Highlights
-
•
Work ability index (WAI) following SARS-CoV-2 in a population-based study is estimated.
-
•
Medical treatment during acute infection and female sex are associated with low WAI.
-
•
Fatigue and neurocognitive impairment were strongest risk factors for low WAI.
-
•
Symptom clusters associated with low work ability differ according to age.
-
•
Physically and mentally demanding work only slightly modify the associations.
Keywords: Employment, Long Covid, Mental Health, Post Covid, SARS-CoV-2, Work Ability
Abstract
Objectives
Evidence on the work-related societal impact of long-term health-related consequences following SARS-CoV-2 is emerging. We characterize the modified work ability index (mWAI) of employees 6 to 12 months after an acute infection compared to pre-infection.
Methods
Analyses were based on a population-based, multi-center cross-sectional study including employees aged 18-65 years with positive SARS-CoV-2 polymerase chain reaction (tested between October 2020-April 2021 in defined geographic regions in Germany). Prevalences and results of adjusted logistic regression analyses were given.
Results
In 9752 employees (mean age 45.6 years, 58% females, response 24%), n = 1217 (13.1%) participants were regarded as having low mWAI compared to pre-infection. Outpatient medical treatment, inpatient treatment, and admission to intensive care during infection were associated with mWAI <15th percentile (P15, each odds ratio [OR] >3.0). Post-COVID symptom clusters most strongly linked to mWAI <P15 were neurocognitive impairment and fatigue, but varying according to different age groups, to a lesser extent according to different work demands. Associations for pre-existing mental disorders (OR 3.6 [95% confidence interval 3.0; 4.3]) and mental disorders during/after infection (OR 8.0 [95% confidence interval 6.1; 10.4]) with mWAI <P15 were found.
Conclusion
Our findings revealed risk factors of mWAI <P15 and associations of post-COVID symptom clusters with WAI <P15, which could be potentially prioritized for targeting rehabilitation measures.
Introduction
Evidence suggests long-term sequelae on health and quality of life following an acute infection with the SARS-CoV-2. A post-COVID-19 condition or post-COVID syndrome (PCS) is considered if symptoms occur within 3 months from the acute infection, last at least 2 months and cannot be explained by an alternative diagnosis [1]. These adverse long-term health sequelae include a variety of symptoms (details see, e.g., reviews [2,3]) requiring multimodal medical management and rehabilitation. The estimated prevalence of PCS may be substantial, with a reported frequency of up to 43% of those infected, 34% in outpatients, and increasing to 54% after hospitalization [4].
According to comprehensive reviews [5,6], about 10% of the current literature provides information on changes in work or occupation following PCS. Yet, evidence on work-related impacts is inconclusive as the studies differ substantially in their methods, including outcome measurements, sample sizes, and follow-up periods. In a German study of 30950 patients diagnosed with COVID-19, the prevalence of sick leave for at least 4 weeks was 5.8% [7] whereas in a Swedish register-based study of 11955 people who received sickness benefits for COVID-19, 9.0% remained on sick leave for 4 months due to PCS [8]. In addition, for those who return to work, it is often necessary to adapt to the work environment and employees’ tasks including reassessment of working hours and the volume of work.
Risk factors for return to work following PCS were e.g. female sex, older age and belonging to a risk group – including pre-existing chronic illness and some specific COVID-19 symptoms during infection like shortness of breath and fatigue [9]. However, only few research studies on return to work or work ability after COVID-19 have employed standardized instruments. In this context, work ability (WAI) [10] as a measure to assess the degree to which an individual is physically and mentally able to cope with demands at work, might provide additional information. The questionnaire is most commonly used in work ability research [11]. It is a reasonably good predictor of long-term sick leave during the following year [12] and of receiving a work disability pension during a follow-up period of 4 to 11 years [13]. Application in COVID-19 research is rare yet.
The aim of our study is to describe work ability 6 to 12 months after acute infection (compared to pre-infection work ability as reference) in employees aged 18-65 years in a large multi-center population-based study. We also evaluated risk factors for low work ability, including the potential role of comorbidity and involved post-COVID-19 symptom clusters.
Methods
Study design and study population
EPILOC (Epidemiology of Long COVID) is a population-based cross-sectional study in four administratively and geographically defined regions in the Federal State of Baden-Württemberg (Southwest Germany). The study included persons aged 18-65 years who had a diagnosis of COVID-19, confirmed by positive polymerase chain reaction for SARS-CoV-2 between October 01, 2020 and April 01, 2021. According to the German Infection Protection Act all SARS-CoV-19 infections had to be reported to the local public health authorities. The included authorities were responsible for four distinct regions with a total population of 2.7 million combined around the university cities of Heidelberg, Freiburg, Tübingen, and Ulm including adjacent rural areas. Surviving persons were contacted by the local public health authorities via postal mail between late August and September 2021. All study materials (i.e., participant information, informed consent form, and a standardized questionnaire) were included in the letter. Participants were asked to provide written informed consent and to send the study materials (postage-paid) to the trustee office of the study center at the Freiburg University Medical Centre. The trustee separated the declaration of informed consent from the completed questionnaire and forwarded the latter to the data management center at Ulm University. The study was conducted according to the Declaration of Helsinki and ethical approval was obtained from the respective ethical review boards of the study centers in Freiburg (21/1484) and Ulm (337/21). It is registered with DRKS (“Deutsches Register Klinischer Studien”) (DRKS 00027012).
Further details and the overall study population are described in detail elsewhere [14]. In brief, of the 50457 eligible with confirmed SARS-CoV-2 infection invited to participate, N=12053 persons responded (response 24%). After excluding 343 participants of unknown age or undetermined sex, the resulting cohort consisted of 11710 participants. Only participants gainfully employed at the time before the acute infection and included in the German pension fund (“Deutsche Rentenversicherung”) were considered in the current analysis. Thus, we excluded 657 (6%) who were non-employed, 767 (7%) who studied or did training on the job, and 621 (5%) who were civil servants. In Germany, civil servants differ largely from the general population in terms of access to health care and characteristics of pension funds. We excluded further 93 (1%) participants who did not provide information on working capacity regained.
Data collection and measurements
Sociodemographic characteristics including birth sex (self-reported by the participant), were part of the standardized questionnaire, as were lifestyle factors, medical information on the acute SARS-CoV-2 infection (date of testing), and medical treatment during COVID-19. Participants were asked for the following occupation-related information: Occupation prior to infection, current sick leave, retraining since COVID-19, change of working hours, and the direction as well as the reason for the change. Thirty specific symptoms were requested prior to, during acute infection, and upon completion of the questionnaire (i.e., 6 to 12 months after infection). The response category for each of these questions was yes/no. Further symptoms could be added as free text. If one of the symptoms was present at the time of the survey, we asked for related medical treatment (yes/no) and whether and to which grade each symptom impaired daily life or activities using a four-point Likert scale (no such symptom, light, moderate, or strong). Current symptom clusters (not present before the acute SARS-CoV-2 infection) were identified using exploratory polychoric factor analysis (with oblimin rotation) based on symptom severity (not present, no impairment, light impairment, moderate impairment, or strong impairment; for further details see [14]).
WAI has been an important concept in occupational health research. The questionnaire comprises seven items, of which we used three established items to keep the questionnaire short: A self-assessment of current working capacity compared to the lifetime best (labelled as WAI1 in the original questionnaire), self-assessed work ability in relation to the mental (WAI2a) and physical demands (WAI2b), and the number of comorbid diseases (referring to different organ systems). For the current study, WAI was adapted to the COVID-19 situation through the following wording: WAI1: “What percentage of your original working capacity (before your infection) have you regained today?” The answer was possible on a 10-point Likert scale (10% steps from 0% to 100%). After assessing the demands of the work (response categories: mainly mental, roughly equally mental and physical, mainly physical), participants were asked to assess their actual work ability in relation to these demands on a five-point Likert scale (categories very good, good, moderate, poor, very poor) as follows: ”How would you assess your work ability according to your physical tasks now?”, and separately asked, “How would you assess your work ability according to your mental tasks now?” WAI2 was calculated by weighting the work ability according to the demands of work. If either WAI1 or WAI2 was missing, the summative index was set to a missing value (n = 253, 3%). Adapted to WAI, we further asked for comorbidities: “Which of the following conditions have been diagnosed by a physician? Please indicate whether this diagnosis was prior to or during/after the infection?” and provided categories such as “musculoskeletal diseases”, “cardiovascular diseases”, “mental disorders”, etc., with examples, according to the structure of the work ability index questionnaire. For each comorbidity, we asked if it was diagnosed prior to (yes/no) or during/after the SARS-CoV-2 infection (yes/no). Correlation and loadings of all variables on WAI were tested, showing moderate correlations and loadings (similar to those indicated for WAI as originally drafted). A summative score of WAI1 and WAI2 has shown to be strongly correlated to WAI [15] and was therefore used in the study as a modified work ability index (mWAI). To analyze the impact of physician-diagnosed comorbidities on mWAI, we did not include them in the index. A potential association of the following work-related and individual factors with WAI is discussed (see review [11]): sex, age, socioeconomic status, marital status, smoking, and obesity which led us to consider these variables as confounders. We used mWAI <15th percentile as an outcome variable for multivariable analyses as done by others [16].
Statistical analysis
Characteristics of the study population are shown. Quantitative variables are presented by means and standard deviation (SD) or median and IQR (interquartile range). We depict the severity of PCS symptom clusters (not present before the acute SARS-CoV-2 infection) in participants with mWAI <P15 by sex. Moreover, adjusted logistic regression with mWAI <P15 as outcome was calculated. To avoid multicollinearity, the following models were used: Model 1 comprised the established risk factors for low WAI (see review [11]), work demands, and medical treatment during acute infection. Model 2 further included the seven most prevalent PCS clusters (i.e., fatigue, neurocognitive impairment, chest symptoms, anxiety/depression, headache/dizziness, musculoskeletal pain, impaired smell/taste, details in [14]). Results according to age groups were depicted in the Supplement (please see Supplement). Model 3 consisted of Model 1 and physician-diagnosed comorbidities prior to or during/after the acute infection (please see Supplement). R2 was calculated. In the Supplement, the distribution of WAI1 is further given (patterns of working capacity regained after COVID-19 infection compared to pre-COVID). To account for a possible participation bias, we calculated the minimum possible prevalence of low WAI1, assuming that all non-responders had WAI1 of 100%, i.e., no loss of work ability compared to pre-infection work ability. The distribution of WAI1 according to age and sex is shown, along with models 2 and 3 stratified by work demands. Statistical procedures were performed with the SAS statistical software package (release 9.4 SAS Institute Inc.) and R version 4.1.2.
Results
The available study population comprised 9572 employees, among them 5548 (58%) women (details in Table 1). The average time since the infection was 8.5 months (SD: 1.6). Overall, 1906 (20%) employees received outpatient medical treatment, 273 (3%) inpatient treatment, and 73 (1%) were admitted to the intensive care unit (ICU) during acute infection.
Table 1.
Description of the study population (N = 9572) 9-12 months after the SARS-CoV-2-infection: sociodemographic and lifestyle factors, and COVID-19-related variables.
| Variables | Study population (N = 9572) | Men (n = 4024) | Women (n = 5548) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Sociodemographic characteristics | |||
| Age [years] | |||
| <30 | 1528 (16) | 555 (14) | 973 (18) |
| 30-<40 | 1827 (19) | 784 (20) | 1043 (19) |
| 40-<50 | 1817 (19) | 765 (19) | 1052 (19) |
| 50-<60 | 3104 (32) | 1278 (32) | 1826 (33) |
| ≥60 | 1296 (14) | 642 (16) | 654 (12) |
| Marital status | |||
| Single | 2390 (25) | 981 (25) | 1409 (26) |
| Married/living together | 6572 (70) | 2859 (72) | 3713 (68) |
| Living apart | 320 (3) | 90 (2) | 230 (4) |
| Widowed | 111 (1) | 18 (1) | 93 (2) |
| Duration of school education [years] ≥12 | 4917 (52) | 1989 (50) | 2928 (53) |
| Nationality German | 9019 (94) | 3756 (94) | 5263 (95) |
| Living area | |||
| Mostly urban | 5869 (63) | 2473 (63) | 3396 (63) |
| Partly urban | 1492 (16) | 629 (16) | 863 (16) |
| Mostly rural | 1955 (21) | 825 (21) | 1130 (21) |
| Lifestyle | |||
| Smoking status | |||
| Current | 992 (10) | 409 (10) | 583 (11) |
| Former | 2527 (27) | 1234 (31) | 1293 (23) |
| Never | 6029 (63) | 2368 (59) | 3661 (66) |
| Obesity (body mass index >30 kg/m2) | 1877 (20) | 867 (22) | 1010 (18) |
| COVID-19-related factors | |||
| Time since infection [months] Mean, Standard deviation | 8.5, 1.6 | 8.5, 1.7 | 8.6, 1.6 |
| Median (interquartile range) | 8.7 (7.5-9.7) | 8.7 (7.4-9.7) | 8.7 (7.6-9.7) |
| Medical treatment during COVID-19 infection | |||
| No | 7236 (76) | 3121 (78) | 4115 (75) |
| Outpatient treatment | 1906 (20) | 669 (17) | 1237 (23) |
| Inpatient treatment | 273 (3) | 159 (4) | 114 (2) |
| Admission to intensive care unit | 73 (1) | 51 (1) | 22 (0) |
A total of 6141 (65%) of the employees worked full-time prior to their infection (Table 2). The occupation at the time of completing the questionnaire (6 to 12 months after acute infection) reflected as much as a 5%-decrease in full-time employment. In total, 417 employees (4%) reported changed working hours due to health-related issues.
Table 2.
Description of the study population (N = 9572) 9-12 months after the SARS-CoV-2 infection: work- and COVID-19-related factors and average mWAI.
| Variables | Study population (N = 9572) |
Men (n = 4024) |
Women (n = 5548) |
|||
|---|---|---|---|---|---|---|
| n (%) | Median (IQR) | n (%) | Median (IQR) | n (%) | Median (IQR) | |
| Occupation pre-COVID-19 | ||||||
| Full-time | 6141 (65) | 18.0 (16.0-20.0) | 3611 (90) | 18.0 (16.0-20.0) | 2530 (46) | 18.0 (15.0-20.0) |
| Part-time | 2720 (29) | 17.0 (15.0-19.0) | 243 (6) | 18.0 (16.0-20.0) | 2477 (45) | 17.0 (15.0-19.0) |
| Mini-job | 639 (7) | 18.0 (16.0-20.0) | 143 (4) | 19.0 (17.0-20.0) | 496 (9) | 18.0 (16.0-20.0) |
| Current occupation | ||||||
| Not employed | 480 (5) | 16.0 (11.0-18.0) | 173 (4) | 16.0 (11.0-18.0) | 307 (6) | 16.0 (11.0-19.0) |
| Full-time | 5705 (60) | 18.0 (16.0-20.0) | 3416 (85) | 18.0 (16.0-20.0) | 2289 (42) | 18.0 (16.0-20.0) |
| Part-time | 2725 (29) | 17.0 (15.0-19.0) | 254 (6) | 18.0 (15.0-20.0) | 2471 (45) | 17.0 (15.0-19.0) |
| Mini-job | 469 (5) | 18.0 (16.0-20.0) | 106 (3) | 18.0 (16.0-20.0) | 363 (7) | 18.0 (16.0-20.0) |
| Study/training | 151 (2) | 18.0 (17.0-20.0) | 60 (2) | 19.5 (18.0-20.0) | 91 (2) | 18.0 (16.0-20.0) |
| Work demands | ||||||
| Mainly mental | 4546 (48) | 18.0 (16.0-20.0) | 2051 (52) | 18.0 (17.0-20.0) | 2495 (46) | 18.0 (16.0-20.0) |
| Mental and physical | 3946 (42) | 17.0 (15.0-19.0) | 1465 (37) | 18.0 (15.0-19.0) | 2481 (46) | 17.0 (15.0-19.0) |
| Mainly physical | 899 (10) | 17.0 (15.0-19.0) | 421 (11) | 18.0 (15.0-20.0) | 478 (9) | 17.0 (14.0-18.0) |
| Currently on sick leave | ||||||
| No | 9139 (96) | 18.0 (16.0-20.0) | 3859 (97) | 18.0 (16.0-20.0) | 5280 (96) | 18.0 (15.0-20.0) |
| Continuously since infection | 118 (1) | 7.0 (3.5-11.0) | 47 (1) | 8.0 (5.0-11.0) | 71 (1) | 7.0 (3.0-10.0) |
| Again | 241 (3) | 12.0 (8.0-16.0) | 91 (2) | 12.0 (7.0-17.0) | 150 (3) | 12.0 (8.0-15.0) |
| Change of working hours (compared to pre-COVID-19) | ||||||
| No | 7964 (85) | 18.0 (16.0-20.0) | 3417 (87) | 18.0 (16.0-20.0) | 4547 (84) | 18.0 (15.0-20.0) |
| Yes, due to other reasons | 965 (10) | 18.0 (16.0-20.0) | 353 (9) | 18.0 (16.0-20.0) | 612 (11) | 18.0 (16.0-20.0) |
| Yes, due to health issues | 414 (4) | 12.5 (10.0-15.0) | 165 (4) | 15 (10.0-15.5) | 249 (5) | 12.0 (10.0-15.0) |
| Direction of change | ||||||
| Decreased | 805 (62) | 15.0 (12.0-18.0) | 312 (63) | 15.0 (12.0-18.0) | 493 (61) | 15.0 (12.0-18.0) |
| Increased | 499 (38) | 18.0 (16.0-20.0) | 186 (37) | 18.0 (16.0-20.0) | 313 (39) | 18.0 (16.0-20.0) |
| Rehabilitation due to COVID-19 infection | ||||||
| No | 8862 (93) | 18.0 (16.0-20.0) | 3751 (94) | 18.0 (16.0-20.0) | 5111 (93) | 18.0 (16.0-20.0) |
| No, but planned | 430 (5) | 13.0 (10.0-15.0) | 149 (4) | 13.0 (10.0-16.0) | 281 (5) | 13.0 (11.0-15.0) |
| Yes, outpatient | 66 (1) | 15.0 (12.0-18.0) | 34 (1) | 17.0 (12.5-18.5) | 32 (1) | 15.0 (12.0-16.0) |
| Yes, inpatient | 163 (2) | 11.0 (7.0-14.0) | 70 (2) | 11.0 (7.0-15.0) | 93 (2) | 11.0 (7.0-14.0) |
IQR: Interquartile range, mWAI: Work ability index modified, low values indicate low work ability
The mWAI (with a possible score from zero to 20 points), filled out 6 to 12 months after acute infection and describing current work ability compared to pre-infection work ability, ranged from 2 to 20 points with an average of 16.9 (SD: 3.2), and a median of 18.0 points (IQR: 15.0-20.0) As shown in Table 2, the mWAI was lower in non- or part-time employed than in full-time employed or mini-jobbers. By categorizing mWAI at the 15th percentile (13.0 points), n = 1217 (13%) participants were regarded as having low work ability. Considering our response of 24% (and the potential of response bias) and under the extreme assumption that all non-responders had a maximum mWAI, this would correspond to a minimum possible prevalence of 2% for mWAI <P15.
Overall 52% of our analysis population reported having regained 100% of their working capacity compared to pre-COVID 6 to 12 months after acute infection, while 27% indicated a regained working capacity of ≤80% (see Supplement Figure 1). The minimum possible prevalence (accounting for potential response bias) was 5%. Women ≤40 years were more often affected by reduced working capacity compared to men and older age categories were also more affected (see Supplement Figure 2).
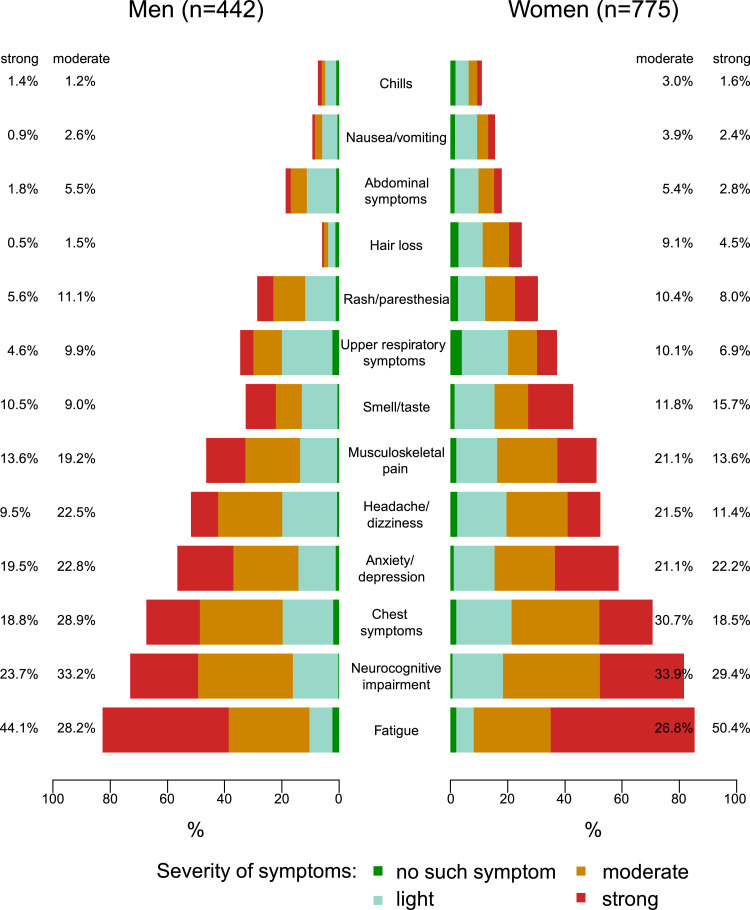
Fatigue (scored as moderate or strong) was the most common symptom cluster likewise in women and men with mWAI <P15 (see Figure 1), followed by neurocognitive impairment, chest symptoms and anxiety/depression with percentages of those affected moderately or strongly of >40% each.
Figure 1.
Severity of post-COVID syndrome symptom clusters among infected with low work ability (mWAI <P15, n = 1217) 9-12 months after the SARS-Cov2-infection. mWAI: Work ability index modified; P15: 15th percentile, current symptom clusters (not present before the acute SARS-CoV-2 infection).
Table 3 shows the association of established risk factors with low work ability (mWAI <P15). In a mutually adjusted model, medical treatment during acute infection was the factor most strongly related to mWAI <P15, with the highest odds ratio (OR) of 6.7 (95% confidence interval [CI] 4.0; 11.2) when admission to ICU had been reported. Age, smoking, obesity, and physical work demands (as opposed to mainly cognitive demands) were also associated with an increased OR for a mWAI <P15 (each OR ≥1.5). Nevertheless, with a R2 = 7.5%, the model fit was rather weak, but enhanced by introducing current PCS symptom clusters (R2 = 21.9%).
Table 3.
Prevalence difference and adjusted associations of potential risk factors with low work ability (mWAI <P15)a among the study population 9-12 months after the SARS-Cov2-infection.
| Risk factors | mWAI <P15 n/N | Pre-valence [%] | Prevalence difference[%] | Odds ratio (95% confidence interval) | |
|---|---|---|---|---|---|
| Sex | Male | 442/3912 | 11.3 | 0 | 1 |
| Female | 775/5407 | 14.3 | 3.0 | 1.3 (1.2; 1.5) | |
| Age [years] | <30 | 102/1490 | 6.8 | 0 | 1 |
| 30-<40 | 154/1789 | 8.6 | 1.7 | 1.2 (0.9; 1.6) | |
| 40-<50 | 243/1766 | 13.8 | 6.9 | 1.9 (1.4; 2.5) | |
| 50-<60 | 471/3027 | 15.6 | 8.7 | 1.9 (1.5; 2.5) | |
| ≥60 | 247/1247 | 19.8 | 13.0 | 2.6 (1.9; 3.4) | |
| Education [years] | <12 | 441/4525 | 16.2 | 6.5 | 1.2 (1.0; 1.3) |
| ≥12 | 774/4769 | 9.7 | 0 | 1 | |
| Marital status | Married/living together | 846/6403 | 13.2 | 0 | 1 |
| Single | 258/2323 | 11.1 | -2.1 | 1.2 (1.0; 1.4) | |
| Living apart | 68/314 | 21.7 | 8.5 | 1.4 (1.0; 1.9) | |
| Widowed | 21/107 | 19.6 | 6.4 | 1.0 (0.6; 1.7) | |
| Nationality | German | 1128/8801 | 12.8 | 0 | 1 |
| Other | 89/507 | 17.6 | 4.7 | 1.4 (1.1; 1.8) | |
| Smoking | Never | 670/5860 | 11.4 | 0 | 1 |
| Current | 152/968 | 15.7 | 4.3 | 1.2 (1.1; 1.4) | |
| Former | 391/2468 | 15.8 | 4.4 | 1.5 (1.2; 1.9) | |
| Obese (body mass index >30 kg/m2) | No | 846/7425 | 11.4 | 0 | 1 |
| Yes | 362/1822 | 19.9 | 8.5 | 1.5 (1.3; 1.7) | |
| Work demands | Mainly mental | 446/4494 | 9.9 | 0 | 1 |
| Mental and physical | 624/3932 | 16.5 | 6.0 | 1.5 (1.3; 1.7) | |
| Mainly physical | 147/893 | 15.9 | 6.5 | 1.5 (1.2; 1.8) | |
| Medical treatment during acute infection | No treatment | 598/7044 | 8.5 | 0 | 1 |
| Outpatient Treatment | 501/1861 | 26.9 | 18.4 | 3.6 (3.1; 4.1) | |
| Inpatient Treatment | 76/265 | 28.7 | 20.2 | 3.7 (2.8; 4.9) | |
| Admission to intensive care unit | 31/69 | 44.9 | 36.4 | 6.7 (4.0; 11.2) |
mWAI: work ability index modified; P15: 15th percentile for the model we only included the most prevalent seven symptom clusters, irrespective of mWAI<P15; clusters of symptoms (not present before the acute SARS-Cov-2 infection); bold letters indicate statistical significance P <0.05.
a: adjusted for sex, age, education, marital status, nationality, smoking, obesity, work demands, and medical treatment during acute infection; a prevalence difference of 0 indicates the comparison group.
Table 4 shows, that both symptom clusters fatigue (OR 2.8 [95% CI 2.2; 3.4]) and neurocognitive impairment (OR 3.3 [95% CI 2.7; 4.0]) were highly associated with mWAI <P15. Also, the symptom clusters chest symptoms and anxiety/depression nearly doubled the odds of mWAI <P15. However, as depicted in the Supplement Figure 3, the association between anxiety/depression and mWAI <P15 differ according to age groups with high odds of mWAI <P15 in the lowest and surprisingly, oldest age category.
Table 4.
Prevalence difference and mutually adjusted associations of post-COVID symptom clusters with low work ability (mWAI <P15)a among the study population 9-12 months after the SARS-Cov2-infection.
| Post-COVID symptom clusters | mWAI<P15 n/N |
Pre-valence[%] | Prevalence difference[%] | Odds ratio (95% confidence interval) | |
|---|---|---|---|---|---|
| Fatigue | No | 183/5505 | 3.3 | 0 | 1 |
| Yes | 987/3501 | 28.2 | 24.9 | 2.8 (2.2; 3.4) | |
| Neurocognitive impairment | No | 250/6081 | 4.1 | 0 | 1 |
| Yes | 913/2977 | 30.7 | 26.6 | 3.3 (2.7; 4.0) | |
| Chest symptoms | No | 362/6267 | 5.8 | 0 | 1 |
| Yes | 825/2822 | 29.2 | 23.5 | 1.9 (1.6; 2.2) | |
| Anxiety/depression | No | 500/7140 | 7.0 | 0 | 1 |
| Yes | 687/2010 | 34.2 | 27.2 | 1.9 (1.6; 2.3) | |
| Headache/dizziness | No | 557/7180 | 7.8 | 0 | 1 |
| Yes | 606/1885 | 32.1 | 24.4 | 1.6 (1.3; 1.9) | |
| Musculoskeletal pain | No | 593/7411 | 8.0 | 0 | 1 |
| Yes | 577/1620 | 35.6 | 27.6 | 1.6 (1.3; 1.8) | |
| Smell/taste | No | 704/6822 | 10.3 | 0 | 1 |
| Yes | 452/2147 | 21.1 | 10.7 | 1.4 (1.2; 1.6) |
mWAI: Work ability index modified; P15: 15th percentile; for the model we only included the most prevalent seven clusters of symptoms (not present before the acute SARS-Cov-2 infection), irrespective of WAI<P15; bold letters indicate statistical significance at P <0.05
a: adjusted for sex, age, education, marital status, nationality, smoking, obesity, work demands, medical treatment during acute infection, and the post-COVID symptom clusters indicated in the table; a prevalence difference of 0 indicates the comparison group.
Physician-diagnosed mental disorders during or after infection were highly associated with mWAI <P15 (OR: 8.0 [95% CI 6.1; 10.4], Supplement Table 1), similar to mental disorders already present prior to COVID-19 (OR: 3.6 [95% CI 3.0; 4.3]). Further comorbidities associated with mWAI <P15 (OR ≥2.0) in descending order were hormonal and metabolic disorders during/after infection and neurological and sensory disorders during/after infection.
After stratification for working tasks, neurocognitive impairment as PCS symptom cluster strongly impacted the work ability in mainly mentally working employees compared to employees with mental and physical or mainly physical work demands (OR mWAI <P15: 5.4 [95% CI 3.9; 7.6] vs OR 2.5 [95% CI 2.0; 3.2]), see Supplement Table 2. And, as might be expected, there is a higher burden of musculoskeletal diseases in employees with mWAI <P15 with mental and physical or mainly physical demands (OR mWAI <P15: 2.3 [95% CI 1.5; 3.5] vs OR 1.1 [95% CI 0.6; 2.0]) (see Supplement Table 3).
Discussion
In this large population-based study of employees aged 18-65 years evaluated 6 to 12 months after acute SARS-CoV-2 infection, risk factors for low work ability compared to pre-infection were female gender, age ≥60 years, mainly physical work demands, and medical treatment during acute infection. The most important symptom clusters (not present before the acute SARS-CoV-2 infection) associated with a low working ability (mWAI <P15) were neurocognitive impairment (OR 3.3) and fatigue (OR 2.8). These symptom clusters linked to low WAI are shown to be however age-dependent with higher mental health issues in the youngest age group. These PCS symptom clusters as well as physician-diagnosed mental disorders showed a high risk for low work ability and may be prioritized for targeting rehabilitation measures.
Kerksieck et al estimated the proportion of PCS patients who did not regain the full work capacity after 1 year to be 5.8% [17], a finding similar to our minimal positive result when we consider subjects with work ability ≤80%. Additionally, in line with our results, recently conducted research has shown that women had a larger reduction in work ability or later return to work due to PCS than men, (e.g., [18], [19], [20], [21], [22], [23]). Age had a non-linear relationship with the outcome in one study, with those who were younger or older having a higher likelihood of recovery whereas poor recovery was associated with middle age (40-59 years) [21]. Other research, like ours, however supported that older age might be associated with prolonged return to work or lower work ability (e.g., [7,8,19]). The differing results may be attributed to the workloads and the working conditions (e.g., manually vs cognitive) of the populations included or the different age ranges under study. Other predictors, such as organizational or work-organizational factors of return to work are less frequently analyzed. We observed lower mWAI in part-time or non-employed compared to full-time employees. However, women are more likely to work part-time which might explain this sex-effect related to part- vs full-time workers. Furthermore, a proportion of employees in our study changed their working hours for non-health-related issues. Mainly physical work demands corresponded to lower mWAI which is in line with the evidence that physicians had better improvement in physical health-related quality of life than nurses and healthcare assistants after COVID-19 in a study conducted in Italy [24]. Whether hospitalization or admission to ICU during acute infection (in terms of disease severity) may serve as predictors for low work ability or related outcomes is still under debate. In their recent review, Dirican and Bal [25] did not find an association between the initial COVID-19 disease severity and work ability except however for dyspnea and fatigue. The opposite was found in other studies (e.g., [8,[26], [27], [28]]).
Leading work ability obstacles comprised the post-COVID-symptom fatigue, the interaction between symptoms and job, lack of control over job pressures, and the organizational culture [29]. Lack of control over job pressure might further explain the lower work ability in healthcare assistants vs physicians. Fernández-de-Las-Peñas et al. identified the post-COVID symptoms, fatigue and dyspnea as diminishing functional ability [18]. Interestingly, post-COVID cognitive impairment [30] or post-COVID fatigue and a reduced cognitive function were associated with not being able to return to work in 77 employees [31], yet no similar association was found with post-COVID depression and anxiety [31]. There was also strong evidence for a difference in the association (i.e., effect modification) of the presence of self-reported COVID-19 related symptoms with current work ability between participants aged 40–64 years and those aged 18–39 years, with a higher reduction in work ability in the older group [17].
Further vulnerable subgroups for low work ability after SARS-CoV-2 infection may persist, specifically those affected by prior mental disorders. Higher age and a history of psychiatric diagnosis were associated with more substantial reductions in post-COVID work ability [17]. Since symptoms of PCS are unpredictable, cyclic and fluctuating, anxiety, stress, and uncertainty may potentially be provoked. Similarly, depression, anxiety, perceived stress, loneliness, and worry about COVID-19 were prospectively associated with a 1.3- to 1.5-fold increased risk of self-reported post–COVID-19 conditions, as well as increased risk of daily life impairment related to post–COVID-19 conditions [32].
During the pandemic, working from home has emerged as the ‘new normal’ especially for knowledge workers [33] with twice as many workers who worked from home full-time compared to prepandemic [34]. Thus, albeit recent efforts to reverse this development and implement suitable hybrid models, the workforce has changed considerably. This refers to, e.g., the frequency of meetings, emails, coaching from supervisors, and face-to-face interactions (see review [35]). Working from home might reduce physical and psychological work stress but promote presenteeism [36]. Flowingly, our findings concerning the employees’ work ability observed in an early stage of the pandemic might not easily be applied to the future course or to possible subsequent pandemics. Yet, our results might be generalized to populations with low rates of hospitalization since only 4% of our study population was hospitalized. However, this is in line with the data from the State Department of Health Protection, Infection Control and Epidemiology (State of Baden-Württemberg), in which the rate of PCR-positive tested patients who needed hospitalization during the first wave equaled 4%.
Nevertheless, the following limitations should be noted: The work ability index is assessed using a slightly modified questionnaire and we restricted our analyses to WAI1 and WAI2. Yet, a sum of these two variables (similar to WAI1 alone) is shown to be highly correlated to WAI [15]. A lack of medical validation of the self-reported nature of symptoms and comorbidities is evident. Also, recall bias must be considered, especially in subjects with neurocognitive impairments. Furthermore, we had a limited response with the possibility of selection bias (e.g., the potential for overestimation of prevalence measures). However, even with the extreme assumption that all non-respondents were not affected by low working capacity, the minimum possible prevalence of a working capacity less than 80% was 5%. In general, response was better in females compared to males and increased with age categories. However, weighting estimates for non-responders resulted in only marginally different estimates [14]. This still can result in a huge societal burden given the high number of infected individuals. As our study cohort was infected mainly with the wild type of SARS-CoV-2, generalizability to other variants is not warranted even if it has to be kept in mind that the risk of PCS decreased and return to work was more likely with each variant [9]. Overall, the prevalence of long-COVID was higher in individuals infected with the first variant compared to those infected with the Alpha, Delta or Omicron variants (see review [37]). Additionally, evidence suggests that vaccination before SARS-CoV-2 infection could reduce the risk of subsequent long-COVID (see reviews [38,39]). For instance, persons who were vaccinated twice reported significantly lowered risks of fatigue, headache, weakness of limbs, and persistent muscle pain [40].
Overall, post-COVID-related clusters such as neurocognitive impairment, fatigue, as well as chest symptoms, and anxiety and depression, most prominently, showed a high risk of low work ability compared to pre-infection as reference. The results may help to identify and target specific rehabilitation needs in order to early intervene and restore work ability.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This work was supported by Baden-Württemberg Federal State Ministry of Science and Art and the German pension fund (“Deutsche Rentenversicherung”) Baden-Württemberg ([grant numbers not applicable]). The funders did not influence the study design, collection, analysis, interpretation of data, writing of the manuscript, or the decision to submit the paper.
Author contributions
WVK led the study conceptualization and the development of the research question, supported by HGK, and AN. RSP, WVK, AN, and DR supervised the study. SOB and GK contributed to the methodology. RSP, AN, and DR were involved in data curation. SB, RSP, AN, and DR did the formal analysis. SOB, SG, UM, and JMS contributed to the validation. SB and RSP visualized the data. All authors were involved in drafting or critically revising the manuscript, and all authors approved the final version. SB and RSP contributed equally. DR and WVK contributed equally. SB and RSP have accessed and verified data. All had full access to all the data in the study and accept responsibility to submit for publication.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request with publication (see email address of the corresponding author).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2023.11.015.
Appendix. Supplementary materials
References
- 1.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11:269. doi: 10.3390/pathogens11020269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226:1593–1607. doi: 10.1093/infdis/jiac136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franco JVA, Garegnani LI, Oltra GV, Metzendorf MI, Trivisonno LF, Sgarbossa N, et al. Long-term health symptoms and sequelae following SARS-CoV-2 infection: an evidence map. Int J Environ Res Public Health. 2022;19:9915. doi: 10.3390/ijerph19169915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokolevich ZM, Crowe M, Mendez D, Biros E, Reznik JE. Most common long COVID physical symptoms in working age adults who experienced mild COVID-19 infection: a scoping review. Healthcare (Basel) 2022;10:2577. doi: 10.3390/healthcare10122577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacob L, Koyanagi A, Smith L, Tanislav C, Konrad M, van Der Beck S, et al. Prevalence of, and factors associated with, long-term COVID-19 sick leave in working-age patients followed in general practices in Germany. Int J Infect Dis. 2021;109:203–208. doi: 10.1016/j.ijid.2021.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westerlind E, Palstam A, Sunnerhagen KS, Persson HC. Patterns and predictors of sick leave after Covid-19 and long Covid in a national Swedish cohort. BMC Public Health. 2021;21:1023. doi: 10.1186/s12889-021-11013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aben B, Kok RN, de Wind A. Return-to-work rates and predictors of absence duration after COVID-19 over the course of the pandemic. Scand J Work Environ Health. 2023;49:182–192. doi: 10.5271/sjweh.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilmarinen J. The work ability index (WAI) Occup Med (Lond) 2006;57:160. doi: 10.1093/occmed/kqm008. [DOI] [Google Scholar]
- 11.van den Berg TIJ, Elders LAM, de Zwart BCH, Burdorf A. The effects of work-related and individual factors on the Work Ability Index: a systematic review. Occup Environ Med. 2009;66:211–220. doi: 10.1136/oem.2008.039883. [DOI] [PubMed] [Google Scholar]
- 12.Kujala V, Tammelin T, Remes J, Vammavaara E, Ek E, Laitinen J. Work ability index of young employees and their sickness absence during the following year. Scand J Work Environ Health. 2006;32:75–84. doi: 10.5271/sjweh.979. [DOI] [PubMed] [Google Scholar]
- 13.Tuomi K, Ilmarinen J, Seitsamo J, Huuhtanen P, Martikainen R, Nygård CH, et al. Summary of the Finnish research project (1981–1992) to promote the health and work ability of aging workers. Scand J Work Environ Health. 1997;23:66–71. [PubMed] [Google Scholar]
- 14.Peter RS, Nieters A, Kräusslich HG, Brockmann SO, Göpel S, Kindle G, et al. Post-acute sequelae of Covid-19 six to 12 months after infection: population based study. BMJ. 2022;379 doi: 10.1136/bmj-2022-071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebener M, Hasselhorn HM. Validation of short measures of work ability for research and employee surveys. Int J Environ Res Public Health. 2019;16:3386. doi: 10.3390/ijerph16183386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laitinen J, Näyhä S, Kujala V. Body mass index and weight change from adolescence into adulthood, waist-to-hip ratio and perceived work ability among young adults. Int J Obes (Lond) 2005;29:697–702. doi: 10.1038/sj.ijo.0802936. [DOI] [PubMed] [Google Scholar]
- 17.Kerksieck P, Ballouz T, Haile SR, Schumacher C, Lacy J, Domenghino A, et al. Post COVID-19 condition, work ability and occupational changes in a population-based cohort. Lancet Reg Health Eur. 2023;31 doi: 10.1016/j.lanepe.2023.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández-de-las-Peñas C, Palacios-Cena D, Gomez-Mayordomo V, Palacios-Ceña M, Rodríguez-Jiménez J, de-la-Llave-Rincón AI, et al. Fatigue and dyspnoea as main persistent post-COVID-19 symptoms in previously hospitalized patients: related functional limitations and disability. Respiration. 2021;101:132–141. doi: 10.1159/000518854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sansone D, Tassinari A, Valentinotti R, Kontogiannis D, Ronchese F, Centonze S, et al. Persistence of symptoms 15 months since COVID-19 diagnosis: prevalence, risk factors and residual work ability. Life (Basel) 2022;13:97. doi: 10.3390/life13010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taboada M, Moreno E, Cariñena A, Rey T, Pita-Romero R, Leal S, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth. 2021;126:e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans RA, McAuley H, Harrison EM, Shikotra A, Singapuri A, Sereno M, et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med. 2021;9:1275–1287. doi: 10.1016/S2213-2600(21)00383-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heightman M, Prashar J, Hillman TE, Marks M, Livingston R, Ridsdale HA, et al. Post-COVID-19 assessment in a specialist clinical service: a 12-month, single-centre, prospective study in 1325 individuals. BMJ Open Respir Res. 2021;8 doi: 10.1136/bmjresp-2021-001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen PA, Andersen MP, Gislason G, Phelps M, Butt JH, Køber L, et al. Return to work after COVID-19 infection – A Danish nationwide registry study. Public Health. 2022;203:116–122. doi: 10.1016/j.puhe.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grazzini M, Lulli LG, Mucci N, Paolini D, Baldassarre A, Gallinoro V, et al. Return to work of healthcare workers after SARS-CoV-2 infection: determinants of physical and mental health. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph19116811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dirican E, Bal T. COVID-19 disease severity to predict persistent symptoms: a systematic review and meta-analysis. Prim Health Care Res Dev. 2022;23:e69. doi: 10.1017/S1463423622000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodgson CL, Higgins AM, Bailey MJ, Mather AM, Beach L, Bellomo R, et al. The impact of COVID-19 critical illness on new disability, functional outcomes and return to work at 6 months: a prospective cohort study. Crit Care. 2021;25:382. doi: 10.1186/s13054-021-03794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skyrud K, Telle K, Magnusson K. Impacts of mild and severe COVID-19 on sick leave. Int J Epidemiol. 2021;50:1745–1747. doi: 10.1093/ije/dyab182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taboada M, Cariñena A, Moreno E, Rodríguez N, Domínguez MJ, Casal A, et al. Post-COVID-19 functional status six-months after hospitalization. J Infect. 2021;82:e31–e33. doi: 10.1016/j.jinf.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunt J, Hemming S, Burton K, Elander J, Baraniak A. What workers can tell us about post-COVID workability. Occup Med (Lond) 2022:kqac086. doi: 10.1093/occmed/kqac086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miskowiak KW, Johnsen S, Sattler SM, Nielsen S, Kunalan K, Rungby J, et al. Cognitive impairments four months after COVID-19 hospital discharge: pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/j.euroneuro.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delgado-Alonso C, Cuevas C, Oliver-Mas S, Díez-Cirarda M, Delgado-Álvarez A, Gil-Moreno MJ, et al. Fatigue and cognitive dysfunction are associated with occupational status in post-COVID syndrome. Int J Environ Res Public Health. 2022;19:13368. doi: 10.3390/ijerph192013368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Quan L, Chavarro JE, Slopen N, Kubzansky LD, Koenen KC, et al. Associations of depression, anxiety, worry, perceived stress, and loneliness prior to infection with risk of post–COVID-19 conditions. JAMA Psychiatry. 2022;79:1081–1091. doi: 10.1001/jamapsychiatry.2022.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hopkins J, Bardoel A. The future is hybrid: how organisations are designing and supporting sustainable hybrid work models in post-pandemic Australia. Sustainability. 2023;15:3086. doi: 10.3390/su15043086. [DOI] [Google Scholar]
- 34.Bick A, Blandin A, Mertens K. Work from home before and after the COVID-19 outbreak. Am Econ J Macroecon. 2023;15:1–39. doi: 10.1257/mac.20210061. [DOI] [Google Scholar]
- 35.McPhail R, Chan XW, May R, Wilkinson AA. Post-COVID remote working and its impact on people, productivity, and the planet: an exploratory scoping review. Int J Hum Resour Manag. 2023;0:1–29. doi: 10.1080/09585192.2023.2221385. [DOI] [Google Scholar]
- 36.Shimura A, Yokoi K, Ishibashi Y, Akatsuka Y, Inoue T. Remote work decreases psychological and physical stress responses, but full-remote work increases presenteeism. Front Psychol. 2021;12 doi: 10.3389/fpsyg.2021.730969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fernández-de-las-Peñas C, Notarte KI, Peligro PJ, Velasco JV, Ocampo MJ, Henry BM, et al. Long-COVID symptoms in individuals infected with different SARS-CoV-2 variants of concern: a systematic review of the literature. Viruses. 2022;14:2629. doi: 10.3390/v14122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marjenberg Z, Leng S, Tascini C, Garg M, Misso K, El Guerche Seblain C, et al. Risk of long COVID main symptoms after SARS-CoV-2 infection: a systematic review and meta-analysis. Sci Rep. 2023;13:15332. doi: 10.1038/s41598-023-42321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Notarte KI, Catahay JA, Velasco JV, Pastrana A, Ver AT, Pangilinan FC, et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. EClinicalmedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuodi P, Gorelik Y, Zayyad H, Wertheim O, Wiegler KB, Abu Jabal K, et al. Association between BNT162b2 vaccination and reported incidence of post-COVID-19 symptoms: cross-sectional study 2020-21, Israel. npj Vaccines. 2022;7:101. doi: 10.1038/s41541-022-00526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request with publication (see email address of the corresponding author).