Abstract
Energy-based surgical instruments produce surgical smoke, which contains harmful byproducts, such as polycyclic aromatic hydrocarbons, volatile organic compounds, particulate matter, and viable microorganisms. The research setting has shifted from the laboratory to the operating room. However, significant heterogeneity in the methods of detection and placement of samplers, diversity in the tissue operated on, and types of surgeries tested has resulted in variability in detected levels and composition of surgical smoke. State regulation limiting surgical smoke exposure through local evacuators is expanding but has yet to reach the national regulatory level. However, most studies have not shown levels above standard established limits but relatively short bursts of high concentrations of these harmful by-products. This review highlights the limitations of the current research and unsupported conclusions while also suggesting further areas of interest that need more focus to improve Occupational Safety and Health Administration guidelines.
Keywords: surgical smoke, surgical plume, smoke evacuation, particulate matter, volatile organic compound, polyaromatic hydrocarbon
Surgical smoke (or plume) is produced by electrosurgical tools, lasers, ultrasonic scalpels, and other energy-based surgical devices (Casey and McNamara, 2023). These instruments apply thermal energy through different properties for tissue cutting and coagulation, which controls bleeding and expedites procedure time (Casey and McNamara, 2023; Ismail et al., 2017). While primarily composed of water vapor, surgical smoke has been shown to contain chemicals, cellular fragments, pathogens, and inactive particles (Ulmer, 2008). According to the United States Occupational Safety and Health Administration (OSHA), at least 500 000 healthcare workers are exposed to surgical smoke, including surgeons, perioperative nurses, surgical technologists and assistants, and anesthetists (Occupational Safety and Health Administration, 2023). However, staff remains hesitant to adopt smoke mitigation practices, perceived as inconveniences, due to a lack of concern for the harms of surgical smoke and limited data regarding its clinical and long-term effects (Michaelis et al., 2020). Current research has been conflicting in measuring toxic compounds in surgical smoke below or above exposure limits (ELs). Permissible exposure limits (PELs) are legally enforceable limits set by OSHA and usually higher than other ELs. Recommended exposure limits (RELs) and threshold limit values (TLVs) are nonenforceable and typically stricter recommendations set by the National Institute for Occupational Safety and Health (NIOSH) and the American Conference of Governmental Industrial Hygienists (ACGIH), respectively. Although 15-min short-term exposure limits (STELs) exist, most ELs consider the amount of exposure averaged over an 8-h workday (U.S. Environmental Protection Agency, 2021). Using benzene as an example, OSHA PEL is 1 part of benzene vapor per million parts of air (ppm), whereas NIOSH REL and ACGIH TLV are 0.1 and 0.5 ppm, respectively. The OSHA 15-min STEL is 5 ppm, whereas NIOSH’s and ACHIH’s are 1 and 2.5 ppm, respectively (U.S. Department of Labor, 2021). As toxic compounds detected in surgical smoke have been linked to cardiorespiratory disease and cancer, it is crucial to accurately measure surgical staffs’ daily exposures to these chemicals for potentially hours at a time in the context of these evidence-based limits. Additionally, by carefully selecting biomarkers of toxicity more systematically, our understanding of potential clinical health outcomes could be better assessed.
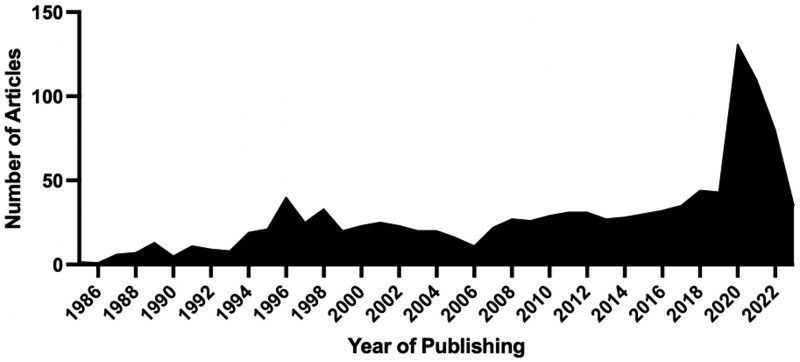
Research interest grew in the setting of the COVID-19 outbreak, with a focus on the viral transmission of the SARS-CoV-2 virus (Figure 1). Concurrently, evacuation systems specifically designed to evacuate surgical smoke and regulations regarding their use have been increasingly emerging based on current hazards of surgical smoke and extrapolation of its long-term effects (AORN, 2020). Considering the rapid increase in state regulation with mandated structural changes in the operating room and emerging focus by federal organizations, this contemporary review summarizes up-to-date research on surgical smoke, with a focus on research over the past 5 years and additional areas that need further focus. The full search strategy has been included as Supplementary File and highlights that more than half of the articles related to surgical smoke included in this contemporary review were published in the past 5 years.
Figure 1.
Database search in PubMed and Embase of published articles regarding surgical, laser, diathermy, or electrocautery smoke or plume displaying an increase in publications over the past 3 years.
Surgical smoke components
A nonexhaustive list of the known components of surgical smoke is presented in Figure 2. Until recently, most studies were limited to laboratory and animal studies. Given the predominance of nonhuman studies, correlating findings to operating room staffs’ exposures was difficult. As analyte detection methods improved, researchers could obtain samples directly from live human surgeries in the operating room. However, these studies have been hampered by sterility requirements and minimal disruptions during operations, which complicate the ability to obtain accurate samples of the staff’s exposure. Furthermore, studies have appreciable heterogeneity in detection instruments, placement of instruments, analytes and their unit measurement, types of heat-producing tools tested, and tissues operated on (Supplementary Table 1). Many of these studies highlight instances of high exposures to known harmful byproducts and extrapolate these measurements over time to set ELs by national and international health organizations.
Figure 2.
Different components detected in surgical smoke.
Volatile organic compounds
Volatile organic compounds (VOCs) have been associated with acute and chronic respiratory symptoms and are a known cancer risk in cases of exposure to specific VOCs, including benzene and formaldehyde (Sarigiannis et al., 2011). Although initial studies of surgical smoke were limited solely to the detection of VOCs (Al Sahaf et al., 2007; Hensman et al., 1998; Sagar et al., 1996), more recent studies have been attempting to quantify the concentration of VOCs within the operating room. A comprehensive, well-designed study funded by (NIOSH) used personal sampling methods, such as passive badges, evacuated air canisters, and personal sampling pumps within surgical technicians’ breathing zones. These samplers detected only a few VOCs, but the levels were <1% of OSHA’s PELs and under NIOSH’s RELs (Benson et al., 2019). In area sampling during breast surgery, 4 VOCs were detected but, again, lower than ELs (Cheng et al., 2021). Metabolites of toluene and xylene were detected at low levels below NIOSH’s RELs in urine from 120 operating room staff. Interestingly, administrative, unexposed nurses had higher levels of one metabolite, whereas surgical nurses had higher levels of the other (Chiu et al., 2021). Six VOCs were measured in smoke directly from the abdomen during laparoscopic surgery. All VOCs, besides methylpropene, showed low levels comparable with ambient urban air and much lower than cigarette smoke inhalation (Fitzgerald et al., 2012). Although several recent studies have noted short periods of elevated VOC levels and highlighted the different compounds detected, these studies on both animal tissue models and operating room samples of different surgeries detected known hazardous VOCs at levels below established ELs (Gioutsos et al., 2022; Lee et al., 2018; Liu et al., 2021; Markowska et al., 2020; O’Brien et al., 2020; Stewart et al., 2021; Tokuda et al., 2020; Van Gestel et al., 2020).
In contrast, maximum total VOC concentrations higher than ELs were measured during minimally invasive liver surgery but not open surgery. However, sampling was measured close to the surgical field without accounting for any filtration or general dispersion among the operating room ventilation (Hsu et al., 2022). A comparable study measuring directly within the surgeon’s breathing zone via a specialized face mask showed that total VOC levels are higher in open surgery as compared with laparoscopic surgery. Still, in both instances, levels are much lower than ELs. These authors extrapolated that surgeons are exposed to about 1-10th of an unfiltered cigarette during each procedure based on detected acetaldehyde and butadiene levels in the surgical smoke (Kocher et al., 2022). Total VOC concentration was elevated in samples collected directly above the surgical instrument for 11 laparoscopic uterine surgeries, but none of the individual VOCs reached the ELs (Yan et al., 2022). Measurement of breathing zones of all staff during a breast surgery found benzene levels higher than ELs in some of the staff members, but not the surgeon (Koze et al., 2022). Over 3 weeks, indoor air samples in an operating room contained elevated total VOC concentrations and benzene levels compared with the Environmental Protection Agency’s Safe Green Building standard but lower than ELs for OSHA and NIOSH (Soysal et al., 2023). Collecting smoke samples directly through the surgical incisions for laparoscopic uterine surgery, all VOCs besides formaldehyde were below ELs. The level of formaldehyde exceeded NIOSH’s short-term EL but not OSHA’s (Ha et al., 2019). Another study evaluating the smoke in otolaryngologic surgeries detected high levels of formaldehyde near the end of the cases higher than the OSHA’s STEL (Li et al., 2022). Other possible contributors to the presence of VOCs in the operating room are cleaning products used in between cases and skin disinfectants applied before the start of surgery (Benson et al., 2019; Lee et al., 2018; Stewart et al., 2021), which need to be considered in the contribution of surgical smoke in VOC exposures in the operating room.
Polycyclic aromatic hydrocarbons
Polycyclic aromatic hydrocarbons (PAHs) can cause acute irritative symptoms and poor fetal development and have been linked to several different types of cardiopulmonary diseases and cancers, especially in the lung (Patel et al., 2020). In the previously mentioned 2016 NIOSH study, the only PAH detected was fluorene, which measured well below ELs (Benson et al., 2019). Samples from 30 obstetric and gynecologic cases showed the highest levels of PAH for naphthalene and its variants, but the highest average concentrations were several magnitudes less than ELs (Li et al., 2020). A sampling of the surgeons’ breathing zones in 40 peritonectomy cases identified naphthalene, acenaphthylene, acenaphthene, phenanthrene, and fluorene as the most abundant PAHs, and all were well below Els, with the carcinogenic PAHs among the lowest compounds detected (Naslund Andreasson et al., 2012). Conversely, a 70-year cancer risk based on PAH exposure in the breathing zones of surgeons and anesthetic technologists during 10 breast surgeries was calculated as 117 × 10−6 and 270 × 10−6, respectively, which are significantly higher than the benchmark set by the EPA at 1 × 10−6 (Tseng et al., 2014). Although the surgeons’ exposures were higher, the risk for the technologist was greater due to longer working hours exposed to surgical smoke compared with surgeons (Figure 3).
Figure 3.
Operating room staff roles and general estimate surgical smoke exposure by proximity. Number of “+” increases with greater exposure but does not denote any quantitative value. Created using Biorender.com.
Particulate matter
Particulate matter (PM) can deposit throughout the respiratory system in various locations depending on its size. PM < 10 μm in diameter (PM10, coarse) mainly settles in the upper airway and trachea; PM < 2.5 μm (PM2.5, fine) in the terminal bronchioles and alveoli; and PM <0.1 μm (PM0.1, ultrafine) has been shown to enter the bloodstream through the alveoli-blood barrier (Li et al., 2019). PM2.5 has been shown to increase the risk for pulmonary infection and lung cancer, exacerbate and cause new cardiac and respiratory diseases, and is associated with worsened cognition and early dementia (Thangavel et al., 2022). In a controlled laboratory environment, dissection of animal tissues through ultrasonic cutting, monopolar electrosurgery, and bone sawing showed that 90% of particles were <10 μm and that in most conditions, 50% were <2.5 μm (Casey et al., 2021). In direct measurement of smoke in 100 laparoscopic surgeries, unhealthy concentrations of PM2.5 (157.73 µg/m3) were measured, but the measurement duration was not reported, which restricts comparisons with established standards. Ultrasonic cautery was noted to produce the lowest concentrations (Feng et al., 2023). During a series of different types of laparoscopic surgeries, smoke 10 cm from the patient had elevated particle number concentrations, especially PM10 and PM2.5. As in other studies, they found that the highest concentrations were during the ventilation of smoke through the surgical trocars to clear the surgical view (Hardy et al., 2021; Wang et al., 2015). However, open abdominal surgery has been found to have higher levels than laparoscopic surgery (Kameyama et al., 2022; Taweerutchana et al., 2021). Extremely high levels of PM2.5 were measured while operating on the liver (approximately 303 ± 109 μg/m3), which was higher compared with surgeries involving muscle, adipose tissue, and blood vessels. Contrary to previous studies, ultrasonic scalpels produced high concentrations of PM (Tan et al., 2019). Although unclear where sampling was measured, 1 study showed that the average PM2.5 concentration was 63.88 ± 26.99 μg/m3 in obstetric open and laparoscopic surgery, well above the EPA limit (Li et al., 2020). Conversely, in several studies of varying surgeries, the maximum particle mass concentration at brief intervals was over the EPA’s healthy air quality index but was within normal limits when averaged over the entire case (Kameyama et al., 2022; Okoshi et al., 2022; Stewart et al., 2021; Tanaka et al., 2023; Wang et al., 2015). One of these studies, covering samplers by the head of bed with surgical masks, concluded that the PM levels detected were comparable with levels measured in the office and magnitudes less than cigarette smoke (Stewart et al., 2021).
Ultrafine particle (UFP) size sampling in a surgeon’s breathing zone (both inside and outside of the respiratory/mask) during animal tissue cutting showed particle number concentration around 1000 times higher than background (Gao et al., 2016). Other researchers detected UFP in the operating room for a variety of surgeries using different samplers either located by the anesthetist side of the table (Bruske-Hohlfeld et al., 2008) or by the surgeon’s breathing zone (Bruske-Hohlfeld et al., 2008; Carr et al., 2020). Much lower particle number concentrations were measured but extremely high peaks at short intervals were recorded, especially when cutting at higher power levels (Carr et al., 2020). These findings of brief high concentrations have also been seen in larger particle sizes around the operating table in otolaryngologic surgical cases (Li et al., 2022). However, another study on nasal surgeries on cadavers found extremely low particle number concentrations compared with previous studies (Sharma et al., 2021). Because the current understanding of UFP is limited, no set standards have been developed to determine unsafe levels.
Microorganisms
Although scant evidence has shown the viability of bacteria in surgical smoke through in vitro models (Capizzi et al., 1998; Schultz, 2015), greater focus has been on the viability and transmission of viruses through surgical smoke. Low levels of evidence have shown RNA or DNA of SARS-CoV-2, hepatitis B, and human immunodeficiency virus, but this has not shown to be viable or transmissible (Cheruiyot et al., 2021; Hirota et al., 2022; Hu et al., 2021; Kwak et al., 2016; Matta et al., 2022; Yokoe et al., 2021). Human papillomavirus (HPV) particles have been variably detected in surgical smoke, but evidence for transmission is more controversial (Palma et al., 2021). HPV DNA was detected in nasal epithelial cells of surgeons performing ablative surgery on HPV+ patients much more frequently than surgeons who do not conduct these types of operations. However, with a notable loss of follow-up, all became negative at 2 years (Palma et al., 2021). The highest level of evidence and most cited studies have been case reports of laser surgeons diagnosed with HPV+ laryngeal papillomatosis (n = 1) and tonsillar cancer (n = 2), as well as an operating room nurse with papillomatosis who was frequently exposed to ablative excision of anogenital warts (Calero and Brusis, 2003; Hallmo and Naess, 1991; Rioux et al., 2013).
Malignant cells
Early research in the 20th century on the viability of malignant cells in surgical smoke had been largely conflicting, and more recent research has been scarce. In 1 study, several cancer cell lines were targeted with radiofrequency ablation, ultrasonic scalpel, and electrocautery. The smoke was collected 5–10 cm away. Only the ultrasonic scalpel group showed viable cells, predominately when collected 5 cm away. The sediment of this smoke was injected into the left and right backs of 20 mice, and 16/40 injection sites revealed tumor development (In et al., 2015). A similar study of mice injected with cervical cancer cells had tumors resected over 12 min with an ultrasonic scalpel and smoke collected 2 cm away. This smoke was then cultured and injected into nude mice. However, no viable malignant cells were identified on culture, and none of the inoculation sites grew tumors after 3 months. However, HPV DNA was detected in the surgical smoke and cross-matched with the cervical cancer cells used. Normal cells were then cultured in surgical smoke-filtered media, which maintained HPV positivity, suggesting viability yet no transmission (Yan et al., 2022).
Biological effects of surgical smoke
A summary of relevant studies evaluating surgical smoke effects is provided in Table 1. Research on the cellular and immunologic alterations associated with surgical smoke has been primarily limited to laboratory and animal exposure studies. Mouse macrophages and human small airway epithelial cells were exposed to surgical smoke collected 5 cm away from the cauterization of breast tissue. Following 24 h of exposure, less than half of the cells underwent cellular death and had produced elevated levels of lactate dehydrogenase, a marker for cytotoxicity. Reactive oxygen species, another marker of cell stress and inflammation, was not found to be elevated (Sisler et al., 2018). PM2.5 from surgical smoke produced a cytotoxic effect on embryonic stem cells and cardiac muscle cells (Zhou et al., 2021). Early laboratory studies have shown suggestions of mutagenicity via the Ames test on strains of Salmonella (Gatti et al., 1992; Tomita et al., 1981). However, a more recent study using gamma Histone 2A family member X to visualize double-stranded DNA breaks did not support this claim (Stewart et al., 2021). Sheep ventilated with laser smoke were shown to have decreased mucus velocity time, impaired gas exchange function, and increased neutrophils on bronchial lavage (Freitag et al., 1987), suggesting airway inflammation and mucociliary dysfunction. Larynges of rats exposed to surgical smoke from sheep liver (1 h each day for 4 weeks) showed a higher density of inflammation microscopically but no changes indicative of metaplasia, hyperplasia, or cellular proliferation compared with controls (Atar et al., 2017). Rats exposed to a smoke chamber for 4 min, 4 times a day for 2 weeks, had histopathological changes of the nasal mucosa, including markers of acute inflammation, vascular congestion, and necrotic cells, compared with controls. Interestingly, these findings were less pronounced when rats were exposed for 4 or 7 days (Sarkarizi et al., 2020). Yet, long-term inhalation of laser smoke in rats showed signs of alveolar damage, resulting in interstitial pneumonia, bronchiolitis, and emphysema (Baggish and Elbakry, 1987; Wenig et al., 1993).
Table 1.
Biological and health effects associated with surgical smoke
| Study (authors, year) | Type of study | Exposure | Experimental system | Length of exposure | Significant findings |
|---|---|---|---|---|---|
| Sisler et al., 2018 | In vitro | Smoke collected 5 cm from the electrocautery site (15 min of cautery of recently resected breast) and loaded into cell medium | Mouse macrophage (MM) and human small airway epithelial (SAEC) cells | 24 h of incubation in surgical smoke cell medium |
|
| Zhou et al., 2021 | In vitro | PM2.5 from the smoke in an operating room collected with a filter over several days and dissolved in cell medium | Embryonic cardiomyocytes | 10 days in surgical smoke cell medium |
|
| Tomita et al., 1981 | In vitro | Smoke condensate from lasered and electrocauterized canine tongue (1 min of smoke produced) | Salmonella typhimurium TA100 and TA98 | 2 days of incubation in smoke condensates | - Mutagenicity equivalent to 3 (laser smoke) or 6 (electrocautery smoke) filtered cigarette smoke |
| Freitag et al., 1987 | Animal/in vivo | Ventilated, chambered smoke from lasered sheep bronchial tissue | Sheep | 10 min (single exposure or repeated 3 times) |
|
| Baggish and Elbakry, 1987 | Animal/in vivo | Smoke from laser of pigskin diffused upwards to ventilated smoke chamber with exhaust hole | Rats | Varied: 2–4 min of exposure followed by 2 min rest; 4 times daily for 4–14 days |
|
| Wenig et al., 1993 | Animal/in vivo | Smoke from laser and electrocautery of pigskin diffused upwards to ventilated smoke chamber with exhaust hole | Rats | Varied: 2–4 min of exposure followed by 2 min rest; 4 times daily for 4–14 days |
|
| Atar et al., 2017 | Animal/in vivo | Smoke from cautery of sheep liver diffused upwards to ventilated plexiglass cabin where subjects were stored | Rats | 60 min a day for 4 weeks |
|
| Sarkarizi et al., 2020 | Animal/in vivo | Smoke from cautery of a rat diffused upwards to smoke chamber with exhaust hole | Rats | Varied: 2–4 min of exposure followed by 2 min rest; 4 times daily for 4–14 days |
|
| Navarro et al., 2016 | Human prospective cohort | Cumulative smoke exposure (not directly measured) in the operating room for surgical residents | Surgical and nonsurgical medical residents | 4 years | - Nasal mucosa hyperplasia (most common) and squamous metaplasia |
| Canicoba and Poveda, 2022 a | Cross-sectional surveys | Smoke exposure (not directly measured) in the operating room | Operating room staff (surgeons, nurses, etc.) | N/A |
|
| Dobrogowski et al., 2014 | Human observational study | Smoke produced in the abdomen during laparoscopic gallbladder removal | Patients | Length of surgery | - Increased levels of benzene and toluene in urine post-operatively compared with pre-operatively |
| Gates et al., 2007; Le Moual et al., 2013; Xie et al., 2021 | Human longitudinal survey | Occupation in the operating room | Operating room nurses | Unclear |
|
Systematic review summarizing 7 cross-sectional surveys regarding the symptoms in the operative room.
When examining direct effects on humans, the evidence is more limited but indicates potential relationships between surgical smoke exposure and adverse health effects. Researchers obtained nasal biopsies from surgical and nonsurgical healthy physicians in training with no known environmental exposures at the beginning of their training and 4 years after to assess for changes following surgical smoke exposure in the surgical physicians. Although patients had normal mucosa initially, after 4 years, 16/23 surgical residents had hyperplasia (most predominant) or squamous metaplasia. Conversely, only 1/20 nonsurgical residents had hyperplasia (Navarro et al., 2016). Symptoms reported by operating room staff during and following surgical smoke exposure are most commonly listed as headache, eye irritation and watering, upper airway irritation and cough, and odors in the hair (Canicoba and Poveda, 2022). Associations with asthma and chronic obstructive pulmonary disease (COPD) risk have been reported within the U.S. Nurses’ Health Study, an ongoing prospective cohort that started in 1976 with biennial surveys. However, this relationship is determined based on operating room employment at the time of enrollment in the survey and disease outcome 10–20 years later (Le Moual et al., 2013; Xie et al., 2021). Female surgeons have higher rates of infertility and reproductive issues compared with the general population, but no stratification of risk factors was possible (Anderson and Goldman, 2020). Lastly, exposure is not only limited to the staff, as patients who are awake during procedures report unpleasant odors from electrosurgery (Golda et al., 2018; Yonan and Ochoa, 2017). Elevated levels of toluene and benzene have been detected in the urine of surgical patients, indicating exposure and absorption of these compounds (Dobrogowski et al., 2014).
The Ames test is a well-established assay to evaluate the mutagenicity of agents, such as specific chemicals or mixtures like tobacco smoke. The assays are based on mutated Salmonella typhomurium strains that cannot synthesize histidine, a required molecule for the strains to grow. If a mutagen is added that can reverse the strains’ histidine-producing mutations, then the strains will grow. As a result, mutagenicity is calculated as the number of revertants (ie, colonies) per unit of exposure. For example, using this assay, the mutagenicity of filtered cigarette smoke condensates was calculated (Sato et al., 1977). A 1981 study collected laser and electrocautery smoke from canine tongues in a closed box system. Using the cigarette smoke mutagenicity data collected in the previous study, they calculated that the mutagenicity of 1 g of tissue ablated was equivalent to 3 or 6 cigarettes (laser and electrocautery, respectively) (Tomita et al., 1981). In 2012, plastic surgeons ablated human and porcine tissue to determine the tissue mass destroyed over 5 min. By reviewing the total electrosurgery activation time over 44 days in their operating room, the researchers concluded, based on the 1 g per 6 cigarettes assumption, that daily electrosurgery produced the equivalent of secondhand smoke from “between 27 and 30 unfiltered cigarettes” (Hill et al., 2012). Although the data are based on the 1977 study that studied filtered cigarettes (not unfiltered), this analogy has been heavily cited throughout the literature and marketed by smoke evacuation device manufacturers (Dispomed, 2021). However, this misleading equivalency is based on calculations of mutagenicity based on smoke produced in a closed-system smoke chamber, which is not equivalent to the operating room exposure (eg, distance from the surgical site, operating room ventilation, masking). Through the NHS cohort focusing on 87 000 nurses with and without operating room experience in 1984, adjusted Cox proportional hazards regression showed no increase in lung cancer incidence in the follow-up years up to 2000. Interestingly, the nurses with the longest operating room experience had significantly less incidence of lung cancer on follow-up, even when controlling for cigarette smoking history (Gates et al., 2007).
Prevention and regulation
Surgical masks are the first line of defense for operating room staff against exhaled large-particle droplets, blood particles, and other splashes/sprays. In addition to variable filtration of fine particles, these masks are typically worn loosely in the operating room and bypass the filtration. Respirator masks (eg, N95, FFP2, FFP3) are tight-fitting and certified to filter particles 0.3 μm in size at variable levels at 94%–99.97% efficiency. Due to the discomfort of these masks, their use is typically limited to HPV+ anogenital and laryngeal papillomatosis surgeries for concerns of viral transmission, even though these masks have been proven to reduce the amount of exposure to fine particles and VOCs in surgical smoke (Elmashae et al., 2018; Gao et al., 2016).
Operating room air ventilation varies by institution depending on factors such as type of airflow, temperature, number of personnel, door openings, and air changes per hour (Sadrizadeh et al., 2021). This variability also limits the generalizability of research based on an operating room’s ventilation layout. The American Society of Heating, Refrigerating and Air-Conditioning Engineers recommends the use of positive pressure rooms, a minimum air change rate of 20 air changes per hour, unidirectional laminar flow, and the use of high-efficiency (HEPA) or ultra-low penetration (ULPA) filters with frequent replacement (ANSI/ASHRAE/ASHE, 2017).
Local smoke evacuation has been the primary focus of smoke mitigation in the past decade, especially during the COVID-19 outbreak. Traditionally, a surgical assistant would hold a suction catheter connected to the wall, even though this method is intended to eliminate liquids. The assistant would hold this catheter over the smoke as the surgeon is operating and attempt to catch the visible smoke as it is produced. It is recommended to hold this suction around 2 inches away from the surgical field (NIOSH, 1996). The main limitations of this approach include the attentiveness of the assistant, obstruction of the surgical field, clogging by liquids and tissue, poor suctioning power, and lack of filtration. In-line filtration can be added to the wall suction, but the other limitations remain, and filtration can worsen the suctioning flow rates. Another option is using the same suction catheter but with higher-quality stand-alone filtration systems and canisters with increased flow rates that can detect and separate smoke and liquid components. Several medical system manufacturers have recently developed smoke evacuation devices connected to electrosurgical tools, such as the electrosurgical unit pencil. These instruments do not rely on an assistant to “catch” the smoke and have been shown to be more effective in removing surgical smoke (Gioutsos et al., 2022; Seipp et al., 2018; Tanaka et al., 2023). During the COVID-19 outbreak, alternative cheaper options were devised that taped small urethral catheters to the electrosurgical device, though their effectiveness was not tested objectively (Andrade et al., 2020; Ekci, 2020). Besides cost, other reasons for not including an integrated smoke evacuation device include: bulkiness and interference with view, lack of concern for surgical smoke risk, minimal surgical smoke produced, and noise level (Michaelis et al., 2020). The noise levels of multiple systems have been evaluated to be below the set standards for occupation noise exposure and do not put the staff at risk for hearing loss. However, the noise level may be high enough to affect the concentration of the operator (Gioutsos et al., 2022; Grigoryan and Kampp, 2021; Seipp et al., 2018). Exposure to intraperitoneal smoke in laparoscopic surgery is caused by either leaking from the trocar sites or being directly released through a relief valve by the surgeon to clear the surgical field. The latter method produces a rapid accumulation of smoke released in a short period (Hardy et al., 2021; Wang et al., 2015). Built-in filter ports have been designed to reduce the risk of surgical smoke exposure, but crucially, flow rates must be less than the carbon dioxide insufflation rate to keep the abdominal cavity inflated (Ha et al., 2019; Hahn et al., 2017).
Although improving, compliance for “safe smoke practices” has been historically low (Ball, 2010; Chavis et al., 2016; Michaelis et al., 2020; Soysal et al., 2023). Many factors contribute to poor compliance: lack of concern about surgical smoke health risks, burdens of different types of smoke evacuation devices, and infrastructure and logistic barriers within the hospital or surgery center system. To address poor compliance, the Association of periOperative Registered Nurses (AORN), supported by Medtronic, a smoke evacuation device manufacturer, has developed a surgical smoke-free recognition program to implement in hospital systems. The keys to improving compliance focus on leadership integration, education programs on the hazards of surgical smoke, support from the surgeon, and identifying gaps in barriers towards success (Chavis et al., 2016). Currently, there are no mandated regulations on the federal level. NIOSH and OSHA recognize the potential hazards of surgical smoke (ie, components of surgical smoke and risk of acute irritative symptoms) and recommend local smoke evacuation. However, OSHA has no specific mandated regulations for surgical smoke evacuation (Administration; NIOSH, 1996). AORN has been a major proponent in lobbying for federal and state regulations for surgical smoke in the United States. As of July 6, 2023, 15 states have passed legislation requiring surgical smoke evacuation systems for energy-producing procedures (AORN, 2023).
Conclusions
Energy-based surgical instruments produce surgical smoke, which has been shown to contain harmful compounds, such as VOCs and PAHs, PM, and viable microorganisms. Studies on surgical smoke exposure have significant heterogeneity in the tissues operated on, placement and type of analyte detection instruments, and the energy-based surgical instruments tested. Although high concentrations of these compounds and PM are detected in short bursts, generally, these levels are lower than established limits by recognized occupational and environmental health bodies. In conjunction with general operating room ventilation and high-filtration masking, local smoke evacuation systems have been shown to reduce surgical smoke exposure.
Future directions
As regulation is increasing across the state level, federal regulation for surgical smoke has yet to be established. Successful exposure reduction measures are already emerging, yet recommendations for regulatory control may be premature since exposure and, more importantly, its effects still need to be fully realized. However, devices such as smoke evacuation systems present themselves as a protective measure until these gaps in knowledge are filled. Although much research was initially being conducted on animal tissue, recent experiments have shifted to the live operating room, producing conflicting results on the components and levels of exposure to the staff. Due to surgical sterility and invasiveness of detection instruments in the staffs’ breathing zones, heterogeneity in surgical design has limited the conclusions that can be drawn and incorporated into implementing regulations. Sampling methods differ in the type of equipment used and their respective sensitivities, placement of samplers, flow rates, and the types of chemicals being studied, which limit conclusions on actual occupation exposure and reproducibility in other operating rooms. Future directions should focus on validating detection methods that can best capture breathing zone exposure without interfering with the operation, such as wearable personal sensors that can be placed on the collar or within the surgical mask (Benson et al., 2019; Elmashae et al., 2018). With a validated detection method, further research on areas of interest, such as levels of exposure for different surgical roles, types of surgical instruments and settings, surgeries, tissues operated, and distance from the surgical field, can be conducted. Additionally, other dangerous byproducts, such as toxic metals or inhaled anesthetic gases, within surgical smoke have yet to be evaluated.
Moreover, the biological and health effects of surgical smoke have yet to be fully elucidated, as research has been primarily in in vitro and animal studies. Human studies have yet to show a direct link between surgical smoke exposure and poor health outcomes besides acute irritative symptoms such as headache and throat irritation. Future research would benefit from evaluating biomarkers of immunological and other adverse health effects of surgical smoke in humans in vivo as well as mechanistic studies using more controlled in vitro or animal models. Additionally, updated and more robust prospective cohort studies on surgical staff and disease outcomes could delineate the long-term health risks of surgical smoke. Specifically, given retrospective surveys showing possible associations with asthma and COPD, this population needs more focused attention to evaluate any link to either the development or exacerbations of respiratory disease with more objective outcome measures, such as spirometry. Lastly, other at-risk populations have yet to be studied—staff operating on laboratory animals and veterinarian surgeons have not been evaluated for exposure to surgical smoke.
Supplementary Material
Acknowledgments
We would like to thank the members of the Jasper Lab for their helpful discussion regarding this topic.
Contributor Information
Ezer H Benaim, Center for Environmental Medicine, Asthma and Lung Biology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA; Department of Otolaryngology-Head and Neck Surgery, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.
Ilona Jaspers, Center for Environmental Medicine, Asthma and Lung Biology, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA; Curriculum in Toxicology & Environmental Medicine, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA; Center for Environmental Medicine, Asthma, and Lung Biology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA; Department of Pediatrics, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA.
Supplementary data
Supplementary data are available at Toxicological Sciences online.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
National Institute on Deafness and Other Communication Disorders branch of the National Institutes of Health (Award Number T32 DC005360 to E.H.B.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Al Sahaf O. S., Vega-Carrascal I., Cunningham F. O., McGrath J. P., Bloomfield F. J. (2007). Chemical composition of smoke produced by high-frequency electrosurgery. Ir. J. Med. Sci. 176, 229–232. [DOI] [PubMed] [Google Scholar]
- Anderson M., Goldman R. H. (2020). Occupational reproductive hazards for female surgeons in the operating room: A review. JAMA Surg. 155, 243–249. [DOI] [PubMed] [Google Scholar]
- Andrade W. P., Goncalves G. G., Medeiros L. C., Araujo D. C. M., Pereira G. T. G., Moraes D. M. P., Spencer R. (2020). Low-cost, safe, and effective smoke evacuation device for surgical procedures in the covid-19 age. J. Surg. Oncol. 122, 844–847. [DOI] [PubMed] [Google Scholar]
- ANSI/ASHRAE/ASHE (2017). Standard 170-2017, ventilation of healthcare facilities. Surgical smoke evacuation legislation status. Available at: https://www.aorn.org/get-involved/government-affairs/policy-agenda/surgical-smoke-free-or/smoke-bills. Accessed September 2023.
- AORN (2023). Surgical smoke evacuation legislation status. Available at: https://www.aorn.org/getinvolved/government-affairs/policy-agenda/surgical-smoke-free-or/smoke-bills. Accessed September 2023.
- AORN (2020). Surgical smoke: Oversight and regulations. AORN 112, 25–26. [DOI] [PubMed] [Google Scholar]
- Atar Y., Salturk Z., Kumral T. L., Uyar Y., Cakir C., Sunnetci G., Berkiten G. (2017). Effects of smoke generated by electrocautery on the larynx. J. Voice 31, e380–387.e389. [DOI] [PubMed] [Google Scholar]
- Baggish M. S., Elbakry M. (1987). The effects of laser smoke on the lungs of rats. Am. J. Obstet. Gynecol. 156, 1260–1265. [DOI] [PubMed] [Google Scholar]
- Ball K. (2010). Compliance with surgical smoke evacuation guidelines: Implications for practice. AORN J. 92, 142–149. [DOI] [PubMed] [Google Scholar]
- Benson S. M., Maskrey J. R., Nembhard M. D., Unice K. M., Shirley M. A., Panko J. M. (2019). Evaluation of personal exposure to surgical smoke generated from electrocautery instruments: A pilot study. Ann. Work Expo. Health. 63, 990–1003. [DOI] [PubMed] [Google Scholar]
- Bruske-Hohlfeld I., Preissler G., Jauch K. W., Pitz M., Nowak D., Peters A., Wichmann H. E. (2008). Surgical smoke and ultrafine particles. J. Occup. Med. Toxicol. 3, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero L., Brusis T. (2003). Laryngeal papillomatosis—First recognition in Germany as an occupational disease in an operating room nurse. Laryngorhinootologie 82, 790–793. [DOI] [PubMed] [Google Scholar]
- Canicoba A. R. B., Poveda V. B. (2022). Surgical smoke and biological symptoms in healthcare professionals and patients: A systematic review. J. Perianesth. Nurs. 37, 130–136. [DOI] [PubMed] [Google Scholar]
- Capizzi P. J., Clay R. P., Battey M. J. (1998). Microbiologic activity in laser resurfacing plume and debris. Lasers Surg. Med. 23, 172–174. [DOI] [PubMed] [Google Scholar]
- Carr M. M., Patel V. A., Soo J. C., Friend S., Lee E. G. (2020). Effect of electrocautery settings on particulate concentrations in surgical plume during tonsillectomy. Otolaryngol. Head Neck Surg. 162, 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey V. J., Martin C., Curtin P., Buckley K., McNamara L. M. (2021). Comparison of surgical smoke generated during electrosurgery with aerosolized particulates from ultrasonic and high-speed cutting. Ann. Biomed. Eng. 49, 560–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey V. J., McNamara L. M. (2023). Instrumental in surgery: A narrative review on energy-based surgical cutting devices and surgical smoke. Ann. Surg. 278, e457–e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis S., Wagner V., Becker M., Bowerman M. I., Jamias M. S. (2016). Clearing the air about surgical smoke: An education program. AORN J. 103, 289–296. [DOI] [PubMed] [Google Scholar]
- Cheng M. H., Chiu C. H., Chen C. T., Chou H. H., Pao L. H., Wan G. H. (2021). Sources and components of volatile organic compounds in breast surgery operating rooms. Ecotoxicol. Environ. Saf. 209, 111855. [DOI] [PubMed] [Google Scholar]
- Cheruiyot I., Sehmi P., Ngure B., Misiani M., Karau P., Olabu B., Henry B. M., Lippi G., Cirocchi R., Ogeng’o J. (2021). Laparoscopic surgery during the covid-19 pandemic: Detection of SARS-COV-2 in abdominal tissues, fluids, and surgical smoke. Langenbecks. Arch. Surg. 406, 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. H., Chen C. T., Cheng M. H., Pao L. H., Wang C., Wan G. H. (2021). Use of urinary hippuric acid and o-/p-/m-methyl hippuric acid to evaluate surgical smoke exposure in operating room healthcare personnel. Ecotoxicol. Environ. Saf. 217, 112231. [DOI] [PubMed] [Google Scholar]
- Dispomed (2021). Did you know that daily exposure to surgical smoke plume is equivalent to smoking 27 to 30 cigarettes a day? Available at: https://www.dispomed.com/did-you-know-that-daily-exposure-to-surgical-smoke-plume-is-equivalent-to-smoking-27-to-30-cigarettes-a-day/. Accessed November 2023.
- Dobrogowski M., Wesolowski W., Kucharska M., Sapota A., Pomorski L. S. (2014). Chemical composition of surgical smoke formed in the abdominal cavity during laparoscopic cholecystectomy—Assessment of the risk to the patient. Int. J. Occup. Med. Environ. Health 27, 314–325. [DOI] [PubMed] [Google Scholar]
- Ekci B. (2020). Easy-to-use electrocautery smoke evacuation device for open surgery under the risk of the Covid-19 pandemic. J. Int. Med. Res. 48, 300060520949772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmashae Y., Koehler R. H., Yermakov M., Reponen T., Grinshpun S. A. (2018). Surgical smoke simulation study: Physical characterization and respiratory protection. Aerosol. Sci. Technol. 52, 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X., Ding B., Shen Y. (2023). The feasibility and efficacy of a novel nano filter in reducing the hazards of surgical smoke exposure during gynecological laparoscopic surgery. Surg. Innov. 30, 654–656. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J. E., Malik M., Ahmed I. (2012). A single-blind controlled study of electrocautery and ultrasonic scalpel smoke plumes in laparoscopic surgery. Surg. Endosc. 26, 337–342. [DOI] [PubMed] [Google Scholar]
- Freitag L., Chapman G. A., Sielczak M., Ahmed A., Russin D. (1987). Laser smoke effect on the bronchial system. Lasers Surg. Med. 7, 283–288. [DOI] [PubMed] [Google Scholar]
- Gao S., Koehler R. H., Yermakov M., Grinshpun S. A. (2016). Performance of facepiece respirators and surgical masks against surgical smoke: Simulated workplace protection factor study. Ann. Occup. Hyg. 60, 608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates M. A., Feskanich D., Speizer F. E., Hankinson S. E. (2007). Operating room nursing and lung cancer risk in a cohort of female registered nurses. Scand. J. Work. Environ. Health. 33, 140–147. [DOI] [PubMed] [Google Scholar]
- Gatti J. E., Bryant C. J., Noone R. B., Murphy J. B. (1992). The mutagenicity of electrocautery smoke. Plast Reconstr. Surg. 89, 781–784. [PubMed] [Google Scholar]
- Gioutsos K., Nguyen T. L., Biber U., Enderle M. D., Koss A., Kocher G. J. (2022). Surgical smoke: Modern mobile smoke evacuation systems improve occupational safety in the operating theatre. Interact. Cardiovasc. Thorac. Surg. 34, 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golda N., Huber A., Gole H. (2018). Determining the impact of intraoperative smoke evacuation on the patient experience during outpatient surgery: A randomized controlled trial. J. Am. Acad. Dermatol. 78, 1007–1009. [DOI] [PubMed] [Google Scholar]
- Grigoryan K. V., Kampp J. T. (2021). Noise associated with surgical smoke evacuators during dermatologic surgery. Dermatol. Surg. 47, 726–727. [DOI] [PubMed] [Google Scholar]
- Ha H. I., Choi M. C., Jung S. G., Joo W. D., Lee C., Song S. H., Park H. (2019). Chemicals in surgical smoke and the efficiency of built-in-filter ports. JSLS 23, e2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn K. Y., Kang D. W., Azman Z. A. M., Kim S. Y., Kim S. H. (2017). Removal of hazardous surgical smoke using a built-in-filter trocar: A study in laparoscopic rectal resection. Surg. Laparosc. Endosc. Percutan. Tech. 27, 341–345. [DOI] [PubMed] [Google Scholar]
- Hallmo P., Naess O. (1991). Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur. Arch. Otorhinolaryngol. 248, 425–427. [DOI] [PubMed] [Google Scholar]
- Hardy N., Dalli J., Khan M. F., Nolan K., Cahill R. A. (2021). Aerosols, airflow, and airspace contamination during laparoscopy. Br. J. Surg. 108, 1022–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensman C., Baty D., Willis R. G., Cuschieri A. (1998). Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment: An in vitro study. Surg. Endosc. 12, 1017–1019. [DOI] [PubMed] [Google Scholar]
- Hill D. S., O’Neill J. K., Powell R. J., Oliver D. W. (2012). Surgical smoke—A health hazard in the operating theatre: A study to quantify exposure and a survey of the use of smoke extractor systems in UK plastic surgery units. J. Plast. Reconstr. Aesthet. Surg. 65, 911–916. [DOI] [PubMed] [Google Scholar]
- Hirota M., Takahashi H., Miyazaki Y., Takahashi T., Kurokawa Y., Yamasaki M., Eguchi H., Doki Y., Nakajima K. (2022). Surgical plume from tissue infected with human hepatitis B virus can contain viral substances. Minim. Invasive Ther. Allied Technol. 31, 728–736. [DOI] [PubMed] [Google Scholar]
- Hsu F. L., Ho T. W., Chang C., Wu J. M., Lin M. T. (2022). Chemical composition of smoke produced by open versus laparoscopic surgery for cholecystectomy. HPB (Oxford) 24, 1335–1340. [DOI] [PubMed] [Google Scholar]
- Hu X., Zhou Q., Yu J., Wang J., Tu Q., Zhu X. (2021). Prevalence of HPV infections in surgical smoke exposed gynecologists. Int. Arch. Occup. Environ. Health. 94, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In S. M., Park D. Y., Sohn I. K., Kim C. H., Lim H. L., Hong S. A., Jung D. Y., Jeong S. Y., Han J. H., Kim H. J. (2015). Experimental study of the potential hazards of surgical smoke from powered instruments. Br. J. Surg. 102, 1581–1586. [DOI] [PubMed] [Google Scholar]
- Ismail A., Abushouk A. I., Elmaraezy A., Menshawy A., Menshawy E., Ismail M., Samir E., Khaled A., Zakarya H., El-Tonoby A., et al. (2017). Cutting electrocautery versus scalpel for surgical incisions: A systematic review and meta-analysis. J. Surg. Res. 220, 147–163. [DOI] [PubMed] [Google Scholar]
- Kameyama H., Otani T., Yamazaki T., Iwaya A., Uehara H., Harada R., Hirai M., Komatsu M., Kubota A., Katada T., et al. (2022). Comparison of surgical smoke between open surgery and laparoscopic surgery for colorectal disease in the Covid-19 era. Surg. Endosc. 36, 1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher G. J., Koss A. R., Groessl M., Schefold J. C., Luedi M. M., Quapp C., Dorn P., Lutz J., Cappellin L., Hutterli M., et al. (2022). Electrocautery smoke exposure and efficacy of smoke evacuation systems in minimally invasive and open surgery: A prospective randomized study. Sci. Rep. 12, 4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koze B. S., MYv G., Yeniay L. (2022). Quality of air in the operating room: Surgical smoke-a descriptive study. Turkiye Klinikleri J. Med. Sci. 42, 282–288. [Google Scholar]
- Kwak H. D., Kim S. H., Seo Y. S., Song K. J. (2016). Detecting hepatitis b virus in surgical smoke emitted during laparoscopic surgery. Occup. Environ. Med. 73, 857–863. [DOI] [PubMed] [Google Scholar]
- Le Moual N., Varraso R., Zock J. P., Henneberger P., Speizer F. E., Kauffmann F., Camargo C. A. Jr (2013). Are operating room nurses at higher risk of severe persistent asthma? The nurses’ health study. J. Occup. Environ. Med. 55, 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Soo J. C., LeBouf R. F., Burns D., Schwegler-Berry D., Kashon M., Bowers J., Harper M. (2018). Surgical smoke control with local exhaust ventilation: Experimental study. J. Occup. Environ. Hyg. 15, 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. I., Chou Y. H., Pai J. Y., Chen C. H., Chiang M. C. (2022). Investigating surgical smoke in otolaryngology operating rooms. Sci. Rep. 12, 1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. I., Pai J. Y., Chen C. H. (2020). Characterization of smoke generated during the use of surgical knife in laparotomy surgeries. J. Air Waste Manag. Assoc. 70, 324–332. [DOI] [PubMed] [Google Scholar]
- Li D., Li Y., Li G., Zhang Y., Li J., Chen H. (2019). Fluorescent reconstitution on deposition of PM(2.5) in lung and extrapulmonary organs. Proc. Natl. Acad. Sci. U.S.A. 116, 2488–2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhao M., Shao Y., Yan L., Zhu X. (2021). Chemical composition of surgical smoke produced during the loop electrosurgical excision procedure when treating cervical intraepithelial neoplasia. World J. Surg. Oncol. 19, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska M., Krajewski A., Maciejewska D., Jeleń H., Kaczmarek M., Stachowska E. (2020). Qualitative analysis of surgical smoke produced during burn operations. Burns 46, 1356–1364. [DOI] [PubMed] [Google Scholar]
- Matta I., Lagana A. S., Ghabi E., Bitar L., Ayed A., Petousis S., Vitale S. G., Sleiman Z. (2022). Covid-19 transmission in surgical smoke during laparoscopy and open surgery: A systematic review. Minim. Invasive Ther. Allied Technol. 31, 690–697. [DOI] [PubMed] [Google Scholar]
- Michaelis M., Hofmann F. M., Nienhaus A., Eickmann U. (2020). Surgical smoke-hazard perceptions and protective measures in German operating rooms. Int. J. Environ. Res. Public Health 17, 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslund Andreasson S., Mahteme H., Sahlberg B., Anundi H. (2012). Polycyclic aromatic hydrocarbons in electrocautery smoke during peritonectomy procedures. J. Environ. Public Health. 2012, 929053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M. C., González R., Aldrete M. G., Carmona D. E. (2016). Cambios en la mucosa nasal de los médicos por exposición al humo por electrocoagulación. Rev. Fac. Nac. Salud Pública 34, 135–144. [Google Scholar]
- NIOSH (1996). Control of smoke from laser/electric surgical procedures. DHHS (NIOSH) Publication Number 96–128.
- O’Brien D. C., Lee E. G., Soo J., Friend S., Callaham S., Carr M. M. (2020). Surgical team exposure to cautery smoke and its mitigation during tonsillectomy. Otolaryngol. Head. Neck Surg. 163, 508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occupational Safety and Health Administration (2023). Laser/electrosurgery plume. Available at: https://www.osha.gov/laser-electrosurgery-plume. Accessed November 2023.
- Occupational Safety and Health Administration-U.S. Department of Labor (2023). Smoke plume. Available at: https://www.osha.gov/etools/hospitals/surgical-suite/smoke-plume. Accessed September 2023.
- Okoshi K., Hida K., Kinoshita K., Morishima T., Nagai Y., Tomizawa Y., Yorozuya K., Nishida T., Matsumoto H., Yamato H. (2022). Measurement of particulate matter 2.5 in surgical smoke and its health hazards. Surg. Today. 52, 1341–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma S., Gnambs T., Crevenna R., Jordakieva G. (2021). Airborne human papillomavirus (HPV) transmission risk during ablation procedures: A systematic review and meta-analysis. Environ. Res. 192, 110437. [DOI] [PubMed] [Google Scholar]
- Patel A. B., Shaikh S., Jain K. R., Desai C., Madamwar D. (2020). Polycyclic aromatic hydrocarbons: Sources, toxicity, and remediation approaches. Front. Microbiol. 11, 562813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux M., Garland A., Webster D., Reardon E. (2013). HPV positive tonsillar cancer in two laser surgeons: Case reports. J. Otolaryngol. Head Neck Surg. 42, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrizadeh S., Aganovic A., Bogdan A., Wang C., Afshari A., Hartmann A., Croitoru C., Khan A., Kriegel M., Lind M., et al. (2021). A systematic review of operating room ventilation. J. Build. Eng. 40, 102693. [Google Scholar]
- Sagar P. M., Meagher A., Sobczak S., Wolff B. G. (1996). Chemical composition and potential hazards of electrocautery smoke. Br. J. Surg. 83, 1792. [DOI] [PubMed] [Google Scholar]
- Sarkarizi, H. K., Salimnejad, R., Jafarian, A. H., Fani, M., Khajavian, N., Nourmohamadi, E., and Sazegar, G. (2020). Effects of electrocauterization smoke on nasal mucosa in rats. Crescent J. Medical Biol. Sci. 7, 34–39. [Google Scholar]
- Sarigiannis D. A., Karakitsios S. P., Gotti A., Liakos I. L., Katsoyiannis A. (2011). Exposure to major volatile organic compounds and carbonyls in European indoor environments and associated health risk. Environ. Int. 37, 743–765. [DOI] [PubMed] [Google Scholar]
- Sato S., Seino Y., Ohka T., Yahagi T., Nagao M., Matsushima T., Sugimura T. (1977). Mutagenicity of smoke condensates from cigarettes, cigars and pipe tobacco. Cancer Lett. 3, 1–8. [DOI] [PubMed] [Google Scholar]
- Schultz L. (2015). Can efficient smoke evacuation limit aerosolization of bacteria? AORN J. 102, 7–14. [DOI] [PubMed] [Google Scholar]
- Seipp H. M., Steffens T., Weigold J., Lahmer A., Maier-Hasselmann A., Herzog T., Herzog-Niescery J. (2018). Efficiencies and noise levels of portable surgical smoke evacuation systems. J. Occup. Environ. Hyg. 15, 773–781. [DOI] [PubMed] [Google Scholar]
- Sharma D., Ye M. J., Campiti V. J., Rubel K. E., Higgins T. S., Wu A. W., Shipchandler T. Z., Sim M. W., Burgin S. J., Illing E. A., et al. (2021). Mitigation of aerosols generated during rhinologic surgery: A pandemic-era cadaveric simulation. Otolaryngol. Head Neck Surg. 164, 433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler J. D., Shaffer J., Soo J. C., LeBouf R. F., Harper M., Qian Y., Lee T. (2018). In vitro toxicological evaluation of surgical smoke from human tissue. J. Occup. Med. Toxicol. 13, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soysal G. E., Ilce A., Lakestani S., Sit M., Avcioglu F. (2023). Comparison of the effects of surgical smoke on the air quality and on the physical symptoms of operating room staff. Biol. Res. Nurs. 25, 444–453. [DOI] [PubMed] [Google Scholar]
- Stewart C. L., Raoof M., Lingeman R., Malkas L., Flores V., Caldwell K., Fong Y., Melstrom K. (2021). A quantitative analysis of surgical smoke exposure as an occupational hazard. Ann. Surg. 274, 306–311. [DOI] [PubMed] [Google Scholar]
- Tan W., Zhu H., Zhang N., Dong D., Wang S., Ren F., Xiang J., Wu R., Lv Y. (2019). Characterization of the PM2.5 concentration in surgical smoke in different tissues during hemihepatectomy and protective measures. Environ. Toxicol. Pharmacol. 72, 103248. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Sawakami K., Shoji H., Segawa H., Ishikawa S., Kameyama H., Ohashi M., Watanabe K., Kawashima H. (2023). Dynamics of surgical smoke in the operating room during spinal surgery: Comparison of particulate matter 2.5-air concentration between the electric scalpel with and without a smoke evacuation pencil: A cross-sectional study. J. Orthop. Sci. 28, 740–744. [DOI] [PubMed] [Google Scholar]
- Taweerutchana V., Suwatthanarak T., Methasate A., Akaraviputh T., Swangsri J., Phalanusitthepha C., Trakarnsanga A., Parakonthun T., Srisuworanan N., Tawantanakorn T., et al. (2021). Laparoscopic surgery produced less surgical smoke and contamination comparing with open surgery: The pilot study in fresh cadaveric experiment in covid-19 pandemic. BMC Surg. 21, 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel P., Park D., Lee Y. C. (2022). Recent insights into particulate matter (PM(2.5))-mediated toxicity in humans: An overview. Int. J. Environ. Res. Public Health 19, 7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuda Y., Okamura T., Maruta M., Orita M., Noguchi M., Suzuki T., Matsuki H. (2020). Prospective randomized study evaluating the usefulness of a surgical smoke evacuation system in operating rooms for breast surgery. J. Occup. Med. Toxicol. 15, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y., Mihashi S., Nagata K., Ueda S., Fujiki M., Hirano M., Hirohata T. (1981). Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat. Res. 89, 145–149. [PubMed] [Google Scholar]
- Tseng H. S., Liu S. P., Uang S. N., Yang L. R., Lee S. C., Liu Y. J., Chen D. R. (2014). Cancer risk of incremental exposure to polycyclic aromatic hydrocarbons in electrocautery smoke for mastectomy personnel. World J. Surg. Oncol. 12, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer B. C. (2008). The hazards of surgical smoke. AORN J. 87, 721–734. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Labor (2021). Benzene. Available at: https://www.osha.gov/chemicaldata/491. Accessed January 2, 2024.
- U.S. Environmental Protection Agency (2021). Health effects notebook glossary. Available at: https://www.epa.gov/haps/health-effects-notebook-glossary. Accessed November 2023.
- Van Gestel E. A. F., Linssen E. S., Creta M., Poels K., Godderis L., Weyler J. J., De Schryver A., Vanoirbeek J. A. J. (2020). Assessment of the absorbed dose after exposure to surgical smoke in an operating room. Toxicol. Lett. 328, 45–51. [DOI] [PubMed] [Google Scholar]
- Wang H. K., Mo F., Ma C. G., Dai B., Shi G. H., Zhu Y., Zhang H. L., Ye D. W. (2015). Evaluation of fine particles in surgical smoke from an urologist's operating room by time and by distance. Int. Urol. Nephrol. 47, 1671–1678. [DOI] [PubMed] [Google Scholar]
- Wenig B. L., Stenson K. M., Wenig B. M., Tracey D. (1993). Effects of plume produced by the Nd:YAG laser and electrocautery on the respiratory system. Lasers Surg. Med. 13, 242–245. [DOI] [PubMed] [Google Scholar]
- Xie W., Dumas O., Varraso R., Boggs K. M., Camargo C. A. Jr,, Stokes A. C. (2021). Association of occupational exposure to inhaled agents in operating rooms with incidence of chronic obstructive pulmonary disease among us female nurses. JAMA Netw. Open. 4, e2125749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Liu Y., Zhang J., Chen X., Li J., Zhu X. (2022). In vivo and in vitro study of the potential hazards of surgical smoke during cervical cancer treatment with an ultrasonic scalpel. Gynecol. Oncol. 164, 587–595. [DOI] [PubMed] [Google Scholar]
- Yokoe T., Kita M., Odaka T., Fujisawa J., Hisamatsu Y., Okada H. (2021). Detection of human coronavirus RNA in surgical smoke generated by surgical devices. J. Hosp. Infect. 117, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonan Y., Ochoa S. (2017). Impact of smoke evacuation on patient experience during Mohs surgery. Dermatol. Surg. 43, 1363–1366. [DOI] [PubMed] [Google Scholar]
- Zhou R., Xia M., Zhang L., Cheng Y., Yan J., Sun Y., Wang J., Jiang H. (2021). Fine particles in surgical smoke affect embryonic cardiomyocyte differentiation through oxidative stress and mitophagy. Ecotoxicol. Environ. Saf. 217, 112259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.