Abstract
Background
In the initial treatment of venous thromboembolism (VTE) low molecular weight heparin (LMWH) is administered once or twice daily. A once daily treatment regimen is more convenient for the patient and may optimise home treatment. However, it is not clear whether a once daily treatment regimen is as safe and effective as a twice daily treatment regimen. This is the second update of a review first published in 2003.
Objectives
To compare the efficacy and safety of once daily versus twice daily administration of LMWH.
Search methods
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (last searched May 2013) and CENTRAL (2013, Issue 4).
Selection criteria
Randomised clinical trials in which LMWH given once daily is compared with LMWH given twice daily for the initial treatment of VTE.
Data collection and analysis
Two review authors assessed trials for inclusion and extracted data independently.
Main results
Five studies were included with a total of 1508 participants. The pooled data showed no statistically significant difference in recurrent VTE between the two treatment regimens (OR 0.82, 0.49 to 1.39; P = 0.47). A comparison of major haemorrhagic events (OR 0.77, 0.40 to 1.45; P = 0.41), improvement of thrombus size (OR 1.41, 0.66 to 3.01; P = 0.38) and mortality (OR 1.14, 0.62 to 2.08; P = 0.68) also showed no statistically significant differences between the two treatment regimens. None of the five included studies reported data on post‐thrombotic syndrome.
Authors' conclusions
Once daily treatment with LMWH is as effective and safe as twice daily treatment with LMWH.
Keywords: Humans; Anticoagulants; Anticoagulants/administration & dosage; Anticoagulants/adverse effects; Drug Administration Schedule; Hemorrhage; Hemorrhage/chemically induced; Heparin, Low‐Molecular‐Weight; Heparin, Low‐Molecular‐Weight/administration & dosage; Heparin, Low‐Molecular‐Weight/adverse effects; Pulmonary Embolism; Pulmonary Embolism/drug therapy; Randomized Controlled Trials as Topic; Recurrence; Thromboembolism; Thromboembolism/drug therapy; Venous Thrombosis; Venous Thrombosis/drug therapy
Plain language summary
Once versus twice daily injections of low molecular weight heparin for the initial treatment of blood clots in the veins
Blood clots in the veins (venous thromboembolism (VTE)) can develop spontaneously or after surgery or bed rest. Venous thromboembolism can be life threatening if clots travel to the lungs. Blood‐thinning drugs such as heparin are used to dissolve clots. Low molecular weight heparin (LMWH) can be given by injection, enabling people to leave hospital. The usual treatment is two injections a day, but once a day would be more convenient. This review included five studies with a combined total of 1508 participants. The combined data showed no statistically significant difference in recurrent VTE between the once daily and twice daily treatment regimens. A comparison of major bleeding events, improvement of the blood clot size and death also showed no statistically significant difference between the two treatment regimens. None of the five included studies reported information on post‐thrombotic syndrome (ongoing swelling of the affected leg, pain, and skin changes). One daily injection with LMWH is therefore as effective and safe as twice daily injections.
Background
Description of the condition
Venous thromboembolism (VTE) is a common disease with an annual incidence of between two and three cases per 1000 inhabitants (Anderson 1991; Nordstrom 1992). Risk factors for VTE can be acquired through trauma, surgery or periods of immobilisation (Heit 2000) or can be inherited, e.g. Factor V Leiden mutation or protein C deficiency (Bertina 1994; Heijboer 1990). The disease requires immediate anticoagulant therapy, as left untreated, VTE has a high morbidity and can be fatal.
Description of the intervention
Intravenous administration of unfractionated heparin (UFH) for approximately one week has been the standard initial treatment for VTE for decades (Hirsh 1991). A group of anticoagulants derived from the unfractionated form of heparin has become available, namely low molecular weight heparins (LMWHs). Low molecular weight heparins have pharmacokinetic advantages over UFH, including a longer half‐life (the compounds remain active within the body for longer), and a more predictable anticoagulant response (the dose does not have to be adjusted continually to maintain the desired level of coagulability) (Hirsh 1992). Hence, a fixed, body‐weight‐adjusted dose of LMWH can be administered subcutaneously without the need for laboratory monitoring. This facilitates the initial treatment and leads to a shorter hospitalisation period for people with VTE, as treatment can take place partially or entirely at home (Erkens 2010; Koopman 1996).
How the intervention might work
In the trials that established the efficacy of LMWH for the initial treatment of VTE, LMWH was usually given twice a day (Bratt 1990; Harenberg 1997; Prandoni 1992) but there are also trials in which LMWH was administered once a day (Fiessinger 1996; Lindmarker 1994). In the past few years, head to head comparisons of once versus twice daily LMWH regimens have been performed (Charbonnier 1998; Partsch 1996).
Why it is important to do this review
A single daily injection of LMWH is more convenient for people and may optimise home treatment. In addition, the appeal on economic resources is lower in a once daily administration regimen. However, it is conceivable that twice daily LMWH results in a more stable level of anticoagulation and thus in fewer complications.
This review evaluates the relative efficacy and safety of LMWH administered once daily versus twice daily.
Objectives
To assess the relative efficacy (in terms of recurrent venous thromboembolism) and safety (i.e. major haemorrhagic events) of once daily low molecular weight heparin (LMWH) versus twice daily LMWH administration in the initial treatment of people with venous thromboembolism.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised trials with an intention‐to‐treat analysis were included. Quasi‐randomised trials were not included. Studies were excluded if they were duplicate reports; or preliminary reports of data later presented in full; and if they were dose‐finding studies, in which the efficacy and safety can be under‐ or overestimated; or if the difference in initial treatment was confounded by differences in concomitant medication or long‐term medication.
Types of participants
People with VTE, i.e. deep vein thrombosis (DVT) or pulmonary embolism (PE) or both, confirmed by objective tests were included. The following criteria were accepted for the diagnosis of VTE:
the suspected DVT was confirmed by either venography or compression ultrasound if venography was not feasible;
the suspected PE was confirmed by pulmonary angiography or a high probability ventilation/perfusion lung scan;
an associated DVT was proven by either venography or compression ultrasound.
Types of interventions
Once versus twice daily administration of a fixed dose of subcutaneous LMWH as the initial treatment for VTE. Brands, doses and duration of treatment medication were registered but were not criteria for excluding trials.
Types of outcome measures
Primary outcomes
The primary outcome measures were:
symptomatic recurrent VTE, i.e. DVT or PE, or both during the initial treatment and during follow up;
major haemorrhagic episodes during initial treatment or within 48 hours after treatment cessation.
A diagnosis of recurrent deep venous thrombosis was accepted if one of the following criteria was met:
a new constant intraluminal filling defect was found which was not present on the last available venogram, or extension of the thrombus on ultrasound,
if the venogram was not diagnostic: either an abnormal 125I‐fibrinogen leg scan or abnormal impedance plethysmogram or ultrasound result that had been normal before the suspected recurrent episode (Büller 1991).
A diagnosis of pulmonary embolism was accepted if one of the following criteria was met:
a segmental defect was found on the perfusion lung scan unmatched on the previous ventilation scan or chest roentgenogram;
a positive pulmonary angiography or spiral computed tomography (CT);
pulmonary embolism at autopsy.
Haemorrhagic events were considered to be major if they were intracranial, retroperitoneal, led directly to death, necessitated transfusion, warranted interruption of antithrombotic treatment or required operation. All other bleeding events were classified as minor.
Secondary outcomes
The main secondary outcome was extension of the thrombus size. In addition, where data on overall mortality and incidence of the post‐thrombotic syndrome were presented, these data were evaluated as well.
Search methods for identification of studies
There were no language restrictions.
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched May 2013) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 4, part of The Cochrane Library, (www.thecochranelibrary.com). See (Appendix 1) for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the (Specialised Register) section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
Searching other resources
The reference lists of articles retrieved by electronic searches were searched for additional citations.
Data collection and analysis
Selection of trials
Two review authors (for the original review MM, CVD and for the update SB, PW) independently evaluated the eligibility and methodological quality of the trials. Disagreements were resolved through discussion and consensus.
Quality of trials
Studies were evaluated to extract information on study details including route of administration, intensity of heparin therapy, and intensity of oral anticoagulant therapy. The adequacy of concealment of allocation prior to randomisation and blinding of the outcome measurement was assessed, based indirectly on the criteria of Jadad (Jadad 1996). Trials without adequate concealment of allocation and/or without blinded outcome measurement were excluded. For future updates of the review, where the information in the original article is not clear, we will contact the authors for clarification. To date this has not been necessary.
Data extraction
For the original review, two review authors (MM, CVD) extracted data independently. The following information was collected: patient characteristics (age, gender, co‐morbidity); incidence of recurrent VTE; incidence of haemorrhagic events; incidence of thrombus size improvement; and, additionally, mortality and the incidence of a post‐thrombotic syndrome. Disagreements were resolved according to the same procedure used for the selection of trials. No authors were contacted for additional information.
Statistical analysis
The following comparisons were made between once and twice daily LMWH:
incidence of symptomatic recurrent DVT and PE during the initial treatment and during follow up;
number of people in each group with improved venographic score;
frequency of major haemorrhagic episodes during initial treatment;
overall mortality at the end of follow up;
incidence of people suffering from a post‐thrombotic syndrome at the end of follow up.
An odds ratio (OR) for all outcome measurements within each study was calculated (an OR of less than one favours once daily). Subsequently, a chi‐square test for statistical heterogeneity was done for each of the comparisons to assess whether differences in treatment effect over individual trials were consistent with natural variation around a constant effect (Collins 1987). Finally, the ORs were combined across studies giving weight to the number of events in each of the two treatment groups in each separate study using the Mantel‐Haenszel procedure (Mantel 1959).
When unfractionated heparin (UFH) is used against placebo, the risk of recurrent VTE is reduced from 20 to 6.7 percent (relative risk reduction (RRR) 67%) (Brandjes 1992). The use of LMWH (mostly twice daily) at least maintains this benefit (upper limit of 95% CI of the OR of LMWH versus UFH = 1.01) (van den Belt 1999). Consequently, taking into account the changes of the comparator drug, LMWH twice daily, (OR below one means that once daily LMWH is better), the upper limit of the 95% CI of the primary analysis should not exceed one by more than 0.5, to show that at least 75% of the effect of LMWH twice daily is maintained.
Results
Description of studies
Results of the search
For this update 18 additional citations relating to 11 studies were retrieved from the search of the Specialised Register. No additional studies were found from the CENTRAL search which were not in the Specialised Register. Five citations were considered to be not relevant for this review from reading the titles and abstracts and a further six studies (12 citations) (Bellosta 2007; Buller 2004; Cosmi 2012; Leizorovicz 2011; Narin 2008; Schellong 2010) were excluded. One citation (Holmström 1990) was an additional publication of the included study Holmström 1992.
Included studies
The five included studies (Charbonnier 1998; Holmström 1992; Merli 2001; Partsch 1996; Siegbahn 1989) incorporated a total of 1508 participants.
One of the five included studies included people with PE and DVT (Merli 2001). The other four studies included only people with DVT. The five included studies used four brands of low molecular weight heparin (LMWH) (enoxaparin, tinzaparin, dalteparin and nadroparin). The manufacturer recommends twice daily administration for nadroparin and enoxaparin. Once daily administration is recommended for tinzaparin and dalteparin. In all the included studies the same generic compounds were used in the head‐to‐head comparison of a once and a twice daily regimen. No authors were contacted for additional information.
Excluded studies
See (Characteristics of excluded studies).
For this update there were an additional six studies (12 citations) (Bellosta 2007; Buller 2004; Cosmi 2012; Leizorovicz 2011; Narin 2008; Schellong 2010) excluded making a total of 41 excluded studies.
Twenty‐six trials did not compare once against twice daily administration of LMWH (Alhenc‐Gelas 1994; Andersen 1997; Bara 1992; Beckman 2003; Belcaro 1999; Bratt 1988; Buller 2004; Fiessinger 1996; Harenberg 1990; Harenberg 1997; Holmström 1997; Hull 1997; Hull 2000a; Hull 2000b; Leroyer 1998; Lindmarker 1994; Luomanmaki 1996; Meyer 1995; Offord 2004; Pini 1994; Sandset 1990; Schellong 2010; Simonneau 1993; Simonneau 1997; Stricker 1999; Wartski 2000). Nine did not feature VTE as the initial event (Agnelli 1995; Boneu 1998; Bratt 1990; Cosmi 2012; Erikson 2002; Erikson 2003; Mismetti 1995; Petilla 2002; Turpie 2002). One study involved the use of unfractionated heparin for the first five days (Leizorovicz 2011). In three studies (Breddin 2001; Breddin 2003; Kakkar 2002), there were differences in concomitant medication whereby vitamin K antagonists were administered to participants in only one treatment group or treatment with vitamin K antagonists was started a few weeks later in one treatment group compared with the other group. One other study was excluded because it used different LMWH, with once daily versus twice daily for one month only and following that, the twice daily treatment arm reverted to once daily prophylactic dose. This makes comparing once daily versus twice daily regimens untenable as there were no results for effectiveness of the regimens at one month (Bellosta 2007). One trial was excluded as it was a retrospective study (Narin 2008).
Risk of bias in included studies
All five included studies were randomised clinical trials. Two studies had a double‐blind design (Charbonnier 1998; Merli 2001). Two other studies were single blind (Holmström 1992; Siegbahn 1989). One study did not mention blinding (Partsch 1996). There were no indications from any of the studies that data were not analysed on an intention‐to‐treat basis. Participants were lost to follow up in only two studies. In one study, one person (0.3%) was lost to follow up in the twice daily group (Charbonnier 1998). In the other study (Merli 2001), seven participants (2.3%) from the group treated once daily were lost to follow up, and seven participants (2.2%) from the group treated twice daily were lost to follow up. There were no disagreements between the two review authors regarding the issue of internal validity.
Effects of interventions
Incidence of recurrent venous thromboembolism (VTE)
Three of the five included studies reported on the recurrence of symptomatic VTE (Charbonnier 1998; Merli 2001; Siegbahn 1989). In the smallest of these (Siegbahn 1989), no recurrent events were reported in either treatment group, hence an OR could not be calculated. In another study (Charbonnier 1998), a statistically non‐significant lower incidence of recurrent VTE was shown in participants receiving LMWH once daily compared with those who received LMWH twice daily. While in the third study a lower incidence of VTE could be observed, which was also not statistically significant, in participants treated with LMWH twice daily (Merli 2001). When the results of these two studies (Charbonnier 1998; Merli 2001) were combined, 26 (4.2%) of the 624 participants treated with LMWH once daily and 33 (5.0%) of the 657 participants treated with LMWH twice daily had a recurrent thromboembolic event. Analysis of the pooled data showed no statistically significant difference in the incidence of recurrent thromboembolic events between LMWH once daily compared with LMWH twice daily (OR 0.82, 0.49 to 1.39; Analysis 1.1). Thus, the a priori determined criterion for equivalence was satisfied. The test for statistical heterogeneity was negative (P = 0.07), although borderline. Visual inspection does not give the impression of heterogeneity.
1.1. Analysis.
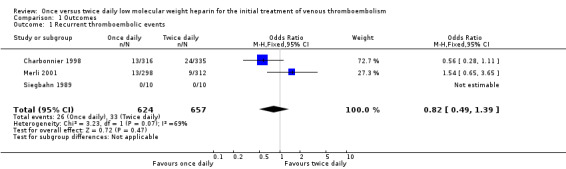
Comparison 1 Outcomes, Outcome 1 Recurrent thromboembolic events.
Incidence of haemorrhagic events
All the included studies reported on the occurrence of major haemorrhagic events. In one study none of the participants had a haemorrhagic event so an OR could not be calculated (Siegbahn 1989). Two studies showed a statistically non‐significant lower incidence of haemorrhagic events in people treated with LMWH once daily (Charbonnier 1998; Holmström 1992). The other two studies showed a non‐significant lower risk of major haemorrhage in people treated with LMWH twice daily compared with people treated once daily (Merli 2001; Partsch 1996). When data were combined it could be seen that 16 (2.2%) out of a total 742 participants in the once daily treatment groups suffered a haemorrhagic event compared with 22 (2.9%) events in the 766 participants in the twice daily treatment groups. Pooled analysis of the study results showed a non‐significant lower incidence in haemorrhagic events in people treated with LMWH once daily compared with those who had a regimen of twice daily administration (OR 0.77, 0.40 to 1.45; Analysis 1.2). The statistical test for heterogeneity was negative (P = 0.63).
1.2. Analysis.
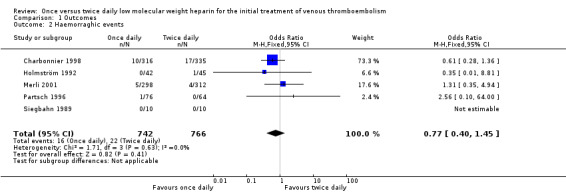
Comparison 1 Outcomes, Outcome 2 Haemorraghic events.
Extension of thrombus size
Data on change in thrombus size could be extracted from two studies (Holmström 1992; Siegbahn 1989). In the larger of these (Holmström 1992), interpretable repeat phlebography was available for only 87 of 101 participants. The number of people in whom an improvement of the thrombus size was found was not statistically significant. The thrombus size improved in 23 (54.8%) of the 42 participants treated with LMWH once daily and 23 (51.1%) of the 45 treated twice daily (OR 1.16, 0.50 to 2.69). The other study (Siegbahn 1989) reported that in the once daily group the thrombus size improved in six out of 10 participants; in the twice daily group the thrombus size improved in three out of 10 participants (OR 3.50, 0.55 to 22.30). Therefore, a combined OR could be calculated which showed no statistically significant difference between the treatment groups (OR 1.41, 0.66 to 3.01; Analysis 1.3). The test for heterogeneity was negative (P = 0.29).
1.3. Analysis.
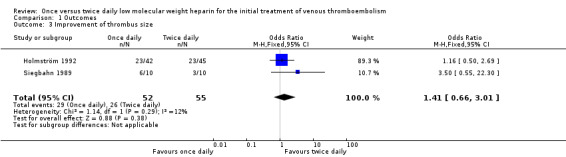
Comparison 1 Outcomes, Outcome 3 Improvement of thrombus size.
Mortality
Four studies reported data on overall mortality (Charbonnier 1998; Merli 2001; Partsch 1996; Siegbahn 1989). In the smallest study (Siegbahn 1989), the mortality in both treatment groups was zero. In another study (Charbonnier 1998), there were fewer deaths amongst the people treated with LMWH once daily, however, this difference was not statistically significant. In the two other studies (Merli 2001; Partsch 1996), a statistically non‐significant lower number of deaths was observed in people who received LMWH twice daily compared with people who received LMWH once daily. Combining these results showed that 23 (3.3%) out of a total of 700 in the once daily groups and 21 (2.9%) out of a total of 721 in the twice daily groups died. A pooled analysis of the data showed that there was no statistically significant difference in mortality between people who are treated with LMWH twice daily compared with people treated with LMWH once daily (OR 1.14, 0.62 to 2.08; Analysis 1.4). The test for statistical heterogeneity on mortality was negative (P = 0.34).
1.4. Analysis.
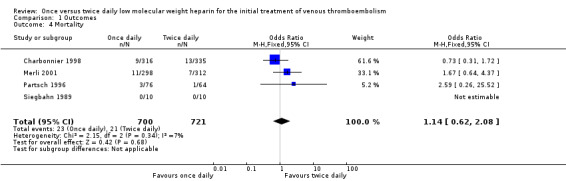
Comparison 1 Outcomes, Outcome 4 Mortality.
Post‐thrombotic syndrome
None of the five included studies reported data on post‐thrombotic syndrome.
Discussion
In this systematic review we assessed the relative efficacy and safety of once daily administration of low molecular weight heparin (LMWH) compared with a twice daily treatment regimen. Five studies comprising a total of 1508 participants were included. Procedures of randomisation and blinded outcome assessment assured reliable estimates of the pooled ORs (Schulz 1995). We found no statistically significant difference in efficacy with respect to recurrent thromboembolic events between the once daily and twice daily treatment regimens. The predefined criterion for equivalence was met since the confidence interval of the pooled OR for recurrence of VTE did not exceed 1.5. In fact, the upper limit of the 95% confidence interval was 1.39, which indicates that at least approximately 80% of the efficacy of the twice daily regimen was maintained by the once daily regimen. The observed clinical equivalence with regard to efficacy was accompanied by similar rates of bleeding complications with both the once and twice daily regimens. Also, mortality rates were low and similar in both groups. No data were available for the incidence of the development of post‐thrombotic syndrome.
With regard to our two main outcome events, recurrent VTE and major haemorrhage, the following should be stated: although the pooled OR (OR 0.82, 0.49 to 1.39) for recurrent thromboembolic events was based on only two studies, it is likely that it is a reliable estimate since the methodological quality of these two largest studies (including 1261 of the total of 1508 participants) was high. Moreover, the two studies that evaluated the change in thrombus size confirm the absence of an important difference in efficacy (Holmström 1992; Siegbahn 1989). In Holmström's study, a relatively large number of the repeat venographs (14 out of 101) were not available. However, the numbers between the two groups (six in the twice daily and eight in the once daily) were comparable. Therefore, it is unlikely that the lack of available venographs in the analysis has biased the results. Data on the other main outcome, risk for major haemorrhagic events, could be derived from all studies. The observed OR indicates at least equal safety for the once daily regimen.
In meta‐analyses of studies comparing UFH with LMWH in relation to recurrent VTE and bleeding outcomes, it appeared that LMWH is at least as effective and safe as UFH (Dolovich 2000; Erkens 2010; Gould 1999). In addition, in all three studies it was concluded that LMWH shows a statistically significant decrease in overall mortality compared with UFH. Frequency of administration was beyond the main scope of these studies. In only one of these meta‐analyses a comparison was made between once daily and twice daily administration of LMWH and it was concluded that once daily administration of LMWH is as effective and safe as a twice daily treatment regimen (Dolovich 2000). This comparison was made across studies, rather than based on direct randomised comparisons. Therefore, the conclusion drawn by the authors can be potentially biased by group differences. However, the results are in agreement with our findings.
This systematic review demonstrates equivalence in efficacy and safety, in the short term, between once and twice daily administration of LMWH for VTE. It should be noted that there are no data available on the effect of dosing frequency on long‐term recurrent thromboembolic events and the development of the post‐thrombotic syndrome. Further research will be required to answer these clinically relevant questions definitively. However, an important difference in these outcomes seems implausible based on the short duration of the initial treatment regimens and their fully comparable efficacy at that stage.
It is questionable whether the results obtained from the small number of PE patients included in this systematic review can be extrapolated to all people with PE. However, if we consider DVT and PE as different manifestations of the same disease, VTE, we can conclude from the evidence presented in this systematic review, that a once daily treatment regimen is not significantly different ‐ with respect to efficacy and safety ‐ to a twice daily regimen in people treated for an first episode of DVT. Therefore, we have no reason to suppose that the recurrence risk in people with PE is increased. However, further research should be done to give more insight in the impact of different LMWH regimens in people with PE.
In the studies of Charbonnier (Charbonnier 1998) and Merli (Merli 2001), different LMWH compounds were used (nadroparin and enoxaparin, respectively). A meta‐analysis (van der Heijden 2000) concluded that safety and efficacy of LMWH is comparable for different compounds of LMWH used in the initial treatment of VTE. Therefore, we believe that different LMWH compounds do not differ with respect to safety and efficacy in relation to a once or twice daily regimen. The best available evidence is presented in this systematic review but further research should be performed to elucidate whether the safety and efficacy of different LMWH compounds are comparable in a once or twice daily regimen.
Authors' conclusions
Implications for practice.
This review has shown that once daily administration of LMWH for the initial treatment of venous thromboembolism is as safe and effective as a twice daily regimen. Either regimen is therefore acceptable in clinical practice.
Implications for research.
Further research should be performed to investigate whether the safety and efficacy of different LMWH compounds are comparable in a once or twice daily regimen. These studies should also focus on the impact of different LMWH regimens in people with PE. A large randomised trial of at least two years' duration should be performed to determine the effects of dosing frequency on long‐term sequelae of venous thromboembolism, such as the development of the post‐thrombotic syndrome.
Feedback
Anticoagulant feedback, 14 February 2011
Summary
Feedback received on this review, and other reviews and protocols on anticoagulants, is available on the Cochrane Editorial Unit website at http://www.editorial‐unit.cochrane.org/anticoagulants‐feedback.
Feedback, 28 August 2014
Summary
My colleagues and I read with interest your Cochrane Review, "Once versus twice daily low molecular weight heparin for the initial treatment of venous thromboembolism." We would like to address a few points from this review.
First, we would like to acknowledge that an important outcome presented in the review is mortality. We noted that the Holmoström 19921 trial did not report on mortality, and was therefore not included in the mortality analysis. Because this outcome takes precedence over all others in determining if one treatment is better than another, we believe that it would be important to attain as much data on it as possible. This can be achieved by contacting the authors of the original article. Furthermore, as the odds ratio confidence interval was so wide for this outcome [(OR 1.14, 0.62 to 2.08)], it is not possible to determine whether a clinically significant difference in mortality is seen when comparing treatments. Therefore, the statement in the review that "there was a statistically non‐significant difference in mortality in favour of people who are treated with LMWH twice daily compared with people treated with LMWH once daily" can be reworded to "there was a statistically non‐significant difference in mortality, but a clinically significant increase or decrease cannot be ruled out." This would allow readers to put the available data into clinical context. In our opinion, the wide confidence interval for mortality limits the ability to conclude with certainty that "once daily treatment with LMWH is as effective and safe as twice daily treatment with LMWH."
We would also like to address the protocol of the review regarding the types of studies that were to be included. The protocol was to include only those that were randomized with an intention‐to‐treat analysis, and that had adequate allocation concealment and blinded outcome measurement. We do not believe that trials should be excluded from a systematic review due to any form of bias. Rather, biases should be outlined in the bias reports, and sensitivity analyses should be done as necessary. Regardless, based on the table of excluded studies, all reasons for exclusion appear to be reasonable. However, it should be clarified in the review that biases did not serve as exclusion criteria, which would aid in strengthening the validity of the review.
We performed a risk of bias assessment for all the included trials, and noted that missing data was present in the Merli 20012 trial, and that Holmström 19921 was not an intention‐to‐treat trial. Missing data is important to address, as analyses using the missing data may yield different results from those presented in the review. For example, in the Merli 20012 trial, 34 patients in the once daily group and 37 in the twice daily group were lost to follow‐up, and no sensitivity analyses were done. Sensitivity analyses assuming hemorrhagic event worst case scenarios for patients lost to follow‐up either in the once or twice daily group would yield statistically significant different results: the odds ratio would increase to 2.39 (1.45, 3.93) if assuming all lost to follow‐up patients in the once daily group had hemorrhagic events, and decrease to 0.28 (0.16, 0.48) if the same is done for the twice daily group.
Thank you for conducting this review; we believe that this is an important issue, as it affects convenience, patient comfort, and cost. We hope that you consider our suggestions, and we appreciate your time.
Sincerely
Hilary Wu (BSc.Pharm), Mark Ho (BSc.Pharm), Karen Hong (BSc.Pharm), and Yin Gong (BSc.Pharm)
References 1. Holmström M, Berglund MC, Granquist S, Bratt G, Törnebohm E, Lockner D. Fragmin once or twice daily subcutaneously in the treatment of deep venous thrombosis of the leg. Thromb Res. 1992 Jul 1;67(1):49–55. 2. Merli G, Spiro TE, Olsson CG, Abildgaard U, Davidson BL, Eldor A, et al. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Ann Intern Med. 2001 Feb 6;134(3):191–202.
Reply
A reply from the review authors is awaited.
Contributors
Feedback: Hilary Wu, Clinical Pharmacist, Providence Health Care, Canada I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Feedback, 24 April 2015
Summary
I think we found a mistake in the table of haemorraghic events. In the Charbonnier´s study 10 major events are reported in the once daily regimen and 17 events in the twice daily regimen. Looking at the original source, 4 events are reported in each regimen as it´s also stated in the Couturaud meta‐analysis (Thromb Haemost 2001; 86: 980‐4). Could you tell us if that is just a mistake or if you have other information not published.
Reply
A reply from the review authors is awaited.
Contributors
Feedback: Andres Aizman, Faculty, Pontificia Universidad Catolica de Chile I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
What's new
| Date | Event | Description |
|---|---|---|
| 24 April 2015 | Feedback has been incorporated | Feedback was submitted for this review |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 28 August 2014 | Feedback has been incorporated | Feedback was submitted for this review |
| 23 May 2013 | New search has been performed | Searches rerun. Review was updated with six additional excluded studies. |
| 23 May 2013 | New citation required but conclusions have not changed | New authors have taken over this review. Review was updated with six additional excluded studies. Abstract and plain language summary expanded; minor copy edits made. No changes to the conclusions. |
| 14 February 2011 | Amended | Link to anticoagulant feedback added |
| 27 October 2008 | Amended | Converted to new review format. |
| 15 May 2005 | New citation required but conclusions have not changed | Review updated. No changes to the conclusions. |
| 15 May 2005 | New search has been performed | Searches re‐run. Review was updated by the addition of eight new excluded studies. |
Acknowledgements
The review authors would like to thank Carlo van Dongen (CVD), Melvin R MacGillavry (MM) and Martin H Prins (MP) for their work on previous versions of this review.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Thrombosis] this term only | 1176 |
| #2 | MeSH descriptor: [Thromboembolism] this term only | 992 |
| #3 | MeSH descriptor: [Venous Thromboembolism] this term only | 275 |
| #4 | MeSH descriptor: [Venous Thrombosis] explode all trees | 2164 |
| #5 | (thromboprophyla* or thrombus* or thrombotic* or thrombolic* or thromboemboli* or thrombos* or embol*):ti,ab,kw | 11677 |
| #6 | MeSH descriptor: [Pulmonary Embolism] explode all trees | 857 |
| #7 | PE or DVT or VTE:ti,ab,kw | 2158 |
| #8 | ((vein* or ven*) near thromb*):ti,ab,kw | 4960 |
| #9 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 | 13368 |
| #10 | MeSH descriptor: [Heparin] explode all trees | 3959 |
| #11 | heparin* | 7829 |
| #12 | UFH or UH or LMWH | 1337 |
| #13 | nadroparin* or fraxiparin* or enoxaparin | 1423 |
| #14 | Clexane or klexane or lovenox | 81 |
| #15 | dalteparin or Fragmin or ardeparin | 592 |
| #16 | normiflo or tinzaparin or logiparin | 231 |
| #17 | Innohep or certoparin or sandoparin or reviparin | 189 |
| #18 | clivarin* or danaproid or danaparoid | 96 |
| #19 | antixarin or ardeparin* or bemiparin* | 64 |
| #20 | Zibor or cy 222 or embolex or monoembolex | 74 |
| #21 | parnaparin* or rd 11885 or RD1185 | 41 |
| #22 | tedelparin or Kabi‐2165 or Kabi 2165 | 68 |
| #23 | emt‐966 or emt‐967 or pk‐10 169 or pk‐10169 or pk10169 | 19 |
| #24 | fr‐860 or cy‐216 or cy216 | 80 |
| #25 | seleparin* or tedegliparin or seleparin* or tedegliparin* | 12 |
| #26 | wy90493 or "wy 90493" | 9 |
| #27 | ("kb 101" or kb101 or lomoparan or orgaran) | 64 |
| #28 | parnaparin or fluxum or lohepa or lowhepa | 49 |
| #29 | op 2123 or parvoparin | 13 |
| #30 | ave 5026 | 3 |
| #31 | calciparin* | 29 |
| #32 | #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 | 8758 |
| #33 | #9 and #32 in Trials | 3324 |
Data and analyses
Comparison 1. Outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent thromboembolic events | 3 | 1281 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.39] |
| 2 Haemorraghic events | 5 | 1508 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.40, 1.45] |
| 3 Improvement of thrombus size | 2 | 107 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.66, 3.01] |
| 4 Mortality | 4 | 1421 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.62, 2.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Charbonnier 1998.
| Methods | Study design: Randomised, multicentre, double blind trial. Method of randomisation: not stated. No. of exclusion post‐randomisation: not stated. Lost to follow up: 1 (twice daily group). |
|
| Participants | Country: 70 centres in Europe. Setting: Hospital. No. of participants: 316 once daily group, 335 twice daily group. Age (mean): 59 ± 17 years once daily group; 60 ± 17 twice daily group. Gender: 56% male once daily group; 53% male twice daily group. Inclusion criteria: 18 years and older with acute symptomatic proximal DVT in popliteal vein or above documented by venography. Exclusion criteria: history of VTE within past two years; thrombosis extending into the vena cava; clinical symptoms at entry suggestive of PE; received full dose heparin treatment for more than 24h; surgery within the last 5 days; actively bleeding; either a known haemorrhagic diathesis or such a tendency detected by the initial pre‐treatment coagulation tests (prothrombin ratio < 60%; platelet count = 150,000 mm3; patient aPTT/control aPTT = 1.4 with no anticoagulant treatment). Other reasons for exclusion were uncontrolled hypertension; severe hepatic or renal failure and short life expectancy (< 6 months). |
|
| Interventions | Once daily nadroparin 20,500 (AXa IU/ml) and one injection of a placebo drug compared with twice daily nadroparin 10,250 (AXa IU/ml). Nadroparin treatment continued for at least 5 days. Warfarin therapy was initiated the same day or the day after and continued for 3 months. The warfarin dose was adjusted to maintain an INR of 2 to 3. | |
| Outcomes | Symptomatic recurrent VTE, including symptomatic worsening or recurrence of the initial VTE; occurrence of a DVT in the contralateral leg; occurrence of symptomatic PE or death, certainly or possibly related to PE; major or minor bleeding; total mortality. Major bleeding was defined as overt and associated with either a decrease in the haemoglobin level (at least 2.0 g per 100 ml); a need for transfusion (2 or more units of blood); retroperitoneal or intracranial bleeding; or if bleeding led to the treatment being discontinued permanently. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Holmström 1992.
| Methods | Study design: Open randomised,
single blind (for outcome assessment), controlled study. Method of randomisation: not stated. No. of exclusions post‐randomisation: 14 excluded from efficacy analysis for various reasons. Lost to follow up: not stated. |
|
| Participants | Country: Sweden. Setting: Hospital. No. of participants: 101; 50 once daily group, 51 twice daily group. Age (mean): 60.0 years (range 24 to 90) once daily group; 62.9 years (range 20 to 90) twice daily group. Gender: 33 M : 17 F once daily group; 24 M : 27 F twice daily group. Inclusion criteria: Patients with a first occurrence of DVT in the lower limb, confirmed with phlebography. Exclusion criteria: thrombosis extended over 2/3rds of the femoral vein; previous ipsilateral thrombosis; heparin treatment > 24 hours; pregnant; impaired coagulation; dementia; psychosis; renal insufficiency; allergy to contrast media; alcoholic. |
|
| Interventions | Prior to Fragmin an i.v. bolus of 5,000 U porcine sodium heparin (UFH) followed by a continuous infusion UFH (not exceeding 24 hours) adjusted to maintain the APTT at 2 to 3 times normal, was given. Subsequently patients received s.c. once daily Fragmin (generic name dalteparin) 200 U (anti‐FXa)/kg or twice daily 100 U (anti‐FXa)/kg. Administration of Fragmin was continued for at least 5 days. Patients were mobilised with compression stockings from day 2. | |
| Outcomes | Marder Score based on phlebography, major and minor bleeding complications. The definition of major bleeding was not specified. The one instance of major bleeding that occurred was firstly characterised as rectal and subsequently as an epistaxis; the haemoglobin concentration fell from 123 to 94 g/litre, and two units of erythrocyte concentrate were administered. | |
| Notes | Once daily treatment group included more calf vein thrombi. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Merli 2001.
| Methods | Study design: Randomised controlled,
double blinded, international, multicentre trial. Method of randomisation: without stratification, in blocks of six according to ascending randomisation number. Numbers affixed to sealed treatment kits containing study medication. No. of exclusions post‐randomisation: 34 in once daily group and 36 in twice daily group discontinued study medication but were still followed up for 3 months as per protocol. Lost to follow up: 1 'missing data' in twice daily group. |
|
| Participants | Country: 16 countries across Europe, United States of America and Australia. Setting: Hospital. No. of participants: 900; 298 in once daily group, 312 in twice daily group. (290 in third group given UFH) Age (mean): 60.7 years (range 19 to 91) in once daily group, 60.7 years (range 18 to 92) in twice daily group. Gender: 161 M : 137 F once daily group, 181 M : 131 F twice daily group. Inclusion criteria: > 18 years with a symptomatic lower‐extremity DVT confirmed by venography or ultrasonography (including 287 patients with confirmed PE). Exclusion criteria: more than 24 hours of previous treatment with heparin or warfarin; need for thrombolytic therapy; known haemorrhagic risk, including active haemorrhage, active intestinal ulcerative disease, known angiodysplasia; or eye, spinal or central nervous system surgery within the previous month; renal or hepatic insufficiency; allergy to heparin, protamine, porcine products, iodine, or contrast media; history of heparin associated thrombocytopenia or heparin‐ or warfarin‐associated skin necrosis; treatment with other investigational therapeutic agents within the previous 4 weeks; inferior vena cava interruption; known pregnancy or lactation. |
|
| Interventions | S.c. enoxaparin at fixed dosages of 1.0 mg/kg of body weight twice daily compared with 1.5 mg/kg body weight once daily and a injection with a placebo drug. Oral anticoagulation was started within 72 hours (INR 2 to 3) and continued for at least 3 months. | |
| Outcomes | Recurrence of DVT or PE, major bleeding and mortality. Major bleeding defined as being associated with at least one of the following: a decrease in haemoglobin level of at least 20 g/litre; need for transfusion of at least two units of blood; retroperitoneal, intracranial, or intraocular bleeding; other associated serious clinical events; need for surgical or medical intervention; or death. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Partsch 1996.
| Methods | Study design: Randomised clinical trial (blinding not reported). Method of randomisation: not stated. No. of exclusions post‐randomisation: not stated. Lost to follow up: not stated. |
|
| Participants | Country: Austria. Setting: Hospital. No. of participants: 140; 76 once daily group, 64 twice daily group. Age (mean): 69.13 ± 17.06 years once daily group; 72.21 ± 13.21 years twice daily group. Gender: 28 M : 48 F once daily group; 34 M : 30 F twice daily group. Inclusion criteria: Consecutive patients presented with DVT extending into the iliofemoral segment diagnosed by duplex ultrasonography. Exclusion criteria: previous fibrinolytic treatment; thrombectomy; complete bed rest for > 3 days within 36 hours of admission to hospital; been previously immobilised in other departments as a result of surgery, trauma or internal diseases because of inability to ambulate; pregnancy. |
|
| Interventions | Fragmin administered 200 IU/kg once daily or 100 IU/kg twice daily started immediately after randomisation for at least 7 days. Coumarin treatment was initiated approximately 10 days after diagnoses and continued for at least 3 months. | |
| Outcomes | Decrease in frequency of PE as judged by the difference between the second V/Q scan and the initial baseline scan, major and minor bleeding, and mortality. The definition of major bleeding was not specified, but the one that occurred was characterised as "requiring 2 U of blood transfusion, gastrointestinal". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Siegbahn 1989.
| Methods | Study design: Randomised, single blinded trial. Method of randomisation: not stated. No. of exclusions post‐randomisation: not stated. Lost to follow up: not stated. |
|
| Participants | Country: Sweden and Denmark. Setting: Hospital. No. of participants: 20; 10 once daily group, 10 twice daily group. Age (mean): 65.5 years (range 48 to 75) once daily group, 63.4 years (range 49 to 77) twice daily group. Gender: 7 M : 3 F once daily group, 6 M : 4 F twice daily group. Inclusion criteria: over 21 years with a venographically confirmed episode of DVT. Exclusion criteria: evidence of haemorraghic disorder; known hypersensitivity against heparin; systemic hypertension; renal insufficiency; a history of earlier ipsilateral DVT; surgery within the last month; history of intracranial bleeding; pregnancy; already on anticoagulant treatment. |
|
| Interventions | Once daily logiparin 150 XaI U/kg compared with twice daily logiparin 75 XaI U/kg. Patients received warfarin therapy from the first day of heparin treatment. | |
| Outcomes | Recurrent VTE; change in thrombus size; and major bleeding. The definition of major bleeding was not specified. The change in thrombus size was depicted as a change in Marder score. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
APTT: activated partial thromboplastin time DVT: deep vein thrombosis INR: International Normalised Ratio IU: international unit i.v.: intravascular PE: pulmonary embolism s.c.: subcutaneous UFH: unfractionated heparin VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agnelli 1995 | Trial participants did not suffer from VTE as initial event. |
| Alhenc‐Gelas 1994 | Did not compare once daily against twice daily treatment with LMWH. |
| Andersen 1997 | Did not compare once daily against twice daily treatment with LMWH. |
| Bara 1992 | Did not compare once daily against twice daily treatment with LMWH. |
| Beckman 2003 | Did not compare once daily against twice daily treatment with LMWH. |
| Belcaro 1999 | Did not compare once daily against twice daily treatment with LMWH. |
| Bellosta 2007 | Used different LMWH, with once daily versus twice daily for one month only and following that, the twice daily treatment arm reverted to once daily prophylactic dose. This makes comparing once daily versus twice daily regimens untenable as there were no results for effectiveness of the regimens at one month. |
| Boneu 1998 | Trial participants did not suffer from VTE as initial event. |
| Bratt 1988 | Did not compare once daily against twice daily treatment with LMWH. |
| Bratt 1990 | Trial participants did not suffer from VTE as initial event. |
| Breddin 2001 | Patients treated with LMWH once daily received a vitamin K antagonist from day 21 onwards, while for those treated with LMWH twice daily, administration of vitamin K antagonists was started at day one. |
| Breddin 2003 | Patients treated with LMWH once daily received a vitamin K antagonist from day 21 onwards, while for those treated with LMWH twice daily, administration of vitamin K antagonists was started at day one. |
| Buller 2004 | Did not compare once daily against twice daily treatment with LMWH. |
| Cosmi 2012 | Superficial vein thrombosis not DVT. The study compared different doses of LMWH and not once versus twice daily regimens. |
| Erikson 2002 | Trial participants did not suffer from VTE as initial event. |
| Erikson 2003 | Trial participants did not suffer from VTE as initial event. |
| Fiessinger 1996 | Did not compare once daily against twice daily treatment with LMWH. |
| Harenberg 1990 | Did not compare once daily against twice daily treatment with LMWH. |
| Harenberg 1997 | Did not compare once daily against twice daily treatment with LMWH. |
| Holmström 1997 | Did not compare once daily against twice daily treatment with LMWH. |
| Hull 1997 | Did not compare once daily against twice daily treatment with LMWH. |
| Hull 2000a | Did not compare once daily against twice daily treatment with LMWH. |
| Hull 2000b | Did not compare once daily against twice daily treatment with LMWH. |
| Kakkar 2002 | Patients treated with LMWH once daily received a vitamin K antagonist from day 21 onwards, while for those treated with LMWH twice daily, administration of vitamin K antagonists was started at day one. |
| Leizorovicz 2011 | Did not compare once daily against twice daily treatment with LMWH. Compared once daily LMWH with twice daily heparin for acute treatment of DVT in elderly patients with renal impairment. |
| Leroyer 1998 | Did not compare once daily against twice daily treatment with LMWH. |
| Lindmarker 1994 | Did not compare once daily against twice daily treatment with LMWH. |
| Luomanmaki 1996 | Did not compare once daily against twice daily treatment with LMWH. |
| Meyer 1995 | Did not compare once daily against twice daily treatment with LMWH. |
| Mismetti 1995 | Trial participants did not suffer from VTE as initial event. |
| Narin 2008 | Retrospective study involving treatment of DVT in pregnant patients. Initial treatment involved unfractionated heparin for five days before starting LMWH. |
| Offord 2004 | Did not compare once daily against twice daily treatment with LMWH. |
| Petilla 2002 | Trial participants did not suffer from VTE as initial event. |
| Pini 1994 | Did not compare once daily against twice daily treatment with LMWH. |
| Sandset 1990 | Did not compare once daily against twice daily treatment with LMWH. |
| Schellong 2010 | Did not compare once daily against twice daily treatment with LMWH. |
| Simonneau 1993 | Did not compare once daily against twice daily treatment with LMWH. |
| Simonneau 1997 | Did not compare once daily against twice daily treatment with LMWH. |
| Stricker 1999 | Did not compare once daily against twice daily treatment with LMWH. |
| Turpie 2002 | Trial participants did not suffer from VTE as initial event. |
| Wartski 2000 | Did not compare once daily against twice daily treatment with LMWH. |
DVT: deep vein thrombosis LMWH: low molecular weight heparin VTE: venous thromboembolism
Contributions of authors
2013 update: SB and PW assessed the studies for eligibility and updated the review accordingly, and expanded the abstract and plain language summary.
2005 update and original review: Conceiving the review: MP Performing the review: CVD, MM Writing the review: CVD, MM, MP Coordinating the review: CVD
The Peripheral Vascular Diseases Review Group assisted with searching for trials.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
The PVD Group editorial base is supported by a programme grant from the NIHR.
Declarations of interest
None known
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Charbonnier 1998 {published data only}
- Charbonnier BA, Fiessinger JN, Banga JD, Wenzel E, d'Azemar P, Sagnard L. Comparison of a once daily with a twice daily subcutaneous low molecular weight heparin regimen in the treatment of deep vein thrombosis. FRAXODI group. Thrombosis and Haemostasis 1998;79(5):897‐901. [PubMed] [Google Scholar]
Holmström 1992 {published data only}
- Holmström M, Berglund MC, Granqvist S, Bratt G, Törnebohm E, Lockner D. Fragmin once or twice daily subcutaneously in the treatment of deep venous thrombosis of the leg. Thrombosis Research 1992;67(1):49‐55. [DOI] [PubMed] [Google Scholar]
- Holmström M, Trönebohm E, Berglund MC, Granqvist S, Lockner D. Fragmin (KABI) subcutaneously once or twice daily in the treatment of deep venous thrombosis (DVT). Journal of Internal Medicine Supplement 1990;733:31. [Google Scholar]
Merli 2001 {published data only}
- Merli G, Spiro TE, Olsson CG, Abildgaard U, Davidson BL, Eldor A, et al. Subcutaneous enoxaparin once or twice daily compared with intravenous unfractionated heparin for treatment of venous thromboembolic disease. Annals of Internal Medicine 2001;134(3):191‐202. [DOI] [PubMed] [Google Scholar]
Partsch 1996 {published data only}
- Partsch H, Kechavarz B, Mostbeck A, Köhn H, Lipp C. Frequency of pulmonary embolism in patients who have iliofemoral deep vein thrombosis and are treated with once‐ or twice‐daily low‐molecular‐weight heparin. Journal of Vascular Surgery 1996;24(5):774‐82. [DOI] [PubMed] [Google Scholar]
Siegbahn 1989 {published data only}
- Siegbahn A, Y‐Hassan S, Boberg J, Bylund H, Neerstrand HS, Østergaard P, et al. Subcutaneous treatment of deep venous thrombosis with low molecular weight heparin. A dose finding study with LMWH‐Novo. Thrombosis Research 1989;55(6):767‐78. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agnelli 1995 {published data only}
- Agnelli G, Iorio A, Renga C, Boschetti E, Nenci GG, Ofosu FA, et al. Prolonged antithrombin activity of low‐molecular‐weight heparins: Clinical implications for the treatment of thromboembolic diseases. Circulation 1995;92(10):2819‐24. [DOI] [PubMed] [Google Scholar]
Alhenc‐Gelas 1994 {published data only}
- Alhenc‐Gelas M, Jestin‐Le Guernic C, Vitoux JF, Kher A, Aiach M, Fiessinger JN. Adjusted versus fixed doses of the low‐molecular‐weight heparin fragmin in the treatment of deep vein thrombosis. Fragmin‐Study Group. Thrombosis and Haemostasis 1994;71(6):698‐702. [PubMed] [Google Scholar]
Andersen 1997 {published data only}
- Andersen BS. Postoperative activation of the haemostatic system‐‐influence of prolonged thromboprophylaxis in patients undergoing total hip arthroplasty. Haemostasis 1997;27(5):219‐27. [DOI] [PubMed] [Google Scholar]
Bara 1992 {published data only}
- Bara L, Leizorovicz A, Picolet H, Samama M. Correlation between anti‐Xa and occurrence of thrombosis and haemorrhage in post‐surgical patients treated with either Logiparin (LMWH) or unfractionated heparin. Post‐surgery Logiparin Study Group. Thrombosis Research 1992;65(4‐5):641‐50. [DOI] [PubMed] [Google Scholar]
Beckman 2003 {published data only}
- Beckman JA, Dunn K, Sasahara AA, Goldhaber SZ. Enoxaparin monotherapy without oral anticoagulation to treat acute symptomatic pulmonary embolism. Thrombosis and Haemostasis 2003;89(6):953‐8. [PubMed] [Google Scholar]
Belcaro 1999 {published data only}
- Belcaro G, Nicolaides AN, Cesarone MR, Laurora G, Sanctis MT, Incandela L, et al. Comparison of low‐molecular‐weight heparin, administered primarily at home, with unfractionated heparin, administered in hospital, and subcutaneous heparin, administered at home for deep‐vein thrombosis. Angiology 1999;50(10):781‐7. [DOI] [PubMed] [Google Scholar]
Bellosta 2007 {published data only}
- Bellosta R, Ferrari P, Luzzani L, Carugati C, Cossu L, Talarico M, et al. Home therapy with LMWH in deep vein thrombosis: Randomized study comparing single and double daily administrations. Angiology 2007;58(3):316‐22. [DOI] [PubMed] [Google Scholar]
Boneu 1998 {published data only}
- Boneu B, Navarro C, Cambus JP, Caplain H, d'Azemar P, Necciari J, et al. Pharmacodynamics and tolerance of two nadroparin formulations (10,250 and 20,500 anti Xa IU x ml(‐1)) delivered for 10 days at therapeutic dose. Thrombosis and Haemostasis 1998;79(2):338‐41. [PubMed] [Google Scholar]
Bratt 1988 {published data only}
- Bratt GA, Törnebohm E, Johanson M, Aberg W, Granqvist S, Lockner D. Clinical experiences in the administration of a low molecular weight heparin (Fragmin, Kabi‐Vitrum) to healthy volunteers and in the treatment of established deep venous thrombosis. Acta Chirurgica Scandinavica 1988;154(Supplement 543):96‐100. [PubMed] [Google Scholar]
Bratt 1990 {published data only}
- Bratt G, Aberg W, Johansson M, Törnebohm E, Granqvist S, Lockner D. Two daily subcutaneous injections of Fragmin as compared with intravenous standard heparin in the treatment of deep venous thrombosis (DVT). Thrombosis and Haemostasis 1990;64(4):506‐10. [PubMed] [Google Scholar]
Breddin 2001 {published data only}
- Breddin HK, Hach‐Wunderle V, Nakov R, Kakkar VV. For the CORTES Investigators. Effects of a low‐molecular‐weight heparin on thrombus regression and recurrent thromboembolism in patients with deep‐vein thrombosis. New England Journal of Medicine 2001;344(9):626‐31. [DOI] [PubMed] [Google Scholar]
Breddin 2003 {published data only}
- Breddin HK, Kadziola Z, Scully M, Nakov R, Misselwitz F, Kakkar VV. Risk factors and coagulation parameters in relationship to phlebographic response and clinical outcome in the treatment of acute deep vein thrombosis. Thrombosis and Haemostasis 2003;89(2):272‐9. [PubMed] [Google Scholar]
Buller 2004 {published data only}
- Buller H, Davidson B, Decousus H, Gallus A, Gent M, Piovella F. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis. A randomized trial. Revue de Medecine Interne 2005;26(1):82‐3. [DOI] [PubMed] [Google Scholar]
- Buller HR, Davidson BL, Decousus H, Gallus A, Gent M, Piovella F, et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Annals of Internal Medicine 2004;140(11):867‐73. [DOI] [PubMed] [Google Scholar]
- Buller HR, The Matisse Investigators. Initial outpatient treatment of venous thromboembolism with fondaparinux (Arixtra(R)): The Matisse trials. Blood 2004;104(11):Abstract 705. [Google Scholar]
- The Matisse Investigators. The MATISSE‐DVT trial, a randomized, double‐blind study comparing once‐daily fondaparinux (ATRIXA) with the low‐molecular‐weight heparin (LMWH) enoxaparin, twice daily, in the initial treatment of symptomatic deep‐vein thrombosis (DVT). Journal of Thrombosis and Haemostasis 2003; Vol. 1, issue Supplement 1:Abstract OC332.
Cosmi 2012 {published data only}
- Cosmi B, Filippini M, Tonti D, Avruscio G, Ghirarduzzi A, Bucherini E, et al. STEFLUX Investigators. A randomized double‐blind study of low‐molecular‐weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). Journal of Thrombosis and Haemostasis 2012;10(6):1026‐35. [DOI] [PubMed] [Google Scholar]
Erikson 2002 {published data only}
- Eriksson BI, Bergqvist D, Kalebo P, Dahl OE, Lindbratt S, Bylock A, et al. Melagatran for thrombin inhibition in orthopaedic surgery. Ximelagatran and melagatran compared with dalteparin for prevention of venous thromboembolism after total hip or knee replacement: the METHRO II randomised trial. Lancet 2002;360(9344):1441‐7. [DOI] [PubMed] [Google Scholar]
Erikson 2003 {published data only}
- Eriksson BI, Agnelli G, Cohen AT, Dahl OE, Mouret P, Rosencher N, et al. Direct thrombin inhibitor melagatran followed by oral ximelagatran in comparison with enoxaparin for prevention of venous thromboembolism after total hip or knee replacement. Thrombosis and Haemostasis 2003;89(2):288‐96. [PubMed] [Google Scholar]
Fiessinger 1996 {published data only}
- Fiessinger JN, Lopez‐Fernandez M, Gatterer E, Granqvist S, Kher A, Olsson CG, et al. Once‐daily subcutaneous dalteparin, a low molecular weight heparin, for the initial treatment of acute deep vein thrombosis. Thrombosis and Haemostasis 1996;76(2):195‐9. [PubMed] [Google Scholar]
Harenberg 1990 {published data only}
- Harenberg J, Huck K, Bratsch H, Stehle G, Dempfle CE, Mall K, et al. Therapeutic application of subcutaneous low‐molecular‐weight heparin in acute venous thrombosis. Haemostasis 1990;20(Supplement 1):205‐19. [DOI] [PubMed] [Google Scholar]
Harenberg 1997 {published data only}
- Harenberg J, Stehle G, Blauth M, Huck K, Mall K, Heene DL. Dosage, anticoagulant, and antithrombotic effects of heparin and low‐molecular‐weight heparin in the treatment of deep vein thrombosis. Seminars in Thrombosis and Hemostasis 1997;23(1):83‐90. [DOI] [PubMed] [Google Scholar]
Holmström 1997 {published data only}
- Holmström M, Lindmarker P, Granqvist S, Johnsson H, Lockner D. A 6‐month venographic follow‐up in 164 patients with acute deep vein thrombosis. Thrombosis and Haemostasis 1997;78(2):803‐7. [PubMed] [Google Scholar]
Hull 1997 {published data only}
- Hull RD, Raskob GE, Brant RF, Pineo GF, Valentine KA. The importance of initial heparin treatment on long‐term clinical outcomes of antithrombotic therapy. The emerging theme of delayed recurrence. Archives of Internal Medicine 1997;157(20):2317‐21. [PubMed] [Google Scholar]
Hull 2000a {published data only}
- Hull RD, Pineo GF, Francis C, Bergqvist D, Fellenius C, Soderberg K, et al. For the North American Fragmin Trial Investigators. Low‐molecular‐weight heparin prophylaxis using dalteparin in close proximity to surgery vs warfarin in hip arthroplasty patients: a double‐blind, randomized comparison. Archives of Internal Medicine 2000;160(14):2199‐207. [DOI] [PubMed] [Google Scholar]
Hull 2000b {published data only}
- Hull RD, Raskob GE, Brant RF, Pineo GF, Elliott G, Stein PD, et al. For the American‐Canadian Thrombosis Study Group. Low‐molecular‐weight heparin vs heparin in the treatment of patients with pulmonary embolism. Archives of Internal Medicine 2000;160(2):229‐36. [DOI] [PubMed] [Google Scholar]
Kakkar 2002 {published data only}
- Kakkar VV, Hoppenstead DA, Fareed J, Kadziola Z, Scully M, Nakov R, et al. Randomized trial of different regimens of heparins and in vivo thrombin generation in acute deep vein thrombosis. Blood 2002;99(6):1965‐70. [DOI] [PubMed] [Google Scholar]
Leizorovicz 2011 {published data only}
- Leizorovicz A. IRIS an ongoing, international, multicentre, open, centrally randomised, parallel group study with tinzaparin or unfractionated heparin (ufh) administered subcutaneously (sc) to patients with deep vein thrombosis (DVT) or pulmonary embolism (PE). XXIst Congress of the International Society on Thrombosis and Haemostasis; 2007 Jul 6‐12; Geneva 2007:Abstract no: P‐M‐673.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐the IRIS Trial. Blood 2008; Vol. 112.
- Leizorovicz A. Tinzaparin compared to unfractionated heparin for initial treatment of deep vein thrombosis in very elderly patients with renal insufficiency‐the IRIS Trial [Abstract No. 434]. ASH 2009; Vol. 112, issue 11:166.
- Leizorovicz A, Siguret V, Mottier D. Safety profile of tinzaparin versus subcutaneous unfractionated heparin in elderly patients with impaired renal function treated for acute deep vein thrombosis: The Innohep((R)) in Renal Insufficiency Study (IRIS). Thrombosis Research 2011;128(1):27‐34. [DOI] [PubMed] [Google Scholar]
Leroyer 1998 {published data only}
- Leroyer C, Bressollette L, Oger E, Mansourati J, Cheze‐Le Rest C, Nonent M, et al. For the ANTENOX Study Group. Early versus delayed introduction of oral vitamin K antagonists in combination with low‐molecular‐weight heparin in the treatment of deep vein thrombosis. A randomized clinical trial. Haemostasis 1998;28(2):70‐7. [DOI] [PubMed] [Google Scholar]
Lindmarker 1994 {published data only}
- Lindmarker P, Holmström M, Granqvist S, Johnsson H, Lockner D. Comparison of once‐daily subcutaneous Fragmin(TM) with continuous intravenous unfractionated heparin in the treatment of deep vein thrombosis. Thrombosis and Haemostasis 1994;72(2):186‐90. [PubMed] [Google Scholar]
Luomanmaki 1996 {published data only}
- Luomanmaki K, Grankvist S, Hallert C, Jauro I, Ketola K, Kim HC, et al. A multicentre comparison of once‐daily subcutaneous dalteparin (low molecular weight heparin) and continuous intravenous heparin in the treatment of deep vein thrombosis. Journal of Internal Medicine 1996;240(2):85‐92. [DOI] [PubMed] [Google Scholar]
Meyer 1995 {published data only}
- Meyer G, Brenot F, Pacouret G, Simonneau G, Gillet Juvin K, Charbonnier B, et al. Subcutaneous low‐molecular‐weight heparin Fragmin versus intravenous unfractionated heparin in the treatment of acute non massive pulmonary embolism: an open randomized pilot study. Thrombosis and Haemostasis 1995;74(6):1432‐5. [PubMed] [Google Scholar]
Mismetti 1995 {published data only}
- Mismetti P, Reynaud J, Tardy‐Ponce B, Laporte‐Simitsidis S, Scully M, Goodwyn C, et al. Chrono‐pharmacological study of once daily curative dose of a low molecular weight heparin (200 IU antiXa/kg of Dalteparin) in ten healthy volunteers. Thrombosis and Haemostasis 1995;74(2):660‐6. [PubMed] [Google Scholar]
Narin 2008 {published data only}
- Narin C, Reyhanoglu H, Tulek B, Onoglu R, Ege E, Sarigul A, et al. Comparison of different dose regimens of enoxaparin in deep vein thrombosis therapy in pregnancy. Advances in Therapy 2008;25(6):585‐94. [DOI] [PubMed] [Google Scholar]
Offord 2004 {published data only}
- Offord R, Lloyd AC, Anderson P, Bearne A. Economic evaluation of enoxaparin for the prevention of venous thromboembolism in acutely ill medical patients. Pharmacy World and Science 2004;26(4):214‐20. [DOI] [PubMed] [Google Scholar]
Petilla 2002 {published data only}
- Pettila V, Leinonen P, Markkola A, Hiilesmaa V, Kaaja R. Postpartum bone mineral density in women treated for thromboprophylaxis with unfractionated heparin or LMW heparin. Thrombosis and Haemostasis 2002;87(2):182‐6. [PubMed] [Google Scholar]
Pini 1994 {published data only}
- Pini M, Aiello S, Manotti C, Pattacini C, Quintavalla R, Poli T, et al. Low molecular weight heparin versus warfarin in the prevention of recurrences after deep vein thrombosis. Thrombosis and Haemostasis 1994;72(2):191‐7. [PubMed] [Google Scholar]
Sandset 1990 {published data only}
- Sandset PM, Dahl T, Stiris M, Rostad B, Scheel B, Abildgaard U. A double‐blind and randomized placebo‐controlled trial of low molecular weight heparin once daily to prevent deep‐vein thrombosis in acute ischemic stroke. Seminars in Thrombosis and Hemostasis 1990;16(Supplement):25‐33. [PubMed] [Google Scholar]
Schellong 2010 {published data only}
- Schellong SM, Haas S, Greinacher A, Schwanebeck U, Sieder C, Abletshauser C, et al. An open‐label comparison of the efficacy and safety of certoparin versus unfractionated heparin for the prevention of thromboembolic complications in acutely ill medical patients: CERTAIN. Expert Opinion on Pharmacotherapy 2010;11(18):2953‐61. [DOI] [PubMed] [Google Scholar]
Simonneau 1993 {published data only}
- Simonneau G, Charbonnier B, Decousus H, Planchon B, Ninet J, Sie P, et al. Subcutaneous low‐molecular‐weight heparin compared with continuous intravenous unfractionated heparin in the treatment of proximal deep vein thrombosis. Archives of Internal Medicine 1993;153(13):1541‐6. [PubMed] [Google Scholar]
Simonneau 1997 {published data only}
- Simonneau G, Sors H, Charbonnier B, Page Y, Laaban J‐P, Azarian R, et al. For the THESEE Study Group. A comparison of low‐molecular‐weight heparin with unfractionated heparin for acute pulmonary embolism. New England Journal of Medicine 1997;337(10):663‐9. [DOI] [PubMed] [Google Scholar]
Stricker 1999 {published data only}
- Stricker H, Marchetti O, Haeberli A, Mombelli G. Hemostatic activation under anticoagulant treatment: a comparison of unfractionated heparin vs. nadroparin in the treatment of proximal deep vein thrombosis. Thrombosis and Haemostasis 1999;82(4):1227‐31. [PubMed] [Google Scholar]
Turpie 2002 {published data only}
- Turpie AG, Bauer KA, Eriksson BI, Lassen MR, PENTATHALON 2000 Study Steering Committee. Postoperative fondaparinux versus postoperative enoxaparin for prevention of venous thromboembolism after elective hip‐replacement surgery: a randomised double‐blind trial. Lancet 2002;359(9319):1721‐6. [DOI] [PubMed] [Google Scholar]
Wartski 2000 {published data only}
- Wartski M, Collignon MA. Incomplete recovery of lung perfusion after 3 months in patients with acute pulmonary embolism treated with antithrombotic agents. THESEE Study Group. Tinzaparin ou Heparin Standard: Evaluation dans l'Embolie Pulmonaire Study. Journal of Nuclear Medicine 2000;41(6):1043‐8. [PubMed] [Google Scholar]
Additional references
Anderson 1991
- Anderson FA Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population‐based perspective of the hospital incidence and case‐fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Archives of Internal Medicine 1991;151(5):933‐8. [PubMed] [Google Scholar]
Bertina 1994
- Bertina RM, Koeleman BP, Koster T, Rosendaal FR, Dirven RJ, Ronde H, et al. Mutation in blood coagulation factor V associated with resistance to activated protein C. Nature 1994;369(6475):64‐7. [DOI] [PubMed] [Google Scholar]
Brandjes 1992
- Brandjes DPM, Heijboer H, Büller HR, Rijk M, Jagt H, Cate JW. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal‐vein thrombosis. New England Journal of Medicine 1992;327(21):1485‐9. [DOI] [PubMed] [Google Scholar]
Büller 1991
- Büller HR, Lensing AWA, Hirsch J, Cate JW. Deep vein thrombosis: new non‐invasive diagnostic tests. Thrombosis and Haemostasis 1991;66(1):133‐7. [PubMed] [Google Scholar]
Collins 1987
- Collins R, Gray R, Godwin J, Peto R. Avoidance of large biases and large random errors in the assessment of moderate treatment effects: the need for systematic overviews. Statistics in Medicine 1987;6(3):245‐54. [DOI] [PubMed] [Google Scholar]
Dolovich 2000
- Dolovich LR, Ginsberg JS, Douketis JD, Holbrook AM, Cheah G. A meta‐analysis comparing low‐molecular‐weight heparins with unfractionated heparin in the treatment of venous thromboembolism: examining some unanswered questions regarding location of treatment, product type, and dosing frequency. Archives of Internal Medicine 2000;160(2):181‐8. [DOI] [PubMed] [Google Scholar]
Erkens 2010
- Erkens PMG, Prins MH. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD001100.pub3] [DOI] [PubMed] [Google Scholar]
Gould 1999
- Gould MK, Dembitzer AD, Doyle RL, Hastie TJ, Garber AM. Low‐molecular‐weight heparins compared with unfractionated heparin for treatment of acute deep venous thrombosis. A meta‐analysis of randomized, controlled trials. Annals of Internal Medicine 1999;130(10):800‐9. [DOI] [PubMed] [Google Scholar]
Heijboer 1990
- Heijboer H, Brandjes DPM, Büller HR, Sturk A, Cate JW. Deficiencies of coagulation‐inhibiting and fibrinolytic proteins in outpatients with deep‐vein thrombosis. New England Journal of Medicine 1990;323(22):1512‐6. [DOI] [PubMed] [Google Scholar]
Heit 2000
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population‐based case‐control study. Archives of Internal Medicine 2000;160(6):809‐15. [DOI] [PubMed] [Google Scholar]
Hirsh 1991
- Hirsh J. Drug therapy: Heparin. New England Journal of Medicine 1991;324(22):1565‐74. [DOI] [PubMed] [Google Scholar]
Hirsh 1992
- Hirsh J, Levine MN. Low molecular weight heparin. Blood 1992;79(1):1‐17. [PubMed] [Google Scholar]
Holmström 1990
- Holmström M, Tornebohm E, Berglund MC, Granqvist S, Lockner D. Fragmin (KABI) subcutaneously once or twice daily in the treatment of deep venous thrombosis (DVT). Journal of Internal Medicine Supplement 1990;733:31. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Koopman 1996
- Koopman MM, Prandoni P, Piovella F, Ockelford PA, Brandjes DP, Meer J, et al. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low‐molecular‐weight heparin administered at home. The Tasman Study Group. New England Journal of Medicine 1996;334(11):682‐7. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PubMed] [Google Scholar]
Nordstrom 1992
- Nordstrom M, Lindblad B, Bergqvist D, Kjellstrom T. A prospective study of the incidence of deep‐vein thrombosis within a defined urban population. Journal of Internal Medicine 1992;232(2):155‐60. [DOI] [PubMed] [Google Scholar]
Prandoni 1992
- Prandoni P, Lensing AWA, Büller HR, Carta M, Cogo A, Vigo M, et al. Comparison of subcutaneous low‐molecular‐weight heparin with intravenous standard heparin in proximal deep‐vein thrombosis. Lancet 1992;339(8791):441‐5. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
van den Belt 1999
- Belt AGM, Prins MH, Lensing AW, Castro AA, Clark OA, Atallah AN, et al. Fixed dose subcutaneous low molecular weight heparins versus adjusted dose unfractionated heparin for venous thromboembolism. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD001100] [DOI] [PubMed] [Google Scholar]
van der Heijden 2000
- Heijden JF, Prins MH, Büller HR. For the initial treatment of venous thromboembolism: are all low‐molecular‐weight heparin compounds the same?. Thrombosis Research 2000;100(2):V121‐30. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Van Dongen 2002
- van Dongen CJ, Mac Gillavry MR, Prins MH. Once versus twice daily LMWH for the initial treatment of venous thromboembolism. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003074] [DOI] [PubMed] [Google Scholar]
Van Dongen 2005
- Dongen CJ, Mac Gillavry MR, Prins MH. Once versus twice daily low molecular weight heparin for the initial treatment of venous thromboembolism. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003074.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


