Abstract
Background
AML1/ETO fusion confers favorable prognosis in acute myeloid leukemia (AML) treated with intensive chemotherapy (IC). However, the impact of AML1/ETO fusion on the efficacy of venetoclax in the treatment of AML is unclear.
Objective
The aim of this study was to evaluate the efficacy of venetoclax plus hypomethylating agents (VEN/HMAs) in patients with AML1/ETO-positive AML.
Patients and Methods
Patients with newly diagnosed AML in two centers were reviewed and divided into three cohorts: AML1/ETO-positive AML treated with frontline VEN/HMA (Cohort A), AML1/ETO-negative AML treated with frontline VEN/HMA (Cohort B), or AML1/ETO-positive AML treated with frontline IC (Cohort C). The response and survival were compared between the cohorts.
Results
A total of 260 patients were included in the study. Patients in Cohort A had a significantly lower overall response rate (ORR) than patients in Cohort B (40.9% vs 71.2%, p = 0.005). The median event-free survival (EFS) in Cohort A and Cohort B was 2.7 months and 7.7 months, respectively, with no significant difference. The ORR and median EFS in Cohort C were 80.8% and 14.9 months, respectively, which were significantly superior to those in Cohort A, and the advantages remained significant after propensity score matching. ORR and EFS in KIT-mutated patients with AML1/ETO-positive AML receiving VEN/HMA were much inferior to those in KIT wild-type patients (ORR 0.0% vs 81.8%, p = 0.001; EFS 1.2 months vs not reached, p < 0.001).
Conclusions
Newly diagnosed AML patients with AML1/ETO fusion had a poor response to frontline VEN/HMA treatment. When determining induction therapy for patients with AML1/ETO-positive AML, IC should be preferred over VEN/HM.
Key Points
| Patients with newly diagnosed acute myeloid leukemia (AML) with AML1/ETO fusion had a poor response to frontline venetoclax plus hypomethylating agents (VEN/HMAs). |
| KIT mutations were associated with poor prognosis in AML1/ETO-positive AML patients treated with frontline VEN/HMA. |
Introduction
AML1/ETO fusion has been found in approximately 8% of newly diagnosed adult acute myeloid leukemia (AML), and approximately 90% of patients with AML1/ETO fusion have the French-American-British (FAB) AML-M2 subtype [1, 2]. AML1/ETO-positive AML had a high complete remission (CR) rate and prolonged survival, especially following consolidation chemotherapy with high-dose cytarabine [3, 4]. Venetoclax (VEN) is an oral BCL-2 inhibitor that was approved in 2018 by the US Food and Drug Administration. The combination of venetoclax with hypomethylating agents (HMAs) showed promising efficacy in AML and has been adopted as a new standard for older patients or those unfit for chemotherapy [5–8]. Because of the good prognosis of AML1/ETO-positive AML with intensive chemotherapy (IC), few patients received VEN/HMA therapy, and the efficacy of VEN/HMA on AML1/ETO-positive AML has not been reported in the literature. In the 3 years since 2019, China has borne the impact of coronavirus disease 2019 (COVID-19). To reduce the likely fatal injury caused by COVID-19, some ‘fit’ patients received VEN/HMA as first-line treatment, providing additional records of AML1/ETO-positive AML treated with VEN/HMA. We performed a retrospective study to analyze the impact of AML1/ETO fusion on the efficacy of VEN/HMA treatment in newly diagnosed AML and compare the outcomes of VEN/HMA and IC in patients with AML1/ETO-positive AML.
Patients and Methods
Study Design and Participants
Adult patients (aged ≥18 years) who were newly diagnosed with AML at the First Affiliated Hospital of Zhejiang University School of Medicine and Ningbo Medical Center Li huili Hospital from January 2020 to June 2023 were reviewed. Patients were divided into three cohorts: AML1/ETO-positive AML treated with frontline VEN/HMA therapy (Cohort A), AML1/ETO-negative AML treated with frontline VEN/HMA therapy (Cohort B), or AML1/ETO-positive AML treated with frontline IC (Cohort C). Venetoclax should be taken for at least 14 days per course. HMAs included azacytidine and decitabine. IC included cytarabine + daunorubicin/idarubicin, cytarabine + homoharringtonine ± aclacinomycin, and cladribine-based regimens. Patients were required to accept at least one cycle of therapy and were follow up to a response assessment or death. The following patients were excluded: (a) patients with acute promyelocytic leukemia; (b) patients who received prior venetoclax therapy; and (c) patients for whom the treatment strategy was changed when a partial response was achieved after one cycle of induction therapy.
The baseline and clinical characteristics were collected, including age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), type of HMAs in patients receiving VEN/HMA therapy, bone marrow blast percentage at diagnosis, FAB categories [9], secondary AML, European LeukemiaNet (ELN) 2022 risk groups [10], targeted PCR-based sequencing of somatic mutations (including KIT mutations, FLT ITD/TKD mutations, TP53 mutations, DNMT3A mutations, and TET2 mutations), salvage therapies after refractory or progressive disease, and allogeneic hematopoietic stem cell transplantation (allo-HSCT) in the following treatment. The differences in baseline characteristics were compared between Cohort A and Cohort B and between Cohort A and Cohort C.
Outcomes
The overall response rate (ORR) included CR, CR with incomplete recovery of blood counts, and morphologic leukemia-free state, and measurable residual disease (MRD) negativity was defined as <0.1% of CD45-expressing cells with the target immunophenotype according to ELN guidelines [10]. Event-free survival (EFS) was defined as the time from the start of therapy to refractory disease, progression, or death. Overall survival (OS) was defined as the time from the start of therapy to death.
Statistical Analysis
Absolute numbers and percentages were used for categorical variables, and the difference between groups was analyzed by the chi-square test or Fisher’s exact test. EFS and OS were evaluated by the Kaplan–Meier method with the log-rank test. A propensity score matching (PSM) method [11] with a 1:1 matching ratio via nearest neighbor and a caliper width of 0.05 was conducted to adjust the imbalanced baseline characteristics. p-Values ≤0.05 were considered statistically significant. SPSS v.25 statistical software was used for analyses, and GraphPad Prism was used for graphing.
Differential Expressing Genes Analysis
RNA-seq datasets (BEATAML1.0-COHORT and TCGA-LAML) and simple nucleotide variation (SNV) datasets (TCGA-LAML and GENIE-UHN) were downloaded from GDC (Genomic Data Commons Data Portal: https://portal.gdc.cancer.gov/). Patients with PML/RARA fusion were excluded from analysis. Differential expressing genes were performed with R package DESeq2 1.40.2 between AML1/ETO-positive and -negative patients [12]. R package clusterpProfiler 4.8.3 was used for pathway analysis [13]. AML1/ETO fusion information was collected from clinical data provided by GDC. SNV files were handled with R packages maftools 2.16.0 [14].
Results
Patient Characteristics
Between January 2020 and June 2023, 161 newly diagnosed AML patients received frontline VEN/HMA treatment, among whom 22 patients had AML1/ETO fusion. Baseline characteristics are listed in Table 1. All AML1/ETO-positive AML patients treated with VEN/HMA (Cohort A) were in the ELN 2022 favorable risk group, while 57% of AML1/ETO-negative patients treated with VEN/HMA (Cohort B) were in the ELN 2022 adverse risk group (p < 0.001). Patients in Cohort A had more concomitant KIT mutations (50% vs 3.6%, p < 0.001) and fewer concomitant DNMT3A mutations (0.0% vs 29.5%, p = 0.003) than those in Cohort B. In addition, patients in Cohort A were likely to be younger than those in Cohort B, but the difference was not significant. Other characteristics between the two cohorts were similar.
Table 1.
Baseline characteristics of patients
|
AML1/ETO-positive AML with VEN/HMA (Cohort A, n = 22) |
AML1/ETO-negative AML with VEN/HMA (Cohort B, n = 139) |
p value* |
AML1/ETO-positive AML with IC (Cohort C, n = 99) |
p value # | |
|---|---|---|---|---|---|
| Age, y | 0.054 | < 0.001 | |||
| < 65 | 13 (59.1%) | 52 (37.4%) | 92 (92.9%) | ||
| ≥ 65 | 9 (40.9%) | 87 (62.6%) | 7 (7.1%) | ||
| Sex | 0.178 | 0.086 | |||
| Male | 8 (36.4%) | 72 (51.8%) | 56 (56.6%) | ||
| Female | 14 (63.6%) | 67 (48.2%) | 43 (43.4%) | ||
| ECOG PS | 0.669 | 0.003 | |||
| ≤ 2 | 10 (45.5%) | 70 (50.4%) | 76 (76.8%) | ||
| > 2 | 12 (54.5%) | 69 (49.6%) | 23 (23.2%) | ||
| Type of HMA | 0.533 | – | |||
| Azacitidine | 19 (86.4%) | 126 (90.6%) | |||
| Decitabine | 3 (13.6%) | 13 (9.4%) | |||
| Bone marrow blast | 0.145 | 0.284 | |||
| < 50% | 13 (59.1%) | 59 (42.4%) | 46 (46.5%) | ||
| ≥ 50% | 9 (40.9%) | 80 (57.6%) | 53 (53.5%) | ||
| FAB-M5 | 6 (27.3%) | 56 (40.3%) | 0.244 | 26 (26.3%) | 0.923 |
| Secondary AML | 1 (4.5%) | 28 (20.1%) | 0.141 | 2 (2.0%) | 0.455 |
| ELN 2022 risk group | < 0.001 | 1.000 | |||
| Favorable | 22 (100%) | 34 (24.5%) | 91/95 (95.8%) | ||
| Intermediate | 0 (0.0%) | 25 (18.0%) | 1/95 (1.1%) | ||
| Adverse | 0 (0.0%) | 80 (57.6%) | 3/95 (3.2%) | ||
| Missing | 0 | 0 | 4 | ||
| KIT mutation | 11 (50%) | 5 (3.6%) | < 0.001 | 47/95 (49.5%) | 0.965 |
| FLT3-ITD/TKD mutation | 4 (18.2%) | 25 (18.0%) | 1.000 | 13/95 (13.7%) | 0.839 |
| TP53 mutation | 0 (0.0%) | 20 (14.4%) | 0.120 | 1/95 (1.1%) | 1.000 |
| ASXL1 mutation | 4 (18.2%) | 21 (15.1%) | 0.958 | 10/95 (10.5%) | 0.527 |
| DNMT3A mutation | 0 (0.0%) | 41 (29.5%) | 0.003 | 2 (2.1%) | 1.000 |
| TET2 mutation | 1 (4.5%) | 20 (14.4%) | 0.351 | 8 (8.4%) | 0.864 |
| Salvage therapy | 14/16 (87.5%) | 74/95 (84.1%) | 0.587 | 42/53 (79.2%) | 0.707 |
| Allo-HSCT | 3 (13.6%) | 19 (13.7%) | 1.000 | 38 (38.4%) | 0.027 |
Allo-HSCT allogeneic hematopoietic stem cell transplantation, AML acute myeloid leukemia, HMA hypomethylating agent, IC intensive chemotherapy, ECOG PS Eastern Cooperative Oncology Group performance status, ELN European LeukemiaNet, FAB French, American, and English, VEN venetoclax
*Comparation between Cohort A and Cohort B
#Comparation between Cohort A and Cohort C
During the same study period, we included 99 patients who had AML1/ETO-positive AML and received frontline IC therapy in Cohort C. Four patients in Cohort C had no information on concomitant gene mutations and were unable to be classified into European ELN 2022 risk groups. Patients in Cohort C were younger (p < 0.001) and had better performance status (p = 0.003) than those in Cohort A. Furthermore, a greater proportion of patients underwent allo-HSCT during follow-up in Cohort C than in Cohort A (38.4% vs 12.6%, p = 0.027). Other characteristics between Cohort A and Cohort C were similar (Table 1).
Outcomes of Patients Treated with VEN/HMA According to AML1/ETO Fusion Status
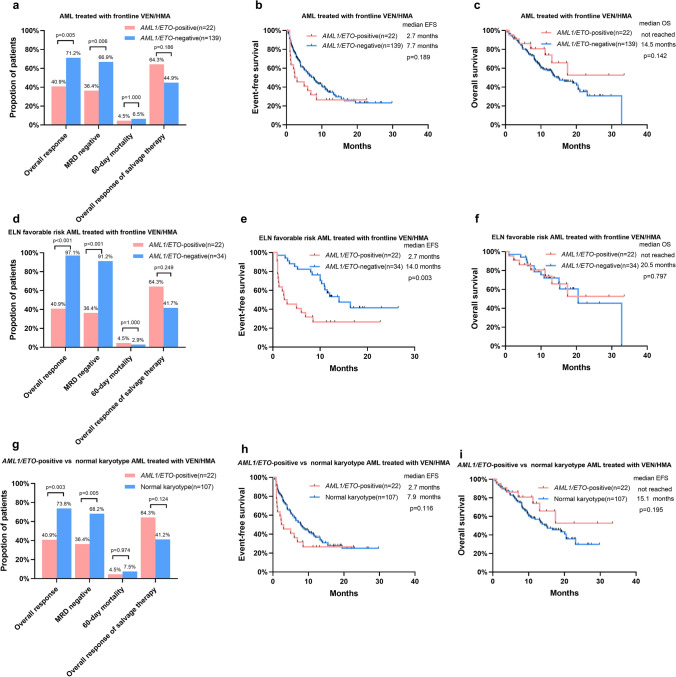
When treated with frontline VEN/HMA, patients with AML1/ETO-positive AML had a significantly lower ORR than patients with AML1/ETO-negative AML (40.9% vs 71.2%, p = 0.005), as well as a lower MRD-negative rate (36.4% vs 66.9%, p = 0.006). The 60-day mortality rates were similar in the two cohorts. The ORR of salvage therapy for patients who were primarily resistant to frontline therapy or relapsed after remission was 64.3% in AML1/ETO-positive AML and 44.9% in AML1/ETO-negative AML, with no significant difference (Fig. 1a). Of the 11 patients who were primarily resistant to VEN/HMA, 8 (72.7%) patients responded to the follow-up salvage chemotherapy. The median EFS and OS in AML1/ETO-positive AML patients were 2.7 months and not reached, respectively, which were not significantly different from those in AML1/ETO-negative patients (Fig. 1b, c).
Fig. 1.
Outcomes in patients treated with frontline VEN/HMA according to AML1/ETO fusion status. a Response and early death, b EFS, and c OS in all patients. d Response and early death, e EFS, and f OS in patients in the ELN favorable risk group. g Response and early death, h EFS, and i OS in AML1/ETO-positive patients and patients with normal karyotype. AML acute myeloid leukemia, EFS event-free survival, ELN European LeukemiaNet, HMA hypomethylating agent, MRD measurable residual disease, OS overall survival, VEN venetoclax
AML1/ETO-positive AML patients were all in the ELN 2022 favorable risk group, while most AML1/ETO-negative AML patients were in the ELN 2022 adverse risk group, which may lead to bias in survival. Thus, we further analyzed the treatment outcomes of patients in the ELN 2022 favorable risk group, including 22 patients with AML1/ETO-positive AML and 34 patients with AML1/ETO-negative AML. The ORR was 97.1% and the MRD-negative rate was 91.2% in AML1/ETO-negative AML patients with an ELN favorable risk, which were both much higher than those in AML1/ETO-positive AML patients (Fig. 1d). The median EFS in AML1/ETO-positive AML patients with ELN favorable risk was significantly shorter than that in AML1/ETO-negative AML patients (2.7 months vs 14 months, p = 0.003, Fig. 1e). The median OS in AML1/ETO-negative patients with an ELN favorable risk was 20.5 months, with no significant difference compared with that in AML1/ETO-positive patients (Fig. 1f).
We also compared the 22 AML1/ETO-positive patients with the normal karyotype subgroup of the 139 AML1/ETO-negative patients receiving VEN/HMA. The ORR was 73.8% and the MRD-negative rate was 68.2% in AML1/ETO-negative AML patients with normal karyotype, which were much higher than those in AML1/ETO-positive AML patients (Fig. 1g). No significant differences were found in EFS and OS between the two groups (Fig 1h, i).
Outcomes of Patients with AML1/ETO-Positive AML According to Frontline Treatment Strategies
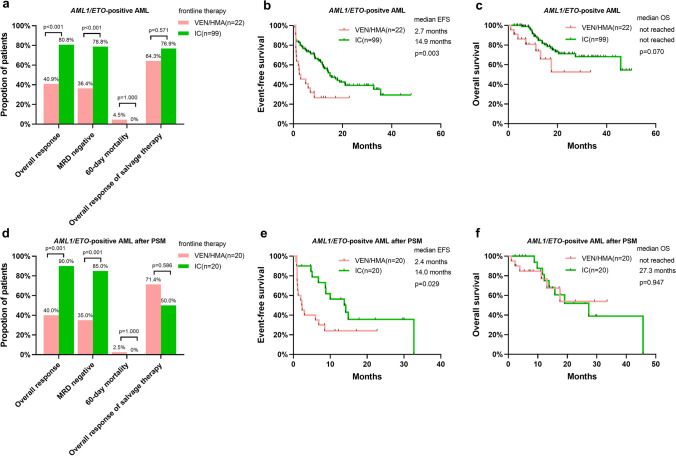
We analyzed the response and survival of AML1/ETO-positive patients treated with frontline VEN/HMA (n = 22) or frontline IC (n = 99). The ORR and MRD-negative rate in AML1/ETO-positive AML patients treated with frontline IC were 80.8% and 78.8%, respectively, which were much higher than those in AML1/ETO-positive patients treated with frontline VEN/HMA (p < 0.001; Fig. 2a). The 60-day mortality and ORR of salvage therapy were similar in the two cohorts (Fig. 2a). The median EFS in patients treated with VEN/HMA was significantly shorter than that in patients treated with IC (2.7 months vs 14.9 months, p = 0.003; Fig. 2b). The median OS in both cohorts was not reached, with no significant difference (p = 0.070, Fig. 2c).
Fig. 2.
Outcomes in patients with AML1/ETO-positive AML according to frontline treatment strategies. a Response and early death, b EFS and c OS in all patients. d Response and early death, e EFS, and f OS in patients after propensity score matching for age, Eastern Cooperative Oncology Group performance status and follow-up allogeneic hematopoietic stem cell transplantation. AML acute myeloid leukemia, EFS event-free survival, HMA hypomethylating agent, IC intensive chemotherapy, MRD measurable residual disease, OS overall survival, VEN venetoclax
However, there were significant differences in the baseline characteristics, such as age, performance status, and follow-up allo-HSCT, between patients treated with VEN/HMA and patients treated with IC. Thus, we analyzed the outcomes of patients after propensity matching for age, ECOG PS and follow-up HSCT. After PSM, 40 patients, including 20 patients with frontline VEN/HM treatment, were matched by a 1:1 matching ratio, and all the baseline characteristics were similar between the two matched cohorts (Table 2). The response and EFS in the propensity-matched VEN/HMA cohort were significantly inferior to those in the propensity-matched IC cohort (ORR 40.0% vs 90.0%, p = 0.001; MRD-negative rate 35.5% vs 85.0%, p = 0.001; median EFS 2.4 months vs 14.0 months, p = 0.029; Fig. 2d and e). No significant OS differences were found between the two propensity-matched cohorts (Fig. 2f).
Table 2.
Baseline characteristics of patients with AML1/ETO-positive AML after propensity score matching
|
AML1/ETO-positive AML treated with VEN/HMA (n = 20) |
AML1/ETO-positive AML treated with IC (n = 20) |
p value | |
|---|---|---|---|
| Age | 1.000 | ||
| < 65 | 13 (65.0%) | 13 (65.0%) | |
| ≥ 65 | 7 (35.0%) | 7 (35.0%) | |
| Sex | 0.337 | ||
| Male | 7 (35.0%) | 10 (50.0%) | |
| Female | 13 (65.0%) | 10 (50.0%) | |
| ECOG PS | 0.752 | ||
| ≤ 2 | 10 (50.0%) | 11 (55.0%) | |
| > 2 | 10 (50.0%) | 9 (45.0%) | |
| Bone marrow blast | 0.525 | ||
| < 50% | 12 (60.0%) | 10 (50.0%) | |
| ≥ 50% | 8 (40.0%) | 10 (50.0%) | |
| FAB-M5 | 6 (30.0%) | 4 (20.0%) | 0.465 |
| Secondary AML | 1 (5.0%) | 1 (5.0%) | 1.000 |
| ELN 2022 risk group | |||
| Favorable | 20 (100%) | 18 (100%) | |
| Intermediate | 0 (0.0%) | 0 (0.0%) | |
| Adverse | 0 (0.0%) | 0 (0.0%) | |
| Missing | 0 | 2 | |
| KIT mutation | 10 (50.0%) | 11/18 (61.1%) | 0.492 |
| FLT-ITD/TKD mutation | 4 (20.0%) | 1/18 (5.6%) | 0.404 |
| TP53 mutation | 0 (0.0%) | 0/18 (0.0%) | |
| ASXL1 mutation | 4 (20.0%) | 2/18 (11.1%) | 0.761 |
| DNMT3A mutation | 0 (0.0%) | 1/18 (5.6%) | 0.474 |
| TET2 mutation | 1 (5.0%) | 3/18 (16.7%) | 0.522 |
| Salvage treatment | 14/15 (93.3%) | 8/12 (66.7%) | 0.203 |
| Allo-HSCT | 3 (15.0%) | 5 (25.0%) | 0.693 |
Allo-HSCT allogeneic hematopoietic stem cell transplantation, AML acute myeloid leukemia, HMA hypomethylating agent, IC intensive chemotherapy, ECOG PS Eastern Cooperative Oncology Group performance status, ELN European LeukemiaNet, FAB French, American, and English, VEN venetoclax
Subgroup Analysis Stratified by KIT Mutation Status
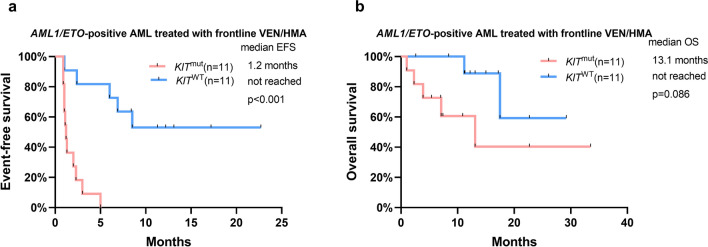
KIT mutations have been reported to be associated with a poor prognosis in core binding factor (CBF)-AML patients receiving IC [15–18]. We therefore performed analyses stratified by KIT mutation status. We first analyzed the impact of KIT mutations on the outcomes of patients with AML1/ETO-positive AML receiving VEN/HMA. The ORR and EFS in KIT-mutated patients with AML1/ETO-positive AML receiving VEN/HMA were much inferior to those in KIT wild-type patients (ORR 0.0% vs 81.8%, p = 0.001; EFS 1.2 months vs not reached, p < 0.001, Table 3, Fig. 3a). OS between the two cohorts was not significantly different (Fig. 3b).
Table 3.
Overall response rates for patients
| AML1/ETO-positive AML with VEN/HMA | AML1/ETO-negative AML with VEN/HMA | p Value * | AML1/ETO-positive AML with IC | p Value # | |
|---|---|---|---|---|---|
| KIT wild-type | 9/11 (81.8%) | 96/134 (71.6%) | 0.708 | 40/48 (83.1%) | 1.000 |
| KIT-mutated | 0/11 (0.0%) | 3/5 (60.0%) | 0.018 | 37/47 (78.7%) | < 0.001 |
| p Value$ | 0.001 | 0.951 |
AML acute myeloid leukemia, HMA hypomethylating agent, IC intensive chemotherapy, VEN venetoclax
*Comparation between AML1/ETO-positive AML with VEN/HMA and AML1/ETO-negative AML with VEN/HMA
#Comparation between AML1/ETO-positive AML with VEN/HMA and AML1/ETO-positive AML with IC
$Comparation between KIT wild-type group and KIT-mutated group
Fig. 3.
a EFS and b OS in AML1/ETO-positive patients treated with frontline VEN/HMA according to KIT mutation status. AML acute myeloid leukemia, EFS event-free survival, HMA hypomethylating agent, OS overall survival, VEN venetoclax
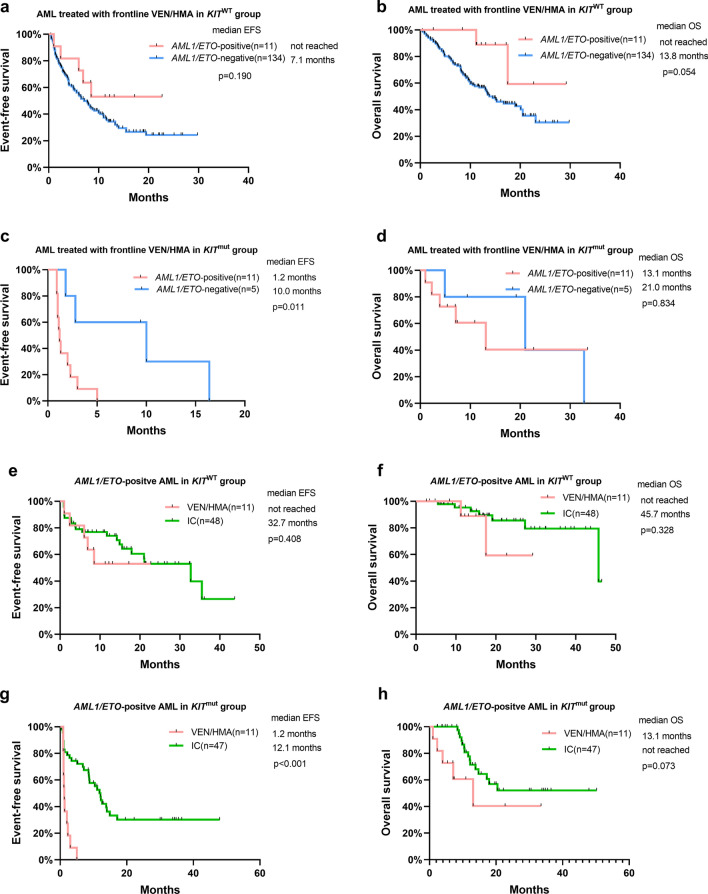
We then conducted subgroup analysis by KIT mutation status. In KIT wild-type patients treated with frontline VEN/HMA, no significant differences in ORR and EFS were found between patients with AML1/ETO-positive AML and AML1/ETO-negative AML (ORR 81.8% vs 70.8%, p = 0.708; median EFS not reached vs 7.1 months, p = 0.190; Table 3, Fig. 4a). In KIT-mutated patients treated with frontline VEN/HMA, the ORR and EFS for patients with AML1/ETO-positive AML were significantly worse than those for AML1/ETO-negative AML (ORR 0.0% vs 60.0%, p = 0.018; median EFS 1.2 months vs 10.0 months, p = 0.011; Table 3, Fig. 4c). In AML1/ETO-positive AML without KIT mutations, no significant differences in ORR and EFS were found between patients treated with VEN/HMA and IC (Table 3, Fig. 4e). In AML1/ETO-positive AML with concomitant KIT mutations, the ORR and EFS for patients treated with VEN/HMA were significantly worse than those for patients treated with IC (ORR 0.0% vs 78.7%, p < 0.001; median EFS 1.2 months vs 12.1 months, p < 0.001; Table 3, Fig. 4g). The OS between cohorts was not significantly different (Fig. 4b, d, f, h).
Fig. 4.
Outcomes in patients stratified by KIT mutation. a EFS and b OS in patients treated with frontline VEN/HMA in the KIT wild-type group according to AML1/ETO fusion status. c EFS and d OS in patients treated with frontline VEN/HMA in the KIT-mutated group according to AML1/ETO fusion status. e EFS and f OS in patients with AML1/ETO-positive AML in the KIT wild-type group according to frontline treatment strategies. g EFS and h OS in patients with AML1/ETO-positive AML in the KIT-mutated group according to frontline treatment strategies. AML acute myeloid leukemia, EFS event-free survival, HMA hypomethylating agent, IC intensive chemotherapy, OS overall survival, VEN venetoclax
Different Gene Expressing Analysis, Pathway Analysis and SNV Analysis of Patients with AML in Datasets
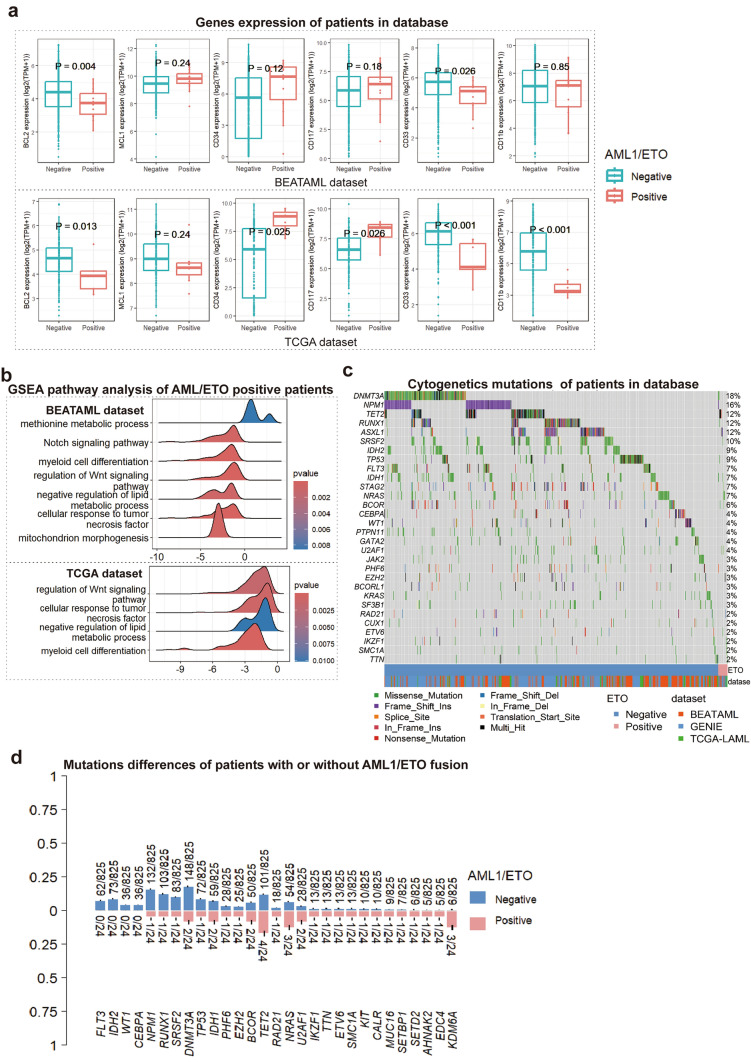
VEN/HMA did not show a satisfactory efficacy for patients with AML1/ETO-positive AML. We analyzed open-source RNA-seq datasets and SNV datasets to explore the reason for poor results in these patients. The differential expressing genes analysis showed that AML1/ETO-positive patients expressed significantly lower BCL2 than AML1/ETO-negative patients (Fig. 5a), which indicated low dependency on BCL2 of AML1/ETO-positive patients. Meanwhile, AML1/ETO-positive patients expressed higher CD34/CD117 and lower CD33/CD11b, which indicated less differentiation (Fig. 5a). Gene set enrichment analysis shows the down-regulation of the mitochondrion morphogenesis pathway in the BEATAML data set (Fig. 5b).
Fig. 5.
Different gene expressing analysis, pathway analysis, and SNV analysis of AML patients in datasets. a BCL2 expression between AML patients with and without AML1/ETO fusion. Expression was in logarithm. p-Value was calculated by DESeq2. b Pathway enrichment result. X axis value presented the quantitative results of pathway enrichment. Down-regulated pathways were at the left side of zero. c Gene mutations and d mutation abundance of AML patients with and without AML1/ETO fusion. AML acute myeloid leukemia, GSEA gene set enrichment analysis, SNV simple nucleotide variation
Concomitant gene mutations in patients with and without AML1/ETO fusion are shown in Fig. 5c. Unfortunately, the differences in gene mutations between AML1/ETO-positive patients and AML1/ETO-negative patients were not significant, which may be the result of a small sample size of AML1/ETO-positive patients. We paid special attention to the mutations in genes involved in DNA methylation such as DNMT3A and TET2. The analysis showed that although lacking statistical significance, relatively fewer patients with AML1/ETO fusion had DNMT3A mutations compared with patients without AML1/ETO fusion (8.3% vs 17.9%, p = 0.287). The probabilities of TET2 mutations were similar in AML1/ETO-positive patients and AML1/ETO-negative patients (16.7% vs 12.3%, p = 0.527, Fig. 5d).
Discussion
AML1/ETO-positive AML patients usually have a good prognosis following intensive chemotherapy, but there is still a subset of patients who are ‘unfit’ for IC. The combination of venetoclax and hypomethylating agents has been an effective strategy for ‘unfit’ AML. However, the assessment of ‘fitness’ is imperfect, and the determination of ‘fit’ or ‘unfit’ for IC is somewhat subjective and may be influenced by many factors [19, 20]. For instance, during the COVID-19 epidemic, the emphasis on low-intensity therapies made it possible for ‘fit’ patients to receive VEN/HMA therapy [21]. Therefore, it is necessary to understand the impact of VEN/HMA in AML patients with AML1/ETO fusion and determine whether AML1/ETO-positive AML prefers IC or VEN/HMA.
To our knowledge, this is the first cohort study to report the efficacy of VEN/HMA treatment in patients with AML1/ETO-positive AML. Our study suggested that patients with AML1/ETO-positive AML had a significantly lower ORR with VEN/HMA frontline therapy than those who had AML1/ETO-negative AML, and they also had a significantly shorter EFS after being balanced for ELN risk. Moreover, for AML1/ETO-positive AML, the ORR and EFS were worse in patients treated with frontline VEN/HMA than in those treated with frontline IC.
In this study, most patients who relapsed or were refractory to VEN/HMA received intensive salvage chemotherapies, and 64.3% of them reached complete remission with a prolonged duration of response. Moreover, intensive salvage chemotherapy can still achieve a high response rate in AML1/ETO-positive patients with primary resistance to VEN/HMA. These results suggested that AML1/ETO-positive AML patients who failed VEN/HMA therapies still have a good prognosis after salvage treatments with IC, which may be one of the reasons explaining the lack of significant differences in overall survival between cohorts.
KIT mutations were found in 12.8–46.8% of AML1/ETO-positive AML [15, 22]. In patients with AML1/ETO-positive AML, KIT mutations were associated with poor survival [16]. In our study, similar to previous data from intensive therapies, KIT mutations were associated with lower ORR and shorter EFS in AML1/ETO-positive AML patients treated with frontline VEN/HMA. The subgroup analysis showed that in the KIT-mutated subgroup, AML1/ETO positivity had a significant effect on the poor response and survival of AML patients treated with frontline VEN/HMA, while the effect was not significant in the KIT wild-type subgroup. Of the 11 patients with coexisting AML1/ETO fusion and KIT mutations who were treated with frontline VEN/HMA, none achieved CR, suggesting that VEN/HMA therapies should be used with great caution in this subgroup of patients. The analysis is subject to some bias because of the small sample size. Further studies with larger sample sizes are needed to confirm the results.
Resistance to venetoclax arises through various mechanisms, including dysregulation of BCL-2 family apoptotic proteins, p53 inactivation, activating kinase mutations, and altered mitochondrial structure [23, 24]. Database analysis in our study showed that AML1/ETO-positive patients have lower BCL2 expression and down-regulation of the mitochondrion morphogenesis pathway, which may lead to resistance to venetoclax. Previous studies have showed that AML with mature differentiation (such as monocytic AML) primarily relies on MCL-1 for survival instead of BCL-2 and is more resistant to venetoclax than primitive AML [25, 26]. However, AML1/ETO-positive patients presented less differentiation, indicating that the resistance is not due to the blast maturation state. Previous studies have shown that activating kinase mutations play an important role in venetoclax resistance [24]. FLT3 mutations and mutations that activate the Ras/Raf/MEK/ERK pathway may drive expressions of MCL-1 [27, 28]. Co-mutations (e.g., KIT, FLT, RAS) are common in AML1/ETO-positive AML, which may possibly result in activated signal transduction pathways and lead to venetoclax resistance. Further studies of venetoclax resistance are urgently needed in patients with AML1/ETO fusion, as well as in patients with inv(16), MLL rearrangement, inv(3), or other more rare fusions, who are also likely to be resistant to VEN/HMA.
Hypomethylating agents exert anti-tumor effects by reversing DNA methylation. Mutations in genes involved in DNA methylation such as DNMT3A and TET2 may predict good prognosis with HMAs in patients with myeloid malignancies [29–31]. Previous studies reported that in patients with AML1/ETO-positive AML, the incidence of a DNMT3A mutation is about 3–6%, and the incidence of TET2 mutations is about 7–11% [32, 33]. In our study, the mutation rate of DNMT3A in AML1/ETO-positive patients was significantly lower than that in AML1/ETO-negative patients. In the SNV analysis of AML patients in datasets, we also found a low probability of DNMT3A mutations in AML1/ETO-positive patients, which may contribute to the poor response of AML1/ETO-positive patients to HMAs.
Our study demonstrated the importance of using IC in AML1/ETO-positive AML, even in relatively older patients, because of the vastly superior outcomes compared with VEN/HMA. However, in the truly elderly or frail patients who are unable to tolerate IC, lower intensity strategies other than VEN/HMA should be explored. Targeted therapy (e.g., FLT 3 inhibitors, IDH inhibitors) is an option for patients with targetable mutations. However, treatment for patients without targeted mutations is a great challenge. Low-dose cytarabine (LDAC) has shown low CR rates, ranging from 7 to 32% [34]. Glasdegib, a hedgehog inhibitor, was approved to be used in combination with LDAC in older or unfit patients with AML based on a phase II trial showing better efficacy than LDAC alone [35]. However, the CR rate (17%) and OS (8.8 months) demonstrated by the combination therapy are not very satisfactory. Nucleoside analogs have been shown to improve the outcomes in older AML patients. In a phase II study of older patients with AML treated with cladribine plus LDAC alternating with decitabine, a CR rate of 58% was achieved with a median OS of 13.8 months [36]. In another phase II study, LDAC and cladribine combined with venetoclax alternating with azacitidine demonstrated a CR rate of 93% in older patients [37]. In previous studies, the effect of low-intensity treatment in the subgroup of AML1/ETO-positive AML had not been described separately. Combination therapy with cladribine may be a choice for this group of patients based on the available data. Further clinical trials are urgently needed in older patients with AML1/ETO fusion.
Our study also affirmed that ELN risk stratification, which was developed from intensively treated patients, may not be suitable for VEN/HMA-treated patients. Patients treated with VEN/HMA need their own risk stratification criteria, which is an important issue that future studies need to address.
There were a few limitations of the current study. First, because it was a retrospective study, the baseline characteristics between cohorts were not completely comparable. Although propensity score matching reduces the bias, it further reduces the number of patients included in the analysis. Then, because of the lack of prospective design, the combinations and dosages of induction therapies for patients in the same treatment subgroup varied, and the treatment strategies after achieving remission differed. Lastly, more accurate subgroup analyses were limited by the small sample size of patients with AML1/ETO-positive AML receiving VEN/HMA. Larger and better matched cohort studies are needed to validate our results.
Conclusions
Our study suggested that newly diagnosed AML with AML1/ETO fusion had a poor response to frontline VEN/HMA treatment, especially in the KIT-mutated subgroup. When determining induction therapy for patients with AML1/ETO-positive AML, intensive chemotherapy should be preferred over VEN/HMA therapy.
Acknowledgments
We thank the patients for cooperating with our investigation and acknowledge all investigators who participated in this study, including physicians, nurses, and laboratory technicians.
Declarations
Funding
This work was supported by National Natural Science Foundation of China (81770217, 81872322), Natural Science Foundation of Zhejiang Province (LY22H080003, LQ22H080008), and Key Research and Development Project of Zhejiang Province (2020C03014).
Conflict of Interest
Dian Jin, Haoguang Chen, Jingsong He, Yi Li, Gaofeng Zheng, Yang Yang, Yi Zhao, Jing Le, Wenxiu Shu, Donghua He, and Zhen Cai declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethics Approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethical Review Committee of The First Affiliated Hospital of Zhejiang University School of Medicine (approval No. IIT20230774A).
Consent to Participate
The requirement for written informed consent was waived as it was a retrospective study.
Consent for Publication
Not applicable.
Availability of Data and Material
Data archiving is not mandated but data will be made available upon reasonable request.
Code Availability
Not applicable.
Author Contributions
Conceptualization and funding acquisition: Donghua He, Zhen Cai; Methodology and Writing - original draft preparation: Dian Jin, Haoguang Chen; Formal analysis: Jingsong He; Investigation: Yi Zhao; Writing - review and editing: Yi Li, Gaofeng Zheng; Resources: Yang Yang, Jing Le, Wenxiu Shu.
Footnotes
Dian Jin and Haoguang Chen contributed equally to this work.
Contributor Information
Donghua He, Email: hedonghua@zju.edu.cn.
Zhen Cai, Email: caiz@zju.edu.cn.
References
- 1.Mrozek K, Heinonen K, Bloomfield CD. Clinical importance of cytogenetics in acute myeloid leukaemia. Best Pract Res Clin Haematol. 2001;14:19–47. doi: 10.1053/beha.2000.0114. [DOI] [PubMed] [Google Scholar]
- 2.Trujillo JM, Cork A, Ahearn MJ, Youness EL, McCredie KB. Hematologic and cytologic characterization of 8/21 translocation acute granulocytic leukemia. Blood. 1979;53:695–706. doi: 10.1182/blood.V53.4.695.695. [DOI] [PubMed] [Google Scholar]
- 3.Mrozek K, Prior TW, Edwards C, Marcucci G, Carroll AJ, Snyder PJ, et al. Comparison of cytogenetic and molecular genetic detection of t(8;21) and inv(16) in a prospective series of adults with de novo acute myeloid leukemia: a Cancer and Leukemia Group B Study. J Clin Oncol. 2001;19:2482–2492. doi: 10.1200/JCO.2001.19.9.2482. [DOI] [PubMed] [Google Scholar]
- 4.Bloomfield CD, Shuma C, Regal L, Philip PP, Hossfeld DK, Hagemeijer AM, et al. Long-term survival of patients with acute myeloid leukemia: a third follow-up of the Fourth International Workshop on Chromosomes in Leukemia. Cancer. 1997;80:2191–2198. doi: 10.1002/(SICI)1097-0142(19971201)80:11+<2191::AID-CNCR5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 5.DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–228. doi: 10.1016/S1470-2045(18)30010-X. [DOI] [PubMed] [Google Scholar]
- 6.DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in previously untreated acute myeloid Leukemia. N Engl J Med. 2020;383:617–629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 7.Pollyea DA, Pratz K, Letai A, Jonas BA, Wei AH, Pullarkat V, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96:208–217. doi: 10.1002/ajh.26039. [DOI] [PubMed] [Google Scholar]
- 8.DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 10.Dohner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–1377. doi: 10.1182/blood.2022016867. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z. Propensity score method: a non-parametric technique to reduce model dependence. Ann Transl Med. 2017;5:7. doi: 10.21037/atm.2016.08.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS. 2012;16:284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayakonda A, Lin DC, Assenov Y, Plass C, Koeffler HP. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res. 2018;28:1747–1756. doi: 10.1101/gr.239244.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padmakumar D, Chandraprabha VR, Gopinath P, Vimala Devi ART, Anitha GRJ, Sreelatha MM, et al. A concise review on the molecular genetics of acute myeloid leukemia. Leuk Res. 2021;111:106727. doi: 10.1016/j.leukres.2021.106727. [DOI] [PubMed] [Google Scholar]
- 16.Cairoli R, Beghini A, Grillo G, Nadali G, Elice F, Ripamonti CB, et al. Prognostic impact of c-KIT mutations in core binding factor leukemias: an Italian retrospective study. Blood. 2006;107:3463–3468. doi: 10.1182/blood-2005-09-3640. [DOI] [PubMed] [Google Scholar]
- 17.Schnittger S, Kohl TM, Haferlach T, Kern W, Hiddemann W, Spiekermann K, et al. KIT-D816 mutations in AML1-ETO-positive AML are associated with impaired event-free and overall survival. Blood. 2006;107:1791–1799. doi: 10.1182/blood-2005-04-1466. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa Y, Kawashima N, Atsuta Y, Sugiura I, Sawa M, Dobashi N, et al. Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11. Blood Adv. 2020;4:66–75. doi: 10.1182/bloodadvances.2019000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen EC, Garcia JS. Does patient fitness play a role in determining first-line treatment of acute myeloid leukemia? Hematol Am Soc Hematol Educ Program. 2020;2020:41–50. doi: 10.1182/hematology.2020000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klepin HD. Definition of unfit for standard acute myeloid leukemia therapy. Curr Hematol Malig Rep. 2016;11:537–544. doi: 10.1007/s11899-016-0348-8. [DOI] [PubMed] [Google Scholar]
- 21.Cherry EM, Abbott D, Amaya M, McMahon C, Schwartz M, Rosser J, et al. Venetoclax and azacitidine compared with induction chemotherapy for newly diagnosed patients with acute myeloid leukemia. Blood Adv. 2021;5:5565–5573. doi: 10.1182/bloodadvances.2021005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cioccio J, Claxton D. Therapy of acute myeloid leukemia: therapeutic targeting of tyrosine kinases. Expert Opin Investig Drugs. 2019;28:337–349. doi: 10.1080/13543784.2019.1584610. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan GP, Flanagan L, Rodrigues DA, Ni Chonghaile T. The path to venetoclax resistance is paved with mutations, metabolism, and more. Sci Transl Med. 2022;14:eabo6891. doi: 10.1126/scitranslmed.abo6891. [DOI] [PubMed] [Google Scholar]
- 24.Dhakal P, Bates M, Tomasson MH, Sutamtewagul G, Dupuy A, Bhatt VR. Acute myeloid leukemia resistant to venetoclax-based therapy: What does the future hold? Blood Rev. 2023;59:101036. doi: 10.1016/j.blre.2022.101036. [DOI] [PubMed] [Google Scholar]
- 25.Kuusanmaki H, Leppa AM, Polonen P, Kontro M, Dufva O, Deb D, et al. Phenotype-based drug screening reveals association between venetoclax response and differentiation stage in acute myeloid leukemia. Haematologica. 2020;105:708–720. doi: 10.3324/haematol.2018.214882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pei S, Pollyea DA, Gustafson A, Stevens BM, Minhajuddin M, Fu R, et al. Monocytic subclones confer resistance to venetoclax-based therapy in patients with acute myeloid leukemia. Cancer Discov. 2020;10:536–551. doi: 10.1158/2159-8290.CD-19-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Nakauchi Y, Kohnke T, Stafford M, Bottomly D, Thomas R, et al. Integrated analysis of patient samples identifies biomarkers for venetoclax efficacy and combination strategies in acute myeloid leukemia. Nat Cancer. 2020;1:826–839. doi: 10.1038/s43018-020-0103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh Mali R, Zhang Q, DeFilippis RA, Cavazos A, Kuruvilla VM, Raman J, et al. Venetoclax combines synergistically with FLT3 inhibition to effectively target leukemic cells in FLT3-ITD+ acute myeloid leukemia models. Haematologica. 2021;106:1034–1046. doi: 10.3324/haematol.2019.244020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter AM, Komrokji RS, Yun S, Al Ali N, Chan O, Song J, et al. Baseline and serial molecular profiling predicts outcomes with hypomethylating agents in myelodysplastic syndromes. Blood Adv. 2021;5:1017–1028. doi: 10.1182/bloodadvances.2020003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metzeler KH, Walker A, Geyer S, Garzon R, Klisovic RB, Bloomfield CD, et al. DNMT3A mutations and response to the hypomethylating agent decitabine in acute myeloid leukemia. Leukemia. 2012;26:1106–1107. doi: 10.1038/leu.2011.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coombs CC, Sallman DA, Devlin SM, Dixit S, Mohanty A, Knapp K, et al. Mutational correlates of response to hypomethylating agent therapy in acute myeloid leukemia. Haematologica. 2016;101:e457–e460. doi: 10.3324/haematol.2016.148999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qin W, Chen X, Shen HJ, Wang Z, Cai X, Jiang N, et al. Comprehensive mutation profile in acute myeloid leukemia patients with RUNX1-RUNX1T1 or CBFB-MYH11 fusions. Turk J Haematol. 2022;39:84–93. doi: 10.4274/tjh.galenos.2022.2021.0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jahn N, Terzer T, Strang E, Dolnik A, Cocciardi S, Panina E, et al. Genomic heterogeneity in core-binding factor acute myeloid leukemia and its clinical implication. Blood Adv. 2020;4:6342–6352. doi: 10.1182/bloodadvances.2020002673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsouqi A, Geramita E, Im A. Treatment of acute myeloid leukemia in older adults. Cancers (Basel) 2023;15(22):5409. doi: 10.3390/cancers15225409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33:379–389. doi: 10.1038/s41375-018-0312-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadia TM, Cortes J, Ravandi F, Jabbour E, Konopleva M, Benton CB, et al. Cladribine and low-dose cytarabine alternating with decitabine as front-line therapy for elderly patients with acute myeloid leukaemia: a phase 2 single-arm trial. Lancet Haematol. 2018;5:e411–e421. doi: 10.1016/S2352-3026(18)30132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadia TM, Reville PK, Wang X, Rausch CR, Borthakur G, Pemmaraju N, et al. Phase II study of venetoclax added to cladribine plus low-dose cytarabine alternating with 5-azacitidine in older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2022;40:3848–3857. doi: 10.1200/JCO.21.02823. [DOI] [PMC free article] [PubMed] [Google Scholar]