Abstract
Background
Chronic hepatic encephalopathy (CHE) has been reported both in patients with congenital porto‐systemic shunts (CPSS) and chronic liver disease. CHE is difficult to recognize in children as there is no clear definition and its manifestations are highly variable. CHE is associated with variations in brain volumes and metabolites that have already been demonstrated using 1.5‐3T MRI systems. However, the in‐depth study of brain metabolism requires the high spectral resolution of high magnetic fields.
Objectives and Methods
We analyzed the neurometabolic profile, brain volumes and T1 relaxation times of a child with a CPSS using high field proton magnetic resonance spectroscopy (1H MRS, 7T) combined with MRI and compared it to an age‐matched control group. We also evaluated the impact of shunt closure on neurocognitive symptoms using adapted neuropsychological tests.
Results
7T MRS revealed a significant increase in glutamine compared to controls, a decrease in brain osmolytes, and a slight elevation in NAA concentrations. 7T MRI scans showed morphological abnormalities but no changes in the signal intensity of the globus pallidus. Neurocognitive testing revealed attention deficit disorder, language difficulties, and mild intellectual disability. Most of these areas improved after shunt closure.
Conclusions
In this paediatric case of type B HE with normal fasting ammonia, neurometabolic profile was compatible with what has been previously shown in chronic liver disease, while also demonstrating an isolated glutamine peak. In addition, neurocognitive function partially improved after shunt closure, arguing strongly for shunt closure in both presymptomatic and symptomatic patients.
Keywords: chronic liver disease, congenital porto‐systemic shunts, hepatic encephalopathy, proton magnetic resonance spectroscopy
What is Known
In adults with Chronic Liver Disease (CLD), brain concentrations in glutamine and glutamate increase while myo‐inositol, taurine and total choline decrease.
Plasma ammonia is accepted to be a major metabolic player in hepatic encephalopathy, generating an increase in cerebral glutamine.
What is New
Use of 7 Tesla MRI and 1H MRS to quantify brain volumes and metabolites in detail is feasible in a child.
Congenital porto‐systemic shunts may be associated with decreased estimated volume of some deep brain structures compared to age‐matched controls.
High field MRS enabled glutamine and glutamate peaks to be isolated.
1. INTRODUCTION
In both adults and children, type B and C hepatic encephalopathy (HE) are often grouped together for purposes of studies or clinical management. 1 Only, the major difference between the two is the presence of associated liver disease in type C. Type B HE is less well studied, as isolated portosystemic shunting‐ surgical or congenital‐ is rare. Recently, increasing awareness of congenital shunts in children and adults has created opportunities to study isolated portosystemic bypass in greater detail, without the noise of associated liver disease. 2 , 3 , 4 , 5 , 6 The rationale for studying type B HE on its own is that cirrhosis is associated with elevated plasma ammonia, accepted to be a major metabolic player in HE, and circulating vasoactive substances, immune mediators and other neuroactive substances that are now accepted to contribute to the neurometabolic and neurocognitive abnormalities observed in patients with chronic liver disease or cirrhosis. 7 Not only are congenital portosystemic shunts typically not associated with any significant liver disease, but fasting plasma ammonia levels are not very elevated in most patients, creating a unique opportunity to study the isolated effect of portosystemic shunting on the brain. 3
The improvement of imaging techniques has allowed the identification of brain abnormalities related to HE, especially T1 hypersignal of the globus pallidus on MRI. 8 The signal intensity of this region is more pronounced in older children, suggesting that the duration of Chronic Liver Disease (CLD), is related to HE. 9 Other signs of HE have been reported using MRI such as cortical atrophy or ventricular enlargement, and alterations in metabolite concentrations on 1H MR Spectroscopy (1H MRS). 8 , 10 , 11 , 12 In addition, the enhanced resolution of high field magnets has dramatically improved the understanding of neurometabolism, including in HE. 8 , 13 , 14 , 15 , 16 Typically, neurometabolic changes found in adult studies with CLD demonstrate an increase of the sum glutamine (Gln) and glutamate (Glu) (at low magnetic field the sum is reported as Glx=Gln+Glu) and a decrease of myo‐Inositol (Ins), taurine (Tau), and total choline (tCho). 8 , 17 , 18 , 19 These changes have also been documented in some children, 20 but the number of studies remains small and the spectral resolution for 1H MRS is often limited by the strength of the magnetic field used.
In this report, we summarize the neurometabolic and neuroimaging (brain morphometry and T1 mapping) findings of an adolescent female with late diagnosis of a congenital portosystemic shunt (CPSS), making use of the unique advantages of highly resolved MRS and MRI performed on a 7T MRI scanner. We show that the typical neurometabolic findings associated with CHE are found in congenital portosystemic shunting.
1.1. Clinical case
A 11‐year‐old girl was admitted to hospital for loss of consciousness, dyspnea and signs of right sided overload on electrocardiogram. Recent history was remarkable for repeated episodes of dizziness without loss of consciousness after exercise. Her past medical history was significant for cognitive, psychomotor and speech delay as well as resolved neonatal cholestasis, and hypoglycemia in infancy. At admission, clinical exam showed a right ventricular heave, a loud second heart sound and unsteadiness while walking. Measurements were: weight 31 Kg (P25), height 147 cm (P50‐P75), BMI 14.5 kg/m2 (P3‐P10). The lab results showed a slight elevation of aminotransferase levels without cholestasis (ALT 37U/I, gGT 85U/I). Plasma ammonia and alpha‐fetoprotein were within normal range.
An echocardiogram showed severe dilatation of the right ventricle, pulmonary trunk and pulmonary arteries without intracardiac shunt, with signs of severe pulmonary arterial hypertension (PAH). Subsequent cardiac catheterization confirmed severe PAH with a mean pulmonary artery pressure (mPAP) of 70 mmHg (norm: ≤20 mmHg) and indexed pulmonary vascular resistance (PVR) of 17.6 WUm2 (norm: <3 WUm2). 21 Treatment for PAH was initiated with macitentan and tadalafil. Angio‐computed tomography scan confirmed a 11 mm diameter porto‐systemic shunt between the spleno‐mesenteric confluence and the retrohepatic vena cava with a hypoplastic intrahepatic portal vein (Figure 1).
Figure 1.
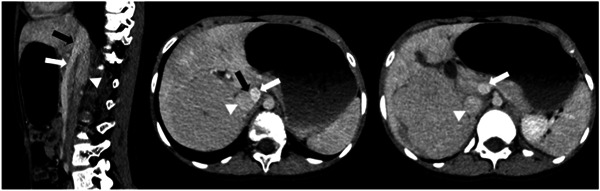
Left: sagittal plane through the shunt, the spleno‐mesenteric confluence and the inferior vena cava. Middle: axial plane through the shunt. Right: lower axial plane through the inferior vena cava and the spleno‐mesenteric confluence. Black arrow: portosystemic shunt between the spleno‐mesenteric confluence and the inferior vena cava. White arrow: spleno‐mesenteric confluence. Arrowhead: inferior vena cava.
Five liver nodules were identified on Gadolinium enhanced MRI: two compatibles with regenerative nodules, two suggestive of focal nodular hyperplasia and one nodule of unknown nature. The two most significant nodules were biopsied: 8.6 cm in segments VI–VII, and 1.9 cm in segment IV. Nodule biopsy confirmed one inflammatory hepatocellular adenoma, with a somatic IL6ST gene mutation, and one mixed hepatocellular adenoma, with both an exon 3 CTNNB1 mutation, and an IL6ST mutation (mixed bex3 IHCA). Of note, the IL6ST mutations were different in the two nodules. Liver biopsy of the non‐nodular liver showed hypoplastic portal veins, with proliferation and dilatation of portal lymphatic vessels. Architectural changes, albeit insufficient for the diagnosis of nodular regenerative hyperplasia (NRH), and sinusoidal distension were seen.
Closure of the shunt was indicated based on the severe clinical presentation combining neurological impairment, PAH and nodules. Before shunt closure and after written and informed consent, she was enrolled in a pilot study analyzing neurometabolism and imaging together with neuropsychological testing in children with chronic liver disease or portosystemic shunting, 22 the results of which are reported herein.
2. METHODS
After written and informed consent, the patient underwent 7T MR scans (MAGNETOM 7T; Siemens Healthcare) for absolute volume and average T1 values (3D MP2RAGE sequence, TR = 6 s, TE = 2.05 ms, TI1 = 0.8 s, TI2 = 2.7 s, α1 = 4°, α2 = 5°, 0.6 × 0.6 × 0.6 mm3 resolution, 320 × 320 × 256 matrix size, TA = 10 min) especially in the globus pallidus using the MorphoBox prototype, 23 while short echo time 1H MR spectra were acquired in a voxel located in gray matter dominated prefrontal cortex (20 × 20 × 25 mm3), as described previously. 24 Briefly, the semi‐adiabatic SPECIAL sequence at short echo‐time (16 ms) was used (TR = 6500 ms, 2 × 50 averages (i.e., acquisitions per spectrum), spectral width of 4000 Hz, 2048 points in FID). LCModel 25 was used for metabolite fitting, and metabolite ratios to tCr were reported as the water content and metabolite T2 relaxation times are not yet known for the age range investigated in the present study at 7T. The following metabolites were simulated using published values of J‐coupling constants, chemical shifts and included in LCModel basis set: alanine (Ala), ascorbate (Asc), aspartate (Asp), glycerophosphocholine (GPC), phosphocholine (PCho), creatine (Cr), phosphocreatine (PCr), γ‐aminobutyric acid (GABA), glutamine (Gln), glutamate (Glu), glutathione (GSH), glycine (Gly), inositol (Ins), lactate (Lac), N‐acetylaspartate (NAA), N‐acetylaspartylglutamate (NAAG), phosphoethanolamine (PE), taurine (Tau), glucose (Glc), scyllo‐inositol (Scyllo), and serine (Ser). PCho and GPC were expressed only as tCho (PCho + GPC) due to better accuracy in the estimation of their concentration as a sum. An in vivo acquired macromolecules spectrum was also included in LCModel basis set. 26 Supporting Information summarizes the minimum reporting standards in MRS. 27 The study was approved by the institutional review board (CCER 2017‐01854). Accordingly, she underwent neuropsychological assessment before and after shunt closure by age‐appropriate neurocognitive test and by Wechsler Intelligent Scale for children. 24 MRI and MRS results were compared to an age matched control group 24 and neuropsychological testing to normative data.
3. RESULTS
High field MRI revealed several structural abnormalities (Figure 2A): skull deformation with plagiocephaly, rarefaction of white matter, superior vermian atrophy with enlarged transverse fissures.
Figure 2.
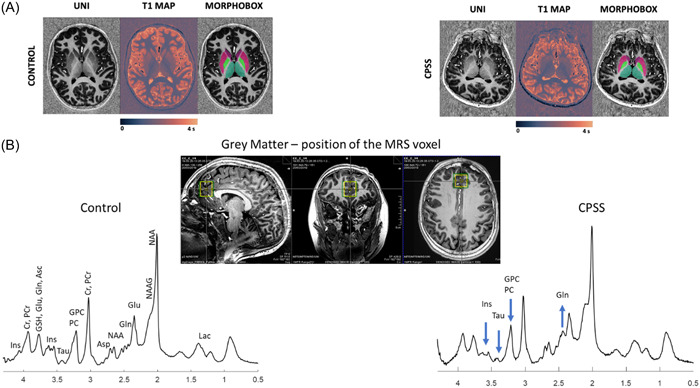
(A) Representative axial slices MP2RAGE uniform contrast (left), T1maps (middle) and corresponding basal ganglia nuclei segmentation masks (right) of a female CTR (left) and a female CPSS (right). (B) Representative 1H MRS spectra with corresponding voxel position and size on anatomical images (green voxel for shimming, yellow voxel for 1H MRS) acquired at 7T in a voxel located in GM (20 × 20 × 25 mm3) dominated prefrontal cortex. Acquisition parameters: semiadiabatic SPECIAL sequence, TE = 16 ms, TR = 6500 ms, 2 × 50 averages, spectral width of 4000 Hz, 2048 points in FID. No postprocessing was applied except for B0 drift and eddy current corrections. The main metabolites are labeled on the spectrum acquired in the GM CTR while for the other spectrum only the main metabolites changing are labeled (Gln +3.4 fold increase; Ins −17% decrease, Tau −54% decrease, GPC+PCho −26% decrease). GM, gray matter dominant.
1H MRS spectra showed a marked increase of glutamine (Gln) (241%) and to a lesser extent of N‐acetylaspartate (NAA) (14%) compared to the age‐matched control group (Figure 2B). Other metabolites decreased: myo‐Inositol (Ins) (−17%), taurine (Tau) (−54%), total choline (tCho, glycerophosphocholine and phosphocholine (GPC + PCho)) (−26%) and γ‐aminobutyrate (GABA) (−50%) as shown in Figure 3A,B and Supporting Information S1: Figure 2.
Figure 3.
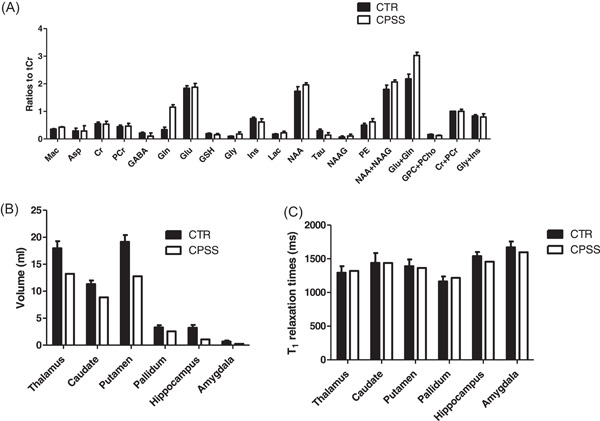
Brain metabolite changes in GM dominant voxel positioned in prefrontal cortex between CTR (black bars, mean ± SD) and CPSS (white bars, mean ± CRLB) (A), absolute brain volumes in milliliters (mL) (B) and T1 relaxation times in milliseconds (ms) (C) between CTR (black bars) and CPSS (white bars) in six different brain regions. CPSS, congenital porto‐systemic shunts.
Brain volume (Figure 3B) was abnormally low compared to age‐matched control group and heterogenous between the different anatomical regions investigated: hippocampus (−67%), amygdala (−64%), putamen (−33%), thalamus (−26%), caudate (−22%), and pallidum (−21%). 7T brain MRI did not reveal the expected hyperintensity in the globus pallidus, as illustrated by comparable mean values in T1 relaxation time (Figure 3C) between the case and the control group. The maximal variation between the CPSS patient and CTR group was 5%.
Extensive neuropsychological assessment before shunt closure showed attention deficit disorder, slowness in the execution of tasks, dyspraxia, language difficulties with a limited vocabulary, and mild intellectual disability with an intellectual quotient (IQ) of 69. Blood workup at the time of neuropsychological evaluation showed mild GGT elevation at 67U/I and ammonia within normal range at 27 μmol/I.
Follow up after shunt closure:
Neurocognition: 5 months after shunt closure, the patient underwent a subset of the neuropsychological tests she had undergone before closure. They targeted IQ, receptive language, concentration, and memory. There was an overall improvement in all scores, especially in attention and concentration skills, reflected by an IQ score of 86 (low normal). Anecdotally, both parents and school reported a much more reactive child, intellectually interested and receptive to learning. Despite the overall improvement, residual difficulties persist in verbal and written expression as well as in memory encoding abilities.
Cardiovascular: She initially had a marked PAH improvement, with functional class I and cardiac catheterization 9 months after shunt closure showing a decrease in mPAP (30 mmHg) and indexed PVR (5.2 WUm2). Four years after shunt closure, PAH has progressed (mPAP: 64 mmHg, PVR: 17WU, indexed PVR: 25 WUm2 and NYHA III) in the context of persistent portal hypertension. At the time of print the patient is on triple PAH therapy including continuous IV epoprostenol with the view to perform liver transplant if haemodynamic criteria are reached.
Follow‐up MRI 2 years after shunt closure revealed a decrease in the beta‐catenin mutated nodule (5.4 cm × 4.7 cm × 6.2 cm vs. 7.1 cm × 7.8 cm × 8 cm). Two nodules remain, reduced in size in segment V and VI/VII. All other nodules have completely regressed, including the CRP positive nodule.
4. DISCUSSION
To the best of our knowledge, this study is the first to quantify brain volumes and metabolites in detail in a child with CPSS using 7 Tesla MRI and 1H MRS. In this patient, the high resolution provided by the 7T MRI highlighted reduced brain volumes and changes in the neurometabolic profile characterized by an increase of Gln and a decrease of tCho (GPC+PCho), Tau and Ins compared to age‐matched controls (CTR). In addition, NAA concentrations were slightly above the range of an age‐matched population, 24 and in keeping with what has been reported with children with compensated chronic liver disease. 24 Neurocognitive assessment performed before and after shunt closure highlighted marked improvement within the first year after shunt closure, although we were unable to confirm that this improvement correlated with changes in1H MRS or brain imaging.
This case raises four points in regard to type B HE: brain metabolic profile, brain volumes, normal plasma ammonia, and the relationship between portosystemic bypass and neurocognition.
First, the neurometabolic profile in the present report is compatible with what has been previously shown in chronic liver disease in both adults and children, raising the question of the contribution of portosystemic shunting in type C HE, rather than liver dysfunction alone. Classically, it is held from high magnetic field spectroscopy studies in animals that administration of ammonium is associated with a rapid and linear increase in brain glutamine. 11 In type C HE animal models 15 , 16 , 28 , 29 , 30 and CPSS mice, 31 the Gln increase generates an osmolarity change in the brain, which in turn releases the main brain osmolytes (Ins, Tau, tCho) to counter the osmotic load. 15 , 18 These findings have been also observed in humans with type C HE. 32 However, few paediatric studies have analyzed cerebral metabolic profiles associated with type B or C HE at high magnetic field. Clinical magnets at 1.5 or 3T offer insufficient resolution for 1H MRS to reliably distinguish the different metabolic peaks, especially between glutamine and glutamate. That said, an increase of Gln+Glu and decrease in Ins, Tau and tCho has been reported in children with CLD. 9 , 11 , 19 In the present study, not only did we demonstrate an isolated Gln peak by separating Gln from Glu, but we also showed that unlike previous reports, NAA was slightly elevated, in keeping with another report in children with CLD. 24 The significance of this novel finding remains to be elucidated.
Second, the novelty of this report is the volumetric analysis. In adults, an inverse correlation between the progression of type C HE and the volumetry of the brain has been reported, 33 while there is no such report in children. Yet, child development is characterized by rapid brain growth, especially in the first 2 years of life during which the structural bases of cognition are formed. 34 It follows that if adults develop brain atrophy in cirrhosis, structural and functional brain development in children may be hindered by congenital portosystemic shunting, impacting cognition and working memory among others, two functions of the prefrontal cortex. 35 This is aligned with the observation that spatial learning is impaired in rats with porto‐systemic shunts and prefrontal cortex involvement. 36 In human subjects, the reversibility of brain atrophy has been shown in two clinical scenarios: anorexia nervosa and biliary atresia. 34 , 37 Whether this extends to patients born with congenital portosystemic shunts is something ripe for exploration.
What is intriguing in this case, is that fasting plasma ammonia measurements were normal, raising two possibilities. The first, and most likely, is that postprandial ammonia may be elevated, that this was missed by measuring fasting ammonia only, and the osmotic changes are in fact associated with postprandial peaks in plasma ammonia rather than chronically elevated plasma concentrations. The second is that other metabolic pathways lead to elevated brain Gln and osmotic stress in the brain of patients with CPSS, which would add to our understanding of the relative role of PS shunting in type C HE.
Finally, although in the present case the brain was exposed to the absence of hepatic first pass during essential neurodevelopmental windows, there clearly was some degree of functional reversibility as revealed by the neurocognitive progress after shunt closure, arguing strongly for shunt closure in both presymptomatic and symptomatic patients.
Although this report brings a novel perspective to the study and understanding of the neurometabolic and neurocognitive repercussions of portosystemic bypass, it does present several limitations. First, the neurometabolic profile was only studied in the gray matter of the PFC, opening the field for further exploration of white matter and other brain regions. Second, the patient did have multiple comorbidities requiring treatment, raising the question of their relative contribution in the neurometabolic profile. Third, CPSS have been observed in multiple syndromes; although none was identified in this patient, it is not out of the question that her relative microcephaly and morphological abnormalities may be partly syndromic in nature rather than entirely attributable to portosystemic bypass. 38 Although shunt closure resulted in a significant improvement in cognition after shunt closure, this cannot be correlated with neurometabolic profile as further imaging was refused by the family. Larger paediatric studies are needed to characterize the cerebral alterations observed in type B HE and their evolution after shunt closure.
In a paediatric case of type B HE with normal fasting ammonia, neurometabolism was characterized by elevated Gln, decreased Ins, Tau and tCho, and elevated NAA in the gray matter dominated PFC, in keeping with reports in adults and children with type C HE. In addition, brain morphology was abnormal as shown by volume estimates lower to age‐matched control group in some deep brain structures. This unique neurometabolic and morphological profile was associated with significant cognitive impairment which improved partially after shunt closure. Further studies are necessary to further our understanding of portosystemic bypass on brain development. Specific points of focus should include: longitudinal analysis of postprandial ammonia levels in patients with CPSS, use of metabolomics of peripheral molecules reaching the brain in portosystemic bypass, brain regional vulnerabilities to portosystemic bypass, susceptible developmental windows, and how to optimize neurocognitive response to shunt closure.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors thank the administrative and nursing staff who participated in patient recruitment and management. We are indebted to the enthusiastic volunteers for their time and interest. Special thanks go to Andrea Gropman and to Lijing Xin for helpful discussions. We thank Tobias Kober from Siemens Healthineers for use of the MP2RAGE WIP 944 and for his thoughtful review of the manuscript. This works was supported by the Swiss National Science Foundation award n° 310030_173222 and 310030_201218 and by the Center for Biomedical Imaging of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards, and Jeantet Foundations.
Chabbey I, Cudalbu C, Barras E, et al. Neurometabolism and brain morphometry in an adolescent female with an extra‐hepatic congenital portosystemic shunt. JPGN Rep. 2024;5:35‐42. 10.1002/jpr3.12035
Contributor Information
Isaline Chabbey, Email: isaline.chabbey@hcuge.ch.
Valérie A. McLin, Email: valerie.mclin@hcuge.ch.
REFERENCES
- 1. McLin VA, D'Antiga L. The current pediatric perspective on type B and C hepatic encephalopathy. Anal Biochem. 2022;643:114576. [DOI] [PubMed] [Google Scholar]
- 2. International registry of Congenital Porto‐Sysemic Shunts . Web site. https://ircpss.com/
- 3. McLin VA, Franchi Abella S, Debray D, et al. Congenital portosystemic shunts: current diagnosis and management. J Pediatr Gastroenterol Nutr. 2019;68(5):615‐622. [DOI] [PubMed] [Google Scholar]
- 4. Korff S, Mostaguir K, Beghetti M, et al. International registry of congenital porto‐systemic shunts: a multi‐centre, retrospective and prospective registry of neonates, children and adults with congenital porto‐systemic shunts. Orphanet J Rare Dis. 2022;17(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernard O, Franchi‐Abella S, Branchereau S, Pariente D, Gauthier F, Jacquemin E. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis. 2013;32(4):273‐287. [DOI] [PubMed] [Google Scholar]
- 6. Franchi‐Abella S, Branchereau S, Lambert V, et al. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr. 2010;51(3):322‐330. [DOI] [PubMed] [Google Scholar]
- 7. Mallet M, Desplats V, Bouzbib C, et al. Blood ammonia in patients with chronic liver diseases: a better defined role in clinical practice. Anal Biochem. 2022;657:114873. [DOI] [PubMed] [Google Scholar]
- 8. Cudalbu C, Taylor‐Robinson SD. Brain edema in chronic hepatic encephalopathy. J Clin Exp Hepatol. 2019;9(3):362‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanquinet S, Morice C, Courvoisier DS, et al. Globus pallidus MR signal abnormalities in children with chronic liver disease and/or porto‐systemic shunting. Eur Radiol. 2017;27(10):4064‐4071. [DOI] [PubMed] [Google Scholar]
- 10. Alonso J, Córdoba J, Rovira A. Brain magnetic resonance in hepatic encephalopathy. Semin Ultrasound CT MRI. 2014;35(2):136‐152. [DOI] [PubMed] [Google Scholar]
- 11. Cudalbu C. In vivo studies of brain metabolism in animal models of hepatic encephalopathy using 1H magnetic resonance spectroscopy. Metab Brain Dis. 2013;28(2):167‐174. [DOI] [PubMed] [Google Scholar]
- 12. Lanz B, Rackayova V, Braissant O, Cudalbu C. MRS studies of neuroenergetics and glutamate/glutamine exchange in rats: extensions to hyperammonemic models. Anal Biochem. 2017;529:245‐269. [DOI] [PubMed] [Google Scholar]
- 13. Öz G, Alger JR, Barker PB, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270(3):658‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perdue MV, DeMayo MM, Bell TK, et al. Changes in brain metabolite levels across childhood. Neuroimage. 2023;274:120087. [DOI] [PubMed] [Google Scholar]
- 15. Braissant O, Rackayová V, Pierzchala K, Grosse J, McLin VA, Cudalbu C. Longitudinal neurometabolic changes in the hippocampus of a rat model of chronic hepatic encephalopathy. J Hepatol. 2019;71(3):505‐515. [DOI] [PubMed] [Google Scholar]
- 16. Rackayova V, Braissant O, Rougemont AL, Cudalbu C, McLin VA. Longitudinal osmotic and neurometabolic changes in young rats with chronic cholestatic liver disease. Sci Rep. 2020;10(1):7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monfort P, Cauli O, Montoliu C, et al. Mechanisms of cognitive alterations in hyperammonemia and hepatic encephalopathy: therapeutical implications. Neurochem Int. 2009;55(1‐3):106‐112. [DOI] [PubMed] [Google Scholar]
- 18. Grover VP, Tognarelli JM, Massie N, et al. The why and wherefore of hepatic encephalopathy. Int J Gen Med. 2015;8:381‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foerster BR, Conklin LS, Petrou M, Barker PB, Schwarz KB. Minimal hepatic encephalopathy in children: evaluation with proton MR spectroscopy. AJNR Am J Neuroradiol. 2009;30(8):1610‐1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baker EH, Basso G, Barker PB, Smith MA, Bonekamp D, Horská A. Regional apparent metabolite concentrations in young adult brain measured by (1)H MR spectroscopy at 3 Tesla. J Magn Reson Imaging. 2008;27(3):489‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wacker J, Beghetti M. Paediatric pulmonary hypertension. Cardiovasc Med. 2023;26:50‐54. [Google Scholar]
- 22. Cudalbu C, Xin L, Marechal B, et al. High field brain proton magnetic resonance spectroscopy and volumetry in children with chronic, compensated liver disease—a pilot study. Anal Biochem. 2023;675:115212. [DOI] [PubMed] [Google Scholar]
- 23. Morel B, Piredda GF, Cottier JP, et al. Normal volumetric and T1 relaxation time values at 1.5 T in segmented pediatric brain MRI using a MP2RAGE acquisition. Eur Radiol. 2021;31(3):1505‐1516. [DOI] [PubMed] [Google Scholar]
- 24. Cudablu C, Marechal B, Lachat S, et al. High‐field brain proton magnetic resonance spectroscopy and volumetry in children with chronic, compensated liver disease. Anal Biochem. 2023;675:115212. [DOI] [PubMed] [Google Scholar]
- 25. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NBM. 2001;14(4):260‐264. [DOI] [PubMed] [Google Scholar]
- 26. Cudalbu C, Behar KL, Bhattacharyya PK, et al. Contribution of macromolecules to brain (1) H MR spectra: experts' consensus recommendations. NBM. 2021;34(5):e4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin A, Andronesi O, Bogner W, et al. Minimum reporting standards for in vivo magnetic resonance spectroscopy (MRSinMRS): experts' consensus recommendations. NBM. 2021;34(5):e4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rackayová V, Flatt E, Braissant O, et al. Probiotics improve the neurometabolic profile of rats with chronic cholestatic liver disease. Sci Rep. 2021;11(1):2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flatt E, McLin VA, Braissant O, et al. Probiotics combined with rifaximin influence the neurometabolic changes in a rat model of type C HE. Sci Rep. 2021;11(1):17988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simicic D, Rackayova V, Braissant O, et al. Neurometabolic changes in a rat pup model of type C hepatic encephalopathy depend on age at liver disease onset. Metab Brain Dis. 2023;38:1999‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cudalbu C, McLin VA, Lei H, et al. The C57BL/6J mouse exhibits sporadic congenital portosystemic shunts. PLoS One. 2013;8(7):e69782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rudler M, Weiss N, Perlbarg V, et al. Combined diffusion tensor imaging and magnetic resonance spectroscopy to predict neurological outcome before transjugular intrahepatic portosystemic shunt. Aliment Pharmacol Ther. 2018;48(8):863‐874. [DOI] [PubMed] [Google Scholar]
- 33. Chavarria L, Cordoba J. Magnetic resonance of the brain in chronic and acute liver failure. Metab Brain Dis. 2014;29(4):937‐944. [DOI] [PubMed] [Google Scholar]
- 34. Fitsiori A, McLin V, Toso S, Vargas MI. Reversible brain atrophy after liver transplantation for biliary atresia in childhood. Neurol Clin Pract. 2021;11(6):e923‐e925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Srivastava A, Chaturvedi S, Gupta RK, et al. Minimal hepatic encephalopathy in children with chronic liver disease: prevalence, pathogenesis and magnetic resonance‐based diagnosis. J Hepatol. 2017;66(3):528‐536. [DOI] [PubMed] [Google Scholar]
- 36. Méndez M, Méndez‐López M, López L, Aller MA, Arias J, Arias JL. Portosystemic hepatic encephalopathy model shows reversal learning impairment and dysfunction of neural activity in the prefrontal cortex and regions involved in motivated behavior. J Clin Neurosci. 2011;18(5):690‐694. [DOI] [PubMed] [Google Scholar]
- 37. Boto J, Gkinis G, Roche A, et al. Evaluating anorexia‐related brain atrophy using MP2RAGE‐based morphometry. Eur Radiol. 2017;27(12):5064‐5072. [DOI] [PubMed] [Google Scholar]
- 38. Sokollik C, Bandsma RHJ, Gana JC, van den Heuvel M, Ling SC. Congenital portosystemic shunt: characterization of a multisystem disease. J Pediatr Gastroenterol Nutr. 2013;56(6):675‐681. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


