Abstract
Background
Some women who have threatened to give birth prematurely, subsequently settle. They may then take oral tocolytic maintenance therapy to prevent preterm birth and to prolong gestation.
Objectives
To assess the effects of oral betamimetic maintenance therapy after threatened preterm labour for preventing preterm birth.
Search methods
We updated the search of the Cochrane Pregnancy and Childbirth Group's Trials Register on 9 November 2012.
Selection criteria
Randomised controlled trials comparing oral betamimetic with alternative tocolytic therapy, placebo or no therapy, for maintenance following treatment of threatened preterm labour.
Data collection and analysis
Two review authors independently applied the selection criteria and carried out data extraction and quality assessment of studies.
Main results
We did not identify any new trials from the updated search so the results remain unchanged as follows.
We included 13 randomised controlled trials (RCTs) with a total of 1551 women. We found no differences for admission to the neonatal intensive care unit when betamimetics were compared with placebo (risk ratio (RR) 1.28, 95% confidence interval (CI) 0.68 to 2.41; two RCTs of terbutaline with 2600 women) or with magnesium (RR 0.80, 95% CI 0.43 to 1.46; one RCT of 137 women). The rate of preterm birth (less than 37 weeks) showed no significant difference in six RCTs, four comparing ritodrine with placebo/no treatment and two comparing terbutaline with placebo/no treatment (RR 1.11, 95% CI 0.91 to 1.35; 644 women). We observed no differences between betamimetics and placebo, no treatment or other tocolytics for perinatal mortality and morbidity outcomes. Some adverse effects such as tachycardia were more frequent in the betamimetics groups than the groups allocated to placebo, no treatment or another type of tocolytic.
Authors' conclusions
Available evidence does not support the use of oral betamimetics for maintenance therapy after threatened preterm labour.
Keywords: Female; Humans; Pregnancy; Administration, Oral; Adrenergic beta‐2 Receptor Agonists; Adrenergic beta‐2 Receptor Agonists/administration & dosage; Indomethacin; Indomethacin/administration & dosage; Magnesium Compounds; Magnesium Compounds/administration & dosage; Maintenance Chemotherapy; Maintenance Chemotherapy/methods; Obstetric Labor, Premature; Obstetric Labor, Premature/drug therapy; Obstetric Labor, Premature/prevention & control; Randomized Controlled Trials as Topic; Ritodrine; Ritodrine/administration & dosage; Terbutaline; Terbutaline/administration & dosage; Tocolytic Agents; Tocolytic Agents/administration & dosage
Plain language summary
Oral betamimetics for maintenance therapy after threatened preterm labour
A substantial proportion of women who have an episode of threatened preterm labour (before 37 weeks) are actively treated with agents that stop the uterine contractions (tocolytic therapy) and they do not progress to give birth. After being successfully treated for an episode of threatened preterm birth, women may then take medication (tocolytics) to prolong gestation so that their baby is not born too early. Medications used for this purpose include betamimetics, magnesium sulphate, calcium channel blockers and COX inhibitors.
Oral betamimetics for maintenance therapy after threatened preterm labour do not prevent preterm labour. This conclusion is based on 13 randomised controlled trials with a total of 1551 women. In this review, the betamimetics ritodrine and terbutaline did not reduce the rate of preterm birth (eight trials), or prevent problems with babies that required admission to the neonatal intensive care unit (two trials), when compared with placebo, no treatment or other tocolytic drugs. Betamimetics may cause pregnant women to have an increased heart rate (palpitations) and rate of breathing, low blood pressure, nausea and vomiting, and high blood sugar concentrations as side effects.
Background
Preterm birth occurs before 37 completed weeks' gestation and may arise from spontaneous preterm labour or from a decision to end the pregnancy early due to concerns regarding maternal or fetal wellbeing. Preterm birth is the principal cause of early neonatal mortality and causes both significant immediate morbidity and substantial long‐term morbidity in a proportion of survivors. Prevention of preterm birth remains a goal in obstetrics, and a variety of therapeutic tocolytic agents have been used to inhibit preterm labour (Keirse 1989). Tocolytic drugs act through a variety of mechanisms to relax the uterus and prevent uterine contractions. While tocolytic drugs have been shown to reduce the occurrence of preterm birth (Anotayanonth 2004; King 1988), this has not translated into a reduction in adverse perinatal outcomes, such as low birthweight, respiratory complications or perinatal death (Gyetvai 1999; Keirse 1995a). A variety of tocolytic drugs have been used to inhibit preterm labour, including betamimetics (such as salbutamol or terbutaline), calcium channel blockers (such as nifedipine (King 2003)) and COX inhibitors (King 2005).
Betamimetic drugs have side effects for the woman and, as they are able to cross the placenta, may affect the infant (Gyetvai 1999; Keirse 1995a). Adverse effects for the woman include tachycardia (increased heart rate), tachypnoea (increased rate of breathing), hypotension (low blood pressure), nausea and vomiting, hyperglycaemia (high blood sugar levels) and pulmonary oedema (fluid accumulation in the lungs) (Gyetvai 1999; Keirse 1989).
A substantial proportion of women who have an episode of threatened preterm labour and are actively treated with intravenous tocolytic therapy do not progress to giving birth. For these women, the use of oral tocolytic maintenance medication (usually taken daily for a variable period of time after the initial episode of threatened preterm labour has settled) has been advocated to reduce the risk of recurrence of preterm labour and to prolong gestation to prevent preterm birth. Different forms of maintenance therapy have been suggested, including betamimetics (Nanda 2002), magnesium sulphate (Crowther 1998), calcium channel blockers (Gaunekar 2004) and COX inhibitors. This review examines the evidence for oral betamimetic maintenance therapy.
Objectives
To assess the effects of oral betamimetic maintenance therapy after threatened preterm labour for preventing preterm birth.
Methods
Criteria for considering studies for this review
Types of studies
Available data from published randomised controlled trials (RCTs) with reported data which compare outcomes in women and infants who receive an oral betamimetic agent following treatment of threatened preterm labour with outcomes in controls who are administered an alternative tocolytic therapy, placebo or no therapy. We have not included quasi‐randomised studies and those presented only in abstract form.
Types of participants
Women who have had at least one episode of threatened preterm labour that settled without preterm birth.
Types of interventions
Women administered an oral betamimetic compared with an alternative tocolytic or no tocolytic treatment for maintenance therapy following threatened preterm labour. We excluded trials in which women are administered an oral betamimetic in combination with another tocolytic agent.
Types of outcome measures
Primary outcomes
Very preterm birth (less than 34 weeks' gestation);
low birthweight (less than 2500 grams);
need for admission to the neonatal intensive care unit;
perinatal mortality;
serious neonatal or infant morbidity ‐ defined as growth restriction (birthweight less than the third centile); chronic lung disease; seizures; birth asphyxia defined by trialists; neonatal encephalopathy; disability in childhood);
maternal death or serious maternal morbidity (e.g. complications related to tocolytic medication and hospital admission).
Secondary outcomes
These relate to outcomes for the infant, measures of effectiveness, women's views, women's measures of satisfaction, and costs.
Outcomes for infant
Apgar score less than seven at five minutes;
preterm birth (less than 37 weeks);
extremely preterm birth (less than 28 weeks);
respiratory distress syndrome;
use of mechanical ventilation;
parameters of birth asphyxia (neonatal irritability; neonatal seizures; neonatal hypotonia; abnormal level of consciousness; neonatal apnoea; tube feeding greater than 48 hours);
neonatal encephalopathy;
intraventricular haemorrhage;
neonatal jaundice requiring phototherapy;
neonatal hypoglycaemia;
disability at childhood follow‐up.
Outcomes for women
Side effects sufficient to stop therapy;
mild side effects (e.g. tachycardia; tachypnoea; hypotension; nausea; vomiting; hyperglycaemia; hypokalaemia);
severe side effects (e.g. pulmonary oedema; myocardial ischaemia).
Measures of effectiveness
Preterm birth within 24, 48 and 72 hours, and one week of commencing maintenance therapy;
maternal antenatal readmission to hospital;
randomisation to birth interval.
Women's views and measures of satisfaction
Woman not satisfied (including anxiety during pregnancy and postnatal depression);
caregiver not satisfied;
woman's preferences for care;
caregiver's preferences for care.
Costs, including
Costs associated with maintenance therapy versus no therapy;
costs associated with maternal hospitalisation and length of stay;
costs associated with neonatal hospitalisation and length of stay.
Outcomes are included in the analysis if data were available according to original allocation and reasonable measures taken to minimise observer bias. Only outcomes with available data appeared in the analysis tables. Results from analyses that were not prespecified were extracted and reported but clearly labelled as not prespecified. The possibility has to be borne in mind that such outcomes are only reported because the difference between the groups, which as a result of chance, has reached conventional levels of statistical significance. In order to minimise the risk of bias the conclusions are based solely on the prestated outcomes.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (9 November 2012).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
For details of searching carried out in the previous version of the review, see:Appendix 1.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 2.
For this update we used the following methods when assessing the trials identified by the updated search (Kumru 2004; Matijevic 2006; Sakamoto 1985; Sophonsritsuk 2000).
Selection of studies
Two review authors will independently assess for inclusion all the potential studies we identify as a result of the search strategy. We will resolve any disagreement through discussion or, if required, we will consult a third person.
Data extraction and management
We will design a form to extract data. For eligible studies, two review authors will extract the data using the agreed form. We will resolve discrepancies through discussion or, if required, we will consult a third person. We will enter data into Review Manager software (RevMan 2008) and check for accuracy.
When information regarding any of the above is unclear, we will attempt to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors will independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We will resolve any disagreement by discussion or by involving a third assessor.
(1) Sequence generation (checking for possible selection bias)
We will describe for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We will assess the method as:
adequate (any truly random process, e.g. random number table; computer random number generator);
inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We will describe for each included study the method used to conceal the allocation sequence and determine whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We will assess the methods as:
adequate (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
We will describe for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We will consider that studies are at low risk of bias if they were blinded, or if we judge that the lack of blinding could not have affected the results. We will assess blinding separately for different outcomes or classes of outcomes.
We will assess the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We will describe for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We will state whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We will consider less than 20% losses to follow‐up as adequate. Where sufficient information is reported, or can be supplied by the trial authors, we will re‐include missing data in the analyses which we undertake. We will assess methods as:
adequate;
inadequate;
unclear.
(5) Selective reporting bias
We will describe for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We will assess the methods as:
adequate (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We will describe for each included study any important concerns we have about other possible sources of bias.
We will assess whether each study was free of other problems that could put it at risk of bias:
yes;
no;
unclear.
(7) Overall risk of bias
We will make explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2008). With reference to (1) to (6) above, we will assess the likely magnitude and direction of the bias and whether we consider it is likely to impact on the findings. We will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we will present results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We will include cluster‐randomised trials in the analyses along with individually randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Handbook using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity or subgroup analyses to investigate the effects of the randomisation unit.
Crossover trials are not considered to be a valid study design for the evaluation of this intervention.
Dealing with missing data
For included studies, we will note levels of attrition. We will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we will carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we will attempt to include all participants randomised to each group in the analyses, and analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial will be the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We will assess statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We will regard heterogeneity as substantial if T² is greater than zero and either I² is greater than 50% or there is a low P‐value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually, and use formal tests for funnel plot asymmetry. For continuous outcomes we will use the test proposed by Egger 1997, and for dichotomous outcomes we will use the test proposed by Harbord 2006. If we detect asymmetry in any of these tests or by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We will carry out statistical analysis using the Review Manager software (RevMan 2008). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if we detect substantial statistical heterogeneity, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. We will treat the random‐effects summary as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with its 95% confidence interval, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
If we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses:
dosage administered;
type of betamimetic agent;
gestational age maintenance therapy given;
type of therapy (other tocolytic agent) in control group.
For fixed‐effect inverse variance meta‐analyses we assess differences between subgroups by interaction tests. For random‐effects and fixed‐effect meta‐analyses using methods other than inverse variance, we will assess differences between subgroups by inspection of the subgroups’ confidence intervals; non‐overlapping confidence intervals indicate a statistically significant difference in treatment effect between the subgroups.
Sensitivity analysis
We will conduct sensitivity analyses to evaluate the effect of risk of bias associated with the quality of the included trials, and to evaluate statistical heterogeneity.
Results
Description of studies
Of the 32 trials we identified as being potentially eligible, we included 13, involving 1551 women.
Included studies
We have provided detailed descriptions of the trials found in the Characteristics of included studies table.
We included 13 randomised trials describing a total of 1551 women in this review. Ten studies were from the United States of America, one from the Netherlands (Holleboom 1996), one from Japan (Sakamoto 1985), and one from Croatia (Matijevic 2006).
Ten trials of 1307 women examined maintenance therapy for threatened preterm labour with betamimetics versus placebo or no treatment. Five of these trials used the betamimetic terbutaline (Brown 1981; How 1995; Lewis 1996; Parilla 1993; Rust 1996), while five trials used ritodrine (Creasy 1980; Holleboom 1996; Matijevic 2006; Ricci 1991; Sakamoto 1985). One trial of 71 women compared maintenance therapy with terbutaline to indomethacin (Bivins 1993). Three trials compared maintenance therapy with betamimetics to oral magnesium in 383 women; two of these used the betamimetic terbutaline (Ridgway 1990; Rust 1996) and one used ritodrine (Ricci 1991). One trial of 113 women compared maintenance therapy with terbutaline to ritodrine (Kopelman 1989).
Five studies stated that they included only singleton pregnancies (Bivins 1993; Kopelman 1989; Matijevic 2006; Ricci 1991; Sakamoto 1985) and eight studies indicated that some multiple births occurred (Brown 1981; Creasy 1980; Holleboom 1996; How 1995; Lewis 1996; Parilla 1993; Ridgway 1990; Rust 1996).
Excluded studies
We have provided the reasons for exclusion for these studies in more detail in the Characteristics of excluded studies table.
We excluded nine studies because the interventions did not meet the predefined criteria for inclusion in the review. Of these, we excluded six studies because women were administered oral betamimetic in combination with another tocolytic agent. In five of the studies (Besinger 1991; Garite 1987; Ingemarsson 1976; Spellacy 1979; W‐De Casparis 1971) the additional tocolytic agent was an intravenous betamimetic, while in two of the studies the additional agent was an intramuscular betamimetic (Larsen 1986; Spellacy 1979). We excluded one study because the betamimetic for maintenance therapy was administered intravenously (Beall 1985), one because the betamimetic was given intramuscularly (Caritis 1984) and one study compared long‐ and short‐acting formulations of the same betamimetic (Hagay 1994).
We excluded one study (Martin 1988) because the allocation of the women to treatment and control groups was according to staff preference. Thus the trial was not a randomised controlled trial.
Levy 1985 reported on the management of prelabour rupture of membranes with betamimetics, rather than premature labour, and thus we excluded this study from the review.
The outcomes measured by Penney 1980 were not prespecified in the protocol as 'Types of outcomes measured' and therefore we have not included this study in the review.
Risk of bias in included studies
Allocation concealment
Four randomised controlled trials (RCTs) used third‐party allocation through a pharmacy and thus we considered them to have adequate allocation concealment (Brown 1981; Holleboom 1996; Rust 1996; Sakamoto 1985). For the remaining eight RCTs, allocation concealment was coded B: sealed envelopes (How 1995; Kopelman 1989; Matijevic 2006; Parilla 1993; Ricci 1991); random number tables (Bivins 1993; Lewis 1996) or method of randomisation and allocation concealment not described (Creasy 1980; Ridgway 1990).
Blinding
Women and care providers were blinded in six trials (Brown 1981; Creasy 1980; Lewis 1996; Matijevic 2006; Rust 1996; Sakamoto 1985). In Ricci 1991 the examining physician was blinded for some aspects of weekly assessments. There was no description of blinding in the remaining seven trials, although the use of a placebo in Holleboom 1996 indicates that the women were blinded to treatment allocation. Blinding of women and care providers would not have been possible in How 1995 where the control group was allocated to bed rest; or in Parilla 1993 in which the control group was not treated.
Losses to follow up
Holleboom 1996, Matijevic 2006 and Lewis 1996 reported losses to follow‐up of less than 3% of participants following randomisation, and three trials reported between 3% and 9.9% of participants lost following randomisation (Bivins 1993; Brown 1981; Sakamoto 1985). Four trials reported losses to follow‐up of between 10% and 19.9% (How 1995; Kopelman 1989; Ridgway 1990; Rust 1996). In Creasy 1980, 21.4% of women were lost to follow‐up. Parilla 1993 and Ricci 1991 did not comment on any losses to follow‐up.
There was no description of sample size calculation for any of the studies.
Effects of interventions
Of the 13 included RCTs, 10 compared a betamimetic with placebo or no treatment, one compared a betamimetic with indomethacin, one compared two different betamimetics and three RCTs compared betamimetics with magnesium (some RCTs had three arms).
Although all the data prespecified in the protocol were dichotomous, some papers reported birthweight and number of days stay in the neonatal intensive care unit. As this information may be important in making clinical decisions, we chose to include these results in the analysis.
Maintenance therapy with a betamimetic versus placebo or no treatment (eight trials)
Primary outcomes
We observed no differences for very preterm birth (Analysis 1.1: one trial comparing ritodrine with placebo; risk ratio (RR) 2.81, 95% confidence interval (CI) 0.30 to 26.22, 120 women), low birthweight (Analysis 1.2: one trial comparing ritodrine with placebo; RR 0.15, 95% CI 0.02 to 1.25, 140 women), neonatal intensive care unit (NICU) admission (Analysis 1.3: one trial comparing terbutaline with placebo; RR 1.28, 95% CI 0.68 to 2.41, 260 women) or for perinatal mortality (Analysis 1.4: three trials comparing ritodrine with no treatment and three trials comparing terbutaline with no treatment; RR 2.41, 95% CI 0.86 to 6.74, 681 infants overall). No results were reported for maternal death or serious maternal morbidity.
1.1. Analysis.
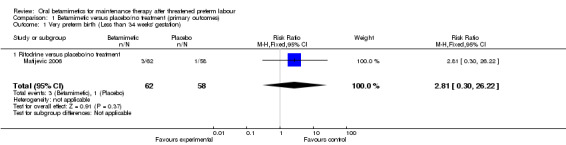
Comparison 1 Betamimetic versus placebo/no treatment (primary outcomes), Outcome 1 Very preterm birth (Less than 34 weeks' gestation).
1.2. Analysis.
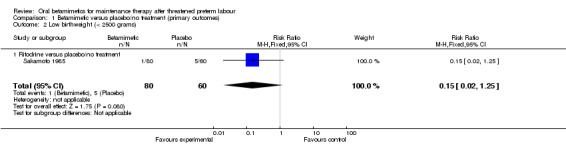
Comparison 1 Betamimetic versus placebo/no treatment (primary outcomes), Outcome 2 Low birthweight (< 2500 grams).
1.3. Analysis.
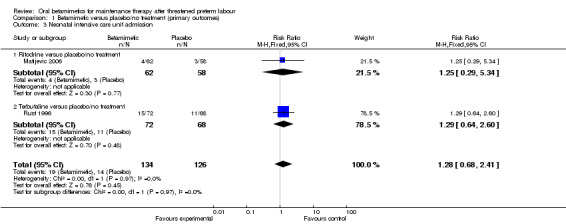
Comparison 1 Betamimetic versus placebo/no treatment (primary outcomes), Outcome 3 Neonatal intensive care unit admission.
1.4. Analysis.
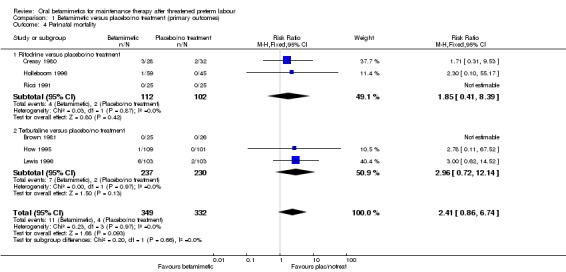
Comparison 1 Betamimetic versus placebo/no treatment (primary outcomes), Outcome 4 Perinatal mortality.
Secondary outcomes
The rate of preterm birth (less than 37 weeks) showed no significant difference (Analysis 2.1: four trials comparing ritodrine with placebo/no treatment and two trials comparing terbutaline with placebo/no treatment; RR 1.11, 95% CI 0.91 to 1.35, 644 women). We observed no differences between betamimetics and placebo/no treatment for birthweight (Analysis 2.2: mean difference (MD) 4.13 g, 95% CI ‐91.89 to 100.16, seven trials) or any category of infant morbidity that was reported:
2.1. Analysis.
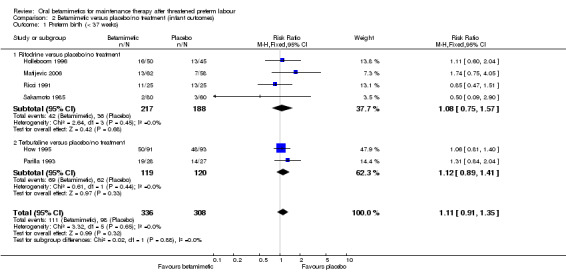
Comparison 2 Betamimetic versus placebo/no treatment (infant outcomes), Outcome 1 Preterm birth (< 37 weeks).
2.2. Analysis.
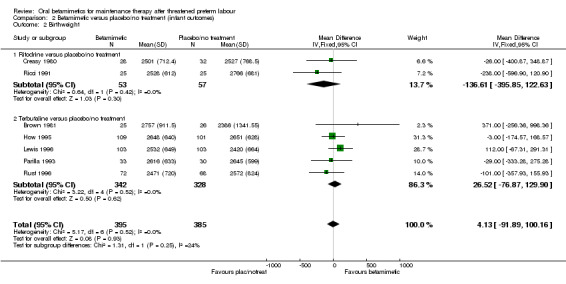
Comparison 2 Betamimetic versus placebo/no treatment (infant outcomes), Outcome 2 Birthweight.
respiratory distress syndrome; Analysis 2.3: RR 1.10, 95% CI 0.61 to 1.98 (six trials);
necrotising enterocolitis; Analysis 2.4: RR 0.98, 95% CI 0.22 to 4.28 (two trials);
intraventricular haemorrhage; Analysis 2.5: RR 0.97, 95% CI 0.27 to 3.58 (three trials);
neonatal jaundice; Analysis 2.6: RR 1.67, 95% CI 0.71 to 3.89 (one trial).
2.3. Analysis.

Comparison 2 Betamimetic versus placebo/no treatment (infant outcomes), Outcome 3 Respiratory distress syndrome.
2.4. Analysis.
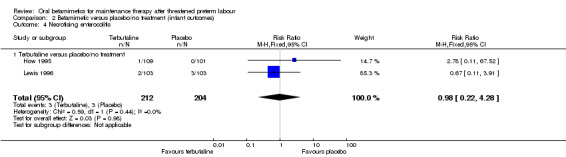
Comparison 2 Betamimetic versus placebo/no treatment (infant outcomes), Outcome 4 Necrotising enterocolitis.
2.5. Analysis.
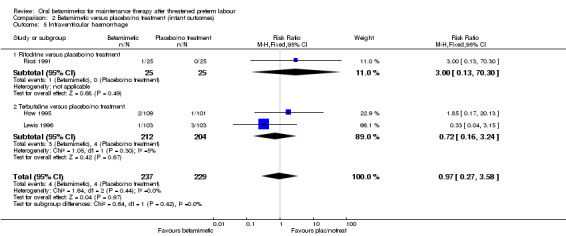
Comparison 2 Betamimetic versus placebo/no treatment (infant outcomes), Outcome 5 Intraventricular haemorrhage.
2.6. Analysis.
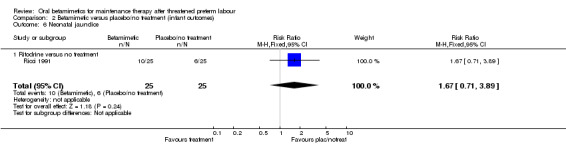
Comparison 2 Betamimetic versus placebo/no treatment (infant outcomes), Outcome 6 Neonatal jaundice.
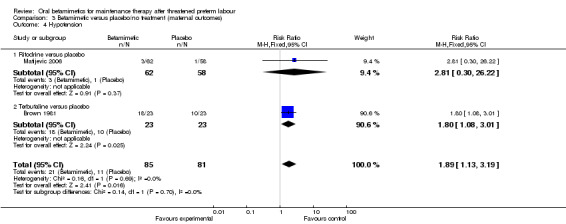
Two trials reported on maternal side effects severe enough to stop therapy (Analysis 3.1: one trial of ritodrine and one of terbutaline versus placebo/no treatment), with no significant differences seen (RR 2.71, 95% CI 0.11 to 64.79, 141 women). However, some individual side effects were significantly more common in the betamimetic group than the placebo or no treatment group:
3.1. Analysis.
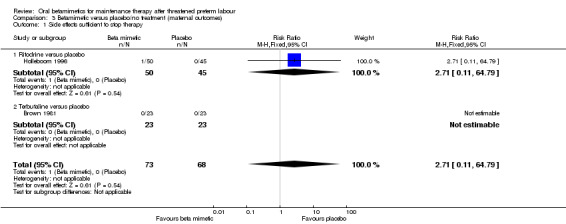
Comparison 3 Betamimetic versus placebo/no treatment (maternal outcomes), Outcome 1 Side effects sufficient to stop therapy.
tachycardia; Analysis 3.2: RR 2.13, 95% CI 1.52 to 2.98 (four trials, three ritodrine and one terbutaline);
tachypnoea; Analysis 3.3: RR 3.52, 95% CI 1.20 to 10.33 (two trials, one ritodrine and one terbutaline)
hypotension; Analysis 3.4: RR 1.89, 95% CI 1.13 to 3.19 (two trials, one ritodrine and one terbutaline);
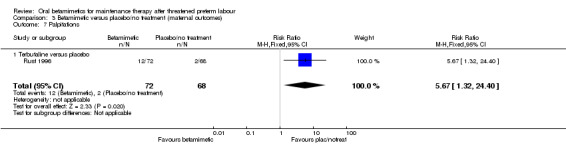
palpitations; Analysis 3.7: RR 5.67, 95% CI 1.32 to 24.40 (one trial of terbutaline).
3.2. Analysis.
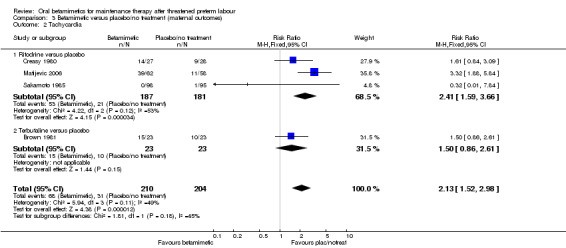
Comparison 3 Betamimetic versus placebo/no treatment (maternal outcomes), Outcome 2 Tachycardia.
3.3. Analysis.
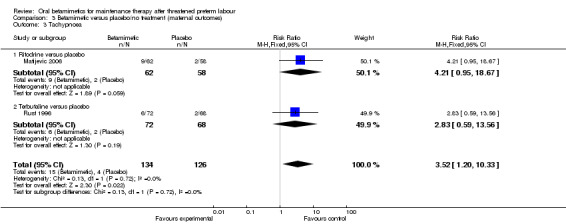
Comparison 3 Betamimetic versus placebo/no treatment (maternal outcomes), Outcome 3 Tachypnoea.
3.4. Analysis.
Comparison 3 Betamimetic versus placebo/no treatment (maternal outcomes), Outcome 4 Hypotension.
3.7. Analysis.
Comparison 3 Betamimetic versus placebo/no treatment (maternal outcomes), Outcome 7 Palpitations.
We observed no differences between the betamimetic group and the placebo or no treatment group for:
tachypnoea; Analysis 3.3: RR 2.83, 95% CI 0.59 to 13.56 (one trial of terbutaline);
nausea; Analysis 3.5: RR 0.95, 95% CI 0.43 to 2.13 (two trials of terbutaline);
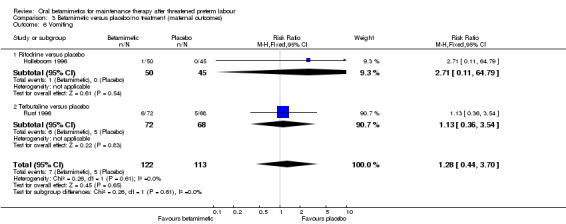
vomiting; Analysis 3.6: RR 1.28, 95% CI 0.44 to 3.70 (one trial each of ritodrine and terbutaline);
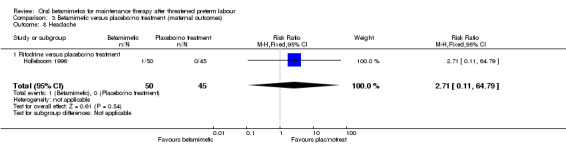
headache; Analysis 3.8: RR 2.71, 95% CI 0.11 to 64.79 (one trial of ritodrine).
3.5. Analysis.

Comparison 3 Betamimetic versus placebo/no treatment (maternal outcomes), Outcome 5 Nausea.
3.6. Analysis.
Comparison 3 Betamimetic versus placebo/no treatment (maternal outcomes), Outcome 6 Vomiting.
3.8. Analysis.
Comparison 3 Betamimetic versus placebo/no treatment (maternal outcomes), Outcome 8 Headache.
We found no difference for the outcomes of preterm birth within 24 hours, 48 hours or one week, or maternal readmission to hospital:
preterm birth within 24 hours; Analysis 4.1: RR 0.67, 95% CI 0.12 to 3.62 (one trial of terbutaline);
preterm birth within 48 hours; Analysis 4.2: RR 0.78, 95% CI 0.30 to 2.01 (one trial of terbutaline);
preterm birth within one week; Analysis 4.3: RR 0.67, 95% CI 0.40 to 1.13 (one trial each of ritodrine and terbutaline);
maternal antenatal readmission to hospital; Analysis 4.4: RR 1.11, 95% CI 0.76 to 1.62 (one trial of ritodrine, three trials of terbutaline).
4.1. Analysis.
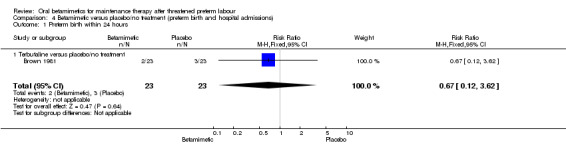
Comparison 4 Betamimetic versus placebo/no treatment (preterm birth and hospital admissions), Outcome 1 Preterm birth within 24 hours.
4.2. Analysis.
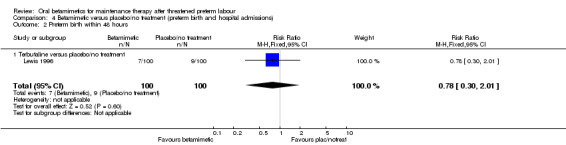
Comparison 4 Betamimetic versus placebo/no treatment (preterm birth and hospital admissions), Outcome 2 Preterm birth within 48 hours.
4.3. Analysis.

Comparison 4 Betamimetic versus placebo/no treatment (preterm birth and hospital admissions), Outcome 3 Preterm birth within 1 week.
4.4. Analysis.
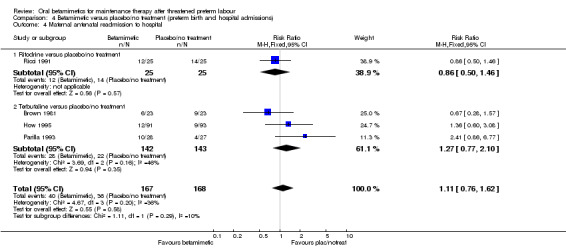
Comparison 4 Betamimetic versus placebo/no treatment (preterm birth and hospital admissions), Outcome 4 Maternal antenatal readmission to hospital.
Maintenance therapy with a betamimetic (terbutaline) versus prostaglandin synthesis inhibitor (indomethacin)
Only one RCT of 65 women made this comparison.
Primary outcomes
We observed no significant differences for very preterm birth (less than 34 weeks) between terbutaline and indomethacin (Analysis 5.1: RR 0.64, 95% CI 0.24 to 1.76). There were no neonatal deaths reported in either the terbutaline or indomethacin group (Analysis 5.2).
5.1. Analysis.
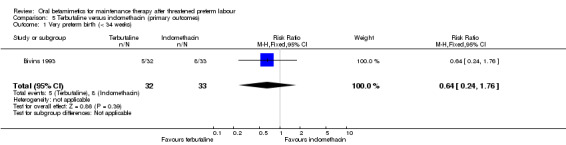
Comparison 5 Terbutaline versus indomethacin (primary outcomes), Outcome 1 Very preterm birth (< 34 weeks).
5.2. Analysis.
Comparison 5 Terbutaline versus indomethacin (primary outcomes), Outcome 2 Neonatal mortality.
Secondary outcomes
We found no differences for the reported infant outcomes:
birthweight; Analysis 6.1: MD 52.00 g, 95% CI ‐202.54 to 306.54;
need for mechanical ventilation; Analysis 6.2: RR 0.34, 95% CI 0.01 to 8.13;
neonatal intensive care unit stay; Analysis 6.3: MD ‐1.17 days, 95% CI ‐2.93 to 0.59;
no intraventricular haemorrhages were reported for either the terbutaline or the indomethacin group (Analysis 6.4).
6.1. Analysis.
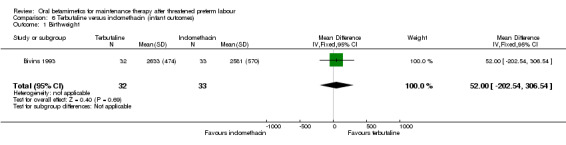
Comparison 6 Terbutaline versus indomethacin (infant outcomes), Outcome 1 Birthweight.
6.2. Analysis.

Comparison 6 Terbutaline versus indomethacin (infant outcomes), Outcome 2 Required mechanical ventilation.
6.3. Analysis.
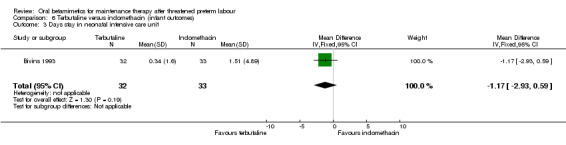
Comparison 6 Terbutaline versus indomethacin (infant outcomes), Outcome 3 Days stay in neonatal intensive care unit.
6.4. Analysis.
Comparison 6 Terbutaline versus indomethacin (infant outcomes), Outcome 4 Intraventricular haemorrhage.
For maternal outcomes, we observed no significant difference for side effects sufficient to stop therapy (Analysis 7.1: RR 3.09, 95% CI 0.13 to 73.19). We found no differences for preterm birth within one week (Analysis 8.1: RR 0.26, 95% CI 0.03 to 2.18) or need for maternal antenatal readmission to hospital (Analysis 8.2: RR 0.60, 95% CI 0.34 to 1.05).
7.1. Analysis.
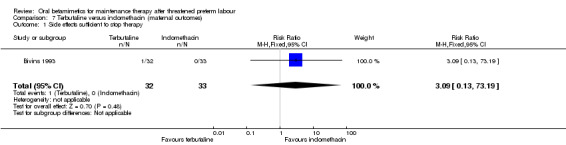
Comparison 7 Terbutaline versus indomethacin (maternal outcomes), Outcome 1 Side effects sufficient to stop therapy.
8.1. Analysis.
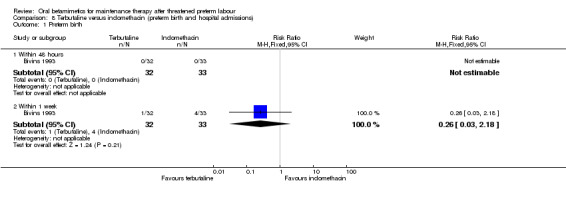
Comparison 8 Terbutaline versus indomethacin (preterm birth and hospital admissions), Outcome 1 Preterm birth.
8.2. Analysis.
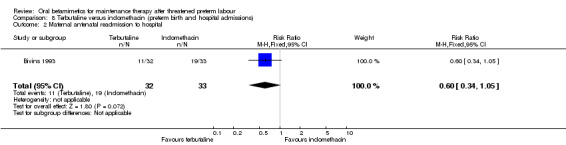
Comparison 8 Terbutaline versus indomethacin (preterm birth and hospital admissions), Outcome 2 Maternal antenatal readmission to hospital.
Maintenance therapy comparing two betamimetics (terbutaline versus ritodrine)
Only one RCT of 91 women made this comparison.
Primary outcomes
We observed no significant differences for very preterm birth (less than 34 weeks) between terbutaline and ritodrine (Analysis 9.1: RR 0.29, 95% CI 0.01 to 6.86).
9.1. Analysis.
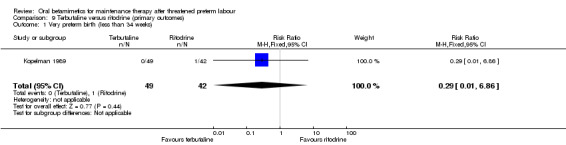
Comparison 9 Terbutaline versus ritodrine (primary outcomes), Outcome 1 Very preterm birth (less than 34 weeks).
Secondary outcomes
Similarly we found no significant differences for:
preterm birth (less than 37 weeks); Analysis 10.1: RR 0.80, 95% CI 0.44 to 1.46;
birthweight; Analysis 10.2: MD 38.30 g, 95% CI ‐210.97 to 287.57;
hyperbilirubinaemia (neonatal jaundice requiring phototherapy); Analysis 10.3: RR 1.45, 95% CI 0.84 to 2.51;
maternal tachycardia; Analysis 11.1: RR 0.57, 95% CI 0.22 to 1.47;
maternal tachypnoea; Analysis 11.2: RR 2.57, 95% CI 0.55 to 12.07;
nausea or vomiting; Analysis 11.3: RR 0.57, 95% CI 0.17 to 1.89;
need for maternal readmission to hospital; Analysis 12.1: RR 1.71, 95% CI 0.56 to 5.29.
10.1. Analysis.
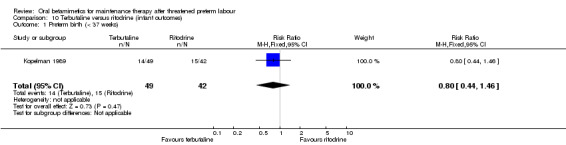
Comparison 10 Terbutaline versus ritodrine (infant outcomes), Outcome 1 Preterm birth (< 37 weeks).
10.2. Analysis.
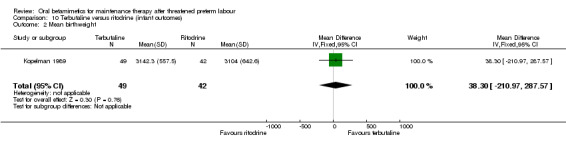
Comparison 10 Terbutaline versus ritodrine (infant outcomes), Outcome 2 Mean birthweight.
10.3. Analysis.
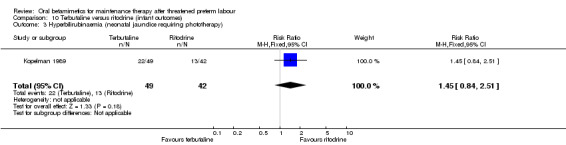
Comparison 10 Terbutaline versus ritodrine (infant outcomes), Outcome 3 Hyperbilirubinaemia (neonatal jaundice requiring phototherapy).
11.1. Analysis.
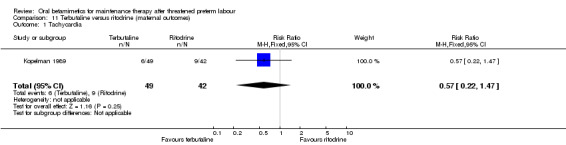
Comparison 11 Terbutaline versus ritodrine (maternal outcomes), Outcome 1 Tachycardia.
11.2. Analysis.
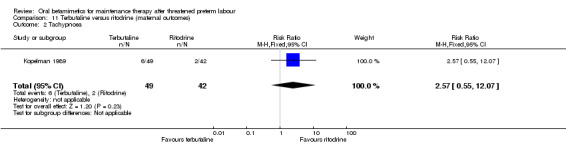
Comparison 11 Terbutaline versus ritodrine (maternal outcomes), Outcome 2 Tachypnoea.
11.3. Analysis.
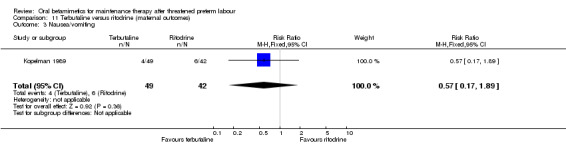
Comparison 11 Terbutaline versus ritodrine (maternal outcomes), Outcome 3 Nausea/vomiting.
12.1. Analysis.
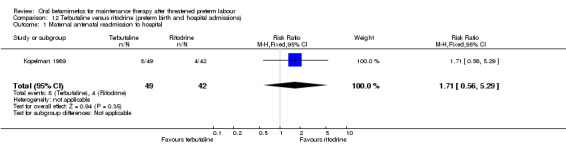
Comparison 12 Terbutaline versus ritodrine (preterm birth and hospital admissions), Outcome 1 Maternal antenatal readmission to hospital.
Maintenance therapy with a betamimetic versus magnesium
Primary outcomes
In one RCT no difference was found for NICU admissions when terbutaline was compared with magnesium; Analysis 13.1: RR 0.80, 95% CI 0.43 to 1.46, 137 women (adjusted for twin gestations). In another RCT comparing ritodrine with magnesium, there were no significant differences for perinatal mortality; Analysis 13.2: RR 0.20, 95% CI 0.01 to 3.97, 50 infants.
13.1. Analysis.
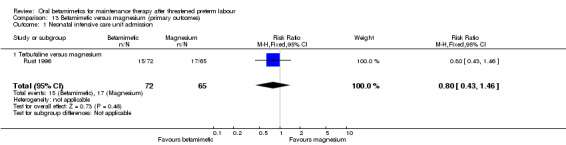
Comparison 13 Betamimetic versus magnesium (primary outcomes), Outcome 1 Neonatal intensive care unit admission.
13.2. Analysis.
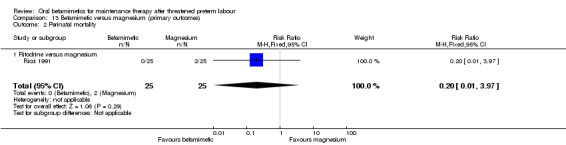
Comparison 13 Betamimetic versus magnesium (primary outcomes), Outcome 2 Perinatal mortality.
Secondary outcomes
We found no differences for preterm birth (less than 37 weeks) in two RCTs (one using ritodrine and one using terbutaline as the betamimetic); Analysis 14.1: RR 1.02, 95% CI 0.58 to 1.79, 100 infants; or for other infant outcomes:
14.1. Analysis.
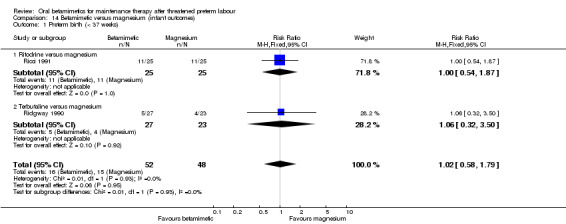
Comparison 14 Betamimetic versus magnesium (infant outcomes), Outcome 1 Preterm birth (< 37 weeks).
birthweight; Analysis 14.2: MD ‐28.80 g, 95% CI ‐187.41 to 129.81, three RCTs with a total of 239 infants;
respiratory distress syndrome; Analysis 14.3: RR 2.00, 95% CI 0.19 to 20.67, one RCT of 50 infants;
intraventricular haemorrhage; Analysis 14.4: RR 1.00, 95% CI 0.07 to 15.12, one RCT of 50 infants;
neonatal jaundice; Analysis 14.5: RR 0.91, 95% CI 0.47 to 1.75, one RCT of 50 infants.
14.2. Analysis.
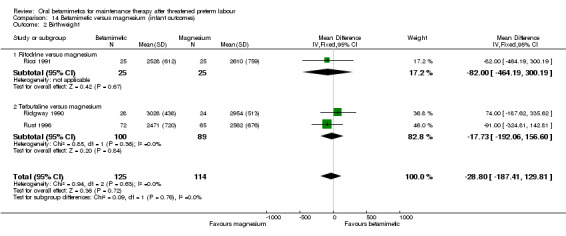
Comparison 14 Betamimetic versus magnesium (infant outcomes), Outcome 2 Birthweight.
14.3. Analysis.

Comparison 14 Betamimetic versus magnesium (infant outcomes), Outcome 3 Respiratory distress syndrome.
14.4. Analysis.
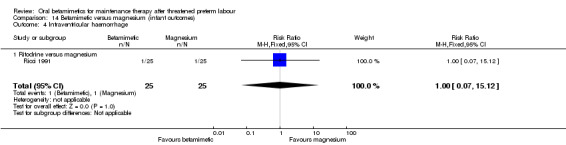
Comparison 14 Betamimetic versus magnesium (infant outcomes), Outcome 4 Intraventricular haemorrhage.
14.5. Analysis.
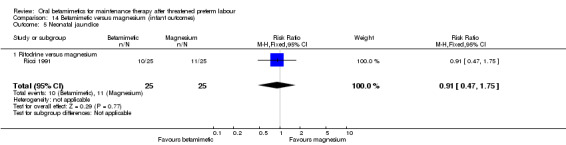
Comparison 14 Betamimetic versus magnesium (infant outcomes), Outcome 5 Neonatal jaundice.
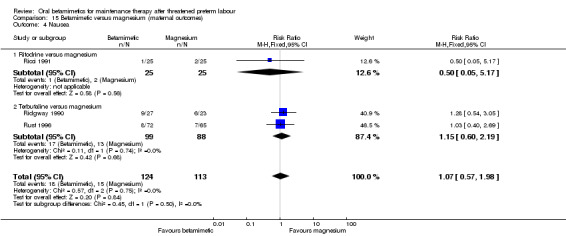
For maternal outcomes, tachycardia or palpitations were more frequent after betamimetics (either ritodrine and terbutaline) than after magnesium; Analysis 15.2: RR 5.61, 95% CI 2.41 to 13.04, three RCTs with a total of 237 women.
15.2. Analysis.
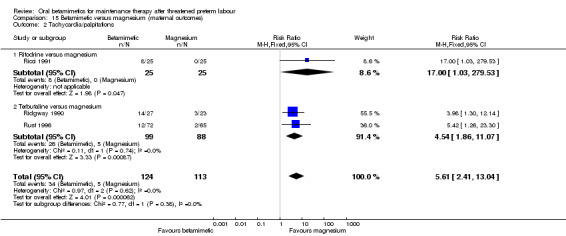
Comparison 15 Betamimetic versus magnesium (maternal outcomes), Outcome 2 Tachycardia/palpitations.
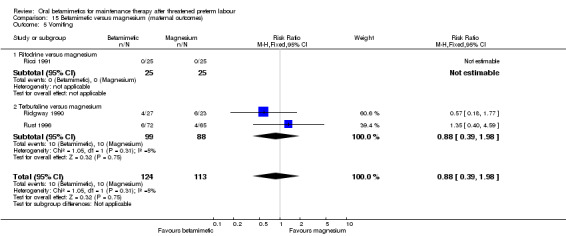
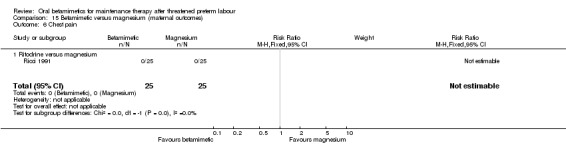
None of the other maternal outcomes showed statistically significant differences:
side effects sufficient to stop therapy; Analysis 15.1: RR 0.90, 95% CI 0.24 to 3.46, two RCTs;
tachypnoea; Analysis 15.3: RR 1.35, 95% CI 0.40 to 4.59, one RCT;
nausea; Analysis 15.4: RR 1.07, 95% CI 0.57 to 1.98, three RCTs;
vomiting; Analysis 15.5: RR 0.88, 95% CI 0.39 to 1.98, three RCTs;
chest pain; Analysis 15.6: no women in an RCT using ritodrine reported chest pain;
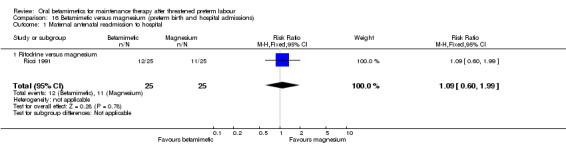
maternal antenatal readmission to hospital; Analysis 16.1: RR 1.09, 95% CI 0.60 to 1.99.
15.1. Analysis.
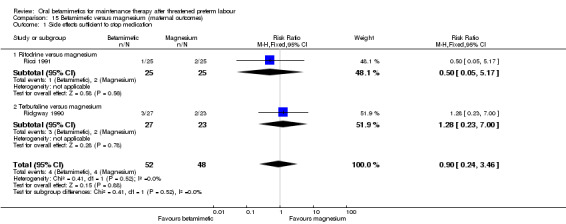
Comparison 15 Betamimetic versus magnesium (maternal outcomes), Outcome 1 Side effects sufficient to stop medication.
15.3. Analysis.
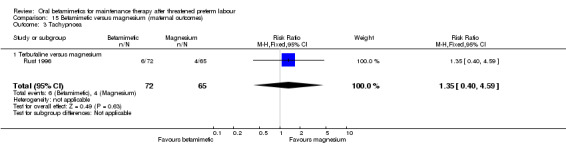
Comparison 15 Betamimetic versus magnesium (maternal outcomes), Outcome 3 Tachypnoea.
15.4. Analysis.
Comparison 15 Betamimetic versus magnesium (maternal outcomes), Outcome 4 Nausea.
15.5. Analysis.
Comparison 15 Betamimetic versus magnesium (maternal outcomes), Outcome 5 Vomiting.
15.6. Analysis.
Comparison 15 Betamimetic versus magnesium (maternal outcomes), Outcome 6 Chest pain.
16.1. Analysis.
Comparison 16 Betamimetic versus magnesium (preterm birth and hospital admissions), Outcome 1 Maternal antenatal readmission to hospital.
Discussion
Results from this review do not support the use of oral betamimetic maintenance therapy after threatened preterm labour. When betamimetics were compared to placebo or no treatment, prostaglandin synthesis inhibitors or magnesium, we observed no differences in the incidence of preterm birth or for most of the other outcomes except for some adverse effects, such as tachycardia. No results were available for women's views and measures of satisfaction or costs. Although the trials were relatively small, the results were relatively consistent between trials and the various comparisons. Systematic reviews have reported some benefit in the use of betamimetics to arrest preterm labour, in particular to delay birth and thus decrease the frequency of preterm birth and low birthweight (Anotayanonth 2004; King 1988). However, reviews assessing the use of various forms of maintenance tocolytic therapy have generally concluded there to be no benefit (Crowther 1998; Gaunekar 2004; Macones 1995; Meirowitz 1999; Nanda 2002; Sanchez‐Ramos 1999). A large number of obstetricians surveyed in Belgium and Holland reported that they would use betamimetics to prevent relapses after an acute episode had been overcome, as well as for prophylactic prevention of preterm labour (Keirse 1984a; Keirse 1984b) and it is likely that many hospitals are still using betamimetics for maintenance therapy.
It has been proposed that in efforts to prevent preterm labour, or maintain resolution of an acute episode of preterm labour, attention should be paid to understanding and treating the risk factors and causes of preterm labour. This may include treatment of vaginal infections and anaemia, and management of social issues such as nutrition, smoking, depression and maternal age (Keirse 1995b).
Oral betamimetics as maintenance therapy after an episode of threatened preterm labour have not been shown to reduce the chance of a woman giving birth early; and they are associated with an increased chance of adverse effects for the woman.
Authors' conclusions
Implications for practice.
Available evidence does not support the use of oral betamimetics for maintenance therapy after threatened preterm labour.
Implications for research.
Given the lack of beneficial effects demonstrated in the use of oral betamimetic therapy to reduce the chances of a woman giving birth preterm, and the risk of maternal adverse effects, research efforts should be directed towards other interventions that may reduce the occurrence of preterm birth. However, should future betamimetic maintenance studies be contemplated, outcome measures should include:
very preterm birth (less than 34 weeks' gestation);
low birthweight (less than 2500 g);
neonatal mortality or serious neonatal or infant morbidity;
maternal death, or serious maternal morbidity.
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2013 | Amended | We have made a minor edit to outcome 2.2 (birthweight). We have changed the graph labels around. There are no implications for the text of the review. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 12 November 2012 | New search has been performed | Search updated. |
| 12 November 2012 | New citation required but conclusions have not changed | No new trial reports identified by the updated search. |
| 25 January 2011 | New search has been performed | Search updated. Two new studies included (Matijevic 2006; Sakamoto 1985). |
| 1 October 2009 | Amended | Search updated. Four reports added to Studies awaiting classification (Kumru 2004; Matijevic 2006; Sakamoto 1985; Sophonsritsuk 2000). |
| 20 September 2008 | Amended | Converted to new review format. |
Acknowledgements
Marianna Dare for her contribution to previous versions of this review.
As part of the pre‐publication editorial process for this update, the Group's Statistical Adviser commented on the review and a member of the Consumer Network commented on the Plain Language Summary.
Many thanks to Neil Hansford for providing a translation for Sakamoto 1985.
Appendices
Appendix 1. Search strategy used in previous version of the review
Authors searched MEDLINE (1966 to August 2003) using the following search terms: preterm labo(u)r; premature labo(u)r; labor, premature (MeSH); beta‐mimetic*; betamimetic*; beta‐agonist*; betaagonist*; beta 2‐mimetic*; beta‐adrenomimetic*; beta‐sympathomimetic*; betasympathomimetic*; tocolytic‐agents (MeSH); tocolysis (MeSH); maintenance.
Appendix 2. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Bivins 1993; Brown 1981; Creasy 1980; Holleboom 1996; How 1995; Kopelman 1989; Lewis 1996; Parilla 1993; Ricci 1991; Ridgway 1990; Rust 1996.
Two review authors evaluated the potentially eligible trials against the selection criteria, with any discrepancies resolved by discussion.
We assigned quality scores for concealment of allocation using the criteria described in Section six of the Cochrane Reviewers' Handbook (Alderson 2004). A = adequate; B = unclear; C = inadequate; D = not used.
We also assessed completeness of follow up and blinding (of investigators, women or outcome assessors).
Two authors separately extracted and double‐entered the data. We resolved discrepancies by discussion. There was no blinding of authorship. Categorical data were compared using relative risks and 95% confidence intervals using a fixed‐effect model. Analyses were based on intention to treat principles. Sensitivity analyses were planned to evaluate the effect of trial quality. Statistical heterogeneity across trial results was evaluated using the I2 statistic.
We planned to conduct the following subgroup analyses:
dosage administered;
type of betamimetic agent;
gestational age maintenance therapy given;
type of therapy (other tocolytic agent) in control group.
However, there were insufficient data to do this in a meaningful way.
Data and analyses
Comparison 1. Betamimetic versus placebo/no treatment (primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Very preterm birth (Less than 34 weeks' gestation) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.30, 26.22] |
| 1.1 Ritodrine versus placebo/no treatment | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.30, 26.22] |
| 2 Low birthweight (< 2500 grams) | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.25] |
| 2.1 Ritodrine versus placebo/no treatment | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.25] |
| 3 Neonatal intensive care unit admission | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.68, 2.41] |
| 3.1 Ritodrine versus placebo/no treatment | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.29, 5.34] |
| 3.2 Terbutaline versus placebo/no treatment | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.64, 2.60] |
| 4 Perinatal mortality | 6 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.86, 6.74] |
| 4.1 Ritodrine versus placebo/no treatment | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.41, 8.39] |
| 4.2 Terbutaline versus placebo/no treatment | 3 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.72, 12.14] |
| 5 Maternal death or serious maternal morbidity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Betamimetic versus placebo/no treatment (infant outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth (< 37 weeks) | 6 | 644 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.35] |
| 1.1 Ritodrine versus placebo/no treatment | 4 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.75, 1.57] |
| 1.2 Terbutaline versus placebo/no treatment | 2 | 239 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.89, 1.41] |
| 2 Birthweight | 7 | 780 | Mean Difference (IV, Fixed, 95% CI) | 4.13 [‐91.89, 100.16] |
| 2.1 Ritodrine versus placebo/no treatment | 2 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐136.61 [‐395.85, 122.63] |
| 2.2 Terbutaline versus placebo/no treatment | 5 | 670 | Mean Difference (IV, Fixed, 95% CI) | 26.52 [‐76.87, 129.90] |
| 3 Respiratory distress syndrome | 6 | 770 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.61, 1.98] |
| 3.1 Ritodrine versus placebo/no treatment | 3 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.57, 3.73] |
| 3.2 Terbutaline versus placebo/no treatment | 3 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.43, 1.98] |
| 4 Necrotising enterocolitis | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.22, 4.28] |
| 4.1 Terbutaline versus placebo/no treatment | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.22, 4.28] |
| 5 Intraventricular haemorrhage | 3 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.27, 3.58] |
| 5.1 Ritodrine versus placebo/no treatment | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 5.2 Terbutaline versus placebo/no treatment | 2 | 416 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.16, 3.24] |
| 6 Neonatal jaundice | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.71, 3.89] |
| 6.1 Ritodrine versus no treatment | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.71, 3.89] |
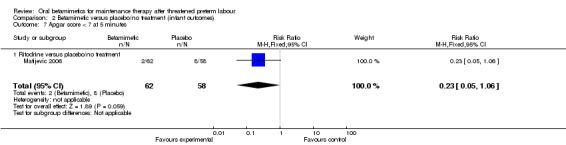
| 7 Apgar score < 7 at 5 minutes | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 1.06] |
| 7.1 Ritodrine versus placebo/no treatment | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.05, 1.06] |
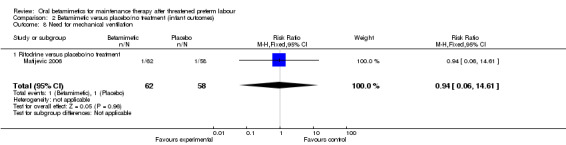
| 8 Need for mechanical ventilation | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 14.61] |
| 8.1 Ritodrine versus placebo/no treatment | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.06, 14.61] |
2.7. Analysis.
Comparison 2 Betamimetic versus placebo/no treatment (infant outcomes), Outcome 7 Apgar score < 7 at 5 minutes.
2.8. Analysis.
Comparison 2 Betamimetic versus placebo/no treatment (infant outcomes), Outcome 8 Need for mechanical ventilation.
Comparison 3. Betamimetic versus placebo/no treatment (maternal outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Side effects sufficient to stop therapy | 2 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.11, 64.79] |
| 1.1 Ritodrine versus placebo | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.11, 64.79] |
| 1.2 Terbutaline versus placebo | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Tachycardia | 4 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.52, 2.98] |
| 2.1 Ritodrine versus placebo | 3 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [1.59, 3.66] |
| 2.2 Terbutaline versus placebo | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.86, 2.61] |
| 3 Tachypnoea | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [1.20, 10.33] |
| 3.1 Ritodrine versus placebo | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.21 [0.95, 18.67] |
| 3.2 Terbutaline versus placebo | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.59, 13.56] |
| 4 Hypotension | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [1.13, 3.19] |
| 4.1 Ritodrine versus placebo | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.30, 26.22] |
| 4.2 Terbutaline versus placebo | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [1.08, 3.01] |
| 5 Nausea | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.43, 2.13] |
| 5.1 Terbutaline versus placebo | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.43, 2.13] |
| 6 Vomiting | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.44, 3.70] |
| 6.1 Ritodrine versus placebo | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.11, 64.79] |
| 6.2 Terbutaline versus placebo | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.36, 3.54] |
| 7 Palpitations | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.67 [1.32, 24.40] |
| 7.1 Terbutaline versus placebo | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.67 [1.32, 24.40] |
| 8 Headache | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.11, 64.79] |
| 8.1 Ritodrine versus placebo/no treatment | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.11, 64.79] |
Comparison 4. Betamimetic versus placebo/no treatment (preterm birth and hospital admissions).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth within 24 hours | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.62] |
| 1.1 Terbutaline versus placebo/no treatment | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.12, 3.62] |
| 2 Preterm birth within 48 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.30, 2.01] |
| 2.1 Terbutaline versus placebo/no treatment | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.30, 2.01] |
| 3 Preterm birth within 1 week | 2 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.40, 1.13] |
| 3.1 Ritodrine versus placebo | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.94] |
| 3.2 Terbutaline versus placebo | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.44, 1.29] |
| 4 Maternal antenatal readmission to hospital | 4 | 335 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.76, 1.62] |
| 4.1 Ritodrine versus placebo/no treatment | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.50, 1.46] |
| 4.2 Terbutaline versus placebo/no treatment | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.77, 2.10] |
Comparison 5. Terbutaline versus indomethacin (primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Very preterm birth (< 34 weeks) | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.24, 1.76] |
| 2 Neonatal mortality | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Terbutaline versus indomethacin (infant outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Birthweight | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | 52.0 [‐202.54, 306.54] |
| 2 Required mechanical ventilation | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 3 Days stay in neonatal intensive care unit | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.17 [‐2.93, 0.59] |
| 4 Intraventricular haemorrhage | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Terbutaline versus indomethacin (maternal outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Side effects sufficient to stop therapy | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.13, 73.19] |
Comparison 8. Terbutaline versus indomethacin (preterm birth and hospital admissions).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Within 48 hours | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Within 1 week | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.18] |
| 2 Maternal antenatal readmission to hospital | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.34, 1.05] |
Comparison 9. Terbutaline versus ritodrine (primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Very preterm birth (less than 34 weeks) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.01, 6.86] |
Comparison 10. Terbutaline versus ritodrine (infant outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth (< 37 weeks) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.44, 1.46] |
| 2 Mean birthweight | 1 | 91 | Mean Difference (IV, Fixed, 95% CI) | 38.30 [‐210.97, 287.57] |
| 3 Hyperbilirubinaemia (neonatal jaundice requiring phototherapy) | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.84, 2.51] |
Comparison 11. Terbutaline versus ritodrine (maternal outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tachycardia | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.22, 1.47] |
| 2 Tachypnoea | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [0.55, 12.07] |
| 3 Nausea/vomiting | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.17, 1.89] |
Comparison 12. Terbutaline versus ritodrine (preterm birth and hospital admissions).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal antenatal readmission to hospital | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.56, 5.29] |
Comparison 13. Betamimetic versus magnesium (primary outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neonatal intensive care unit admission | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.46] |
| 1.1 Terbutaline versus magnesium | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.43, 1.46] |
| 2 Perinatal mortality | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.97] |
| 2.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.97] |
Comparison 14. Betamimetic versus magnesium (infant outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth (< 37 weeks) | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.79] |
| 1.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.54, 1.87] |
| 1.2 Terbutaline versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.32, 3.50] |
| 2 Birthweight | 3 | 239 | Mean Difference (IV, Fixed, 95% CI) | ‐28.80 [‐187.41, 129.81] |
| 2.1 Ritodrine versus magnesium | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐82.0 [‐464.19, 300.19] |
| 2.2 Terbutaline versus magnesium | 2 | 189 | Mean Difference (IV, Fixed, 95% CI) | ‐17.73 [‐192.06, 156.60] |
| 3 Respiratory distress syndrome | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.67] |
| 3.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.67] |
| 4 Intraventricular haemorrhage | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| 4.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
| 5 Neonatal jaundice | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.47, 1.75] |
| 5.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.47, 1.75] |
Comparison 15. Betamimetic versus magnesium (maternal outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Side effects sufficient to stop medication | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.24, 3.46] |
| 1.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
| 1.2 Terbutaline versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.23, 7.00] |
| 2 Tachycardia/palpitations | 3 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.61 [2.41, 13.04] |
| 2.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.0 [1.03, 279.53] |
| 2.2 Terbutaline versus magnesium | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.54 [1.86, 11.07] |
| 3 Tachypnoea | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.40, 4.59] |
| 3.1 Terbutaline versus magnesium | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.40, 4.59] |
| 4 Nausea | 3 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.57, 1.98] |
| 4.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
| 4.2 Terbutaline versus magnesium | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.60, 2.19] |
| 5 Vomiting | 3 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.39, 1.98] |
| 5.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Terbutaline versus magnesium | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.39, 1.98] |
| 6 Chest pain | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. Betamimetic versus magnesium (preterm birth and hospital admissions).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal antenatal readmission to hospital | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.60, 1.99] |
| 1.1 Ritodrine versus magnesium | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.60, 1.99] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bivins 1993.
| Methods | RCT. Randomisation by using a list from a random number table. Blinding: not stated. Losses to follow‐up: 6/71 (8.4%) women randomised were excluded after randomisation because of no follow‐up (3), multifetal gestation (1), and fetal anomaly (2), and their outcomes were not included in the analysis. | |
| Participants | 71 women with successful tocolysis (for at least 12 hours) after treatment with magnesium sulphate who met the following inclusion criteria: 26‐32 weeks' gestation; single, live uterine pregnancy; preterm labour (at least 4 contractions in 20 minutes and progressive cervical change OR single examination with cervix at least 2 cm dilated or at least 80% effaced); amniotic membranes intact. Exclusion criteria: multifetal gestations, suspected chorioamnionitis, abruptio placentae, placenta praevia, fetal anomaly, premature rupture of membranes, pre‐eclampsia, intrauterine growth restriction, oligohydramnios, allergy to aspirin, diabetes. | |
| Interventions | After successful tocolysis with IV magnesium sulphate women were randomised to receive either oral indomethacin 25 mg every 6 hours or terbutaline sulphate 5 mg every 4 hours. Terbutaline = 30 mg/day. | |
| Outcomes | Birth < 34 weeks, mean birthweight, mean length of stay in NICU, neonatal death, mean Apgar score at 5 min, baby requiring mechanical ventilation, intraventricular haemorrhage, side effects sufficient to cease medication, preterm birth within 48 hours, preterm birth within 1 week. | |
| Notes | 2 women in the terbutaline group stopped their medication, but their outcome data were included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8.4% losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Brown 1981.
| Methods | RCT. Randomisation was by the chief pharmacist 'randomly assigning' the participants to treatment groups. Blinding: women and medical attendants were blinded. Losses to follow‐up: 5 exclusions after randomisation (9.8% excluded); 2 in the terbutaline group and 3 in the placebo group due to uncontrolled labour. | |
| Participants | 51 women who met the following inclusion criteria: premature labour between 24 and 36 weeks of gestation, painful regular uterine contractions at intervals of < 5 min. Exclusion criteria: abnormal fetal heart rate pattern; abruptio placentae; heavy vaginal bleeding from placenta praevia; maternal/fetal complications requiring immediate delivery; diabetes and chronic hypertensive disorders; IUGR; cervical dilatation; bulging or ruptured membranes. | |
| Interventions | All women received ethanol IV for 12 hours and compazine. 2 hours before the ethanol dose ended, women were randomised to receive terbutaline sulphate 5 mg or placebo orally. Treatment was continued every 6 hours until the 38th week of gestation. Terbutaline = 20 mg/day. | |
| Outcomes | Mean birthweight, respiratory distress syndrome, perinatal death, tachycardia, low blood pressure, nausea. | |
| Notes | 2 twin pregnancies in the terbutaline group and 3 twin pregnancies in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Pharmacist "Randomly assigning participants to treatment groups". |
| Allocation concealment (selection bias) | Low risk | Allocation through pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women and medical attendants blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9.8% losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Creasy 1980.
| Methods | RCT (method of randomisation unclear). Authors claim double blinding was present throughout the trial. Losses to follow‐up: 1/70 left the hospital (from placebo group); 10 women gave birth within 24 hours. Of the remaining 59 women, 4 were continued on intramuscular treatment, leaving 55 (78.6%) who were placed on oral treatment. | |
| Participants | 55 women who had been successfully treated with IM ritodrine and met the inclusion criteria. Inclusion criteria: gestation 20‐36 weeks, live fetus < 2500 g, intact membranes, 3‐4 contractions per 20 min, progressive cervical effacement or dilatation, informed consent. Exclusion criteria: abnormal vaginal bleeding, ROM, cervical dilatation > 3‐4 cm, fever of unknown origin, erythroblastosis fetalis, cardiovascular or hypertensive disease, active thyroid disease, diabetes mellitus, known drug addiction. | |
| Interventions | All women received bedrest, monitoring and IM ritodrine. If uterine activity was then controlled, the women were given ritodrine tablets (10 to 20 mg every 3 to 4 hours) or placebo until 37 to 38 weeks' gestation. Ritodrine = 30‐80 mg/day. | |
| Outcomes | Mean birthweight, mean Apgar score at 5 min, preterm birth within 24 hours, preterm birth within 1 week, palpitations and flushing, perinatal death. | |
| Notes | 1 twin gestation in the ritodrine group and 4 twin gestations in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated to be "Double blinded". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21.4% losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Holleboom 1996.
| Methods | RCT (multicentre). Randomisation: capsules were distributed in pharmacy coded drug boxes. Blinding: not stated but control consisted of placebo tablets. Losses to follow‐up: 1 mother could not be traced after moving. | |
| Participants | 95 women who met the following inclusion criteria: participated in a randomised comparison of 2 schedules of intravenous ritodrine administration (described elsewhere) where the arrest of active preterm labour occurred. Exclusion criteria: treatment with indomethacin, rupture of membranes, severe side‐effects during intravenous treatment, > 34 weeks' gestation. | |
| Interventions | At the start of maintenance treatment, all women had been on an intravenous dose of 50 microg/min ritodrine for 12 to 24 hours following successful arrest of contractions. Maintenance therapy consisted of 2 40 mg ritodrine sustained released capsules or 2 identical placebo capsules 3 times per day for 7 days. Ritodrine = 240 mg/day. | |
| Outcomes | Perinatal death, birth at < 37 weeks, side‐effects sufficient to stop medication, vomiting, preterm birth within 1 week. | |
| Notes | 7 twin pregnancies and 1 triplet pregnancy in the ritodrine group; no multiple pregnancies in the placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Medication was distributed in pharmacy coded drug boxes. |
| Allocation concealment (selection bias) | Low risk | Allocation through pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women blinded to treatment (use of placebo capsule). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
How 1995.
| Methods | RCT. Randomisation was by sealed envelopes, generated from a table of random numbers. Blinding: not stated. Losses to follow up: 28/212 women (13%) were excluded because of non‐compliance (15 in the terbutaline group and 13 in the bed rest group). | |
| Participants | 212 women who met the following inclusion criteria: between 24 and 35 completed weeks of gestation, with at least one of the following: persistent uterine contractions (at least 6 contractions per hour), progressive cervical dilatation and/or effacement, dilatation at least 2 cm and 50% effacement on the initial cervical examination in the presence of uterine contractions. Exclusion criteria: premature rupture of membranes on initial examination, preterm termination for obstetric indications, inability to reliably assess cervical change. | |
| Interventions | All women were treated with intramuscular betamethasone and intravenous magnesium sulphate until uterine quiescence was achieved for 12‐24 hours, then treated with oral terbutaline for 24‐48 hours. The women were randomised to either terbutaline or bed rest and then divided into 4 groups: group 1: those with a Bishop score of at least 5 with oral terbutaline; group 2: those with a Bishop score at least 5 without oral terbutaline; group 3: those with a Bishop score < 5 with oral terbutaline; group 4: those with a Bishop score < 5 without oral terbutaline. For the purpose of this review, the groups 1 and 3 have been combined, and groups 2 and 4 have been combined, so that treatment of terbutaline is compared with no treatment, regardless of the Bishop score. Terbutaline dose = 5‐10 mg every 4‐6 hours (20‐60 mg/day). | |
| Outcomes | Neonatal death, respiratory distress syndrome, intraventricular haemorrhage, maternal readmission to hospital. | |
| Notes | 166 singleton gestations and 18 multiple gestations (11 twin pregnancies in the terbutaline group and 6 twin and 1 triplet pregnancy in the bed rest group). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated (although blinding of women randomised to bed rest not possible). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Kopelman 1989.
| Methods | RCT. Randomisation from a random number table via sealed envelopes. Blinding: not stated. Losses to follow‐up: 22 women out of 113 (19.4%) were excluded after randomisation; 6 of these were for non‐compliance. | |
| Participants | 113 women who met the following inclusion criteria: singleton pregnancy; gestational age 20‐35 weeks; irregular uterine contractions with clear evidence of cervical dilatation and effacement; persistent, regular uterine contractions (min frequency 8/hr) with or without cervical change. Exclusion criteria: known lethal fetal anomalies, chorioamnionitis, advanced cervical effacement and dilation (completely effaced and > 5 cm dilated). | |
| Interventions | Parenteral tocolysis was given to all women (subcutaneous terbutaline and intravenous ritodrine) then women were randomly assigned to maintenance therapy with oral terbutaline (begun at 2.5 mg every 2 hours for 24 hours, then adjusted to 5 mg every 4 hours) or ritodrine (begun at 10 mg every 2 hours for 24 hours then adjusted to 20 mg every 4 hours). Terbutaline = 30 mg/day, ritodrine = 120 mg/ day. | |
| Outcomes | Mean birthweight, perinatal deaths, hyperbilirubinaemia, tachycardia, tachypnoea, nausea/vomiting, antenatal readmission. | |
| Notes | 1 woman in each group was switched to the alternate drug because of intolerable side effects. In addition, there were 6 noncompliant women ‐ 4 from the ritodrine group and 2 from the terbutaline group. 2 of the 6 women chose to give birth elsewhere and the remaining 4 elected not to continue their medication because of side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Lewis 1996.
| Methods | RCT. Randomisation was by a computer‐generated table of random numbers. Blinding: women blinded to allocation through use of a placebo. Losses to follow up: of the 203 women entered in the study, 3 were lost to follow‐up (1.5%). | |
| Participants | 203 women who met the following inclusion criteria: admitted to the labour and delivery suite between December 1990 and June 1995 with the diagnosis of preterm labour (regular uterine contractions and documented cervical change). Women had been successfully treated with parenteral tocolysis. Exclusion criteria: chorioamnionitis; vaginal bleeding suggesting abruptio placentae; medical history contraindicating use of terbutaline; premature rupture of membranes; maternal or fetal indication for delivery. | |
| Interventions | Following successful parenteral tocolysis, women were randomly assigned to terbutaline (5 mg 5 times per day) or placebo. Terbutaline = 25 mg/day. | |
| Outcomes | Perinatal death, respiratory distress syndrome, intraventricular haemorrhage, mean birthweight, birth within 48 hours, birth within 1 week. | |
| Notes | 3 twin pregnancies in each of the terbutaline and placebo groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women were blinded through the use of a placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.5% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Matijevic 2006.
| Methods | Randomisation was by a computer‐generated table of random numbers. Allocation concealment through sealed opaque envelopes. Blinding: women blinded to allocation through use of a placebo. Losses to follow up: none reported. |
|
| Participants | 120 women who met the following inclusion criteria: singleton pregnancy; gestational age 24‐34 weeks; > 5 contractions per hour for 2 hours; modified bishop score > 3. | |
| Interventions | Following successful parenteral tocolysis, women were randomly assigned to ritodrine (80 mg 3 times per day) or placebo. | |
| Outcomes | Preterm birth < 34 weeks; preterm birth < 37 weeks; preterm birth in 72 hours; infant birthweight; perinatal mortality; NICU admission; Apgar score < 7 at 5 minutes; mechanical ventilation; maternal side effects (pulmonary oedema, tremor, tachycardia, shortness of breath). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No reported losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Parilla 1993.
| Methods | RCT. Randomisation was based on a computer‐generated number table with use of opaque sealed envelopes. Blinding: not stated. Losses to follow‐up: not stated, but do not appear to have been any. | |
| Participants | 55 women who met the following inclusion criteria: admitted for preterm labour between 28 and 35 weeks' gestation with the following cervical changes: at least 1 cm decrease in length and 1 cm increase in dilation; at least 1 cm increase in dilation if the cervix is already completely effaced; at least 2 cm decrease in length without dilation; or at least 2 cm increase in dilation at the internal os without effacement. Labour was successfully arrested with magnesium sulphate. Exclusion criteria: ruptured membranes, abnormal bleeding, suspected chorioamnionitis, pre‐eclampsia, severe IUGR, fetal anomalies incompatible with life, non reassuring fetal heart rate pattern and cervical dilatation > 4 cm. | |
| Interventions | Intravenous magnesium sulphate was continued for 12 hours after the uterine contractions ceased. Women were then randomised to receive either no treatment or oral terbutaline (dose unspecified) (the first dose of which was given 30 minutes before the discontinuation of IV magnesium sulphate). | |
| Outcomes | Mean birthweight, preterm birth (< 37 weeks). | |
| Notes | 5 twin pregnancies in the terbutaline group and 3 twin pregnancies in the no treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated; do not appear to be any losses to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Ricci 1991.
| Methods | RCT. Women were randomised to 1 of 3 groups, using sealed envelopes. Blinding: examining physician was blinded for some aspects of weekly assessments. Losses to follow‐up: not stated. | |
| Participants | 75 women who met the following inclusion criteria: admitted with a diagnosis of preterm labour (initial pelvic exam of 2 cm dilatation in conjunction with 2 or more contractions per 10 minutes of at least 30 seconds' duration or a change in cervical examination detected by the same examiner over a 1‐hour period of at least 30 seconds' duration). Exclusion criteria: cervical dilatation equal to or more than 4 cm; ruptured membranes; obstetric haemorrhage; chorioamnionitis; pre‐eclampsia; eclampsia; fetal death; lethal congenital anomaly. | |
| Interventions | All women were given IV magnesium sulphate and randomised to 1 of 3 groups following a 12‐hour contraction‐free period. Group 1: 10 mg oral ritodrine every 2 hours for 24 hours, then changed to 20 mg every 4 hours; ritodrine = 120 mg/day. Group 2: 535 mg SLOW MAG (enteric‐coated magnesium chloride) every 4 hours. Group 3: observation only. | |
| Outcomes | Preterm birth (< 36 weeks), headache, tachycardia, nausea, vomiting, chest pain. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of some physician visits. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Ridgway 1990.
| Methods | RCT. Randomisation: method not described. Blinding: not stated. Losses to follow up: 10 women (16.7%) ‐ 4 in terbutaline group and 6 in the magnesium group. | |
| Participants | 60 women who met the following inclusion criteria: preterm labour successfully arrested with parenteral treatment. Preterm labour is defined as: gestational age between 25 and 35 weeks, 3 contractions in 20 minutes that persisted despite IV hydration, or any cervical change. | |
| Interventions | Once there had been uterine quiescence for 12 to 24 hours, the women were allocated to 2 groups. Group 1: magnesium oxide 200 mg orally every 3‐4 hours. Group 2: 2.5‐5 mg of terbutaline sulphate orally every 3‐4 hours. Terbutaline = 15‐40 mg/ day. | |
| Outcomes | Mean birthweight, birth at < 36 weeks, tachypnoea, nausea, vomiting. | |
| Notes | One set of twins in each group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16.7% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Rust 1996.
| Methods | RCT. Randomisation: computer‐generated table at the pharmacy. Only the medical professionals responsible for medication distribution had access to group assignment. Blinding: women and care providers were blinded to treatment group for care and outcomes assessment. Losses to follow‐up: of the 248 women randomised, 39 (17%) delivered prior to discharge and were thus not included as part of the results, and a further 4 were lost to follow‐up (17.3% in total). | |
| Participants | 248 women who met the following inclusion criteria: preterm labour (defined by gestational age 24‐34 weeks, regular contractions of > 4 per hour, documented cervical change on serial digital exams, intact membranes and absence of any medical or obstetric complications requiring delivery); arrest of preterm labour with parenteral tocolysis; uterine quiescence documented by tocodymometry; absence of further cervical change. | |
| Interventions | Following arrest of preterm labour with parenteral tocolysis the women were randomised to 1 of 3 groups: oral magnesium chloride (128 mg every 4 hours) ‐ 65 women, 69 infants; oral terbutaline sulphate (5 mg every 4 h) ‐ 72 women, 82 infants; placebo‐68 women, 71 infants. Terbutaline = 30 mg/ day. | |
| Outcomes | NICU, tachypnoea, nausea, vomiting, mean birthweight. | |
| Notes | Some results reported as being adjusted to account for multiple gestation (but no further details provided). Compliance: 55% magnesium, 64% terbutaline, 62% placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table. |
| Allocation concealment (selection bias) | Low risk | Medication distributed through pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women and caregivers blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 17.3% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
Sakamoto 1985.
| Methods | Randomisation: "allocation of drugs carried out at random by the controlled with 2 subjects from each group for a total of 6 subjects per 1 set". Blinding: women and care providers were blinded to treatment group through the use of placebo. Losses to follow‐up: 15 women were excluded and lost to follow‐up (7.7% in total). | |
| Participants | 291 women who met the following inclusion criteria: preterm labour (defined by gestational age 24‐37 weeks, diagnosis of labour, dilation cervix < 3.5 cm and < 80% effacement); arrest of preterm labour with parenteral tocolysis. | |
| Interventions | Following arrest of preterm labour with parenteral tocolysis the women were randomised to 1 of 3 groups: oral ritodrine (15 mg daily) ‐ 98 women; oral medroxyprogesterone acetate (15 mg daily) ‐ 98 women (not included in this review); placebo ‐ 95 women. | |
| Outcomes | Preterm birth < 37 weeks; infant birthweight < 2500 grams; respiratory distress syndrome; maternal tachycardia, nausea. | |
| Notes | Comparison with medroxyprogesterone acetate not included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocation of drugs carried out at random by the controlled with 2 subjects from each group for a total of 6 subjects per 1 set." |
| Allocation concealment (selection bias) | Low risk | Medication distributed by pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women and caregivers through use of placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7.7% loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unable to assess. |
| Other bias | Low risk | |
h: hour IM: intramuscular IUGR: intrauterine growth restriction IV: intravenous min: minute NICU: neonatal intensive care unit RCT: randomised controlled trial ROM: rupture of membranes w: weeks
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beall 1985 | The intervention is the use of IV, not oral, tocolytic therapy. |
| Besinger 1991 | The trial compared IV and oral ritodrine to oral indomethacin. |
| Cabero 1988 | The women in this study were randomised to oral betamimetics for treatment of an initial episode of threatened preterm labour not for maintenance therapy. |
| Caritis 1984 | Study compared 2 betamimetic agents (terbutaline and ritodrine) given intravenously rather than orally. |
| Forster 1987 | The women in this study were randomised to oral betamimetics for treatment of an initial episode of threatened preterm labour not for maintenance therapy. |
| Garite 1987 | Arrest of premature labour was randomised, but use of oral ritodrine as maintenance therapy was not. |
| Hagay 1994 | The trial compares 2 formulations of the same betamimetic tocolytic (ritodrine), rather than comparing a betamimetic with an alternative therapy. |
| Ingemarsson 1976 | The trial compared IV and oral terbutaline to IV and oral placebo. |
| Larsen 1986 | The study assessed the effect of IM ritodrine on the arrest of premature labour, as well as the effect of oral ritodrine as maintenance therapy. The women had thus received "other treatment" in addition to the oral betamimetic. |
| Levy 1985 | The population studied are women with premature rupture of membranes, not preterm labour. |
| Martin 1988 | The women in this study were randomised to the ritodrine and magnesium gluconate groups according to attending staff preference. Thus it is not a RCT. |
| Penney 1980 | Outcomes reported by the study were not those specified in the review protocol. |
| Smit 1983 | The women in this study were randomised to oral betamimetics for treatment of an initial episode of threatened preterm labour not for maintenance therapy. |
| Spellacy 1979 | The study assessed the effect of IV and IM ritodrine as well as the effect of oral ritodrine as maintenance therapy. |
| W‐De Casparis 1971 | The trial compared IV and oral ritodrine to IV and oral placebo. |
| Weisbach 1986 | The women in this study were randomised to oral betamimetics for treatment of an initial episode of threatened preterm labour not for maintenance therapy. |
| Wenstrom 1997 | The women in this study were randomised to oral betamimetics for treatment of an initial episode of threatened preterm labour not for maintenance therapy. |
IM: intramuscular IV: intravenous RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Kumru 2004.
| Methods | Stated that women were "randomly divided into two groups". |
| Participants | Women presenting with preterm labour whose contractions were controlled with parenteral ritodrine. |
| Interventions | Oral ritodrine versus no treatment. |
| Outcomes | Prolongation of pregnancy. |
| Notes | No results presented in abstract; awaiting full translation of manuscript. |
Sophonsritsuk 2000.
| Methods | Stated to be a "randomized controlled trial". |
| Participants | Women between 28 and 34 weeks' gestation presenting with preterm uterine contractions who have been successfully treated with parenteral terbutaline. |
| Interventions | Oral terbutaline (2.5 mg 4 times daily) versus no treatment. |
| Outcomes | Recurrence preterm labour; prolongation of pregnancy; birthweight; preterm birth; 5‐minute Apgar score; congenital anomaly. |
| Notes | Abstract only; recurrent preterm labour presented as percentage only; no other results presented. |
Contributions of authors
J Dodd (JD) and CA Crowther (CAC) wrote the protocol. JD, M Dare and P Middleton applied selection criteria, extracted data and drafted the review. CAC commented on drafts of the review.
Sources of support
Internal sources
Discipline of Obstetrics and Gynaecology, The University of Adelaide, Australia.
External sources
Australian Department of Health and Ageing, Australia.
-
NHMRC Neil Hamilton Fairley Fellowship, Australia.
Fellowship support (JD)
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bivins 1993 {published data only}
- Bivins H, Newman R, Fyfe D, Campbell B, Stramm S. Randomized trial of oral indomethacin and terbutaline sulfate for the long‐term suppression of preterm labor. American Journal of Obstetrics and Gynecology 1993;169:1065‐70. [DOI] [PubMed] [Google Scholar]
Brown 1981 {published data only}
- Brown S, Tejani N. Terbutaline sulfate in the prevention of recurrence of premature labor. Obstetrics & Gynecology 1981;57:22‐5. [PubMed] [Google Scholar]
Creasy 1980 {published data only}
- Creasy R, Golbus M, Laros R, Parer J, Roberts J. Oral ritodrine maintenance in the treatment of preterm labour. American Journal of Obstetrics and Gynecology 1980;137:212‐9. [DOI] [PubMed] [Google Scholar]
Holleboom 1996 {published data only}
- Holleboom C, Merkus J, Elferen L, Keirse M. Double‐blind evaluation of ritodrine sustained released for oral maintenance of tocolysis after active preterm labour. British Journal of Obstetrics and Gynaecology 1996;103:702‐5. [DOI] [PubMed] [Google Scholar]
How 1995 {published data only}
- How H, Allen S, Vogel B, Gall S, Spinnato J. Oral terbutaline in the outpatient management of preterm labor. American Journal of Obstetrics and Gynecology 1994;170:390. [DOI] [PubMed] [Google Scholar]
- How H, Hughes S, Vogel R, Gall S, Spinnato J. Oral terbutaline in the outpatient management of preterm labor. American Journal of Obstetrics and Gynecology 1995;173:1518‐22. [DOI] [PubMed] [Google Scholar]
Kopelman 1989 {published data only}
- Kopelman J, Duff P, Read J. Randomized comparison of oral terbutaline and ritodrine for preventing recurrent preterm labor. Journal of Reproductive Medicine 1989;34(3):225‐30. [PubMed] [Google Scholar]
Lewis 1996 {published data only}
- Lewis R, Mercer B, Salama M, Walsh M, Sibai B. Oral terbutaline after parenteral tocolysis: a randomized, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):315. [DOI] [PubMed] [Google Scholar]
- Lewis R, Mercer B, Salama M, Walsh M, Sibai B. Oral terbutaline after parenteral tocolysis: a randomized, double‐blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1996;175:834‐7. [DOI] [PubMed] [Google Scholar]
Matijevic 2006 {published data only}
- Matijevic R, Grgic O, Vasilj O. Ritodrine in oral maintenance of tocolysis after active preterm labor: randomized controlled trial. Croatian Medical Journal 2006;47:25‐31. [PMC free article] [PubMed] [Google Scholar]
Parilla 1993 {published data only}
- Parilla B, Dooley S, Minogue J, Socol M. The efficacy of oral terbutaline after intravenous tocolysis. American Journal of Obstetrics and Gynecology 1993;169:965‐9. [DOI] [PubMed] [Google Scholar]
- Parilla B, Dooley S, Socol M. The efficacy of oral terbutaline following parenteral tocolysis for preterm labor. American Journal of Obstetrics and Gynecology 1993;168:376. [DOI] [PubMed] [Google Scholar]
Ricci 1991 {published data only}
- Ricci J, Hariharan S, Helfgott A, Reed K, O'Sullivan M. Oral tocolysis with magnesium chloride: a randomized controlled prospective clinical trial. American Journal of Obstetrics and Gynecology 1991;165:603‐10. [DOI] [PubMed] [Google Scholar]
Ridgway 1990 {published data only}
- Ridgway L, Muise K, Wright J, Patterson R, Newton E. A prospective randomised comparison of oral terbutaline and magnesium oxide for the maintenance of tocolysis. American Journal of Obstetrics and Gynecology 1990;163:879‐82. [DOI] [PubMed] [Google Scholar]
- Ridgway LE, Muise K, Patterson RM, Wright JW, Newton E, Gibbs RS. A prospective randomized comparison of oral terbutaline and magnesium oxide for the maintenance of tocolysis. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 January 23‐27; Houston, Texas, USA. 1990:170. [DOI] [PubMed]
Rust 1996 {published data only}
- Rust O, Bofill J, Arriola R, Andrew M, Morrison J. The clinical efficacy of oral tocolytic therapy. American Journal of Obstetrics and Gynecology 1996;175:838‐42. [DOI] [PubMed] [Google Scholar]
- Rust OA, Bofil JA, Andrew M, Arriola R, Morrison JC. The clinical efficacy of oral tocolytic therapy. American Journal of Obstetrics and Gynecology 1996;174:316. [DOI] [PubMed] [Google Scholar]
Sakamoto 1985 {published data only}
- Sakamoto S. Effectiveness of oral ritodrine hydrochloride on preventing tocolysis: a multicentre double‐blinded trial. Igaku no Ayumi 1985;133(10):734‐51. [Google Scholar]
References to studies excluded from this review
Beall 1985 {published data only}
- Beall M, Edgar B, Paul R, Smith‐Wallace T. A comparison of ritodrine, terbutaline, and magnesium sulfate for the suppression of preterm labour. American Journal of Obstetrics and Gynecology 1985;153:854‐9. [DOI] [PubMed] [Google Scholar]
Besinger 1991 {published data only}
- Besinger R, Niebyl J, Keyes W, Johnson T. Randomized comparative trial of indomethacin and ritodrine for the long‐term treatment of preterm labor. American Journal of Obstetrics and Gynecology 1991;164:981‐8. [DOI] [PubMed] [Google Scholar]
Cabero 1988 {published data only}
- Cabero L, Del‐Solar J, Parra J, Salamero F, Esteban‐Altirriba J. Ritodrine retard. A new approach to treatment of threatening premature labour. 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23‐28; Rio de Janeiro, Brazil. 1988:225.
Caritis 1984 {published data only}
- Caritis S, Toig G, Heddinger L, Ashmead G. A double‐blind study comparing ritodrine and terbutaline in the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1984;150(7):7‐14. [DOI] [PubMed] [Google Scholar]
Forster 1987 {published data only}
- Forster F, During R. Comparison of the effectivity of Partusisten® and ethanol tocolysis. Part III: Comparison of long term and short term tocolysis, using Partusisten® or ethanol. Zentralblatt fur Gynakologie 1987;109:1177‐84. [PubMed] [Google Scholar]
Garite 1987 {published data only}
- Garite T, Keegan K, Freeman R, Nageotte M. A randomized trial of ritodrine tocolysis versus expectant management in patients with premature rupture of membranes at 25 to 30 weeks of gestation. American Journal of Obstetrics and Gynecology 1987;157:388‐93. [DOI] [PubMed] [Google Scholar]
Hagay 1994 {published data only}
- Hagay Z, Epstein M, Goldchmit R, Gotlib Z, Blickstein I, Zalel Y, et al. A prospective randomized clinical trial comparing a new oral sustained‐release ritodrine with conventional tablets. European Journal of Obstetrics & Gynecology and Reproductive Biology 1994;56:83‐7. [DOI] [PubMed] [Google Scholar]
- Hagay Z, Epstein M, Gotlib Z, Goldchmit C, Blickstein I, Mazkel A, et al. A prospective randomized clinical trial comparing a new oral sustained release ritodrine vs conventional ritodrine tablets. American Journal of Obstetrics and Gynecology 1992;166:366. [DOI] [PubMed] [Google Scholar]
Ingemarsson 1976 {published data only}
- Ingemarsson I. Effect of terbutaline on premature labor. A double‐blind placebo controlled study. American Journal of Obstetrics and Gynecology 1976;125(4):521‐4. [DOI] [PubMed] [Google Scholar]
Larsen 1986 {published data only}
- Larsen JF, Eldon K, Lange A, Leegaard M, Osler M, Olsen S, et al. Ritodrine in the treatment of preterm labor: second Danish Multicenter Study. Obstetrics & Gynecology 1986;67(5):607‐13. [DOI] [PubMed] [Google Scholar]
Levy 1985 {published data only}
- Levy D, Warsof S. Oral ritodrine and preterm premature rupture of membranes. Obstetrics & Gynecology 1985;66:621‐3. [PubMed] [Google Scholar]
Martin 1988 {published data only}
- Martin R, Martin J, Pryor J, Gaddy D, Wiser W, Morrison J. Comparison of oral ritodrine and magnesium gluconate for ambulatory tocolysis. American Journal of Obstetrics and Gynecology 1988;158:1440‐5. [DOI] [PubMed] [Google Scholar]
Penney 1980 {published data only}
- Penney L, Daniell W. Estimation of success in treatment of premature labor: applicability of prolongation index in a double‐blind, controlled, randomized trial. American Journal of Obstetrics and Gynecology 1980;138(3):345‐6. [DOI] [PubMed] [Google Scholar]
Smit 1983 {published data only}
- Smit DA. Efficacy of orally administered ritodrine after initial intravenous therapy [MD thesis]. Limburg: University of Limburg, 1983. [Google Scholar]
Spellacy 1979 {published data only}
- Spellacy W, Cruz A, Birk S, Buhi W. Treatment of premature labor with ritodrine: a randomized controlled study. Obstetrics & Gynecology 1979;54(2):220‐3. [PubMed] [Google Scholar]
W‐De Casparis 1971 {published data only}
- Wesselius‐De Casparis A, Thiery M, Yo Le Sian A, Baumgarten K, Brosens I, Gamisans O, et al. Results of double‐blind, multicentre study with ritodrine in premature labour. BMJ 1971;3:144‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisbach 1986 {published data only}
- Weisbach W, Wagner F, Jager KH. Oral long term tocolysis in threatening preterm delivery with Clenbuterol (Contraspasmin®, Spiropent®). A randomized clinical trial. Zentralblatt fur Gynakologie 1986;108:419‐23. [PubMed] [Google Scholar]
Wenstrom 1997 {published data only}
- Wenstrom KD, Weiner CP, Merrill D, Niebyl J. A placebo‐controlled randomized trial of the terbutaline pump for prevention of preterm delivery. American Journal of Perinatology 1997;14(2):87‐91. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kumru 2004 {published data only}
- Kumru S, Gurates B, Parmaksiz C. Efficacy of oral ritodrine treatment versus no treatment following suppression of uterine contractions by intravenous ritodrine in cases of preterm labor [Erken dogum tehdidi olgularinda ritodrinin sadece intravenoz verilmesi ile intravenoz tedaviden sonra oral idame uygulamalarinin karsilastirilmasi]. Jinekoloji Ve Obstetrik Dergisi 2004;18(3):158‐61. [Google Scholar]
Sophonsritsuk 2000 {published data only}
- Sophonsritsuk A, Jittacharoen A. The efficacy of oral terbutaline after parenteral tocolysis for prevention of recurrent preterm labour. Thai Journal of Obstetrics and Gynaecology 2000;12(4):362. [Google Scholar]
Additional references
Alderson 2004
- Alderson P, Green S, Higgins JPT, editors. Cochrane Reviewers' Handbook 4.2.2 [updated March 2004]. In: The Cochrane Library, Issue 1, 2004. Chichester, UK: John Wiley & Sons, Ltd.
Anotayanonth 2004
- Anotayanonth S, Subhedar NV, Neilson JP, Harigopal S. Betamimetics for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004352.pub2] [DOI] [PubMed] [Google Scholar]
Crowther 1998
- Crowther CA, Moore V. Magnesium maintenance therapy for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000940] [DOI] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaunekar 2004
- Gaunekar NN, Crowther CA. Maintenance therapy with calcium channel blockers for preventing preterm birth after threatened preterm labour. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD004071.pub2] [DOI] [PubMed] [Google Scholar]
Gyetvai 1999
- Gyetvai K, Hannah ME, Hodnett ED, Ohlsson A. Tocolytics for preterm labor: a systematic review. Obstetrics & Gynecology 1999;94:869‐77. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Keirse 1984a
- Keirse M J. Betamimetic drugs in the prophylaxis of preterm labour: extent and rationale of their use. British Journal of Obstetrics and Gynaecology 1984;91:431‐7. [DOI] [PubMed] [Google Scholar]
Keirse 1984b
- Keirse MJ. A survey of tocolytic drug treatment in preterm labour. British Journal of Obstetrics and Gynaecology 1984;91:424‐30. [DOI] [PubMed] [Google Scholar]
Keirse 1989
- Keirse MJNC, Grant A, King JF. Preterm labour. In: Chalmers I, Enkin M, Keirse M editor(s). Effective care in pregnancy and childbirth. Vol. 1, Oxford: Oxford University Press, 1989:694‐745. [Google Scholar]
Keirse 1995a
- Keirse MJNC. Betamimetic tocolytics in preterm labour. The Cochrane Pregnancy and Childbirth Database, The Cochrane Collaboration 1995, issue 2.
Keirse 1995b
- Keirse MJ. New perspectives for the effective treatment of preterm labour. American Journal of Obstetrics and Gynecology 1995;173:618‐28. [DOI] [PubMed] [Google Scholar]
King 1988
- King JF, Grant A, Keirse MJNC, Chalmers I. Betamimetics in preterm labour: an overview of the randomized controlled trials. British Journal of Obstetrics and Gynaecology 1988;95:211‐22. [DOI] [PubMed] [Google Scholar]
King 2003
- King JF, Flenady VJ, Papatsonis DNM, Dekker GA, Carbonne B. Calcium channel blockers for inhibiting preterm labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002255] [DOI] [PubMed] [Google Scholar]
King 2005
- King JF, Flenady V, Cole S, Thornton S. Cyclo‐oxygenase (COX) inhibitors for treating preterm labour. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001992.pub2] [DOI] [PubMed] [Google Scholar]
Macones 1995
- Macones G, Berlin M, Berlin J. Efficacy of oral beta‐agonist maintenance therapy in preterm labour: a meta analysis. Obstetrics & Gynecology 1995;85:313‐7. [DOI] [PubMed] [Google Scholar]
Meirowitz 1999
- Meirowitz N, Ananth C, Smulian J, Vintzileos A. Value of maintenance therapy with oral tocolytics: a systematic review. Journal of Maternal‐Fetal Medicine 1999;8:177‐83. [DOI] [PubMed] [Google Scholar]
Nanda 2002
- Nanda K, Cook LA, Gallo MF, Grimes DA. Terbutaline pump maintenance therapy after threatened preterm labor for preventing preterm birth. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003933] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sanchez‐Ramos 1999
- Sanchez‐Ramos L, Kaunitz A, Gaudier F, Delke I. Efficacy of maintenance therapy after acute tocolysis: a meta‐analysis. American Journal of Obstetrics and Gynecology 1999;181:484‐90. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Dodd 2006
- Dodd JM, Crowther CA, Dare MR, Middleton P. Oral betamimetics for maintenance therapy after threatened preterm labour. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003927.pub2] [DOI] [PubMed] [Google Scholar]


