Abstract
Background
The aesthetic reconstruction of disfiguring cranio-facial defects after tumour excision can be quite challenging to the neurosurgeon with limited resources. The choice of cranioplasty implant, intraoperative technicalities and the patients’ postoperative appearance are critical considerations in management. There are a number of synthetic materials available for cranioplasty, however, the customised implants are not readily available in our practice setup. They are also mostly constructed and contoured after the bony defect has been created or require sophisticated software construction pre-operatively.
Methods
Eight patients with cranio-facial tumour pathologies who presented to our neurosurgical service, and had titanium mesh cranioplasty for the correction of cosmetically disfiguring cranio-facial tumours.
Results
There were 6 females, and 2 male patients respectively, with an age range between 28 and 74years. The histological diagnoses were meningioma, frontal squamous cell carcinoma, fibrous dysplasia, frontal mucocoele, cemeto-ossifying fibroma, osteoma, and naso-ethmoidal squamous cell carcinoma. The patient with naso-ethmoidal squamous cell carcinoma had post-operative subgaleal empyema which was amenable to incision and drainage procedure. The patient with a frontal cemento-ossifyng fibroma had a transient immediate post-operative mechanical ptosis, which resolved completely in 3months. All of the total eight patients (100%) had satisfactory cosmetic outlook at a minimum follow up period of 1month post-operatively (Numeric Rating Scale of at least 7/10). One of the patients required a revision surgery on account of implant displacement.
Conclusion
Cranioplasty is a common reconstructive neurosurgical procedure. It is important to the neurosurgeon for its neuro-protective function, and in the restoration of intra-cranial CSF dynamics. However, the cosmetic outlook appears to be more important to patients in the absence of pain and/or neurological deficits. Titanium mesh reconstruction is commonly used globally, and is becoming the preferred choice in low resource settings.
Keywords: Cranioplasty, Titanium mesh, Cranio-facial tumours, Cosmesis
1. Introduction
Neuro-cranial defects following excision of tumour involved bone requires repair and reconstruction of the cranium for brain protection and cosmesis. This can be achieved by the use of a variety of synthetic materials, as well as autologous bone grafts.1
Cranioplasty also serves as a therapeutic measure to control alterations in cerebrospinal fluid (CSF), cerebral blood flow, and the metabolic demands of the brain.1, 2, 3, 4 It also facilitates neurological rehabilitation, and improves neurological outcome.5
The skull can become infiltrated by tumour cells, necessitating removal of the hypertrophied or thinned out bone to achieve a complete tumour resection, depending on the underlying pathology. Hyperostosis of the bone overlying meningiomas has been reported in literature to be as many as 50% of cases.3
Unfortunately, cosmetic reconstruction of wide bone defects can pose a significant challenge intraoperatively, and customized cranioplasty implants are quite costly.3The use of autologous bone grafts may impact on the patients’ morbidity, and has been shown to have a significantly higher re-operation rates when compared to synthetic materials (titanium and polymehtylmethacrylate were the most commonly used synthetic materials). These re-operations rates are mostly due to bone resorption, with a rate as high as 20% 5and is largely underreported in literature5
A recent systematic review of various cranioplasty implants (titanium mesh, polyether ether ketone (PEEK), polymethyl methacrylate (PMMA), and Norian implants), showed that titanium mesh had the least infection rate (6.02%).6
2. Methodology
A retrospective clinical series of eight adult patients with cosmetically disfiguring cranio-facial tumours who presented at our neurosurgical service from January 2018 to November 2022. These patients had tumour excision, immediate or delayed cranioplasty with titanium mesh.
Intraoperatively, the mesh was cut, contoured, and refashioned to conform to the cranial defect's geometry (length, breadth, and natural contours). These participants were followed up for a minimum of 3months. Satisfactory cosmetic outlook (Numeric rating scale score) and wound complication incidence were the outcome measures.
3. Results
There were 6 females, and 2 male patients respectively, with an age range of 28 and 74years (Table 1). The histological diagnoses were meningioma (Fig. 1), fibrous dysplasia (Fig. 2), squamous cell carcinoma (Fig. 3), frontal mucocoele (Fig. 4), cemeto-ossifying fibroma, osteoma, and naso-ethmoidal squamous cell carcinoma (Table 1, Table 2).
Table 1.
Patients’ demographics and clinico-pathological features.
| S/n | Sex | Age | Symptom duration | Region | Clinical Diagnosis |
|---|---|---|---|---|---|
| 1 | M | 35 | 3months | Naso-ethmoidal | Naso-ethmoidal tumour |
| 2 | F | 74 | 24months | Frontal | Mucocoele |
| 3 | F | 28 | 18months | Frontal | Mucocoele |
| 4 | M | 46 | 22months | Frontal | Mucocoele |
| 5 | F | 55 | 5years | Frontal | Meningioma |
| 6 | F | 55 | 40years | Fronto-orbito-ethmoidal | Fibrous dysplasia |
| 7 | F | 37 | 18months | Frontal | Frontal Squamous cell ca |
| 8 | F | 48 | 30months | Fronto-parietal | Osteoma |
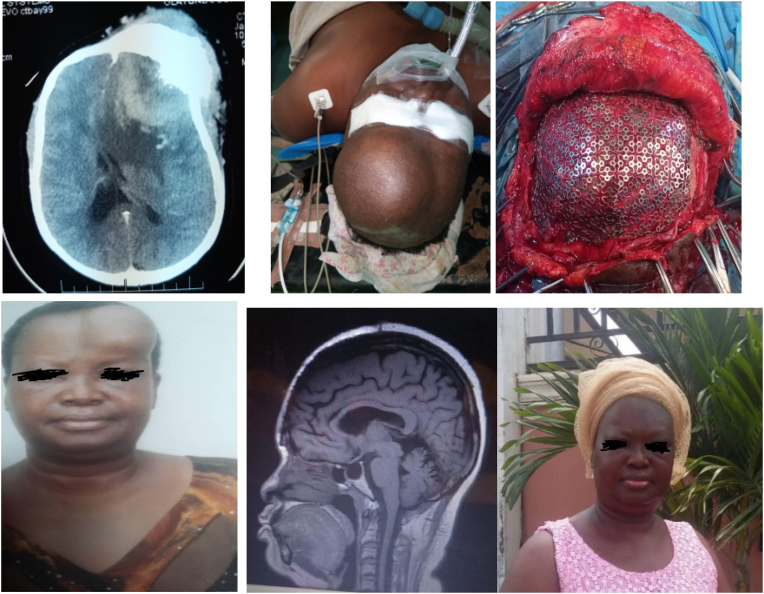
Fig. 1.
a–f a) axial cranial CT scan showing a contrast enhancing frontal mass, with adjacent hyperostosis; b) massive, and disfiguring frontal mass; c) titanium mesh implanted intra-op; d) pre-operative portrait; e and f) 1 year postoperative MRI showing a restored frontal contour.
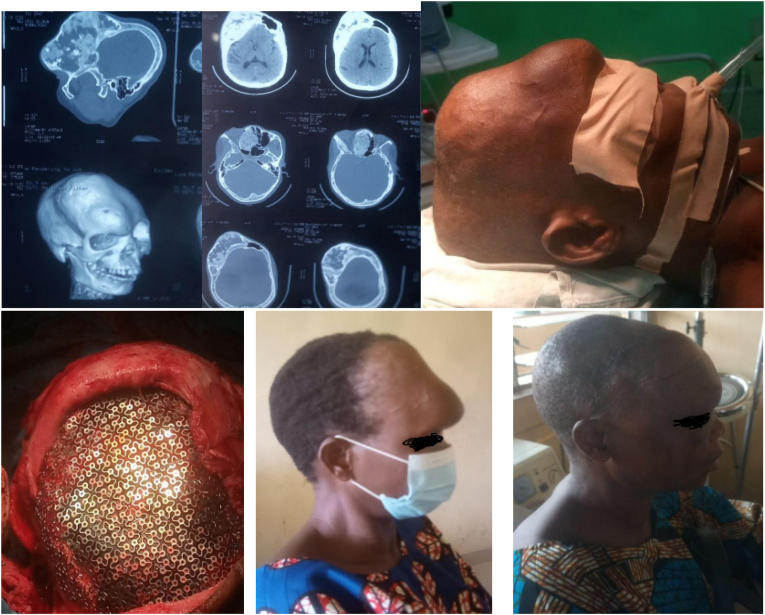
Fig. 2.
a–f a,b) CT scan showing a right parieto-fronto-orbito-ethmoidal bony tumour with a ground-glass appearance. c) Horn-like fronto-orbital mass. d) intra-op, titanium mesh anchored. e) Pre-operative appearance. f) 2weeks post-operative appearance.
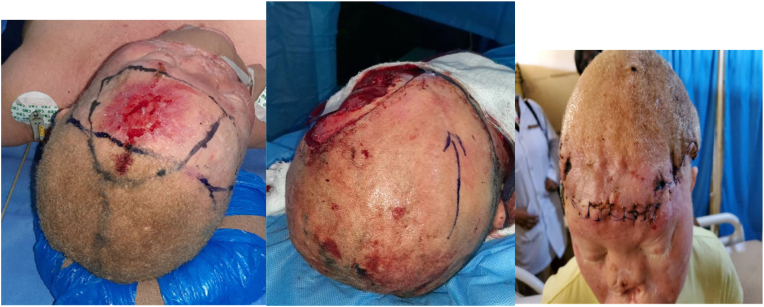
Fig. 3.
a–ca,b) left frontal ulcer, and markings for wide local excision and transposition scalp flap. c) 1week post-operative appearance.
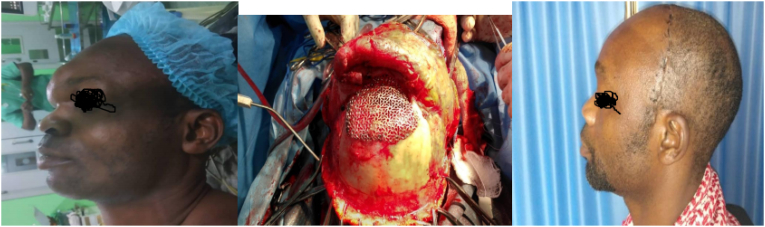
Fig. 4.
a–ca) Pre-operative appearance. b) Intra-operative, titanium mesh implanted. c) Post-operative appearance.
Table 2.
Pathological and surgical indices.
| S/n | Diagnosis | Surgery | Timing | Complication | NRS score |
|---|---|---|---|---|---|
| 1 | Naso-ethmoidal SCC | Excision, skull base repair + Cranioplasty | Immediate | Subgaleal empyema | 7 |
| 2 | Frontal mucocoele | Excision + Cranioplasty | Immediate | – | 8 |
| 3 | Frontal Ossifying Fibroma | Excision + Cranioplasty | Immediate | – | 7 |
| 4 | Frontal Mucocoele | Excision + Cranioplasty | Immediate | – | 8 |
| 5 | Frontal Meningioma | Simpson I excision + Cranioplasty | Delayed | – | 10 |
| 6 | Fronto-orbito-ethmoidal Fibrous Dysplasia | Excision + Cranioplasty | Immediate | Implant displacement | 9 |
| 7 | Frontal Squamous cell ca | Wide local excision + Cranioplasty + Transposition Scalp flap | Immediate | – | 8 |
| 8 | Fronto-parietal Osteoma | Excision + Cranioplasty | Immediate | – | 9 |
The patient with naso-ethmoidal squamous cell carcinoma had post-operative subgaleal empyema. The patient with a frontal ossifyng fibroma had a transient immediate post-operative mechanical ptosis, which resolved completely in 3months.
All of the total eight patients (100%) had satisfactory cosmetic outlook at a minimum follow up period of 1month post-operatively (Numeric Rating Scale Score of at least 7/10). One of the patients had a 10/10 cosmetic satisfaction (Fig. 1a–f), while one required a revision surgery on account of implant displacement (Fig. 2a, b, and 2c).
4. Discussion
In our series, majority of the patients were females (75%), and neoplastic tumours accounted for 5 of the 8 patients. The overall complication rate was 25% which is comparable to 27.8% by Thein et al.7
Our postoperative infection rate was 12.5%, and occurred in patient with a malignant tumour and a Center for Disease Control (CDC) class 2 surgical wound. He was also on long-term steroid use for vasogenic peri-lesional oedema. He had a frontal subgaleal empyema which was entirely amenable to percutaneous drainage, and antibiotics.
The patient with fibrous dysplasia had the longest duration of symptoms before presentation (40years), and had the largest and most cosmetically disfiguring tumour (Fig. 2c). She also had a revision surgery which was due to technical difficulty in reconstructing the supra-orbital ridge.
The patient with frontal meningioma had a delayed cranioplasty (after 3months) on account of financial limitations. She however, had the best cosmetic outcome (NRS score 10/10), and no implant complication (Fig. 1f).
The female patient with frontal squamous cell carcinoma is an albino. She had a wide local excision, cranioplasty, and a transposition scalp flap by the plastic surgeon (Fig. 3b).
Overall, all the patients expressed their satisfaction with the cosmetic outlook, with the least NRS score of 7/10. The NRS is a common and validated tool8, 9, 10 and has been widely used in the assessment of pain and aesthetic outcome in post-operative patients. It has also been demonstrated in literature to have a statistically significant concordance with the Visual Analogue Score (VAS) with p < 0.001.8
The choice of material for cranioplasty range from autologous bone graft (full and split-thickness) to synthetic (monomers or polyners) materials such as Polyethyl ether ketone (PEEK), Titanium mesh, Polymethyl methylacrylate (PMMA), Hydroxyapatite (HA), Ceramic, Porous polyethylene, etc. These materials could either be plain, manually or 3D pre-constructed.5,1,11,12 Autologous bone graft is being replaced with synthetic, notably due to high rates of resorption,5 which has also been underreported in literature.
PMMA and Titanium mesh have been compared in prospective studies13 and both found to have comparable cosmetic outcome, and no difference in complication rate of statistical significance. There is also a recent multicenter clinical trial evaluating PEEK and Titanium mesh cranioplasty, with the primary outcome measure of infection or implant exposure within 6months of surgery.11
Infection and cost are notable problems encountered in resource-limited settings. Titanium mesh appears to be gaining wide application in low resource countries. This may be attributed to concerns of postoperative infection, and the socio-economic burden of its treatment, and revision surgeries. A systematic review by Oliver et al6 documented a relatively lower infection rate with the use of titanium mesh. The cost effectiveness of various synthetic cranioplasty materials has also been studied in the literature, in favour of Titanium mesh,14 and with statistical significance (p = 0.013).
This study is limited by its small sample size, being a case series, and a short term duration of follow-up.
5. Conclusion
The subject of healthcare cost cannot be overemphasized in a low resource setting. Consequently, neurosurgeons and craniofacial surgeons in these climes may be limited with cranioplasty reconstruction options that possess both efficacy and cost-effectiveness.
The cosmetic outlook appears to be more important to patients in the absence of pain and/or neurological deficits. Titanium mesh reconstruction is commonly used globally, and may be recommended in low resource settings.
CRediT authorship contribution statement
C.O. Anele: Conceptualization. S.A. Balogun: Resources. C.O. Ezeaku: Data curation. T.O. Ajekwu: Data curation. H.E. Omon: Resources. G.O. Ejembi: Supervision. E.O. Komolafe: Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
- NRS
Numeric Rating Score
- CSF
Cerebro-Spinal Fluid
- PEEK
Polyether ether ketone
- PMMA
Polymethyl Methacrylate
- SCC
Squamous cell carcinoma
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- CDC
Centers for Disease Control
References
- 1.Lau D., Mcdermott M.W. 2015. (A Method for Combining Thin and Thick Malleable Titanium Mesh in the Repair of Cranial Defects). 7(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comprehensive C.A., Aspects S. Literature review. World Neurosurg. 2020;139:445–452. doi: 10.1016/j.wneu.2020.04.211. [DOI] [PubMed] [Google Scholar]
- 3.Bloch O., Mcdermott M.W. In situ cranioplasty for hyperostosing meningiomas of the cranial vault. Published online. 2021:59–64. doi: 10.1017/s0317167100011082. [DOI] [PubMed] [Google Scholar]
- 4.Li A., Azad T.D., Veeravagu A., Bhatti I. World Neurosurg.; 2017. Cranioplasty Complications and Costs: A National Population-Level Analysis Using the MarketScan Longitudinal Database. Published online. [DOI] [PubMed] [Google Scholar]
- 5.Malcolm J.G., Mahmooth Z., Rindler R.S., et al. Literature review autologous cranioplasty is associated with increased reoperation rate : a systematic review and meta-analysis. World Neurosurg. 2018;116:60–68. doi: 10.1016/j.wneu.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Oliver J.D., Banuelos J., Abu-ghname A., Vyas K.S., Sharaf B. 2019. pp. 289–294. (A Systematic Review Comparing Outcomes with Titanium Mesh , in 3591 Adult Patients). 82(May) [DOI] [PubMed] [Google Scholar]
- 7.Thien A., King N.K.K., Ang T., Wang E., Ng I. Peer-review reports comparison of polyetheretherketone and titanium cranioplasty after decompressive craniectomy. World Neurosurg. 2014;83(2):176–180. doi: 10.1016/j.wneu.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Rosas S., Paço M., Lemos C., Pinho T. Comparison between the visual analog scale and the numerical rating scale in the perception of esthetics and pain. Int Orthod. 2017;15(4):543–560. doi: 10.1016/j.ortho.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Huang L.C., Chen D.Z., Chen L.W., Xu Q.C., Zheng Z.H., Dai X.F. The use of the Scar Cosmesis Assessment and rating scale to evaluate the cosmetic outcomes of totally thoracoscopic cardiac surgery. J Cardiothorac Surg. 2020;15(1):1–8. doi: 10.1186/s13019-020-01294-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Opdam K.T.M., Zwiers R., Vroemen J., Sierevelt I.N., Wiegerinck J.I., van Dijk C.N. High patient satisfaction and good long-term functional outcome after endoscopic calcaneoplasty in patients with retrocalcaneal bursitis. Knee Surg Sports Traumatol Arthrosc. 2021;29(5):1494–1501. doi: 10.1007/s00167-020-06167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J., Sun T., Yuan Y., Li X., Yu H., Guan J. Evaluation of titanium mesh cranioplasty and polyetheretherketone cranioplasty: protocol for a multicentre, assessor-blinded, randomised controlled trial. BMJ Open. 2019;9(12):1–6. doi: 10.1136/bmjopen-2019-033997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teleanu D.M., Cristescu A., Bogaciu S., Teleanu R.I., Ciurea A.V. Titanium mesh implants - alternative for cranial bone defects. Key Eng Mater. 2017;752 KEM:105–110. doi: 10.4028/www.scientific.net/KEM.752.105. [DOI] [Google Scholar]
- 13.Youssef E., Seleem D., Yahia M. Aesthetic and psychological outcomes of cranioplasty, polymethyl methacrylate versus titanium mesh. Peruvian J Neurosurg. 2019;1(1):9–20. doi: 10.53668/2019.pjns11153. [DOI] [Google Scholar]
- 14.Binhammer A., Jakubowski J., Antonyshyn O., Binhammer P. Comparative cost-effectiveness of cranioplasty implants. Plast Surg. 2020;28(1):29–39. doi: 10.1177/2292550319880922. [DOI] [PMC free article] [PubMed] [Google Scholar]