Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that causes the death of motor neurons and consequent muscle paralysis. Despite many efforts to address it, current therapy targeting ALS remains limited, increasing the interest in complementary therapies. Over the years, several herbal preparations and medicinal plants have been studied to prevent and treat this disease, which has received remarkable attention due to their blood-brain barrier penetration properties and low toxicity. Thus, this review presents the therapeutic potential of a variety of medicinal herbs and their relationship with ALS and their physiopathological pathways.
Keywords: Central nervous system, reactive oxygen species, ALS, mtDNA, heat shock proteins, medicinal herbs
1. INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that causes neuron death in the cerebral motor cortex, brainstem, and spinal cord, restricting voluntary movements. Besides, ALS patients present difficulty in speaking, swallowing, and breathing caused by muscle paralysis [1]. Under normal conditions, the nervous impulse transmits messages from brain neurons (upper motor neurons) to spinal cord motor neurons (lower motor neurons) and posteriorly to each muscle [2]. In ALS, both upper and lower neurons degenerate or die and stop sending messages to the muscles, which leads to a gradual weakening of the musculature, which twitches until it atrophies. In cases involving the diaphragm and chest wall, the patient loses the ability to breathe and may experience respiratory failure, often fatal [3, 4]. There is no treatment available to cure and effectively reverse the progression of the disease, which may be related to its multi-faceted physiopathological mechanism [5]. Current treatment options are Riluzole and Edavarone, based on symptoms and respiratory support with only modest benefits. Significant genetic components and variants that cause or predispose ALS development remain unknown, which causes further delays in the development of new treatments [6]. With this obscure perspective, several alternatives have been taken into account in searching for new agents that could act on this disease. Interestingly, natural products have gained extensive importance as alternative therapeutic, as in most cases, they have low toxicity and high efficiency [7, 8]. Natural products originating from plants, animals, marine organisms, and microorganisms have long been an invaluable potential resource for discovering lead compounds and novel drugs [9]. These present many oxygen atoms, sp3-hybrid bridged or spiro-ring carbon atoms, and chiral centers, which differ from the structural backbones of synthetic compounds [10, 11]. These characteristics make modulation of multiple cellular signaling pathways easier and bind to multiple targets with selectivity [12]. As for neurodegeneration, when compared with synthetic and small molecules, a large number of natural products inhibit neuroinflammation and degeneration but also participate in the promotion of injured-neuron repair in some cases [13, 14]. The investigation of natural products can finally provide relevant structural information to be added to studies of rational planning of new drugs. Furthermore, new therapeutic approaches, such as adjuvant treatments in conjunction with synthetic molecules, may provide better results than those observed with isolated molecules [15, 16]. Currently, a variety of mechanisms are associated with the progression of neuronal degeneration in ALS. However, oxidative stress, excitatory amino acid toxicity, neuroinflammation, and calcium cytotoxicity are more frequently associated with natural products active against ALS.
2. OXIDATIVE STRESS AND ALS
The production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) is mainly secondary to enzymatic reactions involved in the respiratory chain, Cytochrome P-450 (CYP450) activity, prostaglandin synthesis, and phagocytosis [17]. Not surprisingly, the production of ROS is a key factor in the etiology of several neurodegenerative diseases [18]. However, a moderate increase of ROS secondary to mitochondrial activity produces preconditioning, which leads to a neuroprotective function against harmful agents [19, 20]. The role of cellular protection in response to ROS accumulation has been reported, and the signal of preconditioning that leads to this protection was related to Hormesis, which means a dose-response relationship of ROS, at low levels, generates a stimulus of neurodegenerative diseases, while its accumulation (high concentration) generates an inhibition of the effect through preconditioning [21-23].
These reactive species play an important role in redox signaling, in particular, ROS (O2- and H2O2), where through structural changes in the cysteine residues of proteins andiron-sulfur cluster proteins, it promotes the functioning of physiological signaling pathways that regulate processes of immunity, thermogenesis, aging, cognition, steroidogenesis, development and proliferation [24, 25].
Although, the problem lies in impaired redox homeostasis, which causes damage to the cell membrane, compromising the viability and integrity of the central nervous system (CNS). Mitochondrial damage compromises the ATP energy supply to neurons and calcium homeostasis. It leads to increased ROS levels, which in turn promotes mitochondrial DNA (mtDNA) mutation and lipid peroxidation of neuronal membranes [26, 27]. The accumulation of mtDNA causes an increase in oxidative damage, which leads to a decrease in the energetic rate and the production of more ROS (Fig. 1). This entire process of mitochondrial dysfunction is responsible for a series of neuronal damage, genetic mutations, and metabolic stress, which invariably can lead to apoptosis [28]. With regard specifically to ALS pathogenesis, it was discussed the role of antioxidant enzymes (i.e., metalloenzymes) as an inactivating agent of free oxygen radicals, converting them into a less harmful substance. The antioxidant enzyme SOD1 is mutated in the familial form of ALS and up to 7% of sporadic ALS [29].
Fig. (1).
Oxidative stress is caused by an imbalance between antioxidant defenses and RONS. OS and mitochondrial dysfunction have been identified as mechanisms involved in the pathogenesis of ALS and are related to neuroinflammation, aggregation of TDP-43, Lipoperoxidation, mutations in the mtDNA, aggregation of SOD1, and reduction of ATP to neurons.
Biomarkers of oxidative stress (OS) and high levels of ROS were observed in the CNS in ALS patients [30], in addition to certain types of ROS (H2O2 and O2•-) in affected cells [31]. Mutant forms of SOD1, an antioxidant enzyme that catalyzes the conversion of O2•- to H2O2 and O2, which is critical in OS regulation [32, 33], have been associated with loss of oxidative control and excessive production of ROS [34-36]. Thus, OS contributes to SOD1 aggregation, increasing mitochondrial dysfunction [37, 38]. Other significant oxidative changes, such as low levels of reduced glutathione (GSH) in erythrocytes and the motor cortex [39, 40] and a systemic pro-inflammatory state [41] in ALS patients, have been observed. Other studies have shown that oxidative damage to nerve cells, a hallmark of ALS, was related to neurodegeneration [42]. High levels of carbonylated proteins and advanced oxidation products were measured in the motor cortex and plasma of ALS patients [43, 44]. In addition, the relationship between vitagene, oxidative damage, and biological resilience mechanisms was also noted as important in neurodegenerative diseases [45-47]. The importance of vitagenes lies in their participation in the nuclear factor erythroid 2-related factor (Nrf2) antioxidant pathway. They play a crucial role in tissue maintenance and repair by releasing antioxidant proteins known as heat shock proteins (Hsp), including Hsp32, Hsp70, thioredoxin, and the sirtuin protein system. These proteins effectively combat different types of stress, such as oxidative and proteotoxic stress, as well as environmental and neurotoxic effects caused by reactive nitrogen species (RNS) or nitric oxide (NO) [21, 22, 45, 48].
Phytotherapeutic oxidizing agents can regulate oxidative stress, improve the antioxidant activity of various enzymatic and non-enzymatic systems, and maintain the expression and regulation of genes involved in ALS [49, 50]. Curcumin (Fig. 2) is a natural and liposoluble polyphenolic dye obtained from the crude rhizome of Curcuma longa. An expressive amount of studies on the anti-inflammatory and neuroprotective effects have been explored [51, 52]. An important study by Jiang et al. demonstrated that curcumin activates the nuclear factor Nrf2, which is a master transcriptional regulator of phase II antioxidant genes. The authors reported that curcumin led to a significant induction of phase II enzymes, which play an important role in protecting cells against stress through the removal of free radicals and detoxication. Thus, the activation of Nrf2 target genes promoted a reduction in the levels of ROS and attenuated oxidative damage and mitochondrial dysfunction [53]. It was later displayed that curcumin can even eliminate the excitability induced by the TAR DNA-binding protein 43 (TDP-43) [54]. Mutation of this protein is detected in patients with familial and sporadic ALS (ALSf and ALSs) [55, 56]. Dimethoxy Curcumin (DMC) (Fig. 2) also demonstrated significant effects on the TDP-43 protein, improving mitochondrial dysfunction in mutated TDP-43 stably transfected cell lines [57]. As already mentioned, ALS has a direct relationship with the pathological deposition of superoxide dismutase (SOD1). Thus, a strategy to combat ALS consists of inhibiting the formation of SOD1. With this objective, the study by Bhatia et al. demonstrated that curcumin inhibits DTT-induced SOD1 fibrillation and favors the formation of smaller and disordered SOD1 aggregates, in addition to a reduction in SOD1-mediated toxicity [58]. Recent work showed the wild-type TDP-43 increased the firing frequency of action potentials, and the mutant Q331K TDP-43 enhanced the firing frequency and decreased the threshold of action potentials to a higher level. Also, the mutant and wild-type TDP-43 induced a more rapid speed of recovery from fast and slow inactivation of the Nav channel, reducing the voltage dependency of slow inactivation [54]. DMC and curcumin significantly decrease the abnormities of action potentials and Nav channels [54]. Curcumin has shown positive neuroprotection results in preclinical in vitro and in vivo studies [59]. However, its low bioavailability due to limited absorption and rapid metabolism results in low serum concentrations and has been a critical point in its clinical development [60, 61]. To overcome this limitation, Tripodo et al. propose a drug delivery system formed by a carrier-in-carrier device for systemic delivery, targeting hydrophobic drugs mediated by micelle-loaded mesenchymal stromal cells to be administered by intravenous injection [62]. The curcumin-loaded micelles, sterilized by filtration, reached the maximum loading in mesenchymal stromal cells in a few minutes, and compared to free curcumin, an evident reduction of cytotoxicity on mesenchymal stromal cells was detected, demonstrating that this is an innovative drug delivery system. A study where rats were exposed to 500 ppm curcumin through diet for 4 weeks showed that supplementation with curcumin in the diet reduced the action of oxidative stress and might counteract the deleterious effects of traumatic brain injury [63]. Other studies have also been successful in improving the bioavailability and pharmacokinetic characteristics of curcumin [64, 65]. In a double-blind, randomized, placebo-controlled trial conducted for 12 months, patients with a definite or probable ALS diagnosis were randomly assigned to receive either nano curcumin (80 mg per day) or placebo, in add-on therapy to Riluzole. The treatment showed a reduction in death or dependence on mechanical ventilation (18% difference compared to the placebo group), which suggests that nano curcumin might improve the probability of survival as an add-on treatment, especially in patients with existing bulbar symptoms [66]. In another double-blind therapeutic trial, curcumin treatment shows encouraging results, demonstrating a slight slowdown in disease progression, improving aerobic metabolism and oxidative damage [50]. The effect of curcumin on microglial cells has also been studied and deserves notable attention, especially in tissue repair [67].
Fig. (2).
Phytochemicals antioxidant agents in ALS: Curcumin, DMC, Resveratrol, EGCG, 7,8-DHF, Quercetin, Fisetin, and Ampelopsin.
Continuing our discussion of polyphenols, these can be classified into different classes according to the number of phenolic rings in their structure and the substituents linked to the rings. There are four groups: phenolic acids, phenolic diterpenes, flavonoids, and volatile oils [68]. Resveratrol (3,5,4′-trihydroxystilbene) (Fig. 2) is a stilbenoid analog found in grapes, berries, peanuts, and others. Despite its low oral bioavailability, resveratrol has a variety of biological actions, including antioxidant and neuroprotective. It has been shown that resveratrol has positive effects by up-regulating sirtuin 1 (SIRT1) expression in the mutant SOD1G93A-bearing motor neuron-like culture model of ALS, improving the cell viability and ATP levels and preventing cell apoptosis [69]. Similarly, the antioxidant activity of resveratrol has previously been reported to be protective against ALS [70]. Mancuso et al. demonstrated that resveratrol exerts potent therapeutic actions in the SOD1G93A model of ALS [71]. The treatment significantly delayed disease onset and extended animal survival. Resveratrol exerted neuroprotective effects mainly through increasing the expression of SIRT1, suppressing oxidative stress, and downregulating p53 and its related apoptotic pathway [72]. In another complementary study, the effect of the combination of resveratrol and a sigma-1R agonist selective agent was evaluated. The combined treatment significantly ameliorated SOD1G93A mice, but it did not show a synergistic effect compared to treatment with isolated substances [73]. Similarly, Srinivasan et al. demonstrated that resveratrol treatment attenuated motor neuron loss, relieving muscle atrophy, and improved the mitochondrial function of muscle fibers in the SOD1G93A mouse model of ALS. The interaction between resveratrol and the mutant form SOD1G93A has also been evaluated by quantum chemical and molecular mechanics approaches [74]. In another study, researchers revealed that bone marrow mesenchymal stem cells extracted from ALS patients exhibited a decrease in AMPK/SIRT1 signaling, which was subsequently restored through resveratrol treatment [75]. Recently, it was observed that the resveratrol treatment significantly reduced thimerosal-induced neurotoxicity in SOD1G93A cells [76]. Hydroxytyrosol (HT) has received notable attention for its antioxidant properties [77-79]. This important component of the Mediterranean diet has been associated with increased survival and motor performance in transgenic mice that overexpress the human variant SOD1G93A [80]. This result was associated with the expression of myogenic factors and autophagy markers, in addition to a decrease in endoplasmic reticulum stress [80, 81]. Recently, a review addressed the potential of Genus Boswellia in the study of neurodegenerative disorders [82]. Although its direct relationship with ALS is new, Boswellic acid has been associated with anti-oxidant and anti-inflammatory properties that may be useful in ALS-related studies [83, 84]. Recently, Boswellic acid has been linked to neuroprotective effects against an experimental model of ALS induced by methylmercury (MeHg). It achieves this by modulating the Nrf2/HO-1 signaling pathway and facilitating the release of antioxidant and anti-inflammatory proteins. Consequently, it helps alleviate neuroinflammation and promotes remyelination [85].
Epigallocatechin gallate (EGCG) (Fig. 2) is another water-soluble polyphenol isolated from green tea [86]. Several pharmacological activities have been attributed to this compound, such as anticancer, antioxidant, and neuroprotective effects. These attributes are attributed to its capacity to traverse the blood-brain barrier and regulate the mitochondrial response to oxidative stress (OS) [87]. One of the first insights was reached by the group of Hockenbery et al. It was observed that the upregulation of the Bcl-2 gene, which inhibits most types of apoptotic cells, and the downregulation of the Bax gene, which can promote apoptosis, were detected in cells treated with EGCG [88]. It was later observed that EGCG promotes protection against lipid peroxidation, highlighting its in vitro antioxidant effects [89]. The neuroprotective potential of EGCG against OS-induced cell death was also reported through the restored reduced protein kinase C (PKC) and extracellular signal-regulated kinases (ERK1/2) activities [90, 91]. In another oxidative stress-induced apoptosis model, 6-Hydroxydopamine (6-OHDA) was used as an inducer in catecholaminergic PC-12 cells, and significant neuroprotective effects against apoptosis were reported, even better than the green tea polyphenol mixture [92]. This result was later reinforced in another study with the same cells [93]. Equally important, it has been shown that the EGCG can prevent OS-induced death of mutant SOD1 motor neuron cells by alteration of cell survival and death signals [94, 95]. In their first study, Koh et al. observed that EGCG demonstrates neuroprotective effects by upregulating PI3K/Akt and GSK-3 pathways in G93A mutant cells [96]. Later, the group evaluated the effect of ECGC in ALS model mice with the mutated gene SOD1G93A. Treatment was observed to significantly prolong symptom onset and lifespan, in addition to preserving signs of survival and reducing signs of death [97]. Similarly, a study performed by Xu et al. demonstrated the neuroprotective effects of EGCG in a transgenic mouse model of ALS SOD1G93A. Oral administration of EGCG from a pre-symptomatic stage has been shown to delay disease onset and prolong life span, in addition to showing an increased number of motor neurons, decreased microglial activation, reduced immunohistochemical reaction of NF-κB, and cleaved caspase-3, as well as, reduced protein levels of iNOS and NF-κB in the spinal cord [98]. More recently, a computational study was performed to examine the inhibitory action of EGCG against native and mutant SOD1, demonstrating that EGCG reduced the formation of toxic aggregates after mutation [99].
Many studies have been carried out to highlight the potential of flavonoids and polyphenols as antioxidant agents [100-104], including those for neurodegenerative diseases [105, 106]. In this class, 7,8-dihydroxyflavone (7,8-DHF) (Fig. 2) showed neuroprotective and neuromuscular transmission regulatory properties. This flavonoid selectively activates enhanced neuromuscular transmission via TrkB activation in the diaphragm muscle [107]. Later, the neuroprotective effects of 7,8-DHF in a transgenic ALS mouse model SOD1G93A were evaluated, demonstrating that chronic administration of 7,8-DHF significantly improved motor deficits and preserved spinal motor neurons count and dendritic spines [108]. Similarly, Quercetin (Fig. 2) has been shown to reduce OS-induced mitochondrial damage. As demonstrated in the work of Sharma et al., quercetin was able to reduce aluminum-induced oxidative stress in the rat hippocampus. In addition, they demonstrated that quercetin also prevents aluminum-induced translocation of cytochrome c, upregulates Bcl-2, down-regulates Bax, p53, and caspase-3 activation, and reduces DNA fragmentation [109]. It was later shown that quercetin and its derivative quercetin-3-β-D-glucoside were found as inhibitors of misfolding and aggregation in SOD1-associated ALS [110]. Fisetin (Fig. 2) was also recently cited as a new protector against ROS damage. Research has indicated that it offers neuroprotection with an improved survival rate, attenuated motor impairment, reduced ROS damage, and regulated redox homeostasis [111]. Furthermore, fisetin increased the expression of phosphorylated ERK, increased the expression of antioxidant factors, and reduced the levels of mutant and wild-type SOD1 [111].
Ampelopsin (Fig. 2) is a flavonoid isolated from Ampelopsis grossedentata, which is known to have antioxidant properties that can be studied in neurodegenerative processes [112, 113]. A pioneering study by Kou et al. demonstrated the potent antioxidant activity of Ampelopsin in PC-12 cells that underwent H2O2-induced apoptosis. The 1-hour treatment reduced vitality loss, inhibited ROS formation and prevented H2O2-induced p38 activation. Additionally, the treatment was effective in upregulating heme oxygenase-1 (HO-1) due to the activation of ERK and Akt signaling pathways [114]. Other natural oxidants related to the treatment of ALS have been reported. Ginkgo biloba L. or maidenhair is a well-known plant belonging to the Ginkgoaceae family, which has in its extract some flavonoid glycosides such as myricetin, kaempferol, and the aforementioned quercetin [115]. A standardized extract of Ginkgo biloba showed protective effects against mitochondrial damage and OS in a transgenic mouse model of ALS. The extract significantly improved motor performance and survival and protected against a loss of spinal-cord anterior motor horn neurons in male mutant transgenic ALS mice but not in littermate females [116]. Other studies have also reported the effects of Ginseng [117] and Genistein [118] in a transgenic mouse model of ALS.
Another OS-modulating substance is Vitamin E (α-tocopherol) (Fig. 3) [119]. A preliminary study showed that dietary supplementation with α-tocopherol delays the onset of clinical disease and slows progression in transgenic mice that express mutated copies of the gene encoding SOD1 [120]. In a double-blind, placebo-controlled, randomized clinical trial, it was shown that after 12 months of treatment, vitamin E administration did not influence survival and motor function in ALS. The utilization of an association with riluzole for 3 months allowed us to verify changes in the levels of biochemical markers for oxidative stress [121]. Later, regular use of vitamin E supplements was associated with a lower risk of dying from ALS [122-124]. However, the study performed by Graf et al. demonstrated no significant difference between placebo and treatment groups (treated with α-tocopherol) [125]. Furthermore, there are not enough results to state that megadoses of vitamin E can effectively reduce the progression of ALS [125-127]. Thus, further studies are needed to analyze the relationship between vitamin E and ALS. Carotenes such as β-carotene, astaxanthin, and lycopene have also been related to trials in which the intake of these pigments was associated with a reduced risk of ALS [128-131]. However, further studies should be performed for more conclusive results.
Fig. (3).
Phytochemicals antioxidant agents in ALS: Vitamin E, AST-IV, Madecassoside, Morroniside, Picroside-II, and DATS.
Some structurally diverse substances have also been reported as potent regulators of ROS in neurodegenerative processes. Astragaloside IV (AST-IV) (Fig. 3) is a saponin isolated from Radix astragali, often cited for its antioxidant properties [132, 133]. The study with a traditional Chinese formulation containing Radix astragali was carried out by Rong et al. The authors mentioned that the formulation's actives act by a cytoprotective mechanism against OS and that Radix astragali would be one of those responsible for the induction of HO-1 [134]. Later, it was reported that AST-IV could increase the activity and content of SOD in the cytoplasm, which is important in ALS [135]. AST-IV pretreatment was shown to attenuate the H2O2-induced loss of SH-SY5Y human neuronal cells in addition to decreasing apoptosis and attenuating ROS overproduction [136]. Recently, the protective effect against neuronal damage caused by microglia has been studied. AST-IV protected microglia from lipopolysaccharide-evoked death and down-regulated the release of pro-inflammatory mediators, including interleukin IL-1β, IL-6, tumor necrosis factor α (TNF-α), and nitric oxide, as well as, expression of Toll-like receptors 4 (TLR4), MyD88, and NF-κB [137].
A saponin associated with antioxidant activity against ALS is Madecassoside (Fig. 3). It is isolated from Centella Asiatica. This triterpenoid saponin was evaluated in an assay in mice expressing SOD1G93A, in which it was possible to observe a significant reduction in the decline in motor strength of mice during the slowest decline stage to the final stage of the disease [138]. In a model of hippocampal neuron injury induced by chronic aluminum intoxication, Madecassoside was shown to reduce neuronal damage caused by OS and inhibit apoptosis [139]. Recently, it was reported that the expression of pro-neuroinflammatory genes such as inducible nitric oxide synthase, cyclooxygenase-2, signal transducer, an activator of transcription 1, and nuclear factor-κB, was significantly downregulated in a dose-independent manner following treatment with Madecassoside [140]. In another study, Madecassoside was evaluated in an in vivo model for its potential in lipopolysaccharide-induced cognitive impairment and neuroinflammation, revealing that treatment for 14 days with 120 mg/kg reduced neurotoxicity, cognitive impairments and the production of inflammatory cytokine agents such as IL-1β, TNF-α, and IL-6 by a mechanism of activation of Nrf2 signaling [141].
Natural iridoid glycosides such as Morroniside and Picroside-II (Fig. 3) have been extensively studied as potentially useful antioxidants in treating neurodegenerative diseases, including ALS. They can be isolated from the dry ripe fruit of Cornus officinalis Sieb. et Zucc. (Cornaceae) and Pseudolysimachion rotundum var. subintegrum, respectively [142, 143]. As demonstrated in the studies performed by Wang et al., morronoside demonstrated inhibitory effects on ROS formation and activation of caspase-3 and 9, in addition to up-regulating Bcl-2 in SH-SY5Y cells of a model of H2O2-induced toxicity [144]. Using the same model, the group also verified that the morronoside reduced the intracellular accumulation of Ca2+ and decreased the mitochondrial membrane potential caused by the addition of H2O2. Additionally, morronoside significantly inhibited SOD1 activity and the percentage of cells in apoptosis in a dose-dependent manner [145]. After that, the group further reported on the potential of morronoside to protect the brain against damage induced by focal cerebral ischemia, probably related to its antioxidant activity in the brain [146]. A model of H2O2-induced cell death in SK-N-SH human neuroblastoma cells was evaluated, in which morronoside protected cells from oxidative damage by inhibiting ROS production while suppressing Bcl-2-associated X protein (Bax), stimulated the expression of Bcl-2 and blocking apoptosis [147].
On the other hand, Picroside-II was reported to be responsible for the marked increase in neurite outgrowth in PC-12 cells, activity attributed to picroside-II nerve growth factor (NGF) [148-150]. Two other models were evaluated, one in vitro (in PC-12 cells treated with glutamate) and another in vivo (in male mice treated with AlCl3). In the first, picroside II enabled an increase in cell viability and a reduction in glutamate-induced ROS. While in the in vivo model, picroside II markedly improved learning and memory dysfunctions. The authors attribute the positive results to the increase in SOD in the mouse brain, relating this substance to the potential against OS [151].
Diallyl trisulfide (DATS) (Fig. 3) is an organic sulfide derived from the Liliaceae allium plant [152]. It is known for its ability to cross the blood-brain barrier (BBB). DATS treatment activated HO-1, demonstrating a protective role, especially against oxidative and inflammatory damage [153]. DATS has been shown to cause activation of Nrf2 and Nrf2 target genes in rat spinal cord explants and protect motor neurons against glutamate-induced excitotoxicity [154]. Neuroprotective effects were also reported in a model of SOD1G93A transgenic mice. Oral administration of DATS-induced acid protein prolonged duration and extended life span for about one week, in addition to HO-1 and reduced glial fibril expression in the lumbar spinal cord of these transgenic mice [155]. In another work, Liu et al. demonstrated the action of DATS against motor neuron-like NSC34 cells overexpressing TDP-43, a pathological and biochemical marker for ALS. The treatment led to induced neuronal autophagy and lysosomal clearance of TDP-43 and C-terminal TDP-43 fragments. It was also observed that the Nrf2 was accumulated in the nucleus, and the expression of HO-1 and NAD(P)H: quinone oxidoreductase was increased [156]. Similarly, activation of Nrf2 was also demonstrated in an in vivo cerebral ischemia model, with increased SOD1 in the nucleus, SOD2, glutathione S-transferase and peroxidase in the cortex, and increased activity of catalase in the striatum, evidencing the protective effects of DATS [157]. All this evidence suggests the important role of antioxidant agents in neurodegenerative diseases, especially those associated with ALS [158, 159].
3. NEUROINFLAMMATION AND ALS
Foran extended period, neuroinflammation was considered only a consequence of motor neuron deathNowadays, it is established as an important factor for several neurodegenerative diseases. Neuroinflammation is mediated by activated glial cells and infiltrating lymphocytes, accompanied by the production of pro-inflammatory cytokines and neurotoxic or neuroprotective molecules [160]. During the initial periods of disease progression (neuroprotective phase), the immune system acts as a protector with glia and T cells, especially M2 macrophages/microglia, T helper 2 cells, and regulatory T-cells, providing anti-inflammatory factors that sustain motor neuron viability. During the period of rapid progression (cytotoxic phase), however, neuroinflammation presents a strong proinflammatory state, characterized by M1 macrophages/microglia and proinflammatory T-cells. In this phase, neuroprotective regulatory T-cells significantly decrease, and neurotoxicity predominates (Fig. 4) [161]. Numerous studies have highlighted the relationship between disease progression and markers of neuroinflammation [162]. Post-mortem analysis of cerebrospinal fluid and spinal cord samples in ALS cases revealed increased microglial activation and lymphocyte permeation [163, 164]. Other studies demonstrate that astrocytes have toxic properties, contributing to motorneuron death, while T-lymphocytes control the microglial response in a neuroprotective mode [161, 165]. These and various other mechanisms related to neuroinflammatory processes have been extensively described [162, 166]. Additionally, several compounds with anti-inflammatory properties have been reported to enhance motor neuron survival in transgenic mice.
Fig. (4).
Representation of the neuroprotective and cytotoxic phase of neuroinflammation. During the initial periods of disease progression (neuroprotective phase), the compensatory response is governed by T helper 2 cells (Th2)/regulatory T-lymphocytes (Tregs), Microglia M2, and astrocytes secreting neurotrophic factors and decreasing neuronal stress. During the rapid progression (cytotoxic phase), the motor neuron becomes damaged, and the inflammatory response becomes harmful. Created with BioRender.com.
Some agents against OS are also frequently reported as modulators of neuroinflammatory processes, such as the aforementioned curcumin [167-171] and resveratrol [172, 173]. In addition to these, other anti-inflammatory and anti-oxidative agents will be mentioned herein (Fig. 5). The first of these is Celastrol, which has already been reported in studies on its therapeutic potential in traditional Chinese medicine [174]. This triterpenoid pigment is extracted from Tripterygium wilfordii Hook F. and it was previously mentioned as an inhibitor of cancer cell proliferation, in addition to acting in the suppression of autoimmune and anti-inflammatory processes [175]. Kiaei et al. administered celastrol in the diet of SOD1G93A mice from their 30th day of life. The treatment promoted improvement in motor performance and delayed the onset of the disease. At the cellular level, lumbar spinal cord neuron cell counts demonstrated a protective effect. Additionally, celastrol down-regulated the expression of TNF-α and iNOS and the immunoreactivity of CD40 and GFAP (a marker of microglia and astrocytes, respectively) [176]. Later, another group reported that the excessive production of NO, TNF-α, and IL-1β induced by lipopolysaccharide in BV-2 cells was inhibited by celastrol. The underlying mechanisms associated were the inhibition of ERK1/2 MAPK phosphorylation and the DNA binding activity of NF-κB [177]. More recently, celastrol has also attenuated cadmium-induced neuronal apoptosis via the calcium-dependent Akt/mTOR pathway [178]. Paeonol is another important antioxidant and anti-inflammatory agent [179]. In a model of injury based on glutamate-induced apoptosis of pheochromocytoma cells, Paeonol prevents, in a dose-dependent manner, cell death and mitochondrial injury, in addition to reducing caspase-3 and p-ERK activity [180]. In another model, inflammation in microglia was induced by lipopolysaccharides, in which paeonol attenuated the overexpression of NO-synthase and cyclooxygenase 2, promoting the reduction of NO and prostaglandin E2 (PGE2). Additionally, paeonol reduced the production of ROS and upregulated HO-1. Also, in this work, the authors observed that in cortical neurons treated with 6-hydroxydopamine, paeonol attenuated the production of ROS, increased the activity of SOD1 and the expression of the antiapoptotic protein B-cell lymphoma 2 [181]. Most of these results were re-reported later [182].
Fig. (5).
Anti Neuroinflammatory agents in ALS: Celastrol, Paeonol, Obovatol, IRN, Ginkgolide.
Obovatol is a neolignan monomer and has been isolated from Magnolia officinalis fresh leaves [183]. In an important study conducted by Ock et al. in lipopolysaccharide-stimulated BV-2 microglial cells. After the administration of obovatol at a concentration of 10 mM, inhibition of NO production and microglial expression of pro-inflammatory cytokines were observed. In addition to the inhibition of multiple signaling pathways, such as NF-κB, STAT1 (signal transducers and activators of transcription 1), and MAPK (mitogen-activated protein kinase). In addition to these results, the authors confirmed that obovatol protects neurons from microglial toxicity and inhibits neuroinflammation in an in vivo model [184]. In this study, the molecular target of obovatol in microglia peroxiredoxin 2 (Prx2) was identified by affinity chromatography, and it was demonstrated that obovatol enhanced the ROS-scavenging activity of Prx2 in vitro, suppressing proinflammatory signaling pathways of microglia [184]. The importance of Prdx2 in neurodegenerative diseases has been recently reviewed [185].
In a work by Yuan et al. Isorhynchophylline (IRN), a component isolated from Uncaria rhynchophylla was presented as an attenuating agent of proinflammatory cytokines production such as TNF-α and IL-1β as well as NO in mouse N9 microglial cells, with potent inhibition of microglial activation. The authors report that the potential molecular mechanism for IRN-mediated attenuation was implicated in suppressions of iNOS protein level, phosphorylation of ERK and p38 MAPKs, and degradation of IκBα [186]. Wogonin is an active ingredient from Scutellaria roots. This flavonoid has been shown to diminish lipopolysaccharide-induced TNF-α, IL-1β, and NO production in a dose-dependent manner. This last one was accompanied by suppression of iNOS induction and NF-κB activation in BV-2 microglia. Additionally, the inhibition of microglial activation by Wogonin promotes the reduction in microglial cytotoxicity in PC-12 cells. Finally, in vivo, experiments demonstrated that wogonin was protective against experimental brain injury [187].
Ginkgolide is a diterpene lactone extracted from Ginkgo biloba leaves that can present in forms A, B, C, J, and M. It has been proposed that Ginkgolide A and B can inhibit NO production in lipopolysaccharide-stimulated microglia [188]. Several studies report the role of this class in the modulation of neuroinflammation caused by neurodegenerative processes [189-192]. In addition, Chrysin and inflexin have also been reported as potential agents in neuroinflammation [193, 194].
4. EXCITATORY AMINO ACID TOXICITY AND ALS
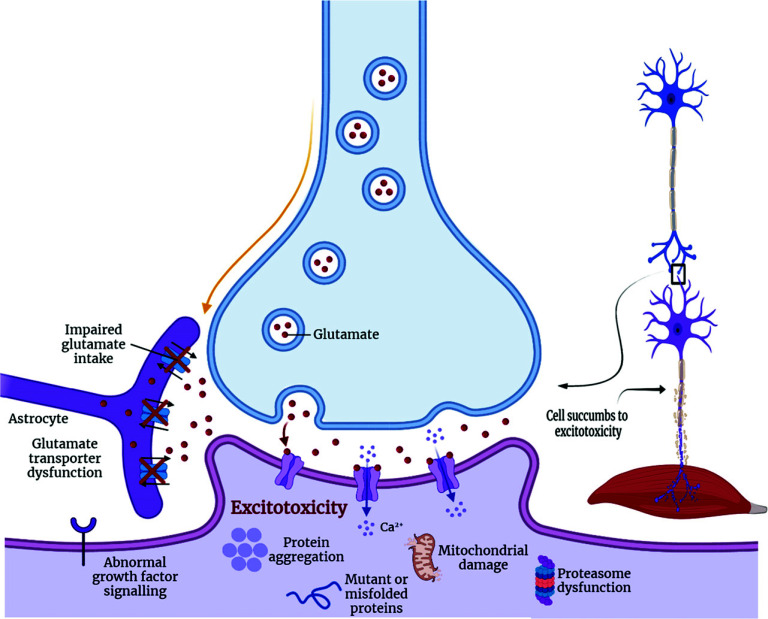
The amino acid glutamate plays an important role within the CNS, acting as an excitatory neurotransmitter in addition to protein biosynthesis [195]. When released into the synaptic cleft, glutamate activates a family of ligand-gated ion channels called ionotropic receptors (named NMDA, AMPA, and KA). Upon termination of excitatory activity, glutamate is reuptake by specific Na+-dependent systems, located mainly in surrounding astrocytes, known as excitatory amino acid transporter-2 (EAAT-2) in humans and glutamate transporter-1 in rodents (GLT-1). Finally, glutamate is converted to glutamine, which has no neurotransmitter properties [196]. The excitotoxicity event is caused by excessive and unregulated activation of glutamate receptors. Prolonged exposure of these receptors to high or persistently increased concentrations of glutamate can lead to cell death [197]. Receptor hypersensitivity causes an influx of calcium through ionotropic receptors, leading to the activation of degradative enzymes, including phospholipase A2, proteases, and iNOS, which directly cause cell death and tissue damage. Elevations in intracellular calcium can disrupt mitochondrial function, leading to free radical production and impaired ATP production (Fig. 6) [198]. The relationship between excitotoxicity and ALS can be exemplified by the finding that the cerebrospinal fluid of patients with the disease had three-fold higher concentrations of glutamate, aspartate, N-acetyl-aspartyl glutamate, and N-acetyl aspartate when compared to healthy controls [199]. In addition, increased plasma glutamate levels have been reported in ALS patients [200], as well as decreased neurotransmitter uptake and reduced levels of EAAT-2 transporter expression [201]. The pathogenic role of glutamate is even more evident as the only current treatment for ALS, riluzole, is based on glutamatergic activity in the CNS.
Fig. (6).
Glutamatergic transmission in ALS under hyperexcitability conditions. The release of glutamate by the presynaptic neuron stimulates the receptors on the postsynaptic neuron generating excitatory postsynaptic potentials. In ALS, presynaptic hypersensitivity causes excessive glutamate release. Created with BioRender.com.
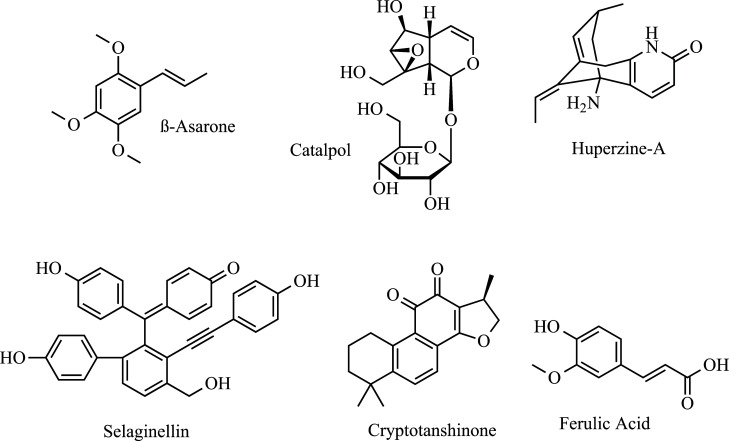
Several natural products are reported to act on the mechanism of excitotoxicity (Fig. 7). β-Asarone can be isolated from Acorus tatarinowii Schott of Araceae plants. A study conducted by Cho et al. demonstrated that β-asarone exhibits neuroprotective action against NMDA or glutamate-induced excitotoxicity by blocking NMDA receptor function [202]. This result was later reported again, in addition to promoting decreased LDH leakage [203]. Catalpol is an iridoid glucoside that is isolated from the roots of Rehmannia glutinosa. Using a cell line of apoptosis induced by H2O2, it was observed that catalpol was able to suppress the down-regulation of Bcl-2, up-regulation of Bax, and the release of mitochondrial cytochrome c to the cytosol, in addition to attenuating the activation of caspase-3, PARP cleavage, and protect against apoptosis [204]. Huperzine A (Hup-A), an alkaloid from Huperzia serrata, reduces glutamate-mediated Ca2+ signaling [205]. Hup-A has been shown to attenuate excitatory amino acid toxicity by blocking the NMDA ion channel and subsequent mobilization of Ca2+ at or near PCP and MK-801 ligand sites [206]. Hemendinger et al. proposes that Hup-A modulates Ca2+ levels through the Cdk5 activation pathway, which activates anti-apoptotic factors [207]. Selaginellin, a pigment extracted from Saussurea pulvinata, acts against glutamate-induced toxicity in PC-12 cells, reducing ROS levels and regulating the klotho gene [208]. Cryptotanshinone is a derivative from Salvia miltiorrhiza acting on glutamate-induced toxicity, protecting primary cortical neurons from neurotoxicity through activation of the phosphoinositide 3-kinase/Akt (PI3K/Akt) pathway [209, 210].
Fig. (7).
Anti excitatory amino acid agents in ALS: β-Asarone, Catalpol, Huperzine-A, Selaginellin, Cryptotanshinone, and Ferulic Acid.
Ferulic acid is a compound found in a variety of herbs and is of neuropharmacological interest as it readily penetrates the BBB [211]. In a culture of cortical neurons with glutamate-induced apoptosis, sodium ferulate activated caspase-3 protein expression and PARP cleavage and inhibited the upregulation of glutamate-induced μ-calpain protein level. The mechanism appears to be via the PI3K/Akt/p70S6K and MEK/ERK1/2 pathways. In addition, there was inhibition of glutamate-induced reduction in Bcl-2 expression [212]. It was later reported that this cinnamic derivative reduced hippocampal neuronal apoptosis and oxidative stress and protected PC-12 cells against the influx of Ca2+, malondialdehyde, and production of glutathione peroxidase. The mechanism appears to be inhibition of the Toll-like receptor/myeloid differentiation factor 88 (TLR4/MyD88) signaling pathway [213]. Recently, ferulic acid has been associated with a protective effect against H2O2-induced apoptosis through the inhibition of phosphorylation of the extracellular signal-regulated kinase (ERK) [214]. Finally, some species can be cited as potential candidates for treatment due to glutamate excitotoxicity, such as saponins from Acanthopanax senticosus [215] and Sea cucumber (morina root) [216].
5. CALCIUM CYTOTOXICITY AND ALS
A particular feature of ALS-affected motor neurons is related to their vulnerability to calcium overload, especially due to the high expression of Ca2+ permeable AMPA receptors [217, 218]. As already mentioned in excitatory amino acid toxicity, disturbances in Ca2+ homeostasis and protein folding are essential features of neurodegeneration since the correct protein folding is driven by folding proteins regulated by intracellular levels of Ca2+ [219]. Under physiological conditions, the release of Ca2+ in the endoplasmic reticulum is controlled by ryanodine receptors (RyR), channels controlled by inositol 1,4,5-triphosphate receptors (IP3R), and the translocon [220]. Internal control is performed by the Sarco/endoplasmic reticulum Ca2+ATPase (SERCA) and in the plasma membrane by sodium/Ca2+ and Ca2+ATPase. In mitochondria, Ca2+ uptake is performed by mUP, also controlled by cytosolic Ca2+ and by Ca2+/calmodulin. Ca2+ in the mitochondria can be ejected back into the cytosol via Na+/Ca2+ and 2H+/Ca2+ exchangers. When the concentration of Ca2+ within the mitochondria rises to a considerable level, the mPTP channel opens, leading to cell death by either apoptosis or necrosis [219]. Abnormalities of Ca2+ homeostasis, endoplasmic reticulum, and mitochondria are associated with motor neuron toxicity in ALS (Fig. 8) [221]. It is also known that both protein misfolding and Ca2+ overload can induce apoptosis through Bcl-2-dependent mechanisms [221].
Fig. (8).
The endoplasmic reticulum mitochondria Ca2+ cycle and receptors involved in calcium transport.
Paeoniflorin (Fig. 9) is the main component of Paeoniae radix and appears to play an important role in neuroprotection. Mao et al. reported that glutamate-induced neurotoxicity in PC-12 cells was evaluated. It was observed that paeoniflorin increased cell viability, inhibited apoptosis, decreased intracellular ROS and malondialdehyde levels, and increased SOD1 activity. Additionally, the treatment enabled a reduction in the Ca2+ overload [222]. Later, the same group demonstrated that the monoterpene glycoside decreased lactate dehydrogenase release in NMDA-treated PC-12 cells [223, 224]. Gastrodin (Fig. 9), another plant-derived compound, has been investigated for its ability to traverse the blood-brain barrier (BBB). This Gastrodia elata derivative showed potential in reducing intracellular Ca2+ levels via voltage-gated Ca2+ channels [225]. Muscone (Fig. 9), derived from natural musk, inhibited Glu-induced apoptosis in PC-12 cells by a mechanism related to the inhibition of intracellular Ca2+ overload and maintenance of mitochondrial membrane potential [226]. Finally, Ligustrazine (Fig. 9) demonstrated inhibitory effects on L-type calcium current in SH-SY5Y human neuroblastoma [227].
Fig. (9).
Anti-calcium cytotoxicity compounds: Paeoniflorin, Gastrodin, Muscone, and Ligustrazine.
CONCLUSION
While there is still no effective therapy for curing ALS, using herbs and plant-based products has become a promising alternative (Table 1). Traditional Chinese medicine has brought several complementary possibilities for treating the disease and has contributed significantly to the search for new modulators of the disease. Through what has been exposed, it is possible to understand that the treatment of ALS can benefit from herbal medicine. Additionally, tests with monomers or active substances isolated in vivo and in vitro must be performed; the mechanisms must be conclusively elucidated, and, in the future, clinical trials will be important for the safe clinical use of these substances.
Table 1.
Summary of natural compounds and activities related to the treatment of amyotrophic lateral sclerosis (ALS).
| Class of Pharmacological Activity and Compound Name | Evidence of Pharmacological Activity Related to ALS |
|---|---|
| Oxidative Stress | |
| Curcumin and Dimethoxy Curcumin (DMC) | Activate Nrf2; protect cells against stress through the removal of free radicals and detoxication; eliminate the excitability induced by TDP-43. Inhibits DTT-induced SOD1 fibrillation and favors the formation of smaller and disordered SOD1 aggregates, reduction in SOD1-mediated toxicity; assessed in In vivo and in vitro studies, with clinical trials. |
| Resveratrol | Up-regulating SIRT1 expression in the mutant SOD1G93A-bearing motor neuron-like culture model of ALS, improving the cell viability and ATP levels and preventing cell apoptosis. |
| Epigallocatechin gallate (EGCG) | Inhibits most types of the apoptotic cell by up-regulation of Bcl-2, downregulating the Bax gene; protecting against lipid peroxidation; preventing OS-induced death of mutant SOD1; upregulating PI3K/Akt and GSK-3 pathways in G93A mutant cells; assess in In vivo and in vitro studies. |
| 7,8-dihydroxyflavone (7,8-DHF) | Enhanced neuromuscular transmission via TrkB activation in diaphragm muscle preserves spinal motor neuron count, and dendritic spines; assessed in In vitro studies. |
| Quercetin | Prevents aluminum-induced translocation of cytochrome c, upregulates Bcl-2, down-regulates Bax, p53, and caspase-3 activation, and reduces DNA fragmentation; assess in In vivo and in vitro studies. |
| Firestin | Increased the expression of phosphorylated ERK, increased the expression of antioxidant factors, and reduce the levels of mutant and wild-type SOD1. |
| Ampelopsin | Prevented H2O2-induced p38 activation; Upregulation of HO-1 due to the activation of ERK and Akt signaling pathways; assessed in In vitro and in vivo studies. |
| Vitamin E | Using it as an adjuvant treatment with riluzole allowed us to verify changes in the levels of biochemical markers for oxidative stress; assessed in In vivo studies. |
| Astragaloside IV | Attenuate the H2O2-induced loss; Decrease apoptosis and attenuate ROS overproduction; Protects microglia from lipopolysaccharide-evoked death and down-regulated the release of pro-inflammatory mediators IL-1β, IL-6, TNF-α, and NO, and the expression of TLR4, MyD88, and NF-κB. |
| Madecassoside | Reduce neuronal damage caused by OS and inhibit apoptosis; reduce neurotoxicity, cognitive impairments, and the production of inflammatory cytokine agents (IL-1β, TNF-α, and IL-6 by activation of Nrf2 signaling); assessed in in vitro and in vivo studies. |
| Morronoside | Inhibitory effects on ROS formation and activation of caspase-3 and 9, up-regulate Bcl-2 in SH-SY5Y cells of a model of H2O2-induced toxicity. Reduce the intracellular accumulation of Ca2+ and decrease the mitochondrial membrane potential caused by the addition of H2O2. |
| Picroside-II | Increase in neurite outgrowth in PC-12 cells. Enable an increase in cell viability and a reduction in glutamate-induced ROS; assess in In vitro and In vivo studies. |
| Dially Trissufide | DATS treatment activated HO-1. Cause activation of Nrf2 and Nrf2 target gene in rat spinal cord, protected motor neurons against glutamate-induced excitotoxicity. Induces neuronal autophagy and lysosomal clearance of TDP-43 and C-terminal TDP-43 fragments. |
| Neuroinflammation | |
| Celastrol | Down-regulates the expression of TNF-α and iNOS and the immunoreactivity of CD40 and GFAP; Inhibition of excessive production of NO, TNF-α, and IL-1β induced by lipopolysaccharide in BV-2 cells; attenuates cadmium-induced neuronal apoptosis via the calcium-dependent Akt/mTOR pathway. |
| Paeonol | Prevent cell death and mitochondrial injury; Reduces caspase-3 and p-ERK activity; Attenuates the overexpression of NO-synthase and cyclooxygenase 2; Reduced the production of ROS-upregulated HO-1. |
| Obovatrol | Inhibition of NO production, microglial expression of pro-inflammatory cytokines, multiple signaling pathways (NF-κB, STAT1, and MAPK); Protect neurons from microglial toxicity; inhibit neuroinflammation; assess in In vivo studies. |
| Isorhynchophylline | Attenuates proinflammatory cytokines production such as TNF-α and IL-1β as well as NO in mouse N9 microglial cells; Suppression of iNOS protein level, phosphorylation of ERK and p38 MAPKs, and degradation of IκBα. |
| Wogonin | Diminishes lipopolysaccharide-induced TNF-α, IL-1β, and NO production in a dose-dependent manner; Promotes the reduction in microglial cytotoxicity in PC-12 cells; Protective against experimental brain injury (in vivo). |
| Ginkgolide A | Inhibits NO production in lipopolysaccharide-stimulated microglia; Modulation of neuroinflammation caused by neurodegenerative processes. |
| Excitatory Amino Acid Toxicity | |
| β-asarone | Neuroprotective action against NMDA or glutamate-induced excitotoxicity by blocking NMDA receptor function; Promotes a decrease in LDH leakage. |
| Catalpol | Suppress the down-regulation of Bcl-2 and up-regulation of Bax; Release mitochondrial cytochrome c to the cytosol; Attenuates the activation of caspase-3 and PARP cleavage and protects against apoptosis. |
| Huperzine-A | Reduces glutamate-mediated Ca2+ signaling; Attenuates excitatory amino acid toxicity; Modulates Ca2+ levels through the Cdk5 activation pathway. |
| Selaginellin | Acts against glutamate-induced toxicity in PC-12 cells, reducing ROS levels and regulating the Klotho gene. |
| Cryptotanshinone | Acts on glutamate-induced toxicity, protecting primary cortical neurons from neurotoxicity through activation of the phosphoinositide 3-kinase/Akt. |
| Ferulic Acid | Activated caspase-3 protein expression and PARP cleavage and inhibited the upregulation of glutamate-induced μ-calpain protein level; Inhibition of glutamate-induced reduction in Bcl-2 expression. |
| Anti-calcium Cytotoxicity | |
| Paeoniflorin | Increases cell viability, inhibits apoptosis, decreases intracellular ROS and malondialdehyde levels, and increases SOD1 activity. |
| Gastrodin | Reduces intracellular Ca2+ level via voltage-gated Ca2+ channels. |
| Muscone | Inhibits Glu-induced apoptosis in PC-12 cells by a mechanism related to inhibition of intracellular Ca2+ overload and maintenance of mitochondrial membrane potential. |
| Ligustrazine | Inhibits L-type calcium current in SH-SY5Y human neuroblastoma. |
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ALS
Amyotrophic Lateral Sclerosis
- BBB
Blood-brain Barrier
- CNS
Central Nervous System
- DATS
Diallyl Trisulfide
- DMC
Dimethoxy Curcumin
- EGCG
Epigallocatechin Gallate
- HT
Hydroxytyrosol
- NO
Nitric Oxide
- OS
Oxidative Stress
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- RyR
Ryanodine Receptors
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Taylor J.P., Brown R.H., Jr, Cleveland D.W. Decoding ALS: From genes to mechanism. Nature. 2016;539(7628):197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zarei S., Carr K., Reiley L., Diaz K., Guerra O., Altamirano P., Pagani W., Lodin D., Orozco G., Chinea A. A comprehensive review of amyotrophic lateral sclerosis. Surg. Neurol. Int. 2015;6(1):171. doi: 10.4103/2152-7806.169561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gil J., Funalot B., Verschueren A., Danel-Brunaud V., Camu W., Vandenberghe N., Desnuelle C., Guy N., Camdessanche J.P., Cintas P., Carluer L., Pittion S., Nicolas G., Corcia P., Fleury M.C., Maugras C., Besson G., Le Masson G., Couratier P. Causes of death amongst French patients with amyotrophic lateral sclerosis: A prospective study. Eur. J. Neurol. 2008;15(11):1245–1251. doi: 10.1111/j.1468-1331.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 4.Spataro R., Lo Re M., Piccoli T., Piccoli F., La Bella V. Causes and place of death in Italian patients with amyotrophic lateral sclerosis. Acta Neurol. Scand. 2010;122(3):217–223. doi: 10.1111/j.1600-0404.2009.01290.x. [DOI] [PubMed] [Google Scholar]
- 5.Turner M.R., Hardiman O., Benatar M., Brooks B.R., Chio A., de Carvalho M., Ince P.G., Lin C., Miller R.G., Mitsumoto H., Nicholson G., Ravits J., Shaw P.J., Swash M., Talbot K., Traynor B.J., Van den Berg L.H., Veldink J.H., Vucic S., Kiernan M.C. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12(3):310–322. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mejzini R., Flynn L.L., Pitout I.L., Fletcher S., Wilton S.D., Akkari P.A. ALS genetics, mechanisms, and therapeutics: Where are we now? Front. Neurosci. 2019;13:1310. doi: 10.3389/fnins.2019.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torquato H., Goettert M., Justo G., Paredes-Gamero E. Anti-cancer phytometabolites targeting cancer stem cells. Curr. Genomics. 2017;18(2):156–174. doi: 10.2174/1389202917666160803162309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J., Lee H.J., Lee K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010;112(6):1415–1430. doi: 10.1111/j.1471-4159.2009.06562.x. [DOI] [PubMed] [Google Scholar]
- 9.Lahlou M. The success of natural products in drug discovery. Pharmacol. & Pharm. 2013;04:17–31. [Google Scholar]
- 10.Henkel T., Brunne R.M., Müller H., Reichel F. Statistical investigation into the structural complementarity of natural products and synthetic compounds. Angew. Chem. Int. Ed. 1999;38(5):643–647. doi: 10.1002/(SICI)1521-3773(19990301)38:5<643:AID-ANIE643>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Lee M.L., Schneider G. Scaffold architecture and pharmacophoric properties of natural products and trade drugs: Application in the design of natural product-based combinatorial libraries. J. Comb. Chem. 2001;3(3):284–289. doi: 10.1021/cc000097l. [DOI] [PubMed] [Google Scholar]
- 12.Jin X., Liu M.Y., Zhang D.F., Zhong X., Du K., Qian P., Gao H., Wei M.J. Natural products as a potential modulator of microglial polarization in neurodegenerative diseases. Pharmacol. Res. 2019;145:104253. doi: 10.1016/j.phrs.2019.104253. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z., Ran Y., Huang S., Wen S., Zhang W., Liu X., Ji Z., Geng X., Ji X., Du H., Leak R.K., Hu X. Curcumin protects against ischemic stroke by titrating microglia/macrophage polarization. Front. Aging Neurosci. 2017;9:233. doi: 10.3389/fnagi.2017.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Paolo M., Papi L., Gori F., Turillazzi E. Natural products in neurodegenerative diseases: A great promise but an ethical challenge. Int. J. Mol. Sci. 2019;20(20):5170. doi: 10.3390/ijms20205170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva J.M., Nobre M.S.C., Albino S.L., Lócio L.L., Nascimento A.P.S., Scotti L., Scotti M.T., Oshiro-Junior J.A., Lima M.C.A., Mendonça-Junior F.J.B., Moura R.O. Secondary metabolites with antioxidant activities for the putative treatment of amyotrophic lateral sclerosis (ALS): “Experimental evidences. Oxid. Med. Cell. Longev. 2020;2020:1–22. doi: 10.1155/2020/5642029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao J.W., Jiang J.L., Zou J.J., Yang M.Y., Chen F.M., Zhang Y.J., Jia L. Therapeutic potential of ginsenosides on diabetes: From hypoglycemic mechanism to clinical trials. J. Funct. Foods. 2020;64:103630. doi: 10.1016/j.jff.2019.103630. [DOI] [Google Scholar]
- 17.Pizzino G., Irrera N., Cucinotta M., Pallio G., Mannino F., Arcoraci V., Squadrito F., Altavilla D., Bitto A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017;2017:1–13. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cenini G., Lloret A., Cascella R. Oxidative stress in neurodegenerative diseases: From a mitochondrial point of view. Oxid. Med. Cell. Longev. 2019;2019:1–18. doi: 10.1155/2019/2105607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirnagl U., Meisel A. Endogenous neuroprotection: Mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55(3):334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Dirnagl U., Becker K., Meisel A. Preconditioning and tolerance against cerebral ischaemia: From experimental strategies to clinical use. Lancet Neurol. 2009;8(4):398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J. Vitagenes, cellular stress response, and acetylcarnitine: Relevance to hormesis. Biofactors. 2009;35(2):146–160. doi: 10.1002/biof.22. [DOI] [PubMed] [Google Scholar]
- 23.Calabrese E.J., Iavicoli I., Calabrese V. Hormesis: Why it is important to biogerontologists. Biogerontology. 2012;13(3):215–235. doi: 10.1007/s10522-012-9374-7. [DOI] [PubMed] [Google Scholar]
- 24.Sies H., Belousov V.V., Chandel N.S., Davies M.J., Jones D.P., Mann G.E., Murphy M.P., Yamamoto M., Winterbourn C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022;23(7):499–515. doi: 10.1038/s41580-022-00456-z. [DOI] [PubMed] [Google Scholar]
- 25.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 26.Van Houten B., Woshner V., Santos J.H. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair. 2006;5(2):145–152. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Selvaraji S., Poh L., Natarajan V., Mallilankaraman K., Arumugam T.V. Negative conditioning of mitochondrial dysfunction in age-related neurodegenerative diseases. Cond. Med. 2019;2(1):30–39. [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y., Xu E., Musich P.R., Lin F. Mitochondrial dysfunction in neurodegenerative diseases and the potential countermeasure. CNS Neurosci. Ther. 2019;25(7):816–824. doi: 10.1111/cns.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemerková P., Vališ M. Role of oxidative stress in the pathogenesis of amyotrophic lateral sclerosis: Antioxidant metalloenzymes and therapeutic strategies. Biomolecules. 2021;11(3):437. doi: 10.3390/biom11030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golenia A., Leśkiewicz M., Regulska M., Budziszewska B., Szczęsny E., Jagiełła J., Wnuk M., Ostrowska M., Lasoń W., Basta-Kaim A., Słowik A. Catalase activity in blood fractions of patients with sporadic ALS. Pharmacol. Rep. 2014;66(4):704–707. doi: 10.1016/j.pharep.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Tabrizi S. Neurodegenerative diseases neurobiology pathogenesis and therapeutics. J. Neurol. Neurosurg. Psychiatry. 2006;77(2):284–284. doi: 10.1136/jnnp.2005.072710. [DOI] [Google Scholar]
- 32.Angelova P.R., Abramov A.Y. Role of mitochondrial ROS in the brain: From physiology to neurodegeneration. FEBS Lett. 2018;592(5):692–702. doi: 10.1002/1873-3468.12964. [DOI] [PubMed] [Google Scholar]
- 33.Pehar M., Beeson G., Beeson C.C., Johnson J.A., Vargas M.R. Mitochondria-targeted catalase reverts the neurotoxicity of hSOD1G⁹³A astrocytes without extending the survival of ALS-linked mutant hSOD1 mice. PLoS One. 2014;9(7):e103438. doi: 10.1371/journal.pone.0103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson K., Allen S.P., Mortiboys H., Grierson A.J., Wharton S.B., Ince P.G., Shaw P.J., Heath P.R. The effect of SOD1 mutation on cellular bioenergetic profile and viability in response to oxidative stress and influence of mutation-type. PLoS One. 2013;8(6):e68256. doi: 10.1371/journal.pone.0068256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahtoniemi T., Jaronen M., Keksa-Goldsteine V., Goldsteins G., Koistinaho J. Mutant SOD1 from spinal cord of G93A rats is destabilized and binds to inner mitochondrial membrane. Neurobiol. Dis. 2008;32(3):479–485. doi: 10.1016/j.nbd.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Xiao Y., Karam C., Yi J., Zhang L., Li X., Yoon D., Wang H., Dhakal K., Ramlow P., Yu T., Mo Z., Ma J., Zhou J. ROS-related mitochondrial dysfunction in skeletal muscle of an ALS mouse model during the disease progression. Pharmacol. Res. 2018;138:25–36. doi: 10.1016/j.phrs.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruijn L.I., Miller T.M., Cleveland D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27(1):723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 38.Vijayvergiya C., Beal M.F., Buck J., Manfredi G. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J. Neurosci. 2005;25(10):2463–2470. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babu G.N., Kumar A., Chandra R., Puri S.K., Singh R.L., Kalita J., Misra U.K. Oxidant-antioxidant imbalance in the erythrocytes of sporadic amyotrophic lateral sclerosis patients correlates with the progression of disease. Neurochem. Int. 2008;52(6):1284–1289. doi: 10.1016/j.neuint.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Ikawa M., Okazawa H., Tsujikawa T., Matsunaga A., Yamamura O., Mori T., Hamano T., Kiyono Y., Nakamoto Y., Yoneda M. Increased oxidative stress is related to disease severity in the ALS motor cortex: A PET study. Neurology. 2015;84(20):2033–2039. doi: 10.1212/WNL.0000000000001588. [DOI] [PubMed] [Google Scholar]
- 41.Ehrhart J., Smith A.J., Kuzmin-Nichols N., Zesiewicz T.A., Jahan I., Shytle R.D., Kim S.H., Sanberg C.D., Vu T.H., Gooch C.L., Sanberg P.R., Garbuzova-Davis S. Humoral factors in ALS patients during disease progression. J. Neuroinflammation. 2015;12(1):127. doi: 10.1186/s12974-015-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollari E., Goldsteins G., Bart G., Koistinaho J., Giniatullin R. The role of oxidative stress in degeneration of the neuromuscular junction in amyotrophic lateral sclerosis. Front. Cell. Neurosci. 2014;8:131. doi: 10.3389/fncel.2014.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan W., Pasinelli P., Trotti D. Role of mitochondria in mutant SOD1 linked amyotrophic lateral sclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2014;1842(8):1295–1301. doi: 10.1016/j.bbadis.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LoGerfo A., Chico L., Borgia L., Petrozzi L., Rocchi A., D’Amelio A., Carlesi C., Ienco E., Mancuso M., Siciliano G. Lack of association between nuclear factor erythroid-derived 2-like 2 promoter gene polymorphisms and oxidative stress biomarkers in amyotrophic lateral sclerosis patients. Oxid. Med. Cell. Longev. 2014;2014:1–9. doi: 10.1155/2014/432626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Giuffrida Stella A.M. Nitric oxide in the central nervous system: Neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 46.Mancuso C., Pani G., Calabrese V. Bilirubin: An endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006;11(5):207–213. doi: 10.1179/135100006X154978. [DOI] [PubMed] [Google Scholar]
- 47.Drake J., Sultana R., Aksenova M., Calabrese V., Butterfield D.A. Elevation of mitochondrial glutathione by? -glutamylcysteine ethyl ester protects mitochondria against peroxynitrite-induced oxidative stress. J. Neurosci. Res. 2003;74(6):917–927. doi: 10.1002/jnr.10810. [DOI] [PubMed] [Google Scholar]
- 48.Siracusa R., Scuto M., Fusco R., Trovato A., Ontario M.L., Crea R., Di Paola R., Cuzzocrea S., Calabrese V. Anti-inflammatory and anti-oxidant activity of hidrox® in rotenone-induced parkinson’s disease in mice. Antioxidants. 2020;9(9):824. doi: 10.3390/antiox9090824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Forni C., Facchiano F., Bartoli M., Pieretti S., Facchiano A., D’Arcangelo D., Norelli S., Valle G., Nisini R., Beninati S., Tabolacci C., Jadeja R.N. Beneficial role of phytochemicals on oxidative stress and age-related diseases. BioMed Res. Int. 2019;2019:1–16. doi: 10.1155/2019/8748253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chico L., Ienco E.C., Bisordi C., Lo Gerfo A., Petrozzi L., Petrucci A., Mancuso M., Siciliano G. Amyotrophic lateral sclerosis and oxidative stress: A double-blind therapeutic trial after curcumin supplementation. CNS Neurol. Disord. Drug Targets. 2018;17(10):767–779. doi: 10.2174/1871527317666180720162029. [DOI] [PubMed] [Google Scholar]
- 51.Kim D.S., Kim J.Y., Han Y. Curcuminoids in neurodegenerative diseases. Recent Patents CNS Drug Discov. 2012;7(3):184–204. doi: 10.2174/157488912803252032. [DOI] [PubMed] [Google Scholar]
- 52.Darvesh A.S., Carroll R.T., Bishayee A., Novotny N.A., Geldenhuys W.J., Van der Schyf C.J. Curcumin and neurodegenerative diseases: A perspective. Expert Opin. Investig. Drugs. 2012;21(8):1123–1140. doi: 10.1517/13543784.2012.693479. [DOI] [PubMed] [Google Scholar]
- 53.Jiang H., Tian X., Guo Y., Duan W., Bu H., Li C. Activation of nuclear factor erythroid 2-related factor 2 cytoprotective signaling by curcumin protect primary spinal cord astrocytes against oxidative toxicity. Biol. Pharm. Bull. 2011;34(8):1194–1197. doi: 10.1248/bpb.34.1194. [DOI] [PubMed] [Google Scholar]
- 54.Dong H., Xu L., Wu L., Wang X., Duan W., Li H., Li C. Curcumin abolishes mutant TDP-43 induced excitability in a motoneuron-like cellular model of ALS. Neuroscience. 2014;272:141–153. doi: 10.1016/j.neuroscience.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 55.Janssens J., Kleinberger G., Wils H., Van Broeckhoven C. The role of mutant TAR DNA-binding protein 43 in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. Biochem. Soc. Trans. 2011;39(4):954–959. doi: 10.1042/BST0390954. [DOI] [PubMed] [Google Scholar]
- 56.Mackenzie I.R.A., Rademakers R. The role of transactive response DNA-binding protein-43 in amyotrophic lateral sclerosis and frontotemporal dementia. Curr. Opin. Neurol. 2008;21(6):693–700. doi: 10.1097/WCO.0b013e3283168d1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu J., Duan W., Guo Y., Jiang H., Li Z., Huang J., Hong K., Li C. Mitochondrial dysfunction in human TDP-43 transfected NSC34 cell lines and the protective effect of dimethoxy curcumin. Brain Res. Bull. 2012;89(5-6):185–190. doi: 10.1016/j.brainresbull.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Bhatia N.K., Srivastava A., Katyal N., Jain N., Khan M.A.I., Kundu B., Deep S. Curcumin binds to the pre-fibrillar aggregates of Cu/Zn superoxide dismutase (SOD1) and alters its amyloidogenic pathway resulting in reduced cytotoxicity. Biochim. Biophys. Acta. Proteins Proteomics. 2015;1854(5):426–436. doi: 10.1016/j.bbapap.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 59.Strong R., Miller R.A., Astle C.M., Baur J.A., de Cabo R., Fernandez E., Guo W., Javors M., Kirkland J.L., Nelson J.F., Sinclair D.A., Teter B., Williams D., Zaveri N., Nadon N.L., Harrison D.E. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68(1):6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 61.Liu W., Zhai Y., Heng X., Che F.Y., Chen W., Sun D., Zhai G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016;24(8):694–702. doi: 10.3109/1061186X.2016.1157883. [DOI] [PubMed] [Google Scholar]
- 62.Tripodo G., Chlapanidas T., Perteghella S., Vigani B., Mandracchia D., Trapani A., Galuzzi M., Tosca M.C., Antonioli B., Gaetani P., Marazzi M., Torre M.L. Mesenchymal stromal cells loading curcumin-INVITE-micelles: A drug delivery system for neurodegenerative diseases. Colloids Surf. B Biointerfaces. 2015;125:300–308. doi: 10.1016/j.colsurfb.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 63.Wu A., Ying Z., Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Exp. Neurol. 2006;197(2):309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 64.Ullah F., Liang A., Rangel A., Gyengesi E., Niedermayer G., Münch G. High bioavailability curcumin: An anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch. Toxicol. 2017;91(4):1623–1634. doi: 10.1007/s00204-017-1939-4. [DOI] [PubMed] [Google Scholar]
- 65.Rakotoarisoa M., Angelova A. Amphiphilic nanocarrier systems for curcumin delivery in neurodegenerative disorders. Medicines. 2018;5(4):126. doi: 10.3390/medicines5040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmadi M., Agah E., Nafissi S., Jaafari M.R., Harirchian M.H., Sarraf P., Faghihi-Kashani S., Hosseini S.J., Ghoreishi A., Aghamollaii V., Hosseini M., Tafakhori A. Safety and efficacy of nanocurcumin as add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: A pilot randomized clinical trial. Neurotherapeutics. 2018;15(2):430–438. doi: 10.1007/s13311-018-0606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghasemi F., Bagheri H., Barreto G.E., Read M.I., Sahebkar A. Effects of curcumin on microglial cells. Neurotox. Res. 2019;36(1):12–26. doi: 10.1007/s12640-019-00030-0. [DOI] [PubMed] [Google Scholar]
- 68.Handique J.G., Baruah J.B. Polyphenolic compounds: An overview. React. Funct. Polym. 2002;52(3):163–188. doi: 10.1016/S1381-5148(02)00091-3. [DOI] [Google Scholar]
- 69.Wang J., Zhang Y., Tang L., Zhang N., Fan D. Protective effects of resveratrol through the up-regulation of SIRT1 expression in the mutant hSOD1-G93A-bearing motor neuron-like cell culture model of amyotrophic lateral sclerosis. Neurosci. Lett. 2011;503(3):250–255. doi: 10.1016/j.neulet.2011.08.047. [DOI] [PubMed] [Google Scholar]
- 70.Barber S.C., Higginbottom A., Mead R.J., Barber S., Shaw P.J. An in vitro screening cascade to identify neuroprotective antioxidants in ALS. Free Radic. Biol. Med. 2009;46(8):1127–1138. doi: 10.1016/j.freeradbiomed.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mancuso R., del Valle J., Modol L., Martinez A., Granado-Serrano A.B., Ramirez-Núñez O., Pallás M., Portero-Otin M., Osta R., Navarro X. Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics. 2014;11(2):419–432. doi: 10.1007/s13311-013-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song L., Chen L., Zhang X., Li J., Le W. Resveratrol ameliorates motor neuron degeneration and improves survival in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. BioMed Res. Int. 2014;2014:1–10. doi: 10.1155/2014/483501. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Mancuso R., del Valle J., Morell M., Pallás M., Osta R., Navarro X. Lack of synergistic effect of resveratrol and sigma-1 receptor agonist (PRE-084) in SOD1G93A ALS mice: Overlapping effects or limited therapeutic opportunity? Orphanet J. Rare Dis. 2014;9(1):78. doi: 10.1186/1750-1172-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Srinivasan E., Rajasekaran R. Quantum chemical and molecular mechanics studies on the assessment of interactions between resveratrol and mutant SOD1 (G93A) protein. J. Comput. Aided Mol. Des. 2018;32(12):1347–1361. doi: 10.1007/s10822-018-0175-1. [DOI] [PubMed] [Google Scholar]
- 75.Yun Y.C., Jeong S., Kim S.H., Cho G. Reduced sirtuin 1/adenosine monophosphate-activated protein kinase in amyotrophic lateral sclerosis patient-derived mesenchymal stem cells can be restored by resveratrol. J. Tissue Eng. Regen. Med. 2018;13(1):110–115. doi: 10.1002/term.2776. [DOI] [PubMed] [Google Scholar]
- 76.Laudati G., Mascolo L., Guida N., Sirabella R., Pizzorusso V., Bruzzaniti S., Serani A., Di Renzo G., Canzoniero L.M.T., Formisano L. Resveratrol treatment reduces the vulnerability of SH-SY5Y cells and cortical neurons overexpressing SOD1-G93A to Thimerosal toxicity through SIRT1/DREAM/PDYN pathway. Neurotoxicology. 2019;71:6–15. doi: 10.1016/j.neuro.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 77.Caplliure-Llopis J., Peralta-Chamba T., Carrera-Juliá S., Cuerda-Ballester M., Drehmer-Rieger E., López-Rodriguez M.M., Rubia Ortí J.E. Therapeutic alternative of the ketogenic Mediterranean diet to improve mitochondrial activity in Amyotrophic Lateral Sclerosis (ALS): A Comprehensive Review. Food Sci. Nutr. 2020;8(1):23–35. doi: 10.1002/fsn3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu T., He X.W., Jiang J.G., Xu X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014;62(7):1449–1455. doi: 10.1021/jf405820v. [DOI] [PubMed] [Google Scholar]
- 79.de Pablos R.M., Espinosa-Oliva A.M., Hornedo-Ortega R., Cano M., Arguelles S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019;143:58–72. doi: 10.1016/j.phrs.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 80.Oliván S., Martínez-Beamonte R., Calvo A.C., Surra J.C., Manzano R., Arnal C., Osta R., Osada J. Extra virgin olive oil intake delays the development of amyotrophic lateral sclerosis associated with reduced reticulum stress and autophagy in muscle of SOD1G93A mice. J. Nutr. Biochem. 2014;25(8):885–892. doi: 10.1016/j.jnutbio.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 81.Kalaiselvan I., Samuthirapandi M., Govindaraju A., Sheeja Malar D., Kasi P.D. Olive oil and its phenolic compounds (hydroxytyrosol and tyrosol) ameliorated TCDD-induced heptotoxicity in rats via inhibition of oxidative stress and apoptosis. Pharm. Biol. 2016;54(2):338–346. doi: 10.3109/13880209.2015.1042980. [DOI] [PubMed] [Google Scholar]
- 82.Rajabian A., Sadeghnia H., Fanoudi S., Hosseini A. Genus Boswellia as a new candidate for neurodegenerative disorders. Iran. J. Basic Med. Sci. 2020;23(3):277–286. doi: 10.22038/IJBMS.2020.35288.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ammon H. Boswellic acids in chronic inflammatory diseases. Planta Med. 2006;72(12):1100–1116. doi: 10.1055/s-2006-947227. [DOI] [PubMed] [Google Scholar]
- 84.Siddiqui A., Shah Z., Jahan R.N., Othman I., Kumari Y. Mechanistic role of boswellic acids in Alzheimer’s disease: Emphasis on anti-inflammatory properties. Biomed. Pharmacother. 2021;144:112250. doi: 10.1016/j.biopha.2021.112250. [DOI] [PubMed] [Google Scholar]
- 85.Minj E., Upadhayay S., Mehan S. Nrf2/HO-1 signaling activator acetyl-11-keto-beta boswellic acid (AKBA)-mediated neuroprotection in methyl mercury-induced experimental model of ALS. Neurochem. Res. 2021;46(11):2867–2884. doi: 10.1007/s11064-021-03366-2. [DOI] [PubMed] [Google Scholar]
- 86.Landis-Piwowar K.R., Huo C., Chen D., Milacic V., Shi G., Chan T.H., Dou Q.P. A novel prodrug of the green tea polyphenol (-)-epigallocatechin-3-gallate as a potential anticancer agent. Cancer Res. 2007;67(9):4303–4310. doi: 10.1158/0008-5472.CAN-06-4699. [DOI] [PubMed] [Google Scholar]
- 87.Bedlack R.S., Joyce N., Carter G.T., Paganoni S., Karam C. Complementary and alternative therapies in amyotrophic lateral sclerosis. Neurol. Clin. 2015;33(4):909–936. doi: 10.1016/j.ncl.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hockenbery D.M., Oltvai Z.N., Yin X.M., Milliman C.L., Korsmeyer S.J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75(2):241–251. doi: 10.1016/0092-8674(93)80066-N. [DOI] [PubMed] [Google Scholar]
- 89.Terao J., Piskula M., Yao Q. Protective effect of epicatechin, epicatechin gallate, and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophys. 1994;308(1):278–284. doi: 10.1006/abbi.1994.1039. [DOI] [PubMed] [Google Scholar]
- 90.Levites Y., Amit T., Youdim M.B.H., Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J. Biol. Chem. 2002;277(34):30574–30580. doi: 10.1074/jbc.M202832200. [DOI] [PubMed] [Google Scholar]
- 91.Mandel S.A., Avramovich-Tirosh Y., Reznichenko L., Zheng H., Weinreb O., Amit T., Youdim M.B.H. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals. 2005;14(1-2):46–60. doi: 10.1159/000085385. [DOI] [PubMed] [Google Scholar]
- 92.Nie G., Cao Y., Zhao B. Protective effects of green tea polyphenols and their major component, (-)-epigallocatechin-3-gallate (EGCG), on 6-hydroxydopamine-induced apoptosis in PC12 cells. Redox Rep. 2002;7(3):171–177. doi: 10.1179/135100002125000424. [DOI] [PubMed] [Google Scholar]
- 93.Reznichenko L., Amit T., Youdim M.B.H., Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate induces neurorescue of long-term serum-deprived PC12 cells and promotes neurite outgrowth. J. Neurochem. 2005;93(5):1157–1167. doi: 10.1111/j.1471-4159.2005.03085.x. [DOI] [PubMed] [Google Scholar]
- 94.Mandel S., Weinreb O., Amit T., Youdim M.B.H. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: Implications for neurodegenerative diseases. J. Neurochem. 2004;88(6):1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- 95.Panickar K.S., Polansky M.M., Anderson R.A. Green tea polyphenols attenuate glial swelling and mitochondrial dysfunction following oxygen-glucose deprivation in cultures. Nutr. Neurosci. 2009;12(3):105–113. doi: 10.1179/147683009X423300. [DOI] [PubMed] [Google Scholar]
- 96.Koh S., Kwon H., Kim K.S., Kim J., Kim M.H., Yu H.J., Kim M., Lee K.W., Do B.R., Jung H.K., Yang K.W., Appel S.H., Kim S.H. Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. Toxicology. 2004;202(3):213–225. doi: 10.1016/j.tox.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 97.Koh S.H., Lee S.M., Kim H.Y., Lee K.Y., Lee Y.J., Kim H.T., Kim J., Kim M.H., Hwang M.S., Song C., Yang K.W., Lee K.W., Kim S.H., Kim O.H. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci. Lett. 2006;395(2):103–107. doi: 10.1016/j.neulet.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 98.Xu Z., Chen S., Li X., Luo G., Li L., Le W. Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem. Res. 2006;31(10):1263–1269. doi: 10.1007/s11064-006-9166-z. [DOI] [PubMed] [Google Scholar]
- 99.Srinivasan E., Rajasekaran R. Probing the inhibitory activity of epigallocatechin-gallate on toxic aggregates of mutant (L84F) SOD1 protein through geometry based sampling and steered molecular dynamics. J. Mol. Graph. Model. 2017;74:288–295. doi: 10.1016/j.jmgm.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 100.Cao G., Sofic E., Prior R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activit relationships. Free Radic. Biol. Med. 1997;22(5):749–760. doi: 10.1016/S0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 101.Esposito E., Rotilio D., Dimatteo V., Digiulio C., Cacchio M., Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol. Aging. 2002;23(5):719–735. doi: 10.1016/S0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 102.Vauzour D., Vafeiadou K., Rodriguez-Mateos A., Rendeiro C., Spencer J.P.E. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008;3(3-4):115–126. doi: 10.1007/s12263-008-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.He L., He T., Farrar S., Ji L., Liu T., Ma X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017;44(2):532–553. doi: 10.1159/000485089. [DOI] [PubMed] [Google Scholar]
- 104.Kim T.Y., Leem E., Lee J.M., Kim S.R. Control of reactive oxygen species for the prevention of parkinson’s disease: The possible application of flavonoids. Antioxidants. 2020;9(7):583. doi: 10.3390/antiox9070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Solanki I., Parihar P., Mansuri M.L., Parihar M.S. Flavonoid-based therapies in the early management of neurodegenerative diseases. Adv. Nutr. 2015;6(1):64–72. doi: 10.3945/an.114.007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mansuri M.L., Parihar P., Solanki I., Parihar M.S. Flavonoids in modulation of cell survival signalling pathways. Genes Nutr. 2014;9(3):400. doi: 10.1007/s12263-014-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mantilla C.B., Ermilov L.G. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve. 2012;45(2):274–276. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Korkmaz O.T., Aytan N., Carreras I., Choi J.K., Kowall N.W., Jenkins B.G., Dedeoglu A. 7,8-Dihydroxyflavone improves motor performance and enhances lower motor neuronal survival in a mouse model of amyotrophic lateral sclerosis. Neurosci. Lett. 2014;566:286–291. doi: 10.1016/j.neulet.2014.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharma D.R., Wani W.Y., Sunkaria A., Kandimalla R.J., Sharma R.K., Verma D., Bal A., Gill K.D. Quercetin attenuates neuronal death against aluminum-induced neurodegeneration in the rat hippocampus. Neuroscience. 2016;324:163–176. doi: 10.1016/j.neuroscience.2016.02.055. [DOI] [PubMed] [Google Scholar]
- 110.Ip P., Sharda P.R., Cunningham A., Chakrabartty S., Pande V., Chakrabartty A. Quercitrin and quercetin 3-β-d-glucoside as chemical chaperones for the A4V SOD1 ALS-causing mutant. Protein Eng. Des. Sel. 2017;30(6):431–440. doi: 10.1093/protein/gzx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang T.H., Wang S.Y., Wang X.D., Jiang H.Q., Yang Y.Q., Wang Y., Cheng J.L., Zhang C.T., Liang W.W., Feng H.L. Fisetin exerts antioxidant and neuroprotective effects in multiple mutant hsod1 models of amyotrophic lateral sclerosis by activating ERK. Neuroscience. 2018;379:152–166. doi: 10.1016/j.neuroscience.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 112.Ye L., Wang H., Duncan S.E., Eigel W.N., O’Keefe S.F. Antioxidant activities of Vine Tea (Ampelopsis grossedentata) extract and its major component dihydromyricetin in soybean oil and cooked ground beef. Food Chem. 2015;172:416–422. doi: 10.1016/j.foodchem.2014.09.090. [DOI] [PubMed] [Google Scholar]
- 113.Murakami T., Miyakoshi M., Araho D., Mizutani K., Kambara T., Ikeda T., Chou W.H., Inukai M., Takenaka A., Igarashi K. Hepatoprotective activity of tocha, the stems and leaves of Ampelopsis grossedentata, and ampelopsin. Biofactors. 2004;21(1-4):175–178. doi: 10.1002/biof.552210136. [DOI] [PubMed] [Google Scholar]
- 114.Kou X., Shen K., An Y., Qi S., Dai W.X., Yin Z. Ampelopsin inhibits H2O2-induced apoptosis by ERK and Akt signaling pathways and up-regulation of heme oxygenase-1. Phytother. Res. 2012;26(7):988–994. doi: 10.1002/ptr.3671. [DOI] [PubMed] [Google Scholar]
- 115.Singh B., Kaur P. Gopichand; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79(6):401–418. doi: 10.1016/j.fitote.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 116.Ferrante R.J., Klein A.M., Dedeoglu A., Beal M.F. Therapeutic efficacy of EGb761 (Gingko biloba extract) in a transgenic mouse model of amyotrophic lateral sclerosis. J. Mol. Neurosci. 2001;17(1):89–96. doi: 10.1385/JMN:17:1:89. [DOI] [PubMed] [Google Scholar]
- 117.Jiang F., DeSilva S., Turnbull J. Beneficial effect of ginseng root in SOD-1 (G93A) transgenic mice. J. Neurol. Sci. 2000;180(1-2):52–54. doi: 10.1016/S0022-510X(00)00421-4. [DOI] [PubMed] [Google Scholar]
- 118.Trieu V.N., Uckun F.M. Genistein is neuroprotective in murine models of familial amyotrophic lateral sclerosis and stroke. Biochem. Biophys. Res. Commun. 1999;258(3):685–688. doi: 10.1006/bbrc.1999.0577. [DOI] [PubMed] [Google Scholar]
- 119.Orrell R., Lane J., Ross M. In: Cochrane Database of Systematic Reviews. Chichester, UK: John Wiley & Sons, Ltd; 2004. Antioxidant treatment for amyotrophic lateral sclerosis/motor neuron disease. [DOI] [Google Scholar]
- 120.Gurney M.E., Cutting F.B., Zhai P., Doble A., Taylor C.P., Andrus P.K., Hall E.D. Benefit of vitamin E, riluzole, and gababapentin in a transgenic model of familial amyotrophic lateral sclerosis. Ann. Neurol. 1996;39(2):147–157. doi: 10.1002/ana.410390203. [DOI] [PubMed] [Google Scholar]
- 121.Desnuelle C., Dib M., Garrel C., Favier A. A double-blind, placebo-controlled randomized clinical trial of α-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2001;2(1):9–18. doi: 10.1080/146608201300079364. [DOI] [PubMed] [Google Scholar]
- 122.Ascherio A., Weisskopf M.G., O’Reilly E.J., Jacobs E.J., McCullough M.L., Calle E.E., Cudkowicz M., Thun M.J., Vitamin E. Vitamin E intake and risk of amyotrophic lateral sclerosis. Ann. Neurol. 2005;57(1):104–110. doi: 10.1002/ana.20316. [DOI] [PubMed] [Google Scholar]
- 123.Veldink J.H., Kalmijn S., Groeneveld G-J., Wunderink W., Koster A., de Vries J.H.M., van der Luyt J., Wokke J.H.J., Van den Berg L.H. Intake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry. 2006;78(4):367–371. doi: 10.1136/jnnp.2005.083378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang H., O’Reilly E.J., Weisskopf M.G., Logroscino G., McCullough M.L., Schatzkin A., Kolonel L.N., Ascherio A., Vitamin E. Vitamin E intake and risk of amyotrophic lateral sclerosis: A pooled analysis of data from 5 prospective cohort studies. Am. J. Epidemiol. 2011;173(6):595–602. doi: 10.1093/aje/kwq416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Graf M., Ecker D., Horowski R., Kramer B., Riederer P., Gerlach M., Hager C., Ludolph A.C., Becker G., Osterhage J., Jost W.H., Schrank B., Stein C., Kostopulos P., Lubik S., Wekwerth K., Dengler R., Troeger M., Wuerz A., Hoge A., Schrader C., Schimke N., Krampfl K., Petri S., Zierz S., Eger K., Neudecker S., Traufeller K., Sievert M., Neundörfer B., Hecht M. High dose vitamin E therapy in amyotrophic lateral sclerosis as add-on therapy to riluzole: Results of a placebo-controlled double-blind study. J. Neural Transm. 2005;112(5):649–660. doi: 10.1007/s00702-004-0220-1. [DOI] [PubMed] [Google Scholar]
- 126.Michal Freedman D., Kuncl R.W., Weinstein S.J., Malila N., Virtamo J., Albanes D. Vitamin E serum levels and controlled supplementation and risk of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 2013;14(4):246–251. doi: 10.3109/21678421.2012.745570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Galbussera A., Tremolizzo L., Brighina L., Testa D., Lovati R., Ferrarese C., Cavaletti G., Filippini G. Vitamin E intake and quality of life in amyotrophic lateral sclerosis patients: A follow-up case series study. Neurol. Sci. 2006;27(3):190–193. doi: 10.1007/s10072-006-0668-x. [DOI] [PubMed] [Google Scholar]
- 128.Longnecker M.P., Kamel F., Umbach D.M., Munsat T.L., Shefner J.M., Lansdell L.W., Sandler D.P. Dietary intake of calcium, magnesium and antioxidants in relation to risk of amyotrophic lateral sclerosis. Neuroepidemiology. 2000;19(4):210–216. doi: 10.1159/000026258. [DOI] [PubMed] [Google Scholar]
- 129.Nieves J.W., Gennings C., Factor-Litvak P., Hupf J., Singleton J., Sharf V., Oskarsson B., Fernandes Filho J.A.M., Sorenson E.J., D’Amico E., Goetz R., Mitsumoto H. Association between dietary intake and function in amyotrophic lateral sclerosis. JAMA Neurol. 2016;73(12):1425–1432. doi: 10.1001/jamaneurol.2016.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fitzgerald K.C., O’Reilly É.J., Fondell E., Falcone G.J., McCullough M.L., Park Y., Kolonel L.N., Ascherio A. Intakes of vitamin C and carotenoids and risk of amyotrophic lateral sclerosis: Pooled results from 5 cohort studies. Ann. Neurol. 2013;73(2):236–245. doi: 10.1002/ana.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okamoto K., Kihira T., Kobashi G., Washio M., Sasaki S., Yokoyama T., Miyake Y., Sakamoto N., Inaba Y., Nagai M. Fruit and vegetable intake and risk of amyotrophic lateral sclerosis in Japan. Neuroepidemiology. 2009;32(4):251–256. doi: 10.1159/000201563. [DOI] [PubMed] [Google Scholar]
- 132.Ma Z., Yang Z. Scavenging effects of Astragalus and Gynostemma pentaphyllum with its product on O2-. and. OH. Zhong Yao Cai. 1999;22(6):303–306. [PubMed] [Google Scholar]
- 133.Shahzad M., Shabbir A., Wojcikowski K., Wohlmuth H., Gobe G.C. The antioxidant effects of radix astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr. Drug Targets. 2016;17(12):1331–1340. doi: 10.2174/1389450116666150907104742. [DOI] [PubMed] [Google Scholar]
- 134.Rong J., Cheung C., Lau A., Shen J., Tam P., Cheng Y.C. Induction of heme oxygenase-1 by traditional Chinese medicine formulation ISF-1 and its ingredients as a cytoprotective mechanism against oxidative stress. Int. J. Mol. Med. 2008;21(4):405–411. doi: 10.3892/ijmm.21.4.405. [DOI] [PubMed] [Google Scholar]
- 135.Hu J.Y., Han J., Chu Z.G., Song H.P., Zhang D.X., Zhang Q., Huang Y.S. Astragaloside IV attenuates hypoxia-induced cardiomyocyte damage in rats by upregulating superoxide dismutase-1 levels. Clin. Exp. Pharmacol. Physiol. 2009;36(4):351–357. doi: 10.1111/j.1440-1681.2008.05059.x. [DOI] [PubMed] [Google Scholar]
- 136.Liu X., Zhang J., Wang S., Qiu J., Yu C., Astragaloside I.V. Astragaloside IV attenuates the H2O2-induced apoptosis of neuronal cells by inhibiting α-synuclein expression via the p38 MAPK pathway. Int. J. Mol. Med. 2017;40(6):1772–1780. doi: 10.3892/ijmm.2017.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu J., Guo M., Li Y., Zhang H., Chai Z., Wang Q., Yan Y., Yu J., Liu C., Zhang G., Cungen M. Astragaloside IV protects neurons from microglia-mediated cell damage through promoting microglia polarization. Folia Neuropathol. 2019;57(2):170–181. doi: 10.5114/fn.2019.86299. [DOI] [PubMed] [Google Scholar]
- 138.Liu Y. Therapeutic potential of madecassoside in transgenic mice of amyotrophic lateral sclerosis. Chin. Tradit. Herbal Drugs. 2006;37:718–720. [Google Scholar]
- 139.Bai J-R., Liu Y-J., Song Y. The mechanism of interfere effects of madecassoside (MC) on neurodegeneration in mice. Zhongguo Laonianxue Zazhi. 2008;28:2297–2300. [Google Scholar]
- 140.Sasmita A.O., Ling A.P.K., Voon K.G.L., Koh R.Y., Wong Y.P. Madecassoside activates anti neuroinflammatory mechanisms by inhibiting lipopolysaccharide induced microglial inflammation. Int. J. Mol. Med. 2018;41(5):3033–3040. doi: 10.3892/ijmm.2018.3479. [DOI] [PubMed] [Google Scholar]
- 141.Liu S., Li G., Tang H., Pan R., Wang H., Jin F., Yan X., Xing Y., Chen G., Fu Y., Dong J. Madecassoside ameliorates lipopolysaccharide-induced neurotoxicity in rats by activating the Nrf2-HO-1 pathway. Neurosci. Lett. 2019;709:134386. doi: 10.1016/j.neulet.2019.134386. [DOI] [PubMed] [Google Scholar]
- 142.Lee K., Choi J., Choi B.K., Gu Y.M., Ryu H.W., Oh S.R., Lee H.J., Picroside I.I., Picroside II. Isolated from Pseudolysimachion rotundum var. subintegrum inhibits glucocorticoid refractory serum amyloid A (SAA) Expression and SAA-induced IL-33 secretion. Molecules. 2019;24(10):2020. doi: 10.3390/molecules24102020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li B., Lei S., Xiong S., Chen S., Zhang Z. Pharmacokinetics and pharmacodynamics of morroniside: A review. Nat. Prod. Commun. 2019. p. 2019. [DOI]
- 144.Wang W., Huang W., Li L., Ai H., Sun F., Liu C., An Y. Morroniside prevents peroxide-induced apoptosis by induction of endogenous glutathione in human neuroblastoma cells. Cell. Mol. Neurobiol. 2008;28(2):293–305. doi: 10.1007/s10571-007-9168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang W., Sun F., An Y., Ai H., Zhang L., Huang W., Li L. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur. J. Pharmacol. 2009;613(1-3):19–23. doi: 10.1016/j.ejphar.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 146.Wang W., Xu J., Li L., Wang P., Ji X., Ai H., Zhang L., Li L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res. Bull. 2010;83(5):196–201. doi: 10.1016/j.brainresbull.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 147.Zhang J.X., Wang R., Xi J., Shen L., Zhu A.Y., Qi Q., Wang Q.Y., Zhang L.J., Wang F.C., Lü H.Z., Hu J.G. Morroniside protects SK-N-SH human neuroblastoma cells against H2O2-induced damage. Int. J. Mol. Med. 2017;39(3):603–612. doi: 10.3892/ijmm.2017.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li P., Matsunaga K., Ohizumi Y. Nerve growth factor-potentiating compounds from Picrorhizae Rhizoma. Biol. Pharm. Bull. 2000;23(7):890–892. doi: 10.1248/bpb.23.890. [DOI] [PubMed] [Google Scholar]
- 149.Cao Y., Liu J.W., Yu Y.J., Zheng P.Y., Zhang X.D., Li T., Guo M.C. Synergistic protective effect of picroside II and NGF on PC12 cells against oxidative stress induced by H2O2. Pharmacol. Rep. 2007;59(5):573–579. [PubMed] [Google Scholar]
- 150.Guo N., Jin C., Shen L., Wu F., Lin X., Feng Y. Chemical components, pharmacological actions, and clinical applications of Rhizoma picrorhizae. Phytother. Res. 2020;34(5):1071–1082. doi: 10.1002/ptr.6591. [DOI] [PubMed] [Google Scholar]
- 151.Li T., Liu J.W., Zhang X.D., Guo M.C., Ji G. The neuroprotective effect of picroside II from hu-huang-lian against oxidative stress. Am. J. Chin. Med. 2007;35(4):681–691. doi: 10.1142/S0192415X0700517X. [DOI] [PubMed] [Google Scholar]
- 152.Gong X., Su X., Liu H. Diallyl trisulfide, the antifungal component of garlic essential oil and the bioactivity of its nanoemulsions formed by spontaneous emulsification. Molecules. 2021;26(23):7186. doi: 10.3390/molecules26237186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Calò L.A., Fusaro M., Davis P.A. HO-1 attenuates hypertension-induced inflammation/oxidative stress: Support from Bartter’s/Gitelman’s patients. Am. J. Hypertens. 2010;23(9):936–936. doi: 10.1038/ajh.2010.130. [DOI] [PubMed] [Google Scholar]
- 154.Sun M.M., Bu H., Li B., Yu J.X., Guo Y.S., Li C.Y. Neuroprotective potential of phase II enzyme inducer diallyl trisulfide. Neurol. Res. 2009;31(1):23–27. doi: 10.1179/174313208X332959. [DOI] [PubMed] [Google Scholar]
- 155.Guo Y., Zhang K., Wang Q., Li Z., Yin Y., Xu Q., Duan W., Li C. Neuroprotective effects of diallyl trisulfide in SOD1-G93A transgenic mouse model of amyotrophic lateral sclerosis. Brain Res. 2011;1374:110–115. doi: 10.1016/j.brainres.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 156.Liu C., Leng B., Li Y., Jiang H., Duan W., Guo Y., Li C., Hong K. Diallyl trisulfide protects motor neurons from the neurotoxic protein TDP-43 via activating lysosomal degradation and the antioxidant response. Neurochem. Res. 2018;43(12):2304–2312. doi: 10.1007/s11064-018-2651-3. [DOI] [PubMed] [Google Scholar]
- 157.Silva-Islas C.A., Chánez-Cárdenas M.E., Barrera-Oviedo D., Ortiz-Plata A., Pedraza-Chaverri J., Maldonado P.D. Diallyl trisulfide protects rat brain tissue against the damage induced by ischemia-reperfusion through the Nrf2 pathway. Antioxidants. 2019;8(9):410. doi: 10.3390/antiox8090410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhu J., Shen L., Lin X., Hong Y., Feng Y. Clinical research on traditional chinese medicine compounds and their preparations for amyotrophic lateral sclerosis. Biomed. Pharmacother. 2017;96:854–864. doi: 10.1016/j.biopha.2017.09.135. [DOI] [PubMed] [Google Scholar]
- 159.Kumar V., Gupta P., Hassan M.I. Mechanism and implications of traditional chinese medicine in amyotrophic lateral sclerosis therapy. J. Proteins Proteomics. 2019;2019:1–17. doi: 10.1007/s42485-019-00009-7. [DOI] [Google Scholar]
- 160.Komine O., Yamanaka K. Neuroinflammation in motor neuron disease. Nagoya J. Med. Sci. 2015;77(4):537–549. [PMC free article] [PubMed] [Google Scholar]
- 161.Hooten K.G., Beers D.R., Zhao W., Appel S.H. Protective and toxic neuroinflammation in amyotrophic lateral sclerosis. Neurotherapeutics. 2015;12(2):364–375. doi: 10.1007/s13311-014-0329-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu J., Wang F. Role of neuroinflammation in amyotrophic lateral sclerosis: Cellular mechanisms and therapeutic implications. Front. Immunol. 2017;8:1005. doi: 10.3389/fimmu.2017.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Süssmuth S., Brettschneider J., Ludolph A., Tumani H. Biochemical markers in CSF of ALS patients. Curr. Med. Chem. 2008;15(18):1788–1801. doi: 10.2174/092986708785133031. [DOI] [PubMed] [Google Scholar]
- 164.Philips T., Robberecht W. Neuroinflammation in amyotrophic lateral sclerosis: role of glial activation in motor neuron disease. Lancet Neurol. 2011;10(3):253–263. doi: 10.1016/S1474-4422(11)70015-1. [DOI] [PubMed] [Google Scholar]
- 165.Peric M., Mitrecic D., Andjus P.R. Targeting astrocytes for treatment in amyotrophic lateral sclerosis. Curr. Pharm. Des. 2018;23(33):23. doi: 10.2174/1381612823666170615110446. [DOI] [PubMed] [Google Scholar]
- 166.Liu E., Karpf L., Bohl D. Neuroinflammation in amyotrophic lateral sclerosis and frontotemporal dementia and the interest of induced pluripotent stem cells to study immune cells interactions with neurons. Front. Mol. Neurosci. 2021;14:767041. doi: 10.3389/fnmol.2021.767041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang C., Zhang X., Fan H., Liu Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009;1282:133–141. doi: 10.1016/j.brainres.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 168.Sikora E., Scapagnini G., Barbagallo M. Curcumin, inflammation, ageing and age-related diseases. Immun. Ageing. 2010;7(1):1. doi: 10.1186/1742-4933-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chico L., Ienco E., Bisordi C., Gerfo A., Schirinzi E., Siciliano G. Curcumin as an ROS scavenger in amyotrophic lateral sclerosis. React. Oxyg. Species. 2016;2(5) doi: 10.20455/ros.2016.861. [DOI] [Google Scholar]
- 170.Bedlack R. ALSUntangled 44: curcumin. Amyotroph. Lateral Scler. Frontotemporal Degener. 2018;19(7-8):623–629. doi: 10.1080/21678421.2018.1440738. [DOI] [PubMed] [Google Scholar]
- 171.Adami R., Bottai D. Curcumin and neurological diseases. Nutr. Neurosci. 2022;25(3):441–461. doi: 10.1080/1028415X.2020.1760531. [DOI] [PubMed] [Google Scholar]
- 172.Bi X.L., Yang J.Y., Dong Y.X., Wang J.M., Cui Y.H., Ikeshima T., Zhao Y.Q., Wu C.F. Resveratrol inhibits nitric oxide and TNF-α production by lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2005;5(1):185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 173.Meng X.L., Yang J.Y., Chen G.L., Wang L.H., Zhang L.J., Wang S., Li J., Wu C.F. Effects of resveratrol and its derivatives on lipopolysaccharide-induced microglial activation and their structure-activity relationships. Chem. Biol. Interact. 2008;174(1):51–59. doi: 10.1016/j.cbi.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 174.Morita T. Celastrol: A new therapeutic potential of traditional Chinese medicine. Am. J. Hypertens. 2010;23(8):821–821. doi: 10.1038/ajh.2010.87. [DOI] [PubMed] [Google Scholar]
- 175.Venkatesha S.H., Dudics S., Astry B., Moudgil K.D. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog. Dis. 2016;74(6):ftw059. doi: 10.1093/femspd/ftw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kiaei M., Kipiani K., Petri S., Chen J., Calingasan N.Y., Beal M.F. Celastrol blocks neuronal cell death and extends life in transgenic mouse model of amyotrophic lateral sclerosis. Neurodegener. Dis. 2005;2(5):246–254. doi: 10.1159/000090364. [DOI] [PubMed] [Google Scholar]
- 177.Jung H.W., Chung Y.S., Kim Y.S., Park Y.K. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-κB in LPS-stimulated BV-2 microglial cells. Exp. Mol. Med. 2007;39(6):715–721. doi: 10.1038/emm.2007.78. [DOI] [PubMed] [Google Scholar]
- 178.Zhang R., Zhu Y., Dong X., Liu B., Zhang N., Wang X., Liu L., Xu C., Huang S., Chen L. Celastrol attenuates cadmium-induced neuronal apoptosis via inhibiting Ca2+-CaMKII-Dependent Akt/mTOR pathway. J. Cell. Physiol. 2017;232(8):2145–2157. doi: 10.1002/jcp.25703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jin X., Wang J., Xia Z.M., Shang C.H., Chao Q.L., Liu Y.R., Fan H.Y., Chen D.Q., Qiu F., Zhao F. Anti-inflammatory and anti-oxidative activities of paeonol and its metabolites through blocking MAPK/ERK/p38 signaling pathway. Inflammation. 2016;39(1):434–446. doi: 10.1007/s10753-015-0265-3. [DOI] [PubMed] [Google Scholar]
- 180.Wang X., Zhu G., Yang S., Wang X., Cheng H., Wang F., Li X., Li Q. Paeonol prevents excitotoxicity in rat pheochromocytoma PC12 cells via downregulation of ERK activation and inhibition of apoptosis. Planta Med. 2011;77(15):1695–1701. doi: 10.1055/s-0030-1271033. [DOI] [PubMed] [Google Scholar]
- 181.Tseng Y.T., Hsu Y.Y., Shih Y.T., Lo Y.C. Paeonol attenuates microglia-mediated inflammation and oxidative stress-induced neurotoxicity in rat primary microglia and cortical neurons. Shock. 2012;37(3):312–318. doi: 10.1097/SHK.0b013e31823fe939. [DOI] [PubMed] [Google Scholar]
- 182.He L.X., Tong X., Zeng J., Tu Y., Wu S., Li M., Deng H., Zhu M., Li X., Nie H., Yang L., Huang F. Paeonol suppresses neuroinflammatory responses in LPS-activated microglia cells. Inflammation. 2016;39(6):1904–1917. doi: 10.1007/s10753-016-0426-z. [DOI] [PubMed] [Google Scholar]
- 183.Vu V.T., Liu X.Q., Nguyen M.T., Lin Y.L., Kong L.Y., Luo J.G. New obovatol trimeric neolignans with NO inhibitory activity from the leaves of Magnolia officinalis var. biloba. Bioorg. Chem. 2020;96:103586. doi: 10.1016/j.bioorg.2020.103586. [DOI] [PubMed] [Google Scholar]
- 184.Ock J., Han H.S., Hong S.H., Lee S.Y., Han Y.M., Kwon B.M., Suk K. Obovatol attenuates microglia-mediated neuroinflammation by modulating redox regulation. Br. J. Pharmacol. 2010;159(8):1646–1662. doi: 10.1111/j.1476-5381.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Liu J., Su G., Gao J., Tian Y., Liu X., Zhang Z. Effects of peroxiredoxin 2 in neurological disorders: A review of its molecular mechanisms. Neurochem. Res. 2020;45(4):720–730. doi: 10.1007/s11064-020-02971-x. [DOI] [PubMed] [Google Scholar]
- 186.Yuan D., Ma B., Yang J., Xie Y., Wang L., Zhang L., Kano Y., Wu C. Anti-inflammatory effects of rhynchophylline and isorhynchophylline in mouse N9 microglial cells and the molecular mechanism. Int. Immunopharmacol. 2009;9(13-14):1549–1554. doi: 10.1016/j.intimp.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 187.Lee H., Kim Y.O., Kim H., Kim S.Y., Noh H.S., Kang S.S., Cho G.J., Choi W.S., Suk K. Flavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003;17(13):1–21. doi: 10.1096/fj.03-0057fje. [DOI] [PubMed] [Google Scholar]
- 188.Du Z.Y., Li X.Y. Inhibitory effects of ginkgolides on nitric oxide production in neonatal rat microglia in vitro. Chung Kuo Yao Li Hsueh Pao. 1998;19(5):467–470. [PubMed] [Google Scholar]
- 189.Wang L., Lei Q., Zhao S., Xu W., Dong W., Ran J., Shi Q., Fu J., Ginkgolide B. Ginkgolide B maintains calcium homeostasis in hypoxic hippocampal neurons by inhibiting calcium influx and intracellular calcium release. Front. Cell. Neurosci. 2021;14:627846. doi: 10.3389/fncel.2020.627846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Huang L., Shi Y., Zhao L., Ginkgolide B. Alleviates learning and memory impairment in rats with vascular dementia by reducing neuroinflammation via regulating NF-ₖB pathway. Front. Pharmacol. 2021:12. doi: 10.3389/fphar.2021.676392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sun M., Sheng Y., Zhu Y., Ginkgolide B. Ginkgolide B alleviates the inflammatory response and attenuates the activation of LPS induced BV2 cells in vitro and in vivo. Exp. Ther. Med. 2021;21(6):586. doi: 10.3892/etm.2021.10018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Briones M.R.S., Snyder A.M., Ferreira R.C., Neely E.B., Connor J.R., Broach J.R. A possible role for platelet-activating factor receptor in amyotrophic lateral sclerosis treatment. Front. Neurol. 2018;9:39. doi: 10.3389/fneur.2018.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ko H.M., Koppula S., Kim B.W., Kim I.S., Hwang B.Y., Suk K., Park E.J., Choi D.K. Inflexin attenuates proinflammatory responses and nuclear factor-κB activation in LPS-treated microglia. Eur. J. Pharmacol. 2010;633(1-3):98–106. doi: 10.1016/j.ejphar.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 194.Ha S.K., Moon E., Kim S.Y. Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neurosci. Lett. 2010;485(3):143–147. doi: 10.1016/j.neulet.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 195.Grewer C., Rauen T. Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J. Membr. Biol. 2005;203(1):1–20. doi: 10.1007/s00232-004-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Foran E., Trotti D. Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis. Antioxid. Redox Signal. 2009;11(7):1587–1602. doi: 10.1089/ars.2009.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Choi D.W. Glutamate receptors and the induction of excitotoxic neuronal death. Prog. Brain Res. 1994;100:47–51. doi: 10.1016/S0079-6123(08)60767-0. [DOI] [PubMed] [Google Scholar]
- 198.Cheah B.C., Vucic S., Krishnan A., Kiernan M.C. Riluzole, neuroprotection and amyotrophic lateral sclerosis. Curr. Med. Chem. 2010;17(18):1942–1959. doi: 10.2174/092986710791163939. [DOI] [PubMed] [Google Scholar]
- 199.Rothstein J.D., Tsai G., Kuncl R.W., Clawson L., Cornblath D.R., Drachman D.B., Pestronk A., Stauch B.L., Coyle J.T. Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann. Neurol. 1990;28(1):18–25. doi: 10.1002/ana.410280106. [DOI] [PubMed] [Google Scholar]
- 200.Plaitakis A., Constantakakis E. Altered metabolism of excitatory amino acids, N-acetyl-aspartate and N-acetyl-aspartylglutamate in amyotrophic lateral sclerosis. Brain Res. Bull. 1993;30(3-4):381–386. doi: 10.1016/0361-9230(93)90269-H. [DOI] [PubMed] [Google Scholar]
- 201.Ferrarese C., Sala G., Riva R., Begni B., Zoia C., Tremolizzo L., Galimberti G., Millul A., Bastone A., Mennini T., Balzarini C., Frattola L., Beghi E. Decreased platelet glutamate uptake in patients with amyotrophic lateral sclerosis. Neurology. 2001;56(2):270–272. doi: 10.1212/WNL.56.2.270. [DOI] [PubMed] [Google Scholar]
- 202.Cho J., Ho Kim Y., Kong J.Y., Ha Yang. C.; Gook Park, C. Protection of cultured rat cortical neurons from excitotoxicity by asarone, a major essential oil component in the rhizomes of Acorus gramineus. Life Sci. 2002;71(5):591–599. doi: 10.1016/S0024-3205(02)01729-0. [DOI] [PubMed] [Google Scholar]
- 203.Chen Y.Z., Wang Q.W., Liang Y., Fang Y.Q. Protective effects of beta-asarone on cultured rat cortical neurons damage induced by glutamate. Zhong Yao Cai. 2007;30(4):436–439. [PubMed] [Google Scholar]
- 204.Jiang B., Liu J.H., Bao Y.M., An L.J. Catalpol inhibits apoptosis in hydrogen peroxide-induced PC12 cells by preventing cytochrome c release and inactivating of caspase cascade. Toxicon. 2004;43(1):53–59. doi: 10.1016/j.toxicon.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 205.Ved H.S., Koenig M.L., Dave J.R., Doctor B.P. Huperzine A, a potential therapeutic agent for dementia, reduces neuronal cell death caused by glutamate. Neuroreport. 1997;8(4):963–967. doi: 10.1097/00001756-199703030-00029. [DOI] [PubMed] [Google Scholar]
- 206.Gordon R.K., Nigam S.V., Weitz J.A., Dave J.R., Doctor B.P., Ved H.S. The NMDA receptor ion channel: A site for binding of huperzine A. J. Appl. Toxicol. 2001;21(S1):S47–S51. doi: 10.1002/jat.805. [DOI] [PubMed] [Google Scholar]
- 207.Hemendinger R.A., Armstrong E.J., III, Persinski R., Todd J., Mougeot J.L., Volvovitz F., Rosenfeld J. Huperzine a provides neuroprotection against several cell death inducers using in vitro model systems of motor neuron cell death. Neurotox. Res. 2008;13(1):49–61. doi: 10.1007/BF03033367. [DOI] [PubMed] [Google Scholar]
- 208.Wang C.J., Hu C.P., Xu K.P., Yuan Q., Li F.S., Zou H., Tan G.S., Li Y.J. Protective effect of selaginellin on glutamate-induced cytotoxicity and apoptosis in differentiated PC12 cells. Naunyn Schmiedebergs Arch. Pharmacol. 2010;381(1):73–81. doi: 10.1007/s00210-009-0470-4. [DOI] [PubMed] [Google Scholar]
- 209.Zhang F., Zheng W., Pi R., Mei Z., Bao Y., Gao J., Tang W., Chen S., Liu P. Cryptotanshinone protects primary rat cortical neurons from glutamate-induced neurotoxicity via the activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. Exp. Brain Res. 2009;193(1):109–118. doi: 10.1007/s00221-008-1600-9. [DOI] [PubMed] [Google Scholar]
- 210.Kanekura K., Hashimoto Y., Kita Y., Sasabe J., Aiso S., Nishimoto I., Matsuoka M.A. Rac1/phosphatidylinositol 3-kinase/Akt3 anti-apoptotic pathway, triggered by AlsinLF, the product of the ALS2 gene, antagonizes Cu/Zn-superoxide dismutase (SOD1) mutant-induced motoneuronal cell death. J. Biol. Chem. 2005;280(6):4532–4543. doi: 10.1074/jbc.M410508200. [DOI] [PubMed] [Google Scholar]
- 211.Chang M.X., Xu L.Y., Tao J.S., Feng Y. Metabolism and pharmacokinetics of ferulic acid in rats. Zhongguo Zhongyao Zazhi. 1993;18(5):300–302, 319. [PubMed] [Google Scholar]
- 212.Jin Y., Yan E., Fan Y., Guo X., Zhao Y., Zong Z., Liu Z. Neuroprotection by sodium ferulate against glutamate-induced apoptosis is mediated by ERK and PI3 kinase pathways. Acta Pharmacol. Sin. 2007;28(12):1881–1890. doi: 10.1111/j.1745-7254.2007.00634.x. [DOI] [PubMed] [Google Scholar]
- 213.Ren Z., Zhang R., Li Y., Li Y., Yang Z., Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017;40(5):1444–1456. doi: 10.3892/ijmm.2017.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nakayama H., Nakahara M., Matsugi E., Soda M., Hattori T., Hara K., Usami A., Kusumoto C., Higashiyama S., Kitaichi K. Protective effect of ferulic acid against hydrogen peroxide induced apoptosis in PC12 cells. Molecules. 2020;26(1):90. doi: 10.3390/molecules26010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Yingzhu CHEN., Yongjian GU., Shiyao BAO. Protective effects of acanthopanax senticousus saponins on cortical neuronal ischemia-hypoxia injury. J. Clin. Neurol. 1988;6:84–87. [Google Scholar]
- 216.Diao H-X., Song S-L., Liang H., Wang Y-S., Wang W-L., Ji A-G. Protective effect of polysaccharides from sea cucumber on glu-induced neurotoxicity in PC12 cells. Zhong Yao Cai. 2009;32(3):398–400. [PubMed] [Google Scholar]
- 217.Guatteo E., Carunchio I., Pieri M., Albo F., Canu N., Mercuri N.B., Zona C. Altered calcium homeostasis in motor neurons following AMPA receptor but not voltage-dependent calcium channels’ activation in a genetic model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2007;28(1):90–100. doi: 10.1016/j.nbd.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 218.Van Den Bosch L., Vandenberghe W., Klaassen H., Van Houtte E., Robberecht W. Ca2+-permeable AMPA receptors and selective vulnerability of motor neurons. J. Neurol. Sci. 2000;180(1-2):29–34. doi: 10.1016/S0022-510X(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 219.Prell T., Lautenschläger J., Grosskreutz J. Calcium-dependent protein folding in amyotrophic lateral sclerosis. Cell Calcium. 2013;54(2):132–143. doi: 10.1016/j.ceca.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 220.Hammadi M., Oulidi A., Gackière F., Katsogiannou M., Slomianny C., Roudbaraki M., Dewailly E., Delcourt P., Lepage G., Lotteau S., Ducreux S., Prevarskaya N., Van Coppenolle F. Modulation of ER stress and apoptosis by endoplasmic reticulum calcium leak via translocon during unfolded protein response: Involvement of GRP78. FASEB J. 2013;27(4):1600–1609. doi: 10.1096/fj.12-218875. [DOI] [PubMed] [Google Scholar]
- 221.Grosskreutz J., Van Den Bosch L., Keller B.U. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47(2):165–174. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 222.Mao Q.Q., Zhong X.M., Feng C.R., Pan A.J., Li Z.Y., Huang Z. Protective effects of paeoniflorin against glutamate-induced neurotoxicity in PC12 cells via antioxidant mechanisms and Ca2+ antagonism. Cell. Mol. Neurobiol. 2010;30(7):1059–1066. doi: 10.1007/s10571-010-9537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mao Q.Q., Zhong X.M., Li Z.Y., Huang Z. Paeoniflorin protects against NMDA-induced neurotoxicity in PC12 cells via Ca2+ antagonism. Phytother. Res. 2011;25(5):681–685. doi: 10.1002/ptr.3321. [DOI] [PubMed] [Google Scholar]
- 224.You J., Tan T., Kuang A., Zhong Y., He S. Biodistribution and metabolism of 3h-gastrodigenin and 3H-gastrodin in mice. J. West China Univ. Med. Sci. 1994;25:325–328. [PubMed] [Google Scholar]
- 225.Chen W.D., Lu X.L. Effect of gastrodin on release of glutamate from cultured nerve cells induced by potassium chloride. Chin. J. Nat. Med. 2000;2:8–10. [Google Scholar]
- 226.Sun R., Zhang Z., Huang W., Lv L., Yin J. Protective effects and machanism of muskone on pheochromocytoma cell injure induced by glutamate. Zhongguo Zhongyao Zazhi. 2009;34(13):1701–1704. [PubMed] [Google Scholar]
- 227.Shu-li S. Effects of ligustrazine on L-type calcium current in SH-SY5Y human neuroblastoma. Chinese J. Neuroimmunol. Neurol. 2004;11:43–45. [Google Scholar]