Abstract
One major risk for recipients undergoing allogeneic hematopoietic stem cell transplants (allo-HSCTs) is infection with the human cytomegalovirus (HCMV). For HCMV treatment, it is especially crucial to be able to differentiate between recipients who are at high risk of reactivation and those who are not. In this study, HCMV-DNA was collected from 60 HLA-A*02 allo-HSCT recipients before and after transplantation. After transplantation, the release of interferon (IFN)-γ by T cells specific to HCMV was assessed using the enzyme-linked immunospot assay (ELISPOT). The results show that the median viral load (VL) was significantly higher in the HCMV persistent-infection group compared to the non-persistent-infection group (p = 0.002), and that the late-infection rate was considerably higher in the high-VL group compared to the low-VL group (p = 0.014). The uninfected group had a considerably higher median IFN-γ spot‐forming cell (SFC) count than the persistent-infection group (p = 0.001), and IFN-γ SFC counts correlated negatively and linearly with VLs (r = −0.397, p = 0.002). The immune-response groups showed significantly difference in median VL (p = 0.018), and the high immune response group had a reduced late-infection rate than the no/low immune response groups (p = 0.049). Our study showed that allo-HSCT recipients with a high VL at an early transplantation stage were at high risk for late HCMV infection. Further HCMV reactivation can be prevented by HCMV-specific T cells secreting enough IFN-γ.
Keywords: Allogeneic hematopoietic stem cell transplantation, HLA‐A*02, Human cytomegalovirus DNA, Interferon‐γ
Highlights
-
•
High HCMV-DNA viral load early after transplant is a risk factor for late infection.
-
•
Higher levels of IFN-γ secretion are associated with better control of HCMV.
-
•
Assessing the immune response reduces the risk of HCMV reactivation.
1. Introduction
Reactivation of human cytomegalovirus (HCMV) is a major cause of morbidity and mortality in recipients of allogeneic stem cell transplantation (allo-HSCT) [1]. The immunosuppressive status of recipients after transplantation increases their risk of HCMV infection/reactivation [2]. HCMV infection/reactivation, especially persistent infection/reactivation, can lead to serious illness, including pneumonia, gastrointestinal disorders, retinitis, and diseases of central nervous system [3]. High HCMV-DNA viral loads (VLs) also raises the risk of death following transplantation [4]. Although preventive or preemptive antiviral therapy has radically decreased the risk of HCMV-related disease [5], it delays the HCMV immune reconstitution and increases the incidence of late HCMV reactivation and disease [6]. However, allo-HSCT recipients with immune recovery are at risk of excessive antiviral treatment [2].
Cellular immunity plays a crucial role in controlling HCMV reactivation and related disease. The reconstitution of specific T cellular immunity has been shown to impact HCMV reactivation and disease prognosis in allo-HSCT recipients [7]. The T cell immune response specific to HCMV is closely associated with HCMV-DNA VLs, HCMV reactivation, and antiviral therapy [8,9]. Allo-HSCT recipients with relatively strong specific T cellular immunity are less likely to experience HCMV reactivation, whereas those with a deficient or delayed immune response, and especially those with higher VLs, may suffer from late HCMV reactivation and disease [[10], [11], [12]]. Interferon (IFN)-γ is a key cytokine controlling HCMV reactivation [10,13]. Current assays used to quantify IFN-γ production by T cells specific to HCMV consist of enzyme-linked immunosorbent assay (ELISA), tetramer staining, flow cytometry with intracellular cytokine staining (ICS) and enzyme-linked immunospot assay (ELISPOT) [[14], [15], [16]]. ELISPOT assay IFN-γ secretion has proven effective in predicting HCMV reactivation after transplantation, which offers high sensitivity and easy operation and is suitable for detecting low cytokine levels [14,17,18]. HLA‐A*02‐restricted pp65495-503 NLVPMVATA(NLV) antigen peptide has been widely used in the study of T cell immune response specific to HCMV [19].
In our previous study [20], we analyzed the relationship between T cellular immune response specific to HCMV and HCMV infection indicators (HCMV-DNA, HCMV-IgG/M, HCMV-pp65) during the initial phases of transplantation. The findings demonstrated a strong relationship between the IFN-γ secretion of T cells and the indicators of HCMV infection. To further study the function of IFN-γ-in predicting late HCMV reactivation, more HLA-A*02 recipients were included for current report.
According to the HCMV infection status and immune response of allo-HSCT recipients, assessment of the relationship between HCMV infection and IFN-γ secreted by T cells specific to HCMV might provide a theoretical basis for evaluating the HCMV reactivation risk and the need for antiviral treatment after transplantation.
2. Materials and methods
2.1. Patients and specimens
This study enrolled 60 HLA-A*02 recipients who received allo-HSCT from March 2016 to April 2022. The exclusion criteria included recipients with hepatitis B virus, human immunodeficiency virus, or herpes simplex virus infection; recipients who received chimeric antigen receptor T cells; as well as those who underwent autologous hematopoietic stem cell transplantation or secondary or multiple transplantation. The characteristics of the 60 HLA-A*02 recipients can be found in Table 1. Approval for this study was granted by the Ethics Committee of the First Affiliated Hospital at the Medical School of Zhejiang University (Approval number: 2019484). Written informed consent was obtained from recipients in accordance to the principles of the Declaration of Helsinki.
Table 1.
Characteristics of the 60 HLA-A*02 allo-HSCT recipients.
| Feature | Quantity |
|---|---|
| Gender (M/F) | 33/27 |
| Age | 40(11–65) |
| Underlying disease | |
| ALL | 18 |
| AML | 36 |
| MDS | 3 |
| NHL | 1 |
| HPS | 1 |
| AA | 1 |
| Source of stem cell | |
| Peripheral | 60 |
| Pretreatment scheme | |
| AraC + BuCy + MECCNU | 5 |
| AraC + BuCy + MECCNU + CTX + ATG | 5 |
| AraC + BuCy + CTX + MECCNU | 10 |
| AraC + BuCy + ATG + MeCCNU | 12 |
| AraC + BuCy + ATG | 8 |
| Flu + BuCy + ATG | 10 |
| AraC + Bucy + ATG + CTX | 4 |
| AraC + CTX + ATG | 2 |
| BuCy + MECCNU | 2 |
| Flu + BuCy | 1 |
| Flu + CTX + ATG | 1 |
| GVHD prophylaxis | |
| CSA + MMF + MTX | 50 |
| CSA + MTX | 10 |
| Type of donor | |
| HLA-identical sibling | 6 |
| Mismatched family member | 50 |
| Matched unrelated | 4 |
| GVHD | |
| None | 52 |
| AcuteI-II | 6 |
| Acute III-IV | 2 |
| Chronic | 0 |
Abbreviations:ALL, acute lymphoblastic leukemia; AML, acute myelocytic leukemia; MDS, myelodysplastic syndromes; NHL, non-Hodgkin's lymphoma; HPS, hemophagocytic lymphohistiocytosis; AA, aplastic anemia; AraC, cytarabine; Bu, baixiaoan; CSA, cyclosporine A; CTX, cyclophosphamide; Flu, Fludarabine; MeCCNU, simustine; MTX, methotrexate; MMF, mycophenolate mofetil ethyl ester; ATG, anti-human thymocyte globulin; HLA, human leukocyte antigen; GVHD, graft-versus-host disease.
HCMV-DNA levels were measured before and after transplantation, with weekly monitoring of HCMV-DNA for a duration of 3 months after transplantation, followed by monthly assessments. The mean HCMV-DNA VL values within 3 months were used for statistical analyses. The IFN-γ secretion by T cells specific to HCMV was evaluated using ELISPOT assay at 3 months after transplantation.
2.2. Detection of HCMV-DNA
HCMV-DNA VL was detected using an HCMV nucleic acid quantitative detection kit (DAAN GENE, Cat No: DA0121) [20]. The quantification of VLs was performed using real-time fluorescence quantitative PCR (Stratagene Mx3000p; Agilent Technologies). The lower limit of detection was 2.7 log10 copies/ml; VL ≥ 2.7 log10 copies/ml was defined as HCMV-DNA-positive.
2.3. IFN-γ secretion by T cells specific to HCMV
ELISPOT assays (Mabtech, Code: 3420-2AST-2) were used to detect the IFN-γ secretion by T cells specific to HCMV. The assays were conducted following the manufacturer's instruction [21]. Briefly, ELISPOT plates were washed four times with 200 μl of PBS and then blocked with RPMI 1640 medium containing 10% fetal bovine serum for 30 min. Subsequently, 3 × 104 peripheral blood mononuclear cells (PBMCs)/well were added to the plates, which were precoated with anti‐IFN‐γ antibody. The HLA‐A*02‐restricted synthetic HCMV‐pp65495–503 epitope NLVPMVATV (HLA-A*02/NLV) was added at a final concentration of 10 μg/ml with a purity of 98%. RPMI 1640 (PAN Biotech GmbH, Cat No: P04-18047) and irrelevant peptide ILKEPVHGV were added as the negative controls, and anti‐CD3 monoclonal antibody (Mabtech, Code: 3420-2AST-2) was added as positive control. Following an 18–21 h incubated at 37 °C in a 5.0% CO2 equilibrated incubator, the plates were washed and then incubated at room temperature for 2 h with 100 μl of ALP-conjugated secondary antibody (1:200 dilution) in each well. Following that, 200 μl of substrate solution filtered through a 0.45 μm filter was added to each well to induce spot development. Finally, spots were counted using an automated ImmunoSpot S5 UV Analyzer (Cellular Technology Ltd.). Assay results were the average of triplicate values and expressed as spot‐forming cell (SFC)/3 × 104 PBMCs.
2.4. Definitions
Based on the results of the HCMV-DNA determinations, HCMV-DNA positivity for more than 4 weeks was defined as persistent infection, and persistence of HCMV-DNA positivity or recurrence of positivity 3 months post-transplantation was defined as late HCMV infection. A VL < 3 log10 copies/ml was defined as a low VL, and a VL > 3 log10 copies/ml was defined as a high VL. Based on IFN-γ SFC counts, SFCs = 0 was defined as no immune response, SFCs <100/3 × 104 PBMCs as a low immune response, and SFCs >100/3 × 104 PBMCs as a high immune response [10].
2.5. Statistical analyses
GraphPad Prism 8.0 software (GraphPad Software Inc.) was used to conduct the statistical analyses. The chi-squared test or Fisher's exact test was used to analyze categorical variables such as HCMV-DNA-positive rate, IFN-γ-positive rate, late HCMV infection rate. The t-test or Mann-Whitney U test was used to analyze differences in IFN-γ SFC counts and HCMV-DNA VLs between two groups, and the Kruskal-Wallis test was used to analyze differences in IFN-γ SFC counts and HCMV-DNA VLs among three groups. Spearman's correlation was used to analyze correlations. A p-value <0.05 was deemed to be statistical significance.
3. Results
3.1. HCMV infection status
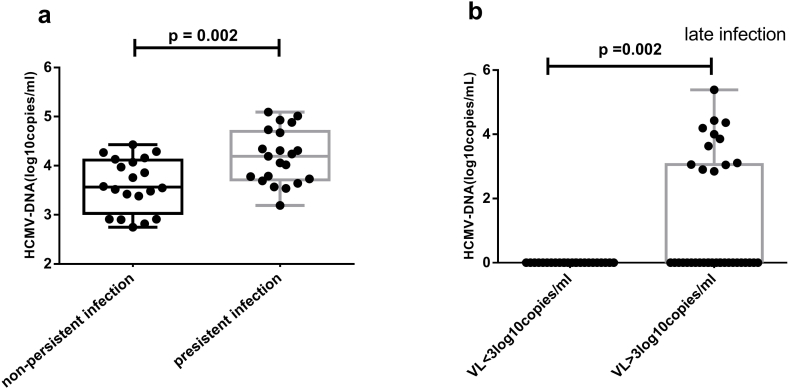
This study included a total of 60 HLA-A*02 recipients who underwent allo-HSCT. All were HCMV-DNA-negative before transplantation, but 41 were HCMV-DNA-positive after transplantation. Twelve recipients developed late infection by 3 months post-transplantation. The recipients were divided into three groups according to the results of the HCMV-DNA assays: uninfected (n = 19), non-persistent infection (n = 20), and persistent infection (n = 21). The mean VLs in the non-persistent-infection and persistent-infection groups were 3.61 ± 0.12 log10 copies/ml and 4.18 ± 0.12 log10 copies/ml, respectively. The mean VL was higher in the persistent-infection group compared to the non-persistent-infection group (p = 0.002, Fig. 1 a)
Fig. 1.
Comparing of different HCMV infection groups. (a) The VL in persistent infection group was significantly higher compared to the non-persistent infection group using t-test; (b) The late infection rates in high VL group were considerably higher than those in low VL group using Mann-Whitney U test.
Recipients were also subdivided into low-VL (n = 24) and high-VL (n = 36) groups. The former group comprised recipients who were HCMV-uninfected or who had non-persistent infection. No late infections were observed among recipients with low-VL, whereas 12 recipients with high-VL developed late infection. The significant difference was found between low-VL group and high-VL group (p = 0.002) (Fig. 1 b).
3.2. IFN‐γ secretion by T cells specific to HCMV
IFN‐γ secretion by T cells specific to HCMV was assessed in all recipients using ELISPOT assay at 3 months post-transplantation (Fig. 2). IFN-γ secretion was determined in 68% (41/60) of recipients but not in the remaining 32% (19/60). The mean IFN‐γ SFC count in the former group was 311/3 × 104 PBMCs (range: 2–990/3 × 104 PBMCs).
Fig. 2.
ELISPOT assay measures the IFN-γ secretion by T cells specific to HCMV. (a) Positive control: CD3 antibody stimulated PBMCs of allo-HSCT recipients; (b) HCMV specific antigen peptide NLVPMVATA stimulated PBMCs of allo-HSCT recipients; (c) Negative control: Irrelevant peptide ILKEPVHGV stimulated PBMCs of allo-HSCT recipients; (d) Negative control: RPMI 1640 co-cultured with PBMCs of allo-HSCT recipients.
Recipients were subdivided into a no-immune-response group (n = 19; SFCs = 0), a low-immune-response group (n = 14; SFCs <100/3 × 104 PBMCs) and a high-immune-response group (n = 27; SFCs >100/3 × 104 PBMCs) based on their IFN-γ SFC counts [19]. The median IFN-γ SFC counts in the low-immune-response group was 12/3 × 104 PBMCs and in the high-immune-response group it was 402/3 × 104 PBMCs. The IFN-γ SFC counts differed significantly among the three immune-response groups (all p < 0.001, Fig. 3).
Fig. 3.
Comparing of IFN-γ SFC counts in different immune response group of recipients at 3 months post transplantation using Mann-Whitney U test and Kruskal-Wallis test.
3.3. Relationship between HCMV infection and IFN‐γ SFC count
3.3.1. IFN‐γ secretion in the different infection groups
Among allo-HSCT recipients, IFN‐γ secretion was detected in 84% of those in the uninfected group, 70% of those in the non-persistent-infection group, and 52% of those in the persistent-infection group, with a gradual decrease seen in the percentage of IFN‐γ secretion in the three groups. The differences in median IFN‐γ SFC counts among the three infection groups showed a trend toward significance (p = 0.059, Fig. 4 a), whereas the median IFN‐γ SFC count was notably reduced in the persistent-infection group in contrast to the uninfected group (p = 0.014, Fig. 4 a).
Fig. 4.
The relationship between HCMV infection and IFN‐γ SFC counts. (a) The median IFN-γ SFC counts in uninfected group were significantly higher than that in persistent group using Mann-Whitney U test; (b) The median IFN-γ SFC counts in low VL group were significantly higher than that in high VL group using Mann-Whitney U test; (c) The IFN-γ SFC counts was negatively linear correlated with VL using Spearman's correlation; (d) The median VL in three immune response groups were significantly difference using Mann-Whitney U test and Kruskal-Wallis test; (e) The median VL in no immune response group was significantly higher than that in high immune response group using Mann-Whitney U test; (f) The median VL in high response group was lower than that in no/low response group using Mann-Whitney U test.
3.3.2. Relationship between HCMV-DNA VL and IFN‐γ SFC count
The median IFN-γ SFC counts were 343/3 × 104 PBMCs in the low-VL group and 8.5/3 × 104 PBMCs in the high-VL group; the difference was significant (p = 0.001, Fig. 4 b). VLs correlated negatively and linearly with IFN-γ SFC counts (r = −0.397, p = 0.002) (Fig. 4 c).
Median VLs differed significantly among the no-immune-response (4.02 log10 copies/ml), low-immune-response (3.66 log10 copies/ml) and high-immune-response (2.82 log10 copies/ml) groups (p = 0.018, Fig. 4 d), with a significantly higher median VL seen in the no-immune-response group than in the high-immune-response group (p = 0.005, Fig. 4 e).
3.3.3. Relationship between late infection and IFN-γ SFC count
Late infection developed in 12 recipients by 3 months post-transplantation, including 6 in the no-immune-response group, 4 in the low-immune-response group and 2 in the high-immune-response group. The late-infection rates were 32% (6/19), 29% (4/14), and 7% (2/27), respectively, and the differences among the three groups showed a trend toward significance (p = 0.051). The late-infection rate was lower in the high-immune-response group than in the no/low-immune-response groups (p = 0.049). The median VL in the high-immune-response group was lower than that in the no/low-immune-response groups (p = 0.042, Fig. 4 f).
4. Discussion
HCMV infection is one of the most common reactivations in recipients of allo-HSCT [22], as these individuals are immunodeficient after transplantation and thus at high risk of HCMV reactivation/reinfection [2]. Although prophylactic antiviral drugs reduce the mortality associated with HCMV disease, antiviral-drug resistance is increasing [23]. All allo-HSCT recipients in this study were given preemptive therapy, but 68% developed HCMV reactivation after transplantation, and one-third of the HCMV-DNA-positive recipients developed persistent infection. Previous studies have shown that persistent infection is an important factor in the morbidity and mortality of allo-HSCT recipients [24]. The development of first-time HCMV-DNA positivity in allo-HSCT recipients with persistent infection varied from 2 to 9 weeks post-transplantation, and the duration ranged from 1 to several months. Although all allo-HSCT recipients with persistent infection were treated with antiviral therapy, their VLs remained high, implying that a high VL is not easily cleared and that recipients with a high VL will develop persistent infection. Therefore, recipients with a high VL are more likely to develop late HCMV infection and should thus be closely monitored in terms of their VLs and drug resistance [25].
IFN-γ exerts antiviral effects in a large number of viral infections [26]. In this study, IFN-γ secretion was detected in two-thirds of the recipients (both uninfected and infected). The IFN-γ SFC counts were higher in uninfected recipients than in those with persistent infection, implying that HCMV infection/reactivation is not a prerequisite for the initiation of an immune response. Donor T cells may rapidly recover and initiate an immune response to prevent HCMV-DNAemia [27], whereas persistent infection may inhibit the recovery of T cells. Most of the one-third of recipients lacking IFN-γ secretion were in the high-VL group. Because a high VL may cause immunosuppression in allo-HSCT recipients [25], decreasing the VL during the early stage of transplantation is crucial for immune recovery.
IFN-γ is an important cytokine secreted by T cells [13,15,28], which inhibits HCMV replication and reduces the risk of HCMV recurrence [29]. In current study, IFN-γ SFC counts correlated negatively and linearly with HCMV-DNA VLs and were significantly higher in the low-VL group compared to the high-VL group. The recipients in the low-VL group maintained a negative HCMV-DNA status at 3 months post-transplantation. These findings suggest that IFN-γ secreted by T cells specific to HCMV may lead to the elimination of HCMV-DNAemia if the HCMV VL is low [24,30]. Among the 69% (25/36) of recipients with a high VL who were in the no/low-immune-response groups, 48% (12/25) developed persistent infection/reactivation by 3 months post-transplantation. Late-infection rates were higher in the no/low-immune-response groups (<100/3 × 104 PBMCs) than in the high-immune-response group, indicative of a close relationship between HCMV-DNA VL and an HCMV-specific T cellular immune response. Previous studies showed that a high VL influences the recovery of a T cellular immune response, with an insufficient response leading to persistent infection/reactivation [31,32].
Our findings indicated that the immune response of recipients with low VL were better than those with high VL during the initial phases of transplantation. Recipients with high VL, especially those with persistent infections were at higher risk of late HCMV infection, who should be intensity monitoring of HCMV-DNA and increase duration of antiviral therapy. Although preventive or preemptive antiviral therapy basing HCMV-DNA monitoring has decreased the risk of HCMV related-disease, it raises the frequency of late HCMV reactivation and disease. More recipients with undetected IFN-γ secretion occurred late HCMV reactivation. The recipients lack of specific T cellular immune response should be treated to improve immune recovery. T cellular immune response assay was benefit for distinguish recipients at higher risk of HCMV reactivation from those who are not. Monitoring immune response of recipients in early stage of transplantation may hold predictive values for allo-HSCT outcomes.
One limitation of our study was that only HLA-A*02-restricted-NLV antigen peptide was selected. HCMV encodes over 200 potential proteins [33]. As studies reported that CD4+ and CD8+ T cell responses target HCMV canonical proteins, which include the 65-kDA phosphoprotein (UL83/pp65), tegument protein pp150 (UL32), envelope glycoprotein B (UL55) and viral transcription factor IE2 (UL122) [33,34]. Both CD4+ and CD8+ T cells play a role in defending against HCMV infection. In our future study, more immunodominant peptides or peptides pool derived from HCMV are merited to assay specific T cell response. Another limitation was IFN-γ secreted by PBMCs stimulated with NLV antigenic peptide was assayed to evaluate specific T cellular immune response in this study. ELISPOT assay was used to detected IFN-γ secretion, which couldn't distinguish IFN-γ secretion from CD4+ or CD8+ T cells. Although pp65495-503 NLV peptide was HLA-A*02 restricted, previous studies reported that memory T cells specific to viruses can identify foreign HLA molecules directly [[35], [36], [37]]. However, the purpose of this study was to evaluate IFN-γ secretion as a predictor of delayed HCMV reactivation following transplantation.
In conclusion, this study found that a high HCMV-DNA VL in allo-HSCT recipients during the early transplantation stages is a risk factor for late infection. The IFN-γ secretion assay can aid in identification of patients at high risk for HCMV reactivation. A sufficient level of IFN-γ secretion by T cells specific to HCMV can prevent further HCMV reactivation.
Data availability statement
Data are available on reasonable request.
Ethics declarations
This study was reviewed and approved by the Ethics Committee of the First Affiliated Hospital at the Medical School of Zhejiang University, with the approval number: 2019484.
CRediT authorship contribution statement
Hanying Liang: Writing – original draft, Resources, Conceptualization. Shengnan Gong: Methodology. Genyong Gui: Methodology. Huiqi Wang: Software. Lili Jiang: Formal analysis. Xuejie Li: Data curation. Jun Fan: Writing – review & editing, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research received no external funding.
References
- 1.Barron M.A., Gao D., Springer K.L., Patterson J.A., Brunvand M.W., McSweeney P.A., Zeng C., Baron A.E., Weinberg A. Relationship of reconstituted adaptive and innate cytomegalovirus (CMV)-specific immune responses with CMV viremia in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49(12):1777–1783. doi: 10.1086/648423. [DOI] [PubMed] [Google Scholar]
- 2.Avetisyan G., Larsson K., Aschan J., Nilsson C., Hassan M., Ljungman P. Impact on the cytomegalovirus (CMV) viral load by CMV-specific T-cell immunity in recipients of allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38(10):687–692. doi: 10.1038/sj.bmt.1705507. [DOI] [PubMed] [Google Scholar]
- 3.Goncalves C., Cipriano A., Videira Santos F., Abreu M., Mendez J., Sarmento E.C.R. Cytomegalovirus acute infection with pulmonary involvement in an immunocompetent patient. IDCases. 2018;14 doi: 10.1016/j.idcr.2018.e00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green M.L., Leisenring W., Xie H., Mast T.C., Cui Y., Sandmaier B.M., et al. Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol. 2016;3(3):e119–e127. doi: 10.1016/S2352-3026(15)00289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boeckh M., Ljungman P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Einsele H., Hebart H., Kauffmann-Schneider C., Sinzger C., Jahn G., Bader P., et al. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant. 2000;25(7):757–763. doi: 10.1038/sj.bmt.1702226. [DOI] [PubMed] [Google Scholar]
- 7.Espigado I., de la Cruz-Vicente F., BenMarzouk-Hidalgo O.J., Gracia-Ahufinger I., Garcia-Lozano J.R., Aguilar-Guisado M., et al. Timing of CMV-specific effector memory T cells predicts viral replication and survival after allogeneic hematopoietic stem cell transplantation. Transpl. Int. 2014;27(12):1253–1262. doi: 10.1111/tri.12406. [DOI] [PubMed] [Google Scholar]
- 8.Zhou W., Longmate J., Lacey S.F., Palmer J.M., Gallez-Hawkins G., Thao L., et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113(25):6465–6476. doi: 10.1182/blood-2009-02-203307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gimenez E., Blanco-Lobo P., Munoz-Cobo B., Solano C., Amat P., Perez-Romero P., et al. Role of cytomegalovirus (CMV)-specific polyfunctional CD8+T-cells and antibodies neutralizing virus epithelial infection in the control of CMV infection in an allogeneic stem-cell transplantation setting. J. Gen. Virol. 2015;96(9):2822–2831. doi: 10.1099/vir.0.000203. [DOI] [PubMed] [Google Scholar]
- 10.Nesher L., Shah D.P., Ariza-Heredia E.J., Azzi J.M., Siddiqui H.K., Ghantoji S.S., Marsh L.Y., Michailidis L., Makedonas G., Rezvani K., et al. Utility of the enzyme-linked immunospot interferon-gamma-release assay to predict the risk of cytomegalovirus infection in hematopoietic cell transplant recipients. J. Infect. Dis. 2016;213(11):1701–1707. doi: 10.1093/infdis/jiw064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gratama J.W., van Esser J.W., Lamers C.H., Tournay C., Lowenberg B., Bolhuis R.L., et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood. 2001;98(5):1358–1364. doi: 10.1182/blood.V98.5.1358. [DOI] [PubMed] [Google Scholar]
- 12.Ganepola S., Gentilini C., Hilbers U., Lange T., Rieger K., Hofmann J., et al. Patients at high risk for CMV infection and disease show delayed CD8+T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39(5):293–299. doi: 10.1038/sj.bmt.1705585. [DOI] [PubMed] [Google Scholar]
- 13.El Haddad L., Ariza-Heredia E., Shah D.P., Jiang Y., Blanchard T., Ghantoji S.S., El Chaer F., El-Haddad D., Prayag A., Nesher L., et al. The ability of a cytomegalovirus ELISPOT assay to predict outcome of low-level CMV reactivation in hematopoietic cell transplant recipients. J. Infect. Dis. 2019;219(6):898–907. doi: 10.1093/infdis/jiy592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yong M.K., Cameron P.U., Slavin M., Morrissey C.O., Bergin K., Spencer A., et al. Identifying cytomegalovirus complications using the quantiferon-CMV assay after allogeneic hematopoietic stem cell transplantation. J. Infect. Dis. 2017;215(11):1684–1694. doi: 10.1093/infdis/jix192. [DOI] [PubMed] [Google Scholar]
- 15.Boadi Amissah Obed, Chen Wenfang, de Dieu Habimana Jean, Sun Yirong, Lin Lihui, Liu Yujie, Wang Ling, Liu Zhaoming, Omar Mukama, Basnet Rajesh, Liu Hohua, Li Junyi, Ding Xuanyan, Lv Lingshuang, Chen Min, Liang Yalin, Huang Rongqi, Li Zhiyuan. NY-ESO-1-specifc T cell receptor-engineered T cells and Tranilast, a TRPV2 antagonist bivalent treatment enhances the killing of esophageal cancer: a dual-targeted cancer therapeutic route. Cancer Cell Int. 2024;24:64. doi: 10.1186/s12935-024-03249-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Yangbing, Bennett Alan D., Zheng Zhili, Wang Qiong J., Robbins Paul F., Yu Lawrence Y.L., Li Yi, Molloy Peter E., Dunn Steven M., Jakobsen Bent K., Rosenberg Steven A., Morgan Richard A. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J. Immunol. 2007;179(9):5845–5854. doi: 10.4049/jimmunol.179.9.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tey S.K., Kennedy G.A., Cromer D., Davenport M.P., Walker S., Jones L.I., et al. Clinical assessment of anti-viral CD8+T cell immune monitoring using QuantiFERON-CMV(R) assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0074744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson A.C., Martin J.N., Younger S.R., Bredt B.M., Epling L., Ronquillo R., et al. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J. Immunol. Methods. 2003;283:141–153. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Liu A., Ma Y., Wu W., Chen X., Huang Y., Hu J., Liang H., Wang H., Yang R., Fan J. Evaluation of human cytomegalovirus-specific CD8+ T-cells in allogeneic haematopoietic stem cell transplant recipients using pentamer and interferon-gamma-enzyme-linked immunospot assays. J. Clin. Virol. 2013;58(2):427–431. doi: 10.1016/j.jcv.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Liang Hanying, Xia Jintao, Zhang Runan, Yang Bing, Wu Jian, Gui Genyong, Huang Yaping, Chen Xiaoming, Yang Rong, Wang Huiqi, Gong Shengnan, Fan Jun. ELISPOT assay of interferon-γ secretion for evaluating human cytomegalovirus reactivation risk in allo-HSCT recipients. J. Med. Virol. 2021;93:6301–6308. doi: 10.1002/jmv.27120. [DOI] [PubMed] [Google Scholar]
- 21.Zhang R., Zhang Y., Hu J., Wu W., Chen X., Lu Z., Yang R., Huang Y., Fan J. Specific T-cell receptor gene transfer enhances immune response: a potential therapeutic strategy for the control of human cytomegalovirus infection in immunocompromised patients. Cell. Immunol. 2019;336:58–65. doi: 10.1016/j.cellimm.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Ciaurriz M., Zabalza A., Beloki L., Mansilla C., Perez-Valderrama E., Lachen M., Bandres E., Olavarria E., Ramirez N. The immune response to cytomegalovirus in allogeneic hematopoietic stem cell transplant recipients. Cell. Mol. Life Sci. 2015;72(21):4049–4062. doi: 10.1007/s00018-015-1986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El Chaer Firas, Shah Dimpy P., Chemaly Roy F. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood. 2016;128(23):2624–2636. doi: 10.1182/blood-2016-06-688432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Kong J., Chang Y.J., et al. Patients with refractory cytomegalovirus (CMV) infection following allogeneic haematopoietic stem cell transplantation are at high risk for CMV disease and non-relapse mortality. Clin. Microbiol. Infect. 2015;21:1121 e9–e15. doi: 10.1016/j.cmi.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Schubert Axel, Ehlert Karoline, Schuler-Luettmann Susanne, Gentner Eva, Mertens Thomas, Michel Detlef. Fast selection of maribavir resistant cytomegalovirus in a bone marrow transplant recipient. BMC Infect. Dis. 2013;19(13):330. doi: 10.1186/1471-2334-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arcssjjp Maciejewski. Apoptosis induced by human cytomegalovirus infection can be enhanced by cytokines to limit the spread of virus. Exp. Hematol. 1999;27:1194–1203. doi: 10.1016/S0301-472X(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 27.Daniel Burkea J., Young H.A. IFN-Γ: a cytokine at the right time, is in the right place. Semin. Immunol. 2019;43 doi: 10.1016/j.smim.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur Kawaljit, Jewett Anahid. Osteoclasts and probiotics mediate significant expansion, functional activation and supercharging in NK, γδ T, and CD3+ T cells: use in cancer immunotherapy. Cells. 2024;13(3):213. doi: 10.3390/cells13030213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douglas A.P., Yu L., Sundararajan V., Szer J., Ritchie D., Slavin M.A., Sasadeusz J., Visvanathan K. The QuantiFERON Monitor((R)) assay is predictive of infection post allogeneic hematopoietic cell transplantation. Transpl. Infect. Dis. 2020;22(3) doi: 10.1111/tid.13786. [DOI] [PubMed] [Google Scholar]
- 30.Krawczyk A., Ackermann J., Goitowski B., Trenschel R., Ditschkowski M., Timm J., Ottinger H., Beelen D.W., Gruner N., Fiedler M. Assessing the risk of CMV reactivation and reconstitution of antiviral immune response post bone marrow transplantation by the QuantiFERON-CMV-assay and real time PCR. J. Clin. Virol. 2018;99–100:61–66. doi: 10.1016/j.jcv.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Wagner-Drouet E., Teschner D., Wolschke C., Janson D., Schafer-Eckart K., Gartner J., Mielke S., Schreder M., Kobbe G., Kondakci M., et al. Standardized monitoring of cytomegalovirus-specific immunity can improve risk stratification of recurrent cytomegalovirus reactivation after hematopoietic stem cell transplantation. Haematologica. 2021;106(2):363–374. doi: 10.3324/haematol.2019.229252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douglas A.P., Yu L., Sundararajan V., Szer J., Ritchie D., Slavin M.A., Sasadeusz J., Visvanathan K. The QuantiFERON Monitor((R)) assay is predictive of infection post allogeneic hematopoietic cell transplantation. Transpl. Infect. Dis. 2020;22(3) doi: 10.1111/tid.13786. [DOI] [PubMed] [Google Scholar]
- 33.Dhanwani Rekha, Kumar Dhanda Sandeep, Pham John, Williams Gregory P., Sidney John, Grifoni Alba, Picarda Gaelle, Lindestam Arlehamn Cecilia S., Sette Alessandro, Benedicta Chris A. Profiling human cytomegalovirus-specific T cell responses reveals novel immunogenic open reading frames. J. Virol. 2021 Nov;95(21) doi: 10.1128/jvi.00940-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sylwester Andrew W., Mitchell Bridget L., Edgar John B., Taormina Cara, Pelte Christian, Ruchti Franziska, Sleath Paul R., Grabstein Kenneth H., Hosken Nancy A., Kern Florian, Nelson Jay A., Picker Louis J. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 2005 Sep 5;202(5):673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Orsogna L., van den Heuvel H., van Kooten C., Heidt S., Claas F.H.J. Infectious pathogens may trigger specific allo‐HLA reactivity via multiple mechanisms. Immunogenetics. 2017;69(8–9):631–641. doi: 10.1007/s00251-017-0989-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Heuvel H., Heutinck K.M., van der Meer‐Prins E.P., et al. Detection of virus‐specific CD8+ T cells with cross‐reactivity against alloantigens: potency and flaws of present experimental methods. Transplant Direct. 2015;1(10):e40. doi: 10.1097/TXD.0000000000000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Link C.S., Eugster A., Heidenreich F., et al. Abundant cytomegalovirus (CMV) reactive clonotypes in the CD8(+) T cell receptor alpha repertoire following allogeneic transplantation. Clin. Exp. Immunol. 2016;184(3):389–402. doi: 10.1111/cei.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.