Abstract
Despite little progress in survival rates with regular therapies, which do not provide complete care for curing pediatric brain tumors (PBTs), there is an urgent need for novel strategies to overcome the toxic effects of conventional therapies to treat PBTs. The co-inhibitory immune checkpoint molecules, e.g., CTLA-4, PD-1/PD-L1, etc., and epigenetic alterations in histone variants, e.g., H3K27me3 that help in immune evasion at tumor microenvironment have not gained much attention in PBTs treatment. However, key epigenetic mechanistic alterations, such as acetylation, methylation, phosphorylation, sumoylation, poly (ADP)-ribosylation, and ubiquitination in histone protein, are greatly acknowledged. The crucial checkpoints in pediatric brain tumors are cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed cell death protein-1 (PD-1) and programmed death-ligand 1 (PD-L1), OX-2 membrane glycoprotein (CD200), and indoleamine 2,3-dioxygenase (IDO). This review covers the state of knowledge on the role of multiple co-inhibitory immunological checkpoint proteins and histone epigenetic alterations in different cancers. We further discuss the processes behind these checkpoints, cell signalling, the current scenario of clinical and preclinical research and potential futuristic opportunities for immunotherapies in the treatment of pediatric brain tumors. Conclusively, this article further discusses the possibilities of these interventions to be used for better therapy options.
Keywords: Epigenetics, histones, pediatric brain tumor, immune checkpoints, immunotherapy, cell signalling
1. INTRODUCTION
Despite the rarity, the incidence of brain tumors (BT) is found to be higher in children compared to adults. However, there are also tumors other than solid tumors in children. Moreover, it is the second most form of cancer, which represents about 23% of total cancer incidents and is also a leading cause of death among all pediatric cancer patients in the United States. It is reported that the rate of occurrence of brain and nervous system cancer is about 1/161 individuals, and 23,770 new cases of both genders have been diagnosed recently, with 16,050 deaths. Despite the advancement in brain cancer therapy, the death rate has remained the same for the past 30 years, and the accurate cause of BTs development is not yet fully understood. PBTs are entirely different from adult BTs in their location and behaviour as children differ due to alterations in physique and anatomy of the brain, which are still in developing mode. In addition, the treatment options are also to be varied with respect to their age because children show relatively better prognoses than adults under similar conditions.
Based on cell structure, composition, growth rate, and other biological characteristics, BTs are classified into astrocytoma, medulloblastoma (MB), ependymoma, atypical teratoid rhabdoid tumor (ATRT), brain stem glioma, choroid plexus carcinoma, choroid plexus papilloma, craniopharyngioma, diffuse intrinsic pontine glioma (DIPG), desmoplastic infantile astrocytoma, cysts, germ cell tumors, neurofibromatosis, oligodendroglioma, optic glioma, primitive neuroectodermal tumor (PNET), etc. However, their names and classification might be altered over time with respect to the availability of data regarding alterations in the tumors.
Local tumors escape from antigen-stimulated primary immune response mediated by major histocompatibility complex (MHC) and T-cell receptor (TCR), followed by co-stimulatory secondary T-cell response mediated by CD28 and CD80/CD86 that can be regulated by inhibitory immune checkpoints by inducing FoxP3+Tregs as reported in different adult malignancies [1, 2]. Apart from FoxP3+ Tregs, myeloid-derived suppressor cells (MDSCs) [3] and tumor-infiltrating Tregs [4] contribute to the cessation of classic and anti-tumor immunities and facilitates the secretion of soluble membrane-bound immunosuppressive molecules [3]. Epigenetics plays an important role in affecting the progression of tumor pathogenesis. Generally, the immune system differentiates the abnormal cells inside the body; eventually, the immune cells identify and eliminate the foreign entity. This process of selection is done by the immune checkpoints [5]. These immune checkpoints act as the control entity that protects the normal cells from damage caused by the immune cells. During tumors, these checkpoints get disturbed and foster the normal immune reaction. The epigenetic modulation of the immune checkpoints can be regulated by various mechanisms like covalent modification, histone modification, and microRNA [6]. The epigenetic functional classification system classifies cancer genes into epigenetic mediators, i.e., progenitor cells, modifiers, and modulators, which affect the tumor environment inside the body. Immune modifiers can act on the genes and regulate them on and off [7]. The modulation of the immune checkpoints by epigenetics thus can be a major therapeutic target. In the development of PBT, the epigenetics of histone H3 plays a vital role. Similar to histone H3, numerous important amino acids, such as serine and lysine-14, undergo alterations, including ubiquitination and phosphorylation, thus affecting the progression of the tumor [8]. However, this concept is poorly understood regarding how they contribute to tumor formation. Together, the initiation, metabolism, angiogenesis, and metastasis of cancer genes are influenced by histone 3 lysine modification [9]. The characteristics and results of PBT carcinogenesis can be significantly altered by altering the histone 3 lysine. This novel concept has been used in clinical trials and may possibly result in effective treatments for tumor patients [10].
Moreover, these checkpoints can actively participate in peripheral immune tolerance and also demonstrate a promising role in immune evasion in tumor microenvironments. However, limited research is focused on the potential benefits of these inhibitors against solid tumors in children.
Furthermore, epigenetic regulations in protein-coding genes at both DNA and expression levels determine cell fate as well as organism development. Though epigenetic changes inherit somatically, they are quite reversible and also ensure the timely expression of genes that are naturally involved in the development and differentiation of cells. Also, they promptly express a version of unnatural genes that are not encoded by DNA in response to environmental stimuli for adaptation. They bring notable molecular and structural changes in DNA without altering the sequence [11]. Among the different epigenetic regulations, DNA methylation is a well-studied process that mainly occurs in cytosine residues of CpG islands by DNA methyltransferases (DNMTs). DNA methylation in DNA repeats aids chromosomal stability and inhibits homologous recombination [12], whereas its episode in the genes prevents turbulent transcriptional initiation [13]. Furthermore, global DNA hypomethylation in gene bodies and hypermethylation of CpG islands in the tumor suppressor genes (TSGs) located in the promoter regions are ultimately responsible for the development of cancer [14]. In normal tissues, which are actively involved in the development and differentiation, the CpG islands of most of the gene promoters remain unaltered [15] except for the inactivation of the X-chromosome [16]. However, hypermethylation of CpG islands initially recruits methyl-CpG binding domain (MBD) proteins, which further promotes the recruitment of histone-modifying and chromatin-remodelling complexes to the methylated promoter region, which in turn, silences the target gene [17, 18].
In addition, histone proteins are the hotspots and are highly susceptible to a wide range of epigenetic changes, which, in turn, regulate the chromatin structure and dynamics. These histone proteins (H2A, H2B, H3, and H4) are highly conserved from primitive eukaryote yeast to evolutionarily high-ranked organisms, such as humans. In the nucleus, histone octamer (two of each histone protein) is wrapped by DNA and forms a nucleosome core, which allows the packing of large DNA into the compact chromatin. In this process, the nucleosomal histone core exposes its amino-terminal tail called “histone code”, which is highly prone to endure an array of posttranslational modifications (PTMs) and subsequently reports the genes to express or silence. Aberrant mutations in such vital proteins drive the development of cancer.
Recently, a novel concept has been proposed that the aberrant epigenetic alterations inducing chromatin structural deformities may exhibit acquired chemo-resistance to some extent in cancer patients [19]. To oppose this, the Food and Drug Administration (FDA) has approved different epigenetic therapies for cancers, such as leukemia, which include DNA methylating agents and histone deacetylase inhibitors (HDIs). Moreover, their combinations were also tested in clinical trials [20]. However, in the treatment of glioblastoma (GBM), only HDIs entered into clinical trials but not demethylating agents. Probably, this is because of methylated O6-methylguanine DNA-methyltransferase (MGMT) promoter (typically involved in DNA repair) sensitizes the tumors to alkylating agents like temozolomide (TMZ), a standard drug for GBM [21]. In general, MGMT neutralizes TMZ, which, in turn, leads to the development of resistance in GBM. However, this mechanism is not yet fully understood in children [22-25]. Later, it was predicted that a large frequency of MGMT methylation might be attributed to the H3.3 G34R/V (glycine to arginine or valine substitution at position 34 on histone H3.3 variant) subtype but not to K27M (lysine to methionine substitution at position 27) mutated tumors [26], which may aid in poor clinical response to TMZ in most of the high-grade gliomas (HGGs), including DIPG [27-30]. Moreover, demethylating agents may activate oncogenes besides the TSGs because of their non-specific mechanism of action. Furthermore, they may affect genome stability by inducing demethylation in gene bodies and hypomethylation in repetitive sequences. Despite the reluctance of conventional therapies against pediatric HGGs, epigenetic modifying agents that targets histone demethylases (HDMs) and HDIs remain effective against DIPG [31, 32] by inducing the restoration of H3K27me3 in alternative ways, highlighting their synergistic effect in combination [31]. With this brief literature, here, we have critically reviewed different immune checkpoints and their inhibitors in clinical trials as well as the epigenetic landscape of histone proteins (H3) and possible epigenetic therapies for PBTs collectively.
2. IMMUNE CHECKPOINTS AND PRECLINICAL STUDIES
2.1. PD-1 and PD-L1
PD-L1 is a 40 kDa glycoprotein that belongs to type-1 transmembrane glycoproteins and is located on the surface of hematopoietic and parenchymal cells [33]. It is a co-stimulatory molecule belonging to B7-family and is also named B7-H1. It typically interacts with the PD-1 protein, a receptor on activated T and B-cells to regulate both humoral and cell-mediated immunities [1]. PD-1 plays a crucial role in autoimmunity and peripheral immune tolerance. Induction of PD-L1 enables tumor growth by causing immune evasion at the tumor microenvironment [1] and further promotes metastasis and the refractory state of the disease in adult malignancies [34, 35]. PD-L1 expression and its blocking responses vary based on the stage and subtype of the tumor [36, 37]. The low PD-L1 expression in human primary osteosarcoma tumors and high PD-L1 expression in metastatic disease neutralize the response of tumor-infiltrating lymphocytes (TILs) effector by interacting with PD-1+, CD8+, and T-cells [36]. However, targeting PD-L1 with specific antibodies showed better survival benefits in the murine model by restoring lymphocyte anti-tumor functions. Likewise, in a study on a murine model of MB subtypes, the PD-1 blockade was more efficient in group-3 MB with a higher CD8+T to PD-1+T-cells ratio compared to Shh-subtype MB [37], indicating the crucial role of immune divergence in disease outcome or therapy (Fig. 1).
Fig. (1).
Epigenetic control of PD-1 and the PD-L1 involved in the immunogenic checkpoints in the cancer. Legend: Figure showing an underlying mechanism of epigenetic alteration of PD-1 and PD-L1 involving the immunogenic checkpoints in cancer. (A) The priming phase, where the T-cells (CD-8) get activated by the dendritic cells; then, the infiltration of the T-cells occurs. (B) An interaction between cancer cells and the T-cells, where cancer cells disturb the regulation of PD-L1. Durvalumab blocks the expression of PD-L1; on the other hand, activation of T-cells epigenetically affects the expression of PD-1. Novolumab acting as a blocker and normalizing the expression of PD-1 in T-cells.
In pediatric cancers, the differential expressions of PD-L1 between moderate to high [38-42] are due to variations in the available PD-L1 antibody specificity [43]. Moreover, these expressions are positively correlated with survival rates [39]. In preclinical studies on pediatric retinoblastoma and neuroblastoma patient cells, the PD-L1 expression was significantly upregulated in the response to immune stimuli [44, 45]. However, PD-L1/PD-1 specific antibody therapy alone was insufficient to induce an anti-tumor T-cell response in neuroblastoma of murine model, but in combination with BLZ945, a selective inhibitor of colony stimulating factor (CSF)-1 receptor, it was able to block MDSCs induced tumor progression [46], suggesting the reliability of combinational therapies.
FDA approved multiple mAbs against PD-1 and PD-L1, which have survival benefits in a wide spectrum of malignancies [47-51]. Nivolumab, a PD-1 inhibitor, was approved by FDA in spite of a few immune-related adverse events (IRAEs) linked to grade 3-4 toxicities in 16% of melanoma patients. However, with the combination of ipilimumab, which is an inhibitor of cytotoxic T-lymphocyte antigen (CTLA)-4, this toxicity was further raised to 55% [51]. Hence, phase I/II trials are in progress to elucidate the safety dosages of nivolumab alone or in combination with ipilimumab for anti-tumor activity. Due to unresponsiveness to the treatment, the PD-L1 levels are not at all considered for tumor prognosis [47, 52]. Despite the lack of clinical studies on testing the potentiality of blockade of PD-1/PD-L1 in pediatric intracranial solid tumors, a recent report has shown the therapeutic efficacy of nivolumab in recurrent multifocal GBM and biallelic mismatch repair deficiency in children [53]. Phase II clinical trials (NCT02550249) in Spain have been testing an adjuvant role of nivolumab in primary or recurrent GBM patients of >1 year of age. In addition, the European basket trial (NCT02813135) has been testing nivolumab in combination with cyclophosphamide against refractory tumors in patients with ≤ 18 years of age, in the presence or absence of radiation. Similarly, phase I/II clinical trials have been initiated by Children’s Oncology group for testing the safety of nivolumab in combination with ipilimumab against refractory solid tumors in pediatric patients (NCT02304458). In Canada, phase II clinical trials have been planned to test the safety and efficacy of durvalumab (PD-L1 inhibitor) in combination with tremelimumab (CTLA-4 inhibitor) against advanced rare tumors in ≥16 years aged patients (NCT02879162). Likewise, another PD-1 inhibitor, pembrolizumab, was also tested in phase II clinical trials for its safety and dosage limits against recurrent or refractory HGG and DIPGs and was found to be active via the PBT consortium. However, there are other clinical trials testing nivolumab alone or in different combinations, such as phase III trials of nivolumab (Opdivo®) + ipilimumab (Yervoy®) against recurrent GBM (NCT02017717) and nivolumab + radiation vs. TMZ (Temodar®) in newly-diagnosed GBM (NCT02617589), etc. Finally, Ludwig Cancer Research has been sponsored for phase II trials to test the efficacy of durvalumab (MEDI4736) against GBM (NCT02336165) and phase I trials to test the durability of nivolumab with the combination of a dendritic cell (DC), a vaccine against recurrent glioma, astrocytoma, or GBM (NCT02529072).
2.2. Cytotoxic T-lymphocyte Antigen-4
It is a homodimeric intracellular glycoprotein belonging to the immunoglobulin (Ig) gene superfamily. However, in response to continuous stimuli and abundant levels of IL-2, CTLA-4 can be exported to the cell surface of different subsets of T-cells, including CD4+, CD8+, and Tregs. CTLA-4 extracellular domain has shared 30% homology with CD28, which is expressed on the surface of T-cells; therefore, it competes with CD28 to interact with B7 family proteins like CD80 (B7-1) or CD86 (B7-2) expressed on antigen-presenting cells (APCs) with high affinity [54, 55]. In this manner, CTLA-4 hampers the production of IL-2, IL-4, IFN-γ, and expression of IL-2R, which results in the depletion of T-cell activation and expansion. Furthermore, it also attenuates the cell cycle to induce death in activated T-cells [56]. CTLA-4 substantially regulates immune tolerance and homeostasis via circulatory Tregs, while its deficiency causes systemic lymphoproliferation [57].
However, its direct inhibition with anti-CTLA-4 antibodies dramatically reduced Tregs count and induced expansion of effector CD8+ T-cells at the tumor microenvironment in colon adenocarcinoma [58], further unveiling the therapeutic potential of targeting CTLA-4 [59]. Similarly, when osteosarcoma cells were incubated with recombinant B7-1 or B7-2 ligands, CTLA-4 directed caspase-dependent apoptosis in these cells, further exploring its sensitivity to be targeted by anti-cancer agents. Polymorphism in the CTLA-4 gene (rs231775) increased malignancy in osteosarcoma and Ewing sarcoma since the affinity with B7-1 molecule was significantly increased and concurrently, effector T-cell function was diminished [60, 61]. A large number of preclinical studies have targeted CTLA-4 in adult malignancies, but it remains unexplored in pediatric tumors [62-64]. A report has sown an upregulated expression of CTLA-4 on the surface and cytoplasmic compartments of different pediatric cancer cells of neuroblastoma, osteosarcoma, and rhabdomyosarcoma [65].
However, ipilimumab was the first checkpoint inhibitor studied in phase I trials against 33 different pediatric solid tumor patients (melanoma, sarcoma, renal/bladder carcinoma, and neuroblastoma) aged between 2.4 to 21 years [66], out of which the majority of patients developed immune-related toxicities as described in adult pancreatitis, pneumonitis, colitis, endocrinopathies, and transaminitis, but the overall survival rate was improved by 93.0%. In continuation, ipilimumab and tremelimumab alone or in combination with standard drugs have increased survival benefits by 87.8% in advanced melanoma [49, 51, 67, 68]. However, it demonstrated grade 1-2 IRAEs in the skin and gastrointestinal tract (GST) and grade 3-4 IRAEs in GST, liver, and endocrine system in 1/3 of patients, and death in 1-2% of overall patients. Recently, a phase II study of ipilimumab against stage III/IV melanoma of pediatrics aged between 12-17 years (NCT01696045) was terminated due to underperformance.
2.3. CD200/OX2
CD200 is another immune checkpoint abundantly expressed in lymphoid and neuronal tissues [69]. It is a type I transmembrane protein belonging to the B7-family and is involved in T-cell signaling via its receptor CD200R, which is expressed on APCs and T-cells. CD200 signaling in macrophages impedes IL-2 and IFN-γ production [70, 71] and reduces effector T-cell response by inducing Tregs [72, 73]. Its expression on the surface of tumor cells directs their immune evasion [73, 74]. Moreover, its overexpression in acute myeloid leukemia was coupled with increased Tregs and reduced natural killer (NK) cells, ultimately resulting in a poor prognosis [75, 76]. Macrophage lineage cells, including brain microglia, displayed an active phenotype and were more abundant in the absence of CD200. Upon facial nerve transection, CD200-deficient neurons induced a rapid microglial response. Furthermore, CD200R agonists reduced mouse and human myeloid cell activity in vitro, establishing a dose-response relationship between receptor expression and cellular suppression [70, 71].
The profiling of its expression was studied in different PBTs and it was reported that CD200 protein levels were elevated in ependymoma, MB, and DIPG compared to normal brain tissue [77]. Likewise, its mRNA levels were found to be upregulated in supratentorial ependymoma and group 4 MB subtypes compared to posterior fossa ependymoma and Shh/group 3 MB subtypes, respectively. Preclinical studies on pediatric neuroblastoma patient samples have confirmed that the high expression of CD200 on their cell surface was correlated with reduced effector Th1 response, which was attributed to low IL-2 and IFN-γ levels [78]. Upon anti-CD200 antibody treatment, the Th1 response was restored, thus suggesting the tumor-inducing property of CD200. Similarly, upon CD200 antagonist (OVA+poly-ICLC) treatment, the antigen-specific T-cell mediated immunity and survival rates were substantially enhanced in the murine glioma model [77]. However, there is a gap in currently active clinical trials for both pediatric and adult targeting CD200. An early report in 2011 has shown moderate efficacy of anti-CD200 mAb (samalizumab) against adult B-cell chronic lymphocytic leukemia or multiple myeloma, in spite of few side effects like fatigue, fever, rash, and neutropenia [79].
2.4. Indoleamine 2,3-dioxygenase (IDO)
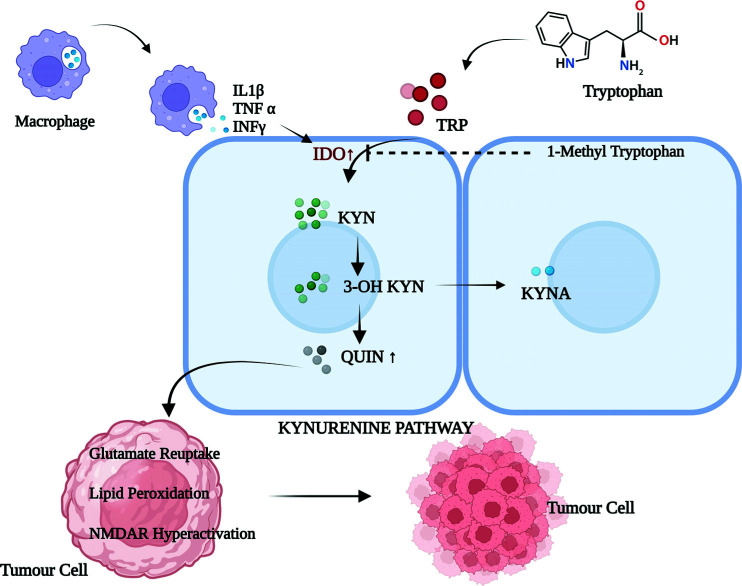
IDO is a rate-limiting enzyme in L-tryptophan catabolism, widely expressed in a variety of cells, and likely contributes to immune suppression and peripheral tolerance in mammals [80, 81]. IDO catalyses the first reaction in the kynurenine pathway [82] and produces a series of metabolic intermediates like quinolinic acid (QUIN), L-kynurenine, and picolinic acid (PIC), which can inhibit T and NK cells proliferation [83] Another intermediate substance, 3-hydrozykynurenine (3-HK), was found to induce the production of free radicals, which, in turn, directed the immunomodulation. However, a further increase in the catabolism of L-tryptophan by inflammatory IFN-γ induced intracellular IDO in monocytes and tumor cells, thus restraining the functions of NK and T-cells effectors [84]. Similarly, IDO overexpression in inflammatory bowel disease prevents the tissue-damaging activity of activated T-cells, unveiling its anti-inflammatory functions [85]. Furthermore, IDO expression in the placenta prevents allogenic fetus rejection during early pregnancy [86]. Likewise, IDO expression in tumor cells of the immunogenic murine model did not tolerate the rejection in the mice model, which was preimmunized with tumor antigen, due to the reduced T-cell infiltration to the tumor site [87] (Fig. 2). However, IDO inhibition by an oral drug, 1-methyl-tryptophan or indoximod remarkably suppressed tumor succession. It is the only drug available against IDO and is currently under phase I/II clinical trials against complex extracranial solid tumors. Indoximod with the combination of conventional chemotherapies or radiation and with other checkpoint inhibitors, such as ipilimumab, pembrolizumab, or nivolumab, has shown grade 2 IRAE hypophysitis in 6% of pre-treated patients [88]. Likewise, other phase I/II trials have been testing indoximod against recurrent glioma (NCT02052648).
Fig. (2).
IDO as a checkpoint in the Kynurenine pathway. Legend: The IDO is the rate-limiting metabolite of the tryptophan metabolism, which is up-regulated by the proinflammatory cytokines IL 1β, TNF α, and INF γ, thus generating the toxic metabolites (Quinolinic acid) affecting the cancer metabolism. Abbreviations: (TRP: Tryptophan, KYNA: Kynurenic Acid, QUIN: Quinolinic Acid, IL 1β: Interlukine 1β, TNF α: Tumor Necrosis Factor α and INF γ: Interferon γ).
Though preclinical studies on IDO and indoximod against PBTs are limited, currently a phase I trial (NCT02502708) is focused on testing indoximod in combination with TMZ against primary malignant brain tumors of patients aged between 3-21 years. During this trial, the dosage of indoximod (12.8 to 22.4 mg/kg/dose) and one dose of TMZ (200 mg/m2) for a total of five days were given to measure the toxicity, responsiveness, pharmacokinetics, and progression-free survival benefits. Previously, IDO was examined in pediatric extracranial solid tumors, such as osteosarcoma, in which, patients’ samples scored ≥ 4 IDO expression measured by immunohistochemistry (IHC). Moreover, it demonstrated poor metastasis-free survival in 53% of patients compared to less-scored patients with 81% metastasis-free survival [89]. Furthermore, IDO expression was also noticed in 85% (17/20) of patients with pediatric Hodgkin lymphoma and Ewing sarcoma.
2.5. B7-H3
B7-H3 (CD276), an important immune checkpoint, belongs to the B7-CD28 family, having 28% homology with other molecules of the same family. Its coding gene is located on chromosome 15q23-q24 in humans and chromosome 9 in mice. It is a type-I transmembrane protein consisting of 534 amino acids with a molecular weight of 57 kDa. B7-H3 was first identified in humans [90], followed by mice [91]. However, its expression is universal across the species. The extracellular domain of B7-H3 is composed of a pair of Ig variable (V) and constant (C) domains without exon duplication in mice (2Ig B7-H3 isoform) and two identical pairs of IgV-IgC domains in humans (4Ig B7-H3 isoform; a predominant splicing variant) with exon duplication. It has no intracellular signalling motif in spite of its short tail [92-95]. Detectable levels of a soluble splicing variant of CD276 without a transmembrane domain were observed in human sera, which might be due to its cleavage from the surface by matrix metallopeptidase (MMP) or alternative splicing of introns [96]. B7-H3 mRNA levels were ubiquitously found in many tissues, like the liver, heart, prostate, spleen, and thymus. However, its protein expression was found to be limited at a steady state, suggesting the involvement of key post-transcriptional control mechanisms [97]. Furthermore, B7-H3 is likely to play a key role in the interaction between tumors and the immune system. It is a ligand for unknown T-cell receptors and binds to the T-cells with its FG-loop of the Ig variable domain.
Over the years, it has been speculated that B7-H3 may show functional dualism on T-cell mediated immune response by interacting with counter-receptors on T-cells [98-100]. B7-H3 has shown co-stimulatory functions like T-cell proliferation, cytokine production, and cytotoxicity by interacting with TREM (triggering receptor expressed on myeloid cell), like transcript 2 (TLT2) [101], which is a counter-receptor on CD8+ T-cells in mice but not in humans [101]. However, the majority of the outcomes have been correlated with its co-inhibitory function on T-cells. In this concern, B7-H3 expression on tumor cells targets NK-cells to suppress their mediated lysis of the tumor. Its wide expression on APCs inhibits T-cell function by diminishing the activity of NFAT, NF-kB, and AP-1 transcription factors [102]. Its expression on DCs stimulated by CD4+CD25+ Tregs, which impairs effector T-cell function [103], is evidence for its co-inhibitory role. Despite the controversial findings regarding its interaction with the other proteins and functions [104], it is still a challenge to identify its functional receptors in humans for better elucidation of CD276 downstream signaling in immune responses and tumor evasion [105, 106].
B7-H3 overexpression has been observed in a wide variety of human solid tumors, which correlates with negative or poor cancer prognosis. Moreover, inducible CD276 expression relatively correlates with the aggression of tumorigenesis and metastasis. Previous reports demonstrated the overexpression of B7-H3 in human malignancies, such as cutaneous melanoma [107], acute leukemia [108], breast cancer [109], prostate cancer [110], ovarian cancer [111], pancreatic cancer [112], colorectal cancer [113], GBM [114], and other cancers. B7-H3 is found on the membrane, in the cytoplasm, or within the nucleus of cancer cells, e.g. osteosarcoma [115] and colon cancer [116]. Interestingly, it was also found in tumor-associated vasculature in renal cell carcinoma [117]. Its overexpression increases the risk of disease spread during surgery, cancer recurrence, and ultimately death in prostate cancer patients [110]. Most recently, in breast cancer, B7-H3 has been successfully used as a biomarker for ultrasound molecular imaging [118]. Due to high differential expression between tumors and normal tissues, B7-H3 has emerged as a promising target for breast cancer therapy, including triple-negative breast cancer (TNBC) [119-122]. Breast cancer patients with B7-H3high/Foxp3high tumors have shorter recurrence-free survival [123]. Abnormal glycosylation, mostly high rate of fucosylation in B7-H3 during oral cancer, may lead to DCs tolerance because membrane proteins like DC-specific ICAM-3-grabbing non-integrin (DC-SIGN) and Langerin of DCs have a better affinity with CD276 [124]. It has been observed that the relative expression of B7-H3 mRNA was higher in melanoma tissues [107] and blood specimens of gastric cancer patients compared to their healthy counterparts [125]. Additionally, an increased B7-H3 expression reduced TILs number and elevated lymph node metastasis, suggesting immune evasion and tumorigenesis in lung cancer [126]. Furthermore, tumor-associated macrophages (TAMs) derived IL-10 attenuated T-cell anti-tumor immunity in lung cancer mice model, which was correlated with increased expression of B7-H3 on the membrane [127]. Fibroblast-associated B7-H3 expression correlated with the shorter metastasis-free survival and the endothelial B7-H3 expression significantly correlated with poor outcome of colorectal cancer [116, 128]. However, its silencing by RNA interference (RNAi) technology was found to increase chemosensitivity of Ara-C and apparently reduce mRNAs of both MMP-2 and MMP-9 in acute myeloid leukemic cell line (U937), indicating B7-H3 involvement in transcriptional regulation of these two genes [129], which plays a central role in tumor migration, invasion, and metastasis [130].
The molecular mechanism behind the mode of action of B7-H3 remains unknown. However, the non-immunological functions, such as invasion, metastasis, and adhesion to fibronectin [131] of B7-H3 mediated by Jak2/Stat3/MMP-9 signaling [132], received great attention over its co-stimulatory or co-inhibitory effects on immune cells and the anti-tumor response in early studies. B7-H3 has no definite role in cell growth or proliferation [107]. In breast and colorectal cancers, overexpression of B7-H3 derails the apoptosis process by inducing Jak2/Stat3/Slug signalling, which further increases resistance to paclitaxel. Conversely, its knockdown in breast cancer reduces the phosphorylation of STAT3 Tyr705 residue via the inactivation of Jak2 [133, 134]. In hepatoma cells, B7-H3 induces epithelial-mesenchymal transition (EMT) via Jak2/Stat3/Slug signalling pathway [135]. In melanoma cells, B7-H3 alters the expression of metastasis markers like MMP-9, tissue inhibitor of metalloproteinase (TIMP)-1 and 2, STAT-3, and IL-8 [115, 131, 136, 137]. However, its knockdown downregulates pSTAT3 and cyclin D1, which are key molecules in the cell cycle [107]. The relationship between B7-H3 and metabolism was unveiled by identifying reduced glycolytic efficacy at low B7-H3 expression, which further increased the sensitivity of breast cancer cells to Akt/mTOR inhibitors [138]. Concurrently, another report demonstrated that B7-H3 promotes the Warburg effect (i.e., increased glucose uptake and lactate production) in breast cancer cells by inducing hypoxia-inducible factor (HIF)-1α and its downstream enzymes, e.g. lactate dehydrogenase (LDH)-A and pyruvate dehydrogenase kinase 1 (PDK1) of the glycolytic pathway, resulting in tumor growth [139].
Over the decade, vascular endothelial-specific B7-H3 expression on tumor cells becomes an attractive target for cancer immunotherapy. In this context, an unconventional mAb (Enoblituzumab/MGA271), which can operate antibody-dependent cell-mediated cytotoxicity (ADCC), was tested against B7-H3 in phase-I clinical trials on different cancers [122, 140, 141]. Likewise, a weekly dosage of MGA271 controlled the tumor growth and exhibited an Fc-mediated effect in renal and bladder carcinoma xenografts [122]. Its anti-tumor activity was also mediated by increasing T-cell repertoire clonality in peripheral blood.
8H9 is an antibody-drug conjugate that specifically binds to the FG loop of B7-H3 and has shown clinical success in patients with metastatic CNS neuroblastoma [142, 143]. Using small interfering (si)-RNA technology, Chen et al. demonstrated that downregulation of human B7-H3 reduced the cell adhesion to fibronectin, migration, and Matrigel invasion of melanoma and breast cancer cells. Other studies have also reported that B7-H3 was important in regulating the adhesion, migration, and invasion characteristics of pancreatic and prostate cancers, GBM, cutaneous melanoma, and osteosarcoma. These studies suggest that it may be a potential therapeutic target for the tumors that overexpress B7-H3. Small-hairpin (sh) RNA targeting B7-H3 in combination with the Ara-c drug was found to upregulate antineoplastic activity and cause 80% tumor reduction in histiocytic lymphoma compared to 40% with Ara-c alone [129]. Similarly, shRNA of B7-H3, together with paclitaxel, caused an 80% tumor reduction in breast cancer, thereby indicating shRNA-induced chemosensitivity and apoptosis in tumor cells [133]. Most recently, Shi et al. identified that the mAb Y4F11 was able to interact with the antigen B7-H3 on different immune cells from malignant pleural effusions of lung cancer patients grafted to BALB/c mice [144]. However, further therapeutic approaches like blocking mAbs, bispecific mAbs, chimeric-antigen receptor (CAR) T-cells, small molecule inhibitors, and synergistic options (chemo/radiation/ anti-CTLA4/anti-PD-1 together with anti-B7-H3) need to be evaluated in targeting B7-H3 for different cancers, including PBTs. MicroRNA (miR)-29a, 29b, and 29c inversely correlate with B7-H3 protein expression, and these miRNAs typically target 3’-UTR of B7-H3 for silencing. However, they were found to be downregulated in solid tumors, including neuroblastoma [145], melanoma (only miR-29c) [107], sarcomas, brain tumors, and tumor cell lines. However, the role of miRNAs in MB development is not yet studied and B7-H3 may have diversified effects based on the type of tumor origin.
Apart from these immune checkpoints, most recently, several other molecules have emerged due to their immunomodulatory properties in human cancers. The lymphocyte activation gene 3 (LAG-3) [146], fibrinogen-like protein 2 (FGL2) [147], colony stimulating factor-1 receptor (CSF-1R), T-cell immunoglobulin mucin domain (TIM)-3 [148], V-domain Ig suppressor of T cell activation (VISTA) [149], inducible co-stimulator (ICOS) [150], OX40 [151], and 4-1BB [152] have entered into clinical trials. The current combination immunotherapy in pediatric brain tumors is presented in Table 1. However, further evaluation is required to register them in pediatric cancer therapy [153-164]. There is a requirement for concrete and robust research on tumor microenvironment and host-tumor interactions in pediatric cancers.
Table 1.
Drugs and their mechanism as immune checkpoint inhibitors.
| S. No. | Drug | Class | Rationale of Uses | References |
|---|---|---|---|---|
| 1. | Nivolumab (Opdivo) | PD-1 inhibitors | Gastro-esophageal adenocarcinoma | [153] |
| 2. | Cemiplimab (Libtayo) | PD-1 inhibitors | Advance cutaneous squamous-cell carcinoma | [154] |
| 3. | Atezolizumab (Tecentriq) | PD-L1 inhibitors | Small- cell lung cancer | [155] |
| 4. | Avelumab (Bavencio) | PD-L1 inhibitors | Advanced urothelial carcinoma | [156] |
| 5. | Durvalumab (Imfinzi) | PD-L1 inhibitors | Biliary tract cancer | [157] |
| 6. | Ipilimumab (Yervoy) | Cytotoxic T-lymphocyte antigen-4 (CTLA-4) | Advanced melanoma | [158] |
| 7. | Tremelimumab (Imjuno) | Cytotoxic T-lymphocyte antigen-4 (CTLA-4) | Hepatocellular carcinoma | [159] |
| 8. | Relatlimab | Lymphocyte Activation Gene-3 (LAG3; CD223) | Chronic lymphocytic leukemia (CLL) | [160] |
| 9. | Opdualag | Lymphocyte Activation Gene-3 (LAG3; CD223) | Melanoma | [161] |
| 10. | Indoximod | Indoleamine 2,3-dioxygenase (IDO-1) inhibitors | Advanced or metastatic melanoma | [162] |
| 11. | Epacadostat | Indoleamine 2,3-dioxygenase (IDO-1) inhibitors | Renal cell carcinoma (RCC) | [163] |
| 12. | Navoximod | Indoleamine 2,3-dioxygenase (IDO-1) inhibitors | Recurrent advanced solid tumors | [164] |
3. IMMUNOTHERAPY OF BRAIN CANCER AND CLINICAL TRIALS
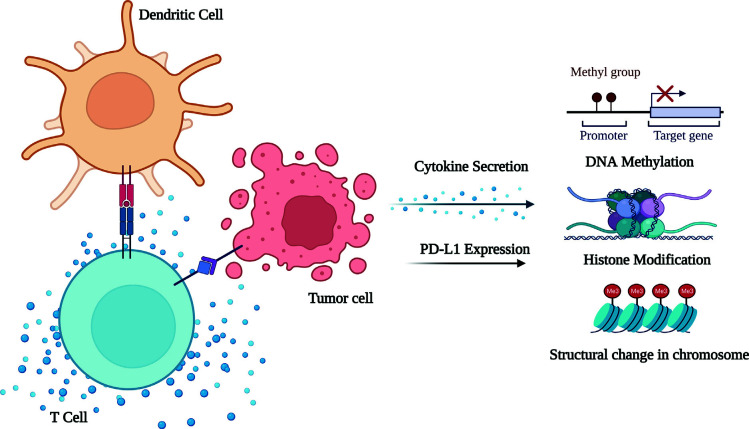
It is known that tumors can escape from immune surveillance by exhibiting immune tolerance. However, this can be surpassed efficiently by different approaches: (I) Using mAbs [165] against immune checkpoints and tumor-specific antigens (TSAs), e.g. targeting epithelial growth factor receptor (EGFR)/EGFRvIII in GBM with ABT-414, an antibody-drug conjugate in phase I (NCT01800695) and II (NCT02573324) clinical trials and (II) Using tumor-specific or tumor-associated antigen vaccines [166], e.g. tumor-derived heat shock protein/protein complex (HSPPC)-96 vaccine, recently tested in phase II trials (NCT01814813) against surgically removable recurrent glioma and revealed its clinical benefit [167]. SL-701, a vaccine comprising multiple synthetic peptides, is in phase I/II trials, testing against recurrent GBM (NCT02078648). ICT-121, a CD133 pulsed-dendritic cell vaccine, is in phase I trials, testing against recurrent GBM (NCT02049489). A dendritic cell vaccine combined with imiquimod, which is a Toll-like receptor (TLR)-7/8 agonist, is in phase I trials, testing against adult and pediatric glioma (NCT01808820) as well as pediatric brain cancer (NCT01902771). Imiquimod combined with GBM-6, which can target brain tumor-initiating cells (BTICs), is in phase I trials, testing against pediatric DIPG (NCT01400672). ADU-623 targets EGFRvIII and NY-ESO-1 antigens and is in phase I trials, testing against recurrent grade III/IV astrocytomas (NCT01967758). Further pilot studies have been testing glioma antigen peptides in combination with imiquimod and Poly-ICLC (Hiltonol®), which is a TLR-3 agonist against recurrent ependymomas (NCT01795313) and pediatric glioma (NCT01130077), respectively. Apart from these, glioma-associated antigens like EphA2, IL-13Rα2, and Survivin can be potential vaccine candidates against pediatric brainstem and non-brainstem glioma [166]. (III) Targeting cytokines and inflammatory pathways [168], e.g. targeting IL-2, IL-4, IFN-γ, TNF-α, and others that are reluctant to treat brain tumors [169] (Fig. 3). (IV) Adoptive cell therapy, in which genetically altered immune cells are reinfused into the patients with the target of improving anti-tumor immunity, such as testing anti-EGFRvIII CAR T-cells against malignant glioma (NCT01454596) and GBM (NCT02209376) is in phase I/II clinical trials.
Fig. (3).
Cytokines influencing the epigenetics and cancer metabolism. Legend: After T-cells activation by the dendritic cells, they interact with the nearby cancerous cell and release cytokines. These cytokines then affect the epigenetics, such as DNA modification, histone modification, change in the structure of chromatin, etc.
4. EPIGENETIC LANDSCAPE OF HISTONE H3 AND POSSIBLE ROLE IN BRAIN TUMORS
The nuclear chromatin packing is finely coordinated by histone proteins via certain PTMs, including acetylation, methylation, phosphorylation, sumoylation, poly (ADP)-ribosylation, and ubiquitination. With these modifications, histones become highly dynamic in regulating replication, transcription, and DNA repair. For instance, histone H3 trimethylation of lysine 4 (H3K4me3) and acetylation of lysine 9 (H3K9ac) are associated with biologically active genes. Conversely, histone H3 dimethylation of lysine 9 (H3K9me2), H3K9me3, H3K27me2, and H3K27me3 is linked with inactive genes, denoting the overlapping pattern of histone methylation. In embryonic stem cells, the bivalent histone marks are speculated to maintain the genes that remain transcriptionally active. They are mediated by inducing epigenetic silencing via DNA hypermethylation in a few heritably regulatory genes, which may result in malignant transformation or tumor succession [170]. In fact, these modification patterns are regulated in a manner by the enzymes, e.g. histone acetyltransferases (HATs) and histone methyltransferases (HMTs), which typically transfer acetyl and methyl groups from the sources, such as acetyl-CoA and S-adenosyl methionine (SAM), respectively. Conversely, histone deacetylases (HDACs) and HDMs excise acetyl and methyl groups, respectively [171, 172]. The dysregulation of these enzymes in cancer becomes a potential target for epigenetic therapy. For example, altered expression of class II and IV HDACs seems to be potential targets for GBM treatment [173] (Fig. 4). Furthermore, high-throughput large-scale gene sequencing of GBM patients revealed other mutations in several protein-coding genes, which are typically involved in epigenetic regulation, e.g. HDAC 2 and 9, HDMs (JMJD 1A and 1B), and HMTs (SET 7 and SETD7, MLL and MLL4, and MBD1). However, their role in gliomagenesis is not yet established [174]. Mutations in PTMs of histones, for example, significant down-regulation of the MLL-p27 (Kip1) pathway in pituitary adenomas and up-regulation of BMI1, which is a component of polycomb repressive complex 1 (PRC1) in MB, are known to have a high rate of mortality or poor survival [175, 176].
Fig. (4).
The epigenetics of the histone protein modification. Legend: Histone protein modification caused by the hypermethylation, mutation, and reduced acetylation affecting the SWI/SFN complex is responsible for alteration in the structure of the chromatin, thereby affecting the cancer metabolism Abbreviations: (SWI/SEN: switch/sucrose non-fermentable complex, HDMs: Histone demethylase, DNMT: DNA Methyl Transferase, HMTs: Histone demethyltransferase, HAT: Histone acetyltransferase, HDAC: Histone deacetylase, TET: Ten Eleven Translocation).
Until mid of this decade, studies on histone mutations in human cancers are very limited. Later, they were studied exclusively in PBTs but not in the GBM of elderly patients [177]. In non-brainstem pediatric GBM (non-BSPG) and DIPG, the genes encoding histone variants, such as H3.3 (H3F3A) and H3.1 (HIST1H3B and 3C) [178], have experienced remarkable recurrent somatic heterozygous mutations. In which, mostly the amino acid substitutions occurred; for example, K27M (lysine to methionine at position 27) in both H3.1 and H3.3 variants and G34V/G34R (glycine to valine or arginine at position 34) in H3.3 were identified in 78% of DIPGs and 36% of non-BSPGs (Fig. 5). Interestingly, H3.3K27M mutations were largely detected in the thalamus, brainstem, cerebellum, and spinal cord, H3.3G34R/V mutations were exclusively distributed in cerebral hemispheres (cortex), and H3.1K27M mutations were confined to the pons; these are the exploring divergent transcriptional profiles and microenvironments engaged by different H3 mutations [179-182]. Despite the structural similarity between these two histone variants, they differ in five amino acids and their time of action. Since H3.1 plays a crucial role in the S-phase of cell cycle and is defined as replication-dependent, whereas, H3.3 is committed to being involved throughout the cell cycle and substitutes old H3 variants at selective loci throughout the genome [183] (Table 2).
Fig. (5).
Epigenetic landscape of histone H3 in the pediatric brain tumor. Legend: The epigenetic modification in the various genes reacting with the H3 histone protein, thus affecting the tumor progression. Different classes of histone protein H3 affecting different tumors. AML: Acute myeloid leukaemia, MSI: Microsatellite instability.
Table 2.
Epigenetic landscape of histone 3 in the pediatric brain tumor.
| Enzyme Category | Gene Name | Histone Substrates | Defect | Tumor Type | References |
|---|---|---|---|---|---|
| HAT | CBP (KAT3A) | H3K18, H3K14 | Translocation | AML | [184] |
| pCAF (KAT2B) | H3K9, H3K14, H3K18 | Mutation | Epithelial Cancer | [185] | |
| HMT | EZH2 (KMT6) | H3K27 | Amplification Mutation | Prostate Lymphoma | [186] |
| MLL1 (KMT2A) | H3K4 | Translocation | Leukemia | [187] | |
| NSD1 (KMT3B) | H3K36 | CpG hypermethylation | Neuroblastoma, glioma | [188] | |
| RIZ1 (KMT8) | H3K9 | CpG hypermethylation | Breast | [189] | |
| Histone demethylase (HDMTs) | GASC1 (KDM4C) | H3K9, H3K36 | Amplification | ESCS; lung; breast | [190, 191] |
| LSD1 ((KDM1) | H3K4, H3K9 | Amplification | Prostate; bladder; lung; CRC | [192] | |
| Histone deacetylases (HDACs) | HDAC2 | Many acetyl residues (except H4K16) | Mutation | MSI | [193] |
The predominance of selective mutations in histone variants in a large group of patients endows the importance of those amino acid residues in transcriptional regulation. Here, K27 of histone H3 has a key role in normal brain development, which frequently undergoes trimethylation (H3K27me3) using methyltransferase activity of enhancer zeste human homolog 2 (EZH2) of PRC2, which directs to inhibit the transcription of various genes (e.g. X-chromosome) involved in lineage assertion, differentiation, and devising of anterior-posterior ends [194, 195]. Although the H3K27M mutation in these PBTs competitively inhibits EZH2 methyltransferase activity, the PRC-mediated transcriptional silencing of a variety of genes is aborted [196]. Obliquely, EZH2 may be capable of regulating DNA methylation by serving as a platform to recruit DNMTs [197]. Furthermore, EZH2 overexpression has been reported in various brain tumors, including astrocytoma, oligodendroglioma, and also in GBM [198]. Another plausible similar mutation was identified, H3K27I, in which, the transversion occurs from ‘AAA’ to ‘ATA’ codon, which codes for inosine instead of lysine and exhibits slightly inhibitory activities on PRC2 and H3K27me3. However, further investigations are still required into PBTs [199].
Conversely, H3G34R/V mutation causes functional aberrations, which is not yet well understood, but it could affect the methylation pattern of H3K36 by negatively regulating the SETD2 methyltransferase domain of EZH2, which is specific to H3K36 [196]. Due to the proximity between G34 and K36 of histone H3, the H3G34R/V mutation alters the transcriptional process of certain target genes. Both these mutations of H3 (H3K27M and H3G34R/V) have different gene expression patterns themselves and when compared to the normal brain tissue. During these mutations, transcriptional activation of certain oncogenes or oncomiRs and repression of TSGs takes place, which, in turn, induces tumorigenesis. Likewise, other variants of similar mutations are also reported in rare childhood bone tumors, such as H3F3A G34W/L (glycine to tryptophan/leucine at position 34) in giant cell tumors and H3F3B K36M in chondroblastoma [200]. It has been observed that both histone H3.3 and H3.1 mutations substantially reduce the DNA methylation right through the epigenome. The K27M mutation reduces DNA methylation globally and the G34R/V mutation predominantly reduces DNA methylation in subtelomeric regions [201].
Besides these histone H3 mutations, there are several protein-coding genes, e.g. ATRX and DAXX, which are involved in chromatin remodelling. These protein-coding genes frequently undergo mutations, which results in their inactivation [202]. Furthermore, they interfere with H3.3 incorporation at the heterochromatic loci of pericentric and telomeric regions of chromosomes in a replication-independent manner, ultimately resulting in the loss of structural integrity of chromosomes. Parenthetically, during ATRX mutation, the length of the telomere sequence is successfully maintained, though there is no participation of telomerase. Hence, it is called alternative lengthening of telomeres (ALT), which facilitates genome stability and growth in tumor cells [202, 203]. Similar mutations were also noticed in pediatric and adult GBMs, neuroblastomas, and pancreatic neuroendocrine tumors.
5. HDIs IN THE TREATMENT OF BRAIN CANCER
Indeed, acetylation and deacetylation modifications of histones regulate chromatin remodelling. However, in cancer progression, abnormal deacetylation causes transcriptional inactivation of TSGs due to chromatin condensation, resulting in epigenetic silencing of TSGs. However, HDIs can able to nullify this process in different types of tumors by promoting the re-expression of various genes, which are involved in differentiation and growth arrest or apoptosis [204]. Concurrently, several HDIs, including vorinostat or SAHA (suberoylanilide hydroxamic acid), depsipeptide, and valproic acid, have entered into clinical trials. The SAHA was shown to arrest the cell cycle at the G2/M transition phase, increase pro-apoptotic and anti-proliferative genes expression (e.g. DR5, TNF-α, p21WAF1, p27KIP1), and decrease anti-apoptotic and pro-growth genes expression (e.g. CDK2, CDK4, cyclin D1, cyclin D2) in GBM cells [205]. Children’s Oncology Group conducted phase I clinical trials and demonstrated that vorinostat was safely tolerable when used alone or in combination with 13-cis retinoic acid (RA) [206]. SAHA combined with RA had an additive effect on the induction of BMP-2 transcription, p38MAPK independent apoptosis in MB, and cisplatin sensitivity because both drugs have the ability to cross the blood-brain barrier (BBB) [207]. Other preclinical studies on vorinostat have demonstrated its anti-tumor activity in MB [207-209] and HGGs [205]. However, further clinical trials on vorinostat with the combination of chemo/radiotherapy are in progress against pediatric gliomas, DIPG, and MB.
Valproic acid was initially recommended as an anti-epileptic drug and further enrolled by HDI activity. It was found to have a potential role in adjuvant radio- and chemo-therapy against HGGs and was safely tolerable [210-212]. Simultaneously, it improved histone acetylation in PMBCs of 50% of the patients at the dosage of 75-100 μg/mL. Though it has shown pulmonary embolism as an adverse effect, it thrives for better survival in anaplastic astrocytoma compared to GBM. The utility of depsipeptide in pediatric tumors remains to be unknown because it does not exert an anti-tumor response but improves histone acetylation, as studied in an in-vivo cancer model [213]. Likewise, romidepsin was also inefficient against recurrent GBM at a single standard dose and schedule [214]. Other HDIs, including entinostat, panobinostat, and phenyl-butyrate, are currently in a clinical evaluation state against HGGs or refractory PBTs and neuroblastoma. In addition, HDIs can induce sensitivity in drug-resistant cells, suggesting that drug resistance can be reversed by HDIs. At the end of 2015, a phase I clinical trial was started by PBT Consortium (PBTC047) to test panobinostat (HDI) against children with DIPG. On the other hand, drugs targeting histone methylation are in preclinical standard, which are likely to be interfering with PRC2 components. The SL11144 drug targets lysine (K)-specific demethylase 1A (KDM1A) and DZNep, which inhibits S-adenosyl homocysteine hydrolase [20]. Likewise, a phase II study is currently investigating 131-iodine (I131) conjugated mAb (Cotara) against DNA-histone H1 complexes via an advanced delivery system in recurrent GBM [215]. Moreover, it has been observed that HDI inhibitors act as a therapeutic treatment for pediatric brain cancer (Table 3).
Table 3.
Current status of selected drugs, HDI inhibitors, for pediatric brain cancer.
| S. No. | Drug | Developer | Country of Origin | Rationale of Use | Current Status | References |
|---|---|---|---|---|---|---|
| 1. | CUDC-907 | Dana-Farber Cancer Institute/Boston Children's Hospital, Boston | United States | Solid tumors, CNS tumors, and Lymphomas | Phase 1 | [216] |
| 2. | Marizomib+ Panobinostat | Boston Children's Hospital Boston, Massachusetts, United States Dana-Farber Cancer Institute Boston, Massachusetts, United States |
United States | Diffuse Intrinsic Pontine Glioma Pediatric Brainstem Glioma Acute myeloid leukemia |
Phase 1 | [217] |
| 3. | Panobinostat | Department of Neurology, Stanford University, Palo Alto, CA 94305, USA | United States | Diffuse Intrinsic Pontine Glioma | Phase 2 | [218] |
| 4. | Entinostat | Children's Hospital of Alabama, United states | United States | Brain Stem Neoplasm Pineal Region Neoplasm | Phase 1 | [219] |
| 5. | Valproic acid | Texas Children's Cancer Center, Texas Children's Hospital, Baylor College of Medicine | United States | Medulloblastoma and supratentorial primitive neuroectodermal tumor | Phase1 | [220] |
| 6. | Sodium butyrate | Brain Tumor Research Laboratory, Department of Neurosurgery, University of Illinois at Chicago | United States | Gliomas | Pre-clinical | [221] |
| 7. | Trichostatin A (TCA) | Genome Stability Laboratory, Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Republic of Singapore | Republic of Singapore | Medulloblastoma | Pre-clinical | [222] |
| 8. | Suberoyl anilide hydroxamic acid (vorinostat) | Texas Children’s Cancer Center, Baylor College of Medicine, Houston, Texas, USA; | USA | Diffuse intrinsic pontine glioma | Phase 2 | [223] |
| 9. | Belinostat | Huntsman Cancer Institute | USA | Brain tumor | Phase 1 | [224] |
| 10. | Romidepsin | Children's Cancer Centre Research Laboratory, Murdoch Children's Research Institute, Royal Children's Hospital, Parkville, Australia | Australia | Atypical teratoid rhabdoid tumor | Pre-clinical | [225] |
6. CURRENT STATUS OF SELECTED DRUGS FOR PEDIATRIC BRAIN CANCER
According to clinical research, the majority of pediatric brain tumors still lack a viable treatment. The gap in research is due to the limited understanding of the biology of brain tumors. The translation of these drugs from the preclinical stage to clinical testing has been challenging due to similar reasons [226]. The network for research on brain tumors is being unified in nascent stages. Over a long period of time, the international pediatric brain tumor community has shown remarkable collaborative activity. Currently, the community is working to identify the genomic subtypes of pediatric brain tumors and to create multidisciplinary teams to conduct preclinical studies that will better guide the design of clinical trials [227]. Such trials may offer potential possibilities to change the current research direction and incorporate the examination of new ideas (Table 4) [228]. Despite these obstacles, the community's immediate goal is to improve the way clinical trials are now conducted. International collaborative clinical trials are essential in the setting of limited, gnomically defined patient subgroups. It is, thus, necessary to conduct a thorough analysis of the present administrative, legal, and financial barriers that prevent the start of such trials [229].
Table 4.
Current status of selected drugs for pediatric brain cancer.
| S. No. | Drug | Developer | Country of Origin | Rationale of Use | Current Status | References |
|---|---|---|---|---|---|---|
| 1. | Dabrafenib | Pediatric Oncology Unit, UCL Great Ormond Street Institute of Child Health, London, United Kingdom | London, United Kingdom | In low-grade gliomas | Phase I/II trial | [230] |
| 2. | Vemurafenib | Department of Neurological Surgery, University of California, San Francisco, San Francisco, CA, USA | San Francisco, CA, USA | Metastatic melanoma | Phase I | [231] |
| 3. | Indoximod | Augusta University, Georgia Cancer Center | Georgia, United States | Glioblastoma, Medulloblastoma, Ependymoma, Diffuse Intrinsic Pontine Glioma | Phase 2 | [232] |
| 4. | MGMT(P140K)gene therapy | Children’s Cancer Research Unit, Kid’s Research Institute, The Children’s Hospital at Westmead, NSW, Australia | Australia | Pediatric brain tumor | Phase I | [233] |
| 5. | Procarbazine, lomustine and vincristine | Department of Internal Medicine, University of Kentucky College of Medicine, Lexington, Kentucky, USA | Kentucky, USA | High-grade glioma | Phase I | [234] |
| 6. | Nelarabine | Carilion Clinic, Pediatrics, Roanoke | USA | T-cell Malignancy | Phase 2 | [235] |
| 7. | Blinatumomab | Johns Hopkins University, Department of Oncology, Baltimore, USA | USA | Pediatric B-Precursor Acute Lymphoblastic Leukemia (B-ALL) | Phase 3 | [236] |
| 8. | Quinapril | St. Jude Children's Research Hospital, Pathology, Memphis, USA | USA | Acute Lymphoblastic Leukemia | Phase 1 | [237] |
| 9. | Mafosfamide | Departments of Pediatrics, Neurosurgery, Neuropathology and Radiotherapy, University of Vienna, Vienna, Austria | Austria | Embryonal tumors | Phase 1 | [238] |
| 10. | Volasertib | Institute Curie and University Paris Descartes, Pediatric, Adolescent and Young Adults Oncology Department, Paris, France | Paris, France | Acute Leukemia and Advanced Solid Tumors | Phase 1 | [239] |
CONCLUSION
These checkpoints provide distinct insights into auto immunity and robust antitumor response. The significant evidence points to immunological check point inhibitors as a promising therapeutic option for pediatric cancers based on considerable responses reported in a number of trials examining their effects on pediatric malignancies. Similarly, checkpoint inhibitors, alone or in conjunction with drugs that block the histones' epigenetic landscape, might be more effective in treating pediatric refractive solid brain tumors. Immune checkpoint inhibition is a possible treatment for pediatric cancers based on strong responses shown in a variety of pediatric malignancies and early data showing that many pediatric solid tumors express checkpoint molecules. Studies on adult checkpoint inhibitors revealed some notable, potentially fatal autoimmune side effects, with combined PD-1 and CTLA-4 blockers exhibiting greater toxicity. Even though histone deacetylase inhibitors have shown therapeutic efficacy in mitigating pediatric brain tumors, the hypothesis of using histone deacetylase inhibitors needs further exploration using preclinical and clinical studies. On a similar aspect, the use of immunotherapy and other histone deacetylase inhibitors providing therapeutic efficacy in mitigating pediatric brain tumors should be further explored in this field. In this concern, we speculate that more research is necessary for evaluating the efficacy and safety of combined targeting B7-H3 and H3K27me3 in recurrent PBTs for future prospects.
ACKNOWLEDGEMENTS
The authors would like to thank the Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, govt. of India, for providing the necessary support to carry out this study.
All the figures in this article are made in Biorender.com.
LIST OF ABBREVIATIONS
- PBTs
Pediatric Brain Tumors
- CTLA-4
Cytotoxic T-lymphocyte-associated Antigen
- PD-1
Programmed Death
- H3K27ac
Histone 3 Lysine 27 Acetylation
- BT
Brain Tumors
- MB
Medulloblastoma
- ATRT
Atypical Teratoid Rhabdoid Tumor
- DIPG
Diffuse Intrinsic Pontine Glioma
- PNET
Primitive Neuroectodermal Tumor
- MHC
Major Histocompatibility Complex
- TCR
T-cell Receptor
- MDSCs
Myeloid-derived Suppressor Cells
- DNMTs
DNA Methyltransferases
- TSG
Tumor Suppressor Gene
- MBD
Methyl-CpG Binding Domain
- PTMs
Posttranslational Modifications
- FDA
Food and Drug Administration
- HDIs
Histone Deacetylase Inhibitors
- GBM
Glioblastoma
- MGMT
Methylated O6-methylguanine DNA-methyl-transferase
- TMZ
Temozolomide
- GST
Gastrointestinal Tract
- IDO
Indoleamine 2,3-dioxygenase
- TREM
Triggering Receptor Expressed on Myeloid Cell
- TAMs
Tumor-associated Macrophages
- TNBC
Triple-negative Breast Cancer
- TIMP
Tissue Inhibitor of Metalloproteinase
- LDH
Lactate Dehydrogenase
- HIF
Hypoxia-inducible Factor
- PDK1
Pyruvate Dehydrogenase Kinase 1
- ADCC
Antibody-dependent Cell-mediated Cytotoxicity
- CAR
Chimeric-antigen Receptor
- LAG-3
Lymphocyte Activation Gene 3
- FGL-2
Fibrinogen-like Protein 2
- VISTA
V-domain Ig Suppressor of T-cell Activation
- EGFR
Epithelial Growth Factor Receptor
- HATs
Histone Acetyltransferases
- HDACs
Histone Deacetylases
- BTICs
Brain Tumor-initiating Cells
- PRC1
Polycomb Repressive Complex 1
- EZH2
Enhancer Zeste Human Homolog 2
- ALT
Alternative Lengthening of Telomeres
- SAHA
Suberoylanilide Hydroxamic Acid
- BBB
Blood-brain Barrier
AUTHORS’ CONTRIBUTIONS
Sarasa Meenakshi contributed to writing the original draft and manuscript revision. Krushna Ch Maharana formulated the figures and revised the manuscript. Lokesh Nama also participated in formulating the figures. V Udaya Kumar participated in writing the original draft. Sameer Dhingra, V Ravichandiran, and Nitesh Kumar* contributed to reviewing and editing. Krishna Murti* contributed to conceptualization and validation.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K., Lennon V.A., Celis E., Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 2.Topalian S.L., Drake C.G., Pardoll D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vignali D.A.A., Collison L.W., Workman C.J. How regulatory T cells work. Nat. Rev. Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Facciabene A., Motz G.T., Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baylin S.B., Esteller M., Rountree M.R., Bachman K.E., Schuebel K. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum. Mol. Genet. 2001;10(7):687–692. doi: 10.1093/hmg/10.7.687. [DOI] [PubMed] [Google Scholar]
- 6.Héninger E., Krueger T.E., Lang J.M. Augmenting antitumor immune responses with epigenetic modifying agents. Front. Immunol. 2015;6:49. doi: 10.3389/fimmu.2015.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feinberg A.P., Koldobskiy M.A., Göndör A.J.N.R.G. Epigenetic modulators, modifiers and mediators in cancer aetiology and progression. Nat. Rev. Genet. 2016;17(5):284–299. doi: 10.1038/nrg.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He C., Xu J., Zhang J., Xie D., Ye H., Xiao Z. High expression of trimethylated histone H3 lysine 4 is associated with poor prognosis in hepatocellular carcinoma. Hum. Pathol. 2012;43(9):1425–1435. doi: 10.1016/j.humpath.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi A., Yamauchi N., Shibahara J., Kimura H., Morikawa T., Ishikawa S., Nagae G., Nishi A., Sakamoto Y., Kokudo N., Aburatani H., Fukayama M. Concurrent activation of acetylation and tri-methylation of H3K27 in a subset of hepatocellular carcinoma with aggressive behavior. PLoS One. 2014;9(3):e91330. doi: 10.1371/journal.pone.0091330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li D. Zeng, ZJBR Epigenetic regulation of histone H3 in the process of hepatocellular tumorigenesis. Biosci. Rep. 2019;39(8):BSR20191815. doi: 10.1042/BSR20191815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger S.L., Kouzarides T., Shiekhattar R., Shilatifard A. An operational definition of epigenetics. Genes Dev. 2009;23(7):781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu G.L., Bestor T.H., Bourc’his D., Hsieh C.L., Tommerup N., Bugge M., Hulten M., Qu X., Russo J.J., Viegas-Péquignot E. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402(6758):187–191. doi: 10.1038/46052. [DOI] [PubMed] [Google Scholar]
- 13.Portela A., Esteller M. Epigenetic modifications and human disease. Nat. Biotechnol. 2010;28(10):1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 14.Baylin S.B., Ohm J.E. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer. 2006;6(2):107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki M.M., Bird A. DNA methylation landscapes: provocative insights from epigenomics. Nat. Rev. Genet. 2008;9(6):465–476. doi: 10.1038/nrg2341. [DOI] [PubMed] [Google Scholar]
- 16.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16(1):6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 17.Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum. Mol. Genet. 2007;16(R1):R50–R59. doi: 10.1093/hmg/ddm018. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Serra L., Esteller M. Proteins that bind methylated DNA and human cancer: reading the wrong words. Br. J. Cancer. 2008;98(12):1881–1885. doi: 10.1038/sj.bjc.6604374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma S.V., Lee D.Y., Li B., Quinlan M.P., Takahashi F., Maheswaran S., McDermott U., Azizian N., Zou L., Fischbach M.A., Wong K.K., Brandstetter K., Wittner B., Ramaswamy S., Classon M., Settleman J. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly T.K., De Carvalho D.D., Jones P.A. Epigenetic modifications as therapeutic targets. Nat. Biotechnol. 2010;28(10):1069–1078. doi: 10.1038/nbt.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J.B., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., Hau P., Brandes A.A., Gijtenbeek J., Marosi C., Vecht C.J., Mokhtari K., Wesseling P., Villa S., Eisenhauer E., Gorlia T., Weller M., Lacombe D., Cairncross J.G., Mirimanoff R.O. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 22.Broniscer A., Chintagumpala M., Fouladi M., Krasin M.J., Kocak M., Bowers D.C., Iacono L.C., Merchant T.E., Stewart C.F., Houghton P.J., Kun L.E., Ledet D., Gajjar A. Temozolomide after radiotherapy for newly diagnosed high-grade glioma and unfavorable low-grade glioma in children. J. Neurooncol. 2006;76(3):313–319. doi: 10.1007/s11060-005-7409-5. [DOI] [PubMed] [Google Scholar]
- 23.Cohen K.J., Pollack I.F., Zhou T., Buxton A., Holmes E.J., Burger P.C., Brat D.J., Rosenblum M.K., Hamilton R.L., Lavey R.S., Heideman R.L. Temozolomide in the treatment of high-grade gliomas in children: a report from the Children’s Oncology Group. Neuro-oncol. 2011;13(3):317–323. doi: 10.1093/neuonc/noq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lashford L.S., Thiesse P., Jouvet A., Jaspan T., Couanet D., Griffiths P.D., Doz F., Ironside J., Robson K., Hobson R., Dugan M., Pearson A.D.J., Vassal G., Frappaz D. Temozolomide in malignant gliomas of childhood: A united kingdom children’s cancer study group and french society for pediatric oncology intergroup study. J. Clin. Oncol. 2002;20(24):4684–4691. doi: 10.1200/JCO.2002.08.141. [DOI] [PubMed] [Google Scholar]
- 25.Ruggiero A., Cefalo G., Garré M.L., Massimino M., Colosimo C., Attinà G., Lazzareschi I., Maurizi P., Ridola V., Mazzarella G., Caldarelli M., Rocco C.D., Madon E., Abate M.E., Clerico A., Sandri A., Riccardi R. Phase II trial of temozolomide in children with recurrent high-grade glioma. J. Neurooncol. 2006;77(1):89–94. doi: 10.1007/s11060-005-9011-2. [DOI] [PubMed] [Google Scholar]
- 26.Korshunov A., Ryzhova M., Hovestadt V., Bender S., Sturm D., Capper D., Meyer J., Schrimpf D., Kool M., Northcott P.A., Zheludkova O., Milde T., Witt O., Kulozik A.E., Reifenberger G., Jabado N., Perry A., Lichter P., von Deimling A., Pfister S.M., Jones D.T.W. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015;129(5):669–678. doi: 10.1007/s00401-015-1405-4. [DOI] [PubMed] [Google Scholar]
- 27.Chassot A., Canale S., Varlet P., Puget S., Roujeau T., Negretti L., Dhermain F., Rialland X., Raquin M.A., Grill J., Dufour C. Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. J. Neurooncol. 2012;106(2):399–407. doi: 10.1007/s11060-011-0681-7. [DOI] [PubMed] [Google Scholar]
- 28.Cohen K.J., Heideman R.L., Zhou T., Holmes E.J., Lavey R.S., Bouffet E., Pollack I.F. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro-oncol. 2011;13(4):410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hargrave D., Bartels U., Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7(3):241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 30.Rizzo D., Scalzone M., Ruggiero A., Maurizi P., Attinà G., Mastrangelo S., Lazzareschi I., Ridola V., Colosimo C., Caldarelli M., Balducci M., Riccardi R. Temozolomide in the treatment of newly diagnosed diffuse brainstem glioma in children: a broken promise? J. Chemother. 2015;27(2):106–110. doi: 10.1179/1973947814Y.0000000228. [DOI] [PubMed] [Google Scholar]
- 31.Grasso C.S., Tang Y., Truffaux N., Berlow N.E., Liu L., Debily M.A., Quist M.J., Davis L.E., Huang E.C., Woo P.J., Ponnuswami A., Chen S., Johung T.B., Sun W., Kogiso M., Du Y., Qi L., Huang Y., Hütt-Cabezas M., Warren K.E., Le Dret L., Meltzer P.S., Mao H., Quezado M., van Vuurden D.G., Abraham J., Fouladi M., Svalina M.N., Wang N., Hawkins C., Nazarian J., Alonso M.M., Raabe E.H., Hulleman E., Spellman P.T., Li X.N., Keller C., Pal R., Grill J., Monje M. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat. Med. 2015;21(6):555–559. doi: 10.1038/nm.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashizume R., Andor N., Ihara Y., Lerner R., Gan H., Chen X., Fang D., Huang X., Tom M.W., Ngo V., Solomon D., Mueller S., Paris P.L., Zhang Z., Petritsch C., Gupta N., Waldman T.A., James C.D. Pharmacologic inhibition of histone demethylation as a therapy for pediatric brainstem glioma. Nat. Med. 2014;20(12):1394–1396. doi: 10.1038/nm.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keir M.E., Liang S.C., Guleria I., Latchman Y.E., Qipo A., Albacker L.A., Koulmanda M., Freeman G.J., Sayegh M.H., Sharpe A.H. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J. Exp. Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohigashi Y., Sho M., Yamada Y., Tsurui Y., Hamada K., Ikeda N., Mizuno T., Yoriki R., Kashizuka H., Yane K., Tsushima F., Otsuki N., Yagita H., Azuma M., Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin. Cancer Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 35.Patel S.P., Kurzrock R. PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol. Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 36.Lussier D.M., O’Neill L., Nieves L.M., McAfee M.S., Holechek S.A., Collins A.W., Dickman P., Jacobsen J., Hingorani P., Blattman J.N. Enhanced T-cell immunity to osteosarcoma through antibody blockade of PD-1/PD-L1 interactions. J. Immunother. 2015;38(3):96–106. doi: 10.1097/CJI.0000000000000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pham C.D., Flores C., Yang C., Pinheiro E.M., Yearley J.H., Sayour E.J., Pei Y., Moore C., McLendon R.E., Huang J., Sampson J.H., Wechsler-Reya R., Mitchell D.A. Differential immune microenvironments and response to immune checkpoint blockade among molecular subtypes of murine medulloblastoma. Clin. Cancer Res. 2016;22(3):582–595. doi: 10.1158/1078-0432.CCR-15-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aoki T., Hino M., Koh K., Kyushiki M., Kishimoto H., Arakawa Y., Hanada R., Kawashima H., Kurihara J., Shimojo N., Motohashi S. Low frequency of programmed death ligand 1 expression in pediatric cancers. Pediatr. Blood Cancer. 2016;63(8):1461–1464. doi: 10.1002/pbc.26018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chowdhury F., Dunn S., Mitchell S., Mellows T., Ashton-Key M., Gray J.C. PD-L1 and CD8 + PD1 + lymphocytes exist as targets in the pediatric tumor microenvironment for immunomodulatory therapy. OncoImmunology. 2015;4(10):e1029701. doi: 10.1080/2162402X.2015.1029701. [DOI] [Google Scholar]
- 40.Kim C., Kim E.K., Jung H., Chon H.J., Han J.W., Shin K.H., Hu H., Kim K.S., Choi Y.D., Kim S., Lee Y.H., Suh J.S., Ahn J.B., Chung H.C., Noh S.H., Rha S.Y., Kim S.H., Kim H.S. Prognostic implications of PD-L1 expression in patients with soft tissue sarcoma. BMC Cancer. 2016;16(1):434. doi: 10.1186/s12885-016-2451-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majzner R.G., Simon J.S., Grosso J.F., Martinez D., Pawel B., Santi-Vincini M., Merchant M.S., Sorensen P., Mackall C.L., Maris J.M. Abstract 249: Assessment of PD-L1 expression and tumor-associated lymphocytes in pediatric cancer tissues. Cancer Res., 2015, 75(15_Supplement)(Suppl.), 249. [DOI]
- 42.Routh J.C., Ashley R.A., Sebo T.J., Lohse C.M., Husmann D.A., Kramer S.A., Kwon E.D. B7-H1 expression in Wilms tumor: correlation with tumor biology and disease recurrence. J. Urol. 2008;179(5):1954–1960. doi: 10.1016/j.juro.2008.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhaijee F., Anders R.A. PD-L1 Expression as a Predictive Biomarker. JAMA Oncol. 2016;2(1):54–55. doi: 10.1001/jamaoncol.2015.3782. [DOI] [PubMed] [Google Scholar]
- 44.Boes M., Meyer-Wentrup F. TLR3 triggering regulates PD-L1 (CD274) expression in human neuroblastoma cells. Cancer Lett. 2015;361(1):49–56. doi: 10.1016/j.canlet.2015.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Usui Y., Okunuki Y., Hattori T., Takeuchi M., Kezuka T., Goto H., Usui M. Expression of costimulatory molecules on human retinoblastoma cells Y-79: functional expression of CD40 and B7H1. Invest. Ophthalmol. Vis. Sci. 2006;47(10):4607–4613. doi: 10.1167/iovs.06-0181. [DOI] [PubMed] [Google Scholar]
- 46.Mao Y., Eissler N., Blanc K.L., Johnsen J.I., Kogner P., Kiessling R. Targeting suppressive myeloid cells potentiates checkpoint inhibitors to control spontaneous neuroblastoma. Clin. Cancer Res. 2016;22(15):3849–3859. doi: 10.1158/1078-0432.CCR-15-1912. [DOI] [PubMed] [Google Scholar]
- 47.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E.E., Poddubskaya E., Antonia S., Pluzanski A., Vokes E.E., Holgado E., Waterhouse D., Ready N., Gainor J., Arén Frontera O., Havel L., Steins M., Garassino M.C., Aerts J.G., Domine M., Paz-Ares L., Reck M., Baudelet C., Harbison C.T., Lestini B., Spigel D.R. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Motzer R.J., Escudier B., McDermott D.F., George S., Hammers H.J., Srinivas S., Tykodi S.S., Sosman J.A., Procopio G., Plimack E.R., Castellano D., Choueiri T.K., Gurney H., Donskov F., Bono P., Wagstaff J., Gauler T.C., Ueda T., Tomita Y., Schutz F.A., Kollmannsberger C., Larkin J., Ravaud A., Simon J.S., Xu L.A., Waxman I.M., Sharma P. Nivolumab versus everolimus in advanced renal-cell carcinoma. N. Engl. J. Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robert C., Schachter J., Long G.V., Arance A., Grob J.J., Mortier L., Daud A., Carlino M.S., McNeil C., Lotem M., Larkin J., Lorigan P., Neyns B., Blank C.U., Hamid O., Mateus C., Shapira-Frommer R., Kosh M., Zhou H., Ibrahim N., Ebbinghaus S., Ribas A. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg J.E., Hoffman-Censits J., Powles T., van der Heijden M.S., Balar A.V., Necchi A., Dawson N., O’Donnell P.H., Balmanoukian A., Loriot Y., Srinivas S., Retz M.M., Grivas P., Joseph R.W., Galsky M.D., Fleming M.T., Petrylak D.P., Perez-Gracia J.L., Burris H.A., Castellano D., Canil C., Bellmunt J., Bajorin D., Nickles D., Bourgon R., Frampton G.M., Cui N., Mariathasan S., Abidoye O., Fine G.D., Dreicer R. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valsecchi M.E. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373(13):1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 52.Carbognin L., Pilotto S., Milella M., Vaccaro V., Brunelli M., Caliò A., Cuppone F., Sperduti I., Giannarelli D., Chilosi M., Bronte V., Scarpa A., Bria E., Tortora G. Differential activity of nivolumab, pembrolizumab and MPDL3280A according to the tumor expression of programmed death-ligand-1 (PD-L1): Sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One. 2015;10(6):e0130142. doi: 10.1371/journal.pone.0130142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouffet E., Larouche V., Campbell B.B., Merico D., de Borja R., Aronson M., Durno C., Krueger J., Cabric V., Ramaswamy V., Zhukova N., Mason G., Farah R., Afzal S., Yalon M., Rechavi G., Magimairajan V., Walsh M.F., Constantini S., Dvir R., Elhasid R., Reddy A., Osborn M., Sullivan M., Hansford J., Dodgshun A., Klauber-Demore N., Peterson L., Patel S., Lindhorst S., Atkinson J., Cohen Z., Laframboise R., Dirks P., Taylor M., Malkin D., Albrecht S., Dudley R.W.R., Jabado N., Hawkins C.E., Shlien A., Tabori U. Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J. Clin. Oncol. 2016;34(19):2206–2211. doi: 10.1200/JCO.2016.66.6552. [DOI] [PubMed] [Google Scholar]
- 54.Alegre M.L., Noel P.J., Eisfelder B.J., Chuang E., Clark M.R., Reiner S.L., Thompson C.B. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J. Immunol. 1996;157(11):4762–4770. doi: 10.4049/jimmunol.157.11.4762. [DOI] [PubMed] [Google Scholar]
- 55.Linsley P.S., Bradshaw J., Greene J., Peach R., Bennett K.L., Mittler R.S. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4(6):535–543. doi: 10.1016/S1074-7613(00)80480-X. [DOI] [PubMed] [Google Scholar]
- 56.Noel P.J., Boise L.H., Green J.M., Thompson C.B. CD28 costimulation prevents cell death during primary T cell activation. J. Immunol. 1996;157(2):636–642. doi: 10.4049/jimmunol.157.2.636. [DOI] [PubMed] [Google Scholar]
- 57.Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322(5899):271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 58.Selby M.J., Engelhardt J.J., Quigley M., Henning K.A., Chen T., Srinivasan M., Korman A.J. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 59.Peggs K.S., Quezada S.A., Chambers C.A., Korman A.J., Allison J.P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009;206(8):1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun T., Zhou Y., Yang M., Hu Z., Tan W., Han X., Shi Y., Yao J., Guo Y., Yu D., Tian T., Zhou X., Shen H., Lin D. Functional genetic variations in cytotoxic T-lymphocyte antigen 4 and susceptibility to multiple types of cancer. Cancer Res. 2008;68(17):7025–7034. doi: 10.1158/0008-5472.CAN-08-0806. [DOI] [PubMed] [Google Scholar]
- 61.Yu F., Miao J. Significant association between cytotoxic T lymphocyte antigen 4 +49G>A polymorphism and risk of malignant bone tumors. Tumour Biol. 2013;34(6):3371–3375. doi: 10.1007/s13277-013-0908-7. [DOI] [PubMed] [Google Scholar]
- 62.Hurwitz A.A., Yu T.F.Y., Leach D.R., Allison J.P. CTLA-4 blockade synergizes with tumor-derived granulocyte- macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc. Natl. Acad. Sci. USA. 1998;95(17):10067–10071. doi: 10.1073/pnas.95.17.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kwon E.D., Hurwitz A.A., Foster B.A., Madias C., Feldhaus A.L., Greenberg N.M., Burg M.B., Allison J.P. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl. Acad. Sci. USA. 1997;94(15):8099–8103. doi: 10.1073/pnas.94.15.8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 65.Contardi E., Palmisano G.L., Tazzari P.L., Martelli A.M., Falà F., Fabbi M., Kato T., Lucarelli E., Donati D., Polito L., Bolognesi A., Ricci F., Salvi S., Gargaglione V., Mantero S., Alberghini M., Ferrara G.B., Pistillo M.P. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int. J. Cancer. 2005;117(4):538–550. doi: 10.1002/ijc.21155. [DOI] [PubMed] [Google Scholar]
- 66.Merchant M.S., Wright M., Baird K., Wexler L.H., Rodriguez-Galindo C., Bernstein D., Delbrook C., Lodish M., Bishop R., Wolchok J.D., Streicher H., Mackall C.L., Phase I. Clinical Trial of Ipilimumab in Pediatric Patients with Advanced Solid Tumors. Clin. Cancer Res. 2016;22(6):1364–1370. doi: 10.1158/1078-0432.CCR-15-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eggermont A.M.M., Chiarion-Sileni V., Grob J.J., Dummer R., Wolchok J.D., Schmidt H., Hamid O., Robert C., Ascierto P.A., Richards J.M., Lebbé C., Ferraresi V., Smylie M., Weber J.S., Maio M., Konto C., Hoos A., de Pril V., Gurunath R.K., de Schaetzen G., Suciu S., Testori A. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 68.Ribas A., Kefford R., Marshall M.A., Punt C.J.A., Haanen J.B., Marmol M., Garbe C., Gogas H., Schachter J., Linette G., Lorigan P., Kendra K.L., Maio M., Trefzer U., Smylie M., McArthur G.A., Dreno B., Nathan P.D., Mackiewicz J., Kirkwood J.M., Gomez-Navarro J., Huang B., Pavlov D., Hauschild A. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 2013;31(5):616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wright G.J., Jones M., Puklavec M.J., Brown M.H., Barclay A.N. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102(2):173–179. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoek R.M., Ruuls S.R., Murphy C.A., Wright G.J., Goddard R., Zurawski S.M., Blom B., Homola M.E., Streit W.J., Brown M.H., Barclay A.N., Sedgwick J.D. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science. 2000;290(5497):1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 71.Jenmalm M.C., Cherwinski H., Bowman E.P., Phillips J.H., Sedgwick J.D. Regulation of myeloid cell function through the CD200 receptor. J. Immunol. 2006;176(1):191–199. doi: 10.4049/jimmunol.176.1.191. [DOI] [PubMed] [Google Scholar]
- 72.Gorczynski R.M., Lee L., Boudakov I. Augmented Induction of CD4+CD25+ Treg using monoclonal antibodies to CD200R. Transplantation. 2005;79(9):1180–1183. doi: 10.1097/01.TP.0000152118.51622.F9. [DOI] [PubMed] [Google Scholar]
- 73.McWhirter J.R., Kretz-Rommel A., Saven A., Maruyama T., Potter K.N., Mockridge C.I., Ravey E.P., Qin F., Bowdish K.S. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc. Natl. Acad. Sci. USA. 2006;103(4):1041–1046. doi: 10.1073/pnas.0510081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kretz-Rommel A., Qin F., Dakappagari N., Ravey E.P., McWhirter J., Oltean D., Frederickson S., Maruyama T., Wild M.A., Nolan M.J., Wu D., Springhorn J., Bowdish K.S. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J. Immunol. 2007;178(9):5595–5605. doi: 10.4049/jimmunol.178.9.5595. [DOI] [PubMed] [Google Scholar]
- 75.Coles S.J., Hills R.K., Wang E.C.Y., Burnett A.K., Man S., Darley R.L., Tonks A. Increased CD200 expression in acute myeloid leukemia is linked with an increased frequency of FoxP3+ regulatory T cells. Leukemia. 2012;26(9):2146–2148. doi: 10.1038/leu.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Damiani D., Tiribelli M., Raspadori D., Sirianni S., Meneghel A., Cavalllin M., Michelutti A., Toffoletti E., Geromin A., Simeone E., Bocchia M., Fanin R. Clinical impact of CD200 expression in patients with acute myeloid leukemia and correlation with other molecular prognostic factors. Oncotarget. 2015;6(30):30212–30221. doi: 10.18632/oncotarget.4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moertel C.L., Xia J., LaRue R., Waldron N.N., Andersen B.M., Prins R.M., Okada H., Donson A.M., Foreman N.K., Hunt M.A., Pennell C.A., Olin M.R. CD200 in CNS tumor-induced immunosuppression: the role for CD200 pathway blockade in targeted immunotherapy. J. Immunother. Cancer. 2014;2(1):46. doi: 10.1186/s40425-014-0046-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Siva A., Xin H., Qin F., Oltean D., Bowdish K.S., Kretz-Rommel A. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol. Immunother. 2008;57(7):987–996. doi: 10.1007/s00262-007-0429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Alexander W. American society of hematology, 52nd annual meeting and exposition. PT. 2011;36(2):100–103. [PMC free article] [PubMed] [Google Scholar]
- 80.Dai X., Zhu B.T. Indoleamine 2,3-dioxygenase tissue distribution and cellular localization in mice: implications for its biological functions. J. Histochem. Cytochem. 2010;58(1):17–28. doi: 10.1369/jhc.2009.953604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guillemin G.J., Smythe G., Takikawa O., Brew B.J. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49(1):15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- 82.Takikawa O. Biochemical and medical aspects of the indoleamine 2,3-dioxygenase-initiated l-tryptophan metabolism. Biochem. Biophys. Res. Commun. 2005;338(1):12–19. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 83.Frumento G., Rotondo R., Tonetti M., Damonte G., Benatti U., Ferrara G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002;196(4):459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Werner-Felmayer G., Werner E.R., Fuchs D., Hausen A., Reibnegger G., Wachter H. Characteristics of interferon induced tryptophan metabolism in human cells in vitro. Biochim. Biophys. Acta Mol. Cell Res. 1989;1012(2):140–147. doi: 10.1016/0167-4889(89)90087-6. [DOI] [PubMed] [Google Scholar]
- 85.Wolf A., Wolf D., Rumpold H., Moschen A.R., Kaser A., Obrist P., Fuchs D., Brandacher G., Winkler C., Geboes K., Rutgeerts P., Tilg H. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin. Immunol. 2004;113(1):47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 86.Munn D.H., Zhou M., Attwood J.T., Bondarev I., Conway S.J., Marshall B., Brown C., Mellor A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281(5380):1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 87.Uyttenhove C., Pilotte L., Théate I., Stroobant V., Colau D., Parmentier N., Boon T., Van den Eynde B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 2003;9(10):1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 88.Soliman H.H., Minton S.E., Han H.S., Ismail-Khan R., Neuger A., Khambati F., Noyes D., Lush R., Chiappori A.A., Roberts J.D., Link C., Vahanian N.N., Mautino M., Streicher H., Sullivan D.M., Antonia S.J. A phase I study of indoximod in patients with advanced malignancies. Oncotarget. 2016;7(16):22928–22938. doi: 10.18632/oncotarget.8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Urakawa H., Nishida Y., Nakashima H., Shimoyama Y., Nakamura S., Ishiguro N. Prognostic value of indoleamine 2,3-dioxygenase expression in high grade osteosarcoma. Clin. Exp. Metastasis. 2009;26(8):1005–1012. doi: 10.1007/s10585-009-9290-7. [DOI] [PubMed] [Google Scholar]
- 90.Chapoval A.I., Ni J., Lau J.S., Wilcox R.A., Flies D.B., Liu D., Dong H., Sica G.L., Zhu G., Tamada K., Chen L. B7-H3: A costimulatory molecule for T cell activation and IFN-γ production. Nat. Immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 91.Sun J., Fu F., Gu W., Yan R., Zhang G., Shen Z., Zhou Y., Wang H., Shen B., Zhang X. Origination of new immunological functions in the costimulatory molecule B7-H3: the role of exon duplication in evolution of the immune system. PLoS One. 2011;6(9):e24751. doi: 10.1371/journal.pone.0024751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ling V., Wu P.W., Spaulding V., Kieleczawa J., Luxenberg D., Carreno B.M., Collins M. Duplication of primate and rodent B7-H3 immunoglobulin V- and C-like domains: divergent history of functional redundancy and exon loss. Genomics. 2003;82(3):365–377. doi: 10.1016/S0888-7543(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 93.Steinberger P., Majdic O., Derdak S.V., Pfistershammer K., Kirchberger S., Klauser C., Zlabinger G., Pickl W.F., Stöckl J., Knapp W. Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J. Immunol. 2004;172(4):2352–2359. doi: 10.4049/jimmunol.172.4.2352. [DOI] [PubMed] [Google Scholar]
- 94.Sun M., Richards S., Prasad D.V.R., Mai X.M., Rudensky A., Dong C. Characterization of mouse and human B7-H3 genes. J. Immunol. 2002;168(12):6294–6297. doi: 10.4049/jimmunol.168.12.6294. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Y.H., Chen Y.J., Ma Z.Y., Xu L., Wang Q., Zhang G.B., Xie F., Ge Y., Wang X.F., Zhang X.G. 4IgB7-H3 is the major isoform expressed on immunocytes as well as malignant cells. Tissue Antigens. 2007;70(2):96–104. doi: 10.1111/j.1399-0039.2007.00853.x. [DOI] [PubMed] [Google Scholar]
- 96.Zhang G., Hou J., Shi J., Yu G., Lu B., Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123(4):538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Picarda E., Ohaegbulam K.C., Zang X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin. Cancer Res. 2016;22(14):3425–3431. doi: 10.1158/1078-0432.CCR-15-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carreno B.M., Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002;20(1):29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 99.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004;4(5):336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 100.Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu. Rev. Immunol. 2005;23(1):515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 101.Hashiguchi M., Kobori H., Ritprajak P., Kamimura Y., Kozono H., Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc. Natl. Acad. Sci. USA. 2008;105(30):10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prasad D.V.R., Nguyen T., Li Z., Yang Y., Duong J., Wang Y., Dong C. Murine B7-H3 is a negative regulator of T cells. J. Immunol. 2004;173(4):2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 103.Mahnke K., Ring S., Johnson T.S., Schallenberg S., Schönfeld K., Storn V., Bedke T., Enk A.H. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: Role of B7-H3 expression and antigen presentation. Eur. J. Immunol. 2007;37(8):2117–2126. doi: 10.1002/eji.200636841. [DOI] [PubMed] [Google Scholar]
- 104.Leitner J., Klauser C., Pickl W.F., Stöckl J., Majdic O., Bardet A.F., Kreil D.P., Dong C., Yamazaki T., Zlabinger G., Pfistershammer K., Steinberger P. B7-H3 is a potent inhibitor of human T-cell activation: No evidence for B7-H3 and TREML2 interaction. Eur. J. Immunol. 2009;39(7):1754–1764. doi: 10.1002/eji.200839028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hofmeyer K.A., Ray A., Zang X. The contrasting role of B7-H3. Proc. Natl. Acad. Sci. USA. 2008;105(30):10277–10278. doi: 10.1073/pnas.0805458105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petroff M.G., Kharatyan E., Torry D.S., Holets L. The immunomodulatory proteins B7-DC, B7-H2, and B7-H3 are differentially expressed across gestation in the human placenta. Am. J. Pathol. 2005;167(2):465–473. doi: 10.1016/S0002-9440(10)62990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang J., Chong K.K., Nakamura Y., Nguyen L., Huang S.K., Kuo C., Zhang W., Yu H., Morton D.L., Hoon D.S.B. B7-H3 associated with tumor progression and epigenetic regulatory activity in cutaneous melanoma. J. Invest. Dermatol. 2013;133(8):2050–2058. doi: 10.1038/jid.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu Y., Lv X., Wu Y., Xu J., Wang L., Chen W., Zhang W., Li J., Zhang S., Qiu H. Expression of costimulatory molecule B7-H3 and its prognostic implications in human acute leukemia. Hematology. 2015;20(4):187–195. doi: 10.1179/1607845414Y.0000000186. [DOI] [PubMed] [Google Scholar]
- 109.Sun J., Guo Y., Li X., Zhang Y., Gu L., Wu P., Bai G., Xiao Y. B7-H3 expression in breast cancer and upregulation of VEGF through gene silence. OncoTargets Ther. 2014;7:1979–1986. doi: 10.2147/OTT.S63424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zang X., Thompson R.H., Al-Ahmadie H.A., Serio A.M., Reuter V.E., Eastham J.A., Scardino P.T., Sharma P., Allison J.P. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc. Natl. Acad. Sci. USA. 2007;104(49):19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zang X., Sullivan P.S., Soslow R.A., Waitz R., Reuter V.E., Wilton A. Tumor associated endothelial expression of B7-H3 predicts survival in ovarian carcinomas. Modern pathology: an official journal of the United States and Canadian Academy of Pathology. Inc. 2010;23(8):1104–1112. doi: 10.1038/modpathol.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen Y., Zhao H., Zhu D. zhi; He, S.; Kuang, Y.; Li, D.; Zhang, Z.; Song, S.; Zhang, L.; Sun, J. The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. OncoTargets Ther. 2014;7:1465–1472. doi: 10.2147/OTT.S66809. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Ingebrigtsen V.A., Boye K., Nesland J.M., Nesbakken A., Flatmark K., Fodstad Ø. B7-H3 expression in colorectal cancer: associations with clinicopathological parameters and patient outcome. BMC Cancer. 2014;14(1):602. doi: 10.1186/1471-2407-14-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lemke D., Pfenning P.N., Sahm F., Klein A.C., Kempf T., Warnken U., Schnölzer M., Tudoran R., Weller M., Platten M., Wick W. Costimulatory protein 4IgB7H3 drives the malignant phenotype of glioblastoma by mediating immune escape and invasiveness. Clin. Cancer Res. 2012;18(1):105–117. doi: 10.1158/1078-0432.CCR-11-0880. [DOI] [PubMed] [Google Scholar]
- 115.Wang L., Zhang Q., Chen W., Shan B., Ding Y., Zhang G., Cao N., Liu L., Zhang Y. B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One. 2013;8(8):e70689. doi: 10.1371/journal.pone.0070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ingebrigtsen V.A., Boye K., Tekle C., Nesland J.M., Flatmark K., Fodstad Ø. B7-H3 expression in colorectal cancer: Nuclear localization strongly predicts poor outcome in colon cancer. Int. J. Cancer. 2012;131(11):2528–2536. doi: 10.1002/ijc.27566. [DOI] [PubMed] [Google Scholar]
- 117.Qin X., Zhang H., Ye D., Dai B., Zhu Y., Shi G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. OncoTargets Ther. 2013;6:1667–1673. doi: 10.2147/OTT.S53565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bachawal S.V., Jensen K.C., Wilson K.E., Tian L., Lutz A.M., Willmann J.K. Breast cancer detection by B7-H3-targeted ultrasound molecular imaging. Cancer Res. 2015;75(12):2501–2509. doi: 10.1158/0008-5472.CAN-14-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Arigami T., Narita N., Mizuno R., Nguyen L., Ye X., Chung A., Giuliano A.E., Hoon D.S.B. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Ann. Surg. 2010;252(6):1044–1051. doi: 10.1097/SLA.0b013e3181f1939d. [DOI] [PubMed] [Google Scholar]
- 120.Kraan J., van den Broek P., Verhoef C., Grunhagen D.J., Taal W., Gratama J.W., Sleijfer S. Endothelial CD276 (B7-H3) expression is increased in human malignancies and distinguishes between normal and tumour-derived circulating endothelial cells. Br. J. Cancer. 2014;111(1):149–156. doi: 10.1038/bjc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu C., Liu J., Wang J., Liu Y., Zhang F., Lin W., Gao A., Sun M., Wang Y., Sun Y. B7-H3 expression in ductal and lobular breast cancer and its association with IL-10. Mol. Med. Rep. 2013;7(1):134–138. doi: 10.3892/mmr.2012.1158. [DOI] [PubMed] [Google Scholar]
- 122.Loo D., Alderson R.F., Chen F.Z., Huang L., Zhang W., Gorlatov S., Burke S., Ciccarone V., Li H., Yang Y., Son T., Chen Y., Easton A.N., Li J.C., Rillema J.R., Licea M., Fieger C., Liang T.W., Mather J.P., Koenig S., Stewart S.J., Johnson S., Bonvini E., Moore P.A. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin. Cancer Res. 2012;18(14):3834–3845. doi: 10.1158/1078-0432.CCR-12-0715. [DOI] [PubMed] [Google Scholar]
- 123.Maeda N., Yoshimura K., Yamamoto S., Kuramasu A., Inoue M., Suzuki N., Watanabe Y., Maeda Y., Kamei R., Tsunedomi R., Shindo Y., Inui M., Tamada K., Yoshino S., Hazama S., Oka M. Expression of B7-H3, a potential factor of tumor immune evasion in combination with the number of regulatory T cells, affects against recurrence-free survival in breast cancer patients. Ann. Surg. Oncol. 2014;21(S4) Suppl. 4:546–554. doi: 10.1245/s10434-014-3564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen J.T., Chen C.H., Ku K.L., Hsiao M., Chiang C.P., Hsu T.L., Chen M.H., Wong C.H. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response. Proc. Natl. Acad. Sci. USA. 2015;112(42):13057–13062. doi: 10.1073/pnas.1516991112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Biglarian A., Hajizadeh E., Kazemnejad A., Zayeri F. Determining of prognostic factors in gastric cancer patients using artificial neural networks. APJCP. 2010;11(2):533–536. [PubMed] [Google Scholar]
- 126.Sun Y., Wang Y., Zhao J., Gu M., Giscombe R., Lefvert A.K., Wang X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53(2):143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 127.Chen C., Shen Y., Qu Q.X., Chen X.Q., Zhang X.G., Huang J.A. Induced expression of B7-H3 on the lung cancer cells and macrophages suppresses T-cell mediating anti-tumor immune response. Exp. Cell Res. 2013;319(1):96–102. doi: 10.1016/j.yexcr.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 128.Lupu C.M., Eisenbach C., Kuefner M.A., Schmidt J., Lupu A.D., Stremmel W., Encke J. An orthotopic colon cancer model for studying the B7-H3 antitumor effect in vivo. J. Gastrointest. Surg. 2006;10(5):635–645. doi: 10.1007/BF03239969. [DOI] [PubMed] [Google Scholar]
- 129.Zhang W., Wang J., Wang Y., Dong F., Zhu M., Wan W., Li H., Wu F., Yan X., Ke X. B7-H3 silencing by RNAi inhibits tumor progression and enhances chemosensitivity in U937 cells. OncoTargets Ther. 2015;8:1721–1733. doi: 10.2147/OTT.S85272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shapiro S.D. Matrix metalloproteinase degradation of extracellular matrix: biological consequences. Curr. Opin. Cell Biol. 1998;10(5):602–608. doi: 10.1016/S0955-0674(98)80035-5. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y.W., Tekle C., Fodstad O. The immunoregulatory protein human B7H3 is a tumor-associated antigen that regulates tumor cell migration and invasion. Curr. Cancer Drug Targets. 2008;8(5):404–413. doi: 10.2174/156800908785133141. [DOI] [PubMed] [Google Scholar]
- 132.Liu F., Zhang T., Zou S., Jiang B., Hua D. B7-H3 promotes cell migration and invasion through the Jak2/Stat3/MMP9 signaling pathway in colorectal cancer. Mol. Med. Rep. 2015;12(4):5455–5460. doi: 10.3892/mmr.2015.4050. [DOI] [PubMed] [Google Scholar]
- 133.Liu H., Tekle C., Chen Y.W., Kristian A., Zhao Y., Zhou M., Liu Z., Ding Y., Wang B., Mælandsmo G.M., Nesland J.M., Fodstad O., Tan M. B7-H3 silencing increases paclitaxel sensitivity by abrogating Jak2/Stat3 phosphorylation. Mol. Cancer Ther. 2011;10(6):960–971. doi: 10.1158/1535-7163.MCT-11-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang T., Jiang B., Zou S.T., Liu F., Hua D. Overexpression of B7-H3 augments anti-apoptosis of colorectal cancer cells by Jak2-STAT3. World J. Gastroenterol. 2015;21(6):1804–1813. doi: 10.3748/wjg.v21.i6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kang F., Wang L., Jia H., Li D., Li H., Zhang Y., Sun D. B7-H3 promotes aggression and invasion of hepatocellular carcinoma by targeting epithelial-to-mesenchymal transition via JAK2/STAT3/Slug signaling pathway. Cancer Cell Int. 2015;15(1):45. doi: 10.1186/s12935-015-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tekle C., Nygren M.K., Chen Y.W., Dybsjord I., Nesland J.M., Maelandsmo G.M., Fodstad Ø. B7-H3 contributes to the metastatic capacity of melanoma cells by modulation of known metastasis-associated genes. Int. J. Cancer. 2012;130(10):2282–2290. doi: 10.1002/ijc.26238. [DOI] [PubMed] [Google Scholar]
- 137.Xu L., Ding X., Tan H., Qian J. Correlation between B7-H3 expression and matrix metalloproteinases 2 expression in pancreatic cancer. Cancer Cell Int. 2013;13(1):81. doi: 10.1186/1475-2867-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nunes-Xavier C.E., Karlsen K.F., Tekle C., Pedersen C., Øyjord T., Hongisto V., Nesland J.M., Tan M., Sahlberg K.K., Fodstad Ø. Decreased expression of B7-H3 reduces the glycolytic capacity and sensitizes breast cancer cells to AKT/mTOR inhibitors. Oncotarget. 2016;7(6):6891–6901. doi: 10.18632/oncotarget.6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lim S., Liu H., da Silva L.M., Arora R., Liu Z., Phillips J.B., Schmitt D.C., Vu T., McClellan S., Lin Y., Lin W., Piazza G.A., Fodstad O., Tan M. Immunoregulatory protein B7-H3 reprograms glucose metabolism in cancer cells by ROS-mediated stabilization of HIF1α. Cancer Res. 2016;76(8):2231–2242. doi: 10.1158/0008-5472.CAN-15-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luther N., Zhou Z., Zanzonico P., Cheung N.K., Humm J., Edgar M.A. The potential of theragnostic (1)(2)(4)I-8H9 convection-enhanced delivery in diffuse intrinsic pontine glioma. Neuro-oncol. 2014;16(6):800–806. doi: 10.1093/neuonc/not298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Modak S., Guo H.F., Humm J.L., Smith-Jones P.M., Larson S.M., Cheung N.K.V. Radioimmunotargeting of human rhabdomyosarcoma using monoclonal antibody 8H9. Cancer Biother. Radiopharm. 2005;20(5):534–546. doi: 10.1089/cbr.2005.20.534. [DOI] [PubMed] [Google Scholar]
- 142.Ahmed M., Cheng M., Zhao Q., Goldgur Y., Cheal S.M., Guo H.F., Larson S.M., Cheung N.K.V. Humanized Affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7-H3. J. Biol. Chem. 2015;290(50):30018–30029. doi: 10.1074/jbc.M115.679852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kramer K., Kushner B.H., Modak S., Pandit-Taskar N., Smith-Jones P., Zanzonico P., Humm J.L., Xu H., Wolden S.L., Souweidane M.M., Larson S.M., Cheung N.K.V. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J. Neurooncol. 2010;97(3):409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shi Z., Hu Y., Chen H., Fu F., Zhang X. Identification and characterization of monoclonal antibody Y4F11 against human B7-H3. XiBao Yu FenZi Mian Yi X ue ZaZ hi. 2016;32(10):1402–1406. [PubMed] [Google Scholar]
- 145.Xu H., Cheung I.Y., Guo H.F., Cheung N.K.V. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69(15):6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li B., VanRoey M., Triebel F., Jooss K. Lymphocyte activation gene-3 fusion protein increases the potency of a granulocyte macrophage colony-stimulating factor-secreting tumor cell immunotherapy. Clin. Cancer Res. 2008;14(11):3545–3554. doi: 10.1158/1078-0432.CCR-07-5200. [DOI] [PubMed] [Google Scholar]
- 147.Yan J., Kong L.Y., Hu J., Gabrusiewicz K., Dibra D., Xia X., Heimberger A.B., Li S. FGL2 as a multimodality regulator of tumor-mediated immune suppression and therapeutic target in gliomas. J. Natl. Cancer Inst. 2015;107(8):djv137. doi: 10.1093/jnci/djv137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., Anderson A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang L., Rubinstein R., Lines J.L., Wasiuk A., Ahonen C., Guo Y., Lu L.F., Gondek D., Wang Y., Fava R.A., Fiser A., Almo S., Noelle R.J. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011;208(3):577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fan X., Quezada S.A., Sepulveda M.A., Sharma P., Allison J.P. Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J. Exp. Med. 2014;211(4):715–725. doi: 10.1084/jem.20130590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Curti B.D., Kovacsovics-Bankowski M., Morris N., Walker E., Chisholm L., Floyd K., Walker J., Gonzalez I., Meeuwsen T., Fox B.A., Moudgil T., Miller W., Haley D., Coffey T., Fisher B., Delanty-Miller L., Rymarchyk N., Kelly T., Crocenzi T., Bernstein E., Sanborn R., Urba W.J., Weinberg A.D. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73(24):7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Melero I., Shuford W.W., Newby S.A., Aruffo A., Ledbetter J.A., Hellström K.E., Mittler R.S., Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997;3(6):682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 153.Janjigian Y.Y., Shitara K., Moehler M., Garrido M., Salman P., Shen L., Wyrwicz L., Yamaguchi K., Skoczylas T., Campos Bragagnoli A., Liu T., Schenker M., Yanez P., Tehfe M., Kowalyszyn R., Karamouzis M.V., Bruges R., Zander T., Pazo-Cid R., Hitre E., Feeney K., Cleary J.M., Poulart V., Cullen D., Lei M., Xiao H., Kondo K., Li M., Ajani J.A. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Migden M.R., Rischin D., Schmults C.D., Guminski A., Hauschild A., Lewis K.D., Chung C.H., Hernandez-Aya L., Lim A.M., Chang A.L.S., Rabinowits G., Thai A.A., Dunn L.A., Hughes B.G.M., Khushalani N.I., Modi B., Schadendorf D., Gao B., Seebach F., Li S., Li J., Mathias M., Booth J., Mohan K., Stankevich E., Babiker H.M., Brana I., Gil-Martin M., Homsi J., Johnson M.L., Moreno V., Niu J., Owonikoko T.K., Papadopoulos K.P., Yancopoulos G.D., Lowy I., Fury M.G. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2018;379(4):341–351. doi: 10.1056/NEJMoa1805131. [DOI] [PubMed] [Google Scholar]
- 155.Cho B.C., Abreu D.R., Hussein M., Cobo M., Patel A.J., Secen N., Lee K.H., Massuti B., Hiret S., Yang J.C.H., Barlesi F., Lee D.H., Ares L.P., Hsieh R.W., Patil N.S., Twomey P., Yang X., Meng R., Johnson M.L. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022;23(6):781–792. doi: 10.1016/S1470-2045(22)00226-1. [DOI] [PubMed] [Google Scholar]
- 156.Powles T., Park S.H., Voog E., Caserta C., Valderrama B.P., Gurney H., Kalofonos H. Radulović S.; Demey, W.; Ullén, A.; Loriot, Y.; Sridhar, S.S.; Tsuchiya, N.; Kopyltsov, E.; Sternberg, C.N.; Bellmunt, J.; Aragon-Ching, J.B.; Petrylak, D.P.; Laliberte, R.; Wang, J.; Huang, B.; Davis, C.; Fowst, C.; Costa, N.; Blake-Haskins, J.A.; di Pietro, A.; Grivas, P. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020;383(13):1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 157.Rizzo A., Ricci A.D., Brandi G. Durvalumab: an investigational anti-PD-L1 antibody for the treatment of biliary tract cancer. Expert Opin. Investig. Drugs. 2021;30(4):343–350. doi: 10.1080/13543784.2021.1897102. [DOI] [PubMed] [Google Scholar]
- 158.Wolchok J.D., Kluger H., Callahan M.K., Postow M.A., Rizvi N.A., Lesokhin A.M., Segal N.H., Ariyan C.E., Gordon R.A., Reed K., Burke M.M., Caldwell A., Kronenberg S.A., Agunwamba B.U., Zhang X., Lowy I., Inzunza H.D., Feely W., Horak C.E., Hong Q., Korman A.J., Wigginton J.M., Gupta A., Sznol M. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kelley R.K., Sangro B., Harris W., Ikeda M., Okusaka T., Kang Y.K., Qin S., Tai D.W.M., Lim H.Y., Yau T., Yong W.P., Cheng A.L., Gasbarrini A., Damian S., Bruix J., Borad M., Bendell J., Kim T.Y., Standifer N., He P., Makowsky M., Negro A., Kudo M., Abou-Alfa G.K. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J. Clin. Oncol. 2021;39(27):2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sordo-Bahamonde C., Lorenzo-Herrero S., González-Rodríguez A.P., Payer Á.R., González-García E., López-Soto A., Gonzalez S. LAG-3 blockade with relatlimab (BMS-986016) restores anti-leukemic responses in chronic lymphocytic leukemia. Cancers (Basel) 2021;13(9):2112. doi: 10.3390/cancers13092112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chocarro L., Bocanegra A., Blanco E., Fernández-Rubio L., Arasanz H., Echaide M., Garnica M., Ramos P., Piñeiro-Hermida S., Vera R., Escors D., Kochan G. Cutting-Edge: preclinical and clinical development of the first approved Lag-3 inhibitor. Cells. 2022;11(15):2351. doi: 10.3390/cells11152351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kumar S., Jaipuri F.A., Waldo J.P., Potturi H., Marcinowicz A., Adams J., Van Allen C., Zhuang H., Vahanian N., Link C., Jr, Brincks E.L., Mautino M.R. Discovery of indoximod prodrugs and characterization of clinical candidate NLG802. Eur. J. Med. Chem. 2020;198:112373. doi: 10.1016/j.ejmech.2020.112373. [DOI] [PubMed] [Google Scholar]
- 163.Long G.V., Dummer R., Hamid O., Gajewski T.F., Caglevic C., Dalle S., Arance A., Carlino M.S., Grob J.J., Kim T.M., Demidov L., Robert C., Larkin J., Anderson J.R., Maleski J., Jones M., Diede S.J., Mitchell T.C. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20(8):1083–1097. doi: 10.1016/S1470-2045(19)30274-8. [DOI] [PubMed] [Google Scholar]
- 164.Nayak-Kapoor A., Hao Z., Sadek R., Dobbins R., Marshall L., Vahanian N.N., Jay Ramsey W., Kennedy E., Mautino M.R., Link C.J., Lin R.S., Royer-Joo S., Liang X., Salphati L., Morrissey K.M., Mahrus S., McCall B., Pirzkall A., Munn D.H., Janik J.E., Khleif S.N. Phase Ia study of the indoleamine 2,3-dioxygenase 1 (IDO1) inhibitor navoximod (GDC-0919) in patients with recurrent advanced solid tumors. J. Immunother. Cancer. 2018;6(1):61. doi: 10.1186/s40425-018-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ferris R.L., Jaffee E.M., Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J. Clin. Oncol. 2010;28(28):4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Okada H., Low K.L., Kohanbash G., McDonald H.A., Hamilton R.L., Pollack I.F. Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J. Neurooncol. 2008;88(3):245–250. doi: 10.1007/s11060-008-9566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yang M., Xie W., Mostaghel E., Nakabayashi M., Werner L., Sun T., Pomerantz M., Freedman M., Ross R., Regan M., Sharifi N., Figg W.D., Balk S., Brown M., Taplin M.E., Oh W.K., Lee G.S.M., Kantoff P.W. SLCO2B1 and SLCO1B3 may determine time to progression for patients receiving androgen deprivation therapy for prostate cancer. J. Clin. Oncol. 2011;29(18):2565–2573. doi: 10.1200/JCO.2010.31.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu Z., Zhong S., Shen Z. Targeting the inflammatory pathways to enhance chemotherapy of cancer. Cancer Biol. Ther. 2011;12(2):95–105. doi: 10.4161/cbt.12.2.15952. [DOI] [PubMed] [Google Scholar]
- 169.Curtin J., King G., Candolfi M., Greeno R., Kroeger K., Lowenstein P., Castro M. Combining cytotoxic and immune-mediated gene therapy to treat brain tumors. Curr. Top. Med. Chem. 2005;5(12):1151–1170. doi: 10.2174/156802605774370856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ohm J.E., McGarvey K.M., Yu X., Cheng L., Schuebel K.E., Cope L., Mohammad H.P., Chen W., Daniel V.C., Yu W., Berman D.M., Jenuwein T., Pruitt K., Sharkis S.J., Watkins D.N., Herman J.G., Baylin S.B. A stem cell-like chromatin pattern may predispose tumor suppressor genes to DNA hypermethylation and heritable silencing. Nat. Genet. 2007;39(2):237–242. doi: 10.1038/ng1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Haberland M., Montgomery R.L., Olson E.N. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 2009;10(1):32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat. Rev. Genet. 2007;8(11):829–833. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- 173.Lucio-Eterovic A.K.B., Cortez M.A.A., Valera E.T., Motta F.J.N., Queiroz R.G.P., Machado H.R., Carlotti C.G., Jr, Neder L., Scrideli C.A., Tone L.G. Differential expression of 12 histone deacetylase (HDAC) genes in astrocytomas and normal brain tissue: class II and IV are hypoexpressed in glioblastomas. BMC Cancer. 2008;8(1):243. doi: 10.1186/1471-2407-8-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Parsons D.W., Jones S., Zhang X., Lin J.C.H., Leary R.J., Angenendt P., Mankoo P., Carter H., Siu I.M., Gallia G.L., Olivi A., McLendon R., Rasheed B.A., Keir S., Nikolskaya T., Nikolsky Y., Busam D.A., Tekleab H., Diaz L.A., Jr, Hartigan J., Smith D.R., Strausberg R.L., Marie S.K.N., Shinjo S.M.O., Yan H., Riggins G.J., Bigner D.D., Karchin R., Papadopoulos N., Parmigiani G., Vogelstein B., Velculescu V.E., Kinzler K.W. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Leung C., Lingbeek M., Shakhova O., Liu J., Tanger E., Saremaslani P., van Lohuizen M., Marino S. Bmi1 is essential for cerebellar development and is overexpressed in human medulloblastomas. Nature. 2004;428(6980):337–341. doi: 10.1038/nature02385. [DOI] [PubMed] [Google Scholar]
- 176.Milde T., Oehme I., Korshunov A., Kopp-Schneider A., Remke M., Northcott P., Deubzer H.E., Lodrini M., Taylor M.D., von Deimling A., Pfister S., Witt O. HDAC5 and HDAC9 in medulloblastoma: novel markers for risk stratification and role in tumor cell growth. Clin. Cancer Res. 2010;16(12):3240–3252. doi: 10.1158/1078-0432.CCR-10-0395. [DOI] [PubMed] [Google Scholar]
- 177.Sturm D., Bender S., Jones D.T.W., Lichter P., Grill J., Becher O., Hawkins C., Majewski J., Jones C., Costello J.F., Iavarone A., Aldape K., Brennan C.W., Jabado N., Pfister S.M. Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge. Nat. Rev. Cancer. 2014;14(2):92–107. doi: 10.1038/nrc3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wu G., Broniscer A., McEachron T.A., Lu C., Paugh B.S., Becksfort J., Qu C., Ding L., Huether R., Parker M., Zhang J., Gajjar A., Dyer M.A., Mullighan C.G., Gilbertson R.J., Mardis E.R., Wilson R.K., Downing J.R., Ellison D.W., Zhang J., Baker S.J. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 2012;44(3):251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Buczkowicz P., Hoeman C., Rakopoulos P., Pajovic S., Letourneau L., Dzamba M., Morrison A., Lewis P., Bouffet E., Bartels U., Zuccaro J., Agnihotri S., Ryall S., Barszczyk M., Chornenkyy Y., Bourgey M., Bourque G., Montpetit A., Cordero F., Castelo-Branco P., Mangerel J., Tabori U., Ho K.C., Huang A., Taylor K.R., Mackay A., Bendel A.E., Nazarian J., Fangusaro J.R., Karajannis M.A., Zagzag D., Foreman N.K., Donson A., Hegert J.V., Smith A., Chan J., Lafay-Cousin L., Dunn S., Hukin J., Dunham C., Scheinemann K., Michaud J., Zelcer S., Ramsay D., Cain J., Brennan C., Souweidane M.M., Jones C., Allis C.D., Brudno M., Becher O., Hawkins C. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat. Genet. 2014;46(5):451–456. doi: 10.1038/ng.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fontebasso A.M., Papillon-Cavanagh S., Schwartzentruber J., Nikbakht H., Gerges N., Fiset P.O., Bechet D., Faury D., De Jay N., Ramkissoon L.A., Corcoran A., Jones D.T.W., Sturm D., Johann P., Tomita T., Goldman S., Nagib M., Bendel A., Goumnerova L., Bowers D.C., Leonard J.R., Rubin J.B., Alden T., Browd S., Geyer J.R., Leary S., Jallo G., Cohen K., Gupta N., Prados M.D., Carret A.S., Ellezam B., Crevier L., Klekner A., Bognar L., Hauser P., Garami M., Myseros J., Dong Z., Siegel P.M., Malkin H., Ligon A.H., Albrecht S., Pfister S.M., Ligon K.L., Majewski J., Jabado N., Kieran M.W. Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat. Genet. 2014;46(5):462–466. doi: 10.1038/ng.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Taylor K.R., Mackay A., Truffaux N., Butterfield Y.S., Morozova O., Philippe C., Castel D., Grasso C.S., Vinci M., Carvalho D., Carcaboso A.M., de Torres C., Cruz O., Mora J., Entz-Werle N., Ingram W.J., Monje M., Hargrave D., Bullock A.N., Puget S., Yip S., Jones C., Grill J. Recurrent activating ACVR1 mutations in diffuse intrinsic pontine glioma. Nat. Genet. 2014;46(5):457–461. doi: 10.1038/ng.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wu G., Diaz A.K., Paugh B.S., Rankin S.L., Ju B., Li Y., Zhu X., Qu C., Chen X., Zhang J., Easton J., Edmonson M., Ma X., Lu C., Nagahawatte P., Hedlund E., Rusch M., Pounds S., Lin T., Onar-Thomas A., Huether R., Kriwacki R., Parker M., Gupta P., Becksfort J., Wei L., Mulder H.L., Boggs K., Vadodaria B., Yergeau D., Russell J.C., Ochoa K., Fulton R.S., Fulton L.L., Jones C., Boop F.A., Broniscer A., Wetmore C., Gajjar A., Ding L., Mardis E.R., Wilson R.K., Taylor M.R., Downing J.R., Ellison D.W., Zhang J., Baker S.J. The genomic landscape of diffuse intrinsic pontine glioma and pediatric non-brainstem high-grade glioma. Nat. Genet. 2014;46(5):444–450. doi: 10.1038/ng.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Henikoff S., Smith M.M. Histone variants and epigenetics. Cold Spring Harb. Perspect. Biol. 2015;7(1):a019364. doi: 10.1101/cshperspect.a019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Panagopoulos I., Fioretos T., Isaksson M., Samuelsson U., Billström R., Strömbeck B., Mitelman F., Johansson B. Fusion of the MORF and CBP genes in acute myeloid leukemia with the t(10;16)(q22;p13). Hum. Mol. Genet. 2001;10(4):395–404. doi: 10.1093/hmg/10.4.395. [DOI] [PubMed] [Google Scholar]
- 185.Zhu C., Qin Y-R., Xie D., Chua D.T.T., Fung J.M., Chen L., Fu L., Hu L., Guan X-Y. Characterization of tumor suppressive function of P300/CBP-associated factor at frequently deleted region 3p24 in esophageal squamous cell carcinoma. Oncogene. 2009;28(31):2821–2828. doi: 10.1038/onc.2009.137. [DOI] [PubMed] [Google Scholar]
- 186.Bracken A.P., Pasini D., Capra M., Prosperini E., Colli E., Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22(20):5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Miremadi A., Oestergaard M.Z., Pharoah P.D.P., Caldas C. Cancer genetics of epigenetic genes. Hum. Mol. Genet. 2007;16(R1):R28–R49. doi: 10.1093/hmg/ddm021. [DOI] [PubMed] [Google Scholar]
- 188.Jaju R.J., Fidler C., Haas O.A., Strickson A.J., Watkins F., Clark K., Cross N.C., Cheng J.F., Aplan P.D., Kearney L., Boultwood J., Wainscoat J.S. A novel gene, NSD1, is fused to NUP98 in the t(5;11)(q35;p15.5) in de novo childhood acute myeloid leukemia. Blood. 2001;98(4):1264–1267. doi: 10.1182/blood.V98.4.1264. [DOI] [PubMed] [Google Scholar]
- 189.Du Y., Carling T., Fang W., Piao Z., Sheu J.C., Huang S. Hypermethylation in human cancers of the RIZ1 tumor suppressor gene, a member of a histone/protein methyltransferase superfamily. Cancer Res. 2001;61(22):8094–8099. [PubMed] [Google Scholar]
- 190.Italiano A., Attias R., Aurias A., Pérot G., Burel-Vandenbos F., Otto J., Venissac N., Pedeutour F. Molecular cytogenetic characterization of a metastatic lung sarcomatoid carcinoma: 9p23 neocentromere and 9p23-p24 amplification including JAK2 and JMJD2C. Cancer Genet. Cytogenet. 2006;167(2):122–130. doi: 10.1016/j.cancergencyto.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 191.Liu G., Bollig-Fischer A., Kreike B., van de Vijver M.J., Abrams J., Ethier S.P., Yang Z.Q. Genomic amplification and oncogenic properties of the GASC1 histone demethylase gene in breast cancer. Oncogene. 2009;28(50):4491–4500. doi: 10.1038/onc.2009.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kahl P., Gullotti L., Heukamp L.C., Wolf S., Friedrichs N., Vorreuther R., Solleder G., Bastian P.J., Ellinger J., Metzger E., Schüle R., Buettner R. Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence. Cancer Res. 2006;66(23):11341–11347. doi: 10.1158/0008-5472.CAN-06-1570. [DOI] [PubMed] [Google Scholar]
- 193.Ropero S., Fraga M.F., Ballestar E., Hamelin R., Yamamoto H., Boix-Chornet M., Caballero R., Alaminos M., Setien F., Paz M.F., Herranz M., Palacios J., Arango D., Orntoft T.F., Aaltonen L.A., Schwartz S., Jr, Esteller M. A truncating mutation of HDAC2 in human cancers confers resistance to histone deacetylase inhibition. Nat. Genet. 2006;38(5):566–569. doi: 10.1038/ng1773. [DOI] [PubMed] [Google Scholar]
- 194.Faria C.M.C., Rutka J.T., Smith C., Kongkham P. Epigenetic mechanisms regulating neural development and pediatric brain tumor formation. J. Neurosurg. Pediatr. 2011;8(2):119–132. doi: 10.3171/2011.5.PEDS1140. [DOI] [PubMed] [Google Scholar]
- 195.Grossniklaus U., Paro R. Transcriptional silencing by polycomb-group proteins. Cold Spring Harb. Perspect. Biol. 2014;6(11):a019331. doi: 10.1101/cshperspect.a019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lewis P.W., Müller M.M., Koletsky M.S., Cordero F., Lin S., Banaszynski L.A., Garcia B.A., Muir T.W., Becher O.J., Allis C.D. Inhibition of PRC2 activity by a gain-of-function H3 mutation found in pediatric glioblastoma. Science. 2013;340(6134):857–861. doi: 10.1126/science.1232245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Viré E., Brenner C., Deplus R., Blanchon L., Fraga M., Didelot C., Morey L., Van Eynde A., Bernard D., Vanderwinden J.M., Bollen M., Esteller M., Di Croce L., de Launoit Y., Fuks F. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439(7078):871–874. doi: 10.1038/nature04431. [DOI] [PubMed] [Google Scholar]
- 198.Zheng S., Houseman E.A., Morrison Z., Wrensch M.R., Patoka J.S., Ramos C., Haas-Kogan D.A., McBride S., Marsit C.J., Christensen B.C., Nelson H.H., Stokoe D., Wiemels J.L., Chang S.M., Prados M.D., Tihan T., Vandenberg S.R., Kelsey K.T., Berger M.S., Wiencke J.K. DNA hypermethylation profiles associated with glioma subtypes and EZH2 and IGFBP2 mRNA expression. Neuro-oncol. 2011;13(3):280–289. doi: 10.1093/neuonc/noq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lewis P.W., Allis C.D. Poisoning the “histone code” in pediatric gliomagenesis. Cell Cycle. 2013;12(20):3241–3242. doi: 10.4161/cc.26356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Behjati S., Tarpey P.S., Presneau N., Scheipl S., Pillay N., Van Loo P., Wedge D.C., Cooke S.L., Gundem G., Davies H., Nik-Zainal S., Martin S., McLaren S., Goody V., Robinson B., Butler A., Teague J.W., Halai D., Khatri B., Myklebost O., Baumhoer D., Jundt G., Hamoudi R., Tirabosco R., Amary M.F., Futreal P.A., Stratton M.R., Campbell P.J., Flanagan A.M. Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat. Genet. 2013;45(12):1479–1482. doi: 10.1038/ng.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sturm D., Witt H., Hovestadt V., Khuong-Quang D.A., Jones D.T.W., Konermann C., Pfaff E., Tönjes M., Sill M., Bender S., Kool M., Zapatka M., Becker N., Zucknick M., Hielscher T., Liu X.Y., Fontebasso A.M., Ryzhova M., Albrecht S., Jacob K., Wolter M., Ebinger M., Schuhmann M.U., van Meter T., Frühwald M.C., Hauch H., Pekrun A., Radlwimmer B., Niehues T., von Komorowski G., Dürken M., Kulozik A.E., Madden J., Donson A., Foreman N.K., Drissi R., Fouladi M., Scheurlen W., von Deimling A., Monoranu C., Roggendorf W., Herold-Mende C., Unterberg A., Kramm C.M., Felsberg J., Hartmann C., Wiestler B., Wick W., Milde T., Witt O., Lindroth A.M., Schwartzentruber J., Faury D., Fleming A., Zakrzewska M., Liberski P.P., Zakrzewski K., Hauser P., Garami M., Klekner A., Bognar L., Morrissy S., Cavalli F., Taylor M.D., van Sluis P., Koster J., Versteeg R., Volckmann R., Mikkelsen T., Aldape K., Reifenberger G., Collins V.P., Majewski J., Korshunov A., Lichter P., Plass C., Jabado N., Pfister S.M. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. doi: 10.1016/j.ccr.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 202.Schwartzentruber J., Korshunov A., Liu X.Y., Jones D.T.W., Pfaff E., Jacob K., Sturm D., Fontebasso A.M., Quang D.A.K., Tönjes M., Hovestadt V., Albrecht S., Kool M., Nantel A., Konermann C., Lindroth A., Jäger N., Rausch T., Ryzhova M., Korbel J.O., Hielscher T., Hauser P., Garami M., Klekner A., Bognar L., Ebinger M., Schuhmann M.U., Scheurlen W., Pekrun A., Frühwald M.C., Roggendorf W., Kramm C., Dürken M., Atkinson J., Lepage P., Montpetit A., Zakrzewska M., Zakrzewski K., Liberski P.P., Dong Z., Siegel P., Kulozik A.E., Zapatka M., Guha A., Malkin D., Felsberg J., Reifenberger G., von Deimling A., Ichimura K., Collins V.P., Witt H., Milde T., Witt O., Zhang C., Castelo-Branco P., Lichter P., Faury D., Tabori U., Plass C., Majewski J., Pfister S.M., Jabado N. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482(7384):226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]
- 203.Lovejoy C.A., Li W., Reisenweber S., Thongthip S., Bruno J., de Lange T., De S., Petrini J.H.J., Sung P.A., Jasin M., Rosenbluh J., Zwang Y., Weir B.A., Hatton C., Ivanova E., Macconaill L., Hanna M., Hahn W.C., Lue N.F., Reddel R.R., Jiao Y., Kinzler K., Vogelstein B., Papadopoulos N., Meeker A.K. Loss of ATRX, genome instability, and an altered DNA damage response are hallmarks of the alternative lengthening of telomeres pathway. PLoS Genet. 2012;8(7):e1002772. doi: 10.1371/journal.pgen.1002772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Marks P.A., Xu W.S. Histone deacetylase inhibitors: Potential in cancer therapy. J. Cell. Biochem. 2009;107(4):600–608. doi: 10.1002/jcb.22185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yin D., Ong J.M., Hu J., Desmond J.C., Kawamata N., Konda B.M., Black K.L., Koeffler H.P. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin. Cancer Res. 2007;13(3):1045–1052. doi: 10.1158/1078-0432.CCR-06-1261. [DOI] [PubMed] [Google Scholar]
- 206.Fouladi M., Park J.R., Stewart C.F., Gilbertson R.J., Schaiquevich P., Sun J., Reid J.M., Ames M.M., Speights R., Ingle A.M., Zwiebel J., Blaney S.M., Adamson P.C. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children’s Oncology Group phase I consortium report. J. Clin. Oncol. 2010;28(22):3623–3629. doi: 10.1200/JCO.2009.25.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Spiller S.E., Ditzler S.H., Pullar B.J., Olson J.M. Response of preclinical medulloblastoma models to combination therapy with 13-cis retinoic acid and suberoylanilide hydroxamic acid (SAHA). J. Neurooncol. 2008;87(2):133–141. doi: 10.1007/s11060-007-9505-1. [DOI] [PubMed] [Google Scholar]
- 208.Furchert S.E., Lanvers-Kaminsky C., Juürgens H., Jung M., Loidl A., Frühwald M.C. Inhibitors of histone deacetylases as potential therapeutic tools for high-risk embryonal tumors of the nervous system of childhood. Int. J. Cancer. 2007;120(8):1787–1794. doi: 10.1002/ijc.22401. [DOI] [PubMed] [Google Scholar]
- 209.Spiller S.E., Ravanpay A.C., Hahn A.W., Olson J.M. Suberoylanilide hydroxamic acid is effective in preclinical studies of medulloblastoma. J. Neurooncol. 2006;79(3):259–270. doi: 10.1007/s11060-006-9142-0. [DOI] [PubMed] [Google Scholar]
- 210.Masoudi A., Elopre M., Amini E., Nagel M.E., Ater J.L., Gopalakrishnan V., Wolff J.E. Influence of valproic acid on outcome of high-grade gliomas in children. Anticancer Res. 2008;28(4C):2437–2442. [PubMed] [Google Scholar]
- 211.Su J.M., Li X.N., Thompson P., Ou C.N., Ingle A.M., Russell H., Lau C.C., Adamson P.C., Blaney S.M. Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumors: a children’s oncology group report. Clin. Cancer Res. 2011;17(3):589–597. doi: 10.1158/1078-0432.CCR-10-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wolff J.E.A., Kramm C., Kortmann R.D., Pietsch T., Rutkowski S., Jorch N., Gnekow A., Driever P.H. Valproic acid was well tolerated in heavily pretreated pediatric patients with high-grade glioma. J. Neurooncol. 2008;90(3):309–314. doi: 10.1007/s11060-008-9662-x. [DOI] [PubMed] [Google Scholar]
- 213.Graham C., Tucker C., Creech J., Favours E., Billups C.A., Liu T., Fouladi M., Freeman B.B., III, Stewart C.F., Houghton P.J. Evaluation of the antitumor efficacy, pharmacokinetics, and pharmacodynamics of the histone deacetylase inhibitor depsipeptide in childhood cancer models in vivo. Clin. Cancer Res. 2006;12(1):223–234. doi: 10.1158/1078-0432.CCR-05-1225. [DOI] [PubMed] [Google Scholar]
- 214.Iwamoto F.M., Lamborn K.R., Robins H.I., Mehta M.P., Chang S.M., Butowski N.A., DeAngelis L.M., Abrey L.E., Zhang W.T., Prados M.D., Fine H.A. Phase II trial of pazopanib (GW786034), an oral multi-targeted angiogenesis inhibitor, for adults with recurrent glioblastoma (North American Brain Tumor Consortium Study 06-02). Neuro-oncol. 2010;12(8):855–861. doi: 10.1093/neuonc/noq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Hdeib A., Sloan A.E. Convection-enhanced delivery of 131 I-chTNT-1/B mAB for treatment of high-grade adult gliomas. Expert Opin. Biol. Ther. 2011;11(6):799–806. doi: 10.1517/14712598.2011.579097. [DOI] [PubMed] [Google Scholar]
- 216.Chilamakuri R., Agarwal S.J.C. Dual Targeting of PI3K and HDAC by CUDC-907 inhibits pediatric neuroblastoma growth. Cancers (Basel) 2022;14(4):1067. doi: 10.3390/cancers14041067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Corrales-Medina F.F., Manton C.A., Orlowski R.Z., Chandra J.J. Efficacy of panobinostat and marizomib in acute myeloid leukemia and bortezomib-resistant models. Leuk. Res. 2015;39(3):371–379. doi: 10.1016/j.leukres.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Nagaraja S., Vitanza N.A., Woo P.J., Taylor K.R., Liu F., Zhang L. Transcriptional dependencies in diffuse intrinsic pontine glioma. Cancer Cell. 2017;31(5):635–652. doi: 10.1016/j.ccell.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Knipstein J., Gore L. Entinostat for treatment of solid tumors and hematologic malignancies. Expert Opin. Investig. Drugs. 2011;20(10):1455–1467. doi: 10.1517/13543784.2011.613822. [DOI] [PubMed] [Google Scholar]
- 220.Li X-N., Shu Q., Su J.M-F., Perlaky L., Blaney S.M., Lau C.C.J. Valproic acid induces growth arrest, apoptosis, and senescence in medulloblastomas by increasing histone hyperacetylation and regulating expression of p21Cip1, CDK4, and CMYC. Mol. Cancer Ther. 2005;4(12):1912–1922. doi: 10.1158/1535-7163.MCT-05-0184. [DOI] [PubMed] [Google Scholar]
- 221.Engelhard H.H., Duncan H.A., Kim S., Criswell P.S., Van Eldik L.J.N. Therapeutic effects of sodium butyrate on glioma cells in vitro and in the rat C6 glioma model. Neurosurgery. 2001;48(3):616–624. doi: 10.1097/00006123-200103000-00035. [DOI] [PubMed] [Google Scholar]
- 222.Rahman R., Osteso-Ibanez T., Hirst R.A., Levesley J., Kilday J-P., Quinn S. Histone deacetylase inhibition attenuates cell growth with associated telomerase inhibition in high-grade childhood brain tumor cells. Mol. Cancer Ther. 2010;9(9):2568–2581. doi: 10.1158/1535-7163.MCT-10-0272. [DOI] [PubMed] [Google Scholar]
- 223.Su J.M., Kilburn L.B., Mansur D.B., Krailo M., Buxton A., Adekunle A., Gajjar A., Adamson P.C., Weigel B., Fox E., Blaney S.M., Fouladi M. Phase I/II trial of vorinostat and radiation and maintenance vorinostat in children with diffuse intrinsic pontine glioma: A Children’s Oncology Group report. Neuro-oncol. 2022;24(4):655–664. doi: 10.1093/neuonc/noab188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Agarwal N., McPherson J.P., Bailey H., Gupta S., Werner T.L., Reddy G., Bhat G., Bailey E.B., Sharma S. A phase I clinical trial of the effect of belinostat on the pharmacokinetics and pharmacodynamics of warfarin. Cancer Chemother. Pharmacol. 2016;77(2):299–308. doi: 10.1007/s00280-015-2934-1. [DOI] [PubMed] [Google Scholar]
- 225.Algar E.M., Muscat A., Dagar V., Rickert C., Chow C.W., Biegel J.A., Ekert P.G., Saffery R., Craig J., Johnstone R.W., Ashley D.M. Imprinted CDKN1C is a tumor suppressor in rhabdoid tumor and activated by restoration of SMARCB1 and histone deacetylase inhibitors. PLoS One. 2009;4(2):e4482. doi: 10.1371/journal.pone.0004482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nimmervoll B.V., Boulos N., Bianski B., Dapper J., DeCuypere M., Shelat A. Establishing a preclinical multidisciplinary board for brain tumors. Clin. Cancer Res. 2018;24(7):1654–1666. doi: 10.1158/1078-0432.CCR-17-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Northcott P.A., Jones D.T., Kool M., Robinson G.W., Gilbertson R.J., Cho Y-J. Medulloblastomics: The end of the beginning. Nat. Rev. Cancer. 2012;12(12):818–834. doi: 10.1038/nrc3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Alexander B.M., Ba S., Berger M.S., Berry D.A., Cavenee W.K., Chang S.M., Cloughesy T.F., Jiang T., Khasraw M., Li W., Mittman R., Poste G.H., Wen P.Y., Yung W.K.A., Barker A.D. Adaptive global innovative learning environment for glioblastoma: GBM AGILE. Clin. Cancer Res. 2018;24(4):737–743. doi: 10.1158/1078-0432.CCR-17-0764. [DOI] [PubMed] [Google Scholar]
- 229.Chow S-C. Adaptive clinical trial design. Annu. Rev. Med. 2017;65:405–415. doi: 10.1146/annurev-med-092012-112310. [DOI] [PubMed] [Google Scholar]
- 230.Hargrave D.R., Bouffet E., Tabori U., Broniscer A., Cohen K.J., Hansford J.R., Geoerger B., Hingorani P., Dunkel I.J., Russo M.W., Tseng L., Dasgupta K., Gasal E., Whitlock J.A., Kieran M.W. Efficacy and safety of dabrafenib in pediatric patients with BRAF V600 mutation-positive relapsed or refractory low-grade Glioma: Results from a phase I/IIa study. Clin. Cancer Res. 2019;25(24):7303–7311. doi: 10.1158/1078-0432.CCR-19-2177. [DOI] [PubMed] [Google Scholar]
- 231.Nicolaides T., Nazemi K., Crawford J., Kilburn L., Minturn J., Gajjar A., Gauvain K., Leary S., Dhall G., Aboian M., Robinson G., Molinaro A., Mueller S., Prados M. Pdct-19. A safety study of vemurafenib, an oral inhibitor of braf(V600e), in children with recurrent/refractory braf(V600e) mutant brain tumors: Pnoc-002. Neuro-oncol. 2017;19(Suppl. 6):vi188. doi: 10.1093/neuonc/nox168.761. [DOI] [Google Scholar]
- 232.Dobbins R., MacDonald T.J., Munn D.H., Johnson T.S. Pediatric trial of indoximod with chemotherapy and radiation for relapsed brain tumors or newly diagnosed DIPG. p. NCT04049669.
- 233.Kramer B., Singh R., Wischusen J., Dent R., Rush A., Middlemiss S., Ching Y.W., Alexander I.E., McCowage G. Clinical Trial of MGMT (P140K). Hum. Gene Ther. 2018;29(8):874–885. doi: 10.1089/hum.2017.235. [DOI] [PubMed] [Google Scholar]
- 234.Parasramka S., Talari G., Rosenfeld M., Guo J. Procarbazine, lomustine and vincristine for recurrent high-grade glioma. Cochrane Database Syst. Rev. 2017;7(7):CD011773. doi: 10.1002/14651858.CD011773.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Dunsmore K.P., Winter S., Devidas M., Wood B.L., Esiashvili N., Eisenberg N. COG AALL0434: A randomized trial testing nelarabine in newly diagnosed t-cell malignancy. J. Clin. Oncol. 2018;36(15):10500. doi: 10.1200/JCO.20.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Stein A., Franklin J.L., Chia V.M., Arrindell D., Kormany W., Wright J. Benefit-risk assessment of blinatumomab in the treatment of relapsed/refractory B-cell precursor acute lymphoblastic leukemia. Drug Saf. 2019;42(5):587–601. doi: 10.1007/s40264-018-0760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Amin N.L. Osteonecrosis and bone health in children, teenagers and young people with leukaemia. PhD thesis, University of Leeds. 2019. [Google Scholar]
- 238.Blaney S.M., Boyett J., Friedman H., Gajjar A., Geyer R., Horowtiz M., Hunt D., Kieran M., Kun L., Packer R., Phillips P., Pollack I.F., Prados M., Heideman R. Phase I clinical trial of mafosfamide in infants and children aged 3 years or younger with newly diagnosed embryonal tumors: a pediatric brain tumor consortium study (PBTC-001). J. Clin. Oncol. 2005;23(3):525–531. doi: 10.1200/JCO.2005.06.544. [DOI] [PubMed] [Google Scholar]
- 239.Doz F., Locatelli F., Baruchel A., Blin N., De Moerloose B., Frappaz D. Phase I dose-escalation study of volasertib in pediatric patients with acute leukemia or advanced solid tumors. Pediatr. Blood Cancer. 2019;66(10):e27900. doi: 10.1002/pbc.27900. [DOI] [PubMed] [Google Scholar]