Abstract
Background:
Magnetic resonance imaging of peripheral nerves in the wrist and palm is challenging due to the small size, tortuous course, complex surrounding tissues, and accompanying blood vessels. The occurrence of carpal palmar lesions leads to edema, swelling, and mass effect, which may further interfere with the display and identification of nerves.
Objective:
To evaluate whether contrast-enhanced magnetic resonance neurography (ceMRN) improves the visualization of the morphology and pathology of the median, ulnar nerves, and their small branches in the wrist and palm.
Design:
An observational study.
Methods:
In total 57 subjects, including 36 volunteers and 21 patients with carpal palmar lesions, were enrolled and underwent ceMRN and non-contrast MRN (ncMRN) examination at 3.0 Tesla. The degree of vascular suppression, nerve visualization, diagnostic confidence, and lesion conspicuity was qualitatively assessed by two radiologists. Kappa statistics were obtained for inter-reader agreement. The signal-to-noise ratio, contrast ratio (CR), and contrast-to-noise ratio (CNR) of the median nerve were measured. The subjective ratings and quantitative measurements were compared between ncMRN and ceMRN.
Results:
The inter-reader agreement was excellent (k > 0.8) for all qualitative assessments and visualization assessment of each nerve segment. Compared with ncMRN, ceMRN significantly improved vascular suppression in volunteers and patients (both p < 0.001). The ceMRN significantly enhanced nerve visualization of each segment (all p < 0.05) and diagnostic confidence in volunteers and patients (both p < 0.05). The ceMRN improved lesion conspicuity (p = 0.003) in patients. Quantitatively, ceMRN had significantly higher CRs of nerve versus subcutaneous fat, bone marrow, and vessels and CNR of nerve versus vessel than ncMRN (all p < 0.05).
Conclusion:
The ceMRN significantly improves the visualization of peripheral nerves and pathology in the wrist and palm by robustly suppressing the signals of fat, bone marrow, and especially vessels in volunteers and patients.
Keywords: common palmar digital nerve, magnetic resonance imaging, magnetic resonance neurography, median nerve, palm, proper digital nerve, thenar muscular branch, ulnar nerve, wrist
Plain language summary
Study on the improvement of magnetic resonance imaging and lesion display of small nerves in the wrist and palm using contrast agents
Why was the study done? Because the nerves and branches in the wrist and palm are numerous, small, tortuous, and surrounded by muscles, fat, bones, blood vessels and other tissues, it is difficult to show their complete shape with conventional magnetic resonance imaging. Hand lesions often lead to swelling, edema and masses, which interfere with the display of nerves. Therefore, it is difficult to directly diagnose the relationship between the lesions and nerves in clinical practice. What did the researchers do? The research team used contrast agent plus three-dimensional high-resolution magnetic resonance sequence to display the nerves of volunteers and patients with hand lesions, and used subjective and objective evaluation methods to compare the display effect of the sequence on the nerves before and after the use of contrast agent. What did the researchers find? The imaging method of contrast agent plus three-dimensional high-resolution magnetic resonance sequence can reduce the interference of fat, blood vessels, etc. on nerve display, improve the display effect of each nerve segment of the wrist and palm, increase readers’ confidence in identifying nerves, and improve the detection of lesions. What do the findings mean? This study verified the feasibility and advantages of using contrast agents for magnetic resonance imaging of nerves in the wrist and palm. It provides a new method for clinical and imaging diagnosis of hand lesions, which can simultaneously display the morphological characteristics of nerves and lesions, reducing the difficulty of clinical diagnosis and improving the efficiency of imaging diagnosis.
Introduction
Peripheral nerves in the distal upper extremity are commonly injured by many causes such as trauma, compression, ischemia, infection, tumor, and metabolic disorders. 1 Early diagnosis and proper treatment are vital to improve the prognosis of patients with peripheral neuropathies. However, accurate localization of nerve lesions and evaluation of the severity are still challenging due to the complex anatomy and thin branches, especially in the wrist and palm. Despite its superior soft tissue contrast and high spatial resolution, routine magnetic resonance imaging (MRI)2,3 is still unable to delineate the gradually thinner nerve branches running in tortuous pathways, accompanied by vessels of similar caliber and signal intensity (SI). 4 An imaging method precisely showing the anatomy of the nerves and branches is needed by surgeons to make timely diagnoses and proper treatment decisions. Recently, high-resolution magnetic resonance (MR) sequences such as 3D fast imaging employing steady-state acquisition (3D FIESTA), 3D reversed fast imaging with steady state free precession (3D PSIF), and 3D double-echo steady-state with water excitation (3D-DESS-WE) have been applied for locally displaying the thin nerve branches in the maxillofacial region and extremity.5–10 In addition, diffusion-weighted magnetic resonance neurography (DW-MRN) and diffusion tensor imaging have shown potential in imaging the panorama of peripheral nerves. DW-MRN could be used to obtain an overview image of the median and ulnar nerves. 11 3D delineation of peripheral nerves was achievable by post-processing diffusion-weighted data with maximum intensity projection (MIP) and diffusion tensor tractography (DTT).12,13 These advanced techniques made it possible to highlight nerves and differentiate nerves from other tissues. However, DW-MRN was hardly able to display the two proper digital nerves (PDNs) to the thumb and the thenar muscular branch (TMB) and was also difficult to delineate nerves where vessels with similar hyperintensity travel alongside. 11 Image distortion is another concern when using DTT to track nerve fibers. 13 Imaging the nerves and tiny branches in the wrist and palm with continuous, high-resolution, and panoramic view remains challenging.
Maintaining good spatial resolution and tissue contrast is critical for visualizing small nerve branches in the wrist and palm. In previous studies, contrast-enhanced 3D turbo spin echo (TSE) short tau inversion recovery (STIR) sequence or contrast-enhanced magnetic resonance neurography (ceMRN) was developed to visualize the peripheral nervous system, including the extracranial nerves, brachial plexus, lumbosacral plexus, and their distal branches, providing satisfactory high-resolution high-contrast MR images.4,14–16 By combining the characteristics of heavily T2-weighted and high-resolution imaging of 3D TSE STIR sequence and the effect of shortening T2 relaxation time of paramagnetic contrast agents, ceMRN effectively suppressed the signals of tissues other than peripheral nerves, especially the accompanying blood vessels. ceMRN has advantages in showing the anatomy of peripheral nerves and neuropathies such as tumors, trauma, chronic inflammatory demyelinating polyradiculoneuropathy, and nerve root compression.14,17–19 Therefore, ceMRN is a promising method to visualize the nerves in the wrist and palm.
In this study, the assessment of vessel suppression, nerve visualization, diagnostic confidence, lesion conspicuity, and measurements of image quality were compared between ceMRN and non-contrast MRN (ncMRN). The aim was to evaluate the feasibility and advantages of ceMRN for visualizing the morphology of the median nerve, ulnar nerve, and their branches and the lesions in the wrist and palm.
Materials and methods
Subjects
This is an observational study. From June 2021 to April 2022, a total of 57 subjects (36 women, 21 men; aged 19–60 years, mean 38.3 years) were recruited by convenience sampling approach. Inclusion criteria were patients who complained of symptoms such as pain, numbness, swelling, mobility impairment, and discomfort in the wrist and palm. Exclusion criteria included (1) patients with any MRI contraindications; (2) patients with severe renal insufficiency (estimated glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2); (3) patients who were unable to undergo MRI examinations or had poor images with severe artifacts. A total of 21 patients were enrolled. After clinical and radiological diagnosis, nine cases were carpal tunnel syndrome (CTS), seven cases were synovitis, two cases were ganglion cysts, two cases were hemangioma along nerves, and one case was edema of hypothenar muscle. In addition, 36 volunteers with no history or symptoms of neuropathy in the wrist and palm were included in this study.
MR image acquisition
MRI scanning was performed with a 3.0-T MRI system (Philips Ingenia CX, Best, Netherlands). An 8-channel wrist coil was used. MRI protocol included coronal T1-weighted imaging (T1WI), coronal proton density-weighted imaging (PDWI), axial PDWI, and coronal 3D TSE STIR sequence before and after gadolinium contrast injection. Gadolinium contrast (Magnevist; Bayer AG, Leverkusen, Germany) of 0.3 ml/kg was injected through an indwelling venous catheter with a high-pressure injector at a speed of 1.5 ml/s, followed by a 15 ml saline at the same rate. The scan of the 3D TSE STIR sequence started after a delay of 1–1.5 min.
The parameters of 3D TSE STIR sequence were as follows: repetition time (TR) = 2200 ms, echo time (TE) = 185 ms, inversion time (TI) = 210 ms, TSE factor = 40, integrated parallel acquisition techniques (iPAT) acceleration factor = 2, field of view (FOV) = 150 × 100 mm2, slice thickness = 1.4 mm, gap = 0.7 mm, acquisition matrix = 215 × 145, in-plane resolution = 0.69 × 0.69 mm2, average = 1, bandwidth (BW) = 432 Hz/pixel. The sequence acquisition time was 6 min and 21 s.
The coronal T1WI, PDWI, and axial PDWI were obtained by the parameters: TR/TE, 510/10, 1930/27, 2890/27 ms, respectively, FOV = 120 × 50 mm2, 120 × 50 mm2, 120 × 50 mm2, respectively, acquisition time = 54 s, 1 min 52 s, 2 min, respectively. The overall scanning time was 17 min 28 s.
Qualitative evaluation
Built-in 3D post-processing methods including MIP, multiplanar reconstruction (MPR), and curved range thin-slab MIP in the workstation (ISP, Philips Healthcare) were used on all subjects’ ncMRN and ceMRN 3D data. The ncMRN and ceMRN images were extracted from the patient’s imaging files and anonymized. Two radiologists (W.W. with 7 years of experience and J.K. with 4 years of experience) who were blinded to the sequences evaluated all images at random on a PACS workstation (Carestream, Shanghai, China) for (1) the degree of vascular (mainly vein) signal suppression in volunteers; (2) the visibility to the median nerve, ulnar nerve, and their branches (including the TMB, PDNs to the thumb, one PDN to the radial side of index finger, the second and third common palmar digital nerves (CPDNs), and the ulnar nerve superficial branch) in volunteers; (3) the diagnostic confidence in differentiating nerves from pathology in patients; and (4) the identification of pathology (lesion conspicuity) in patients. A 4-score scale (scores 0–3) was used for the qualitative evaluation (Table 1). The final score was determined by a senior neuroradiologist (L.W. with 19 years of experience).
Table 1.
Scales utilized for qualitative evaluation.
| Score | Vascular suppression | Nerve visualization | Diagnostic confidence | Lesion conspicuity |
|---|---|---|---|---|
| 0 | No vessel’s signal is suppressed | Nerve course is unidentifiable | Low confidence in evaluating nerves | The border of the lesion is not identifiable |
| 1 | Small-caliber vessel’s signal is suppressed | Only the proximal or distal portion is visible | Intermediate confidence in evaluating nerves | The border of the lesion is partially identifiable |
| 2 | Middle and small-caliber vessels’ signal is suppressed | Discontinuous proximal and distal portions are visible | High confidence in evaluating nerves | The border of the lesion is mostly identifiable |
| 3 | Most vessel’s signal is suppressed | The complete course of the nerve is visible | – | The lesion is fully identified |
Quantitative measurement
As the largest and most visible nerve, the median nerve at the level of pisiform bone and its adjacent tissue (e.g. bone marrow, tendon, muscle, subcutaneous fat, small blood vessel) were chosen as regions of interest (ROIs) for acquisition of tissue signal intensity (SI) and background noise standard deviation (SD). Among the 36 volunteers who underwent both ncMRN and ceMRN, signal-to-noise ratio (SNR), contrast-to-noise ratio (CNR), and contrast ratio (CR) were calculated. These metrics were then compared between ncMRN and ceMRN. ROIs were drawn on the ncMRN and ceMRN source images using the workstation (ISP, Philips Healthcare). The formulas for the SNR, CNR, and CR were as follows:
| (1) |
| (2) |
| (3) |
in which ‘tissue’ represents other tissues (e.g. bone marrow, tendon, muscles, subcutaneous fat, small blood vessels). The factor of 0.655 corrects for the fact that the MR background signal has a Rayleigh distribution, not a Gaussian distribution. 20
Statistical analysis
All data were expressed as average ± standard deviation. For qualitative data, the inter-reader agreement of the vascular suppression, nerve visualization, diagnostic confidence, and lesion conspicuity scores on both ncMRN and ceMRN was assessed using kappa analysis. Wilcoxon signed-rank test was used to compare the differences in the scores between ncMRN and ceMRN. For quantitative data, a paired t-test was used to compare differences in image measurements between ncMRN and ceMRN. All analyses were performed using the statistical software SPSS 26.0 (SPSS, Inc., Chicago, IL, USA). A p value < 0.05 was used to determine statistical significance.
Results
Qualitative analysis
The inter-reader agreement for qualitative evaluation including vascular suppression, nerve visualization (overall), diagnostic confidence, and lesion conspicuity on both ceMRN and ncMRN images was excellent (all k > 0.8, Table 2). ceMRN improved the inter-reader agreement for diagnostic confidence compared with ncMRN. The inter-reader agreement of nerve visualization of each segment on both ceMRN and ncMRN images was excellent (all k > 0.8, Table 3).
Table 2.
Inter-reader agreement of qualitative evaluation.
| k | Vascular suppression | Nerve visualization (overall) | Diagnostic confidence | Lesion conspicuity |
|---|---|---|---|---|
| ceMRN | 0.877 | 0.831 | 0.882 | 0.834 |
| ncMRN | 1 | 0.826 | 0.868 | 0.858 |
ceMRN, contrast-enhanced magnetic resonance neurography; ncMRN, non-contrast MRN.
Table 3.
Inter-reader agreement of nerve visualization of each segment.
| k | MN | UN | TMB | Thumb PDN | Index finger PDN | CPDN #2 | CPDN #3 | UN superficial branch |
|---|---|---|---|---|---|---|---|---|
| ceMRN | 0.879 | 0.84 | 0.804 | 0.841 | 0.83 | 0.835 | 0.812 | 0.807 |
| ncMRN | 0.861 | 0.839 | 0.812 | 0.801 | 0.811 | 0.82 | 0.84 | 0.825 |
ceMRN, contrast-enhanced magnetic resonance neurography; CPDN, common palmar digital nerve; MN, median nerve; ncMRN, non-contrast MRN; PDN, proper digital nerve; TMB, thenar muscular branch; UN, ulnar nerve.
For vascular suppression, the scores of ceMRN were significantly higher than those of ncMRN in volunteers and patients (both p < 0.001). The nerve visualization scores of all segments on ceMRN were significantly higher than those on ncMRN in volunteers and patients. In both groups, ceMRN improved diagnostic confidence in identifying nerves compared with ncMRN (p < 0.001, p = 0.005). In patients, the lesion conspicuity scores on ceMRN were significantly higher than those on ncMRN (p = 0.003; Table 4). Figure 1 compares nerve visualization scores of each segment between ncMRN and ceMRN in all subjects. A comparison of vascular suppression, diagnostic confidence, and lesion conspicuity scores between ncMRN and ceMRN in all subjects is shown in Figure 2.
Table 4.
Comparisons of qualitative metrics between ncMRN and ceMRN in 36 volunteers and 21 patients.
| Metrics | Volunteers (n = 36) | Patients (n = 21) | ||||
|---|---|---|---|---|---|---|
| ncMRN | ceMRN | p | ncMRN | ceMRN | p | |
| Vascular suppression | 0 | 2.06 ± 0.333 | <0.001** | 0 | 2 ± 0.548 | <0.001** |
| Nerve visualization | ||||||
| MN | 2.33 ± 0.676 | 2.86 ± 0.351 | <0.001** | 1.86 ± 0.573 | 2.81 ± 0.402 | <0.001** |
| UN | 1.44 ± 1.027 | 2.08 ± 0.806 | 0.005* | 1.24 ± 0.436 | 1.95 ± 0.669 | <0.001** |
| TMB | 1.35 ± 0.774 | 2.18 ± 0.904 | <0.001** | 1 ± 0.649 | 2.1 ± 0.968 | 0.001* |
| Thumb PDN | 1.76 ± 0.89 | 2.47 ± 0.788 | 0.001* | 1.63 ± 0.831 | 2.37 ± 0.895 | 0.014* |
| Index finger PDN | 0.97 ± 0.797 | 1.85 ± 0.784 | <0.001** | 0.84 ± 0.765 | 1.68 ± 0.82 | 0.004* |
| CPDN #2 | 1.25 ± 0.95 | 1.91 ± 0.928 | 0.009* | 0.68 ± 0.82 | 1.53 ± 1.02 | 0.011* |
| CPDN #3 | 1.47 ± 0.983 | 2.16 ± 0.92 | 0.007* | 0.89 ± 0.875 | 1.79 ± 1.228 | 0.019* |
| UN superficial branch | 1.35 ± 0.95 | 2.09 ± 0.712 | <0.001** | 1.16 ± 0.765 | 2.26 ± 0.806 | <0.001** |
| Diagnostic confidence | 1.61 ± 0.494 | 2 ± 0.239 | <0.001** | 1.24 ± 0.436 | 1.95 ± 0.218 | 0.005* |
| Lesion conspicuity | – | – | – | 2 ± 0.678 | 2.62 ± 0.498 | 0.003* |
Data are expressed as average ± standard deviation.
p < 0.05. **p < 0.001.
ceMRN, contrast-enhanced magnetic resonance neurography; CPDN, common palmar digital nerve; MN, median nerve; ncMRN, non-contrast MRN; PDN, proper digital nerve; TMB, thenar muscular branch; UN, ulnar nerve.
Figure 1.

Nerve visualization scores between ncMRN and ceMRN in all subjects.
ceMRN, contrast-enhanced magnetic resonance neurography; CPDN, common palmar digital nerve; MN, median nerve; ncMRN, non-contrast MRN; PDN, proper digital nerve; TMB, thenar muscular branch; UN, ulnar nerve.
Figure 2.
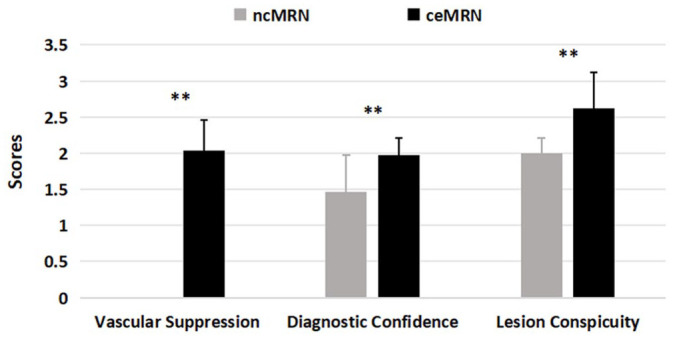
Comparison of vascular suppression, diagnostic confidence, and lesion conspicuity scores between ncMRN and ceMRN in all subjects.
ceMRN, contrast-enhanced magnetic resonance neurography; ncMRN, non-contrast MRN.
Quantitative assessment
The SNR(nerve), SNR(thenar muscle), SNR(bone marrow), SNR(tendon), SNR(subcutaneous fat), and SNR(vessel) on ceMRN were lower than those on ncMRN in both volunteers and patients. As for CR, CR(nerve to bone marrow) and CR(nerve to vessel) on ceMRN were significantly higher than those on ncMRN (p = 0.043 and p < 0.001) in volunteers. CR(nerve to fat) and CR(nerve to vessel) on ceMRN were significantly higher than those on ncMRN (both p = 0.001) in patients. The CNR(nerve to vessel) on ceMRN was significantly higher than that on ncMRN in both volunteers and patients(p < 0.001 and p = 0.005). The CNR(nerve to tendon) on ceMRN was significantly lower than that on ncMRN in patients (p = 0.026; Table 5).
Table 5.
Comparisons of SNR, CR, and CNR between ncMRN and ceMRN in 36 volunteers and 21 patients.
| Metrics | Volunteers | Patients | ||||
|---|---|---|---|---|---|---|
| ncMRN (n = 36) | ceMRN (n = 36) | p | ncMRN (n = 21) | ceMRN (n = 21) | p | |
| SNR | ||||||
| Nerve | 250.35 ± 148.04 | 217 ± 110.94 | 0.069 | 256.58 ± 119.93 | 204.49 ± 119.25 | 0.014* |
| Thenar muscles | 181.16 ± 109.86 | 159.42 ± 94.23 | 0.076 | 167.36 ± 68.20 | 138.63 ± 99.44 | 0.137 |
| Bone marrow | 53.97 ± 38.75 | 36.20 ± 24.18 | 0.002* | 46.77 ± 18.31 | 31.16 ± 21.22 | 0.004* |
| Tendon | 20.29 ± 14.64 | 16.40 ± 9.08 | 0.079 | 21.90 ± 15.82 | 14.23 ± 10.57 | 0.014* |
| Subcutaneous fat | 76.17 ± 58.34 | 57.28 ± 67.85 | 0.023* | 79.32 ± 43.26 | 38.31 ± 27.18 | <0.001** |
| Vessel | 414.95 ± 279.03 | 157.41 ± 193.89 | <0.001** | 442.28 ± 344.28 | 178.34 ± 128.42 | 0.002* |
| CR | ||||||
| Nerve versus muscle | 0.15 ± 0.15 | 0.17 ± 0.16 | 0.538 | 0.18 ± 0.20 | 0.19 ± 0.18 | 0.964 |
| Nerve versus bone marrow | 0.65 ± 0.15 | 0.71 ± 0.16 | 0.043* | 0.66 ± 0.14 | 0.71 ± 0.14 | 0.137 |
| Nerve versus tendon | 0.84 ± 0.08 | 0.85 ± 0.06 | 0.509 | 0.83 ± 0.08 | 0.86 ± 0.07 | 0.057 |
| Nerve versus subcutaneous fat | 0.55 ± 0.24 | 0.61 ± 0.34 | 0.063 | 0.52 ± 0.19 | 0.68 ± 0.16 | 0.001* |
| Nerve versus vessel | −0.16 ± 0.35 | 0.29 ± 0.37 | <0.001** | −0.21 ± 0.30 | 0.13 ± 0.39 | 0.001* |
| CNR | ||||||
| Nerve versus muscle | 105.63 ± 131.57 | 87.90 ± 102.81 | 0.288 | 136.21 ± 158.65 | 100.54 ± 113.49 | 0.14 |
| Nerve versus bone marrow | 299.81 ± 198.36 | 276.02 ± 155.48 | 0.379 | 320.32 ± 171.37 | 264.61 ± 162.35 | 0.054 |
| Nerve versus tendon | 351.23 ± 211.75 | 306.25 ± 160.32 | 0.088 | 358.30 ± 172.04 | 290.47 ± 172.46 | 0.026* |
| Nerve versus subcutaneous fat | 265.92 ± 203.60 | 243.84 ± 203.80 | 0.386 | 270.63 ± 162.19 | 253.71 ± 153.09 | 0.599 |
| Nerve versus vessel | −251.31 ± 376.06 | 90.98 ± 281.94 | <0.001** | −283.51 ± 478.78 | 39.92 ± 187.92 | 0.005* |
p < 0.05. **p < 0.001.
ceMRN, contrast-enhanced magnetic resonance neurography; CNR, contrast-to-noise ratio; CR, contrast ratio; ncMRN, non-contrast MRN; SNR, signal-to-noise ratio.
Normal and pathological findings on ceMRN images
The median, ulnar nerves and their small branches including TMB, CPDNs, and PDNs in the wrist and palm are clearly delineated (Figures 3 and 4 and Supplemental Figure). All neuropathies and associated lesions, including CTS, ganglion cysts, hemangioma, and edema of hypothenar muscle, were depicted on both ncMRN and ceMRN images. In the case of CTS, the median nerve exhibits a focal hypointensity at the level of entrapment on ceMRN images (Figure 5). In the case of hemangioma, the lesion grows along the median nerve with an unclear demarcation on the ncMRN image. Meanwhile, the distal nerve and vessel branches are distinguished through signal suppression of malformed vessels on the ceMRN image (Figure 6). In the case of hypothenar muscle edema, the diffuse hyperintensity of edema obscured the nerves on the ncMRN image. Meanwhile, the contour of the median nerve, ulnar nerve, and their branches are clearly delineated by suppressing the signal of edema on the ceMRN image (Figure 7).
Figure 3.
Volunteer, 45-year-old, female. The ncMRN (a) and ceMRN (b) show coronal views of the median and ulnar nerves and their branches in the wrist and palm. The visualization of the nerves is significantly improved on the ceMRN image. The median nerve passes through the transverse carpal ligament and gives off the common palmar digital nerves (white circle), and the proper digital nerves (arrowhead) of the thumb. The superficial ulnar nerve passes through the palmar carpal ligament and gives off the common digital nerves (long arrow, white ★) and communication branch to the median (short arrow). The signal of the palmar vessel (stubby arrows) is significantly suppressed on the ceMRN image. (c) ceMRN delineates the first common palmar digital nerve further divided into two proper digital nerves to the thumb and one proper digital nerve to the radial side of the index finger at a distal level (short arrows). The proper digital nerves to the thumb include the medial and lateral branches (d).
ceMRN, contrast-enhanced magnetic resonance neurography; ncMRN, non-contrast MRN.
Figure 4.
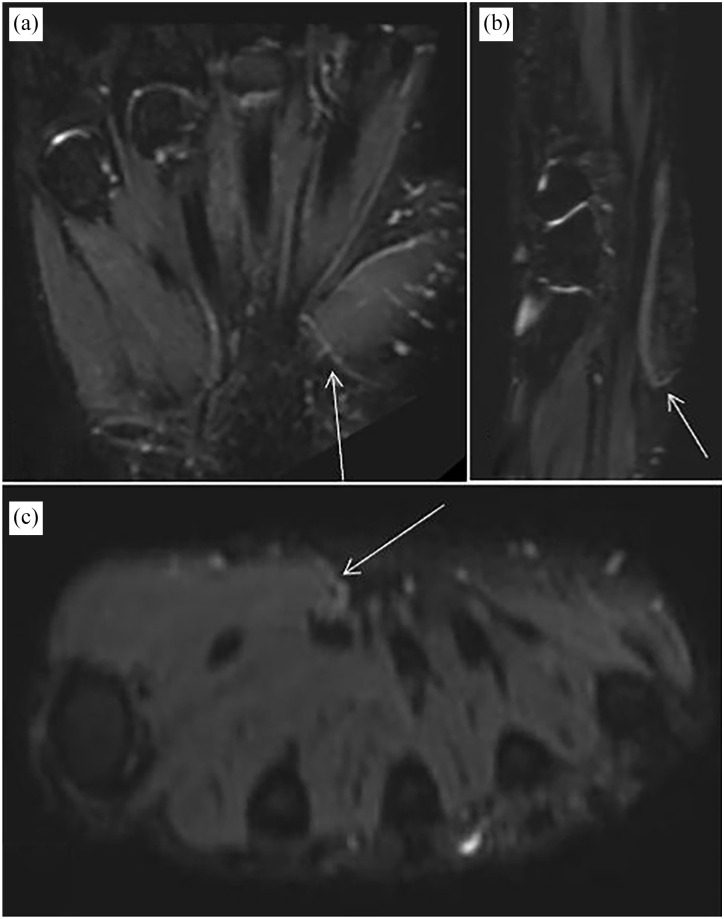
Volunteer, 29-year-old, male. The coronal image (a), sagittal image (b), and axial image (c) of ceMRN show the TMB of the median nerve.
ceMRN, contrast-enhanced magnetic resonance neurography; TMB, thenar muscular branch.
Figure 5.
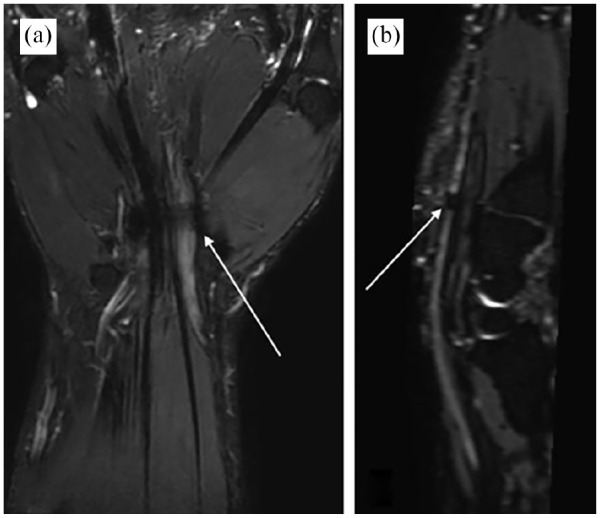
Images of carpal tunnel syndrome obtained with ceMRN. The coronal (a) and sagittal (b) images show a hypointense lesion of the median nerve at the level of the distal edge of the transverse carpal ligament (arrow), which seems to be interrupted.
ceMRN, contrast-enhanced magnetic resonance neurography.
Figure 6.
An example of hemangioma along the median nerve on ncMRN (a) and ceMRN (b) images shows the relationship between lesions and median nerves. (a) The hemangioma surrounding the median nerve was spindle shaped and hyperintense on the ncMRN image. The hemangioma encapsulates the nerve with ill-defined borders. (b) On the ceMRN image, the malformed vessel exhibits hypointense (long arrow), and the vascular signal of the superficial palmar arch (short arrow) is suppressed, distinguishing it from the proper digital nerve (thick arrow).
ceMRN, contrast-enhanced magnetic resonance neurography; ncMRN, non-contrast MRN.
Figure 7.
An example of edema of the hypothenar on ncMRN (a) and ceMRN (b) images. (a) The median nerve (long arrow) and ulnar nerve (short arrow) are obscured by the hyperintensity of edema and vessels on the ncMRN image. (b) The edema signal in the thenar region is significantly reduced (thick arrow) and the delineation of the median nerve branches in the carpometacarpal region (circle) and the ulnar nerve at the entrance of the carpal tunnel (short arrow) are significantly improved on ceMRN image.
ceMRN, contrast-enhanced magnetic resonance neurography; ncMRN, non-contrast MRN.
Discussions
Visualization of nerves in the wrist and palm using MRI remains challenging due to the tiny branches, complex structure, and susceptibility to interference from adjacent tissues, especially blood vessels.21,22 Recently, the application of ceMRN to visualize the peripheral nerves, especially the small branches, has attracted the attention of clinicians.23,24 Robust signal suppression of soft tissues such as vessels and fat facilitates the imaging of anatomy and neuropathies.14–16 In this study, we evaluated the feasibility and advantages of ceMRN in visualizing nerves and lesions in this region from both qualitative and quantitative perspectives and found that the median nerve, ulnar nerve, and small branches and nerve-associated lesions obtained excellent visualization and identification by ceMRN.
The anatomy of the median nerve, ulnar nerve, and their small branches can be clearly delineated using ceMRN. Notably, the TMB, a main motor branch of the median nerve dominating the movements of the thumb, which has multiple variations and is hard to be imaged by routine sequences,25–28 can be well displayed by ceMRN. This study used coronal scanning of the wrist and palm, with an in-plane spatial resolution of 0.69 × 0.69 mm2. Combined with MIP, it can clearly display the complete course of small nerves such as TMB. To shorten the scanning time, the slice thickness was set to 1.4 mm and the inter-slice gap was set to 0.7 mm, which was sufficient for sagittal and axial reconstruction by MPR. If clinically necessary, the inter-slice resolution can be further improved. Good preoperative visualization of the small branches such as the TMB may aid in the planning of CTS decompression surgery. Potentially, ceMRN can provide indications for preoperative planning.
In addition, some specific imaging signs were revealed by ceMRN in patients with nerve-associated lesions in the wrist and palm. We found that the signal of the median nerve at the compression site was reduced in some patients with CTS. We speculate that the compression by the transverse carpal ligament induces blood-nerve barrier (BNB) damage and leads to the entrance of the contrast agent into the nerve. Interestingly, a similar phenomenon was found in a patient with hypothenar edema. Compared with ncMRN, the diffuse hyperintensity of edema was significantly reduced using ceMRN, and the shape of the nerves was clearly displayed. We speculate that the hypothenar edema increases vascular permeability, and the infiltration of contrast agent leads to a reduced signal. In addition, we also found a signal reduction of lesions in a patient with hemangioma growing around the median nerve on ceMRN. The retention of contrast agents in the malformed vessels is supposed to be the cause of signal reduction. Thus, ceMRN helps identify neuropathy and distinguish lesions from nerves.
Combining 3D TSE STIR sequence and paramagnetic contrast agent, ceMRN provides us with a new option to clarify the relationship between nerves and lesions visually. The application of a paramagnetic contrast agent acts to reduce the signal of soft tissues other than nerves, especially vessels. In addition to shortening the T1 relaxation time, paramagnetic contrast agents can also shorten the T2 relaxation time. On the T2-weighted ceMRN images, tissues that uptake or retain contrast agents appear as hypointensity. For example, the vascular signal is significantly decreased because of slow blood flow and high concentration of intravascular contrast agent. Meanwhile, nerves find it difficult to uptake contrast agents due to BNB, so the hyperintensity is maintained. Similarly, contrast-uptaking lesions also manifest hypointense and can be well differentiated from nerves. Compared with other vascular suppression techniques such as motion-sensitive driven equilibrium, 29 contrast-enhanced technique appeared to produce a stronger vascular suppression effect while providing visualization of nerves and enhancement of lesions characteristics simultaneously. Therefore, considering the adverse effects of contrast agents, we believe that ceMRN is particularly suitable for patients who require enhanced examination to evaluate both nerves and lesions.
High contrast of nerves and other tissues and high spatial resolution are required for panoramic imaging of peripheral nerves. On routine MRI, vessels with similar signals make nerve identification difficult, and inhomogeneous or insufficient fat suppression also obscures the course of the nerve. As for the usage of contrast agents, a high-pressure injector is recommended to better control the speed and timing of injection and to make the concentration of contrast agents in the blood vessels high enough. By comparing the image quality of ncMRN and ceMRN, we found that ceMRN significantly improved tissue contrast while sacrificing a small amount of SNR. We attribute the superior nerve imaging on ceMRN to efficient vascular and fat signal suppression. Our further subjective assessment also showed that ceMRN did better in suppressing vascular signals, visualizing nerves, and branches, and identifying lesions than ncMRN. With high contrast and spatial resolution, ceMRN enables the display of tiny branches while providing panoramic images of nerves.
We acknowledge that this study had several limitations. First, we did not compare ceMRN with other high-resolution and vascular suppression sequences such as PSIF and DESS, which have the advantage of locally displaying tiny nerve structures. Second, this study did not carry out a power analysis for sample size calculation, and the sample size was small, so it was difficult to determine the conditions for the application of ceMRN. In general, ceMRN is indicated for patients who require routine enhanced MRI examination and peripheral nerve evaluation. Third, the manifestation of different lesions involving nerves such as trauma and tumors was not revealed in this study. Studies with larger sample sizes and comparisons with other advanced sequences will further confirm the value of ceMRN in the visualization and diagnosis of peripheral nerve diseases.
Conclusion
In conclusion, the ceMRN significantly improves the visualization of peripheral nerves and pathology in the wrist and palm by robustly suppressing the signals of fat, bone marrow, and especially vessels in volunteers and patients.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864241239739 for Improved visualization of median, ulnar nerves, and small branches in the wrist and palm using contrast-enhanced magnetic resonance neurography by Jiamin Kang, Wenjun Wu, Xiangchuang Kong, Yu Su, Dingxi Liu, Chungao Li, Nan Gao, Youzhi Wang, Chuansheng Zheng, Yuxiong Weng and Lixia Wang in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
ORCID iD: Wenjun Wu  https://orcid.org/0000-0001-9594-7026
https://orcid.org/0000-0001-9594-7026
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jiamin Kang, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Department of Radiology, Wuhan No. 1 Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Wenjun Wu, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China.
Xiangchuang Kong, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China.
Yu Su, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Dingxi Liu, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China.
Chungao Li, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China.
Nan Gao, Department of Hand Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Youzhi Wang, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Chuansheng Zheng, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China.
Yuxiong Weng, Department of Hand Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Lixia Wang, Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, No. 1277 Jiefang Avenue, Wuhan 430022, China; Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China.
Declarations
Ethics approval and consent to participate: This study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. UHCT21809, approval date 2021-01-04). Written informed consent to participate was obtained from all subjects.
Consent for publication: Written informed consent to publish was obtained from all subjects.
Author contributions: Jiamin Kang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Visualization; Writing – original draft.
Wenjun Wu: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Resources; Software; Validation; Visualization; Writing – review & editing.
Xiangchuang Kong: Methodology; Resources; Writing – review & editing.
Yu Su: Data curation; Methodology; Software; Writing – review & editing.
Dingxi Liu: Methodology; Software; Writing – review & editing.
Chungao Li: Data curation; Investigation; Methodology; Visualization; Writing – review & editing.
Nan Gao: Data curation; Investigation; Methodology; Writing – review & editing.
Youzhi Wang: Data curation; Investigation; Software; Writing – review & editing.
Chuansheng Zheng: Resources; Software; Supervision; Writing – review & editing.
Yuxiong Weng: Conceptualization; Project administration; Resources; Software; Supervision; Writing – review & editing.
Lixia Wang: Conceptualization; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Hubei Province key research and development project (2022BCA034). Scientific Research Foundation of Wuhan Union Hospital (02.03.2018-224, 02.03.2019-131, F016.02004.21003.103).
The authors declare that there is no conflict of interest.
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Pridmore MD, Glassman GE, Pollins AC, et al. Initial findings in traumatic peripheral nerve injury and repair with diffusion tensor imaging. Ann Clin Transl Neurol 2021; 8: 332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chhabra A, Madhuranthakam AJ, Andreisek G. Magnetic resonance neurography: current perspectives and literature review. Eur Radiol 2018; 28: 698–707. [DOI] [PubMed] [Google Scholar]
- 3. Filler AG, Howe FA, Hayes CE, et al. Magnetic resonance neurography. Lancet 1993; 341: 659–661. [DOI] [PubMed] [Google Scholar]
- 4. Sneag DB, Daniels SP, Geannette C, et al. Post-contrast 3D inversion recovery magnetic resonance neurography for evaluation of branch nerves of the brachial plexus. Eur J Radiol 2020; 132: 109304. [DOI] [PubMed] [Google Scholar]
- 5. Fujii H, Fujita A, Yang A, et al. Visualization of the peripheral branches of the Mandibular division of the trigeminal nerve on 3D double-echo steady-state with water excitation sequence. Am J Neuroradiol 2015; 36: 1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chhabra A, Soldatos T, Subhawong TK, et al. The application of three-dimensional diffusion-weighted PSIF technique in peripheral nerve imaging of the distal extremities. J Magn Reson Imaging 2011; 34: 962–967. [DOI] [PubMed] [Google Scholar]
- 7. Dessouky R, Xi Y, Zuniga J, et al. Role of MR neurography for the diagnosis of peripheral trigeminal nerve injuries in patients with prior molar tooth extraction. Am J Neuroradiol 2018; 39: 162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chalian M, Behzadi AH, Williams EH, et al. High-resolution magnetic resonance neurography in upper extremity neuropathy. Neuroimaging Clin N Am 2014; 24: 109–125. [DOI] [PubMed] [Google Scholar]
- 9. Cassetta M, Pranno N, Pompa V, et al. High resolution 3-T MR imaging in the evaluation of the trigeminal nerve course. Eur Rev Med Pharmacol Sci 2014; 18: 257–264. [PubMed] [Google Scholar]
- 10. Zhang Z, Meng Q, Chen Y, et al. 3-T imaging of the cranial nerves using three-dimensional reversed FISP with diffusion-weighted MR sequence. J Magn Reson Imaging 2008; 27: 454–458. [DOI] [PubMed] [Google Scholar]
- 11. Bao H, Wang S, Wang G, et al. Diffusion-weighted MR neurography of median and ulnar nerves in the wrist and palm. Eur Radiol 2017; 27: 2359–2366. [DOI] [PubMed] [Google Scholar]
- 12. Ding WQ, Zhou XJ, Tang JB, et al. Three-dimensional display of peripheral nerves in the wrist region based on MR diffusion tensor imaging and maximum intensity projection post-processing. Eur J Radiol 2015; 84: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 13. Jeon T, Fung MM, Koch KM, et al. Peripheral nerve diffusion tensor imaging: overview, pitfalls, and future directions. J Magn Reson Imaging 2018; 47: 1171–1189. [DOI] [PubMed] [Google Scholar]
- 14. Wu W, Wu F, Liu D, et al. Visualization of the morphology and pathology of the peripheral branches of the cranial nerves using three-dimensional high-resolution high-contrast magnetic resonance neurography. Eur J Radiol 2020; 132: 109137. [DOI] [PubMed] [Google Scholar]
- 15. Wang L, Niu Y, Kong X, et al. The application of paramagnetic contrast-based T2 effect to 3D heavily T2W high-resolution MR imaging of the brachial plexus and its branches. Eur J Radiol 2016; 85: 578–584. [DOI] [PubMed] [Google Scholar]
- 16. Zhang Y, Kong X, Zhao Q, et al. Enhanced MR neurography of the lumbosacral plexus with robust vascular suppression and improved delineation of its small branches. Eur J Radiol 2020; 129: 109128. [DOI] [PubMed] [Google Scholar]
- 17. Su X, Kong X, Lu Z, et al. Use of magnetic resonance neurography for evaluating the distribution and patterns of chronic inflammatory demyelinating polyneuropathy. Korean J Radiol 2020; 21: 483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhai H, Lv Y, Kong X, et al. Magnetic resonance neurography appearance and diagnostic evaluation of peripheral nerve sheath tumors. Sci Rep 2019; 9: 6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang X, Li M, Guan J, et al. Evaluation of the sacral nerve plexus in pelvic endometriosis by three-dimensional MR neurography. J Magn Reson Imaging 2017; 45: 1225–1231. [DOI] [PubMed] [Google Scholar]
- 20. American College of Radiology. Magnetic resonance imaging quality control manual. Reston, VA: ACR, 2015, pp. 97–100. [Google Scholar]
- 21. Dos Santos Silva J, de Barros LFP, de Freitas Souza R, et al. ‘Million dollar nerve’ magnetic resonance neurography: first normal and pathological findings. Eur Radiol 2022; 32: 1154–1162. [DOI] [PubMed] [Google Scholar]
- 22. Chhabra A, Subhawong TK, Bizzell C, et al. 3T MR neurography using three-dimensional diffusion-weighted PSIF: technical issues and advantages. Skeletal Radiol 2011; 40: 1355–1360. [DOI] [PubMed] [Google Scholar]
- 23. Deshmukh S, Tegtmeyer K, Kovour M, et al. Diagnostic contribution of contrast-enhanced 3D MR imaging of peripheral nerve pathology. Skeletal Radiol 2021; 50: 2509–2518. [DOI] [PubMed] [Google Scholar]
- 24. Xu Z, Zhang T, Chen J, et al. Combine contrast-enhanced 3D T2-weighted short inversion time inversion recovery MR neurography with MR angiography at 1.5 T in the assessment of brachial plexopathy. MAGMA 2021; 34: 229–239. [DOI] [PubMed] [Google Scholar]
- 25. Henry BM, Zwinczewska H, Roy J, et al. The prevalence of anatomical variations of the median nerve in the carpal tunnel: a systematic review and meta-analysis. PLoS One 2015; 10: e136477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riegler G, Pivec C, Platzgummer H, et al. High-resolution ultrasound visualization of the recurrent motor branch of the median nerve: normal and first pathological findings. Eur Radiol 2016; 27: 2941–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ahn DS, Yoon ES, Koo SH, et al. A prospective study of the anatomic variations of the median nerve in the carpal tunnel in Asians. Ann Plast Surg 2000; 44: 282–287. [DOI] [PubMed] [Google Scholar]
- 28. Petrover D, Bellity J, Vigan M, et al. Ultrasound imaging of the thenar motor branch of the median nerve: a cadaveric study. Eur Radiol 2017; 27: 4883–4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. De Paepe KN, Higgins DM, Ball I, et al. Visualizing the autonomic and somatic innervation of the female pelvis with 3D MR neurography: a feasibility study. Acta Radiol 2020; 61: 1668–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864241239739 for Improved visualization of median, ulnar nerves, and small branches in the wrist and palm using contrast-enhanced magnetic resonance neurography by Jiamin Kang, Wenjun Wu, Xiangchuang Kong, Yu Su, Dingxi Liu, Chungao Li, Nan Gao, Youzhi Wang, Chuansheng Zheng, Yuxiong Weng and Lixia Wang in Therapeutic Advances in Neurological Disorders