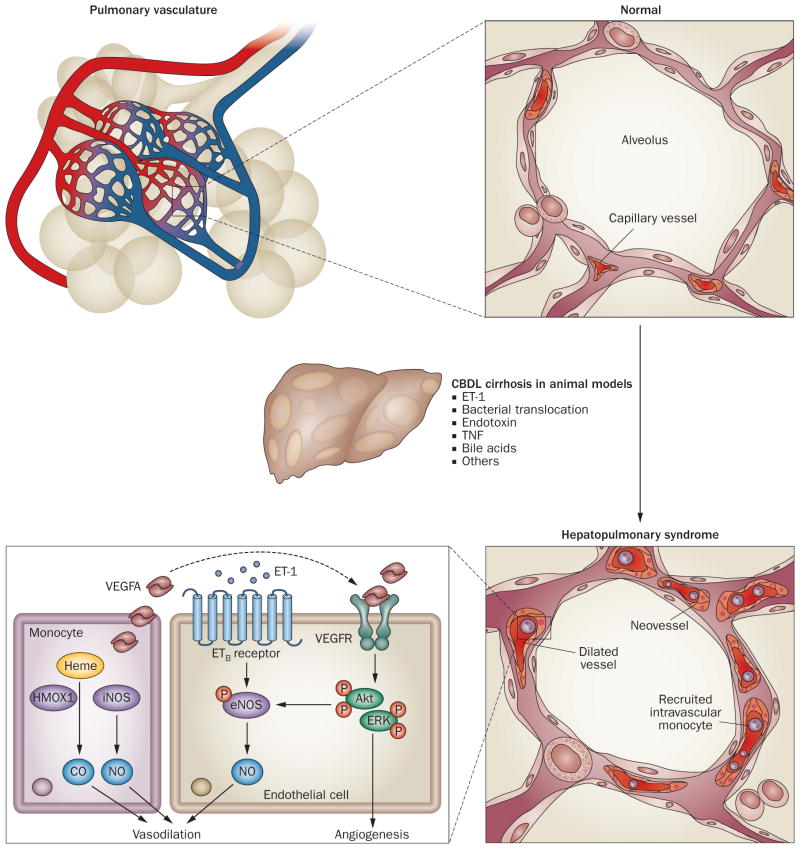
The most well-described alteration in HPS is dilations in the precapillary and postcapillary pulmonary vasculature, resulting in impaired oxygenation of venous blood as it passes through the lung.1,75,76 These changes result from decreased precapillary arteriolar tone and also seem to involve additional mechanisms including angiogenesis, vascular remodelling and vasculogenesis.77,78 Our current understanding of the pathogenesis of HPS is mainly drawn from experimental studies using animal models (Figure 1). Less is known about the pathogenesis of human HPS and how the mechanisms identified in the development of experimental HPS contribute to human disease.
Figure 1.
Working model of pathogenic mechanisms in experimental HPS. The key pathophysiological features of experimental HPS induced by CBDL cirrhosis are pulmonary microvascular alterations, including vasodilation, intravascular monocyte accumulation and angiogenesis. Pulmonary vasodilation is triggered by excessive NO production through ET-1/ETB receptor-driven eNOS activation and iNOS induction in intravascular monocytes, as well as the altered CO production (caused by altered levels of HMOX1) in monocytes. Moreover, monocytes adhered to the pulmonary vasculature produce growth factors such as VEGFA, which contribute to the development of angiogenesis by activating angiogenic signalling pathways including Akt and ERK in endothelial cells. Abbreviations: Akt, protein kinase B; CBDL, common bile duct ligation; CO, carbon monoxide; eNOS, endothelial nitric oxide synthase; ERK, extracellular signal-regulated protein kinase; ET-1, endothelin-1; ETB receptor, endothelin B receptor; HPS, hepatopulmonary syndrome; HMOX1, heme oxygenase 1 (also known as HO1); iNOS, inducible nitric oxide synthase; NO, nitric oxide; TNF, tumour necrosis factor; VEGFA, vascular endothelial growth factor A; VEGFR, vascular endothelial growth factor receptor.
Experimental HPS
Animal models
Defining well characterized and easily accessible animal models that mimic human diseases is critical for exploring pathogenic features and mechanisms of disease, and for developing effective therapeutic strategies for HPS. To date, chronic common bile duct ligation (CBDL) in the rat is the only established experimental model of human HPS.
CBDL induces biliary fibrosis, which results in a reduction in pulmonary vascular resistance and gas exchange abnormalities similar to human HPS.79–82 Direct measurement of pulmonary microvascular size and arterial blood gases show that there is a progressive increase in the size of the pulmonary microvasculature and in the AaPO2 that begins within 2 weeks after CBDL in the absence of light-level pulmonary histological abnormalities.83–85 During this time period, onset of bile duct proliferation, bridging biliary fibrosis and a hyperdynamic state with early portal hypertension occur.83,84,86,87 Therefore, HPS develops prior to the full development of cirrhosis and portal hypertension after CBDL. These observations support a concept drawn from human studies in which the presence or development of HPS does not require advanced and long-standing liver disease.6,67
As experimental controls, two additional liver disease models have been evaluated and compared with the CBDL model for the development of HPS. Partial portal vein ligation results in prehepatic portal hypertension without cirrhosis, accompanied by hyperdynamic circulation, splanchnic vasodilation and portal–systemic shunts.88–90 Chronic thioacetamide administration results in toxic hepatocellular injury that leads to nonbiliary micronodular cirrhosis and portal hypertension within 8 weeks.86,91–96 HPS does not develop in either model. Together, these observations document that CBDL, relative to the partial portal vein ligation or the thioacetamide model, triggers unique alterations that lead to the development of HPS.
Vasodilation
The pathogenic hallmark of human and experimental HPS is microvascular alterations within the pulmonary arterial circulation. Both human and animal studies support the hypothesis that excess pulmonary production of gaseous vasodilators, including nitric oxide (NO) and carbon monoxide (CO), contributes to vasodilatation in the lung.
We and others have identified increased pulmonary vascular endothelial nitric oxide synthase (eNOS) as a major source of pulmonary NO production in CBDL,83,85,92,97,98 and have demonstrated that inhibition of the eNOS–NO pathway using NG-nitro-L-arginine methylester (L-NAME) or methylene blue improve hypoxaemia after CBDL.98–101 One important trigger for pulmonary eNOS activation and vascular dilation is the increased hepatic production and release of endothelin-1.85,91,97,102 This effect is mediated by an increase in expression of pulmonary vascular endothelial endothelin B (ETB) receptor, which augments endothelial NO production in response to endothelin-1.92,103 Accordingly, selective ETB receptor inhibition or genetic ETB receptor depletion decreases pulmonary endothelial eNOS–NO activation and markedly improves HPS after CBDL.104,105 An increase in expression of inducible nitric oxide synthase (iNOS) in the lungs of CBDL animals (transient in some studies) can also contribute to local NO production during the progression of HPS.86,106 Together, these observations document that CBDL recapitulates the physiological findings in human HPS, and support a role for NO in experimental HPS.
Pulmonary production of CO is also increased as experimental HPS progresses. CO seems to derive, in part, from intravascular macrophages that progressively accumulate after CBDL. These cells transiently produce iNOS and progressively produce heme oxygenase 186,107 and contribute to vasodilatation through production of iNOS-derived NO and CO derived from heme oxygenase 1. Accordingly, in vivo inhibition of heme oxygenase 1 activity ameliorates gas exchange abnormalities and intrapulmonary vascular dilatation in CBDL animals.86 Further studies indicate that crosstalk occurs between the eNOS–NO and heme oxygenase 1–CO systems and has a role in the progression of experimental HPS.86,107
Angiogenesis
Several lines of evidence suggest that the pathophysiology of human and experimental HPS might involve factors in addition to intrapulmonary vascular dilatation. In humans, early autopsy studies found increased capillary density abutting alveoli in the arterial microvasculature in cirrhosis, suggesting the presence of what is now considered angiogenesis.62 This concept is supported by the finding that acute inhibition of NOS in general has failed to reliably improve oxygenation in human HPS77 and that the syndrome might take more than 1 year to resolve after liver transplantation in some patients. Moreover, single nucleotide polymorphisms in certain genes important in angiogenesis occur more commonly in patients with cirrhosis who have HPS than in control patients with cirrhosis without HPS.108 In experimental HPS, we and others have expanded on earlier work showing increased pulmonary microvessel density by electron microscopy after CBDL,75 by documenting the development of pulmonary angiogenesis and activation of vascular endothelial growth factor A (VEGFA)-dependent angiogenic signalling pathways, including downstream Akt (protein kinase B) and eNOS.96,99,109 A similar role for VEGFA-mediated splanchnic and hepatic angiogenesis has been observed during the onset of cirrhosis and the development of portosystemic vascular collaterals in experimental models.110–116 The importance of pulmonary angiogenesis in the development of HPS has been confirmed by studies showing that the inhibition of angiogenesis improves gas exchange abnormalities in experimental HPS.96 Interestingly, one major source of VEGFA production in experimental HPS is monocytes adhered to the pulmonary vasculature.96 Therefore, understanding the specific signals and mediators that drive pulmonary angiogenesis in HPS, including how monocytes home to the pulmonary microvasculature, could provide critical insights for developing effective medical therapies.
Intravascular macrophages
The observation that monocytes adhere to the lung microvasculature in experimental HPS and could be important in pathogenesis was made in the initial studies of experimental HPS.81 These studies used electron microscopy and quantification of lung uptake of radioactive particles to show that phagocytically active pulmonary intravascular macrophages are detectable between 2 weeks and 3 weeks after CBDL.81 These cells do not seem to migrate into the lung parenchyma over time, and no reliable accumulation is found in other organs. Further studies have revealed that accumulation of pulmonary intravascular macrophages and/or monocytes is an early event in response to CBDL.86 In addition, modulation of monocyte infiltration can alter intrapulmonary vasodilation and angiogenesis, and inhibition of angiogenesis decreases monocyte accumulation in experimental HPS.96,109,117 The precise mechanisms that drive the accumulation and activation of macrophages in the lung remain undefined. However, studies suggest that circulating tumour necrosis factor (TNF; owing to an immune response to translocation of bacteria or bacterial endotoxins), endothelin-1 and possibly monocyte-directed chemokines contribute to intravascular accumulation of monocytes in the lung.91,102–104,118–120
Human HPS
Three mechanisms for the development of hypoxaemia have been described in human HPS: ventilation–perfusion mismatch (increased capillary blood flow possibly attributable to vasodilatation), diffusion–perfusion mismatching (impaired passage of oxygen from the alveolus into the vasculature possibly as a result of vasodilatation or angiogenesis) and anatomic arteriovenous shunting (possibly because of vasodilatation or angiogenesis).1,121,122 The relative contribution of these mechanisms to gas exchange abnormalities seem to vary based on the severity of HPS.31,122 In line with the concept that ‘physiological’ rather than ‘anatomic’ shunting of blood through the alveolar microcirculation is the major mechanism of hypoxaemia in HPS, many patients have a substantial increase in PaO2 (to >300 mmHg) when breathing 100% oxygen.123
Pulmonary vascular dilatations in human HPS have been attributed to excess production of vasodilators, particularly NO.124–126 Exhaled NO levels—reflecting pulmonary production—are increased in HPS and return to normal levels after liver transplantation, as HPS regresses. However, what modulates pulmonary NO production and how it relates to the severity of liver injury and portal hypertension remain uncertain. Observations show that inhibition of NO production or action does not reliably improve HPS and that increased NO production is not unique to HPS,43–45 supporting the concept that factors other than NOS-derived NO modulate pulmonary vascular tone. Heme oxygenase 1-derived CO production does seem to be selectively increased in human HPS, although whether this increased production derives from lung production or influences the vasculature is not known.127 In addition, the fact that HPS occurs across a spectrum of aetiologies, diseases and severities of portal hypertension, and develops in <50% of patients with cirrhosis, suggests that HPS develops in patients with an underlying predisposition to the disease. That variation in genes associated with vascular growth and development is associated with the risk of HPS raises the possibility that genetic susceptibility to angiogenesis might be one predisposing factor.108 Finally, whether monocytes adhere to the lung vasculature in human HPS and whether inhibition of TNF or bacterial translocation across the gastrointestinal tract alters the severity of HPS are poorly defined.109
Natural history and clinical features
The natural history of HPS is incompletely characterized, although quality of life and survival are adversely affected by its presence.6 Over time, the majority of patients seem to develop progressive intrapulmonary vascular dilatation and worsening gas exchange, and spontaneous improvement, though reported, is rare.3 Mortality in patients with HPS is increased twofold relative to unaffected patients with cirrhosis.3,6,51 In addition, many patients with moderate to severe HPS have comparatively well-preserved hepatic synthetic function, making it probable that the presence of HPS will contribute to poor outcomes.3,6,65
The majority of patients with HPS are either asymptomatic, particularly if diagnosed during evaluation for liver transplantation, or develop the insidious onset of dyspnoea.128 Classically, dyspnoea (platypnea) and hypoxaemia (orthodeoxia) increase in the upright position in HPS owing to the predominance of vasodilatation in the lung bases and the increased blood flow through these regions when sitting upright.129 These findings are highly suggestive of HPS but are not present in the majority of patients and are therefore of limited diagnostic utility.78,130 Several other clinical features, including spider angiomata, clubbing and cyanosis are also commonly described in HPS, but are also not reliable diagnostic indicators.30 In addition, respiratory symptoms are common in cirrhosis owing to poor physical condition, smoking, ascites and/or intrinsic lung disease.54 The presence of HPS might, therefore, be difficult to discern and the diagnosis delayed and identified only after severe arterial hypoxaemia has ensued. Finally, sleep-time oxygen desaturation also frequently occurs in patients with HPS and can worsen hypoxaemia at night.131
Chest radiography, chest CT and/or pulmonary function tests (PFTs) are often performed to evaluate dyspnoea in cirrhosis and during evaluations for liver transplantation. Commonly, chest radiograph findings are normal in HPS, even when hypoxaemia is severe.6,132 However, lower lobe interstitial markings resulting from dilated vessels can be present and are often confused with pulmonary fibrosis.133 Dilated vessels, as well as fibrotic lung disease, are visible on high-resolution chest CT, but its role in the diagnosis of HPS has not been established. PFTs typically demonstrate well-preserved spirometry and lung volumes. The diffusing capacity for CO is often reduced and can indicate a positive diagnosis, although a decrease in this parameter frequently occurs in cirrhosis in the absence of HPS, limiting diagnostic utility.134–136
HPS also affects children, although few prospective studies are reported. Overall, it seems to be less common in children than in adults (3–19%).40,137,138 Most frequently, the disease is found in common causes of paediatric liver disease requiring liver transplantation (biliary atresia), but is also reported in congenital disorders that alter portal venous blood flow through the liver (Abernethy malformation, polysplenia with interrupted inferior vena cava).39–42 Findings of liver disease can be minimal or absent in these syndromes, requiring a high degree of clinical suspicion of HPS to make the diagnosis.40 Compared with adults, whether children have a higher frequency of type 2 angiographic features resulting in improved sensitivity for 99mTcMAA scanning relative to contrast echocardiography in the diagnosis of HPS is not resolved.139
Management
No clearly effective medical therapy for HPS is available although a number of compounds have been studied in experimental and human disease (Table 1). Supplemental oxygen therapy is appropriate in hypoxaemic patients with HPS, although no studies have evaluated survival benefit. Somatostatin, almitrine, indometacin, norfloxacin, inhaled (nebulized) L-NAME, aspirin and plasma exchange have all been tried in patients with HPS without clear benefit.1,77,101,133,140 A small open-label clinical trial, several case reports and a prospective trial using garlic have shown some benefit in HPS.141–144 Moreover, pentoxifylline—a phosphodiesterase inhibitor with known mild inhibitory effects on TNF and NO—has been linked to improved oxygenation in experimental HPS.118,120,145,146 However, in human HPS, results with pentoxifylline are conflicting. In one study, tolerability of the drug was poor and no oxygenation benefit was observed.145 In another study, tolerability to pentoxyfylline was not the limiting factor and there was an overall improvement in PaO2 of >10 mmHg.146 No studies have explored whether endothelin-receptor antagonists or angiogenesis inhibitors, which have benefit in experimental HPS, are effective in human disease. A number of case reports have suggested a beneficial effect of other interventions, including inhaled prostacyclin derivatives to improve ventilation–perfusion matching,147,148 withdrawal of chronic methadone149 and lowering of portal pressure with transjugular intrahepatic portosystemic shunt (TIPS) on HPS. Although several reports of using TIPS to treat HPS reported marked improvement, no benefit has been found in others, which makes assessments of utility difficult.150–154 These reports highlight the need to identify and target probable pathogenic mechanisms and undertake randomized, multicentre trials of sufficient size to determine efficacy. Finally, ligation of congenital portosystemic shunts in patients with Abernethy malformation associated with HPS has resulted in increased hepatic blood flow to the pulmonary arterial bed and resolution of HPS.42,155
Table 1.
Selected compounds used in studies of experimental and human HPS
| Agents | Mechanisms of action | Effects in HPS | |
|---|---|---|---|
| Experimental | Human | ||
| NG-nitro-L-arginine methyl ester (L-NAME) | Inhibitor of NO synthesis | Decreases iNOS-mediated NO production in lung intravascular macrophages;100 improves intrapulmonary shunting and gas exchange98,100 | Decreases NO production; intrapulmonary shunt and arterial deoxygenation remain unchanged;77 increases arterial oxygen pressure in one case report101 |
| Methylene blue | Oxidizing agent that blocks NO stimulation of soluble guanylate cyclase | Improves arterial gas exchange and angiogenesis99 | Improves intrapulmonary shunt, gas exchange and haemodynamic abnormalities;169,170 worsening of pulmonary gas exchange in a case report171 |
| Garlic | Unknown, effects might be attributable to an improvement in perfusion ventilation (V/Q) mismatch (redistribution of pulmonary blood flow) | Unknown | Improves intrapulmonary shunts and arterial oxygenation141–144 |
| Pentoxifylline | Nonspecific phosphodiesterase inhibitor that decreases TNF production | Decreases TNF and NO levels, improves intrapulmonary shunting and gas exchange;96,118,120 inhibits lung intravascular monocyte accumulation and angiogenesis96,118 | Arterial deoxygenation remains unchanged; gastrointestinal toxicity reported;145 decreases TNF levels; improves gas exchange146 |
| Norfloxacin | Antibiotic | Decreases iNOS-mediated NO production in lung intravascular macrophages; improves intrapulmonary shunting and gas exchange119 | Improves hypoxaemia in a case report;58 no major effect on gas exchange140 |
| BQ788 | ETB receptor antagonist | Decreases eNOS and NO production and intravascular monocyte accumulation; improves intrapulmonary shunting and gas exchange104 | Unknown |
| Tin protoporphyrin (SnPP) | Inhibitor for HMOX1 activity | Decreases HMOX1-mediated CO production; improves intrapulmonary shunting and gas exchange86 | Unknown |
| Angiostatin/endostatin | Antiangiogenic agents | Decreases eNOS and NO production; inhibits lung intravascular monocyte accumulation and angiogenesis; improves gas exchange96 | Unknown |
| Caffeic acid phenethyl ester (CAPE) | Free radical scavenger | Decreases NO levels and vessel diameter172 | Unknown |
| Quercetin | Dietary flavonoid with antioxidant effects | Decreases lung production of NO, iNOS, receptor; blocks eNOS, HMOX1 and ETB monocyte accumulation; improves gas exchange and vessel dilation173 | Unknown |
| Gadolinium (GdCl3) or clodronate-liposome | Depletion of lung vascular monocytes | Decreases iNOS levels; inhibits dilation and angiogenesis; improves gas exchange109 | Unknown |
Abbreviations: CO, carbon monoxide; eNOS, endothelial nitric oxide synthase; ETB, endothelin receptor B; HMOX1, heme oxygenase 1; HPS, hepatopulmonary syndrome; iNOS, inducible nitric oxide synthase; NO, nitric oxide; TNF, tumour necrosis factor.
Currently, liver transplantation is the only effective treatment for patients with HPS and complete resolution of gas exchange abnormalities is reported in >80% of such patients.1,50 Both living donor and deceased donor liver transplantation have been reported to be effective. 156–158 However, an early prospective study found that those with severe HPS (preoperative PaO2 of ≤50 mmHg and 99mTcMAA shunt fraction ≥20%) had a marked increase in postoperative mortality, in part attributable to prolonged mechanical ventilation and the development of unique postoperative complications (such as worsening hypoxaemia and embolic intracerebral haemorrhage) recognized in these patients.59 These findings support the current practice of providing model for end-stage liver disease (MELD) exception points to patients with cirrhosis who have HPS and a PaO2 <60 mmHg listed to undergo liver transplantation. Strategies including the use of inhaled NO and frequent repositioning of patients have been reported to be beneficial in improving oxygenation during recovery after liver transplantation.159–162 Since the initial prospective study reporting HPS outcomes after liver transplantation,4 a number of additional small studies and an analysis of the Scientific Registry of Transplant Recipients data have found 1–3 year mortality after liver transplantation in patients with HPS to range widely from 5% to 42%.3,4,51,156,163–165 These studies highlight the need to more precisely define the influence of HPS on liver transplantation outcomes to guide MELD exception policy.
Conclusions
Over the past 15 years, HPS has been increasingly recognized as an important clinical entity that influences survival and liver transplant candidacy in affected patients. No effective medical therapies exist. The pathogenesis of HPS remains incompletely understood, although ongoing studies in the CBDL animal model and in human disease suggest that vascular remodelling might have a central role in its development. One working hypothesis is that pulmonary vascular alterations in HPS represent a variation of inflammatory or tumour angiogenesis166– 168 in which homing and activation of inflammatory cells (including monocytes) results in paracrine production of mediators that drive a local angiogenic response. Evaluating the mechanisms underlying experimental HPS provides a pathogenic framework for investigating human disease and for developing and testing potential novel and effective therapies.
Key points.
HPS is a common finding in patients with cirrhosis that increases mortality in this context
HPS is defined by the triad of liver disease with intrapulmonary vascular dilatation causing abnormal oxygenation
No effective medical therapies for HPS exist and liver transplantation is the only treatment option
Chronic common bile duct ligation in the rat is the only established experimental model of human HPS
Excess lung production of gaseous vasodilators, nitric oxide and carbon monoxide contributes to vasodilatation in human and experimental HPS
Pulmonary angiogenesis has an additive role in the development of experimental HPS
Review criteria.
A search for original articles published between 1990 and 2012 and focusing on hepatopulmonary syndrome was performed in MEDLINE and PubMed. The search terms used were “hepatopulmonary syndrome”, “intrapulmonary vasodilation”, “intrapulmonary shunting”, “hypoxaemia”, “gas exchange”, “cirrhosis”, “portal hypertension”, “pathogenesis” and “common bile duct ligation” alone and/or in combination. All articles identified were English-language, full-text papers. We also searched the reference lists of identified articles for further relevant papers.
Acknowledgments
The authors acknowledge the support of NIH grant 5DKR01DK056804 (M. B. Fallon) and an award from the American Heart Association (J. Zhang).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
Both authors contributed equally to all aspects of this article.
References
- 1.Rodriguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome—a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378–2387. doi: 10.1056/NEJMra0707185. [DOI] [PubMed] [Google Scholar]
- 2.Schenk P, et al. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51:853–859. doi: 10.1136/gut.51.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swanson K, Wiesner R, Krowka M. Natural history of hepatopulmonary syndrome: impact of liver transplantation. Hepatology. 2005;41:1122–1129. doi: 10.1002/hep.20658. [DOI] [PubMed] [Google Scholar]
- 4.Arguedas M, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003;37:192–197. doi: 10.1053/jhep.2003.50023. [DOI] [PubMed] [Google Scholar]
- 5.Arguedas M, Singh H, Faulk D, Fallon MB. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol. 2007;5:749–754. doi: 10.1016/j.cgh.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Fallon MB, et al. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168–1175. doi: 10.1053/j.gastro.2008.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krowka MJ, Cortese DA. Hepatopulmonary syndrome: an evolving perspective in the era of liver transplantation. Hepatology. 1990;11:138–141. doi: 10.1002/hep.1840110123. [DOI] [PubMed] [Google Scholar]
- 8.Battaglia SE, Pretto JJ, Irving LB, Jones RM, Angus PW. Resolution of gas exchange abnormalities and intrapulmonary shunting following liver transplantation. Hepatology. 1997;25:1228–1232. doi: 10.1002/hep.510250527. [DOI] [PubMed] [Google Scholar]
- 9.Benjaminov FS, et al. Portopulmonary hypertension in decompensated cirrhosis with refractory ascites. Gut. 2003;52:1355–1362. doi: 10.1136/gut.52.9.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castro M, et al. Frequency and clinical implications of increased pulmonary artery pressures in liver transplantation. Mayo Clin Proc. 1996;71:543–551. doi: 10.4065/71.6.543. [DOI] [PubMed] [Google Scholar]
- 11.Hadengue A, Benhayoun M, Lebrec D, Benhamou J. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520–528. doi: 10.1016/0016-5085(91)90225-a. [DOI] [PubMed] [Google Scholar]
- 12.Hoeper MM, Krowka MJ, Strassburg CP. Portopulmonary hypertension and hepatopulmonary syndrome. Lancet. 2004;363:1461–1468. doi: 10.1016/S0140-6736(04)16107-2. [DOI] [PubMed] [Google Scholar]
- 13.Mantz F, Craige E. Portal axis thrombosis with spontaneous portocaval shunt and resultant cor pulmonale. AMA Arch Pathol. 1951;52:91–97. [PubMed] [Google Scholar]
- 14.McDonnell P, Toye P, Hutchins G. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis. 1983;127:437–441. doi: 10.1164/arrd.1983.127.4.437. [DOI] [PubMed] [Google Scholar]
- 15.Plevak D, Krowka M, Rettke S, Dunn W, Southorn P. Successful liver transplantation in patients with mild to moderate pulmonary hypertension. Transplant Proc. 1993;25:1840. [PubMed] [Google Scholar]
- 16.Rodriguez-Roisin R, et al. Pulmonary–hepatic vascular disorders (PHD) Eur Respir J. 2004;24:861–880. doi: 10.1183/09031936.04.00010904. [DOI] [PubMed] [Google Scholar]
- 17.Austin MJ, et al. Safety and efficacy of combined use of sildenafil, bosentan, and iloprost before and after liver transplantation in severe portopulmonary hypertension. Liver Transpl. 2008;14:287–291. doi: 10.1002/lt.21310. [DOI] [PubMed] [Google Scholar]
- 18.Chua R, Keogh A, Miyashita M. Novel use of sildenafil in the treatment of portopulmonary hypertension. J Heart Lung Transplant. 2005;24:498–500. doi: 10.1016/j.healun.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Halank M, et al. Use of oral endothelin-receptor antagonist bosentan in the treatment of portopulmonary hypertension. Transplantation. 2004;77:1775–1776. doi: 10.1097/01.tp.0000122420.86904.89. [DOI] [PubMed] [Google Scholar]
- 20.Minder S, et al. Intravenous iloprost bridging to orthotopic liver transplantation in portopulmonary hypertension. Eur Respir J. 2004;24:703–707. doi: 10.1183/09031936.04.00133203. [DOI] [PubMed] [Google Scholar]
- 21.Gough MS, White RJ. Sildenafil therapy is associated with improved hemodynamics in liver transplantation candidates with pulmonary arterial hypertension. Liver Transpl. 2009;15:30–36. doi: 10.1002/lt.21533. [DOI] [PubMed] [Google Scholar]
- 22.Ioachimescu OC, Mehta AC, Stoller JK. Hepatopulmonary syndrome following portopulmonary hypertension. Eur Respir J. 2007;29:1277–1280. doi: 10.1183/09031936.00140306. [DOI] [PubMed] [Google Scholar]
- 23.Jones FD, et al. The coexistence of portopulmonary hypertension and hepatopulmonary syndrome. Anesthesiology. 1999;90:626–629. doi: 10.1097/00000542-199902000-00041. [DOI] [PubMed] [Google Scholar]
- 24.Aucejo F, et al. Pulmonary hypertension after liver transplantation in patients with antecedent hepatopulmonary syndrome: a report of 2 cases and review of the literature. Liver Transpl. 2006;12:1278–1282. doi: 10.1002/lt.20830. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Palli G, et al. Severe portopulmonary hypertension after liver transplantation in a patient with preexisting hepatopulmonary syndrome. J Hepatol. 1999;31:1075–1079. doi: 10.1016/s0168-8278(99)80321-3. [DOI] [PubMed] [Google Scholar]
- 26.Mal H, et al. Pulmonary hypertension following hepatopulmonary syndrome in a patient with cirrhosis. J Hepatol. 1999;31:360–364. doi: 10.1016/s0168-8278(99)80236-0. [DOI] [PubMed] [Google Scholar]
- 27.Pham DM, Subramanian R, Parekh S. Coexisting hepatopulmonary syndrome and portopulmonary hypertension: implications for liver transplantation. J Clin Gastroenterol. 2010;44:e136–e140. doi: 10.1097/MCG.0b013e3181da76fc. [DOI] [PubMed] [Google Scholar]
- 28.Abrams G, Nanda N, Dubovsky E, Krowka M, Fallon M. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology. 1998;114:305–310. doi: 10.1016/s0016-5085(98)70481-0. [DOI] [PubMed] [Google Scholar]
- 29.Martinez G, et al. Hepatopulmonary syndrome associated with cardiorespiratory disease. J Hepatol. 1999;30:882–889. doi: 10.1016/s0168-8278(99)80143-3. [DOI] [PubMed] [Google Scholar]
- 30.Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283–1288. doi: 10.1016/0016-5085(95)90589-8. [DOI] [PubMed] [Google Scholar]
- 31.Martinez G, et al. Hepatopulmonary syndrome in candidates for liver transplantation. J Hepatol. 2001;34:756–758. doi: 10.1016/s0168-8278(00)00108-2. [DOI] [PubMed] [Google Scholar]
- 32.Abrams G, Fallon M. The hepatopulmonary syndrome. Clin Liver Dis. 1997;1:185–200. doi: 10.1016/s1089-3261(05)70263-4. [DOI] [PubMed] [Google Scholar]
- 33.Binay K, et al. Hepatopulmonary syndrome in inferior vena cava obstruction responding to cavoplasty. Gastroenterology. 2000;118:192–196. doi: 10.1016/s0016-5085(00)70428-8. [DOI] [PubMed] [Google Scholar]
- 34.Gupta D, et al. Prevalence of hepatopulmonary syndrome in cirrhosis and extrahepatic portal venous obstruction. Am J Gastroenterol. 2001;96:3395–3399. doi: 10.1111/j.1572-0241.2001.05274.x. [DOI] [PubMed] [Google Scholar]
- 35.Fuhrmann V, et al. Hepatopulmonary syndrome in patients with hypoxic hepatitis. Gastroenterology. 2006;131:69–75. doi: 10.1053/j.gastro.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Regev A, et al. Transient hepatopulmonary syndrome in a patient with acute hepatitis A. J Viral Hep. 2001;8:83–86. doi: 10.1046/j.1365-2893.2001.00270.x. [DOI] [PubMed] [Google Scholar]
- 37.Teuber G, et al. Pulmonary dysfunction in noncirrhotic patients with chronic viral hepatitis. Eur J Intern Med. 2002;13:311–318. doi: 10.1016/s0953-6205(02)00066-3. [DOI] [PubMed] [Google Scholar]
- 38.Lee D, Lepler L. Severe intrapulmonary shunting associated with metastatic carcinoid. Chest. 1999;115:1203–1207. doi: 10.1378/chest.115.4.1203. [DOI] [PubMed] [Google Scholar]
- 39.Law YM, et al. Cardiopulmonary manifestations of portovenous shunts from congenital absence of the portal vein: pulmonary hypertension and pulmonary vascular dilatation. Pediatr Transplant. 2011;15:E162–E168. doi: 10.1111/j.1399-3046.2010.01355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta NA, et al. Pediatric hepatopulmonary syndrome is seen with polysplenia/interrupted inferior vena cava and without cirrhosis. Liver Transpl. 2007;13:680–686. doi: 10.1002/lt.21113. [DOI] [PubMed] [Google Scholar]
- 41.Kinane TB, Westra SJ. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises Case 31–2004 A four-year-old boy with hypoxemia. N Engl J Med. 2004;351:1667–1675. doi: 10.1056/NEJMcpc049023. [DOI] [PubMed] [Google Scholar]
- 42.O’Leary JG, Rees CR, Klintmalm GB, Davis GL. Inferior vena cava stent resolves hepatopulmonary syndrome in an adult with a spontaneous inferior vena cava–portal vein shunt. Liver Transpl. 2009;15:1897–1900. doi: 10.1002/lt.21884. [DOI] [PubMed] [Google Scholar]
- 43.McFaul R, Tajik A, Mair D, Danielson G, Seward J. Development of pulmonary arteriovenous shunt after superior vena cavaright pulmonary artery (glenn) anastamosis. Report of four cases. Circulation. 1977;55:212–216. doi: 10.1161/01.cir.55.1.212. [DOI] [PubMed] [Google Scholar]
- 44.Srivastava D, et al. Hepatic venous blood and the development of pulmonary arteriovenous malformations in congenital heart disease. Circulation. 1995;92:1217–1222. doi: 10.1161/01.cir.92.5.1217. [DOI] [PubMed] [Google Scholar]
- 45.Duncan BW, Desai S. Pulmonary arteriovenous malformations after cavopulmonary anastomosis. Ann Thorac Surg. 2003;76:1759–1766. doi: 10.1016/s0003-4975(03)00450-8. [DOI] [PubMed] [Google Scholar]
- 46.Lee J, Menkis AH, Rosenberg HC. Reversal of pulmonary arteriovenous malformation after diversion of anomalous hepatic drainage. Ann Thorac Surg. 1998;65:848–849. doi: 10.1016/s0003-4975(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Roison R, Agusti AG, Roca J. The hepatopulmonary syndrome: new name, old complexities. Thorax. 1992;47:897–902. doi: 10.1136/thx.47.11.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lange PA, Stoller JK. The hepatopulmonary syndrome. Ann Intern Med. 1995;122:521–529. doi: 10.7326/0003-4819-122-7-199504010-00008. [DOI] [PubMed] [Google Scholar]
- 49.Fallon M, Mulligan D, Gish R, Krowka M. Model for end-stage liver disease (MELD) exception for hepatopulmonary syndrome. Liver Transpl. 2006;12:s105–s107. doi: 10.1002/lt.20971. [DOI] [PubMed] [Google Scholar]
- 50.Krowka MJ, et al. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl. 2004;10:174–182. doi: 10.1002/lt.20016. [DOI] [PubMed] [Google Scholar]
- 51.Schenk P, et al. Prognostic significance of the hepatopulmonary syndrome in patients with cirrhosis. Gastroenterology. 2003;125:1042–1052. doi: 10.1016/s0016-5085(03)01207-1. [DOI] [PubMed] [Google Scholar]
- 52.Abrams GA, Sanders MK, Fallon MB. Utility of pulse oximetry in the detection of arterial hypoxemia in liver transplant candidates. Liver Transpl. 2002;8:391–396. doi: 10.1053/jlts.2002.32252. [DOI] [PubMed] [Google Scholar]
- 53.Palma DT, Fallon MB. The hepatopulmonary syndrome. J Hepatol. 2006;45:617–625. doi: 10.1016/j.jhep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Crapo RO, Jensen RL, Hegewald M, Tashkin DP. Arterial blood gas reference values for sea level and an altitude of 1,400 meters. Am J Respir Crit Care Med. 1999;160:1525–1531. doi: 10.1164/ajrccm.160.5.9806006. [DOI] [PubMed] [Google Scholar]
- 55.Cardús J, et al. Increase in pulmonary ventilation-perfusion inequality with age in healthy individuals. Am J Respir Crit Care Med. 1997;156:648–653. doi: 10.1164/ajrccm.156.2.9606016. [DOI] [PubMed] [Google Scholar]
- 56.Harris E, Kenyon A, Nisbet H, Seelye E, Whitlock R. The normal alveolar-arterial oxygen-tension gradient in man. Clin Sci Mol Med. 1974;46:89–104. doi: 10.1042/cs0460089. [DOI] [PubMed] [Google Scholar]
- 57.Krowka MJ, Dickson ER, Cortese DA. Hepatopulmonary syndrome. Clinical observations and lack of therapeutic response to somatostatin analogue. Chest. 1993;104:515–521. doi: 10.1378/chest.104.2.515. [DOI] [PubMed] [Google Scholar]
- 58.Anel RM, Sheagren JN. Novel presentation and approach to management of hepatopulmonary syndrome with use of antimicrobial agents. Clin Infect Dis. 2001;32:E131–E136. doi: 10.1086/320149. [DOI] [PubMed] [Google Scholar]
- 59.Poterucha JJ, et al. Failure of hepatopulmonary syndrome to resolve after liver transplantation and successful treatment with embolotherapy. Hepatology. 1995;21:96–100. [PubMed] [Google Scholar]
- 60.Molleston JP, et al. Brain abscess in hepatopulmonary syndrome. J Pediatr Gastroenterol Nutr. 1999;29:225–226. doi: 10.1097/00005176-199908000-00024. [DOI] [PubMed] [Google Scholar]
- 61.Stickland MK, et al. Intra-pulmonary shunt and pulmonary gas exchange during exercise in humans. J Physiol. 2004;561:321–329. doi: 10.1113/jphysiol.2004.069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berthelot P, Walter JG, Sherlock S, Reid L. Arterial changes in the lungs in cirrhosis of the liver-lung spider nevi. N Engl J Med. 1966;274:291–298. doi: 10.1056/NEJM196602102740601. [DOI] [PubMed] [Google Scholar]
- 63.Herve P, et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J. 1998;11:1153–1166. doi: 10.1183/09031936.98.11051153. [DOI] [PubMed] [Google Scholar]
- 64.Roberts DN, Arguedas MR, Fallon MB. Cost-effectiveness of screening for hepatopulmonary syndrome in liver transplant candidates. Liver Transpl. 2007;13:206–214. doi: 10.1002/lt.20931. [DOI] [PubMed] [Google Scholar]
- 65.Krowka M, et al. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, pao2 response to 100% oxygen, and brain uptake after 99mTc MAA lung scanning. Chest. 2000;118:615–624. doi: 10.1378/chest.118.3.615. [DOI] [PubMed] [Google Scholar]
- 66.Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994;105:1528–1537. doi: 10.1378/chest.105.5.1528. [DOI] [PubMed] [Google Scholar]
- 67.Sussman NL, Kochar R, Fallon MB. Pulmonary complications in cirrhosis. Curr Opin Organ Transplant. 2011;16:281–288. doi: 10.1097/MOT.0b013e32834664df. [DOI] [PubMed] [Google Scholar]
- 68.Aller R, et al. Diagnosis of hepatopulmonary syndrome with contrast transesophageal echocardiography: advantages over contrast transthoracic echocardiography. Dig Dis Sci. 1999;44:1243–1248. doi: 10.1023/a:1026657114256. [DOI] [PubMed] [Google Scholar]
- 69.Vedrinne JM, et al. Comparison of transesophageal and transthoracic contrast echocardiography for detection of an intrapulmonary shunt in liver disease. Chest. 1997;111:1236–1240. doi: 10.1378/chest.111.5.1236. [DOI] [PubMed] [Google Scholar]
- 70.Fischer CH, et al. Role of contrast-enhanced transesophageal echocardiography for detection of and scoring intrapulmonary vascular dilatation. Echocardiography. 2010;27:1233–1237. doi: 10.1111/j.1540-8175.2010.01228.x. [DOI] [PubMed] [Google Scholar]
- 71.Ryu JK, Oh JH. Hepatopulmonary syndrome: angiography and therapeutic embolization. Clin Imaging. 2003;27:97–100. doi: 10.1016/s0899-7071(02)00511-9. [DOI] [PubMed] [Google Scholar]
- 72.Saad NEA, Lee DE, Waldman DL, Saad WEA. Pulmonary arterial coil embolization for the management of persistent type I hepatopulmonary syndrome after liver transplantation. J Vasc Interv Radiol. 2007;18:1576–1580. doi: 10.1016/j.jvir.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Koksal D, et al. Evaluation of intrapulmonary vascular dilatations with high-resolution computed thorax tomography in patients with hepatopulmonary syndrome. J Clin Gastroenterol. 2006;40:77–83. doi: 10.1097/01.mcg.0000190775.57903.86. [DOI] [PubMed] [Google Scholar]
- 74.Suga K, Kawakami Y, Iwanaga H, Tokuda O, Matsunaga N. Findings of hepatopulmonary syndrome on breath-hold perfusion spect-ct fusion images. Ann Nucl Med. 2009;23:413–419. doi: 10.1007/s12149-009-0250-8. [DOI] [PubMed] [Google Scholar]
- 75.Schraufnagel D, Malik R, Goel V, Ohara N, Chang S. Lung capillary changes in hepatic cirrhosis in rats. Am J Physiol. 1997;272:L139–L147. doi: 10.1152/ajplung.1997.272.1.L139. [DOI] [PubMed] [Google Scholar]
- 76.Schraufnagel D, Kay J. Structural and pathologic changes in the lung vasculature in chronic liver disease. Clin Chest Med. 1996;17:1–15. doi: 10.1016/s0272-5231(05)70295-1. [DOI] [PubMed] [Google Scholar]
- 77.Gómez F, et al. Effects of nebulized ng-nitro-larginine methyl ester in patients with hepatopulmonary syndrome. Hepatology. 2006;43:1084–1091. doi: 10.1002/hep.21141. [DOI] [PubMed] [Google Scholar]
- 78.Gómez F, et al. Gas exchange mechanism of orthodeoxia in hepatopulmonary syndrome. Hepatology. 2004;40:660–666. doi: 10.1002/hep.20358. [DOI] [PubMed] [Google Scholar]
- 79.Chang SW, O’Hara N. Increased pulmonary vascular permeability in rats with biliary cirrhosis: role of thomboxane A2. Am J Physiol. 1993;264:L245–L252. doi: 10.1152/ajplung.1993.264.3.L245. [DOI] [PubMed] [Google Scholar]
- 80.Chang SW, O’Hara N. Pulmonary circulatory dysfunction in rats with biliary cirrhosis. An animal model of the hepatopulmonary syndrome. Am Rev Respir Dis. 1992;145:798–805. doi: 10.1164/ajrccm/145.4_Pt_1.798. [DOI] [PubMed] [Google Scholar]
- 81.Chang SW, O’Hara N. Chronic biliary obstruction induces pulmonary intravascular phagocytosis and endotoxin sensitivity in rats. J Clin Invest. 1994;94:2009–2019. doi: 10.1172/JCI117554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chang SW, O’Hara N. Pulmonary circulatory dysfunction in rats with biliary cirrhosis. Am Rev Respir Dis. 1992;148:798–805. doi: 10.1164/ajrccm/145.4_Pt_1.798. [DOI] [PubMed] [Google Scholar]
- 83.Fallon MB, et al. The role of endothelial nitric oxide synthase in the pathogenesis of a rat model of hepatopulmonary syndrome. Gastroenterology. 1997;113:606–614. doi: 10.1053/gast.1997.v113.pm9247483. [DOI] [PubMed] [Google Scholar]
- 84.Fallon MB, Abrams GA, McGrath JW, Hou Z, Luo B. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol. 1997;272:G779–G784. doi: 10.1152/ajpgi.1997.272.4.G779. [DOI] [PubMed] [Google Scholar]
- 85.Luo B, Abrams GA, Fallon MB. Endothelin-1 in the rat bile duct ligation model of hepatopulmonary syndrome: correlation with pulmonary dysfunction. J Hepatol. 1998;29:571–578. doi: 10.1016/s0168-8278(98)80152-9. [DOI] [PubMed] [Google Scholar]
- 86.Zhang J, et al. Analysis of pulmonary heme oxygenase-1 and nitric oxide synthase alterations in experimental hepatopulmonary syndrome. Gastroenterology. 2003;125:1441–1451. doi: 10.1016/j.gastro.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 87.Georgiev P, et al. Characterization of time-related changes after experimental bile duct ligation. Br J Surg. 2008;95:646–656. doi: 10.1002/bjs.6050. [DOI] [PubMed] [Google Scholar]
- 88.Groszmann RJ, Vorobioff J, Riley E. Splanchnic hemodynamics in portal-hypertensive rats: measurement with gamma-labeled microspheres. Am J Physiol. 1982;242:G156–G160. doi: 10.1152/ajpgi.1982.242.2.G156. [DOI] [PubMed] [Google Scholar]
- 89.Vorobioff J, Bredfeldt J, Grosszmann RJ. Hyperdynamic circulation in a portal hypertensive rat model: a primary factor for maintenance of chronic portal hypertension. Am J Physiol. 1983;244:G52–G57. doi: 10.1152/ajpgi.1983.244.1.G52. [DOI] [PubMed] [Google Scholar]
- 90.Abraldes JG, et al. Mild increases in portal pressure upregulate vascular endothelial growth factor and endothelial nitric oxide synthase in the intestinal microcirculatory bed, leading to a hyperdynamic state. Am J Physiol Gastrointest Liver Physiol. 2006;290:G980–G987. doi: 10.1152/ajpgi.00336.2005. [DOI] [PubMed] [Google Scholar]
- 91.Luo B, et al. ET-1 and TNF-α in HPS: analysis in prehepatic portal hypertension and biliary and nonbiliary cirrhosis in rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G294–G303. doi: 10.1152/ajpgi.00298.2003. [DOI] [PubMed] [Google Scholar]
- 92.Luo B, et al. Increased pulmonary vascular endothelin b receptor expression and responsiveness to endothelin-1 in cirrhotic and portal hypertensive rats: a potential mechanism. [DOI] [PubMed] [Google Scholar]
- 93.Geerts A, et al. Comparison of three research models of portal hypertension in mice: Macroscopic, histological and portal pressure evaluation. Int J Exp Pathol. 2008;89:251–263. doi: 10.1111/j.1365-2613.2008.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mejias M, et al. Beneficial effects of sorafenib on splanchnic, intrahepatic, and portocollateral circulations in portal hypertensive and cirrhotic rats. Hepatology. 2009;49:1245–1256. doi: 10.1002/hep.22758. [DOI] [PubMed] [Google Scholar]
- 95.Laleman W, et al. A stable model of cirrhotic portal hypertension in the rat: thioacetamide revisited. Eur J Clin Invest. 2006;36:242–249. doi: 10.1111/j.1365-2362.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 96.Zhang J, et al. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology. 2009;136:1070–1080. doi: 10.1053/j.gastro.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang M, Luo B, Chen SJ, Abrams GA, Fallon MB. Endothelin-1 stimulation of endothelial nitric oxide synthase in the pathogenesis of hepatopulmonary syndrome. Am J Physiol. 1999;277:G944–G952. doi: 10.1152/ajpgi.1999.277.5.G944. [DOI] [PubMed] [Google Scholar]
- 98.Zhang XJ, et al. Intrapulmonary vascular dilatation and nitric oxide in hypoxemic rats with chronic bile duct ligation. J Hepatol. 2003;39:724–730. doi: 10.1016/s0168-8278(03)00430-6. [DOI] [PubMed] [Google Scholar]
- 99.Miyamoto A, et al. Effect of chronic methylene blue administration on hypoxemia in rats with common bile duct ligation. Hepatol Res. 2010;40:622–632. doi: 10.1111/j.1872-034X.2010.00640.x. [DOI] [PubMed] [Google Scholar]
- 100.Nunes H, et al. Role of nitric oxide in hepatopulmonary syndrome in cirrhotic rats. Am J Respir Crit Care Med. 2001;164:879–885. doi: 10.1164/ajrccm.164.5.2009008. [DOI] [PubMed] [Google Scholar]
- 101.Brussino L, et al. Effect on dyspnoea and hypoxaemia of inhaled ng-nitro-l-arginine methyl ester in hepatopulmonary syndrome. Lancet. 2003;362:43–44. doi: 10.1016/S0140-6736(03)13807-X. [DOI] [PubMed] [Google Scholar]
- 102.Luo B, et al. Cholangiocyte endothelin-1 and transforming growth factor-beta1 production in rat experimental hepatopulmonary syndrome. Gastroenterology. 2005;129:682–695. doi: 10.1016/j.gastro.2005.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang L, et al. Modulation of pulmonary endothelial endothelin b receptor expression and signaling: Implications for experimental hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1467–L1472. doi: 10.1152/ajplung.00446.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ling Y, et al. The role of endothelin-1 and the endothelin b receptor in the pathogenesis of experimental hepatopulmonary syndrome. Hepatology. 2004;39:1593–1602. doi: 10.1002/hep.20244. [DOI] [PubMed] [Google Scholar]
- 105.Zhang J, et al. Attenuation of experimental hepatopulmonary syndrome in endothelin b receptor-deficient rats. Am J Physiol Gastrointest Liver Physiol. 2009;296:G704–G708. doi: 10.1152/ajpgi.90627.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schroeder RA, Ewing CA, Sitzmann JV, Kuo PC. Pulmonary expression of inos and ho-1 protein is upregulated in a rat model of prehepatic portal hypertension. Dig Dis Sci. 2000;45:2405–2410. doi: 10.1023/a:1005651327654. [DOI] [PubMed] [Google Scholar]
- 107.Carter EP, et al. Regulation of heme oxygenase-1 by nitric oxide during hepatopulmonary syndrome. Am J Physiol Lung Cell Mol Physiol. 2002;283:L346–L353. doi: 10.1152/ajplung.00385.2001. [DOI] [PubMed] [Google Scholar]
- 108.Roberts KE, et al. Genetic risk factors for hepatopulmonary syndrome in patients with advanced liver disease. Gastroenterology. 2010;139:130–139e124. doi: 10.1053/j.gastro.2010.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thenappan T, et al. A central role for CD68+ macrophages in hepatopulmonary syndrome. Am J Respir Crit Care Med. 2011;183:1080–1091. doi: 10.1164/rccm.201008-1303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bosch J. Vascular deterioration in cirrhosis: the big picture. J Clin Gastroenterol. 2007;41:S247–S253. doi: 10.1097/MCG.0b013e3181572357. [DOI] [PubMed] [Google Scholar]
- 111.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 112.Dimmeler S, Zeiher AM. Akt takes center stage in angiogenesis signaling. Circ Res. 2000;86:4–5. doi: 10.1161/01.res.86.1.4. [DOI] [PubMed] [Google Scholar]
- 113.Fernandez M, et al. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology. 2007;46:1208–1217. doi: 10.1002/hep.21785. [DOI] [PubMed] [Google Scholar]
- 114.Tugues S, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–1926. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 115.Lee J, Semela D, Iredale J, Shah V. Sinusoidal remodeling and angiogenesis: a new function for the liver-specific pericyte? Hepatology. 2007;45:817–825. doi: 10.1002/hep.21564. [DOI] [PubMed] [Google Scholar]
- 116.Fernandez LG, et al. Differential vascular growth in postpneumonectomy compensatory lung growth. J Thorac Cardiovasc Surg. 2007;133:309–316. doi: 10.1016/j.jtcvs.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 117.Gill S, et al. Role of pulmonary intravascular macrophages in endotoxin-induced lung inflammation and mortality in a rat model. Respir Res. 2008;9:69. doi: 10.1186/1465-9921-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang J, et al. Pentoxifylline attenuation of experimental hepatopulmonary syndrome. J Appl Physiol. 2007;102:949–955. doi: 10.1152/japplphysiol.01048.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rabiller A, et al. Prevention of gram-negative translocation reduces the severity of hepatopulmonary syndrome. Am J Respir Crit Care Med. 2002;166:514–517. doi: 10.1164/rccm.200201-027OC. [DOI] [PubMed] [Google Scholar]
- 120.Sztrymf B, et al. Prevention of hepatopulmonary syndrome by pentoxifylline in cirrhotic rats. Eur Respir J. 2004;23:752–758. doi: 10.1183/09031936.04.00080404. [DOI] [PubMed] [Google Scholar]
- 121.Hemprich U, Papadakos PJ, Lachmann B. Respiratory failure and hypoxemia in the cirrhotic patient including hepatopulmonary syndrome. Curr Opin Anaesthesiol. 2010;23:133–138. doi: 10.1097/ACO.0b013e328335f024. [DOI] [PubMed] [Google Scholar]
- 122.Agusti AGN, Roca J, Rodriguez-Roisin R. Mechanisms of gas exchange impairment in patients with liver cirrhosis. Clin Chest Med. 1996;17:49–66. doi: 10.1016/s0272-5231(05)70298-7. [DOI] [PubMed] [Google Scholar]
- 123.Krowka MJ, et al. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO2 response to 100% oxygen, and brain uptake after 99mTc MAA lung scanning. Chest. 2000;118:615–624. doi: 10.1378/chest.118.3.615. [DOI] [PubMed] [Google Scholar]
- 124.Cremona G, et al. Elevated exhaled nitric oxide in patients with hepatopulmonary syndrome. Eur Respir J. 1995;8:1883–1885. doi: 10.1183/09031936.95.08111883. [DOI] [PubMed] [Google Scholar]
- 125.Rolla G, Brussino L, Colagrande P. Exhaled nitric oxide and impaired oxygenation in cirrhotic patients before and after liver transplantation. Ann Intern Med. 1998;129:375–378. doi: 10.7326/0003-4819-129-5-199809010-00005. [DOI] [PubMed] [Google Scholar]
- 126.Rolla G, et al. Exhaled nitric oxide and oxygenation abnormalities in hepatic cirrhosis. Hepatology. 1997;26:842–847. doi: 10.1053/jhep.1997.v26.pm0009328302. [DOI] [PubMed] [Google Scholar]
- 127.Arguedas MR, Drake BB, Kapoor A, Fallon MB. Carboxyhemoglobin levels in cirrhotic patients with and without hepatopulmonary syndrome. Gastroenterology. 2005;128:328–333. doi: 10.1053/j.gastro.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 128.Sood G, et al. Utility of a dyspnea–fatigue index for screening liver transplant candidates for hepatopulmonary syndrome. Hepatology. 1998;28:2319. [Google Scholar]
- 129.Robin ED, Laman D, Horn BR, Theodore J. Platypnea related to orthodeoxia caused by true vascular lung shunts. N Engl J Med. 1976;294:941–943. doi: 10.1056/NEJM197604222941711. [DOI] [PubMed] [Google Scholar]
- 130.Martinez GP, et al. Hepatopulmonary syndrome in candidates for liver transplantation. J Hepatol. 2001;34:651–657. doi: 10.1016/s0168-8278(00)00108-2. [DOI] [PubMed] [Google Scholar]
- 131.Palma DT, Philips GM, Arguedas MR, Harding SM, Fallon MB. Oxygen desaturation during sleep in hepatopulmonary syndrome. Hepatology. 2008;47:1257–1263. doi: 10.1002/hep.22143. [DOI] [PubMed] [Google Scholar]
- 132.McAdams HP, et al. The hepatopulmonary syndrome: radiologic findings in 10 patients. Am J Roentgenol. 1996;166:1379–1385. doi: 10.2214/ajr.166.6.8633451. [DOI] [PubMed] [Google Scholar]
- 133.Fallon M, Abrams G. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859–865. doi: 10.1053/jhep.2000.7519. [DOI] [PubMed] [Google Scholar]
- 134.Lima B, et al. Frequency, clinical characteristics, and respiratory parameters of hepatopulmonary syndrome. Mayo Clin Proc. 2004;79:42–48. doi: 10.4065/79.1.42. [DOI] [PubMed] [Google Scholar]
- 135.Yigit I, Hacievliyagil S, Seckin Y, Öner R, Karincaoglu M. The relationship between severity of liver cirrhosis and pulmonary function tests. Dig Dis Sci. 2008;53:1951–1956. doi: 10.1007/s10620-007-0100-2. [DOI] [PubMed] [Google Scholar]
- 136.Møller S, Krag A, Madsen JL, Henriksen JH, Bendtsen F. Pulmonary dysfunction and hepatopulmonary syndrome in cirrhosis and portal hypertension. Liver Int. 2009;29:1528–1537. doi: 10.1111/j.1478-3231.2009.02103.x. [DOI] [PubMed] [Google Scholar]
- 137.Tumgor G, et al. Childhood cirrhosis, hepatopulmonary syndrome and liver transplantation. Pediatr Transplant. 2008;12:353–357. doi: 10.1111/j.1399-3046.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 138.Whitworth JR, Ivy DD, Gralla J, Narkewicz MR, Sokol RJ. Pulmonary vascular complications in asymptomatic children with portal hypertension. J Pediatr Gastroenterol Nutr. 2009;49:607–612. doi: 10.1097/MPG.0b013e3181a5267d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.El-Shabrawi MH, et al. 99mTechnetiummacroaggregated albumin perfusion lung scan versus contrast enhanced echocardiography in the diagnosis of the hepatopulmonary syndrome in children with chronic liver disease. Eur J Gastroenterol Hepatol. 2010;22:1006–1012. doi: 10.1097/MEG.0b013e328336562e. [DOI] [PubMed] [Google Scholar]
- 140.Gupta S, et al. Norfloxacin therapy for hepatopulmonary syndrome: a pilot randomized controlled trial. Clin Gastroenterol Hepatol. 2010;8:1095–1098. doi: 10.1016/j.cgh.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 141.Abrams GA, Fallon MB. Treatment of hepatopulmonary syndrome with allium sativum l (garlic): a pilot trial. J Clin Gastroenterol. 1998;27:232–235. doi: 10.1097/00004836-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 142.Sani MN, Kianifar HR, Kianee A, Khatami G. Effect of oral garlic on arterial oxygen pressure in children with hepatopulmonary syndrome. World J Gastroenterol. 2006;12:2427–2431. doi: 10.3748/wjg.v12.i15.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De BK, et al. The role of garlic in hepatopulmonary syndrome: a randomized controlled trial. Can J Gastroenterol. 2010;24:183–188. doi: 10.1155/2010/349076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Akyüz F, et al. Is there any medical therapeutic option in hepatopulmonary syndrome? A case report. Eur J Intern Med. 2005;16:126–128. doi: 10.1016/j.ejim.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 145.Tanikella R, Philips G, Faulk D, Kawut S, Fallon M. Pilot study of pentoxifylline in hepatopulmonary syndrome. Liver Transpl. 2008;14:1199–1203. doi: 10.1002/lt.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gupta LB, et al. Pentoxifylline therapy for hepatopulmonary syndrome: a pilot study. Arch Intern Med. 2008;168:1820–1823. doi: 10.1001/archinte.168.16.1820. [DOI] [PubMed] [Google Scholar]
- 147.Iqbal CW, et al. Liver transplantation for pulmonary vascular complications of pediatric end-stage liver disease. J Pediatr Surg. 2008;43:1813–1820. doi: 10.1016/j.jpedsurg.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 148.Krug S, Seyfarth HJ, Hagendorff A, Wirtz H. Inhaled iloprost for hepatopulmonary syndrome: Improvement of hypoxemia. Eur J Gastroenterol Hepatol. 2007;19:1140–1143. doi: 10.1097/MEG.0b013e328220ed72. [DOI] [PubMed] [Google Scholar]
- 149.Lau EMT, McCaughan G, Torzillo PJ. Improvement in hepatopulmonary syndrome after methadone withdrawal: a case report with implications for disease mechanism. Liver Transpl. 2010;16:870–873. doi: 10.1002/lt.22081. [DOI] [PubMed] [Google Scholar]
- 150.Reigler JL, Lang KA, Johnson SP, Westerman JH. Transjugular intrahepatic portosystemic shunt improves oxygenation in hepatopulmonary syndrome. Gastroenterology. 1995;109:978–983. doi: 10.1016/0016-5085(95)90409-3. [DOI] [PubMed] [Google Scholar]
- 151.Selim KM, Akriviadis EA, Zuckerman E, Chen D, Reynolds TB. Transjugular intrahepatic portosystemic shunt: a successful treatment for hepatopulmonary syndrome. Am J Gastroenterol. 1998;93:455–458. doi: 10.1111/j.1572-0241.1998.00455.x. [DOI] [PubMed] [Google Scholar]
- 152.Allgaier HP, et al. Hepatopulmonary syndrome: Successful treatment by transjugular intrahepatic portosystemic stent-shunt (tips) J Hepatol. 1995;23:102–105. doi: 10.1016/0168-8278(95)80318-1. [DOI] [PubMed] [Google Scholar]
- 153.Paramesh A, et al. Improvement of hepatopulmonary syndrome after transjugular intrahepatic portasystemic shunting: case report and review of literature. Pediatr Transplant. 2003;7:157–162. doi: 10.1034/j.1399-3046.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- 154.Corley DA, Scharschmidt B, Bass N, Somberg K, Gold W. Lack of efficacy of tips for hepatopulmonary syndrome. Gastroenterology. 1997;113:728–731. doi: 10.1053/gast.1997.v113.agast971130728. [DOI] [PubMed] [Google Scholar]
- 155.Morikawa N, et al. Resolution of hepatopulmonary syndrome after ligation of a portosystemic shunt in a pediatric patient with an abernethy malformation. J Pediatr Surg. 2008;43:e35–e38. doi: 10.1016/j.jpedsurg.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 156.Gupta S, et al. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10:354–363. doi: 10.1111/j.1600-6143.2009.02822.x. [DOI] [PubMed] [Google Scholar]
- 157.Motomura T, et al. Living donor liver transplantation for end-stage liver disease with severe hepatopulmonary syndrome: report of a case. Surg Today. 2011;41:436–440. doi: 10.1007/s00595-009-4260-x. [DOI] [PubMed] [Google Scholar]
- 158.Chen K, Li B. Reversal of severe hepatopulmonary syndrome in chronic hepatic cirrhosis by living donor liver transplantation: Report of two cases. Surg Today. 2011;41:441–443. doi: 10.1007/s00595-009-4219-y. [DOI] [PubMed] [Google Scholar]
- 159.Schiller O, et al. Nitric oxide for post-liver-transplantation hypoxemia in pediatric hepatopulmonary syndrome: case report and review. Pediatr Transplant. 2011;15:E130–E134. doi: 10.1111/j.1399-3046.2010.01313.x. [DOI] [PubMed] [Google Scholar]
- 160.Durand P, et al. Reversal of hypoxemia by inhaled nitric oxide in children with severe hepatopulmonary syndrome, type 1, during and after liver transplantation. Transplantation. 1998;65:437–439. doi: 10.1097/00007890-199802150-00026. [DOI] [PubMed] [Google Scholar]
- 161.Taniai N, et al. Reversal of hypoxemia by inhaled nitric oxide in a child with hepatopulmonary syndrome after living-related liver transplantation. Transplant Proc. 2002;34:2791–2792. doi: 10.1016/s0041-1345(02)03415-2. [DOI] [PubMed] [Google Scholar]
- 162.Meyers C, Low L, Kaufman L, Druger G, Wong L. Trendelenburg positioning and continuous lateral rotation improve oxygenation in heaptopulmonary syndrome after liver transplantation. Liver Transpl Surg. 1998;6:510–512. doi: 10.1002/lt.500040608. [DOI] [PubMed] [Google Scholar]
- 163.Taille C, et al. Liver transplantation for hepatopulmonary syndrome: a ten-year experience in Paris, France. Transplantation. 2003;79:1482–1489. doi: 10.1097/01.TP.0000061612.78954.6C. [DOI] [PubMed] [Google Scholar]
- 164.Deberaldini M, et al. Hepatopulmonary syndrome: morbidity and survival after liver transplantation. Transplant Proc. 2008;40:3512–3516. doi: 10.1016/j.transproceed.2008.08.134. [DOI] [PubMed] [Google Scholar]
- 165.Schiffer E, et al. Hepatopulmonary syndrome increases the postoperative mortality rate following liver transplantation: a prospective study in 90 patients. Am J Transplant. 2006;6:1430–1437. doi: 10.1111/j.1600-6143.2006.01334.x. [DOI] [PubMed] [Google Scholar]
- 166.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:545–550. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 167.Elsheikh E, et al. Only a specific subset of human peripheral-blood monocytes has endothelial-like functional capacity. Blood. 2005;106:2347–2355. doi: 10.1182/blood-2005-04-1407. [DOI] [PubMed] [Google Scholar]
- 168.Venneri MA, et al. Identification of proangiogenic tie2-expressing monocytes (TEMS) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 169.Schenk P, Madl C, Rezale-Majd S, Lehr S, Muller C. Methylene blue improves the hepatopulmonary syndrome. Ann Intern Med. 2000;133:701–706. doi: 10.7326/0003-4819-133-9-200011070-00012. [DOI] [PubMed] [Google Scholar]
- 170.Rolla G, Bucca C, Brussino L. Methylene blue in the hepatopulmonary syndrome. N Engl J Med. 1994;331:1098. doi: 10.1056/NEJM199410203311617. [DOI] [PubMed] [Google Scholar]
- 171.Almeida JA, et al. Deleterious effect of nitric oxide inhibition in chronic hepatopulmonary syndrome. Eur J Gastroenterol Hepatol. 2007;19:341–346. doi: 10.1097/MEG.0b013e328014a3bf. [DOI] [PubMed] [Google Scholar]
- 172.Tekin A, et al. Effects of caffeic acid phenethyl ester (cape) on hepatopulmonary syndrome. Inflammation. 2011;34:614–619. doi: 10.1007/s10753-010-9270-8. [DOI] [PubMed] [Google Scholar]
- 173.Tieppo J, et al. Quercetin administration ameliorates pulmonary complications of cirrhosis in rats. J Nutr. 2009;139:1339–1346. doi: 10.3945/jn.109.105353. [DOI] [PubMed] [Google Scholar]