Abstract
Background
Beyond glycemic control, glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and sodium-glucose cotransporter 2 inhibitors (SGLT2is) have been proposed to reduce the risk of cardiovascular events. The aim of the present systematic review and meta-analysis is to demonstrate the effects of GLP-1 RA and SGLT2is on intima-media thickness (IMT).
Methods
PubMed, EMBASE, Web of Science, SCOPUS, and Google Scholar databases were searched from inception to September 9, 2023. All interventional and observational studies that provided data on the effects of GLP-1 RAs or SGLT2is on IMT were included. Critical appraisal was performed using the Joanna Briggs Institute checklists. IMT changes (preintervention and postintervention) were pooled and meta-analyzed using a random-effects model. Subgroup analyses were based on type of medication (GLP-1 RA: liraglutide and exenatide; SGLT2i: empagliflozin, ipragliflozin, tofogliflozin, and dapagliflozin), randomized clinical trials (RCTs), and diabetic patients.
Results
The literature search yielded 708 related articles after duplicates were removed. Eighteen studies examined the effects of GLP-1 RA, and eleven examined the effects of SGLT2i. GLP-1 RA and SGLT2i significantly decreased IMT (MD = −0.123, 95% CI (-0.170, -0.076), P < 0.0001, I2 = 98% and MD = −0.048, 95% CI (-0.092, -0.004), P = 0.031, I2 = 95%, respectively). Metaregression showed that IMT change correlated with baseline IMT, whereas it did not correlate with gender, duration of diabetes, and duration of treatment.
Conclusions
Treatment with GLP-1 RA and SGLT2i can lower IMT in diabetic patients, and GLP-1 RA may be more effective than SGLT2i.
1. Introduction
Glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) and sodium/glucose cotransporter 2 inhibitors (SGLT2is) have been introduced for the treatment of type 2 diabetes mellitus (T2DM). Their promising results in glycemic control and weight loss, as well as their low risk of hypoglycemia, less adverse events, and favourable renocardiovascular effects have made them desirable therapies for the treatment of T2DM and its concomitant diseases and complications [1]. In addition to their putative target, numerous molecular targets for GLP-1s have been identified, justifying their potential for broader medical applications, including autophagy, oxidative stress, platelet function, lipid metabolism, and inflammation [2–9]. GLP-1 RA causes an increase in insulin secretion and a decrease in glucagon levels in response to glucose and delays gastric emptying, thereby suppressing postprandial hyperglycemia and appetite, resulting in a decrease in total energy intake and body weight [10]. SGLT2is act independently of insulin; they block renal glucose reabsorption mediated by SGLT2 expressed along proximal tubules and cause glucosuria [7].
Carotid intima-media thickness (IMT) is a quick and noninvasive ultrasound marker that indicates the thickness of the two innermost layers of the carotid artery. It is a risk stratification tool used as a surrogate marker for atherosclerosis in numerous studies to assess the risk of cardiovascular events [11–13]. We are interested in comparing the effects of GLP-1 RA and SGLT2i therapies on IMT, which may reflect the cardioprotective effects of these drugs. A direct comparison of the cardioprotective benefits of two second-line therapies in T2DM could help us find a better strategy for glycemic control. However, no systematic comparison has been performed for GLP-1 RA or SGLT2i therapies in terms of their effect on IMT. Therefore, we performed a comprehensive systematic review and meta-analyses to determine the effects of GLP-1 RA and SGLT2i drugs on IMT.
2. Methods
This systematic review is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [14]. The study protocol was registered with the International Prospective Registry of Systematic Reviews (PROSPERO).
2.1. Data Sources and Searching Strategy
To identify potentially relevant studies, searches were conducted in the following four databases (since inception to September 9, 2023): PubMed, EMBASE, Web of Science, and SCOPUS, with two reviewers (A.A. and S.H.) working independently and in parallel. Citations of all included studies and relevant published papers were reviewed by hand search. “Google Scholar” was also searched to find potentially relevant articles. Studies were found by searching for three main terms and their synonyms, including “IM,” “SGLT2i,” and “GLP-1 RA.” The complete search strategy for each term is shown in Table S1. The search was not limited by time, type of article, or language. We used reference management software (EndNote X8) to import references, remove duplicates, and review the literature.
2.2. Selection Criteria
Inclusive criteria for this systematic review were studies that investigated IMT in groups of patients treated with GLP-1 RA or SGLT2i. Eligible studies that met the following criteria were included in the meta-analysis: (1) the studies reported mean IMT at baseline and final or mean change in IMT after GLP-1 RA or SGLT2i therapy, and (2) the follow-up period was at least 2 weeks. Two authors (A.A. and S.H.), working independently and in parallel, reviewed the abstract and included the paper reporting the effects of GLP-1 RA or SGLT2i on IMT. Subsequently, A.A. and S.H. independently assessed the full text of the papers and made the final decision. Disagreements in study selection were adjudicated by a third reviewer.
2.3. Quality Assessment
Two authors (L.H. and S.H.) independently assessed the quality of studies using the JBI checklists [15]. The JBI checklist assessed bias in selection, measurement, and analysis. If there were disagreements, they were resolved by discussion or referral to another investigator to achieve consensus. The checklist questions were answered “yes,” “no,” “unclear,” or “not applicable.” For each “yes” answer, 1 point is awarded, and after adding the points, the final score is calculated.
2.4. Data Extraction
The two investigators (A.A. and S.H.) independently extracted the following data: first name, year in which studies were conducted (if no data were provided, the year of study publication was considered), groups, dosage, population, size, gender, age, location, study design, follow-up, IMT at baseline, IMT at end, and disease duration.
2.5. Publication Bias and Statistical Analysis
Publication bias was examined using funnel plots, Egger's test, and Duval and Tweedie's trim and fill test. Pre- and postintervention IMT values were recorded to calculate the mean difference (MD) and 95% confidence interval (CI). Subgroup analyses were performed based on drug classes, and sensitivity analyses were performed based on effect models (random to fixed or vice versa), RTCs, T2DM patients, and R values (0.3, 0.5, and 0.8). The Cochrane Q statistic was used to assess heterogeneity, and if it was less than 0.05, a random-effects model was used for analysis. Metaregression was performed to determine the correlation between IMT changes and disease duration, gender, follow-up period, and baseline IMT. A P value of less than 0.05 was considered statistically significant for the outcome and heterogeneity analyses. Data analysis was performed using Comprehensive Meta-Analysis software (CMA) V.3.
3. Results
The literature search yielded 708 related articles after duplicates were removed. Eighteen studies examined the effects of GLP-1 RA [11–13, 16–31], and eleven examined the effects of SGLT2i on IMT [32–42]. Studies that did not provide IMT results [43–49], duplicate data [50–56], combination therapies without apparent GLP-1 RA effects [57], or assessed IMT of arteries other than the carotid artery were excluded [58–60]. The study selection process is shown in Figure 1.
Figure 1.

PRISMA flow diagram of the systematic review process.
3.1. Characteristics of the Included Studies
Three different GLP-1 RA drugs were investigated in the included studies: liraglutide [11, 12, 16, 18, 23–29], semaglutide [21], and exenatide [19, 22, 30, 31]. Also, five different SGLT2i drugs were studied, including empagliflozin [32, 36, 38, 42], ipragliflozin [33, 39, 41], tofogliflozin [34, 40], dapagliflozin [35–37, 42], and luseogliflozin [40]. The range of intervention periods for GLP-1 RA trials ranged from 4 months [12, 13, 21, 26] to 3 years [29] and for SGLT2i trials was from 2 weeks [38] to 3.6 years [36]. All SGLT2i studies [32–42] and fifteen GLP-1 RA studies included T2DM patients [11, 12, 17, 19–27, 29–31]. Italy (n = 7) was the country with the largest number of published articles for GLP-1 RA and Japan for SGLT2i (n = 5). Characteristics of the evaluated studies are presented in Table 1.
Table 1.
Characteristics of included studies.
| Study, year | Groups, dosage (per day) | Size, population | Male | Age (years) | Location | Study design | Follow-up | Baseline CIMT (mm) | Final CIMT (mm) | Disease duration (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| GLP-1 RA | ||||||||||
| Rizzo et al., 2012 [26] | Liraglutide 1.2 mg add on metformin 1500 mg | 121 T2DM | 59% | 62 ± 9 | Italy | Uncontrolled clinical trial | 18 months | 0.97 ± 0.18 | 0.78 ± 0.20 | 9 ± 8 |
| Dejgaard et al., 2016 [16] | Liraglutide 1.8 mg Placebo |
50 T1DM 50 T1DM |
60% 70% |
47 ± 13 49 ± 12 |
Denmark | Randomized, double-blinded clinical trial | 24 weeks | ND | 0.01 (-0.01, 0.03)/p = 0.361 | 20 ± 12 25 ± 12 |
| Nikolic, 2021 [13] | Liraglutide 1.2 mg add on metformin | 62 T2DM | 50% | 61 ± 9 | Italy | Uncontrolled clinical trial | 4 months | 1.13 ± 0.29 | 0.92 ± 0.24 | 9 ± 8 |
| Patti et al., 2019 [22] | Semaglutide 0.50 mg/week add on routine treatment | 40 T2DM | 65% | 66 ± 10 | Italy | Retrospective | 4 months | 1.04 ± 0.16 | 0.90 ± 0.14 | 14 ± 10 |
| Giglio, 2014 [11] | Liraglutide add on metformin 1500-3000 mg | 29 T2DM + NAFLD 29 T2DM |
55% 55% |
61 ± 10 61 ± 8 |
Italy | Clinical trial | 8 months | 1.00 ± 0.30 0.91 ± 0.23 |
0.90 ± 0.10 0.85 ± 0.15 |
10 ± 9 9 ± 8 |
| Patti, 2023 [21] | Exenatide LAR 2 mg/week add on metformin 1500-3000 mg | 60 T2DM | 68% | 60 ± 10 | Italy | Uncontrolled clinical trial | 8 months | 0.98 ± 0.14 | 0.87 ± 0.15 | 9 ± 8 |
| Hopkins, 2013 [17] | Exenatide 20 μg Liraglutide 1.2 mg |
9 T2DM + obesity 2 T2DM + obesity |
63.6% | 55 ± 8 | United Kingdom | Uncontrolled clinical trial | 6 months | 0.76 ± 0.07 | 0.76 ± 0.11 | 8.3 ± 4.7 |
| Kahal et al., 2015 [18] | Liraglutide 1.8 mg | 13 PCOS 12 controls |
0% | 33.9 ± 6.7 33.5 ± 7.1 |
United Kingdom | Clinical trial | 6 months | 0.51 ± 0.05 0.48 ± 0.06 |
0.51 ± 0.05 0.48 ± 0.06 |
ND |
| Köseoğlu, 2021 [19] | Exenatide 20 μg | 45 T2DM + obesity | 8.8% | 47.91 ± 7.30 | Turkey | Uncontrolled clinical trial | 6 months | 1.04 ± 0.11 | 0.75 ± 0.12 | ND |
| Zhang, 2022 [31] | Exenatide Insulin |
27 T2DM 32 T2DM |
70.0% 43.7% |
58.85 ± 12.54 58.03 ± 13.32 |
China | Randomized, open-label clinical trial | 52 weeks | ND | -0.1 0.02 (change from baseline) |
6.59 ± 5.32 7.81 ± 6.02 |
| Luna-Marco et al., 2023 [20] | GLP-1 RA Non-GLP-1 RA Control |
59 T2DM 196 T2DM 175 Control |
63% 55% 54% |
56.5 ± 9.9 59.9 ± 10.5 54.9 ± 13.5 |
Spain | Cross-sectional | ND | ND | 0.630 ± 0.742 0.750 ± 0.238 0.516 ± 0.070 |
13.8 ± 8.7 10.4 ± 8.1 |
| Meng, 2023† [12] | Liraglutide Metformin and sulfonylurea |
38 T2DM 40 T2DM |
60.5% 45% |
56 ± 11 59 ± 7 |
China | Randomized clinical trial | 16 weeks | 1.14 ± 0.10 1.13 ± 0.13 |
0.85 ± 0.08 1.05 ± 0.10 |
0.5-16.0 1.0-16.0 |
| Patti et al., 2013 [23] | Liraglutide 1.2 mg add on metformin | 64 T2DM | 50% | 63 ± 8 | Italy | Uncontrolled clinical trial | 8 months | 1.19 ± 0.47 | 0.95 ± 0.21 | 9 ± 8 |
| Ripa, 2021 [24] | Liraglutide 1.8 mg Placebo |
50 T2DM 48 T2DM |
88.2% 80.4% |
65.9 ± 8.6 66.9 ± 7.8 |
Denmark | Randomized, double-blinded clinical trial | 26 weeks | 0.77 ± 0.17 0.75 ± 0.14 |
0.76 ± 0.17 0.75 ± 0.14 |
12.2 ± 3.2 11.3 ± 3.4 |
| Rizzo et al., 2012 [26] | Liraglutide 1.2 mg add on metformin 1500 mg | 33 T2DM | 58% | 59 ± 9 | Italy | Uncontrolled clinical trial | 4 months | 1.55 ± 0.45 | 1.36 ± 0.31 | ND |
| Sun, 2023 [28] | Liraglutide 1.2 mg Lifestyle interventions |
17 IGT + overweight 22 IGT + overweight |
35.3% 31.9% |
44.92 ± 14.69 48.91 ± 10.12 |
China | Randomized, double-blinded clinical trial | 6 months | 0.91 ± 0.25 0.91 ± 0.23 |
0.70 ± 0.16 0.92 ± 0.18 |
ND |
| Yoshida et al., 2018 [29]∗‡ | Liraglutide 0.9 mg Linagliptin |
34 T2DM | 23.5% | 75.7 ± 7.8 | Japan | Clinical trial | 3 years | 2.42 ± 1.65 2.25 ± 1.19 |
2.14 ± 1.40 2.19 ± 1.01 |
ND |
| Yoshida et al., 2012 [30]∗ | Exenatide 20 μg add on routine treatment Routine treatment |
56 T2DM 50 T2DM |
44.6% 44.6% |
63.8 ± 11.0 63.8 ± 11.0 |
Japan | Clinical trial | 12 months | 1.09 ± 0.33 1.08 ± 0.27 |
1.02 ± 0.31 1.13 ± 0.34 |
ND |
| SGLT2i | ||||||||||
| Irace et al., 2018 [32] | Empagliflozin Incretin-based therapy |
40 T2DM 30 T2DM |
75% 80% |
58 ± 9 60 ± 7 |
Italy | Prospective cohort | 3 months | 0.831 ± 0.156 0.890 ± 0.146 |
0.766 ± 0.127 0.841 ± 0.109 |
15 ± 9 17 ± 10 |
| Kang, 2023 [33] | Ipragliflozin 50 mg Sitagliptin 100 mg |
70 T2DM 70 T2DM |
ND | ND | South Korea | Randomized, open-label clinical trial | 24 weeks | 0.900 ± 0.420 0.830 ± 0.230 |
0.900 ± 0.360 0.840 ± 0.250 |
ND |
| Katakami, 2022 [34] | Tofogliflozin 20 mg Conventional therapy |
169 T2DM 171 T2DM |
58.3% 58.0% |
61.4 ± 9.3 60.8 ± 9.7 |
Multicenter (Japan) | Randomized, open-label clinical trial | 104 weeks | 0.870 ± 0.160 0.860 ± 0.150 |
0.740 ± 0.140 0.720 ± 0.130 |
12.1 ± 8.4 12.4 ± 8.2 |
| Korzh et al., 2020 [35]∗ | Dapagliflozin 10 mg | 35 T2DM | ND | ND | Ukraine | Clinical trial | 12 weeks | ND | Decreased significantly from baseline | ND |
| Kourtidou, 2023 [36] | Empagliflozin/dapagliflozin Standard care |
15 T2DM 25 T2DM |
73.3% 68% |
68.9 ± 7.3 73.2 ± 9.6 |
Greece | Cross-sectional | 3.6 ± 1.2 years | ND | 0.7 ± 0.2 0.9 ± 0.2 (no significant difference) |
12.6 ± 9.1 13.3 ± 7.1 |
| Lamaida, 2022∗ [37] | Dapagliflozin Standard care |
20 T2DM 20 T2DM |
ND | 55 ± 10 50 ± 10 |
Italy | Clinical trial | 2.0 years | ND | Decreased significantly from baseline | ND |
| Murakami and Mizuno, 2014 [38]∗ | SGLT2i Standard care |
10 T2DM 10 T2DM |
ND | ND | ND | Randomized clinical trial | 2 weeks | 0.340 ± 0.080 0.350 ± 0.080 |
0.310 ± 0.060 0.360 ± 0.050 |
ND |
| Nomiyama et al., 2018 [39] | Ipragliflozin 50 mg | 134 T2DM | 52% | 53.9 ± 10.5 | Japan | Clinical trial | 52 weeks | 0.760 ± 0.160 | 0.750 ± 0.150 | 8.2 ± 7.9 |
| Sakai, 2019 [40] | Empagliflozin 10-25 mg Luseogliflozin 2.5-5 mg Tofogliflozin 20 mg |
59 HfpEF + T2DM 63 HfpEF + T2DM 62 HfpEF + T2DM |
61.5% 42.9% 78.6% |
62.0 ± 9.4 70.3 ± 11.4 66.0 ± 9.8 |
Japan | Clinical trial | 12 weeks | 0.860 ± 0.700 0.840 ± 0.700 0.870 ± 0.500 |
0.780 ± 0.200 0.940 ± 0.400 0.730 ± 0.300 |
ND |
| Tanaka, 2023 [41] | Ipragliflozin 50 mg Standard care |
241 T2DM 215 T2DM |
69.4% 67.2% |
67 (60, 72) 68 (60, 73) |
Multicenter (Japan) | Randomized, open-label clinical trial | 24 months | 0.8200 ± 0.037 0.8400 ± 0.037 |
0.815 ± 0.034 0.836 ± 0.039 |
9.1 ± 6.8 8.1 ± 6.9 |
| Yamagishi et al., 2016 [42] ∗ | SGLT2i | 31 T2DM + obesity | ND | 53 | Japan | Clinical trial | 12 months | 1.340 ± 0.480 | 1.220 ± 0.437 | ND |
∗Conference papers. †Chinese language. ‡Max MIT. Abbreviations: HfpEF: heart failure with preserved ejection fraction; T2DM: type 2 diabetes mellitus; PCOS: polycystic ovary syndrome; NAFLD: nonalcoholic fatty liver disease; IGT: impaired glucose tolerance; ND: not determined.
3.2. GLP-1 RA
Nineteen GLP-1 RA-treated groups with a total population of 790 subjects were included in the meta-analysis. Figure 2 shows that GLP-1 RA significantly reduced IMT (MD = −0.123, 95% CI (-0.170, -0.076), P < 0.0001, I2 = 98%). A sensitivity analysis on studies that included only T2DM patients showed a higher potential of GLP-1 RA to reduce IMT (MD = −0.145, 95% CI (-0.196, -0.094), P < 0.0001, I2 = 98%) (Figure S1). In addition, a sensitivity analysis based on 5 RCTs reached the same conclusion (MD = −0.119, 95% CI (-0.219, -0.018), P = 0.021, I2 = 99%) (Figure 3). A subgroup analysis on liraglutide and exenatide trials significantly reduced IMT (liraglutide: MD = −0.127, 95% CI (-0.201, -0.054), P = 0.001, I2 = 99%; exenatide: MD = −0.144, 95% CI (-0.240, -0.047), P = 0.003, I2 = 99%) (Figure S2). Metaregression showed that IMT change was significantly correlated with baseline IMT (coefficient = −0.246, P = 0.0001) but not significantly correlated with duration of treatment, duration of diabetes, and gender (coefficient = −0.003, P = 0.635; coefficient = 0.009, P = 0.108; and coefficient = 0.001, P = 0.849, respectively) (Figure 4).
Figure 2.

Forest plot displaying pre-post difference and 95% confidence interval for the impact of GLP-1 RA on IMT.
Figure 3.

Forest plot displaying pre-post difference and 95% confidence interval for the impact of GLP-1 RA on IMT based on randomized clinical trials.
Figure 4.

Meta-regression plots of the association between IMT with gender, follow-up, duration of diabetes, and baseline IMT for GLP-1 RA studies.
Meta-analysis of 4 studies (n = 343) showed a significant reduction in the GLP-1 RA group compared with the placebo/control group (MD = −0.398, 95% CI (-0.792, -0.004), P = 0.048, I2 = 68%) (Figure 5).
Figure 5.

Forest plot displaying mean difference and 95% confidence interval for the impact of GLP-1 RA compared to control groups on IMT.
3.3. SGLT2i
Ten groups treated with SGLT2i with a total population of 879 subjects were included in the meta-analysis. Figure 6 shows that SGLT2i could significantly reduce IMT (MD = −0.048, 95% CI (-0.092, -0.004), P = 0.031, I2 = 95%). In addition, a sensitivity analysis based on 4 RCTs reached the same conclusion (MD = −0.043, 95% CI (-0.119, 0.034), P = 0.274, I2 = 98%) (Figure 7), but the sensitivity analysis based on the change from random to fixed effects showed a significant reduction in IMT (MD = −0.067, 95% CI (-0.077, -0.057), P = 0.0001). A subgroup analysis on empagliflozin and tofogliflozin trials significantly reduced IMT (empagliflozin: MD = −0.066, 95% CI (-0.094, -0.037), P < 0.0001, I2 = 0%; tofogliflozin: MD = −0.130, 95% CI (-0.145, -0.116), P < 0.0001, I2 = 0%), whereas ipragliflozin failed to reduce IMT (MD = −0.007, 95% CI (-0.019, 0.004), P = 0.222, I2 = 0%) (Figure S3). Metaregression showed that IMT change was not significantly correlated with baseline IMT, treatment duration, diabetes duration, and gender (coefficient = −0.092, P = 0.363; coefficient = −0.001, P = 0.623; coefficient = −0.012, P = 0.178, and coefficient = −0.004, P = 0.171, respectively) (Figure 8).
Figure 6.

Forest plot displaying pre-post difference and 95% confidence interval for the impact of SGLT2i on IMT.
Figure 7.
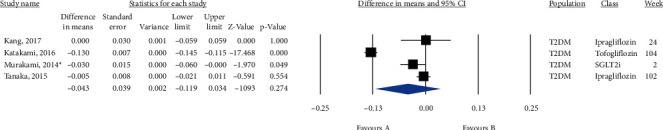
Forest plot displaying pre-post difference and 95% confidence interval for the impact of SGLT2i on IMT based on randomized clinical trials.
Figure 8.

Metaregression plots of the association between IMT with gender, follow-up, duration of diabetes, and baseline IMT for SGLT2i studies.
All comparisons were repeated by changing the R value to 0.3, 0.5, or 0.8, but no differences were found. A sensitivity analysis in which the random-effects analysis was replaced by a fixed-effects analysis also confirmed the results, except as noted in the manuscript.
3.4. Quality Assessment and Publication Bias
Quality assessment using the JBI checklist and final scores for cohort studies, cross-sectional studies, RCTs, and nonrandomized clinical trials are described in detail in Table S2. All funnel plots of all analyses are shown in the figures. Figure 9 shows the funnel plot of the pre-post comparison of GLP-1 RA and SGLT2i treatment, and Figure S4 shows the funnel plots of the sensitivity analysis. In addition, the results of the Egger test and the trim-and-fill method of Duval and Tweedie, which indicate no significant publication bias, are shown in Table 2 (Table 2).
Figure 9.

Funnel plot displaying the impact of GLP-1 RA and SGLT2i on IMT.
Table 2.
Publication bias evaluation by Egger's regression test and Duval and Tweedie trim and fill test.
| Finding/distribution pattern | Egger's test | Trim and fill method | |||
|---|---|---|---|---|---|
| Egger's intercept | P value | Number of trimmed studies | Point estimate after trim | Change after trim | |
| Baseline-final GLP-1 RA | -1.191 | 0.706 | 1 | -0.113 | 0.010 |
| Baseline-final GLP-1 RA (RCTs) | -1.172 | 0.917 | 0 | -0.119 | 0.000 |
| Baseline-final GLP-1 RA (T2DM) | -1.685 | 0.565 | 0 | -0.196 | 0.000 |
| Baseline-final liraglutide | -2.523 | 0.590 | 0 | -0.127 | 0.000 |
| Baseline-final exenatide | -5.933 | 0.581 | 0 | -0.240 | 0.000 |
| GLP-1 RA compared to control | -6.759 | 0.085 | 0 | -0.398 | 0.000 |
| Baseline-final SGLT2i | 0.621 | 0.816 | 0 | -0.048 | 0.000 |
| Baseline-final SGLT2i (RCTs) | 5.190 | 0.629 | 0 | -0.043 | 0.000 |
RCT: randomized clinical trial; T2DM: type 2 diabetes mellitus; GLP-1 RA: glucagon-like peptide-1 receptor agonists; SGLT2i: sodium-glucose cotransporter 2 inhibitors.
4. Discussion
T2DM is associated with a high prevalence of cardiovascular risk, and pharmacotherapies have been introduced to reduce the risk of atherosclerosis in various ways, including glycemic control, lipid balance, uric acid lowering, and blood pressure control [61]. Our meta-analysis showed a significant reduction in IMT, a surrogate atherosclerosis marker, after GLP-1 RA or SGLT2i therapy; however, it appears that GLP-1 RA is more effective in reducing IMT. Similarly, a recent meta-analysis of RCTs showed that GLP-1 RAs were effective in preventing serious adverse cardiovascular events in T2DM patients with obesity (relative risk = 0.88, 95% CI (0.81, 0.96)), whereas SGLT2i marginally prevented serious adverse cardiovascular events (relative risk = 0.91, 95% CI (0.83, 1.00)) [62]. In contrast to a recent review showing cardiovascular benefits for liraglutide and semaglutide but not for exenatide, we demonstrated that exenatide can also reduce IMT [63]. It appears that the effects of GLP-1 RA are not class-dependent, whereas the effects of SGLT2i are.
Consistent with our findings, previous studies reported that GLP-1 RA was effective in reducing major adverse events associated with cardiac events regardless of gender. In contrast, in terms of reducing major adverse events associated with cardiac events, SGLT2i was effective in men but not in women [64]. This inconsistency may be due to different outcome measures. Metaregression analysis showed that the effects of GLP-1 RA and SGLT2i persisted with long-term treatment, suggesting that these drugs do not induce tolerance. In contrast to a previous study claiming that the duration of T2DM might influence efficacy, the metaregression showed no significant correlation between the duration of diabetes and change in IMT [65]. However, the metaregression showed that higher baseline IMT leads to greater IMT reduction. A study by Kahal et al. showed that GLP-1 RA was not significantly effective in patients with polycystic ovary syndrome whose baseline IMT was lower than that of T2DM patients [18]. In these cases, confounding factors and heterogeneity may affect the results, so well-designed RCTs are warranted.
A meta-analysis by Song et al. evaluated the efficacy of GLP-1-based therapies and concluded that IMT was not significantly reduced. Insufficient studies, heterogeneity, and pooling other GLP-1-based therapy other than GLP-1 RA including dipeptidyl peptidase-4 inhibitors may lead to different results compared with our findings [66]. They also showed that brain natriuretic peptide, a marker of atherosclerosis, decreased significantly with GLP-1-based therapies. Furthermore, in a prospective study of elderly people in Sweden, it was observed that higher serum GLP-1 levels correlated with lower IMT [67].
Prior studies suggest that liraglutide can regulate the NLRP3 inflammasome and NF-κB signaling pathway, which causes the inflammatory state [28, 68, 69]. It has also been shown that GLP-1 RA protects cardiomyocytes from IL 1β-induced metabolic dysfunction and mitochondrial dysfunction [70]. Previous studies have shown that GLP-1 RA therapy lowers both systolic and diastolic blood pressure [71], improves endothelial dysfunction [72, 73], and reduces macrophage foam cell formation and atherosclerosis [74]. Qu and Qu reviewed evidence from epidemiological and human studies that low-density lipoprotein cholesterol (LDL-C) is an important regulator in the development of atherosclerosis [75]. A previous meta-analysis by Zhao et al. showed a significant reduction in LDL-C following GLP-1 RA therapy [76], whereas Sánchez-García et al. did not achieve a significant reduction after SGLT2is [77]. Another mechanism described for GLP-1 RA agonists is that these drugs increase antioxidant enzymes (superoxide dismutase and glutathione reductase) and decrease reactive oxygen species and malondialdehyde levels [78]. In vivo studies have shown that GLP-1 RA reduces atherosclerosis by suppressing endoplasmic reticulum stress, macrophage apoptosis, and microvesicle production [79]. Hyperglycemia has also been shown to lead to a decrease in endothelial nitric oxide function via a decrease in synthesis and an increase in degradation and to play a role in endothelial dysfunction, with liraglutide effectively restoring endothelial nitric oxide synthase activity in the diabetic mouse model [80, 81]. The same mechanism involving amelioration of inflammation, insulin resistance, endothelial dysfunction, dislipidemia, hyperglycemia, and oxidative state has been proposed for SGLT2is [82–86]. Previous studies have demonstrated the importance of AT1R/NADPH oxidase/SGLT1 and 2 signaling pathways in promoting atherosclerosis [87–89]. They showed that atherosclerotic plaques have higher SGLT2 expression [87–89]. A recent meta-analysis summarizing data from 9 RCTs and 2 cohorts concluded that SGLT2i improves flow-mediated dilation but not pulse wave velocity [90].
The paucity of high-quality randomized clinical trials in this systematic review is one of the major limitations of the current study. Lacking sufficient studies, we could not evaluate the effects of different classes, different doses, or patient characteristics of GLP-1 RA or SGLT2i on IMT. Also, there were insufficient studies to compare SGLT2i with control groups. There was too much heterogeneity, which could be due to different inclusion criteria, different types and dosages of GLP-1 RA or SGLT2i, follow-up time, and study design. Despite these aforementioned biases, sensitivity analyses yielded nearly consistent results.
In conclusion, GLP-1 RA and SGLT2i may reduce IMT. Among the different GLP-1 RAs, liraglutide was the most studied and had a significant effect on IMT reduction. In addition, GLP-1 RAs might be more effective than SGLT2is in lowering IMT.
Acknowledgments
We thank the Student Research Committee of the Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran, and the cardiologists of Ghaem Hospital, Mashhad University of Medical Sciences, Mashhad, Iran, for their scientific support.
Data Availability
Data is available on request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Table S1: search strategy of GLP-1 RA and SGLT2i. Table S2: quality assessment table for randomized clinical trial studies, cohort studies, cross-sectional studies, and nonrandomized studies based on JBI Critical Appraisal. Figure S1: GLP-1RA and IMT (sensitivity analysis on T2DM patients). Figure S2: liraglutide and exenatide effects on IMT. Figure S3: empagliflozin, tofogliflozin, and ipragliflozin effects on IMT. Figure S4: all analysis funnel plots (baseline-final comparison of GLP-1 RA (RCTs), baseline-final comparison of GLP-1 RA (T2DM), and baseline-final comparison of SGLT2i (RCTs)).
References
- 1.Brown E., Wilding J. P. H., Barber T. M., Alam U., Cuthbertson D. J. Weight loss variability with SGLT2 inhibitors and GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: mechanistic possibilities. Obesity Reviews . 2019;20(6):816–828. doi: 10.1111/obr.12841. [DOI] [PubMed] [Google Scholar]
- 2.Yaribeygi H., Maleki M., Butler A. E., Jamialahmadi T., Sahebkar A. The impact of incretin-based medications on lipid metabolism. Journal of Diabetes Research . 2021;2021:10. doi: 10.1155/2021/1815178.1815178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yaribeygi H., Maleki M., Atkin S. L., Jamialahmadi T., Sahebkar A. Impact of incretin-based therapies on adipokines and adiponectin. Journal of Diabetes Research . 2021;2021:9. doi: 10.1155/2021/3331865.3331865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaribeygi H., Lhaf F., Sathyapalan T., Sahebkar A. Effects of novel antidiabetes agents on apoptotic processes in diabetes and malignancy: implications for lowering tissue damage. Life Sciences . 2019;231, article 116538 doi: 10.1016/j.lfs.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Radbakhsh S., Sathyapalan T., Banach M., Sahebkar A. Incretins and microRNAs: interactions and physiological relevance. Pharmacological Research . 2020;153, article 104662 doi: 10.1016/j.phrs.2020.104662. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafizadeh M., Yaribeygi H., Atkin S. L., Sahebkar A. Effects of newly introduced antidiabetic drugs on autophagy. Diabetes & Metabolic Syndrome . 2019;13(4):2445–2449. doi: 10.1016/j.dsx.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Akbari A., Rafiee M., Sathyapalan T., Sahebkar A. Impacts of Sodium/Glucose Cotransporter-2 Inhibitors on Circulating Uric Acid Concentrations: A Systematic Review and Meta-Analysis. Journal of Diabetes Research . 2022;2022:17. doi: 10.1155/2022/7520632.7520632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yaribeygi H., Atkin S. L., Butler A. E., Sahebkar A. Sodium–glucose cotransporter inhibitors and oxidative stress: an update. Journal of Cellular Physiology . 2019;234(4):3231–3237. doi: 10.1002/jcp.26760. [DOI] [PubMed] [Google Scholar]
- 9.Yaribeygi H., Atkin S. L., Jamialahmadi T., Sahebkar A. A review on the effects of new anti-diabetic drugs on platelet function. Endocrine, Metabolic & Immune Disorders Drug Targets . 2020;20(3):328–334. doi: 10.2174/1871530319666191014110414. [DOI] [PubMed] [Google Scholar]
- 10.Yanai H., Adachi H., Hakoshima M., Katsuyama H. Glucagon-like peptide 1 receptor agonists versus sodium-glucose cotransporter 2 inhibitors for atherosclerotic cardiovascular disease in patients with type 2 diabetes. Cardiology Research . 2023;14(1):12–21. doi: 10.14740/cr1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giglio R. V., Rizzo M., Patti A. M., et al. Liraglutide improves carotid intima-media thickness in patients with type 2 diabetes and non-alcoholic fatty liver disease: an 8-month prospective pilot study. Diabetologia . 2014;57(1):p. S369. doi: 10.1517/14712598.2015.1067299. [DOI] [PubMed] [Google Scholar]
- 12.Meng X. M., Hao Y. P., Yu S., Ren R. Z., Yu X., Tang Y. X. Effect of liraglutide on platelet distribution width and carotid intima-media thickness in type 2 diabetic mellitus patients with obesity. Zhonghua Yi Xue Za Zhi . 2023;103(17):1316–1322. doi: 10.3760/cma.j.cn112137-20220924-02018. [DOI] [PubMed] [Google Scholar]
- 13.Nikolic D., Giglio R. V., Rizvi A. A., et al. Liraglutide reduces carotid intima-media thickness by reducing small dense low-density lipoproteins in a real-world setting of patients with type 2 diabetes: a novel anti-atherogenic effect. Diabetes Therapy . 2021;12(1):261–274. doi: 10.1007/s13300-020-00962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page M. J., McKenzie J. E., Bossuyt P. M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Revista Espanola de Cardiologia . 2021;74(9):790–799. doi: 10.1016/j.recesp.2021.06.016. [DOI] [PubMed] [Google Scholar]
- 15.Ma L.-L., Wang Y.-Y., Yang Z.-H., Huang D., Weng H., Zeng X.-T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Military Medical Research . 2020;7(1):p. 7. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dejgaard T. F., Johansen N. B., Frandsen C. S., et al. Cardiovascular effects of liraglutide in patients with type 1 diabetes: a randomised, double-blinded placebo-controlled trial (Lira-1) Diabetologia . 2016;59(1):S356–S357. [Google Scholar]
- 17.Hopkins N. D., Cuthbertson D. J., Kemp G. J., et al. Effects of 6 months glucagon-like peptide-1 receptor agonist treatment on endothelial function in type 2 diabetes mellitus patients. Diabetes, Obesity & Metabolism . 2013;15(8):770–773. doi: 10.1111/dom.12089. [DOI] [PubMed] [Google Scholar]
- 18.Kahal H., Aburima A., Ungvari T., et al. The effects of treatment with liraglutide on atherothrombotic risk in obese young women with polycystic ovary syndrome and controls. BMC Endocrine Disorders . 2015;15(1) doi: 10.1186/s12902-015-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Köseoğlu D., Koparal S. S., Başer Ö. Ö., Berker D. Exenatide improves cardiovascular risk factors in obese patients with type 2 diabetes mellitus: a prospective study. Turkish Journal of Medical Sciences . 2021;51(1):167–174. doi: 10.3906/sag-2004-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luna-Marco C., de Marañon A. M., Hermo-Argibay A., et al. Effects of GLP-1 receptor agonists on mitochondrial function, inflammatory markers and leukocyte-endothelium interactions in type 2 diabetes. Redox Biology . 2023;66, article 102849 doi: 10.1016/j.redox.2023.102849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patti A. M., Giglio R. V., Allotta A., et al. Effect of semaglutide on subclinical atherosclerosis and cardiometabolic compensation: a real-world study in patients with type 2 diabetes. Biomedicine . 2023;11(5):p. 1362. doi: 10.3390/biomedicines11051362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patti A. M., Nikolic D., Magan-Fernandez A., et al. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: an 8-month prospective study. Diabetes Research and Clinical Practice . 2019;149:163–169. doi: 10.1016/j.diabres.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Patti A. M., Rizzo M., Di Bartolo V., et al. Beneficial effects of liraglutide on carotid intima-media thickness in patients with type-2 diabetes: a 8-month prospective study. Cardiology . 2013;126:p. 290. [Google Scholar]
- 24.Ripa R. S., Zobel E. H., von Scholten B. J., et al. Effect of Liraglutide on arterial inflammation assessed as [18F]FDG uptake in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. Circulation: Cardiovascular Imaging . 2021;14(7, article e012174) doi: 10.1161/CIRCIMAGING.120.012174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizzo M., Chandalia M., Patti A. M., et al. Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovascular Diabetology . 2014;13(1) doi: 10.1186/1475-2840-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizzo M., Patti A. M., Di Bartolo V., Giglio R. V., Montalto G., Rizvi A. A. Effect of liraglutide on carotid intima-media thickness in patients with type-2 diabetes: a 4-month prospective study. Diabetes . 2012;61:p. A109. [Google Scholar]
- 27.Rizzo M., Rizvi A. A., Patti A. M., et al. Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: an 18-month prospective study. Cardiovascular Diabetology . 2016;15(1):p. 162. doi: 10.1186/s12933-016-0480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L., Yuan Y., Li Y., Rao X. Effect of liraglutide on atherosclerosis in patients with impaired glucose tolerance: a double-blind, randomized controlled clinical trial. Experimental and Therapeutic Medicine . 2023;25(6):p. 249. doi: 10.3892/etm.2023.11948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshida M., Morimoto T., Oh E., et al. Possibility of liraglutide for prevention of dementia progression in patients with type 2 diabetes. Diabetes . 2018;67(Supplement_1):p. A296. doi: 10.2337/db18-1110-P. [DOI] [Google Scholar]
- 30.Yoshida M., Yamamoto N., Saeki A., et al. Exenatide therapy reduced intina-media thickness of carotid artery in patients with type 2 diabetes. Diabetologia . 2012;55:S322–S323. [Google Scholar]
- 31.Zhang J., Xian T.-Z., Wu M.-X., et al. Comparing the effects of twice-daily exenatide and insulin on renal function in patients with type 2 diabetes mellitus: secondary analysis of a randomized controlled trial. Journal of Investigative Medicine . 2022;70(7):1529–1535. doi: 10.1136/jim-2021-002237. [DOI] [PubMed] [Google Scholar]
- 32.Irace C., Casciaro F., Scavelli F. B., et al. Empagliflozin influences blood viscosity and wall shear stress in subjects with type 2 diabetes mellitus compared with incretin-based therapy. Cardiovascular Diabetology . 2018;17(1):p. 52. doi: 10.1186/s12933-018-0695-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang S. M., Yun H. M., Sohn M., Lim S. Vascular and metabolic effects of ipragliflozin versus sitagliptin (IVS) in type 2 diabetes treated with sulphonylurea and metformin: IVS study. Diabetes, Obesity and Metabolism . 2023;25(7):1922–1931. doi: 10.1111/dom.15056. [DOI] [PubMed] [Google Scholar]
- 34.Katakami N., Mita T., Maeda N., et al. Evaluation of the effect of tofogliflozin on the tissue characteristics of the carotid wall—a sub-analysis of the UTOPIA trial. Cardiovascular Diabetology . 2022;21(1):p. 19. doi: 10.1186/s12933-022-01451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korzh O., Titkova A., Krasnokutskiy S. Dapagliflozin improves endothelial function and microvascular reactivity in type 2 diabetes mellitus. American Heart Journal . 2020;229:p. 168. doi: 10.1016/j.ahj.2020.10.029. [DOI] [Google Scholar]
- 36.Kourtidou C., Rafailidis V., Varouktsi G., et al. Effects of sodium-glucose co-transporter-2 inhibitors on markers of vascular damage. Journal of Personalized Medicine . 2023;13(3):p. 536. doi: 10.3390/jpm13030536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lamaida N., Cerciello A. Dapaglifozin and intima-mediatickness in type II diabetic patients. European Heart Journal Supplements . 2022;24:p. K144. [Google Scholar]
- 38.Murakami T., Mizuno S. Abstract 13577: A Selective SGLT2 Inhibitor Early Resolutes Metabolic and Arterial Disturbances in Type-2 Diabetics With Coronary Artery Disease. Circulation . 2014;130, article A13577(Supplement_2) [Google Scholar]
- 39.Nomiyama T., Shimono D., Horikawa T., et al. Efficacy and safety of sodium-glucose cotransporter 2 inhibitor ipragliflozin on glycemic control and cardiovascular parameters in Japanese patients with type 2 diabetes mellitus; Fukuoka study of ipragliflozin (FUSION) Endocrine Journal . 2018;65(8):859–867. doi: 10.1507/endocrj.EJ18-0022. [DOI] [PubMed] [Google Scholar]
- 40.Sakai T., Miura S. Effects of sodium-glucose cotransporter 2 inhibitor on vascular endothelial and diastolic function in heart failure with preserved ejection fraction-novel prospective cohort study. Circulation Reports . 2019;1(7):286–295. doi: 10.1253/circrep.CR-19-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka A., Sata M., Okada Y., et al. Effect of ipragliflozin on carotid intima-media thickness in patients with type 2 diabetes: a multicenter, randomized, controlled trial. European Heart Journal - Cardiovascular Pharmacotherapy . 2023;9(2):165–172. doi: 10.1093/ehjcvp/pvac059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamagishi T., Hasegawa H., Kanai H. [PP.05.19] Effects of SGLT2 inhibitors on blood pressure, BAPWV, carotid arterial IMT and elastic modulus in type 2 diabetic patients with obesity. Journal of Hypertension . 2016;34, article e145(Supplement 2) doi: 10.1097/01.hjh.0000491731.92423.b9. [DOI] [Google Scholar]
- 43.D’Onofrio N., Sardu C., Trotta M. C., et al. Sodium-glucose co-transporter2 expression and inflammatory activity in diabetic atherosclerotic plaques: effects of sodium-glucose co-transporter2 inhibitor treatment. Molecular Metabolism . 2021;54, article 101337 doi: 10.1016/j.molmet.2021.101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakai T., Miura S. Effect of sodium-glucose cotransporter 2 inhibitor on vascular endothelial function and diastolic function in patients with heart failure with preserved ejection fraction (HFpEF) European Heart Journal Cardiovascular Imaging . 2017;18, article iii222 [Google Scholar]
- 45.Sposito A. C., Breder I., Soares A. A. S., et al. Dapagliflozin effect on endothelial dysfunction in diabetic patients with atherosclerotic disease: a randomized active-controlled trial. Cardiovascular Diabetology . 2021;20(1):p. 74. doi: 10.1186/s12933-021-01264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Xian T., Wu M., et al. Effects of exenatide and insulin aspart 30 on vascular function in diabetic patients with different baseline characteristics. Chinese Journal of Diabetes Mellitus . 2021;13(2):156–161. [Google Scholar]
- 47.Maloberti A., Cimino E., Casati M., et al. PP.05.27: arterial structural and functional effects of incretin therapy in type 2 diabetes mellitus patients: preliminary results. Journal of Hypertension . 2015;33(Supplement 1):e175–e176. doi: 10.1097/01.hjh.0000467868.87943.c2. [DOI] [Google Scholar]
- 48.Rasmussen I. K. B., Zobel E. H., Ripa R. S., et al. Liraglutide reduces cardiac adipose tissue in type 2 diabetes: a secondary analysis of the LIRAFLAME randomized placebo-controlled trial. Diabetes, Obesity and Metabolism . 2021;23(12):2651–2659. doi: 10.1111/dom.14516. [DOI] [PubMed] [Google Scholar]
- 49.Ren W., Guo J., Liu J., Xi G. Effect of liraglutide on cardiovascular markers in type 2 diabetes mellitus. Diabetes . 2015;64:p. A339. [Google Scholar]
- 50.Barone M., Cutruzzolà A., Parise M., et al. Empagliflozin influences hemorheology, arterial morphology AD function. Giornale Italiano di Cardiologia . 2019;20(12):p. 128S. [Google Scholar]
- 51.Lamaida N., De Luca E., Cutolo M., Cerciello A. P190 carotid doppler ultrasonographic and dapaglifozin in type 2 diabetic patient. European Heart Journal Supplements . 2022;24, article C126(Supplement_C) doi: 10.1093/eurheartj/suac012.182. [DOI] [Google Scholar]
- 52.Al Busaidi N., Rizvi A. A., Montalto G., et al. Liraglutide improves metabolic parameters and reduces carotid intima-media thickness in patients with type 2 diabetes: a 2-year prospective study. Diabetes . 2015;64:A289–A290. [Google Scholar]
- 53.Chianetta R., Patti A. M., Castellino G., et al. Exenatide LAR reduces subclinical carotid atherosclerosis and improves endothelial function in patients with type 2 diabetes: an 8-month prospective study, diabetes. Stoffwechsel und Herz . 2017;26(6):p. 356. [Google Scholar]
- 54.Adamo A., Chianetta R., Giglio R. V., et al. Liraglutide improves metabolic parameters and reduces intima-media thickness in obese vs. non-obese patients with type 2 diabetes: 18-month prospective study. Diabetes, Stoffwechsel und Herz . 2017;26(6):356–357. [Google Scholar]
- 55.Nikolic D., Patti A. M., Giglio R. V., et al. Liraglutide improved cardiometabolic parameters more in obese than in non-obese patients with type 2 diabetes: a real-world 18-month prospective study. Diabetes Therapy . 2022;13(3):453–464. doi: 10.1007/s13300-022-01217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rizzo M., Ferlita A., Nikolic D., et al. Effects of liraglutide on metabolic parameters and carotid intima-media thickness in patients with the metabolic syndrome: a 12-month prospective pilot study. Diabetes . 2014;63:p. A261. [Google Scholar]
- 57.Abdul-Ghani M., Puckett C. L., Triplitt C. L., et al. Durable HbA1c reduction with initial combination therapy with metformin/pioglitazone/exenatide in subjects with new-onset diabetes-six-year follow-up of the edict study. Diabetes . 2018;67(Supplement_1):A32–A33. doi: 10.2337/db18-123-OR. [DOI] [Google Scholar]
- 58.Murakami T., Ohsato K., Niwa K. Dapagliflozin provides multiple ultrasonic-evaluated antiatherosclerotic effects independent of diabetic improvement for type-2 diabetics with coronary artery disease receiving statin or sartan. Circulation . 2016;134 [Google Scholar]
- 59.Murakami T., Ohsato K. Long-term antidiabetic treatment by dapagliflozin provides multiple antiatherosclerotic effects independent of diabetic improvement for type-2 diabetics with coronary artery disease receiving statin or sartan. Circulation . 2019;140 [Google Scholar]
- 60.Sardu C., Trotta M. C., Sasso F. C., et al. SGLT2-inhibitors effects on the coronary fibrous cap thickness and MACEs in diabetic patients with inducible myocardial ischemia and multi vessels non-obstructive coronary artery stenosis. Cardiovascular Diabetology . 2023;22(1):p. 80. doi: 10.1186/s12933-023-01814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akbari A., Razmi M., Rafiee M., Watts G. F., Sahebkar A. The effect of statin therapy on serum uric acid levels: a systematic review and meta-analysis. Current Medicinal Chemistry . 2024;31(13):1726–1739. doi: 10.2174/0929867330666230207124516. [DOI] [PubMed] [Google Scholar]
- 62.Uneda K., Kawai Y., Yamada T., et al. Systematic review and meta-analysis for prevention of cardiovascular complications using GLP-1 receptor agonists and SGLT-2 inhibitors in obese diabetic patients. Scientific Reports . 2021;11(1):p. 10166. doi: 10.1038/s41598-021-89620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sachinidis A., Nikolic D., Stoian A. P., et al. Cardiovascular outcomes trials with incretin-based medications: a critical review of data available on GLP-1 receptor agonists and DPP-4 inhibitors. Metabolism . 2020;111:p. 154343. doi: 10.1016/j.metabol.2020.154343. [DOI] [PubMed] [Google Scholar]
- 64.Singh A. K., Singh R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: a systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes & Metabolic Syndrome: Clinical Research & Reviews . 2020;14(3):181–187. doi: 10.1016/j.dsx.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Blonde L., Pencek R., MacConell L. Association among weight change, glycemic control, and markers of cardiovascular risk with exenatide once weekly: a pooled analysis of patients with type 2 diabetes. Cardiovascular Diabetology . 2015;14(1):p. 12. doi: 10.1186/s12933-014-0171-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song X., Jia H., Jiang Y., et al. Anti-atherosclerotic effects of the glucagon-like peptide-1 (GLP-1) based therapies in patients with type 2 diabetes mellitus: a meta-analysis. Scientific Reports . 2015;5(1, article 10202) doi: 10.1038/srep10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jujić A., Nilsson P. M., Atabaki-Pasdar N., et al. Glucose-dependent insulinotropic peptide in the high-normal range is associated with increased carotid intima-media thickness. Diabetes Care . 2021;44(1):224–230. doi: 10.2337/dc20-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiraki A., Oyama J.-i., Komoda H., et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis . 2012;221(2):375–382. doi: 10.1016/j.atherosclerosis.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 69.Xia J., Li Q., Liu Y., et al. A GLP-1 analog liraglutide reduces intimal hyperplasia after coronary stent implantation via regulation of glycemic variability and NLRP3 inflammasome/IL-10 signaling in diabetic swine. Frontiers in Pharmacology . 2020;11:p. 372. doi: 10.3389/fphar.2020.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang L., Tian J., Diao S., Zhang G., Xiao M., Chang D. GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1β-induced metabolic disturbance and mitochondrial dysfunction. Chemico-Biological Interactions . 2020;332:p. 109252. doi: 10.1016/j.cbi.2020.109252. [DOI] [PubMed] [Google Scholar]
- 71.Robinson L. E., Holt T. A., Rees K., Randeva H. S., O'Hare J. P. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open . 2013;3(1, article e001986) doi: 10.1136/bmjopen-2012-001986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nyström T., Gutniak M. K., Zhang Q., et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. American Journal of Physiology-Endocrinology and Metabolism . 2004;287(6):E1209–E1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 73.Ceriello A., Esposito K., Testa R., Bonfigli A. R., Marra M., Giugliano D. The possible protective role of glucagon-like peptide 1 on endothelium during the meal and evidence for an "endothelial resistance" to glucagon-like peptide 1 in diabetes. Diabetes Care . 2011;34(3):697–702. doi: 10.2337/dc10-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tashiro Y., Sato K., Watanabe T., et al. A glucagon-like peptide-1 analog liraglutide suppresses macrophage foam cell formation and atherosclerosis. Peptides . 2014;54:19–26. doi: 10.1016/j.peptides.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 75.Qu B., Qu T. Causes of changes in carotid intima-media thickness: a literature review. Cardiovascular Ultrasound . 2015;13(1):p. 46. doi: 10.1186/s12947-015-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhao Y., Zhao W., Bu H., Toshiyoshi M., Zhao Y. Liraglutide on type 2 diabetes mellitus with nonalcoholic fatty liver disease: a systematic review and meta-analysis of 16 RCTs. Medicine . 2023;102(6, article e32892) doi: 10.1097/MD.0000000000032892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sánchez-García A., Simental-Mendía M., Millán-Alanís J. M., Simental-Mendía L. E. Effect of sodium-glucose co-transporter 2 inhibitors on lipid profile: a systematic review and meta-analysis of 48 randomized controlled trials. Pharmacological Research . 2020;160:p. 105068. doi: 10.1016/j.phrs.2020.105068. [DOI] [PubMed] [Google Scholar]
- 78.Bułdak Ł., Łabuzek K., Bułdak R. J., Machnik G., Bołdys A., Okopień B. Exenatide (a GLP-1 agonist) improves the antioxidative potential of in vitro cultured human monocytes/macrophages. Naunyn-Schmiedeberg's Archives of Pharmacology . 2015;388(9):905–919. doi: 10.1007/s00210-015-1124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J., Liu X., Fang Q., Ding M., Li C. Liraglutide attenuates atherosclerosis via inhibiting ER-induced macrophage derived microvesicles production in T2DM rats. Diabetology & Metabolic Syndrome . 2017;9(1):p. 94. doi: 10.1186/s13098-017-0289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aljada A., Dandona P. Effect of insulin on human aortic endothelial nitric oxide synthase. Metabolism . 2000;49(2):147–150. doi: 10.1016/S0026-0495(00)91039-4. [DOI] [PubMed] [Google Scholar]
- 81.Zhou S. J., Bai L., Lv L., et al. Liraglutide ameliorates renal injury in streptozotocin-induced diabetic rats by activating endothelial nitric oxide synthase activity via the downregulation of the nuclear factor-κB pathway. Molecular Medicine Reports . 2014;10(5):2587–2594. doi: 10.3892/mmr.2014.2555. [DOI] [PubMed] [Google Scholar]
- 82.Han J. H., Oh T. J., Lee G., et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE−/− mice fed a western diet. Diabetologia . 2017;60(2):364–376. doi: 10.1007/s00125-016-4158-2. [DOI] [PubMed] [Google Scholar]
- 83.Pennig J., Scherrer P., Gissler M. C., et al. Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice. Scientific Reports . 2019;9(1):p. 17937. doi: 10.1038/s41598-019-54224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ganbaatar B., Fukuda D., Shinohara M., et al. Empagliflozin ameliorates endothelial dysfunction and suppresses atherogenesis in diabetic apolipoprotein E-deficient mice. European Journal of Pharmacology . 2020;875:p. 173040. doi: 10.1016/j.ejphar.2020.173040. [DOI] [PubMed] [Google Scholar]
- 85.Khat D. Z., Husain M. Molecular mechanisms underlying the cardiovascular benefits of SGLT2i and GLP-1RA. Current Diabetes Reports . 2018;18(7):p. 45. doi: 10.1007/s11892-018-1011-7. [DOI] [PubMed] [Google Scholar]
- 86.Park S. H., Farooq M. A., Gaertner S., et al. Empagliflozin improved systolic blood pressure, endothelial dysfunction and heart remodeling in the metabolic syndrome ZSF1 rat. Cardiovascular Diabetology . 2020;19(1):p. 19. doi: 10.1186/s12933-020-00997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park S. H., Belcastro E., Hasan H., et al. Angiotensin II-induced upregulation of SGLT1 and 2 contributes to human microparticle-stimulated endothelial senescence and dysfunction: protective effect of gliflozins. Cardiovascular Diabetology . 2021;20(1):p. 65. doi: 10.1186/s12933-021-01252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Z., Ma X., Ilyas I., et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: from pharmacology to pre-clinical and clinical therapeutics. Theranostics . 2021;11(9):4502–4515. doi: 10.7150/thno.54498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alshnbari A. S., Millar S. A., O’Sullivan S. E., Idris I. Effect of sodium-glucose cotransporter-2 inhibitors on endothelial function: a systematic review of preclinical studies. Diabetes Therapy . 2020;11(9):1947–1963. doi: 10.1007/s13300-020-00885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei R., Wang W., Pan Q., Guo L. Effects of SGLT-2 inhibitors on vascular endothelial function and arterial stiffness in subjects with type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Frontiers in Endocrinology . 2022;13, article 826604 doi: 10.3389/fendo.2022.826604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: search strategy of GLP-1 RA and SGLT2i. Table S2: quality assessment table for randomized clinical trial studies, cohort studies, cross-sectional studies, and nonrandomized studies based on JBI Critical Appraisal. Figure S1: GLP-1RA and IMT (sensitivity analysis on T2DM patients). Figure S2: liraglutide and exenatide effects on IMT. Figure S3: empagliflozin, tofogliflozin, and ipragliflozin effects on IMT. Figure S4: all analysis funnel plots (baseline-final comparison of GLP-1 RA (RCTs), baseline-final comparison of GLP-1 RA (T2DM), and baseline-final comparison of SGLT2i (RCTs)).
Data Availability Statement
Data is available on request from the corresponding author.


