Abstract
Awareness is growing that human health cannot be considered in isolation but is inextricably woven with the health of the environment in which we live. It is, however, under-recognized that the sustainability of human activities strongly relies on preserving the equilibrium of the microbial communities living in/on/around us. Microbial metabolic activities are instrumental for production, functionalization, processing, and preservation of food. For circular economy, microbial metabolism would be exploited to produce building blocks for the chemical industry, to achieve effective crop protection, agri-food waste revalorization, or biofuel production, as well as in bioremediation and bioaugmentation of contaminated areas. Low pH is undoubtedly a key physical–chemical parameter that needs to be considered for exploiting the powerful microbial metabolic arsenal. Deviation from optimal pH conditions has profound effects on shaping the microbial communities responsible for carrying out essential processes. Furthermore, novel strategies to combat contaminations and infections by pathogens rely on microbial-derived acidic molecules that suppress/inhibit their growth. Herein, we present the state-of-the-art of the knowledge on the impact of acidic pH in many applied areas and how this knowledge can guide us to use the immense arsenal of microbial metabolic activities for their more impactful exploitation in a Planetary Health perspective.
Keywords: antimicrobial, biohydrogen, phytopathogen, organic acids; food preservation, waste valorization
This review highlights how acidic pH, by impacting microbial metabolism, affects many areas of applied sciences, and how research can open avenues for an impactful exploitation of microbial activities at low pH in a Planetary Health perspective.
Abbreviations
- AMR
Antimicrobial resistance
- DF
Dark fermentations
- GAD
Glutamic acid decarboxylase
- GABA
γ-aminobutyric acid
- GRAS
Generally recognized as safe
- LAB
Lactic acid bacteria
- MEC
Microbial electrolysis cells
- PAW
Plasma activated water
- PHA
Polyhydroxyalkanoates
- ROS
Reactive oxygen species
- VFA
Volatile fatty acids
- VS
Volatile solids
Introduction
The extractive and polluting nature of the linear economy (take–make–consume–waste) has by far passed the limits of environmental sustainability (Despoudi et al. 2021). In the last two centuries, especially since the second industrial revolution, the anthropocentric perspective has prevailed over that of the planet and the environments in which we live (Baporikar 2020). Though Nature is resilient and has an incredible ability for self-renewal, the rate at which humankind pollute and the kind of waste generated has now reached a point of no return, i.e. by far exceeding that of Earth’s self-regeneration (Folke et al. 2021). A circular bioeconomy model (i.e. to stop waste accumulation and aiming at reduce–reuse–recycle) would be more sustainable, and the development of such an economy is now a stated target of governments and companies worldwide (Neves and Marques 2022). The circular bioeconomy model incorporates two important notions: generation of renewable energy and production of chemicals that are less-toxic and, most of all, recyclable (Tan and Lamers 2021). In a circular bioeconomy a fundamental role can be played by micro-organisms (archea, bacteria, and fungi), which are capable of colonizing the most disparate environments and niches on our planet and possess a very broad range of metabolic activities (Sauer 2022). Exploiting waste material is therefore a fundamental component of the circular bioeconomy and its main aim is to generate high-value products and bioenergy from waste streams (Priya et al. 2023). For its practical realization to large scale waste material refining, a considerable effort of interdisciplinary teams is needed. This also applies to bioremediation and bioaugmentation when it comes to polluted sites.
As we will discuss in this review, micro-organisms have the potential to be extremely valuable in regard to the above because of their very broad range of different metabolic activities, many of which have not yet been exploited (O’Connor 2021). Synthetic biology is paving the way to microbial cell factories that will meet human needs in a greener way than current processes do (Sauer 2022).
Key physical–chemical parameters that need to be understood and, when needed, manipulated for the full exploitation of the microbial metabolism include the presence/absence of molecular oxygen, the pH, the salinity, the osmotic pressure, and the temperature (Breznak and Costilow 2014). This is true regardless of whether single species and microbial community are being considered. In this review, we highlight the role and the importance of acidic pH (low pH) in many areas of applied sciences that can contribute to the circular bioeconomy.
Acidic pH greatly impacts foods shelf life and safety because it reduces spoilage and inhibits pathogens growth (Lund et al. 2020), Acidity can be imposed by the addition of acidic molecules (for the most part organic) during food processing or generated by the natural metabolic activities of beneficial micro-organisms that are present in food. As we will discuss in the following section, the substances produced by microbial processes at acidic pH, mostly driven by fermentation, play a vital role not only in food production, preservation, and shelf life, but also in increasing the final nutritional value, functional properties (i.e. benefits beyond basic nutrition) and sensory quality of the final food products. Fermented foods are “foods made through desired microbial growth and enzymatic conversions of food components” (Marco et al. 2021), most of which are intrinsically acidic. Fermented acid foods, including many traditional food and drinks (e.g. yogurt, cheese, sour krauts, pickled vegetables, kefir, and different types of fermented milks), are the result of the biotransformations performed by micro-organisms and provide additional health benefits for human and animal health.
However, the role and key importance of microbial activities at low pH go beyond food safety, and plant, animal, and human health and disease. The acidification of soil and oceans is for example a key parameter to monitor and ideally manage, because it shapes the microbial communities living in these environments and negatively impacts on the microbial biodiversity (Peixoto et al. 2022), with inevitable adverse consequences on food chains. Even in clouds acidic pH is a key parameter that, in combination with sunlight, may influence the survival of bacteria and affect their metabolism and ability to degrade organic acids in clouds (Liu et al. 2023).
On the other hand, weak organic acids, such as itaconic acid, lactic acid, and succinic acid, represent important building blocks with the potential for microbial production under low pH, as it will be discussed in one of the following sections. Itaconic acid, for example, is a platform chemical the microbial production of which can be improved by strain development and process optimization at low pH. Lactic acid, the most commonly used term for 2-hydroxypropionic acid, is mostly produced today by fermentation: its demand has increased significantly due to its utilization as a monomer for production of poly-lactides and poly-coglycolates. These polymers are thermostable, biocompatible, and biodegradable and also suitable for biomedical applications and food packaging with significant advantages over petroleum-based polymers for the mentioned applications (Djukić-Vuković et al. 2019, Magalhães Júnior et al. 2021). Bio-based production of succinic acid as a building block has the potential to replace monomers obtained from fossil oil in the production, for example, of polybutylene succinate, a biodegradable polymer of the polyesters family, suitable for the production of disposable items (Mancini et al. 2020). Routes for microbial production of succinic acid are still not sufficiently developed to make it competitive with the currently dominant petroleum-based production, however, initiatives are active in the European market (https://www.european-bioplastics.org/).
As the above examples show, understanding and ultimately enhancing the activity at low pH of neutralophilic micro-organisms and acidophilic micro-organisms, through appropriate biotechnological applications and strategies can be channeled into the needs of the circular bioeconomy. This review aims to provide an updated account of where we are in many applied science fields that exploit microbial responses to low pH to enhance both our and planet health.
Low pH as a key parameter in food preservation, processing, and protection: the impact of microbial acid stress responses on food safety, quality, and functionalization
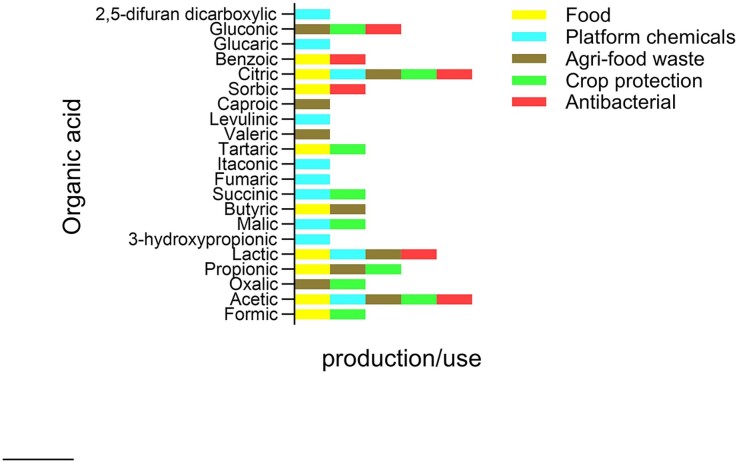
Acidity is an inherent property of some foods, such as citrus fruits and juices, and can be also caused by fermentation processes by autochthonous micro-organisms or intentionally added starter cultures (Pérez-Díaz et al. 2017). Acidophilic micro-organisms, like certain strains of lactic acid bacteria (LAB), generate organic acids that effectively inhibit the growth of pathogens and spoilage microbes. Hydrogen peroxide and bacteriocins are other antimicrobial substances produced by LAB and also greatly slow down the growth of organisms that cause food spoilage (Singh 2018). In addition, many ingredients and additives are added to food to reduce pH of the end product, which have beneficial effects on shelf life and hence safety. Among them, several organic acids, including acetic, citric, formic, lactic, propionic, sorbic, ascorbic, and benzoic acid, are routinely used in food products mainly as acidity regulators, but also (for some of them) as flavor enhancers, antioxidants or substances with direct antimicrobial activity (Coban 2020). Figure 1 summarizes the applied sectors in which the above, as well as other organic acids discussed in this review, are more prominently employed or produced.
Figure 1.
Organic acids of microbial origin that find important applications and/or are produced in different fields. The graph mainly reflects the molecules discussed in this review. Created with GraphPad Prism 10.0.
Acidic pH and food safety
Acidification is one of the most important pretreatments in some food industries, such as the canning industry, where it is commonly applied before the thermal treatment to reduce the heat resistance and inhibit the germination of bacterial spores, therefore allowing the reduction of the intensity of the thermal treatments applied (Derossi et al. 2011). Acidification by the food industry in such cases can be applied through blanching or immersion of the food ingredients in acidified aqueous solutions, direct batch acidification, or addition of acid raw materials to low-acid foods in controlled proportion to conform with specific formulations (Derossi et al. 2011).
Acidic pH also plays an important role contributing to the antimicrobial effect of some recent technological interventions developed to improve food safety. An example of such novel technology is plasma-activated water (PAW), generated through the direct exposure of water to the action of nonthermal atmospheric plasma. During its production PAW is rapidly acidified, reaching values close to pH 3.0. This is mainly due to the dissociation of water caused by the plasma treatment, and to the formation of nitric acid and nitrous acid, if nitrogen is present, either from ambient air or from the gas used in the process (Oliveira et al. 2022). Recent studies have shown that the microbial responses to PAW are quite similar to those triggered by acid pH (Fernández-Gómez et al. 2023). Several reactive species with strong antimicrobial activity are present in PAW. These include ozone, atomic oxygen, reactive oxygen species (ROS, such as singlet oxygen, hydrogen peroxide, hydroperoxide, hydroxyl, and superoxide anion radicals), nitrogen oxides (NO2, N2O3, N2O5, and N2O4), and nitric oxide derivatives, such as nitrates, nitrites, and peroxynitrites (Tian et al. 2015, Risa Vaka et al. 2019, Xu et al. 2020, Hou et al. 2021). Although acidification is not the primary cause of microbial inactivation by PAW, it has been suggested that its low pH could contribute to (i) the stabilization of some reactive chemical species present in PAW, (ii) the formation of new antimicrobial compounds, and/or (iii) the penetration of the reactive species through the cell wall, thus enhancing the antimicrobial effects attained (Naïtali et al. 2010, Oehmigen et al. 2010, Julák et al. 2012). Considering the strong bactericidal properties of PAW, it is being currently proposed as an attractive alternative for the pretreatment or washing of foods, or food-contact surfaces, taking also into account its potential for sustainable production with low energy input, offsite generation, and storability under refrigeration (Herianto et al. 2021).
The micro-organisms that occur in acidic foods or that are exposed to acidic environments in the food industry are endowed with mechanisms to respond to low pH conditions. When acid stress responses are activated in spoilage and pathogenic microbes, food quality and safety can be compromised, because the long-term survival/growth of these microbes during food processing and storage can lead to increased food waste and outbreaks of disease, even in high income countries (European Centre for Disease and Control 2023, Lakicevic et al. 2022, Yang et al. 2017). On the other hand, the enhanced growth and/or survival abilities provided by low pH responses are considered desirable attributes in the case of beneficial microbes (micro-organisms added to food for technological, sensorial, or functionalization purposes) (Lund et al. 2020). Indeed, high acid tolerance or an ability to respond to acidic pH is indispensable for industrial strains that are intentionally added to food and has become one of the most important standards for strain screening (Ko et al. 2022). Considering the competence provided by enhanced acid tolerance, engineering strategies have also been applied to improve the survival and metabolic activity of beneficial microbes under acid stress. Some of these engineering approaches involve random mutation evolution in the presence of the stressor (i.e. acid pH), where parental strains are repeatedly exposed to acidic pH while the pH is lowered gradually, and then selecting for strains with enhanced capabilities under acidity relative to the parental strain (Zhu et al. 2010).
Acidic pH and food quality
Preadaptation to acid stress has been proposed as a valuable strategy to improve the survival of beneficial microbes, such as LAB, under low pH conditions, where microbial cultures are pretreated (preculturing) at a sublethal acid stress condition prior to exposure to a harsher or more lethal acid environment (Upadrasta et al. 2011). In bifidobacteria, the enhanced survival of acid-adapted cultures has been described to be mediated by a physiological acid tolerance response which can include, analogously to other bacterial species (Lund et al. 2014): (i) pH homeostasis by proton-translocating F1FO-ATPase, (ii) the alteration of cell membrane properties by modifications in the fatty acid composition, (iii) an increase in the alkalinity of the cytoplasm by the activity of amino acid decarboxylase systems, and (iv) the production of several stress proteins (Ruiz et al. 2011). As an example, Settachaimongkon et al. (2015) demonstrated that the adaptation of the probiotic strains Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB12 to sublethal acid pH conditions (pH 4.5 or pH 5.0) improved their viability in yogurt during refrigerated storage. Likewise, the exploitation of acid stress responses, through strain engineering or acid stress adaptation of cultures, can increase the robustness in the acidic environment of the stomach upon ingestion of probiotics (Sánchez et al. 2012).
Apart from having an impact on the survival of microbes in food and the gastrointestinal tract upon ingestion, microbial acid stress responses also involve important alterations in the metabolic activity of the microbial cells, which can lead to substantial changes in their technological and functional potential, and even in the biochemical and organoleptic characteristics of fermented foods (Serrazanetti et al. 2009). As an example, Settachaimongkon et al. (2015), in the aforementioned study, showed how culturing the probiotic strains L. rhamnosus GG and B. animalis subsp. lactis BB12 under sublethal acid stress conditions changed the relative abundance of various aroma compounds, mainly of volatiles derived from the catabolism of branched-chain amino acids and sulfur-containing amino acids in yogurt. These compounds included 1-methoxy-2-propanol (Val), 2-methyl-1-butanol (Ile/Leu), 3-methyl-2-butenal (Ile/Leu), 3-methyl-butanoic acid (Leu), 2-methyl-propanoic acid (Val), and sulfur-containing compounds (Cys/Met), which can considerably influence the organoleptic quality of the product (Settachaimongkon et al. 2015).
A paradigmatic case of both the negative and positive impacts that the metabolic changes induced by acid stress responses may have on human health outcomes is exemplified by the various amino acid decarboxylases that are induced by cell exposure to acid pH. Decarboxylation pathways are activated in acid conditions as they catalyze the conversion of an amino acid into a biogenic amine, generating carbon dioxide and consuming a proton, thus contributing to the maintenance of the intracellular pH and improved survival at low pH (Pennacchietti et al. 2016, Lund et al. 2020). On the negative side, some biogenic amines produced by micro-organisms through the action of decarboxylases, such as histamine (from His), tyramine (from Tyr), 2-phenylethylamine (from Phe), tryptamine (from Trp), putrescine (from ornithine or through the agmatine deiminase pathway, which follows the decarboxylation of arginine to agmatine), and cadaverine (from Lys), can cause several adverse reactions to consumers, such as tingling tongue, rash, vomiting, diarrhea, burning sensation, headache and dizziness, nausea, palpitations, or breathing difficulties. Thus, while on one hand the acid stress causes a beneficial growth delay, it can also increase the contents of putrescine, histamine, and cadaverine. Indeed, it has been extensively demonstrated that the transcription of genes of many decarboxylase clusters involved in biogenic amine synthesis are induced by low pH (Gardini et al. 2016). On the positive side, in some micro-organisms under acidic conditions, including several LAB strains, the glutamate decarboxylase (GAD) system catalyzes a reaction producing GABA (γ-aminobutyric acid), a metabolite associated with several physiological functions in humans, such as strengthening of blood vessels, insulin secretion modulation, increased blood cholesterol prevention, or mitigation of emotional unrest, among others (Rashmi et al. 2018, Strandwitz et al. 2018). Various research initiatives are currently exploring the possibility of exploiting strains of LAB with capability to produce GABA to obtain GABA-enriched dairy products with health promoting properties. In this respect pH is an important factor influencing the yield of GABA in the fermented foods, given that the GAD enzyme of LAB is only active under acidic conditions and sharply loses activity at pH values higher than 5.0 (Yang et al. 2008, Li et al. 2010, Renes et al. 2017, 2019). The recent studies on GAD in LAB confirm that some biochemical features are shared with GAD from Escherichia coli, which is the most extensively characterized GAD at the biochemical level (De Biase and Pennacchietti 2012). Detailed characterization of decarboxylase systems is important for the selection of appropriate live cultures for food industry applications, to counteract the accumulation of biogenic amines in foods or to identify strains with desired functionalities. Moreover, modulation of their activity through exogenous addition of their substrates (e.g. glutamate in the case of the GAD system) or the strict control of the culture environment (pH, temperature, salinity, and so on) can facilitate the production of safer fermented foods with improved functional attributes.
Many substances of interest for food industries, as well as novel foods, are or will be soon industrially produced through microbial synthesis processes in bioreactors or fermenters, where organic acids accumulate as either products or by-products of fermentation, negatively affecting the productivity and yield along the process (Yáñez et al. 2008, Wang and Yang 2013, Ghaffar et al. 2014, Jiang et al. 2015). In the food industry, some of these organic acids, like propionic acid or lactic acid, are used for acidifying or regulating the pH of food, reducing its water activity and enhancing the effect of some antioxidants such as ascorbic acid (Sun et al. 2020a). In addition, organic acids can be added to food for their direct antimicrobial activity in fresh or semiprocessed products, or used for the decontamination of carcasses or meat cuts (BIOHAZ 2011). As it will be discussed in more detail in the section "Relevance of low pH in the production of valuable organic acids as building blocks for the chemical industry", acid-resistant strains can be regarded as potential cell factories for these biotechnological processes.
Acidic pH and food properties
With regard to flavor development, pH conditions can influence the production of volatile compounds during microbial fermentation processes, leading to the development of unique flavors and aromas in foods (Sharma et al. 2020b). Acidophilic micro-organisms, such as certain LAB strains and yeasts, produce specific flavors that enhance the taste profile of fermented foods and beverages (Hu et al. 2022). In addition to improving the digestibility and taste of the food, metabolic activities by these organisms may also add pharmacological and nutritional benefits to food (Xiang et al. 2019, Han et al. 2022). A wide range of micro-organisms that are derived from the raw material, starter cultures, machinery, and processing environments participate in fermentation (Tamang et al. 2016, Maicas 2020). In fermented foods, the principal role of LAB is the fermentation of carbohydrates into lactic acid, which, in addition to acidifying the food matrix increasing shelf life and thereby microbiological safety (Wang et al. 2022), also contributes in developing their flavors (Anal 2019). Volatile compounds including alcohols, organic acids, aldehydes, heterocycles, esters, ketones, terpenes, sulfur, and nitrogen compounds, have been detected in fermented foods (Dai et al. 2018, Tian et al. 2022).
During the fermentation of meat products, a large number of beneficial micro-organisms are produced that control growth of pathogenic and spoilage bacteria while also lowering the amount of toxic compounds like nitrite. Zhong et al. (2021) investigated the relationship between microbial communities and flavor in conventionally fermented sour meat. Lactobacillus, Weissella, Staphylococcus, Kodamaea, Hyphopichia, and Yarrowia were the core micro-organisms in fermented sour meat. These dominant micro-organisms correlated with flavor substances. Similar results identifying potential links between microorganism and flavor were also reported for dry sausages (Hu et al. 2020), dry-cured grass carp (Zhao et al. 2022), and Suan zuo rou, a fermented meat from China (Wang et al. 2021). Thus, besides increasing the safety of meat products, these beneficial bacteria can also enhance the flavor.
As for texture modification, the role of acidophilic micro-organisms in food is widely exploited in the food industry. The best examples are yogurt and cheese production. Yogurt, for example, is made from milk fermented typically with Lactobacillus delbrueckii subsp. bulgaricus, Lactococcus lactis, and Streptococcus thermophilus. These bacteria are generally used as starter cultures of yogurt (Chang et al. 2021) because they ensure consistency, product quality and safety (Ibrahim et al. 2021). The fermentation of lactose, the natural sugar present in milk, into lactic acid by lowering the pH causes proteins to coagulate and form a gel-like structure. This acid-induced coagulation contributes to the characteristic thick and creamy texture of yogurt (Nagaoka 2019, Kamal-Eldin et al. 2020, Wang et al. 2022).
Acidophilic bacteria, along with yeasts, are also responsible for the fermentation process in sourdough bread. In a recent study Sevgili et al. (2023) investigated LAB and yeast of 36 home-made traditional sourdoughs. They reported that sourdough containing Lactobacillus brevis, Leuconostoc mesenteroides subsp. mesenteroides, Pedicoccus acidilactici, Lactiplantibacillus plantarum, Saccharomyces cerevisiae, and Kluyveromyces marxianus gave the most preferred bread. The sour taste, high volume, easy swallowing and chewiness, minimal hardness and moisture content were all attributed to the activity of the micro-organisms involved.
In addition to the above processes, acidophilic bacteria are involved in the fermentation of vegetables and fruits such as sauerkraut and kimchi (Ashaolu and Reale 2020). The increased acidity not only enhances the flavor but also affects the texture of the vegetables, resulting in a crispy and crunchy texture as well as enhancing the bioavailability of amino acids, vitamins, bioactive peptides, and phytochemicals.
Thus, acidic fermentation is an important biotechnological tool for enhancing the safety, and health-promoting properties of bread, different kind of fermented meat, dairy and vegetable products (Melini et al. 2019).
Acidic pH and probiotics activities
There is also a strong link between low pH and the enhancement of functional properties when it comes to the addition of probiotics in food. Probiotics are defined as “live micro-organisms which when administered in adequate amounts confer a health benefit on the host” (Hill et al. 2014). They can be present in the food matrix, but also in some beverages or supplements or even medical food, and have the potential to greatly benefit human health. Resistance to low pH is particularly important for probiotics. In fact, in order to exert positive effects, they should be capable of preserving viability during storage and of colonizing gut, oral cavity or vaginal mucosae after oral administration. Indeed, the criteria for the selection of probiotic strains include in vitro tests for resistance of strains to pH of 2.5, typically encountered in the stomach, as well as in the presence of bile salts (Hill et al. 2014). These in vitro conditions therefore mimic gastric passage and allow to select strains suitable to colonize the gut. However, anaerobic conditions, prevailing in the distal gut, in contrast with the aerobic ones of the proximal gut as well as in food production and processing, present additional obstacles to selection, cultivation, and application of many probiotic candidates. This limits research on applications of strains naturally present in the gut but not cultivable in the lab yet. Therefore, micro-organisms capable of producing and tolerating organic acids and oxygen like LAB, yeasts and some Bifidobacterium spp. are currently among those most represented in both functional food and probiotic formulations in supplements (De Filippis et al. 2020). Due to the acidic pH-induced activity of GAD mentioned above, GABA-producing strains are also interesting for the functional upgrading of food, such as GABA-enriched Bifidobacterium adolescentis fermented milk (Tames et al. 2023). In addition to this probiotic, industrially relevant and food-related probiotic strains belonging to former Lactobacillus spp. (i.e, L. rhamnosus, L. plantarum, L. casei, and L. sakei) have been reported as good GABA producers (Diez-Gutiérrez et al. 2020).
Recently, the tremendous expansion in the research on gut microbiota and the decrease in the pricing for metagenomics sequencing (a valuable culture-independent technique) allowed to discover very promising new probiotic strains from species like Akkermansia muciniphila, Roseburia intestinalis, Faecalibacterium prausnitzii, and different Eubacterium spp. or Bacteroides spp. (De Filippis et al. 2020, Cunningham et al. 2021). Metabolomic data from fecal samples helped to gain insight into the mechanisms of probiotic’s action. In this respect, the composition of the microbial communities along the gastrointestinal tract and their effects on host cells, has been shown to be strongly dependent on pH, which is known to change significantly along the gastrointestinal tract (Fallingborg 1999, De Biase and Lund 2015). Probiotics able to hydrolyzing bile acids into deconjugated bile acids are also receiving attention. Primary bile acids released into the proximal gut lumen by the bile duct are digestive surfactants that cause a stress to transiting bacteria. Probiotics belonging to the Lactobacillus and Bifidobacterium genera possess the enzyme bile salt hydrolase, which catalyzes hydrolysis of the amide bond between the steroid nucleus and the glycine/taurine moiety (Prete et al. 2020, Hernandez-Gomez et al. 2021, Ruiz et al. 2021). Bile salt hydrolase is maximally active at acidic pH and its activity offers a survival advantage in the small intestine, a prerequisite to persistence in the gut. The composition of the gut microbiota and the amount of one or other type of bile acids metabolites changes insulin signaling, lipid metabolism in the liver and energy management in the organism (Winston and Theriot 2020). Also, the composition of bile acids shapes the intestinal microbiota favoring some cohorts, which can be linked to the occurrence of diabetes and obesity-related pathologies in the population (Fiorucci and Distrutti 2015, Cunningham et al. 2021).
In general, many novel probiotics strains and gut microbiota produce conventional volatile fatty acids (VFAs; which include acetic, propionic, and butyric acid) and unconventional branched short chain fatty acids in mM concentration, mainly in the colon. Being important modulators of immune response, they affect differentiation of regulatory T cells and IgA levels with numerous effects on host metabolism and health (Guo et al. 2019).
Altogether the abovementioned examples indicate an important role for low pH tolerating bacteria in improving food nutritional, sensory, and health promoting properties.
Exploiting low pH in waste management and in the revalorization of agricultural/food waste
Valorization of waste material is a fundamental component of the circular economy, i.e. getting significant support from governments and companies worldwide. This concept aims to generate high-value products and bioenergy from waste streams (Priya et al. 2023). A considerable effort of many scientists representing interdisciplinary teams is needed for the practical realization of this vision and of the current ideas for waste material refining.
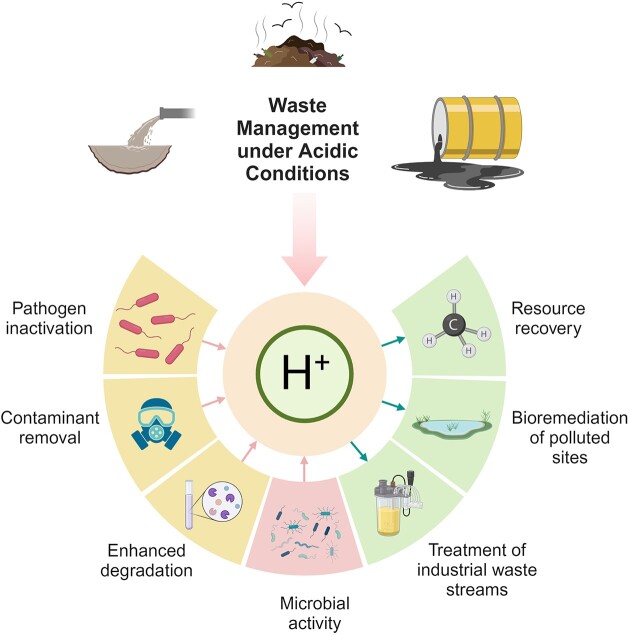
Harnessing the benefits of acidic conditions, the exploitation of acidophiles in waste management, and the revalorization of agricultural/food waste offer an innovative approach that holds great potential for effective waste treatment and resource recovery. Acidic pH environments provide unique opportunities for various waste management applications, including the degradation of organic matter, removal of contaminants, and the transformation of waste materials into valuable products (Mallick and Das 2023, Razia et al. 2023). This approach not only offers sustainable waste management solutions but also holds an enormous potential to the circular economy by reconsidering agricultural and food waste into valuable resources as illustrated in Fig. 2.
Figure 2.
Overview of the main benefits of exploiting low pH in waste management and revalorization of waste streams. Pink arrows signify areas where input issues exist, which can be effectively addressed through the application of low pH. Conversely, the blue arrows represent the outcomes that can be obtained as a result of these treatments. Created with BioRender.com.
In the previous section, how the adaptation in acidic environments allows micro-organisms’ exploitation in the food sector was discussed, while in the following section, the biotransformations for the production of valuable chemicals are discussed. As for the main molecular mechanisms that allow growth and/or survival at low pH, it is possible to refer to excellent reviews previously published (Krulwich et al. 2011, Kanjee and Houry 2013, Lund et al. 2014, Schwarz et al. 2022). In this section of the review, the acid stress response of micro-organisms is viewed in the perspective of how it can be harnessed in waste management.
Organic waste materials are in different aggregate states, which represents a first factor to take into consideration when selecting appropriate waste refining strategies. Liquid waste streams are more suitable for conducting various types of fermentations, as for example for biopolymer production. Solid waste material can be used in anaerobic processes for biogas production (see the section “Production of fuels under low pH conditions”) and can also be preprocessed using physical or enzymatic treatment to extract macro- and micro-elements for further revalorization (Fisgativa et al. 2016).
Food and agricultural waste from households, restaurants, schools, markets, and farms represent a source of different organic compounds which can be used per se or after pretreatment for valuable chemicals and materials production. However, the main challenge in refining food and agricultural waste material is their heterogeneous chemical composition, which varies depending on source, region, and seasons and ability to support the growth of specific microbial groups (Ren et al. 2018, Sindhu et al. 2019, Mengqi et al. 2023). In addition, food and agricultural waste has often low pH (< 4.0) possibly caused by anaerobic fermentation during storage and transport but also during controlled bioprocess development (Zhan et al. 2022). Starter cultures can direct the bioprocess into the desired metabolic conversion, but the substrate must be usually pretreated using high temperature, UV or chemicals, to inhibit or suppress the natural microbiota, a step that represents a cost burden in waste material valorization (Sharma et al. 2020a).
Low pH in the management of agriculture and food waste material
In this section, the management of agricultural and food waste will be considered from three perspectives: (i) microbial management of low pH waste material of food and agricultural origin, (ii) management of food and agricultural waste material that become acidified during fermentation, and (iii) low pH medium caused by chemical pretreatment of food/agricultural waste material for final microbial revalorization.
As for microbial management of low pH waste material of food and agricultural origin, bioethanol production from food waste, and feedstock is certainly one of the best-established biotechnological processes, of which the global market is still under an increase in trend. Since a large part of the product cost depends on the substrate, a low-value nonsterile organic waste material enables more cost-effective ethanol fermentation. The high-yield ethanol-producing microbial communities must first be selected from complex native microbial consortia. The ethanol producers, belonging to the Zymomonas genus and different yeasts species, compete for carbohydrates with VFA and lactic acid-producing LAB. In this environment, the pH < 5 enables the establishment of an effective consortium of LAB and homo-ethanol producers, leading to robust and competitive ethanol yields (Moscoviz et al. 2021).
A fungal fermentation was used to valorize cheese whey permeate into oil-rich fungal biomass as a potential feedstock for biofuel production and nutraceutical applications (Chan et al. 2018). Triacylglycerides were the major lipid class (92%), containing predominantly oleic (41%), palmitic (23%), linoleic (11%), and γ-linolenic acid (9%). The pH 4.5 in combination with a temperature of 33.6°C, yielded the highest biomass content of the oleaginous fungal strain Mucor circinelloides f. lusitanicus. Another example of cheese whey valorization is the production of polyhydroxyalkanoates (PHA) as a sustainable and promising alternatives to petrochemical plastics. PHA are secondary products of the microbial metabolism of some heterotrophic and photosynthetic bacteria or mixed microbial cultures. The composition of PHA can vary by controlling the pH in the acidic phase of the anaerobic bioprocess working with a microbial consortium originating from a waste treatment plant. At pH 4 the LAB prevailed, with Lactococcus being dominant over Lactobacillus, while the opposite occurred at pH 4.5 and 5.0. The microbial consortium was very robust, meaning that by varying the pH, without restarting the bioprocess, the fermentation profile necessary for particular PHA composition can be easily manipulated (Gouveia et al. 2017). This provides an excellent example of how low pH can be used to fine modulate the community composition for different purposes.
The high amount of soluble and insoluble carbohydrates in the citrus peel makes it also an attractive bioprocessing substrate. It can be utilized directly to produce hydrolytic enzymes (El-Sheekh et al. 2009). A straightforward and cost-effective medium was prepared for exopolygalacturonase production by Aspergillus sojae (Buyukkileci et al. 2015). The medium comprised orange peel, an industrial food by-product, and an inorganic nitrogen source. The submerged process, under uncontrolled pH, successfully ran at pH from 4.3 down to 2.5. The low pH was also advantageous to decrease the risk of bacterial contamination (Buyukkileci et al. 2015), which is indeed a general feature of the low pH over the many applications described in this review.
Notably, diverse organic food and agricultural waste material become acidified during fermentation and this is instrumental for the process. The following are some interesting examples.
Chinese cabbage is the most widely produced vegetable in China. However, about 30% of its yield represents waste for which anaerobic fermentation by LAB has been established for organic acid production, primarily lactic and acetic acids (Li et al. 2023b). The acids cause pH to decrease to values around 4.0. The study showed that fructose and molasses addition enriched the acid-tolerant bacteria Lactobacillus paralimentarius and Lactobacillus heilongjiangensis, promoting fiber degradation and inhibiting undesired bacteria, such as pathogenic and biofilm-forming strains. The selected strains are thus directing the cabbage waste revalorization into efficient acid production in a safe microbiological bioprocess (Li et al. 2023a). Another example is that of biohydrogen fermentation liquor produced from rice-straw, with pH 4 (due to the presence of acetate, propionate, butyrate, and valerate) successfully used by Bacillus fusiformis, Bacillus subtilis, and Bacillus flexis for polysaccharides production with flocculation capacities of kaolin suspension (Siddeeg et al. 2020). Citric acid is another acid, widely used in beverages, detergents, foods, and cosmetics. The choice of the substrate for its production plays an important role in reducing costs. Different food and agricultural waste material, such as coffee husk, rice bran, wheat bran, carrot waste, cassava bagasse, banana peel, vegetable wastes, brewery wastes, decaying fruits pineapple peel, or pomaces of grapes, among others can be used by citric acid-producing micro-organisms. The filamentous fungi Aspergillus niger is the workhorse for its production. The acidic pH in these processes is important for inhibiting oxalic and gluconic acids production and to prevent contamination by other micro-organisms (Behera et al. 2021). In recent years, a product named “Agricultural Jiaosu,” with high enzymatic activity was produced from one or more food wastes (Gao et al. 2022). The product has biocatalytic and antimicrobial activities and has been used, for example, in wastewater treatment, soil remediation, compost quality improvement, and so on. The product has low pH (∼3), high content of beneficial organisms, and of different organic acids, besides other metabolites (Gao et al. 2023).
A low pH medium can also be the result of a chemical pretreatment of food/agricultural waste material. It is the case of the production of β-carotene, used as a nutrition supplement and in medicine due to its antioxidant and pro-vitamin A activities, and to its anticancer effect, respectively. In order to enhance its yield and reduce the cost of its production by microbial fermentation, food wastes containing significant amounts of sugars can be used as a medium for microbial growth (Cheng and Yang 2016). However, the sugars must be first extracted by water or acid hydrolysis. A recent study presented an efficient sequential two-step process for β-carotene production with yeast Rhodotorula glutinis in a growth medium obtained from orange and grape wastes (Uğurlu et al. 2023). Both steps resulted in acidic pH: water extraction at pH 5.5–6.0, and acid hydrolysis with H2SO4 at pH 5.5. The β-carotenes in these media are produced very efficiently, i.e. without using any additional nutrients, which is extremely positive for the economic feasibility of the process and waste management perspective. In another study, the yeast Sporobolomyces roseus was used for the coproduction of lipids and carotenoids using pasta processing waste. The sugars were released from wastes after enzymatic treatment using Aspergillus luchuensis. After centrifugation and filtration, the substrate was adjusted to pH 5.0, supplemented with selected salts and successfully used for simultaneous lipids and carotenoid production (Villegas-Méndez et al. 2023). A highly demanded product in medicine, cosmetology, and pharmacy is bacterial cellulose membrane. The acid and thermal hydrolysate of grape pomace was successfully used for bacterial cellulose production by Komagataeibacter melomenusus solely in a grape pomace extract at pH 4.5 (Gorgieva et al. 2023).
During the degradation of organic waste through fermentation by acidogenic micro-organisms, organic waste streams are converted into carboxylic acids such as VFA and lactic acid (Kim et al. 2015, Kibler et al. 2018). As said, these carboxylic acids can be used in many different ways, such as the production of bio-based materials like biogas, bioplastics, medium-chain fatty acids, and microbial proteins (as discussed in the following sections). Furthermore, these carboxylic acids can also be recovered as raw materials for several industries, such as the chemical industry, personal care, medical, and animal feed (Strazzera et al. 2018). Importantly, the acidic environment during fermentation plays a crucial role in increasing the hydrolysis rate of waste, resulting in higher end product yields, and also influences the product profile based on the intended application (Zhou et al. 2018, Luo et al. 2021). However, as mentioned before, the production of carboxylic acids during the fermentation process leads to a further decrease in pH, requiring the addition of alkali to maintain pH stability, and therefore avoid halting of the metabolic activities (Chen et al. 2015). Approximately 15% of the operational costs are imputable to this practice (Joglekar et al. 2006). On the other hand, acidic conditions can facilitate the separation and purification of target products, as precipitation or separation of specific compounds are favored (Tang et al. 2016).
The benefits of acidic conditions for carboxylic acid production is exemplified by the study of Pau et al. (2022) who investigated the effects of hydraulic retention time and organic loading rate on lactic acid production from food waste. They found that an acidic pH < 3.5 favored lactic acid production by inhibiting the production of VFAs and enhancing the performance of Lactobacillus strains. The highest lactic acid production (8.72 g l−1), with an 82% conversion rate, was attained at pH 3.11, at 14 days hydraulic retention time and 2.14 g volatile solids (VS) l−1 day−1 (Pau et al. 2022).
Low pH and the ability to manipulate product profiles
In the process of anaerobic digestion for organic waste streams, the breakdown of the carbon source into methane typically occurs within a pH range of 6.5–8.2. However, the acidic pH inhibits the activity of methanogens, which are responsible for the production of methane. Qui et al. (2023) demonstrated a complete suppression of methanogenesis at pH 4.0, in contrast to neutral pH conditions (pH 7.0), and attributed to the acidic environment the observed reduction in abundance of acetoclastic methanogens. This strategy can be employed to inhibit methanogenesis and increase the production yield of carboxylic acids.
Atasoy and Cetecioglu (2022) demonstrated that acidic conditions (pH 5.0) enhanced VFA (also known as short chain fatty acids) production by 4.4-fold compared to neutral pH (pH 7) conditions using cheese production wastewater. Moreover, adjusting the pH of the system toward acidic conditions not only influences the product spectrum, but also drives the selection of the predominant acid type. In a study by Candry et al. (2020), it was observed that when the pH level increased from below pH 6 to above it, the product distribution distinctly switched from a medium chain fatty acids mixture (primarily composed of butyric, valeric, and caproic acids) to a mixture dominated by acetic and propionic acids.Thus, by understanding and utilizing the acid stress response of micro-organisms, the waste management processes can be optimized for improved efficiency and the production of valuable products. For this, it is crucial to consider the specific requirements of the fermentation pathways and microbial communities involved to achieve the desired outcomes. Moreover, as already mentioned, an acidic environment creates unfavorable conditions for the survival and proliferation of pathogenic micro-organisms (Lund et al. 2014, Li et al. 2022) and this is extremely important because acid-tolerant micro-organisms present in waste can outcompete and suppress the growth of pathogens, leading to a reduction in their overall abundance and activity (Mokoena et al. 2021). This strategy can be used for microbial protein production from waste streams. Microbial protein, also known as single-cell protein, holds great promise as a sustainable and protein-rich source, offering versatility in terms of feedstock selection. Its unique advantages, including independence from climate, soil characteristics, and weather conditions, as well as reduced greenhouse gas emissions and nitrogen losses, rapid production with a small ecological footprint, and abundant protein, carbohydrate, vitamin, mineral, and nucleic acid contents (Anupama and Ravindra 2000, Matassa et al. 2016), make it an attractive option. However, the cost of substrates remains a significant limitation for achieving sustainable and profitable microbial protein production. To address this challenge, waste streams have emerged as appealing substrate sources for microbial protein production. Nevertheless, the presence of pathogens poses a major concern (Lee et al. 2015, Khoshnevisan et al. 2019, Zhou et al. 2019). In this regard, the fermentation of food waste under acidic conditions offers a 2-fold solution: (i) it provides a rich substrate source for microbial protein production, thereby contributing to enhanced productivity and (ii) the inherent acidity of the environment acts as a protective barrier, reducing to a significant extent pathogen contamination during the fermentation process. By utilizing food waste as a substrate and creating acidic conditions, microbial protein production can effectively capitalize on waste resources while mitigating the risk of pathogen-related issues. This approach not only maximizes the economic viability of microbial protein production but also aligns with sustainability goals by reducing waste and ensuring the safety and quality of the final product (Anupama and Ravindra 2000, Raziq 2020). However, it is important to note that the effectiveness of acidic environments for pathogen inactivation varies, depending on factors such as the specific pathogen types, their tolerance to acidic conditions, and the intricacies of the waste management process (Li et al. 2022). Consideration of parameters like pH levels, contact time, and the nature of the waste materials being treated is, therefore crucial to ensure optimal pathogen inactivation while minimizing any potential adverse effects on the environment or beneficial micro-organisms.
Overall, exploiting the low pH conditions in waste management and harnessing the potential of acidophilic micro-organisms present a promising pathway toward sustainable waste treatment and resource recovery. This approach offers innovative solutions for efficient waste management and for the generation of valuable products from waste materials, fully in line with circular bioeconomy model.
Relevance of low pH in the production of valuable organic acids as building blocks for the chemical industry
Production of building blocks, i.e. organic compounds that are used for the synthesis of more complex molecules, from renewable substrates by microbial biotransformation is an alternative to petroleum-based chemicals. Currently, bio-based chemicals represent around 3% of the global market for chemical compounds (Spekreijse et al. 2019). Support of policymakers for transition toward bio-based chemicals, including through microbial production, grew in recent decades in the framework of Sustainable Development Goals. Recent legislation in the EU-identified bio-based products as a key enabling technology and a priority area for development (Spekreijse et al. 2019). Many microbial bio-based building blocks are still not economically competitive with the chemical synthesis processes using hydrocarbons, which are fossil fuel-based, but significant growth is expected. Among bio-based chemicals lactic acid production is already economically competitive, i.e. 1.17 EUR kg−1 for bio-based versus 1.75 EUR kg−1 for fossil-based (Spekreijse et al. 2021). Spekreijse et al. (2019) from the European Joint Research Centre analyzed the current status of the market and projected the annual growth rate for platform chemicals in the EU at 10%. A similar annual growth rate of 9%–10% is predicted globally for the next 5 years (Fortune 2021, Estimates 2022). It is expected that the demand from the chemical industry for biomass will grow until 2050 with a staggering rate of 327% for wheat only (Nong et al. 2020), which could have a huge effect on ecosystems globally and could question the sustainability of the transition to bio-based chemicals if it is not implemented responsibly, i.e. considering the planet health. This emphasizes the role of efficient biocatalysts, robust micro-organisms capable to convert substrates with high efficiency and minimal wasting of resources.
Bio-based chemicals are produced either by direct fermentation with the final product obtained by microbial transformation using whole cell-based process from renewable substrate or part of the synthesis is done by fermentation and derivatives are obtained by different routes (Bozell and Petersen 2010). Although whole-cell microbial production of many bio-based chemicals is modest in scale, microbial products have significant potential for derivatization into commercially important chemicals that are currently petroleum-based. Microbial production of chemicals also has the advantage of being stereospecific, while chemical synthesis results in racemic mixtures, which require additional separation of isomers for downstream applications. This is particularly important when microbial products are used for polymerization, since the ratio of isomers defines the physical–chemical properties of polymers (Abdel-Rahman et al. 2013).
Organic acids, which could be produced by micro-organisms, are recognized as being among the most promising bio-based chemicals obtained from biomasses. They included: dicarboxylic acids (fumaric, 2,5 furan, glucaric, itaconic, malic, and succinic acid), 3-hydroxy propionic, levulinic, glutamic, and aspartic acids (Werpy and Petersen 2004). A later revised list addressed advances in microbial and biochemical production of building blocks and included, by similar criteria, among the top ten promising bio-based chemicals, five organic acids, i.e. lactic, succinic, 3-hydroxy propionic, 2,5 furan dicarboxylic, and levulinic acid (Bozell and Petersen 2010). In the 28-members EU market, acetic and lactic acid are the most represented organic acids among bio-based platform chemicals (Spekreijse et al. 2019).
The main reasons for the low market share of bio-based chemicals lay in low process productivity on second and third generation substrates and the high costs related to substrate pretreatment and final product extraction and purification (Magalhães Júnior et al. 2021). Cheaper biomass substrates like by-products and wastes from the agri-food industry and lignocellulose are very complex and often contain many inhibitory substances, such as metals in molasses. Inhibitors are also generated during substrate pretreatment, hydrolysis, and sterilization. End-products induce media acidification during the process and affect the productivity, viability, and recovery of produced organic acids. Thus, robust micro-organisms capable of achieving high yields in the presence of multiple stressors, including low pH, are needed.
To keep costs lower for extraction of organic acids from fermentation media, it is preferable to run the process at low pH, as this avoids neutralization and production of excessive amounts of salts during purification steps. The use of micro-organisms that are natural producers of acids, like wildtype strains of LAB for lactic acid, Aspergillus terreus for itaconic acid and other Aspergillus spp. for malic and citric acid or Rhizopus strains for fumaric acid (Lee et al. 2011) is a clear advantage because they already possess several mechanisms to cope with low pH and other stresses, as previously explained. However, yields and profiles of biotransformation in media are still greatly affected by deviations in pH and concentrations of acids in media (Djukić-Vuković et al. 2012, Burgé et al. 2015a, Asunis et al. 2019). For example, Lactobacillus reuteri produces 3-hydroxypropionic acid naturally and tolerates concentrations of up to 2.5 g l−1 of a by-product, 3-hydroxypropionic aldehyde, when grown on glycerol and at pH above 5.0; in contrast below pH 5.0, this compound is toxic (Burgé et al. 2015b). In general, neutral or mildly acidic conditions favor a dissociated form of acids and make it more difficult for the acid to enter microbial cells and hinder viability and productivity (Guan and Liu 2020, Lund et al. 2020). The pKa of the carboxylic groups in lactic acid (3.8), itaconic acid (3.8 and 5.5), fumaric acid (3.1 and 4.4), and similar pKa values for other microbial organic acids, require that the majority of industrial fermentations for organic acid production is carried out at pH values between 5.0 and 7.0, with pH control, so as to avoid affecting cell viability and productivity. Neutralization of media, usually with the addition of CaCO3, NaOH, or Ca(OH)2 during fermentation, leads to the generation of large quantities of side streams of salts like gypsum, thus adding a negative environmental footprint of the process (Salek et al. 2015, Shi et al. 2015).
To overcome this problem heterologous and nonconventional strains, engineered by adaptive evolution and genetic manipulation to tolerate low pH, can be used (Thorwall et al. 2020). These genetic manipulations can be coupled with alternatives in process design like different substrate feeding strategies (Hosseinpour Tehrani et al. 2019a), reactive extraction of organic acids or electrochemical pH swings, which can be well coupled with engineering of strains for high yield of specific products (Becker et al. 2020, Gausmann et al. 2021).
Itaconic acid production
Itaconic acid is an unsaturated dicarboxylic acid that can be used as a building block for polymers production (in alternative to acrylate), following its transformation into esters. Itaconic acid-derived polymers can be used in corrosion prevention but also in dental and drug delivery applications (Robert and Friebel 2016). The advances in strain and process engineering have been studied in detail for the production of itaconic acid, because this acid is an important precursor for applications in the pharmaceutical industry (Steiger et al. 2013, 2016, Hosseinpour Tehrani et al. 2019a, b, c, Trivedi et al. 2020). Aspergillus terreus is the dominant industrial microorganism for itaconic acid production because it provides high yields, and we have a good know-how in process development with this microorganism (Steiger et al. 2016). A techno-economic assessment estimated costs for itaconic acid fermentation by A. terreus at 1.13 US$ kg−1 and compared costs of different downstream processing strategies. Costs were estimated at 0.63 US$ kg−1 for adsorption, at 0.88 US$ kg−1 for reactive extraction, while electrodialysis-based process adds 1.50 US$ kg−1 (Magalhães et al. 2019). Downstream processing significantly affects process profitability and Magalhães et al. (2019) identified cheap carbon sources and fermentation with high itaconic acid titres as critical steps to make the process financially viable, emphasizing the importance of resilient production micro-organisms. Additionally, changes in the morphology of micro-organisms, and the solubility of end product, plus its interaction with other compounds present in media, particularly in the case of cheaper waste substrates, depend on pH during production and processing and affect upscaling, due to technical difficulties like clogging, mixing, mass transfer and aeration during the process (Alonso et al. 2015, Trivedi et al. 2020). Although highly productive, A. terreus is susceptible to impurities which are present in cheaper substrates used for itaconic acid production (Steiger et al. 2013, 2016). To overcome this limitation, Becker et al. (2020) optimized nonconventional Ustillago maydis strain MB215 to produce itaconic acid at theoretical maximum levels, at pH 4.0, in shaken culture by cutting off carbon flux into the side product pathways, favoring yeast-like morphology and enabling transport of metabolites within basidiospore compartments to prevent bottlenecks in the main metabolic pathway.
Another nonconventional strain, Ustilago cynodontis has a low-pH resistance and after engineering and deletion of the fuz7 gene, it achieved 82.9 g l−1 concentration of itaconic acid at pH 3.6 while keeping the yeast-like morphology (Hosseinpour Tehrani et al. 2019b) proving the applicability of the proposed strategy on a different strain. Engineered strains achieved itaconic acid concentrations of up to 220 g l−1 with process engineering and optimization, showing that there is great potential for commercial implementation and scaling up of the production (Hosseinpour Tehrani et al. 2019a). These significant advancements in production of itaconic acid will probably soon increase the share of microbial itaconic acid in the market.
Lactic acid production
Lactic acid is a very good example of a bio-based platform chemical already produced by fermentation on large scale today. Over 90% of all available lactic acid on the market today is produced by fermentation and it is widely used in bulk, as an acidulant, to enhance flavor, and as preservative in the food, beverage and cosmetic industry or even as a descaling agent in the marine industry or as a platform chemical for the production of poly-lactides (Abdel-Rahman et al. 2013, Djukić-Vuković et al. 2019, Sauer and Han 2021). Innovations in lactic acid production and the development of very productive processes have been driven by the expanding range of applications of lactic acid polymers in medicine and packaging (Castro-Aguirre et al. 2016, Murariu and Dubois 2016).
Competition of lactic acid polymers with petroleum-based polymers on the market set maximal costs for lactic acid production and pushed research efforts into the development of novel, more productive strains. A number of natural lactic acid producing bacteria use the homofermentative route for the conversion of glucose into l- or d-lactic acid with yields of over 95%. However, pH control is still required and lactic acid concentrations in batch fermentation are most often below 90 g l−1 when natural lactic acid producing bacteria are used (Chen and Nielsen 2016, Djukić-Vuković et al. 2019). The potential of LAB as cell factories is huge and was extensively reviewed (Upadhyaya et al. 2014, Sauer et al. 2017, Börner et al. 2019, Sauer and Han 2021) including the benefits of the systems biology approach (Liu et al. 2019). Other than LAB, Rhizopus oryzae ranks among the top natural lactic acid producing strains, with concentrations of up to 230 g l−1 but also at a medium pH above 4.5 (Yamane and Tanaka 2013).
The robustness of yeasts to survive at very low pH qualified them as potentially very good hosts for lactic acid production. Some yeasts, like Kluyveromyces thermotolerans, have genes for lactic acid production at low pH, although productivity is low,. As in the case of other organic acids, lactic acid yields are higher at neutral pH, but extraction and purification are much more expensive when neutralizing agents are used, with the generation of salts and consumption of acids used later to release free lactic acid. This is not financially viable and production of free lactic acid at low pH is preferred.
Adaptive laboratory evolution has been widely used to improve acid resistance among both natural lactic acid producers and engineered strains (Singhvi et al. 2015, 2018, Liang et al. 2018, Börner et al. 2019, Cubas-Cano et al. 2019), and it is also a convenient strategy for adaptation to nonconventional carbon sources, like xylose, which are present in cheaper lignocellulose or waste substrates (Cubas-Cano et al. 2019, Mladenović et al. 2019). Adaptive evolution was combined with genome shuffling in S. cerevisiae for lactic acid production (Wang et al. 2018), while error-prone whole genome amplification was used in the case of a Lactobacillus pentosus stain, enabling its growth at pH 3.6 and the production of lactic acid with 95% yield during 25 subcultures (Ye et al. 2013). Bacillus spp. were also reported as good and robust lactic acid producing micro-organisms. They are, suitable for low-pH lactic acid production on very complex substrates with mixed carbon sources to avoid catabolic repression or even in open fermentations, with Bacillus coagulans being the most promising candidate (Ma et al. 2014, 2016, Zhang et al. 2014a, Glaser and Venus 2018, Wang et al. 2018, 2019, Alexandri et al. 2020).
Industrial lactic acid production processes are mostly performed by engineered strains. Cargill, one of the largest lactic acid producers globally, reported that the engineered yeast strain CB1 is capable to produce over 135 g l−1 of lactic acid at pH 3, with 90% of free lactic acid, decreasing extraction and purification costs significantly. Screening of promising strains was first performed based on the resistance of over 1200 yeast isolates to low pH and temperature. Following the screening, the nine most promising candidates were engineered by deleting pyruvate decarboxylase and introducing lactate dehydrogenase genes, together with introducing random and targeted mutations for further optimization (Miller et al. 2011).
The potential of natural LAB strains as probiotics, their status of generally recognized as safe (GRAS) micro-organisms and their wide utilization in the food industry were considered positively in some processes for lactic acid production because the microbial biomass after the fermentation can be used as added value feed product (Mladenović et al. 2018, Sadiq et al. 2019) or for silage to improve the cost-effectiveness of the process (Hatti-Kaul et al. 2018). However, the need for valorizations of the remaining microbial biomass in feed production is accompanied by aversion toward genetically modified micro-organisms in food and feed chains. This has affected to some extent the development of engineering tools for LAB and has limited commercial genome manipulation of LAB even for the production of lactic acid as a chemical (Börner et al. 2019).
Succinic acid production
Succinic acid is an organic acid mostly produced chemically from glucose and is very important as a platform chemical for the production of oil-based polymers. Its natural producers are very limited. Biosuccinic acid can be produced by fermentation from second-generation feedstocks by Acinobacillus succinogenes or by engineered E. coli with yields of around 30 g l−1 (Mancini et al. 2020, Putri et al. 2020, Magalhães Júnior et al. 2021). The limited availability of suitable micro-organisms in terms of productivity is currently a key factor that limits a wider adoption of biosuccinic acid production. However, the importance of succinic acid for the production of a large number of chemicals makes it an attractive candidate for the development of microbial producers of biosuccinic acid.
Costs in all phases of fermentative production of platform chemicals vary significantly and depend on the number of parameters. Techno-economic analyses were done for some processes (Kwan et al. 2015, Gezae Daful and Görgens 2017, Magalhães et al. 2019) and should be performed more often for the assessment of improvements in industrial micro-organisms and process engineering. The majority of these studies considered fermentative production on renewable biomass as a more sustainable option, however, critical assessment reveals that the substitution of synthetic chemicals with bio-based ones at the estimated increase in demand requires huge consumption of biomass as substrates. This would cause deforestation, arable land deterioration and possibly loss of biodiversity and it argues against the overall sustainability and improved CO2 footprint of bio-based chemicals production. (Morone and D’Amato 2019, Nong et al. 2020). However, significant transition toward circular economy approach, as emphasized earlier, considers utilization of waste streams of one industry as raw materials for other. The metabolic potential and role of micro-organisms in this respect is unprecedented and still not fully exploited. Diversity, versatility, and adaptability of micro-organisms, as well as additional spectra of biotransformation, which can become available through metabolic and genetic engineering of strains are among the most powerful tools on our planet for resource recovery. Even highly polluting fossil-based polymers, which are not biodegradable in reasonable time, could be degraded, under the specific conditions, by Aspergillus spp., Pseudomonas spp., Brevibacillus spp., Mucor spp., and so on (Ru et al. 2020). A circular design concept, that involves the design of new polymers based on the knowledge on metabolic capabilities of micro-organisms and suitability of specific bonds for microbial cleavage in order to enable degradation and resource recovery (Schink et al. 1992), could indeed play an important role for really circular and sustainable solutions in the future.
Harnessing microbial activity in contaminated acidic environments: biotechnological approaches and strategies
The application of biotechnological methods that harness the benefits of acidic environments in microbial communities holds significant potential for optimizing resource recovery, pollutant remediation, and ecosystem restoration. Various techniques have been employed to leverage the acid stress response of microbial communities, as well as benefit of the metabolic activity of acidophile. These techniques include bioremediation, bioaugmentation, bioleaching, chemotaxis, synthetic biology, and genetic engineering, depending on the aim of the study (Zhang et al. 2018, Calero and Nikel 2019, Muter 2023).
Enhancing metabolic capabilities could significantly improve bioremediation, a widely employed technique, which offers immense potential to combat environmental contaminations. Bioremediation harnesses the power of micro-organisms or their metabolic processes to degrade contaminants or convert them into less detrimental/toxic substances (Calero and Nikel 2019). Bioremediation strategies can be employed to stimulate indigenous acidophilic micro-organisms naturally present in contaminated sites, thereby amplifying their activity and effectiveness. This approach encompasses various methods such as bioaugmentation, biostimulation, bioattenuation, and biosparging, tailored to in situ and ex situ applications (Vishwakarma et al. 2020, Shweta et al. 2021).
Bioaugmentation and biostimulation represent essential subgroups within the field of bioremediation. Biostimulation focuses on the enhancement of indigenous micro-organisms already present in the environment, harnessing their existing metabolic capabilities to achieve remediation objectives via the addition of one or more limiting nutrients or other specific compounds to the system (Kouzuma and Watanabe 2011, Yagnik et al. 2023). Bioaugmentation on the other hand entails the deliberate introduction of specific micro-organisms or microbial groups into a system to enhance targeted applications such as pollutant degradation or transformation, as well as the amplification of desired metabolites production (Herrero and Stuckey 2015, Butler and Hung 2016, Nzila et al. 2016, Raper et al. 2018). Bioaugmentation can be delivered via several methods including direct injection of micro-organisms, construction of biogranulation (i.e. microbial aggregation), biocurtains (biobarriers), or gene-mediated methods (Gough and Nielsen 2016). By utilizing the natural adaptability and abilities of acidophiles to survive in acidic environments, bioaugmentation can be designed for remediation of acidic mine drainage (Anekwe and Isa 2023), removal of metal contaminants from acidic sites (Ayangbenro et al. 2018), treatment of acidic industrial wastewater (e.g. acidic food processing, acidic metal planting, and acidic petrochemical wastewater treatment) (Nzila et al. 2016, Anh et al. 2021), remediation and restoration of acidic soil/sediment and water bodies (Kumar et al. 2018, Adetunji and Anani 2021), and recovery of valuable products from waste streams under acidic conditions (Zhang et al. 2022b).
Zhang et al. (2022b) developed a bioaugmentation approach aimed at enriching autochthonous acid-tolerant LAB, enhancing the production of lactic acid from source-sorted organic household waste at pH 4. The findings revealed that bioaugmentation with a specific acid-tolerant strain (L. reuteri DTUAT 04) resulted in a 29% increase in lactic acid production at pH 4 compared to the non-bioaugmented control group (Zhang et al. 2022b).
Additionally, Sánchez-Andrea et al. (2012) devised a bioremediation approach to address the treatment of acid mine drainage in conjunction with domestic wastewater at bioreactors under pH 5. The study employed sediments sourced from the Tinto River in Spain, characterized by an exceedingly acidic environment and a high concentration of metals, as the inoculum. Through this strategy, the researchers accomplished the successful removal of dissolved metals exceeding 99% (with the exception of Mn), sulfate removal by over 75%, and iron removal surpassing 85% (Sánchez-Andrea et al. 2012).
Similarly, Anekwe and Isa (2022) implemented an innovative bioremediation strategy to address acid mine drainage-contaminated soils. Their approach involved the combination of biostimulation, bioventing (i.e. injection of air or molecular oxygen into the system), and bioattenuation (i.e. utilization of native micro-organisms) techniques. In a microcosm experiment utilizing 1 kg of polluted soil, the researchers employed domestic and brewery wastewaters for biostimulation to enhance the microbial community through nutrient and carbon source enrichment. For bioventing, atmospheric air was introduced alongside wastewater as a form of biostimulation, while for bioattenuation, the researchers focused on monitoring the growth rate of the microbial communities without additional interventions. The results demonstrated that the highest efficiency in metal removal was achieved through the synergistic combination of bioventing and biostimulation, yielding a removal rate of 56%–70%. Biostimulation alone resulted in a removal rate of 50%–66%, while bioattenuation achieved a removal rate of 12%–31%. This study highlights the significance of employing multiple approaches in tandem, as their combined effects can lead to enhanced degradation efficiency (Anekwe and Isa 2022).
In addition to the growing interest in the development of synthetic cultures for targeted pollutant degradation, several studies have successfully patented bioaugmentation strategies employing micro-organisms. Notably, BioTigerTM, a consortium of 12 aerobic bacteria isolated from an oil refinery in Poland, has demonstrated promising results in the bioremediation of hexanoic acid and phenanthrene present in oil sands. It is important here to recall that hexanoic acid, a naphthenic acid, and phenanthrene, a polycyclic aromatic hydrocarbon, are recognized as the principal toxic compounds found in oil refinery wastewater. Within a 24-hour timeframe, BioTigerTM exhibited significant degradation capabilities, with ~55% degradation of hexanoic acid and ~80% degradation of phenanthrene (Reddy et al. 2020).
Bioaugmentation strategies are commonly applied in resource recovery approaches, facilitated by the controllable environment of bioreactors and for ex situ applications targeting the degradation of pollutant sites. Nonetheless, despite its inherent complexity, bioaugmentation can also be effectively implemented in situ within ecosystems. Dybas et al. (2002) conducted a study in Schoolcraft, MI, USA, focusing on the remediation of a carbon tetrachloride and nitrate-impacted aquifer. They implemented a combined approach of bioaugmentation and biostimulation, utilizing a biocurtain. In this method, they introduced Pseudomonas stutzeri KC strain, a denitrifying bacterium capable of cometabolically degrading carbon tetrachloride without generating chloroform, for bioaugmentation. Additionally, the wells were stimulated through the external addition of an electron donor (acetate), phosphorus, and pH adjustment using a base. Over a span of 4 years, their findings demonstrated that the biocurtain achieved a carbon tetrachloride removal rate exceeding 99% and successful colonization of the bioaugmented species, i.e. P. stutzeri KC (Dybas et al. 2002). Although in this specific case the developed solution did not involve the application of acidic conditions, it paves the way to the implementation of a bioaugmentation strategy for remediating in situ acid mine drainage or rehabilitating acidic soil, sediments, and aquatic ecosystems.
In situ applications of bioremediation strategies have demonstrated success on contaminated sites; however, the complexities inherent in in situ ecosystems present significant challenges. These challenges include complex environmental factors, contamination heterogeneity, limited nutrient availability, the presence of inhibitory substances, and long-term monitoring requirements. Such complexities make the application of bioremediation more challenging compared to controlled laboratory or ex situ environments. To overcome these difficulties, the integration of modeling approaches into bioremediation strategies can provide valuable insights into the long-term effects of bioaugmentation or biostimulation. Genome-scale models have emerged as powerful tools for understanding microbial interactions and metabolic networks, primarily in bioreactor settings. By utilizing genome-scale models, researchers can gain valuable insights into the interactions of microbial communities and ecosystem processes. As suggested by Wang et al. (2023a), the combination of mathematical models with the construction of synthetic microbial communities offers a quantitative approach to validate theoretical studies of microbial ecology. These modeling approaches have the potential to ease the difficulties associated with in situ bioremediation, providing a foundation for predicting and optimizing the outcomes of bioaugmentation or biostimulation strategies when dealing with complex environmental settings (Wang et al. 2023b).
Based on all the above, it is possible to conclude that the application of acidic environments in microbial communities through various approaches, such as bioremediation, bioaugmentation, bioleaching, and more, has been used effectively for pollutant remediation, ecosystem restoration, and resource recovery. These strategies are being further enhanced through the continued development and application of synthetic biology, along with the integration of diverse modeling methods, including metabolic and microbial community models.
Production of fuels under low pH conditions
The transition to a low-emission economy will strongly depend on green fuels, the production of which is both sustainable and economically viable. Among these, a leading role is expected to be played by “green hydrogen,” as hydrogen is a precursor of most synthetic fuels. The global demand for hydrogen is expected to nearly double between 2021 and 2030. In 2021, worldwide demand for hydrogen stood at 94.3 million metric tons per year (https://www.statista.com/statistics/1121206/global-hydrogen-demand). Most hydrogen is consumed in the chemicals and refining sectors and still largely derived from fossil fuels, in the process called natural gas reforming. The US Department of Energy has proposed the goal of producing green hydrogen at a cost of below 2$ kg−1 by 2025 and 1$ kg−1 by 2030. In the near- and mid-term these goals are expected to be fulfilled by electrolysis, however, in a slightly longer perspective waste-to-hydrogen technologies should be included. This expectation gave a strong impulse to new research studies on biohydrogen production in dark fermentation (DF) and also microbial electrolysis cells (MEC) and photofermentation. The number of new research papers related to DF has grown substantially in the last few years (from 3562 articles in 2020 to already 3451 as of July 2023) (source: ScienceDirect.com). When referring to biohydrogen, it is meant hydrogen produced through biological process (e.g. DF process and algae photobiological water splitting).
DF is an anaerobic fermentation with inhibition of methanogenesis, which is the process of methane generation. DF was found from a life-cycle perspective by Estevez et al. (2023) to be significantly more environmentally friendly than other waste management processes, such as composting.
Biohydogen process conditions
As expected, process results strongly depend on substrates and on the microbial consortia applied in fermentation. Table 1 presents the most popular wastes and residues (as well as their characteristics) that are anaerobically digested to produce biohydrogen and other bioproducts. Some wastewaters or activated sewage sludge may need to be cofermented with another substrate in order to increase process efficiency (Yang and Wang 2017). Although sludge might contain high organics and nutrients content, the biohydrogen yield often is lower than 25.2 ml g−1 of VS added (Alemahdi et al. 2015) due to low carbon/nitrogen ratio (C/N ratio 4–9), low carbohydrates content (< 10% dry mass), and the presence of inhibitors (e.g. toxic organics and heavy metals). This is solved by cofermentation of sewage sludge with other organic wastes, such as crude glycerol, crop residue, grass residue, flower waste, food waste, molasses wastewater, tofu residue, or fallen leaves (Yang et al. 2019). This helps to improve the C/N ratio (i.e. 20–30), provide more bioavailable carbohydrates, and dilute the inhibitors. such as ammonia (Grosser and Neczaj 2018). The cofermentation process showed a synergistic effect on biohydrogen production; the optimal mixing ratio of sludge to leaves was 20:80 (VS basis) with a biohydrogen yield 37.8 ml g−1 VS added. The mono-fermentation of sludge gives yields of 10.3 ml g−1 VS added and for leaves 30.5 ml g−1 VS added.
Table 1.
Characteristics and process conditions for some common wastes and residues that can be anaerobically digested to produce valuable chemicals.
| Substrate | Advantages for fuel/organic acids/plastic/other production | Disadvantages for fuel/organic acids/plastic/other production | Products | Substrate pretreatment with acid | References |
|---|---|---|---|---|---|
| Straw | - Easily accessible | - Seasonal product - Price fluctuation, depending on availability - Expensive - Used for other purposes (i.e. bedding) |
- Biogas - Biohydrogen - Biomaterials |
Yes (pH 2–4) | Tan et al. (2021) |
| Activated sludgeand municipal wastewaters | - Readily available - Additional income for collecting it - Stable and huge supply - Codigestion |
- Low C/N ratio (4–9) - Low carbohydrates content (< 10% dry mass) - Possible presence of inhibitors (e.g. toxic organics and heavy metals) - Possible presence of pathogens |
- Biopolymers - Biogas - Biohydrogen |
Yes (pH 3) | Chang et al. (2011) |
| Food waste and municipal wastes | - Readily available - Additional income for collecting it - Stable supply |
- Heterogeneous composition - Presence of pathogens and impurities (especially plastics and glass) |
- Biogas - Biohydrogen - VFA - Medium-chain acids |
Yes (pH 1- 4) | Kim et al. (2014) |
| Industrial organic wastewater | - Readily available - Additional income for collecting it - Stable supply - Homogeneous composition |
- Low-energy - Low-dry mass waste |
- VFA - Medium-chain acids - Biogas - Biohydrogen |
Yes (pH 5–5.5) | Preethi et al. (2019) |
| Industrial by-products (e.g. glycerol) | - Readily available - Additional income for collecting it - Stable and huge supply |
- Possible presence of inhibitors (e.g. toxic organics and heavy metals) - Possible nonadequate C/N ratio |
- Biogas | Yes (0.06% H2SO4) | Pascal et al. (2019) |
| Sugar beet pulp | - High-energy waste | - Local availability | - Biogas - Biohydrogen - VFA - Medium-chain acids |
Yes (75 mM H2SO4) | Chiocchio (2021) |
| Wood chips, sawdust, and bark | - Carbon supplementation for animal waste with high nitrogen content | - High lignin content | - Biogas | Yes (pH 3–4) | del Campo et al. (2006) |
| Vegetable waste | - High availability (field crops and greenhouses) - Cheap waste |
- Heterogeneous composition - High content of fertilizers - Possible presence of pathogens |
- Biogas - VFA - Medium-chain acids |
Yes (0.5% H2SO4) | Mucha (2020) |
In order to increase the biohydrogen yield the substrates should be pretreated. Nissilä et al. (2014) analyzed the effects of various pretreatment methods, including physical, chemical, and physicochemical ones, and identified the best one for corn stover fermentation, namely steam explosion, a physical pretreatment using steam and sudden pressure drop, and diluted sulfuric acid (the hydrogen yield on hexose was 3 mol mol−1). The diluted acid or hydrothermal pretreatments of wheat straw enabled yield of 2.8 mol·mol−1 and 2.6 mol·mol−1, respectively. In a recent study of water-hyacinth DF, it was found that ultrasonic-assisted alkaline pretreatment, which is needed to enhance delignification, can increase hydrogen yield by 350% (Thu Ha Tran and Khanh Thinh Nguyen 2022).
Inoculation of a mixed microbial consortium is preferred for stable operation and control of the process for a wide range of substrates (Dessì et al. 2017). However, mixed consortia, while preferred from a practical point of view, may contain some species, which do not produce hydrogen or even consume hydrogen as a part of their metabolism. In order to inhibit methogenesis and remove those often non-sporulating species from the inoculum a proper pretreatment (called also stressing) is used, which may include heat shock (i.e. boiling or freezing even up to 60 min) (Hernández et al. 2019), sudden change of pH even below 4.0 (Yang et al. 2019), microwaves interaction (Rafieenia et al. 2018), sonication, twisting (which consists in a fast rotation) (Perez-Pimienta et al. 2016), or addition of chemical agents (Hu and Chen 2007). Sporulating species are unfortunately more resistant to such pretreatment, which is the case of the spore-forming thermophilic homoacetogenic bacterium Moorella glycerini (Dessì et al. 2017).
In some cases, e.g. cofermentation of sewage sludge and fallen leaves, microbial community analysis showed that the cofermentation influenced the microbial consortium; cofermentation enriched Clostridium, Bacillus, and Rummeliibacillus genera, which were responsible for the synergistic effect of biohydrogen production. Moreover, Dauptain et al. (2020) showed that some indigenous bacteria from varied organic substrates can also effectively generate H2. Ferraro et al. (2020) proposed inoculum enrichment (i.e. bioaugmentation) using sequential reinoculation, where shorter (24 and 48 h) reinoculum times favored hydrogen and longer (96 h) methane production. Generally, the result of inoculum and substrate pretreatment depends on the interactions of indigenous and exogenous bacteria, suppressing some nonspore-forming bacteria.
There are several important process parameters which determine the process efficiency (Nissilä et al. 2014) e.g. optimal C/N ratio between 20 and 30; 5 < pH < 6, optimal temperature (> 50°C) (Yin et al. 2023) and substrate concentration between 10 and 20 g l−1. Some were confirmed by a recently performed meta-analysis which relates high productivity to 6 ≤ pH ≤ 6.8 and 35°C ≤ T ≤ 37°C and H2 high yield to 5.5 ≤ pH ≤ 7.5 (Lopez-Hidalgo et al. 2022). Much lower pH is postulated in installation implementing patent (Ignaciuk and Ignaciuk 2018).
Another important parameter is hydraulic retention time: if it is too low, wash out of granular bacterial biomass occurs; if it is too high, product inhibition appears due to accumulation of VFAs. Larger cellulose particles, which take longer to decompose into simple sugars, require longer hydraulic retention time. Removal of produced H2 gas from the system prevents its partial pressure to increase and to reduce the formation of unnecessary by-products. The pH value has tendency to evolve (i.e. acidify) during fermentation, but its control and stabilization as well as process microaeration showed increased H2 yield (Cetecioglu et al. 2022).
Challenges/barriers and proposed solutions for biohydrogen production
In order to make the process of biohydrogen production technically and economically feasible one needs to overcome several barriers and challenges which include: (i) the choice of inoculum and its pretreatment (stressing) in order to eliminate hydrogen consuming bacteria; (ii) the choice of substrate and suitable pretreatment methods (they vary for different substrates); (iii) lack of trace elements, especially iron and nickel, essential for hydrogenases (including (Fe), (NiFe)-, and (NiFeSe)-hydrogenase); (iv) high hydrogen partial pressure, which results in the reduction of oxidized ferredoxin, thus hindering hydrogen production; (v) scale-up methods—however, there are cases (still rare) of full-scale installation for biomethane, e.g. Ignaciuk and Ignaciuk (2018), Cetecioglu et al. (2022); and (vi) low hydrogen yield (and inhibition caused by by-products, e.g. acetate, butyrate, and propionate).
As an example, with respect to point (iii), Cieciura-Włoch et al. (2020) showed that Fe2O3 addition greatly enhanced biohydrogen production from sugar beet pulp, whereas iron salts (FeSO4 and FeCl3) were not effective.
As for the low biohydrogen yield, it can be changed into an advantage by the exploitation of the emerging by-products for extra energy/fuel (two stage process) or for bioplastics such as PHAs production, in a biorefinery framework (see also the section “Exploiting low pH in waste management and in the revalorization of agricultural/food waste”). A second stage treatment of the DF effluents helps also to reduce their organic load, hardly decreased during DF, before being discharged into the environment.
Two-stage process for fuel production
DF is a process with a rather low hydrogen yield (chemical oxygen demand—COD; H2/COD feedstock) below 17% (Lee et al. 2022). However, the process rate is much faster (up to 192 m3 H2 (m3_reactor)−1 day−1) than the other MEC or photofermentation (18 m3 H2 (m3_reactor)−1 day−1 in MECs) (Alexandropoulou et al. 2018). DF can utilize complex organics to initiate waste digestion. This is possible due to the diversity and functional redundancy of fermenting bacteria (e.g. Clostridium, Ethanoligens, Escherichia, Citrobacter, and so on). The by-products generated by DF, such as VFAs are the main source for hydrogen production by microbial consortia in photofermentation and MECs, with hydrogen yield as high as 98% (Kim et al. 2019). DF combined with MEC can support up to 105 million metric ton of hydrogen gas from 1.3 billion metric food waste. Production potential is thus 120% higher than the hydrogen demand in 2021 (i.e. ∼ 90 million metric ton) (Lee et al. 2022).
The DF effluents can be also utilized for biomethane production (Nathao et al. 2013, An et al. 2024). As discussed above, the process is already implemented in large scale installation (Cetecioglu et al. 2022).
Bioplastic production
It is important here to recall that different types of wastes and wastewaters can be used for bioplastic production (see also “Exploiting low pH in waste management and in the revalorization of agricultural/food waste”). These wastes often must be treated in order to achieve high concentration of VFAs. Notably, this treatment is not needed in the postprocessed effluents after DF because VFA are already present at a high concentration (Kora et al. 2023).
Summing up, DF may not always provide high biohydrogen yield, however, its economic feasibility increases when DF is combined with follow-up process (related to extra fuel or bioplastics production). Although there are several parameters crucial for DF, such as substrate composition, bacterial consortium, applied pretreatment, temperature, pH, and so on, emerging machine learning procedures could help in the near future to the optimization of the process.
Crops protection: pH-dependent pathogenicity and possible interventions at low pH
Fungal plant pathogens pose significant threats to global food security, causing substantial crop losses and damaging agricultural produce. Few examples of economically impacting phytopathogens include the broad host range Botrytis cinerea causing pre- and post-harvest gray mold in many agronomically important crops, Blumeria graminis causing powdery mildews of wheat and barley, the mycotoxinogenic Fusarium spp. causing the destructive cereal head blight disease, vascular wilt on a wide range of plants and postharvest fruit rot in many important crops, and Colletotrichum, which is one of the most common and important genera of plant-pathogenic fungi for crop plants worldwide (Dean et al. 2012). Fungal pathogens employ various molecular mechanisms to promote infection of their plant host, which include the secretion of degrading enzymes, production of toxins, initiating an oxidative burst, and modulation of environmental pH. These mechanisms perturb the host immunity and induce host cell death, from which the pathogens acquire nutrients for their growth.
The success of fungal infections is affected by environmental factors, particularly pH (Xu et al. 2015, Barda et al. 2020, Han et al. 2021, Jimdjio et al. 2021, Li et al. 2022, Yang et al. 2022). Recent findings indicated that extracellular or cytosolic acidification rapidly induce the highly conserved mitogen-activated protein kinase signaling cascades, which regulate various aspects of fungal pathogenicity and promote infection of host plant (Fernandes et al. 2023). Pathogenic fungi have the ability to manipulate the pH of the surrounding environment when infecting plants, creating a more favorable condition for their growth and reproduction (Kesten et al. 2019). Fungi employ different strategies to manipulate pH in their favor. Acidifying or alkalinizing pathogens decrease or increase the pH around their infection site, respectively (Prusky et al. 2016). While various fungi employ different strategies, the specific fungal response is also much dependent on carbon and nitrogen availability (Bi et al. 2016, Ziv et al. 2020, Van Laethem et al. 2021).
Acidifying fungi
These fungi acidify the colonized tissue by secreting organic acids (pH from 3.6 to 3.0), which subsequently facilitates colonization and pathogenicity (Bi et al. 2016). Oxalic acid, one of the most prevalent organic acids, plays a key role affecting pathogenicity of fungi while in parallel modulating plant host response (Palmieri et al. 2019). The best-studied example is of the broad host range pathogenic fungi Sclerotinia sclerotiorum and B. cinerea, which are known for their oxalic acid production as part of their pathogenic attack (van Kan 2006, Mbengue et al. 2016). In these fungi, oxalic acid serves as a major pH-dependent virulence factor acting through multiple pathways. As a strong acid, oxalate acidifies the environment to stimulate the production and activity of a spectrum of fungal secreted enzymes including pectinases, proteinases, and laccases. This arsenal of enzymes plays an important role in plant tissue maceration and degradation to facilitate fungal colonization and proliferation. In addition, due to its metal-chelating properties oxalate sequesters calcium ions that further weaken the plant cell wall facilitating fungal colonization. Furthermore, oxalate inhibits the host defense-process by reducing the oxidative burst in plant tissues and by directly triggering programmed plant cell death, which facilitates the necrotrophic infection. Altogether, oxalic acid has been shown to play a significant role in the pathogenicity of many fungi and bacteria. However, partial restoration of the virulence phenotype of Sclerotinia mutants that cannot produce oxalic acid, by exogenous acidification of host tissue, suggests that low pH and not a specific organic acid is required for pathogenicity (Xu et al. 2015). Thus, not only oxalic acid can modulate plant host pH, rather this effect can be achieved by other organic acids. As such, it was reported that B. cinerea relies also on the production of citric acid and succinic acid during colonization of sunflower (Mbengue et al. 2016), while other pathogens like Penicillium expansum acidify the colonized tissue by generating d-gluconic acid and citric acid that aid fungal colonization and pathogenicity (Barad et al. 2012). The same strategy has been reported also for Penicullum digitatum and Penicillum italicum (Costa et al. 2019, Kanashiro et al. 2020). Collectively, these reports indicate that production and secretion of organic acids for lowering plant host pH is an important virulence factor of these pathogens.
While plant pathogens can actively modify host tissue pH, their physiology and virulence is in turn much affect by the pH of the infected tissue (Eshel et al. 2002, Sánchez-Rangel et al. 2018, Barda et al. 2020, Li et al. 2022). However, altered pH can also modulate host physiology that subsequently activates its response to the pathogen. In line with that, fruit susceptibility to P. expansum, causing blue mold in apples, was reported to be significantly dependent on pH. Inoculated apple fruits at pH 2.5 and 8.5 improve resistance to P. expansum by modulating ROS metabolism, compared with pHs 5.0 and 7.0. Colonization and pathogenicity of P. expansum in apple fruit was delayed under acidic or basic conditions, possibly due to the activation of fruit antioxidant capacities, namely the upregulation of both the nonenzymatic antioxidants (glutathione and ascorbate), as well as antioxidant activities by various enzymes (such as catalase, superoxide dismutase, and peroxidase), that enhanced the efficient scavenging of ROS metabolites and protected the plant cell (Jimdjio Kouasseu et al. 2023).
Alkalizing fungi
Other pathogens like Colletotrichum spp., Alternaria alternate and Trichothecium roseum alkalize the host tissue to enhance their pathogenicity (Prusky et al. 2001, Eshel et al. 2002, Han et al. 2021). This modulation of host pH is achieved by releasing ammonia, which is produced as a result of protease activity and deamination of amino acids. For example, Colletotrichum gloeosporioides has been reported to turn l-glutamate or glutamine into ammonia and increases the pH of colonized fruit from 5.6 to 8.5 (Vylkova 2017). Other pathogens like the vascular wilt pathogen Fusarium oxysporum, raise the extracellular pH (from pH 5 to 7) by secreting a peptide with homology to plant rapid alkalinizing factors. This pH modulation suppresses host immunity, although its role in virulence is still debated. Nevertheless, rapid alkalinizing factors encoding genes are found in many fungal pathogens, suggesting the prevalence of this mechanism for alkalinizing infection sites and inhibiting host immunity (Rodriguez-Moreno et al. 2018, Sánchez-Rangel et al. 2018). Similarly, Fusarium sulphureum inoculation of muskmelon led to pH increasing of the tissue around the inoculation site, resulting in a weak alkaline environment that increased its pathogenicity by stimulating ROS metabolism (Yang et al. 2022). The pH modulation of host tissue described above is a common strategy employed by necrotrophic fungal pathogens to weaken the host defense, ultimately leading to rapid tissue death and enhancing pathogenicity. However, host tissue pH modulation is not limited to necrotrophic pathogens. In fact, apoplastic alkalinization of leaves during biotrophic interactions was also reported during powdery mildew (B. graminis f. sp. Hordei) infection of barley (Hordeum vulgare), however, this may be the plant response and not an active process by the pathogen (Felle et al. 2004).
Furthermore, the pH modulation of host tissues is not restricted to fungal pathogens but also occurs during interactions with bacterial pathogens. For example, Pseudomonas syringae, which causes lesions on bean leaves, induces apoplastic alkalinization upon infection (Geilfus et al. 2020). This alkalinization facilitates bacterial colonization and leaf lesions by increasing the availability of apoplastic sucrose to the pathogen. Interestingly, foliar application of a synthetic auxin or acidic pH buffer attenuated apoplastic alkalinization and by that reduced the number of colony forming units and area of bacterial lesions on bean leaves.
Because of the above, preharvest application of organic acids was reported to be effective in reducing fungal infections. Citric acid foliar application (30 mM) during Capsicum cultivation was shown to increase fruit epidermis and cuticle thickness, which was suggested to account for the reduced gray mold disease incidences observed on pepper fruits in cold storage (Mekawi et al. 2019). In addition, spraying pepper plants with citric acid increased peroxidase and polyphenol oxidase activities in the fruit that can further increase fruit natural resistance to fungal pathogens. Furthermore, the increased antioxidant capacity of plants treated with malic and oxalic acids as elicitors was accompanied by the accumulation of phenolic compounds with antifungal properties (El-Zaeddi et al. 2017) that can promote plant innate immunity.
While the preharvest application of organic acids to control plant pathogens is rarely implemented, postharvest application to disinfect fruits and vegetables and to extend fresh produce shelf life is well-established (Feliziani et al. 2016). Organic acid as disinfectants and sanitizers of fruits are considered safe and not harmful to humans or the environment (Visconti et al. 2021). As said, acetic, citric, malic, tartaric, and propionic acids are collectively allowed in the food industry as preservatives and are classified by the FDA as GRAS compounds. Similar results for postharvest fungal pathogens inhibition was reported for oxalic acid. These organic acids have been shown to directly modulate fungal physiology (e.g. inhibition of fungal respiration by acetic and malic acid or causing membrane damage and triggering intracellular ROS production by cinnamic acid) or by inducing host defense response and delaying senescence (e.g. inhibition of ripening and chlorophyll degradation by oxalic acid) (Feliziani et al. 2016).
The use of acetic, formic, and propionic acids vapors for the control of postharvest decay of fruit and vegetables is promising as minimal handling is required, thus it has been investigated for the last couple of decades. Encouraging results have been collected on apples, pears, tomato, kiwi, and table grapes, among others. However, care should be taken as high doses may be problematic causing phytotoxic responses (Feliziani et al. 2016, Zhang et al. 2023).
To meet the demand for high-quality, chemical-free fresh produce, nonchemical treatment technologies have gained significance. Among these alternatives, hot water treatments have emerged as promising strategies for postharvest disease control, replacing synthetic fungicides (Fallik et al. 2021). However, when used individually, these techniques may offer limited effectiveness in controlling postharvest diseases. Interestingly, integrating nonchemical approaches as hot water treatments with GRAS organic acid applications can yield reliable and more reproducible outcomes, improving disease control and extending fresh produce shelf life. This was demonstrated by combining hot water treatment and sorbic acid (in the form of potassium sorbate) that effectively controlled gray mold rot in kiwifruit (Ge et al. 2020). The combined treatment inhibited in vitro mycelial growth and eliminated spore germination of B. cinerea and was very effective to suppress lesion expansion caused by the pathogen. Another combined approach of mixing various organic acids with chitosan coating was shown to reduce fruit rot and to increase the shelf life of litchi, plum and grape fruits (Xu et al. 2021). The use of PAW (plasma activated water) is also gaining significant interest in recent years for postharvest treatments of fruits and vegetables (Rahman et al. 2022) and it was shown to be effective in inhibiting conidial germination of Colletotrichum spp. on avocado (Siddique et al. 2021). For more details on PAW and low pH see the section "Low pH as a key parameter in food preservation, processing, and protection: the impact of microbial acid stress responses on food safety, quality, and functionalization”.
A promising new approach in developing improved delivery of antifungal compounds is based on the pH-dependent release of activated compounds/fungicides from nano-capsule for higher antifungal effect and low biotoxicity (Shan et al. 2020, Liang et al. 2021, Xiao et al. 2021, Xu et al. 2021, Zhang et al. 2022a). The release of various fungicides has shown a pH sensitive response due to a weak acidic group in the structure, causing the rate of release to be faster under acid condition (< 7). As said above, at the early stage of infecting the host plant, acidifying fungi like B. cinerea produce and secret acidic substances, such as oxalic acid, which are conducive to acidification and trigger the rapid release of fungicidal compound, which penetrates the fungal pathogen at the onset of the pathogenic attack. The early contact of the fungicide with the pathogen results in a more effective killing, while the biotoxicity is reduced due to a more targeted release of the active compound. This suggested nanoparticles platform for smart control over fungal pathogens, holds a great potential to improve traditional chemical approach to control crop diseases in a more economic and ecological manner.
Collectively, the vast array of mechanisms for pH modulation during interactions between micro-organisms and host plants highlight the importance of pH in shaping the outcome of pathogenic process, and holds promise for its harnessing to improved antifungal applications. Thus, understanding and manipulating pH modulation by fungal and bacterial pathogens can contribute to the development of effective strategies for disease management in agriculture.
Low pH and organic acids in the fight against a silent pandemic
The worrying scenario in front of us is that another pandemic (by some called “silent pandemic”) (Mahoney et al. 2021) may arise because of the lack of antimicrobials to fight the fast spread on our planet of antimicrobial resistance (AMR) in bacterial pathogens (Laxminarayan 2022). This affects not only humans, but also our pets and feedstock. In 2019, six pathogens were to a similar extent responsible for close to 1 million human deaths attributable to AMR, a figure very similar to the sum of HIV deaths and malaria deaths, globally. These pathogens are E. coli, Klebsiella pneumoniae, Staphylococcus aureus, Acinetobacter baumannii, Staphylococcus pneumoniae, and Pseudomonas aeruginosa. All together in 2019 close to 5 million deaths were calculated to be attributable to or associated with AMR (Antimicrobial Resistance 2022). We cannot keep neglecting the problem, which is even exacerbated in low and middle income countries and, in general, affects poor individuals who cannot afford second-line antibiotics that are more expensive than the first-line ones (Laxminarayan 2022).
There are many ways to combat the spread of AMR. The first is to limit the use of antibiotics only to those pathological conditions that specifically require them, something that was even more worryingly neglected during the COVID-19 pandemic, in high-income as well as in low- and middle-income countries (Amarsy et al. 2022, Rizvi and Ahammad 2022, Gul et al. 2023). The second approach is to develop new strategies to combat infectious diseases by applying good practices in the healthcare settings where the risk of becoming infected by a pathogen increases significantly (Frost et al. 2018). The third approach is to develop new strategies by combating the formation of persisters, that are transiently tolerant variants intrinsically resistant to antibiotics. Persister formation makes pathogens impossible to eradicate by antibiotic treatment and on the contrary increases their chance of acquiring antibiotic resistance genes (Van den Bergh et al. 2017), and is hence considered as one of the routes by which AMR can originate (Balaban et al. 2019). Finally, expanding our arsenal with new molecules or improving the efficacy of those already available can potentially combat AMR by hitting different targets or physiological processes in bacteria (Blaskovich et al. 2017). Avenues for developing novel therapeutics and diagnostics have to take into account current (and future) knowledge on how bacteria evade antibiotics and eventually develop resistance to them. In this respect, the bacterial stress responses (including those to low pH) can suggest therapeutic strategies and alternatives to antibiotics as well as possible new targets for novel antibiotics (Dawan and Ahn 2022).
As for the latter two approaches, low pH plays indeed an important role as summarized in Fig. 3.
Figure 3.
Combating AMR by exploiting the properties of acidic compounds and the effect of low pH on microbes. Created with BioRender.com.
Persisters formation
An increased frequency in the formation of persisters (a clonal subpopulation of cells with a “dormant” phenotype highly tolerant to antibiotics and other stresses) has been associated with an acidification of the intracellular pH (i.e. deviation from neutrality) and to the activity of some acid resistance genes (Hong et al. 2012, Van den Bergh et al. 2016, 2022, Goode et al. 2021). In pathogenic E. coli, complex I of the respiratory chain has been found to be a target for spontaneous mutations associated with the formation of persisters, following evolution experiments where pathogenic E. coli UTI89 was cyclically exposed to the antibiotic amikacin (Van den Bergh et al. 2022). The mutants that displayed the highest persister frequency were found to be those with Complex I impaired in its proton translocation activity, which lead to significant cytoplasmic acidification. The studies showed that intracellular acidification induces the persistent state in two ways: through the activation of the regulon of RpoS (the sigma factor of stationary phase RNA polymerase) and, in the case of more significant cytoplasmic acidification, through the arrest of protein synthesis (Van den Bergh et al. 2022). The same authors also provided preliminary evidence that gadC, coding for the glutamate/glutamine-GABA antiporter, an essential structural component of the major acid resistance system in many bacteria, i.e. the AR2(_Q) system (Lund et al. 2014, Pennacchietti et al. 2018), is also a target for mutations that lead to an increased frequency of persisters. Goode et al. (2021) demonstrated that in E. coli persisters have an intrinsically lower intracellular pH than susceptible cells and that this pH does not decrease further during ampicillin treatment. This observation was linked to the activity of tryptophanase (encoded by the gene tnaA) in a growth phase-dependent manner: the loss of production of indole (a well-known signaling molecule) in a ΔtnaA mutant increased ampicillin persisters in stationary phase but not in exponential phase.
Heterogeneity in a population can be the basis for the formation of a clonal subpopulation that has a fitness advantage due to its ability to survive a stress. The connection between intracellular acidification and persisters formation may be part of this bacterial strategy as well as that of activating the acid resistance genes in a bet-hedging perspective of improving fitness (Van Riet et al. 2022, Wang et al. 2023a). Though still at the level of understanding fundamental mechanisms, the cited studies provide a very strong connection between intracellular acidification (with respect to physiological pH) and antibiotic resistance, and the knowledge may pave the way to novel antimicrobial strategies that interfere with persister formation mechanism, one of the routes to AMR (Van den Bergh et al. 2017).
An interesting and different case is that of trimethoprim, the folate biosynthesis inhibitor, which has been shown to activate an acid stress response in E. coli (with increased gadBC expression, the main structural genes of the GAD system) likely prompted by adenine depletion (Mitosch et al. 2017). As observed also by others using gentamycin (Ketcham et al. 2022), the intracellular acidification following antibiotic treatment seem to be a common causal factor in bacterial cell death caused by antibiotics (Goode et al. 2021). In support of this, genetic manipulations that increase intracellular pH also increase resistance (Ketcham et al. 2022). It has not yet fully clarified why intracellular acidification is associated with death in susceptible cells, but not in persisters: this is an interesting open question that highlights the importance of intracellular pH acidification as a parameter in determining antibiotic effectiveness.
Low pH and the efficacy of natural organic acids as antimicrobials
There are already many antimicrobial treatments whose effectiveness is likely to arise from the increased sensitivity of micro-organisms to some chemicals at low pH, and ongoing studies reported in the literature suggest that these have the potential to increase in the future. These range from cases where the treatment is itself an acid, to those where a treatment is pH sensitive and more effective at low pH. Detailed mechanisms of the higher effectiveness at lower pH have often not been fully elucidated.
In the case of bacteria, several anti-infection treatments used as traditional remedies for hundreds or even thousands of years are likely to have low pH as an important component. The most obvious of these is vinegar, whose use against infection was recorded by Hippocrates (Johnston and Gaas 2006). Acetic acid (along with other organic acids, notably citric) is used clinically as a topical agent to prevent or treat infection in wounds, though its use is not widespread, and has significant biocide and antibiofilm effect against pathogens from burns wounds. It also turns out to be effective for decontaminations in hospital settings (Sloss et al. 1993, Nagoba et al. 2012, 2013, Bjarnsholt et al. 2015, Halstead et al. 2015, Stjärne Aspelund et al. 2016, Agrawal et al. 2017). The mechanisms for this have been intensively studied, not least because of the importance of weak organic acids (acetic, sorbic, citric, and benzoic) as food preservatives for prevention of microbial growth (Ng et al. 2023), as previously presented in the section “Low pH as a key parameter in food preservation, processing, and protection: the impact of microbial acid stress responses on food safety, quality, and functionalization”.
Another example is honey, widely used in historical times as an anti-infective. When applied to wounds, honey acidity triggers molecular oxygen release from hemoglobin, which makes the wound environment less favorable for destructive proteases; furthermore, honey high osmolarity extracts fluid out of the wound bed thereby promoting an outflow of lymph, just as occurs when a negative pressure wound therapy is applied (Molan and Rhodes 2015). Acidity is only one component in a complex mixture, but a recent study indicates that synergy between gluconic acid (the main acid in honey) and hydrogen peroxide is of particular significance in honey antibacterial effect. These two components synergize in causing depolarization of cell membrane and cell wall destruction, that eventually halted bacterial growth (Masoura et al. 2020).
The re-emerging interest in natural products, including the above mentioned examples, clearly offer an advantage to researchers, who can take advantage of the “logic of nature” (i.e. optimized by evolution, as it was for penicillin discovery by Fleming) to exploit the immense arsenal of biologically active molecules, including antibiotics (Bachmann et al. 2014).
A class of interesting natural products are phosphonic compounds (Ju et al. 2015, Kayrouz et al. 2020, Shiraishi and Kuzuyama 2021) and phosphinic compounds (De Biase et al. 2020), though the latter occur less frequently in nature. These molecules act by mimicking phosphoric and carboxylic groups (both acidic) that are present in biological molecules. They have the potential to inhibit novel targets of metabolic enzymes and can be used as lead compounds for the development of a variety of drugs. Fosfomycin is a well-known phosphonate, widely used as an antibacterial to treat urinary tract infections, but also frequently used in combination with other antibiotics, with which it acts synergistically, thus effectively reducing the risk of developing AMR (Antonello et al. 2020). A recent and intriguing example includes a study on multidrug resistant K. pneumoniae strains (i.e. resistant to > 10 different antibiotics), isolated from patients, that were inhibited by peptides derived from phosphinothricin (Demiankova et al. 2023). This work also highlights that “repositioning” of some drugs can be an effective strategy.
The pH at which the treatment is done is also a key parameter to be considered when applying treatments. For example, silver has been used as an antibacterial treatment since historical times, but its effectiveness in the form of an alginate gel against a range of pathogens, including those with high antibiotic resistance and in biofilms, is enhanced at pH 5.5 compared to pH 7 (Percival et al. 2014). Further examples of weak organic acids with clinical use and whose antibacterial efficacy has been shown to increase at low pH include the antibiotic fusidic acid, an important alternative treatment in combination therapies against S. aureus infections (Lemaire et al. 2011), and azelaic acid, used as a topical treatment for dermatoses, particularly acne. The pH-dependent mechanism of antibacterial action in the latter case has been carefully investigated, and is associated with dissipation of the trans-membrane pH gradient (Bojar et al. 1994). In general, human skin is slightly acidic, however, wounds cause an alkalinization over time: thus a multifactorial approach toward controlling biofilm should take into account both wound pH and use of appropriate antibacterial agents (together with a knowledge of their dependence on pH for activity). This is an area ripe for further careful study (Wiegand et al. 2015).
As mentioned above and stressed in the literature, the pKa of an organic acid is important (Lund et al. 2020) when it comes to preventing bacterial growth either in food (Ng et al. 2023) or in treating bacterial infections. As for the latter case, some examples were already provided in this section, but many more can be brought to the reader’s attention in areas other than wounds and skins infections. For example, the activity of the prodrug pyrazinamide, an important component of combination therapies used for treating tuberculosis, which is active against persister cells, is also heightened at low pH. This is thought to be because an enzyme in Mycobacterium tuberculosis converts it to pyrazinoic acid, which leaves the cell as a charged molecule but, when the external milieu is acidic, pyrazinoic acid becomes protonated and can freely diffuse back into the cell, where it is thought to act by inhibiting the essential enzyme PanD, required for pantothenate biosynthesis in this organism (Zhang et al. 2014b, Sun et al. 2020b).
There are other reasons for investigating the effects of pH on antibacterial agents, in addition to cases where antibacterial agents are weak acids and hence likely to show a strong pH-dependence in their effects. For example, if the agents are more active at a low pH, tests done at neutral pH will not reflect biological reality and could lead to useful activities being missed. An example of this is the fluoroquinolone antibiotic finafloxacin, which shows higher activity under mild acidic conditions (pH 5.0–6.0), and which may hence be more indicated for use in environments such as skin, respiratory epithelia, and the urinary tract (Stubbings et al. 2011) that are themselves mildly acidic. Another microenvironment in which pH can be acidic is inside some biofilms, typically poorly penetrated by antibiotics, because of their nature and size. For example, another flouroquinolone antibiotic, delafloxacin, a weak acid with a pKa of ∼5.6, in its uncharged species can penetrate the biofilm and this correlates with the acidic biofilm microenvironment (Siala et al. 2014). It is important here to recall that an extracellular low pH is a general trigger to promote biofilm formation, but also the temperature as well as other factors play a role (Toyofuku et al. 2016, Mathlouthi et al. 2018, Lin et al. 2021).
A final example where pH has clinical microbiology relevance is in treatment of uropathogens, as the pH of urine varies with different clinical conditions and is also clinically malleable. A comprehensive study of 24 different antibiotics and their effects on six different uropathogenic species showed that several of them had an increased activity at pH < 6, including tetracycline derivatives, nitrofurantoin, and several β-lactams (Yang et al. 2014). In a preventive approach, the diet can affect the acidity of urine pH thereby exerting protective effects against recurrent urinary tract infections (Chavez et al. 2021). The study by Yang et al. (2014) also showed a different efficacy of antibiotics (increased, decreased, and unaffected) depending on pH, and this further supports the notion that a very sensible way to increase antibiotic efficacy without the need of too high doses (which promote AMR) is to always consider which is the best pH at which the treatment can be applied or the environment in which the treatment is applied (skin, wound, biofilm, bladder, and so on) (Yang et al. 2014).
Conclusions
In this review, we highlighted the importance of the exploitation of the knowledge on the responses and activity at low pH of micro-organisms, both neutralophilic and acidophilic, for the development of effective strategies for disease management in agriculture, implementation of bioprocesses and biotechnological applications in waste valorization, sustainable, and natural preservation of foods and their functionalization.
Figure 4 shows the network analysis based on the literature (titles and abstracts) used in this review visualized with the software VOS (van Eck and Waltman 2010). Using the parameters provided in the legend to Fig. 4, the analysis generated five clusters of terms: the blue cluster includes mostly food-related terms; the green cluster includes terms related to bioproduction of platform chemicals and very close to this cluster there is the yellow cluster which includes waste valorization for biogas production and circular economy; the red cluster includes terms that are more related to crop protection and remediation of polluted environments; finally, the violet cluster includes terms of medical interest. The proximity of “lactic acid” “yield” and “productivity” with “waste,” “value,” and “biogas production” highlights the dedication to the circular economy concept and the consideration of the financial and efficiency aspects. This aspect will need to be acknowledged in future developments of highly productive micro-organisms, efficient in the presence of stressors, including acidic pH. Interestingly “lactic acid bacteria” (LAB) are closely connected to “food” in the blue cluster, however, “lactic acid”, though linked to LAB, belongs to another cluster: this highlights the transition of LAB from only food-relevant micro-organisms to producers of a valuable platform chemical. This offers an elegant model for expanding the use of organic acids microbial producers to the large-scale bioproduction of platform chemicals.
Figure 4.
Occurrence and links of terms found in abstracts and titles of the scientific and review articles cited in this review. The Network is generated using VOSviewer (version 1.6.19) (van Eck and Waltman 2010). Criteria for map generation included 15 minimum occurrences of a term. For network visualization, default settings were used for normalization and layout, except for clustering for which a minimum of six terms was set to generate a cluster. A total of 105 terms were extracted, of which the most relevant terms (60% of total, as set by default) were visualized after manually removing 12 terms (mutant, host, water, response, ability, glucose, paper, factor, microbe, cell, bacterium, and mechanism) that though occurring frequently were much less specific than other terms which could be better visible in the network. A thesaurus file was generated and used to count some terms as the same term (e.g. PAW as plasma activated water; LAB as lactic acid bacteria; and volatile fatty acids as VFA).
As discussed, engineering of highly productive micro-organisms and improvement in process engineering could decrease the environmental footprint of processes, maximizing substrate valorization and minimizing side stream generation. Huge advancements were already made for itaconic acid production and this path is expected to be followed in future for other biobased organic acids. The high-value applications of biodegradable polymers from platform chemicals was the driving force for innovations in fermentations and development of strains for lactic acid and polylactides. A similar trend could be expected in future for others platform chemicals, in particular for biobased production of succinic acid.
In particular the knowledge gathered on the mechanisms of microbial response to acid stress can be applied to the development of more robust acid tolerant industrial strains either through engineering at the genome-scale level to improve the acid stress tolerance or through metabolic engineering by overexpressing acid-resistance elements detected by systems biology approaches (Guan et al. 2016, Deparis et al. 2017).
In recognition of the need for wider application and exploitation of microbial products as probiotics, synbiotics, and so on, a new category of live biotherapeutic products was recently introduced in European and earlier in US legislation (Cordaillat-Simmons et al. 2020). This will hopefully pave the way to innovations and applications for next-generation probiotics and help exploit the potential of microorganism for the benefit of all. As showed in this review, low pH is a key parameter to best exploit the beneficial effect of these micro-organisms.
As we have explained in this review, microbes are challenged by acidic conditions during bioprocesses and these conditions hinder product yields in many bioreactors and in open fermentations. Thus, identifying or improving micro-organisms that can tolerate acidic conditions, coculturing different species and strains, and/or isolating and characterizing acid-tolerant enzymes for revalorizing specific waste material will surely have a positive impact to tackle the current limitation of product development under acidic conditions (Awasthi et al. 2018, Li et al. 2018, Ijoma et al. 2021). Furthermore, the production of specific bioactive compounds on food wastes may be anticipated through artificial neural network modeling (Sabater et al. 2020).
As for biogas production, the microalgal cultivation on DF effluents is a solution intensively studied in recent years (Lacroux et al. 2023). In general, the role of artificial intelligence can indeed be of help for such complex contexts: a machine learning approach, including artificial neural networks to predict biogas production in a biogas plant, was recently employed (Frankowski et al. 2020, Hosseinzadeh-Bandbafha et al. 2022) and the use of nanotechnological solutions to improve biohydrogen production was also proposed (Bosu and Rajamohan 2022, Cao et al. 2022, Vadalà et al. 2023).
As a large community of scientiists working in the areas of research discussed in this review, we believe that interdisciplinary research will surely make possible to develop stronger links between areas of research where scientists communicate less; this is particularly important in medicine, which typically is less connected with the others. Nowadays, the paradigm micro-organism = pathogen has been revolutionized and microbes are now regarded as important (if not fundamental) allies to promote our health as well as that of our planet. Literature, mostly on fundamental knowledge, and network expertise can also be accessed via a dedicated website (https://euromicroph.eu/).
Acknowledgments
All the authors are grateful to the COST (European Cooperation in Science and Technology) for supporting the activities of the Action CA18113 (EuroMicropH) from April 2019 to October 2023.
Contributor Information
Merve Atasoy, UNLOCK, Wageningen University & Research and Technical University Delft, Droevendaalsesteeg 4, 6708 PB,Wageningen, the Netherlands.
Avelino Álvarez Ordóñez, Department of Food Hygiene and Technology and Institute of Food Science and Technology, Universidad de León, Campus de Vegazana s/n, 24071 León, Spain.
Adam Cenian, Institute of Fluid Flow Machinery, Polish Academy of Sciences, Department of Physical Aspects of Ecoenergy, 14 Fiszera St., 80-231 Gdańsk, Poland.
Aleksandra Djukić-Vuković, Department of Biochemical Engineering and Biotechnology, Faculty of Technology and Metallurgy, University of Belgrade, Karnegijeva 4, 11120 Belgrade, Serbia.
Peter A Lund, Institute of Microbiology and Infection, School of Biosciences, University of Birmingham, Birmingham B15 2TT, United Kingdom.
Fatih Ozogul, Department of Seafood Processing and Technology, Faculty of Fisheries, Cukurova University, Balcali, 01330, Adana, Turkey; Biotechnology Research and Application Center, Cukurova University, Balcali, 01330 Adana, Turkey.
Janja Trček, Department of Biology, Faculty of Natural Sciences and Mathematics, University of Maribor, Koroška cesta 160, 2000 Maribor, Slovenia.
Carmit Ziv, Department of Postharvest Science, Agricultural Research Organization – Volcani Center, 68 HaMaccabim Road , P.O.B 15159 Rishon LeZion 7505101, Israel.
Daniela De Biase, Department of Medico-Surgical Sciences and Biotechnologies, Sapienza University of Rome, Corso della Repubblica 79, 04100 Latina, Italy.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
This work was supported by EuroMicropH-COST Action CA18113. This work was also partially supported by Sapienza University of Rome (Progetti Medi di Ateneo n. RM11916B861B9985 and RM120172B6587496 to D.D.B.).
References
- Abdel-Rahman MA, Tashiro Y, Sonomoto K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv. 2013;31:877–902. [DOI] [PubMed] [Google Scholar]
- Adetunji CO, Anani OA. Bioaugmentation: a powerful biotechnological techniques for sustainable ecorestoration of soil and groundwater contaminants. In: Microbial Rejuvenation of Polluted Environment. Berlin: Springer, 2021, 373–98. 10.1007/978-981-15-7447-4_15. [DOI] [Google Scholar]
- Agrawal KS, Sarda AV, Shrotriya Ret al. Acetic acid dressings: finding the Holy Grail for infected wound management. Ind J Plast Surg. 2017;50:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemahdi N, Che Man H, Abd Rahman NAet al. Enhanced mesophilic bio-hydrogen production of raw rice straw and activated sewage sludge by co-digestion. Int J Hydrog Energy. 2015;40:16033–44. [Google Scholar]
- Alexandri M, Blanco-Catalá J, Schneider Ret al. High L(+)-lactic acid productivity in continuous fermentations using bakery waste and lucerne green juice as renewable substrates. Bioresour Technol. 2020;316:123949. [DOI] [PubMed] [Google Scholar]
- Alexandropoulou M, Antonopoulou G, Trably Eet al. Continuous biohydrogen production from a food industry waste: influence of operational parameters and microbial community analysis. J Clean Prod. 2018;174:1054–63. [Google Scholar]
- Alonso S, Rendueles M, Díaz M. Microbial production of specialty organic acids from renewable and waste materials. Crit Rev Biotechnol. 2015;35:497–513. [DOI] [PubMed] [Google Scholar]
- Amarsy R, Trystram D, Cambau Eet al. Surging bloodstream infections and antimicrobial resistance during the first wave of COVID-19: a study in a large multihospital institution in the Paris region. Int J Infect Dis. 2022;114:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Xu Y, Dai X. Biohythane production from two-stage anaerobic digestion of food waste: a review. J Environ Sci. 2024;139:334–49. [DOI] [PubMed] [Google Scholar]
- Anal A. Quality ingredients and safety concerns for traditional fermented foods and beverages from Asia: a review. Fermentation. 2019;5:8. [Google Scholar]
- Anekwe IMS, Isa YM. Bioremediation of acid mine drainage – review. Alexandria Eng J. 2023;65:1047–75. [Google Scholar]
- Anekwe IMS, Isa YM. Bioremediation of acid mine drainage contaminated soils using bioattenuation, wastewater and air-injection system. Biorem J. 2022;6:1047–75. https://doi.org/10.1080/10889868.2022.2130873: 1-19. [Google Scholar]
- Anh HTH, Shahsavari E, Bott NJet al. Bioaugmentation of seafood processing wastewater enhances the removal of inorganic nitrogen and chemical oxygen demand. Aquaculture. 2021;542:736818. [Google Scholar]
- Antimicrobial Resistance Collaborators . Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonello RM, Principe L, Maraolo AEet al. Fosfomycin as partner drug for systemic infection management. A systematic review of its synergistic properties from in vitro and in vivo studies. Antibiotics. 2020;9:500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anupama, Ravindra P. Value-added food. Biotechnol Adv. 2000;18:459–79. [DOI] [PubMed] [Google Scholar]
- Ashaolu T, Reale A. A holistic review on Euro-Asian lactic acid bacteria fermented cereals and vegetables. Microorganisms. 2020;8:1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asunis F, De Gioannis G, Isipato Met al. Control of fermentation duration and pH to orient biochemicals and biofuels production from cheese whey. Bioresour Technol. 2019;289:121722. [DOI] [PubMed] [Google Scholar]
- Atasoy M, Cetecioglu Z. The effects of pH on the production of volatile fatty acids and microbial dynamics in long-term reactor operation. J Environ Manage. 2022;319:115700. [DOI] [PubMed] [Google Scholar]
- Awasthi MK, Wong JWC, Kumar Set al. Biodegradation of food waste using microbial cultures producing thermostable α-amylase and cellulase under different pH and temperature. Bioresour Technol. 2018;248:160–70. [DOI] [PubMed] [Google Scholar]
- Ayangbenro AS, Olanrewaju OS, Babalola OO. Sulfate-reducing bacteria as an effective tool for sustainable acid mine bioremediation. Front Microbiol. 2018;9:1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann BO, Van Lanen SG, Baltz RH. Microbial genome mining for accelerated natural products discovery: is a renaissance in the making?. J Ind Microbiol Biotechnol. 2014;41:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Helaine S, Lewis Ket al. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol. 2019;17:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baporikar N. Handbook of Research on Entrepreneurship Development and Opportunities in Circular Economy: Hershey: IGI Global, 2020. [Google Scholar]
- Barad S, Horowitz SB, Moscovitz Oet al. A Penicillium expansum glucose oxidase–encoding gene, GOX2, is essential for gluconic acid production and acidification during colonization of deciduous fruit. Mol Plant Microbe Interac. 2012;25:779–88. [DOI] [PubMed] [Google Scholar]
- Barda O, Maor U, Sadhasivam Set al. The pH-responsive transcription factor PacC governs pathogenicity and ochratoxin A biosynthesis in Aspergillus carbonarius. Front Microbiol. 2020;11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker J, Hosseinpour Tehrani H, Ernst Pet al. An optimized Ustilago maydis for itaconic acid production at maximal theoretical yield. J Fungi. 2020;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera BC, Mishra R, Mohapatra S. Microbial citric acid: production, properties, application, and future perspectives. Food Front. 2021;2:62–76. [Google Scholar]
- Bi F, Barad S, Ment Det al. Carbon regulation of environmental pH by secreted small molecules that modulate pathogenicity in phytopathogenic fungi. Mol Plant Pathol. 2016;17:1178–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIOHAZ . Scientific Opinion on the evaluation of the safety and efficacy of lactic acid for the removal of microbial surface contamination of beef carcasses, cuts and trimmings. EFSA J. 2011;9. 10.2903/j.efsa.2011.2317. [DOI] [Google Scholar]
- Bjarnsholt T, Alhede M, Jensen PØet al. Antibiofilm properties of acetic acid. Adv Wound Care. 2015;4:363–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskovich Mark AT, Butler Mark S, Cooper Matthew A. Polishing the tarnished silver bullet: the quest for new antibiotics. Essays Biochem. 2017;61:103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojar RA, Cunliffe WJ, Holland KT. Disruption of the transmembrane pH gradient—a possible mechanism for the antibacterial action of azelaic acid in Propionibucterium acnesand Staphylococcus epidermidis. J Antimicrob Chemother. 1994;34:321–30. [DOI] [PubMed] [Google Scholar]
- Börner RA, Kandasamy V, Axelsen AMet al. Genome editing of lactic acid bacteria: opportunities for food, feed, pharma and biotech. FEMS Microbiol Lett. 2019;366:fny291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosu S, Rajamohan N. Nanotechnology approach for enhancement in biohydrogen production- review on applications of nanocatalyst and life cycle assessment. Fuel. 2022;323:124351. [Google Scholar]
- Bozell JJ, Petersen GR. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy's “Top 10” revisited. Green Chem. 2010;12:539. [Google Scholar]
- Breznak JA, Costilow RN. Physicochemical factors in growth. In: Methods for General and Molecular Microbiology, Washington: ASM Press, 2014, 309–29. 10.1128/9781555817497.ch14. [DOI] [Google Scholar]
- Burgé G, Saulou-Bérion C, Moussa Met al. Diversity of Lactobacillus reuteri strains in converting glycerol into 3-hydroxypropionic acid. Appl Biochem Biotechnol. 2015b;177:923–39. [DOI] [PubMed] [Google Scholar]
- Burgé G, Saulou-Bérion C, Moussa Met al. Relationships between the use of Embden Meyerhof pathway (EMP) or Phosphoketolase pathway (PKP) and lactate production capabilities of diverse Lactobacillus reuteri strains. J Microbiol. 2015a;53:702–10. [DOI] [PubMed] [Google Scholar]
- Butler E, Hung Y-T. Bioaugmentation for Water Resources protection. In: Advances in Water Resources Management, Cham: Springer International Publishing, 2016, 339–401. 10.1007/978-3-319-22924-9_5. [DOI] [Google Scholar]
- Buyukkileci AO, Lahore MF, Tari C. Utilization of orange peel, a food industrial waste, in the production of exo-polygalacturonase by pellet forming Aspergillus sojae. Bioprocess Biosyst Eng. 2015;38:749–60. [DOI] [PubMed] [Google Scholar]
- Calero P, Nikel PI. Chasing bacterial chassisfor metabolic engineering: a perspective review from classical to non-traditional microorganisms. Microb Biotechnol. 2019;12:98–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candry P, Radic L, Favere Jet al. Mildly acidic pH selects for chain elongation to caproic acid over alternative pathways during lactic acid fermentation. Water Res. 2020;186:116396. [DOI] [PubMed] [Google Scholar]
- Cao X, Zhao L, Dong Wet al. Revealing the mechanisms of alkali-based magnetic nanosheets enhanced hydrogen production from dark fermentation: comparison between mesophilic and thermophilic conditions. Bioresour Technol. 2022;343:126141. [DOI] [PubMed] [Google Scholar]
- Castro-Aguirre E, Iñiguez-Franco F, Samsudin Het al. Poly(lactic acid)—mass production, processing, industrial applications, and end of life. Adv Drug Deliv Rev. 2016;107:333–66. [DOI] [PubMed] [Google Scholar]
- Cetecioglu Z, Atasoy M, Cenian Aet al. Bio-based processes for material and energy production from waste streams under acidic conditions. Fermentation. 2022;8:115. [Google Scholar]
- Chan LG, Cohen JL, Ozturk Get al. Bioconversion of cheese whey permeate into fungal oil by Mucor circinelloides. J Biol Eng. 2018;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Li J-Z, Liu F. Evaluation of different pretreatment methods for preparing hydrogen-producing seed inocula from waste activated sludge. Renew Energy. 2011;36:1517–22. [Google Scholar]
- Chang YH, Jeong CH, Cheng WNet al. Quality characteristics of yogurts fermented with short-chain fatty acid-producing probiotics and their effects on mucin production and probiotic adhesion onto human colon epithelial cells. J Dairy Sci. 2021;104:7415–25. [DOI] [PubMed] [Google Scholar]
- Chavez JA, Chavez JM, Kuprasertkul Aet al. Prospective evaluation of daily and weekly urine pH variations along with diet intake in postmenopausal women with recurrent urinary tract infections. Female Pelvic Med Reconstr Surg. 2021;27:e352–e9. [DOI] [PubMed] [Google Scholar]
- Chen S, Zhang J, Wang X. Effects of alkalinity sources on the stability of anaerobic digestion from food waste. Waste Manage Res J Sustain Circ Econ. 2015;33:1033–40. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nielsen J. Biobased organic acids production by metabolically engineered microorganisms. Curr Opin Biotechnol. 2016;37:165–72. [DOI] [PubMed] [Google Scholar]
- Cheng Y-T, Yang C-F. Using strain Rhodotorula mucilaginosa to produce carotenoids using food wastes. J Taiwan Inst Chem Eng. 2016;61:270–5. [Google Scholar]
- Chiocchio R. Continuous sugar beet pulp pretreatment and bioconversion in a biorefinery context. Ph.D. Thesis. Department of Biochemical Engineering, University College London, 2021, 1–322. [Google Scholar]
- Cieciura-Włoch W, Borowski S, Domański J. Dark fermentative hydrogen production from hydrolyzed sugar beet pulp improved by iron addition. Bioresour Technol. 2020;314:123713. [DOI] [PubMed] [Google Scholar]
- Coban HB. Organic acids as antimicrobial food agents: applications and microbial productions. Bioprocess Biosyst Eng. 2020;43:569–91. [DOI] [PubMed] [Google Scholar]
- Cordaillat-Simmons M, Rouanet A, Pot B. Live biotherapeutic products: the importance of a defined regulatory framework. Exp Mol Med. 2020;52:1397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JH, Bazioli JM, de Moraes Pontes JGet al. Penicillium digitatum infection mechanisms in citrus: what do we know so far?. Fung Biol. 2019;123:584–93. [DOI] [PubMed] [Google Scholar]
- Cubas-Cano E, González-Fernández C, Tomás-Pejó E. Evolutionary engineering of Lactobacillus pentosus improves lactic acid productivity from xylose-rich media at low pH. Bioresour Technol. 2019;288:121540. [DOI] [PubMed] [Google Scholar]
- Cunningham M, Azcarate-Peril MA, Barnard Aet al. Shaping the future of probiotics and prebiotics. Trends Microbiol. 2021;29:667–85. [DOI] [PubMed] [Google Scholar]
- Dai C, Huang X, Lv Ret al. Analysis of volatile compounds of Tremella aurantialba fermentation viaelectronic nose and HS-SPME-GC-MS. J Food Saf. 2018;38:e12555. [Google Scholar]
- Dauptain K, Trably E, Santa-Catalina Get al. Role of indigenous bacteria in dark fermentation of organic substrates. Bioresour Technol. 2020;313:123665. [DOI] [PubMed] [Google Scholar]
- Dawan J, Ahn J. Bacterial stress responses as potential targets in overcoming antibiotic resistance. Microorganisms. 2022;10:1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase D, Cappadocio F, Pennacchietti Eet al. Enzymatic kinetic resolution of desmethylphosphinothricin indicates that phosphinic group is a bioisostere of carboxyl group. Commun Chem. 2020;3:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase D, Lund PA. The Escherichia coli acid stress response and its significance for pathogenesis. Adv Appl Microbiol. 2015;92:49–88. 10.1016/bs.aambs.2015.03.002. [DOI] [PubMed] [Google Scholar]
- De Biase D, Pennacchietti E. Glutamate decarboxylase-dependent acid resistance in orally acquired bacteria: function, distribution and biomedical implications of the gadBC operon. Mol Microbiol. 2012;86:770–86. [DOI] [PubMed] [Google Scholar]
- De Filippis F, Pasolli E, Ercolini D. The food-gut axis: lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol Rev. 2020;44:454–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R, Van Kan JA, Pretorius ZAet al. The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13:414–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Campo I, Alegría I, Zazpe Met al. Diluted acid hydrolysis pretreatment of agri-food wastes for bioethanol production. Indus Crops Prod. 2006;24:214–21. [Google Scholar]
- Demiankova MV, Giovannercole F, Khomutov MAet al. Antibacterial activity of peptide derivatives of phosphinothricin against multidrug-resistant Klebsiella pneumoniae. Molecules. 2023;28:1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deparis Q, Claes A, Foulquié-Moreno MRet al. Engineering tolerance to industrially relevant stress factors in yeast cell factories. FEMS Yeast Res. 2017;17:fox036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi A, Fiore AG, De Pilli Tet al. A review on acidifying treatments for vegetable canned food. Crit Rev Food Sci Nutr. 2011;51:955–64. [DOI] [PubMed] [Google Scholar]
- Despoudi S, Sivarajah U, Dora M. From Linear to Circular Food Supply Chains: Achieving Sustainable Change. New York: Springer International Publishing, 2021. [Google Scholar]
- Dessì P, Lakaniemi A-M, Lens PNL. Biohydrogen production from xylose by fresh and digested activated sludge at 37, 55 and 70°C. Water Res. 2017;115:120–9. [DOI] [PubMed] [Google Scholar]
- Diez-Gutiérrez L, San Vicente L, Barrón LJRet al. Gamma-aminobutyric acid and probiotics: multiple health benefits and their future in the global functional food and nutraceuticals market. J Funct Foods. 2020;64:103669. [Google Scholar]
- Djukić-Vuković A, Mladenović D, Ivanović Jet al. Towards sustainability of lactic acid and poly-lactic acid polymers production. Renew Sustain Energy Rev. 2019;108:238–52. [Google Scholar]
- Djukić-Vuković AP, Mojović LV, Vukašinović-Sekulić MSet al. Effect of different fermentation parameters on l-lactic acid production from liquid distillery stillage. Food Chem. 2012;134:1038–43. [DOI] [PubMed] [Google Scholar]
- Dybas MJ, Hyndman DW, Heine Ret al. Development, operation, and long-term performance of a full-scale biocurtain utilizing bioaugmentation. Environ Sci Technol. 2002;36:3635–44. [DOI] [PubMed] [Google Scholar]
- El-Sheekh MM, Ismail A-m, El-Abd MAet al. Effective technological pectinases by Aspergillus carneus NRC1 utilizing the Egyptian orange juice industry scraps. Int Biodeterior Biodegrad. 2009;63:12–8. [Google Scholar]
- El-Zaeddi H, Calín-Sánchez Á, Nowicka Pet al. Preharvest treatments with malic, oxalic, and acetylsalicylic acids affect the phenolic composition and antioxidant capacity of coriander, dill and parsley. Food Chem. 2017;226:179–86. [DOI] [PubMed] [Google Scholar]
- Eshel D, Miyara I, Ailing Tet al. pH regulates endoglucanase expression and virulence of Alternaria alternatain persimmon fruit. Mol Plant Microbe Interac. 2002;15:774–9. [DOI] [PubMed] [Google Scholar]
- Estévez S, Rebolledo-Leiva R, Hernández Det al. Benchmarking composting, anaerobic digestion and dark fermentation for apple vinasse management as a strategy for sustainable energy production. Energy. 2023;274:127319. [Google Scholar]
- European Centre for Disease Prevention and Control, EFSA . Multi-country outbreak of Salmonella virchow ST16 infections linked to the consumption of meat products containing chicken meat. Parma, 2023. [Google Scholar]
- Fallik E, Alkalai-Tuvia S, Chalupowicz D. Hot water rinsing and brushing of fresh produce as an alternative to chemical treatment after harvest—the story behind the technology. Agronomy. 2021;11:1653. [Google Scholar]
- Fallingborg J. Intraluminal pH of the human gastrointestinal tract. Danish Med J. 1999;46:183–96. [PubMed] [Google Scholar]
- Feliziani E, Lichter A, Smilanick JLet al. Disinfecting agents for controlling fruit and vegetable diseases after harvest. Postharvest Biol Technol. 2016;122:53–69. [Google Scholar]
- Felle HH, Herrmann A, Hanstein Set al. Apoplastic pH signaling in Barley leaves attacked by the powdery mildew Fungus Blumeria graminisf. Sp. hordei. Mol Plant Microbe Interac. 2004;17:118–23. [DOI] [PubMed] [Google Scholar]
- Fernandes TR, Mariscal M, Serrano Aet al. Cytosolic pH controls fungal MAPK signaling and pathogenicity. mBio. 2023;14:e0028523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Gómez P, Cobo-Díaz JF, Oliveira Met al. Susceptibility and transcriptomic response to plasma-activated water of Listeria monocytogenes planktonic and sessile cells. Food Microbiol. 2023;113:104252. [DOI] [PubMed] [Google Scholar]
- Ferraro A, Massini G, Mazzurco Miritana Vet al. A novel enrichment approach for anaerobic digestion of lignocellulosic biomass: process performance enhancement through an inoculum habitat selection. Bioresour Technol. 2020;313:123703. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Distrutti E. Bile acid-activated receptors, intestinal microbiota, and the treatment of metabolic disorders. Trends Mol Med. 2015;21:702–14. [DOI] [PubMed] [Google Scholar]
- Fisgativa H, Tremier A, Dabert P. Characterizing the variability of food waste quality: a need for efficient valorisation through anaerobic digestion. Waste Manage. 2016;50:264–74. [DOI] [PubMed] [Google Scholar]
- Folke C, Polasky S, Rockström Jet al. Our future in the Anthropocene biosphere. Ambio. 2021;50:834–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune . Bio-based Chemicals market. Pune: 2021. [Google Scholar]
- Frankowski J, Zaborowicz M, Dach Jet al. Biological waste management in the case of a pandemic emergency and other natural disasters. Determination of bioenergy production from floricultural waste and modeling of methane production using deep neural modeling methods. Energies. 2020;13:3014. [Google Scholar]
- Frost I, McKenna N, Chai Set al. Antimicrobial Resistance and Primary Health Care. In: Safety DoSDa (ed.), Geneva: WHO, 2018. [Google Scholar]
- Gao Y, Zhang Y, Cheng Xet al. Agricultural Jiaosu: an eco-friendly and cost-effective control strategy for suppressing fusarium root rot disease in Astragalus membranaceus. Front Microbiol. 2022;13:823704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zheng Z, Cheng Xet al. An innovative way to treat cash crop wastes: the fermentation characteristics and functional microbial community using different substrates to produce agricultural Jiaosu. Environ Res. 2023;227:115727. [DOI] [PubMed] [Google Scholar]
- Gardini F, Özogul Y, Suzzi Get al. Technological factors affecting biogenic amine content in foods: a review. Front Microbiol. 2016;7:1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gausmann M, Kocks C, Pastoors Jet al. Electrochemical pH-T-swing separation of itaconic acid for zero salt waste downstream processing. ACS Sustain Chem Eng. 2021;9:9336–47. [Google Scholar]
- Ge M, Zhang L, Ai Jet al. Effect of heat shock and potassium sorbate treatments on gray mold and postharvest quality of ‘XuXiang’ kiwifruit. Food Chem. 2020;324:126891. [DOI] [PubMed] [Google Scholar]
- Geilfus C-M, Wang L, Wu Jet al. The pH of the leaf apoplast is critical for the formation of Pseudomonas syringae-induced lesions on leaves of the common bean (Phaseolus vulgaris). Plant Sci. 2020;290:110328. [DOI] [PubMed] [Google Scholar]
- Gezae Daful A, Görgens JF. Techno-economic analysis and environmental impact assessment of lignocellulosic lactic acid production. Chem Eng Sci. 2017;162:53–65. [Google Scholar]
- Ghaffar T, Irshad M, Anwar Zet al. Recent trends in lactic acid biotechnology: a brief review on production to purification. J Radiat Res Appl Sci. 2014;7:222–9. [Google Scholar]
- Glaser R, Venus J. Co-fermentation of the main sugar types from a beechwood organosolv hydrolysate by several strains of Bacillus coagulans results in effective lactic acid production. Biotechnol Rep. 2018;18:e00245–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Market Estimates . Global market estimates. Brooklyn: 2022. [Google Scholar]
- Goode O, Smith A, Zarkan Aet al. Persister Escherichia coli cells have a lower intracellular pH than susceptible cells but maintain their pH in response to antibiotic treatment. mBio. 2021;12:e0090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgieva S, Jančič U, Cepec Eet al. Production efficiency and properties of bacterial cellulose membranes in a novel grape pomace hydrolysate by Komagataeibacter melomenusus AV436T and Komagataeibacter xylinus LMG 1518. Int J Biol Macromol. 2023;244:125368. [DOI] [PubMed] [Google Scholar]
- Gough HL, Nielsen JL. Bioaugmentation. In: Springer Protocols Handbooks. Berlin: Springer, 2016, 105–15. 10.1007/8623_2016_205. [DOI] [Google Scholar]
- Gouveia AR, Freitas EB, Galinha CFet al. Dynamic change of pH in acidogenic fermentation of cheese whey towards polyhydroxyalkanoates production: impact on performance and microbial population. New Biotechnol. 2017;37:108–16. [DOI] [PubMed] [Google Scholar]
- Grosser A, Neczaj E. Sewage sludge and fat rich materials co-digestion - performance and energy potential. J Cleaner Prod. 2018;198:1076–89. [Google Scholar]
- Guan N, Li J, Shin H-det al. Metabolic engineering of acid resistance elements to improve acid resistance and propionic acid production of Propionibacterium jensenii. Biotechnol Bioeng. 2016;113:1294–304. [DOI] [PubMed] [Google Scholar]
- Guan N, Liu L. Microbial response to acid stress: mechanisms and applications. Appl Microbiol Biotechnol. 2020;104:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gul B, Sana M, Saleem Aet al. Antimicrobial dispensing practices during COVID-19 and the implications for Pakistan. Antibiotics. 2023;12:1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C-J, Allen BM, Hiam KJet al. Depletion of microbiome-derived molecules in the host using Clostridiumgenetics. Science. 2019;366:eaav1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead FD, Rauf M, Moiemen NSet al. The antibacterial activity of acetic acid against biofilm-producing pathogens of relevance to burns patients. PLoS ONE. 2015;10:e0136190–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Kong T, Wang Qet al. Regulation of microbial metabolism on the formation of characteristic flavor and quality formation in the traditional fish sauce during fermentation: a review. Crit Rev Food Sci Nutr. 2022;63:7564–83. https://doi.org/10.1080/10408398.2022.2047884: 1-20. [DOI] [PubMed] [Google Scholar]
- Han Z, Wang Z, Bi Yet al. The effect of environmental pH during Trichothecium roseum (Pers.:fr.) link inoculation of apple fruits on the host differential reactive oxygen species metabolism. Antioxidants. 2021;10:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatti-Kaul R, Chen L, Dishisha Tet al. Lactic acid bacteria: from starter cultures to producers of chemicals. FEMS Microbiol Lett. 2018;365:30169778. [DOI] [PubMed] [Google Scholar]
- Herianto S, Hou CY, Lin CMet al. Nonthermal plasma-activated water: a comprehensive review of this new tool for enhanced food safety and quality. Comprehen Rev Food Sci Food Saf. 2021;20:583–626. [DOI] [PubMed] [Google Scholar]
- Hernández C, Alamilla-Ortiz ZL, Escalante AEet al. Heat-shock treatment applied to inocula for H2 production decreases microbial diversities, interspecific interactions and performance using cellulose as substrate. Int J Hydrog Energy. 2019;44:13126–34. 10.1016/j.ijhydene.2019.03.124. [DOI] [Google Scholar]
- Hernandez-Gomez JG, Lopez-Bonilla A, Trejo-Tapia Get al. In vitro bile salt hydrolase (BSH) activity screening of different probiotic microorganisms. Foods. 2021;10:674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M, Stuckey DC. Bioaugmentation and its application in wastewater treatment: a review. Chemosphere. 2015;140:119–28. [DOI] [PubMed] [Google Scholar]
- Hill C, Guarner F, Reid Get al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11:506–14. [DOI] [PubMed] [Google Scholar]
- Hong SH, Wang X, O'Connor HFet al. Bacterial persistence increases as environmental fitness decreases. Microb Biotechnol. 2012;5:509–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinpour Tehrani H, Becker J, Bator Iet al. Integrated strain- and process design enable production of 220 g L−1 itaconic acid with Ustilago maydis. Biotechnol Biofuels. 2019a;12:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinpour Tehrani H, Saur K, Tharmasothirajan Aet al. Process engineering of pH tolerant Ustilago cynodontis for efficient itaconic acid production. Microb Cell Fact. 2019b;18:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinpour Tehrani H, Tharmasothirajan A, Track Eet al. Engineering the morphology and metabolism of pH tolerant Ustilago cynodontis for efficient itaconic acid production. Metab Eng. 2019c;54:293–300. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh-Bandbafha H, Kumar D, Singh Bet al. Biodiesel antioxidants and their impact on the behavior of diesel engines: a comprehensive review. Fuel Process Technol. 2022;232:107264. [Google Scholar]
- Hou C-Y, Lai Y-C, Hsiao C-Pet al. Antibacterial activity and the physicochemical characteristics of plasma activated water on tomato surfaces. LWT. 2021;149:111879. [Google Scholar]
- Hu B, Chen S. Pretreatment of methanogenic granules for immobilized hydrogen fermentation. Int J Hydrog Energy. 2007;32:3266–73. [Google Scholar]
- Hu Y, Zhang L, Liu Qet al. The potential correlation between bacterial diversity and the characteristic volatile flavour of traditional dry sausages from Northeast China. Food Microbiol. 2020;91:103505. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang L, Wen Ret al. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: a review. Crit Rev Food Sci Nutr. 2022;62:2741–55. [DOI] [PubMed] [Google Scholar]
- Ibrahim SA, Ayivi RD, Zimmerman Tet al. Lactic acid bacteria as antimicrobial agents: food safety and microbial food spoilage prevention. Foods. 2021;10:3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignaciuk H, Ignaciuk J. Method and Installation for Biogas and Hydrogen Production, and Fertilizers Containing Chelates Obtained by this Method. Munich: European Patent Office, 2018. https://patentscopewipoint/search/en/detailjsf?docId=EP231424619&recNum=171&docAn=17164444&queryString=FP:(vaccin*%20or%20immunis*%20or%20immuniz*)%20&maxRec=102720(August 2023, date last accessed). [Google Scholar]
- Ijoma GN, Adegbenro G, Rashama Cet al. Peculiar response in the co-culture fermentation of Leuconostoc mesenteroides and Lactobacillus plantarum for the production of ABE solvents. Fermentation. 2021;7:212. [Google Scholar]
- Jiang L, Cui H, Zhu Let al. Enhanced propionic acid production from whey lactose with immobilized Propionibacterium acidipropionici and the role of trehalose synthesis in acid tolerance. Green Chem. 2015;17:250–9. [Google Scholar]
- Jimdjio CK, Xue H, Bi Yet al. Effect of ambient pH on growth, pathogenicity, and patulin production of Penicillium expansum. Toxins. 2021;13:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimdjio Kouasseu C, Yang X, Xue Het al. Reactive oxygen species metabolism modulation on the quality of apple fruits inoculated with Penicillium expansum under different ambient pHs. Horticulturae. 2023;9:538. [Google Scholar]
- Joglekar HG, Rahman I, Babu Set al. Comparative assessment of downstream processing options for lactic acid. Sep Purif Technol. 2006;52:1–17. [Google Scholar]
- Johnston CS, Gaas CA. Vinegar: medicinal uses and antiglycemic effect. Medscape Gen Med. 2006;8:61–75. [PMC free article] [PubMed] [Google Scholar]
- Ju K-S, Gao J, Doroghazi JRet al. Discovery of phosphonic acid natural products by mining the genomes of 10,000 actinomycetes. Proc Natl Acad Sci. 2015;112:12175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julák J, Scholtz V, Kotúčová Set al. The persistent microbicidal effect in water exposed to the corona discharge. Phys Med. 2012;28:230–9. [DOI] [PubMed] [Google Scholar]
- Kamal-Eldin A, Alhammadi A, Gharsallaoui Aet al. Physicochemical, rheological, and micro-structural properties of yogurts produced from mixtures of camel and bovine milks. NFS J. 2020;19:26–33. [Google Scholar]
- Kanashiro AM, Akiyama DY, Kupper KCet al. Penicillium italicum: an underexplored postharvest pathogen. Front Microbiol. 2020;11:606852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjee U, Houry WA. Mechanisms of acid resistance in Escherichia coli. Annu Rev Microbiol. 2013;67:65–81. [DOI] [PubMed] [Google Scholar]
- Kayrouz CM, Zhang Y, Pham TMet al. Genome mining reveals the phosphonoalamide natural products and a new route in phosphonic acid biosynthesis. ACS Chem Biol. 2020;15:1921–9. [DOI] [PubMed] [Google Scholar]
- Kesten C, Gámez-Arjona FM, Menna Aet al. Pathogen-induced pH changes regulate the growth-defense balance in plants. EMBO J. 2019;38:e101822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketcham A, Freddolino PL, Tavazoie S. Intracellular acidification is a hallmark of thymineless death in E. coli. PLos Genet. 2022;18:e1010456–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnevisan B, Tsapekos P, Zhang Yet al. Urban biowaste valorization by coupling anaerobic digestion and single cell protein production. Bioresour Technol. 2019;290:121743. [DOI] [PubMed] [Google Scholar]
- Kibler KM, Reinhart D, Hawkins Cet al. Food waste and the food-energy-water nexus: a review of food waste management alternatives. Waste Manage. 2018;74:52–62. [DOI] [PubMed] [Google Scholar]
- Kim D-H, Jang S, Yun Y-Met al. Effect of acid-pretreatment on hydrogen fermentation of food waste: Microbial community analysis by next generation sequencing. Int J Hydrog Energy. 2014;39:16302–9. [Google Scholar]
- Kim K-Y, Habas SE, Schaidle JAet al. Application of phase-pure nickel phosphide nanoparticles as cathode catalysts for hydrogen production in microbial electrolysis cells. Bioresour Technol. 2019;293:122067. [DOI] [PubMed] [Google Scholar]
- Kim M, Chowdhury MMI, Nakhla Get al. Characterization of typical household food wastes from disposers: fractionation of constituents and implications for resource recovery at wastewater treatment. Bioresour Technol. 2015;183:61–9. [DOI] [PubMed] [Google Scholar]
- Ko HI, Jeong CH, Hong SWet al. Optimizing conditions in the acid tolerance test for potential probiotics using response surface methodology. Microbiol Spectr. 2022;10:e0162522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kora E, Patrinou V, Antonopoulou Get al. Dark fermentation of expired fruit juices for biohydrogen production followed by treatment and biotechnological exploitation of effluents towards bioplastics and microbial lipids. Biochem Eng J. 2023;195:108901. [Google Scholar]
- Kouzuma A, Watanabe K. Molecular approaches for the analysis of natural attenuation and bioremediation. In: Comprehensive Biotechnology. Amsterdam: Elsevier, 2011, 107–18. 10.1016/B978-0-444-64046-8.00337-2. [DOI] [Google Scholar]
- Krulwich TA, Sachs G, Padan E. Molecular aspects of bacterial pH sensing and homeostasis. Nat Rev Microbiol. 2011;9:330–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Shahi SK, Singh S. Bioremediation: an eco-sustainable approach for restoration of contaminated sites. In: Microbial Bioprospecting for Sustainable Development. Singapore: Springer, 2018, 115–36. 10.1007/978-981-13-0053-0_6. [DOI] [Google Scholar]
- Kwan TH, Pleissner D, Lau KYet al. Techno-economic analysis of a food waste valorization process via microalgae cultivation and co-production of plasticizer, lactic acid and animal feed from algal biomass and food waste. Bioresour Technol. 2015;198:292–9. [DOI] [PubMed] [Google Scholar]
- Lacroux J, Llamas M, Dauptain Ket al. Dark fermentation and microalgae cultivation coupled systems: outlook and challenges. Sci Total Environ. 2023;865:161136. [DOI] [PubMed] [Google Scholar]
- Lakicevic BZ, Den Besten HMW, De Biase D. Landscape of stress response and virulence genes among Listeria monocytogenes strains. Front Microbiol. 2022;12:738470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R. The overlooked pandemic of antimicrobial resistance. Lancet North Am Ed. 2022;399:606–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-S, Xin W, Katakojwala Ret al. Microbial electrolysis cells for the production of biohydrogen in dark fermentation – a review. Bioresour Technol. 2022;363:127934. [DOI] [PubMed] [Google Scholar]
- Lee JW, Kim HU, Choi Set al. Microbial production of building block chemicals and polymers. Curr Opin Biotechnol. 2011;22:758–67. [DOI] [PubMed] [Google Scholar]
- Lee JZ, Logan A, Terry Set al. Microbial response to single-cell protein production and brewery wastewater treatment. Microb Biotechnol. 2015;8:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire S, Van Bambeke F, Pierard Det al. Activity of fusidic acid against extracellular and intracellular Staphylococcus aureus: influence of pH and comparison with Linezolid and Clindamycin. Clin Infect Dis. 2011;52:S493–503. [DOI] [PubMed] [Google Scholar]
- Li C, Gao S, Li Xet al. Efficient metabolic evolution of engineered Yarrowia lipolytica for succinic acid production using a glucose-based medium in an in situ fibrous bioreactor under low-pH condition. Biotechnol Biofuels. 2018;11:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Qiu T, Huang Get al. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb Cell Fact. 2010;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Jia S, Ma Det al. Effects of citric acid and heterofermentative inoculants on anaerobic co-fermentation of Chinese cabbage waste and wheat bran. Bioresour Technol. 2023a;377:128942. [DOI] [PubMed] [Google Scholar]
- Li J, Ma D, Tian Jet al. The responses of organic acid production and microbial community to different carbon source additions during the anaerobic fermentation of Chinese cabbage waste. Bioresour Technol. 2023b;371:128624. [DOI] [PubMed] [Google Scholar]
- Li M, Song G, Liu Ret al. Inactivation and risk control of pathogenic microorganisms in municipal sludge treatment: a review. Front Environ Sci Eng. 2022;16:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S, Gao D, Liu Het al. Metabolomic and proteomic analysis of D-lactate-producing Lactobacillus delbrueckiiunder various fermentation conditions. J Ind Microbiol Biotechnol. 2018;45:681–96. [DOI] [PubMed] [Google Scholar]
- Liang W, Xie Z, Cheng Jet al. A light-triggered pH-responsive metal-organic framework for smart delivery of fungicide to control sclerotinia diseases of oilseed rape. ACS Nano. 2021;15:6987–97. [DOI] [PubMed] [Google Scholar]
- Lin Q, Pilewski JM, Di YP. Acidic microenvironment determines antibiotic susceptibility and biofilm formation of Pseudomonas aeruginosa. Front Microbiol. 2021;12. 10.3389/fmicb.2021.747834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Chan SHJ, Chen Jet al. Systems biology – a guide for understanding and developing improved strains of lactic acid bacteria. Front Microbiol. 2019;10. 10.3389/fmicb.2019.00876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Lim CK, Shen Zet al. Effects of pH and light exposure on the survival of bacteria and their ability to biodegrade organic compounds in clouds: implications for microbial activity in acidic cloud water. Atmos Chem Phys. 2023;23:1731–47. [Google Scholar]
- Lopez-Hidalgo AM, Smoliński A, Sanchez A. A meta-analysis of research trends on hydrogen production via dark fermentation. Int J Hydrog Energy. 2022;47:13300–39. [Google Scholar]
- Lund P, Tramonti A, De Biase D. Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev. 2014;38:1091–125. [DOI] [PubMed] [Google Scholar]
- Lund PA, De Biase D, Liran Oet al. Understanding how microorganisms respond to acid pH is central to their control and successful exploitation. Front Microbiol. 2020;11:556140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Wang F, Cheng Xet al. Metatranscriptomic insights of the metabolic process enhancement during food wastes fermentation driven by linear alkylbenzene sulphonates. J Clean Prod. 2021;315:128145. [Google Scholar]
- Ma K, Hu G, Pan Let al. Highly efficient production of optically pure l-lactic acid from corn stover hydrolysate by thermophilic Bacillus coagulans. Bioresour Technol. 2016;219:114–22. [DOI] [PubMed] [Google Scholar]
- Ma K, Maeda T, You Het al. Open fermentative production of l-lactic acid with high optical purity by thermophilic Bacillus coagulans using excess sludge as nutrient. Bioresour Technol. 2014;151:28–35. [DOI] [PubMed] [Google Scholar]
- Magalhães AI, de Carvalho JC, Thoms JFet al. Techno-economic analysis of downstream processes in itaconic acid production from fermentation broth. J Clean Prod. 2019;206:336–48. [Google Scholar]
- Magalhães Júnior AI, Soccol CR, Camara MCet al. Challenges in the production of second-generation organic acids (potential monomers for application in biopolymers). Biomass Bioenergy. 2021;149:106092. [Google Scholar]
- Mahoney AR, Safaee MM, Wuest WMet al. The silent pandemic: emergent antibiotic resistances following the global response to SARS-CoV-2. iScience. 2021;24:102304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maicas S. The role of yeasts in fermentation processes. Microorganisms. 2020;8:1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick S, Das S. Acid-tolerant bacteria and prospects in industrial and environmental applications. Appl Microbiol Biotechnol. 2023;107:3355–74. [DOI] [PubMed] [Google Scholar]
- Mancini E, Mansouri SS, Gernaey KVet al. From second generation feed-stocks to innovative fermentation and downstream techniques for succinic acid production. Crit Rev Environ Sci Technol. 2020;50:1829–73. [Google Scholar]
- Marco ML, Sanders ME, Gänzle Met al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol. 2021;18:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoura M, Passaretti P, Overton TWet al. Use of a model to understand the synergies underlying the antibacterial mechanism of H2O2-producing honeys. Sci Rep. 2020;10:17692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matassa S, Boon N, Pikaar Iet al. Microbial protein: future sustainable food supply route with low environmental footprint. Microb Biotechnol. 2016;9:568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathlouthi A, Pennacchietti E, De Biase D. Effect of temperature, pH and plasmids on in vitro biofilm formation in Escherichia coli. Acta Naturae. 2018;10:129–32. [PMC free article] [PubMed] [Google Scholar]
- Mbengue M, Navaud O, Peyraud Ret al. Emerging trends in molecular interactions between plants and the broad host range fungal pathogens Botrytis cinerea and sclerotinia sclerotiorum. Front Plant Sci. 2016;7:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekawi EM, Khafagi EY, Abdel-Rahman FA. Effect of pre-harvest application with some organic acids and plant oils on antioxidant properties and resistance to Botrytis cinerea in pepper fruits. Sci Hortic. 2019;257:108736. [Google Scholar]
- Melini F, Melini V, Luziatelli Fet al. Health-promoting components in fermented foods: an up-to-date systematic review. Nutrients. 2019;11:1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengqi Z, Shi A, Ajmal Met al. Comprehensive review on agricultural waste utilization and high-temperature fermentation and composting. Biomass Conver Bioref. 2023;13:5445–68. [Google Scholar]
- Miller C, Fosmer A, Rush Bet al. Industrial production of lactic acid. In: Comprehensive Biotechnology. Amsterdam: Elsevier, 2011, 179–88. 10.1016/B978-0-08-088504-9.00177-X. [DOI] [Google Scholar]
- Mitosch K, Rieckh G, Bollenbach T. Noisy response to antibiotic stress predicts subsequent single-cell survival in an acidic environment. Cell Syst. 2017;4:393–403. [DOI] [PubMed] [Google Scholar]
- Mladenović D, Pejin J, Kocić-Tanackov Set al. Enhanced lactic acid production by adaptive evolution of Lactobacillus paracasei on agro-industrial substrate. Appl Biochem Biotechnol. 2019;187:753–69. [DOI] [PubMed] [Google Scholar]
- Mladenović D, Pejin J, Kocić-Tanackov Set al. Lactic acid production on molasses enriched potato stillage by Lactobacillus paracasei immobilized onto agro-industrial waste supports. Ind Crops Prod. 2018;124:142–8. [Google Scholar]
- Mokoena MP, Omatola CA, Olaniran AO. Applications of lactic acid bacteria and their bacteriocins against food spoilage microorganisms and foodborne pathogens. Molecules. 2021;26:7055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molan P, Rhodes T. Honey: a biologic wound dressing. Wounds. 2015;27:141–51. [PubMed] [Google Scholar]
- Morone P, D'Amato D. The role of sustainability standards in the uptake of bio-based chemicals. Curr Opin Green Sustain Chem. 2019;19:45–9. [Google Scholar]
- Moscoviz R, Kleerebezem R, Rombouts JL. Directing carbohydrates toward ethanol using mesophilic microbial communities. Curr Opin Biotechnol. 2021;67:175–83. [DOI] [PubMed] [Google Scholar]
- Mucha JW. Pre-extraction of wood components. Mild hydrothermal methods for a future materials biorefinery. Ph.D. Thesis, Department of Chemistry and Chemical Engineering, Chalmers University of Technology, 2020, 1–102. [Google Scholar]
- Murariu M, Dubois P. PLA composites: from production to properties. Adv Drug Deliv Rev. 2016;107:17–46. [DOI] [PubMed] [Google Scholar]
- Muter O. Current trends in bioaugmentation tools for bioremediation: a critical review of advances and knowledge gaps. Microorganisms. 2023;11:710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka S. Yogurt production. Methods Mol Biol. 2019;1887:45–54. 10.1007/978-1-4939-8907-2_5. [DOI] [PubMed] [Google Scholar]
- Nagoba BS, Selkar SP, Wadher BJet al. Acetic acid treatment of pseudomonal wound infections – A review. J Infect Public Health. 2013;6:410–5. [DOI] [PubMed] [Google Scholar]
- Nagoba BS, Wadher BJ, Rao Aet al. Treatment of lepromatous ulcers using citric acid as a sole antimicrobial agent. Int Wound J. 2012;9:553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naïtali M, Kamgang-Youbi G, Herry J-Met al. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl Environ Microbiol. 2010;76:7662–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathao C, Sirisukpoka U, Pisutpaisal N. Production of hydrogen and methane by one and two stage fermentation of food waste. Int J Hydrog Energy. 2013;38:15764–9. [Google Scholar]
- Neves SA, Marques AC. Drivers and barriers in the transition from a linear economy to a circular economy. J Clean Prod. 2022;341:130865. [Google Scholar]
- Ng K-S, Bambace MF, Schwab C. Microbially produced short-chain carboxylic acids are ancient food biopreservatives with complex mode of action. Curr Opin Food Sci. 2023;52:101066. [Google Scholar]
- Nissilä ME, Lay C-H, Puhakka JA. Dark fermentative hydrogen production from lignocellulosic hydrolyzates – a review. Biomass Bioenergy. 2014;67:145–59. [Google Scholar]
- Nong D, Escobar N, Britz Wet al. Long-term impacts of bio-based innovation in the chemical sector: a dynamic global perspective. J Clean Prod. 2020;272:122738. [Google Scholar]
- Nzila A, Razzak S, Zhu J. Bioaugmentation: an emerging strategy of industrial wastewater treatment for reuse and discharge. Int J Environ Res Public Health. 2016;13:846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor KE. Microbiology challenges and opportunities in the circular economy. Microbiology. 2021;167:001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehmigen K, Hähnel M, Brandenburg Ret al. The role of acidification for antimicrobial activity of atmospheric pressure plasma in liquids. Plasma Processes Polym. 2010;7:250–7. [Google Scholar]
- Oliveira M, Fernández-Gómez P, Álvarez-Ordóñez Aet al. Plasma-activated water: a cutting-edge technology driving innovation in the food industry. Food Res Int. 2022;156:111368. [DOI] [PubMed] [Google Scholar]
- Palmieri F, Estoppey A, House GLet al. Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. Adv Appl Microbiol. 2019;106:49–77. 10.1016/bs.aambs.2018.10.001. [DOI] [PubMed] [Google Scholar]
- Pascal K, Ren H, Sun FFet al. Mild acid-catalyzed atmospheric glycerol organosolv pretreatment effectively improves enzymatic hydrolyzability of lignocellulosic biomass. ACS Omega. 2019;4: 20015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau S, Tan LC, Arriaga Set al. Lactic acid fermentation of food waste at acidic conditions in a semicontinuous system: effect of HRT and OLR changes. Biomass Convers Bioref. 2022. 10.1007/s13399-022-03201-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto RS, Voolstra CR, Sweet Met al. Harnessing the microbiome to prevent global biodiversity loss. Nat Microbiol. 2022;7:1726–35. [DOI] [PubMed] [Google Scholar]
- Pennacchietti E, D'Alonzo C, Freddi Let al. The glutaminase-dependent acid resistance system: qualitative and quantitative assays and analysis of its distribution in enteric bacteria. Front Microbiol. 2018;9:2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchietti E, Giovannercole F, De Biase D. Acid survival mechanisms in neutralophilic bacteria. In: Stress and Environmental Regulation of Gene Expression and Adaptation in Bacteria, Hoboken: John Wiley & Sons, Inc, 2016, 911–26. 10.1002/9781119004813.ch89. [DOI] [Google Scholar]
- Percival SL, Finnegan S, Donelli Get al. Antiseptics for treating infected wounds: efficacy on biofilms and effect of pH. Crit Rev Microbiol. 2014;42. https://doi.org/10.3109/1040841X.2014.940495: 1-17. [DOI] [PubMed] [Google Scholar]
- Pérez-Díaz IM, Altuntas EG, Juneja VK. Microbial fermentation in food preservation. In: Microbial Control and Food Preservation. New York: Springer, 2017, 281–98. 10.1007/978-1-4939-7556-3_13. [DOI] [Google Scholar]
- Perez-Pimienta JA, Flores-Gómez CA, Ruiz HAet al. Evaluation of agave bagasse recalcitrance using AFEX™, autohydrolysis, and ionic liquid pretreatments. Bioresour Technol. 2016;211:216–23. [DOI] [PubMed] [Google Scholar]
- Preethi, Usman TMM, Rajesh Banu Jet al. Biohydrogen production from industrial wastewater: An overview. Bioresour Technol Rep. 2019;7: 100287. [Google Scholar]
- Prete R, Long SL, Gallardo ALet al. Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci Rep. 2020;10:1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priya AK, Alagumalai A, Balaji Det al. Bio-based agricultural products: a sustainable alternative to agrochemicals for promoting a circular economy. RSC Sustain. 2023;1:746–62. [Google Scholar]
- Prusky D, Barad S, Ment Det al. The pH modulation by fungal secreted molecules: a mechanism affecting pathogenicity by postharvest pathogens. Israel J Plant Sci. 2016;63:22–30. [Google Scholar]
- Prusky D, McEvoy JL, Leverentz Bet al. Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Mol Plant Microbe Interac. 2001;14:1105–13. [DOI] [PubMed] [Google Scholar]
- Putri DN, Sahlan M, Montastruc Let al. Progress of fermentation methods for bio-succinic acid production using agro-industrial waste by Actinobacillus succinogenes. Energy Rep. 2020;6:234–9. [Google Scholar]
- Qiu S, Zhang X, Xia Wet al. Effect of extreme pH conditions on methanogenesis: methanogen metabolism and community structure. Sci Total Environ. 2023;877:162702. [DOI] [PubMed] [Google Scholar]
- Rafieenia R, Lavagnolo MC, Pivato A. Pre-treatment technologies for dark fermentative hydrogen production: current advances and future directions. Waste Manage. 2018;71:734–48. [DOI] [PubMed] [Google Scholar]
- Rahman M, Hasan MS, Islam Ret al. Plasma-activated water for Food safety and quality: a review of recent developments. Int J Environ Res Public Health. 2022;19:6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper E, Stephenson T, Anderson DRet al. Industrial wastewater treatment through bioaugmentation. Process Saf Environ Prot. 2018;118:178–87. [Google Scholar]
- Rashmi D, Zanan R, John Set al. γ-aminobutyric acid (GABA): biosynthesis, role, commercial production, and applications. Stud Nat Prod Chem. 2018;57:413–52. 10.1016/B978-0-444-64057-4.00013-2. [DOI] [Google Scholar]
- Razia S, Hadibarata T, Lau SY. Acidophilic microorganisms in remediation of contaminants present in extremely acidic conditions. Bioprocess Biosyst Eng. 2023;46:341–58. [DOI] [PubMed] [Google Scholar]
- Raziq A. Single cell protein (SCP) production and potential substrates: a comprehensive review. Pure Appl Biol. 2020;9:1743–54. [Google Scholar]
- Reddy DO, Milliken CE, Foreman Ket al. Bioremediation of hexanoic acid and phenanthrene in oil sands tailings by the Microbial Consortium BioTiger™. Bull Environ Contam Toxicol. 2020;104:253–8. [DOI] [PubMed] [Google Scholar]
- Ren Y, Yu M, Wu Cet al. A comprehensive review on food waste anaerobic digestion: research updates and tendencies. Bioresour Technol. 2018;247:1069–76. [DOI] [PubMed] [Google Scholar]
- Renes E, Ladero V, Tornadijo MEet al. Production of sheep milk cheese with high γ-aminobutyric acid and ornithine concentration and with reduced biogenic amines level using autochthonous lactic acid bacteria strains. Food Microbiol. 2019;78:1–10. [DOI] [PubMed] [Google Scholar]
- Renes E, Linares DM, González Let al. Production of conjugated linoleic acid and gamma-aminobutyric acid by autochthonous lactic acid bacteria and detection of the genes involved. J Funct Foods. 2017;34:340–6. [Google Scholar]
- Risa Vaka M, Sone I, García Álvarez Ret al. Towards the next-generation disinfectant: composition, storability and preservation potential of plasma activated water on baby spinach leaves. Foods. 2019;8:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SG, Ahammad SZ. COVID-19 and antimicrobial resistance: a cross-study. Sci Total Environ. 2022;807:150873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert T, Friebel S. Itaconic acid – a versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016;18:2922–34. [Google Scholar]
- Rodriguez-Moreno L, Ebert MK, Bolton MDet al. Tools of the crook- infection strategies of fungal plant pathogens. Plant J. 2018;93:664–74. [DOI] [PubMed] [Google Scholar]
- Ru J, Huo Y, Yang Y. Microbial degradation and valorization of plastic wastes. Front Microbiol. 2020;11:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz L, Ruas-Madiedo P, Gueimonde Met al. How do bifidobacteria counteract environmental challenges? Mechanisms involved and physiological consequences. Genes Nutr. 2011;6:307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz L, Sanchez B, Margolles A. Determination of bile salt hydrolase activity in bifidobacteria. Methods Mol Biol. 2021;2278:149–55. [DOI] [PubMed] [Google Scholar]
- Sabater C, Ruiz L, Delgado Set al. Valorization of vegetable food waste and by-products through fermentation processes. Front Microbiol. 2020;11:581997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadiq FA, Yan B, Tian Fet al. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: a comprehensive review. Comprehen Rev Food Sci Food Saf. 2019;18:1403–36. [DOI] [PubMed] [Google Scholar]
- Salek SS, van Turnhout AG, Kleerebezem Ret al. pH control in biological systems using calcium carbonate. Biotechnol Bioeng. 2015;112:905–13. [DOI] [PubMed] [Google Scholar]
- Sánchez B, Ruiz L, Gueimonde Met al. Toward improving technological and functional properties of probiotics in foods. Trends Food Sci Technol. 2012;26:56–63. [Google Scholar]
- Sánchez-Andrea I, Triana D, Sanz JL. Bioremediation of acid mine drainage coupled with domestic wastewater treatment. Water Sci Technol. 2012;66:2425–31. [DOI] [PubMed] [Google Scholar]
- Sánchez-Rangel D, Hernández-Domínguez E-E, Pérez-Torres C-Aet al. Environmental pH modulates transcriptomic responses in the fungus Fusarium sp. associated with KSHB Euwallacea sp. near fornicatus. BMC Genomics. 2018;19:721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer M, Han NS. Lactic acid bacteria: little helpers for many human tasks. Essays Biochem. 2021;65:163–71. [DOI] [PubMed] [Google Scholar]
- Sauer M, Russmayer H, Grabherr Ret al. The efficient clade: lactic acid bacteria for industrial chemical production. Trends Biotechnol. 2017;35:756–69. [DOI] [PubMed] [Google Scholar]
- Sauer M. Microbiology and bio-economy – sustainability by nature. In: Good Microbes in Medicine, Food Production, Biotechnology, Bioremediation, and Agriculture. Hoboken: Wiley, 2022, 237–46. 10.1002/9781119762621.ch19. [DOI] [Google Scholar]
- Schink B, Janssen PH, Frings J. Microbial degradation of natural and of new synthetic polymers. FEMS Microbiol Rev. 1992;9:311–6. [DOI] [PubMed] [Google Scholar]
- Schwarz J, Schumacher K, Brameyer Set al. Bacterial battle against acidity. FEMS Microbiol Rev. 2022;46:fuac037. [DOI] [PubMed] [Google Scholar]
- Serrazanetti DI, Guerzoni ME, Corsetti Aet al. Metabolic impact and potential exploitation of the stress reactions in lactobacilli. Food Microbiol. 2009;26:700–11. [DOI] [PubMed] [Google Scholar]
- Settachaimongkon S, van Valenberg HJF, Winata Vet al. Effect of sublethal preculturing on the survival of probiotics and metabolite formation in set-yoghurt. Food Microbiol. 2015;49:104–15. [DOI] [PubMed] [Google Scholar]
- Sevgili A, Can C, Ceyhan DIet al. Molecular identification of LAB and yeasts from traditional sourdoughs and their impacts on the sourdough bread quality characteristics. Curr Res Food Sci. 2023;6:100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y, Xu C, Zhang Het al. Polydopamine-modified metal–organic frameworks, NH2-Fe-MIL-101, as pH-sensitive nanocarriers for controlled pesticide release. Nanomaterials. 2020;10:2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Gaur VK, Kim S-Het al. Microbial strategies for bio-transforming food waste into resources. Bioresour Technol. 2020a;299:122580. [DOI] [PubMed] [Google Scholar]
- Sharma R, Garg P, Kumar Pet al. Microbial fermentation and its role in quality improvement of fermented foods. Fermentation. 2020b;6:106. [Google Scholar]
- Shi S, Kang L, Lee YY. Production of lactic acid from the mixture of softwood pre-hydrolysate and paper mill sludge by simultaneous saccharification and fermentation. Appl Biochem Biotechnol. 2015;175:2741–54. [DOI] [PubMed] [Google Scholar]
- Shiraishi T, Kuzuyama T. Biosynthetic pathways and enzymes involved in the production of phosphonic acid natural products. Biosci Biotechnol Biochem. 2021;85:42–52. [DOI] [PubMed] [Google Scholar]
- Shweta N, Samatha S, Keshavkant S. Mechanisms, types, effectors, and methods of bioremediation: the universal solution. In: Microbial Ecology of Wastewater Treatment Plants. Amsterdam: Elsevier, 2021, 41–72. 10.1016/B978-0-12-822503-5.00010-2. [DOI] [Google Scholar]
- Siala W, Mingeot-Leclercq M-P, Tulkens PMet al. Comparison of the antibiotic activities of Daptomycin, Vancomycin, and the investigational fluoroquinolone Delafloxacin against biofilms from Staphylococcus aureus clinical isolates. Antimicrob Agents Chemother. 2014;58:6385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddeeg SM, Tahoon MA, Ben Rebah F. Agro-industrial waste materials and wastewater as growth media for microbial bioflocculants production: a review. Mater Res Expr. 2020;7:012001. [Google Scholar]
- Siddique SS, Hardy GESJ, Bayliss KL. Plasma-activated water inhibits in vitro conidial germination of Colletotrichum alienum, a postharvest pathogen of avocado. Plant Pathol. 2021;70:367–76. [Google Scholar]
- Sindhu R, Gnansounou E, Rebello Set al. Conversion of food and kitchen waste to value-added products. J Environ Manage. 2019;241:619–30. [DOI] [PubMed] [Google Scholar]
- Singh VP. Recent approaches in food bio-preservation - a review. Open Vet J. 2018;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhvi M, Gurjar G, Gupta Vet al. Biocatalyst development for lactic acid production at acidic pH using inter-generic protoplast fusion. RSC Adv. 2015;5:2024–31. [Google Scholar]
- Singhvi M, Zendo T, Sonomoto K. Free lactic acid production under acidic conditions by lactic acid bacteria strains: challenges and future prospects. Appl Microbiol Biotechnol. 2018;102:5911–24. [DOI] [PubMed] [Google Scholar]
- Sloss JM, Cumberland N, Milner SM. Acetic acid used for the elimination of Pseudomonas aeruginosa from burn and soft tissue wounds. J R Army Med Corps. 1993;139:49–51. [DOI] [PubMed] [Google Scholar]
- Spekreijse J, Lammens T, Parisi Cet al. Insights into the European Market for Bio-Based Chemicals, Luxembourg: Publications Office of the European Union, 2019. 10.2760/18942. [DOI] [Google Scholar]
- Spekreijse J, Vikla K, Vis Met al. Bio-based value chains for chemicals, plastics and pharmaceuticals. Luxembourg: Publications Office of the European Union, 2021, [Google Scholar]
- Steiger MG, Blumhoff ML, Mattanovich Det al. Biochemistry of microbial itaconic acid production. Front Microbiol. 2013;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger MG, Wierckx N, Blank LMet al. Itaconic acid - an emerging building block. In: Industrial Biotechnology, Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2016, 453–72. 10.1002/9783527807833.ch15. [DOI] [Google Scholar]
- Stjärne Aspelund A, Sjöström K, Olsson Liljequist Bet al. Acetic acid as a decontamination method for sink drains in a nosocomial outbreak of metallo-β-lactamase-producing Pseudomonas aeruginosa. J Hosp Infect. 2016;94:13–20. [DOI] [PubMed] [Google Scholar]
- Strandwitz P, Kim KH, Terekhova Det al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol. 2018;4:396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzera G, Battista F, Garcia NHet al. Volatile fatty acids production from food wastes for biorefinery platforms: a review. J Environ Manage. 2018;226:278–88. [DOI] [PubMed] [Google Scholar]
- Stubbings W, Leow P, Yong GCet al. In vitro spectrum of activity of finafloxacin, a novel, pH-activated fluoroquinolone, under standard and acidic conditions. Antimicrob Agents Chemother. 2011;55:4394–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Gong M, Lv Xet al. Current advance in biological production of short-chain organic acid. Appl Microbiol Biotechnol. 2020a;104:9109–24. [DOI] [PubMed] [Google Scholar]
- Sun Q, Li X, Perez LMet al. The molecular basis of pyrazinamide activity on Mycobacterium tuberculosis PanD. Nat Commun. 2020b;11:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang JP, Shin D-H, Jung S-Jet al. Functional properties of microorganisms in fermented foods. Front Microbiol. 2016;7:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tames H, Sabater C, Margolles Aet al. Production of GABA in milk fermented by Bifidobacterium adolescentis strains selected on the bases of their technological and gastrointestinal performance. Food Res Int. 2023;171:113009. [DOI] [PubMed] [Google Scholar]
- Tan ECD, Lamers P. Circular bioeconomy concepts—a perspective. Front Sustain. 2021;2. 10.3389/frsus.2021.701509. [DOI] [Google Scholar]
- Tan J, Li Y, Tan Xet al. Advances in pretreatment of straw biomass for sugar production. Front Chem. 2021;9:696030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Wang X, Hu Yet al. Lactic acid fermentation from food waste with indigenous microbiota: effects of pH, temperature and high OLR. Waste Manage. 2016;52:278–85. [DOI] [PubMed] [Google Scholar]
- Thorwall S, Schwartz C, Chartron JWet al. Stress-tolerant non-conventional microbes enable next-generation chemical biosynthesis. Nat Chem Biol. 2020;16:113–21. [DOI] [PubMed] [Google Scholar]
- Thu Ha Tran T, Khanh Thinh Nguyen P. Enhanced hydrogen production from water hyacinth by a combination of ultrasonic-assisted alkaline pretreatment, dark fermentation, and microbial electrolysis cell. Bioresour Technol. 2022;357:127340. [DOI] [PubMed] [Google Scholar]
- Tian Y, Ma R, Zhang Qet al. Assessment of the physicochemical properties and biological effects of water activated by non-thermal plasma above and beneath the water surface. Plasma Processes Polym. 2015;12:439–49. [Google Scholar]
- Tian Z, Ameer K, Shi Yet al. Characterization of physicochemical properties, microbial diversity and volatile compounds of traditional fermented soybean paste in Henan province of China. Food Biosci. 2022;50:102045. [Google Scholar]
- Toyofuku M, Inaba T, Kiyokawa Tet al. Environmental factors that shape biofilm formation. Biosci Biotechnol Biochem. 2016;80:7–12. [DOI] [PubMed] [Google Scholar]
- Trivedi J, Bhonsle AK, Atray N. Processing food waste for the production of platform chemicals. In: Refining Biomass Residues for Sustainable Energy and Bioproducts. Amsterdam: Elsevier, 2020, 427–48. 10.1016/B978-0-12-818996-2.00019-3. [DOI] [Google Scholar]
- Uğurlu Ş, Günan Yücel H, Aksu Z. Valorization of food wastes with a sequential two-step process for microbial β-carotene production: a zero waste approach. J Environ Manage. 2023;340:118003. [DOI] [PubMed] [Google Scholar]
- Upadhyaya BP, DeVeaux LC, Christopher LP. Metabolic engineering as a tool for enhanced lactic acid production. Trends Biotechnol. 2014;32:637–44. [DOI] [PubMed] [Google Scholar]
- Upadrasta A, Stanton C, Hill Cet al. Improving the stress tolerance of probiotic cultures: recent trends and future directions. In: Stress Responses of Lactic Acid Bacteria, Boston: Springer, 2011, 395–438. 10.1007/978-0-387-92771-8_17. [DOI] [Google Scholar]
- Vadalà M, Kröll E, Küppers Met al. Hydrogen production via dark fermentation by bacteria colonies on porous PDMS-scaffolds. Int J Hydrog Energy. 2023;48:25274–84. [Google Scholar]
- Van den Bergh B, Fauvart M, Michiels J. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol Rev. 2017;41:219–51. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B, Michiels JE, Wenseleers Tet al. Frequency of antibiotic application drives rapid evolutionary adaptation of Escherichia coli persistence. Nat Microbiol. 2016;1:16020. [DOI] [PubMed] [Google Scholar]
- Van den Bergh B, Schramke H, Michiels JEet al. Mutations in respiratory complex I promote antibiotic persistence through alterations in intracellular acidity and protein synthesis. Nat Commun. 2022;13:546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kan JAL. Licensed to kill: the lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006;11:247–53. [DOI] [PubMed] [Google Scholar]
- Van Laethem S, Frans M, Aerts Ret al. pH modulation of the environment by Stagonosporopsis cucurbitacearum, an important pathogen causing fruit rot in Cucurbitaceae. Eur J Plant Pathol. 2021;159:235–45. [Google Scholar]
- Van Riet S, Tadesse W, Mortier Jet al. Heterogeneity and evolutionary tunability of Escherichia coli resistance against extreme acid stress. Microbiol Spectr. 2022;10:e0375722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas-Méndez MÁ, Montañez J, Contreras-Esquivel JCet al. Scale-up and fed-batch cultivation strategy for the enhanced co-production of microbial lipids and carotenoids using renewable waste feedstock. J Environ Manage. 2023;339:117866. [DOI] [PubMed] [Google Scholar]
- Visconti V, Coton E, Rigalma Ket al. Effects of disinfectants on inactivation of mold spores relevant to the food industry: a review. Fung Biol Rev. 2021;38:44–66. [Google Scholar]
- Vishwakarma GS, Bhattacharjee G, Gohil Net al. Current status, challenges and future of bioremediation. In: Bioremediation of Pollutants. Amsterdam: Elsevier, 2020, 403–15. 10.1016/B978-0-12-819025-8.00020-X:. [DOI] [Google Scholar]
- Vylkova S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017;13:e1006149–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Lin AE, Yuan Jet al. Single-cell massively-parallel multiplexed microbial sequencing (M3-seq) identifies rare bacterial populations and profiles phage infection. Nat Microbiol. 2023a;8:1846–62. https://doi.org/10.1038/s41564-023-01462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Su W, Mu Yet al. Correlation between microbial diversity and volatile flavor compounds of Suan zuo rou, a fermented meat product from Guizhou, China. Front Microbiol. 2021;12. 10.3389/fmicb.2021.736525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wang X, Wu Het al. Metabolic modeling of synthetic microbial communities for bioremediation. Crit Rev Environ Sci Technol. 2023b;53:1–20. 10.1080/10643389.2023.2212569. [DOI] [Google Scholar]
- Wang Y, Cao W, Luo Jet al. Exploring the potential of lactic acid production from lignocellulosic hydrolysates with various ratios of hexose versus pentose by Bacillus coagulans IPE22. Bioresour Technol. 2018;261:342–9. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cao W, Luo Jet al. One step open fermentation for lactic acid production from inedible starchy biomass by thermophilic Bacillus coagulans IPE22. Bioresour Technol. 2019;272:398–406. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang C, Liu Fet al. Ecological succession and functional characteristics of lactic acid bacteria in traditional fermented foods. Crit Rev Food Sci Nutr. 2022;63:1–15. 10.1080/10408398.2021.2025035. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang S-T. Propionic acid production in glycerol/glucose co-fermentation by Propionibacterium freudenreichii subsp. Shermanii. Bioresour Technol. 2013;137:116–23. [DOI] [PubMed] [Google Scholar]
- Werpy T, Petersen G. Top value added chemicals from biomass. In: Results of Screening for Potential Candidates From Sugars and Synthesis Gas. Vol. I. Golden: National Renewable Energy Laboratory, 2004. 10.2172/15008859. [DOI] [Google Scholar]
- Wiegand C, Abel M, Ruth Pet al. In vitro assessment of the antimicrobial activity of wound dressings: influence of the test method selected and impact of the pH. J Mater Sci Mater Med. 2015;26:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H, Sun-Waterhouse D, Waterhouse GINet al. Fermentation-enabled wellness foods: a fresh perspective. Food Sci Hum Wellness. 2019;8:203–43. [Google Scholar]
- Xiao D, Cheng J, Liang Wet al. Metal-phenolic coated and prochloraz-loaded calcium carbonate carriers with pH responsiveness for environmentally-safe fungicide delivery. Chem Eng J. 2021;418:129274. [Google Scholar]
- Xu C, Cao L, Bilal Met al. Multifunctional manganese-based carboxymethyl chitosan hydrogels for pH-triggered pesticide release and enhanced fungicidal activity. Carbohydr Polym. 2021;262:117933. [DOI] [PubMed] [Google Scholar]
- Xu H, Ma R, Zhu Yet al. A systematic study of the antimicrobial mechanisms of cold atmospheric-pressure plasma for water disinfection. Sci Total Environ. 2020;703:134965. [DOI] [PubMed] [Google Scholar]
- Xu L, Xiang M, White Det al. pH dependency of sclerotial development and pathogenicity revealed by using genetically defined oxalate-minus mutants of Sclerotinia sclerotiorum. Environ Microbiol. 2015;17:2896–909. [DOI] [PubMed] [Google Scholar]
- Yagnik SM, Arya PS, Raval VH. Microbial enzymes in bioremediation. In: Biotechnology of Microbial Enzymes. Amsterdam: Elsevier, 2023, 685–708. 10.1016/B978-0-443-19059-9.00010-4. [DOI] [Google Scholar]
- Yamane T, Tanaka R. Highly accumulative production of l(+)-lactate from glucose by crystallization fermentation with immobilized Rhizopus oryzae. J Biosci Bioeng. 2013;115:90–5. [DOI] [PubMed] [Google Scholar]
- Yáñez R, Marques S, Gírio FMet al. The effect of acid stress on lactate production and growth kinetics in Lactobacillus rhamnosus cultures. Process Biochem. 2008;43:356–61. [Google Scholar]
- Yang G, Hu Y, Wang J. Biohydrogen production from co-fermentation of fallen leaves and sewage sludge. Bioresour Technol. 2019;285:121342. [DOI] [PubMed] [Google Scholar]
- Yang G, Wang J. Co-fermentation of sewage sludge with ryegrass for enhancing hydrogen production: performance evaluation and kinetic analysis. Bioresour Technol. 2017;243:1027–36. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang K, Li Het al. The influence of urinary pH on antibiotic efficacy against bacterial uropathogens. Urology. 2014;84:731. [DOI] [PubMed] [Google Scholar]
- Yang L, Xue H, Liu Zet al. The effects of different ambient pH on the pathogenicity of Fusarium sulphureum and reactive oxygen species metabolism in F. sulphureum inoculation muskmelon fruits. Physiol Mol Plant Pathol. 2022;122:101893. [Google Scholar]
- Yang S-C, Lin C-H, Aljuffali IAet al. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch Microbiol. 2017;199:811–25. [DOI] [PubMed] [Google Scholar]
- Yang SY, Lü FX, Lu ZXet al. Production of γ-aminobutyric acid by Streptococcus salivarius subsp. Thermophilus Y2 under submerged fermentation. Amino Acids. 2008;34:473–8. [DOI] [PubMed] [Google Scholar]
- Ye L, Zhao H, Li Zet al. Improved acid tolerance of Lactobacillus pentosus by error-prone whole genome amplification. Bioresour Technol. 2013;135:459–63. [DOI] [PubMed] [Google Scholar]
- Yin T, Wang W, Zhuo Set al. Thermophilic dark fermentation fast start-up of hydrogen production with substrate concentration regulation and moderate pretreatment inoculum. Fuel. 2023;334:126748. [Google Scholar]
- Zhan Y, Chang Y, Tao Yet al. Insight into the dynamic microbial community and core bacteria in composting from different sources by advanced bioinformatics methods. Environ Sci Pollut Res. 2022;30:8956–66. [DOI] [PubMed] [Google Scholar]
- Zhang S, Merino N, Okamoto Aet al. Interkingdom microbial consortia mechanisms to guide biotechnological applications. Microb Biotechnol. 2018;11:833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Jiang Y, Zhang Z. The role of different natural organic acids in postharvest fruit quality management and its mechanism. Food Front. 2023;4:1127–43. [Google Scholar]
- Zhang X, Tang X, Zhao Cet al. A pH-responsive MOF for site-specific delivery of fungicide to control citrus disease of Botrytis cinerea. Chem Eng J. 2022a;431:133351. [Google Scholar]
- Zhang Y, Chen X, Qi Bet al. Improving lactic acid productivity from wheat straw hydrolysates by membrane integrated repeated batch fermentation under non-sterilized conditions. Bioresour Technol. 2014a;163:160–6. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shi W, Zhang Wet al. Mechanisms of Pyrazinamide Action and Resistance. Microbiol Spectr. 2014b;2:MGM2–0023-2013. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Tsapekos P, Alvarado-Morales Met al. Improving lactic acid production via bio-augmentation with acid-tolerant isolates from source-sorted organic household waste. Biomass Conver Bioref. 2022b;12:4449–61. [Google Scholar]
- Zhao D, Hu J, Chen W. Analysis of the relationship between microorganisms and flavour development in dry-cured grass carp by high-throughput sequencing, volatile flavour analysis and metabolomics. Food Chem. 2022;368:130889. [DOI] [PubMed] [Google Scholar]
- Zhong A, Chen W, Duan Yet al. The potential correlation between microbial communities and flavors in traditional fermented sour meat. LWT. 2021;149:111873. [Google Scholar]
- Zhou M, Yan B, Wong JWCet al. Enhanced volatile fatty acids production from anaerobic fermentation of food waste: a mini-review focusing on acidogenic metabolic pathways. Bioresour Technol. 2018;248:68–78. [DOI] [PubMed] [Google Scholar]
- Zhou Y-M, Chen Y-P, Guo J-Set al. Recycling of orange waste for single cell protein production and the synergistic and antagonistic effects on production quality. J Clean Prod. 2019;213:384–92. [Google Scholar]
- Zhu Y, Li J, Tan Met al. Optimization and scale-up of propionic acid production by propionic acid-tolerant Propionibacterium acidipropionici with glycerol as the carbon source. Bioresour Technol. 2010;101:8902–6. [DOI] [PubMed] [Google Scholar]
- Ziv C, Kumar D, Sela Net al. Sugar-regulated susceptibility of tomato fruit to Colletotrichum and Penicillium requires differential mechanisms of pathogenicity and fruit responses. Environ Microbiol. 2020;22:2870–91. [DOI] [PubMed] [Google Scholar]