Abstract
Autoimmune enteropathy is a rare cause of chronic intractable diarrhea and is present in <1 in 100,000 infants. We report the case of a 9‐month‐old boy who presented with intractable diarrhea and vomiting. Genetic panel testing revealed a STAT3 heterozygous mutation in exon 6, suggesting infantile‐onset multisystem autoimmune disease‐1. The patient was initially treated with steroids and sulfasalazine. However, on tapering steroids, he had another episode of diarrhea and was subsequently put on baricitinib to which he responded.
Keywords: baricitinib, gastroenteritis, malabsorption, STAT3, STAT3 mutation
1. INTRODUCTION
A rare cause of chronic, intractable diarrhea is autoimmune enteropathy (AIE) which presents in <1 in 100,000 infants. 1 It falls under the spectrum of Infantile‐onset multisystem autoimmune disease‐1. 2 We present a 9‐month‐old male who presented with intractable diarrhea and vomiting resistant to dietary modifications and common treatment modalities.
2. CASE SUMMARY
A 9‐month‐old male, second by birth order, born out of nonconsanguineous marriage presented with diarrhea for 1 month and vomiting for 2 weeks. On presentation to us, weight was 5.8 kg (<3rd Standard Deviation [SD] as per WHO growth chart), and length was 70 cm (0 to −1 SD as per WHO growth chart). General and systemic examination was normal including no hepatosplenomegaly, except for perianal rash which was suggestive of osmotic diarrhea. The child had been weaned onto solids including gluten‐containing products before presentation. The child was then diagnosed with persistent diarrhea with severe acute malnutrition (SAM). Blood investigations are depicted in Table 1. Stool routine microscopy and stool culture sensitivity were normal. Blood culture was negative. Tissue transglutaminase IgA was negative (1.69 AU/mL, normal up to 8 AU/mL) and had normal levels of immunoglobulins IgM, IgG, IgA, and IgE.
Table 1.
Investigations of the patient.
| Investigations | Observed value | Normal values |
|---|---|---|
| Hemoglobin (gm/dL) | 11.8 | 12–15.5 |
| Total leukocyte count (cells/cumm) | 21,500 | 4500–11,000 |
| Platelet (cells/cumm) | 660,000 | 150,000–450,000 |
| SGOT (U/L) | 35.7 | <40 |
| SGPT (U/L) | 14.5 | <40 |
| GGT (U/L) | 10 | 5–40 |
| BUN (mg/dL)/Creatinine (mg/dL) | 8.9/0.35 | 7–30/0.7–1.2 |
| pH | 7.18 | 7.35–7.45 |
| HCO3− (mEq/L) | 6 | 22–26 |
| C‐reactive protein (mg/L) | 1.7 | |
| Sodium (mmol/L) | 143 | 135–145 |
| Potassium (mmol/L) | 3.4 | 3.5–5 |
| Chloride (mmol/L) | 111 | 95–105 |
| Free T3 (pg/mL) | 2.17 | 2.0–4.4 |
| Free T4 (ng/dL) | 0.977 | 0.8–1.8 |
| TSH (uIU/mL) | 1.43 | 0.2–4.2 |
| HbA1C | 4.5% | <6% |
| Immunoglobulin G (g/l) | 2.35 | 2.46–9.04 |
| Immunoglobulin A (g/l) | 0.72 | 0.16–0.5 |
| Immunoglobulin M (g/l) | 0.9 | 0.32–1.32 |
| Immunoglobulin E (IU/ml) | 17.80 | 0–34 |
| Absolute B Lymphocytes (cumm) | 1156 | 610–2600 |
| Absolute T Lymphocytes (cumm) | 4752 | 1900–5900 |
| Absolute Th Lymphocytes (cumm) | 2312 | 1400–4300 |
| Absolute Tc Lymphocytes (cumm) | 1991 | 500–1700 |
| Absolute NK cell (cumm) | 450 | 160–950 |
Abbreviations: BUN, blood urea nitrogen; GGT, gamma‐glutamyl transferase; NK, natural killer; SGOT, serum glutamic‐oxaloacetic transaminase; SGPT, serum glutamate pyruvate transaminas; TSH, thyroid stimulating hormone.
The patient was started on intravenous (IV) antibiotics, IV fluids, and starter formula for SAM (75 kcal and 0.9 g protein/100 mL of milk) diet, on which the diarrhea did not subside, hence shifted to a low lactose diet and multivitamins. Despite that, the patient had loose stools, so stool multiplex polymerase chain reaction was sent (which came back negative) and an amino‐acid‐based formula was started along with IV fluids while stopping all sugar‐containing syrups.
Upper and lower gastrointestinal endoscopy showed diffuse whitish discoloration in the mucosa of the second part of the duodenum along with absence of villi and scalloping sign (Figure 1) and diffuse edematous colonic mucosa, and histopathology showed chronic active inflammation of the duodenum with moderate villous blunting (Figure 2) and mild chronic active colitis. Sulphamethoxazole and trimethoprim (Septran) combination was started with the continuation of an amino acid‐based formula due to suspicion of tropical sprue. Diarrhea started decreasing and he was discharged from the hospital 2 months later, had a weight of 6.5 kg at the time of discharge, and passed two to three stools per day. He was continued on an amino‐acid‐based formula. The patient was monitored weekly for weight gain, food input, and output.
Figure 1.
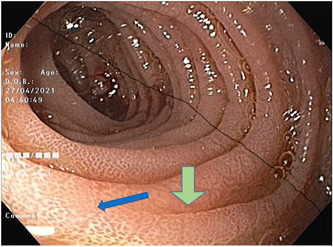
Upper gastrointestinal scopy showing diffuse whitish discoloration in D2 mucosa with the blue arrow pointing at an area of absent villi. Green arrow showing scalloping sign.
Figure 2.
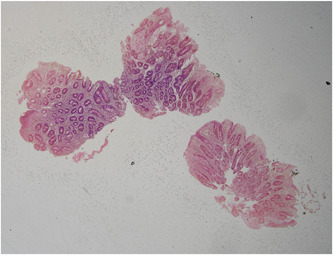
Histopathology of duodenum showing chronic active inflammation of the duodenum with moderate villous blunting. Surface epithelium shows focal areas of erosion. No significant number of intraepithelial lymphocytes (<15 IEL/100 enterocytes). Focal cryptitis noted. No crypt abscess seen. Lamina propria shows moderate to dense inflammatory infiltrate comprising of many polymorphs, lymphocytes, plasma cells, and few histiocytes. No evidence of Helicobacter pylori, parasite, granuloma, atypia or malignancy, apoptotic bodies. CD3 not done.
The patient presented 2 months later, when he was 13 months, with intractable diarrhea for a week and static weight after the introduction of beetroot with signs of dehydration and hypovolemic shock. He was managed in the intensive care unit with mechanical ventilation for 5 days. Persistent diarrhea prompted a clinical exome sequencing, and a heterozygous mutation in Signal Transducer and Activator of Transcription (STAT) 3 (ENST0000677421.1) in exon 6, variant c.487 A > G (p. Lys163Glu) suggesting Infantile Onset Multisystem Autoimmune Disease‐1 (autosomal dominant) was found. A diagnosis of AIE was reached. The result came in 4 weeks. Thyroid function tests and glycosylated hemoglobin were normal. The patient was started on oral prednisolone at 1 mg/kg/day of steroids for 10 days followed by 2 mg/kg/day for the next 13 days but even after that, the patient showed persistent diarrhea, presence of fat globules in the stool, and a lack of weight gain. The patient was then started on oral sulphasalazine (50 mg/kg/day). After a month on the above therapy, there was good clinical response with resolution of diarrhea and consistent weight gain at 15 months of age. Steroids were slowly tapered and stopped after 10 months of initiation and sulfasalazine was continued but diarrhea relapsed 1 month after stopping steroid at 2 years of age and there was weight loss. Oral prednisolone at 2 mg/kg/day was restarted immediately and baricitinib (a Janus Kinase [JAK] inhibitor) at a dose of 0.08 mg/kg/day was introduced. The steroid was tapered after 3 weeks and after 3 months of starting baracitinib, weight was 9.7 kg and diarrhea was under control.
3. DISCUSSION
The clinical manifestations of AIE included intractable diarrhea and malabsorption with unresponsiveness to diet modifications. 3 The diagnostic criteria have been expanded in a recent study of adults with AIE to include chronic intractable diarrhea (lasting more than 6 weeks) with malabsorption and partial or total blunting of the small bowel villi, deep crypt lymphocytosis, increased crypt apoptotic bodies, minimal intraepithelial lymphocytosis, and the exclusion of other causes of villous atrophy. 4 As seen in our patient, the workup for pathogenic bacteria and parasites is negative, and the diarrhea is typically nonbloody and frequently accompanied by steatorrhea. 5 In a study by Ahmed et al., no single set of antibodies was found to be pathognomonic for the diagnosis of AIE. 6 The majority of cases involve the duodenum and have moderate‐to‐severe villous atrophy, extensive lymphoplasmacytic infiltration, and neutrophilic cryptitis with or without crypt abscess as the most typical histopathological findings. 7 Gastrointestinal mucosal abnormalities were identified beyond the small intestine in 24 out of 25 cases with AIE, where the stomach was damaged in 86% of cases, followed by the colon (64%), and the esophagus (28%). 7 This was evident in our patient who had duodenitis and colitis. AIE due to STAT3 GOF mutation can be a part of Infantile‐onset multisystem autoimmune disease‐1 with extra‐gastrointestinal diseases like insulin‐dependent diabetes mellitus, autoimmune hematologic disorders, and hypothyroidism. 2 This disorder is characterized by a STAT3 heterozygous gain of function mutation in exon 6, 8 as was evident in the patient.
First‐line treatment is usually the application of steroids followed by other forms of immunosuppressive therapy like mycophenolate mofetil (MMF), cyclosporine, and tacrolimus. 5 But MMF (2/5), Tacrolimus (1/4), Azathioprine (0/4), and Rituximab (1/3) all have poor treatment outcomes, according to a retrospective study analyzing published case reports and smaller case series and thus were not considered for management of our patient. 9
STAT 3 is a transcription factor which regulates cellular survival, proliferation, differentiation, and effector function. 10 Cytokines activate JAKs, which causes phosphorylation, dimerization, and nuclear translocation of STAT3 which leads to expression of target genes. 11 Thus, inhibition of JAK signaling has a role in treating STAT 3 GOF mutations. JAK inhibitors (JAKinibs) have been used to treat severe immune dysregulation in patients with either STAT1 or STAT3 gain of function mutations 2 with significant clinical improvement seen in 10 out of 13 patients being treated with JAKinibs. 12
Hematopoietic stem cell transplantation (HSCT) has been used as a curative therapy for individuals with AIE, and its function is expanding. 6 In patients with progressing disease where pharmaceutical therapy has not achieved optimal control of the disease, HSCT is taken into consideration. 10 By engrafting donor CD4 + CD25 + FOXP3+ regulatory T cells, which can effectively suppress autoreactive T and B cells, symptoms are resolved by HSCT. 6 The published longitudinal data are currently insufficient to finally determine the role and longterm outcome of HSCT considering the multisystem expression of STAT3. 13 This together with aspects of transplant‐related morbidity will be carefully reviewed as part of a multidisciplinary discussion including the parents.
The degree of symptoms (including fecal output), the extent and severity of histological lesions along the gastrointestinal tract, and the presence of extra‐intestinal involvement all affect the prognosis of AIE. 1
4. CONCLUSION
The patient presented in this report, who had persistent intractable diarrhea, was found to have infantile‐onset multisystem autoimmune disease‐1 on genomic studies, after having tested negative for multiple causes and being unresponsive to various treatments. Monogenetic immunologic causes, like STAT3 GOF mutations in our case, should be considered in cases of protracted diarrhea of infancy refractory to conventional treatment. Access to modern genomic technologies (whole exome/genome sequencing and targeted disease‐specific panels) increasingly allows early identification of disease etiology and pathway‐targeted treatments.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS STATEMENT
Informed parental consent was obtained for publication of the case details.
ACKNOWLEDGMENTS
The authors have no funding to report.
Tetarbe S, Shah K, Shah I. Intractable diarrhea in an infant—autoimmune enteropathy: a case report. JPGN Rep. 2024;5:70‐73. 10.1002/jpr3.12038
Contributor Information
Kasvi Shah, Email: kasvishah03@gmail.com.
Ira Shah, Email: irashah@pediatriconcall.com.
REFERENCES
- 1. Montalto M, D'Onofrio F, Santoro L, Gallo A, Gasbarrini A, Gasbarrini G. Autoimmune enteropathy in children and adults. Scand J Gastroenterol. 2009;44(9):1029‐1036. [DOI] [PubMed] [Google Scholar]
- 2. Leiding JW, Vogel TP, Santarlas VGJ. Monogenic early‐onset lymphoproliferation and autoimmunity: natural history of STAT3 gain‐of‐function syndrome. J Allergy Clin Immunol. 2022;151:1081‐1095. https://www.jacionline.org/article/S0091-6749(22)01182-4/fulltext#back-bib3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kinnunen U, Vuopala K, Kaukinen K. [Autoimmune enteropathy]. Duodecim. 2015;131(6):533‐540. [PubMed] [Google Scholar]
- 4. Akram S, Murray JA, Pardi DS, et al. Adult autoimmune enteropathy: Mayo Clinic Rochester experience. Clin Gastroenterol Hepatol. 2007;5(11):1282‐1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gentile NM, Murray JA, Pardi DS. Autoimmune enteropathy: a review and update of clinical management. Curr Gastroenterol Rep. 2012;14(5):380‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahmed Z, Imdad A, Connelly JA, Acra S. Autoimmune enteropathy: an updated review with special focus on stem cell transplant therapy. Dig Dis Sci. 2019;64(3):643‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Francalanci P, Cafferata B, Alaggio R, et al. Pediatric autoimmune disorders with gastrointestinal expressions: from bench to bedside. Pathologica. 2022;114(1):32‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X‐linked (IPEX) and IPEX‐related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25(6):708‐714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Faletti L, Ehl S, Heeg M. Germline STAT3 gain‐of‐function mutations in primary immunodeficiency: impact on the cellular and clinical phenotype. Biomed J. 2021;44(4):412‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vogel TP, Leiding JW, Cooper MA, Forbes Satter LR. STAT3 gain‐of‐function syndrome. Front Pediatr. 2023;10:770077. 10.3389/fped.2022.770077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mackie J, Ma CS, Tangye SG, Guerin A. The ups and downs of STAT3 function: too much, too little and human immune dysregulation. Clin Exp Immunol. 2023;212(2):107‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hadjadj J, Frémond ML, Neven B. Emerging place of JAK inhibitors in the treatment of inborn errors of immunity. Front Immunol. 2021;12:717388. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8484879/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Goldilocks Principle of JAKs and STATs: Gain‐of‐function mutations, loss‐of‐function mutations, and their clinical consequences [Internet]. 2022. https://www.researchsquare.com


