Abstract
Objective:
Stimulant medications are the main treatment for Attention Deficit Hyperactivity Disorder (ADHD), but overall treatment efficacy in adults has less than a 60% response rate. This study aimed to identify neural and cognitive markers predictive of longitudinal improvement in response to stimulant treatment in drug-naïve adults with ADHD.
Method:
We used diffusion tensor imaging (DTI) and executive function measures with 36 drug-naïve adult ADHD patients in a prospective study design.
Results:
Structural connectivity (measured by fractional anisotropy, FA) in striatal regions correlated with ADHD clinical symptom improvement following stimulant treatment (amphetamine or methylphenidate) in better medication responders. A significant positive correlation was also found between working memory performance and stimulant-related symptom improvement. Higher pre-treatment working memory scores correlated with greater response.
Conclusion:
These findings provide evidence of pre-treatment neural and behavioral markers predictive of longitudinal treatment response to stimulant medications in adults with ADHD.
Keywords: ADHD, diffusion tensor imaging, working memory, drug response, stimulants
Introduction
Attention Deficit Hyperactivity Disorder (ADHD), which affects an estimated 11% of children and adolescents and 4.4% of adults (Spencer et al., 2005; Weisler et al., 2006), is the most common neurodevelopmental disorder that results in significantly reduced quality of life. This disorder not only impairs a broad range of cognitive and behavioral functions, but in about 50 to 80% of sufferers, the ADHD persists into their adulthood (Kuperman et al., 2001; Kooij et al., 2004; Weisler et al., 2006). Currently, stimulant medicine (including options for methylphenidate [MPH] and amphetamine [AMPH]) is the main FDA-approved treatment for treating ADHD. A meta-analysis of ADHD studies on children, adolescents, and adults found that, in general, adults with ADHD showed significant response efficacy with stimulant medications compared with placebo; however, the response rate in adults was weaker and more varied than that in children and adolescents (Cortese et al., 2018). Overall response rates are less than 60% among treated adult patients (Adler et al., 2008; Ginsberg & Lindefors, 2012; Kooij et al., 2004; Spencer et al., 2001, 2005; Weiss & Hechtman, 2006). Further, up to 41% of medication-naïve adults diagnosed with ADHD who started stimulant treatment needed to change their initially prescribed stimulant drug to an alternative stimulant family due to low tolerance of the drug’s side effects (Biederman, DiSalvo, Green, Woodworth, Gilfix, et al., 2021; Biederman, DiSalvo, Green, Woodworth, Law, et al., 2021).
To date, however, there is no scientific evidence available to physicians on how to predict which individual patient is more or less likely to respond to a stimulant treatment plan. Meta-analyses have concluded that demographic and clinical measures are unable to predict stimulant response (MPH; (Pagnier, 2023)). As a result, treating physicians have to make decisions based on a trial-and-error fashion. An unsuccessful initial treatment could be a discouraging experience for ADHD patients who often struggle with motivational difficulties, and therefore could prevent these patients from continuing their efforts to seek alternative treatment options.
The goal of this study was to identify sensitive neural and behavioral measures that predict longitudinal response to stimulant treatment in adults with ADHD. This predictive approach is an example of personalized or precision medicine, with the hope that specific neurobiological and behavioral profiles can identify individual patient characteristics that predict patient responses to a specific pharmacological treatment.
In this study, we employed a case-control, prospective study design and used diffusion tensor imaging (DTI), combined with an executive-function measure, to search for pre-treatment characteristics that were predictive of the longitudinal stimulant-related response efficacy in 36 drug-naïve adult patients diagnosed with ADHD. Neuroimaging and behavioral characterization occurred prior to treatment. Patients were then prescribed with one of the two most common medications for adult ADHD, MPH or AMPH, in random order, and were behaviorally assessed at two follow-up visits after 2 to 4 months of the initiation of medication. We hypothesized that striatal connectivity and executive function would be implicated in predicting longitudinal response to stimulant treatment.
ADHD neuroimaging research has attempted to identify biomarkers associated with stimulant medication treatment efficacy. One study across a broad age range of patients (6–42 years of age) found that variation in striatal (left putamen) and precuneus grey matter was associated with a response to MPH (Chang et al., 2021). Another study used a form of machine learning, support vector machines (SVMs), applied to DTI measures in children and adolescents with ADHD to predict response to MPH and found a relation between pre-treatment measures, especially in the right supramarginal gyrus, and response to treatment (Griffiths et al., 2021). One more study compared responders versus non-responders and found that responders had higher incentive salience and hedonic experience scores than non-responders and that such scores correlated with resting-state functional magnetic resonance imaging (fMRI) connectivity in the ventral striatum (Rode et al., 2023). The findings that pre-treatment striatal structure and function were related to treatment response are consistent with considerable structural and functional imaging evidence pointing toward the role of striatal regions associated with receiving ADHD stimulant medication across child, adolescent, and adult ADHD cohorts (Berberat et al., 2021; Bouziane et al., 2019; Schrantee et al., 2016; Schweren et al., 2016; Vaidya et al., 1998).
To our knowledge, the current study is the first study combining DTI technique and cognitive profiles to predict stimulant-related longitudinal symptom improvement in adult patients with ADHD who had no prior medication history. The findings of this study may not only suggest a mechanistic understanding of the neural-behavioral characteristics that are predictive of clinical outcomes in response to pharmacological treatments, but may contribute to improved clinical decision-making for treatment selection for adult ADHD.
Methods
Participants
Participants include forty medication-naïve adult patients diagnosed with ADHD consecutively after referral to the Massachusetts General Hospital (MGH) Adult ADHD Program (Table 1). Participants were assessed by the study clinicians and were confirmed that they had the diagnosis of ADHD by meeting the DSM-V criteria. Participants were excluded if they had concurrent active psychiatric or neurological comorbidities that required clinical attention, or if they had neuroimaging contraindications. Participants with mild symptoms or a history of psychiatric conditions that are commonly presented in ADHD patients such as anxiety and depression that did not require clinical attention were not excluded. The study was approved by both the MGH Institutional Review Board and the Committee on the Use of Humans as Experimental Subjects at the Massachusetts Institute of Technology (MIT).
Table 1.
ADHD Participant Demographics.
| Better responders | Worse responders | t-Test p value | |
|---|---|---|---|
| Number | 18 | 18 | |
| Gender (male/female) | 10/8 | 6/12 | |
| Age (years) | 32.3 (±6.9) | 30.1 (±7.6) | 0.55 |
| IQ (WASI) | 110.2 (±14.5) | 110.3 (±16.0) | 0.92 |
Note. Age and IQ scores did not differ between the two ADHD groups. Standard deviation in parentheses; WASI = Wechsler Abbreviated Scale of Intelligence.
Brain scanning was conducted at baseline assessment at MIT before initiation of treatment at MGH. After obtaining the brain scan, within 2 weeks, study participants were randomly assigned to receive either MPH or AMPH (N of MPH = 25; N of AMPH = 11). After the initiation of treatment, patients were naturalistically followed in the clinic for two follow-up assessment visits for an average of 51 days (mid-point follow-up) and 121 days (final follow-up). The clinical decisions on medication management including the dosing, the type of the methylphenidate or amphetamine prescribed (i.e., short-acting vs. long-acting), and switching to another stimulant medication in the case where the participants reported poor response or adverse events to the original medication were made by the study clinicians following a natural treatment course. By the end of the follow-up period, there were 15 participants receiving MPH and 21 receiving AMPH.
ADHD Clinical Progression Measurement
At baseline and at each follow up visit, participants were assessed with the NIH clinician-assessed ADHD Clinical Global Impression (CGI) Scale of Severity (CGI-S: 1 minimally ill-7 extremely ill) (Guy, 1976). All clinical assessments were conducted by the treating clinician (the senior co-author, JB) blindly to any data analysis or neuroimaging data collection. We calculated symptom improvement percentage changes from the baseline to each follow-up visit as well as between the two follow-up visits by calculating score changes over time relative to the score at the reference time point for example, subtracting the final follow-up CGI-S score from the baseline CGI-S score and then dividing this difference score by the baseline CGI-S score (Table 1). These percentage change scores were used for all analyses related to treatment efficacy, including the non-parametric whole-brain DTI tests, and the remaining ROI and post-hoc tests. All ADHD participants were categorized into one of two groups, the Better Responder Group and the Worse Responder Group (the category split cut-off score was the median score of changes in CGI-S from baseline to final follow-up). Brain-behavioral analyses were carried out across groups and within groups.
Behavior Rating Inventory of Executive Function (BRIEF-Adult Version)
We administered the BRIEF-A executive function measure, which included eight self-report clinical scales (Inhibit, Shift, Emotional Control, Initiate, Working Memory, Plan/Organize, Organization of Materials, Monitor) and an overall score of the Global Executive Composite (Roth et al., 2005). We also examined whether any of the BRIEF-A measures correlated with treatment response independent of neuroimaging.
Neuroimaging
All participants underwent MRI/fMRI scanning, including T1-weighted whole-head anatomical and diffusion-weighted imaging scans at the baseline assessment visit at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research located at the MIT campus. Imaging data were acquired on a 3 Tesla Siemens Trio scanner (Siemens, Erlangen, Germany) using a 32-channel head coil. T1 MPRAGE sequence parameters included 1.0 × 1.0 mm2 in-plane resolution, 1.0 mm slice thickness, field of view (FOV) = 256 × 256 mm2, matrix = 256 × 256, 176 slices, single echo sequence with TE = 2.34 milliseconds, TR = 2.53 seconds, an Inversion time of TI = 900 milliseconds and a read out bandwidth of 190 Hz/pixel. Simultaneous Multi Slice (SMS) BOLD was used increase the total number of slices while maintaining a TR of 1 second using SliceAcc = 6, TE = 38 milliseconds, FOV = 208 × 208 mm2, Matrix size 104 × 104 pixels, and 66 mm2 slices. The diffusion-weighted scan sequence included 1 non-diffusion weighted reference volume (b = 0) and 2 shells of 64 diffusion directions (b = 1,000 and 3,000 seconds/mm2) with acquisition parameters: 2.0 × 2.0 mm2 in-plane resolution, 2.0 mm slice thickness, FOV = 220 × 220 mm2 66 slices, SMS = 3, chosen for full brain coverage, matrix = 110 × 110, TE = 67 milliseconds, and TR = 3.0 seconds.
DTI Data Processing
Diffusion data were processed by the pre-processing algorithm of TRACULA (TRActs Constrained by UnderLying Anatomy) (Yendiki et al., 2011). The TRACULA-processed data were then fed into TBSS algorithm (Track-Based Spatial Statistics) (Smith et al., 2006). The fractional anisotropy (FA) data were non-linearly aligned to a common space. FMRIB58_FA image was used as the target image for a linear registration to the standard space. Each participant’s mean diffusion measure image was generated and thinned to create an alignment-invariant tract representation (e.g., the “mean FA skeleton”) representing the centers of all tracts common to the group. All participants’ diffusion data were then aligned on the skeleton space as 4D series and set at a threshold of 0.2 before statistical testing.
Quality Assurance
As quality control, four DTI motion measures were derived by TRACULA, including the average translation score, rotation score, signal drop-out score (percentage of bad slices), and signal drop-out severity (Yendiki et al., 2011). All participants’ translation scores were below 2 mm and rotation scores below 0.01°. Normal signal drop-out percentage (~0%) and severity (~1) were observed across all participants. None of these motion measure showed a significant correlation with the CGI change scores.
Voxelwise Brain-Symptom Analyses
Across all ADHD participants, a whole-brain voxelwise analysis was first carried out on the skeletonized FA map in TBSS using general linear models (GLM) by regressing the CGI-S change score against the FA, to identify cross-group brain regions significantly correlated with ADHD symptom improvement over time (indexed by CGI-S percentage change scores; Table 1). Then, a whole-brain two-way interaction test was carried out to identify differences of brain-behavior relationship between the two ADHD subgroups in terms of FA’s association with the longitudinal ADHD clinical severity changes (measured by CGI-S percentage changes); in other words, we identified an interaction effect between the ADHD subgroup and the level of longitudinal ADHD clinical improvement on structural brain connectivity measure. In all analyses, individual IQ, gender, ADHD onset age, and time-lapse (days) between the two CGI assessment visits were used as co-variates to control for individual variances from potential confounding factors. Non-parametric randomized permutation test was performed (number of permutations = 5,000) (Winkler et al., 2016), correcting for multiple comparisons using the threshold-free cluster enhancement method (Smith & Nichols, 2009) and controlling for the family-wise error rate (p < .1). For result presentation, the JHU DTI-based atlases were used (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/Atlases) to determine white-matter locations of significant results. Significant TBSS result images were filled into the mean FA space for presentation purposes. For any TBSS-significant findings, individual mean FA values averaged across all significant voxels in the standard space were calculated for further SPSS tests and visualization.
Assessment of Brain-Behavior Associations
To determine whether any cognitive measure of the executive functions predicted the medication-related longitudinal ADHD symptom improvement, linear correlation tests were carried out between the CGI symptom improvement score (between baseline to final follow up) and each of the BRIEF subtest scores.
Results
The final sample consisted of 36 ADHD adult patients (mean age = 31.2; 16 males and 20 females; see Table 1 for participant demographics). Four participants were excluded due to incomplete follow-up data collection or low-quality imaging data that are unanalyzable. The clinical assessment of ADHD symptom changes for each ADHD group (Table 2) showed that both groups demonstrated the largest symptom improvement from baseline to final follow-up. The Better Responder Group exhibited significant and continued ADHD symptom improvements from baseline to mid-point follow-up and from mid-point follow-up to final follow-up time points (p < .05, Paired t-tests). In contrast, the Worse Responder Group showed an initial symptom improvement from baseline to mid-point follow-up (p < .05) but not from mid-point follow-up to final follow-up (Table 3).
Table 2.
Clinical ADHD Symptom Severity at Each Time Point (CGI-S Score; N = 36).
| CGI severity score | Baseline | Mid-point follow-up | Final follow-up |
|---|---|---|---|
| Better Responders | 5.33 (±0.59) | 3.56 (±1.04) | 2.33 (±0.59) |
| Worse Responders | 5.11 (±0.47) | 4.06 (±1.26) | 4.11 (±0.90) |
Note. Higher CGI-S score reflects worse symptom severity.
Table 3.
Longitudinal ADHD Symptom Improvement (CGI % Change; N = 36).
| CGI-S change score (%) | Baseline to mid-point follow-up | Baseline to final follow-up | Mid-point to final follow-up |
|---|---|---|---|
| Better Responders | 33 (±19) % | 56 (±13) % | 30 (±21) % |
| Worse Responders | 20 (±20) % | 20 (±16) % | 8 (±41) % |
Note. Higher CGI-S percentage change score reflects greater symptom improvement over time. Better/Worse Responders were categorized based on the cut-off score using the median CGI change score from baseline to the second follow-up (score = 40%).
Neuromarkers of ADHD Symptom Improvement
The voxelwise correlation analysis between DTI measures and longitudinal ADHD symptom improvement did not reveal any significant correlations, either across groups or within each group. However, analysis of the brain-behavioral interaction tests showed a significant two-way interaction effect on the FA, between the responder group (Better/Worse Responders) and the CGI-S change score, localized to the striatal regions (p < .05, corrected; Figure 1a). Specifically, the relationships (slopes) between the FA and the longitudinal ADHD symptom improvement significantly differed between the Better and the Worse Responder Groups, with a lower degree of relationship in the Better Responder Group than in the Worse Responder Group (Figure 1b). The significant brain location is the right striatal white matter region in the posterior limb of the internal capsule along the corticospinal tract (CST). Within-group examination using the mean FA extracted from the TBSS-identified region suggests that the interaction effect was driven by a significant negative relationship between the FA and the CGI-S change in the Better Responder Group (ρ = −0.71, p < .05, Spearman’s correlation; Figure 1b), whereas in the Worse Responder Group, no significant correlation was observed.
Figure 1.
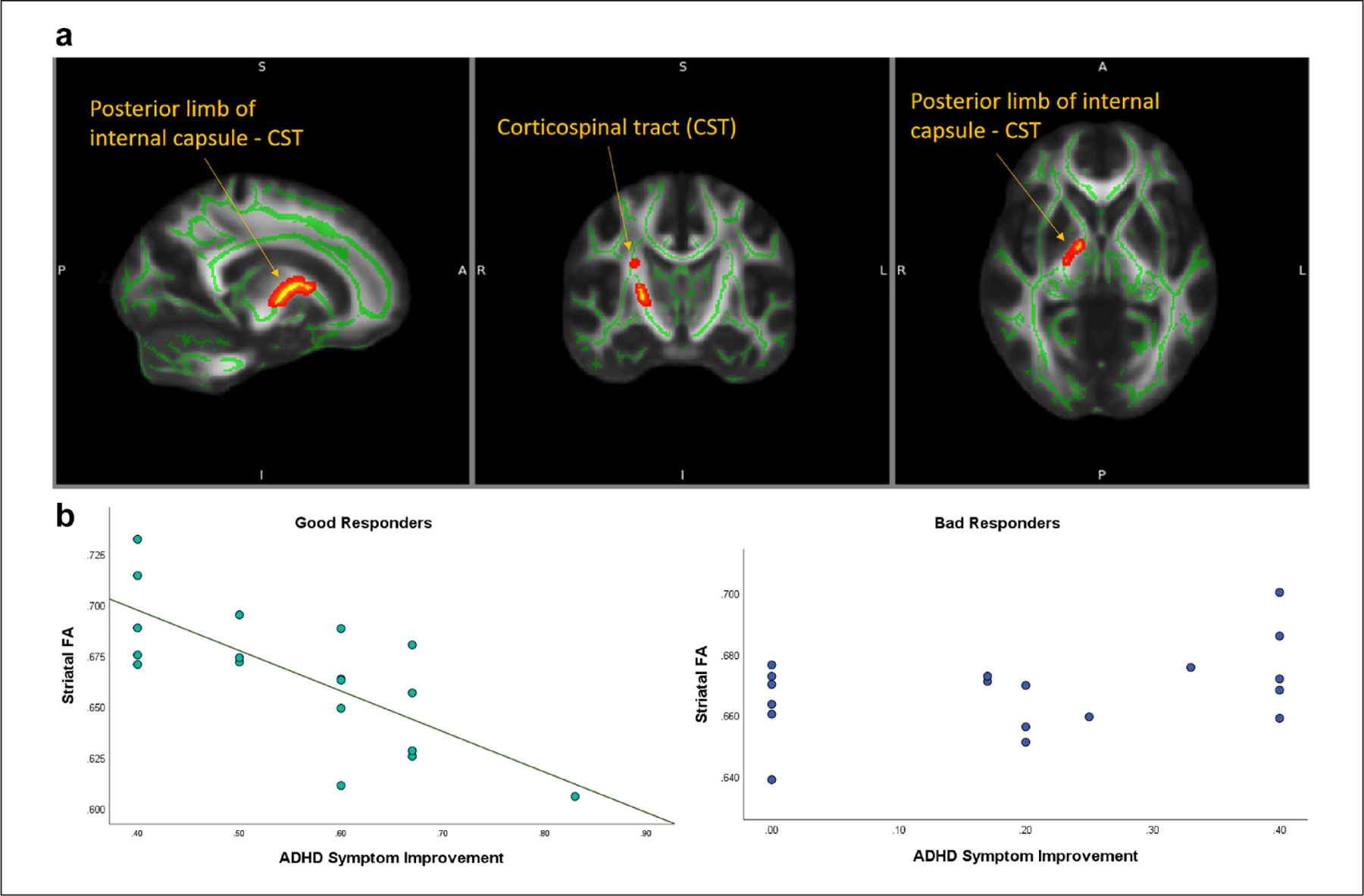
Significant striatal regions showing an interaction effect of differences in brain-symptom connections between the Better and Worse Responder Groups. (a) Whole-brain voxelwise statistics show that the degree of the relationship (slope) between the FA in the right striatal region and the longitudinal ADHD symptom improvement (CGI-S change) is significantly lower in the Better Responder Group than in the Worse Responder Group. (b) Individual mean FAs extracted from the identified striatal regions (Y-axis) are plotted against the ADHD symptom improvement (% of CGI-S change from baseline to final follow-up; X-axis). The within-group correlation assessments suggest that the identified interaction effect is driven by a significant negative relationship found between the FA and ADHD symptom improvement in the Better Responder Group.
Brain-Behavior Associations Between Executive Function and Medication Efficacy.
There was a significant positive correlation between the BRIEF Working Memory scores and the CGI improvement scores across all participants (N = 36, r = 0.42, p < .05; Spearman’s correlation test; Figure 2). Higher Working Memory scores before treatment were associated with greater medication efficacy.
Figure 2.
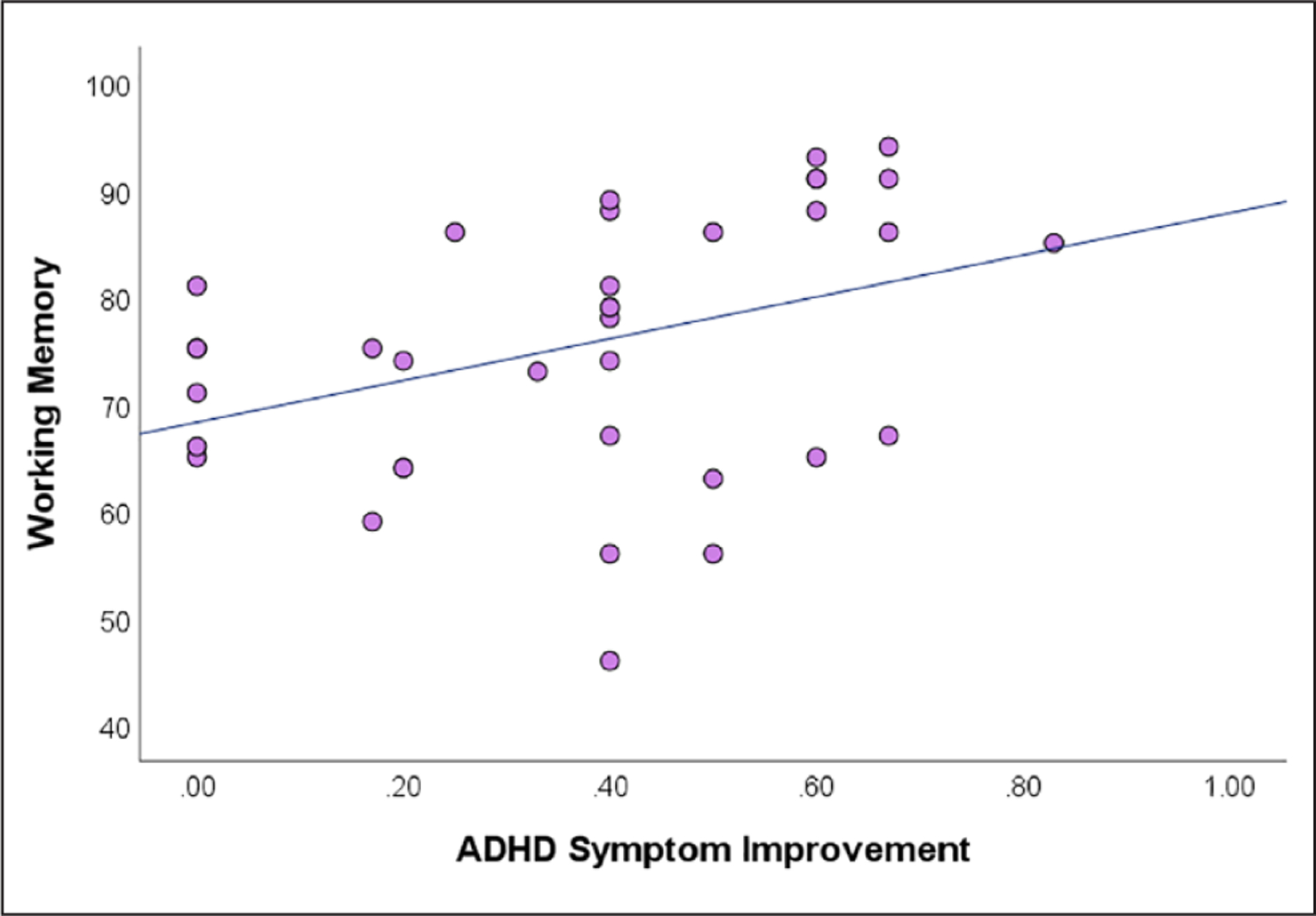
Pre-treatment working memory scores significantly correlated with the medication-induced ADHD symptom improvement across all patients.
Discussions
In drug-naïve ADHD adult patients, ADHD symptom improvement due to stimulant treatment among better responders, but not worse responders, correlated with pretreatment strength of anatomic white-matter connectivity in the striatum, with weaker connectivity associated with greater clinical improvement. Furthermore, this effect was modulated by the working memory score from the BRIEF-A measure of executive functions. The finding of this association in the striatum is consistent with prior studies noting stimulant treatment effects in the striatum of patients with ADHD (Berberat et al., 2021; Bouziane et al., 2019; Schrantee et al., 2016; Schweren et al., 2016; Vaidya et al., 1998) as well as two studies reporting that pre-treatment structural (Chang et al., 2021) or functional (Rode et al., 2023) neuroimaging measures of the striatum were associated with stimulant treatment efficacy in ADHD. Further, higher pre-treatment Working Memory scores were associated with greater treatment efficacy across all participants.
For any pre-treatment measure to support personalized or precision clinical decision making, such as whether or not to prescribe a stimulant or which stimulant to prescribe for a patient with ADHD, that measure must be informative prior to knowledge about clinical response. The DTI measures in the present study were not associated with overall stimulant response. That could have occurred for several reasons. DTI measures may simply not be predictive of response. Other sorts of neuroimaging measures, especially fMRI, have often yielded predictive information about pharmacological or behavioral treatments of mood and anxiety disorders (Doehrmann et al., 2013; Fonzo et al., 2019; Klumpp & Fitzgerald, 2018; Picó-Pérez et al., 2023; Whitfield-Gabrieli et al., 2016). In one study, DTI measures, together with fMRI and clinical measures, yielded high accuracy in predicting response to cognitive behavioral therapy in patients with social anxiety disorder (Whitfield-Gabrieli et al., 2016). Thus, DTI measures have the potential to be combined with other measures, perhaps using machine learning approaches, to enhance predictive accuracy.
Further, predictive measures of alternative treatments would be especially valuable because the clinician and patient need to know what among reasonable alternative treatments is most likely to be helpful for that individual patient. One study reported that fMRI activation in the striatum (caudate) during an inhibitory control task was associated with differential response to MPH and atomoxetine (Schulz et al., 2017). Future studies might include multiple neuroimaging, clinical, and behavioral measures to enhance predictive accuracy for individual treatments and comparative efficacy among alternative treatments.
Nevertheless, the observation that pre-treatment variation in striatal white matter was relevant to treatment efficacy is consistent with evidence about a critical role for the striatum in response to treatment. First, cross-sectional studies on ADHD have reported that smaller striatal volumes were observed in worse relative to better ADHD stimulant responders across different age groups (Chang et al., 2021; Frodl & Skokauskas, 2012; Moreno et al., 2014). Also, lower striatal dopamine transporter availability was linked to greater ADHD symptom improvement after 10 weeks of MPH treatment (Krause et al., 2005), and striatal fMRI connectivity with frontal and limbic areas was found to be lower in better MPH stimulant responders (Hong et al., 2015). It need not be the case that brain predictors of response are the same brain measures that mediate response, but it is possible that they are highly related.
The only measure that was associated with better treatment response across all patients was the Working Memory score from the BRIEF-A. Higher pre-treatment scores were associated with greater symptom reduction. These behavioral differences may also reflect brain differences because the presence or absence of working memory deficits in adult ADHD patients was associated with fMRI differences in multiple brain regions (Mattfeld et al., 2016). This finding is broadly consistent with two studies in youth reporting that another behavioral measure of executive function, performance on the Stroop color-word interference test, was a predictor of response to MPH in youth (Kim et al., 2015) and that fMRI activation in response to a Go/No-Go inhibitory task was predictive of response to MPH and atomoxetine (Schulz et al., 2017). Thus, executive functions may be useful predictors of treatment response.
The current pilot findings can be considered in the context of limitations and future directions. First, the value of studying newly diagnosed adults with ADHD is that the findings were not confounded with treatment history and were applicable to the adult age group that most often shows limited benefits of stimulant treatment. The results, however, cannot speak directly to younger patients. Second, patients were followed for a limited time. However, given the frequent non-response in adult ADHD patients in response to any particular stimulant (Adler et al., 2008; Kooij et al., 2004; Spencer et al., 2001, 2005; Ginsberg & Lindefors, 2012; Weiss & Hechtman, 2006) and the high rate of needing to switch adult patients from an initially prescribed stimulant (Biederman, DiSalvo, Green, Woodworth, Gilfix, et al., 2021; Biederman, DiSalvo, Green, Woodworth, Law, et al., 2021), it will be valuable to conduct more comprehensive studies of brain (and perhaps genetic) biomarkers and demographic and clinical measures to optimize the likelihood that ADHD patients can receive effective initial treatment. Third, treatment selection was based on individualized patient needs and profiles. On the one hand, this reflects actual clinical practice, but on the other hand, treatment regimens varied across patients.
Overall, this pilot study contributes toward development of behavioral and brain markers that may predict, to some degree, the likelihood that stimulant treatment is effective on behalf of individual patients with ADHD. Additional research is needed, but the high rate of treatment ineffectiveness in adults ought to inspire larger and more comprehensive studies to improve patient outcome.
Acknowledgments
All authors contributed to the design and execution of the study, and the writing of the paper. Dr. Biederman was the principal investigator overseeing this project.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Drs. John Gabrieli and Yuwen Hung were partially supported by Poitras Center for Psychiatric Disorders Research at the McGovern Institute for Brain Research at MIT. Dr. Mai Uchida is partially supported by the NIH K award, grant number 1K23MH122667-01. The funders had no role in the design, analysis, interpretation, or publication of this study.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Joseph Biederman has received research support from the following sources: AACAP, Feinstein Institute for Medical Research, Food & Drug Administration, Genentech, Headspace Inc., NIDA, Pfizer Pharmaceuticals, Roche TCRC Inc., Sunovion Pharmaceuticals Inc., Takeda/Shire Pharmaceuticals Inc., Tris, and NIH. Joseph Biederman’s program has received departmental royalties from a copyrighted rating scale used for ADHD diagnoses, paid by Biomarin, Bracket Global, Cogstate, Ingenix, Medavent Prophase, Shire, Sunovion, and Theravance; these royalties were paid to the Department of Psychiatry at MGH. Joseph Biederman has a US Patent (#14/027,676) for a non-stimulant treatment for ADHD, a US Patent (#10,245,271 B2) on a treatment of impaired cognitive flexibility, and a patent pending (#61/233,686) on a method to prevent stimulant abuse. Joseph Biederman was a consultant for Akili, Avekshan, Jazz Pharma, and Shire/Takeda. He received research support from Lundbeck AS and Neurocentria Inc. Through MGH CTNI, he participated in a scientific advisory board for Supernus. Dr. Mai Uchida received honoraria from Mochida Pharmaceuticals and American Physician’s Institute, received royalties for the book, “Ask The Geniuses About The Future” (Magazine House Publishing) and provided consultations to Guidepoint and Moderna. Dr. Hung, Ms. Green, Ms. Kelberman, Ms. Gaillard, Mr. Cappella, Ms. Rudberg, and Dr. Gabrieli has no disclosures to report.
Biographies
Yuwen Hung was a post-doctoral research fellow at the Massachusetts Institute of Technology.
Allison Green was a clinical research coordinator at the MGH pediatric psychopharmacology program and is currently a doctoral student in clinical psychology at Indiana University.
Caroline Kelberman was a clinical research coordinator at the MGH pediatric psychopharmacology program and is currently a doctoral student in clinical psychology at the University of Maine.
Schuyler Gaillard was a research coordinator at the Massachusetts Institute of Technology and is currently a medical student at Georgetown University School of Medicine.
James Capella was a research coordinator at the Massachusetts Institute of Technology and is currently a doctoral student in developmental psychology at the University of North Carolina—Chapel Hill.
Nicole Rudberg is a research coordinator for Dr. Yuwen Hung.
John D. E. Gabrieli is a neuroscientist and the Grover Hermann Professor of Health Sciences and Technology and Cognitive Neuroscience at the Massachusetts Institute of Technology.
Joseph Biederman was a child and adolescent psychiatrist, Professor of Psychiatry at Harvard Medical School, and Director of the Clinical and Research Program in Pediatric Psychopharmacology and Adult ADHD at Massachusetts General Hospital.
Mai Uchida is a child and adolescent psychiatrist, Associate Professor of Psychiatry at Harvard Medical School, and Director of Pediatric Depression at Massachusetts General Hospital.
References
- Adler LA, Goodman DW, Kollins SH, Weisler RH, Krishnan S, Zhang Y, & Biederman J; 303 Study Group. (2008). Double-blind, placebo-controlled study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder. The Journal of Clinical Psychiatry, 69(9), 1364–1373. 10.4088/JCP.v69n0903 [DOI] [PubMed] [Google Scholar]
- Berberat J, Huggenberger R, Montali M, Gruber P, Pircher A, Lövblad K, Killer HE, & Remonda L (2021). Brain activation patterns in medicated versus medication-naïve adults with attention-deficit hyperactivity disorder during fMRI tasks of motor inhibition and cognitive switching. BMC Medical Imaging, 21(1), 53. 10.1186/s12880-021-00579-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J, DiSalvo M, Green A, Woodworth KY, Gilfix T, Law C, Gabrieli J, & Faraone SV (2021). Rates of switching stimulants in consecutively referred medication naïve adults with ADHD. Acta Psychiatrica Scandinavica, 144(6), 626–634. 10.1111/acps.13370 [DOI] [PubMed] [Google Scholar]
- Biederman J, DiSalvo M, Green A, Woodworth KY, Law C, Gabrieli JDE, & Faraone SV (2021). How frequent is switching from an initial stimulant family to the alternative one in the clinical setting?: A pilot study of 49 consecutively referred medication-naive adults with attention-deficit/hyperactivity disorder. Journal of Clinical Psychopharmacology, 41(3), 310–314. 10.1097/JCP.0000000000001374 [DOI] [PubMed] [Google Scholar]
- Bouziane C, Filatova OG, Schrantee A, Caan MWA, Vos FM, & Reneman L (2019). White matter by diffusion MRI following methylphenidate treatment: A randomized control trial in males with attention-deficit/hyperactivity disorder. Radiology, 293(1), 186–192. 10.1148/radiol.2019182528 [DOI] [PubMed] [Google Scholar]
- Chang J, Lin H, Lv J, Tseng WI, & Gau SS (2021). Correction to: Regional brain volume predicts response to methylphenidate treatment in individuals with ADHD. BMC Psychiatry, 21(1), 102. 10.1186/s12888-021-03096-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese S, Adamo N, Del Giovane C, Mohr-Jensen C, Hayes AJ, Carucci S, Atkinson LZ, Tessari L, Banaschewski T, Coghill D, Hollis C, Simonoff E, Zuddas A, Barbui C, Purgato M, Steinhausen HC, Shokraneh F, Xia J, & Cipriani A (2018). Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. The Lancet. Psychiatry, 5(9), 727–738. 10.1016/S2215-0366(18)30269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F, Keshavan A, Triantafyllou C, Saygin ZM, Whitfield-Gabrieli S, Hofmann SG, Pollack M, & Gabrieli JD (2013). Predicting treatment response in social anxiety disorder from functional magnetic resonance imaging. Archives of General Psychiatry, 70(1), 87–97. 10.1001/2013.jamapsychiatry.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonzo GA, Etkin A, Zhang Y, Wu W, Cooper C, Chin-Fatt C, Jha MK, Trombello J, Deckersbach T, Adams P, McInnis M, McGrath PJ, Weissman MM, Fava M, & Trivedi MH (2019). Brain regulation of emotional conflict predicts antidepressant treatment response for depression. Nature Human Behaviour, 3(12), 1319–1331. 10.1038/s41562-019-0732-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, & Skokauskas N (2012). Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica, 125(2), 114–126. 10.1111/j.1600-0447.2011.01786.x [DOI] [PubMed] [Google Scholar]
- Ginsberg Y, & Lindefors N (2012). Methylphenidate treatment of adult male prison inmates with attention-deficit hyperactivity disorder: Randomised double-blind placebo-controlled trial with open-label extension. British Journal of Psychiatry, 200(1), 68–73. 10.1192/bjp.bp.111.092940 [DOI] [PubMed] [Google Scholar]
- Griffiths KR, Braund TA, Kohn MR, Clarke S, Williams LM, & Korgaonkar MS (2021). Structural brain network topology underpinning ADHD and response to methylphenidate treatment. Translational Psychiatry, 11(1), 150. 10.1038/s41398-021-01278-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy W (1976). ECDEU assessment manual for psychopharmacology / william guy. U.S. Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976. https://hdl.handle.net/2027/uc1.31210000126621 [Google Scholar]
- Hong S-B, Harrison BJ, Fornito A, Sohn C-H, Song I, & Kim J-W (2015). Functional dysconnectivity of corticostriatal circuitry and differential response to methylphenidate in youth with attention-deficit/hyperactivity disorder. Journal of Psychiatry & Neuroscience, 40(1), 46–57. 10.1503/jpn.130290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Sharma V, & Ryan ND (2015). Predicting methylphenidate response in ADHD using machine learning approaches. International Journal of Neuropsychopharmacology, 18(11), pyv052. 10.1093/ijnp/pyv052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpp H, & Fitzgerald JM (2018). Neuroimaging predictors and mechanisms of treatment response in social anxiety disorder: An overview of the amygdala. Current Psychiatry Reports, 20(10), 89. 10.1007/s11920-018-0948-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooij JJS, Burger H, Boonstra AM, Van Der Linden PD, Kalma LE, & Buitelaar JK (2004). Efficacy and safety of methylphenidate in 45 adults with attention-deficit/hyperactivity disorder. A randomized placebo-controlled double-blind cross-over trial. Psychological Medicine, 34(6), 973–982. 10.1017/S0033291703001776 [DOI] [PubMed] [Google Scholar]
- Krause J, la Fougere C, Krause K, Ackenheil M, & Dresel SH (2005). Influence of striatal dopamine transporter availability on the response to methylphenidate in adult patients with ADHD. European Archives of Psychiatry and Clinical Neuroscience, 255(6), 428–431. 10.1007/s00406-005-0602-x [DOI] [PubMed] [Google Scholar]
- Kuperman S, Perry PJ, Gaffney GR, Lund BC, Bever-Stille KA, Arndt S, Holman TL, Moser DJ, & Paulsen JS (2001). Bupropion SR vs. methylphenidate vs. placebo for attention deficit hyperactivity disorder in adults. Annals of Clinical Psychiatry, 13(3), 129–134. 10.3109/10401230109148958 [DOI] [PubMed] [Google Scholar]
- Mattfeld AT, Whitfield-Gabrieli S, Biederman J, Spencer T, Brown A, Fried R, & Gabrieli JD (2016). Dissociation of working memory impairments and attention-deficit/hyper-activity disorder in the brain. NeuroImage: Clinical, 10(C), 274–282. 10.1016/j.nicl.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno A, Duñó L, Hoekzema E, Picado M, Martín LM, Fauquet J, Vives-Gilabert Y, Bulbena A, & Vilarroya O (2014). Striatal volume deficits in children with ADHD who present a poor response to methylphenidate. European Child & Adolescent Psychiatry, 23(9), 805–812. 10.1007/s00787-013-0510-y [DOI] [PubMed] [Google Scholar]
- Pagnier M (2023). Predicting the response of children and adolescents with ADHD to methylphenidate: A systematic review. Journal of Attention Disorders, 27, 1377–1392. 10.1177/10870547231177234 [DOI] [PubMed] [Google Scholar]
- Picó-Pérez M, Fullana MA, Albajes-Eizagirre A, Vega D, Marco-Pallarés J, Vilar A, Chamorro J, Felmingham KL, Harrison BJ, Radua J, & Soriano-Mas C (2023). Neural predictors of cognitive-behavior therapy outcome in anxiety-related disorders: A meta-analysis of task-based fMRI studies. Psychological Medicine, 53(8), 3387–3395. 10.1017/S0033291721005444 [DOI] [PubMed] [Google Scholar]
- Rode J, Runnamo R, Thunberg P, & Msghina M (2023). Salience and hedonic experience as predictors of central stimulant treatment response in ADHD – A resting state fMRI study. Journal of Psychiatric Research, 163, 378–385. 10.1016/j.jpsychires.2023.05.073 [DOI] [PubMed] [Google Scholar]
- Roth RM, Isquith PK, & Gioia GA (2005). BRIEF-A: Behavior rating inventory of executive function–adult version: Professional manual. Psychological Assessment Resources. [Google Scholar]
- Schrantee A, Tamminga HGH, Bouziane C, Bottelier MA, Bron EE, Mutsaerts HMM, Zwinderman AH, Groote IR, Rombouts SA, Lindauer RJ, Klein S, Niessen WJ, Opmeer BC, Boer F, Lucassen PJ, Andersen SL, Geurts HM, & Reneman L (2016). Age-dependent effects of methylphenidate on the human dopaminergic system in young vs adult patients with attention-deficit/hyperactivity disorder: A randomized clinical trial. JAMA Psychiatry (Chicago, Ill.), 73(9), 955–962. 10.1001/jamapsychiatry.2016.1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KP, Bédard AV, Fan J, Hildebrandt TB, Stein MA, Ivanov I, Halperin JM, & Newcorn JH (2017). Striatal activation predicts differential therapeutic responses to methylphenidate and atomoxetine. Journal of the American Academy of Child and Adolescent Psychiatry, 56(7), 602–609.e2. 10.1016/j.jaac.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Schweren LJS, Hartman CA, Zwiers MP, Heslenfeld DJ, Franke B, Oosterlaan J, Buitelaar JK, & Hoekstra PJ (2016). Stimulant treatment history predicts frontal-striatal structural connectivity in adolescents with attention-deficit/hyperactivity disorder. European Neuropsychopharmacology, 26(4), 674–683. 10.1016/j.euroneuro.2016.02.007 [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, & Behrens TE (2006). Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage (Orlando, Fla.), 31(4), 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Smith SM, & Nichols TE (2009). Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage (Orlando, Fla.), 44(1), 83–98. 10.1016/j.neuroimage.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Doyle R, Surman C, Prince J, Mick E, Aleardi M, Herzig K, & Faraone S (2005). A large, double-blind, randomized clinical trial of methylphenidate in the treatment of adults with attention-deficit/hyperactivity disorder. Biological Psychiatry (1969), 57(5), 456–463. 10.1016/j.biopsych.2004.11.043 [DOI] [PubMed] [Google Scholar]
- Spencer T, Biederman J, Wilens T, Faraone S, Prince J, Gerard K, Doyle R, Parekh A, Kagan J, & Bearman SK (2001). Efficacy of a mixed amphetamine salts compound in adults with attention-deficit/hyperactivity disorder. Archives of General Psychiatry, 58(8), 775–782. 10.1001/archpsyc.58.8.775 [DOI] [PubMed] [Google Scholar]
- Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, & Gabrieli JD (1998). Selective effects of methylphenidate in attention deficit hyperactivity disorder: A functional magnetic resonance study. Proceedings of the National Academy of Sciences USA, 95(24), 14494–14499. 10.1073/pnas.95.24.14494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisler RH, Biederman J, Spencer TJ, Wilens TE, Faraone SV, Chrisman AK, Read SC, & Tulloch SJ (2006). Mixed amphetamine salts extended-release in the treatment of adult ADHD: A randomized, controlled trial. CNS Spectrums, 11(8), 625–639. 10.1017/S1092852900013687 [DOI] [PubMed] [Google Scholar]
- Weiss M, & Hechtman L (2006). A randomized double-blind trial of paroxetine and/or dextroamphetamine and problem-focused therapy for attention-deficit/hyperactivity disorder in adults. The Journal of Clinical Psychiatry, 67(4), 611–619. 10.4088/JCP.v67n0412 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ghosh SS, Nieto-Castanon A, Saygin Z, Doehrmann O, Chai XJ, Reynolds GO, Hofmann SG, Pollack MH, & Gabrieli JD (2016). Brain connectomics predict response to treatment in social anxiety disorder. Molecular Psychiatry, 21(5), 680–685. 10.1038/mp.2015.109 [DOI] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Brooks JC, Tracey I, Smith SM, & Nichols TE (2016). Non-parametric combination and related permutation tests for neuroimaging. Human Brain Mapping, 37(4), 1486–1511. 10.1002/hbm.23115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T, Jbabdi S, Gollub R, & Fischl B (2011). Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Frontiers in Neuroinformatics, 5, 23. 10.3389/fninf.2011.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]


