Abstract
Background:
Clonal mast cell disorders and elevated BST of unknown cause(s) are associated with severe Hymenoptera venom-triggered anaphylaxis (HVA). However, some individuals with clonal disease have normal BST (<11.4 ng/mL).
Objective:
To evaluate whether screening for KIT p.D816V in the blood is a useful clinical tool to risk-stratify patients with venom allergy.
Methods:
We prospectively recruited 374 patients with Hymenoptera allergy and no overt signs of mastocytosis referred to our center in the years 2018–19. KIT p.D816V was determined in the peripheral blood with qPCR and tryptase genotyping was performed by droplet-digital PCR.
Results:
351 patients (93.9%) had normal levels of BST and KIT p.D816V was detected in 8% of patients (28/351), predominantly in patients with the most severe Mueller grade IV anaphylaxis (18.2%[24/132] vs 1.8%[4/88 in grade III; 0/131 in other grades] in lower grades; P<0.001). In grade IV patients with normal BST, KIT p.D816V was associated with more severe symptoms including a significantly higher frequency of loss of consciousness (58.3%[14/24] vs 34.3%[37/108]; P=0.03) and absence of skin symptoms (41.7%[10/24] vs 15.7%[17/108]; P=0.004). Among patients with normal BST, KIT p.D816V (OR [95%CI]: 10.25[3.75–36.14]; P<0.0001) was the major risk factor associated with severe HVA. Hereditary α-tryptasemia (HαT), due to increased germline copies of TPSAB1 encoding α-tryptase was the most common cause (65.2%; 15/23) of elevated BST in patients with HVA and together with KIT p.D816V accounted for 90% (20/23) of BST elevations in HVA patients.
Conclusion:
These results indicate that routine KIT p.D816V screening identifies clonal disease in high-risk HVA patients regularly missed using BST alone.
Keywords: Anaphylaxis, venom, tryptase, mast cells, KIT p.D816V, hereditary alpha-tryptasemia
Capsule summary
Presence of the KIT p.D816V is most commonly associated with normal BST, while HαT is the most common cause of elevated BST, among individuals with severe HVA and should be considered clinically to risk-stratify patients with venom allergy.
Graphical Abstract
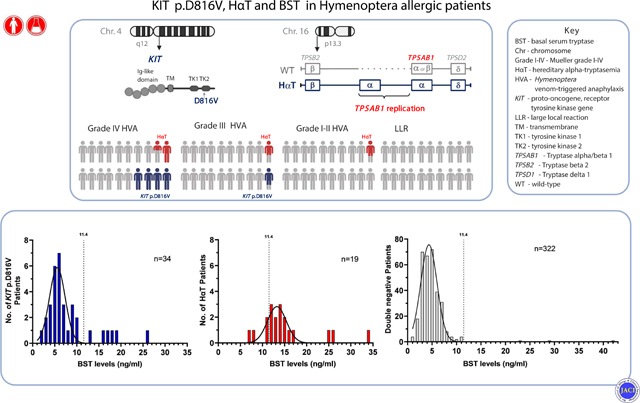
INTRODUCTION:
In a recent study describing hereditary alpha-tryptasemia (HαT) as the first common heritable risk factor for severe anaphylaxis, Lyons et al.1 found a surprisingly high frequency (23%) of peripheral blood KIT p.D816V missense variant carriers among individuals with severe (Mueller grade IV) Hymenoptera venom-triggered anaphylaxis (HVA). The vast majority (74% [36/49] with grade IV of Slovenian cohort) of KIT p.D816V positive patients had normal (<11.4 ng/mL) basal serum tryptase (BST) levels.1 Another recent report suggested that the KIT p.D816V missense variant was detectable in peripheral blood of 5% (6/123) of patients with anaphylaxis and normal BST.2 In order to expand on these retrospective findings, we recruited a large cohort of sequentially referred patients with venom allergy to prospectively evaluate whether routine screening for KIT p.D816V in the blood is a useful clinical tool to identify those individuals at risk for severe HVA.
RESULTS and DISCUSSION:
We prospectively recruited 374 patients (219 male, age 20–81 years) with Hymenoptera venom allergy referred to University Clinic Golnik in the years 2018 and 2019 (Table 1). Our center is the only referral center for adults with venom allergy in Slovenia, and none of the patients referred to our center underwent prior screening, and thus were not selected for clonal mast cell (MC) disease prior to evaluation. None of the subjects recruited were reported in the previous study.1 Complete histories and physical examinations were performed, and reaction grades were assigned based upon the Mueller grading system.3 BST levels and clinically indicated specific IgE testing to Hymenoptera species were obtained in all study participants. Likewise, in all subjects the KIT p.D816V missense variant was assayed in peripheral blood by quantitative PCR (qPCR) as described.1,4,5 All individuals with BST ≥ 6 ng/mL underwent tryptase genotyping by ddPCR as described1,6; no individual has ever been reported or observed with HαT and BST< 6 ng/mL.1,6–11 Unpaired t-tests, Mann-Whitney tests, and Chi-square tests were employed as appropriate in order to test the significance of associations; logistic regression analysis was used to evaluate the predictors of severity of reactions, and frequency distribution to displays the frequency of outcomes in a sample. Ethical approval was obtained from the Slovenian National Medical Ethics Committee (150/09/13) and all subjects provided written informed consent. Details of methodology are described in the Online supplement.
Table 1.
Characteristics of study participants stratified by BST level.
| Patients with normal BST* (n=351) | Patients with elevated BST (n=23) | P-value | |
|---|---|---|---|
|
| |||
| Age - median (range) | 49 (20–81) | 57 (21–76) | 0.09 |
|
| |||
| Gender - n (%) | |||
| Female | 146 (41.6) | 9 (39.1) | 0.82 |
| Male | 205 (58.4) | 14 (60.9) | |
|
| |||
| Reaction severity grade† - n (%) | |||
| LLR | 21 (6.0) | 0 ( 0) | 0.22 |
| I | 40 (11.4) | 2 (8.7) | 0.69 |
| II | 70 (19.9) | 1 (4.3) | 0.06 |
| III | 88 (25.1) | 2 (8.7) | 0.08 |
| IV | 132 (37.6) | 18 (78.3) | <0.001 |
|
| |||
| Culprit history- n (%) | |||
| Honey bee | 109 (31.1) | 4 (17.4) | 0.17 |
| Vespinae spp. | 174 (49.6) | 13 (56.5) | 0.52 |
| Honey bee and Vespinae spp. | 3 (0.9) | 0 (0) | 0.66 |
| Unknown Hymenoptera | 65 (18.4) | 6 (26.1) | 0.37 |
|
| |||
| Specific IgE (>0.35 kU/L)- n (%) | |||
| Honey bee (i1) | 67 (19.1) | 5 (17.4) | 0.75 |
| Yellow jacket (i3) | 123 (35.0) | 8 (34.7) | 0.6 |
| Double (i1 and i3) | 161 (45.9) | 10 (43.5) | 0.82 |
|
| |||
| KIT p.D816V - n (%) | |||
| Positive | 28 (8.0) | 6 (26.1) | 0.003 |
| Negative | 323 (92.0) | 17 (73.9) | |
|
| |||
| HαT -n (%) ‡ | |||
|
| |||
| Positive | 4 (1.1) | 15 (65.2) | <0.001 |
|
| |||
| Negative | 347 (98.9) | 8 (34.8) | |
|
| |||
| BST - median (range) | 4.62 (<1–11.3) | 16.1 (11.8–41.8) | na |
The normal range in serum for total tryptase is considered 1–11.4 ng/mL.
Grades were assigned based upon the Mueller grading system.3
High prevalence of KIT p.D816V in patients with severe venom anaphylaxis and normal BST
In total, 93.9% (351 of 374; 205 male, age 20–81 years,) of patients with Hymenoptera venom allergy had normal levels of BST (median [IQR]: 4.62 ng/mL [2.57]) and the KIT p.D816V variant was detected in the blood of 8% (28/351) of patients with normal BST (<11.4 ng/mL) and 26.1% (6/23) of those with elevated BST (Table 1). None of those identified by qPCR had cutaneous findings such as urticaria pigmentosa or other systemic findings such as organomegaly that would have suggested the presence of clonal mast cell disease. KIT p.D816V was almost exclusively present in patients with the most severe (Mueller grade IV) HVA (Figure 1). Consequently, the frequency of KIT p.D816V was 18.2% (24/132) in patients with grade IV venom anaphylaxis and normal BST compared to 1.8% (4/219) in patients with lower grades and normal BST (4.6%[4/88] in patients with grade III venom anaphylaxis and none [0/131] in other lower grades, P<0.001; Figure 1). Of those individuals with KIT p.D816V and normal BST 85.7% (24/28) had a grade IV and 14.3% (4/28) grade III venom anaphylaxis. Moreover, in grade IV patients with normal BST, KIT p.D816V was associated with more severe symptoms including significantly higher frequencies of loss of consciousness (58.3%[14/24] vs 34.3%[37/108]; P=0.03) and a paucity of skin symptoms (41.7%[10/24] vs 15.7%[17/108]; P=0.004) during an anaphylactic episode (Table 2). Furthermore, KIT p.D816V was also associated with more severe symptoms in grade IV patients with elevated BST (Table E1). Patients with KIT p.D816V and BST within the normal range also showed marginally, but significantly higher BST values (5.9 vs 5.0 ng/mL, P=0.007; Table 2). Our results confirm that the presence of KIT p.D816V is highly associated with severe anaphylactic reactions. However, 82% (28/34; Table E2) of these individuals would have been missed by the current standard practice of screening with BST alone. Therefore, by adding routine screening for KIT p.D816V regardless of BST level, the fraction of individuals correctly identified to be at risk for severe HVA – and thus to receive life-long venom immunotherapy – is roughly doubled from ~10% to ~20%.
Figure 1.
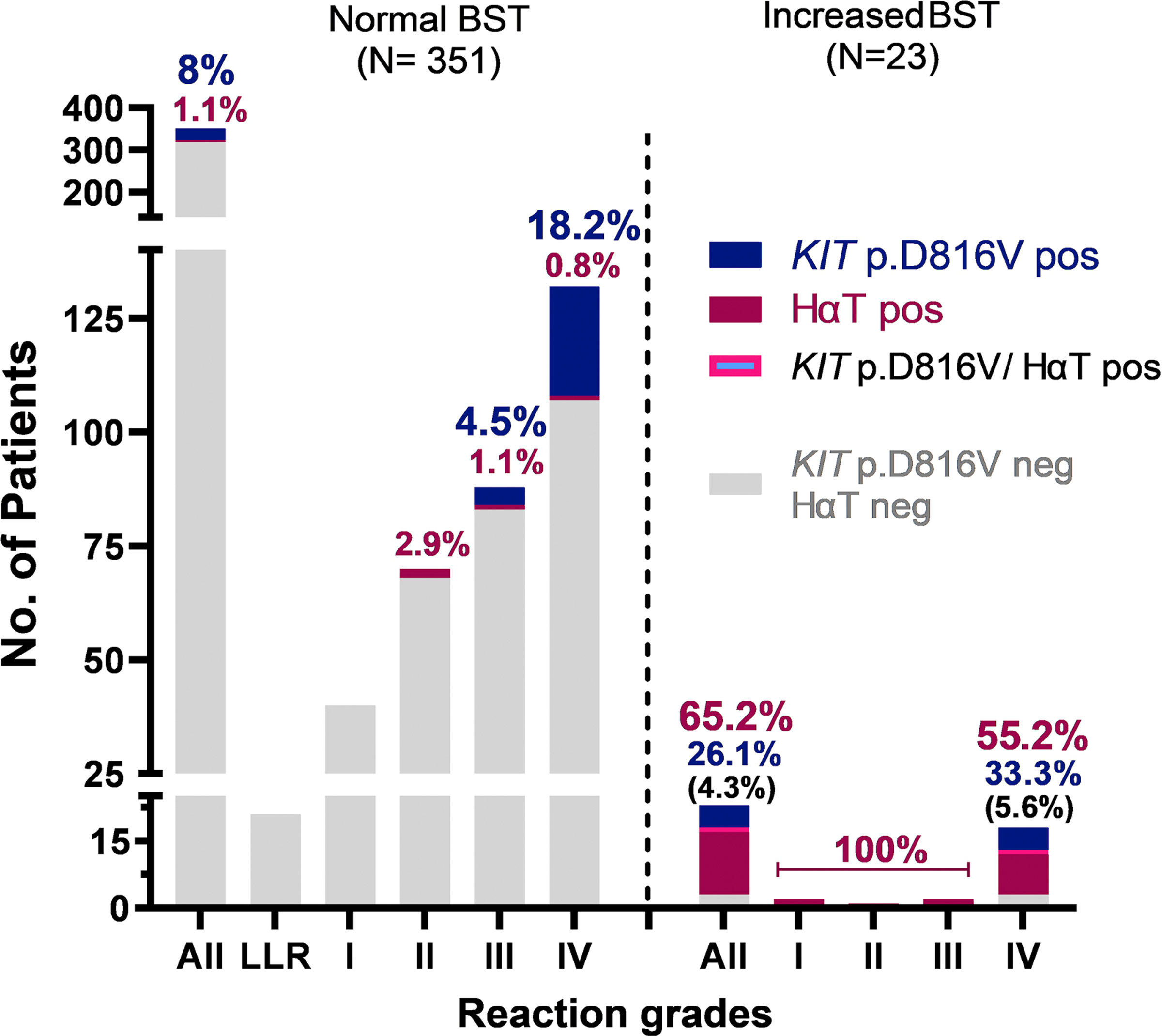
Prevalence of peripheral blood KIT p.D816V missense variant carriers and hereditary α-tryptasemia (HαT), according to the severity of Hymenoptera sting reaction, and normal and elevated BST level.
Legend: BST, basal serum tryptase; the normal range is considered 1–11.4 ng/mL. LLR, large local reaction. Grades were assigned based upon the Mueller grading system.3
Table 2.
Characteristics of patients with grade IV venom anaphylaxis and normal BST, grouped according to the presence of KIT p.D816V.
| Patients with grade IV venom anaphylaxis and normal BST | |||
|---|---|---|---|
| KIT p.D816V pos (n=24) | KIT p.D816V neg (n=108) | P-value | |
|
| |||
| Age - median (range) | 55 (25.73) | 54 (21.81) | 1 |
|
| |||
| Gender male - n (%) | 15 (62.5) | 63 (58.3) | 0.88 |
|
| |||
| BST - median (range) | 5.9 (3.3–10.3) | 5.0 (<1–11.3) | 0.007 |
|
| |||
| Concomitant CVD - n (%) | 12 (50) | 33 (30.6) | 0.07 |
|
| |||
| Culprit history- n (%) | |||
| Honey bee | 6 (25) | 29 (26.9) | 0.85 |
| Vespinae spp. | 10 (43.5) | 67 (62.0) | 0.07 |
| Unknown Hymenoptera | 8 (30.3) | 12 (11.1) | 0.006 |
|
| |||
| Specific IgE (>0.35 kU/L)- n (%) | |||
| Honey bee (i1) | 6 (25) | 9 (8.3) | 0.02 |
| Yellow jacket (i3) | 8 (33.3) | 46 (42.6) | 0.4 |
| Double (i1 and i3) | 10 (41.7) | 53 (49.1) | 0.5 |
|
| |||
| Skin symptoms - n (%) | |||
| No | 10 (41.7) | 17 (15.7) | 0.004 |
| Yes | 10 (41.7) | 71 (65.7) | 0.03 |
| Unknown | 4 (16.7) | 20 (18.5) | 0.83 |
|
| |||
| Unconsciousness - n (%) | 14 (58.3) | 37 (34.3) | 0.03 |
BST, basal serum tryptase; the normal range is considered 1–11.4 ng/mL. CVD, cardiovascular diseases. Grade was assigned based upon the Mueller grading system.3
HαT – the most common cause of elevated BST in patients with venom anaphylaxis
Nineteen individuals (15 with elevated BST and 4 with normal [2 grade II, 1 grade III and 1 grade IV] BST) of 111 patients (BST ≥ 6 ng/mL) who underwent tryptase genotyping were found to have increased germline α-tryptase encoding copies at TPSAB1 (Figure 1, Table 1, E2 and E3). Thus, HαT was the most common (65.2%; 15/23) cause of elevated BST in patients with HVA, with the frequency of 55.5% (10/18) among those with grade IV HVA and 100% (5/5) among lower grades (2 grade I, 1 grade II and 2 grade III) (Figure 1). In one individual with grade IV we also detected a concomitant KIT p.D816V variant. Consequently, in patients with HVA HαT and/or KIT p.D816V accounted for elevated BST in 90% (20/23) identified.
KIT p.D816V – an independent and major predictor of severe venom anaphylaxis in patients with normal BST
We next sought to identify independent predictors of severe HVA (grade IV vs lower grades) in the 351 Hymenoptera venom allergy patients with normal BST. Despite excluding individuals with elevated BST, serum levels still correlated significantly with venom reaction severity when examined using a univariate log-regression (OR [95% CI]: 1.16[1.04–1.30], P=0.01; Table 3). However, when KIT p.D816V and age12, two other major risk factors for severe venom anaphylaxis, were included in the prediction model, BST levels lost their statistical significance (OR [95%CI]: 1.07[0.94–1.22], P=0.30), whereas KIT p.D816V (OR [95%CI]: 10.25[3.75 −36.14], P<0.0001) and older age (OR [95%CI] for each year: 1.03[1.01–1.05], P=0.002) remained as significant predictors of severe HVA (Table 3). KIT p.D816V (OR [95%CI]: 10.29 [3.81–36.01] P<0.0001) was also the most signifant risk factor of severe HVA when all patients (normal and elevated BST) were included in the prediction model (Table E4).
Table 3.
Predictors of grade IV venom anaphylaxis in patients with normal BST.
| Univarate log-regression | Multivariate log-regression | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI (OR) | P-value | OR | 95% CI (OR) | P-value | |
| BST | 1.16 | 1.04–1.30 | 0.01 | 1.07 | 0.94–1.22 | 0.30 |
| KIT p.D816V | 11.94 | 4.04–35.30 | <0.0001 | 10.25 | 3.75–36.14 | <0.0001 |
| HαT | 0.55 | 0.03–4.34 | 0.61 | 0.65 | 0.03–5.87 | 0.73 |
| Age | 1.03 | 1.02–1.05 | <0.0001 | 1.03 | 1.01–1.05 | 0.002 |
| Gender (male) | 1.05 | 0.68–1.62 | 0.88 | 0.98 | 0.62–1.58 | 0.95 |
BST, basal serum tryptase; the normal range is considered 1–11.4 ng/mL.OR, odds ratio; CI, confidence interval. Grades were assigned based upon the Mueller grading system.3 BST and age (years) were treated as continuous variables.
We next compared the frequencies of KIT p.D816V in patients with grade IV venom anaphylaxis and different levels of normal BST. KIT p.D816V was not found in a subgroup (0/8) with the lowest BST levels (<2.5 ng/mL); however 11.6% (8/69) of individuals with BST levels of 2.5–5.4 ng/mL, 23.9% (11/46) of individuals with BST levels of 5.5–8.4 ng/mL, and 55.6% (5/9) of individuals with BST levels 8.5–11.4 ng/mL, were identified with KIT p.D816V (Figure E1). These observations demonstrated that 33.3% (8/24) of patients with KIT p.D816V, normal BST, and grade IV venom anaphylaxis have BST levels of less than 5.5 ng/mL and 79.2% (19/24) had levels of less than 8.5 ng/mL. We then performed frequency distribution analysis to explore the distribution of KIT p.D816V in patients based upon BST levels (Figure 2A). We analyzed all 34 patients with KIT p.D816V. The arithmetic mean of BST levels among individuals with KIT p.D816V was 5.6 ng/mL ([95%CI]: 5.2–6.1 ng/mL) with standard deviation of 1.7 ([95%CI]: 1.1–2.6 ng/mL), and a robust correlation coefficient for a Gaussian fit, despite the identified outliers (R2: 0.77) (Figure 2A). We went on to perform the same analysis for the 49 KIT p.D816V positive patients with grade IV venom anaphylaxis from the Lyons et al. study (publicly available data of Slovenian cohort from Table E2 within the Online Repository).1 In those individuals the arithmetic mean for BST levels was 4.3 ng/mL ([95%CI]: <1–5.5 ng/mL) with a standard deviation of 3.9 ([95%CI]: 2.7–10.8 ng/mL), when using a Gaussian fit (R2: 0.6) (Figure E2). On the other hand, the arithmetic mean of BST levels among individuals with HαT was 13.3 ng/mL ([95%CI]: 12.7–13.8 ng/mL) with a standard deviation of 2.1 ([95%CI]: 1.6–2.9 ng/mL), when using a Gaussian fit (R2: 0.69) (Figure 2B). In all other patients the arithmetic mean for BST levels was 4.4 ng/mL ([95%CI]: 4.2–4.5 ng/mL) with a standard deviation of 1.7 ([95%CI]: 1.5–1.8 ng/mL), when using a Gaussian fit (R2: 0.96) (Figure 2C).
Figure 2.
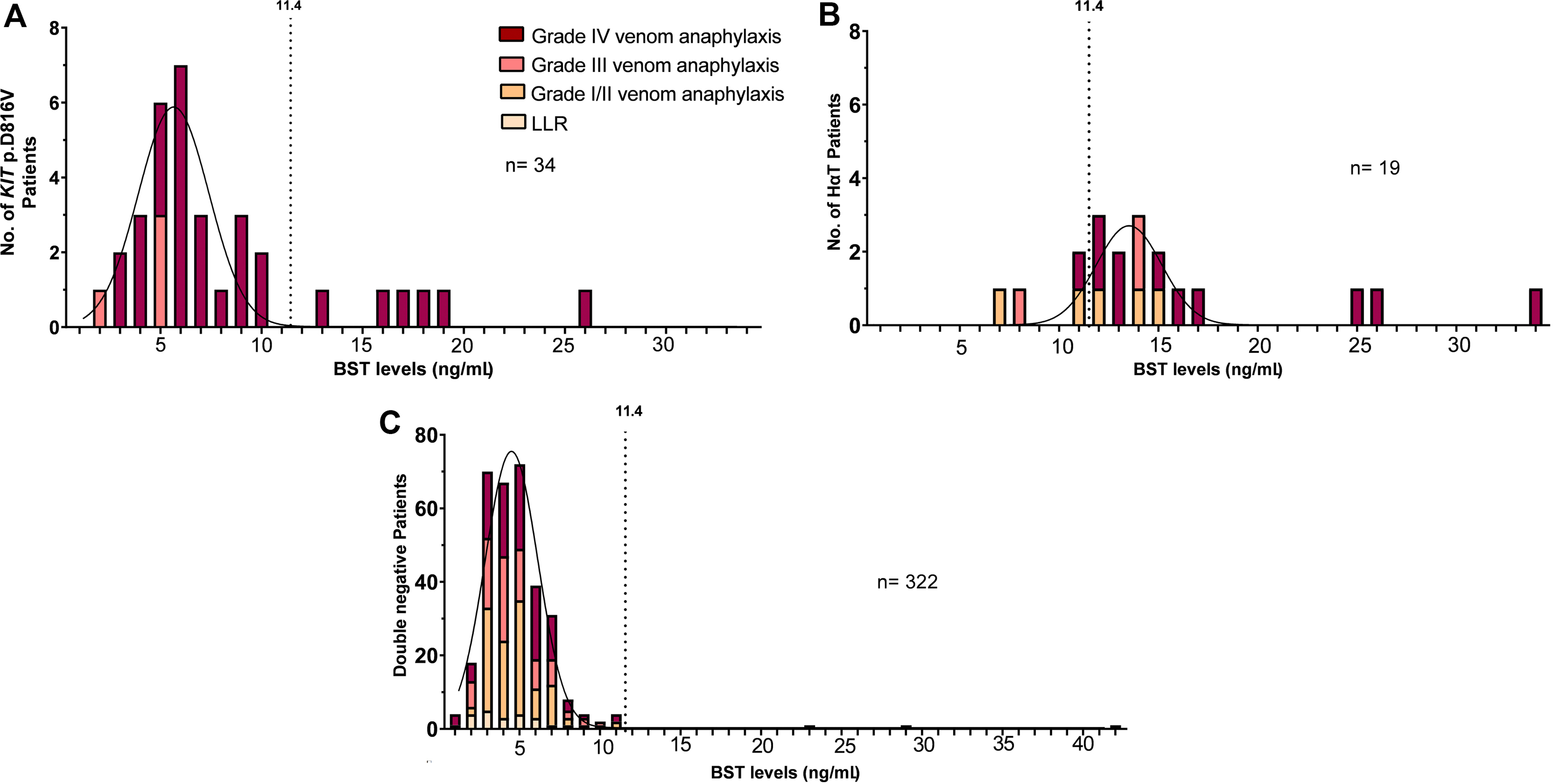
A-C. Frequency distribution of the A KIT p.D816V missense variant in peripheral blood, B hereditary α-tryptasemia (HαT) and C negtive patients, based upon BST level.
Legend: BST, basal serum tryptase; the normal range is considered 1–11.4 ng/mL. LLR, large local reaction. The curve represents Gaussian Least squares fit. Grades were assigned based upon the Mueller grading system.3
Whereas previous studies have suggested that BST levels of at least 5 ng/mL13 to 8 ng/mL12,14 are associated with a higher risk for severe anaphylactic reactions, our data show that using even these cut-offs below what is considered elevated BST (>11.4 ng/mL) would miss the majority of individuals with clonal MC disease at high risk for severe anaphylaxis. Taken together, our data demonstrate that KIT p.D816V is the major testable risk factor for severe anaphylaxis among individuals with normal BST levels and venom allergy, and it is associated with a greater likelihood of severe HVA independent of BST levels.
Peripheral blood KIT p.D816V screening of individuals with severe venom anaphylaxis and normal BST identifies occult clonal mast cell disease
In all patients we have identified with severe venom anaphylaxis and KIT p.D816V in peripheral blood, venom immunotherapy (VIT) was initiated (Table E2) and the planned duration increased from 5-years to life-long.15 Two individuals with normal BST and 3 with elevated BST who had finished 5-years of yellow jacket VIT and/or 5–10 years of honeybee VIT prior to inclusion in this study, relapsed with grade IV venom anaphylaxis (Table E2). Therefore all patients are being treated in concordance with the presumed underlying diagnosis of ISM16 or MMAS17, including initiation of regular blood count and bone scan.
To better characterize and define the underlying disorder(s) associated with a finding of KIT p.D816V in peripheral blood, bone marrow (BM) assessment was accepted by five individuals, one individual with normal BST and 4 with elevated BST. While none of the KIT p.D816V positive patients exhibited urticaria pigmentosa or other clinical cutaneous lesions indicative of mastocytosis18 and none had organomegaly16,19 that would suggest a clonal disorder prior to evaluation, all 5 met criteria for the diagnosis of indolent SM (Table E2). These findings are consistent with similar results in other reports. Zanotti et al.20 reported identifying ISM in 15/22 and MMAS in 1/22 patients with severe HVA and normal BST; none were reported to have had cutaneous involvement. Dölle-Bierke et al.2 also reported an absence of skin involvement in 4/4 KIT p.D816V positive HVA patients with normal BST.
In conclusion, the ability to risk stratify individuals with venom allergy, and identify those at risk for severe anaphylaxis is important for both anticipatory counseling of patients, but also guides therapy, as life-long VIT is currently recommended for individuals with clonal MC disease and HVA. Here we prospectively demonstrated the value of routinely screening all individuals, regardless of BST for the presence of KIT p.D816V in peripheral blood and tryptase genotyping in all individuals with elevated BST. Importantly among those with clonal disease, 82% in this cohort would have been missed using BST alone, all of whom had grade III-IV anaphylaxis. Among individuals with normal BST, KIT p.D816V and age were the only two risk factors identified to be associated with severe venom anaphylaxis. HαT was the most common cause of elevated BST in HVA and was together with KIT p.D816V associated with elevated BST in up to 90% of HVA patients. Taken together, these findings demonstrate the importance of screening for KIT p.D816V and tryptase genotypes in the peripheral blood of individuals with moderate to severe HVA and support the routine use of this test in the work-up of individuals with venom allergy.
Supplementary Material
Key messages:
KIT p.D816V missense variant is common (18.2%) in patients with Mueller grade IV HVA and normal basal serum tryptase.
Increased germline copy number of α-tryptase-encoding sequences at TPSAB1 is the most common (65.2%) cause of elevated BST in patients with HVA.
KIT p.D816V is associated with more severe symptoms including more frequent loss of consciousness and absence of cutaneous symptoms.
Screening all individuals with history of moderate to severe HVA for KIT p.D816V identifies the majority of those with clonal disease who would have been missed using BST alone and provides an important risk stratification tool.
Acknowledgments
Funding: This study was supported in part by the Slovenian Research Agency (P3–0360) and by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, NIH.
Abbreviations
- BM
bone marrow
- BST
basal serum tryptase
- ddPCR
droplet-digital PCR
- HαT
hereditary alpha tryptasemia
- HVA
Hymenoptera venom-triggered anaphylaxis
- ISM
indolent systemic mastocytosis
- LLR
large local reaction
- MC
mast cell
- MMAS
Monoclonal mast cell activation syndrome
- qPCR
quantitative PCR
- SM
systemic mastocytosis
- TPSAB1
Tryptase alpha/beta 1
- YJ
yellow jacket
Footnotes
Disclosure of conflicts of interest
The authors have no relevant conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lyons JJ, Chovanec J, O’Connell MP, Liu Y, Šelb J, Zanotti R, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2021;147:622–32. [DOI] [PubMed] [Google Scholar]
- 2.Dölle-Bierke S, Siebenhaar F, Burmeister T, Worm M. Detection of KIT D816V mutation in patients with severe anaphylaxis and normal basal tryptase—first data from the Anaphylaxis Registry (NORA). J Allergy Clin Immunol. 2019;144:1448–1450. [DOI] [PubMed] [Google Scholar]
- 3.Mueller Ulrich R. Insect Sting Allergy: Clinical picture, diagnosis and treatment. Gustav Fischer Verlag. 1990;183 p. [Google Scholar]
- 4.Kristensen T, Vestergaard H, Møller MB. Improved detection of the KIT D816V mutation in patients with systemic mastocytosis using a quantitative and highly sensitive real-time qPCR assay. J Mol Diagnostics. 2011;13:180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristensen T, Vestergaard H, Bindslev-Jensen C, Møller MB, Broesby-Olsen S. Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol. 2014;89:493–8. [DOI] [PubMed] [Google Scholar]
- 6.Lyons JJ, Yu X, Hughes JD, Le QT, Jamil A, Bai Y, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greiner G, Sprinzl B, Górska A, Ratzinger F, Gurbisz M, Witzeneder N, et al. Hereditary alpha tryptasemia is a valid genetic biomarker for severe mediator-related symptoms in mastocytosis. Blood. 2021;137:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyons JJ, Sun G, Stone KD, Nelson C, Wisch L, O’Brien M, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabato V, Chovanec J, Faber M, Milner JD, Ebo D, Lyons JJ. First Identification of an Inherited TPSAB1 Quintuplication in a Patient with Clonal Mast Cell Disease. J Allergy Clin Immunol. 2018;38:457–459. [DOI] [PubMed] [Google Scholar]
- 10.Robey RC, Wilcock A, Bonin H, Beaman G, Myers B, Grattan C, et al. Hereditary Alpha-Tryptasemia: UK Prevalence and Variability in Disease Expression. J Allergy Clin Immunol Pract. 2020;8:3549–56. [DOI] [PubMed] [Google Scholar]
- 11.Giannetti MP, Akin C, Hufdhi R, Hamilton MJ, Weller E, van Anrooij B, et al. Patients with mast cell activation symptoms and elevated baseline serum tryptase level have unique bone marrow morphology. J Allergy Clin Immunol. 2020; Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Worm M, Francuzik W, Renaudin JM, Bilo MB, Cardona V, Scherer Hofmeier K, et al. Factors increasing the risk for a severe reaction in anaphylaxis: An analysis of data from The European Anaphylaxis Registry. Allergy Eur J Allergy Clin Immunol. 2018;73:1322–30. [DOI] [PubMed] [Google Scholar]
- 13.Ruëff F, Przybilla B, Biló MB, Müller U, Scheipl F, Aberer W, et al. Predictors of severe systemic anaphylactic reactions in patients with Hymenoptera venom allergy: importance of baseline serum tryptase-a study of the European Academy of Allergology and Clinical Immunology Interest Group on Insect Venom Hypersensitivity. J Allergy Clin Immunol 2009;124:1047–54. [DOI] [PubMed] [Google Scholar]
- 14.Francuzik W, Ruëff F, Bauer A, Bilò MB, Cardona V, Christoff G, et al. Phenotype and risk factors of venom-induced anaphylaxis: A case-control study of the European Anaphylaxis Registry. J Allergy Clin Immunol. 2020;147:653–662. [DOI] [PubMed] [Google Scholar]
- 15.Golden DBK, Demain J, Freeman T, Graft D, Tankersley M, Tracy J, et al. Stinging insect hypersensitivity: A practice parameter update 2016. Ann Allergy, Asthma Immunol. 2017;118:28–54. [DOI] [PubMed] [Google Scholar]
- 16.Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017;129:1420–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valent P, Akin C, Bonadonna P, Hartmann K, Brockow K, Niedoszytko M, et al. Proposed Diagnostic Algorithm for Patients with Suspected Mast Cell Activation Syndrome. Vol. 7, Journal of Allergy and Clinical Immunology: In Practice. 2019;7:1125–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; The American Academy of Allergy, Asthma & Immunology; And the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35–45. [DOI] [PubMed] [Google Scholar]
- 19.Pardanani A. Systemic mastocytosis in adults: 2019 update on diagnosis, risk stratification and management. Am J Hematol. 2019;94:363–77. [DOI] [PubMed] [Google Scholar]
- 20.Zanotti R, Lombardo C, Passalacqua G, Caimmi C, Bonifacio M, De Matteis G, et al. Clonal mast cell disorders in patients with severe Hymenoptera venom allergy and normal serum tryptase levels. J Allergy Clin Immunol. 2015;136:135–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


