Abstract
Several viral determinants were shown to play a role in the ability of human immunodeficiency virus type 1 (HIV-1) to infect nondividing cells. In particular, Vpr and Gag matrix (MA) were recognized to be involved in the nuclear transport of the viral preintegration complex. The goal of the present study was to evaluate the ability of isogenic HIV-1 viruses harboring different vpr and gag genes to infect nondividing cells. Surprisingly, our results reveal that the introduction of mutations in the MA nuclear localization signal marginally affected the ability of proviruses to establish infection in growth-arrested HeLa or MT4 cells. In contrast, we show that in our experimental system, the absence of Vpr expression leads to a reduction in viral infectivity and production which correlates with a decrease in the synthesis and nuclear transport of proviral DNA as determined by PCR analysis. Moreover, our data demonstrate that this reduction of viral replication is also observed with proviruses containing different mutated Vpr alleles. In particular, the Vpr Q65E mutant, which contains a substitution in the second predicted amphipathic alpha-helical structure located in the central region of the protein, is associated with an impairment of the protein nuclear localization and a concomitant reduction of the nuclear transport of proviral DNA. The results of this study provide evidence that a putative amphipathic alpha-helical structure in the central region of Vpr contains a determinant involved in the nuclear translocation of the preintegration complex in nondividing cells.
In contrast to onco-retroviruses, the lentivirus human immunodeficiency virus type 1 (HIV-1) has the capacity to infect nondividing cells (14, 36). HIV-1 infection of nondividing cells, such as quiescent T lymphocytes and terminally differentiated dendritic macrophages and microglial cells, is believed to play an important role in the establishment and evolution of HIV-1-related diseases (46, 57).
The in vitro establishment of productive infection in nonproliferating cells was demonstrated to be dependent on the physiological status of the cells. Indeed, cells arrested at different phases of the cell cycle, including HeLa cells growth arrested at the G2/M phase (35) and primary monocyte-derived macrophages (MDM) or activated T lymphocytes growth arrested at the G1/S phase (3, 47), could sustain HIV-1 viral production. In contrast, quiescent T lymphocytes and G1-arrested MDM are refractory to productive infection. The absence of viral production in these systems results from a block of the viral replicative cycle at levels which differ between studies. These included a block at the level of proviral DNA synthesis (47, 60, 62), proviral DNA transport into the cell nucleus (49), or viral gene expression (48). In metabolically inactive quiescent T lymphocytes, the low levels of ribonucleotide reductase activity obtained by limiting the deoxynucleotide pool could participate in the restriction of the de novo reverse transcription of the viral RNA (23).
The HIV-1 proviral DNA is transported into the nuclei of infected cells as part of a large complex comprised of viral nucleic acids and proteins, including integrase, reverse transcriptase (RT), viral protein R (Vpr), matrix (MA), and nucleocapsid (4, 22). In cells which do not go through mitosis, the nuclear transport of the large viral preintegration complex (PIC) is an active process which is independent of nuclear membrane disassembly and at such constitutes a limiting step in viral replication. The presence of a nuclear transport signal in the basic region of the MA protein has been reported to enable the virus to actively transport its PIC into the nuclei of nondividing cells (2). Moreover, a functional nuclear localization signal (NLS) in MA was shown to be required for infection of primary macrophages (56). Gallay et al. (20) reported that the NLS in MA is indeed recognized by Rch1, a member of the cellular karyopherin-α family, which is responsible for targeting NLS-bearing substrates to the nuclear pore. However, the issue of the function and the role of the MA NLS in nondividing cells is still under debate, since other studies have shown that viruses carrying mutations in this region (K26T and K27T) replicate only marginally less than the wild-type virus in nondividing, as well as dividing, cell populations (15–18).
The accessory protein Vpr is a 14-kDa gene product which is well conserved between HIV-1, HIV-2, and simian immunodeficiency virus (SIV) (51, 54). Although Vpr does not contain a canonical NLS, the protein is detected in the nuclei of cells transfected with Vpr expressors (37). This viral protein was shown to prevent proliferation of HIV-1-infected cells by interfering with normal cell cycle control. In fact, cells expressing Vpr accumulate in the G2 phase of the cell cycle (29, 42, 45). During the late stages of the virus life cycle, Vpr is expressed by a Rev-dependent pathway and is incorporated into virions through an interaction mediated by the p6 domain of the Gag p55 precursor (7, 9, 31). The Vpr protein contains four structural regions: the N-terminal region, one central domain containing two putative amphipathic alpha helices (Hα1 and Hα2) as predicted by computer-assisted analysis, and the arginine-rich C-terminal region. Mutagenic studies have shown that the predicted Hα1 is critical for the virion incorporation of the protein (13, 40, 59), whereas both Hα1 and Hα2 are involved in the nuclear localization of the protein (39, 52, 59). The C-terminal basic region appears to be critical for cell cycle arrest (13, 39). The substantial levels of Vpr associated with virions suggest that it may play a significant role in the early events of the viral life cycle. A number of studies have reported that Vpr is involved in the establishment of HIV-1 infection in nondividing cells, including MDM (1, 11, 28, 58). Indeed, Vpr was shown to contribute with the MA protein to the nuclear translocation of the proviral DNA in nondividing cells (28, 50). An NLS peptide corresponding to the prototypic simian virus 40 T-antigen NLS motif (PKKKRKVEDPYC) blocks the MA- but not the Vpr-mediated nuclear import of the HIV-1 PIC (20, 24), suggesting that Vpr governs the nuclear import of the PIC through a distinct pathway.
In the present study, we investigated the requirement of the MA NLS and Vpr for the infection of gamma-irradiated cells that were growth arrested at the G2/M phase of the cell cycle. Our results indicate that, in contrast to Vpr, the MA NLS plays a minor role in the infection of nondividing cells. Data from semiquantitative PCR analyses reveal that the level of viral DNA and its transport to the nucleus are reduced in the absence of Vpr. We also compared the abilities of MA NLS-mutated HIV-1 proviruses expressing different Vpr alleles to infect G2/M arrested cells. Our site-directed mutagenesis analysis indicates that the region containing the putative alpha helix 2 contains a determinant which is responsible for the ability of Vpr to direct the nuclear translocation of the proviral DNA in nondividing cells.
MATERIALS AND METHODS
DNA provirus constructions.
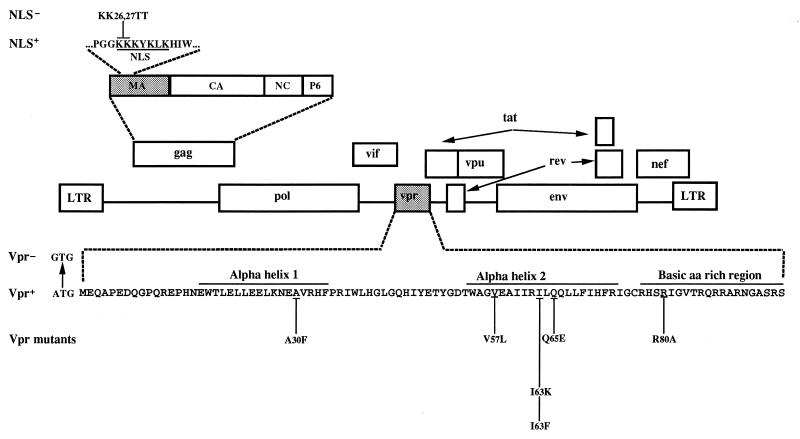
HIV-1 provirus plasmid HxBRU, referred as NLS+ Vpr+ in this study, is a molecular chimeric clone derived from two related proviruses, HxBc2 and BRU/LAI (32, 59). The genotype of this hybrid provirus is 5′-long terminal repeat (LTR) gag+ pol+ vif+ vpr+ tat+ rev+ vpu− env+ nef− 3′-LTR. HxBRUR− (referred to here as NLS+ Vpr−) is an isogenic molecular clone in which the initiation codon (ATG) of Vpr has been mutated to GTG. The HxBRU proviral constructions harboring the Vpr substitution mutations A30F and I63F were previously described (59). Four additional Vpr substitution mutations, V57L, I63K, Q65E, and R80A, were introduced in the HxBRU proviral construct by using a modified two-step PCR-based method described previously (59) and are fully described and characterized elsewhere (52).
Using the same PCR-based method, we also generated an isogenic MA NLS− mutant (described in reference 28) by replacing, in the NLS+ Vpr+ provirus, the two adjacent lysine residues (Lys-26 and -27) of MA with two threonine residues. The nucleotide sequence of the sense mutagenic oligonucleotide of the MA NLS− mutant is 5′-AGGCCAGGGGGTACCACAAAATATAAA-3′. These mutations were then introduced, by cloning the Nar1-Apa1 (nucleotides [nt] 186 and 1555 [+1 = transcription initiation site]) fragment containing the MA NLS− mutation, into all constructions to generate proviruses containing NLS− mutations combined with the different vpr alleles.
Cell lines and antisera.
Cos-7 cells, an African green monkey kidney cell line transformed by an origin-defective mutant of simian virus 40 (25), were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, N.Y.) supplemented with 10% fetal calf serum (FCS). HeLa-CD4-LTR/β-gal cells (obtained from the National Institutes of Health AIDS Research and Reference Reagent Program [30]), which contain one integrated copy of the HIV-1 LTR (nt −138 through +80) linked to the β-galactosidase (β-gal) gene modified by the addition of an NLS, were cultured in DMEM supplemented with 10% FCS. MT4 cells, a human T-lymphoid cell line transformed by human T-cell lymphotropic virus type 1 (26), were maintained in RPMI 1640 medium (Gibco) containing 10% FCS. Cells were incubated at 37°C in a 5% CO2 humidified atmosphere. Human anti-HIV-1 serum no. 162, collected from an HIV-1-seropositive individual, and rabbit anti-Vpr serum raised against Escherichia coli-derived Vpr protein have been previously described (59).
Transfection of cells and virus stock preparation.
Cos-7 cells (106) were seeded into a 10-cm-diameter culture petri dish. After 24 h, the cultures were transfected in parallel with 25 μg of the different proviral DNAs by using the calcium phosphate precipitation technique (59). For the preparation of virus stocks, the culture media were replaced with fresh media at 48 h posttransfection. The culture supernatants were collected 24 h later and clarified by filtration through a 0.45-μm-pore-size filter (Costar, Cambridge, Mass.). The virions were then pelleted by ultracentrifugation and resuspended in RPMI 1640 medium containing 20% FCS. Each virus stock was titrated by end point dilution and kept at −80°C.
Titration of virus stocks.
Virus stocks were serially diluted by two- to threefold in triplicate in 96-well culture plates. MT4 cells (1 × 104 to 5 × 104 per well) were immediately added, and the end point dilution infectivity was determined at 8 days postinfection (p.i.) by the observation of syncytium formation in the culture. The 50% tissue culture infective dose (TCID50) was calculated by using the Reed-Muench method for each virus stock (43).
Gamma irradiation of cells and analysis of cell cycle arrest.
MT4 and HeLa-CD4-LTR/β-gal cells were exposed to 3,000 and 4,000 rads, respectively, with a 60Co source. Typically, the irradiation of proliferating cells produces a cell population arrested mainly in the G2/M phase of the cell cycle.
The G2 state of cells at the time of infection was evaluated by propidium iodide staining of their DNA and flow cytometry analysis. Briefly, the cells were fixed with 70% ethanol, washed, treated with RNase (50 μg/ml) for 1 h at 37°C, and finally resuspended in a solution containing propidium iodide (50 μg/ml) and 0.1% Na citrate. The fluorescence associated with the cells in the normal and irradiated populations was evaluated by cytofluorometry with a FACStar (Becton Dickinson, Mountain View, Calif.) equipped with the Cell-Fit software program (Becton Dickinson).
DNA synthesis was also monitored by [3H]thymidine incorporation. [methyl-3H]thymidine (Du Pont Canada Inc., NEN Products, Montreal, Canada) was added to triplicate nonirradiated and gamma-irradiated MT4 cultures (38). After labeling, cells were harvested on glass fiber filters and the level of [methyl-3H]thymidine incorporation into DNA was evaluated in the presence of a scintillation liquid (Cytoscint; ICN Biomedicals Inc., Toronto, Ontario, Canada) in a scintillation counter (Beckman LS-600 SC).
Cell infection and measurement of virus production.
Equal numbers of infectious units (multiplicity of infection [MOI] ranging from 0.005 to 0.5 TCID50/cell) of the different viruses were used to infect cells in the presence of 10 μg of Polybrene (Sigma Chemical Co., Mississauga, Ontario, Canada) per ml. At 8 h p.i., the cells were washed three times with phosphate-buffered saline and resuspended in culture medium. Virus production from proliferating or gamma-irradiated MT4 cells was evaluated by measuring RT activity or p24 (capsid antigen) levels in culture supernatants. Two hundred microliters of supernatants from duplicate cultures was collected every 2 days or at the indicated time p.i. and replaced with fresh medium. Virus-specific RT activity in each supernatant was measured as described previously (33). The release of capsid antigen was measured by using an enzyme-linked immunosorbent assay (ELISA) kit.
MAGI assay.
Proliferating and gamma-irradiated HeLa-CD4-LTR/β-gal cells were infected in triplicate with different viruses at the same MOI in the presence of 20 μg of DEAE-dextran per ml. The plates were gently rocked every 30 min for 2 h, at which time 1 ml of DMEM was added. The MAGI assay was carried out as described previously (30). Briefly, infection of cells was stopped at 48 h p.i. by removing the culture medium. The infected monolayers were then fixed with a solution of 0.2% glutaraldehyde and 1.0% formaldehyde for 5 min at room temperature. After fixation, the cells were washed with phosphate-buffered saline and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) for 50 min at 37°C. Positive infected cells, which correspond to cells with blue nuclei, were counted under a Zeiss microscope.
Semiquantitative PCR assays.
MT4 or HeLa-CD4-LTR/β-gal cells were infected in parallel with equal numbers of infectious units of viruses. At 8 or 24 h p.i., cells were resuspended (105 cells/300 μl) in a lysis buffer containing 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 0.001% sodium dodecyl sulfate, and 0.001% Triton and incubated at 55°C for 2 h with proteinase K (600 μg/ml). Total DNA was purified from lysates by phenol-chloroform extractions. Before the semiquantitative evaluation of viral DNA content, the samples were diluted serially by 10-fold and subjected to a prequantitative PCR analysis, using primers and probe for the human cellular β-2-adrenergic receptor IC1 (β-AR) gene (GenBank accession no. Y00106), in order to evaluate the total DNA content of each sample. PCRs were carried out in a mixture containing 1× PCR buffer (Perkin-Elmer, Mississauga, Ontario, Canada), 2 mM (each) deoxynucleoside triphosphate, and 2 U of Taq polymerase (5 U/μl) (Perkin-Elmer). Amplification reactions were performed with 30 to 35 sequential cycles of 94°C for 1 min, 50°C for 2 min, and 72°C for 3 min in a DNA Thermal Cycler (Perkin-Elmer). The amplified PCR products were denatured at 94°C for 5 min and hybridized with specific γ-32P-labeled oligonucleotide probes in liquid phase at 55°C for 10 min as described previously (12). The results were analyzed by densitometric analysis of the autoradiograms after electrophoresis on a sodium dodecyl sulfate–8 to 10% polyacrylamide gel. The primer and probe sequences used for the β-AR DNA are as follows: primer 1, 5′-CGTCTACTCCAGGGTCTTTCAG-3′; primer 2, 5′-TAGGCCTTCAAAGAAGACCTGC-3′; and probe, 5′-CATGTCCAGAACCTTAGCCAGGTGGAGGAGGATG-3′.
Equivalent amounts of cellular DNAs of each sample were then serially diluted by threefold. The diluted DNAs were routinely subjected to parallel semiquantitative PCR analyses with primers and probes for β-AR DNA (internal control), for viral linear DNA (total viral DNA), and for 2-LTR circular viral DNA (nucleus-associated viral DNA form) (60). Serial dilutions of ACH-2 cell line DNA, which contains one viral DNA copy per cell (8), and DNA from productively HIV-1-infected MT4 cells collected at 48 h p.i. were also routinely used in parallel as positive PCR controls for linear and 2-LTR circular viral DNA reactions, respectively. The primer and probe DNA sequences for viral linear DNA are as follows: LTR, 5′-CTCTAGCAGTGGCGCCCGAACAGGGAC-3′; Gag, 5′-ACTGACGCTCTCGCACCCATCTCTCTC-3′; and probe, 5′-GAGGAGCTCTCTCGACGCAGGACTCGGCTTGCTGA-3′. The primer and probe DNA sequences for the 2-LTR circular viral DNA are as follows: LTR9, 5′-GCCTCAATAAAGCTTGCCTTG-3′; LTR8, 5′-TCCCAGGCTCAGATCTGGTCTAAC-3′ (20); and probe, 5′-CATCGAGCTTGCTACAAGGGACTTTCCGCT-3′. All of the oligonucleotides were kept at −20°C in Tris-EDTA buffer.
End labeling of oligonucleotide probes.
The oligonucleotide probes used in the semiquantitative PCR analysis were labeled with [γ-32P]ATP (4,500 Ci/mmol; ICN Biomedicals Inc.) by using the T4 polynucleotide kinase. The labeling mixture contained 1 μl of T4 polynucleotide kinase (10 U/μl; Pharmacia Biotech, Baie D’Urfé, Québec, Canada), 4 μl of kinase buffer, 5 μl of [γ-32P]ATP, and 29 μl of double-distilled water. The mixture was incubated at 37°C for 1 h, and the reaction was stopped by the addition of 60 μl of 25 mM EDTA. The labeled probes were purified on a G50 column (Pharmacia). The radioactivity in 1 μl of the labeled probe was counted in 5 ml of scintillation liquid (Cytoscint; ICN Biomedicals Inc.) with a scintillation counter (Beckman LS-600 SC). A specific activity of >105 cpm/μl was routinely obtained.
Densitometric analyses.
Densitometric analysis of the autoradiograms was carried out with a Personal Densitometer (Molecular Dynamics, Sunnyvale, Calif.) with ImageQuantTM software, version 3.22.
RESULTS
Construction of HIV-1 proviruses and characterization of Vpr-associated phenotypes.
Vpr and MA were recognized to share redundant early viral replicative functions in nondividing cells (28). In order to identify the domains associated with Vpr’s functions in growth-arrested cells, we generated a series of HIV proviruses harboring mutated versions of Vpr in combination with MA NLS mutations.
Amino acid substitutions of two positively charged amino acids (Lys-26 and Lys-27 replaced by two Thr residues) (MA NLS−) located in the reported NLS of MA were introduced into infectious HIV proviruses isogenic except for the expression of Vpr (Vpr+ and Vpr−) (59). We also constructed a panel of MA NLS− proviruses that encode mutated versions of Vpr (Fig. 1). The A30F and I63F substitution mutants have been previously described (59). The A30F substitution mutant was constructed to introduce a change in the predicted alpha-helical structure (Hα1) located near the N terminus of Vpr. Wild-type residues Val-57, Ile-63, and Gln-65 were replaced with Leu (V57L), Lys (I63K), and Glu (Q65E), respectively, to introduce structural as well as charge modifications in the predicted amphipathic alpha-helical structure (Hα2) located near the C terminus of Vpr (52). Finally, Arg-80 was replaced by Ala (R80A) to affect the basic character of the C-terminal region. This mutation was previously associated with a loss in the ability of Vpr to induce an arrest in the G2 phase of the cell cycle (13). None of these Vpr mutations modified other open reading frames or known splicing sites in the HIV sequence. Mutated Vpr proteins were characterized for their stability and their abilities to be incorporated into virions and to be translocated into the cell nucleus (52, 59). Briefly, the Vpr stability was evaluated by pulse-chase labeling and immunoprecipitation in transfected Cos-7 cells as described previously (59). As shown in Table 1, the nonconservative replacement of the hydrophobic Ile-63 residue by a Lys leads to an unstable Vpr protein compared to wild-type Vpr. However, the conservative substitutions A30F, V57L, I63F, and Q65E and the nonconservative substitution R80A lead to stable Vpr proteins.
FIG. 1.
HIV-1 genetic organization and construction of molecular clones containing mutations in MA NLS and Vpr. The schematic organization of the HIV-1 genome and the complete amino acid sequence of Vpr are shown. The positions of the predicted alpha-helical structures (αH1 and αH2) and the basic-amino-acid (aa)-rich domain on Vpr are indicated. NLS− mutants were generated by replacement of the indicated two adjacent lysines in the Gag MA NLS by threonines as described previously (28). The Vpr mutations were introduced by site-directed mutagenesis as indicated.
TABLE 1.
Phenotypic variations of the HIV-1 vpr alleles
| Vpr genotype | Vpr-Associated phenotypes
|
|||||
|---|---|---|---|---|---|---|
| Vpr stability | Virion incorporation (%) | Proviral linear DNAa | Nuclear localizationb | Nuclear transport of viral DNAc | Infectivity in growth-arrested cells (%)d | |
| NLS+ Vpr+ | WTe | 100 | WT | n | WT | 100 |
| NLS+ Vpr− | NAf | NA | Reduced | NA | Reduced | 55 |
| NLS− Vpr+ | WT | 100 | WT | n | WT | 85 |
| NLS− Vpr− | NA | NA | Reduced | NA | Reduced | 35 |
| NLS− A30F | WT | 20 | —g | ph | — | 27 |
| NLS− V57L | WT | 75 | — | p | — | 80 |
| NLS− I63F | WT | 75 | — | p | — | 75 |
| NLS− I63K | Reduced | 65 | — | p | — | 38 |
| NLS− Q65E | WT | 100 | WTi | p | Reducedi | 40 |
| NLS− R80A | WT | 100 | Reducedi | n | Reducedi | 25 |
Based on the semiquantitative PCR evaluation of proviral linear DNA in gamma-irradiated infected MT4 cells.
Nuclear immunofluorescence patterns as evaluated by confocal microscopy analysis. n, primarily nuclear; p, primarily perinuclear (52).
Based on the semiquantitative PCR evaluation of the 2-LTR circular proviral DNA in gamma-irradiated infected MT4 cells.
Mean percentage of β-gal-positive gamma-irradiated infected HeLa-CD4-LTR/β-gal cells relative to the number of positive cells obtained following infection with the wild-type virus (from at least two independent experiments).
WT, wild type.
NA, not applicable.
—, not determined.
Unpublished data.
This analysis was performed with gamma-irradiated infected HeLa-CD4-LTR/β-gal cells.
We then determined the level of virion incorporation of the mutated Vpr proteins, as previously described (59). As shown in Table 1 and as already published (59), diminished levels of Vpr were detected in virions obtained from proviruses harboring mutations in the region extending from amino acid 16 to 34 (Hα1; A30F). The mutations V57L, I63K, I63F, Q65E, and R80A lead to the expression of Vpr proteins with almost wild-type capacity (from 65 to 100% of the wild-type level) to be incorporated into progeny virions (52).
Finally, we characterized the karyophilic potential of the Vpr mutants expressed in the absence of other viral proteins. For this purpose, the different Vpr mutants were cloned into eukaryotic expression vectors (59). These plasmids were transfected into Cos-7 cells, and Vpr protein expression was subjected to immunofluorescence detection with Vpr polyclonal antibodies and confocal microscopy analysis as described previously (52). In agreement with published data (13) and as presented in Table 1, the immunofluorescence confocal analysis reveals that the wild-type and the R80A-mutated Vpr proteins are localized predominantly inside the nucleus. In contrast, the substitution mutations A30F, V57L, I63K, I63F, and Q65E lead to substantial reductions of the intranuclear staining pattern, although to different extents (Table 1) (52). These data indicate that both Vpr alpha helix regions are important for the accumulation of Vpr into the nucleus. Mutations affecting alpha helices 1 and 2 lead to Vpr proteins which are still able to be targeted to the nucleus. However, presumably following association with the nuclear pores, these mutated proteins are impaired in their ability either to translocate or to be retained into the cell nucleus.
Viral replication of Vpr and/or MA NLS mutant viruses in dividing MT4 cells.
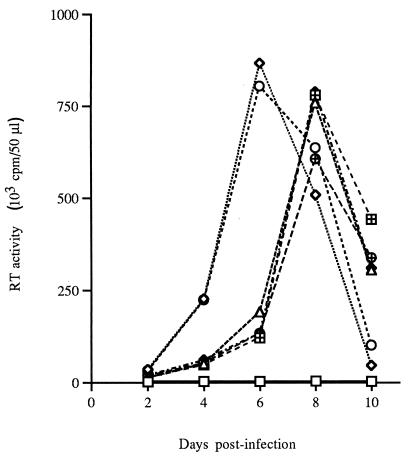
We next evaluated the abilities of Vpr and/or MA NLS mutant viruses to infect and propagate in dividing MT4 cells. As virus stocks can differ in the relative amounts of p24 antigens, RT activity, and infectious virus titer, inocula were standardized on the basis of end point dilution (TCID50) in MT4 cells. Cell-free virions collected from transfected Cos-7 cells were used to infect MT4 cells at an MOI of 0.01 TCID50/cell. The replication of mutant viruses was monitored daily by measurement of virion-associated RT activity in the supernatants of the infected MT4 cell cultures (Fig. 2). The absence of Vpr expression did not affect virus infectivity and production in dividing MT4 cells at the tested MOI. However, viruses harboring substitutions in the MA NLS exhibited a delayed kinetics compared to that of the replication of wild-type MA viruses irrespective of the expression of Vpr (Fig. 2). The kinetics of viral replication of viruses harboring the MA NLS− substitution combined with two tested Vpr mutations (Q65E and R80A) were also determined and were found to be similar to those of the MA NLS− Vpr+ virus. Previous studies have demonstrated that MA plays a key role in retroviral assembly by directing the intracellular transport and membrane association of the Gag precursor polyprotein (p55Gag). Zhou et al. (64) demonstrated that the N-terminal region of HIV-1 Gag contains a bipartite membrane targeting signal which includes the two basic residues Lys-26 and Lys-27. In addition, mutations of these residues were shown to result in a decrease of viral replication (19, 34). Similarly, mutations in the MA basic region could have affected these MA functions, leading to the delay in the kinetics of replication observed with these viruses in our experimental system. Interestingly, Fouchier et al. (16) recently reported that similar MA mutations reduced the rate at which the viral p55Gag is posttranslationally processed by the viral protease.
FIG. 2.
Replication kinetics of Vpr and/or MA NLS mutant viruses in infected dividing MT4 cells. The cells were infected with the different mutant viruses at an MOI of 0.01. Virus production in culture supernatants was monitored every 2 days by measuring RT activity. □, mock; ◊, NLS+ Vpr+; ○, NLS+ Vpr−; ▵, NLS− Vpr+; ⊞, NLS− Vpr−; ⧫, NLS− Q65E; ⊕, NLS− R80A.
Viral replication of Vpr and/or MA NLS mutant viruses in nondividing MT4 cells.
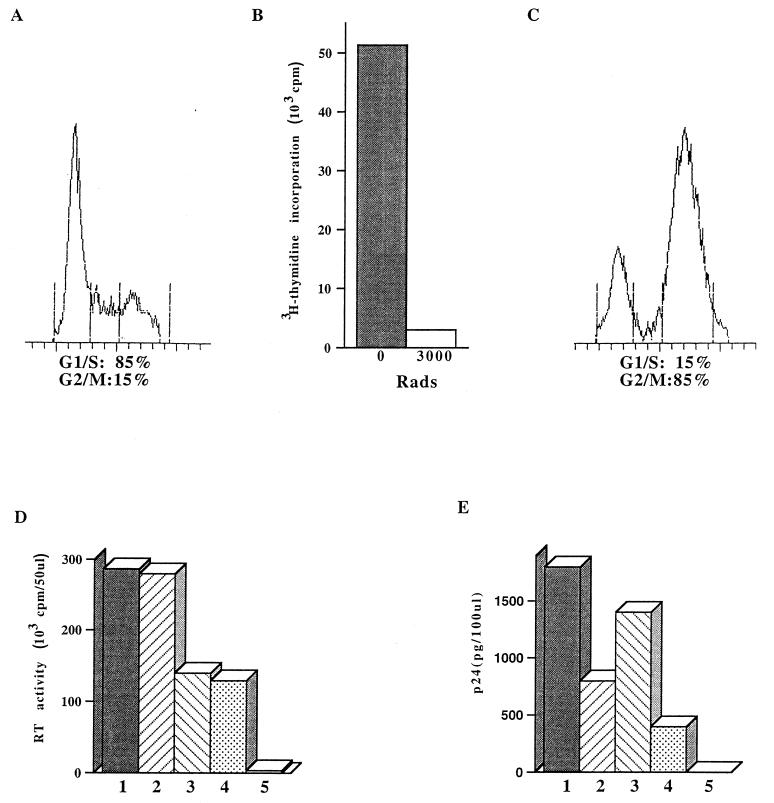
In order to evaluate the respective roles of Vpr and the MA NLS in viral replication in nondividing cells, we next infected in parallel dividing and gamma-irradiated, growth-arrested MT4 cells. As shown in Fig. 3C, gamma irradiation (3,000 rads) of MT4 cells results in the accumulation of a large proportion of cells in the G2/M phase (85%, compared to 15% in control dividing population [Fig. 3A]). The gamma irradiation was also shown to abolish cell proliferation, as measured by the inhibition of [3H]thymidine incorporation (Fig. 3B). Irradiated and nonirradiated cells were infected with the different viruses at an MOI of 0.01 TCID50/cell. For dividing cells, the replication of viruses was monitored by the measurement of virion-associated RT activity. For growth-arrested cells, the viral replication was monitored by using the more sensitive p24 ELISA method. As opposed to the viral replication pattern observed in dividing cells (Fig. 2 and 3D), the viral production from cultures infected with NLS− viruses was only slightly decreased compared to the case for wild-type NLS+ viruses (Fig. 3E, compare bars 1 and 3 and bars 2 and 4). These data indicate that the absence of an intact MA NLS has less impact on viral replication in nondividing cells than in dividing cells. In contrast, and in agreement with published results (28), our data show that although viral replication of Vpr+ and Vpr− viruses is almost the same in dividing cells (Fig. 3D, compare bars 1 and 2 and bars 3 and 4), the viral replication of viruses that do not express Vpr is markedly reduced in nondividing cells (Fig. 3E, compare bars 2 and 1 and bars 4 and 3). Indeed, in gamma-irradiated MT4 cells, a two- to threefold decrease in virus production could be demonstrated following infection with Vpr− mutants compared to the wild type in three independent experiments using two sets of viral stocks. In addition, these results indicate that in nondividing cells, the absence of Vpr results in a reduction of viral replication which is not fully complemented by the presence of an intact MA NLS.
FIG. 3.
Effect of Vpr and/or MA NLS mutations on viral production in dividing and nondividing MT4 cells. (A to C) Dividing (A and B) or gamma-irradiated (B and C) MT4 cells were analyzed by fluorescence-activated cell sorting (A and C) and by [3H]thymidine incorporation (B). (A and C) The numbers of cells in G1/S and G2/M phases are shown as percentages of the total viable cells. (B) Histograms represent the level of cell-associated radioactivity following [3H]thymidine labeling of nonirradiated or irradiated (3,000 rads) MT4 cultures. (D and E) Dividing (D) or G2-growth-arrested (E) MT4 cells were infected with HIV-1 variants containing single or double mutations in the MA NLS and/or Vpr at an MOI of 0.01 TCID50/cell. Virus production in culture supernatants was monitored daily by RT activity or p24 ELISA. Data shown are representative of those for supernatants harvested at 3 days p.i. from three independent experiments and are for noninfected culture (bars 5) or cultures infected with wild-type (bars 1), Vpr− (bars 2), MA NLS− (bars 3), or Vpr− MA NLS− (bars 4) virus.
Infectivity of Vpr and/or MA NLS mutant viruses in nondividing cells.
To further characterize the decrease in viral replication associated with Vpr mutants in nondividing cells, we measured the ability of viruses to activate LTR-driven gene expression in growth-arrested indicator cells (MAGI assay). This assay, which measures the number of cells associated with β-gal gene activation, reflects the ability of viruses to perform steps of the infection cycle occurring prior to viral assembly and virion release.
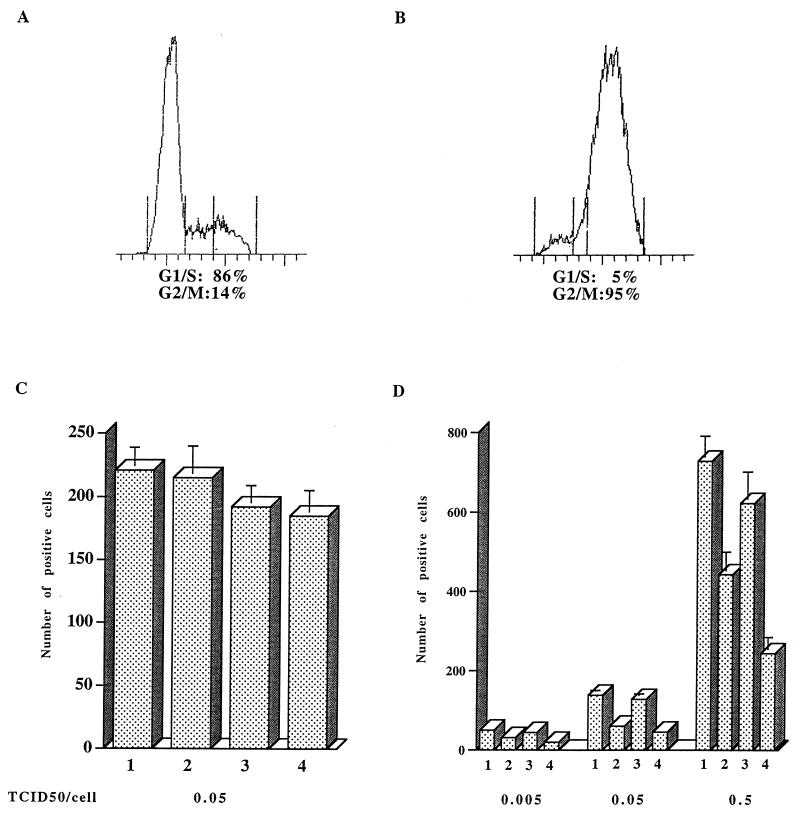
We infected in parallel dividing and growth-arrested HeLa-CD4-LTR/β-gal cells. As shown in Fig. 4B, gamma irradiation (4,000 rads) resulted in the accumulation of 95% of the cells in G2/M phase (compared to 14% with proliferating cells [Fig. 4A]). Irradiated and nonirradiated cells were infected with viruses at increasing MOIs (0.005, 0.05, and 0.5 TCID50/cell). At 48 h p.i., cells were fixed and stained with X-Gal, and the number of β-gal-expressing cells was counted under a microscope. The number of positive cells correlates in this assay with the input of infectious particles as evaluated by end point dilution titration. Hence, as expected, the numbers of positive cells obtained after infection of dividing cells with the four viruses were similar (Fig. 4C).
FIG. 4.
Effect of Vpr and/or MA NLS mutations on infectivity in dividing and nondividing HeLa-CD4-LTR/β-gal cells. (A and B) Dividing (A) or gamma-irradiated (4,000 rads) (B) HeLa-CD4/LTR-β-gal cells were analyzed by fluorescence-activated cell sorting as described in the legend to Fig. 3. (C and D) Dividing (C) or G2-growth-arrested (D) cells were infected with HIV-1 variants containing single or double mutations in the MA NLS and/or Vpr at the indicated MOI. Infectivity was assayed by transactivation of an endogenous LTR/β-gal gene as described previously (30), and histograms represent the mean numbers of X-Gal-positive cells from triplicate measurements from one experiment. Data are for wild-type (bars 1), Vpr− (bars 2), MA NLS− (bars 3), or Vpr− MA NLS− (bars 4) virus. For the infection at an MOI of 0.05 TCID50/cell, the data are representative of four independent experiments. Error bars represent standard deviations.
Interestingly, we found that although no difference could be observed for dividing cells, the numbers of β-gal-positive cells among nondividing cells infected with Vpr+ or Vpr− virus at the same MOI were different (compare Fig. 4C and D). In gamma-irradiated HeLa-CD4-LTR/β-gal cells, a two- to threefold decrease in the number of positive cells was observed following infection with the Vpr− mutant as compared to the wild type, even in the presence of MA NLS+ virus, in four independent experiments using at least two sets of viral stocks. Figure 4D shows in addition that the difference between Vpr+ and Vpr− is MOI independent (representative results from two independent replication experiments are shown). These results, which measure viral infectivity, strongly support the viral production data obtained with nondividing MT4 cells infected with the same viruses. Accordingly, the absence of Vpr during infection of nondividing cells results in a reduction of viral replication, most probably because viral steps occurring prior to virion assembly and release are affected.
Infectivity of proviruses expressing different Vpr alleles in nondividing cells.
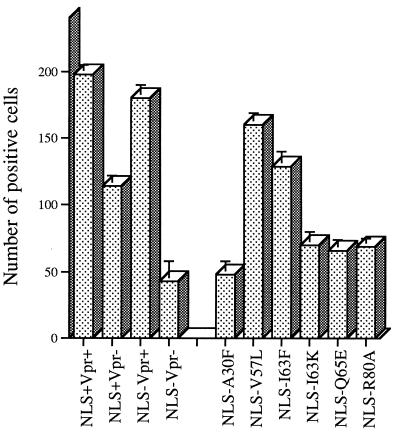
In order to determine the region(s) of Vpr involved in the reduction of viral production in growth-arrested cells, we systematically tested the infectivities of different Vpr alleles by using the MAGI assay. Gamma-irradiated, growth-arrested HeLa-CD4-LTR/β-gal cells were infected with MA NLS− viruses harboring different Vpr alleles at an MOI of 0.05 TCID50/cell. Figure 5 shows a representative set of results from at least two independent experiments (the results are summarized in Table 1). The numbers of β-gal-positive cells counted following inoculation with viruses with V57L and I63F mutations were only marginally reduced compared to control (MA NLS− Vpr+) values. In contrast, viruses harboring A30F, I63K, Q65E, and R80A mutations caused a two- to threefold reduction in the number of β-gal-positive cells. This reduction is similar to the one observed following inoculation of growth-arrested cells with the Vpr− viruses, indicating that these Vpr mutants have lost a Vpr function(s) associated with the ability to infect nondividing cells.
FIG. 5.
Effect of Vpr alleles on viral infectivity in nondividing HeLa-CD4-LTR/β-gal cells. G2-growth-arrested HeLa-CD4-LTR/β-gal cells were infected with HIV-1 variants containing the indicated mutations at an MOI of 0.05 TCID50/cell. Viral infectivity was determined by MAGI assay as described in the legend to Fig. 4.
We have also calculated the viral production in gamma-irradiated MT4 cells infected with MA NLS− viruses harboring different Vpr alleles at an MOI of 0.01 TCID50/cell. At 3 days p.i., we monitored the replication of these viruses by measuring virion-associated p24 in the culture supernatants. A twofold reduction of viral production was observed with the A30F, Q65E, and R80A mutants relative to the wild type (results not shown).
The reduction of infectivity observed in nondividing HeLa-CD4-LTR/β-gal cells infected with the I63K mutant could be attributed to decreases in both the protein stability and virion incorporation (Table 1). Mutation of A30 results in a stable Vpr variant which is mutated in a domain involved in virion incorporation. Although we cannot rule out the involvement of this mutation in a late Vpr function, the simplest explanation in this particular case would be that the viral replication impairment results from the severe reduction in virion incorporation. However, the Q65E and R80A mutants, which are stable proteins incorporated into virions at wild-type level, still exhibit impairment of viral infectivity in nondividing cells.
DNA synthesis in growth-arrested cells infected with Vpr and/or MA NLS mutant viruses.
Previous studies have shown that Vpr is involved in the nuclear transport of the PIC. To determine the ability of the different mutants to perform very early steps of the viral replicative cycle, we thus measured the levels of synthesis of the viral cDNA in growth-arrested MT4 or HeLa-CD4-LTR/β-gal cells infected with MA NLS− viruses harboring Vpr+, Vpr−, Q65E, or R80A vpr genes.
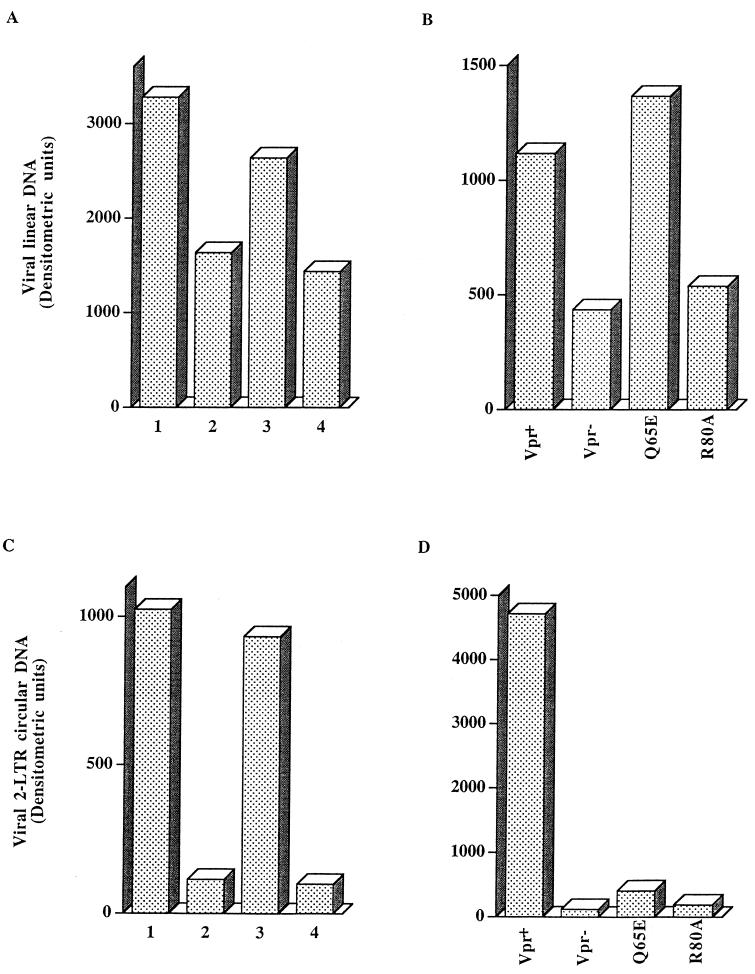
Growth-arrested cells were infected in parallel with the different viruses at an MOI of 0.05 TCID50/cell, and at 8 and 48 h p.i., DNAs were extracted and used to perform semiquantitative PCR analyses. A primer pair specific for the Gag region was chosen to detect full-length or nearly completed viral linear DNA. Only minor differences in the levels of linear DNA made by the NLS+ and NLS− viruses were observed (see Fig. 7A). Surprisingly, we found at both times p.i. a consistent reduction of the linear DNA content in cells infected with viruses which do not express Vpr (Fig. 6, examplified for DNA extracted at 48 h p.i.). Densitometric analysis of the PCR results shows that, in three independent experiments using three sets of viral stocks, the Vpr− mutants are reproducibly associated with a two- to threefold decrease in linear DNA content compared to the case for wild-type Vpr (Fig. 7A shows results from a representative experiment). Interestingly, although the viral linear DNA levels present in growth-arrested HeLa-CD4-LTR/β-gal cells infected with the Q65E mutant were almost similar to those in cells infected with the wild type, the R80A mutant was associated with a reduction of viral DNA synthesis comparable to the one observed with the Vpr− viruses. These data again consistently paralleled the results observed when viral production and infectivity in nondividing cells infected with the same sets of viruses were measured. Thus, the viral replication impairment observed in growth-arrested cells infected with Vpr− and R80A viruses could be attributed, at least in part, to a reduction in the level of proviral DNA synthesis.
FIG. 7.
Densitometric analysis of the synthesis and nuclear translocation of viral DNA in G2-growth-arrested MT4 or HeLa-CD4-LTR/β-gal cells infected with Vpr and/or MA NLS HIV-1 variants. (A and C) Densitometric analysis of the results presented in Fig. 6; data are for wild-type (bars 1), Vpr− (bars 2), MA NLS− (bars 3), or Vpr− MA NLS− (bars 4) virus. (B and D) Densitometric analysis of PCR products obtained following infection of gamma-irradiated HeLa-CD4-LTR/β-gal cells as described in the legend to Fig. 6. Cells were infected at an MOI of 0.05 TCID50/cell with MA NLS− viruses containing the indicated Vpr alleles.
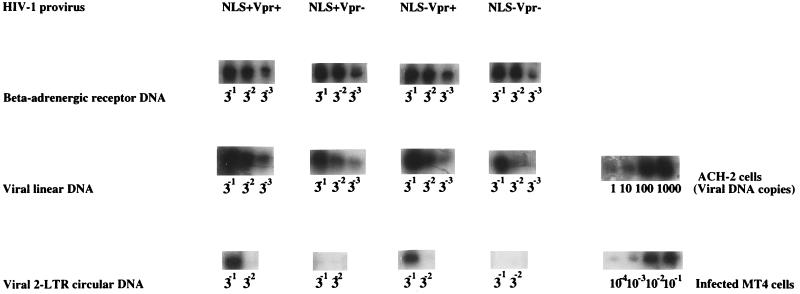
FIG. 6.
Synthesis and nuclear translocation of viral DNA in G2-growth-arrested MT4 cells infected with Vpr and/or MA NLS HIV-1 variants. Gamma-irradiated, growth-arrested MT4 cells were infected with HIV-1 variants containing single or double mutations in the MA NLS and/or Vpr at an MOI of 0.05 TCID50/cell. At 48 h p.i., cell aliquots were harvested for isolation of total DNA and analysis of viral and cellular DNAs by PCR. Serial dilutions of DNA, as indicated, were analyzed by using primers corresponding to late products of reverse transcription (viral linear DNA) and to the 2-LTR circular form of viral DNA, which served as a marker for the nuclear transport of viral DNA (3). Equivalent amounts of cellular DNA were assayed by PCR with primers specific for the β-AR.
Viral DNA transport in growth-arrested cells infected with Vpr and/or MA NLS mutant viruses.
We next measured the ability of the same viruses to translocate the viral DNA in the nucleus of infected, growth-arrested cells. Translocation of viral cDNAs from the cytoplasm to the nuclear compartment was monitored by using primers which span the junction between the 5′-LTR R region and 3′-LTR U5 region as created during formation of the 2-LTR circles, a strategy described by Burkrinsky et al. (3). Such circle dead-end products are formed only after the synthesis and nuclear translocation of the linear viral DNA and thus represent a marker for nuclear PIC transport.
We found a striking and consistent reduction of the 2-LTR circular DNA content in both growth-arrested MT4 and HeLa-CD4-LTR/β-gal cells infected with viruses which do not express Vpr (Fig. 6 and 7C and D) or which express mutated R80A and Q65E Vpr alleles (Fig. 7D). In contrast, the 2-LTR circular DNA content in nondividing MT4 cells infected with the MA NLS− viruses is almost similar to that in cells infected with the wild-type MA NLS+ viruses (Fig. 7C). In three independent experiments, the Vpr− mutants were associated with an approximately 20-fold reduction of the 2-LTR circular DNA content (Table 1 and Fig. 7C and D). This reduction of the 2-LTR circular DNA content could be due in part to the observed decrease in the synthesis of the viral linear DNA content following inoculation of cells with the Vpr− and R80A viruses (Fig. 7B). However, it could in addition be due to a decrease of proviral DNA transport into the cell nucleus. In this respect, it is interesting that although the level of linear DNA associated with the Q65E mutant is similar to that for the wild-type Vpr, its 2-LTR circular DNA content is severely reduced (compare Fig. 7B and D).
DISCUSSION
Lentiviruses are unique among retroviruses, as they can infect nondividing cells. In HIV-1-infected individuals, the viral population represents a mixture of different viral quasispecies which are associated with different biological characteristics. Adaptation to growth in several cells of the immune system which are in a nondividing state can lead to selection of HIV-1 strains harboring genes which facilitate this process. The main purpose of the present study was to evaluate the ability of isogenic HIV-1 viruses harboring different vpr genes to infect nondividing cells.
As a first step in the elaboration of our experimental system, we constructed proviruses harboring mutations in MA and in Vpr, two determinants recognized for their involvement in the nuclear transport of the viral PIC (2, 28). Surprisingly, the introduction of mutations in MA only slightly affected the ability of proviruses to establish infection in growth-arrested cells. Indeed, the viral production observed with mutated MA was only slightly reduced compared to that of wild-type MA in both the presence and absence of Vpr. In addition, the levels of viral DNA synthesis and nuclear transport of the proviral DNA observed with mutated and wild-type MA proviruses were similar. These results show that the two basic residues of the NLS within MA are not major determinants governing the ability of HIV-1 to infect nondividing cells. In contrast, the absence of Vpr expression led to a decrease in infectivity and viral production which correlated well with a decrease in synthesis and nuclear transport of the viral DNA. Taken together, these data show that the level of PIC nuclear transport is dependent mainly on Vpr in our experimental system.
These findings are in agreement with others studies which show that the MA NLS does not play a major role in the infection of nondividing cells (15–17). Freed et al. (18) recently demonstrated that vpr mutation alone lead to a two- to threefold reduction in viral production in MDM. However, these results contrast with those obtained with other experimental systems, where Gag MA and Vpr were reported to provide additive nuclear import functions (28). The discrepancy between the published data could be due to differences in the experimental designs. Indeed, results showing that the presence of wild-type MA can rescue mutated Vpr in nondividing cells were derived from studies in which cells were infected on the basis of the same p24 or RT viral stock contents (2, 4, 28, 56). Trono and Gallay (55) recently showed that the phenotype of the MA- and Vpr-defective strains is directly linked to the MOI. Indeed, they observed that at a high initial inoculum, mutated viruses exhibited a wild-type phenotype, whereas their growth was impaired when the cells were exposed to a lower dose of virus. In addition, several studies have pointed out that viral stocks consisted largely of defective particles. For instance, it was estimated that the proportion of infectious particles in even highly infectious HIV-1 viral stocks is between 1/3,500 and 1/12,300 (30). Accordingly, differences in MOI are expected when independently prepared viral stocks are normalized on the basis of p24 content or RT activity. These observations suggest that the levels of infectivity measured to compare infections which are based on same p24 or RT values may reflect differences in the initial inocula. In this respect, we observed that viral stocks were associated with different proportions of p24 and RT per infectious particle, and accordingly, we performed our infections with viral stocks normalized for the same MOI as evaluated with dividing cells. The infection of nondividing cells under these conditions has consistently revealed that the presence of an intact MA NLS determinant was not sufficient to complement the reduction of nuclear translocation of proviral DNA observed in the absence of Vpr.
The requirement of an intact MA NLS for infection of nondividing cells may vary according to the viral isolate being analyzed. Indeed, in addition to Vpr and MA, several viral products were reported to be present in the PIC and could potentially be involved in its nuclear transport (5, 22). Thus, it is possible that variations in the PIC composition, as the result of viral genetic differences, can account for some specific viral determinant dependencies. In this respect, MA was shown to play a key role in PIC nuclear transport in nondividing cells specifically in the context of Vpr-defective viruses (2, 4, 28, 56). It is possible that a mutation in a not-yet-identified determinant in the viral molecular clone that we used might similarly render the role of Vpr preponderant over that of the MA NLS. Such a determinant could correspond to other viral proteins, including different region of Gag, which might be responsible for activating the NLS signal of MA. During virion formation, cellular protein kinases were shown to be incorporated into budding virions and to mediate the MA phosphorylation necessary to activate the MA NLS (5, 21, 50). Recent data have indicated that Nef could play a role in such a process by mediating the virion incorporation of cellular kinases (50, 53). Interestingly, the prototypical infectious molecular clone used in this study does not express an intact nef gene (41).
By using site-directed mutagenesis, we constructed a series of proviruses that express different Vpr alleles. We then evaluated the ability of these proviruses to infect growth-arrested cells. Four proviruses of the six tested show a reduced viral infectivity in growth-arrested HeLa-CD4-LTR/β-gal cells compared to the wild-type provirus. As observed with viruses which do not express Vpr, viruses harboring the R80A mutation are associated with a two- to threefold reduction in the viral linear DNA content and a concomitant 20-fold reduction in the 2-LTR circular DNA content. The decrease in nuclear transport observed with this mutant could result from its inability to assist the nuclear transport itself. However, several lines of evidence indicate that this is unlikely. The R80A mutation was previously associated with an incapacity to induce the cellular G2 arrest normally observed in the presence of the wild-type Vpr protein (13). Recent observations strongly suggest that the Vpr-mediated nuclear transport and the ability to induce a G2 arrest indeed correspond to distinct Vpr domains. In this regard, Fletcher et al. (15) have recently shown that these two phenotypes segregate on two different genes (i.e., vpr and vpx) in HIV-2 and SIV SM. In addition, the R80A mutation in HIV-1 Vpr is associated with a wild-type capacity of the protein to accumulate in the cell nucleus (13). This makes it unlikely that the region identified by the R80A mutation corresponds to a region also involved in the viral DNA transport into the nucleus.
The reduction of 2-LTR circular DNA content with the R80A mutant may alternatively be a direct consequence of the observed decrease in the level of viral linear DNA. This reduction in the viral linear DNA level could theoretically be attributed to any of the viral steps preceding and including the reverse transcription process itself. The rate of reverse transcription has already been reported to be specifically delayed or restricted in some nonproliferating primary cell systems (10, 46, 48, 60, 61). In addition, mutations in the Vpr gene were similarly shown to result in a two- to threefold reduction in the abundance of full-length cDNA products in SIV agm infection (6). Therefore, the absence of Vpr could affect the level of viral DNA synthesis in nondividing cells, and the substitution at residue R80 might identify a region of Vpr involved in this function. This may not represent an essential Vpr function in dividing cells; however, it could contribute to reduce viral infectivity in nondividing cells. Interestingly, the R80A phenotype suggests that the Vpr function which mediates the G2 arrest could be linked to the Vpr function affecting viral DNA synthesis. The G2 arrest induced by Vpr was shown to result from a dysfunction of kinase/phosphatase pathways involved in cell cycle control (27). Thus, it is possible that, due to the presence of virion-associated Vpr, the reverse transcription process might be influenced by similar physiological changes occurring locally at the site of virus entry.
The Vpr Q65E mutant is associated with a wild-type level of linear DNA but shows otherwise a severe reduction in the 2-LTR circular DNA content. This indicates that the Q65E mutation identifies a Vpr region involved in the nuclear transport of the proviral DNA. Thus, the viral infectivity reduction observed with this mutant likely results from a decrease in the ability of Vpr Q65E to assist the nuclear transport of the viral PIC. Interestingly, the Q65E mutant was also shown to be associated with an important impairment in the capacity to accumulate in the cell nucleus (52). In addition, this mutation is located in a predicted alpha-helical region (Hα2) which was previously shown to be involved in an interaction with a cellular protein designated RIP (63). As suggested, it might be possible that the proper nuclear translocation of Vpr is related to its association with the RIP or with another nucleophilic cellular transporter (44). The recruitment of such a transporter through this Vpr domain may thus contribute to increase the nucleophilic property of the PIC.
The exact mechanism sustaining the function of Vpr in the PIC nuclear translocation has to be further clarified, since V57L and I63F mutations, which lead to an impairment in Vpr nuclear accumulation similar to that for the Q65 mutation, were associated with only a minor decrease of viral infectivity in nondividing HeLa/β-gal cells. Since the nuclear transport is not completely impaired with any of these three mutants, it is possible that subtle differences in their ability to accumulate into the cell nucleus might explain their differential capacity to affect infectivity in nondividing cells. Alternatively, these results might suggest that the ability of Vpr to be targeted to the perinuclear membrane rather than to accumulate into the nucleus, as experimentally shown in the context of a Vpr expressor, may be sufficient to assist nuclear transport of the PIC. The mutation of an uncharged glutamine residue to a charged glutamic acid residue introduces a modification of the hydrophilic interface of the predicted amphipathic alpha helix, whereas the V57L and I63F mutations both involve a modification of the hydrophobic side. Thus, each interface of the alpha helix may be involved in distinct types of molecular interactions. The Q65 mutation may disrupt a molecular interaction, specifically occurring during viral infection, which is essential for the Vpr-mediated nuclear translocation of the PIC. Such an interaction could involve a direct or indirect interaction of Vpr with the PIC.
ACKNOWLEDGMENTS
We appreciate the excellent assistance of Serge Senechal with the fluorescence-activated cell sorter analysis.
E.A.C. is the recipient of a National Health Scientist award from the National Health Research and Development Program (NHRDP) of Canada. This research was supported by grants from the Medical Research Council of Canada and the Fonds pour la Formation de Chercheur et l’Aide à la Recherche to E.A.C.
REFERENCES
- 1.Balliet J W, Kolson D L, Eiger G, Kim F M, McGann K A, Srinivasan A, Collman R. Distincts effects in primary macrophages and lymphocytes of the human immunodeficiency virus type 1 accessory genes vpr, vpu and nef: mutational analysis of a primary HIV-1 isolate. Virology. 1994;200:623–631. doi: 10.1006/viro.1994.1225. [DOI] [PubMed] [Google Scholar]
- 2.Bukrinsky M I, Haggerty S, Dempsey M P, Sharova N, Adzhubel A, Spitz L, Lewis P, Goldfarb D, Emerman M, Stevenson M. A nuclear localization signal within HIV-1 matrix protein that governs infection of non-dividing cells. Nature. 1993;365:666–669. doi: 10.1038/365666a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bukrinsky M I, Sharova N, Dempsey M P, Stanwick T L, Bukrinskaya A G, Haggerty S, Stevenson M. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci USA. 1992;89:6580–6584. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bukrinsky M I, Sharova N, McDonald T L, Pushkarskaya T, Tarpley W G, Stevenson M. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc Natl Acad Sci USA. 1993;90:6125–6129. doi: 10.1073/pnas.90.13.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camaur D, Galley P, Swingler S, Trono D. Human immunodeficiency virus matrix tyrosine phosphorylation: characterization of the kinase and its substrate requirements. J Virol. 1997;71:6834–6841. doi: 10.1128/jvi.71.9.6834-6841.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell B J, Hirsch V M. Vpr of simian immunodeficiency virus of African green monkeys is required for replication in macaque macrophages and lymphocytes. J Virol. 1997;71:5593–5602. doi: 10.1128/jvi.71.7.5593-5602.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Checroune F, Yao X-J, Göttlinger H, Bergeron D, Cohen E A. Incorporation of Vpr into human immunodeficiency virus type 1: role of conserved regions within the P6 domain of Pr55gag. J Acquired Immune Defic Syndr. 1995;10:1–7. [PubMed] [Google Scholar]
- 8.Clouse K A, Powell D, Washington I, Poli G, Strebel K, Farrar W, Barstad P, Kovacs J, Fauci A S, Folks T M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989;142:431–438. [PubMed] [Google Scholar]
- 9.Cohen E A, Dehni G, Sodroski J G, Haseltine W A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collin M, Gordon S. The kinetics of human immunodeficiency virus reverse transcription are slower in primary human macrophages than in a lymphoid cell line. Virology. 1994;200:114–120. doi: 10.1006/viro.1994.1169. [DOI] [PubMed] [Google Scholar]
- 11.Connor R I, Kuan Chen K, Choe S, Landau N. Vpr is required for efficient replication of human immunodeficiency virus type 1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- 12.Dianzani F, Antonelli G, Turriziani O, Dong G, Capobianchi M R, Riva E. In vitro selection of human immunodeficiency virus type 1 resistant to 3′-azido-3′-deoxythymidine. Antiviral Res. 1992;18:39–52. doi: 10.1016/0166-3542(92)90004-o. [DOI] [PubMed] [Google Scholar]
- 13.Di Marzio P, Choe S, Ebright M, Knoblauch R, Landau N R. Mutational analysis of cell cycle arrest, nuclear localization, and virion packaging of human immunodeficiency virus type 1 Vpr. J Virol. 1995;69:7909–7916. doi: 10.1128/jvi.69.12.7909-7916.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emerman M. HIV-1, Vpr and the cell cycle. Curr Biol. 1996;6:1096–103. doi: 10.1016/s0960-9822(02)00676-0. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher T M R, Brichacek B, Sharova N, Newman M A, Stivahtis G, Sharp P M, Emerman M, Hahn B H, Stevenson M. Nuclear import and cell cycle arrest functions of the HIV-1 Vpr protein are encoded by two separate genes in HIV-2/SIV(SM) EMBO J. 1996;15:6155–6165. [PMC free article] [PubMed] [Google Scholar]
- 16.Fouchier R A M, Meyer B E, Simon J H M, Fisher U, Malim M H. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the matrix protein is important for Gag processing but not for post-entry nuclear import. EMBO J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freed E O, Englund G, Martin M. Role of the basic domain of human immunodeficiency virus type matrix in macrophage infection. J Virol. 1995;69:3449–3454. doi: 10.1128/jvi.69.6.3949-3954.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freed E O, Englund G, Maldarelli F, Martin M A. Phosphorylation of residue 131 of HIV-1 matrix is not required for macrophage infection. Cell. 1997;88:171–173. doi: 10.1016/s0092-8674(00)81836-x. [DOI] [PubMed] [Google Scholar]
- 19.Freed E O, Martin M A. HIV-1 infection in non-dividing cells. Nature. 1994;369:107–108. doi: 10.1038/369107b0. [DOI] [PubMed] [Google Scholar]
- 20.Gallay P, Stitt V, Mundy C, Oettinger M, Trono D. Role of the karyopherin pathway in the human immunodeficiency virus type 1 nuclear import. J Virol. 1996;70:1027–1032. doi: 10.1128/jvi.70.2.1027-1032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 22.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 23.Gao W Y, Cara A, Gallo R C, Lori F. Low levels of deoxynucleotides in peripheral blood lymphocytes: a strategy to inhibit human immunodeficiency virus type 1 replication. Proc Natl Acad Sci USA. 1993;90:8925–8928. doi: 10.1073/pnas.90.19.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gulizia J, Dempsey M P, Sharova N, Bukrinsky M I, Spitz L, Goldfarb D, Stevenson M. Reduced nuclear import of human immunodeficiency virus type 1 preintegration complexes in the presence of a prototypic nuclear targeting signal. J Virol. 1994;68:2021–2025. doi: 10.1128/jvi.68.3.2021-2025.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981;23:175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- 26.Harada S, Koyanagi Y, Yamamoto N. Infection of HTLV-III/LAV in HTLV-1-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985;229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 27.He J, Choe S, Walker R, Di Marzio P, Morgan D O, Landau N R. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J Virol. 1995;69:6705–6711. doi: 10.1128/jvi.69.11.6705-6711.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heinzinger N K, Bukrinsky M I, Haggerty S A, Ragland A M, Kewalramani V, Lee M-A, Gendelman H E, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jowett J B M, Planelles V, Poon B, Shah N P, Chen M-L, Chen I S Y. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line based on activation of an integrated beta-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kondo E, Göttlinger H G. A conserved LXXLF sequence is the major determinant in p6gag required for the incorporation of human immunodeficiency virus type 1 Vpr. J Virol. 1996;70:159–164. doi: 10.1128/jvi.70.1.159-164.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavallée C, Yao X Y, Ladha A, Gottlinger H, Haseltine W A, Cohen E A. Requirement of the Pr55 gag precursor for incorporation of Vpr product into human immunodeficiency virus type 1 viral particles. J Virol. 1994;68:1926–1934. doi: 10.1128/jvi.68.3.1926-1934.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee M H, Sano K, Morales F E, Imagawa D T. Sensitive reverse transcriptase assay to detect and quantitate human immunodeficiency virus. J Clin Microbiol. 1987;35:1717–1721. doi: 10.1128/jcm.25.9.1717-1721.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y M, Tang X B, Cimakasky L M, Hildreth J E, Yu X F. Mutations in the matrix protein of human immunodeficiency virus type 1 inhibit surface expression and virion incorporation of viral envelope glycoproteins in CD4+ T lymphocytes. J Virol. 1997;71:1443–1452. doi: 10.1128/jvi.71.2.1443-1452.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis P L, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu Y-L, Spearman P, Ratner L. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J Virol. 1993;67:6542–6550. doi: 10.1128/jvi.67.11.6542-6550.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magnier-Gaubil C, Herbert J M, Quarck R, Papp B, Corvazier E, Wuytack F, Levy-Toledano S, Enouf J. Smooth muscle cell cycle and proliferation. Relationship between calcium influx and sarco-endoplasmic reticulum Ca2+ATPase regulation. J Biol Chem. 1996;271:27788–27794. doi: 10.1074/jbc.271.44.27788. [DOI] [PubMed] [Google Scholar]
- 39.Mahalingam S, Ayyavoo V, Patel M, Kierber-Emmons T, Weiner D B. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J Virol. 1997;71:6339–6347. doi: 10.1128/jvi.71.9.6339-6347.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahalingam S, Khan S A, Jabbar M A, Monken C E, Collman R G, Srinivasan A. Identification of residues in the N-terminal acidic domain of HIV-1 Vpr essential for virion incorporation. Virology. 1995;207:297–302. doi: 10.1006/viro.1995.1081. [DOI] [PubMed] [Google Scholar]
- 41.Ratner L, Haseltine W A, Patarca R, Livak K J, Starcich B, Josephs S F, Doran E R, Rafalski J A, Whitehorn E A, Baumeister K, Inanoff L, Petteway S R, Pearson J A, Lautenberger J A, Papas T S, Ghrayeb J, Chang N T, Gallo R C, Wong-Staal F. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313:277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 42.Re F, Braaten D, Franke E K, Luban J. Human immunodeficiency virus type 1 Vpr arrests the cell cycle in G2 by inhibiting the activation of p34-cdc2-cyclin B. J Virol. 1995;69:6859–6864. doi: 10.1128/jvi.69.11.6859-6864.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed F, Muench R. A simple method of estimated fifty percent end-points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 44.Refaeli Y, Levy D N, Weiner D B. The glucocorticoid receptor type II complex is a target of the HIV-1 vpr gene product. Proc Natl Acad Sci USA. 1995;92:3621–3625. doi: 10.1073/pnas.92.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogel M E, Wu L I, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882–888. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuitemaker H. Macrophage-tropic HIV-1 variants: initiators of infection and AIDS pathogenesis? J Leukocyte Biol. 1994;56:218–224. doi: 10.1002/jlb.56.3.218. [DOI] [PubMed] [Google Scholar]
- 47.Schuitemaker H, Kootstra N A, Fouchier R A, Hooibrink B, Miedema F. Productive HIV-1 infection of macrophages restricted to the cell fraction with proliferative capacity. EMBO J. 1994;13:5929–5936. doi: 10.1002/j.1460-2075.1994.tb06938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spina C A, Guatelli J C, Richman D D. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J Virol. 1995;69:2977–2988. doi: 10.1128/jvi.69.5.2977-2988.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevenson M, Stanwick T L, Dempsey M P, Lamonica C A. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 1990;9:1551–1560. doi: 10.1002/j.1460-2075.1990.tb08274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stevenson M I. Portals of entry: uncovering HIV nuclear transport pathways. Trends Cell Biol. 1996;6:9–15. doi: 10.1016/0962-8924(96)81032-4. [DOI] [PubMed] [Google Scholar]
- 51.Subbramanian R, Cohen E A. Molecular biology of HIV accessory genes. J Virol. 1994;68:6831–6835. doi: 10.1128/jvi.68.11.6831-6835.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subbramanian, R. A., X.-J. Yao, H. Dilhuydy, N. Rougeau, D. Bergeron, Y. Robitaille, and E. A. Cohen. Human immunodeficiency virus type 1 Vpr localization: nuclear transport of a viral protein modulated by a putative amphipathic helical structure and its relevance to biological activity. J. Mol. Biol., in press. [DOI] [PubMed]
- 53.Swingler S, Gallay P, Camaur D, Song J, Abo A, Trono D. The Nef protein of human immunodeficiency virus type 1 enhances serine phosphorylation of the viral matrix. J Virol. 1997;71:4372–4377. doi: 10.1128/jvi.71.6.4372-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tristem M, Marshal C, Karpas A, Hill F. Evolution of the primate lentiviruses: evidence from Vpr and Vpx. EMBO J. 1992;11:3405–3412. doi: 10.1002/j.1460-2075.1992.tb05419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trono D, Gallay P. In response to Freed et al. Cell. 1997;88:173–174. [Google Scholar]
- 56.von Schwedler U, Kornbluth R S, Trono D. The nuclear localization signal of the matrix protein of human immunodeficiency virus type 1 allows the establishment of infection in macrophages and quiescent T lymphocytes. Proc Natl Acad Sci USA. 1994;91:6992–6996. doi: 10.1073/pnas.91.15.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weissman D, Fauci A S. Role of dendritic cells in immunopathogenesis of human immunodeficiency virus infection. Clin Microbiol Rev. 1997;10:358–367. doi: 10.1128/cmr.10.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westervelt P, Henkel T, Towbridge D B, Orenstein J, Heuser J, Gendelman H E, Ratner L. Dual regulation of silent and productive infection in monocytes by distinct human immunodeficiency virus type 1 determinants. J Virol. 1992;66:3925–3931. doi: 10.1128/jvi.66.6.3925-3931.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yao X-J, Subbramanian R, Rougeau N, Boisvert F, Bergeron D, Cohen E A. Mutagenic analysis of HIV-1 Vpr: role of a predicted N-terminal alpha-helical structure on Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zack J A, Arrigo S A, Weitsman S R. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile latent viral structure. Cell. 1990;61:213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 61.Zack J A, Arrigo S J, Chen I S. Control of expression and cell tropism of human immunodeficiency virus type 1. Adv Virus Res. 1990;38:125–146. doi: 10.1016/s0065-3527(08)60861-1. [DOI] [PubMed] [Google Scholar]
- 62.Zack J A, Haislip A M, Krogstad P, Chen I S. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J Virol. 1992;66:1717–1725. doi: 10.1128/jvi.66.3.1717-1725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao L-J, Mukherjee S, Narayan O. Biochemical mechanism of HIV-1 Vpr function: specific interaction with a cellular protein. J Biol Chem. 1994;289:15827–15832. [PubMed] [Google Scholar]
- 64.Zhou W, Parent L J, Wills J W, Resh M D. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]