Abstract
Nonanal, (E)-2-nonenal, (E,E)-2,4-nonadienal, and (E,Z)-2,6-nonadienal were used to study the effect of number and position of the unsaturated bond in aliphatic aldehydes on meat flavorings. Cysteine-Amadori and thiazolidine derivatives were synthesized, identified by UPLC-TOF/MS and NMR, and quantitatively by UPLC-MS/MS. The polyunsaturated aldehydes exhibited higher inhibition than monounsaturated aldehydes, and monounsaturated aldehydes exhibited higher inhibition than saturated aldehydes, mainly manifested by the inhibition of the cysteine-Amadori formation and acceleration of the thiazolidine derivatives formation. The effect of unsaturated bonds position in aliphatic aldehydes on the initial Maillard reaction stage was similar. The cysteine played an important role in catalyzing the reaction of aliphatic aldehydes. A total of 109 volatile compounds derived by heating prepared thiazolidine derivatives degradation were detected by GC-MS. Formation pathways of volatile compounds were proposed by retro-aldol, oxidation, etc. Particularly, a route to form thiazole by the decarboxylation reaction of thiazolidine derivatives which derivatives from formaldehyde reacting with cysteine was proposed.
Keywords: Aliphatic aldehydes, Unsaturated bond, Maillard reaction, Intermediates, Flavor formation
Graphical abstract
Highlights
-
•
Cystine-Amadori and thiazolidine derivatives were synthesized and identified.
-
•
Aldehydes inhibited cystine-Amadori formation in meat flavorings processing.
-
•
Cysteine catalyzed thiazolidine derivatives formation from the aldehydes.
-
•
109 volatile compounds derived by the thiazolidine derivatives degradation.
1. Introduction
Controlling flavor is a key factor for the Maillard reaction meat flavorings. Lipid-controlled the Maillard reaction meat flavorings has been extensively studied. Lipid degradation and the Maillard reaction play a key role in the formation of food aroma during meat flavorings processing (Mottram, 1998). In the Maillard reaction, cysteine is known as an important meaty flavor precursor, which could result in the formation of various sulfur-containing compounds. Aliphatic aldehydes come from the oxidative degradation of fatty acids. In the early period, hydroperoxides were formed through the removal of hydrogen free radical for the alkyl radical, the addition of O 2, and absorption of hydrogen free radical (Du et al., 2019; Whitfield, 1992). For example, the degradation of 9- and 10-hydroperoxides of oleic acid and linoleic acid can lead to nonanal, and (E)-2-nonenal. It was noteworthy that aliphatic aldehydes not only contribute to fatty flavor, but also change the pathway of Maillard reaction flavors (Wang et al., 2020; Zhao et al., 2019a).
According to the Hodge-scheme, early stage, intermediate stage, and final stage had been investigated by researchers since 1950s. In the early stage, carbonyls of aldoses or ketoses (reducing sugars) react with the amino groups of amino acids by nucleophilic reaction to form a reversible aldosylamines or ketosylamines (Schiff base), which is based on ring opening of the reducing sugar moiety. The aldosylamines/ketosylamines undergo Amadori/Heyns rearrangement reaction to form N-substituted 1-amino-2-deoxyketose/N-substituted 2-amino-1-deoxyaldose named Amadori rearrangement products/Heyns rearrangement products, which were based on the π bond (-C N-) of the Schiff base which could move towards the reducing sugar moiety. Dried foods were particularly rich in Amadori rearrangement products/Heyns rearrangement products, comprising as much as several percent of the total weight (Yu et al., 2018). The general result is that inclusion of lipids and lipid oxidation products in the reaction system of cysteine and reducing sugars tends to reduce the level of sulfur-containing compounds derived solely from the Maillard reaction (Du et al., 2020). Due to the electrophilic properties, it is well established that the reaction of cysteine with aldehydes leads to the formation of thiazolidine-4-carboxylic acids (Yoshitake et al., 2019). On the other hand, when cysteine reacts with an α,β-unsaturated aldehyde, the other significant reaction is Michael addition. Briefly, in the interaction between the Maillard reaction and aldehydes, the reducing sugars compete with aliphatic aldehydes to react with cysteine and form nonvolatile intermediates, e.g., 2-threityl-thiazolidine-4-carboxylic acids (TTCA), Amadori compounds, hemiacetals, and thiazolidines (Cui et al., 2018; Fernandez et al., 2001). It was reported that thiazolidine derivatives formed from hexanal, (E)-2-heptenal and (E,E)-2,4-decadienal reacted with glutathione (Wang et al., 2020). According to our former research, the number and position of unsaturated bonds in aliphatic aldehydes affect the volatile compounds of cysteine-glucose Maillard reaction (Du et al., 2023). However, the effect of aldehydes on changes in the content of Amadori compounds and thiazolidine derivatives have not been reported. Moreover, it has great significance to study the number and position of the unsaturated bond in the aliphatic aldehydes to understand the influence on nonvolatile intermediates formation. For instance, Clark and Deed, (2018) identified 3-S-glutathionylhexanal in grape juices of (E)-2-hexenal with GSH (Clark and Deed, 2018). Furthermore, the addition of lipid to the Maillard reaction system inhibits the production of sulfur-containing compounds, resulting in the formation of new alkyl compounds by nucleophilic substitution/addition. It was reported that 2-pentylthiophene was produced from the reaction between (E,E)-2,4-nonadienal and H2S via the Strecker degradation of cysteine (Zhao et al., 2019a). So, it is of great significance to study the compounds’ formation pathway and the influence of aliphatic aldehydes on the Maillard reaction.
This study explored the effect of number and position of unsaturated bond in aliphatic aldehydes on initial Maillard reactions meat flavorings in a stew meat model system. It used the essential lipid degradation products of nonanal, (E)-2-nonenal, (E,E)-2,4-nonadienal and (E,Z)-2,6-nondienal, as these key aroma compounds played a significant effect on food flavor (Bi et al., 2019; Li et al., 2023; Pu et al., 2022; Sohail et al., 2022). Intermediates (cysteine-Amadori and thiazolidine derivatives) were synthesized and identified, as standard compounds quantitative in different reaction systems. This study not only compared the reaction degree of the meat flavorings processing, but also predicted the volatile compound formation pathway by thiazolidine derivatives. This objective of the work is helpful to further understand the reaction pathway involving Maillard reaction with the lipid degradation during controlling savory flavoring or meat foods, and can also provide some guidance for efficient preparation of processed-meat flavorings.
2. Materials and methods
2.1. Chemicals
L-Cysteine, D-glucose, nonanal, (E)-2-nonenal, (E,E)-2,4-nonadienal, (E,Z)-2,6-nonadienal, 1,2-dichlorobenzene, and the n-alkanes (C5–C30) were purchasing from J&K Chemical Ltd. (99%, Beijing, China). Dichloromethane, PBS (phosphate buffer, 0.2 M, pH 6.50), Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Stand compounds including: 1-hexanethiol (98%), 2-hexylthiophene (98%), 2-propylthiophene (98%), 3,4-dimethylthiophene (98%), 2-butylthiophene (98%), 2-pentylthiophene (98%), 2-pentylfuran (95%), 2-methylpyridine (98%), 5-methyl-2-thiophenecarboxaldehyde (99%), thieno[3,2-b]thiophene (98%), thieno[2,3-b]thiophene (98%), 2-propylthiazole (99%), 2-ethylthiazole (98%), 2-thiophenecarboxaldehyde (98%), and other lipid degradation products (J & K Chemical Co., Ltd. Beijing, China).
2.2. Preparation of the intermediate compounds
Cys-Amadori (N-(1-deoxy-D-glucos-1-yl)-L-cysteine) was prepared by L-cysteine (0.02 mol), D-glucose (0.02 mol), was dissolved in 5 mL of PBS, magnetic with stirring in a 15 mL vial, in a water bath, heated at 55 °C for 1 h, and then 85 °C for 6 h, to purify the intermediate product separations were conducted using a preparative column (40 cm × 1.6 cm, Bio-Rad AG 50 W-X 4 (H +) 200–400 mesh) cation exchange resin, UV monitoring at 220 nm. The reaction mixture had entirely loaded in the column and then eluted gradually with water and 0.15 M ammonia solution, and then was collected and freeze-dried (Hou et al., 2017).
Cys-Amadori: UPLC-TOF/MS (ESI+) MS: 284.1158 ([M + H] +). Structure 1: 1 H NMR (500 MHz, (CD 3)2S O, ppm): δ 2.71–2.73 (d, J = 8, CH 2-3), 3.03(s, CH 2-4), 3.36–3.39(m, CH-8), 3.66–3.68 (m, CH-6, CH 2-9), 3.69–3.77 (m, CH-2, CH-7). 13 C NMR (500 MHz, (CD 3)2S O, ppm): δ 25.78 (C-3), 59.16 (C-4), 63.99 (C-9), 70.30 (C-2), 65.90 (C-7), 75.02(C-8), 80.23(C-6), 81.03 (C-5), 173.26 (C-1, COO−). Structure 2: 1 H NMR (500 MHz, (CD 3)2S O, ppm): δ 2.83–2.84 (d, J = 4, CH 2 -3), 3.43–3.47 (m, CH-6, CH-7), 3.66–3.68 (m, CH-5, CH-8), 3.69–3.77(d, CH 2-9), 3.78–3.79(d, J = 4, CH-2), 4.72–4.73 (d, J = 4, CH-4). 13 C NMR (500 MHz, (CD 3)2S O, ppm): δ 22.84 (C-3), 63.86 (C-2), 63.65 (C-8), 64.95 (C-7), 72.27 (C-6), 72.34 (C-9), 72.80 (C-5), 72.22 (C-4), 173.09 (C-1, COO−).
R 1: 2-(octyl)-thiazolidine-4-carboxylic acid; R 2: 2-(1-octenyl)-thiazolidine-4-carboxylic acid; R 3: 2-(1,3-octadienyl)-thiazolidine-4-carboxylic acid; R 4: 2-(1,5-octadienyl)-thiazolidine-4-carboxylic acid. Thiazolidine derivatives were prepared by, i.e., R 1: L-cysteine (2 mmol), nonanal (1 mmol); R 2: L-cysteine (2 mmol), (E)-2-nonenal (1 mmol); R 3: L-cysteine (2 mmol), (E,E)-2,4-nonadienal (1 mmol); R 4: L-cysteine(2 mmol), (E,Z)-2,6-nonadienal (1 mmol). Aliphatic aldehydes were dissolved in 5 mL of ethnaol, cysteine was dissolved in 10 mL of H2O, magnetic with stirring in a 15 mL glass vial with a screw cap, at room temperature for 12 h. The resulting reaction mixture was separated on a column (40 cm × 1.6 cm, polyamide resin, 125–150 mesh Mitsubishi Chemical Corporation, Tokyo, Japan), UV monitoring: 220 nm. The sample had entirely loaded in the column and then eluted gradually with water and ammonium hydroxide (0.04 M), followed by freeze-dried (Wang et al., 2020).
R 1: UPLC-TOF/MS (ESI +) MS: 246.1414 ([M + H] +). 1 H NMR (500 MHz, (CD 3)2S O, ppm): δ 0.84–0.88 (t, J = 16 Hz, CH 3-12), 1.17–1.34 (m, CH 2-11, CH 2-10, CH 2-9, CH 2-8, CH 2-7, CH 2-6), 1.70–1.77 (m, CH 2-5), 2.71–2.77(d, J = 24, CH 2-3), 3.67–3.72(t, J = 20, CH-4). 13 C NMR (500 MHz, (CD 3)2S O, ppm): δ 14.42 (C-12), 22.57 (C-11), 29.09 (C-6), 29.34 (C-9), 29.40 (C-8), 31.19 (C-5), 31.75 (C-10), 35.31(C-3), 65.67 (C-4), 71.52 (C-2), 172.78 (C-1, COO−). R 2: UPLC-TOF/MS (ESI+) MS: 244.1478 ([M + H] +). 1 H NMR (500 MHz, (CD 3)2S O, ppm): δ 0.86 (t, J = 12 Hz, CH 3-12), 1.27–1.44 (m, CH 2-11, CH 2-10, CH 2-9, CH 2-8), 2.05–2.16 (m, CH 2-7), 2.86–2.89 (d, J = 12 Hz, CH 2-3), 3.39–3.49 (t, J = 20 Hz, CH-2), 4.02–4.04 (d, J = 8, CH-4), 5.00–5.27 (m, CH-5, CH-6). 13 C NMR (500 MHz, (CD 3)2S O, ppm): δ 14.31 (C-12), 22.37 (C-11), 27.75 (C-9), 29.71 (C-8), 31.28 (C-10), 31.79 (C-7), 33.89 (C-3), 53.03 (C-4), 55.62 (C-2), 122.89 (C-5), 148.54 (C-6), 167.89 (C-1, COO−). R 3: UPLC-TOF/MS (ESI +) MS: 242.1313 ([M + H] +). 1 H NMR (500 MHz, (CD 3)2S O, ppm): δ 0.87 (t, J = 12 Hz, CH 3-12), 1.28–1.44 (m, CH 2-11, CH 2-10), 2.18–2.22 (dt, J = 8 Hz, CH 2-9), 2.76–2.78 (d, J = 8 Hz, CH 2-3), 3.37–3.41 (t, J = 10 Hz, CH -2), 4.14–4.20 (d, J = 24 Hz, CH-4), 5.23–5.31 (dt, J = 16 Hz, CH-8), 5.91–6.00 (dd, J = 16 Hz, CH-6), 6.33–6.42 (dd, J = 8 Hz, CH-7). 13 C NMR (500 MHz, (CD 3)2S O, ppm): δ 14.20 (C-12), 22.21 (C-11), 29.72 (C-10), 30.64 (C-9), 32.70 (C-3), 55.88 (C-4), 70.89 (C-2), 129.26 (C-5), 130.31 (C-6), 147.63 (C-7), 153.77 (C-8), 194.64 (C-1, COO−). R 4: UPLC-TOF/MS (ESI +) MS: 242.1090 ([M + H] +). 1 H NMR (500 MHz, (CD 3)2S O, ppm): δ 0.91–0.94 (t, J = 12 Hz, CH 3-12), 1.98–2.04 (m, CH 2-11, CH2-8, CH 2-7), 2.69–2.71 (d, J = 12 Hz, CH 3-3), 3.09–3.12 (t, J = 12 Hz, CH-2), 4.45–4.50 (d, J = 20 Hz, CH-4), 5.30–5.33 (m, CH-10, CH-9), 5.90–5.97 (m, CH-6, CH-5). 13 C NMR (500 MHz, (CD 3)2S O, ppm): δ 14.57 (C-12), 20.55 (C-11), 25.44(C-7), 32.59 (C-8), 33.92 (C-3), 53.07 (C-4), 55.69 (C-2), 127.86 (C-5), 132.84 (C-6), 133.19 (C-9), 159.36 (C-10), 194.81 (C-1, COO−).
2.3. UPLC-UV analysis
An Acquity UPLC Ⅰ Class system coupled with a PDA e λ delector (Waters Corporation, Milford, DE, USA) was used. Chromatographic separation was performed by a T 3 column (1.8 μm, 2.1 mm × 100 mm, Waters ACQUITY UPLC HSS T 3, USA). The mobile phase: acetonitrile and ammonium formate (10 mM; 70:30, v/v), flowing in isocratic elution mode: 0.25 mL/min. Column oven temperature at 40 °C. The sample injection volume was 2 μL. The product was subjected to UPLC-UV analyses, with purity ≥95%.
2.4. UPLC–Q/TOF qualitative analysis
An Acquity UPLC Ⅰ Class and Xevo G 2-XS Q Tof system equipped with UPLC and Q/TOF (Waters Corporation, Milford, DE, USA) were used. UPLC conditions were described in the above UPLC-UV analysis, a system equipped with a column T 3 (1.8 μm, 2.1 mm × 10 cm, Waters ACQUITY UPLC HSS T 3, USA). The flowing at 0.25 mL/min. MS E centroid mode, electrospray ionization (ESI) positive mode. The ion source capillary was 3.0 kV. Sample cone 40 v. The capillary temperature was 150 °C. The collision energy 4 v and Ramp collision energy 10 v. The flow rates of the cone gas and desolvation gas: 50 L/Hr and 900 L/Hr, respectively. The full scan range of mass spectra was set over m/z 40–1200 for MS/TOF detection.
2.5. NMR qualitative analysis
The 20 mg product was used with (CD 3)2S O as the solvent. 1 H NMR and 13 C NMR analyses, an AV 500 NMR spectrometer (Bruker, Switzerland).
2.6. UPLC-MS/MS quantitative analysis
An Acquity UPLC H Class PLUS and Xevo TQ - XS system equipped with UPLC and Q/Q/Q (Waters Corporation, Milford, DE, USA) were used. UPLC conditions used were identical to those described in the above UPLC-UV analysis, flowing at 0.25 mL/min. MRM mode and experiment condition are shown in Table S1. Electrospray ionization: ESI, positive ion mode. The ion source capillary was 2.5 kV. Cone was 21 v. The capillary temperature was 250 °C. Flow rates of the cone gas and desolvation gas were 600 L/Hr and 150 L/Hr. Data were acquired with the Xcalibur software system.
2.7. Volatile compounds from the degradation of the intermediates
R 1, R 2, R 3, and R 4 (2 mmol) each were dissolved in 3 mL of PBS (0.2 M, pH 6.50) in a 15 mL pressure vial. The four intermediates model systems were magnetic stirred at 140 °C in an oil bath for 120 min.
SPME (Solid-phase micro-extraction, 50/35 μm Carboxen/polydimethylsiloxane/divinylbenzene fiber, CAR/PDMS/DVB, Supelco Inc., Bellefonte, PA), 1 μL of 1,2-dichlorobenzene solution (internal standard, 100 μg/mL of methanol) was added to intermediates model system containing 3 mL. The pressure vial was equilibrated at 60 °C, for 30 min. The fiber was exposed in the headspace of the pressure vial at 60 °C, for 20 min before GC-MS-O and GC-MS for further analysis.
Gas chromatography-mass spectrometry (GC-MS) and gas chromatography-mass spectrometry-olfactometry (GC-MS-O) analysis. The 7890 B gas chromatograph-5977 A mass spectrometer (Agilent echnology, Santa Clara, CA). The DB-Wax capillary column (30 m × 0.25 mm × 0.25 μm) was used for the flavor compounds, with carrier gas (Helium, 99.999 %) at 1 mL/min. The gas chromatography condition was adjusted with an oven temperature of 40 °C, raised to 205 °C at 3.5 °C/min; and finally raised to 245 °C at 8 °C/min, and the fiber was desorbed for 3.5 min at 230 °C (splitless mode).
The mass detector and the olfactometer (Sniffer 9000, Brechbühler, Schlieren, Switzerland) running model was performed using the method of the literature which we had published before (Du et al., 2023).
2.8. Identification of volatile compounds
The volatile compounds was identified by searching NIST 15 mass spectra library (MS), retention indices (RI, relative to n-alkanes (C 5-C 30) in both GC-MS and GC-MS-O analyses), authentic chemicals (S) and odor characteristics (O).
2.9. Statistical analysis
All statistical analyses were the averages of three replicates. The pathways of the volatile compounds were plotted with ChemDraw 7.0, and Origin 2019 b.
3. Results and discussion
3.1. Quantitation of non-volatile components in the system
UPLC-MS/MS (TQXS) is a specialized non-volatile compound quantitative means with high performance. Based on our previous study, to study the influence of the number and position of the unsaturated bond in aliphatic aldehydes on the Maillard reaction system of “cysteine-glucose”, the conditions of the meat flavorings system were designed (Du et al., 2019).
As described above, a preliminary reaction scheme was presented for the intermediates formation of cysteine, glucose, and aliphatic aldehydes (nonanal, (E)-2-nonenal, (E,E)-2,4-nonadienal, and (E,Z)-2,6-nonadienal). The protonated carbonyl group of aliphatic aldehydes had strong electrophilicity under acidic conditions, and was prone to nucleophilic addition reaction with amino group of cysteine. After dehydration, imidonium salt were formed. The lone pair electrons on sulfhydryl sulfur attacked the carbon of imminium salts, the π bond break and the electrons transferred, and deprotonated after cyclic closure, forming thiazolidane derivatives. As shown in Fig. 1 (1), thiazolidine derivatives prepared by aliphatic aldehydes reacted with cysteine. In addition, the complex reaction not only leads to the formation of thiazolidine derivatives, which was the aforementioned classical carbonyl addition, but also to the Michael addition of sulfhydryl to the conjugated C C double bond of α,β-unsaturated aldehydes (1,4-conjugate addition) (Adams et al., 2011; Yoshitake et al., 2019). Overall, we were interested in the quantification of nucleophilic addition products of thiazolidine derivatives. Additionally, Fig. 1 (2) was presented, the scheme for the reaction of cysteine and glucose to form cysteine-Amadori intermediate compounds.
Fig. 1.
According to the initial reaction stage, the two major pathways to develop thiazolidine derivatives (1) and Amadori compound (2) in reaction systems.
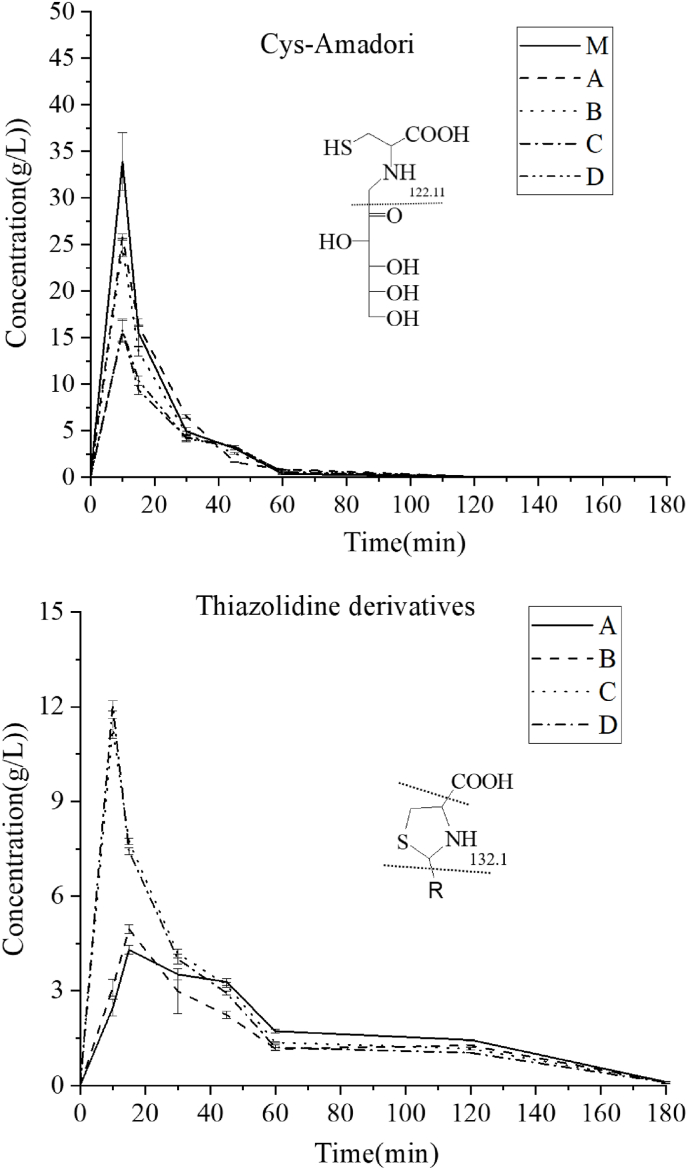
The resulting reaction mixture was analyzed by UPLC-MS/MS, Fig. 2 showed the concentrations of nonvolatile compounds of cysteine-Amadori and thiazolidine derivatives compounds in the five reaction mixture system (system M (cysteine-glucose), system A (cysteine-glucose-nonanal), system B (cysteine-glucose-(E)-2-nonenal), system C (cysteine-glucose-(E,E)-2,4-nonadienal), system D (cysteine-glucose-(E,Z)-2,6-nonadienal)). The change in nonvolatile component content can be studied and compared to the reaction degree. As shown in Fig. 2 (thiazolidine derivatives), concentrations of all the intermediates first rose and then fell with reaction time. Although few reaction products were formed resulting from the condensation of cysteine with aldehydes, the cysteine plays an important role in catalyzing the further reaction of these aliphatic aldehydes, especially unsaturated aldehydes (Adams et al., 2011). Regarding thiazolidine derivatives, the maximum concentration occurred first in systems C and D, then system B, and finally system A. System C and D was the first to reach the maximum concentration. The unsaturated aldehydes (E,E)-2,4-nonadienal and (E,Z)-2,6-nonadienal exhibited high reactivity against glucose for cysteine, yielding higher levels of thiazolidine derivatives than nonanal and (E)-2-nonenal. This phenomenon suggested an acceleration effect of unsaturated bond on rate of the reaction in cysteine-aliphatic aldehydes. Briefly, an amino acid can facilitate the cyclization process by acid catalysis. The zwitterionic form of the amino acid protonates the carbonyl group of the aliphatic aldehydes, thus increasing its electrophilicity, while at the same time the hydroxyl function was deprotonated, increasing its nucleophilicity. This was particularly evident in unsaturated aldehydes. However, the positions of the unsaturated bonds in aliphatic aldehydes in the cysteine-aliphatic aldehydes reaction degree were similar.
Fig. 2.
Concentrations of the detected Amadori compound and thiazolidine derivatives with reaction time in the reaction mixtures (system M (cysteine-glucose), system A (cysteine-glucose-nonanal), system B (cysteine-glucose-(E)-2-nonenal), system C (cysteine-glucose-(E,E)-2,4-nonadienal), system D (cysteine-glucose-(E,Z)-2,6-nonadienal)).
The concentration of intermediate compounds in the reaction mixture of the five systems was determined (as shown in Fig. 2 cysteine-Amadori). At 10 min, system M had the highest cysteine-Amadori content, followed by system A, system B, and system C and D, indicating that aliphatic aldehydes inhibited cysteine-Amadori formation, (E,E)-2,4-nonadienal and (E,Z)-2,6-nonadienal inhibited most markedly. The double bond content in aliphatic aldehydes contributes highly to reaction activity, mainly manifested in inhibition of the initial Maillard reaction and participation in the initial Maillard reaction. These suggested that polyunsaturated aldehydes exhibited higher inhibition on the initial Maillard reaction than monounsaturated aldehydes, monounsaturated aldehydes exhibited higher inhibition on the initial Maillard reaction than saturated aldehydes, because of higher reactivity of the unsaturated aldehydes to react with cysteine against glucose. Other relative differences between the aliphatic aldehydes detected would probably depend on their steric effects.
The concentrations of cysteine-Amadori decreased with reaction time, this was evidenced by their degradation, as obviously seen from Fig. 2. Meanwhile, this dramatic drop suggested that a high rate of amino-carbonyl reaction depleting the amino groups of cysteine occurred at that time, since in the initial reaction period the reactants all had a high concentration. Formation of the cysteine-Amadori component in four systems showed inhibition by the aliphatic aldehydes (nonanal, (E)-2-nonenal, (E,E)-2,4-nonadienal, and (E,Z)-2,6-nonadienal) on cysteine with glucose reaction. Otherwise, according to previous research, the lipid inhibits the Maillard reaction (Yang et al., 2015).
3.2. Structure identification by UPLC-Q/TOF
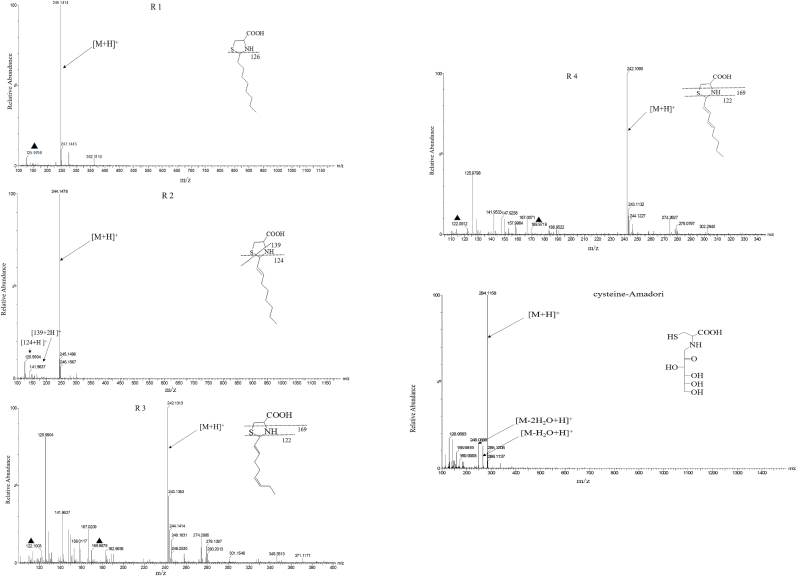
UPLC-Q/TOF is a high-resolution mass spectrometer especially used for the characterization of compounds because of its high precision and structural elucidation process. According to the chemical structures of the intermediates shown in Fig. 3, cys-Amadori and thiazolidine derivatives were identified by the reaction of aliphatic aldehydes and glucose with cysteine.
Fig. 3.
Identified thiazolidine derivatives and Amadori compound by Q/TOF spectra.
As shown in Fig. 3 (cysteine-Amadori), the ions m/z 284.1158 for cysteine-Amadori ([M + H] +), and m/z 248 for cysteine-Amadori with loss of H2O or 2 H2O from the carbohydrate moiety of the respective [M + H] + were revealed with a strong peak. Besides, as shown in Fig. 3 R 1, R 2, R 3, and R 4 (ion m/z 246.1414, 244.1478, 242.1313, 242.1090) due to scission in the carbohydrate chain of the [M + H] + ion all had a good abundance, indicating thiazolidine derivatives be caught in collision-induced dissociation (CID) during MS/TOF analysis. Moreover, the ions m/z 126 for R 1, m/z 124 for R 2, m/z 122 for R 3, and m/z 122 for R 4 could all be acquired by removal of the alkyl chain from the respective [M + H] + ion (shown in Fig. 3). The ion m/z 139 for R 2, m/z 169 for R 3, and m/z 169 for R 4 could be acquired by cleavage of the thiazolidine from the respective [M + H] + ion (shown in Fig. 3).
3.3. Structure identification by NMR
Preparation of cysteine-Amadori and thiazolidine derivatives was performed by isolating the reaction mixture to further elucidate the structures of the intermediates detected by NMR.
The processed NMR data were presented in the previous Section, while the NMR spectra are shown in Fig. S1. As expected, with regards to thiazolidine derivatives (R 1), chemical shifts (ppm) of δ C 65.67(C-4), δ C 35.31 (C-3), and δ C 71.52 (C-2) corresponding to the thiazolidine ring were consistent with the literature (Fernandez et al., 2001; Wang et al., 2020). With regards to R 2 thiazolidine derivatives, the chemical shifts (ppm) of δ C 122.89 (C-5) and δ C 148.54 (C-6) corresponded to an olefinic bond. R 3 thiazolidine derivatives, the chemical shifts (ppm) of δ C 129.26/δ H 6.00 (C-5), δ C 130.31/δ H 5.91 (C-6), δ C 147.63/δ H 6.33 (C-7), and δ C 153.77/δ H 5.23 (C-8) corresponded to an olefinic bond. Moreover, chemical shifts (ppm) of δ C 194.64 (C-1), correspond to carboxylic acid. R 4 thiazolidine derivatives, the chemical shifts (ppm) of δ C 127.86/δ H 5.97 (C-5), δ C 132.84/δ H 5.90 (C-6), δ C 133.19/δ H 5.30 (C-9), and δ C 159.36/δ H 5.33 (C-10) corresponded to an olefinic bond. The chemical shifts in thiazolidine derivatives compounds showed similar reported results (Wang et al., 2020; Fernandez et al., 2001). However, signals of impurities or environmental contaminants were detected, not labeled with chemical shifts.
The structures of ARP were further confirmed by NMR. It was worth noting that the ARP was not found in the NMR data like the reference (Cao et al., 2017; Hou et al., 2017). The isomers contained were detected in ARP compounds. As expected, the six-membered rings in the structures of isomers. The NMR data presented in Fig. S1 showed that structure 1 and structure 2 signals were in the expected chemical shifts and agreed with those reported in the literature. Regarding structure 1, signals (ppm) of δ C 25.78/δ H 2.71 (C-3), δ C 59.16/δ H 3.03 (C-4), δ C 63.99/δ H 3.66 (C-9), δ C 70.30/δ H 3.66 (C-2), δ C 65.90/δ H 3.69 (C-7), δ C 75.02/δ H 3.39 (C-8), δ C 80.23/δ H 3.68 (C-6), δ C 81.03 (C-5), δ C 173.26 (C-1, COO−). Regarding structure 2, signals (ppm) of δ C 22.84/δ H 2.83 (C-3), δ C 72.22/δ H 4.72 (C-4), δ C 72.34/δ H 3.69 (C-9), δ C 63.86/δ H 3.78 (C-2), δ C 64.95/δ H 3.43 (C-7), δ C 63.65/δ H 3.68 (C-8), δ C 72.27/δ H 3.47 (C-6), δ C 72.80/δ H 3.66 (C-5), δ C 173.09 (C-1, COO−). Notably, regarding the carbonyl group, chemical shifts (ppm) of δ C 173.09. Moreover, other signals might be ascribed to the structure of the tautomer. Anyway, the aforementioned tautomerization needs to be further proved, since some NMR signals were not discerned.
3.4. Volatile flavor compounds formed from the degradation of the thiazolidine derivatives
The Maillard reaction model system has always been the classic model reaction to study meat flavorings aroma compounds (Wei et al., 2020). Based on our previous studies, a stew meat-like flavor was produced from thiazolidine derivatives model system at pH 6.5, 140 °C, and 2 h (Du et al., 2023). A total of 109 flavor compounds were identified by GC-MS and GC-MS-O, as shown in Table 1, derived from the R 1, R 2, R 3, and R 4.
Table 1.
Volatile aroma compounds derived from thiazolidine derivatives degradation.
| 1RI | Compounds | 2Odor descriptors |
3Quantity (μg/L) |
4ID | |||
|---|---|---|---|---|---|---|---|
| R 1 | R 2 | R 3 | R 4 | ||||
| thiophenes | |||||||
| 1078 | 3-methylthiophene | sulfur | – | – | – | 32.02 ± 0.76 | RI/MS/O |
| 1225 | 2-ethyl-5-methylthiophene | – | – | – | – | 110.79 ± 2.00 | RI/MS |
| 1233 | 2-propylthiophene | sulfur | – | – | 183.31 ± 2.86 | 853.78 ± 228.92 | RI/MS/O/S |
| 1241 | 2-ethenylthiophene | – | – | 0.48 ± 0.01 | 122.82 ± 6.61 | – | RI/MS |
| 1253 | 3,4-dimethylthiophene | – | – | 136.73 ± 27.77 | – | 53.97 ± 2.00 | RI/MS/S |
| 1340 | 2-butylthiophene | sulfur | 32.40 ± 3.01 | – | – | 30.85 ± 2.00 | RI/MS/O/S |
| 1450 | 2-pentylthiophene | sulfur | – | 630.92 ± 7.00 | 140.15 ± 11.11 | – | RI/MS/O/S |
| 1531 | 2-hexylthiophene | meaty | – | 57.23 ± 7.24 | – | – | RI/MS/O/S |
| 1675 | 2-thiophenecarboxaldehyde | – | 52.89 ± 5.82 | – | 95.57 ± 2.86 | – | RI/MS/S |
| 1652 | 2-heptylthiophene | meaty | 1627.12 ± 66.72 | 24.63 ± 0.60 | – | – | RI/MS/O/S |
| 1785 | 5-methyl-2-thiophenecarboxaldehyde | sulfur | 19.00 ± 5.68 | – | – | 476.37 ± 11.45 | RI/MS/O/S |
| 1817 | 1-(2-thienyl)-1-propanone | – | – | – | 33.68 ± 2.20 | – | RI/MS |
| 1822 | 1-(2-thienyl)-1-butanone | – | – | – | 73.27 ± 5.44 | – | RI/MS |
| 1837 | thieno[3,2-b]thiophene | meaty | – | 46.07 ± 0.78 | – | – | RI/MS/O/S |
| 1840 | thieno[2,3-b]thiophene | meaty | 65.95 ± 2.77 | – | – | – | RI/MS/O/S |
| 1942 | 2-ethyl-5-propylthiophene | – | – | – | 438.71 ± 28.38 | 660.10 ± 57.23 | RI/MS |
| 1951 | 4-ethyl-2-propylthiophene | – | 10.46 ± 0.29 | – | – | – | RI/MS |
| 2132 | 1-(2-thienyl)-1-heptanone | – | – | 57.46 ± 1.91 | – | – | RI/MS |
| 2253 | 2-butyl-5-hexylthiophene | – | – | – | 15.54 ± 1.33 | – | RI/MS |
| sum | 1807.82 | 953.52 | 1103.05 | 2217.88 | |||
| thiazoles | |||||||
| thiazole | – | – | 136.74 ± 12.47 | 371.44 ± 15.49 | 79.95 ± 2.86 | RI/MS/S | |
| 1301 | 4,5-dihydro-2-methylthiazole | – | – | 94.90 ± 9.27 | – | – | RI/MS |
| 1304 | 2-ethylthiazole | roast | 47.01 ± 5.82 | 139.45 ± 5.94 | 146.02 ± 7.91 | 634.83 ± 28.62 | RI/MS/O/S |
| 1363 | 4-methyl-5-ethylthiazole | – | – | – | 1099.48 ± 15.35 | 180.00 ± 5.72 | RI/MS |
| 1374 | 4-propylthiazole | – | – | – | 1248.40 ± 38.28 | – | RI/MS |
| 1376 | 2-propylthiazole | roast | – | 15.29 ± 1.54 | 154.73 ± 12.90 | 48.00 ± 2.00 | RI/MS/O/S |
| 1411 | 5-ethyl-2-methylthiazole | – | – | 240.81 ± 17.10 | – | 242.02 ± 5.72 | RI/MS |
| 1415 | 4-ethyl-5-methylthiazole | – | – | – | 623.36 ± 39.14 | – | RI/MS |
| 1422 | 2-methyl-5-propylthiazole | – | – | 27.93 ± 1.41 | – | 78.35 ± 11.45 | RI/MS |
| 1496 | 4,5-dimethyl-2-propylthiazole | – | – | 33.48 ± 0.32 | – | – | RI/MS |
| 1523 | 2-butylbenzothiazole | – | – | – | 75.27 ± 0.73 | – | MS |
| sum | 47.01 | 688.6 | 3718.7 | 1263.15 | |||
| furans | |||||||
| 955 | 2-ethylfuran | – | – | – | 162.11 ± 2.78 | RI/MS | |
| 1011 | 2-propylfuran | – | – | – | 218.45 ± 1.15 | – | RI/MS/S |
| 1124 | 2-n-butylfuran | – | 4.15 ± 0.82 | – | 83.15 ± 1.09 | – | RI/MS |
| 1152 | 2-butyltetrahydrofuran | – | 600.32 ± 85.87 | – | – | RI/MS | |
| 1231 | 2-pentylfuran | caramel | 38.23 ± 5.81 | 435.36 ± 28.87 | – | – | RI/MS/O/S |
| 1416 | (E)-2-(1-pentenyl)-furan | – | – | – | 184.99 ± 11.27 | – | RI/MS |
| 1751 | 2-hexanoylfuran | – | – | 5.17 ± 0.03 | 196.32 ± 11.02 | – | RI/MS |
| 1797 | dihydro-5-propyl-2(3H)-furanone | caramel | 414.44 ± 2.86 | 71.92 ± 1.40 | – | – | RI/MS/O |
| 1916 | 5-butyldihydro-2(3H)-furanone | – | 211.76 ± 1.02 | – | 85.91 ± 7.21 | – | RI/MS |
| 2028 | dihydro-5-pentyl-2(3H)-furanone | – | 1141.07 ± 8.85 | – | – | – | RI/MS |
| 2132 | 3-hexyldihydro-2(3H)-furanone | caramel | – | – | 262.90 ± 8.58 | – | RI/MS |
| sum | 2409.97 | 512.45 | 1031.72 | 162.11 | |||
| sulfides | |||||||
| 1149 | 1-hexanethiol | garlic | – | 18.99 ± 0.23 | – | – | RI/MS/O/S |
| 1572 | anti-3,5-dimethyl-1,2,4-trithiolane | – | 3119.84 ± 142.43 | 10993.43 ± 790.31 | – | 11519.27 ± 94.43 | RI/MS |
| 1596 | syn-3,5-dimethyl-1,2,4-trithiolane | – | – | – | – | 3515.78 ± 1917.22 | RI/MS |
| 1730 | 1,2,3-trithiolane | – | – | 708.69 ± 8.07 | 371.46 ± 0.34 | 1446.39 ± 34.34 | RI/MS |
| 1750 | 1,2,5-trithiepane | – | 101.70 ± 5.72 | – | 99.51 ± 5.72 | – | RI/MS |
| 1815 | 4-methyl-1,2,3-trithiolane | – | 35.86 ± 2.20 | 128.90 ± 0.60 | – | 992.60 ± 32.05 | RI/MS |
| 1918 | 1,3,5-trithiane | – | – | 114.54 ± 4.01 | – | – | RI/MS |
| 2107 | 3-ethyl-5-methyl-1,2,4-trithiolane | – | – | 501.44 ± 19.75 | – | 5231.98 ± 34.91 | RI/MS |
| 2159 | 2,4,6-trimethyl-1,3,5-trithiane | – | 136.89 ± 67.86 | 1661.01 ± 219.90 | – | 1996.21 ± 50.36 | RI/MS |
| sum | 3394.29 | 14127 | 470.97 | 24702.23 | |||
| pyridines | |||||||
| 1214 | 2-methylpyridine | – | 2.93 ± 0.73 | – | – | 30.08 ± 1.43 | RI/MS/S |
| 1282 | 3-ethylpyridine | – | – | – | – | 726.57 ± 85.85 | RI/MS |
| 1311 | 2-ethylpyridine | – | – | – | – | 73.19 ± 5.72 | RI/MS |
| 1375 | 3-propylpyridine | – | – | 13.44 ± 3.05 | 80.54 ± 2.63 | 769.43 ± 57.23 | RI/MS |
| 1412 | 5-ethyl-2-methylpyridine | – | – | 13.36 ± 1.59 | – | 297.63 ± 11.45 | MS |
| 1469 | 2-butylpyridine | – | 129.28 ± 0.04 | 310.75 ± 18.72 | 1391.54 ± 51.12 | 144.81 ± 11.45 | RI/MS |
| 1572 | 2-pentylpyridine | burnt | – | – | 559.34 ± 342.79 | – | RI/MS/O/S |
| 1758 | 2-hexylpyridine | – | – | 49.91 ± 0.17 | – | – | |
| sum | 132.21 | 387.46 | 2031.42 | 2041.71 | |||
| aldehydes | |||||||
| 961 | 3-methylbutanal | – | – | 12.80 ± 1.26 | – | – | RI/MS |
| 1058 | 2-butenal | – | 72.88 ± 5.72 | – | – | – | RI/MS/S |
| 1097 | hexanal | green | 144.35 ± 5.72 | 463.41 ± 10.80 | 446.22 ± 15.27 | – | RI/MS/O/S |
| 1182 | 5-methylhexanal | – | 122.21 ± 1.00 | – | 296.31 ± 1.54 | – | RI/MS |
| 1184 | heptanal | green | 43.22 ± 4.80 | – | 22.46 ± 4.39 | 318.64 ± 8.58 | RI/MS/O/S |
| 1297 | octanal | green | – | – | 124.32 ± 4.84 | – | RI/MS/O/S |
| 1322 | (E)-2-heptenal | green | 202.00 ± 2.86 | – | 1369.76 ± 13.70 | – | RI/MS/O/S |
| 1391 | nonanal | fatty | – | – | 5311.32 ± 210.40 | – | RI/MS/O/S |
| 1492 | (E,E)-2,4-heptadienal | green | 496.75 ± 5.72 | – | – | – | RI/MS/O/S |
| 1530 | benzaldehyde | almond | 24.97 ± 0.86 | 12.10 ± 0.47 | 619.25 ± 12.55 | – | RI/MS/S |
| 1544 | (E)-2-nonenal | – | – | 167.15 ± 21.27 | 206.28 ± 1.25 | – | RI/MS |
| 1642 | 2-methylbenzaldehyde | – | – | – | 32.99 ± 1.98 | – | RI/MS |
| 1701 | (E,E)-2,4-nonadienal | green | – | – | 1528.30 ± 176.47 | – | RI/MS/O/S |
| 1715 | (E,Z)-2,6-nonadienal | – | – | – | – | 98.34 ± 11.45 | RI/MS/S |
| 1796 | (E,Z)-2,4-decadienal | fatty | 71.06 ± 5.72 | – | – | – | RI/MS/O/S |
| 1836 | 2-methylundecanal | – | 572.98 ± 217.75 | – | – | – | RI/MS |
| 2209 | 2-pentyl-2-nonenal | – | – | – | 347.31 ± 37.25 | – | MS |
| 2301 | 4-butylbenzaldehyde | – | – | – | 1071.85 ± 116.41 | – | MS |
| sum | 1750.42 | 655.46 | 11376.37 | 416.98 | |||
| alcohols | |||||||
| 1125 | 1-butanol | – | – | – | 64.65 ± 1.23 | – | RI/MS |
| 1158 | 1-penten-3-ol | mushroom | 223.43 ± 5.72 | – | – | 281.00 ± 19.45 | RI/MS |
| 1252 | 1-pentanol | – | 39.50 ± 21.07 | 177.43 ± 0.69 | – | – | RI/MS/S |
| 1361 | 1-hexanol | – | 113.25 ± 1.60 | 350.70 ± 4.27 | – | 10.56 ± 0.29 | RI/MS/S |
| 1465 | 1-heptanol | – | 642.48 ± 51.18 | 101.89 ± 3.11 | – | – | RI/MS |
| 1452 | 1-octen-3-ol | mushroom | – | 8.24 ± 1.72 | – | – | RI/MS |
| 1540 | 2-octanol | soap | 1021.90 ± 56.51 | 85.26 ± 2.56 | – | – | RI/MS/O/S |
| 1546 | 1-octanol | green | – | 119.71 ± 19.67 | 240.00 ± 13.04 | 72.14 ± 8.58 | RI/MS/O/S |
| 1673 | 1-nonanol | soap | – | – | 150.76 ± 24.20 | – | RI/MS/O/S |
| 1771 | 1-decanol | – | 5730.43 ± 67.35 | – | – | – | RI/MS |
| 1775 | 5-decanol | – | – | 344.36 ± 41.76 | – | – | RI/MS |
| 1982 | 1-dodecanol | – | – | 28.28 ± 2.09 | 131.21 ± 13.61 | – | RI/MS |
| sum | 7770.99 | 1215.87 | 586.62 | 363.7 | |||
| ketones | |||||||
| 1097 | 2-hexanone | – | – | 7.93 ± 0.47 | – | 415.97 ± 8.58 | RI/MS |
| 1287 | 2-octanone | sweet | 1416.08 ± 97.31 | 2656.21 ± 121.56 | 20.73 ± 2.34 | – | RI/MS/O/S |
| 1317 | 2-octen-4-one | – | – | – | 83.45 ± 2.68 | 383.33 ± 14.31 | RI/MS |
| 1357 | 3-nonanone | – | 62.57 ± 2.86 | 3.20 ± 0.41 | – | – | RI/MS |
| 1390 | 2-nonanone | sweet | 889.95 ± 8.71 | 858.05 ± 26.53 | – | 989.02 ± 114.46 | RI/MS/O/S |
| 1414 | 3-octen-2-one | – | – | – | 1254.32 ± 135.57 | 410.10 ± 9.44 | RI/MS/S |
| 1492 | 5-decanone | – | – | – | 418.54 ± 8.16 | – | RI/MS |
| 1599 | 2-undecanone | – | 527.90 ± 6.84 | – | – | – | RI/MS |
| sum | 2896.50 | 3525.39 | 1777.04 | 2198.42 | |||
| acids | |||||||
| 1638 | 2-propenoic acid | – | 60.31 ± 1.28 | – | – | 37.94 ± 0.29 | RI/MS |
| 1744 | pentanoic acid | acid | – | – | 23.69 ± 2.08 | 128.26 ± 5.72 | RI/MS/O/S |
| 1854 | hexanoic acid | acid | 269.83 ± 16.80 | 44.04 ± 1.17 | – | – | RI/MS/O/S |
| 1960 | heptanoic acid | – | 14.09 ± 1.24 | 177.04 ± 5.08 | 66.97 ± 14.34 | – | RI/MS |
| 2031 | valproic Acid | – | – | 6.81 ± 5.72 | – | – | RI/MS |
| 2066 | octanoic acid | – | 807.60 ± 17.16 | 123.44 ± 2.04 | 235.15 ± 24.95 | 123.79 ± 0.57 | RI/MS/S |
| 2097 | 2-methyloctanoic acid | – | 846.77 ± 59.56 | 73.04 ± 1.74 | – | – | MS |
| 2174 | nonanoic acid | – | 949.62 ± 52.70 | 2161.75 ± 31.41 | 1784.96 ± 52.36 | 3139.34 ± 85.85 | RI/MS/S |
| 2181 | n-decanoic acid | – | – | – | 59.55 ± 2.57 | 241.96 ± 11.45 | RI/MS |
| 2192 | 8-methylnonanoic acid | – | 109.53 ± 20.34 | – | – | – | MS |
| 2281 | n-decanoic acid | – | – | 44.06 ± 3.86 | – | – | RI/MS/S |
| 2325 | dodecanoic acid | – | – | – | 21.27 ± 1.09 | – | MS |
| 2331 | (E)-2-dodecenoic acid | – | – | – | 9.58 ± 0.29 | – | RI/MS |
| 2385 | n-hexadecanoic acid | – | 651.03 ± 8.60 | – | – | – | MS |
| sum | 3708.78 | 2630.18 | 2201.17 | 3671.29 | |||
| total | 22917.99 | 24695.93 | 24297.06 | 37037.47 | |||
1RI, retention indices determined using the DB-Wax capillary column (GC–MS/GC–MS-O analysis).
2The odor characteristics detected by GC-MS-O analysis of the thiazolidine derivatives degradation; “–”, not detected.
3Quantity, the concentrations calculated from the peak area ratio of flavor compounds to the 1,2-dichlorobenzene; Different superscript letters in the same row indicated significant differences (P < 0.05); Note: “–”, not detected.
4Identification methods.
As shown in Table 1, R 1 system had detected 55 compounds, R 2 system had detected 61 compounds, R 3 system had detected 63 compounds, and R 4 system had detected 50 compounds. The four systems produced many lipids oxidative degradation compounds such as alcohols, ketones, aldehydes, and acids (shown in Table 1). This indicated that during heating, these thiazolidine derivatives were reversibly reacted to release the original reactants. Overall, the released aliphatic aldehydes undergo into degradation and polymerization process (shown in Fig. 4 a, b, c, d, e) (Du, et al., 2023; Shakoor et al., 2022). The degradation reaction during the heating of aliphatic aldehydes resulted in cracking the compound to form shorter aliphatic aldehydes. Whereas, in the polymerization process it forms aliphatic aldehydes with longer chains. Moreover, a study shown that enaldehydes also form dienaldehydes through oxidation or aldol condensation reactions (Wei et al., 2020).
Fig. 4.
(a) (b) (c) Formation of new aliphatic aldehydes from (E)-2-nonenal, (E,E)-2,4-nondienal and (E,Z)-2,6-nondienal via hydration and retro-aldol reaction. (d) (e)Formation of 2,4-dienal from 2-enal. (e) (f) (g) Proposed possible formation pathways for the alkyl thiophenes, furans, and pyridines. (h) Possible formation pathways for the thiazole.
R 1 system produced a very high amount of 2-heptylthiophene (meaty flavor). R 4 system produced a very high amount of 2-propylthiophene (sulfur flavor). The content of volatile compounds formed in the R 4 system, R 3 system, R 2 system, and R 1 system was in descending. The double bond content in thiazolidine derivatives contributes highly to reaction activity.
As shown in Table 1, alkyl sulfur-, oxygen-, or nitrogen-containing compounds (e.g., 2-pentylthiophene, 2-hexanoylfuran, and 2-butylpyridine) were found. This indicated that these reaction systems passed in the same way via these intermediates to form the aroma flavors (Mottram, 1998). Fig. 4 (d, e, f, g, h) also shown possible formation pathways of some identified compounds. Thiophenes were the most significant class of meat flavor compounds, and 2-propylthiophene, 2-pentylthiophene, and 2-heptylthiophene were the high-content compounds, where these flavor compounds were essential in meat food (Du et al., 2020; Sohail et al., 2022). As a study shown that 3-methyl-2-thiophenecarboxaldehyde, 2-hexylthiophene, 5-methyl-2-thiophenecarboxaldehyde, 2-pentylthiophene, and 2-thiophenecarboxaldehyde were detected in meat food (Sohail, et al., 2022). R 4 System had the most abundant thiophenes compared with other 3 systems. (E,E)-2,4-Octadienal, (E,E)-2,4-heptadienal, and (E,E)-2,4-decadienal reacting with H2S could form the 2-butylthiophene, 2-propylthiophene, and 2-hexylthiophene, respectively.
The thiazoles with alkyl chains were generated in the 4 systems. This may be due to the degradation products of aliphatic aldehydes involved in the form pathway. With reported that the most commonly identified thiazoles were 4,5-dimethylthiazole and 2,4,5-trimethylthiazole (Du et al., 2020; Sohail et al., 2022). During heating, thermal degradation of the Strecker degradation of cysteine from thiazolidine derivatives induced by the aliphatic aldehyde degradation products can release H2S and NH3 (Shakoor et al., 2022; Zhao et al., 2019b). The high content of thiazole in R 2, R 3, and R4 may be due to the decarboxylation reaction of thiazolidine derivatives formed by the reaction of formaldehyde with cysteine, as shown in Fig. 4 (h).
The high level of 2-pentylfuran could be formed from nonanal and (E)-2-nonenal through alkene epoxidation and ring opening, cyclization, loss of water, and oxidization, as shown in Fig. 4 (f). Similarly, the identified 2-ethylfuran, 2-propylfuran, and 2-n-butylfuran from thiazolidine derivatives could result from cyclization of (E)-2-hexenal, (E)-2-heptenal, and (E)-2-octenal, in turn (Mottram, 1998). As reported in the literature, amino acids have catalytic function to catalyze the cyclization of α,β-unsaturated aldehydes to form alkyl furans (Adams et al., 2011; Du et al., 2023; Xu et al., 2023).
2-Butylpyridine could be generated from aliphatic aldehydes in the same way as 2-pentylthiophene, with NH3 involved instead of H2S, as shown in Fig. 4 (d, e) (Mottram, 1998). The identified 1-hexanethiol in the R 2 system could be formed from the reaction of 1-hexanol and H2S, while the reduction of hexanal could lead to the 1-hexanol (Wang et al., 2020).
4. Conclusions
In conclusion, in the processing of meat flavorings, concentrations of all the intermediates first rose and then fell with the extended reaction time, which was evidenced by their formation and degradation. The unsaturated aliphatic aldehydes contribute highly to reaction activity, mainly manifested in the inhibition of the cysteine-Amadori formation and acceleration of the thiazolidine derivatives formation. The effects of polyunsaturated on the initial Maillard reaction stage were similar. Cysteine as well were able to catalyze thiazolidine derivatives formation from the corresponding aliphatic aldehydes. Heating thiazolidine derivatives resulted in very complex flavor compound generation. Particularly, a route to form thiazole by the decarboxylation reaction of thiazolidine derivatives by cysteine reacting with formaldehyde was proposed. The work helps gain insight into the formation pathway of initial reaction intermediate compounds, and can also provide some guidance for processing of meat flavorings or meat foods.
Funding sources
The present work was supported by the Special National Key Research and Development Plan (2021YFD1600100), the Project of Science and Technology Department of Qinghai Province (2021-NK-A3), and the Shandong Province Key Research and Development Plan(2022CXGC010602).
Informed consent
Not applicable.
CRediT authorship contribution statement
Wenbin Du: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – original draft. Yutang Wang: Conceptualization, Formal analysis, Project administration, Writing – review & editing. Qianli Ma: Data curation, Supervision, Software. Yang Li: Supervision, Resources, Software. Bo Wang: Investigation, Formal analysis, Methodology. Shuang Bai: Data curation, Validation, Visualization. Bei Fan: Conceptualization, Funding acquisition, Project administration, Supervision, Resources, Writing – review & editing. Fengzhong Wang: Funding acquisition, Conceptualization, Formal analysis, Project administration, Writing – review & editing.
Declaration of competing interest
No conflict of interest exits in the submission of this manuscript (entitled “The number and position of unsaturated bonds in aliphatic aldehydes affect meat flavorings system: Insights on initial Maillard reaction stage and meat flavor formation from thiazolidine derivatives”; authors: Wenbin Du, Yutang Wang, Qianli Ma, Yang Li, Bo Wang, Shuang Bai, Bei Fan*, Fengzhong Wang*), and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere. We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Handling Editor: Dr. Xing Chen
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2024.100719.
Contributor Information
Bei Fan, Email: fanbei517@163.com.
Fengzhong Wang, Email: wangfengzhong@sina.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- Adams A., Bouckaert C., Van Lancker F., De Meulenaer B., De Kimpe N. Amino acid catalysis of 2-alkylfuran formation from lipid oxidation-derived α,β-unsaturated aldehydes. J. Agric. Food Chem. 2011;59(20):11058–11062. doi: 10.1021/jf202448v. [DOI] [PubMed] [Google Scholar]
- Bi S., Wang A., Wang Y., Xu X., Luo D., Shen Q., Wu J. Effect of cooking on aroma profiles of Chinese foxtail millet (Setaria italica) and correlation with sensory quality. Food Chem. 2019;289:680–692. doi: 10.1016/j.foodchem.2019.03.108. [DOI] [PubMed] [Google Scholar]
- Cao C., Xie J., Hou L., Zhao J., Chen F., Xiao Q., Zhao M., Fan M. Effect of glycine on reaction of cysteine-xylose: Insights on initial Maillard stage intermediates to develop meat flavor. Food Res. Int. 2017;99(Pt 1):444–453. doi: 10.1016/j.foodres.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Clark A.C., Deed R.C. The chemical reaction of glutathione and trans-2-hexenal in grape juice media to form wine aroma precursors: the impact of pH, temperature, and sulfur dioxide. J. Agric. Food Chem. 2018;66(5):1214–1221. doi: 10.1021/acs.jafc.7b04991. [DOI] [PubMed] [Google Scholar]
- Cui H., Hayat K., Jia C., Duhoranimana E., Huang Q., Zhang X., Ho C.T. Synergistic effect of a thermal reaction and vacuum dehydration on improving xylose-phenylalanine conversion to N-(1-deoxy-d-xylulos-1-yl)-phenylalanine during an aqueous Maillard reaction. J. Agric. Food Chem. 2018;66(38):10077–10085. doi: 10.1021/acs.jafc.8b04448. [DOI] [PubMed] [Google Scholar]
- Du W., Wang Y., Yan Q., Bai S., Huang Y., Li L., Mu Y., Shakoor A., Fan B., Wang F. The number and position of unsaturated bonds in aliphatic aldehydes affect the cysteine-glucose Maillard reaction: formation mechanism and comparison of volatile compounds. Food Res. Int. 2023;173 doi: 10.1016/j.foodres.2023.113337. [DOI] [PubMed] [Google Scholar]
- Du W., Zhao M., Zhen D., Tan J., Wang T., Xie J. Key aroma compounds in Chinese fried food of youtiao. Flavour Fragrance J. 2019;35(1):88–98. doi: 10.1002/ffj.3539. [DOI] [Google Scholar]
- Du W., Zhen D., Wang Y., Cheng J., Xie J. Characterization of the key odorants in grilled mutton shashlik with or without suet brushing during grilling. Flavour Fragrance J. 2020;36(1):111–120. doi: 10.1002/ffj.3621. [DOI] [Google Scholar]
- Fernandez X., Duñach E., Fellous R., Lizzani-Cuvelier L., Loiseau M., Dompe V., Cozzolino F., George G., Rochard S., Schippa C. Identification of thiazolidines in guava: stereochemical studies. Flavour Fragrance J. 2001;16(4):274–280. doi: 10.1002/ffj.996. [DOI] [Google Scholar]
- Hou L., Xie J., Zhao J., Zhao M., Fan M., Xiao Q., Liang J., Chen F. Roles of different initial Maillard intermediates and pathways in meat flavor formation for cysteine-xylose-glycine model reaction systems. Food Chem. 2017;232:135–144. doi: 10.1002/ffj.996. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang W., Zeng X., Xi Y., Li Y., Hui B., Li J. Characterization of the major odor-active off-flavor compounds in normal and lipoxygenase-lacking soy protein isolates by sensory-directed flavor analysis. J. Agric. Food Chem. 2023;71(21):8129–8139. doi: 10.1021/acs.jafc.3c00793. [DOI] [PubMed] [Google Scholar]
- Mottram D.S. Flavour formation in meat and meat products: a review. Food Chem. 1998;62(4):415–425. doi: 10.1016/S0308-8146(98)00076-4. [DOI] [Google Scholar]
- Pu D., Shan Y., Zhang L., Sun B., Zhang Y. Identification and inhibition of the key off-oodorants in duck broth by means of the sensomics approach and binary odor mixture. J. Agric. Food Chem. 2022;70(41):13367–13378. doi: 10.1021/acs.jafc.2c02687. 0.1021/acs.jafc.2c02687. [DOI] [PubMed] [Google Scholar]
- Shakoor A., Zhang C., Xie J., Yang X. Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chem. 2022;393 doi: 10.1016/j.foodchem.2022.133416. [DOI] [PubMed] [Google Scholar]
- Sohail A., Al-Dalali S., Wang J., Xie J., Shakoor A., Asimi S., Shah H., Patil P. Aroma compounds identified in cooked meat: a review. Food Res. Int. 2022;157 doi: 10.1016/j.foodres.2022.111385. [DOI] [PubMed] [Google Scholar]
- Wang T., Zhen D., Tan J., Xie J., Cheng J., Zhao J. Characterization of initial reaction intermediates in heated model systems of glucose, glutathione, and aliphatic aldehydes. Food Chem. 2020;305 doi: 10.1016/j.foodchem.2019.125482. [DOI] [PubMed] [Google Scholar]
- Wei C.K., Ni Z.J., Thakur K., Liao A.M., Huang J.H., Wei Z.J. Aromatic effects of immobilized enzymatic oxidation of chicken fat on flaxseed (Linum usitatissimum L.) derived Maillard reaction products. Food Chem. 2020;306 doi: 10.1016/j.foodchem.2019.125560. [DOI] [PubMed] [Google Scholar]
- Whitfield F.B. Volatiles from interactions of Maillard reactions and lipids. Crit. Rev. Food Sci. Nutr. 1992;31(1–2):1–58. doi: 10.1080/10408399209527560. [DOI] [PubMed] [Google Scholar]
- Xu Y., Bi S., Xiong C., Dai Y., Zhou Q., Liu Y. Identification of aroma active compounds in walnut oil by monolithic material adsorption extraction of RSC18 combined with gas chromatography-olfactory-mass spectrometry. Food Chem. 2023;402 doi: 10.1016/j.foodchem.2022.134303. [DOI] [PubMed] [Google Scholar]
- Yang Z., Xie J., Zhang L., Du R., Cao C., Wang M., Acree T., Sun B. Aromatic effect of fat and oxidized fat on a meat-like model reaction system of cysteine and glucose. Flavour Fragrance J. 2015;30(4):320–329. doi: 10.1002/ffj.3248. [DOI] [Google Scholar]
- Yoshitake J., Shibata T., Shimayama C., Uchida K. 2-Alkenal modification of hemoglobin: Identification of a novel hemoglobin-specific alkanoic acid-histidine adduct. Redox Biol. 2019;23 doi: 10.1016/j.redox.2019.101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Zhang S., Zhang L. Evaluation of the extent of initial Maillard reaction during cooking some vegetables by direct measurement of the Amadori compounds. J. Sci. Food Agric. 2018;98(1):190–197. doi: 10.1002/jsfa.8455. [DOI] [PubMed] [Google Scholar]
- Zhao J., Wang T., Xie J., Xiao Q., Cheng J., Chen F., Wang S., Sun B. Formation mechanism of aroma compounds in a glutathione-glucose reaction with fat or oxidized fat. Food Chem. 2019;270:436–444. doi: 10.1016/j.foodchem.2018.07.106. [DOI] [PubMed] [Google Scholar]
- Zhao J., Wang T., Xie J., Xiao Q., Du W., Wang Y., Cheng J., Wang S. Meat flavor generation from different composition patterns of initial Maillard stage intermediates formed in heated cysteine-xylose-glycine reaction systems. Food Chem. 2019;274:79–88. doi: 10.1016/j.foodchem.2018.08.096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.