Abstract
The NL4.3 T-cell-line-tropic human immunodeficiency virus type 1 strain is sensitive to the CXC chemokine stromal cell-derived factor 1α (SDF-1α), the natural ligand for CXC chemokine receptor 4 (CXCR4); the 50% inhibitory concentration (IC50) in MT-4 cells is 130 ng/ml. We generated resistant virus through passaging of the virus in the presence of increasing concentrations of SDF-1α. After 24 passages, the virus was no longer sensitive to SDF-1α (SDF-1αres virus) (IC50, >2 μg/ml) and became resistant to SDF-1β (IC50, >2 μg/ml) and to a specific CXCR4 monoclonal antibody (IC50, >20 μg/ml). The SDF-1αres virus was about 10-fold less sensitive than the wild-type virus to the bicyclam AMD3100, a specific CXCR4 antagonist. The SDF-1αres virus contained the following mutations in the gp120 molecule: N106K in the V1 loop; S134N and F145L in the V2 loop; F245I in the C2 loop; K269E, Q278H, I288V, and N293D in the V3 loop; a deletion of 5 amino acids (FNSTW) at positions 364 to 368 in the V4 loop; and R378T in the CD4 binding domain. Replication of the NL4.3 wild-type virus and the SDF-1αres virus was demonstrated in U87 cells that coexpressed CD4 and CXCR4 (U87.CD4.CXCR4) but not in U87.CD4.CCR5 cells. Thus, the resistant virus was not able to switch to the CC chemokine receptor 5 (CCR5) coreceptor (the main coreceptor for macrophage-tropic viruses). The SDF-1αres virus replicated in HOS.CD4 cells expressing CCR1, CCR2b, CCR3, CCR4, CCR5, and CXCR4 but also in HOS.CD4.pBABE cells. However, all HOS transfectant cells expressed a low level of CXCR4. Neither of the two virus strains was able to infect HOS.CXCR4 or HOS.CCR5 transfectants, demonstrating the necessity of the CD4 receptor. The T-cell-line-tropic SDF-1αres virus was thus able to overcome the inhibitory effect of SDF-1α through mutations in gp120 but still needed CXCR4 to enter the cells.
CXC chemokine receptor 4 (CXCR4) was recently shown to be a coreceptor used by T-cell-line-tropic (T-tropic) human immunodeficiency virus (HIV) strains to enter target cells (5, 27), whereas CC chemokine receptor 5 (CCR5) allows the entry of macrophage-tropic (M-tropic) HIV strains (2, 9, 16, 20, 21). The CXC chemokine stromal cell-derived factor 1α (SDF-1α), the natural ligand for CXCR4, has been shown to inhibit T-tropic (such as the NL4.3 strain) but not M-tropic viruses and to inhibit primary HIV isolates (6, 33). Also, CXCR4 is used by HIV type 2 (HIV-2) strains to enter cells, even in the absence of the CD4 receptor (23). Monoclonal antibody (MAb) 12G5 specifically binds to CXCR4 and inhibits infection with T-tropic HIV type 1 (HIV-1) strains, dual-tropic HIV-1 strains, and HIV-2 strains (32), although variation in its antiviral activity has been described, depending on the viral strain and the target cells used in the assays (32, 42). A change in coreceptor use from predominantly CCR5 toward CXCR4 is correlated in HIV-1-infected patients with progression to AIDS (40), and this change is also associated with a switch from the non-syncytium-inducing to the syncytium-inducing phenotype (41) and a decrease in CD4+ T-cell counts. During disease progression in patients, the virus expands its coreceptor use to CCR5, CCR3, CCR2b, and CXCR4. The use of these coreceptors is dependent on the sequence of the V3 loop of viral gp120 (10, 44, 45). However, many virus strains are capable of using more than one coreceptor. Typically T-tropic syncytium-inducing viruses not only use CXCR4 to infect cells but also can use other coreceptors, such as CCR5 (8, 39). Recently, several new coreceptors were identified: Bonzo/STRL33 (3, 17, 31), BOB/GPR15 (17, 26), GPR1 (26), and US28 (35); these can also be used by immunodeficiency viruses to enter cells.
We previously reported the development of HIV-1 resistance to the polyanion dextran sulfate (DS) (25), the oligonucleotide AR177 (24), and the bicyclam derivative AMD3100 (18), which are all potent inhibitors of HIV-1 and HIV-2 replication (4, 14, 15, 34). The first two compounds inhibit binding and fusion of the virus (4, 38); the bicyclam does not inhibit virus binding but acts as a specific CXCR4 antagonist and therefore inhibits entry of the virus into the cells (19, 36, 40). For all of the NL4.3 virus strains that were made resistant to DS (DSres virus) (25), AR177 (AR177res virus) (24), or AMD3100 (AMD3100res virus) (18), mutations were always situated in env glycoprotein 120 (gp120).
Here we describe a T-tropic NL4.3 virus strain that was made resistant to the CXC chemokine SDF-1α (the SDF-1αres virus strain). We investigated the pattern of cross resistance of the virus to other inhibitors of virus binding and fusion. The resistant phenotype of the SDF-1αres virus could be attributed to a number of mutations in gp120. The SDF-1αres mutant did not change its coreceptor use.
MATERIALS AND METHODS
Virus stocks and cell lines.
The HIV-1 T-tropic molecular clone NL4.3 (1) was obtained from the National Institute of Allergy and Infectious Disease AIDS reagent program. Human osteosarcoma HOS.CD4 cells, which express human CD4 and the chemokine receptors CCR1, CCR2b, CCR3, CCR4, CCR5, and CXCR4 or pBABE, and HOS cells, which express CCR5 or CXCR4 (12, 17), were obtained from the National Institute of Allergy and Infectious Disease AIDS reagent program. Astroglioma U87.CD4 cells transfected with CXCR4 or CCR5 were kindly provided by Nathaniel R. Landau. The transformed MT-4 T-cell line has been described elsewhere (28). The AMD3100-resistant NL4.3 virus was generated as described previously (18, 19). Cells were infected with different concentrations of virus, and the supernatant was collected 5 to 10 days after infection and stored at −20°C (17, 25). HIV-1 core antigen in the culture supernatant was analyzed with the p24 antigen enzyme-linked immunosorbent assay kit from DuPont (Brussels, Belgium).
Compounds and chemokines.
DS (molecular weight, 5,000), a sulfated polysaccharide, was purchased from Sigma Chemie (Deisenhofen, Germany). The bicyclam derivatives AMD2763 and AMD3100 were synthesized as described previously (7) and kindly provided by Geoffrey Henson (AnorMed, Langley, Canada). Oligonucleotide AR177, also called T30177 or Zintevir, was provided by Robert F. Rando (Aronex Pharmaceuticals, The Woodlands, Tex.). 3′-Azido-3′-deoxythymidine (AZT) was obtained from Wellcome (Beckenham, United Kingdom). The CXC chemokine SDF-1α, SDF-1β, and the anti-CXCR4 MAb 12G5 were obtained from R & D Systems Europe Ltd., Oxon, United Kingdom.
MAbs and flow cytometric analyses.
The anti-gp120 MAb NEA9305 (DuPont), specifically recognizing the V3 loop epitope RIQRGPGRAFVTGK of HIV-1, was used. The anti-CD4 MAb Leu-3a and isotype-matched control MAbs were purchased from Becton Dickinson (Erembodegem, Belgium). The staining protocols were described in detail elsewhere (37, 38). Cells were analyzed with a FACScan (Becton Dickinson Immunocytometry Systems, San Jose, Calif.) flow cytometer. Data were acquired and analyzed with CellQuest software (Becton Dickinson Immunocytometry Systems) on an Apple Macintosh computer.
Selection of HIV-1 NL4.3 mutant strains.
MT-4 cells were infected with HIV-1 NL4.3 in medium containing SDF-1α at 100 ng/ml. Cultures were incubated at 37°C until an extensive cytopathic effect (CPE) was observed (4 to 5 days). The culture supernatant was used for further passage of virus in MT-4 cells in the presence of increasing concentrations of SDF-1α up to 2 μg/ml.
DNA sequence analysis of gp120.
MT-4 cells were infected with wild-type virus or SDF-1αres virus and incubated for 4 days at 37°C. The cells were washed in phosphate-buffered saline, and total DNA was extracted with a QIAamp blood kit (Qiagen, Westburg, The Netherlands). PCR amplification was performed with ULTMA DNA polymerase with proofreading capacity (Perkin-Elmer Cetus, Norwalk, Conn.) according to De Vreese et al. (18). The PCR product was electrophoresed in an agarose gel, and the relevant band was excised and purified with a QIAquick purification kit. DNA sequencing was performed as described in detail by Esté et al. (25), and sequences were analyzed with DNA Navigator software (Perkin-Elmer).
RESULTS
Selection of the SDF-1αres strain.
HIV-1 NL4.3 was passaged in MT-4 cells in the presence of SDF-1α at a starting concentration corresponding to the 50% inhibitory concentration (IC50) (100 ng/ml). Virus replication was monitored microscopically by the appearance of CPE. Every 4 or 5 days, the replicating virus was passaged in fresh uninfected cells in the presence of SDF-1α at the same concentration as in the previous passage or at a twofold higher concentration, depending on the CPE observed. After 24 passages (100 days), virus that was fully able to replicate in MT-4 cells in the presence of 2 μg of SDF-1α per ml was recovered. Virus from passage 20 could grow in the presence of 2 μg of SDF-1α per ml, and the induced CPE was comparable to that of the wild-type virus.
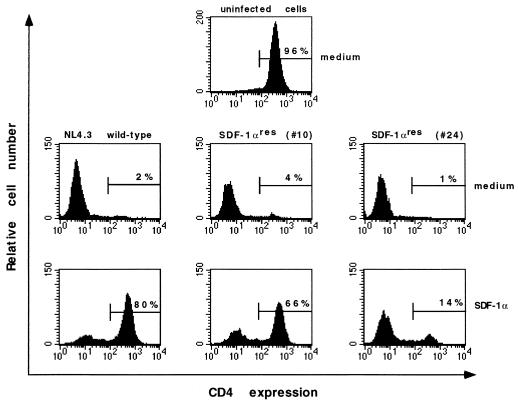
To demonstrate the gradual decrease in the antiviral activity of SDF-1α, MT-4 cells were infected with 100 50% cell culture infective doses (CCID50) of the HIV-1 NL4.3 wild type or NL4.3 SDF-1αres from passage 10 or passage 24, and SDF-1α was added to the cells at different concentrations up to 1 μg/ml. At 5 days after infection, the cells were analyzed for CD4 expression because productive infection of MT-4 cells by T-tropic viruses is accompanied by the disappearance of CD4 from the T-cell surface (13). The uninfected MT-4 cells were 96% CD4+ (Fig. 1, top panel) (37), whereas only 2, 4, and 1% of cells infected with the NL4-3 wild type, NL4-3 SDF-1αres (passage 10), and NL4-3 SDF-1αres (passage 24), respectively, expressed CD4 (Fig. 1, middle panels). As can be seen in the lower panels of Fig. 1, SDF-1α was active against the NL4.3 wild type (80% of the cells still expressed CD4), less active against NL4.3 SDF-1αres (passage 10) (66% CD4+), and virtually inactive against NL4.3 SDF-1αres (passage 24) (14% CD4+). The IC50s of SDF-1α, as calculated from CD4 expression in these cultures, were 150 ng/ml for the wild type, 800 ng/ml for SDF-1αres (passage 10), and >1 μg/ml for SDF-1αres (passage 24). These IC50s were comparable to the IC50s calculated from the p24 antigen contents of these cultures. In all of the further experiments, NL4.3 SDF-1αres virus (passage 24) was used and referred to as SDF-1αres.
FIG. 1.
Effect of SDF-1α (1 μg/ml) on NL4.3 wild-type, SDF-1αres (passage 10), and SDF-1αres (passage 24) HIV-1 replication in MT-4 cells, as monitored by CD4 expression. Cells were infected with the virus strains at 100 CCID50 in the presence or absence of SDF-1α and stained 5 days after infection with MAb Leu-3a directly labeled with phycoerythrin (Becton Dickinson). As a control, uninfected cells also were stained with MAb Leu-3a. The percentage of CD4+ cells (uninfected cells) is indicated in each histogram.
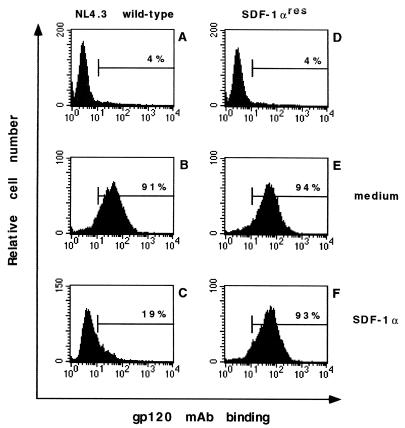
In Fig. 2, MT-4 cells were infected with the wild-type virus and the SDF-1αres virus and analyzed for gp120 expression with an anti-gp120 MAb (NEA9305) 5 days after infection. The expression of gp120 in SDF-1αres virus-infected cells (94%) (Fig. 2E) was comparable to that in wild-type virus-infected cells (91%) (Fig. 2B). SDF-1α at 1 μg/ml was highly protective against the wild-type virus (only 19% of the cells expressed gp120) (Fig. 2C) and inactive against the SDF-1αres virus (93% gp120-positive cells) (Fig. 2F). The IC50s of SDF-1α, as calculated from gp120 expression in these cultures, were 90 ng/ml for the wild-type virus and >1 μg/ml for the SDF-1αres virus. Again, these IC50s were comparable to those calculated from the p24 antigen contents of these cultures.
FIG. 2.
Effect of SDF-1α (1 μg/ml) on NL4.3 wild-type (A, B, and C) and SDF-1αres (D, E, and F) HIV-1 replication in MT-4 cells, as monitored by anti-gp120 MAb binding. Cells were infected with the virus strains at 100 CCID50 and stained 4 days after infection with MAb NEA9305. The percentage of gp120-positive cells (HIV-1-infected cells) is indicated in each histogram. In panels A and D, cells were stained with the secondary antibody only.
Cross-resistance to other compounds.
The wild-type virus that had been grown in MT-4 cells in parallel with the SDF-1αres virus but in the absence of SDF-1α was as sensitive as the original virus stock to SDF-1α (IC50, 130 ng/ml) (Table 1). SDF-1β, which differs from SDF-1α only in four carboxy-terminal amino acids (6, 43), was inactive against the SDF-1αres virus, and it was somewhat less active against the wild-type virus than SDF-1α (IC50, 200 ng/ml). We also examined the effect of the anti-CXCR4 MAb 12G5 on the replication of the wild-type virus and the SDF-1αres virus. MAb 12G5 inhibited the replication of the NL4.3 wild-type by 50% at 8 μg/ml; however, the anti-CXCR4 MAb had no effect whatsoever on the replication of the SDF-1αres virus up to a concentration of 20 μg/ml (Table 1).
TABLE 1.
Anti-HIV activity of SDF-1α, SDF-1β, MAb 12G5, and other compounds against wild-type, SDF-1αres, and AMD3100res viruses in MT-4 cellsa
| Anti-HIV agent | IC50 (μg/ml) for:
|
||
|---|---|---|---|
| Wild-type virus | SDF-1αres virus | AMD3100res virus | |
| SDF-1α | 0.130 | >2 | >2 |
| SDF-1β | 0.200 | >2 | >2 |
| MAb 12G5 | 8 | >20 | >20 |
| AMD3100 | 0.006 | 0.065 | 0.689 |
| AMD2763 | 0.3 | 2.5 | >25 |
| DS | 0.2 | 0.2 | >5 |
| AR177 | 1.1 | 1.5 | >5 |
| AZT | 0.0006 | 0.0008 | 0.0005 |
Virus yield was monitored in the cell-free supernatant from MT-4 cells 4 to 5 days after infection by a viral p24 antigen enzyme-linked immunosorbent assay. Results represent mean values from three to eight separate experiments.
We recently demonstrated that the bicyclams are specific CXCR4 antagonists (36, 40). Therefore, two prototypes, AMD2763 and AMD3100, were tested for their antiviral activity against the SDF-1αres virus. The SDF-1αres virus proved partially cross-resistant to AMD2763 and AMD3100 (10-fold decrease in sensitivity) (Table 1). The SDF-1αres virus was not cross-resistant to the HIV binding or fusion inhibitors AR177 (Zintevir) and DS (Table 1) and the reverse transcriptase inhibitor AZT (Table 1). SDF-1α and AMD2763 were completely inactive against the AMD3100-resistant virus (18, 36) (Table 1), the CXC chemokine SDF-1β and the anti-CXCR4 MAb had no activity against the AMD3100res virus (Table 1), but the AMD3100res virus still retained marked sensitivity to AMD3100 (18) (Table 1).
U87.CD4 transfectants.
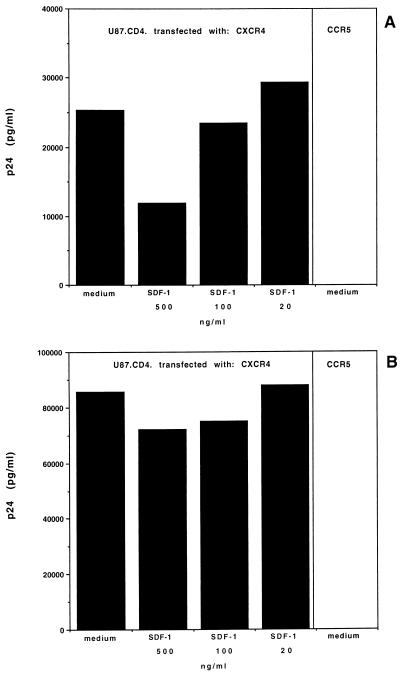
To determine whether the SDF-1αres virus might use a different coreceptor in MT-4 cells, the replication of the SDF-1αres virus was tested in the astroglioma cell line U87 stably expressing CD4 and CXCR4 or CD4 and CCR5 (17). Cells were incubated with 103 pg of p24 from either wild-type or SDF-1αres virus per ml, and the p24 concentrations were measured 6 to 10 days later. Both virus strains were able to infect U87.CD4.CXCR4 at comparable levels (Fig. 3). SDF-1α was active against the wild-type virus in these transfected cells, although to a somewhat lesser extent than in MT-4 cells (Table 1), other CD4+ T-cell lines (data not shown), or peripheral blood mononuclear cells (36). SDF-1α had no significant activity against the SDF-1αres virus (Fig. 3B). The T-tropic NL4.3 wild-type virus was, as expected, not able to infect U87.CD4.CCR5 cells, and the SDF-1αres virus was not able to replicate in these cells either (less than 5 pg of p24 per ml; under the detection limit) (Fig. 3). As controls, the M-tropic HIV-1 BaL strain and simian immunodeficiency virus strain MAC251, known to use CCR5 to enter cells (2, 22), were found to replicate in U87.CD4.CCR5 cells (data not shown). When U87.CD4.CCR5 cells were incubated with 104 pg of p24 from either wild-type or SDF-1αres virus per ml, the concentrations of p24 measured 6 to 10 days later were still below the detection limit. These results suggest that both the wild-type and the SDF-1αres virus strains use CXCR4 for entry and infection and that the SDF-1αres virus cannot switch to CCR5 as a coreceptor for entry.
FIG. 3.
Replication of SDF-1αres virus in U87 cells. U87.CD4.CXCR4 and U87.CD4.CCR5 cells were infected with NL4-3 wild-type (A) and SDF-1αres (B) HIV-1 in the presence of different concentrations of SDF-1α (500, 100, and 20 ng/ml). p24 antigen levels were measured 8 days after infection.
HOS transfectants.
The SDF-1αres virus replicated in HOS.CD4.CCR1, HOS.CD4.CCR2b, HOS.CD4.CCR3, HOS.CD4.CCR4, HOS.CD4.CCR5, and HOS.CD4.CXCR4 cells equally well, whereas the NL4.3 wild-type virus replicated preferentially in HOS.CD4.CXCR4 cells, although viral replication could be measured in the other HOS.CD4 transfectants. In addition, HOS.CD4.pBABE cells were also infected with the SDF-1αres virus. However, all HOS transfectant cells express low amounts of CXCR4 (35a). This finding demonstrates that the SDF-1αres virus still uses CXCR4, although this virus could use a smaller amount of CXCR4 receptors to enter the cells. Neither virus strain, even at 10 ng of p24, was able to replicate in HOS cells expressing CXCR4 or CCR5 (data not shown). Thus, the SDF-1αres virus, like the wild-type virus, needs the CD4 receptor together with the CXCR4 coreceptor to enter target cells.
DNA sequence analysis of the env gene of SDF-1αres.
We identified several mutations in the gp120 gene sequence of the SDF-1αres virus strain that were not present in the wild-type virus strain (Table 2). Four mutations were clustered in the V3 loop region: K269E, Q278H, I288V, and N293D. Other mutations were found in the V1 (N106K), V2 (S134N and F145L), C2 (F245I), and V4 (R378T) regions of the SDF-1αres virus. Remarkably, a deletion of 5 amino acids (FNSTW) at positions 364 to 368 in the V4 loop was found. The F245I mutation in C2, all four mutations in the V3 loop, and the deletion of 5 amino acids were also found in the AMD3100res virus (18).
TABLE 2.
Mutations in gp120 of the SDF-1αres virus
| Amino acid position (region) | Wild-type virus
|
SDF-1αres virus
|
||
|---|---|---|---|---|
| Codon | Amino acid | Codon | Amino acid | |
| 106 (V1) | AAT | N | AAG | K |
| 134 (V2) | AGC | S | AAC | N |
| 145 (V2)a | TTC | F | TTA | L |
| 245 (C2) | TTC | F | ATC | I |
| 269 (V3)a | AAC/AAA | N (K) | GAA | E |
| 278 (V3)a | CAG | Q | CAT | H |
| 288 (V3)a | ATA | I | GTC | V |
| 293 (V3)a | AAT | N | GAT | D |
| Δ364–368a | TTT AAT AGT ACT TGG | FNSTW | Deletion | Deletion |
| 387 (V4) | AGA | R | ACA | T |
Mutations that were also present in the AMD3100res virus (18).
DISCUSSION
A factor allowing the entry of T-tropic HIV-1 strains was identified by genetic complementation of murine CD4+ cells and was named fusin (27). A few months later, this factor was shown to be the receptor for the CXC chemokine SDF-1α, and fusin was renamed CXCR4 (6, 33). This receptor is used by HIV-1 and HIV-2 strains to enter cells (6, 23, 33). It does not allow infection by M-tropic HIV strains, which instead use CCR5 (2, 9, 16, 20, 21). The V3 domain of gp120 was found to be necessary, although other domains of gp120 were also found to play a role in the interaction with CCR5 (44, 45). The role of the V3 domain in the interaction of gp120 with CXCR4 has not been directly demonstrated, but a complete V3 loop substitution of a T-tropic strain with an M-tropic strain resulted in a switch from CXCR4 to CCR5 (11).
It also has been shown by immunoprecipitation that in the presence of CD4, gp120 forms a complex with CXCR4, suggesting that both CXCR4 and CD4 interact directly with the viral envelope (30). Further support for a direct interaction between CXCR4 and gp120 is given by the mutations observed in gp120 of the SDF-1αres virus. Four of the nine mutations in gp120 are located in the V3 domain. Also, mutations were found in other domains of gp120, and one was also present in the CD4 binding domain. Therefore, the CXCR4 binding site is probably not limited to the V3 loop alone.
The SDF-1αres virus is no longer sensitive to the chemokines SDF-1α and SDF-1β, which are the natural ligands for the CXCR4 receptor. The anti-CXCR4 MAb 12G5 is reported to inhibit HIV-1 and HIV-2 infection at 1 to 20 μg/ml, although the ability of this MAb to block infection by T-tropic isolates of HIV-1 is highly dependent on the viral isolate and the target cell (32); MAb 12G5 is even inactive against certain T-tropic viruses, such as the IIIB strain (42). This fact suggests that other cofactors may be involved or that some viruses may use a different epitope of CXCR4 that is not blocked by MAb 12G5. We obtained an IC50 of 8 μg/ml for the NL4.3 strain in MT-4 cells. This MAb also was more active against dual-tropic viruses in MT-4 cells (data not shown), a result which corresponds to what has already been described by other investigators using other T-cell lines (42) and which suggests the usage of different epitopes by dual- and T-tropic viruses. MAb 12G5 completely lost its activity against the SDF-1αres virus, even at a concentration of 20 μg/ml (Table 1).
The two bicyclams, AMD2763 and AMD3100, were only about 10-fold less inhibitory to the SDF-1αres virus than to the wild-type virus. The antiviral activity profile of AMD3100 suggests that it directly interacts with the CXCR4 receptor: AMD3100 inhibits the binding of an anti-CXCR4 MAb to its receptor, blocks infection by T-tropic viruses but not M-tropic viruses, and inhibits intracellular SDF-1α signaling in a concentration-dependent fashion (36, 40). It is therefore reasonable to speculate that the SDF-1αres virus has adapted to use a different binding site on the CXCR4 coreceptor. Of the nine mutations detected in gp120 of the SDF-1αres NL4.3 virus strain, four were located in the V3 domain and all four were also detected in the AMD3100res virus (18).
The SDF-1αres virus was not able to switch to the CCR5 coreceptor. The data obtained with U87.CD4 cells demonstrated this result clearly. U87.CD4 cells are negative for MAb 12G5 staining (23; unpublished data) and do not express CXCR4 mRNA (27). The results obtained with the HOS transfectants were more confusing, due to the low expression of CXCR4 on the parental cells. HOS cells are positive for CXCR4 mRNA (29) and weakly positive when stained with MAb 12G5 (35a). With a higher virus input (104 pg of p24 per ml), the NL4.3 virus was also able to replicate in all of the HOS.CD4 cell lines. Thus, although CXCR4 is expressed in HOS cells, the wild-type virus was not able to use it as avidly as the SDF-1αres virus. The presence of CD4 on the cell membrane was still necessary for the SDF-1αres virus (as for the wild-type virus) to enter the target cells, because HOS cells transfected with only CXCR4 could not be infected (data not shown). However, AMD3100 was still active against the SDF-1αres virus when tested in all HOS.CD4 cell lines at an IC50 comparable to that obtained in MT-4 cells. This finding also demonstrates that the SDF-1αres virus uses CXCR4 as a coreceptor for entry into cells. The results obtained with the transfected cells in the presence of AMD3100 also demonstrated that the SDF-1αres virus does not use CCR1, CCR2b, CCR3, or CCR4 to enter cells. Also, the SDF-1αres virus is not capable of using two newly described chemokine receptors, Bonzo and BOB (17). Because U87.CD4 cells are positive for Bonzo/STRL33 (17), the SDF-1αres virus is able to infect these cells only when CXCR4 is expressed (Fig. 3). The SDF-1αres virus also does not use BOB/GPR15, because although CEMX174 cells are positive for this receptor (17) (but also positive for CXCR4) and the SDF-1αres virus is able to infect CEMX174 cells, the CXCR4 antagonist AMD3100 is able to inhibit SDF-1αres virus infection at an IC50 of 70 ng/ml in these cells (35a).
It took 24 passages in cell cultures (100 days) for the NL4.3 strain to become resistant to SDF-1α (>20-fold resistance). In comparison, it took 17 passages in cell cultures (100 days) for the NL4.3 strain to become resistant to DS (IC50, >125 μg/ml) (>900-fold resistance) (25), 33 passages (182 days) for >200-fold resistance to AR177 (IC50, >125 μg/ml) (24), 25 passages (120 days) for >172-fold resistance to AMD2763 (IC50, >250 μg/ml) (19), and 63 passages (almost 1 year) for 300-fold resistance to AMD3100 (IC50, 546 ng/ml) (18). We never obtained complete resistance against AMD3100 with the NL4.3 strain. The SDF-1αres virus was still sensitive to AMD3100, showing that this compound has a much stronger interaction with CXCR4 than the CXC chemokine itself, a finding which is also reflected in the larger number of mutations present in gp120 of the AMD3100res virus than in gp120 of the SDF-1αres virus (18).
In conclusion, the NL4.3 SDF-1αres virus overcomes the inhibitory effects of SDF-1α by mutations in gp120 but is not able to switch to another coreceptor.
ACKNOWLEDGMENTS
We thank Sandra Claes and Erik Fonteyn for excellent technical assistance. We are grateful to Nathaniel R. Landau for kindly providing the U87.CD4 transfectant cell lines.
This work was supported by grants from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, the Belgian Geconcerteerde Onderzoekacties, the Belgian Fonds voor Geneeskundig Wetenschappelijk Onderzoek, the Janssen Research Foundation, and the Fundació IRSI-CAIXA.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Alkhatib G, Liao F, Berger E A, Farber J M, Peden K W C. A new SIV co-receptor, STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 4.Baba M, Pauwels R, Balzarini J, Arnout J, Desmyter J, De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc Natl Acad Sci USA. 1988;85:6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berson J F, Long D, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–832. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 7.Bridger G J, Skerlj R T, Thornton D, Padmanabhan S, Martellucci S A, Henson G W, Abrams M J, Yamamoto N, De Vreese K, Pauwels R, De Clercq E. Synthesis and structure-activity relationships of phenylenebis(methylene)-linked bis-tetraazamacrocycles that inhibit HIV replication. Effects of macrocyclic ring size and substituents on the aromatic linker. J Med Chem. 1995;38:366–378. doi: 10.1021/jm00002a019. [DOI] [PubMed] [Google Scholar]
- 8.Cheng-Mayer C, Liu R, Landau N R, Stamatatos L. Macrophage tropism of human immunodeficiency virus type 1 and utilization of the CC-CKR5 coreceptor. J Virol. 1997;71:1657–1661. doi: 10.1128/jvi.71.2.1657-1661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 10.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 11.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 12.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1 infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalgleish A G, Beverley P C L, Clapham P R, Crawford D H, Greaves M F, Weiss R A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 14.De Clercq E, Yamamoto N, Pauwels R, Baba M, Schols D, Nakashima H, Balzarini J, Murrer B A, Schwartz D, Thornton D, Bridger G, Fricker S, Henson G, Abrams M, Picker D. Potent and selective inhibition of human immunodeficiency virus (HIV)-1 and HIV-2 replication by a class of bicyclams interacting with a viral uncoating event. Proc Natl Acad Sci USA. 1992;89:5286–5290. doi: 10.1073/pnas.89.12.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Clercq E, Yamamoto N, Pauwels R, Balzarini J, Witvrouw M, De Vreese K, Debyser Z, Rosenwirth B, Peichl P, Datema R, Thornton D, Skerlj R, Gaul F, Padmanabhan S, Bridger G, Henson G, Abrams M. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother. 1994;38:668–674. doi: 10.1128/aac.38.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Marzio P D, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 17.Deng H, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 18.De Vreese K, Kofler-Mongold V, Leutgeb C, Weber V, Vermeire K, Schacht S, Anné J, De Clercq E, Datema R, Werner G. The molecular target of bicyclams, potent inhibitors of human immunodeficiency virus replication. J Virol. 1996;70:689–696. doi: 10.1128/jvi.70.2.689-696.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Vreese K, Reymen D, Griffin P, Steinkasserer A, Werner G, Bridger G J, Esté J, James W, Henson G, Desmyter J, Anné J, De Clercq E. The bicyclams, a new class of potent human immunodeficiency virus inhibitors, block viral entry after binding. Antiviral Res. 1996;29:209–219. doi: 10.1016/0166-3542(95)00837-3. [DOI] [PubMed] [Google Scholar]
- 20.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 21.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 22.Edinger A L, Amedee A, Miller K, Doranz B J, Endres M, Sharron M, Samson M, Lu Z-H, Clements J E, Murphy-Corb M, Peiper S C, Parmentier M, Broder C C, Doms R W. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc Natl Acad Sci USA. 1997;94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 24.Esté, J. A., C. Cabrera, D. Schols, P. Cherepanov, M. Witvrouw, C. Pannecouque, Z. Debyser, R. F. Rando, B. Clotet, J. Desmyter, and E. De Clercq. Human immunodeficiency virus glycoprotein gp120 as the primary target for the antiviral action of AR177 (Zintevir). Mol. Pharmacol., in press. [DOI] [PubMed]
- 25.Esté J A, Schols D, De Vreese K, Van Laethem K, Vandamme A-M, Desmyter J, De Clercq E. Development of resistance of human immunodeficiency virus type 1 to dextran sulfate associated with the emergence of specific mutations in the envelope gp120 glycoprotein. Mol Pharmacol. 1997;52:98–104. doi: 10.1124/mol.52.1.98. [DOI] [PubMed] [Google Scholar]
- 26.Farzan M, Choe H, Martin K, Marcon L, Hofmann W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 28.Harada S, Koyanagi Y, Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985;229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 29.He J, Landau N R. Use of a novel human immunodeficiency virus type 1 reporter virus expressing human placental alkaline phosphatase to detect an alternative viral receptor. J Virol. 1995;69:4587–4592. doi: 10.1128/jvi.69.7.4587-4592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapham C K, Ouyang J, Chandrasekhar B, Nguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 31.Liao F, Alkhatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusion and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 34.Ojwang J O, Buckheit R W, Pommier Y, Mazumder A, DeVreese K, Esté J A, Reymen D, Pallansch L A, Lackman-Smith C, Wallace T L, De Clercq E, McGrath M S, Rando R F. T30177, an oligonucleotide stabilized by an intramolecular guanosine octet, is a potent inhibitor of laboratory strains and clinical isolates of human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1995;39:2426–2435. doi: 10.1128/aac.39.11.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pleskoff O, Tréboute C, Brelot A, Heveker N, Seman M, Alizon M. Identification of a chemokine receptor encoded by human cytomegalovirus as a cofactor for HIV-1 entry. Science. 1997;276:1874–1878. doi: 10.1126/science.276.5320.1874. [DOI] [PubMed] [Google Scholar]
- 35a.Schols, D. Unpublished data.
- 36.Schols D, Esté J A, Henson G, De Clercq E. Bicyclams, a class of potent anti-HIV agents, are targeted at the HIV coreceptor fusin/CXCR-4. Antiviral Res. 1997;35:147–156. doi: 10.1016/s0166-3542(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 37.Schols D, Pauwels R, Baba M, Desmyter J, De Clercq E. Specific interaction of aurintricarboxylic acid with the human immunodeficiency virus/CD4 cell receptor. Proc Natl Acad Sci USA. 1989;86:3322–3326. doi: 10.1073/pnas.86.9.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schols D, Pauwels R, Desmyter J, De Clercq E. Dextran sulfate and other polyanionic anti-HIV compounds specifically interact with the viral GP120 glycoprotein of persistently HIV-1 infected cells. Virology. 1990;175:556–561. doi: 10.1016/0042-6822(90)90440-3. [DOI] [PubMed] [Google Scholar]
- 39.Schols D, Proost P, Van Damme J, De Clercq E. RANTES and MCP-3 inhibit the replication of T-cell-tropic human immunodeficiency virus type 1 strains (SF-2, MN and HE) J Virol. 1997;71:7300–7304. doi: 10.1128/jvi.71.10.7300-7304.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schols D, Struyf S, Van Damme J, Esté J A, Henson G, De Clercq E. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J Exp Med. 1997;186:1383–1388. doi: 10.1084/jem.186.8.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuitemaker H, Koot M, Kootstra N A, Dercksen M W, de Goede R E Y, van Steenwijk R P, Lange J M A, Schattenkerk J K M E, Miedema F, Tersmette M. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strizki J M, Turner J D, Collman R G, Hoxie J, González-Scarano F. A monoclonal antibody (12G5) directed against CXCR-4 inhibits infection with the dual-tropic human immunodeficiency virus type 1 isolate HIV-189.6 but not the T-tropic isolate HIV-1HxB. J Virol. 1997;71:5678–5683. doi: 10.1128/jvi.71.7.5678-5683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 44.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 45.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]