Abstract
In order to investigate if immune responses to the fusion (F) protein of respiratory syncytial virus (RSV) could be influenced by cytokines, recombinant vaccinia viruses (rVV) carrying both the F gene of RSV and the gene for murine interleukin-2 (IL-2), IL-4, or gamma interferon (IFN-γ) were constructed. In vitro characterization of rVV revealed that insertion of the cytokine gene into the VP37 locus of the vaccinia virus genome resulted in 100- to 1,000-fold higher expression than insertion of the same gene into the thymidine kinase (TK) locus. In comparison, only a two- to fivefold difference in the level of expression of the F protein was observed when the gene was inserted into either of these two loci. Mice vaccinated with rVV expressing the F protein and high levels of IL-2 or IFN-γ cleared rVV more rapidly than mice inoculated with a control rVV and developed only low levels of RSV-specific serum antibody. In addition, these recombinants were much less effective at priming RSV-specific memory cytotoxic T lymphocytes (CTL) and IFN-γ production by spleen cells than rVV expressing the F protein alone. In contrast, mice vaccinated with rVV expressing high levels of IL-4 showed signs of delayed rVV clearance. RSV-specific serum antibody responses were biased in favor of immunoglobulin G1 (IgG1) in these mice, as there was a significant reduction in IgG2a antibody responses compared with serum antibody responses in mice vaccinated with rVV expressing the F protein alone. However, vaccination with rVV expressing the F protein together with high levels of IL-4 did not alter the development of RSV-specific memory CTL or IFN-γ production by RSV-restimulated splenocytes.
Infection of inbred mouse strains with a number of pathogens has revealed that the selective differentiation and development of effector T cells have profound implications for disease resistance or disease susceptibility. Th1-like immune responses, producing high levels of interleukin-2 (IL-2) and gamma interferon (IFN-γ) (25, 26, 28), are protective against the intracellular pathogens Leishmania major and Candida albicans (31, 36), whereas hosts that mount Th2-like responses are susceptible to progressive infection. In contrast, Th2 cells which secrete IL-4, IL-5, IL-6, and IL-10 (23, 26) are protective against extracellular pathogens such as Trichuris muris and Borrelia burgdorferi (22, 36), and the induction of Th1 responses is nonprotective. Hence, most pathogens are usually preferentially susceptible to one type of immune response, and the identification of strategies for the induction of specific types of immunity will aid vaccine design.
The development of naive Th cells into Th1- or Th2-like cells is influenced by the cytokine microenvironment upon activation. Thus, IL-4 can direct the development of Th cells into Th2 cells (20, 33, 41) while IL-12 or IFN-γ can induce the development of Th cells into Th1 cells (6, 16, 34). Furthermore, cytokines produced by one Th subset can block the production or activity of cytokines produced by the other subset (13, 40). This feedback mechanism allows the possibility of the use of vector vaccines expressing cytokine genes to manipulate the microenvironment to favor the development of appropriate protective immune responses. Both Th1 and Th2 cells provide B-cell help to antibody-producing B cells, but the differential secretion of IL-4 and IFN-γ can regulate the relative quantities of immunoglobulin G1 (IgG1), IgG2a, and IgE that are made (10).
The BALB/c mouse model of respiratory syncytial virus (RSV) infection has revealed that T lymphocytes and the cytokines that they produce play an important role in determining the outcome of RSV infection (3, 15, 42, 43). Of particular interest is evidence that the fusion (F) and the attachment (G) proteins of RSV prime for different Th-cell responses in BALB/c mice (1). Thus, recombinant vaccinia viruses (rVV) expressing the F protein of RSV prime for cytotoxic T lymphocytes (CTL) and a Th1 response, resulting in a characteristic polymorphonuclear (PMN) efflux in the lungs of mice following RSV challenge. In contrast, rVV expressing the G protein prime for a Th2 response, which induces large numbers of eosinophils in pulmonary exudate following RSV challenge (1, 27). The cytokines produced as a result of these different Th responses are therefore reflected by characteristic changes in pulmonary pathology of vaccinated mice following RSV challenge.
Most studies to date have only examined the effects of vaccinia virus (VV)-expressed cytokines on primary immune responses. In the studies described here, we investigated the effects of cytokines on the establishment of memory to the F glycoprotein of RSV expressed in rVV. The ability of the coexpressed cytokines IL-2, IL-4, and IFN-γ to influence the isotype of RSV-specific antibody, CTL, and Th priming was assessed. This approach provides a model for studying the role of Th subsets and T-lymphocyte–virus interactions and may be a practical approach for the design of effective vaccines.
MATERIALS AND METHODS
Viruses.
rVV were constructed according to standard methods briefly described below. Two parental VVs were used: strain WR and strain vRB12, a strain WR-derived virus defective for plaque formation (5). The vRB12 virus lacks the gene encoding protein VP37; insertion plasmid pRB21 provides a complete copy of the VP37 gene, allowing rVV to be selected on the basis of plaque formation (5). Plasmid pSCF and the corresponding rVV VA-F have been described elsewhere (21) and are referred to here as VSCF. Plasmid pRBF was obtained by subcloning a fragment of plasmid LF1 containing the F gene of the Long strain of RSV (7) into the StuI-HindIII-cleaved pRB21 vector and was used to produce the rVV VRBF. The coding regions for murine cytokine genes were obtained from plasmids pCD-IL-2 for IL-2 (47), pCD2A-E3 for IL-4 (19), and pRB322-IFN-γ for IFN-γ. Cytokine genes were subcloned into the SmaI-cleaved pSC11 plasmid or into the StuI-HindIII-cleaved pRB21 plasmid. rVV were obtained following the transfection of parental VV-infected CV-1 cells with the corresponding recombinant plasmids. At 48 h postinfection, viral progeny were recovered. WR-derived single rVV were selected in HuTK− 143B cells containing 25 μg of bromodeoxyuridine ml−1. vRB12-derived single rVV were selected in CV-1 cells by their ability to plaque. vRB12-derived double rVV expressing the F protein and a given cytokine were selected in HuTK− 143B cells by their ability to produce blue plaques in the presence of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal). The resulting rVV and site of insertion of the RSV F protein gene or the murine cytokine gene within the VV genome are indicated in Table 1.
TABLE 1.
Ability of rVV expressing different levels of murine cytokines to protect mice against RSV challenge 3 weeks after vaccination
| Vaccine | Gene inserted at the following
VV genome locus:
|
Titer of F proteina | Cytokine expression
(ng)b
|
Antibody response to RSVc | Lung RSV titerd | BAL cell counte | ||
|---|---|---|---|---|---|---|---|---|
| TK | VP37 | S/N | C/A | |||||
| VSCF | F | 759 | ND | ND | 5.2 ± 0.3 | <1.7h | 5.8 ± 0.2i | |
| VRBF | F | 1,995 | ND | ND | 5.1 ± 0.4 | <1.7h | ND | |
| VRBmIL-2 | IL-2 | ND | 3,470 | 210 | <1.5 | 4.4 ± 0.3 | ND | |
| VRBF.mIL-2low | IL-2 | F | 1,738 | 5 | 1 | 4.5 ± 0.1g | <1.7h | ND |
| VSCF.mIL-2high | F | IL-2 | 708 | 3,400 | 310 | 4.1 ± 0.1f | <1.7h | 5.8 ± 0.1i |
| VRBmIL-4 | IL-4 | ND | 8,650 | 840 | <1.5 | ND | ND | |
| VRBF.mIL-4low | IL-4 | F | 2,884 | 7 | 1 | 5.1 ± 0.2 | <1.7h | ND |
| VSCF.mIL-4high | F | IL-4 | 776 | 6,700 | 490 | 5.0 ± 0.2 | <1.7h | 5.7 ± 0.1i |
| VRBmIFN-γ | IFN-γ | ND | 3,430 | 315 | <1.5 | 4.2 ± 0.2 | ND | |
| VRBF.mIFN-γlow | IFN-γ | F | 1,548 | BD | BD | 4.5 ± 0.2g | <1.7h | ND |
| VSCF.mIFN-γhigh | F | IFN-γ | 794 | 3,630 | 300 | 3.0 ± 0.2f | <1.7h | 5.6 ± 0.1i |
| VA-βgal | βgal | ND | ND | ND | <1.5 | 4.3 ± 0.2 | 5.2 ± 0.1 | |
Means (n = 3) of titers of F glycoprotein determined by an ELISA. ND, not determined.
Total cytokine levels in 60-mm-diameter petri dish. BD, below detection. S/N, supernatant; C/A, cell associated.
Mean ± SD log10 titer of antibody to RSV determined by an ELISA 3 weeks postvaccination.
Mean ± SD log10 PFU of RSV per gram recovered from lungs of mice 5 days after RSV challenge.
Mean ± SD log10 cells per milliliter 5 days after RSV challenge.
The probabilities of the differences in antibody responses between VSCF-vaccinated mice and VSCF.mIL-2- or VSCF.mIFN-γ-vaccinated mice were <0.02 and <0.0001, respectively, as calculated by Student’s t test.
Antibody responses between VRBF-vaccinated mice and VRBF.mIL-2- or VRBF.mIFN-γ-vaccinated mice were significantly different, as determined by Student’s t test (P < 0.02 and P < 0.06, respectively).
Virus titers between vaccinated and control mice were significantly different, as determined by Student’s t test (P < 0.0001).
BAL cell counts in vaccinated mice were significantly different from those in VA-βgal-treated control mice, as determined by Student’s t test (P < 0.006).
rVV were propagated in CV-1 cells and titrated on HTK cells as described previously (39). The A2 strain of human RSV was grown in fetal calf kidney cells. A single pool of virus that contained approximately 3 × 106 PFU/ml was stored in liquid nitrogen and used in all experiments.
Mice.
Six-week-old specific-pathogen-free BALB/c female mice, obtained from Charles River Breeding Laboratories, were inoculated intraperitoneally (i.p.) with 2 × 106 PFU of rVV. Serum samples were obtained on weeks 1 to 3 postinoculation by bleeding live mice from the tail vein. Three weeks postimmunization, mice were challenged intranasally (i.n.) with approximately 105 PFU of the A2 strain of RSV. Five days after challenge, groups of five mice were killed and the titer of RSV in lung homogenates was determined by a plaque assay (45). Other groups of five mice were killed and subjected to one round of bronchoalveolar lavage (BAL) as described previously (8), but with 1 ml of 12 mM lidocaine in phosphate-buffered saline. The numbers of neutrophils and eosinophils in cytocentrifuge preparations of BAL cells stained with May-Grunwald Giemsa stain were counted. Between 300 and 400 cells per mouse were examined on each slide. BAL cells for use in flow cytometric analysis were isolated in the same manner; lungs were subjected to three successive rounds of lavage, and cells from groups of five mice were pooled.
To examine the kinetics of VV clearance, groups of four mice were inoculated i.p. with 2 × 106 PFU of rVV. At intervals after virus inoculation, mice were killed with an overdose of sodium pentobarbital. Samples of liver and spleen were obtained and homogenized to yield 10 or 20% suspensions as described previously (45). Virus infectivity in tissue samples was assayed on HTK cell monolayers incubated for 48 h at 37°C. Titers of virus were expressed as log10 PFU per gram of tissue.
Flow cytometry.
Two color flow cytometric examinations of isolated BAL cells were done with rat anti-mouse CD4 coupled to fluorescein isothiocyanate (Sigma, Poole, United Kingdom) and biotinylated rat anti-mouse CD8 (Pharmingen, San Diego, Calif.) followed by streptavidin-phycoerythrin (Southern Biotechnology Associates, Birmingham, Ala.). Levels of background staining were assessed with irrelevant isotype-matched monoclonal antibody (MAb) controls (Pharmingen). Staining was analyzed on a FACScan (Becton Dickinson, Mountain View, Calif.).
Antibody assays.
The presence of antibodies to RSV was determined by an enzyme-linked immunosorbent assay (ELISA) as described previously (39). Bound antibody was detected by adding goat anti-mouse IgG serum coupled to horseradish peroxidase (Kirkegaard and Perry Laboratories Inc., Gaithersburg, Md.) or horseradish peroxidase-conjugated rabbit anti-mouse IgG1, IgG2a, or IgG2b (ICN Biomedicals Inc., Thame, United Kingdom).
Cytokine assays.
Splenocytes from mice immunized 3 weeks previously were stimulated with RSV-infected autologous splenocytes as described elsewhere (14). Supernatants were harvested on days 1 to 4 and assessed for cytokine production (see below). Cytokines present in the supernatant or cell-associated material of VV-infected cells were assessed with a cytokine-specific antigen capture ELISA. Maxisorp plates (Nunc, Roskilde, Denmark) were coated overnight with 50 μl of rat anti-mouse IL-2 MAb (JES6-1A12), rat anti-mouse IL-4 MAb (BVDV-1D11), or rat anti-mouse IFN-γ MAb (R4-6A2), (all MAbs from Pharmingen) at 2 μg/ml in 0.1 M NaHCO3 (pH 8.2). After being washed with phosphate-buffered saline containing 0.05% Tween 20 (PBSTw) the plates were blocked for 2 h with PBSTw containing 5% pig serum. Samples or standards (recombinant murine IL-2, IL-4, or IFN-γ; Pharmingen) were added and incubated for 20 h at 4°C. The plates were washed and then incubated with 100 μl of biotinylated anti-IL-2 MAb (JES6-5H4), anti-IL-4 MAb (BVDV-24G2), or anti-IFN-γ MAb (XMG1.2) (all MAbs from Pharmingen) at 1 μg/ml followed by avidin-peroxidase (Sigma) before the addition of 3,3′,5,5′-tetramethylbenzidine and hydrogen peroxide in 0.1 M sodium acetate buffer (pH 6.0) as a substrate. To determine the concentration of cytokine, mean background levels (±2.5 standard deviations [SD]) were subtracted from the mean values for triplicate samples.
CTL assays.
Spleen lymphocytes from mice immunized 4 or 5 weeks earlier with rVV were restimulated in vitro with RSV-infected splenocytes for 5 days as described previously (14) and used as a source of secondary CTL. Target cells for cytotoxicity assays were BCH4 cells, which is a BALB/c fibroblast line (H-2d) persistently infected with the Long strain of RSV (9), BALB/c fibroblasts (44), and the mouse fibroblast line L-929 (H-2k). Cell lines were used uninfected or infected with the A2 strain of RSV and were labelled with 51Cr as described previously (14). Lytic units (LU) were taken as the number of effector cells necessary to cause 33% specific lysis (44).
RESULTS
In vitro expression of the F protein and murine cytokines by rVV.
The construction of double rVV allowed insertion of the F glycoprotein or cytokine gene into either of two loci within the VV genome, namely, the thymidine kinase (TK) or VP37 locus. In order to determine whether the site of insertion and/or promoter usage influenced the levels of expression of the F protein or of the cytokines, supernatants or cell-associated antigens from CV-1 cells infected with an equivalent multiplicity of infection of different rVV were compared. Levels of expression of the F protein under the control of the p7.5 promoter (inserted into the TK locus) were two- to fivefold lower than those in constructs expressing the F protein under the control of a synthetic early or late promoter in the VP37 locus (5). Results of typical endpoint titrations are shown in Table 1. Expression of the IL-2, IL-4, or IFN-γ cytokine genes in the VP37 locus resulted in 100- to 1,000-fold more cytokine in both supernatants and cell-associated material than did expression in the TK locus (Table 1). Furthermore, the level of IFN-γ in both supernatants and cell-associated material from VRBF.mIFN-γ-infected CV-1 cells was below the level of detection in the ELISA. rVV that coexpressed the F protein and high levels of cytokines are referred to hereafter as VV-F.mIL-2high, VV-F.mIL-4high, and VV-F.mIFN-γhigh. Similarly, rVV that expressed low levels of cytokines are given the suffix “low”.
Coexpression of IL-2 or IFN-γ reduces antibody responses to RSV.
The antibody responses induced by the various rVV in mice were examined to determine whether the coexpression of murine cytokines with the F protein could influence antibody responses to RSV. Groups of mice inoculated 3 to 4 weeks previously with rVV expressing the F protein either alone or together with murine cytokines developed RSV-specific antibody, with endpoint titers ranging from log10 3 to 5 (Table 1). Antibody responses induced with VV-F.mIL-4high or VV-F.mIL-4low had titers similar to that induced by VSCF, but significantly lower titers were obtained in mice vaccinated with rVV expressing the F protein together with IL-2 or IFN-γ.
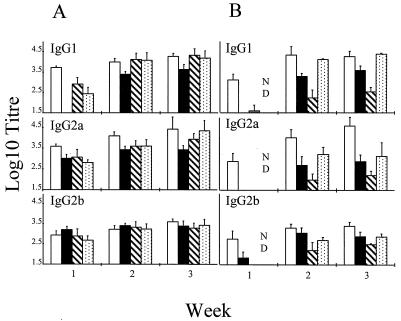
In order to determine the effect of cytokines on the isotype of RSV-specific antibody, sera were collected from immunized mice on a weekly basis and examined by an ELISA with isotype-specific secondary reagents. Mice inoculated with VRBF developed high levels of IgG1 and IgG2a antibodies 1 week after immunization (Fig. 1A). Mice that received rVV expressing F and low levels of IL-2 (VRBF.mIL-2) exhibited a delayed and reduced IgG1 antibody response which was undetectable 1 week after vaccination and which had a titer fourfold lower than that in controls vaccinated with VRBF after 3 weeks. IgG1 antibody titers were also reduced 7 days postvaccination in mice given rVV coexpressing F and low levels of either IFN-γ or IL-4, whereas titers of IgG2a and IgG2b antibodies were similar to those detected in mice vaccinated with VRBF. There was no significant difference in antibody titers by 3 weeks postvaccination (Fig. 1A).
FIG. 1.
Effect of cytokines on isotype-specific serum
antibody responses to RSV. Mice were vaccinated with (A) VRBF (□),
VV-F.mIL-2low (▪), VVF.mIFN-γlow
( ), or VV-F.mIL-4low (
) or
with (B) VSCF (□), VV-F.mIL-2high (▪), VV-F.mIFN-γhigh
(
), or VV-F.mIL-4low (
) or
with (B) VSCF (□), VV-F.mIL-2high (▪), VV-F.mIFN-γhigh
( ), or VV-F.mIL-4high (
). ND,
Not determined. Data are given as log10 mean antibody
titers ± SD.
), or VV-F.mIL-4high (
). ND,
Not determined. Data are given as log10 mean antibody
titers ± SD.
More dramatic effects on antibody responses were observed for mice inoculated with rVV expressing higher levels of murine cytokines from the VP37 locus (Fig. 1B). Thus, 7 days after vaccination with either VV-F.mIL-2high or VV-F.mIFN-γhigh, little or no IgG1, IgG2a, or IgG2b serum antibody was detected, whereas high levels of all three isotypes were detected in sera from VSCF-vaccinated mice. Furthermore, titers of all antibody isotypes were significantly lower in mice given VV-F.mIL-2high and VV-F.mIFN-γhigh than in those given VSCF 3 weeks after vaccination. Serum samples were not taken in the first week from mice immunized with VV-F.mIL-4high. A mortality of 100% was observed for mice immunized with VRBmIL-4, and up to 20% mortality was observed for some groups of mice immunized with VV-F.mIL-4high. These mice appeared ill by 7 days, with a ruffled coat and peritoneal edema, but the majority made a good recovery and showed no signs of ill health 9 to 11 days after immunization. This outcome contrasted with that for mice immunized with rVV expressing low levels of IL-4 (VRBF.mIL-4), which showed no ill effects. There were no significant differences in IgG1 antibody titers in sera from mice vaccinated with VSCF or VV-F.mIL-4high 3 weeks after inoculation. However, a significant reduction in IgG2a antibody titers was detected 2 weeks after vaccination with VV-F.mIL-4high, and IgG2a antibody titers were still significantly lower than those in mice vaccinated with VSCF 3 weeks previously (Fig. 1B).
Although there was evidence that higher levels of the F protein were expressed from the VP37 locus than from the TK locus (Table 1), there were no significant differences in IgG1, IgG2a, or IgG2b serum antibody responses in mice vaccinated with either VSCF or VRBF. These findings indicate that differences in RSV-specific antibody isotypes were due to the effects of the different levels of cytokines produced by the rVV and did not simply reflect differences in the level of expression of the F protein by the recombinant viruses. We therefore chose to further characterize the immune responses elicited by rVV that expressed high levels of cytokines.
High levels of IL-2 or IFN-γ attenuate rVV replication, whereas high levels of IL-4 increase virulence in euthymic mice.
In order to determine the effect of high levels of cytokines on the replication of rVV, the virulence of VV-F.mIL-2high, VV-F.mIFN-γhigh, and VV-F.mIL-4high was compared with that of VSCF after i.p. immunization of mice with 2 × 106 PFU of the various rVV. Samples of liver and spleen were removed from groups of four mice on days 1, 2, 3, 4, and 9 after immunization. Virus was recovered from the spleens of VSCF-immunized mice on days 1 to 4 and from the livers on days 1 and 2 and was cleared from these tissues by day 9 (Table 2). In contrast, virus was recovered from the spleens of three of four VV-F.mIL-2high-immunized mice on day 2 and two of four mice on day 3 but not from the livers at any time after vaccination. Virus was not recovered from the spleens after day 3. Replication of VV-F.mIFN-γhigh appeared to be severely restricted in vivo, as virus was recovered only from the spleen of one of four mice inoculated 24 h previously (Table 2). Although the levels of virus recovered from the spleens of VV-F.mIL-4high-immunized mice were similar to those recovered from the spleens of VSCF-immunized mice (Table 2), the levels of virus recovered from the livers of VV-F.mIL-4high-immunized mice were 10-fold higher. Furthermore, clearance of VV-F.mIL-4high might have been delayed compared with that of VSCF, as virus was still detectable in one of four mice 9 days after inoculation (Table 2). Thus, the resolution of rVV infection was delayed in mice given virus carrying the gene encoding IL-4, whereas rVV carrying the gene encoding IL-2 or IFN-γ was cleared more rapidly from the spleen and liver than was VSCF.
TABLE 2.
Replication of VSCF, VV-F.mIL-2high, VV-F.mIL-4high, and VV-F.mIFN-γhigh in spleens and livers after i.p. inoculation of groups of four BALB/c mice with approximately 2 × 106 PFU of virus
| Day | Mean ± SD
log10 PFU of the indicated virus/g (no. of infected mice)
in:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Spleen
|
Liver
|
|||||||
| VSCF | VV-F.mIL-2 | VV-F.mIFN-γ | VV-F.mIL-4 | VSCF | VV-F.mIL-2 | VV-F.mIFN-γ | VV-F.mIL-4 | |
| 1 | 3.4 ± 0.9 (4) | <1.7 (0) | 2.5 (1) | 2.9 ± 0.4 (3) | 2.9 ± 0.2 (4) | <1.4 (0) | <1.4 (0) | 4.5 (1) |
| 2 | 3.5 ± 0.5 (4) | 2.6 ± 0.4 (3) | <1.7 (0) | 4.2 ± 0.6 (4) | 3.0 ± 0.2 (2) | <1.4 (0) | <1.4 (0) | 2.9 ± 0 (3) |
| 3 | 4.0 ± 0.4 (3) | 2.8 ± 0.4 (2) | <1.7 (0) | 3.7 ± 0.8 (4) | <1.4 (0) | <1.4 (0) | <1.4 (0) | 4.3 ± 0.8 (4) |
| 4 | 3.5 ± 0.5 (4) | <1.7 (0) | <1.7 (0) | 3.6 ± 0.7 (4) | <1.4 (0) | <1.4 (0) | <1.4 (0) | 4.0 ± 0.7 (4) |
| 9 | <1.7 (0) | <1.7 (0) | <1.7 (0) | 2.48 (1) | <1.4 (0) | <1.4 (0) | <1.4 (0) | 2.5 (1) |
Priming of RSV-specific CTL memory is inhibited by rVV expressing high levels of IL-2 and IFN-γ but not by rVV expressing high levels of IL-4.
Studies with rVV expressing individual RSV proteins have shown that the F protein primes BALB/c mice for RSV-specific CTL (2, 29). In order to investigate whether CTL priming by the F protein could be influenced by the cytokines IL-2, IL-4, and IFN-γ, secondary CTL were generated from splenocytes of mice immunized with rVV expressing F together with high levels of cytokines and stimulated with RSV in vitro. In order to estimate the relative RSV-specific CTL activities in spleens from mice vaccinated with the different recombinants, LU/106 cells were calculated.
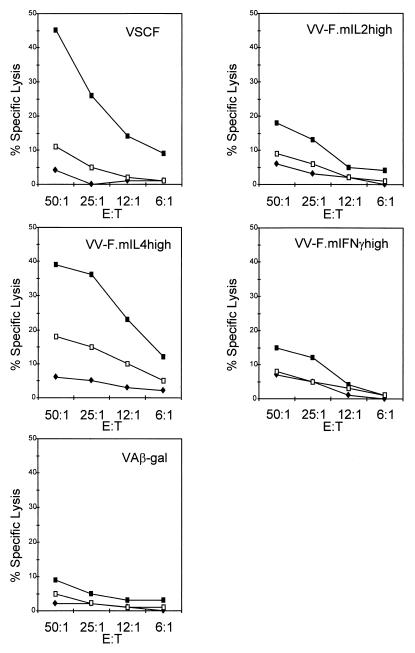
Lymphocytes from mice immunized with VSCF specifically lysed BCH4 cells (H-2d) and RSV-infected BALB/c fibroblasts (data not shown) but not uninfected BALB/c fibroblasts or virus-infected, major histocompatibility complex-mismatched L-929 cells (H-2k) (Fig. 2) and contained 3.3 LU/106 cells. A similar level of RSV-specific CTL activity was observed in splenocytes from VV-F.mIL-4high-immunized mice (3.8 LU/106 cells). However, in comparison with the level of RSV-specific CTL activity in VSCF-immunized mice, the level of CTL activity in mice vaccinated with VV-F.mIL-2high or VV-F.mIFN-γhigh was significantly reduced (Fig. 2) (0.2 LU/106 cells). Nevertheless, the level of CTL activity in splenocytes from these mice was higher than that observed in splenocytes from mice vaccinated with rVV expressing β-galactosidase (VA-βgal) (0.0004 LU/106 cells) (Fig. 2).
FIG. 2.
Effect of cytokines on priming of RSV-specific CTL. Shown is the cytotoxic activity of splenocytes from mice immunized with VSCF, VV-F.mIL-2high, VV-F.mIL-4high, VV-F.mIFN-γhigh, or VA-βgal (VAβ-gal) at different effector cell/target cell (E:T) ratios on BCH4 cells (H-2d) (▪), RSV-infected L-929 cells (H-2k) (□), or uninfected BALB/c fibroblasts (⧫).
Cytokine production is reduced by RSV-specific memory T cells from mice vaccinated with rVV expressing IL-2 or IFN-γ but not IL-4.
In order to study the effect of coexpressed cytokines on Th priming, the levels of cytokines produced by isolated immune splenocytes following in vitro restimulation with RSV were analyzed (Table 3). IL-2 was detectable in all cultures and peaked at 72 h. The highest levels were produced by lymphocytes from VSCF-primed mice, although similar levels were also found in supernatants of restimulated splenocytes from mice primed with VV-F.mIL-2high, VV-F.mIL-4high, and VV-F.mIFN-γhigh. IFN-γ was detectable in supernatants from all restimulated splenocyte cultures and was still increasing on day 4. The highest levels were found in culture supernatants from VSCF-primed mice, and the lowest levels were found in supernatants from mice primed with VV-F.mIL-2high and VA-βgal. VV-F.mIL-2high-primed mice produced almost 8-fold less IFN-γ than did VSCF-primed mice, while VV-F.mIFN-γhigh-primed mice produced only 2.5-fold less. Surprisingly, the level of IFN-γ produced by lymphocytes from mice vaccinated with VV-F.mIL-4high was similar to that produced by lymphocytes from VSCF-primed mice. IL-10 was also found in all culture supernatants, and levels were still increasing on day 4. The highest levels of IL-10 were produced by mice vaccinated with VSCF or VV-F.mIL-4high, and the concentrations were approximately six- to eightfold higher than those produced by mice primed with VV-F.mIL-2high or VV-F.mIFN-γhigh. Only low levels of IL-10 and IL-2 were detected in splenocyte supernatants from VA-βgal-vaccinated mice. IL-4 and IL-5 were not detected in supernatants from any of the lymphocyte cultures.
TABLE 3.
Peak levels of cytokine production from in vitro-restimulated immune splenocytesa
| Vaccine | Production (pg/ml) of:
|
||||
|---|---|---|---|---|---|
| IL-2 (72 h) | IL-4 | IL-5 | IL-10 (96 h) | IFN-γ (96 h) | |
| VSCF | 227 ± 6 | <15 | <10 | 2,423 ± 100 | 24,774 ± 695 |
| VV-F.mIL-2high | 163 ± 1 | <15 | <10 | 411 ± 10 | 3,241 ± 315 |
| VV-F.mIL-4high | 209 ± 6 | <15 | <10 | 2,273 ± 209 | 25,513 ± 1,413 |
| VV-F.mIFN-γhigh | 203 ± 10 | <15 | <10 | 314 ± 10 | 9,641 ± 362 |
| VA-βgal | 118 ± 10 | <15 | <10 | 78 ± 9 | 4,097 ± 174 |
Spleens were removed from mice 3 to 4 weeks after immunization with rVV and restimulated with gamma-irradiated RSV-infected (multiplicity of infection, 1) autologous splenocytes at a 5:1 effector cell/stimulator cell ratio. Supernatants were harvested on a daily basis and assessed for cytokines by an ELISA. Results are given as mean ± SD.
Ability of rVV to protect against RSV challenge.
Protection against RSV challenge was examined 4 weeks after immunization with the various rVV (Table 1). All rVV expressing the F protein of RSV induced protection against a subsequent RSV infection, as virus was not recovered from the lungs of any of the vaccinated mice 5 days after RSV challenge (Table 1). In order to determine if the high levels of cytokines produced by rVV affected RSV titers after challenge, mice were immunized with rVV that did not express the F protein, namely, VRBmIL-2 or VRBmIFN-γ, and challenged. These mice were not protected against RSV infection, and titers of RSV recovered from the lungs of these mice were similar to those in the control (VA-βgal-treated) group (Table 1).
Vaccination with rVV expressing F together with high levels of IL-2, IL-4, or IFN-γ does not have a major impact on the pulmonary inflammatory response after RSV challenge.
To determine the influence of the cytokines IL-2, IL-4, and IFN-γ on the pulmonary cell responses of vaccinated mice 5 days after RSV challenge, animals were subjected to repeated BAL. The numbers of leukocytes recovered from the lungs of mice immunized with VSCF, VV-F.mIL-2high, VV-F.mIL-4high, and VV-F.mIFN-γhigh were increased 5 days after RSV challenge compared with those in mice primed with VA-βgal (Table 1). Analysis of BAL showed that VSCF-vaccinated mice developed neutrophil (PMN) efflux into the lungs (median value, 39 × 103/ml). PMN efflux also occurred in groups of mice inoculated with VV-F.mIL-2high (88 × 103/ml), VV-F.mIL-4high (45 × 103/ml), and VV-F.mIFN-γhigh (52 × 103/ml). Fewer leukocytes were present in BAL from mice immunized with VA-βgal, and this finding was reflected in the decreased numbers of neutrophils in the lungs (18 × 103/ml). Few eosinophils (<1%) were present in BAL from any of the mice.
The effect of immunization with rVV that coexpressed IL-2, IL-4, or IFN-γ with the F protein on the recruitment of CD4+ and CD8+ subsets into the lungs 5 days after RSV challenge was examined by flow cytometry. Although there was a slight reduction in the number of CD4+ cells in BAL from mice immunized with VV-F.mIFN-γhigh or VV-F.mIL-2high 5 days after RSV challenge compared with the number recovered in BAL from VSCF- or VV-F.mIL-4high-immunized mice, there was no significant change in the ratio of CD4+ to CD8+ T cells, and CD8+ T-cell numbers always exceeded CD4+ T-cell numbers (Table 4).
TABLE 4.
Analysis of BAL lymphocyte subsets 5 days after RSV challengea
| Vaccineb | No. of cells/ml
(103)
|
|||
|---|---|---|---|---|
| CD4− CD8− | CD4+ | CD8+ | CD4+ CD8+ | |
| VSCF | 493 | 535 | 779 | 0.7 |
| VV-F.mIL-2high | 246 | 208 | 311 | 0.7 |
| VV-F.mIL-4high | 378 | 330 | 453 | 0.7 |
| VV-F.mIFN-γhigh | 360 | 200 | 407 | 0.5 |
| VA-βgal | 105 | 198 | 178 | 1 |
Cells obtained from repeated BAL of groups of five mice were pooled, and total cells were counted by light microscopy. Cells were stained for CD4+ and CD8+ and analyzed by flow cytometry. The percentage of cells displaying CD4+ or CD8+ was converted into thousands of cells per group of five mice.
Mice were immunized i.p. with 2 × 106 PFU of rVV and challenged i.n. with RSV 3 weeks after vaccination.
DISCUSSION
Immunization of mice with rVV that coexpressed the genes for murine IL-2, IL-4, or IFN-γ and the F protein of RSV allowed us to investigate the influence of these factors on the development of immunity to RSV. The data presented here show that high levels of cytokine expression by rVV containing the gene for IL-2 or IFN-γ in the VP37 locus markedly reduced the replication of the rVV in euthymic mice. This reduction in virus replication resulted in a reduction in all RSV-specific antibody isotypes, a marked reduction in CTL priming, and a reduction in IFN-γ and IL-10 production. In contrast, although IL-4 biased the RSV-specific antibody response in favor of IgG1, priming of RSV-specific CTL and IFN-γ production were not adversely affected.
Previous studies demonstrated that an rVV expressing murine IL-2 was less virulent in immunodeficient mice than a control rVV but did not show any significant attenuation based on the kinetics of virus clearance in euthymic mice (30). However, our findings that IL-2 and IFN-γ resulted in more rapid clearance of rVV from the spleen and liver are similar to those reported previously, where IL-2 and IFN-γ enhanced the clearance of rVV from the ovaries of euthymic mice (17, 18). In contrast, inclusion of the gene encoding IL-4 resulted in greater replication and delayed clearance of rVV from the liver and spleen. This observation is similar to those reported previously, where clearance of rVV from the ovaries and clearance of influenza virus from the lungs were delayed by IL-4 (24, 35).
Infection of mice with rVV expressing IL-4 appears to be lethal in female but not in male CBA/H mice, with a mean time to death of 5 to 7 days (4). This difference in susceptibility to infection has been attributed to the high titers of rVV recovered from the ovaries of infected mice (17). However, others have reported that CBA/H mice are able to clear rVV expressing IL-4 (35). We found that VRBmIL-4 (high levels of IL-4) was lethal in female BALB/c mice. This virus is thymidine kinase positive and is therefore likely to grow to higher titers than VV-F.mIL-4high, which is thymidine kinase negative but which expresses a level of IL-4 similar to that expressed by VRBmIL-4 in vitro. The observed peritoneal edema in mice immunized with an rVV expressing IL-4 (VV-F.mIL-4high) is similar to that reported elsewhere (4); however, we observed no adverse effects or signs of peritonitis in mice immunized with VV-F.mIL-4low. This vascular leak syndrome is similar to that seen following the systemic administration of recombinant murine IL-2 (32), and we also saw increased peritoneal swelling in mice immunized with VV-F.mIL-2high but not in mice immunized with VV-F.mIL-2low. These observations suggest that the high levels of cytokine production in VV-F.mIL-2high, VV-F.mIL-4high, and VV-F.mIFN-γhigh compared with VV-F.mIL-2low, VV-F.mIL-4low, and VV-F.mIFN-γlow act in a systemic rather than a localized fashion.
It is well known that lymphokines can control immunoglobulin isotype selection in vivo. IFN-γ has been shown to enhance IgG2a and inhibit IgG1 production (10, 11). In contrast, IL-4 can induce activated B lymphocytes to secrete IgG1 and IgE (37, 46). Although immunization with rVV expressing F together with low levels of IL-2 or IFN-γ (VRBF.mIL-2 or VRBF.mIFN-γ) induced significantly lower titers of RSV-specific antibody than did immunization with VRBF, we did not find the preferential induction of any particular isotype. Furthermore, antibody titers and isotypes induced by VV-F.mIL-4low- or VRBF-immunized mice were similar. These findings are broadly in agreement with other published data, where the genes encoding IL-2, IL-4, and IFN-γ were inserted into the TK locus (18, 35), but are in contrast to the more dramatic reduction in serum antibody responses to RSV induced by rVV expressing F together with high levels of IL-2 or IFN-γ (VV-F.mIL-2high or VV-F.mIFN-γ high, respectively), where the cytokine genes were inserted into the VP37 locus. However, the reduction in antibody responses was not isotype specific. In contrast, high levels of IL-4 (VV-F.mIL-4high) resulted in a preferential induction of IgG1 antibody. Thus, although the IgG1 titer was similar to that in sera from VSCF-immunized mice, the IgG2a titer was significantly reduced in animals vaccinated with VV-F.mIL-4high. The abilities of IL-4 to induce IgG1 antibody and to inhibit IgG2a antibody in VV-F.mIL-4high-immunized mice but not in VV-F.mIL-4low-immunized mice suggest that high levels of IL-4 are required to exert an effect on B-cell responses during infection with VV.
Although IL-4 biased the antibody isotype in favor of IgG1, therefore reflecting a Th2-like immune response, few eosinophils were detected in the BAL of VV-F.mIL-4high-vaccinated mice following RSV challenge. Cytokines in culture supernatants from spleen cells of mice scarified with rVV expressing the G protein of RSV are typically Th2-like following restimulation with RSV in vitro, and large numbers of eosinophils are present in the BAL following RSV challenge (1, 27). However, the route of vaccination is critical to inducing a pulmonary eosinophilic response following RSV challenge (4a). Hence, vaccination by the i.p. route with rVV expressing the G protein does not result in an eosinophilic response, whereas scarification with the same rVV does. The abilities of VV-F.mIL-4high to induce a Th2-like immune response to the F protein when mice are vaccinated by dermal scarification and to induce a pulmonary eosinophilic response after RSV challenge are currently under investigation.
Perhaps surprisingly, the priming of RSV-specific CTL was not impaired in mice vaccinated with VV-F.mIL-4high. It has been reported that the VV-specific precursor CTL frequency in spleens determined 6 days after infection of mice with an rVV expressing IL-4 was 12-fold lower than that in mice infected with a control rVV and remained suppressed throughout the course of the infection (35). The expression of IL-2, IL-12, and IFN-γ mRNAs was also reduced during the acute phase of rVV infection in these mice (35). This down-regulation of Th1 cytokines and antiviral CTL responses is a likely explanation for the delayed rVV clearance and increased virus titers in mice immunized with VV-F.mIL-4high. However, our findings suggest that the development of Th1 and CTL memory for a coexpressed F protein is not impaired by IL-4 at the time of vaccination. Delayed virus clearance and reduced CTL activity were also observed for IL-4-treated mice during primary influenza virus infection (24) and for mice that constitutively overexpressed IL-4 during RSV infection (12). Our experimental design differs in one significant respect from those of other groups, who assayed CTL activity while virus-expressed or exogenous IL-4 was still present during an acute viral infection (24, 35). We assayed CTL activity from a memory rather than an effector cell population at a time when virus-expressed IL-4 was no longer present in the system. The ability of secondary CTL to lyse RSV-infected target cells in vitro may not necessarily correspond to their ability to clear virus in vivo. CTL generated from influenza virus-infected mice and restimulated in vitro in the presence of IL-4 retained their ability to lyse appropriate targets in vitro but did not clear virus following adoptive transfer to influenza virus-infected mice (24). The ability of RSV-specific CTL from VV-F.mIL-4high-primed mice to clear virus as readily as CTL from VSCF-primed mice following adoptive transfer into infected recipients is yet to be tested.
Splenocytes from VSCF-immunized mice restimulated with RSV-infected autologous splenocytes produce an array of cytokines, including IL-2, IL-3, IL-4, and IL-5, although the latter two are produced at significantly lower levels than those produced by splenocytes from mice vaccinated with an rVV expressing the G protein of RSV (1). We found that the predominant cytokines detectable in supernatants from splenocytes of mice vaccinated with rVV expressing the F protein and restimulated in vitro with RSV were IL-2, IL-10, and IFN-γ. These findings are essentially the same as those reported by Srikiatkhachorn and Braciale (38). However, those investigators found that the effector T-lymphocyte population exhibits a cytokine profile different from that of memory T-lymphocyte precursors (38). We are therefore currently investigating the cytokine profile of restimulated splenocytes obtained from VV-F.mIL-4high-immunized mice 5 days after i.n. challenge with RSV to determine whether the coexpression of IL-4 in rVV can prime for an effector Th2-like response following RSV challenge.
Data obtained from this study indicate that although a Th2 cytokine (IL-4) present at the time of acute rVV infection resulted in delayed virus clearance, there was no effect on the development of RSV-specific memory CTL or IFN-γ production, and mice were resistant to subsequent RSV challenge. It therefore appears that the coexpression of IL-4 with the F protein in a live-virus vector delivery system (rVV) failed to subvert the Th1 memory response on subsequent exposure to RSV. Further studies are in progress to examine the ability of coexpressed cytokines to influence the response to rVV expressing the G protein of RSV, a protein known to induce a Th2-like immune response. These studies suggest that the changes observed in the vaccine-induced immune response are the result of an altered antigen load due to differences in viral vector replication, rather than of a direct effect of the cytokines on the developing immune response. In order to determine if the immune response to the F or G protein of RSV can be influenced by cytokines, further studies with plasmids carrying RSV F or G protein and cytokine genes are currently in progress.
ACKNOWLEDGMENTS
This work was supported by the European Union (grant PL920489) and the Ministry of Agriculture, Fisheries and Food.
REFERENCES
- 1.Alwan W H, Openshaw P J. Distinct patterns of T- and B-cell immunity to respiratory syncytial virus induced by individual viral proteins. Vaccine. 1993;11:431–437. doi: 10.1016/0264-410x(93)90284-5. [DOI] [PubMed] [Google Scholar]
- 2.Alwan W H, Record F M, Openshaw P J M. Phenotypic and functional characterization of T cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993;150:5211–5218. [PubMed] [Google Scholar]
- 3.Alwan W H, Record F M, Openshaw P J M. CD4+ T-cells clear virus but augment disease in mice infected with respiratory syncytial virus. Comparison with the effects of CD8+ T-cells. Clin Exp Immunol. 1992;88:527–536. doi: 10.1111/j.1365-2249.1992.tb06482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrew M E, Coupar B E H. Biological effects of recombinant vaccinia virus-expressed interleukin 4. Cytokine. 1992;4:281–286. doi: 10.1016/1043-4666(92)90068-3. [DOI] [PubMed] [Google Scholar]
- 4a.Bembridge, G. P., et al. Unpublished data.
- 5.Blasco R, Moss B. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene. 1995;158:157–162. doi: 10.1016/0378-1119(95)00149-z. [DOI] [PubMed] [Google Scholar]
- 6.Bradley L M, Dalton D K, Croft M. A direct role for IFN-gamma in regulation of Th1 cell development. J Immunol. 1996;157:1350–1358. [PubMed] [Google Scholar]
- 7.Cristina J, Lopez J A, Albo C, Garcia-Barreno B, Garcia J, Melero J A, Portela A. Analysis of genetic-variability in human respiratory syncytial virus by the RNase A mismatch cleavage method—subtype divergence and heterogeneity. Virology. 1990;174:126–134. doi: 10.1016/0042-6822(90)90061-u. [DOI] [PubMed] [Google Scholar]
- 8.Denny F W, Taylor-Robinson D, Allison A C. The role of the thymus-dependent immunity in Mycoplasma pulmonis infections of mice. J Med Microbiol. 1972;5:327–335. doi: 10.1099/00222615-5-3-327. [DOI] [PubMed] [Google Scholar]
- 9.Fernie B F, Ford E C, Gerin J L. The development of BALB/c cells persistently infected with respiratory syncytial virus: presence of ribonucleoprotein on the cell surface. Proc Soc Exp Biol Med. 1981;167:83–86. doi: 10.3181/00379727-167-41129. [DOI] [PubMed] [Google Scholar]
- 10.Finkelman F D, Holmes J. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 11.Finkelman F D, Katona I M, Mosmann T R, Coffman R L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 1988;140:1022–1027. [PubMed] [Google Scholar]
- 12.Fischer J E, Johnson J E, Kuli-Zade R K, Johnson T R, Aung S, Parker R A, Graham B S. Overexpression of interleukin-4 delays virus clearance in mice infected with respiratory syncytial virus. J Virol. 1997;71:8672–8677. doi: 10.1128/jvi.71.11.8672-8677.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Florentino D F, Bond M W, Mosmann T R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaddum R M, Cook R S, Wyld S G, Lopez J A, Bustos R, Melero J A, Taylor G. Mutant forms of the F protein of human respiratory syncytial (RS) virus induce a cytotoxic T lymphocyte response but not a neutralizing antibody response and only transient resistance to RS virus infection. J Gen Virol. 1996;77:1239–1248. doi: 10.1099/0022-1317-77-6-1239. [DOI] [PubMed] [Google Scholar]
- 15.Graham B S, Henderson G S, Tang Y, Lu X, Neuzil K M, Colley D G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993;151:2032–2040. [PubMed] [Google Scholar]
- 16.Hsieh C-S, Macatonia S E, Tripp C S, Wolf S F, O’Garra A, Murphy K M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 17.Karupiah G, Coupar B, Ramshaw I, Boyle D, Blanden R, Andrew M. Vaccinia virus-mediated damage of murine ovaries and protection by virus-expressed interleukin-2. Immunol Cell Biol. 1990;68:325–333. doi: 10.1038/icb.1990.44. [DOI] [PubMed] [Google Scholar]
- 18.Kohonen-Corish M R J, King N J C, Woodhams C E, Ramshaw I A. Immunodeficient mice recover from infection with vaccinia virus expressing interferon-gamma. Eur J Immunol. 1990;20:157–161. doi: 10.1002/eji.1830200123. [DOI] [PubMed] [Google Scholar]
- 19.Lee F, Yokota T, Otsuka T, Meyerson P, Villaret D, Coffman R, Mosmann T, Rennick D, Roehm N, Smith N, Zlotnik A, Arai K-I. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Nat Acad Sci USA. 1986;83:2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Gros G, Ben-Sasson S Z, Seder R, Finkelman F D, Paul W E. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopez J A, Bustos R, Portela A, Garcia-Barreno B, Melero J A. A point mutation in the F1 subunit of human respiratory syncytial virus blocks its cell surface transport at an early stage of the exocytic pathway. J Gen Virol. 1996;77:649–660. doi: 10.1099/0022-1317-77-4-649. [DOI] [PubMed] [Google Scholar]
- 22.Matyniak J E, Reiner S L. T helper phenotype and genetic susceptibility in experimental Lyme disease. J Exp Med. 1995;181:1251–1254. doi: 10.1084/jem.181.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore K W, O’Garra A, de Waal Maldfyt R, Vieira P, Mosmann T R. Interleukin 10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 24.Moran T M, Isobe H, Fernandez-Sesma A, Schulman J L. Interleukin-4 causes delayed virus clearance in influenza virus-infected mice. J Virol. 1996;70:5230–5235. doi: 10.1128/jvi.70.8.5230-5235.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosmann T R, Cherwinski H, Bond M W, Gredlin M A, Coffman R L. Two types of murine helper T cell clones. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 26.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 27.Openshaw P J, Clarke S L, Record F M. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int Immunol. 1992;4:493–500. doi: 10.1093/intimm/4.4.493. [DOI] [PubMed] [Google Scholar]
- 28.Paul W E, Seder R A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 29.Pemberton R M, Cannon M J, Openshaw P J M, Ball L A, Wertz G W, Askonas B A. Cytotoxic T-cell specificity for respiratory syncytial virus proteins—fusion protein is an important target antigen. J Gen Virol. 1987;68:2177–2182. doi: 10.1099/0022-1317-68-8-2177. [DOI] [PubMed] [Google Scholar]
- 30.Ramshaw I A, Andrew M E, Phillips S M, Boyle D B, Coupar B E H. Recovery of immunodeficient mice from a vaccinia virus/IL-2 recombinant infection. Nature. 1987;329:545–546. doi: 10.1038/329545a0. [DOI] [PubMed] [Google Scholar]
- 31.Romani L, Mencacci A, Cenci E, Spaccapelo R, Mosci P, Puccetti P, Bistoni F. CD4+ subset expression in murine candidiasis. J Immunol. 1993;150:925–931. [PubMed] [Google Scholar]
- 32.Rosenstein M, Ettinghausen S E, Rosenberg S A. Extravasation of intravascular fluid mediated by the systemic administration of recombinant interleukin 2. J Immunol. 1986;137:1735–1742. [PubMed] [Google Scholar]
- 33.Schmitt E, Van Brandwijk R, Fischer H-G, Rude E. Establishment of different T cell sublines using either interleukin 2 or interleukin 4 as growth factors. Eur J Immunol. 1990;20:1709–1715. doi: 10.1002/eji.1830200813. [DOI] [PubMed] [Google Scholar]
- 34.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 35.Sharma D P, Ramsay A J, Maguire D J, Rolph M S, Ramshaw I A. Interleukin-4 mediates down regulation of antiviral cytokine expression and cytotoxic T-lymphocyte responses and exacerbates vaccinia virus infection in vivo. J Virol. 1996;70:7103–7107. doi: 10.1128/jvi.70.10.7103-7107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sher A, Coffman R L. Regulation of immunity to parasites by T cell and T-cell derived cytokines. Annu Rev Immunol. 1992;10:385–409. doi: 10.1146/annurev.iy.10.040192.002125. [DOI] [PubMed] [Google Scholar]
- 37.Snapper C M, Finkelman F D, Paul W E. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J Exp Med. 1988;167:183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srikiatkhachorn A, Braciale T J. Virus-specific memory and effector T lymphocytes exhibit different cytokine responses to antigens during experimental murine respiratory syncytial virus infection. J Virol. 1997;71:678–685. doi: 10.1128/jvi.71.1.678-685.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stott E J, Taylor G, Ball L A, Anderson K, Young K K-Y, King A M Q, Wertz G W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J Virol. 1987;61:3855–3861. doi: 10.1128/jvi.61.12.3855-3861.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Street N E, Schumacher J H, Fong T A, Bass H, Florentino D F, Leverah J A, Mosmann T R. Heterogeneity of mouse helper T cells: evidence from bulk cultures and limiting dilution cloning for precursors of TH1 and TH2 cells. J Immunol. 1990;144:1629–1639. [PubMed] [Google Scholar]
- 41.Swain S L, Weinberg A D, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 42.Tang Y, Graham B S. Anti-IL-4 treatment at immunization modulates cytokine expression, reduces illness, and increases cytotoxic T lymphocyte activity in mice challenged with respiratory syncytial virus. J Clin Invest. 1994;94:1953–1958. doi: 10.1172/JCI117546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Y W, Graham B S. Interleukin-12 treatment during immunization elicits a T-helper cell type 1-like immune response in mice challenged with respiratory syncytial virus and improves vaccine immunogenicity. J Infect Dis. 1995;172:734–738. doi: 10.1093/infdis/172.3.734. [DOI] [PubMed] [Google Scholar]
- 44.Taylor G, Stott E J, Hayle A J. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J Gen Virol. 1985;66:2533–2538. doi: 10.1099/0022-1317-66-12-2533. [DOI] [PubMed] [Google Scholar]
- 45.Taylor G, Stott E J, Hughes M, Collins A P. Respiratory syncytial virus infection in mice. Infect Immun. 1984;43:649–655. doi: 10.1128/iai.43.2.649-655.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitetta E S, Ohara J, Myers C, Layton J, Krammer P H, Paul W E. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B-cell differentiation factor for IgG1. J Exp Med. 1985;161:1726–1731. doi: 10.1084/jem.162.5.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yokota T, Arai N, Lee F, Rennick D, Mosmann T, Arai K-I. Use of a cDNA expression vector for isolation of mouse interleukin-2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci USA. 1985;82:68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]