Abstract
Background:
Reporting of sex-specific analyses in multiple sclerosis (MS) is sparse. Disability accrual results from relapses (relapse-associated worsening) and independent thereof (progression independent of relapses).
Objectives:
A population of MS patients during relapse treated per standard of care was analyzed for sex differences and short-term relapse outcome (3–6 months) as measured by Expanded Disability Status Scale (EDSS) change.
Design:
Single-center retrospective study.
Methods:
We analyzed 134 MS relapses between March 2016 and August 2020. All events required relapse treatment (steroids and/or plasma exchange). Demographic, disease, and paraclinical characteristics [cerebrospinal fluid (CSF) and magnetic resonance imaging (MRI)] were displayed separated by sex. Multivariable linear regression was run to identify factors associated with short-term EDSS change.
Results:
Mean age at relapse was 38.4 years (95% confidence interval: 36.3–40.4) with a proportion of 71.6% women in our cohort. Smoking was more than twice as prevalent in men (65.8%) than women (32.3%). In- and after-relapse EDSSs were higher in men [men: 3.3 (2.8–3.9), women: 2.7 (2.4–3.0); men: 3.0 (1.3–3.6); women: 1.8 (1.5–2.1)] despite similar relapse intervention. Paraclinical parameters revealed no sex differences. Our primary model identified female sex, younger age, and higher EDSS at relapse to be associated with EDSS improvement. A higher immunoglobulin G (IgG) quotient (CSF/serum) was associated with poorer short-term outcome [mean days between first relapse treatment and last EDSS assessment 130.2 (79.3–181.0)].
Conclusion:
Sex and gender differences are important in outcome analyses of MS relapses. Effective treatment regimens need to respect putative markers for a worse outcome to modify long-term prognosis such as clinical and demographic variables, complemented by intrathecal IgG synthesis. Prospective trials should be designed to address these differences and confirm our results.
Keywords: EDSS, gender, MS, plasma exchange, steroid
Plain language summary
An analysis of 134 acute relapses of multiple sclerosis reveal sex differences influencing recovery from relapse
Sex-specific analyses are important in medicine, but more knowledge is still needed. Multiple sclerosis (MS) as an inflammatory disease of the brain and spinal cord mainly affects younger people who are at risk for development of disability. Disability may result from acute relapses of the disease that insufficiently recover. Our analysis aimed to assess sex differences with a special focus on the acute relapse and 3 to 6 months later on average. We collected existing data from our center and identified 134 relapse events with sufficient data for further analysis. All relapses were treated with medical (high-dose steroids) and/or interventional treatment (plasma exchange). We analyzed the influence of sex, age, smoking, relapse severity, relapse treatment and other treatment (immunotherapy) for MS. In a second analysis, cerebrospinal fluid (CSF) and imaging (MRI) parameters were included. Our cohort consisted of 72% women. The mean age was 38 years. Smoking was twice as common in men (66%) than in women (32%). Men also experienced more severe disability in and after the relapse. Several other factors were similar between men and women. Female sex and younger age were associated with lower disability after a relapse. Paradoxically, also higher disability in the relapse was associated with lower disability later on. This might be a statistical phenomenon and partly explained by overall low disability levels in our analysis. It might therefore not be true for more advanced disease stages with higher disability. The presence of a certain CSF marker (intrathecal IgG synthesis) was associated with higher disability after the relapse. Our analysis thus identified markers associated with different relapse recovery, male vs. female sex being one of them.
Introduction
Multiple sclerosis (MS) represents a neurological disorder with rising prevalence worldwide. 1 Most commonly, affected persons suffer from a relapsing onset.2,3 Recent evidence underscores the importance of early neurodegeneration and disability accrual independently of relapses, so-called progression independent of relapses, in relapsing-onset MS.4–6 However, it has been demonstrated that early relapse-associated worsening (RAW) and most importantly early relapse activity per se represent relevant predictors of long-term disability and conversion to secondary progressive disease.2,7,8 Yet, only RAW seemed to be modifiable with early immunomodulatory treatment. 7 Effective relapse treatment will thus remain an important therapeutic tool to lower RAW and modify long-term prognosis in MS.
A more individualized relapse treatment approach based on potential predictors of relapse outcome still represents an unmet need in MS treatment. Risk factors of poor relapse recovery have been described and include male sex, higher age at relapse, longer disease duration, and relapse characteristics such as motor and cerebellar involvement.9–11 Data on paraclinical markers, for example, black holes in brain magnetic resonance imaging (MRI) seem less robust. 10
To pave the way for personalized approaches, the understanding of sex-specific differences seems basic, but crucial. With regard to MS and more specifically relapse outcome, contradictory elements persist. As an example, in relapse-onset MS, male sex has been found to be associated with faster disability accrual in an MSBase analysis, 12 but not in the German MS registry. 3 Likewise, male sex was associated with worse relapse recovery, 10 but not identified as a generalizable indicator of aggressive MS or significantly influencing MS severity scale (MSSS) modeling.13,14 Interestingly, sex differences in relapse presentation seem robust over different study settings with more motor and cerebellar involvement in men and more sensory and visual symptoms in women.3,9 Taken together, these data with poorer relapse recovery and more motor involvement in men might suggest a general effect of male sex on higher disability progression 11 indicating that additional factors must play a role. Among the latter surely are patient age, disease duration, and disease-modifying treatment (DMT),2,10,13 but also individual and possibly gender-influenced behavioral factors, especially smoking15–20 and obesity. 21
We here set out to describe to what extent male and female MS patients during relapse treated per standard of care differ in relapse presentation and severity, demographic, sociocultural, and disease characteristics, and to analyze demographic, clinical, and paraclinical [cerebrospinal fluid (CSF) and MRI] factors potentially associated with short-term relapse outcome (after 3–6 months) as measured by Expanded Disability Status Scale (EDSS) change.
Methods
Study design and participants
In this retrospective study, adult patients with relapsing forms of MS (including clinically isolated syndrome, relapsing-remitting and secondary progressive MS with relapse) were identified from clinical records having received steroid and/or plasma exchange (PLEX) treatment between March 2016 and August 2020. All patients were treated within routine clinical care in the Department of Neurology, Inselspital, Bern University Hospital (Switzerland). PLEX was performed during routine clinical care as described elsewhere by our group. 22
Assessment plan and outcomes
The following parameters were retrospectively identified from the electronic patient files:
- Demographics [sex, age at relapse, smoking status (yes/no)].
- MS disease characteristics [disease course and duration, first relapse (yes/no), prior PLEX treatment (yes/no), DMT at relapse].
- Current relapse characteristics [onset and symptoms of current relapse, characterized by EDSS total score 23 and affected functional systems, visual acuity (in case of optic neuritis) before, at relapse and after each course of relapse treatment, all administered relapse treatments including dosages/number of sessions].
The descriptive analysis aimed to assess potential sex differences of these parameters.
The consecutive primary analysis aimed to detect whether these factors were associated with EDSS change from first relapse assessment to last assessment after relapse treatment.
In exploratory subgroup analyses, we performed the same analysis for mono- and polysymptomatic events, separately, and likewise for mainly visual, brainstem, motor, and sensory events.
For those patients with available CSF and MRI as part of their relapse work-up, the following parameters were additionally assessed:
- Immunoglobulin G (IgG) quotient (CSF/serum), IgM quotient (CSF/serum), IgA quotient (CSF/serum), albumin quotient (CSF/serum), total CSF protein, total CSF white blood cell count, presence of CSF-restricted oligoclonal bands (OCB).
- Brain MRI: presence of leptomeningeal enhancement, presence of any and number of gadolinium-enhancing lesions/paramagnetic rim lesions/central vein sign lesions/black holes/diffusion-restricted lesions.
- Spinal MRI: presence of any and number of gadolinium-enhancing lesions.
- Orbital MRI: presence of gadolinium enhancement or edema of the optic nerve.
- Presence of a gadolinium-enhancing lesion corresponding to the relapse symptoms.
As patient numbers were expectedly lower in these subgroups, these paraclinical factors were investigated as an exploratory outcome in the model, as well.
Statistical analyses
The group comparisons between men and women were performed using Mann–Whitney test or Fisher’s exact test for frequency distributions, respectively. Bonferroni adjustment to control for multiple comparison has been performed for CSF and MRI marker sets in comparison of sex effects, as indicated in Table 1.
Table 1.
Demographic, clinical, and paraclinical characteristics of the cohort.
| Variable | Total cohort (n = 134 events in 113 persons) | Women (n = 96 events in 79 persons) | Men (n = 38 in 34 persons) | p Value |
|---|---|---|---|---|
| (A) Basic demography and disease course | ||||
| Female sex (n, %) | 96/134 (71.6%) | – | – | |
| Age at relapse (years; mean, 95% CI) | 38.4 (36.3–40.4) | 37.0 (34.7–39.3) | 41.8 (37.5–46.1) | 0.06 |
| Current smoking (n, %) | 56/134 (41.8%) | 31/96 (32.3) | 25/38 (65.8) | <0.001 |
| MS disease course (n, %) | ||||
| CIS | 6/134 (4.5%) | 5/96 (5.2%) | 1/38 (2.6%) | 0.68 |
| RMS | 128/134 (95.5%) | 91/96 (94.8%) | 37/38 (97.4%) | |
| MS disease duration at index relapse (years; mean, 95% CI; available for n = 131) | 6.3 (4.9–7.7) | 5.0 (3.6–6.4) | 9.9 (6.5–13.2) | 0.02 |
| First relapse (n, %) | 42/134 (31.3%) | 32/96 (33.3%) | 10/38 (26.3%) | 0.54 |
| Prior PLEX (n, %; available for n = 127) | 18/127 (14.2%) | 16/93 (17.2%) | 2/34 (5.9%) | 0.15 |
| (B) Relapse characteristics | ||||
| - Monosymptomatic relapse (n, %) | 72/132 (54.5%) | 54/94 (57.4%) | 18/38 (47.4%) | 0.34 |
| - Polysymptomatic relapse (n, %) | 60/132 (45.5%) | 40/94 (42.6%) | 20/38 (52.6%) | |
| Main relapse symptom (n, %) | ||||
| Visual | 37 (27.6%) | 30 (31.3%) | 7 (18.4%) | 0.15 |
| Brainstem | 16 (11.9%) | 13 (13.5%) | 3 (7.9%) | |
| Sensory | 32 (23.9%) | 20 (20.8%) | 12 (31.6%) | |
| Pyramidal | 11 (8.2%) | 5 (5.2%) | 6 (15.8%) | |
| Bowel/bladder | 2 (1.5%) | 1 (1.0%) | 1 (2.6%) | |
| Combinations/unclassifiable | 36 (26.9%) | 27 (28.1%) | 9 (23.7%) | |
| If not first relapse: EDSS before current relapse (mean, 95% CI; available for n = 70) | 2.2 (1.8–2.7) | 1.7 (1.3–2.2) | 3.2 (2.4–4.1) | 0.001 |
| EDSS during current relapse before first relapse treatment (mean, 95% CI) | 2.9 (2.6–3.1) | 2.7 (2.4–3.0) | 3.3 (2.8–3.9) | 0.02 |
| EDSS after last relapse treatment (mean, 95% CI) | 2.1 (1.9–2.4) | 1.8 (1.5–2.1) | 3.0 (1.3–3.6) | <0.001 |
| EDSS change during current relapse (before first to after last relapse treatment; mean, 95% CI) | −0.7 [−0.9 to (−0.5)] | −0.9 [−1.1 to (−0.6)] | −0.4 (−0.6 to 0.14) | 0.01 |
| (C) Relapse treatments | ||||
| Time between first symptoms of present relapse and start of first relapse treatment (days; mean, 95% CI; available for n = 131) | 35.0 (2.9–46.1) | 35.2 (22.2–48.2) | 34.5 (12.1–56.9) | 0.99 |
| Time between first relapse treatment and last EDSS assessment (days; mean, 95% CI) | 130.2 (79.3–181.0) | 102.3 (76.4–128.2) | 200.6 (30.1–371.0) | 0.24 |
| Steroid dosage, total (g; mean, 95% CI) | 4.9 (4.4–5.5) | 5.3 (4.6–6.1) | 4.0 (3.2–4.9) | 0.21 |
| PLEX (n, %) | 47/134 (35.1%) | 38/96 (39.6%) | 9/38 (23.7%) | 0.11 |
| (D) Disease-modifying treatment at relapse (n, %) | ||||
| - yes | 49/134 (36.6%) | 34/96 (41.7%) | 15/38 (42.1%) | 0.69 |
| Interferon-beta formulations | 6 | 6 | 0 | |
| Glatiramer acetate | 3 | 3 | 0 | |
| Dimethylfumarate | 16 | 12 | 4 | |
| Teriflunomide | 2 | 2 | 0 | |
| Fingolimod | 16 | 9 | 7 | |
| Ocrelizumab | 3 | 2 | 1 | |
| Rituximab | 3 | 0 | 3 | |
| (E) CSF parameters (Bonferroni adjusted significance level of p value = 0.007) | ||||
| IgG quotient (CSF/serum; *10−3; mean, 95% CI; available for n = 87) | 5.0 (4.3–5.7) | 5.1 (4.1–6.1) | 4.7 (4.0–5.5) | 0.24 |
| IgM quotient (CSF/serum; *10−3; mean, 95% CI; available for n = 59) | 1.0 (0.7–1.4) | 1.1 (0.6–1.5) | 1.0 (0.7–1.3) | 0.04 |
| IgA quotient (CSF/serum; *10−3; mean, 95% CI; available for n = 61) | 1.9 (1.6–2.3) | 1.8 (1.4–2.3) | 2.2 (1.8–2.6) | 0.01 |
| Albumin quotient (CSF/serum; *10−3; mean, 95% CI; available for n = 87) | 5.7 (5.1–6.3) | 5.4 (4.7–6.2) | 6.5 (5.6–7.3) | 0.01 |
| Total CSF protein (g/l; mean, 95% CI; available for n = 90) | 0.4 (0.3–0.4) | 0.4 (0.3–0.4) | 0.4 (0.4–0.5) | 0.01 |
| Total CSF white blood cell count (M/l; mean, 95% CI; available for n = 81) | 16.0 (4.0–28.0) | 19.8 (3.1–36.6) | 6.5 (3.6–9.4) | 0.40 |
| CSF-restricted oligoclonal bands, yes (n, %; available for n = 67) | 61/67 (91.0) | 16/18 (88.9) | 45/49 (91.8) | 0.66 |
| (F) MRI parameters (Bonferroni adjusted significance level of p value = 0.004) | ||||
| Brain: Number of new T2/FLAIR lesions* (mean, 95% CI; available for n = 131) | 9.2 (6.4–12.1) | 9.8 (6.5–13.2) | 7.7 (2.2–13.3) | 0.18 |
| Brain: Number of Gad-enhancing lesions (mean, 95% CI; available for n = 130) | 2.9 (1.3–4.6) | 3.4 (1.2–5.7) | 1.6 (0.6–2.7) | 0.31 |
| Brain: Number of iron rim lesions (mean, 95% CI; available for n = 72) | 0.2 (0.1–0.4) | 0.2 (0.0–0.4) | 0.3 (0.1–0.6) | 0.91 |
| Brain: Number of lesions with central vein sign (mean, 95% CI; available for n = 72) | 2.4 (1.8–3.1) | 2.4 (1.6–3.1) | 2.7 (1.4–3.9) | 0.67 |
| Brain: Number of black holes (mean, 95% CI; available for n = 126) | 9.4 (5.5–13.4) | 8.1 (3.8–12.3) | 12.8 (3.9–21.6) | 0.09 |
| Brain: Number of diffusion-restricted lesions (mean, 95% CI; available for n = 128) | 1.8 (0.2–3.4) | 2.2 (−0.1 to 4.5) | 0.8 (0.2–1.5) | 0.29 |
| Brain: Leptomeningeal enhancement, yes (n, %; available for n = 95) | 7/95 (7.4) | 4/68 (5.9) | 3/27 (11.1) | 0.40 |
| Spinal cord: Number of new T2 lesions* (mean, 95% CI; available for n = 99) | 2.7 (0.7–4.7) | 1.9 (1.3–2.6) | 4.9 (−2.9 to 12.6) | 0.37 |
| Spinal cord: Number of Gad-enhancing lesions (mean, 95% CI; available for n = 98) | 0.4 (0.2–0.5) | 0.3 (0.2–0.4) | 0.6 (0.2–1.0) | 0.33 |
| Orbita: ON Gad-enhancement, yes (n, %; available for n = 35) | 29/35 (82.9) | 6/8 (75.0) | 23/27 (85.2) | 0.60 |
| Orbita: ON edema, yes (n, %; available for n = 35) | 30/35 (85.7) | 7/8 (87.5) | 23/27 (85.2) | 1.00 |
| Gad-enhancing lesion corresponding to relapse symptoms, yes (n, %; available for n = 124) | 81/124 (65.3) | 63/89 (70.8) | 18/35 (51.4) | 0.06 |
Bonferroni adjustment of p value level was performed for the marker sets of CSF and MRI. Significant results displayed in bold.
All lesions were counted as new in case of first performed MRI.
95% CI, 95% confidence interval; CIS, clinically isolated syndrome; CSF, cerebrospinal fluid; EDSS, expanded disability status scale; FLAIR, fluid attenuated inverse recovery; Gad, gadolinium; IgA/IgG/IgM, immunoglobulin A/G/M; MRI, magnetic resonance imaging; MS, multiple sclerosis; ON, optic nerve; PLEX, plasma exchange; RMS, relapsing forms of MS.
A multivariable linear regression (mvReg) was run to analyze the change in EDSS from first assessment at relapse to EDSS after the last relapse treatment for all relapse events (primary outcome) irrespective of the relapse phenotype. Sex, age at relapse, smoking status (yes versus no), total EDSS at relapse, administered steroid dosage (up to 3 g methyl prednisolone equivalent versus more than 3 g reflecting clinical routine of steroid dose escalation), PLEX treatment for current relapses (yes versus no), and disease modifying treatment at relapse (yes versus no) were included as independent variables.
As an exploratory analysis, different relapses phenotypes were separately investigated. The same mvReg model and independent variables were thus applied to monosymptomatic and polysymptomatic relapses as well as relapses classified by main affected functional domain.
In an additional exploratory analysis, CSF and MRI parameters were separately added to the mvReg model and the most promising CSF and MRI candidate parameter each was integrated into the final CSF + MRI model.
Adjusted Nagelkerke’s R2 is reported for each model. The variance inflation factor (VIF) is given in each model to address potential collinearity between variables with VIF of 5 or more indicating an interrelation. p values <0.05 were considered as significant in multivariable analyses.
The software used was SPSS Statistic 25 (IBM Corp., Armonk, NY, USA).
Results
Characteristics of the cohort and comparison of sexes
Expectedly, the cohort consisted of more female (71.6%) than male MS patients. Overall, female and male patients exhibited similar demographic and clinical characteristics (Table 1).
However, remarkable differences were detected for smoking behavior being more than twice as common in men. Men had a longer disease duration than women and a higher EDSS before the index relapse, during and thereafter. The EDSS difference from in- to after-relapse was thus larger in women despite similar relapse treatment patterns and timings. The DMT distribution, CSF, and MRI parameters revealed no significant differences.
Demographic and clinical factors associated with EDSS change after relapse treatment
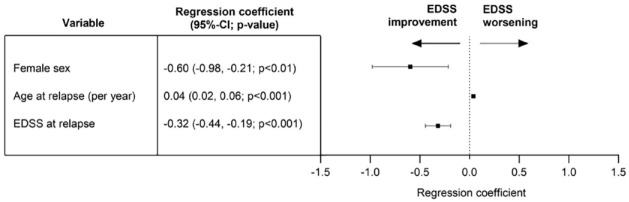
We identified female sex, younger age, and a higher EDSS during relapse as factors associated with a higher chance of EDSS improvement after relapse treatment. Smoking, relapse treatment parameters, and DMT were not associated with the change in EDSS (Figure 1).
Figure 1.
Multivariable linear regression analysis of all relapses (primary analysis) irrespective of relapse phenotype. Dependent variable (outcome): change in EDSS from first assessment at relapse to EDSS after the last relapse treatment. Independent variables: sex, age at relapse, smoking status (yes versus no), total EDSS at relapse, administered steroid dosage (up to 3 g methyl prednisolone equivalent versus more than 3 g), plasma exchange treatment for current relapse (yes versus no), and disease-modifying treatment at relapse (yes versus no). n = 134 events, adjusted Nagelkerke’s R2 0.27, variance inflation factor for all independent variables below 1.8. Significant associations for female sex, age at relapse, and EDSS at relapse, all other investigated independent variables did not demonstrate a significant association with EDSS change (Supplemental Table 1).
95% CI, 95% confidence interval; EDSS, expanded disability status scale; PLEX, plasma exchange.
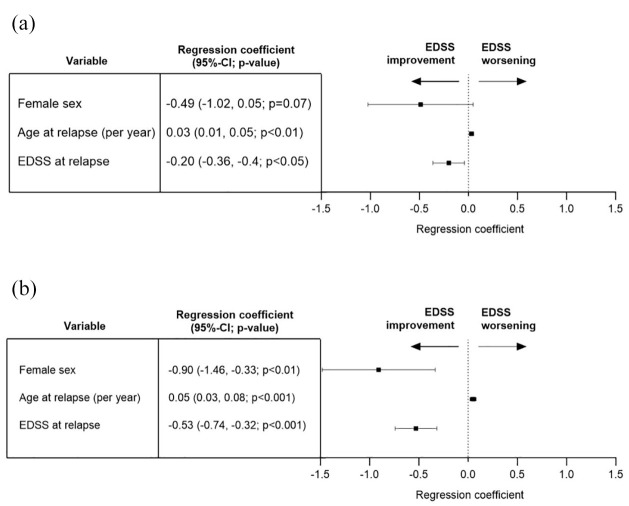
When stratifying relapse events by mono- versus polysymptomatic presentation, thus with lower event numbers in both groups, the sex effect only demonstrated a trend in monosymptomatic relapses whereas all other parts of the analyses display similar results [Figure 2(a) and (b)].
Figure 2.
Multivariable linear regression analysis of mono- and polysymptomatic relapses (exploratory analysis). Dependent variable (outcome): change in EDSS from first assessment at relapse to EDSS after the last relapse treatment. Independent variables: sex, age at relapse, smoking status (yes versus no), total EDSS at relapse, administered steroid dosage (up to 3 g methyl prednisolone equivalent versus more than 3 g), plasma exchange treatment for current relapse (yes versus no), and disease-modifying treatment at relapse (yes versus no). (a) Monosymptomatic: n = 72 events, adjusted Nagelkerke’s R2 0.26, VIF for all independent variables below 1.8. Significant associations for age at relapse, EDSS at relapse and smoking status (not on graph), all other investigated independent variables did not demonstrate a significant association with EDSS change (Supplemental Table 1). (b) Polysymptomatic: n = 60 events, adjusted Nagelkerke’s R2 0.36, VIF for all independent variables below 1.8. Significant associations for female sex, age at relapse, and EDSS at relapse; all other investigated independent variables did not demonstrate a significant association with EDSS change (Supplemental Table 1).
95% CI, 95% confidence interval; EDSS, expanded disability status scale; VIF, variance inflation factor.
The subgroup analyses of different relapse phenotypes with 11–37 events per group did not demonstrate robust findings (Supplemental Table 1, Section 4–7).
CSF- and MRI-related factors associated with EDSS change after relapse treatment
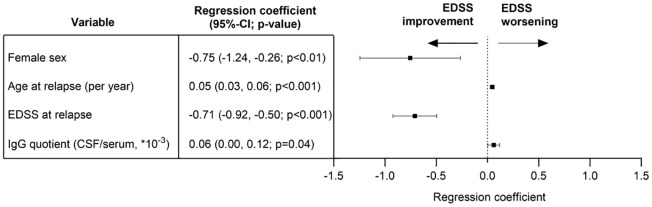
The mvReg model of the primary outcome (Figure 1) was used to add each CSF and MRI parameter, separately, in order to identify potential candidate markers for EDSS change after relapse treatment. The most promising candidate of the CSF set, IgG quotient [CSF/serum; regression coefficient 0.06 (95% CI: 0.00–0.11, p = 0.04)], and of the MRI set, presence of a Gadolinium enhancing lesion corresponding to relapse symptoms [regression coefficient −0.43 (95% CI −0.89 to 0.03, p = 0.06)], were then included in a common mvReg model including CSF + MRI candidates (Figure 3). Herein, only the higher IgG quotient was associated with a higher EDSS after relapse in addition to the clinical markers mentioned afore.
Figure 3.
Multivariable linear regression analysis of all relapses irrespective of relapse phenotype including CSF + MRI candidate (exploratory analysis). Dependent variable (outcome): change in EDSS from first assessment at relapse to EDSS after the last relapse treatment. Independent variables: sex, age at relapse, smoking status (yes versus no), total EDSS at relapse, administered steroid dosage (up to 3 g methyl prednisolone equivalent versus more than 3 g), plasma exchange treatment for current relapse (yes versus no), disease-modifying treatment at relapse (yes versus no), IgG quotient (CSF/serum, ×10−3), and presence of a gadolinium-enhancing lesion corresponding to the relapse symptoms (yes versus no). n = 82 events, adjusted Nagelkerke’s R2 0.47, variance inflation factor for all independent variables below 1.9. Significant associations for female sex, age at relapse, EDSS at relapse, and IgG quotient (CSF/serum); all other investigated independent variables did not demonstrate a significant association with EDSS change (Supplemental Table 1).
95% CI, 95% confidence interval; CSF, cerebrospinal fluid; EDSS, expanded disability status scale; IgG, immunoglobulin G; MRI, magnetic resonance imaging.
Discussion
Sex differences in several neurological disorders are still poorly understood.24,25 The current analysis focused on sex differences in MS relapse and potential factors associated with short-term relapse outcome as measured by EDSS change.
Within our study, we confirm behavioral and thus probably more gender-attributed differences between men and women, reflected by the more than twice as high proportion of smokers in male persons. 19 The two groups exhibited some basic differences, especially longer disease duration and higher EDSS prior to the index event in men. An interrelation of these two factors is likely. However, whether the higher EDSS in men is driven by the longer disease duration or additionally influenced by smoking behavior, as robustly shown before,15–20 cannot be answered by our data. Of note, persons experiencing their first clinical relapse (31.3%) were not included in this pre-relapse EDSS, which is why we refrained from calculating the EDSS difference pre- to during relapse.
Relapse characteristics (mono- versus polysymptomatic or affected functional domains) themselves did not differ between sexes in our cohort unlike other cohorts.3,9 This might be explained by the fact that patients were included due to the initiation of relapse treatment. Untreated relapses are not part of our analysis. Relapses with nondisabling symptoms may thus be underrepresented although of importance for long-term disability. 8
Comparing during and post-relapse disability levels of men and women, both the absolute disability and the dynamics after treatment differ by sex disadvantaging men in line with previous data10,11 although timing and treatment regimen during relapse did not differ between men and women reflecting a monocentric treatment pattern. Respecting that we are discussing effects on a group level, it may cautiously be stated that the EDSS change, as defined for our primary outcome in the regression model reflecting during- to post-relapse EDSS differences, displays a clinically meaningful change with mean EDSS improvements of 0.9 for women and 0.4 for men (Table 1).
CSF and imaging parameters did not display significant sex differences in our analysis. However, there are some interesting numerical differences in MRI parameters between men and women that could be indicative of more progressive features and spinal cord involvement in men as compared to higher disease activity in women. This remains speculative due to the retrospective design and small sample size for these subanalyses in our study. Additional larger-scale data investigating the suggested CSF and MRI parameters will be valuable to further understand sex differences in MS-related pathophysiology.26,27
The primary mvReg analysis underscores the sex-specific difference in EDSS change with an association of EDSS improvement for women. Higher age negatively impacts relapse-associated EDSS change. These factors are in line with previous data.2,10 Bearing in mind the sex differences for disease duration and pre-relapse EDSS, we cannot rule out an impact of these two factors on our primary outcome variable. The pre-relapse EDSS is only available for a subgroup of patients (n = 70) hampering a direct comparison with during- and post-relapse EDSS values. However, as the in-relapse EDSS difference between men and women is smaller than the post-relapse EDSS difference, we still deem that recovery is different between women and men, as measured by our primary outcome, the EDSS change. Potentially influenced by limited sample sizes, the time intervals between first relapse treatment and last EDSS assessment display a wide range, especially in men, whereas the symptom onset to relapse treatment initiation intervals were considerably small with only a small range (Table 1). For neither of these time intervals, a significant sex difference was detected, and most importantly, no additional relapses occurred within this post-relapse observational period.
A higher EDSS during relapse as a factor for improvement rather reflects a statistical phenomenon as the chances for improvement are per se higher within the EDSS range of our cohort. This counterintuitive phenomenon will possibly be mitigated in higher EDSS ranges where the likelihood of change is lower.
We did not detect an influence of the steroid dosage (less or more than 3 g methylprednisolone equivalent) or PLEX (yes or no) on short-term EDSS change. This might indicate that these treatments are able to modify relapse outcomes in the way that more severe relapses with the necessity of higher steroid dosage or PLEX display similar outcomes as the less severe relapses due to, but not despite of treatment. A similar effect has been demonstrated earlier for steroid- versus untreated relapses where steroid treatment was more likely to ‘result in’ disability. 11 Effects of smoking and DMT were not seen in our analysis investigating short-term effects with the last EDSS assessment within 3–6 months after the index event. For both factors, exposure rather seems to be cumulative and thus represent a long-term contributor to worse (smoking) or better (DMT) prognosis.2,8,18–20 Previous smoking behavior was not available for our analysis, but may be a relevant mediating factor for relapse outcome.
As a limitation for our primary analysis, we were not able to derive proper data on previous disease activity, for example, an annualized relapse rate, based on the available retrospective information. In addition, our retrospective approach may not delineate direct causality of the variables included in the regression model, but only describe associations. As an example, it may not be the age at relapse per se that drives the association with relapse recovery, but that age rather is a proxy for other factors mediated or influenced by age, but not available in our retrospective dataset.
Being aware of the shortcomings in using the EDSS as an outcome parameter, for example, due to the limited sensitivity to change – yet, being the one most robustly documented in clinical routine 28 and thus used in this retrospective analysis – we decided to add exploratory analyses on different relapse phenotypes. These did not add major novel insights to the primary analysis and seem less robust due to lower sample sizes of the subgroups. A more granular analysis of recovery of different relapse phenotypes may not only require higher sample sizes, but also more specific outcome parameters, starting with the affected functional system score, visual acuity, or additional specific parameters such as color vision and low contrast visual acuity which were not available in our cohort.
However, in the attempt to find additional paraclinical markers predictive of short-term relapse EDSS change, higher intrathecal IgG synthesis was associated with worse outcome. The presence of intrathecal IgG synthesis has been associated with worse long-term outcome. 29 Our analysis thus hints at a dose-dependent effect of intrathecal IgG synthesis and underscores the additional value of this quantitative measure over the sole detection of CSF-restricted OCBs for prognostic purposes.
Unlike previous associations of black holes with poorer relapse outcome, 10 several MRI parameters were not prognostic in our analysis. Whereas this previous analysis has assessed the question whether the presence or absence of black holes might be associated with outcome, we explored a potential effect of the number of lesions and of the presence of specific lesional patterns. As black holes are indicative of pronounced neuroaxonal damage, the hypothesis of their prognostic value might still be reasonable. Yet, both studies have the limitations of a retrospective design and limited event numbers.
In summary, our data argue for an individualized relapse treatment approach respecting a set of prognostic factors, sex being one of them, yet, mainly with the goal to reduce the threshold for effective relapse treatments than to restrict more aggressive relapse treatments to those populations at highest risk for a worse outcome. Our data underscore that effective relapse treatments including ultra-high-dose steroid (>3 g) and PLEX modulate more severe relapses. Persons with risk factors for poorer prognosis should be promoted to receive escalated relapse treatment regimens early and not only with most severe presentations as the relevance of relapses for long-term prognosis has robustly been demonstrated, also for less disabling events.2,8,11 The contribution of RAW to long-term prognosis can, thus, be influenced by effective relapse treatment and DMT.2,11
The main limitations of our study are the retrospective design and limited sample size, especially relevant for the male sex as this group is expectedly smaller. However, we consider the set of markers investigated here to be valuable for further prospective studies of larger size to corroborate and expand. Additional fluid biomarkers such as serum neurofilament light chain and serum glial fibrillary acidic protein might add to a multidimensional marker set for inflammatory and neurodegenerative features of MS 6 and thus enable individualized decision making. Of note, the gap in the reporting of sex-specific data including potentially different threshold values for men and women needs to be overcome, and trial designs should be tailored and adapted accordingly.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864241237853 for Sex differences in multiple sclerosis relapse presentation and outcome: a retrospective, monocentric study of 134 relapse events by Pauline Thränhardt, Admirim Veselaj, Christoph Friedli, Franca Wagner, Stefanie Marti, Lara Diem, Helly Hammer, Piotr Radojewski, Roland Wiest, Andrew Chan, Robert Hoepner and Anke Salmen in Therapeutic Advances in Neurological Disorders
Acknowledgments
None.
Footnotes
ORCID iDs: Helly Hammer  https://orcid.org/0000-0003-3486-3448
https://orcid.org/0000-0003-3486-3448
Robert Hoepner  https://orcid.org/0000-0002-0115-7021
https://orcid.org/0000-0002-0115-7021
Anke Salmen  https://orcid.org/0000-0002-4751-299X
https://orcid.org/0000-0002-4751-299X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Pauline Thränhardt, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Admirim Veselaj, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Christoph Friedli, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; Department of Neurology, Waikato Hospital, Hamilton, New Zealand.
Franca Wagner, University Department of Diagnostic and Interventional Neuroradiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; Translational Imaging Center, Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland.
Stefanie Marti, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Lara Diem, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Helly Hammer, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Piotr Radojewski, University Department of Diagnostic and Interventional Neuroradiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; Translational Imaging Center, Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland.
Roland Wiest, University Department of Diagnostic and Interventional Neuroradiology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; Translational Imaging Center, Swiss Institute for Translational and Entrepreneurial Medicine, Bern, Switzerland.
Andrew Chan, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Robert Hoepner, Department of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Anke Salmen, Department of Neurology, St Josef-Hospital Bochum, Ruhr-University Bochum, Gudrunstrasse 56, Bochum 44791, GermanyDepartment of Neurology, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Declarations
Ethics approval and consent to participate: Pseudonymized patient data were only included after assessing the patients’ consent status according to the protocol of the NeuroImmunology (NI-) registry [Cantonal ethics committee Bern (KEK-BE), ethics approval registration number KEK-BE 2017-01369, last amendment as of 06-2020].
Consent for publication: Not applicable.
Author contributions: Pauline Thränhardt: Data curation; Formal analysis; Writing – original draft.
Admirim Veselaj: Data curation; Formal analysis; Writing – original draft.
Christoph Friedli: Data curation; Formal analysis; Writing – review & editing.
Franca Wagner: Data curation; Formal analysis; Writing – review & editing.
Stefanie Marti: Data curation; Formal analysis; Writing – review & editing.
Lara Diem: Data curation; Formal analysis; Writing – review & editing.
Helly Hammer: Data curation; Formal analysis; Writing – review & editing.
Piotr Radojewski: Data curation; Formal analysis; Writing – review & editing.
Roland Wiest: Data curation; Formal analysis; Funding acquisition; Writing – review & editing.
Andrew Chan: Data curation; Formal analysis; Funding acquisition; Writing – review & editing.
Robert Hoepner: Conceptualization; Data curation; Formal analysis; Investigation; Writing – review & editing.
Anke Salmen: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Visualization; Writing – original draft.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by a research grant of Baasch Medicus Foundation and from the Medical Faculty of the University of Bern (platform for integrated neuroscience). The funding body was not involved in study design, the collection, analysis, and interpretation of data, or the decision to submit the manuscript.
PT, AV, FW, SM, and PR declare no disclosures relevant to the manuscript. CF received speaker honoraria and/or travel compensation for activities with Biogen, Sanofi Genzyme, Novartis, and Merck and research support from Chiesi, not related to this work. LD received travel grants from Merck, Biogen, Roche, and Bayer Schweiz. She also received speaker’s/advisor honoraria from Biogen, Novartis, Roche, Merck, Lundbeck, Swiss MS Society, and National Swiss Ice Hockey League. She is a member of the Advisory Board of the Swiss MS Society. She also serves as Guest-Co Editor for Brain Sciences (2022/2023) and as Guest-Editor for Journal of Central Nervous System Disease (2022/2023). HH speaker/advisor honorary from Merck, Biogen, Janssen, Teva/Mepha. She received research support within the last 5 years from Biogen. She received travel grants from Biogen, Roche, Janssen, and Merck. RW received speaker/advisor honoraria from Bayer Healthcare, Biogen, Siemens Healthineers. He received research support from Biogen, the Swiss Innovation Agency, the U.S. National Institute of Health, and the Swiss National Foundation. AC received speakers’/board honoraria from Actelion (Janssen/J&J), Almirall, Bayer, Biogen, Celgene (BMS), Genzyme, Merck KGaA (Darmstadt, Germany), Novartis, Roche, and Teva, all for hospital research funds. He received research support from Biogen, Genzyme, and UCB, the European Union, and the Swiss National Foundation. He serves as an associate editor of the European Journal of Neurology, on the editorial board for Clinical and Translational Neuroscience, and as a topic editor for the Journal of International Medical Research. RH received speaker/advisor honorary from Merck, Novartis, Roche, Biogen, Alexion, Sanofi, Janssen, Bristol-Myers Squibb, Teva/Mepha, and Almirall. He received research support within the last 5 years from Roche, Merck, Sanofi, Biogen, Chiesi, and Bristol-Myers Squibb. He also received research grants from the Swiss MS Society and is a member of the Advisory Board of the Swiss MS Society. He also serves as an associated editor for Journal of Central Nervous System disease. AS received speaker honoraria and/or travel compensation for activities with Bristol Myers Squibb, CSL Behring, Novartis, and Roche, and research support by the Baasch Medicus Foundation, the Medical Faculty of the University of Bern, and the Swiss MS Society.
Availability of data and materials: Anonymized data will be shared with qualified investigators upon request to the corresponding author.
References
- 1. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler 2020; 26: 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jokubaitis VG, Spelman T, Kalincik T, et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol 2016; 80: 89–100. [DOI] [PubMed] [Google Scholar]
- 3. Rommer PS, Ellenberger D, Hellwig K, et al. Relapsing and progressive MS: the sex-specific perspective. Ther Adv Neurol Disord 2020; 13: 1756286420956495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol 2020; 77: 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cagol A, Schaedelin S, Barakovic M, et al. Association of brain atrophy with disease progression independent of relapse activity in patients with relapsing multiple sclerosis. JAMA Neurol 2022; 79: 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meier S, Willemse EAJ, Schaedelin S, et al. Serum glial fibrillary acidic protein compared with neurofilament light chain as a biomarker for disease progression in multiple sclerosis. JAMA Neurol 2023; 80: 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dzau W, Sharmin S, Patti F, et al. Risk of secondary progressive multiple sclerosis after early worsening of disability. J Neurol Neurosurg Psychiatry 2023; 94: 984–991. [DOI] [PubMed] [Google Scholar]
- 8. Daruwalla C, Shaygannejad V, Ozakbas S, et al. Early non-disabling relapses are important predictors of disability accumulation in people with relapsing-remitting multiple sclerosis. Mult Scler 2023; 29: 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kalincik T, Buzzard K, Jokubaitis V, et al. Risk of relapse phenotype recurrence in multiple sclerosis. Mult Scler 2014; 20: 1511–1522. [DOI] [PubMed] [Google Scholar]
- 10. Hosny HS, Shehata HS, Ahmed S, et al. Predictors of severity and outcome of multiple sclerosis relapses. BMC Neurol 2023; 23: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart T, Spelman T, Havrdova E, et al. Contribution of different relapse phenotypes to disability in multiple sclerosis. Mult Scler 2017; 23: 266–276. [DOI] [PubMed] [Google Scholar]
- 12. Ribbons KA, McElduff P, Boz C, et al. Male sex is independently associated with faster disability accumulation in relapse-onset MS but not in primary progressive MS. PLoS One 2015; 10: e0122686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Malpas CB, Manouchehrinia A, Sharmin S, et al. Early clinical markers of aggressive multiple sclerosis. Brain 2020; 143: 1400–1413. [DOI] [PubMed] [Google Scholar]
- 14. Zhou Y, Claflin SB, Stankovich J, et al. Redefining the Multiple Sclerosis Severity Score (MSSS): the effect of sex and onset phenotype. Mult Scler 2020; 26: 1765–1774. [DOI] [PubMed] [Google Scholar]
- 15. Alfredsson L, Hillert J, Olsson T, et al. Observed associations between indicators of socioeconomic status and risk of multiple sclerosis in Sweden are explained by a few lifestyle-related factors. Eur J Neurol 2023; 30: 1001–1013. [DOI] [PubMed] [Google Scholar]
- 16. Alshehri E, Cohen JA, Ontaneda D, et al. The impact of cigarette smoking on cognitive processing speed and brain atrophy in multiple sclerosis. Mult Scler 2023; 29: 846–855. [DOI] [PubMed] [Google Scholar]
- 17. Graetz C, Groger A, Luessi F, et al. Association of smoking but not HLA-DRB1*15:01, APOE or body mass index with brain atrophy in early multiple sclerosis. Mult Scler 2019; 25: 661–668. [DOI] [PubMed] [Google Scholar]
- 18. Tanaka E, Watanabe M, Fukumoto S, et al. Effect of smoking on disease activity in multiple sclerosis patients treated with dimethyl fumarate or fingolimod. Mult Scler Relat Disord 2023; 70: 104513. [DOI] [PubMed] [Google Scholar]
- 19. Wingerchuk DM. Smoking: effects on multiple sclerosis susceptibility and disease progression. Ther Adv Neurol Disord 2012; 5: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu J, Olsson T, Hillert J, et al. Influence of oral tobacco versus smoking on multiple sclerosis disease activity and progression. J Neurol Neurosurg Psychiatry 2023; 94: 589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lutfullin I, Eveslage M, Bittner S, et al. Association of obesity with disease outcome in multiple sclerosis. J Neurol Neurosurg Psychiatry 2023; 94: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skorupka N, Miclea A, Jalowiec KA, et al. Visual outcomes of plasma exchange treatment of steroid-refractory optic neuritis: a retrospective monocentric analysis. Transfus Med Hemother 2019; 46: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 24. Moores G, Steadman PE, Momen A, et al. Sex differences in neurology: a scoping review. BMJ Open 2023; 13: e071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rechlin RK, Splinter TFL, Hodges TE, et al. An analysis of neuroscience and psychiatry papers published from 2009 and 2019 outlines opportunities for increasing discovery of sex differences. Nat Commun 2022; 13: 2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castellazzi M, Ferri C, Tecilla G, et al. The sexual dimorphism in cerebrospinal fluid protein content does not affect intrathecal IgG synthesis in multiple sclerosis. J Pers Med 2022; 12: 977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nytrova P, Dolezal O. Sex bias in multiple sclerosis and neuromyelitis optica spectrum disorders: how it influences clinical course, MRI parameters and prognosis. Front Immunol 2022; 13: 933415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meyer-Moock S, Feng YS, Maeurer M, et al. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol 2014; 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gasperi C, Salmen A, Antony G, et al. Association of intrathecal immunoglobulin G synthesis with disability worsening in multiple sclerosis. JAMA Neurol 2019; 76: 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864241237853 for Sex differences in multiple sclerosis relapse presentation and outcome: a retrospective, monocentric study of 134 relapse events by Pauline Thränhardt, Admirim Veselaj, Christoph Friedli, Franca Wagner, Stefanie Marti, Lara Diem, Helly Hammer, Piotr Radojewski, Roland Wiest, Andrew Chan, Robert Hoepner and Anke Salmen in Therapeutic Advances in Neurological Disorders