Abstract
Background
The complexity of leadless pacemaker (LP) implantation varies widely. However, the predictive factors determining this difficulty are poorly understood.
Objective
The purpose of this study was to evaluate the factors influencing LP implantation difficulty, specifically procedural time during right atrial (RA) and right ventricular (RV) manipulation, based on patient background, cardiac function, and anatomic characteristics.
Methods
Analysis included LP implantation cases between 2017 and 2023, excluding the initial 3 implants performed by each operator. The relevance of patient background, cardiac function, and anatomic features on procedural and fluoroscopy times was evaluated.
Results
Fifty-four patients (mean age 82.2 ± 10.0 years; 57.4% male) were included in the study. Median procedural and fluoroscopy time was 45.8 minutes and 16.0 minutes, respectively, with an average of 2.0 ± 1.4 device deployments. Univariate analysis showed associations between procedural time and older age, RA and RV diameter, and severity of tricuspid regurgitation (TR). After adjustment for physician and potential contributing factors, RV dilation (midventricular diameter ≥35 mm) and severe TR were identified as independent predictors of prolonged procedural time. Medical history exhibited no association with procedural time. Consistent results were observed in analyses using fluoroscopy time as the outcome.
Conclusion
RV dilation and severe TR were associated with prolonged procedural time for LP implantation. Anatomic features obtained from preprocedural echocardiography could provide valuable insights into both the safety and efficiency of LP implantation, thereby enhancing tailored treatment strategies for patients undergoing pacemaker implantation.
Keywords: Leadless pacemaker, Procedural time, Right atrium, Right ventricle, Tricuspid regurgitation
Key Findings.
-
▪
Among 54 patients undergoing leadless pacemaker (LP) implantation, right ventricular dilation and severe tricuspid regurgitation were independently associated with prolonged procedural and fluoroscopy times.
-
▪
Although 2 cases of dislodgment occurred, no major complications, including pericardial effusion or cardiac tamponade, were reported, thus underscoring the inherent safety of LP implantation.
-
▪
Evaluation of the anatomic features identified by preprocedural echocardiography could provide important insights into optimizing the safety and efficiency of LP implantation.
Introduction
Leadless pacemakers (LPs) with integrated electrodes and batteries deployed in the right ventricle (RV) have the advantage of reducing the risk of infection, lead fracture, and tricuspid regurgitation (TR). Their indications are expanding with the advent of models that can be synchronized with the atrium.1, 2, 3
Although the safety of the implantation procedure is comparable to that of transvenous pacemakers, the degree of complexity varies greatly depending on the anatomy of the individual case, and the risk of myocardial injury and pericardial effusion increases in complex cases with the number of deployments and procedural time.4, 5, 6 Assessing the complexity of LP implantation is critical to avoid complications; however, little is known about the factors that determine the complexity of LP implantation in individual cases.7
This study aimed to identify the factors that influence the complexity of LP implantation based on patient background, cardiac function, and anatomic characteristics. This should lead to a recommendation as to whether an LP or a transvenous pacemaker is preferable in terms of procedural complexity and safety in patients requiring pacemaker implantation.
Methods
Study design and implantation procedure
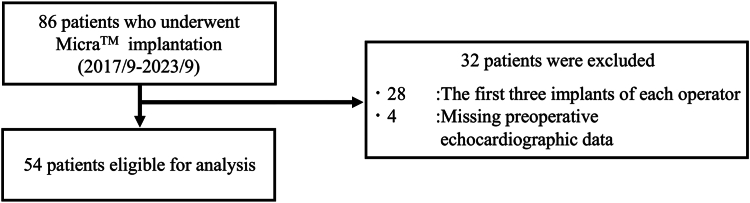
We retrospectively analyzed the data of 86 consecutive patients who underwent implantation of the MicraTM transcatheter pacing system (MC1VR01 or MC1AVR1; Medtronic Inc., Minneapolis, MN) at Keio University Hospital between September 2017 and September 2023. To address the learning curve, we excluded the initial 3 implants performed by each of the 11 operators involved. Among these operators, 4 had not encountered their first 3 cases. Consequently, we excluded 4 patients who lacked preoperative echocardiographic data and 28 patients who corresponded to the first 3 implants by each operator, resulting in a final analysis of 54 patients (Figure 1).
Figure 1.
Analytic cohort. Analysis was conducted of 86 consecutive patients who underwent MicraTM implantation. Four patients without preoperative echocardiographic data and 28 patients with the first 3 implants of each operator were excluded, leaving a total of 54 patients.
All procedures were performed with patients under propofol sedation, supported by noninvasive positive-pressure ventilation when necessary. RV procedural time was defined as the time from insertion of the 23F introducer sheath to cutting of the tether and release of the LP. During the implantation procedure, right ventriculography was performed using a 5F BermanTM angiographic catheter (Teleflex Medical OEM, Plymouth, MN) accessed through a 6F sheath inserted into the right femoral vein to assess RV anatomy before implantation. A 23F hydrophilic coated introducer sheath (Medtronic Inc.) was then placed through the femoral vein into the right atrium (RA). Cardiac angiography was performed using a biplane system in 2 directions (30° right anterior oblique and 55° left anterior oblique views). The steerable delivery system with the LP was advanced through the introducer sheath into the RV. With the tip of the delivery system positioned in the RV septum, contrast was injected through the tip to confirm that the delivery system was firmly attached to the septum, and the LP was deployed. The tether was retracted to confirm that the tines were properly attached to the tissue using fluoroscopy, and electrical measurements (ie, pacing impedance, R-wave amplitude, and pacing thresholds) were checked for any irregularities. If problems were detected, the tether was pulled, the LP was retracted into the system, and the procedure was repeated. After confirming that the device was stable and the device parameters were acceptable for at least 10 minutes after deployment, the procedure was completed by cutting the tether and releasing the LP.
Written informed consent was obtained from all patients before the procedure was performed. Because our study design was retrospective and observational, and it did not involve any deviation from standard clinical procedures or patient contact, the study did not meet the criteria for review by an institutional review board.
Assessment of anatomy and outcomes
Anatomic information on left ventricular (LV) systolic and diastolic dimensions, LV ejection fraction, RV diameter, RA size, and severity of TR was obtained from echocardiography performed before LP implantation. RV diameter was measured at the midportion, and RV dilation was defined as diameter ≥35 mm.8 RA size was measured using the major and minor axis lengths from the apical 4-chamber view. The angles of the inferior vena cava and inferior wall of the RA were assessed using the RV venogram captured at a 30° right anterior oblique projection during LP implantation (Supplemental Figure S1). We evaluated factors contributing to RV procedural and total fluoroscopy times for LP implantation.
Statistical analysis
Data are given as median [interquartile range] or frequency (percentage) depending on whether they are continuous or categorical, respectively. The rate of missing data for patient-level factors was <2%. Univariate linear regression analysis was performed for RV procedural and fluoroscopy times, followed by multivariate linear regression analysis adjusted for physicians and variables that were identified as potential contributing factors in the univariate analysis. Results are given as the values of the beta coefficient and 95% confidence intervals (CIs). All statistical analyses were performed using SPSS Version 27.0 (IBM Corp., Armonk, NY). All reported P values are 2-tailed, and P <.05 was considered significant.
Results
Baseline characteristics
The 54 patients who underwent LP implantation had a mean age of 82.2 ± 10.0 years; 31 (57.4%) were male, and 18 (33.3%) were implanted with the Micra AVTM. Median RV procedural time was 45.8 [30.5–62.7] minutes, and median fluoroscopy time was 16.0 [8.2–28.2] minutes (Table 1). One case of LP dislodgment after the tether was cut required retrieval with a snare and reimplantation with a new delivery system. In another case, the pacing threshold worsened the next day, requiring LP retrieval and reimplantation during the hospital stay. Perioperative pericardial effusion was not observed.
Table 1.
Baseline characteristics and univariate predictors of procedural time for leadless pacemaker implantation
| Variables | Median [25th, 75th percentiles] or n (%) | Univariate analysis |
||
|---|---|---|---|---|
| β | 95% CI | P value | ||
| No. | 54 | |||
| Age (y) | 86 [78, 89] | 1.11 | 0.16 to 2.06 | .023 |
| Female | 23 (42.6) | 1.24 | –18.83 to 21.31 | .90 |
| Height (m) | 1.58 [1.49, 1.64] | –20.33 | –127.43 to 86.77 | .71 |
| Weight (kg) | 54.8 [48.4, 61.6] | –0.41 | –1.40 to 0.58 | .41 |
| BMI (kg/m2) | 22.0 [20.4, 24.3] | –0.99 | –3.83 to 1.85 | .49 |
| BMI <20 | 12 (22.2) | –7.81 | –31.58 to 15.97 | .51 |
| BMI ≥25 | 8 (14.8) | –14.49 | –42.14 to 13.15 | .30 |
| Medical history | ||||
| Hypertension | 34 (63.0) | –2.81 | –23.35 to 17.73 | .79 |
| Diabetes mellitus | 19 (35.2) | 3.23 | 17.53 to 23.99 | .76 |
| CKD (eGFR <60 mL/min) | 45 (83.3) | 2.29 | –24.33 to 28.91 | .86 |
| Hemodialysis | 7 (13.0) | –9.29 | –38.71 to 20.15 | .53 |
| Heart failure | 13 (24.1) | 12.97 | –9.96 to 35.90 | .26 |
| Coronary artery disease | 18 (33.3) | 5.26 | –15.74 to 26.27 | .62 |
| Myocardial infarction | 4 (7.4) | –17.74 | –55.31 to 19.84 | .35 |
| Atral fibrillation | 27 (50.0) | 4.78 | –15.03 to 24.58 | .63 |
| Transcatheter aortic valve implantation | 19 (35.2) | 3.65 | –17.11 to 24.41 | .73 |
| Previous cardiac surgery | 9 (16.7) | –11.84 | –38.26 to 14.59 | .37 |
| Cardiomyopathy | 4 (7.4) | 12.32 | –25.42 to 50.05 | .52 |
| Ischemic cardiomyopathy | 1 (1.9) | |||
| Hypertrophic cardiomyopathy | 1 (1.9) | |||
| Dilated cardiomyopathy | 1 (1.9) | |||
| Amyloidosis | 1 (1.9) | |||
| Micra AVTM | 18 (33.3) | 5.76 | –15.23 to 26.76 | .58 |
| LVEF (%) | 64.8 [58.8, 69.6] | 0.86 | –0.21 to 1.93 | .11 |
| LVEF <50% | 6 (11.1) | –6.94 | –33.15 to 19.26 | .60 |
| LV diastolic dimension (mm) | 45 [42, 49] | 0.22 | –1.37 to 1.81 | .78 |
| LV systolic dimension (mm) | 28 [25, 33] | –0.59 | –2.16 to 0.98 | .45 |
| RV mid-diameter (mm) | 31 [26, 35] | 1.51 | 0.16 to 2.86 | .029 |
| RV mid-diameter ≥35 mm | 15 (27.8) | 25.81 | 4.86 to 46.77 | .017 |
| RA minor axis length (mm) | 40 [33, 44] | 1.36 | 0.32 to 2.41 | .012 |
| RA major axis length (mm) | 52 [48, 57] | 1.43 | 0.61 to 2.25 | <.001 |
| Tricuspid regurgitation | 19.93 | 10.41 to 29.44 | <.001 | |
| None or trivial | 25 (46.2) | |||
| Mild | 19 (35.2) | |||
| Moderate | 7 (13.0) | |||
| Severe | 3 (5.6) | |||
| Angle between IVC and RA inferior (°) | 83 [77, 89] | 0.12 | –0.94 to 1.17 | .83 |
| RV procedural time (min) | 45.8 [30.5, 62.7] | NA | NA | NA |
| Total procedural time (min) | 72.5 [48.0, 93.5] | 0.89 | 0.78 to 0.99 | <.001 |
| Fluoroscopy time (min) | 16.0 [8.2, 28.2] | 1.98 | 1.76 to 2.20 | <.001 |
| No. of deployments | 1 (1, 3) | 13.70 | 7.92 to 19.48 | <.001 |
| Complications | 2 (3.7) | –14.85 | –67.23 to 37.54 | .57 |
| Dislodgment | 2 (3.7) | |||
BMI = body mass index; CI = confidence interval; CKD = chronic kidney disease; eGFR = estimated glomerular filtration rate; IVC = inferior vena cava; LV = left ventricle; LVEF = left ventricular ejection fraction; RA = right atrium; RV = right ventricle.
Predictors of procedural outcomes
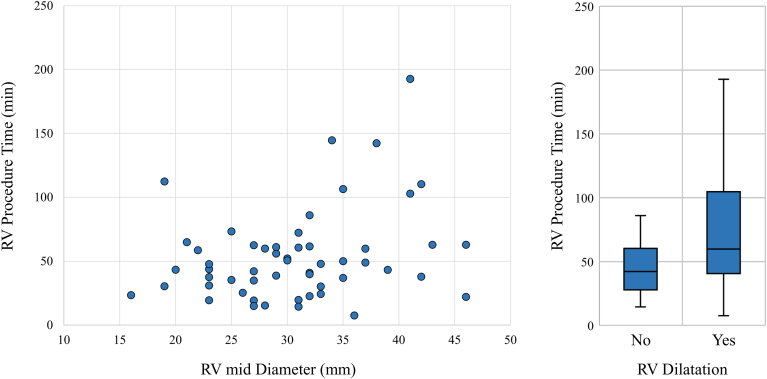
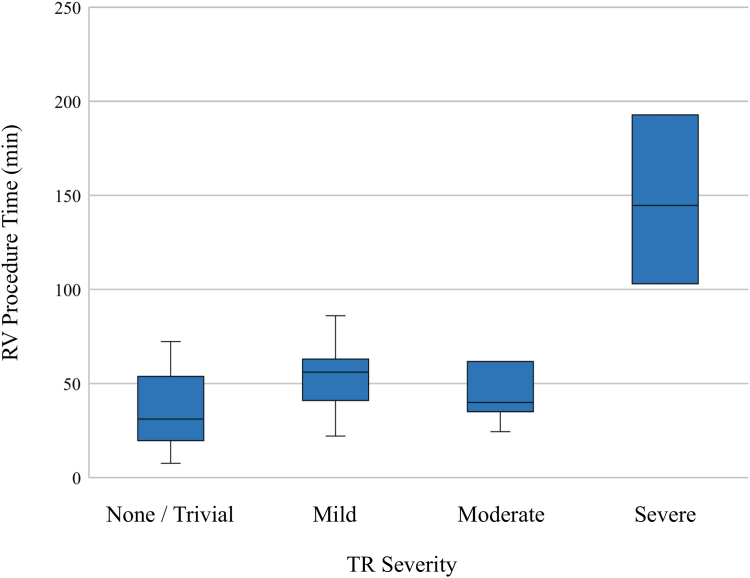
Table 1 and Supplemental Table S1 show the patient characteristics and results of the univariate linear regression analysis for RV procedure and fluoroscopy times, respectively. There was no significant effect of medical history on RV procedural time, whereas older age, larger RV midsection diameter, RV dilation (ie, RV mid-diameter ≥35 mm), greater RA major and minor axis length, and severity of TR were significantly associated with longer RV procedural time (Table 1, and Figures 2 and 3). Consistent relationships were shown for each variable and fluoroscopy time (Supplemental Table S1, and Supplemental Figures S2 and S3).
Figure 2.
Right ventricular (RV) diameter and RV procedural time of leadless pacemaker (LP) implantation. The relationship between RV diameter and RV procedural time of LP implantation is shown. Left: Scatter plot showing a positive correlation between RV diameter and RV procedural time. Right: Comparison of RV procedural times of patients with and those without RV dilation. Those with RV dilation (ie, RV mid-diameter ≥35 mm) had significantly longer RV procedural time than those without.
Figure 3.
Tricuspid regurgitation (TR) severity and right ventricular (RV) procedural time of leadless pacemaker (LP) implantation. The variation in LP implantation time according to the severity of TR is shown. Patients with greater TR tended to demonstrate longer RV procedural time.
Multivariate analysis
After adjusting for physicians and variables that were identified as potential contributing factors in the univariate analysis, RV dilation and severe TR were independent predictors of prolonged RV procedural time (Table 2). The same multivariate model also demonstrated RV dilation (coefficient 11.1; 95% CI 1.0–21.2; P = .032) and severe TR (coefficient 31.6; 95% CI 4.6–58.5; P = .023) as independent predictors of longer fluoroscopy time.
Table 2.
Factors associated with RV procedural time
| Variable | β (95% CI) | P value |
|---|---|---|
| Age (per 1-y increase) | 0.64 (–0.21 to 1.49) | .14 |
| RA minor axis length (mm) | –0.61 (–1.89 to 0.66) | .34 |
| RA major axis length (mm) | –0.31 (–1.51 to 0.89) | .61 |
| RV mid-diameter ≥35 mm | 24.1 (4.95 to 43.2) | .015 |
| Severe TR | 107 (56 to 158) | <.001 |
Dependent variable: RV procedural time. Linear regression analysis adjusted for physician, age, RA minor and major axis lengths, RV dilation (RV mid-diameter ≥35mm), and severe tricuspid regurgitation (TR).
Abbreviations as in Table 1.
Discussion
In this single-center study focusing on LP implantation, our results demonstrated that RV dilation and severe TR independently predicted prolonged RV procedural time, even after accounting for relevant variables and physician influence. Similar trends were observed with regard to fluoroscopy time. These results underscore the critical influence of patient-specific anatomic and functional cardiac characteristics on LP implantation procedures, and emphasize the need for preprocedural assessment and a tailored approach to optimize procedural strategies and device selection.
LPs with an integrated generator and electrodes demonstrate advantages over transvenous pacemakers, particularly in terms of reduced lead fracture risk, lower device infection rates, and minimized interference with the tricuspid valve.9, 10, 11, 12 The safety of the implantation procedure has been shown to be comparable to that for transvenous pacemakers. However, in cases in which the procedure is challenging, longer procedural times can increase the risk of complications.4, 5, 6 With a focus on the risk of complication, Piccini et al6 reported that elderly individuals (≥85 years old), those with a low body mass index (<20 kg/m2), females, patients with a history of heart failure, patients on hemodialysis, and those with non–atrial fibrillation were associated with an increased risk of pericardial effusion after LP implantation. However, our present dataset indicated that none of these factors was associated with a longer RV procedural time, and only anatomic and functional features were related (ie, RV dilation and severe TR). This suggests a distinction between factors determining the complexity of the procedure and the risk of complications, that is, anatomic features vs patient characteristics. Although few reports have specified factors that determine the complexity of the procedure, Garweg et al7 reported that the presence of a prominent septal component of septomarginal trabeculation in the RV is associated with prolonged RV procedural time. However, this anatomic feature is only revealed by right ventriculography during the implantation procedure, making it difficult to predict the complexity preoperatively. In this regard, RV dilation (ie, RV mid-diameter ≥35 mm) and severe TR, which were shown to be independent predictors in this study, are versatile assessment criteria that can be noninvasively and easily estimated by preoperative echocardiography, thus showing usefulness in planning treatment strategies, including device selection (ie, LP or transvenous pacemaker).
There are several relevant considerations regarding the association between RV dilation and LP implantation complexity. During deployment of the LP toward the RV septum using a steerable delivery system, achieving support and stability from the free wall of the RV by positioning the catheter shaft is crucial.13, 14, 15 In cases of RV dilation, obtaining secure support from the free wall becomes challenging, resulting in difficulties in securing the catheter tip. In addition, the severity of TR, identified as an independent predictor of prolonged RV procedural time, can make it difficult to maintain catheter stability. However, it is important to note that TR could be a consequence of RV dilation, acting as a confounding factor for procedural complexity.16, 17, 18 Indeed, in the present population, patients with severe TR showed a larger RV diameter than those without, suggesting a potential interaction. The relevance of this relationship with procedural complexity should be further investigated and evaluated in future studies.
Although the number of patients eligible for LP has increased substantially with the introduction of LPs featuring atrial synchronization, careful evaluation of the procedural complexity is increasingly vital in the selection between LP and transvenous pacemakers to avoid complications.19, 20, 21 Considering the present results, transvenous pacemakers may be an acceptable option for patients exhibiting RV dilation or severe TR. Nonetheless, it is noteworthy that although 2 cases in our study showed threshold elevation during or after LP implantation, no major complications, including pericardial effusion or cardiac tamponade, were reported. Notably, the 2 cases of dislodgment took place during the 9th and 11th procedures, which were conducted by different physicians. These cases exhibited no substantial RV or RA dilation, and TR was mild in both cases. This finding underscores the inherent safety of LP implantation. It is important to take a comprehensive approach for each case, considering the preoperative anatomic information and carefully weighing the safety and efficacy of the implantation procedure to enhance the individualized management of patients undergoing pacemaker implantation.
Study limitations
First, this was a single-center retrospective study, which may have introduced inherent biases related to data selection and limited the generalizability of the findings to a broader population. Second, follow-up data were lacking. A more detailed analysis over a longer follow-up period would provide a more comprehensive assessment of the safety and outcomes of this procedure. Third, the anatomic information was primarily based on transthoracic echocardiography data and less on computed tomography or angiography, which offer more comprehensive anatomic details. Structural evaluations using these modalities hold significant importance, given that the morphology and positioning of the RA, RV, and interventricular septum are pivotal factors during LP implantation. However, it should be noted that echocardiographic information can be obtained noninvasively before pacemaker implantation and is a practical method for evaluating treatment strategies. Finally, given the observational nature of this study, unknown confounders may have influenced the results.
Conclusion
RV dilation and severe TR correlated with prolonged RV procedural and fluoroscopy times. Careful consideration of the anatomic characteristics identified through preprocedural assessment could offer crucial insights into optimizing the safety and efficiency of LP implantation.
Acknowledgment
We are grateful to Editage (www.editage.com) for English language editing.
Funding Sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Patient Consent
Written informed consent was obtained from all patients before the procedure was performed.
Ethics Statement
Because study design was retrospective and observational, institutional review board approval was not required. The research reported in this study was conducted in accordance with the principles of the Declaration of Helsinki.
Data Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hroo.2023.12.004.
Appendix. Supplementary data
References
- 1.Reddy V.Y., Knops R.E., Sperzel J., et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2014;129:1466–1471. doi: 10.1161/CIRCULATIONAHA.113.006987. [DOI] [PubMed] [Google Scholar]
- 2.Knops R.E., Tjong F.V., Neuzil P., et al. Chronic performance of a leadless cardiac pacemaker: 1-year follow-up of the LEADLESS trial. J Am Coll Cardiol. 2015;65:1497–1504. doi: 10.1016/j.jacc.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 3.El-Chami M.F., Al-Samadi F., Clementy N., et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Heart Rhythm. 2018;15:1800–1807. doi: 10.1016/j.hrthm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Cantillon D.J., Dukkipati S.R., Ip J.H., et al. Comparative study of acute and mid-term complications with leadless and transvenous cardiac pacemakers. Heart Rhythm. 2018;15:1023–1030. doi: 10.1016/j.hrthm.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Piccini J.P., El-Chami M., Wherry K., et al. Contemporaneous comparison of outcomes among patients implanted with a leadless vs transvenous single-chamber ventricular pacemaker. JAMA Cardiol. 2021;6:1187–1195. doi: 10.1001/jamacardio.2021.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccini J.P., Cunnane R., Steffel J., et al. Development and validation of a risk score for predicting pericardial effusion in patients undergoing leadless pacemaker implantation: experience with the Micra transcatheter pacemaker. Europace. 2022;24:1119–1126. doi: 10.1093/europace/euab315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garweg C., Vandenberk B., Foulon S., et al. Determinants of the difficulty of leadless pacemaker implantation. Pacing Clin Electrophysiol. 2020;43:551–557. doi: 10.1111/pace.13933. [DOI] [PubMed] [Google Scholar]
- 8.Lang R.M., Badano L.P., Mor-Avi V., et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Ritter P., Duray G.Z., Steinwender C., et al. Early performance of a miniaturized leadless cardiac pacemaker: the Micra Transcatheter Pacing Study. Eur Heart J. 2015;36:2510–2519. doi: 10.1093/eurheartj/ehv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy V.Y., Exner D.V., Cantillon D.J., et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373:1125–1135. doi: 10.1056/NEJMoa1507192. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds D., Duray G.Z., Omar R., et al. A leadless intracardiac transcatheter pacing system. N Engl J Med. 2016;374:533–541. doi: 10.1056/NEJMoa1511643. [DOI] [PubMed] [Google Scholar]
- 12.Duray G.Z., Ritter P., El-Chami M., et al. Long-term performance of a transcatheter pacing system: 12-month results from the Micra Transcatheter Pacing Study. Heart Rhythm. 2017;14:702–709. doi: 10.1016/j.hrthm.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 13.Morita J., Kondo Y., Okada T., Kitai T., Kasai Y., Fujita T. Predictors of pacing capture threshold exacerbation after leadless pacemaker implantation. Int Heart J. 2023;64:602–605. doi: 10.1536/ihj.22-698. [DOI] [PubMed] [Google Scholar]
- 14.Kiani S., Wallace K., Stromberg K., et al. A predictive model for the long-term electrical performance of a leadless transcatheter pacemaker. JACC Clin Electrophysiol. 2021;7:502–512. doi: 10.1016/j.jacep.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Tolosana J.M., Guasch E., San Antonio R., et al. Very high pacing thresholds during long-term follow-up predicted by a combination of implant pacing threshold and impedance in leadless transcatheter pacemakers. J Cardiovasc Electrophysiol. 2020;31:868–874. doi: 10.1111/jce.14360. [DOI] [PubMed] [Google Scholar]
- 16.Dietz M.F., Prihadi E.A., van der Bijl P., et al. Prognostic implications of right ventricular remodeling and function in patients with significant secondary tricuspid regurgitation. Circulation. 2019;140:836–845. doi: 10.1161/CIRCULATIONAHA.119.039630. [DOI] [PubMed] [Google Scholar]
- 17.Badano L.P., Muraru D., Enriquez-Sarano M. Assessment of functional tricuspid regurgitation. Eur Heart J. 2013;34:1875–1885. doi: 10.1093/eurheartj/ehs474. [DOI] [PubMed] [Google Scholar]
- 18.Dreyfus G.D., Martin R.P., Chan K.M., Dulguerov F., Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol. 2015;65:2331–2336. doi: 10.1016/j.jacc.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Crossley G, Longacre C, Higuera L, et al. Outcomes of patients implanted with an atrioventricular synchronous leadless ventricular pacemaker in the Medicare population. Heart Rhythm 2023 Sep 22:S1547-5271(23)02759-5. [DOI] [PubMed]
- 20.Lenormand T., Abou Khalil K., Bodin A., Babuty D., Bisson A., Clementy N. Comparison of first- and second-generation leadless pacemakers in patients with sinus rhythm and complete atrioventricular block. J Cardiovasc Electrophysiol. 2023;34:1730–1737. doi: 10.1111/jce.15981. [DOI] [PubMed] [Google Scholar]
- 21.Chinitz L.A., El-Chami M.F., Sagi V., et al. Ambulatory atrioventricular synchronous pacing over time using a leadless ventricular pacemaker: primary results from the AccelAV study. Heart Rhythm. 2023;20:46–54. doi: 10.1016/j.hrthm.2022.08.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.