Abstract
Biological age, measured via epigenetic clocks, offers a unique and useful tool for prevention scientists to explore the short- and long-term implications of age deviations for health, development, and behavior. The use of epigenetic clocks in pediatric research is rapidly increasing, and there is a need to review the landscape of this work to understand the utility of these clocks for prevention scientists. We summarize the current state of the literature on the use of specific epigenetic clocks in childhood. Using systematic review methods, we identified studies published through February 2023 that used one of three epigenetic clocks as a measure of biological aging. These epigenetic clocks could either be used as a predictor of health outcomes or as a health outcome of interest. The database search identified 982 records, 908 of which were included in a title and abstract review. After full-text screening, 68 studies were eligible for inclusion. While findings were somewhat mixed, a majority of included studies found significant associations between the epigenetic clock used and the health outcome of interest or between an exposure and the epigenetic clock used. From these results, we propose the use of epigenetic clocks as a tool to understand how exposures impact biologic aging pathways and development in early life, as well as to monitor the effectiveness of preventive interventions that aim to reduce exposure and associated adverse health outcomes.
Keywords: Epigenetic age, Biological clock, Biological adaptation, Hannum clock, Horvath clock, PedBE clock
Introduction
Increasingly, research has focused on understanding the distinction between chronological and biological age. Markers of biological aging help characterize underlying changes in health and development occurring at the cellular level, even in the absence of, or alongside, obvious phenotypic changes (Levine et al., 2018; Xia et al., 2017). In adults, links between biological aging, resulting loss of physiological integrity and functioning, and diseases of aging such as cardiovascular disease, neurologic disease, and cancer in adults are well established (Mattson & Arumugam, 2018). Studies of biological aging in children are beginning to accumulate (e.g., Binder et al., 2018; Brody et al., 2016a, b; Hamlat et al., 2021; McEwen et al., 2020; Peng et al., 2019; Shiau et al., 2018). Across the life course, quantitative biomarkers of cellular aging may capture processes including changes to DNA and chromosomes, RNA, and the transcriptome, metabolism, oxidative stress, mitochondrial dysfunction, cell senescence, and inflammation (Xia et al., 2017). Epigenetic age, which captures CpG methylation, is among the most common measures of biological age. Individuals who exhibit more rapid aging based on DNAm than would be predicted by their chronological age have increased risk of disease and mortality (Fransquet et al., 2019). Composite biomarker predictors, and in particular the epigenetic clock, hold promise for understanding heterogeneity of biological aging, as no single candidate biomarker (e.g., blood pressure) has been shown to be a robust indicator of all-cause mortality (Jylhävä et al., 2017). Moreover, the field’s historical focus on adult populations and mortality precludes interpretation of aging as a continual process across the life course and minimizes growing recognition of sensitive periods in epigenetic programming (Marini et al., 2020). The focus of this systematic review is studies utilizing epigenetic clocks in pediatric research, which may help inform or identify sensitive periods in child development that are useful for prevention programming.
Epigenetic clocks are a biomarker of aging based on DNA methylation (DNAm). DNAm is measured for a set of CpG sites, the number and subset of which depend on the specific clock (Bell et al., 2019). Most of the extant literature relies on two clocks: the Hannum clock and the pan-tissue Horvath DNAm (Horvath) clock (Horvath, 2013). The Hannum clock includes 71 CpG sites from the Illumina 450 k methylation array, utilizing DNA from blood and age-related shifts in blood cell composition to determine biological age (Bell et al., 2019). The Horvath clock estimates DNAm age from most tissue and cell types based on methylation at 353 CpG sites (Bell et al., 2019; Horvath, 2013). More recently, several other epigenetic-based clocks in adults have been developed including PhenoAge (Levine et al., 2018), GrimAge (Lu et al., 2019), and DunedinPoAm (Belsky et al., 2017); evidence linking their relationship to health outcomes is an emerging area of research. Specific to infant and pediatric populations, the gestational age (Knight et al., 2016; Lee et al., 2019) and pediatric buccal epigenetic (PedBE) (McEwen et al., 2020) clocks were developed to investigate developmental biologic aging and impact on health outcomes. Accelerated biological aging early in the life course may serve to calibrate developmental processes based on environmental input. For example, accelerated biological aging in childhood is associated with earlier onset of puberty (Binder et al., 2018; Ellis et al., 2009). From an evolutionary standpoint, this serves to maximize opportunity for reproduction (Rickard et al., 2014).
Evaluating DNAm age in children and adolescents offers the opportunity to compare the biological impact of different physical, chemical, and social exposures at various points in development and to understand their potential implications for health and behavioral risk and resilience. However, in children and adolescents, the relationship between chronological and DNAm age as measured by epigenetic clocks developed for use in adults is less clear, in part because DNAm is more dynamic in early relative to later life (McEwen et al., 2020). To date, in studies using adult clocks, correlations between DNAm age and chronological age are the lowest in infancy and increase into adolescence (Simpkin et al., 2017). This lack of pediatric specification in some common clocks presents a challenge when inferring biological age with precision. To address this concern, infant- and pediatric-specific clocks have been developed to assess DNAm age from birth to adolescence (Bohlin et al., 2016; Knight et al., 2016; Lee et al., 2019).
In the current study, we focus on clocks that are easily accessible and replicable for prevention science researchers and have been previously used in pediatric samples (i.e., the Hannum, the pan-tissue Horvath, and the PedBE clock designed for use in pediatric populations). We focus on named epigenetic clocks, which are likely to be more accessible for the average non-epigenetic researcher and have the largest breadth of literature. Further, many named clocks have been manualized, meaning their estimation is easily done using publicly available software code and tutorials. Some studies not included in this review simply utilized a different named clock that has not been widely used in the pediatric literature or created their own metric of epigenetic aging (Bohlin et al., 2016; Knight et al., 2016; Lee et al., 2019). With the abundance of longitudinal data available in many preventive intervention trials along with existing cohort studies with prevention implications, we looked for clocks that were valid to use across multiple developmental periods such that inferences could be made about change over time. While this is an area of rapidly changing research, there is a substantial body of literature utilizing the Horvath and Hannum epigenetic clocks. The PedBE clock is newer (2019), but the focus and validity on pediatric populations were the basis for its inclusion here. The PedBE clock has demonstrated reliability and validity in multiple tissue types, which is common among clocks designed for use in children (Kling et al., 2020). The overall goal of this systematic review is to identify studies utilizing epigenetic clocks in pediatric research, provide some synthesis of their use in these studies, and make suggestions for how prevention scientists can incorporate biological aging into their research program.
Method
Inclusion and Exclusion Criteria
We conducted a systematic review through February 2023 following Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines (Moher et al., 2009). Included studies met the following inclusion criteria: (a) published in English, (b) conducted in humans, (c) used the Hannum, Horvath or PedBE epigenetic clock as a biomarker of biological aging, and (d) the clock was used to measure DNAm in children (0–18 years) or during adulthood to evaluate the long-term correlates of an exposure and/or predictor (e.g., adverse childhood exposures, internalizing symptoms) during childhood (0–18 years). Because we were interested in the broad literature around epigenetic clocks in pediatric research, we did not include search terms for specific exposures. Studies were excluded if they were non-empirical (e.g., review, commentary, letter), they measured DNAm but did not include an epigenetic clock, or if they used clock other than the Horvath, Hannum, or PedBE.
Literature Search Procedure and Parameters
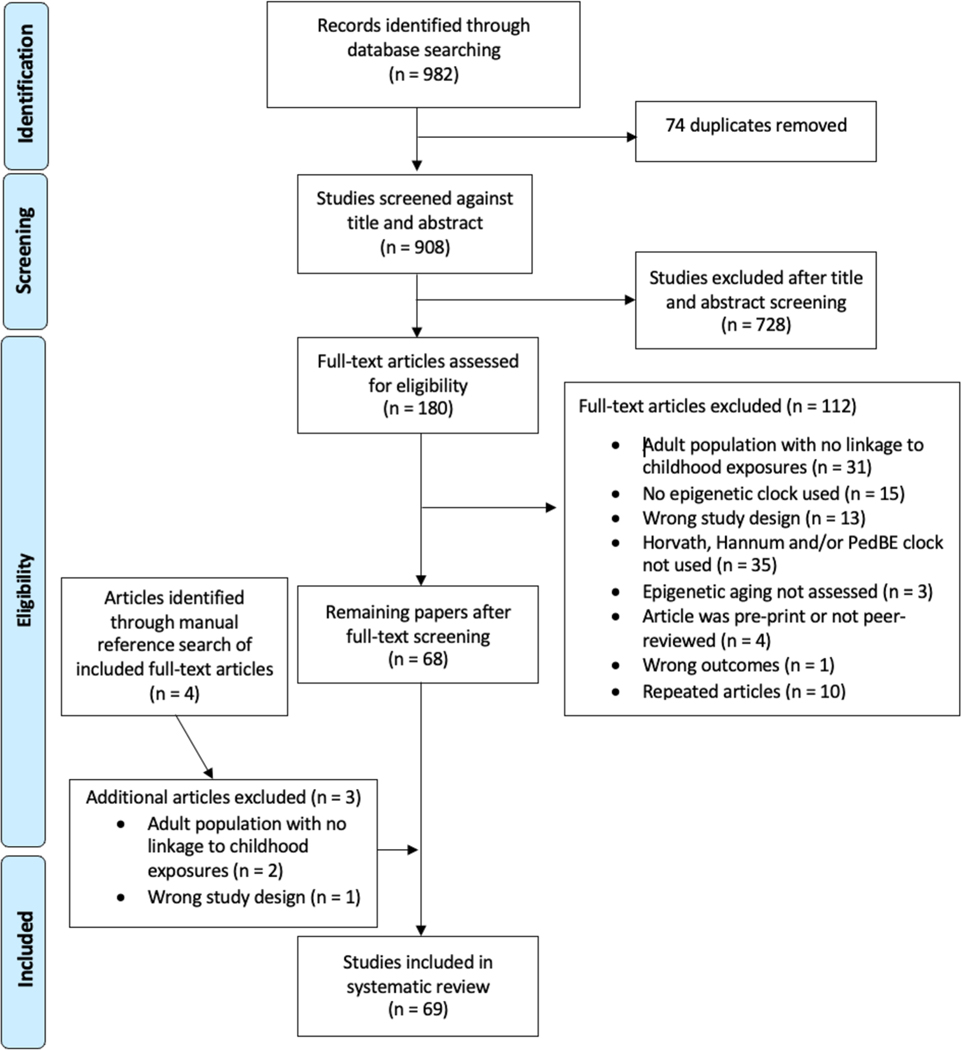
Relevant articles published through May 2021 were identified via a systematic search of PubMed New Interface, PsychINFO, and Google Scholar. A search string that incorporated three concepts, biological aging, DNA methylation, and children, was developed (see Supplemental Materials). Duplicates were identified and removed, and the remaining unique citations were imported into a web-based systematic review manager (Covidence, Veritas Health Innovation, Melbourne, Australia) for further evaluation. Inclusion and exclusion criteria were applied by review of titles first, then abstracts, and finally full text. Screening was conducted by two evaluators independently. Conflicts were resolved by two additional evaluators. Details of the search process and sample construction are summarized in Fig. 1. After the full-text review, a manual reference search was conducted for all included articles following a similar procedure outlined above.
Fig. 1.
PRISMA flow chart of identification and elimination of studies for review. The search was completed through February 2023
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISM Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISM Statement. PLoS Med 6(7): e1000097. doi: 10.1371/journal.pmed1000097
Data Extraction
After full-text screening, the following information was extracted from each study, as applicable: study sample characteristics, study design, exposure(s) and/or predictor(s), outcome measure(s), age at which the epigenetic clock was used, clock type, biospecimen used, covariates, and method of analysis.
Quality Assessment
Studies were assessed for methodological quality using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Cohort or Cross-Sectional Studies (https://joannabriggs.org/). Risk of bias evaluation was used to evaluate the quality of evidence but was not used to exclude articles from the review (Vardell & Malloy, 2013).
Results
Study Design
Figure 1 provides a detailed summary of the screening and inclusion process. Of the 180 articles that were subjected to full-text review, 113 were excluded. A majority were excluded because they did not use an eligible epigenetic clock or focused entirely on an adult population. A total of 68 studies were included in the systematic review after full-text screening. Most of the included studies utilized epigenetic clocks as outcomes of interest, while some used these metrics of biological aging as a predictor in a health outcome model. Because of the complexity of many of the included studies, in this review, we focus on the main analysis of epigenetic age and do not explicitly discuss all analyses in a given study.
Study Sample Characteristics
Included studies were conducted with samples around the world including in Chile (Binder et al., 2018), Australia (Huang et al., 2019), Finland (Suarez et al., 2018a, b), the Republic of the Congo (Gettler et al., 2020), South Africa (Horvath et al., 2018a), and Ireland (Lecorguillé et al., 2023). Several studies used data from the large UK-based prenatal cohort, the Avon Longitudinal Study of Parents and Children (ALSPAC) (Lawn et al., 2018; Marini et al., 2020; Simpkin et al., 2016, 2017; Tang et al., 2020).
In the USA, four studies reported on research conducted in rural Georgia. Brody and colleagues (2016b) used data from two longitudinal studies: the Strong African American Healthy Adult Project (SHAPE) and the Adults in the Making (AIM) project. Chen and colleagues (2016) used data only from SHAPE. whereas Miller et al. used data from AIM. Similarly, Brody and colleagues (2016a) used data from the Strong African American Families (SAAF) study, a randomized prevention trial in rural Georgia. In a separate investigation, Jovanovic and colleagues (2017) used data from a longitudinal study of trauma exposure in children from an urban hospital in Atlanta, Georgia.
Biospecimen Type
A majority of studies (n = 45) extracted DNA from blood. Some used umbilical cord blood (Peng et al., 2019; Simpkin et al., 2016, 2017; Suarez et al., 2018a, b), while others used whole blood (Hamlat et al., 2021, among others) (see Tables 1 and 2). One study used dried blood spots from finger sticks (Gettler et al., 2020). One study used respiratory epithelial cells from nasal swabs (Cardenas et al., 2019). Many studies used saliva/buccal cells (Cerveira de Baumont et al., 2021; Clausing & Non, 2021; Dammering et al., 2021; Davis et al., 2017; Graw et al., 2021; Jovanovic et al., 2017; Mathewson et al., 2021; Nishitani et al., 2021; Phang et al., 2020; Sumner et al., 2019; Tollenaar et al., 2021; Van Lieshout et al., 2021, among others) (see Tables 1 and 2).
Table 1.
Characteristics of included studies when epigenetic clock measures were used as a predictor
| Study | Study design | Study population | Sample size (% female) | Tissue type | Epigenetic clock | Age of clock assessment | Predictors | Outcome |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Binder et al. (2018) | Prospective cohort study | Randomly selected subset of girls from a longitudinal study examining growth and obesity (GOCS) in Chile who had epigenetic data at two waves | 94 (100%) | Peripheral blood | Horvath | Middle childhood to adolescence | Epigenetic age acceleration (DNAm vs. chronological age residuals) | Pubertal timing (i.e.. Tanner staging, breast volume, menarche) |
| Bolhuis et al. (2022) | Prospective cohort study | Dutch low risk mother child dyads | 193 (47.67% of offspring) | Buccal cells | PedBE | Age 6 and age 10 | Attachment insecurity, epigenetic aging (mediator), telomere length (mediator | Pubertal onset, callous unemotional traits, aggression, risk-taking |
| Davis et al. (2017) | Longitudinal study | Adolescent girls who were in a longitudinal study examining familial risk (maternal history of MDD) and development | 46 (100%) (n = 24 high risk, n = 22 low risk) | Saliva | Horvath | Middle childhood to adolescence | Epigenetic age acceleration | Diurnal cortisol, hippocampal volume, amygdala volume |
| Gomaa et al. (2022) | Prospective cohort study | Infants born very pre-term (24–32-week gestation) | 35 (40%) | Buccal cells | PedBE | Within the first 2 weeks of life | Epigenetic age acceleration | Brain growth and neurodevelopmental outcomes (Bayley scales) |
| Graw et al. (2021) | Cross-sectional | Participants from the Neonatal Neurobehavior and Outcomes in Very Preterm Infants (NOVI) study | 542 (44.5%) | Buccal cells | NEOage, Horvath, PedBE | Preterm infants | Epigenetic age | Post-menstrual and postnatal age |
| Hoare et al. (2022) | Prospective cohort study | Baseline data from the Cape Town Adolescent Antiretroviral cohort study | 180 (52.8% female) | Peripheral blood | Horvath | Middle childhood | Epigenetic age and HIV treatment | Brain volume, cortical thickness, cortical surface area, neuronal microstructure |
| Huang et al. (2019) | Prospective cohort study | Youth originally part of a longitudinal study commencing in the prenatal period in Western Australia (Raine study) | 995 (49.6%) | Peripheral blood | Horvath and Hannum | Adolescence | Epigenetic age acceleration (intrinsic and extrinsic) | Adiposity & BMI, cardiovascular disease (CVD) risk factors (ages 17, 20, 22), predicting CVD development 30 years later |
| Manczak et al. (2023) | Multi-wave cohort study | Subset of the BiB study | 154 (49.4% female) | Umbilical cord blood | Horvath | Birth | Epigenetic age and Maternal Hostility | Psychological symptoms |
| Neri de Souza Reis et al. (2021) | Cross-sectional | Mother-child dyads in São Paulo, Brazil. Children had an ASD diagnosis, ages between 3 and 7, and an IQ between 50 and 75 | 67 mothers (100%); 67 children (17.9%) | Whole blood | Horvath | Early childhood | DNA méthylation age acceleration, family social class, maternal schooling, maternal stress during gestation, toxic environmental exposures, familial psychiatric history, gestational complications | Autism Spectrum Disorder |
| Peng et al. (2019) | Prospective cohort study | Children from a longitudinal pre-birth cohort study conducted in Boston, MA (Project Viva) with epigenetic data measured at birth, early childhood, and middle childhood; replicated methods in a subset of children in Costa Rica (Genetics of Asthma in Costa Rica Study) | Project Viva: 408 (49%) Genetics of asthma in Costa Rica: 159 (41%) |
Umbilical cord blood (birth), peripheral blood (early and midchildhood) | Horvath and Hannum | Birth to middle childhood | Epigenetic age and epigenetic age acceleration at birth, early childhood and middle childhood | Allergic phenotypes in middle childhood (e.g., serum IgE, food allergen sensitization, asthma) |
| Shenk et al. (2022) | Accelerated cross-sequential prospective cohort | Subset of the Female Growth and Development Study (FGDS) | 172 (100% female) | Whole blood | Horvath and Hannum | Adulthood | Epigenetic age and childhood sexual abuse | cortisol trajectories |
| Simpkin et al. (2016) | Prospective cohort study | Subset of children from a population-based cohort study in UK (ALSPAC) Replication of findings in a Danish sample of newborns (GOYA study) | ALSPAC: 1022 (52%) GOYA: 981 (not indicated) |
Umbilical or blood (at birth); venous blood | Horvath and Hannum | Birth, childhood and adolescence | Repeated measures of epigenetic aging: raw age acceleration differences and age acceleration residuals | Horvath vs. Hannum performance, BMI, alcohol consumption, smoking, education, maternal health, and child health |
| Simpkin et al. (2017) | Prospective cohort study | Subset of children from a population-based cohort study in the UK (ALSPAC) | 914 (51%) | Cord blood (time 1); venous blood (time 2) | Horvath | Childhood to adolescence | Repeated measures of epigenetic aging: raw age acceleration | Physical characteristics (height, weight, BMI, etc.); pubertal stage/onset age |
| Suarez et al. (2018b) | Prospective cohort study; cross-sectional analysis | Youth originally enrolled in a longitudinal study commencing when they were infants | 239 (51.5%) | Blood | Horvath | Middle childhood to adolescence | Epigenetic age acceleration | Pubertal timing, physical growth, neuroendocrine parameters (i.e., cortisol), psychiatric and cognitive aging-related outcomes |
| Hoare et al. (2020) | Prospective cohort study; Cross-sectional analysis | Adolescents enrolled in Cape Town Adolescent Antiretroviral Cohort (CTAAC) study with epigenetic data | 44 (54.5) | Blood (peripheral blood) | Horvath and Hannum | Middle childhood to adolescence | Epigenetic age acceleration residual and extrinsic epigenetic age acceleration | Cognitive functioning via neuropsychiatric battery of tests, brain structure and integrity via neuroimaging and DTI |
| Shenk et al. (2021) | Case-control study design | Children, with and without PTSD, recruited from child protective services in an urban Midwest county (USA) | 70 (65.7) | Buccal cells | Horvath | Middle childhood to adolescence | Epigenetic age acceleration; lifetime exposure to child adversity | PTSD status (child PTSD symptoms scale) |
| Tollenaar et al. (2021) | Prospective cohort study | Children from an ongoing longitudinal study who were followed up from ages 2.5 to 10 | 148 (46.6) | Buccal cells | Horvath | Childhood | Internalizing and externalizing symptoms (CBCL), epigenetic age acceleration | Epigenetic age acceleration, internalizing and externalizing symptoms |
Table 2.
Characteristics of included studies when epigenetic clock measures were used as an outcome or correlate
| Study | Study design | Study population | Sample size (% female) | Tissue type | Epigenetic clock | Age of clock assessment | Predictors | Outcome |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Allen et al. (2022) | Longitudinal cohort study | Adolescents recruited in grades 7–8 in southeastern USA and followed into adulthood | 154(56.5%) | Blood | Horvath, GrimAge | Midlife | Adolescent peer struggles | Epigenetic age acceleration |
| Austin et al. (2018) | Cross-sectional (2×2 design) | Healthy participants enrolled in a study on early-life and current adult socioeconomic status (SES) on health-related outcomes | 335 (55%) | Blood | Horvath | Adolescence to adulthood | Early-life (0–5 years) and current (adult) socioeconomic status | Epigenetic age acceleration in monocytes |
| Beijers et al. (2023) | Prospective longitudinal cohort | Dutch low-risk community sample | 193 (47.67%) | Buccal | PedBE | Age 6 and age 10 | Cumulative risk measured | Epigenetic age, telomere length |
| Brody et al. (2016a) | Prospective cohort study | Subset of African American youth and families in rural Georgia who were originally a part of a prevention trial (SAAF) | Control: 157 (51%) Intervention: 242 (57%) | Blood | Horvath | Early adulthood | Parental depression; prevention program (moderation); harsh parenting and program effects | Epigenetic aging/age acceleration at age 22 |
| Brody et al. (2016b) | Longitudinal study on two cohorts | African American youth in rural communities in Georgia, USA, who were from two longitudinal studies (SHAPE and AIM) | SHAPE: 322 (57.1%) AIM: 294 (63.6%) | Blood | Hannum | Early adulthood | Perceived racial discrimination and support in the family environment | Epigenetic aging in immune cells at age 22 |
| Cardenas et al. (2019) | Cross-sectional | Children enrolled in a study to investigate nasal cellular epigenome and airway disease and environmental response (Project Viva) | 547 (49.5%) | Respiratory epithelial cells (nasal swabs of anterior nares) | Horvath | Adolescence | Asthma, allergic asthma, biomarkers, allergic rhinitis | Epigenetic age acceleration |
| Cerveira de Baumont et al. (2021) | Observational study; cross-sectional analysis | Children and adolescents from public schools | 234 (61% female) | Saliva | Horvath | Adolescence (time 1; age 13, time 2; age 17) | Anxiety symptoms | Epigenetic age/acceleration |
| Chen et al. (2016) | Prospective cohort study | African American adolescents in Georgia, USA, who were followed from pre-recession to post-recession (2007–2010) to assess impact of macroeconomic conditions on health | 330(53.6) | Blood | Horvath and Hannum | Early adulthood | Economic trajectories across period of Great Recession (2007–2010) | Epigenetic aging (leukocyte DNAm profiles), allostatic load, adolescent self-report of health (2010 at age ~ 19) |
| Clausing and Non (2021) | Longitudinal cohort study | Foreign-born immigrant Latina mothers over the age of 18 and their 6–13-year-old children | 71 mothers (100%<); 71 children (56.3%) | Saliva | Horvath, Hannum, PedBE, skin and blood age | Late childhood (ages 6–13) | Various psychosocial stress and resilience factors | Epigenetic age |
| Dammering et al. (2021) | Prospective cohort study; cross-sectional analysis | Children in the Berlin Longitudinal Study with history of maltreatment exposure with and without internalizing disorders | 158 (46.2); 49 with current internalizing disorder and 109 without current internalizing disorder | Buccal | PedBE and Horvath | Early childhood | Maltreatment exposure; internalizing disorders | Epigenetic age and age acceleration |
| Dong et al. (2022) | Cohort study, matched controls | SJLIFE1 and SJLIFE2 participants enrolled in the St. Jude Lifetime Cohort Study, comprised of childhood cancer survivors. Community controls, with no history of childhood cancer | SJLIFE1: 2138 (47%); SJLIFE2: 502 (46.8%); 282 (51.4%) | Whole blood | Hannum, Horvath, PhenoAge, and GrimAge | Adolescence to midlife | Childhood cancer | Epigenetic age |
| Etzel et al. (2022) | Prospective cohort study; cross-sectional analysis | High-risk (82% investigated for maltreatment) children ages 8–14 years who are part of the prospective cohort Child Health Study (Pennsylvania) | 273 (225 maltreated, 48 comparison; 50.5%) | Blood | HorvathAA, Hannum A A, GrimAgeAA, and PhenoAgeAA, DunedinPoAm | Middle childhood | BMI, exposure to maltreatment | Epigenetic age acceleration |
| Fiorito et al. (2017) | Prospective cohort study | Adults enrolled in independent prospective cohorts in Italy, Australia, and Ireland | 5111 (48) | Peripheral blood | Horvath and Hannum | Adulthood | Early-life and current socioeconomic status | Epigenetic age acceleration (adulthood), noncommunicable diseases |
| George et al. (2021) | Cross-sectional | Socially stratified cohort of singleton births in 1 week of March 1946 in Britain (NSHD) | 1376 (not reported) | Blood | Horvath, Hannum, PhenoAge, and GrimAge | Adulthood (53 years) | Childhood and adulthood social class/socioeconomic position | Epigenetic age acceleration |
| Gettler et al. (2020) | Cross-sectional | Children of Bondongo families in a remote part of northern Republic of Congo who were in a study focused on fathering, family function and wellbeing | 54 (51.9%) | Blood | Horvath and Hannum | Childhood to adolescence | Weight-for-height, height-for-age, antibody titers/inflammation, family environment | Epigenetic age acceleration (Horvath), intrinsic age acceleration (Horvath), extrinsic age acceleration (Hannum) |
| Gomez-Verjan et al. (2021) | Prospective cohort study | Subset of the Tlaltizapan cohort | 39 (61.5% female) | Peripheral blood | Horvath & Hannum | Adulthood (age 57) | Years of schooling | Epigenetic age/acceleration |
| Harris et al. (2022) | Observational; Cross-sectional analysis | Survivors of CNS tumors | 83 (27.7% female) | Whole blood | Horvath | Middle childhood | Primary diagnosis, age at diagnosis | Epigenetic age/acceleration |
| Hamlat et al. (2021) | Cross-sectional analysis | Premenopausal women enrolled in a larger project focused on chronic caregiver stress and aging in the University of California, San Francisco Autism Program | 183 (100) | Whole blood | Horvath, Hannum, PhenoAge, Grim-Age | Adulthood | Early-life trauma (Child Trauma Questionnaire) | Epigenetic age acceleration (adulthood) and pubertal timing (self-reported age at menarche) |
| Henckel et al. (2020) | Longitudinal case-control study | Subgroup of preterm and term born children with longitudinal blood samples in a case-control (LUFT) study | 60 (43.3) | Umbilical cord blood and venous blood | Horvath | Infant to early childhood | Preterm vs. term birth | Epigenetic age change per year over first 2 years of life |
| Herrera-Moreno et al. (2022) | Birth cohort & prospective cohort study; Cross-sectional analysis | Participants in the Programming Research in Obesity, Growth, Environment, and Social Stressors (PROGRESS) study | 507 (44.9% female) | Cord blood | Horvath | Birth | Prenatal lead exposure | Epigenetic age/acceleration |
| Horvath et al., (2018a, 2018b) | Cross-sectional | Perinatally HIV-infected (PHIV+) and HIV-uninfected (control) youth in the Cape Town Adolescent Antiretroviral Cohort (CTAAC) study | PHIV+: 204 (51%) Control: 44 (55%) | Blood | Horvath and Hannum | Middle childhood to adolescence | Perinatal HIV infection; cognitive functioning (neuropsychological test battery) | Epigenetic age acceleration; extrinsic epigenetic age acceleration (FEA A) |
| Hughes et al. (2018) | Longitudinal cohort study | Participants from the UK Household Longitudinal Study | 1099 (57.6%) | Whole blood | Horvath, Hannum | Adulthood | Current SES, childhood SES | Epigenetic age acceleration |
| Javed et al. (2016) | Meta-analysis & cross-sectional réplication | Mother-newborn dyads enrolled in a study examining prenatal exposures and intrauterine environment on newborns | Public dataset: 613 (31%) De novo data: 96 (not indicated) | Cord blood | Horvath | Neonatal | Sociodemographic and newborn characteristics | Epigenetic age acceleration, lymphocyte and immune markers (CD4 + and CDS+ T cell compartments) |
| Jovanovic et al. (2017) | Longitudinal study; cross-sectional analysis | African American children recruited as a part of a longitudinal study on trauma exposure in children (Atlanta, GA) | 101 (54.5%) | Saliva | Horvath | Childhood to adolescence | Neighborhood violence and heart rate response to stressful task | DNAm age; epigenetic age acceleration |
| Joyce et al. (2022) | Prospective cohort studies | Participants from 3 US-based cohorts (CARDIA, FFCWS, PROGRESS) and one Mexico-based cohort (Project Viva) | CARDIA: 1036 (year 15 follow-up; 51.8); 1016 (year 20 follow-up; 50.9); FFCWS: 637 (not reported); Project Viva: 120 in early childhood and 460 in middle childhood); PROGRESS: not reported | Blood | Hannum, Horvath, PhenoAge, GrimAge, and DunedinPoAM | Infancy and childhood | Personal, parental, and neighborhood SES measures (i.e., education) | Epigenetic age and age acceleration |
| Kim et al. (2022) | Longitudinal cohort study | Data captured from the Exploring Perinatal Outcomes in Children (EPOCH) | 179 (49.7%) | Blood | Horvath | Age 10 | Offspring diet, pubertal development, gestational diabetes mellitus exposure | Epigenetic age acceleration |
| Lawn et al. (2018) | Prospective cohort studies | Women in two cohort studies (ALSPAC and MRC National Survey of Health and Development; NHSD) with data on psychosocial adversity in childhood and epigenetic data collected at ages 29 and 47 (ALSPAC) or 53 (NSHD) | ALSPAC: 989 (100%) NHSD: 773 (100%) |
Peripheral blood, buccal cells | Horvath | Adulthood | Psychosocial adversity in childhood | Epigenetic age acceleration in adulthood |
| Lecorguillé et al. (2023) | Prospective family study | The Lifeways Cross-Generation Cohort Study, families were recruited antenatally from two hospitals in Ireland | 374 (244 mother child dyads, 130 father child dyads) | Saliva | PedBE | 9.8 years | Parental dietary scores | Accelerated epigenetic age |
| Maddock et al. (2021) | Prospective cohort study | Subset of the National Child and Development study | 1,376 (52.3% female) | Whole blood | Horvath & Hannum | Adulthood (age 53) | Height/weight | Epigenetic age/acceleration |
| Marini et al. (2020) | Prospective cohort study | Subset of children from a population-based cohort study in UK (ALSPAC) at age 7 | 973 (50.2%) | Venous blood | Horvath and Hannum | Childhood | Adversity | Epigenetic age acceleration using both clocks |
| Mathewson et al. (2021) | Prospective cohort, matched Controls | Participants in the extremely low birthweight group were born in southern Ontario, matched with a normal birthweight group based on age, sex, ethnicity, and SES, based on lists provided by school boards | Extremely low birthweight: 45 (62.2%); normal birth weight controls: 47 (57.4%) | Buccal cells | Horvath | Middle adulthood | Cumulative risks (resting respiratory sinus arrhythmia, blood pressure, basal cortisol, grip strength, body mass index, and self-esteem) | Epigenetic age |
| McCrory et al. (2019) | Prospective cohort study | Subsample of the Irish Longitudinal Study on Ageing (TILDA) who had epigenetic data available | 264 (50.2) | Blood | Horvath, Hannum, and Levine | Adulthood | Life course social class trajectory | Epigenetic age acceleration and allostatic load (immune, cardiovascular, metabolic, renal) in adulthood |
| McEwen et al. (2020) | Secondary analysis of prospective cohorts for clock development | Individuals (ages 0–20 years) from 11 cohorts with DNAm data | Training data: 1032 (52%) Test data: 689 (46.7%) |
Buccal cells | Horvath and PedBE | Birth to young adulthood | Gestational age, birthweight, autism spectrum disorder (ASD) diagnosis | Epigenetic age and epigenetic age acceleration using and comparing both clocks |
| McGill et al. (2022) | Prospective cohort study | Mother-child dyads from two longitudinal cohorts (Basal Influences on Baby Development (BIBO; Netherlands) and Growing Up in Singapore Towards Healthy Outcomes (GUSTO; Singapore)) | 165 (47.3) in BIBO, 340 (49.4) in GUSTO | Buccal cells | PedBE and Horvath | Infancy (3–48 months) and childhood (6, 10 years) | Maternal mood, symptoms of anxiety and depression in prenatal and postnatal follow-up; child mental health | Epigenetic age and age acceleration |
| Miller et al. (2015) | Randomized trial and prospective cohort study | African American adolescents enrolled in a randomized trial focused on substance and alcohol-use prevention (AIM; rural counties in Georgia, USA) | 292 (63.7%) | Antecubital blood | Horvath and Hannum | Adulthood | Trajectories of self-control and socioeconomic disadvantage; BMI and life stress assessed in mediation models | Epigenetic age acceleration using both clocks at age 22 |
| Nishitani et al. (2021) | Cross-sectional, matched controls | Children aged 2–9 years old who were removed from their biological parents by Child Protection Service, matched with children with no history of child maltreatment | Maltreated children: 25 (48%); healthy controls: 31 (58%) | Buccal cells | PedBE, SkinBlood, and Horvath | Early childhood (ages 2–9) | Child maltreatment | Epigenetic age acceleration |
| N wanaj i-Enwerem et al. (2021) | Prospective cohort study | Mexican American mothers and children who were enrolled in the CHAMACOS study followed up at child ages 7, 9, 14 years | 238 children (54–59%-across time) | Blood | Horvath, Hannum, SkinBloodClock, Intrinsic, Extrinsic, PhenoAge, GrimAge | Childhood to adolescence (ages 7, 9, and 14 years) | Maternal preconception adverse childhood experiences | Epigenetic age acceleration |
| Okazaki et al. (2021) | Secondary data analysis | Four publicly available DNAm data sets of children with fetal alcohol spectrum disorder (FASD) and healthy controls | 401 (49.1) | Buccal cells (2 data sets) and peripheral blood (2 datasets) | Horvath, Hannum, SkinBlood Age, PhenoAge, GrimAge | Childhood to middle childhood | Fetal alcohol spectrum disorder | Epigenetic aging measured by 5 clocks |
| Penova-Veselinovic et al. (2021) | Prospective and observational cohort study | Participants from Western Australia in the Growing Up Healthy Study (GUHS) and generation 2 of the Raine Study (RS). GUHS is a cohort of adolescents and young adults conceived through ART, while the Raine Study participants are a cross-section of a longitudinal study with mothers and their biological children | GUHS: 231 (47.2%); RS: 1188 (49.1%) | Whole blood | Horvath, Hanuum, PhenoAge [Levine], and skin Horvath | Adolescence | ART vs. natural birth | Epigenetic age |
| Phang et al. (2020) | Cross-sectional | Mother-newborn dyads enrolled in a study examining implications of maternal diet on newborn fatness and cardiovascular health | 169 (53.2%) | Saliva | Horvath | Neonatal | Maternal dietary and macronutrient intake | DNAm age/predicted biological age at birth, epigenetic age acceleration; aortic intima-media thickness; HRV |
| Popovic et al. (2021) | Longitudinal case-control study | Subset of mothers and infants in the NINFEA Italian Birth Cohort with saliva samples | 139 (43.2) | Saliva | Horvath and PedBE | Infancy | Parental socioeconomic status; pregnancy outcomes | Epigenetic age and age acceleration using both clocks |
| Reimann et al. (2022) | Prospective cohort study | Mothers enrolled from the ENVIRONAGE birth cohort, an ongoing prospective cohort with mother-newborn pairs recruited from East-Limburg Hospital in Genk, Belgium | 190 mothers (100%); newborns: 190 (49.2%) | Cord blood | Horvath | Birth | Global méthylation, mtDNA, cord blood telomere length | Epigenetic age/age acceleration |
| Shiau et al. (2018) | Cross-sectional | HIV-infected, HIV-exposed uninfected (HEU), and HIV-unexposed uninfected (HUU) children who were part of a HIV research cohort study | 178 (51.6%) (220 HIV-infected; 33 HEU; 25 HUU) | Venous blood | Horvath | Childhood | HIV exposure/infection status: HIV-infected, HIV-exposed uninfected, and HIV-unexposed uninfected | Telomere length, epigenetic age acceleration, systemic inflammation (IL-6, TNF-alpha), CD4 count |
| Shiau et al. (2021a) | Prospective cohort study | Children who were part of a HIV research cohort study, some exposed to HIV perinatally and others not exposed | 39 (48.7%) | Peripheral blood | Horvath | Age 10.9 and age 16.8 | HIV exposure/infection status: HIV-infected, HIV-exposed uninfected and HIV-unexposed uninfected | Epigenetic age acceleration |
| Shiau et al. (2021b) | Prospective study | Children of women (with and without prenatal gestational diabetes (GDM) who participated in the Tianjin GDM Prevention Program | 1145 (47.8%) | Whole blood | Horvath and Hannum | Childhood | Exposure to gestational diabetes in utero | Epigenetic age of child |
| Suarez et al. (2018a) | Prospective cohort study | Mother-infant dyads in a cohort study (PREDO) examining prenatal exposures and intrauterine milieu on fetal development | 814(47.2%) | Cord blood | Horvath | Neonatal | Maternal history of depression diagnosed pre-pregnancy; antenatal depression | Epigenetic gestational age (DNAm GA): internalizing/externalizing problems in early childhood |
| Sumner et al. (2019) | Cross-sectional | Children dyads enrolled in a study examining early-life adversity, emotional regulation, and psychopathy | 247 (47.8%) | Saliva | Horvath | Childhood to adolescence | Early-life adversity (threat- and deprivation-related) | Epigenetic age |
| Tang et al. (2020) | Prospective cohort study; cross-sectional analysis | Subset of children from a population-based cohort study in the UK (ALSPAC) with epigenetic data measured in adolescence | 974 (51.3%) | Peripheral blood | Horvath | Adolescence | Adverse childhood experiences from cumulative ACEs and exposure to individual ACEs | Epigenetic age acceleration |
| Van Lieshout et al. (2021) | Case-control study | Extremely low birth weight survivors and matched controls | 92 (59.8% female) | Buccal cells | Horvath | Adulthood (age 32) | Extremely low birth weight, number of chronic health conditions | Epigenetic age/acceleration |
| Verlinden et al. (2023) | Longitudinal follow-up of randomized trial | Participants who were part of the PEPaNIC RCT, which studied children admitted to the PICU, and matched healthy children | 1,210 (N = 392 healthy controls, N = 818 PICU patients) | Buccal cells | PedBE | 3.8 years in healthy controls, 3.4 in PICU participants | Critical illness, early parenteral nutrition vs. late parenteral nutrition | Epigenetic age deviation |
| Zannas et al. (2015) | Cross-sectional analysis | African American adults in the Grady Trauma Project (GTP); Caucasian adults in the Max Planck Institute of Psychiatry (MPIP) cohort | GTP: 393 (70.7) MPIP: 124 (35.5) | Peripheral blood | Horvath | Adulthood | Childhood trauma exposure (retrospective self-report), stressful life events, PTSD, and depression symptomatology | Epigenetic age acceleration in adulthood |
Sample Size
The average sample size of the included studies was 580.7 (SD = 791.7; median = 256.0; range = 35–5111 participants), but sample sizes varied widely. Fifteen studies included data from less than 100 participants (e.g., Binder et al., 2018; Davis et al., 2017; Gettler et al., 2020); 27 included between 100 and less than 500 participants (e.g., Austin et al., 2018; Brody et al., 2016a, b; Chen et al., 2016; Horvath et al., 2018a; Miller et al., 2015; Phang et al., 2020; Shiau et al., 2018; Suarez et al., 2018a, b; Sumner et al., 2019), and 26 studies included more than 500 participants (e.g., Huang et al., 2019; Lawn et al., 2018; Marini et al., 2020; Simpkin et al., 2016, 2017; Tang et al., 2020).
Summary of Epigenetic Clocks Used
Only one study (Brody et al., 2016a, b) relied on the Hannum clock, and some (five) relied on the PedBE clock. The large majority of studies relied solely on the Horvath clock (31 studies) or utilized multiple clocks (31 studies) (see Table 1).
In most studies (75%, n = 51), epigenetic age was the outcome of interest (i.e., Austin et al., 2018; Brody et al., 2016a, b; Cardenas et al., 2019; Chen et al., 2016; Gettler et al., 2020; Javed et al., 2016; Jovanovic et al., 2917; Lawn et al., 2018; Marini et al., 2020; Miller et al., 2015; Phang et al., 2020; Shiau et al., 2018; Suarez et al., 2018a; Sumner et al., 2019; Tang et al., 2020). In these studies, the exposures were heterogeneous including perceived racial discrimination (Brody et al., 2016b), maternal dietary and macronutrient intake (Phang et al., 2020), HIV exposure and infection status (Shiau et al., 2018), and childhood adversity (Lawn et al., 2018; Marini et al., 2020; Sumner et al., 2019; Tang et al., 2020). The goal of these studies was to assess whether a particular exposure in childhood was associated with accelerated epigenetic age.
A minority of studies (25%, n = 17) used epigenetic age as a predictor of a later outcome such as pubertal timing (Binder et al., 2018), cardiovascular disease risk, body mass index (Huang et al., 2019), allergic phenotypes (Peng et al., 2019), or other indicators of growth and development (Davis et al., 2017; Simpkin et al., 2016, 2017) (see Table 1). Most of these studies included epigenetic age as a predictor of a particular health outcome.
In papers that used multiple clocks, researchers were often interested in comparing new epigenetic clocks, often those developed by the research team, to those already in use in the field (Graw et al., 2021; McEwen et al., 2020). For example, Graw and colleagues (2021) developed a novel epigenetic clock (NEOage) focused on age estimation of preterm infants. They compared their clock to more conventional clocks like Horvath and PedBE (McEwen et al., 2020). Many of the included studies qualitatively compared associations between epigenetic aging measured by specific clocks and their outcome of interest (Etzel et al., 2022). Some studies explored correlations between epigenetic age acceleration as measured by different epigenetic clocks. For example, Hamlat and colleagues (2021) found modest correlations between epigenetic age acceleration measured by the Horvath, Hannum, PhenoAge, and Grim age clocks. Please see Table 1 for additional studies that utilized multiple clocks.
Summary of Findings
Almost all studies showed that adverse exposures, in some form, between birth and 18 years were significantly associated with accelerated biological aging, with some exceptions; a study by Shiau and colleagues (2018) found no difference in accelerated biological aging when comparing HIV-infected children to HIV-exposed but uninfected children. Zannas and colleagues (2015) found an association with cumulative lifetime stress but not childhood maltreatment. Sumner and colleagues (2019) found a significant association between accelerated epigenetic age and threat-related early-life adversity but no association between epigenetic age and deprivation-related early-life adversity. Other studies supported the idea that environmental experiences and exposures can get under the skin to impact biological aging and risk of adverse health and developmental outcomes. Further, studies exploring the link between biological aging and another construct (e.g., mental health, physical health) generated mixed findings. For example, Shenk and colleagues (2021) found that accelerated epigenetic age significantly predicted PTSD status, and epigenetic age acceleration was also related to PTSD symptom severity. Huang and colleagues (2019) found that epigenetic age acceleration in adolescence was associated with inflammation, BMI measured 5 years later, and probability of cardiovascular disease in middle age.
Many of the included studies that reported on the correlation between epigenetic age and chronological age found a significant positive correlation between the two (Austin et al., 2018; Binder et al., 2018; Cardenas et al., 2019; Davis et al., 2017; Gettler et al., 2020; Jovanovic et al., 2017; Peng et al., 2019; Shiau et al., 2018; Sumner et al., 2019). Other studies either did not report correlations between epigenetic age and chronological age (e.g., Kim et al., 2022; Neri de Souza Reis et al., 2021 or reported low correlation between the two constructs (e.g., Simpkin et al., 2017; Tollenaar et al., 2021).
Discussion
Epigenetic clocks offer promise for researchers interested in elucidating the biological mechanisms by which environmental exposures early in life shape both short- and long-term health and behavioral outcomes. Epigenetic clocks offer a metric to capture and quantify developing individuals’ adaptation to their environments across genes. For example, Marini and colleagues (2019) found that exposure to adversity in early and middle childhood was significantly associated to the Hannum-based epigenetic aging clock, and Jovanovic and colleagues (2017) found that epigenetic age acceleration in children was significantly associated with violence exposure. Moreover, epigenetic age may be particularly useful in prevention science for investigating potential biological impacts of preventive interventions. Prior research points to the need to incorporate precision approaches in prevention science (August & Gewirtz, 2019; Latendresse et al., 2018; Musci & Schlomer, 2018).
One of the studies included in this systematic review incorporated epigenetic clocks into a prevention trial, making it a particularly illustrative example for prevention scientists. Brody and colleagues (2016a) explored the impact of their Strong African American Families (SAAF) program on epigenetic age and investigated whether that relationship was mediated by reductions in harsh parenting. The authors found that the prevention program was associated with epigenetic age, more specifically, individuals in the intervention group had significantly lower epigenetic age at age 20 compared to those in the control group. This study also demonstrated moderated mediation such that the SAAF program led to lower epigenetic age compared to controls through reductions in harsh parenting among those who had a caregiver with high depressive symptoms. Brody and colleagues (2016a) may serve as a guide for other prevention researchers interested in understanding how their programming may disrupt the relationship between adverse exposures and biological aging. Monitoring program impacts at the level of gene expression can inform not only how environmental exposures impact health at the cellular level, but also point to modifiable intervention targets. Further, prior research demonstrates that DNA methylation is reversible, which suggests that the future of prevention science could include strategies to alter or prevent methylation patterns associated with negative outcomes, including, perhaps even medications that directly target methylation (Szyf et al., 2016).
It is important to note that across the existing literature, the correlation between chronological age and epigenetic age has been variable. This may be explained by differences in environments that may impact methylation patterns among participants in a given sample. Alternatively, this heterogeneity may be explained by features of the epigenetic clock used. Clocks trained on samples that are highly divergent from those in which they are used might generate particularly discrepant results between chronological and epigenetic age (McEwen et al., 2020). For example, the Horvath clock has demonstrated strong correlation between epigenetic age and chronological age in adult samples (Horvath, 2013). Clocks developed in adult samples have demonstrated low correlation in pediatric samples (McEwen et al., 2020). This has led researchers to develop more pediatric-focused epigenetic clocks that can more reliably characterize epigenetic aging in childhood.
Low correlations between epigenetic age as measured by epigenetic clocks developed in adult populations and chronological age may also influence associations between epigenetic age measured in childhood and later outcomes. For example, Simpkin and colleagues (2017) reported low correlations between epigenetic age in childhood and later pubertal status. Similarly, Tollenaar and colleagues (2021) reported low correlations between epigenetic age in childhood and later internalizing and externalizing symptoms. These low correlations were one of the reasons why there has been a recent push to develop pediatric-focused biomarkers of aging such as the PedBE clock (McEwen et al., 2020). While the correlation between these constructs may be an important factor in understanding accelerated aging, for most prevention researchers, it is likely that a lower correlation between epigenetic age and chronological age will not be impactful.
Methodological Considerations
A significant methodological consideration for ongoing research in biological aging is tissue choice and the comparison of clocks that rely on different tissue types. The collection of invasive biospecimens, including blood, can be particularly challenging in children in non-clinical settings. Unwillingness to consent to blood collection is a common reason for parents’ declining participation in pediatric research (Gattuso et al., 2006). Saliva is significantly easier to obtain and more acceptable to study participants. From saliva samples, one can capture several different cell types including epithelial cells and leukocytes (Smith et al., 2015). Saliva and epithelial cells have been used in many epigenetic studies of behavior and psychopathology. Notably, Dempster et al. (2014) used buccal cells in a study of adolescent depression, and work by Lowe and colleagues (2013) suggests that buccal cells are more informative than blood for epigenome-wide association studies, given the closer lineage to neurological systems. The tissue issue also comes into play when researchers are interested in exploring DNA methylation patterns longitudinally; accessible biospecimens may differ depending on the developmental stage of the participants. This is not, of course, the end of the story as much remains uncertain with regard to how tissue type interacts with developmental stage to impact epigenetic age calculations.
Limitations
The current review is limited to studies that used the Hannum, pan-tissue Horvath, and the PedBE epigenetic clocks, given their relevance and popularity. However, new clocks are emerging and are increasingly being tailored for the investigation of specific outcomes or periods of development. For example, the PhenoAge clock has outperformed other clocks in predicting aging outcomes like Alzheimer’s disease (Levine et al., 2018). Other clocks include the Grim-Age clock (Lu et al., 2019) and the Skin and Blood Clock (Horvath et al., 2018a). Our focus on Horvath, Hannum, and PedBE clocks only is certainly a limitation though we see this as an important contribution as prevention researchers explore ways to successfully integrate epigenetic analyses into their current line of research. As more clocks are developed in pediatric samples, studied, and their findings replicated, it will be important for prevention researchers to match the clock they choose to the sample to which they want to generalize.
Considerations for Future Research
There has been a notable increase in the use of epigenetic clocks in pediatric populations. Epigenetic clocks offer great promise for understanding how environmental exposures influence the biologic aging pathway during sensitive periods in development. Use of these clocks can aid prevention researchers interested in understanding the etiology of diseases and disorders, elucidating mechanistic pathways, and informing the creation or improvement of promotion, prevention, intervention, and treatment programming across the life course.
The ability of researchers to use many different types of biospecimens collected by large-scale epidemiology studies to measure DNA methylation as a predictor of biological aging offers the opportunity to retrospectively study the impact of a variety of environmental exposures on health outcomes. The ability to capture epigenetic data from archived or banked biospecimens may reduce participant burden and make studying epigenetics more accessible for prevention researchers. For example, several researchers have used banked dried blood spots initially captured at birth for newborn metabolic screening programs for other purposes such as calculating biological age (Hollegaard et al., 2013; Knight et al., 2016; McClendon-Weary et al., 2020).
In summary, while research is increasingly relying on epigenetic clocks to characterize biological aging, the bulk of the extant literature and methods are focused on adults and aging populations. Epigenetic clocks offer promise for prevention science researchers interested in elucidating the multideterminant etiology of mental, behavioral, and physical health problems. Researchers with an interest in methylation and epigenetics should explore the potential use of these clocks as an avenue to understand mechanisms of environmental exposures and adverse health outcomes.
Supplementary Material
Funding
This study was supported by NIH HD093643 to SBJ and RJM.
Footnotes
Declarations
Ethical Approval All procedures performed in the current study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s11121-023-01576-4.
Consent to Participate Informed consent was not required as no human subjects were used.
Conflict of Interest The authors declare no competing interests.
References
- Allen JP, Danoff JS, Costello MA, Loeb EL, Davis AA, Hunt GL, Gregory SG, Giamberardino SN, & Connelly JJ (2022). Adolescent peer struggles predict accelerated epigenetic aging in midlife. Development and Psychopathology, 1–14. 10.1017/S0954579422000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- August GJ, & Gewirtz A. (2019). Moving toward a precision-based, personalized framework for prevention science: Introduction to the special issue. Prevention Science, 20(1), 1–9. 10.1007/s11121-018-0955-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin MK, Chen E, Ross KM, McEwen LM, Maclsaac JL, Kobor MS, & Miller GE (2018). Early-life socioeconomic disadvantage, not current, predicts accelerated epigenetic aging of monocytes. Psychoneuroendocrinology, 97, 131–134. 10.1016/j.psyneuen.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Beijers R, ten Thije I, Bolhuis E, O’Donnell KJ, Tollenaar MS, Shalev I, Hastings WJ, MacIsaac JL, Lin DTS, Meaney M, Kobor MS, Belsky J, & de Weerth C. (2023). Cumulative risk exposure and child cellular aging in a Dutch low-risk community sample. Psychophysiology, 60(4). 10.1111/psyp.14205 [DOI] [PubMed] [Google Scholar]
- Bell CG, Lowe R, Adams PD, Baccarelli AA, Beck S, Bell JT, Christensen BC, Gladyshev VN, Heijmans BT, Horvath S, Ideker T, Issa J-PJ, Kelsey KT, Marioni RE, Reik W, Relton CL, Schalkwyk LC, Teschendorff AE, Wagner W, & Rakyan VK (2019). DNA methylation aging clocks: Challenges and recommendations. Genome Biology, 20(1), 249. 10.1186/s13059-019-1824-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, & Caspi A. (2017). Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: Do they measure the same thing? American Journal of Epidemiology. 10.1093/aje/kwx346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder AM, Corvalan C, Mericq V, Pereira A, Santos JL, Horvath S, Shepherd J, & Michels KB (2018). Faster ticking rate of the epigenetic clock is associated with faster pubertal development in girls. Epigenetics, 13(1), 85–94. 10.1080/15592294.2017.1414127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin J, Håberg SE, Magnus P, Reese SE, Gjessing HK, Magnus MC, Parr CL, Page CM, London SJ, & Nystad W. (2016). Prediction of gestational age based on genome-wide differentially methylated regions. Genome Biology, 17(1), 207. 10.1186/s13059-016-1063-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis E, Belsky J, Frankenhuis WE, Shalev I, Hastings WJ, Tollenaar MS, O’Donnell KJ, McGill MG, Pokhvisneva I, Lin DTS, MacIsaac JL, Kobor MS, de Weerth C, & Beijers R. (2022). Attachment insecurity and the biological embedding of reproductive strategies: Investigating the role of cellular aging. Biological Psychology, 175, 108446. 10.1016/j.biopsycho.2022.108446 [DOI] [PubMed] [Google Scholar]
- Brody GH, Miller GE, Yu T, Beach SRH, & Chen E. (2016a). Supportive family environments ameliorate the link between racial discrimination and epigenetic aging: A replication across two longitudinal cohorts. Psychological Science, 27(4), 530–541. 10.1177/0956797615626703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody GH, Yu T, Chen E, Beach SRH, & Miller GE (2016b). Family-centered prevention ameliorates the longitudinal association between risky family processes and epigenetic aging. Journal of Child Psychology and Psychiatry, 57(5), 566–574. 10.1111/jcpp.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Sordillo JE, Rifas-Shiman SL, Chung W, Liang L, Coull BA, Hivert M-F, Lai PS, Forno E, Celedón JC, Litonjua AA, Brennan KJ, DeMeo DL, Baccarelli AA, Oken E, & Gold DR (2019). The nasal methylome as a biomarker of asthma and airway inflammation in children. Nature Communications, 10(1), 3095. 10.1038/s41467-019-11058-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveira de Baumont A, Hoffmann MS, Bortoluzzi A, Fries GR, Lavandoski P, Grun LK, Guimarães LSP, Guma FTCR, Salum GA, Barbé-Tuana FM, & Manfro GG (2021). Telomere length and epigenetic age acceleration in adolescents with anxiety disorders. Scientific Reports, 11(1), 7716. 10.1038/s41598-021-87045-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Miller GE, Yu T, & Brody GH (2016). The Great Recession and health risks in African American youth. Brain, Behavior, and Immunity, 53, 234–241. 10.1016/j.bbi.2015.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausing ES, & Non AL (2021). Epigenetics as a mechanism of developmental embodiment of stress, resilience, and cardio-metabolic risk across generations of Latinx immigrant families. Frontiers in Psychiatry, 12, 696827. 10.3389/fpsyt.2021.696827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammering F, Martins J, Dittrich K, Czamara D, Rex-Haffner M, Overfeld J, de Punder K, Buss C, Entringer S, Winter SM, Binder EB, & Heim C. (2021). The pediatric buccal epigenetic clock identifies significant ageing acceleration in children with internalizing disorder and maltreatment exposure. Neurobiology of Stress, 15, 100394. 10.1016/j.ynstr.2021.100394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EG, Humphreys KL, McEwen LM, Sacchet MD, Camacho MC, MacIsaac JL, Lin DTS, Kobor MS, & Gotlib IH (2017). Accelerated DNA methylation age in adolescent girls: Associations with elevated diurnal cortisol and reduced hippocampal volume. Translational Psychiatry, 7(8), e1223–e1223. 10.1038/tp.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster EL, Wong CCY, Lester KJ, Burrage J, Gregory AM, Mill J, & Eley TC (2014). Genome-wide methylomic analysis of monozygotic twins discordant for adolescent depression. Biological Psychiatry, 76(12), 977–983. 10.1016/j.biopsych.2014.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Song N, Qin N, Chen C, Li Z, Sun X, Easton J, Mulder H, Plyler E, Neale G, Walker E, Li Q, Ma X, Chen X, Huang I-C, Yasui Y, Ness KK, Zhang J, Hudson MM, & Wang Z. (2022). Genome-wide association studies identify novel genetic loci for epigenetic age acceleration among survivors of childhood cancer. Genome Medicine, 14(1), 32. 10.1186/s13073-022-01038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, & Schlomer GL (2009). Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Human Nature, 20(2), 204–268. 10.1007/s12110-009-9063-7 [DOI] [PubMed] [Google Scholar]
- Etzel L, Hastings WJ, Hall MA, Heim CM, Meaney MJ, Noll JG, O’Donnell KJ, Pokhvisneva I, Rose EJ, Schreier HMC, Shenk CE, & Shalev I. (2022). Obesity and accelerated epigenetic aging in a high-risk cohort of children. Scientific Reports, 12(1), 8328. 10.1038/s41598-022-11562-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito G, Polidoro S, Dugué P-A, Kivimaki M, Ponzi E, Matullo G, Guarrera S, Assumma MB, Georgiadis P, Kyrtopoulos SA, Krogh V, Palli D, Panico S, Sacerdote C, Tumino R, Chadeau-Hyam M, Stringhini S, Severi G, Hodge AM, & Vineis P. (2017). Social adversity and epigenetic aging: A multi-cohort study on socioeconomic differences in peripheral blood DNA methylation. Scientific Reports, 7(1), 16266. 10.1038/s41598-017-16391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransquet PD, Wrigglesworth J, Woods RL, Ernst ME, & Ryan J. (2019). The epigenetic clock as a predictor of disease and mortality risk: A systematic review and meta-analysis. Clinical Epigenetics, 11(1), 62. 10.1186/s13148-019-0656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattuso J, Hinds P, Tong X, & Srivastava K. (2006). Monitoring child and parent refusals to enrol in clinical research protocols. Journal of Advanced Nursing, 53(3), 319–326. 10.1111/j.1365-2648.2006.03724.x [DOI] [PubMed] [Google Scholar]
- George A, Hardy R, Castillo Fernandez J, Kelly Y, & Maddock J. (2021). Life course socioeconomic position and DNA methylation age acceleration in mid-life. Journal of Epidemiology and Community Health, 75(11), 1084–1090. 10.1136/jech-2020-215608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettler LT, Lin DTS, Miegakanda V, Lew-Levy S, Eick GN, Snodgrass JJ, MacIsaac JL, Ramadori KE, Kobor MS, & Boyette AH (2020). Epigenetic aging in children from a small-scale farming society in the Congo Basin: Associations with child growth and family conflict. Developmental Psychobiology, 62(2), 138–153. 10.1002/dev.21935 [DOI] [PubMed] [Google Scholar]
- Gomaa N, Konwar C, Gladish N, Au-Young SH, Guo T, Sheng M, Merrill SM, Kelly E, Chau V, Branson HM, Ly LG, Duerden EG, Grunau RE, Kobor MS, & Miller SP (2022). Association of pediatric buccal epigenetic age acceleration with adverse neonatal brain growth and neurodevelopmental outcomes among children born very preterm with a neonatal infection. JAMA Network Open, 5(11), e2239796. 10.1001/jamanetworkopen.2022.39796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Verjan JC, Esparza-Aguilar M, Martín-Martín V, Salazar-Perez C, Cadena-Trejo C, Gutierrez-Robledo LM, Martínez-Magaña JJ, Nicolini H, & Arroyo P. (2021). Years of schooling could reduce epigenetic aging: A study of a Mexican cohort. Genes, 12(9), 1408. 10.3390/genes12091408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw S, Camerota M, Carter BS, Helderman J, Hofheimer JA, McGowan EC, Neal CR, Pastyrnak SL, Smith LM, DellaGrotta SA, Dansereau LM, Padbury JF, O’Shea M, Lester BM, Marsit CJ, & Everson TM (2021). NEOage clocks—Epigenetic clocks to estimate post-menstrual and postnatal age in preterm infants. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlat EJ, Prather AA, Horvath S, Belsky J, & Epel ES (2021). Early life adversity, pubertal timing, and epigenetic age acceleration in adulthood. Developmental Psychobiology, dev.22085. 10.1002/dev.22085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RD, Richard MA, Gramatges MMJ, Wilhelm K, Scheurer ME, Lupo PJ, & Brown AL (2022). Epigenetic age acceleration among survivors of pediatric medulloblastoma and primitive neuroectodermal tumor. Pediatric Hematology and Oncology, 1–5. 10.1080/08880018.2022.2101722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henckel E, Landfors M, Haider Z, Kosma P, Hultdin M, Degerman S, & Bohlin K. (2020). Hematopoietic cellular aging is not accelerated during the first 2 years of life in children born preterm. Pediatric Research, 88(6), 903–909. 10.1038/s41390-020-0833-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Moreno JF, Estrada-Gutierrez G, Wu H, Bloomquist TR, Rosa MJ, Just AC, Lamadrid-Figueroa H, Téllez-Rojo MM, Wright RO, & Baccarelli AA (2022). Prenatal lead exposure, telomere length in cord blood, and DNA methylation age in the PROGRESS prenatal cohort. Environmental Research, 205, 112577. 10.1016/j.envres.2021.112577 [DOI] [PubMed] [Google Scholar]
- Hoare J, Stein DJ, Heany SJ, Fouche J-P, Phillips N, Er S, Myer L, Zar HJ, Horvath S, & Levine AJ (2020). Accelerated epigenetic aging in adolescents from low-income households is associated with altered development of brain structures. Metabolic Brain Disease, 35(8), 1287–1298. 10.1007/s11011-020-00589-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J, Stein DJ, Heany SJ, Fouche J-P, Phillips N, Er S, Myer L, Zar HJ, Horvath S, & Levine AJ (2022). Accelerated epigenetic aging in adolescents living with HIV is associated with altered development of brain structures. Journal of NeuroVirology, 28(2), 208–216. 10.1007/s13365-021-00947-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollegaard MV, Grauholm J, Nørgaard-Pedersen B, & Hougaard DM (2013). DNA methylome profiling using neonatal dried blood spot samples: A proof-of-principle study. Molecular Genetics and Metabolism, 108(4), 225–231. 10.1016/j.ymgme.2013.01.016 [DOI] [PubMed] [Google Scholar]
- Horvath S. (2013). DNA methylation age of human tissues and cell types. Genome Biology, 14(10), R115. 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Oshima J, Martin GM, Lu AT, Quach A, Cohen H, Felton S, Matsuyama M, Lowe D, Kabacik S, Wilson JG, Reiner AP, Maierhofer A, Flunkert J, Aviv A, Hou L, Baccarelli AA, Li Y, Stewart JD, & Raj K. (2018a). Epigenetic clock for skin and blood cells applied to Hutchinson Gilford progeria syndrome and ex vivo studies. Aging, 10(7), 1758–1775. 10.18632/aging.101508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Stein DJ, Phillips N, Heany SJ, Kobor MS, Lin DTS, Myer L, Zar HJ, Levine AJ, & Hoare J. (2018b). Perinatally acquired HIV infection accelerates epigenetic aging in South African adolescents. AIDS, 32(11), 1465–1474. 10.1097/QAD.0000000000001854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R-C, Lillycrop KA, Beilin LJ, Godfrey KM, Anderson D, Mori TA, Rauschert S, Craig JM, Oddy WH, Ayonrinde OT, Pennell CE, Holbrook JD, & Melton PE (2019). Epigenetic age acceleration in adolescence associates with BMI, inflammation, and risk score for middle age cardiovascular disease. The Journal of Clinical Endocrinology & Metabolism, 104(7), 3012–3024. 10.1210/jc.2018-02076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, Smart M, Gorrie-Stone T, Hannon E, Mill J, Bao Y, Burrage J, Schalkwyk L, & Kumari M. (2018). Socioeconomic position and DNA methylation age acceleration across the life course. American Journal of Epidemiology, 187(11), 2346–2354. 10.1093/aje/kwy155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed R, Chen W, Lin F, & Liang H. (2016). Infant’s DNA methylation age at birth and epigenetic aging accelerators. BioMed Research International, 2016, 1–10. 10.1155/2016/4515928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Vance LA, Cross D, Knight AK, Kilaru V, Michopoulos V, Klengel T, & Smith AK (2017). Exposure to violence accelerates epigenetic aging in children. Scientific Reports, 7(1), 8962. 10.1038/s41598-017-09235-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce BT, Gao T, Koss K, Zheng Y, Cardenas A, Heiss J, Just A, Zhang K, van Horn L, Allen NB, Greenland P, Cohen S, Gordon-Larsen P, Mitchell C, McLanahan S, Schneper L, Notterman D, Rifas-Shiman SL, Oken E, & Hou L. (2022). Impact of paternal education on epigenetic ageing in adolescence and mid-adulthood: A multi-cohort study in the USA and Mexico. International Journal of Epidemiology, 51(3), 870–884. 10.1093/ije/dyab196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jylhävä J, Pedersen NL, & Hägg S. (2017). Biological Age Predictors. Ebiomedicine, 21, 29–36. 10.1016/j.ebiom.2017.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Harrall KK, Glueck DH, Needham BL, & Dabelea D. (2022). Gestational diabetes mellitus, epigenetic age and offspring metabolism. Diabetic Medicine, 39(11). 10.1111/dme.14925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling T, Wenger A, & Carén H. (2020). DNA methylation-based age estimation in pediatric healthy tissues and brain tumors. Aging, 12(21), 21037–21056. 10.18632/aging.202145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AK, Craig JM, Theda C, Bækvad-Hansen M, Bybjerg-Grauholm J, Hansen CS, Hollegaard MV, Hougaard DM, Mortensen PB, Weinsheimer SM, Werge TM, Brennan PA, Cubells JF, Newport DJ, Stowe ZN, Cheong JLY, Dalach P, Doyle LW, Loke YJ, & Smith AK (2016). An epigenetic clock for gestational age at birth based on blood methylation data. Genome Biology, 17(1), 206. 10.1186/s13059-016-1068-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latendresse SJ, Musci R, & Maher BS (2018). Critical issues in the inclusion of genetic and epigenetic information in prevention and intervention trials. Prevention Science, 19(1), 58–67. 10.1007/s11121-017-0785-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn RB, Anderson EL, Suderman M, Simpkin AJ, Gaunt TR, Teschendorff AE, Widschwendter M, Hardy R, Kuh D, Relton CL, & Howe LD (2018). Psychosocial adversity and socioeconomic position during childhood and epigenetic age: Analysis of two prospective cohort studies. Human Molecular Genetics, 27(7), 1301–1308. 10.1093/hmg/ddy036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecorguillé M, Navarro P, Chen L-W, Murrin C, Viljoen K, Mehegan J, Shivappa N, Hébert JR, Kelleher CC, Suderman M, & Phillips CM (2023). Maternal and paternal dietary quality and dietary inflammation associations with offspring DNA methylation and epigenetic biomarkers of aging in the lifeways cross-generation study. The Journal of Nutrition, S0022316623055785. 10.1016/j.tjnut.2023.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Choufani S, Weksberg R, Wilson SL, Yuan V, Burt A, Marsit C, Lu AT, Ritz B, Bohlin J, Gjessing HK, Harris JR, Magnus P, Binder AM, Robinson WP, Jugessur A, & Horvath S. (2019). Placental epigenetic clocks: Estimating gestational age using placental DNA methylation levels. Aging, 11(12), 4238–4253. 10.18632/aging.102049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson G, Reiner AP, Aviv A, Lohman K, Liu Y, & Ferrucci L. (2018). An epigenetic biomarker of aging for lifespan and healthspan. Aging, 10(4), 573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe R, Gemma C, Beyan H, Hawa MI, Bazeos A, Leslie RD, Montpetit A, Rakyan VK, & Ramagopalan SV (2013). Buccals are likely to be a more informative surrogate tissue than blood for epigenome-wide association studies. Epigenetics, 8(4), 445–454. 10.4161/epi.24362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, Assimes TL, Ferrucci L, & Horvath S. (2019). DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging, 11(2), 303–327. 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock J, Castillo-Fernandez J, Wong A, Ploubidis GB, Kuh D, Bell JT, & Hardy R. (2021). Childhood growth and development and DNA methylation age in mid-life. Clinical Epigenetics, 13(1), 155. 10.1186/s13148-021-01138-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak EM, Scott SR, & Millwood SN (2023). Accelerated epigenetic aging at birth interacts with parenting hostility to predict child temperament and subsequent psychological symptoms. Development and Psychopathology, 35(1), 109–118. 10.1017/S0954579421000614 [DOI] [PubMed] [Google Scholar]
- Marini S, Davis KA, Soare TW, Zhu Y, Suderman MJ, Simpkin AJ, Smith ADAC, Wolf EJ, Relton CL, & Dunn EC (2020). Adversity exposure during sensitive periods predicts accelerated epigenetic aging in children. Psychoneuroendocrinology, 113, 104484. 10.1016/j.psyneuen.2019.104484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KJ, McGowan PO, Vega WC, Morrison KM, Saigal S, Van Lieshout RJ, & Schmidt LA (2021). Cumulative risks predict epigenetic age in adult survivors of extremely low birth weight. Developmental Psychobiology, 63(S1). 10.1002/dev.22222 [DOI] [PubMed] [Google Scholar]
- Mattson MP, & Arumugam TV (2018). Hallmarks of brain aging: Adaptive and pathological modification by metabolic states. Cell Metabolism, 27(6), 1176–1199. 10.1016/j.cmet.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon-Weary B, Putnick DL, Robinson S, & Yeung E. (2020). Little to give, much to gain—What can you do with a dried blood spot? Current Environmental Health Reports, 7(3), 211–221. 10.1007/s40572-020-00289-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory C, Fiorito G, Ni Cheallaigh C, Polidoro S, Karisola P, Alenius H, Layte R, Seeman T, Vineis P, & Kenny RA (2019). How does socio-economic position (SEP) get biologically embedded? A comparison of allostatic load and the epigenetic clock(s). Psychoneuroendocrinology, 104, 64–73. 10.1016/j.psyneuen.2019.02.018 [DOI] [PubMed] [Google Scholar]
- McEwen LM, O’Donnell KJ, McGill MG, Edgar RD, Jones MJ, MacIsaac JL, Lin DTS, Ramadori K, Morin A, Gladish N, Garg E, Unternaehrer E, Pokhvisneva I, Karnani N, Kee MZL, Klengel T, Adler NE, Barr RG, Letourneau N, & Kobor MS (2020). The PedBE clock accurately estimates DNA methylation age in pediatric buccal cells. Proceedings of the National Academy of Sciences, 117(38), 23329–23335. 10.1073/pnas.1820843116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MG, Pokhvisneva I, Clappison AS, McEwen LM, Beijers R, Tollenaar MS, Pham H, Kee MZL, Garg E, de Mendonça Filho EJ, Karnani N, Silveira PP, Kobor MS, de Weerth C, Meaney MJ, & O’Donnell KJ (2022). Maternal prenatal anxiety and the fetal origins of epigenetic aging. Biological Psychiatry, 91(3), 303–312. 10.1016/j.biopsych.2021.07.025 [DOI] [PubMed] [Google Scholar]
- Miller GE, Yu T, Chen E, & Brody GH (2015). Self-control forecasts better psychosocial outcomes but faster epigenetic aging in low-SES youth. Proceedings of the National Academy of Sciences, 112(33), 10325–10330. 10.1073/pnas.1505063112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & The Prisma Group. (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Medicine, 6(7). [PMC free article] [PubMed] [Google Scholar]
- Musci RJ, & Schlomer G. (2018). The implications of genetics for prevention and intervention programming. Prevention Science, 19(1), 1–5. 10.1007/s11121-017-0837-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri de Souza Reis V, Tahira AC, Daguano Gastaldi V, Mari P, Portolese J, Feio dos Santos AC, Lisboa B, Mari J, Caetano SC, Brunoni D, Bordini D, Silvestre de Paula C, Vêncio RZN, Quackenbush J, & Brentani H. (2021). Environmental influences measured by epigenetic clock and vulnerability components at birth impact clinical ASD heterogeneity. Genes, 12(9), 1433. 10.3390/genes12091433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani S, Suzuki S, Ochiai K, Yao A, Fujioka T, Fujisawa TX, & Tomoda A. (2021). Altered epigenetic clock in children exposed to maltreatment. Psychiatry and Clinical Neurosciences, 75(3), 110–112. 10.1111/pcn.13183 [DOI] [PubMed] [Google Scholar]
- Nwanaji-Enwerem JC, Van Der Laan L, Kogut K, Eskenazi B, Holland N, Deardorff J, & Cardenas A. (2021). Maternal adverse childhood experiences before pregnancy are associated with epigenetic aging changes in their children. Aging, 13(24), 25653–25669. 10.18632/aging.203776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki S, Otsuka I, Shinko Y, Horai T, Hirata T, Yamaki N, Sora I, & Hishimoto A. (2021). Epigenetic clock analysis in children with fetal alcohol spectrum disorder. Alcoholism: Clinical and Experimental Research, acer.14532. 10.1111/acer.14532 [DOI] [PubMed] [Google Scholar]
- Peng C, Cardenas A, Rifas-Shiman SL, Hivert M-F, Gold DR, Platts-Mills TA, Lin X, Oken E, Avila L, Celedón JC, Weiss ST, Baccarelli AA, Litonjua AA, & DeMeo DL (2019). Epigenetic age acceleration is associated with allergy and asthma in children in Project Viva. Journal of Allergy and Clinical Immunology, 143(6), 2263–2270.e14. 10.1016/j.jaci.2019.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penova-Veselinovic B, Melton PE, Huang RC, Yovich JL, Burton P, Wijs LA, & Hart RJ (2021). DNA methylation patterns within whole blood of adolescents born from assisted reproductive technology are not different from adolescents born from natural conception. Human Reproduction, 36(7), 2035–2049. 10.1093/humrep/deab078 [DOI] [PubMed] [Google Scholar]
- Phang M, Ross J, Raythatha JH, Dissanayake HU, McMullan RL, Kong Y, Hyett J, Gordon A, Molloy P, & Skilton MR (2020). Epigenetic aging in newborns: Role of maternal diet. The American Journal of Clinical Nutrition, 111(3), 555–561. 10.1093/ajcn/nqz326 [DOI] [PubMed] [Google Scholar]
- Popovic M, Fiano V, Isaevska E, Moccia C, Trevisan M, Rusconi F, De Marco L, Polidoro S, Merletti F, Pizzi C, & Richiardi L. (2021). Determination of saliva epigenetic age in infancy, and its association with parental socio-economic characteristics and pregnancy outcomes. Journal of Developmental Origins of Health and Disease, 12(2), 319–327. 10.1017/S2040174420000380 [DOI] [PubMed] [Google Scholar]
- Reimann B, Martens DS, Wang C, Ghantous A, Herceg Z, Plusquin M, & Nawrot TS (2022). Interrelationships and determinants of aging biomarkers in cord blood. Journal of Translational Medicine, 20(1), 353. 10.1186/s12967-022-03541-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard IJ, Frankenhuis WE, & Nettle D. (2014). Why are childhood family factors associated with timing of maturation? A role for internal prediction. Perspectives on Psychological Science, 9(1), 3–15. 10.1177/1745691613513467 [DOI] [PubMed] [Google Scholar]
- Shenk CE, Felt JM, Ram N, O’Donnell KJ, Sliwinski MJ, Pokhvisneva I, Benson L, Meaney MJ, Putnam FW, & Noll JG (2022). Cortisol trajectories measured prospectively across thirty years of female development following exposure to childhood sexual abuse: Moderation by epigenetic age acceleration at midlife. Psychoneuroendocrinology, 136, 105606. 10.1016/j.psyneuen.2021.105606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk CE, O’Donnell KJ, Pokhvisneva I, Kobor MS, Meaney MJ, Bensman HE, Allen EK, & Olson AE (2021). Epigenetic age acceleration and risk for posttraumatic stress disorder following exposure to substantiated child maltreatment. Journal of Clinical Child & Adolescent Psychology, 1–11. 10.1080/15374416.2020.1864738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau S, Brummel SS, Kennedy EM, Hermetz K, Spector SA, Williams PL, Kacanek D, Smith R, Drury SS, Agwu A, Ellis A, Patel K, Seage GR, Van Dyke RB, & Marsit CJ (2021a). Longitudinal changes in epigenetic age in youth with perinatally acquired HIV and youth who are perinatally HIV-exposed uninfected. AIDS, 35(5), 811–819. 10.1097/QAD.0000000000002805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau S, Strehlau R, Shen J, Violari A, Patel F, Liberty A, Foca M, Wang S, Terry MB, Yin MT, Coovadia A, Abrams EJ, Arpadi SM, & Kuhn L. (2018). Biomarkers of aging in HIV-infected children on suppressive antiretroviral therapy. JAIDS Journal of Acquired Immune Deficiency Syndromes, 78(5), 549–556. 10.1097/QAI.0000000000001714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiau S, Wang L, Liu H, Zheng Y, Drong A, Joyce BT, Wang J, Li W, Leng J, Shen Y, Gao R, Hu G, Hou L, & Baccarelli AA (2021b). Prenatal gestational diabetes mellitus exposure and accelerated offspring DNA methylation age in early childhood. Epigenetics, 16(2), 186–195. 10.1080/15592294.2020.1790924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkin AJ, Hemani G, Suderman M, Gaunt TR, Lyttleton O, Mcardle WL, Ring SM, Sharp GC, Tilling K, Horvath S, Kunze S, Peters A, Waldenberger M, Ward-Caviness C, Nohr EA, Sørensen TIA, Relton CL, & Smith GD (2016). Prenatal and early life influences on epigenetic age in children: A study of mother–offspring pairs from two cohort studies. Human Molecular Genetics, 25(1), 191–201. 10.1093/hmg/ddv456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkin AJ, Howe LD, Tilling K, Gaunt TR, Lyttleton O, McArdle WL, Ring SM, Horvath S, Smith GD, & Relton CL (2017). The epigenetic clock and physical development during childhood and adolescence: Longitudinal analysis from a UK birth cohort. International Journal of Epidemiology, dyw307. 10.1093/ije/dyw307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Kilaru V, Klengel T, Mercer KB, Bradley B, Conneely KN, Ressler K. j., & Binder EB (2015). DNA extracted from saliva for methylation studies of psychiatric traits: Evidence tissue specificity and relatedness to brain. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 168(1), 36–44. 10.1002/ajmg.b.v168.1, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez A, Lahti J, Czamara D, Lahti-Pulkkinen M, Girchenko P, Andersson S, Strandberg TE, Reynolds RM, Kajantie E, Binder EB, & Raikkonen K. (2018a). The epigenetic clock and pubertal, neuroendocrine, psychiatric, and cognitive outcomes in adolescents. Clinical Epigenetics, 10(1), 96. 10.1186/s13148-018-0528-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez A, Lahti J, Czamara D, Lahti-Pulkkinen M, Knight AK, Girchenko P, Hämäläinen E, Kajantie E, Lipsanen J, Laivuori H, Villa PM, Reynolds RM, Smith AK, Binder EB, & Räikkönen K. (2018b). The epigenetic clock at birth: Associations with maternal antenatal depression and child psychiatric problems. Journal of the American Academy of Child & Adolescent Psychiatry, 57(5), 321–328.e2. 10.1016/j.jaac.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, & McLaughlin KA (2019). Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry, 85(3), 268–278. 10.1016/j.biopsych.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyf M, Tang Y-Y, Hill KG, & Musci R. (2016). The dynamic epigenome and its implications for behavioral interventions: A role for epigenetics to inform disorder prevention and health promotion. Translational Behavioral Medicine, 6(1), 55–62. 10.1007/s13142-016-0387-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Howe LD, Suderman M, Relton CL, Crawford AA, & Houtepen LC (2020). Adverse childhood experiences, DNA methylation age acceleration, and cortisol in UK children: A prospective population-based cohort study. Clinical Epigenetics, 12(1), 55. 10.1186/s13148-020-00844-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollenaar MS, Beijers R, Garg E, Nguyen TTT, Lin DTS, MacIsaac JL, Shalev I, Kobor MS, Meaney MJ, O’Donnell KJ, & de Weerth C. (2021). Internalizing symptoms associate with the pace of epigenetic aging in childhood. Biological Psychology, 159, 108021. 10.1016/j.biopsycho.2021.108021 [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, McGowan PO, de Vega WC, Savoy CD, Morrison KM, Saigal S, Mathewson KJ, & Schmidt LA (2021). Extremely low birth weight and accelerated biological aging. Pediatrics, 147(6), e2020001230. 10.1542/peds.2020-001230 [DOI] [PubMed] [Google Scholar]
- Vardell E, & Malloy M. (2013). Joanna Briggs Institute: An evidence-based practice database. Medical Reference Services Quarterly, 32(4), 434–442. 10.1080/02763869.2013.837734 [DOI] [PubMed] [Google Scholar]
- Verlinden I, Coppens G, Vanhorebeek I, Güiza F, Derese I, Wouters PJ, Joosten KF, Verbruggen SC, & Van den Berghe G. (2023). Long-term impact of paediatric critical illness on the difference between epigenetic and chronological age in relation to physical growth. Clinical Epigenetics, 15(1), 8. 10.1186/s13148-023-01424-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Chen W, McDermott J, & Han J-DJ (2017). Molecular and phenotypic biomarkers of aging. F1000Research, 6, 860. 10.12688/f1000research.10692.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Arloth J, Carrillo-Roa T, Iurato S, Röh S, Ressler KJ, Nemeroff CB, Smith AK, Bradley B, Heim C, Menke A, Lange JF, Brückl T, Ising M, Wray NR, Erhardt A, Binder EB, & Mehta D. (2015). Lifetime stress accelerates epigenetic aging in an urban, African American cohort: Relevance of glucocorticoid signaling. Genome Biology, 16(1), 266. 10.1186/s13059-015-0828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.