Abstract
Methyl orange (MO) is commonly used in the textile dyeing industry, posing serious health and environmental hazards due to its carcinogenic, mutagenic properties, and potential for bioaccumulation. Appropriate handling is needed to solve these problems by harnessing the capacity of living microorganisms and the adsorption properties of bentonite clay minerals. Although the conventional approach predominantly depends on free cells, recent study has developed other methods such as immobilization techniques. Therefore, this study aimed to investigate the efficiency of the immobilization matrix comprising sodium alginate (SA), polyvinyl alcohol (PVA), and bentonite by modifying Pseudomonas aeruginosa, Bacillus subtilis, and Ralstonia pickettii for MO removal of 50 mg/L. In the free cell technique, the results showed that the MO decreased to 43.13, 36.61, and 27.45% for each of the bacteria within 10 days at 35 °C. The bacterial immobilization technique, including live immobilized P. aeruginosa (LIPa), live immobilized B. subtilis (LIBs), and live immobilized R. pickettii (LIRp) beads also demonstrated significant efficiency, achieving MO removal rates up to 97.15, 95.65, and 66.63% within 10 days. These synthesized beads showed reusability, with LIPa, LIBs, and LIRp being used up to 4, 4, and 2 cycles, respectively. The external and internal surface conditions were observed using SEM instrument and the results showed that all components were agglomerated. Comparisons using dead bacterial biomass indicated that treatment with live bacteria consistently yielded significantly higher removal rates. These results showed the effectiveness of immobilized bacteria in MO removal, offering a promising potential in reducing pollutants.
Keywords: Bioremediation, Pollution, Immobilization, Bacteria, Methyl orange
1. Introduction
Dyes are materials with several functions and are often used in the textile industry for coloring fabrics and textiles. Various types of dyes have been identified [1], one of which is classified based on charge, depending on the chemical structure. For example, anionic dyes have a negative charge when dissolved in water, including methyl orange (MO), which is usually used for dyeing textiles such as wool, cotton, and silk fabrics [2,3]. Actually, azo dyes (e.g. MO), contribute approximately 50% of global dyes production, approximately 15% of these dyes are wasted in wastewater during dyeing operations [4]. These dyes could be toxic and mutagenic when accumulated in living organisms [5] and can disrupt sunlight penetration in polluted water environments, thereby affecting the photosynthesis process [6]. The Ecological and Toxicological Association of Dyestuffs (ETAD) reported that 90% of the 4000 dyes that are commonly used had severe toxicity, with an LD50 value of about 2 × 103 mg/kg [7,8].
Various methods have been developed for handling dyes, including adsorption, coagulation, advanced oxidation process (AOP), and biodegradation [[9], [10], [11]]. The adsorption process depends on adsorbents to adsorb adsorbates or pollutants. This process was affected by the large adsorption capacity and the interactions occurring between the adsorbent and the adsorbate [12]. Currently, immobilization techniques are widely used in the dye removal process [13], specifically Immobilization Matrix comprising sodium alginate (SA) and polyvinyl alcohol (PVA) [14,15], where crosslinked alginate forms calcium alginate within CaCl2. This matrix is biocompatible, forming beads with a high absorption surface area [16]. Furthermore, a matrix made of PVA is characterized by a high mechanical resistance, rendering it less prone to brittleness [16]. Previous studies had proven that matrix made of SA and PVA were able to absorb several pollutants and dyes, such as methylene blue [17], phenol [18], thiamethoxam [15], polycyclic aromatic hydrocarbon [19], congo red, MO [20], Direct Orange-26 (DO-26), Direct Red-31 (DR-31), Direct Blue-67 (DB-67) and Ever direct Orange-3GL (EDO-3) [21], and crystal violet [22].
The addition of support materials for adsorption such as bentonite, a cation exchange clay mineral with a sheet surface of Si–O has been extensively studied [23,24]. In a previous study, bentonite was used as an adsorbent agent, particularly for cationic dyes, such as MB [25]. The results showed that natural bentonite clay could adsorb cationic dyes (MB) with a maximum adsorption capacity of 256 mg/g [23]. According to Nabilah et al. [25], SA-PVA-bentonite could remove MB by 78.98% of the concentration initial 100 mg/L and anionic MO dye. A hydrophobic interaction occurs between MO and SA-PVA-Bentonite with the organic compounds, allowing MO to enter the bentonite sheet [26,27]. Consequently, MO requires a slightly longer time to be adsorbed in the adsorbent. Several studies have also added bacteria to the adsorption and degradation processes [23,28], including Bacillus subtilis to adsorb MB and obtained a maximum capacity of 37.01 mg/g [28]. The results showed that immobilized bacteria can still work for dye removal, as indicated by the SEM obtained [29,30].
This study further investigated the removal ability of SA-PVA-Bentonite by modifying the addition of bacteria, namely Pseudomonas aeruginosa, B. subtilis, and Ralstonia pickettii, as a biodegradation agent. Previous study only reported that MO removal was only adsorbed with this matrix without any bacteria addition. Meanwhile, in this study the addition of bacteria was applied [26]. The experiment was carried out three times (triplicate) and the immobilized beads used for MO removal were compared with each free bacterial cell treatment. Furthermore, the effect of immobilized dead bacterial/bacterial biomass was investigated and the morphology of each bead was analyzed using SEM instrumentation. This research supposed to be had such an efficiency result on MO removal than without any bacteria addition and eco-friendly residual waste.
2. Materials and methods
2.1. Microbials
The bacteria used were P. aeruginosa NBRC 3080 (2.11 × 108 CFU/mL), B. subtilis NBRC 3009 (2.47 × 108 CFU/mL), and R. pickettii NBRC 102503 (2.46 × 108 CFU/mL). These bacterial strains were sourced from the Laboratory of Microorganism Chemistry, Institut Teknologi Sepuluh Nopember, Surabaya.
2.2. Materials
The materials used for the inoculation of bacteria consisted of nutrient agar (NA, Germany Merck), Luria Bertani broth (LB, Germany Merck), demineralized water (UD. Sumber Ilmiah Persada, Indonesia), alcohol 70% (UD. Sumber Ilmiah Persada, Indonesia). The components for immobilization were sodium alginate for microbiology (SA, India Himedia), polyvinyl alcohol 60,000 molecular weight (PVA, Germany Merck), and bentonite (PT. Bentonite Alam Indonesia), while the dye used was methyl orange (MO, Germany Merck).
2.3. Culture condition
Each of the bacteria used was regenerated using NA media and incubated for 24 h at 37 °C. Subsequently, the cultures were inoculated into LB broth and incubated at 37 °C for 24 h as a pre-culture. The culture was transferred to another LB until the optical density (OD600) touched a value of 1 and incubated at 37 °C to enter a stationary phase (P. aeruginosa: 24 h, B. subtilis: 24 h, and R. pickettii: 44 h). Finally, 50 mL of each bacteria was taken, and centrifuged to obtain the biomass [28,29].
2.4. Hydrogel preparation
Hydrogel preparation was carried out using a w/v ratio of 1:4:1 for each of SA, PVA, and bentonite. In a total volume of 50 mL of demineralized water, 0.5 g of SA was dissolved at 105 °C for 30 min, followed by the addition of 2 g PVA, which was heated until dissolved. Subsequently, 0.5 g of bentonite was added and stirred until homogeneous for 30 min [25]. The hydrogel solution was sterilized using an autoclave before being used for the next process.
2.5. Immobilization of bacteria
Bacterial immobilization was carried out separately for each bacterium by adding centrifuged bacteria to the hydrogel solution. The hydrogel was stirred using a stirrer for up to 30 min and dripped into a cold 4% w/v CaCl2 solution using a syringe injection to form round beads [31]. The beads were immersed in CaCl2 solution for 24 h [32] and stored in a refrigerator at 4 °C for preservation. In this study, immobilization of dead bacterial biomass was also carried out and bacteria were inactivated using high temperatures by autoclaving at 121 °C for 15 min [33]. Subsequently, the same process for immobilizing inactivated bacteria was used for live bacteria.
2.6. MO removal test
All treatment tests were carried out under sterile conditions, with three repetitions to ensure accuracy. The synthesized beads with a mass of 35 g were washed three times using sterile demineralized water and were used for MO removal at an initial concentration of 50 mg/L for 10 days at 35 °C [6,25,34,35]. A similar procedure was also applied for the beads without bacteria and inactivated (dead) bacteria. For comparison within the free cell technique, the biomass of live and dead bacteria was also used for this treatment at the same initial MO concentration at wavelength 465 nm. The MO removal process was analyzed using a UV–Vis Genesys 10S Thermo scientific spectrophotometer. The calculation of the percentage of MO removal used Eq. (1), as expressed below:
| (1) |
where C0 is the initial concentration, while Ct is the final concentration [36].
2.7. Beads viability test
The viability test was carried out to determine the condition of the immobilized bacteria in the beads. The process was conducted by reinserting the sample beads of each of the bacteria that had been synthesized into the sterile LB broth. Subsequently, the growth of bacteria that occurred was analyzed using a UV–Vis spectrophotometer at OD600. From this test, the value of bacterial growth was obtained starting from the lag to the dead phase. In addition to immobilized beads, free bacterial cells were also used for this viability test, serving as a comparison within immobilized beads [37].
2.8. Reusability test
The reusability test was used to determine the times and performance of the synthesized beads. In this test, the concentration of MO remained constant, with the same incubation time for each bacterium. The analysis process also used a UV–Vis spectrophotometer and Eq. (1), which was continued until the synthesized beads were damaged. Experiments on this treatment were also carried out in triplicate under sterile conditions [28].
2.9. Morphological characterization analysis
Freeze-drying preparation was handled before characterization to remove the water content of beads. Subsequently, beads were characterized using Scanning Electron Microscopy (SEM) to determine their morphology and topology, indicating the surface and slicing appearance [28].
2.10. Statistical analysis
The statistical analysis employed Analysis of Variance (ANOVA) to investigate the significant different between each treatment on beads immobilization and free cell, which expressed as mean of three replicates ± SD (standard deviation). The difference between mean values at confidence level of 5% (P < 0.05) were considered as statistically significant between group mean [1,38].
3. Results and discussion
3.1. MO removal
In this study, an anionic synthetic dye MO was treated using a matrix derived from SA, PVA, and Bentonite with the addition of P. aeruginosa, B. subtilis, or R. pickettii, separately. The result obtained is shown in Fig. 1 and the final MO concentrations are presented in Table 1. The figure showed that the beads without bacteria (SPB) had an MO removal percentage of 33.48%, as similarly obtained in other treatments. The percentage of immobilized dead bacteria NIPa, NIBs, and NIRp beads also had a lower percentage compared to the removal of each live bacterium, with final concentrations of 21.16, 32.46, and 17.97 mg/L, respectively. This variation was due to the biosorption properties of live bacteria, which contributed to the adsorption of MO when entering the beads [39].
Fig. 1.
MO removal percentage by immobilized bacteria.
Note. – SPB = SA-PVA-Bentonite; LIPa = Live Immobilized P. aeruginosa; NIPa = Nonlive Immobilized P. aeruginosa; LIBs = Live Immobilized B. subtilis; NIBs = Nonlive Immobilized B. subtilis; LIRp = Live Immobilized R. pickettii; NIRp = Nonlive Immobilized R. pickettii. The result exhibited means values ± SD of three replicate experiments. All relationships are statistically significant (P < 0.05).
Table 1.
Final MO concentration after treatment by immobilized bacteria.
| Sample Name | MO Final Concentration (mg/L) |
|---|---|
| SPB | 33.52 |
| LIPa | 1.41 |
| NIPa | 21.16 |
| LIBs | 2.17 |
| NIBs | 32.46 |
| LIRp | 33.06 |
| NIRp | 17.97 |
Dye removal through bacteria has been widely investigated in previous studies. Nabilah et al. [25] successfully removed methylene blue dye using a mixture of R. pickettii bacteria and Trichoderma viride fungi immobilized into SA-PVA-Bentonite. Rohmah et al. [28] also showed that B. subtilis could decolorize and degrade methylene blue dye, while according to Upendar et al. [31], the bacteria were immobilized into the suitable matrix. Generally, P. aeruginosa is one of the bacteria that is often used in study focused on the degradation of dye waste, indicating its potential to degrade methylene blue, methyl orange, reactive yellow, direct orange, etc [[40], [41], [42]]. Dyes consist of various types and groups, including classification based on charge, which was divided into cation and anion dyes [43].
The principle that works in dead bacteria is biosorption, while live bacteria incorporate both biosorption function and biodegradation [44]. Kishor et al. [40] proved that living P. aeruginosa can degrade MO dye into its metabolite product, including water and carbon dioxide, due to the presence of a ligninolytic enzyme (manganese peroxidase/MnP) secreted by the bacteria. MnP enzyme can also degrade, remove, and mineralize the textile dyes [45]. B. subtilis has shown the ability to degrade azo-mixed dye by producing intracellular azoreductase and laccase [46,47]. The use of non-living bacteria biomass has been reported to be effective in the removal of dyes [25,26,48] through surface adsorption cell and diffusion processes [49].
In the case of immobilized living bacteria, the percentage of MO elimination successively decreased from the smallest to the highest ranging from LIRp, LIBs, and LIPa beads. Based on the results, LIPa beads were removed 50 mg/L within 10 min and reached an almost perfect result of 97.15%, MO final concentration after treatment 1.41 mg/L, followed by LIBs beads of 95.65%. P. aeruginosa and B. subtilis bacteria were able to secrete the enzymes azoreductase and laccase [40,46]. Azoreductase enzyme was included in the biotransformation of azo dyes and catalyzes reactions in the presence of reducing agents such as NADH, NADPH, and FADH2 [50]. However, laccase is an oxidizing enzyme that breaks down dye structure by removing a hydrogen atom from the hydroxyl group of ortho and para-substituted mono and polyphenolic substrates, as well as aromatic amines, leading to depolymerization, demethylation, or quinine formations [50].
R. pickettii bacteria is still rarely investigated for dye removal. R. pickettii can produce lipase enzymes and be immobilized into alginate as an additive to commercial detergents in laundry and in the hydrolysis of oils [11]. A previous study also showed that R. pickettii had resistance to arsenite [51], with promising potential in dye removal for the elimination of methylene blue, due to a combination of alginate, PVA, and bentonite [25].
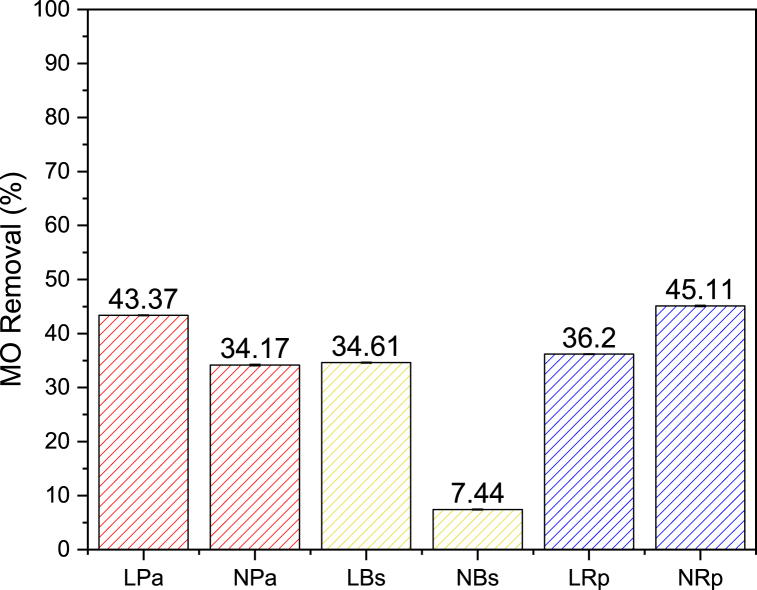
This study also conducted a comparison of MO removal using the free cell technique through bacterial biomass obtained from liquid culture. A total of 50 mL bacterial biomass was taken and applied to 50 mg/L MO solution for 10 days at 35 °C. The results obtained were shown in Fig. 2 and the final concentrations were in Table 2, indicating the percentage removal of MO by each bacterium. Live P. aeruginosa (LPa) bacteria showed a high percentage removal of 43.37%, compared to B. subtilis (LBs) and R. picketti (LRp) with 34.61 and 36.20%. The final MO concentrations after treatment of each bacteria were 26.30, 33.54, and 27.45 mg/L, respectively.
Fig. 2.
MO removal percentage by free cell technique.
Note. –LPa = Live P. aeruginosa; NPa = Nonlive P. aeruginosa; LBs = Live B. subtilis; NBs = Nonlive B. subtilis; LRp = Live R. pickettii; NRp = Nonlive R. pickettii. The result represented means values ± SD of three replicate experiments. All relationships are statistically significant (P < 0.05).
Table 2.
Final MO concentration after treatment by free cell technique.
| Sample Name | MO Final Concentration (mg/L) |
|---|---|
| LPa | 26.30 |
| NPa | 28.40 |
| LBs | 33.54 |
| NBs | 47.37 |
| LRp | 27.45 |
| NRp | 9.59 |
MO removal using dead bacteria to determine the biomass ability was also examined. The results showed that dead bacteria had significantly higher values, when compared to others, except for B. subtilis (NBs). Specifically, inactive R. pickettii (NRp) reduced MO dye up to 45.11%, due to rupture of cell membranes and the loss of intracellular components in dead bacteria caused by high temperatures, which facilitated liquid absorption [52,53]. A similar phenomenon was observed in P. aeruginosa, which has an optimum temperature of approximately 37–40 °C [54]. However, B. subtilis has higher resistance and adaptive ability, which kills at a range temperature of 95–100 °C [55]. This ability could make B. subtilis only show a lower percentage of MO removal compared to others.
3.2. Beads viability analysis
In this study, the immobilized bacteria were analyzed either alive or dead. A bacterial viability test was used on the beads to determine whether the bacteria remained viable [56]. This method was carried out to make a bacterial growth curve, which was measured using the turbidimetric principle at OD600. The measured OD was rated as absorbance at a wavelength of 600 nm because the medium used had a brownish-yellow color [57].
The beads that had been synthesized were put back into the LB medium. When the bacteria immobilized in the beads were still alive, the available carbon source was used for growth to obtain phases of bacterial life [57]. Fig. 3 shows the results of the viability test for P. aeruginosa, B. subtilis, and R. pickettii bacteria in the beads, indicating that each bacterium experienced different growth at certain times due to their unique growth phases [58].
Fig. 3.
Growth of bacteria culture on viability test.
Fig. 3 showed that the viability of each free cell decreased the absorbance due to the direct use of carbon sources in the media, leading to a rapid entry into the death phase. The growth curve of immobilized bacteria was different, as bacteria were more resistant to pressure in their environment, including limited carbon and energy sources. Consequently, bacteria were able to survive, as indicated in the absorbance of immobilized bacteria which continued to increase.
3.3. Beads reusability
One of the advantages of using the immobilization technique is the reusing of synthesized beads maximum capacity is reached. When optimum capacity has not been achieved, the beads continue to reabsorb the substrate solution [59]. The measurement of the percentage of MO removal in each cycle is calculated until it reaches 50% or the beads are not able to absorb optimally [60]. Based on the results, it was found that LIPa, LIBs, and LIRp beads had reuse cycles of up to 4, 4, and 2, respectively, as shown in Fig. 4. P. aeruginosa and B. subtilis bacteria showed a stable decrease in graphic results compared to R. pickettii bacteria. In the 4th cycle, the LIPa and LIBs bacteria absorbed MO up to 41.23 and 26.43%. However, for the LIRp bacteria, in the 2nd cycle, the bacteria experienced a significant decrease, with a percentage MO removal of 20.03%.
Fig. 4.
MO removal depends on the various beads reusability.
3.4. SEM characterization
Characterization analysis was carried out using an SEM instrument to determine the state of the morphology and topology of the beads. Before imagining, the beads were dried using a freeze dryer to remove their water content. The SEM image results showed that all the components that build up the beads were agglomerated, as presented in Fig. 5a, b, c, and d. Furthermore, the bead morphology after MO adsorption treatment was also observed, as illustrated in Fig. 6a, b, c, and d, indicating that MO was adsorbed into the beads.
Fig. 5.
SEM images of (a) SPB, (b) LIPa), (c) LIBs, and (d) LIRp beads before MO removal.
Fig. 6.
SEM images of (a) SPB, (b) LIPa), (c) LIBs, and (d) LIRp beads after MO removal.
This research involved bacteria's role on MO removal. Hence, MO treatment had been carried out by environmentally friendly principle. In addition, it also proven that immobilized bacteria showed high result on MO removal than without bacteria addition. These results could enhance the efficiency level on pollutant remediation, especially anionic dyes. However, bioremediation methods using bacteria still require improvement and development for their application on a wide scale area, because of its limitations on nutrition source and environmental stress.
4. Conclusion
In conclusion, this study successfully immobilized P. aeruginosa, B. subtilis, and R. pickettii in a matrix made of SA, PVA, and bentonite, separately. The Immobilization matrix was used for the removal of anionic MO dyes, leading to a significant reduction in MO compared to non-bacterial and cell-free techniques. The results showed that 50 mg/L MO could be removed by LIPa, LIBs, and LIRp beads within 10 days of incubation with a removal percentage of 97.15, 95.65, and 66.63%, respectively. The use of dead bacteria immobilization was also investigated and the highest percentage of removal was obtained from NIPa beads, amounting to 57.58%. Furthermore, using the free cell technique for each of P. aeruginosa, B. subtilis, and R. pickettii, yielded 43.13, 36.61, and 27.45%. The use of repeated beads was observed for LIPa, LIBs, and LIRp beads and could eliminate MO at 4, 4, and 2 cycles, respectively. These results showed that bacterial immobilization had an effective ability to produce higher removal outcomes in handling MO dye.
CRediT authorship contribution statement
Adi Setyo Purnomo: Writing – review & editing, Writing – original draft, Validation, Supervision, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Frida Wahyu Hairunnisa: Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis. Misdar: Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis. Virda Putri Maria: Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis. Alya Awinatul Rohmah: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Project administration, Formal analysis. Surya Rosa Putra: Writing – original draft, Supervision. Herdayanto Sulistyo Putro: Writing – review & editing, Supervision, Conceptualization. Hamdan Dwi Rizqi: Writing – original draft, Validation, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
Adi Setyo Purnomo reports financial support was provided by Ministry of Education, Culture, Research and Technology, Indonesia.
Acknowledgments
This study was funded by the Directorate of Research, Technology and Community Service, Ministry of Education, Culture, Research and Technology, Indonesia under the Fundamental Research Scheme with Contract Number: 112/E5/PG.02.00. PL/2023 and 1960/PKS/ITS/2023, June 20, 2023.
References
- 1.Zhu Y., Ma L., Hai X., Yang Z., Li X., Chen M., Yuan M., Xiong H., Gao Y., Wang L., Shi F. Adsorption of methyl orange by porous membranes prepared from deep eutectic supramolecular polymer-modified chitosan. Environ. Res. 2023;236 doi: 10.1016/j.envres.2023.116778. [DOI] [PubMed] [Google Scholar]
- 2.Ramutshatsha-Makhwedzha D., Mavhungu A., Moropeng M.L., Mbaya R. Activated carbon derived from waste orange and lemon peels for the adsorption of methyl orange and methylene blue dyes from wastewater. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu S., Xie Z., Wang R., Zou H., Lian S., Guo R. Combined disposal of methyl orange and corn straw via stepwise adsorption-biomethanation-composting. J. Environ. Manag. 2023;344 doi: 10.1016/j.jenvman.2023.118358. [DOI] [PubMed] [Google Scholar]
- 4.Bai Y.N., Wang X.N., Zhang F., Wu J., Zhang W., Lu Y.Z., Fu L., Lau T.C., Zeng R.J. High-rate anaerobic decolorization of methyl orange from synthetic azo dye wastewater in a methane-based hollow fiber membrane bioreactor. J. Hazard Mater. 2020;388 doi: 10.1016/J.JHAZMAT.2019.121753. [DOI] [PubMed] [Google Scholar]
- 5.Cao M., Shen Y., Yan Z., Wei Q., Jiao T., Shen Y., Han Y., Wang Y., Wang S., Xia Y., Yue T. Extraction-like removal of organic dyes from polluted water by the graphene oxide/PNIPAM composite system. Chem. Eng. J. 2021;405 doi: 10.1016/J.CEJ.2020.126647. [DOI] [Google Scholar]
- 6.Mon P.P., Cho P.P., Chandana L., Srikanth V.V.S.S., Madras G., Ch S. Biowaste-derived Ni/NiO decorated-2D biochar for adsorption of methyl orange. J. Environ. Manag. 2023;344 doi: 10.1016/J.JENVMAN.2023.118418. [DOI] [PubMed] [Google Scholar]
- 7.Hussain S., Kamran M., Khan S.A., Shaheen K., Shah Z., Suo H., Khan Q., Shah A.B., Rehman W.U., Al-Ghamdi Y.O., Ghani U. Adsorption, kinetics and thermodynamics studies of methyl orange dye sequestration through chitosan composites films. Int. J. Biol. Macromol. 2021;168:383–394. doi: 10.1016/J.IJBIOMAC.2020.12.054. [DOI] [PubMed] [Google Scholar]
- 8.Jawad A.H., Norrahma S.S.A., Hameed B.H., Ismail K. Chitosan-glyoxal film as a superior adsorbent for two structurally different reactive and acid dyes: adsorption and mechanism study. Int. J. Biol. Macromol. 2019;135:569–581. doi: 10.1016/J.IJBIOMAC.2019.05.127. [DOI] [PubMed] [Google Scholar]
- 9.Cheng X., Jiang D., Chen H., Barati B., Yuan C., Li H., Wang S. Multi-stage adsorption of methyl orange on the nitrogen-rich biomass-derived carbon adsorbent: DFT and MD evaluation. Chemosphere. 2023;338 doi: 10.1016/j.chemosphere.2023.139218. [DOI] [PubMed] [Google Scholar]
- 10.Hidayat A.R.P., Widyanto A.R., Asranudin A., Ediati R., Sulistiono D.O., Putro H.S., Sugiarso D., Prasetyoko D., Purnomo A.S., Bahruji H., Ali B.T.I., Caralin I.S. Recent development of double chamber microbial fuel cell for hexavalent chromium waste removal. J. Environ. Chem. Eng. 2022;10 doi: 10.1016/j.jece.2022.107505. [DOI] [Google Scholar]
- 11.Purnomo A.S., Maulianawati D., Kamei I. Ralstonia pickettii enhance the DDT biodegradation by pleurotus eryngii. J. Microbiol. Biotechnol. 2019;29:1424–1433. doi: 10.4014/jmb.1906.06030. [DOI] [PubMed] [Google Scholar]
- 12.Rashmi S., Michalska M., Krajewski M., Bochenek K., Zaszczynska A., Czeppe T., Rogal L., Jain A. vol. 297. 2023. (Materials Science & Engineering B One-step Synthesis of a Sustainable Carbon Material for High Performance Supercapacitor and Dye Adsorption Applications). [DOI] [Google Scholar]
- 13.Nallapan Maniyam M., Hari M., Yaacob N.S. Enhanced methylene blue decolourization by Rhodococcus strain UCC 0003 grown in banana peel agricultural waste through response surface methodology. Biocatal. Agric. Biotechnol. 2020;23 doi: 10.1016/j.bcab.2019.101486. [DOI] [Google Scholar]
- 14.Ariaeenejad S., Motamedi E. Improved saccharification of rice straw by removing phenolic compounds using a stable immobilized metagenome-derived laccase on sodium alginate-based hydrogel. Biochem. Eng. J. 2023;198 doi: 10.1016/j.bej.2023.109021. [DOI] [Google Scholar]
- 15.Xiang X., Yi X., Zheng W., Li Y., Zhang C., Wang X., Chen Z., Huang M., Ying G.G. Enhanced biodegradation of thiamethoxam with a novel polyvinyl alcohol (PVA)/sodium alginate (SA)/biochar immobilized Chryseobacterium sp H5. J. Hazard Mater. 2023;443 doi: 10.1016/j.jhazmat.2022.130247. [DOI] [PubMed] [Google Scholar]
- 16.Liu C., Liu H., Xiong T., Xu A., Pan B., Tang K. Graphene oxide reinforced alginate/PVA double network hydrogels for efficient dye removal. Polymers. 2018;10 doi: 10.3390/polym10080835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aljar M.A.A., Rashdan S., El-Fattah A.A. Environmentally friendly polyvinyl alcohol−alginate/bentonite semi-interpenetrating polymer network nanocomposite hydrogel beads as an efficient adsorbent for the removal of methylene blue from aqueous solution. Polymers. 2021;13:1–19. doi: 10.3390/polym13224000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan B., Wu P., Chen M., Lai X., Chen L., Yu L., Gong B., Kang C., Dang Z., Shi Z., Liu Z. Immobilization of Sphingomonas sp. GY2B in polyvinyl alcohol–alginate–kaolin beads for efficient degradation of phenol against unfavorable environmental factors. Ecotoxicol. Environ. Saf. 2018;162:103–111. doi: 10.1016/j.ecoenv.2018.06.058. [DOI] [PubMed] [Google Scholar]
- 19.Chen W., Zhang H., Zhang M., Shen X., Zhang X., Wu F., Hu J., Wang B., Wang X. Removal of PAHs at high concentrations in a soil washing solution containing TX-100 via simultaneous sorption and biodegradation processes by immobilized degrading bacteria in PVA-SA hydrogel beads. J. Hazard Mater. 2021;410 doi: 10.1016/j.jhazmat.2020.124533. [DOI] [PubMed] [Google Scholar]
- 20.Zhang M.K., Ling X.H., Zhang X.H., Han G.Z. A novel alginate/PVA hydrogel -supported Fe3O4 particles for efficient heterogeneous Fenton degradation of organic dyes. Colloids Surf., A Physicochem. Eng. Asp. 2022;652 doi: 10.1016/j.colsurfa.2022.129830. [DOI] [Google Scholar]
- 21.Bhatti H.N., Safa Y., Yakout S.M., Shair O.H., Iqbal M., Nazir A. Efficient removal of dyes using carboxymethyl cellulose/alginate/polyvinyl alcohol/rice husk composite: adsorption/desorption, kinetics and recycling studies. Int. J. Biol. Macromol. 2020;150:861–870. doi: 10.1016/j.ijbiomac.2020.02.093. [DOI] [PubMed] [Google Scholar]
- 22.Cheng Y., Lin H.Y., Chen Z., Megharaj M., Naidu R. Biodegradation of crystal violet using Burkholderia vietnamiensis C09V immobilized on PVA-sodium alginate-kaolin gel beads. Ecotoxicol. Environ. Saf. 2012;83:108–114. doi: 10.1016/j.ecoenv.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Ravi L.M. Pandey. Enhanced adsorption capacity of designed bentonite and alginate beads for the effective removal of methylene blue. Appl. Clay Sci. 2019;169:102–111. doi: 10.1016/j.clay.2018.12.019. [DOI] [Google Scholar]
- 24.Racette J., Walker A., Nagasaki S., Yang T.T., Saito T., Vilks P. Influence of Ca–Na–Cl physicochemical solution properties on the adsorption of Se(-II) onto granite and MX-80 bentonite to support the post-closure safety assessment of a used nuclear repository in crystalline rock. Nucl. Eng. Technol. 2023 doi: 10.1016/j.net.2023.06.049. [DOI] [Google Scholar]
- 25.Nabilah B., Purnomo A.S., Prasetyoko D., Rohmah A.A. Methylene Blue biodecolorization and biodegradation by immobilized mixed cultures of Trichoderma viride and Ralstonia pickettii into SA-PVA-Bentonite matrix. Arab. J. Chem. 2023;16 doi: 10.1016/j.arabjc.2023.104940. [DOI] [Google Scholar]
- 26.Belhouchat N., Zaghouane-Boudiaf H., Viseras C. Removal of anionic and cationic dyes from aqueous solution with activated organo-bentonite/sodium alginate encapsulated beads. Appl. Clay Sci. 2017;135:9–15. doi: 10.1016/j.clay.2016.08.031. [DOI] [Google Scholar]
- 27.Hameed B.H. Equilibrium and kinetics studies of 2,4,6-trichlorophenol adsorption onto activated clay. Colloids Surf., A Physicochem. Eng. Asp. 2007;307:45–52. doi: 10.1016/j.colsurfa.2007.05.002. [DOI] [Google Scholar]
- 28.Rohmah A.A., Purnomo A.S., Safitri W.N. Biodecolorization of methylene blue by using Bacillus subtilis immobilized into SA-PVA-bentonite matrix in mineral salt medium and non-nutritious medium. Indones. J. Chem. 2022;22:1637–1650. doi: 10.22146/ijc.76080. [DOI] [Google Scholar]
- 29.Lin C., Gan L., Chen Z., Megharaj M., Naidu R. Biodegradation of naphthalene using a functional biomaterial based on immobilized Bacillus fusiformis (BFN) Biochem. Eng. J. 2014;90:1–7. doi: 10.1016/j.bej.2014.05.003. [DOI] [Google Scholar]
- 30.Lau S.H., Lin I.C., Su C.L., Chang Y.T., Jane W.N. Synthesis of cross-linked magnetic chitosan beads immobilised with bacteria for aerobic biodegrading benzophenone-type UV filter. Chemosphere. 2022;307 doi: 10.1016/j.chemosphere.2022.136010. [DOI] [PubMed] [Google Scholar]
- 31.Upendar G., Dutta S., Bhattacharya P., Dutta A. Bioremediation of methylene blue dye using Bacillus subtilis MTCC 441. Water Sci. Technol. 2017;75:1572–1583. doi: 10.2166/wst.2017.031. [DOI] [PubMed] [Google Scholar]
- 32.Asranudin N., Holilah N., Purnomo A.S., Bahruji H., Allouss D., El Alaoui-Elbalrhiti I., Subagyo R., Rohmah A.A., Prasetyoko D. Hectorite-CTAB-alginate composite beads for water treatment: kinetic, isothermal and thermodynamic studies. RSC Adv. 2023;13:790–801. doi: 10.1039/d2ra06934b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wahyuni S., Khaeruni A., Purnomo A.S., Asranudin, Holilah Fatahu. Characterization of mannanase isolated from corncob waste bacteria. Asian J. Chem. 2017;29:1119–1120. doi: 10.14233/ajchem.2017.20437. [DOI] [Google Scholar]
- 34.Birniwa A.H., Ali U., Jahun B.M., Saleh Al-dhawi B.N., Jagaba A.H. Cobalt oxide doped polyaniline composites for methyl orange adsorption: optimization through response surface methodology. Case Stud. Chem. Environ. Eng. 2024;9 doi: 10.1016/J.CSCEE.2023.100553. [DOI] [Google Scholar]
- 35.Guo T.S., Yang S.D., Cui H.M., Yu Q.F., Li M.F. Synthesis of lignin nanoparticle-manganese dioxide complex and its adsorption of methyl orange. Int. J. Biol. Macromol. 2023;253 doi: 10.1016/J.IJBIOMAC.2023.127012. [DOI] [PubMed] [Google Scholar]
- 36.Alkas T.R., Ediati R., Ersam T., Nawfa R., Purnomo A.S. Fabrication of metal-organic framework Universitetet i Oslo-66 (UiO-66) and brown-rot fungus Gloeophyllum trabeum biocomposite (UiO-66 @ GT) and its application for reactive black 5 decolorization. Arab. J. Chem. 2022;15 doi: 10.1016/j.arabjc.2022.104129. [DOI] [Google Scholar]
- 37.Jakubovskis R., Ivaškė A., Malaiškienė J., Urbonavičius J. Impact of Portland cement type on bacterial viability in biological concrete. Cem. Concr. Compos. 2022;127 doi: 10.1016/j.cemconcomp.2022.104413. [DOI] [Google Scholar]
- 38.Purnomo A.S., Putra S.R., Putro H.S., Hamzah A., Rohma N.A., Rohmah A.A., Rizqi H.D., Asranudin N., Tangahu B.V., Warmadewanthi I.D.A.A., Shimizu K. The application of biosurfactant-producing bacteria immobilized in PVA/SA/bentonite bio-composite for hydrocarbon-contaminated soil bioremediation. RSC Adv. 2023;13:21163–21170. doi: 10.1039/d3ra02249h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Göçenoğlu Sarıkaya A., Erden Kopar E. Biosorption of Sirius Blue azo-dye by Agaricus campestris biomass: batch and continuous column studies. Mater. Chem. Phys. 2022;276 doi: 10.1016/J.MATCHEMPHYS.2021.125381. [DOI] [Google Scholar]
- 40.Kishor R., Purchase D., Saratale G.D., Romanholo Ferreira L.F., Hussain C.M., Mulla S.I., Bharagava R.N. Degradation mechanism and toxicity reduction of methyl orange dye by a newly isolated bacterium Pseudomonas aeruginosa MZ520730. J. Water Process Eng. 2021;43 doi: 10.1016/j.jwpe.2021.102300. [DOI] [Google Scholar]
- 41.Garg N., Garg A., Mukherji S. Eco-friendly decolorization and degradation of reactive yellow 145 textile dye by Pseudomonas aeruginosa and Thiosphaera pantotropha. J. Environ. Manag. 2020;263 doi: 10.1016/j.jenvman.2020.110383. [DOI] [PubMed] [Google Scholar]
- 42.da Silva V.L., Dilarri G., Mendes C.R., Lovaglio R.B., Gonçalves A.R., Montagnolli R.N., Contiero J. Rhamnolipid from Pseudomonas aeruginosa can improve the removal of Direct Orange 2GL in textile dye industry effluents. J. Mol. Liq. 2021;321 doi: 10.1016/j.molliq.2020.114753. [DOI] [Google Scholar]
- 43.Moradi O., Madanpisheh M.A., Moghaddas M. Synthesis of GO/HEMA, GO/HEMA/TiO2, and GO/Fe3O4/HEMA as novel nanocomposites and their dye removal ability. Adv. Compos. Hybrid Mater. 2021;4:1185–1204. doi: 10.1007/s42114-021-00353-7. [DOI] [Google Scholar]
- 44.Chan S.S., Khoo K.S., Chew K.W., Ling T.C., Show P.L. Recent advances biodegradation and biosorption of organic compounds from wastewater: microalgae-bacteria consortium - a review. Bioresour. Technol. 2022;344 doi: 10.1016/J.BIORTECH.2021.126159. [DOI] [PubMed] [Google Scholar]
- 45.Kishor R., Purchase D., Saratale G.D., Saratale R.G., Ferreira L.F.R., Bilal M., Chandra R., Bharagava R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2020.105012. [DOI] [Google Scholar]
- 46.Krithika A., Gayathri · K Veena, Kumar · D Thirumal, George · C., Doss P. Mixed azo dyes degradation by an intracellular azoreductase enzyme from alkaliphilic Bacillus subtilis: a molecular docking study. Arch. Microbiol. 2021;203:3033–3044. doi: 10.1007/s00203-021-02299-2. [DOI] [PubMed] [Google Scholar]
- 47.Muthukumarasamy N.P., Jackson B., Joseph Raj A., Sevanan M. Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agroresidues as a potential substrate. Biochem. Res. Int. 2015;2015 doi: 10.1155/2015/765190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Purnomo A.S., Mawaddah M.O. Biodecolorization of methyl orange by mixed cultures of brown-rot fungus daedalea dickinsii and bacterium pseudomonas aeruginosa. Biodiversitas. 2020;21:2297–2302. doi: 10.13057/biodiv/d210561. [DOI] [Google Scholar]
- 49.Ratnasari A., Syafiuddin A., Zaidi N.S., Hong Kueh A.B., Hadibarata T., Prastyo D.D., Ravikumar R., Sathishkumar P. Bioremediation of micropollutants using living and non-living algae - current perspectives and challenges. Environ. Pollut. 2021;292 doi: 10.1016/j.envpol.2021.118474. [DOI] [PubMed] [Google Scholar]
- 50.Singh R.L., Singh P.K., Singh R.P. Enzymatic decolorization and degradation of azo dyes - a review. Int. Biodeterior. Biodegrad. 2015;104:21–31. doi: 10.1016/j.ibiod.2015.04.027. [DOI] [Google Scholar]
- 51.Ferro P., Vaz-Moreira I., Manaia C.M. Evolution of gentamicin and arsenite resistance acquisition in Ralstonia pickettii water isolates. Res. Microbiol. 2021;172 doi: 10.1016/j.resmic.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Huang H., Zhao Y., Xu Z., Ding Y., Zhang W., Wu L. Biosorption characteristics of a highly Mn(II)resistant Ralstonia pickettii strain isolated from Mn ore. PLoS One. 2018;13:1–17. doi: 10.1371/journal.pone.0203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho B.D., Beech J.P., Tegenfeldt J.O. Cell sorting using electrokinetic deterministic lateral displacement. Micromachines. 2021;12:1–14. doi: 10.3390/mi12010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan F.A., Ullah R., Albadrani G.M., Bayram R., Mohamed H.R.H. 2022. Biological Mineralization of Methyl Orange by Pseudomonas aeruginosa. [Google Scholar]
- 55.Movahedi S., Waites W. Cold shock response in sporulating Bacillus subtilis and its effect on spore heat resistance. J. Bacteriol. 2002;184:5275–5281. doi: 10.1128/JB.184.19.5275-5281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alekseeva O.V., Smirnova D.N., Noskov A.V., Kuznetsov O.Y., Kirilenko M.A., Agafonov A.V. Mesoporous halloysite/magnetite composite: synthesis, characterization and in vitro evaluation of the effect on the bacteria viability. Mater. Today Commun. 2022;32 doi: 10.1016/J.MTCOMM.2022.103877. [DOI] [Google Scholar]
- 57.Masoodi K.Z., Lone S.M., Rasool R.S. Growth of bacterial cultures and preparation of growth curve. Adv. Methods Mol. Biol. Biotechnol. 2021:163–166. doi: 10.1016/B978-0-12-824449-4.00030-X. [DOI] [Google Scholar]
- 58.Pahalagedara A.S.N.W., Gkogka E., Ravn L.W., Hammershøj M. The growth potential and thermal resistance of bacterial spores under conditions relevant for ambient acid dairy-based products. Food Control. 2023;152 doi: 10.1016/J.FOODCONT.2023.109841. [DOI] [Google Scholar]
- 59.Aksu Demirezen D., Demirezen Yılmaz D., Yıldız Y.Ş. Magnetic chitosan/calcium alginate double-network hydrogel beads: preparation, adsorption of anionic and cationic surfactants, and reuse in the removal of methylene blue. Int. J. Biol. Macromol. 2023;239 doi: 10.1016/j.ijbiomac.2023.124311. [DOI] [PubMed] [Google Scholar]
- 60.Girijan S., Kumar M., Gomber S. Starch and powdered activated carbon amended alginate-biomass beads for metronidazole and bulk organic matter removal: synthesis, optimization, reaction kinetics and reusability. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/J.JECE.2021.106102. [DOI] [Google Scholar]