Abstract
Study Design
Systematic review and meta-analysis.
Objectives
In an effort to prevent intraoperative neurological injury during spine surgery, the use of intraoperative neurophysiological monitoring (IONM) has increased significantly in recent years. Using IONM, spinal cord function can be evaluated intraoperatively by recording signals from specific nerve roots, motor tracts, and sensory tracts. We performed a systematic review and meta-analysis of diagnostic test accuracy (DTA) studies to evaluate the efficacy of IONM among patients undergoing spine surgery for any indication.
Methods
The current systematic review and meta-analysis was performed using the Preferred Reporting Items for a Systematic Review and Meta-analysis statement for Diagnostic Test Accuracy Studies (PRISMA-DTA) and was registered on PROSPERO. A comprehensive search was performed using MEDLINE, EMBASE and SCOPUS for all studies assessing the diagnostic accuracy of neuromonitoring, including somatosensory evoked potential (SSEP), motor evoked potential (MEP) and electromyography (EMG), either on their own or in combination (multimodal). Studies were included if they reported raw numbers for True Positives (TP), False Negatives (FN), False Positives (FP) and True Negative (TN) either in a 2 × 2 contingency table or in text, and if they used postoperative neurologic exam as a reference standard. Pooled sensitivity and specificity were calculated to evaluate the overall efficacy of each modality type using a bivariate model adapted by Reitsma et al, for all spine surgeries and for individual disease groups and regions of spine. The risk of bias (ROB) of included studies was assessed using the quality assessment tool for diagnostic accuracy studies (QUADAS-2).
Results
A total of 163 studies were included; 52 of these studies with 16,310 patients reported data for SSEP, 68 studies with 71,144 patients reported data for MEP, 16 studies with 7888 patients reported data for EMG and 69 studies with 17,968 patients reported data for multimodal monitoring. The overall sensitivity, specificity, DOR and AUC for SSEP were 71.4% (95% CI 54.8-83.7), 97.1% (95% CI 95.3-98.3), 41.9 (95% CI 24.1-73.1) and .899, respectively; for MEP, these were 90.2% (95% CI 86.2-93.1), 96% (95% CI 94.3-97.2), 103.25 (95% CI 69.98-152.34) and .927; for EMG, these were 48.3% (95% CI 31.4-65.6), 92.9% (95% CI 84.4-96.9), 11.2 (95% CI 4.84-25.97) and .773; for multimodal, these were found to be 83.5% (95% CI 81-85.7), 93.8% (95% CI 90.6-95.9), 60 (95% CI 35.6-101.3) and .895, respectively. Using the QUADAS-2 ROB analysis, of the 52 studies reporting on SSEP, 13 (25%) were high-risk, 10 (19.2%) had some concerns and 29 (55.8%) were low-risk; for MEP, 8 (11.7%) were high-risk, 21 had some concerns and 39 (57.3%) were low-risk; for EMG, 4 (25%) were high-risk, 3 (18.75%) had some concerns and 9 (56.25%) were low-risk; for multimodal, 14 (20.3%) were high-risk, 13 (18.8%) had some concerns and 42 (60.7%) were low-risk.
Conclusions
These results indicate that all neuromonitoring modalities have diagnostic utility in successfully detecting impending or incident intraoperative neurologic injuries among patients undergoing spine surgery for any condition, although it is clear that the accuracy of each modality differs.
PROSPERO Registration Number: CRD42023384158
Keywords: spinal cord injury, neuro, trauma, intraoperative neurological injury
Introduction
Intraoperative neurological injury is a feared complication in surgical spinal procedures, with significant medical, social and economic consequences. 1 The use of intraoperative neurophysiologic monitoring (IONM) has thus been employed to prevent neurological deficits and identify intraoperative maneuvers that can lead to neurological injury, such as in deformity correction or during intramedullary spinal tumor resections.2,3 IONM in current practice refers to various techniques used to assess neural system integrity intraoperatively, including somatosensory evoked potentials (SSEP), motor evoked potentials (MEP), D-waves, and electromyography (EMG).2–4 The purpose of using IONM is to detect neurophysiological changes during a surgical procedure that could result in neurological deficits.4,5 While the value of using IONM is becoming increasingly recognized, a quantitative assessment of the diagnostic accuracy of various IONM modalities is lacking. Moreover, there is no clear consensus on the use of IONM for spinal surgery.
There have been previous systematic reviews with and without meta-analyses, which have attempted to summarize the role of neurophysiologic monitoring for intraoperative spinal cord injury (ISCI).4,6–16 However, these reviews have focused on a specific question or have only included studies comparing one modality to another. A comprehensive assessment of diagnostic test accuracy (DTA) of neuromonitoring following the PRISMA-DTA guidelines and GRADE guidelines has yet to be performed.
Key Question: What is the accuracy of neurophysiological monitoring for diagnosis of intraoperative spinal cord injury (ISCI) compared with immediate postoperative clinical assessment?
Methods
This systematic review and meta-analysis was performed using the Preferred Reporting Items for Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA). 17 The abstract was drafted using the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) abstract. A comprehensive search was performed using MEDLINE, EMBASE and SCOPUS for all studies assessing the diagnostic accuracy of neuromonitoring, including SSEP, MEP and EMG, either on their own (unimodal) or in combination (multimodal).
Criteria for Inclusion/Exclusion of Studies in the Review
The criteria for inclusion and exclusion of studies for this systematic review were specified a priori for population, interventions, outcomes, reference standard, timing, and settings/studies (PICOTS) and are listed in Table 1. Only studies reporting raw numbers for True Positives (TP), False Negatives (FN), False Positives (FP) and True Negative (TN) either in a 2 × 2 contingency table or in text were included. Moreover, only studies using postoperative neurologic exam as a reference standard were included.
Table 1.
Inclusion and Exclusion Criteria: Population, Interventions, Comparators, Outcomes, Timing, and Study Designs.
| Inclusion | Exclusion | |
|---|---|---|
| Population | • Adolescents (≥12 years to <18 years old) or adults (≥18 years) undergoing any type of spine surgery for any indication or spine-related pathology (including trauma-related pathology, conus injuries, cauda equina injuries) Subpopulations/groups of interest: • Spinal deformity (eg, scoliosis) • Intra-dural tumors vs extra-dural lesions • Degenerative spine lesions |
• Patients <11 years old • Patients with new post-operative compression (eg, hematoma, abscess) • Patients with pedicle screw breach • Patients with neurologic deficits due to cranial pathology (eg, stroke) |
| Intervention | • Neuromonitoring including somatosensory evoked potentials (SSEP), motor evoked potentials (MEP), electromyography (EMG) and multimodal monitoring | |
| Outcomes | • True positives (a change in monitoring corresponding to a change in postoperative neurologic status) • False positive (a change in monitoring corresponding to no change in postoperative neurologic status) • False negative (no change in monitoring, but a change in postoperative neurologic status • True negative (no change in monitoring corresponding to no change in postoperative neurologic status) |
|
| Reference standard | • Postoperative neurological change/recovery (based on validated measures) - AIS grade - Motor score - Frankel grade |
• Pedicle screw breach |
| Timing | • Immediate post-operative period | |
| Study design | The review focus on the evidence at least risk for bias • RCTs or comparative observational studies (comparative cohorts, case control studies) • Case series will be included if comparative studies are not available |
• Case series with ≤10 patients • Animal studies • Abstracts, editorials, letters • Duplicate publications of the same study that do not report on different outcomes • Single reports from multicenter trials • White papers • Narrative reviews • Proceedings/abstracts from meetings • Articles identified as preliminary reports when results are published in later versions |
Study Design
Randomized control trials (RCTs) and high-quality prospective comparative cohort studies that control for confounding and met inclusion criteria were included as the primary evidence source. In the absence of high-quality studies, lower quality studies (eg retrospective observational studies) were considered.
Literature Search Strategies
Literature Databases
MEDLINE®, EMBASE and SCOPUS were searched using an appropriate search strategy. We included studies published in English and kept track of studies with English abstracts but not fully published in English that appeared to be relevant. Citations suggested by the clinical authors and guideline development group were compared against the s criteria for inclusion and exclusion. The search strategy for MEDLINE/EMBASE and SCOPUS is summarized in Table 2.
Table 2.
Search Strategy.
| String # | Search String | Count of articles |
|---|---|---|
| 1 | (neuromonitoring or intraoperative monitoring or neurophysiologic monitoring or neurophsiological monitoring or intraoperative neurophsiologic monitoring or intraoperative neurophysiological monitoring or IONM or SSEP or somatosensory evoked potential or motor evoked potential or MEP or electromyography or electroneuromyography or d-wave or multi-modal monitoring or multimodal neuromonitoring or multimodal intraoperative neuromonitoring or multimodal intraoperative monitoring or multimodal intraoperative neurophysiologic monitoring).mp. [mp = ti, ab, hw, tn, ot, dm, mf, dv, kf, fx, dq, bt, nm, ox, px, rx, ui, sy] | 254696 |
| 2 | Limit 1 to human | 201046 |
| 3 | (Spine surgery or cervical spine or thoracic spine or lumbar spine or lumbosacral spine or thoracolumbar or cervicothoracic or spinal surgery or spin* surg* or spin* deformity or scoliosis or spin* extradural or spin* intradual or extramedullary tumor or intramedullary tumor or spin * tumor or degenerative spine or spin* myelopathy).mp. [mp = ti, ab, hw, tn, ot, dm, mf, dv, kf, fx, dq, bt, nm, ox, px, rx, ui, sy] | 347753 |
| 4 | Limit 3 to human | 291603 |
| 5 | (Sensitivity or sensiti* or specificity or specif* or accuracy* or true positive or true negative or false positive or false negative).mp. [mp = ti, ab, hw, tn, ot, dm, mf, dv, kf, fx, dq, bt, nm, ox, px, rx, ui, sy] | 13186005 |
| 6 | Limit 5 to human | 7681782 |
| 7 | 2 and 4 and 6 | 2574 |
SCOPUS.
(TITLE-ABS-KEY ( “neuromonitoring” OR “intraoperative monitoring” OR “neurophysiologic monitoring” OR “neurophsiological monitoring” OR “intraoperative neurophsiologic monitoring” OR “intraoperative neurophysiological monitoring” OR “IONM” OR “SSEP” OR “somatosensory evoked potential” OR “motor evoked potential” OR “MEP” OR “electromyography” OR “electroneuromyography” OR “d-wave” OR “multi-modal monitoring” OR “multimodal neuromonitoring” OR “multimodal intraoperative neuromonitoring” OR “multimodal intraoperative monitoring” OR “multimodal intraoperative neurophysiologic monitoring”).
AND.
TITLE-ABS-KEY (“spine surgery” OR “cervical spine” OR “thoracic spine” OR “lumbar spine” OR “lumbosacral spine” OR “thoracolumbar” OR “cervicothoracic” OR “spinal surgery” OR “scoliosis” OR “intradual tumor” OR “extramedullary tumor” OR “intramedullary tumor” OR “spinal tumor” OR “degenerative spine”).
AND.
TITLE-ABS-KEY (“sensitivity” OR “specificity” OR “accuracy” OR “true positive” OR “true negative” OR “false positive” OR “false negative”).
Number of Documents: 854.
Embase Classic+Embase <1947 to 2022 November 17>
Ovid MEDLINE(R) ALL <1946 to November 17, 2022>
Publication Date Range
The search included citations from database inception to September 2022.
Hand Searching
Reference lists of included studies, relevant systematic reviews, and pertinent gray literature were also evaluated for eligible studies.
Process for Selecting Studies
All studies retrieved through the search strategy were uploaded to Covidence. 18 The pre-established criteria above were used by 2 reviewers to screen the titles and abstracts of the citations identified through our searches (MAA and AQ). Any citation deemed not relevant for full-text review was reviewed by a second researcher to assure accuracy and completeness. Each full-text article was independently reviewed for eligibility by 2 team members (MAA and NH). Any disagreements were resolved by consensus. A record of studies excluded at the full-text level with reasons for exclusion was maintained (supplemental material).
Some of the included studies presented data for more than one type of IONM modality. For example, a study utilizing multimodal neuromonitoring presented data for SSEP, MEP and EMG separately, and all of these were included in their respective groups. Moreover, some studies reported data using different thresholds to define neurologic injury. For example, a study presented data for MEP using both a 75% and 50% threshold and both were included.
Data Abstraction and Data Management
Abstraction of information related to the key question was limited to information needed to answer the questions. General patient characteristics, relevant surgical information, characteristics of neurophysiological monitoring (including any thresholds) as well as metrics of diagnostic accuracy were abstracted.
After studies were selected for inclusion for the key question, standardized data abstraction included the following (at minimum): patient characteristics (age, sex, comorbidities), completeness (AIS) and level of spinal cord injury (SCI), indication for spine surgery (eg, scoliosis, tumor), clinical/pathology characteristics (eg, myelopathy), surgical procedure characteristics (eg approach, levels, instrumentation), adjunctive treatments (eg, steroids, vasopressors), study-related characteristics (eg, sample size, design, control of confounding, timing of follow-up), intervention characteristics (eg, type, such as MEP, SEPP, timing, thresholds) and outcomes with a focus on the primary outcomes related to neurological recovery and adverse events listed in Table 1.
Assessment of Methodological Risk of Bias of Individual Studies
The risk of bias (ROB) and applicability of included studies was assessed using the quality assessment tool for diagnostic accuracy studies (QUADAS-2). 19 Four primary domains make up QUADAS-2:
• Patient Selection
• Index Test
• Reference Standard
• Flow and Time
Each domain is evaluated for risk of bias, and the first 3 are evaluated for issues about application. Signaling questions are offered to help with the assessment of bias risk. We also created traffic light and summary plots to illustrate risk of bias for each study using the robvis tool.20,21 Each study was classified as either “low risk, some concerns, or high risk.”
Data Synthesis and Statistical Analysis
The summary statistics and summary line from 4 sets of fundamental data—TP, FP, FN and TN—were used to describe the DTA. Sensitivity, specificity, diagnostic odds ratio (DOR), forest plot, and summary receiver operating characteristic (SROC) curve are examples of representative summary statistics and summary curves, respectively. Sensitivity is calculated using the formula (TP/(TP+FN)), while specificity is calculated using the formula (TN/(TN+FP)). Logit-transformed data are more frequently used than raw data for such proportion-type data. The logit transformation is a technique for modifying the distribution of data in accordance with statistical hypotheses. The lowest and upper bounds of the proportion-type data are 0 and 1, respectively. Their upper and lower limits should be freed by conducting multiplication and log transformations, respectively, to make the data suitable for the assumptions of statistics.
As with pairwise meta-analysis, a suitable model should be chosen in order to determine the DTA's summary statistics. The Moses-Littenberg SROC model,22,23 the bivariate model, 24 and the hierarchical SROC (HSROC) model 25 are examples of models that take both sensitivity and specificity into account. The Moses-Littenberg model, a relatively straightforward approach developed early on to compute DTA, uses simple linear regression to estimate the SROC. This is comparable to the fixed-effect model used in pairwise meta-analysis and is unable to evaluate study heterogeneity. Additionally, because this model just offers the SORC curve without providing parameter estimates, standard deviation, or confidence intervals, it can only perform restricted analysis and cannot discriminate between within-study and between-study variations in any variations (CIs). The bivariate model and HSROC model were created based on the hierarchical model to address the shortcomings of the Moses-Littenberg model. 26 When there is no covariate, these 2 models mathematically provide the same value.27,28 This is comparable to the pairwise meta-analysis random-effect model. Both models are capable of estimating the heterogeneity, or the variation of studies both within and between studies. In the bivariate model, the sensitivity and specificity for within-study variations are directly modeled by a binominal distribution, while the sensitivity and specificity for between-study variations are assumed by a bivariate normal distribution. Therefore, we followed a bivariate model for performing pooled DTA analysis. Analyses were performed on R-Studio using the “mada”, 29 “mvtnorm”, 30 “ellipse”, 31 “mvtmeta”, 32 “meta”, 33 “metafor”, 34 “rmeta” 35 packages.
Publication Bias Assessment
DTA meta-analyses differ from conventional intervention meta-analysis in a number of ways, making it more difficult to estimate the likelihood of publication bias. The Egger test is a statistical method for identifying funnel plot asymmetry in conventional meta-analysis. 36 In order to test the global null hypothesis that “all of the univariate funnel plots for multiple outcomes are symmetric,” Hong et al (2020) first proposed an expanded version of this test for multivariate meta-analysis. 37 In comparison to the common univariate publication bias test, this overall test contains various outcome information, and the statistical power is often increased. The Hong's test (also known as MSSET) avoids correlation data among various outcomes that are occasionally absent under some circumstances of multivariate meta-analysis. However, for DTA meta-analysis, the Reitsma's bivariate meta-analysis model has all of the correlation data, and since MSSET does not make use of this data, its statistical power may be wasteful. 24 For the same global null hypothesis, Noma (2020) created alternative generalized Egger tests that successfully take into account the correlation data (called as MSSET2 and MSSET3). Because Noma's tests make use of correlation data, it is anticipated that they will have greater statistical power than the MSSET when applied to DTA meta-analysis. 38 Using this information, we used the “MVPBT” package in R to compute funnel plots and perform statistical tests for asymmetry. 39
Grading the Strength of Evidence for Major Comparisons and Outcomes
The overall quality (strength) of evidence (SOE) for the primary (critical) outcomes of neurological recovery and adverse events was assessed based on the application of GRADE, particularly for DTA. 40 TSOE was initially evaluated by one methodologist and reviewed independently by a second for consistency and validity before the final assessment. Disagreements were resolved by consensus. For a DTA evidence synthesis, RCT and observational prospective/retrospective studies were initially considered to be high quality of evidence; however, the evidence was downgraded based on the aggregate assessment of risk of bias across studies reporting on the outcome, consistency, imprecision, directness, and publication bias. Comparative observational studies begin as low quality of evidence. There are also situations where the observational evidence may be upgraded (eg, large magnitude of effect, presence of dose-response relationship or existence of plausible unmeasured confounders) as described in the AHRQ Methods Guide. 41
The sSOE was computed for the main diagnostic groups (SSEP, MEP, EMG and multimodal) and also for subgroups. The SOE was assigned an overall grade of high, moderate, low, or very low according to a four-level scale by evaluating and weighing the combined results of the above domains (Table 3).
Table 3.
Description of the Quality (Strength) of Evidence Grades.
| Strength of evidence | Description |
|---|---|
| High | We are very confident that the estimate of risk lies close to the true effect for this outcome. The body of evidence has few or no deficiencies. We believe that the findings are stable, ie, another study would not change the conclusions |
| Moderate | We are moderately confident that the estimate of risk lies close to the true effect for this outcome. The body of evidence has some deficiencies. We believe that the findings are likely to be stable, but some doubt remains |
| Low | We have limited/low confidence that the estimate of risk lies close to the true effect for this outcome. The body of evidence has major or numerous deficiencies (or both). We believe that additional evidence is needed before concluding either that the findings are stable or that the estimate of effect is close to the true effect |
| Very low | We have extraordinarily little confidence in the estimate for this outcome. The body of evidence has unacceptable deficiencies |
Results
Study Selection
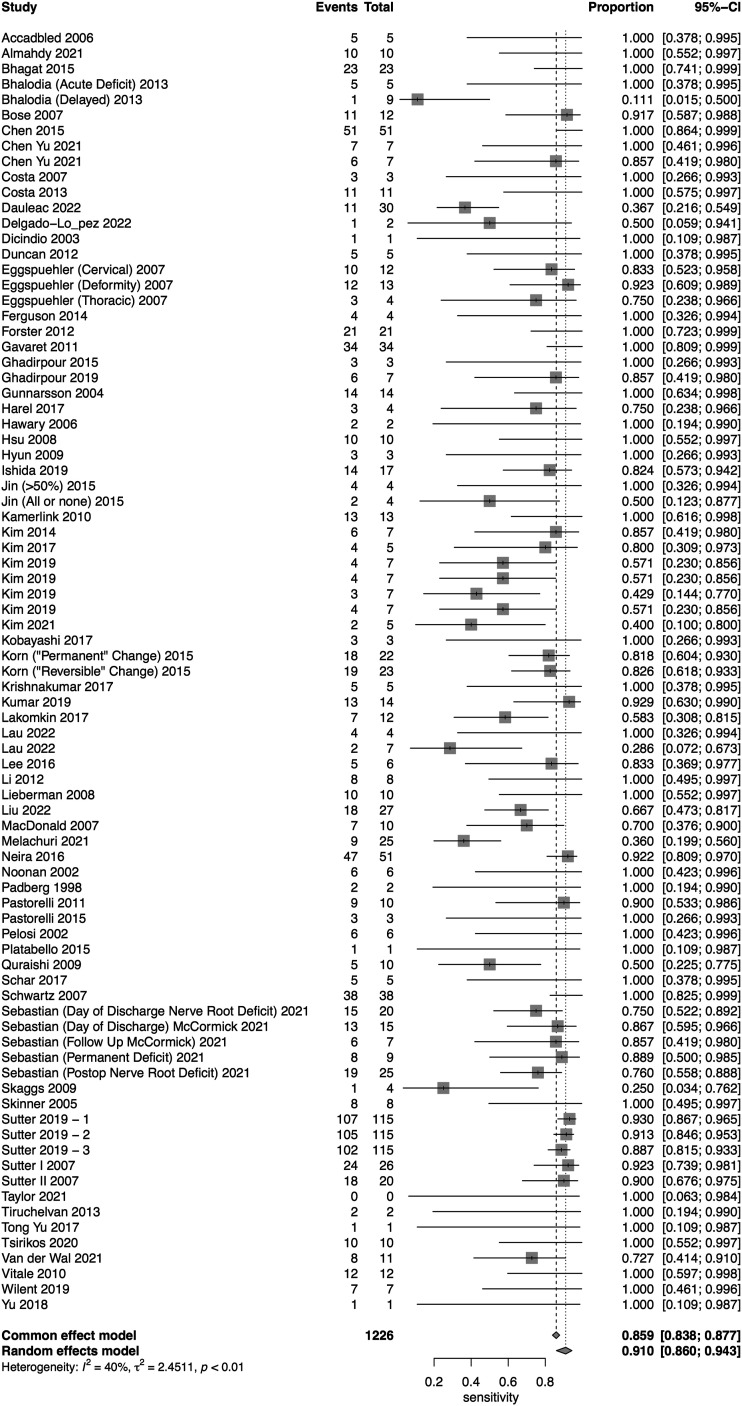
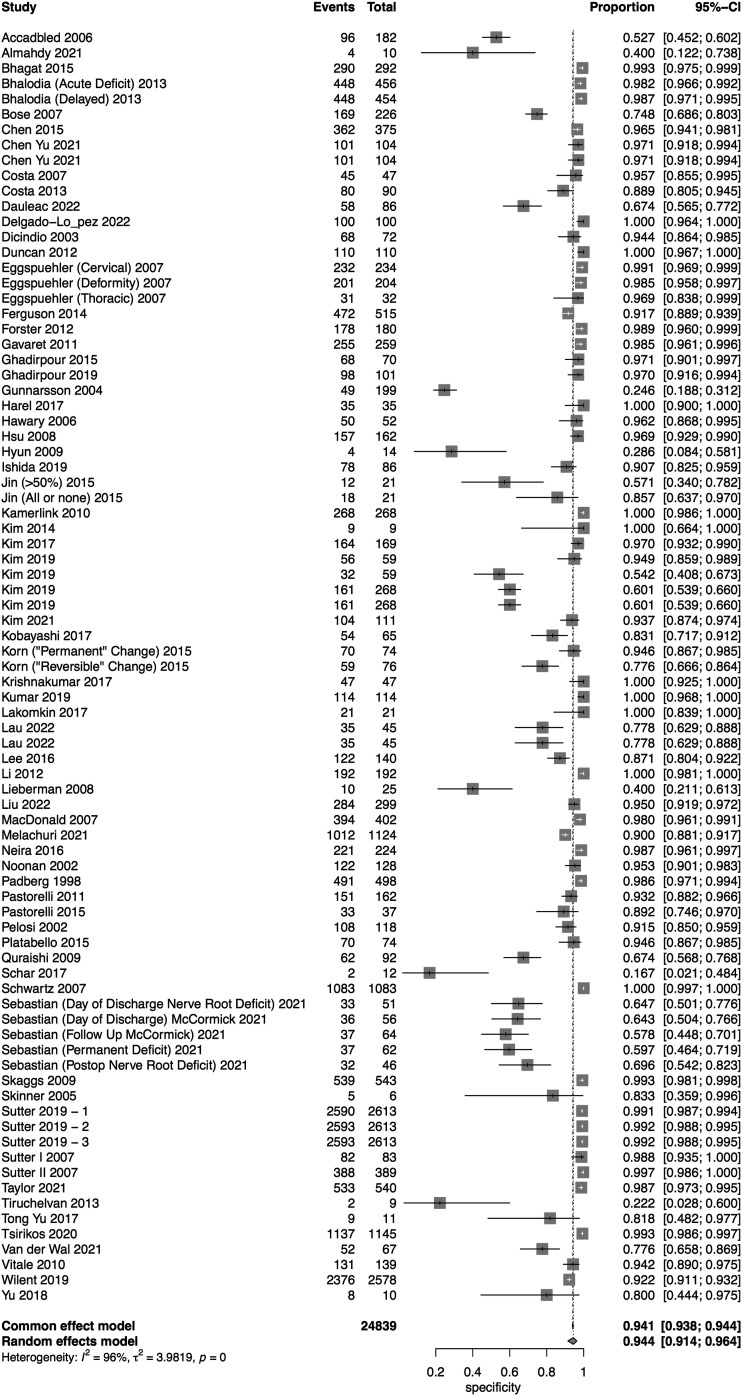
The search strategy using EMBASE, MEDLINE and SCOPUS yielded a total of 2270 articles after removing 305 duplicates. Of these, 1915 abstracts were considered irrelevant. The full texts of the remaining 355 articles were reviewed. Of these, 189 were excluded. A flowchart summarzing the selection of studies is provided in Figure 1. Details related to excluded studies, including reasons for exclusion, are presented in Supplementary Table 1. A total of 164 studies2,5,42–52, 52–62, 62–72, 72–82, 82–92, 92–102, 102–112, 112–122, 122–132, 132–142, 142–152, 152–162, 162–172, 172–182, 182–192, 192–203 consisting of 99937 patients were included. Of the 164 studies, 16 (9.75%) were prospective while 148 (90.25%) were retrospective. In terms of disease group, most studies included patients with mixed pathology (29.87%, n = 49), followed by deformity (26.83%, n = 44), degenerative disease (21.95%, n = 36), tumors (17.68%, n = 29), trauma (1.83%, n = 3), congenital diseases (1.2%, n = 2) and AVM (.6%, n = 1). Most studies featured centers/hospitals from the United States (35.36%, n = 58), followed by Japan (15.85%, n = 26), China (9.1%, n = 15), Korea, UK (5.5% each, n = 9), Canada, Switzerland (4.9% each, n = 8), and others. Several studies consisted of only adult patients (50%, n = 82), while others included both adolescent and adult patients (34.7%, n = 57) or only adolescent patients (9.1%, n = 15). Ten studies (6%) did not specify patient age. Of the 164 studies, 52 studies (31.7%) presented data for SSEP, 75 studies (45.7%) presented data for MEP, 16 studies (9.75%) presented data for EMG, and 69 studies (42.07%) presented data for multimodal neuromonitoring. These study characteristics are summarized in Table 4.
Figure 1.
PRISMA-DTA Flowchart for selection of studies.
Table 4.
Characteristics of included studies.
| First author and year | Study type | Country/Region | Total patient count | Disease type | Procedures | Population (adult, adolescent or both) | Neuromonitoring type | Data presented for |
|---|---|---|---|---|---|---|---|---|
| Abdelkader 2019 | Prospective | Egypt | 50 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Extramedullary manipulations, decompression of an epidural abscess or neoplasm, removal of intramedullary tumor or arteriovenous malformation, or spine correction procedures | Both | SSEP | SSEP |
| Accadbled 2006 | Retrospective | France | 191 | Deformity | Corrective surgery | Both | Multimodal: SSEP and MEP | Multimodal |
| Almahdy 2021 | Retrospective | USA | 50 | Mixed: Tumor, abscess, AVM, degenerative (stenosis, disc), trauma | Non- instrumented posterior cervical (C3-6) laminectomy, tumor resection | Both | Multimodal: SSEP and MEP | Multimodal |
| Avila 2013 | Retrospective | USA | 208 | Tumor: Metastasis | Tumor resection | Both | Multimodal: SSEP, MEP, EMG | MEP |
| Bhagat 2015 | Retrospective | UK | 354 | Deformity | Corrective surgery | Both | Multimodal: SSEP and MEP | Multimodal, MEP, SSEP |
| Bhalodia 2013 | Retrospective | USA | 229 | Mixed | Cervical spine operations involving the C-4 and/or C-5 levels | Adult | Multimodal: SSEP, MEP and EMG | Multimodal, MEP |
| Bir 2021 | Retrospective | India | 31 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Not specified | Adult | Multimodal: MEP, D-wave | MEP |
| Bose 2002 | Retrospective | USA | 61 | Degenerative | Lumbar fusion | Adult | EMG | EMG |
| Bose 2007 | Retrospective | USA | 238 | Degenerative | Anterior cervical spinal procedures included single-level discectomies, multilevel discectomies, corpectomies, and stabilization of odontoid fractures | Adult | Multimodal: SSEP, MEP and EMG | Multimodal, MEP, SSEP, EMG |
| Calancie 1998 | Retrospective | USA | 34 | Mixed: Tumor, spinal deformity (scoliosis, kyphosis, or displaced fracture), arteriovenous fistula, tethered cord | Not specified | Both | Multimodal: MEP and EMG | MEP |
| Calancie 2008 | Retrospective | USA | 859 | Mixed: Tumor, tethered cord, orthopedic, vascular and cyst | Decompression and/or stabilization of the cervical, thoracic, or thoracolumbar spine | Adult | Multimodal: MEP and EMG | Multimodal, MEP |
| CaminoWillhuber 2021 | Retrospective | Argentina | 137 | Degenerative | Percutaneous cement discoplasty | Adult | EMG | EMG |
| Chandanwale 2008 | Retrospective | India | 112 | Trauma | Thoracic or lumbar surgery | Both | SSEP | SSEP |
| Chen 2015 | Retrospective | China | 426 | Mixed: Degenerative, deformity and tumor | Unclear | Both | Multimodal: SSEP and MEP | Multimodal |
| Chen Yu 2021 | Retrospective | China | 113 | Degenerative | TLIF | Adult | Multimodal: SSEP, MEP and EMG; multimodal: MEP and EMG | Multimodal, MEP, EMG, SSEP |
| Choi 2014 | Retrospective | Korea | 76 | Tumor: Intramedullary | Not specified | Adult | Multimodal: SSEP and MEP | MEP |
| Chung 2009 | Prospective | USA | 230 | Degenerative | Lumbar decompression, discectomy and fusion | Adult | SSEP | SSEP |
| Clark 2013 | Retrospective | USA | 140 | Degenerative | Anterior or posterior cervical surgery | Adult | MEP | MEP |
| Clark 2016 | Retrospective | USA | 144 | Mixed: Tumor, infection, trauma, inflammatory | Anterior or posterior cervical surgery | Adult | MEP | MEP |
| Cornips 2017 | Retrospective | Netherlands | 77 | Degenerative: Thoracic disc herniation | Anterior transthoracic surgery | Adult | MEP | MEP |
| Costa 2007 | Retrospective | Italy | 52 | Mixed: Trauma, tumor, degenerative (spondylosis), scoliosis, AVM, echinicoccus | Not specified | Adult | Multimodal: SSEP and MEP | Multimodal |
| Costa 2013 | Retrospective | Italy | 101 | Mixed: Tumor (intra and extra medullary) and degenerative | Not specified | Both | Multimodal: SSEP and MEP | Multimodal |
| Dauleac 2022 | Retrospective | France | 99 | Mixed: Tumor, degenerative, tethered cord | Not specified | Adult | Multimodal:SSEP and MEP | Multimodal |
| Delgado-lópez 2022 | Retrospective | Spain | 103 | Degenerative | Anterior cervical surgery | Adult | Multimodal: SSEP and MEP | Multimodal |
| Deutsch 2000 | Retrospective | USA | 44 | Tumor | Anterior thoracic | Adult | SSEP | SSEP |
| Dicindio 2003 | Retrospective | USA | 68 | Deformity | Not specified | Adolescent | Multimodal: SSEP and MEP | Multimodal |
| Ding 2008 | Retrospective | China | 76 | Degenerative | ACDF and laminoplasty | Adult | SSEP | SSEP |
| Dinner 1986 | Retrospective | USA | 220 | Mixed | Not specified | Adult | SSEP | SSEP |
| Duncan 2012 | Retrospective | USA | 115 | Degenerative | Lumbar fusion | Adult | Multimodal: SSEP, MEP and EMG | Multimodal, SSEP |
| Eggspuehler (cervical) 2007 | Retrospective | Switzerland | 246 | Degenerative | Cervical surgery | Adult | Multimodal: SSEP, MEP and EMG | Multimodal |
| Eggspuehler (deformity) 2007 | Retrospective | Switzerland | 217 | Deformity | Corrective surgery | Adult | Multimodal: SSEP, MEP and EMG | Multimodal |
| Eggspuehler (thoracic) 2007 | Retrospective | Switzerland | 36 | Degenerative | Thoracic surgery | Adult | Multimodal: SSEP, MEP and EMG | Multimodal |
| Feng 2012 | Retrospective | China | 176 | Deformity | Corrective surgery | Both | Multimodal:SSEP and MEP | MEP |
| Ferguson 2014 | Retrospective | USA | 519 | Deformity | Corrective surgery | Adult | Multimodal:SSEP and MEP | Multimodal |
| Feyissa 2015 | Retrospective | USA | 19 | Tumor | Thoracic Laminectomy, Facetectomy, vertebrectomy | Adult | SSEP | SSEP |
| Forster 2012 | Retrospective | Germany | 203 | Tumor | Not specified | Adult | Multimodal: SSEP and MEP | Multimodal, MEP |
| Fujiwara 2016 | Retrospective | Japan | 160 | Degenerative | Laminoplasty | Adult | MEP | MEP |
| Funaba 2021 | Retrospective | Japan | 1176 | Degenerative | Laminoplasty, ACDF, posterior fusion, circumferential fusion | Adult | MEP | MEP |
| Funaba 2022 | Retrospective | Japan | 2476 | Mixed: Tumor, infection, trauma, deformity, inflammatory | Decompression, fusion with instrumentation | Both | MEP | MEP |
| Gavaret 2011 | Retrospective | France | 300 | Mixed: Trauma, tumor, degenerative (spondylosis), scoliosis | Not specified | Both | Multimodal: SSEP and D-wave | SSEP, multimodal |
| Ghadirpour 2015 | Retrospective | Italy | 68 | Tumor: Extramedullary | Tumor resection | Adult | Multimodal: SSEP, MEP and D-wave | Multimodal |
| Ghadirpour 2019 | Retrospective | Italy | 108 | Tumor | Not specified | Adult | Multimodal: SSEP MEP and D-wave | Multimodal |
| Gundanna 2003 | Retrospective | USA | 186 | Mixed: Degenerative, scoliosis | Posterior fusion | Adult | SSEP | SSEP |
| Gunnarsson 2004 | Retrospective | Canada | 213 | Mixed: Tumor, spinal deformity (scoliosis, kyphosis, or displaced fracture), degenerative, tethered cord | Decompression, microdiscectomy, tumor resection/removal, tethered cord release, others, instrumented fusions | Adult | Multimodal: SSEP and EMG | Multimodal |
| Harel 2017 | Retrospective | Israel | 41 | Tumor | Not specified | Adult | Multimodal: SSEP, MEP and EMG | Multimodal |
| Hawary 2006 | Retrospective | Canada | 41 | Deformity | Decompression, release, resection | Adolescent | Multimodal: SSEP and MEP | Multimodal |
| Hilibrand 2004 | Retrospective | USA | 427 | Degenerative | Anterior, posterior and circumferential cervical surgery | Adult | Multimodal: SSEP and MEP | MEP |
| Hsu 2008 | Retrospective | Australia | 144 | Deformity | Correction | Both | MEP and CMAP | Multimodal, MEP |
| Hu 2011 | Retrospective | Hong Kong | 191 | Mixed | Not specified | Adult | SSEP | SSEP |
| Huang 2016 | Retrospective | China | 89 | Deformity | Not specified | Both | SSEP | SSEP |
| Hyun 2009 | Retrospective | Korea | 17 | Tumor | Not specified | Adult | Multimodal: SSEP and MEP | Multimodal, MEP |
| Ille 2021 | Retrospective | Germany | 71 | Tumor | Not specified | Adult | Multimodal: SSEP, MEP and EMG | Multimodal, MEP |
| Ishida 2019 | Retrospective | USA | 103 | Tumor | Not specified | Adult | Multimodal: SSEP, MEP and EMG | Multimodal |
| Ito 2012 | Retrospective | Japan | 884 | Mixed: Tumor, degenerative (spondylosis), scoliosis | Not specified | Not specified | MEP | MEP |
| Ito 2013 | Retrospective | Egypt | 295 | Mixed | Not specified | Adult | Multimodal: SSEP and MEP | MEP, SSEP |
| Izumi 1993 | Retrospective | Japan | 20 | Tumor | Not specified | Not specified | D-wave | MEP |
| Jarvis 2013 | Retrospective | Canada | 37 | Deformity | 3-Column posterior spinal osteotomies | Adolescent | Multimodal: SSEP and MEP | MEP |
| Jin 2015 | Retrospective | Korea | 30 | Tumor | Not specified | Adult | Multimodal: MEP and EMG | Multimodal |
| Kamerlink 2010 | Retrospective | USA | 281 | Deformity | Spinal fusion | Both | Multimodal: SSEP and MEP | Multimodal |
| Kelleher 2008 | Prospective | Canada | 1055 | Mixed | Not specified | Adult | Multimodal: SSEP, MEP and EMG | SSEP, MEP |
| Khan 2006 | Retrospective | USA | 508 | Mixed | Cervical corpectomy | Adult | SSEP | SSEP |
| Kim 2007 | Retrospective | USA | 52 | Degenerative | Cervical ACDF, laminectomy, posterior fusion | Adult | MEP | MEP |
| Kim 2012 | Retrospective | Korea | 209 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Not specified | Adult | MEP | MEP |
| Kim 2014 | Retrospective | Korea | 22 | Tumor: Intramedullary | Not specified | Adult | Multimodal: SSEP and MEP | Multimodal |
| Kim 2017 | Retrospective | Korea | 200 | Degenerative | ACDF | Adult | Multimodal: SSEP and MEP | Multimodal |
| Kim 2019 | Retrospective | USA | 275 | Degenerative | TLIF | Adult | Multimodal: SSEP, MEP and EMG | Multimodal, MEP, EMG, SSEP |
| Kim 2021 | Retrospective | Korea | 196 | Degenerative | ACDF | Adult | Multimodal: SSEP, MEP and EMG | Multimodal, MEP, EMG, SSEP |
| Kobayashi 2014 | Retrospective | Japan | 959 | Mixed: Degenerative (stenosis, disc, OPLL), deformity (scoliosis), tumor | Not specified | Not specified | MEP | MEP |
| Kobayashi 2017 | Prospective | Japan | 68 | Deformity | Corrective surgery | Adolescent | MEP | MEP |
| Kobayashi 2018 | Retrospective | Japan | 83 | Mixed: Degenerative (stenosis, disc, OPLL), deformity (scoliosis), tumor | Not specified | Both | MEP | MEP |
| Kobayashi 2018 | Retrospective | Japan | 70 | Mixed: Degenerative (stenosis, disc, OPLL), deformity (scoliosis), tumor | Not specified | Both | Multimodal: SSEP, MEP and D-wave | MEP, multimodal |
| Kobayashi 2019 | Retrospective | Japan | 159 | Mixed: Degenerative (stenosis, disc, OPLL), tumor | Thoracic surgery | Both | MEP | MEP |
| Kobayashi 2021 | Prospective | Japan | 3560 | Mixed: Degenerative (stenosis, disc, OPLL), deformity (scoliosis), tumor | Not specified | Both | MEP | MEP |
| Kobayashi 2021 | Prospective | Japan | 233 | Tumor: Intramedullary | Not specified | Both | MEP | MEP |
| Kobayashi 2022 | Prospective | Japan | 3625 | Mixed: Degenerative (stenosis, disc, OPLL), deformity (scoliosis), tumor | Not specified | Both | MEP | MEP |
| Koffie 2022 | Retrospective | USA | 498 | Degenerative | Posterior cervical laminectomy or laminoplasty with or without fixation and fusion | Adult | Multimodal: SSEP and MEP | MEP, SSEP |
| Korn 2015 | Retrospective | Israel | 100 | Tumor: Extramedullary | Not specified | Both | Multimodal: SSEP, MEP and EMG | Multimodal |
| Krishnakumar 2011 | Retrospective | India | 52 | Deformity | Not specified | Both | Multimodal: SSEP and MEP | Multimodal, SSEP, MEP |
| Kumar 2019 | Retrospective | UK | 128 | Tumor: Metastasis | Not specified | Adult | Multimodal: SSEP, MEP and EMG | Multimodal |
| Kundnani 2010 | Prospective | Singapore | 354 | Deformity | Corrective surgery | Adolescent | Multimodal: SSEP and MEP | MEP |
| Kurokawa 2017 | Retrospective | Japan | 58 | Tumor: Intramedullary | Not specified | Both | MEP | MEP |
| Lakomkin 2017 | Retrospective | USA | 52 | Tumor | Not specified | Both | Multimodal: SSEP and MEP | Multimodal |
| Langeloo 2003 | Retrospective | Netherlands | 145 | Deformity | Anterior or posterior surgery | Both | MEP | MEP |
| Lau 2019 | Retrospective | USA | 242 | Deformity | Lumbar pedicle subtraction osteotom | Adult | MEP | MEP |
| Lau 2022 | Retrospective | USA | 56 | Deformity | Lower cervical and upper thoracic posterior-based three-column osteotomies | Adult | Multimodal: SSEP, MEP and EMG | Multimodal |
| Lee 2016 | Retrospective | Korea | 146 | Mixed: Degenerative, congenital, tumor, trauma | Goel's technique: Intraoperative CVJ realignment through a rod and screw system | Adult | Multimodal: SSEP and MEP | Multimodal |
| Leung 2005 | Retrospective | UK | 871 | Deformity | Anterior surgery | Not specified | Multimodal: SSEP and MEP | SSEP |
| Li 2012 | Retrospective | USA | 200 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Anterior cervical surgery | Adult | Multimodal: SSEP, MEP and EMG | Multimodal |
| Li 2018 | Retrospective | China | 55 | AVM | Not specified | Both | Multimodal: SSEP, MEP and EMG | Multimodal, MEP, SSEP |
| Lieberman 2008 | Retrospective | USA | 35 | Deformity | Corrective surgery | Both | Multimodal: MEP, D-wave | MEP, multimodal |
| Liu 2022 | Retrospective | China | 326 | Degenerative | Posterior thoracic Decompression surgery |
Adult | Multimodal: SSEP and MEP | Multimodal |
| Loder 1991 | Retrospective | USA | 52 | Deformity | Not specified | Both | SSEP | SSEP |
| Lubitz 1999 | Retrospective | USA | 60 | Deformity | Not specified | Adolescent | MEP | MEP |
| Luk 2001 | Retrospective | Hong Kong | 30 | Deformity | Not specified | Adolescent | Multimodal: CSEP, SSEP, MEP | MEP, SSEP |
| MacDonald 2007 | Retrospective | Saudi Arabia | 206 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Thoracolumbar surgeries | Both | Multimodal: SSEP and MEP | Multimodal |
| Makarov 2012 | Retrospective | USA | 233 | Deformity | External fixation procedures | Adolescent | SSEP | SSEP |
| Manninen 1998 | Retrospective | Canada | 309 | Mixed: Tumor, abscess, degenerative (stenosis, disc), trauma | Corrective surgery, laminectomy, fusion | Both | SSEP | SSEP |
| Melachuri 2017 | Retrospective | USA | 1036 | Degenerative | Posterior spinal fusions | Adult | SSEP | SSEP |
| Melachuri 2019 | Retrospective | USA | 1057 | Degenerative | Lumbar interbody fusions | Adult | SSEP | SSEP |
| Melachuri 2020 | Retrospective | USA | 771 | Mixed | Thoracic decompression and fusion | Adult | SSEP | SSEP |
| Melachuri 2021 | Retrospective | USA | 179 | Mixed | Mixed | Adult | Multimodal: SSEP and EMG | Multimodal, EMG |
| May 2006 | Retrospective | UK | 182 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Anterior or posterior cervical surgery | Adult | SSEP | SSEP |
| Meyer 1988 | Retrospective | USA | 150 | Trauma | Thoracic and lumbar spine internal fixation | Both | SSEP | SSEP |
| Miller 2019 | Retrospective | USA | 125 | Deformity | Not specified | Adult | MEP | MEP |
| Mochida 1997 | Retrospective | Japan | 32 | Mixed: Tumor, AVM, degenerative (OPLL), tethered cord | Thoracic and thoracolumbar surgery | Not specified | MEP | MEP |
| Muramoto 2013 | Retrospective | Japan | 80 | Mixed: Degenerative, tumor, trauma, deformity | Thoracic spine surgery | Adult | Multimodal: SSEP, MEP and D-wave | MEP |
| Muramoto 2014 | Retrospective | Japan | 37 | Tumor: Intramedullary | Various | Both | MEP | MEP |
| Neira 2016 | Retrospective | Canada | 296 | Deformity | Spinal fusion | Adolescent | Multimodal: SSEP and MEP | Multimodal, SSEP |
| Noonan 2002 | Retrospective | USA | 165 | Deformity | Corrective surgery | Adolescent | Multimodal: SSEP and MEP | Multimodal |
| Noordeen 1997 | Retrospective | UK | 99 | Deformity | Not specified | Adolescent | SSEP | SSEP |
| Oya 2017 | Retrospective | USA | 135 | Degenerative | Laminoplasty | Adult | Multimodal: SSEP, MEP and EMG | EMG, MEP |
| Padberg 1996 | Retrospective | USA | 74 | Mixed: Degenerative, congenital, trauma | Anterior, posterior and circumferential fusion | Both | SSEP | SSEP |
| Padberg 1998 | Retrospective | USA | 500 | Deformity | Instrumentation | Both | Multimodal: SSEP and MEP | Multimodal |
| Papastefanou 2000 | Retrospective | UK | 442 | Mixed: Degenerative, trauma, tumor, deformity | Not specified | Both | SSEP | SSEP |
| Paradiso 2006 | Prospective | Canada | 44 | Adult tethered cord | Detethering procedures | Adult | Multimodal: SSEP and EMG | EMG, SSEP, MEP |
| Park 2011 | Retrospective | USA | 29 | Deformity | Anterior, posterior and circumferential fusion | Adult | Multimodal: SSEP, MEP and EMG | EMG, SSEP, MEP |
| Park 2018 | Retrospective | Korea | 34 | Tumor: Intramedullary | Not specified | Both | Multimodal: SSEP, MEP and EMG | SSEP, MEP |
| Pastorelli 2011 | Retrospective | Italy | 172 | Deformity | Posterior fusion with instrumentation surgery, anterior spinal Fusion by thoracotomy, combined anterior and posterior Approach | Adult | Multimodal: SSEP and MEP | Multimodal |
| Pastorelli 2015 | Retrospective | Italy | 40 | Deformity | Posterior fusion with instrumentation surgery | Adolescent | Multimodal: SSEP and MEP | Multimodal |
| Pelosi 2002 | Retrospective | UK | 97 | Mixed: Degenerative, trauma, deformity, infection | Anterior or posterior surgery | Both | Multimodal: SSEP and MEP | Multimodal |
| Platabello 2015 | Retrospective | Spain | 75 | Mixed: Degenerative, tumor, trauma | Anterior and posterior cervical | Adult | Multimodal: SSEP, MEP and EMG | Multimodal |
| Qiu 2022 | Retrospective | China | 87 | Chiari malformation, syringomyelia, split-cord malformation, and tethered cord syndrome | Posterior spinal fusion | Both | Multimodal: SSEP and MEP | SSEP, MEP |
| Quinones 2005 | Retrospective | USA | 28 | Tumor: Intramedullary | Not specified | Adult | MEP | MEP |
| Quraishi 2009 | Retrospective | Canada | 102 | Deformity | Instrumented fusion, Decompression, Osteotomies, PLIF, ALIF |
Adult | Multimodal: SSEP, MEP and EMG | Multimodal, EMG |
| Ruschel 2021 | Retrospective | Brazil | 47 | Tumor: Intramedullary | Not specified | Adult | Multimodal: SSEP, MEP and D-wave | EMG |
| Sakaki 2012 | Retrospective | Japan | 350 | Degenerative | ACDF, laminoplasty, laminectomy, posterior fusion | Not specified | MEP | MEP |
| Schar 2017 | Retrospective | Switzerland | 17 | Degenerative: L5–S1 isthmic spondylolisthesis | Instrumented transforaminal lumbar interbody fusion | Both | Multimodal: SSEP, MEP and EMG | Multimodal |
| Schwartz 2007 | Retrospective | USA | 38 | Deformity | Anterior, posterior and circumferential fusion | Both | Multimodal: SSEP and MEP | Multimodal |
| Skaggs 2009 | Retrospective | USA | 1736 | Deformity | Not specified | Both | Multimodal: SSEP and MEP | Multimodal |
| Skinner 2005 | Retrospective | USA | 13 | Tumor: Intramedullary | Not specified | Both | EMG | EMG |
| Smith 2006 | Retrospective | USA | 577 | Degenerative: Non-myelopathy:cervical stenosis, radiculopathy, herniated nucleus pulposus, junctional stenosis, Or nonunion from prior surgery | ACDF | Adult | SSEP | SSEP |
| Stechison 1995 | Retrospective | USA | 150 | Mixed: Degenerative, trauma, tumor, deformity | Various | Not specified | SSEP | SSEP |
| Sutter 2007 | Retrospective | Switzerland | 1017 | Mixed: Degenerative, deformity and tumor | Various | Both | Multimodal: SSEP, MEP and EMG | Multimodal, EMG |
| Sutter 2019 | Retrospective | Switzerland | 2728 | Mixed: Degenerative, deformity and tumor | Not specified | Both | Multimodal: SSEP, MEP and EMG | Multimodal, MEP |
| Sutter I 2007 | Retrospective | Switzerland | 109 | Tumor | Not specified | Both | Multimodal: SSEP, MEP and EMG | Multimodal |
| Sutter II 2007 | Retrospective | Switzerland | 409 | Degenerative | Not specified | Both | Multimodal: SSEP, MEP and EMG | Multimodal |
| Takahashi 2021 | Prospective | Japan | 1934 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Not specified | Adult | Multimodal: SSEP, MEP and D-wave | MEP |
| Tanaka 2015 | Retrospective | Japan | 196 | Degenerative | ACDF, laminoplasty, lumbar fusion, lumbar laminectomy, discectomy, fenestration | Not specified | MEP | MEP |
| Taylor 2021 | Prospective | USA | 540 | Degenerative | Cervical ACDF, laminectomy, posterior fusion | Not specified | Multimodal: SSEP and MEP | Multimodal |
| Thirumala 2014 | Retrospective | USA | 477 | Deformity | Corrective surgery | Adolescent | SSEP | SSEP |
| Tiruchelvarayan 2013 | Retrospective | Singapore | 13 | Tumor: Intramedullary | Laminectomy with or without fixation | Both | Multimodal: SSEP and MEP | Multimodal |
| Tohmeh 2022 | Prospective | USA | 59 | Mixed: Degenerative, deformity | Transpsoas lateral lumbar interbody fusion | Adult | SSEP | SSEP |
| Tong Yu 2017 | Prospective | China | 12 | Deformity | Corrective surgery | Adolescent | Multimodal: SSEP, MEP and EMG | Multimodal |
| Traba 2018 | Retrospective | Spain | 203 | Degenerative | TLIF, XLIF | Adult | Multimodal: SSEP, MEP and EMG | MEP |
| Tsirikos 2004 | Retrospective | UK | 82 | Trauma | Posterior fusion, cervical laminectomy, anterior corpectomy, ACDF | Both | SSEP | SSEP |
| Tsirikos 2020 | Retrospective | UK | 1155 | Deformity | Posterior closing wedge osteotomies | Both | Multimodal: SSEP and MEP | Multimodal, SSEP |
| Ushirozako 2018 | Retrospective | Japan | 295 | Deformity | Posterior corrective surgery, with 3-column osteotomies, with posterior column resections, after LLIF | Adult | MEP | MEP |
| Ushirozako 2019 | Retrospective | Japan | 703 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Not specified | Adult | MEP | MEP |
| Ushirozako 2020 | Retrospective | Japan | 393 | Deformity | Corrective surgery | Both | MEP | MEP |
| Ushirozako 2021 | Retrospective | Japan | 428 | Tumor | Not specified | Adult | MEP | MEP |
| Van der wal 2021 | Retrospective | Netherlands | 78 | Tumor: Extramedullary | Not specified | Both | Multimodal: SSEP and MEP | Multimodal |
| Velayutham 2016 | Retrospective | India | 300 | Tumor | Not specified | Both | MEP | MEP |
| Vitale 2010 | Retrospective | USA | 162 | Deformity | Not specified | Both | Multimodal: SSEP and MEP | Multimodal |
| Wang 2016 | Retrospective | China | 117 | Deformity | Not specified | Both | MEP | MEP |
| Wang 2016 | Retrospective | China | 86 | Degenerative | Posterior laminoplasty or laminectomy surgery | Both | MEP | MEP |
| Welch 1997 | Retrospective | USA | 32 | Degenerative | Lumbar fusion | Adult | EMG | EMG |
| Wilent 2019 | Retrospective | USA | 40919 | Degenerative | Extradural cervical surgery | Adult | Multimodal: SSEP, MEP and EMG | Multimodal, MEP |
| Wilent 2020 | Retrospective | USA | 4386 | Degenerative | Extradural posterior lumbosacra | Adult | Multimodal: SSEP, MEP and EMG | MEP, EMG, SSEP |
| Xu 2011 | Retrospective | USA | 57 | Degenerative | ACDF | Adult | Multimodal: SSEP and MEP | MEP, SSEP |
| Yang 1994 | Prospective | China | 11 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Various | Adult | Multimodal: SSEP and MEP | MEP, SSEP |
| Yoshida 2018 | Prospective | Japan | 2867 | Mixed: Degenerative (stenosis, disc, OPLL), trauma, deformity (scoliosis), tumor | Various | Not specified | MEP | MEP |
| Yoshida 2022 | Prospective | Japan | 311 | Deformity | ALIF, LLIF, PLIF, TLIF | Adult | MEP | MEP |
| Zhuang 2014 | Retrospective | China | 1162 | Deformity | Pedicle subtraction osteotomy, smith-petersen osteotomy, vertebral column resection | Both | Multimodal: SSEP and MEP | MEP |
| Zuccaro 2017 | Retrospective | USA | 806 | Deformity | Posterior spinal fusion, vertebral body stapling, vertebral column resection | Adolescent | Multimodal: SSEP and MEP | MEP |
SSEP
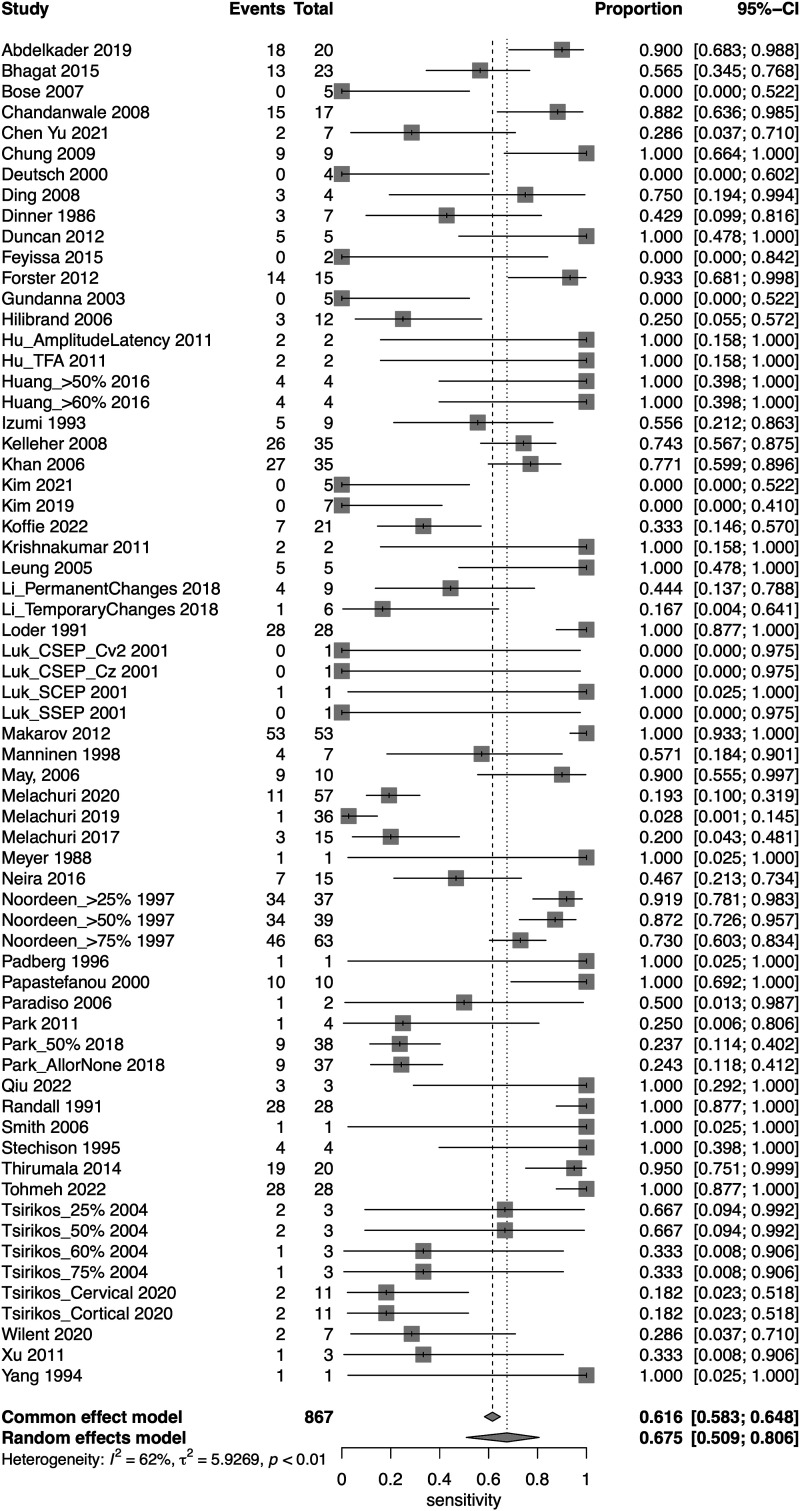
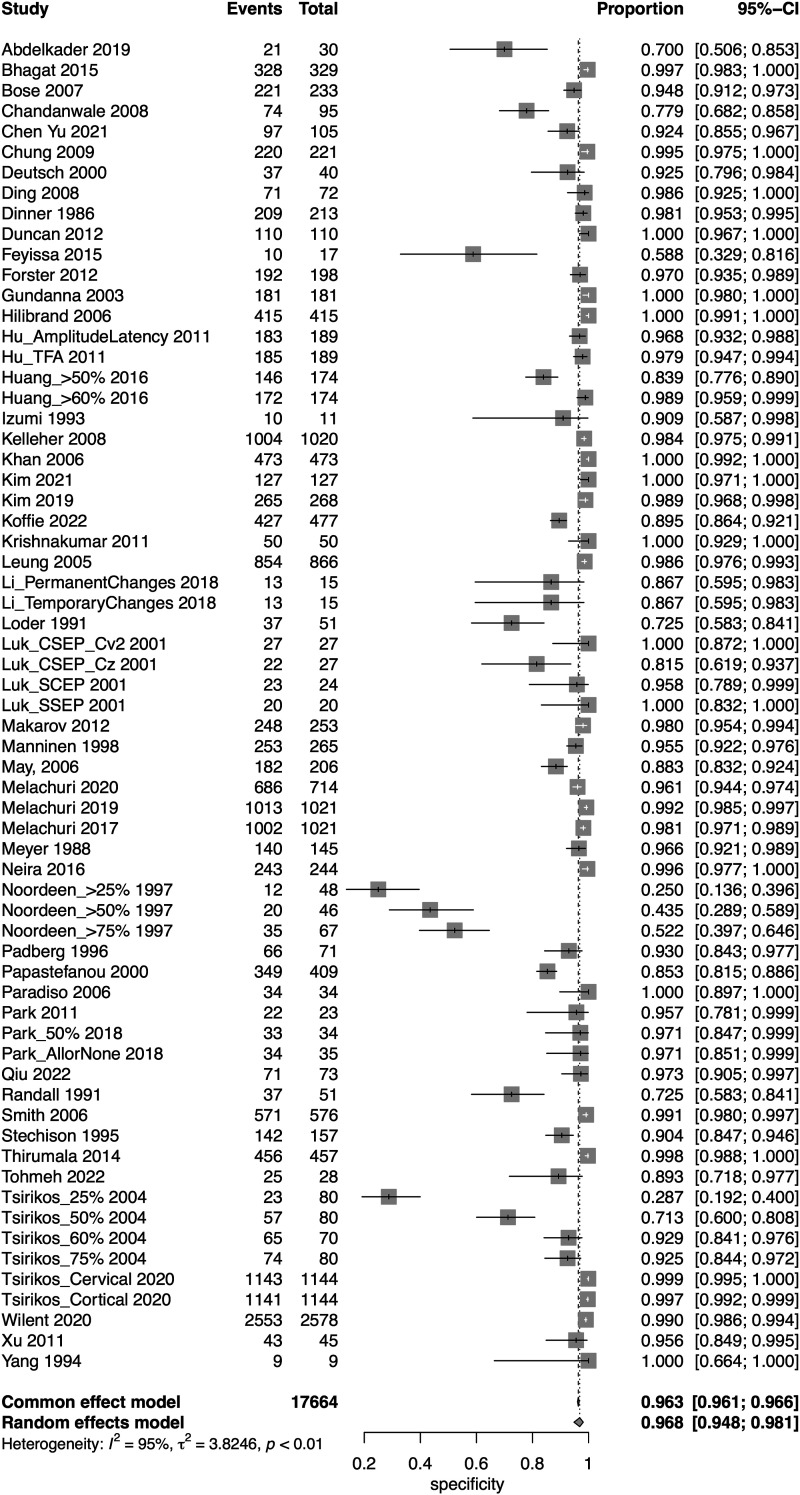
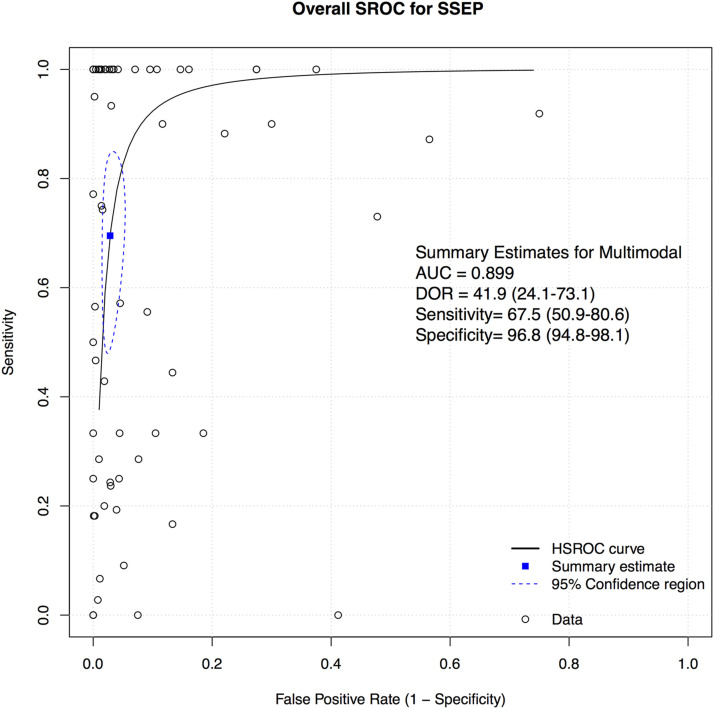
A total of 52 studies presented data for SSEP on a total of 18,076 patients. Overall, the sensitivity of SSEP was 67.5% (95% CI 50.9-80.6, Heterogeneity: I2 = 62%, τ2 = 5.9269, P < .01) (Figure 2), while the specificity was 96.8% (95% CI 94.8-98.1, Heterogeneity: I2 = 95%, τ2 = 3.8246, P < .01) (Figure 3). Overall, the AUC value was .899, while the DOR was 41.9 (95% CI 24.1-73.1) (Figure 4).
Figure 2.
Forest plot for sensitivity of SSEP.
Figure 3.
Forest plot for specificity of SSEP.
Figure 4.
Overall sROC plot for SSEP.
We also performed subgroup analysis for various thresholds for IONM alerts, different reported disease groups, and different regions.
Subgroup Analyses:
1. Thresholds:
The most commonly reported threshold for alert was 50% (n = 43 studies), followed by 60% (n = 4 studies), 25%, 75% (n = 2 studies each) and “all or none” (n = 1 study). Seven studies either reported a different threshold/alert criterion or did not report the actual alert criterion in explicit details; these were classified under “other”. The pooled sensitivities for the 25% threshold, 50% threshold, 60% threshold, 75% threshold, “all or none” alert and other threshold were 90% (95% CI 76.2-96.2, Heterogeneity: I2 = 38%, τ2 = 0, P = .20), 71.6% (95% CI 49.5-86.6 Heterogeneity: I2 = 62%, τ2 = 8.4139, P < .01, Heterogeneity: I2 = 0%, τ2 = 2.5549, P = .99), 62.9% (95% CI 12.9-95.1, Heterogeneity: I2 = 0%, τ2 = 2.55, P = .99), 71.2% (95% CI 59.2-80.8, Heterogeneity: I2 = 45%, τ2 = 0, P = .18), 24.3% (95% CI 11.8-41.2, Heterogeneity: I2 = 45%, τ2 = 0, P = .1) and 41.2% (95% CI 28.6-55, Heterogeneity: I2 = 0%, τ2 = 0, P = .93), respectively (Supplemental Figure 1(a)). The pooled specificities for the 25% threshold, 50% threshold, 60% threshold, 75% threshold, “all or none” alert and other threshold were 27.3% (95% CI 20.3-35.7, Heterogeneity: I2 = 0%, τ2 = 0, P = .65), 97.5% (95% CI 95.6-98.6, Heterogeneity: I2 = 94%, τ2 = 3.5177, P < .01), 98.5% (90.5-99.8, Heterogeneity: I2 = 48%, τ2 = 3.1291, P = .12), 78.4% (95% CI 11.5-88.4, Heterogeneity: I2 = 96%, τ2 = 1.4229, P < .01), 97.1% (95% CI 85.1-99.9) and 95.3% (95% CI 92.3-97.2, Heterogeneity: I2 = 73%, τ2 = .2627, P < .01), respectively (Supplemental Figure 1(b)).
2. Disease Group:
In terms of disease group, most studies presenting data for SSEP consisted of patients with mixed diseases (n = 15), followed by deformity (n = 14), degenerative diseases (n = 13), tumors (n = 5), trauma (n = 3), and others (n = 2). The pooled sensitivity for studies consisting of patients with mixed diseases was 52% (95% CI 26.5-76.5, Heterogeneity: I2 = 78%, τ2 = 3.9806, P < .01), for deformity it was 94% (95% CI 77.6-98.6, Heterogeneity: I2 = 3%, τ2 = 5.2433, P = .42), for degenerative it was 49.9% (95% CI 11.5-88.4 Heterogeneity: I2 = 0%, τ2 = 10.9146, P = .99), for tumor it was 33.2% (95% CI 9.2-70.9, Heterogeneity: I2 = 67%, τ2 = 2.7226, P = .01), for trauma it was 69.4% (95% CI 42.8-87.3, Heterogeneity: I2 = 11%, τ2 = .4518, P = .35), and for other diseases group, it was 35.3% (95% CI 16.8-59.6, Heterogeneity: I2 = 0%, τ2 = 0, P = .52) (Supplemental Figure 2(a)).
The pooled specificity for studies consisting of patients with mixed diseases was 97.6% (95% CI 94.2-99, Heterogeneity: I2 = 93%, τ2 = 2.8975, P < .01), for deformity it was 96% (95% CI 89.7-98.5, Heterogeneity: I2 = 95%, τ2 = 4.698, P < .01), for degenerative it was 99.1% (95% CI 97.6-99.7, Heterogeneity: I2 = 91%, τ2 = 2.1, P < .01), for tumor it was 93.5% (95% CI 83.4-97.6, Heterogeneity: I2 = 81%, τ2 = 1.16, P < .01), for trauma it was 83.4% (95% CI 42.8-87.3, Heterogeneity: I2 = 11%, τ2 = .4518, P = .35), and for other diseases group, it was 35.3% (95% CI 16.8-59.6, Heterogeneity: I2 = 0%, τ2 = .68, P = 1.00) (Supplemental Figure 2(b)).
The AUC for mixed pathology group, deformity, degenerative, tumor and trauma were .911, .908, .948, .791 and .744, respectively (Supplemental Figure 2(c)).
3. Regions
In terms of region of surgery, most studies presenting data for SSEP consisted of patients undergoing surgery for any region (n = 20), followed by surgery in the cervical spine (n = 11), lumbosacral (n = 9), thoracolumbar and cervicothoracic (n = 6 each) segments. The pooled sensitivity for studies consisting of patients with all regions was 77.2% (95% CI 60.4-88.3, Heterogeneity: I2 = 49%, τ2 = 3.27, P < .01), for cervical spine surgery it was 46.6% (95% CI 24.3-70.4, Heterogeneity: I2 = 59%, τ2 = 1.91, P < .01), for thoracolumbar spine surgery it was 99.1% (95% CI 29.1-100. Heterogeneity: I2 = 0%, τ2 = 16.9, P = .99), for lumbosacral it was 49.7% (95% CI 3.5-96.4, Heterogeneity: I2 = 0%, τ2 = 20.5, P = .68) and for cervicothoracic spine it was 24% (95% CI 17.8-31.5, Heterogeneity: I2 = 0%, τ2 = 0, P = .56) (Supplemental Figure 3(a)).
The pooled specificity for studies consisting of patients with surgery for any region was 95.5% (95% CI 90.5-97.9, Heterogeneity: I2 = 95%, τ2 = 4.57, P < .01), for cervical spine it was 98.5% (95% CI 95.2-99.6, Heterogeneity: I2 = 88%, τ2 = 3.3, P < .01), for thoracolumbar surgery it was 88.6% (95% CI 74.1-95.4, Heterogeneity: I2 = 91%, τ2 = 1.7, P < .01), for lumbosacral it was 99% (95% CI 3.5-96.4, Heterogeneity: I2 = 0%, τ2 = 20.5, P < .01), for cervicothoracic it was 96% (95% CI 94.4-97.1, Heterogeneity: I2 = 0%, τ2 = 0, P = .01) (Supplemental Figure 3(b)).
The AUC for cervical spine, cervicothoracic, thoracolumbar, lumbosacral and all regions were .928, .729, .879, .926 and .911, respectively (Supplemental Figure 3(c)).
MEP
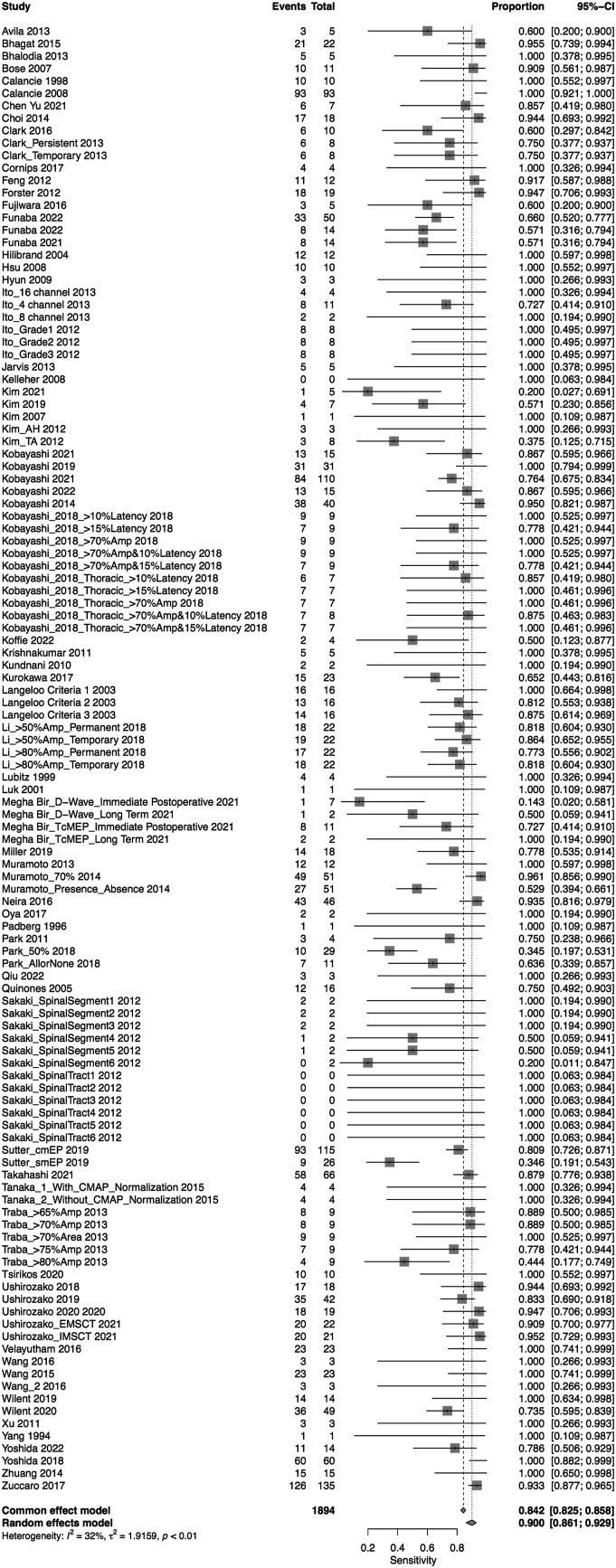
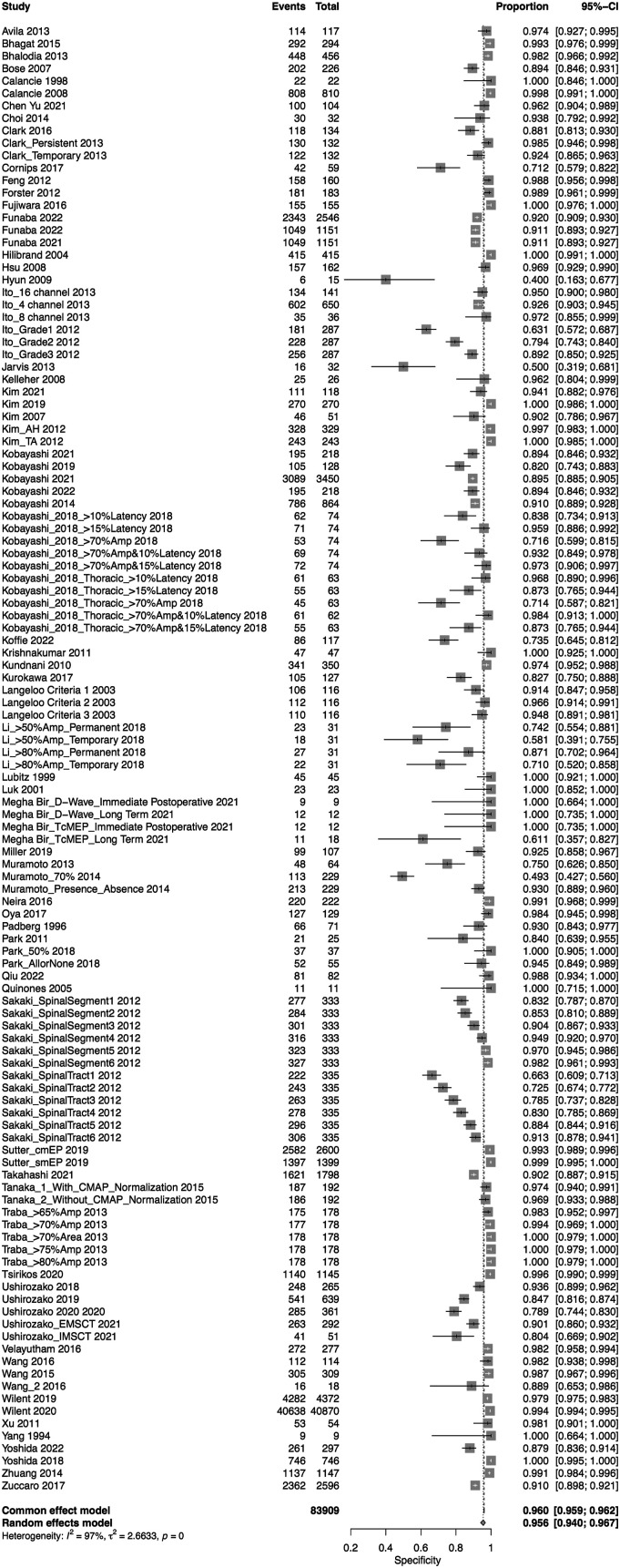
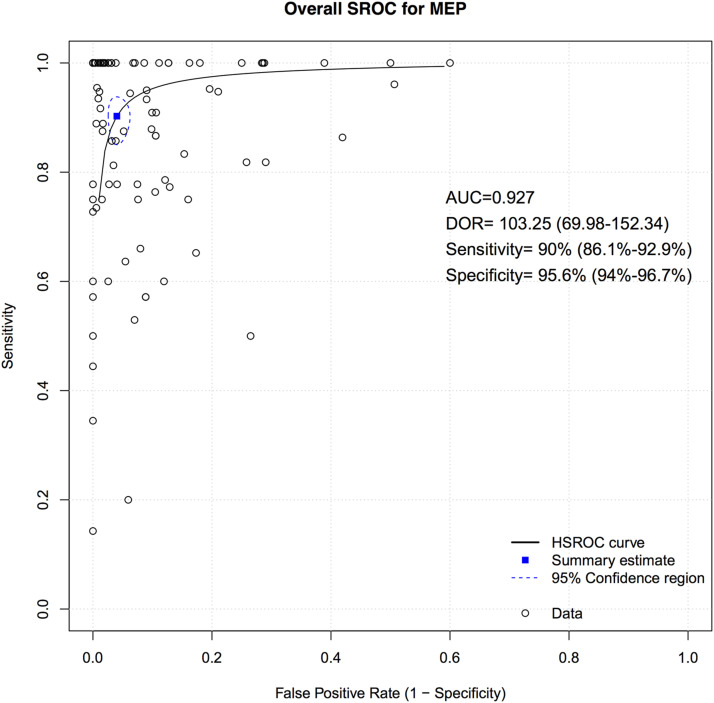
A total of 75 studies presented data for MEP on 79,545 patients. Overall, the sensitivity of MEP was 90% (95% CI 86.1-92.9, Heterogeneity: I2 = 32%, τ2 = 1.91, P < .01) (Figure 5), while the specificity was 95.6% (95% CI 94-96.7, Heterogeneity: I2 = 97%, τ2 = 2.7, P < .01) (Figure 6). Overall, the AUC value was .927, while the DOR was 103.25 (95% CI 69.98—152.34) (Figure 7).
Figure 5.
Forest plot for sensitivity of MEP.
Figure 6.
Forest plot for specificity of MEP.
Figure 7.
Overall sROC plot for MEP.
We also performed subgroup analysis for various thresholds for IONM alerts u, different reported disease groups and different regions.
Subgroup Analyses:
1. Thresholds:
The most commonly reported change in amplitude threshold for alert was 50% (n = 24 studies), followed by 70% (n = 20 studies), 80% (n = 16 studies), “all or none” (n = 5 studies), 65% (n = 5 studies), 75% (n = 3 studies), change in latency of >10% and >15% (n = 2 each), 10%, 20%, 30%, 40%, 50%–65%, 50%–80%, 60% (n = 1 each), and significant change. Nine studies either did not specify the criteria for alarm or reported a method other than amplitude change or latency change. The pooled sensitivities for 50% threshold, 70% threshold, 80% threshold, “all or none” alert, 65% threshold and 75% threshold were 85.4% (95% CI 75-92, Heterogeneity: I2 = 50%, τ2 = 1.47, P < .01), 91.3% (95% CI 85.4-95, Heterogeneity: I2 = 38%, τ2 = 1.16, P = .03), 92.3% (95% CI 79.2-97.4, Heterogeneity: I2 = 0%, τ2 = 2.59, P = .65), 56.8% (95% CI 45.3-67.5, Heterogeneity: I2 = 0%, τ2 = 0, P = .92), 94% (95% CI 85.1-97.7, Heterogeneity: I2 = 0%, τ2 = 0, P = .99) and 89.7% (95% CI 75.7-96.1, Heterogeneity: I2 = 0%, τ2 = 0, P = .43), respectively (Supplemental Figure 4(a)). The pooled specificity for 50% threshold, 70% threshold, 80% threshold, “all or none” alert, 65% threshold and 75% threshold were 94.2% (95% CI 88.2-97.2, Heterogeneity: I2 = 99%, τ2 = 3.42, P < .01), 90.4% (95% CI 85.4-93.9, Heterogeneity: I2 = 94%, τ2 = 1.39, P < .01), 96.7% (95% CI 94.2-98.1, Heterogeneity: I2 = 88%, τ2 = 1.47, P < .01), 96.1% (95% CI 92.7-98, Heterogeneity: I2 = 69%, τ2 = .39, P < .01), 98.2% (95% CI 97.3-98.8, Heterogeneity: I2 = 0%, τ2 = 0, P = .72) and 99% (95% CI 94.1-99.8, Heterogeneity: I2 = 25%, τ2 = 1.39, P = .43), respectively (Supplemental Figure 4(b)).
2. Disease Group:
In terms of disease group, most studies presenting data for MEP consisted of patients with mixed diseases (n = 27), followed by deformity (n = 19), degenerative diseases (n = 17), tumors (n = 12), and others (n = 2). The pooled sensitivities for studies consisting of patients with mixed diseases was 93.9% (95% CI 87.1-97.2, Heterogeneity: I2 = 20%, τ2 = 3.63, P = .12), for deformity it was 92.4% (95% CI 89.3-94.7, Heterogeneity: I2 = 0%, τ2 = 0, P = .95), for degenerative it was 80% (95% CI 66.3-89, Heterogeneity: I2 = 0%, τ2 = 1.0, P = .99), and for tumor it was 85.4% (95% CI 72.9-92.7, Heterogeneity: I2 = 73%, τ2 = 1.58, P < .01) (Supplemental Figure 5(a)).
The pooled specificities for studies consisting of patients with mixed diseases was 97% (95% CI 94.6-98.3, Heterogeneity: I2 = 93%, τ2 = 2.8975, P < .01), for deformity it was 96.1% (95% CI 93-97.9, Heterogeneity: I2 = 93%, τ2 = 3.51, P < .01), for degenerative it was 94.9% (95% CI 91.5-97, Heterogeneity: I2 = 99%, τ2 = 2.1, P < .01) and for tumor it was 85.4% (95% CI 72.9-92.7, Heterogeneity: I2 = 95%, τ2 = 1.94, P < .01) (Supplemental Figure 5(b)).
The AUC for mixed pathology group, deformity, degenerative and tumor were .937, .934, .948, .722 and .915, respectively (Supplemental Figure 5(c)).
3. Regions
In terms of region of surgery, most studies presenting data for MEP consisted of patients undergoing surgery for any region (n = 20), followed by surgery in the cervical spine (n = 11), lumbosacral (n = 9), thoracolumbar and cervicothoracic (n = 6 each) segments. The pooled sensitivity for studies consisting of patients with all regions was found to be 91.6% (95% CI 87.2-94.6, Heterogeneity: I2 = 35%, τ2 = 1.7, P < .01), for cervical spine surgery it was 80.2% (95% CI 64.7-89.9, Heterogeneity: I2 = 0%, τ2 = 1.26, P = 1.00), for thoracic spine surgery it was 100% (95% CI 0-100. Heterogeneity: I2 = 0%, τ2 = 0, P = .99), for lumbosacral it was 74.7% (95% CI 65.1-82.5, Heterogeneity: I2 = 0%, τ2 = 0, P = .78) and for cervicothoracic spine it was 90.6% (95% CI 77.6-96.4, Heterogeneity: I2 = 45%, τ2 = 2.37, P = .02) (Supplemental Figure 6(a)).
The pooled specificity for studies consisting of patients with surgery for any region was 97% (95% CI 95.1-98.2, Heterogeneity: I2 = 93%, τ2 = 3.44, P < .01), for cervical spine, it was 93.3% (95% CI 89.8-95.6, Heterogeneity: I2 = 96%, τ2 = 1.38, P < .01), for thoracic spine, it was 77.6% (95% CI 71.2-82.8, Heterogeneity: I2 = 35%, τ2 = .007, P < .01), for lumbosacral it was 98% (95% CI 91.1-99.6, Heterogeneity: I2 = 99%, τ2 = 2.7, P < .01), for cervicothoracic it was 92.8% (95% CI 87.1-96.1, Heterogeneity: I2 = 92%, τ2 = 1.47, P < .01) (Supplemental Figure 6(b)).
The AUC for cervical spine, cervicothoracic, thoracic, lumbosacral and all regions were .742, .919, .895, .804 and .934, respectively (Supplemental Figure 6(c)).
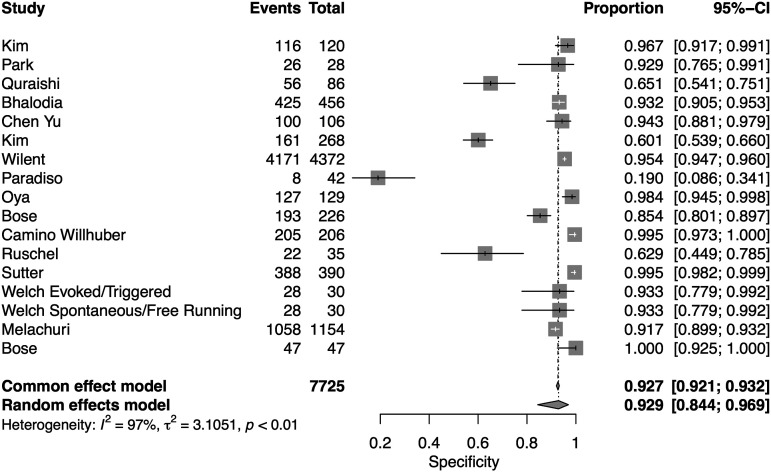
EMG
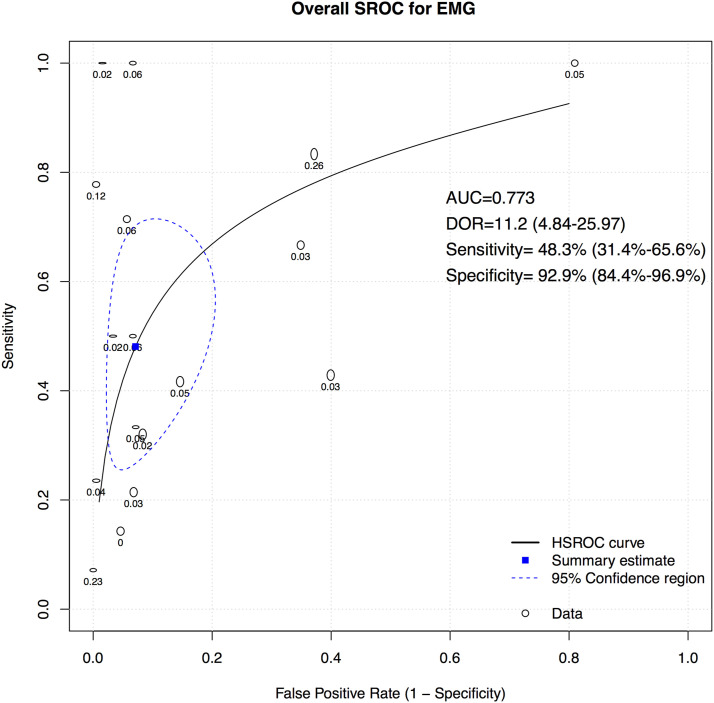
A total of 16 studies presented data for EMG on 7004 patients. Overall, the pooled sensitivity for EMG was 48.3% (95% CI 31.4-65.6, Heterogeneity I2 = 54, τ2 = 1.27, P < .01) (Figure 8), while the pooled specificity was 92.9% (95% CI 84.4-96.9, Heterogeneity I2 = 97, τ2 = 3.1, P < .01) (Figure 9). The AUC was .773 and the DOR was 11.2 (95% CI 4.84-25.97) (Figure 10).
Figure 8.
Forest plot for sensitivity of EMG.
Figure 9.
Forest plot for specificity of EMG.
Figure 10.
Overall sROC plot for EMG.
We also performed subgroup analysis for type of EMG, different reported disease groups, and different regions.
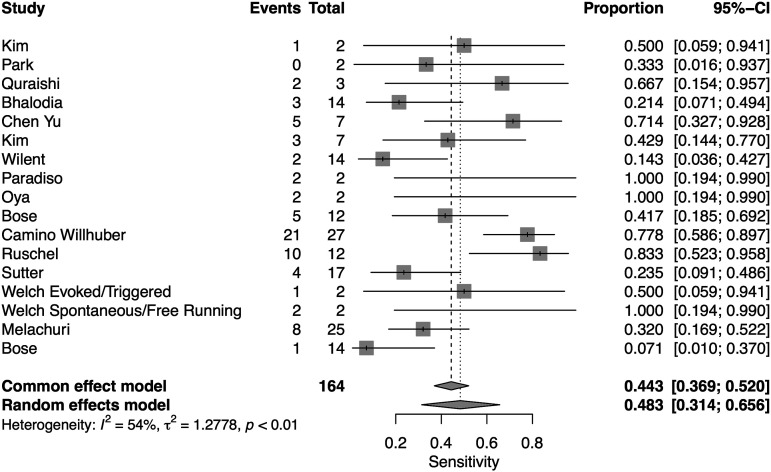
1. Type of EMG
Eleven studies reported on free-running or spontaneous EMG, 2 studies reported on evoked/triggered/stimulated EMG, and 4 studies reported on combined free-running and triggered EMG. The pooled sensitivity for free-running EMG was 54.6% (95% CI 33.8-74, Heterogeneity: I2 = 55%, τ2 = 1.13, P = .02), for evoked/triggered/stimulated EMG it was 33.3% (95% CI 31.4-65.6, Heterogeneity: I2 = 0%, τ2 = 0, P = .61), and for combined free-running and triggered EMG it was 40.7% (95% CI 9.4-82, Heterogeneity: I2 = 60%, τ2 = 2.5, P = .06) (Supplemental Figure 7(a)).
The pooled specificity for free-running EMG was 91.9% (95% CI 82.4-96.5, Heterogeneity: I2 = 98%, τ2 = 1.98, P < .01), for evoked/triggered/stimulated EMG it was 91.7% (95% CI 90-93.2, Heterogeneity: I2 = 0%, τ2 = 0, P = .75), and for combined free-running and triggered EMG it was 96.4% (95% CI 48.2-99, Heterogeneity: I2 = 97%, τ2 = 10.01, P < .01) (Supplemental Figure 7(b)).
The AUC for free-running was .773, for triggered EMG it was .873, and for combined free-running and triggered EMG it was .792 (Supplemental Figure 7(c)).
2. Disease Group
Three studies reported data on EMG for deformity surgery, 5 studies reported data for degenerative diseases and 5 studies reported data for surgery of mixed pathologies. One study each reported data for EMG for detethering, tumor and unspecified. The pooled sensitivity for EMG for deformity studies was found to be 30.2% (95% CI 14.7-52.1, Heterogeneity: I2 = 0%, τ2 = 0, P = .38), for degenerative studies it was found to be 76.2% (95% CI 61.1-86.7, Heterogeneity: I2 = 0%, τ2 = 0, P = .93), while for mixed pathology studies it was found to be 29.2% (95% CI 19.9-40.6, Heterogeneity: I2 = 0%, τ2 = 0, P = .50) (Supplemental Figure 8(a)).
The pooled specificity for EMG for deformity studies was found to be 94.7% (95% CI 64.1-99.4, Heterogeneity: I2 = 95%, τ2 = 3.73, P < .01), for degenerative studies it was found to be 97.3% (95% CI 95.6-98.3, Heterogeneity: I2 = 40%, τ2 = .36, P = .75), while for mixed pathology studies it was found to be 88.6% (95% CI 77.6-94.6, Heterogeneity: I2 = 99%, τ2 = .83, P < .01) (Supplemental Figure 8(b)).
The AUC for deformity was found to be .659, for degenerative it was found to be .888, while for various diseases it was found to be .552 (Supplemental Figure 8(c)).
3. Regions
Eight studies provided data for EMG for lumbosacral surgery, 5 studies provided data for cervicothoracic surgery, 2 studies provided data for EMG for surgery for any region, and one study provided data for EMG for cervical spine surgery.
The pooled sensitivity for lumbosacral surgery was 49.6% (95% CI 26.6-72.8, Heterogeneity: I2 = 63%, τ2 = 1.41, P < .01), for cervicothoracic surgery it was 36.1% (95% CI 16-62.7, Heterogeneity: I2 = 0%, τ2 = .3, P < .01) and for surgery for any region it was 80% (95% CI 53-93.4, Heterogeneity: I2 = 0%, τ2 = 0, P < .01) (Supplemental Figure 9(a)).
The pooled specificity for lumbosacral surgery was 94.7% (95% CI 78.6-98.8, Heterogeneity: I2 = 97%, τ2 = 5.1, P < .01), for cervicothoracic surgery it was 94.9% (95% CI 89.9-97.5, Heterogeneity: I2 = 91%, τ2 = .50, P < .01) and for surgery for any region it was 64.5% (95% CI 55.6-72.5, Heterogeneity: I2 = 0%, τ2 = 0, P < .01) (Supplemental Figure 9(b)).
The AUC values for lumbosacral surgery, cervicothoracic surgery and for surgery for any region were .738, .492 and .655, respectively (Supplemental Figure 9(c)).
Multimodal
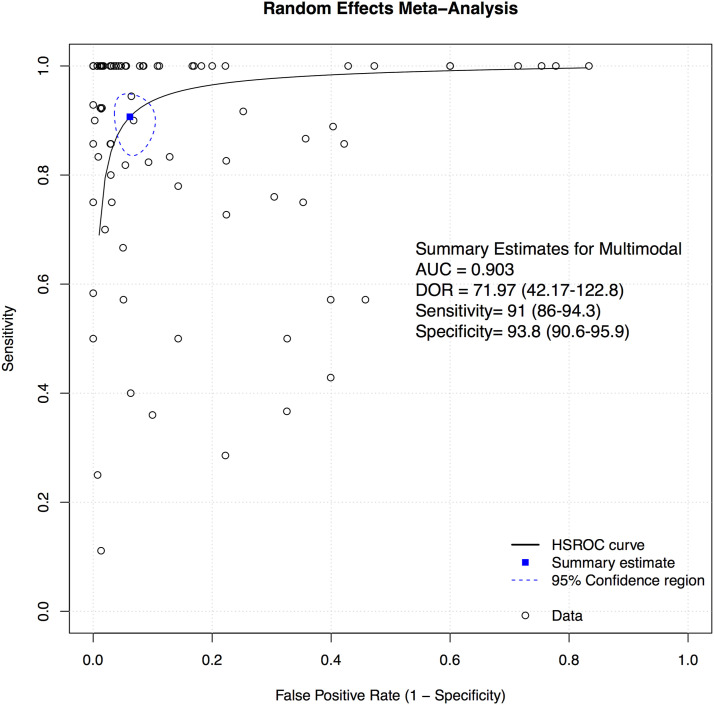
A total of 69 studies presented data for multimodal neuromonitoring on 58,325 patients. Overall, the sensitivity of multimodal neuromonitoring was 91% (95% CI 86%–94.3%) (Figure 11), while the pooled specificity was 93.8% (95% CI 90.6%–95.9%) (Figure 12). The AUC value was .903 while the DOR was 71.97 (95% CI 42.17-122.8) (Figure 13).
Figure 11.
Forest plot for sensitivity of Multimodal Neuromonitoring.
Figure 12.
Forest plot for specificity of Multimodal Neuromonitoring.
Figure 13.
Overall sROC plot for Multimodal Neuromonitoring.
We also performed subgroup analyses based on type of multimodal neuromonitoring, disease subset, and region of surgery.
1. Type of Multimodal Neuromonitoring.
A total of 33 studies presented data for combined SSEP and MEP, 27 studies for combined SSEP, MEP and EMG, 6 studies for combined SSEP, MEP and D-wave, 4 studies for combined MEP and EMG, 3 studies for combined MEP and D-wave, 2 studies for combined SSEP and EMG, and one study for combined SSEP and D-wave. The pooled sensitivity for SSEP and MEP was 93.5% (95% CI 83.1-97.7, Heterogeneity: 0%, τ2 = 3.8, P = .65), for combined SSEP, MEP and EMG it was 87.7% (95% CI 80-92.7, Heterogeneity: 18%, τ2 = 1.22, P = .19), for combined SSEP, MEP and D-wave it was 90.2% (95% CI 63.5-98, Heterogeneity: 63%, τ2 = 2.68, P = .01), for combined MEP and EMG it was 92.3% (95% CI 53.8-99.2, Heterogeneity: 0%, τ2 = 2.06, P = .65), for combined MEP and D-wave it was 90.4% (95% CI 86-94.3, Heterogeneity: 0%, τ2 = 0, P = .81), and for SSEP and EMG it was 90.7% (95% CI 3.6-100, Heterogeneity: 0%, τ2 = 9.91, P = .99) (Supplemental Figure 10(a)).
The pooled specificity for SSEP and MEP was 95.3% (95% CI 90.7-97.7, Heterogeneity: 95%, τ2 = 4.11, P < .01), for combined SSEP, MEP and EMG it was 94.3% (95% CI 88.7-97.2, Heterogeneity: 96%, τ2 = 4.27, P < .01), for combined SSEP, MEP and D-wave it was 93.1% (95% CI 83.6-97.3, Heterogeneity: 90%, τ2 = 1.48, P < .01), for combined MEP and EMG it was 77.2% (95% CI 40-94.5, Heterogeneity: 91%, τ2 = 2.35, P = .65), for combined MEP and D-wave it was 99.2% (95% CI 98.9-99.4, Heterogeneity: 77%, τ2 = 0, P = .01), and for SSEP and EMG it was 63.3% (95% CI 14.6-94.5, Heterogeneity: 100%, τ2 = 2.75, P < .01) (Supplemental Figure 10(b)).
The AUC values for combined SSEP and MEP was found to be .908; .881 for combined SSEP, MEP and EMG; .938 for SSEP, MEP and D-wave, and .848 for MEP and EMG (Supplemental Figure 10(c)).
2. Disease Group
A total of 23 studies presented data for multimodal neuromonitoring for deformity surgery, 17 studies for spinal tumors, 16 studies for various disease groups, 12 studies for degenerative, and 1 study for trauma.
The pooled sensitivity for multimodal neuromonitoring for deformity was 98.8% (95% CI 88.9-99.9, Heterogeneity: I2 = 0%, τ2 = 7.92, P = .51), for degenerative disease it was 74.7% (95% CI 62.3-84, Heterogeneity: I2 = 0%, τ2 = .35, P = .84), for mixed pathology it was 95.6% (95% CI 84.1-98.9, Heterogeneity: I2 = 68%, τ2 = 4.94, P < .01), and for tumor it was 83.9% (95% CI 75.6-89.8, Heterogeneity: I2 = 31%, τ2 = .66, P = .08) (Supplemental Figure 11(a)).
The pooled specificity for multimodal neuromonitoring for deformity was 96% (95% CI 91.4-98.2, Heterogeneity: I2 = 95%, τ2 = 3.78, P < .01) for degenerative disease it was 95.2% (95% CI 86.7-98.3, Heterogeneity: I2 = 96%, τ2 = 4.50, P < .01), for mixed pathology it was f 95.8% (95% CI 90.8-98.1, Heterogeneity: I2 = 98%, τ2 = 3.27, P < .01), and for tumor it was 88.6% (95% CI 77.1-94.7, Heterogeneity: 84%, τ2 = 3.49, P < .01) (Supplemental Figure 11(b)).
The AUC for deformity was .946, for degenerativ disease it was .787, for mixed pathology it was .958, and for tumor it was .844 (Supplemental Figure 11(c)).
3. Regions
Six studies provided data for multimodal neuromonitoring for lumbosacral surgery, 3 studies for cervicothoracic surgery, 37 studies for surgery for any region, 10 studies for cervical spine surgery, 11 studies for thoracolumbar surgery, and 2 studies for thoracic spine surgery.
The pooled sensitivity for lumbosacral surgery was 76.2% (95% CI 54.4-89.6, Heterogeneity: I2 = 32%, τ2 = 1.35, P = .15), for cervicothoracic surgery it was 98.4% (95% CI 6.4-100, Heterogeneity: I2 = 0%, τ2 = 13.02, P = .99), for surgery for any region it was 92.5% (95% CI 86.9-95.8, Heterogeneity: I2 = 41%, τ2 = 1.92, P < .01), for cervical spine surgery it was 81.2% (95% CI 54-94.1, Heterogeneity: 27%, τ2 = 2.4, P = .19), for thoracolumbar surgery it was 96.9% (95% CI 82.6-99.5, Heterogeneity: 0%, τ2 = 1.49, P = .99), and for thoracic surgery it was 67.7% (95% CI 49.7-81.7, Heterogeneity: 0%, τ2 = 0, P = .74) (Supplemental Figure 12(a)).
The pooled specificity for lumbosacral surgery was 91.5% (95% CI 69.3-98.1, Heterogeneity: I2 = 97%, τ2 = 5.78, P < .01), for cervicothoracic surgery it was 59.5% (95% CI 33.7-81, Heterogeneity: I2 = 77%, τ2 = .84, P < .01), for surgery for any region it was 95.2% (95% CI 91.4-97.4, Heterogeneity: I2 = 97%, τ2 = 4.03, P < .01), for cervical spine surgery it was 97.6% (95% CI 94.3-99.1, Heterogeneity: 94%, τ2 = 2, P < .01), for thoracolumbar surgery it was 92.1% (95% CI 81.2-97, Heterogeneity: 96%, τ2 = 2.59, P < .01) and for thoracic surgery it was 95.2% (95% CI 92.3-97, Heterogeneity: 0%, τ2 = 0, P = .64) (Supplemental Figure 12(b)).
The AUC values for cervical surgery, cervicothoracic surgery, thoracic surgery, thoracolumbar, lumbosacral and surgery for any region were found to be .0.928, .718, .845, .89, .791 and .916, respectively (Supplemental Figure 12(c)).
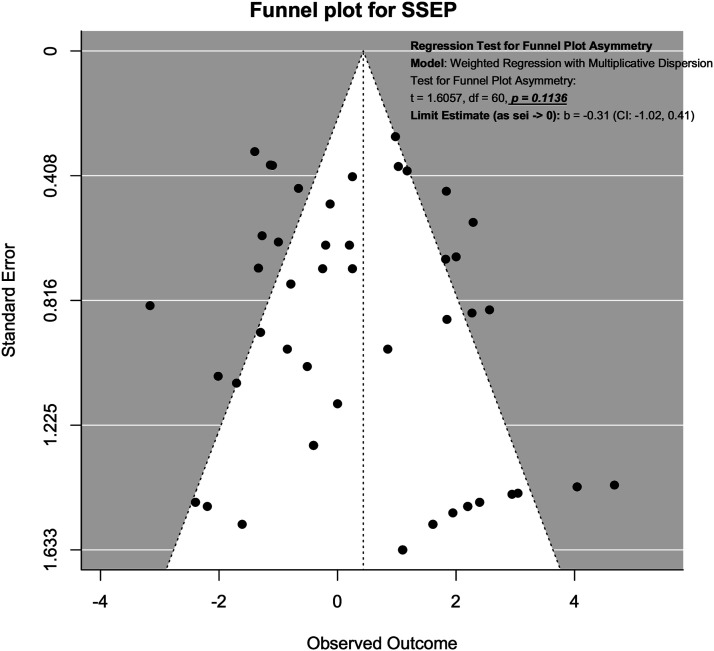
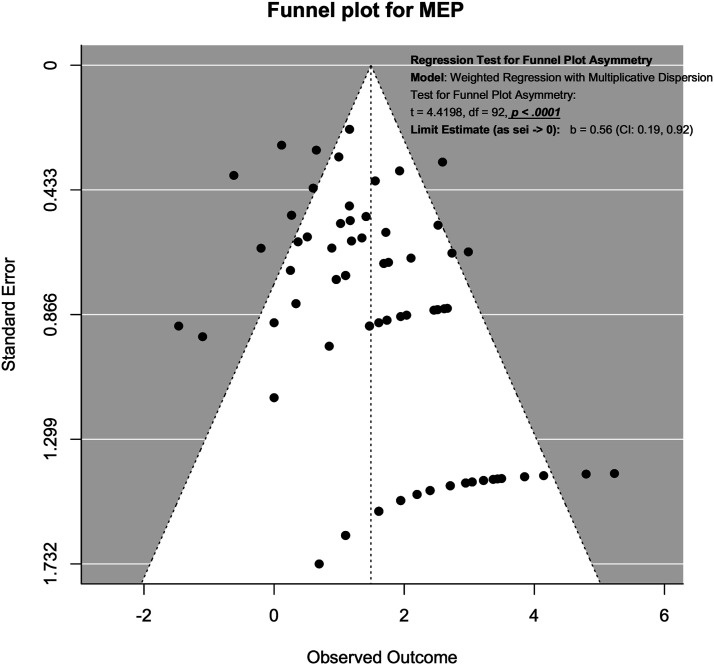
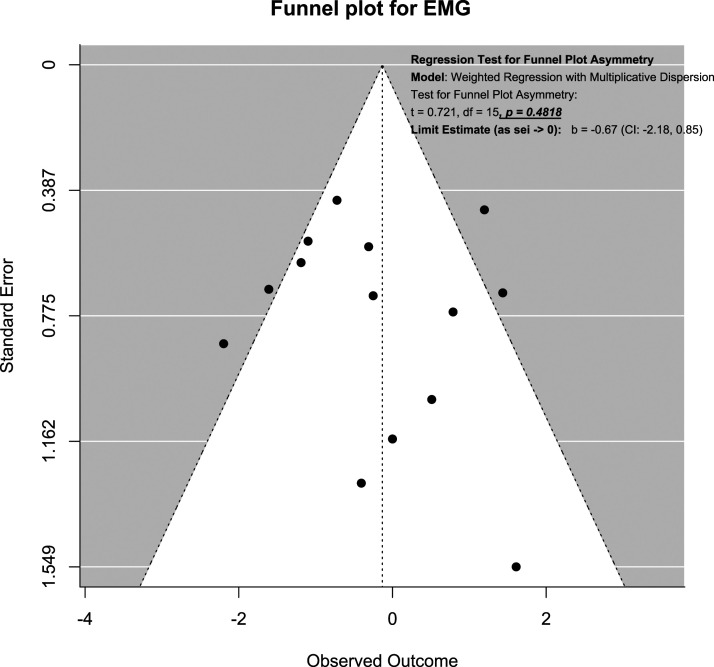
Publication Bias Assessment Using Funnel Plot
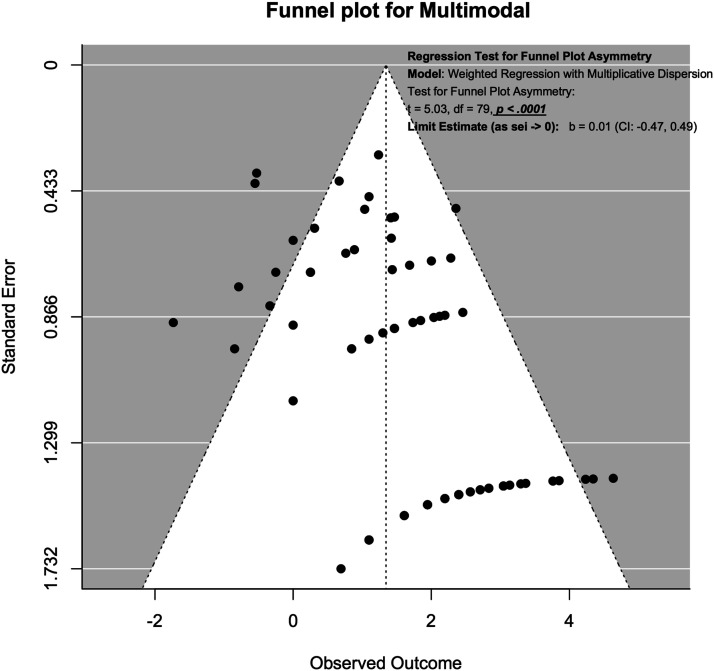
Publication bias was assessed using funnel plots and modified Hong’s test proposed by Noma. 38 For SSEP neuromonitoring, (Figure 14), we observed slight asymmetry but the weighted regression with multiplicative dispersion test for asymmetry was not statistically significant (t = 1.61, df = 60, P = .11). For MEP neuromonitoring, (Figure 15), we observed asymmetry and the weighted regression with multiplicative dispersion test for asymmetry was statistically significant (t = 4.42, df = 92, P < .001). For multimodal neuromonitoring, (Figure 16), we observed asymmetry but the weighted regression with multiplicative dispersion test for asymmetry was not statistically significant (t = .72, df = 15, P = .48). For multimodal neuromonitoring, (Figure 17), we observed asymmetry and the weighted regression with multiplicative dispersion test for asymmetry was statistically significant (t = 5.03, df = 79, P < .001).
Figure 14.
Funnel plot for Assessment of Publication Bias for SSEP.
Figure 15.
Funnel plot for Assessment of Publication Bias for MEP.
Figure 16.
Funnel plot for Assessment of Publication Bias for EMG.
Figure 17.
Funnel plot for Assessment of Publication Bias for Multimodal Neuromonitoring.
Risk of Bias Assessment Using QUADAS Tool
Risk of bias was assessed using the QUADAS tool
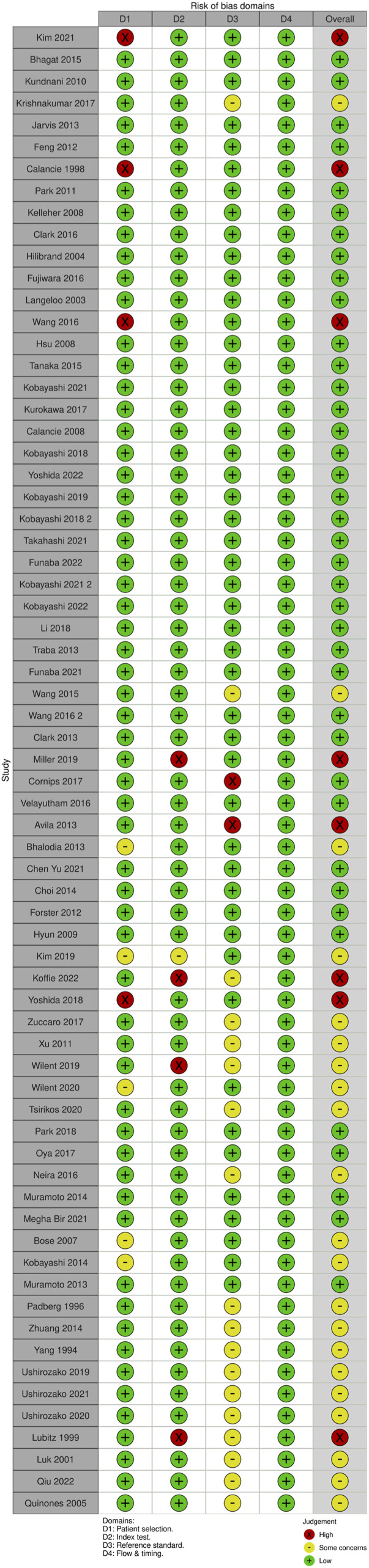
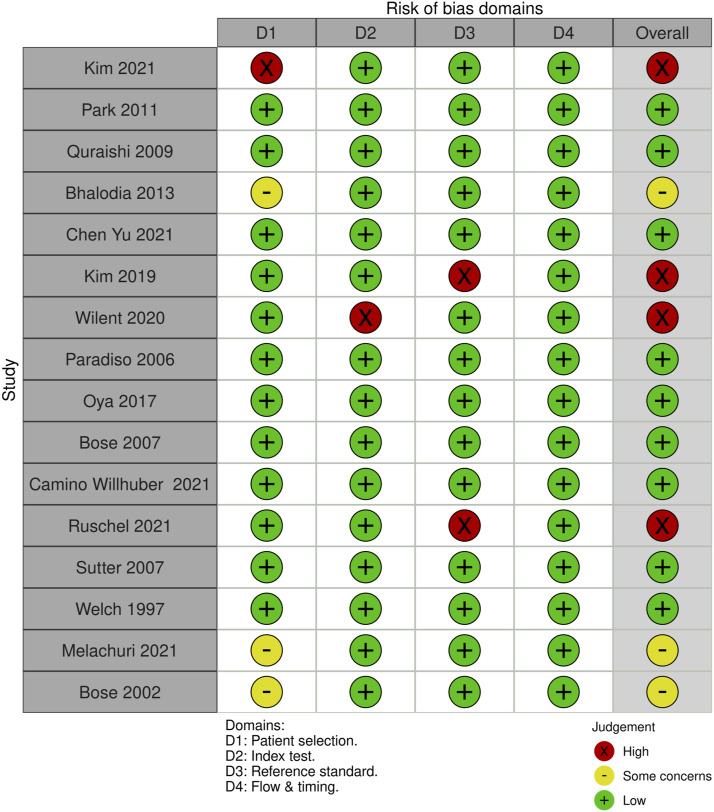
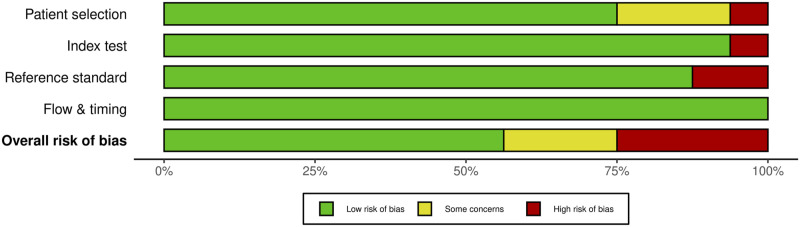
For SSEP monitoring, of the 52 studies, 10 studies (19.2%) had some concerns, 25% (n = 13) were high risk and the remaining 29 studies (55.8%) were low risk. For most of the studies that were graded down, risk of bias was identified in the “reference standard” domain; the reason was lack of specification details of the postoperative examination, or the use of a non-standard exam (Figures 18 and 19).
Figure 18.
QUADAS-2 risk of bias traffic light plot for SSEP.
Figure 19.
QUADAS-2 risk of bias summary plot for SSEP.
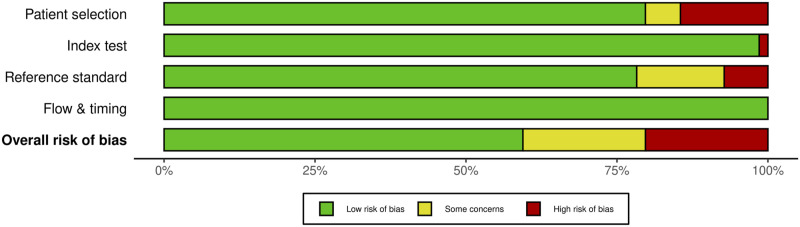
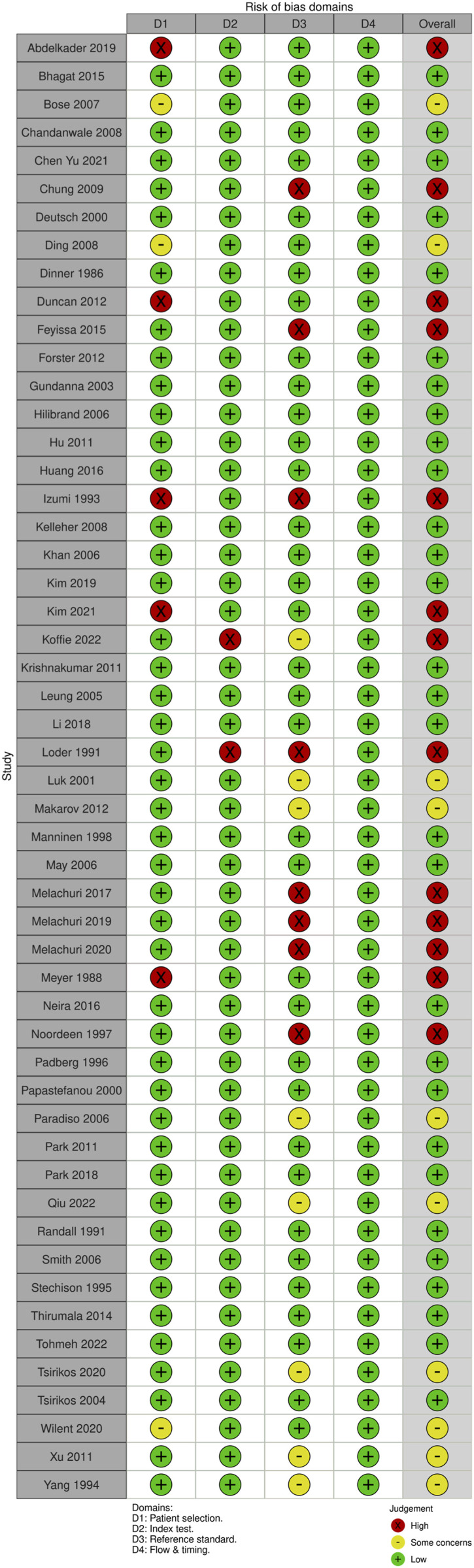
For MEP monitoring, of the 75 studies, 21 studies (28%) had some concerns, 10.7% (n = 8) were high risk and the remaining 46 studies (61.3%) were low risk. For most of the studies that were graded down, risk of bias was identified in the “reference standard” domain (Figures 20 and 21).
Figure 20.
QUADAS-2 risk of bias traffic light plot for MEP.
Figure 21.
QUADAS-2 risk of bias summary plot for MEP.
For EMG monitoring, of the 16 studies, 3 studies (18.75%) had some concerns, 25% (n = 4) were high risk and the remaining 9 studies (56.25%) were low risk. For most of the studies that were graded down, risk of bias was identified in the “index test” test domain; the reason was lack of specification details of the changes in EMG monitoring that were considered an alert (Figures 22 and 23).
Figure 22.
QUADAS-2 risk of bias traffic light plot for EMG.
Figure 23.
QUADAS-2 risk of bias summary plot for EMG.
For multimodal neuromonitoring, of the 69 studies, 14 studies (20.3%) had some concerns, 14 studies (20.3%) were high risk and the remaining 41 studies (59.4%) were low risk. For most of the studies that were graded down, ris of bias was identified in the “index” domain; the reason was lack of specification/details of the criteria that constituted an alert. (Figures 24 and 25).
Figure 24.
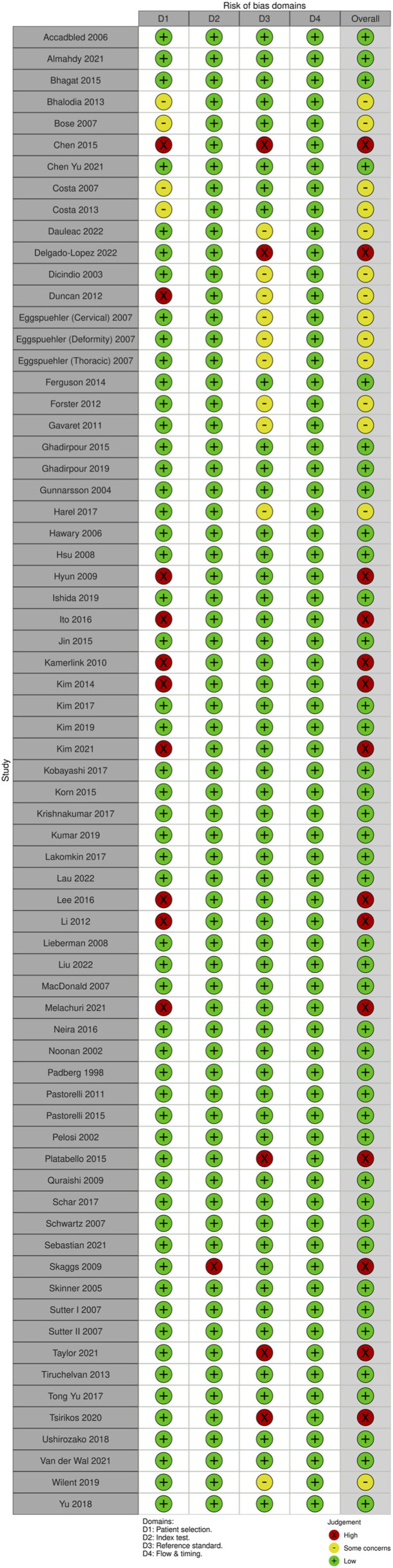
QUADAS-2 risk of bias traffic light plot for multimodal neuromonitoring.
Figure 25.
QUADAS-2 risk of bias summary plot for multimodal neuromonitoring.
GRADE Assessment of Strength of Evidence
We applied the GRADE assessment methodology described by Yang et al to evaluate the strength of evidence for each of the 4 groups:. SSEP, MEP, EMG and multimodal neuromonitoring. These are summarized in Tables 5a, 5b, 5c, and 5d, respectively. For all 4 groups, the final quality of the evidence was “Low”. Evidence was downgraded for “Inconsistency,” “Imprecision” and “Publication Bias.” Studies were downgraded for inconsistency because of differences in included population/pathology type (deformity vs tumor vs degenerative vs mixed population) and because of the use of different “thresholds”. Studies were downgrade for “Imprecision” due to low number of events (TP + FN) resulting in large confidence intervals, particularly for sensitivity. Finally, studies were downgraded for “Publication Bias” due to both observed and statistically significant asymmetry.
Table 5.
GRADE strength of evidence for different neuromonitoring approaches.
| Table 5a: GRADE strength of evidence for SSEP | |||
|---|---|---|---|
| Quality criteria | Rating (circle one for each criterion) | Footnotes (explain reasons for up- or downgrading) | Quality of the evidence (circle one per outcome) |
| Risk of bias | No serious (-1) very serious (-2) | All studies observational with majority of them being low risk of bias | ⊕⊕⊕⊕ High ⊕⊕⊕□ Moderate ⊕⊕□□ Low ⊕□□□ Very low |
| Inconsistency | No serious (-1) very serious (-2) | Inconsistency in results/estimates/effects very likely due to differences in included population/pathology type (deformity vs tumor vs degenerative vs mixed population) | |
| Indirectness | No serious (-1) very serious (-2) | All studies employed a postoperative neurologic examination as a reference standard using which the utility/diagnostic efficacy of neuromonitoring was assessed | |
| Imprecision | No serious (-1) very serious (-2) | Due to low number of events (true positive + false negatives), most studies have very large confidence intervals, particularly for sensitivity | |
| Publication bias | Unlikely likely (-1) very likely (-2) | Test for asymmetry not statistically significant | |
| Large effect | Large (+1) Very large (+2) | No | |
| Dose-response gradient | No Yes (+1) | NA | |
| Plausible confounding would change the effect | No Yes (+1) | NA | |
Discussion
One of the potential benefits of neuromonitoring is that it allows the surgical team to detect a SCI early on and to institute measures that may potentially reverse or minimize the neurologic deficit. The earlier an injury is detected, the more likely it is that corrective action can be taken to prevent or minimize further damage.
Another potential benefit of neuromonitoring is that it may help to reduce the risk of complications during surgery. Neuromonitoring can also help to improve the accuracy and precision of surgical procedures that involve the spinal cord. By providing real-time information about the function of the spinal cord sensory and motor tracts, neuromonitoring can help the surgical team make more informed decisions about how to proceed with the surgery. This may lead to better outcomes and a lower risk of complications. In addition, neuromonitoring can help to reduce the risk of legal liability for the surgical team. If an SCI occurs during surgery, the surgical team may be held responsible if they did not take appropriate precautions to prevent the injury. By using neuromonitoring, the surgical team can demonstrate that they took additional precautions to minimize the risk of injury and protect the patient's health.
In the current systematic review and meta-analysis, the authors sought to comprehensively summarize all available evidence related to the use of neuromonitoring to detect ISCI. Using novel quantitative statistical methods, we found that all neuromonitoring modalities have acceptable test characteristics as evident from the sROC and AUC. Moreover, we were also able to compute diagnostic test accuracy of each neuromonitoring type for specific disease groups and for specific regions of surgery. We discuss briefly the role of neuromonitoring for specific disease groups.
Monitoring for Cervical Degenerative Surgery
IONM has been more commonly used in degenerative cervical spine surgery recently, even though the risk of neurological complications is low. 204 SSEP is currently the IONM modality that is used the most frequently. 205 It is used in cervical spine surgery not only for assessment of the spinal cord and nerve roots following surgical positioning, but also for the monitoring of sensory tracts throughout the procedure. However, SSEP changes during surgery are not necessarily linked to postoperative neurological impairments due to its low specificity, as demonstrated in our analyses. As a result, many experts advise against using it as the sole monitoring modality in complicated cervical surgeries. This is evident from our results given that the sensitivity of using SSEP alone in cervical spine surgery was only 46%.
Numerous studies have proven that MEPs are reliable at detecting probable neurological damage. In their study, Clark et al found that using MEPs for predicting postoperative impairments in patients undergoing surgery for degenerative cervical myelopathy had a sensitivity of 71% and a specificity of 94%. 59 In 427 consecutive patients who underwent cervical spine surgery, Hilibrand et al compared the utilization of both SSEP and transcranial MEP (tcMEP) monitoring. 2 The authors described 12 individuals who had considerable monitoring modifications, 2 of whom were later discovered to have new neurological impairments. Since only one of the 2 patients with a deficiency had SSEP alterations, the authors came to the conclusion that the reported sensitivity and specificity for tcMEP were only 25% and 100%, respectively. However, recent studies have shown more promising results and better diagnostic accuracy for detecting intraoperative injury.47,49,95,97,157,185 The pooled results yielded a net sensitivity of 80.2% for MEP for cervical surgery.
In order to increase the effectiveness of IONM during cervical decompression surgery, a combination of SSEP, MEP and EMG has been explored due to the safety issues with the use of only SSEPs and the limitations of MEPs, as previously stated. While a previous qualitative analysis found an overall sensitivity of 50%, our analyses yielded a pooled sensitivity of 81.2%.
Some experts argue against the use of IONM in non-complex cervical spine surgeries, despite the fact that numerous researchers have shown its value. Traynelis et al 206 concluded that surgical decompression and reconstruction for symptomatic cervical spine disease may be safely carried out without the use of IONM after conducting a retrospective examination of 720 patients. Ajiboye et al 207 likewise discovered no advantage of IONM in the prevention of new postoperative neurological problems following anterior cervical surgery, supporting this study's findings. Our analyses, when restricted to studies investigating the use of multimodal monitoring for cervical spine and non-complex degenerative diseases, yielded a sensitivity of 62.7%. Therefore, there is ongoing debate in the spine community over whether monitoring is necessary for routine, non-complex cases.
Monitoring for Deformity Surgery
Several studies have highlighted the importance of using IONM in spinal deformity surgery. The incidence of neurological problems following scoliosis surgery has decreased dramatically since the 1970s, when SSEP monitoring was first introduced. 208 A large study by Nuwer et al published the findings of a survey by the Scoliosis Research Society (SRS), which asked its members to submit information on the surgical outcomes of patients who had undergone surgery, including the use of IONM. With stated sensitivity of 92% and specificity of 98.9%, SSEP monitoring was used in 51263 of 97586 spinal cases (53%), and positive and negative predictive values were 42% and 99.9%, respectively. However, given that this study did not provide 2 × 2 data for TP, FP, FN and TN, it was not included in our analyses. Nevertheless, our results showed optimum performance of all modalities for detecting ISCI during deformity surgery; 94% sensitivity for SSEP, 92.4% for MEP, and 98.8% for multimodal neuromonitoring.
Degenerative Lumbar Surgery
Although IONM is frequently employed in the treatment of spinal deformity in the present day, its application in degenerative lumbar surgery, particularly in straightforward procedures, is still debatable. 209 Supporters of IONM emphasize the technology’s significance in accurately identifying spinal nerve root damage, particularly in revision and instrumented fusion cases.48,164,210,211 The issue of monitoring spinal nerve root function is still debatable despite developments.209,212 Additionally, although numerous studies have supported the use of IONM in lumbar fusion surgery, it is still unclear whether the improved detection of crisis events intraoperatively translates to a decreased rate of postoperative neurological deficit. 213 Our results indicate that while EMG has poor sensitivity for most other surgeries, its role in nerve root monitoring for degenerative spine surgeries employing pedicle screw fixation and instrumentation in most cases is still valuable, with a pooled sensitivity of 76.2%.
Spinal Tumor Surgery
The value of neuromonitoring for spinal tumor surgery is also well established. A previous systematic review by Rijs et al included 14 studies and reported a pooled sensitivity of 80.8% for SSEP, 83.8% for MEP, and 83.5% for multimodal monitoring. We were able to identify 29 studies reporting the results of various neuromonitoring techniques for tumor surgery. We found the pooled sensitivity of SSEP for tumor surgery to be 33.2% based on 5 studies; 85.4% for MEP based on 12 studies; and 88.6% for multimodal based on 17 studies. Moreover, we were also able to parse out the statistics based on type of tumors, ie intra-medullary vs extra-medullary. Among patients undergoing surgery for intra-medullary tumors, the sensitivity for MEP monitoring was 74% and for multimodal was 81.4%. For extra-medullary tumors, the sensitivity for MEP and multimodal monitoring were 85.9% and 82.9%, respectively.
It is clear that IONM is a tool with significant usefulness when removing spinal cord tumors. It provides the surgeon with crucial knowledge about potential spinal cord damage and has enabled more complete tumor resections. Its important to remember that IONM is a tool and not a cure. Its value varies depending on the particular circumstance and how the surgeon applies the knowledge from the IONM alert to the resection technique. However, it would be much more useful to gather information with a longer follow-up on both the neurologic result and the quality of life in the present era of value-based health care. 11
Conclusion
The present systematic review and meta-analysis has summarized the role of neuromonitoring for detecting ISCI during spine surgery. Our results indicate that there is low level evidence that all neuromonitoring modalities have acceptable performance in terms of detecting ISCI, particularly for high-risk spinal surgery. It is therefore recommended that some form of neuromonitoring be employed, particularly in high-risk spinal surgery.
Supplemental Material
Supplemental Material for Accuracy of Intraoperative Neuromonitoring in the Diagnosis of Intraoperative Neurological Decline in the Setting of Spinal Surgery—A Systematic Review and Meta-Analysis by Mohammed Ali Alvi, Brian K. Kwon, Nader Hejrati, Lindsay A. Tetreault, Nathan Evaniew, Andrea C. Skelly, and Michael G. Fehlings in Global Spine Journal
Acknowledgments
MGF is supported by the Robert Campeau Family Foundation/Dr C.H Tator Chair in Brain and Spinal Cord Research at UHN. BKK is the Canada Research Chair in Spinal Cord Injury and the Dvorak Chair in Spinal Trauma. NH acknowledges support by the Research Fund of the University of Basel for Excellent Junior Researchers. This Focus Issue was reviewed by the Joint Guidelines Review Committee of the American Association of Neurological Surgeons and Congress of Neurological Surgeons as well as the North American Spine Society. However, this review process does not constitute or imply endorsement of this work product by these organizations.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was financially supported by the AO Foundation, AO Spine and Praxis Spinal Cord Institute. This study was jointly organized and funded by AO Foundation through the AO Spine Knowledge Forum Spinal Cord Injury (SCI) (www.aospine.org/kf-sci), a focused group of international SCI experts, and the Praxis Spinal Cord Institute (https://praxisinstitute.org/) through funding from Western Economic Diversification Canada. The funding bodies did not control or influence the editorial content of the articles or the guidelines process. Methodologic and analytic support for this work was provided by Aggregate Analytics, Inc, with funding from the AO Foundation and Praxis Spinal Cord Institute.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Mohammed Ali Alvi https://orcid.org/0000-0002-7131-079X
Nader Hejrati https://orcid.org/0000-0001-8583-9849
Nathan Evaniew https://orcid.org/0000-0003-1974-5224
Michael G. Fehlings https://orcid.org/0000-0002-5722-6364
References
- 1.Ahn H, Fehlings MG. Prevention, identification, and treatment of perioperative spinal cord injury. Neurosurg Focus. 2008;25(5):E15. [DOI] [PubMed] [Google Scholar]
- 2.Hilibrand AS, Schwartz DM, Sethuraman V, Vaccaro AR, Albert TJ. Comparison of transcranial electric motor and somatosensory evoked potential monitoring during cervical spine surgery. J Bone Joint Surg Am. 2004;86(6):1248-1253. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald DB, Al Zayed Z, Khoudeir I, Stigsby B. Monitoring scoliosis surgery with combined multiple pulse transcranial electric motor and cortical somatosensory-evoked potentials from the lower and upper extremities. Spine. 2003;28(2):194-203. [DOI] [PubMed] [Google Scholar]
- 4.Fehlings MG, Brodke DS, Norvell DC, Dettori JR. The evidence for intraoperative neurophysiological monitoring in spine surgery: does it make a difference? Spine. 2010;35(9S):S37. [DOI] [PubMed] [Google Scholar]
- 5.Gunnarsson T, Krassioukov AV, Sarjeant R, FehlingsReal-Time Continuous Intraoperative Electromyographic MG, Evoked S. Potential recordings in spinal surgery: Correlation of clinical and electrophysiologic findings in a prospective, consecutive series of 213 cases. Spine. 2004;29(6):677-684. doi: 10.1097/01.brs.0000115144.30607.e9. [DOI] [PubMed] [Google Scholar]
- 6.Liu Q, Wang Q, Liu H, Wu WKK, Chan MTV. Warning criteria for intraoperative neurophysiologic monitoring. Curr Opin Anaesthesiol. 2017;30(5):557-562. [DOI] [PubMed] [Google Scholar]
- 7.Chang R, Reddy RP, Coutinho DV, et al. Diagnostic accuracy of SSEP changes during lumbar spine surgery for predicting postoperative neurological deficit: A systematic review and meta-analysis. Spine. 2021;46(24):E1343-E1352. [DOI] [PubMed] [Google Scholar]
- 8.Reddy RP, Chang R, Coutinho DV, et al. Triggered electromyography is a useful intraoperative adjunct to predict postoperative neurological deficit following lumbar pedicle screw instrumentation. Global Spine J. 2022;12(5):1003-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thirumala PD, Huang J, Brahme IS, et al. Alarm criteria for motor evoked potentials. Neurol India. 2017;65(4):708-715. [DOI] [PubMed] [Google Scholar]
- 10.Thirumala PD, Huang J, Thiagarajan K, Cheng H, Balzer J, Crammond DJ. Diagnostic accuracy of combined multimodality somatosensory evoked potential and transcranial motor evoked potential intraoperative monitoring in patients with idiopathic scoliosis. Spine. 2016;41(19):E1177-E1184. [DOI] [PubMed] [Google Scholar]
- 11.Rijs K, Klimek M, Scheltens-de Boer M, Biesheuvel K, Harhangi BS. Intraoperative neuromonitoring in patients with intramedullary spinal cord tumor: A systematic review, meta-analysis, and case series. World Neurosurg. 2019;125:498-510. [DOI] [PubMed] [Google Scholar]
- 12.Di Martino A, Papalia R, Caldaria A, Torre G, Denaro L, Denaro V. Should evoked potential monitoring be used in degenerative cervical spine surgery? A systematic review. J Orthop Traumatol. 2019;20(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ajiboye RM, Zoller SD, Sharma A, et al. Intraoperative neuromonitoring for anterior cervical spine surgery: What is the evidence? Spine. 2017;42(6):385-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holdefer RN, Skinner SA. Motor evoked potential recovery with surgeon interventions and neurologic outcomes: A meta-analysis and structural causal model for spine deformity surgeries. Clin Neurophysiol. 2020;131(7):1556-1566. doi: 10.1016/j.clinph.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 15.Daniel JW, Botelho RV, Milano JB, et al. Intraoperative neurophysiological monitoring in spine surgery: A systematic review and meta-analysis. Spine. 2018;43(16):1154-1160. [DOI] [PubMed] [Google Scholar]
- 16.Charalampidis A, Jiang F, Wilson JRF, Badhiwala JH, Brodke DS, Fehlings MG. The use of intraoperative neurophysiological monitoring in spine surgery. Global Spine J. 2020;10(1 Suppl):104S-114S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.PRISMA . 2022. https://www.prisma-statement.org/Extensions/DTA
- 18.Covidence . Covidence Systematic Review Software, Veritas Health Innovation, Melbourne: Australia. 2021. [Google Scholar]
- 19.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529-536. [DOI] [PubMed] [Google Scholar]
- 20.robvis . 2022. https://mcguinlu.shinyapps.io/robvis/
- 21.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55-61. [DOI] [PubMed] [Google Scholar]
- 22.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: Data-analytic approaches and some additional considerations. Stat Med. 1993;12(14):1293-1316. [DOI] [PubMed] [Google Scholar]
- 23.Littenberg B, Moses LE. Estimating diagnostic accuracy from multiple conflicting reports: A new meta-analytic method. Med Decis Making. 1993;13(4):313-321. [DOI] [PubMed] [Google Scholar]
- 24.Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol. 2005;58(10):982-990. [DOI] [PubMed] [Google Scholar]
- 25.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20(19):2865-2884. [DOI] [PubMed] [Google Scholar]
- 26.Shim SR, Kim SJ, Lee J. Diagnostic test accuracy: Application and practice using R software. Epidemiology and Health. 2019;41:e2019007. doi: 10.4178/epih.e2019007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arends LR, Hamza TH, van Houwelingen JC, Heijenbrok-Kal MH, Hunink MGM, Stijnen T. Bivariate random effects meta-analysis of ROC curves. Med Decis Making. 2008;28(5):621-638. [DOI] [PubMed] [Google Scholar]
- 28.Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JAC. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics. 2007;8(2):239-251. [DOI] [PubMed] [Google Scholar]
- 29.Mada . Meta-analysis of diagnostic accuracy. Comprehensive R Archive Network (CRAN). 2022. https://cran.r-project.org/web/packages/mada/index.html [Google Scholar]
- 30.Multivariate Normal and t Distributions [R package mvtnorm version 1.1-3]. 2022. https://cran.r-project.org/web/packages/mvtnorm/index.html [Google Scholar]
- 31.Ellipse . Functions for drawing ellipses and ellipse-like confidence regions. Comprehensive R Archive Network (CRAN). 2022. https://cran.r-project.org/web/packages/ellipse/index.html [Google Scholar]
- 32.mvtmeta . Multivariate meta-analysis. Comprehensive R archive network (CRAN). 2022. https://cran.r-project.org/web/packages/mvtmeta/index.html [Google Scholar]
- 33.Schwarzer G. General Package for Meta-Analysis [R package meta version 6.1-0]. Published online. 2022. https://cran.r-project.org/web/packages/meta [Google Scholar]
- 34.Viechtbauer W. Meta-analysis package for r [R package metafor version 3.8-1]. Published online. 2022. https://cran.r-project.org/web/packages/metafor [Google Scholar]
- 35.rmeta . Meta-analysis. Comprehensive R Archive Network (CRAN). 2022. https://cran.r-project.org/web/packages/rmeta/index.html [Google Scholar]
- 36.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ, 315; 1997:629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong C, Salanti G, Morton SC, et al. Testing small study effects in multivariate meta-analysis. Biometrics. 2020;76(4):1240-1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noma H. Discussion on “testing small study effects in multivariate meta-analysis” by chuan Hong, Georgia salanti, sally morton, richard riley, haitao chu, stephen E. Kimmel, and yong chen. Biometrics. 2020;76(4):1255-1259. [DOI] [PubMed] [Google Scholar]
- 39.Noma H. MVPBT: R package for publication bias tests in meta-analysis of diagnostic accuracy studies. arXiv [statCO]. 2022. http://arxiv.org/abs/2209.07270 [Google Scholar]
- 40.Yang B, Mustafa RA, Bossuyt PM, et al. GRADE Guidance: 31. Assessing the certainty across a body of evidence for comparative test accuracy. J Clin Epidemiol. 2021;136:146-156. [DOI] [PubMed] [Google Scholar]
- 41.Institute of Medicine . Board on health care services, committee on standards for systematic reviews of comparative effectiveness research. Finding what works in health care: standards for systematic reviews. National Academies Press; 2011. [PubMed] [Google Scholar]
- 42.Abdelkader AA, Zohdi A. El gohary AM, el-hadidy RA, AlMahdy RA. Somatosensory evoked potentials as a stand-alone tool during spine surgery: An Egyptian preliminary report. J Clin Neurophysiol. 2019;36(2):161-165. [DOI] [PubMed] [Google Scholar]
- 43.Accadbled F, Henry P, de Gauzy JS, Cahuzac JP. Spinal cord monitoring in scoliosis surgery using an epidural electrode. Results of a prospective, consecutive series of 191 cases. Spine. 2006;31(22):2614-2623. [DOI] [PubMed] [Google Scholar]
- 44.AlMahdy RA, Wahid M, Abdelkader AA, Lotfy M, Soliman MAR. The utility of multimodal intraoperative neuromonitoring in spine surgery: case series from a lower-middle-income country perspective. World Neurosurg. 2021;152:e220-e226. [DOI] [PubMed] [Google Scholar]
- 45.Avila EK, Bradley Elder J, Singh P, Chen X, Bilsky MH. Intraoperative neurophysiologic monitoring and neurologic outcomes in patients with epidural spine tumors. Clin Neurol Neurosurg. 2013;115(10):2147-2152. doi: 10.1016/j.clineuro.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Bhagat S, Durst A, Grover H, et al. An evaluation of multimodal spinal cord monitoring in scoliosis surgery: A single centre experience of 354 operations. Eur Spine J. 2015;24(7):1399-1407. [DOI] [PubMed] [Google Scholar]
- 47.Bhalodia VM, Schwartz DM, Sestokas AK, et al. Efficacy of intraoperative monitoring of transcranial electrical stimulation–induced motor evoked potentials and spontaneous electromyography activity to identify acute-versus delayed-onset C-5 nerve root palsy during cervical spine surgery. J Neurosurg Spine. 2013;19(4):395-402. [DOI] [PubMed] [Google Scholar]
- 48.Bose B, Wierzbowski LR, Sestokas AK. Neurophysiologic monitoring of spinal nerve root function during instrumented posterior lumbar spine surgery. Spine. 2002;27(13):1444-1450. [DOI] [PubMed] [Google Scholar]
- 49.Bose B, Sestokas AK, Schwartz DM. Neurophysiological detection of iatrogenic C-5 nerve deficit during anterior cervical spinal surgery. J Neurosurg Spine. 2007;6(5):381-385. [DOI] [PubMed] [Google Scholar]
- 50.Jaryal A, Bir M, Gupta U, et al. Predictive value of intraoperative D-wave and m-MEP neurophysiological monitoring in patients with preoperative motor deficits in immediate and late postoperative period. J Craniovertebral Junction Spine. 2021;12(1):26. doi: 10.4103/jcvjs.jcvjs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Calancie B, Harris W, Broton JG, Alexeeva N, Green BA. “Threshold-level” multipulse transcranial electrical stimulation of motor cortex for intraoperative monitoring of spinal motor tracts: Description of method and comparison to somatosensory evoked potential monitoring. J Neurosurg. 1998;88(3):457-470. [DOI] [PubMed] [Google Scholar]
- 52.Calancie B, Molano MR. Alarm criteria for motor-evoked potentials: What’s wrong with the “presence-or-absence” approach? Spine. 2008;33(4):406. [DOI] [PubMed] [Google Scholar]
- 53.Camino Willhuber GO, Bendersky M, Vilte C, et al. Accuracy of intraoperative neuromonitoring during percutaneous cement discoplasty. Rev Fac Cien Med Univ Nac Cordoba. 2021;78(3):257-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandanwale AS, Ramteke AA, Barhate S. Intra-operative somatosensory-evoked potential monitoring. J Orthop Surg. 2008;16(3):277-280. [DOI] [PubMed] [Google Scholar]
- 55.Chen B, Chen Y, Yang J, et al. Comparison of the wake-up test and combined TES-MEP and CSEP monitoring in spinal surgery. J Spinal Disord Tech. 2015;28(9):335-340. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y, Luo C, Wang J, et al. Roles of multimodal intra-operative neurophysiological monitoring (IONM) in percutaneous endoscopic transforaminal lumbar interbody fusion: a case series of 113 patients. BMC Musculoskelet Disord. 2021;22(1):989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi I, Hyun SJ, Kang JK, Rhim SC. Combined muscle motor and somatosensory evoked potentials for intramedullary spinal cord tumour surgery. Yonsei Med J. 2014;55(4):1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chung I, Glow JA, Dimopoulos V, et al. Upper-limb somatosensory evoked potential monitoring in lumbosacral spine surgery: A prognostic marker for position-related ulnar nerve injury. Spine J. 2009;9(4):287-295. [DOI] [PubMed] [Google Scholar]
- 59.Clark AJ, Safaee M, Chou D, et al. Comparative sensitivity of intraoperative motor evoked potential monitoring in predicting postoperative neurologic deficits: Nondegenerative versus degenerative myelopathy. Global Spine J. 2016;6(5):452-458. doi: 10.1055/s-0035-1565258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clark AJ, Ziewacz JE, Safaee M, et al. Intraoperative neuromonitoring with MEPs and prediction of postoperative neurological deficits in patients undergoing surgery for cervical and cervicothoracic myelopathy. Neurosurg Focus. 2013;35(1):E7. doi: 10.3171/2013.4.focus13121. [DOI] [PubMed] [Google Scholar]
- 61.Cornips E, Habets J, van Kranen-Mastenbroek V, Bos H, Bergs P, Postma A. Anterior transthoracic surgery with motor evoked potential monitoring for high-risk thoracic disc herniations: Technique and results. World Neurosurg. 2017;105:441-455. [DOI] [PubMed] [Google Scholar]
- 62.Costa P, Bruno A, Bonzanino M, et al. Somatosensory- and motor-evoked potential monitoring during spine and spinal cord surgery. Spinal Cord. 2007;45(1):86-91. [DOI] [PubMed] [Google Scholar]
- 63.Costa P, Peretta P, Faccani G. Relevance of intraoperative D wave in spine and spinal cord surgeries. Eur Spine J. 2013;22(4):840-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dauleac C, Boulogne S, Barrey CY, et al. Predictors of functional outcome after spinal cord surgery: Relevance of intraoperative neurophysiological monitoring combined with preoperative neurophysiological and MRI assessments. Neurophysiol Clin. 2022;52(3):242-251. doi: 10.1016/j.neucli.2022.03.004. [DOI] [PubMed] [Google Scholar]
- 65.Delgado-López PD, Montalvo-Afonso A, Araus-Galdós E, et al. Need for head and neck repositioning to restore electrophysiological signal changes at positioning for cervical myelopathy surgery. Neurocirugia. 2022;33(5):209-218. [DOI] [PubMed] [Google Scholar]
- 66.Deutsch H, Arginteanu M, Manhart K, et al. Somatosensory evoked potential monitoring in anterior thoracic vertebrectomy. J Neurosurg. 2000;92(2 Suppl):155-161. [DOI] [PubMed] [Google Scholar]
- 67.Ding Y, Hu Y, Ruan DK, Chen B. Value of somatosensory evoked potentials in diagnosis, surgical monitoring and prognosis of cervical spondylotic myelopathy. Chin Med J. 2008;121(15):1374-1378. [PubMed] [Google Scholar]
- 68.Dinner DS, Lüders H, Lesser RP, Morris HH, Barnett G, Klem G. Intraoperative spinal somatosensory evoked potential monitoring. J Neurosurg. 1986;65(6):807-814. [DOI] [PubMed] [Google Scholar]
- 69.Duncan JW, Bailey RA, Baena R. Intraoperative decrease in amplitude of somatosensory-evoked potentials of the lower extremities with interbody fusion cage placement during lumbar fusion surgery. Spine. 2012;37(20):E1290-E1295. [DOI] [PubMed] [Google Scholar]
- 70.Eggspuehler A, Sutter MA, Grob D, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring during surgery of spinal deformities in 217 patients. Eur Spine J. 2007;2:S188–S196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eggspuehler A, Sutter MA, Grob D, Porchet F, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring (MIOM) during surgical decompression of thoracic spinal stenosis in 36 patients. Eur Spine J. 2007;2:S216–S220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eggspuehler A, Sutter MA, Grob D, Jeszenszky D, Porchet F, Dvorak J. Multimodal intraoperative monitoring (MIOM) during cervical spine surgical procedures in 246 patients. Eur Spine J. 2007;2:S209–S215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El-Hawary R, Sucato DJ, Sparagana S, McClung A, Van Allen E, Rampy P. Spinal cord monitoring in patients with spinal deformity and neural axis abnormalities: A comparison with adolescent idiopathic scoliosis patients. Spine. 2006;31(19):E698-E706. [DOI] [PubMed] [Google Scholar]
- 74.Feng B, Qiu G, Shen J, et al. Impact of multimodal intraoperative monitoring during surgery for spine deformity and potential risk factors for neurological monitoring changes. J Spinal Disord Tech. 2012;25(4):E108-E114. [DOI] [PubMed] [Google Scholar]
- 75.Ferguson J, Hwang SW, Tataryn Z, Samdani AF. Neuromonitoring changes in pediatric spinal deformity surgery: A single-institution experience. J Neurosurg Pediatr. 2014;13(3):247-254. [DOI] [PubMed] [Google Scholar]
- 76.Feyissa AM, Tummala S. Intraoperative neurophysiologic monitoring with Hoffmann reflex during thoracic spine surgery. J Clin Neurosci. 2015;22(6):990-994. [DOI] [PubMed] [Google Scholar]
- 77.Forster MT, Marquardt G, Seifert V, Szelényi A. Spinal cord tumor surgery—importance of continuous intraoperative neurophysiological monitoring after tumor resection. Spine. 2012;37(16):E1001. [DOI] [PubMed] [Google Scholar]
- 78.Fujiwara Y, Manabe H, Izumi B, Tanaka H, Kawai K, Tanaka N. The efficacy of intraoperative neurophysiological monitoring using transcranial electrically stimulated muscle-evoked potentials (TcE-MsEPs) for predicting postoperative segmental upper extremity motor paresis after cervical laminoplasty. Clinical spine surgery. A Spine Publication. 2016;29(4):E188-E195. doi: 10.1097/bsd.0000000000000311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Funaba M, Kanchiku T, Yoshida G, et al. Efficacy of intraoperative neuromonitoring using transcranial motor-evoked potentials for degenerative cervical myelopathy: A prospective multicenter study by the monitoring committee of the Japanese society for spine surgery and related research. Spine. 2022;47(1):E27-E37. [DOI] [PubMed] [Google Scholar]
- 80.Funaba M, Kanchiku T, Kobayashi K, et al. The utility of transcranial stimulated motor-evoked potential alerts in cervical spine surgery varies based on preoperative motor status. Spine. 2022;47(23):1659-1668. [DOI] [PubMed] [Google Scholar]
- 81.Ghadirpour R, Nasi D, Iaccarino C, et al. Intraoperative neurophysiological monitoring for intradural extramedullary spinal tumors: Predictive value and relevance of D-wave amplitude on surgical outcome during a 10-year experience. J Neurosurg Spine. 2018;30(2):259-267. [DOI] [PubMed] [Google Scholar]
- 82.Ghadirpour R, Nasi D, Iaccarino C, et al. Intraoperative neurophysiological monitoring for intradural extramedullary tumors: Why not? Clin Neurol Neurosurg. 2015;130:140-149. doi: 10.1016/j.clineuro.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 83.Gavaret M, Maillard L, Jung J, High-resolution EEG. (HR-EEG) and magnetoencephalography (MEG). Neurophysiol Clin. 2015;45(1):105-111. [DOI] [PubMed] [Google Scholar]
- 84.Harel R, Schleifer D, Appel S, Attia M, Cohen ZR, Knoller N. Spinal intradural extramedullary tumors: The value of intraoperative neurophysiologic monitoring on surgical outcome. Neurosurg Rev. 2017;40(4):613-619. [DOI] [PubMed] [Google Scholar]
- 85.Hsu B, Cree AK, Lagopoulos J, Cummine JL. Transcranial motor-evoked potentials combined with response recording through compound muscle action potential as the sole modality of spinal cord monitoring in spinal deformity surgery. Spine. 2008;33(10):1100-1106. doi: 10.1097/brs.0b013e31816f5f09. [DOI] [PubMed] [Google Scholar]
- 86.Hyun SJ, Rhim SC, Kang JK, Hong SH, Park BRG. Combined motor- and somatosensory-evoked potential monitoring for spine and spinal cord surgery: Correlation of clinical and neurophysiological data in 85 consecutive procedures. Spinal Cord. 2009;47(8):616-622. [DOI] [PubMed] [Google Scholar]
- 87.Hu Y, Liu H, Luk KD. Time–frequency analysis of somatosensory evoked potentials for intraoperative spinal cord monitoring. J Clin Neurophysiol. 2011;28(5):504. [DOI] [PubMed] [Google Scholar]
- 88.Korn A, Halevi D, Lidar Z, Biron T, Ekstein P, Constantini S. Intraoperative neurophysiological monitoring during resection of intradural extramedullary spinal cord tumors: Experience with 100 cases. Acta Neurochir. 2015;157(5):819-830. [DOI] [PubMed] [Google Scholar]
- 89.Ito Z, Imagama S, Sakai Y, et al. A new criterion for the alarm point for compound muscle action potentials. J Neurosurg Spine. 2012;17(4):348-356. [DOI] [PubMed] [Google Scholar]
- 90.Ito Z, Matsuyama Y, Shinomiya K, et al. Usefulness of multi-channels in intraoperative spinal cord monitoring: Multi-center study by the monitoring committee of the Japanese Society for Spine Surgery and related research. Eur Spine J. 2013;22(8):1891-1896. doi: 10.1007/s00586-013-2722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koyanagi I, Iwasaki Y, Isu T, Abe H, Akino M, Kuroda S. Spinal cord evoked potential monitoring after spinal cord stimulation during surgery of spinal cord tumors. Neurosurgery. 1993;33(3):459–460. [DOI] [PubMed] [Google Scholar]
- 92.Jarvis JG, Strantzas S, Lipkus M, et al. Responding to neuromonitoring changes in 3-column posterior spinal osteotomies for rigid pediatric spinal deformities. Spine. 2013;38(8):E493-E503. [DOI] [PubMed] [Google Scholar]
- 93.Jin SH, Chung CK, Kim CH, Choi YD, Kwak G, Kim BE. Multimodal intraoperative monitoring during intramedullary spinal cord tumor surgery. Acta Neurochir. 2015;157(12):2149-2155. [DOI] [PubMed] [Google Scholar]
- 94.Kamerlink JR, Errico T, Xavier S, et al. Major intraoperative neurologic monitoring deficits in consecutive pediatric and adult spinal deformity patients at one institution. Spine. 2010;35(2):240-245. [DOI] [PubMed] [Google Scholar]
- 95.Kelleher MO, Tan G, Sarjeant R, Fehlings MG. Predictive value of intraoperative neurophysiological monitoring during cervical spine surgery: A prospective analysis of 1055 consecutive patients. J Neurosurg Spine. 2008;8(3):215-221. [DOI] [PubMed] [Google Scholar]
- 96.Khan MH, Smith PN, Balzer JR, et al. Intraoperative somatosensory evoked potential monitoring during cervical spine corpectomy surgery: Experience with 508 cases. Spine. 2006;31(4):E105-E113. [DOI] [PubMed] [Google Scholar]
- 97.Kim DH, Zaremski J, Kwon B, et al. Risk factors for false positive transcranial motor evoked potential monitoring alerts during surgical treatment of cervical myelopathy. Spine. 2007;32(26):3041-3046. [DOI] [PubMed] [Google Scholar]
- 98.Kim SM, Yang H, Park SB, et al. Pattern-specific changes and discordant prognostic values of individual leg-muscle motor evoked potentials during spinal surgery. Clin Neurophysiol. 2012;123(7):1465-1470. [DOI] [PubMed] [Google Scholar]
- 99.Kim JE, Kim JS, Yang S, et al. Neurophysiological monitoring during anterior cervical discectomy and fusion for ossification of the posterior longitudinal ligament. Clin Neurophysiol Pract. 2021;6:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim DG, Son YR, Park YS, et al. Differences in multimodality intraoperative neurophysiological monitoring changes between spinal intramedullary ependymoma and hemangioblastoma. J Clin Neurophysiol. 2016;33(2):120-126. [DOI] [PubMed] [Google Scholar]
- 101.Kim DG, Jo SR, Park YS, et al. Multi-channel motor evoked potential monitoring during anterior cervical discectomy and fusion. Clin Neurophysiol Pract. 2017;2:48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kobayashi S, Matsuyama Y, Shinomiya K, et al. A new alarm point of transcranial electrical stimulation motor evoked potentials for intraoperative spinal cord monitoring: A prospective multicenter study from the spinal cord monitoring working group of the Japanese society for Spine surgery and related research. J Neurosurg Spine. 2014;20(1):102-107. [DOI] [PubMed] [Google Scholar]
- 103.Kobayashi K, Ando K, Shinjo R, et al. A new criterion for the alarm point using a combination of waveform amplitude and onset latency in Br (E)-MsEP monitoring in spine surgery. J Neurosurg Spine. 2018;29(4):435-441. [DOI] [PubMed] [Google Scholar]
- 104.Kobayashi K, Ando K, Shinjo R, et al. Evaluation of a combination of waveform amplitude and peak latency in intraoperative spinal cord monitoring. Spine. 2018;43(17):1231-1237. [DOI] [PubMed] [Google Scholar]
- 105.Kobayashi K, Ando K, Tsushima M, et al. Characteristics of multi-channel Br(E)-MsEP waveforms for the lower extremity muscles in thoracic spine surgery: Comparison based on preoperative motor status. Eur Spine J. 2019;28(3):484-491. doi: 10.1007/s00586-018-5825-4. [DOI] [PubMed] [Google Scholar]
- 106.Kobayashi K, Imagama S, Ando K, et al. Characteristics of Tc-MEP waveforms for different locations of intradural extramedullary tumors: A prospective multicenter study of the monitoring committee of the Japanese society for spine surgery and related research. Spine. 2022;47(2):172-179. [DOI] [PubMed] [Google Scholar]
- 107.Kobayashi K, Ando K, Yoshida G, et al. Characteristics of Tc-MEP waveforms in spine surgery for patients with severe obesity. Spine. 2021;46(24):1738-1747. [DOI] [PubMed] [Google Scholar]
- 108.Kobayashi K, Imagama S, Yoshida G, et al. Effects of preoperative motor status on intraoperative motor-evoked potential monitoring for high-risk spinal surgery: A prospective multicenter study. Spine. 2021;46(12):E694-E700. [DOI] [PubMed] [Google Scholar]
- 109.Krishnakumar R, Srivatsa N. Multimodal intraoperative neuromonitoring in scoliosis surgery: A two-year prospective analysis in a single centre. Neurol India. 2017;65(1):75-79. [DOI] [PubMed] [Google Scholar]
- 110.Kumar N, G V RN, et al. Intraoperative neuromonitoring (IONM): Is there a role in metastatic spine tumor surgery? Spine. 2019;44(4):E219-E224. [DOI] [PubMed] [Google Scholar]
- 111.Kurokawa R, Kim P, Itoki K, et al. False-positive and false-negative results of motor evoked potential monitoring during surgery for intramedullary spinal cord tumors. Oper Neurosurg (Hagerstown). 2018;14(3):279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kundnani VK, Zhu L, Tak H, Wong H. Multimodal intraoperative neuromonitoring in corrective surgery for adolescent idiopathic scoliosis: Evaluation of 354 consecutive cases. Indian J Orthop. 2010;44(1):64-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lakomkin N, Mistry AM, Zuckerman SL, et al. Utility of intraoperative monitoring in the resection of spinal cord tumors: An analysis by tumor location and anatomical region. Spine. 2018;43(4):287-294. [DOI] [PubMed] [Google Scholar]
- 114.Langeloo DD, Lelivelt A, Louis Journée H, Slappendel R, de Kleuver M. Transcranial electrical motor-evoked potential monitoring during surgery for spinal deformity: A study of 145 patients. Spine. 2003;28(10):1043-1050. [DOI] [PubMed] [Google Scholar]
- 115.Lee JJ, Hong JT, Kim IS, Kwon JY, Lee JB, Park JH. Significance of multimodal intraoperative monitoring during surgery in patients with craniovertebral junction pathology. World Neurosurgery. 2018;118:e887-e894. doi: 10.1016/j.wneu.2018.07.092. [DOI] [PubMed] [Google Scholar]
- 116.Lau D, Dalle Ore CL, Reid P, et al. Utility of neuromonitoring during lumbar pedicle subtraction osteotomy for adult spinal deformity. J Neurosurg Spine. 2019;31(3):397-407. doi: 10.3171/2019.3.spine181409. [DOI] [PubMed] [Google Scholar]
- 117.Lau D, Guo L, Deviren V, Ames CP. Utility of intraoperative neuromonitoring and outcomes of neurological complication in lower cervical and upper thoracic posterior-based three-column osteotomies for cervical deformity. J Neurosurg Spine. 2022;36(3):470-478. doi: 10.3171/2021.5.spine202057. [DOI] [PubMed] [Google Scholar]
- 118.Leung YL, Grevitt M, Henderson L, SmithCord Monitoring Changes J, Vessel S. Ligation in the “at risk” cord during anterior spinal deformity surgery. Spine. 2005;30(16):1870. [DOI] [PubMed] [Google Scholar]
- 119.Li F, Gorji R, Allott G, Modes K, Lunn R, Yang ZJ. The usefulness of intraoperative neurophysiological monitoring in cervical spine surgery: A retrospective analysis of 200 consecutive patients. J Neurosurg Anesthesiol. 2012;24(3):185-190. [DOI] [PubMed] [Google Scholar]
- 120.Li X, Zhang HQ, Ling F, et al. Intraoperative neurophysiological monitoring during the surgery of spinal arteriovenous malformation: Sensitivity, specificity, and warning criteria. Clin Neurol Neurosurg. 2018;165:29-37. [DOI] [PubMed] [Google Scholar]
- 121.Lieberman JA, Lyon R, Feiner J, Hu SS, Berven SH. The efficacy of motor evoked potentials in fixed sagittal imbalance deformity correction surgery. Spine. 2008;33(13):E414-E424. [DOI] [PubMed] [Google Scholar]
- 122.Liu T, Yan L, Qi H, et al. Diagnostic value of multimodal intraoperative neuromonitoring by combining somatosensory-with motor-evoked potential in posterior decompression surgery for thoracic spinal stenosis. Front Neurosci. 2022:16. doi: 10.3389/fnins.2022.879435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Loder RT, Thomson GJ, LaMONT RL. Spinal cord monitoring in patients with nonidiopathic spinal deformities using somatosensory evoked potentials. Spine. 1991;16(12):1359-1364. doi: 10.1097/00007632-199112000-00003. [DOI] [PubMed] [Google Scholar]
- 124.Lubitz SE, Keith RW, Crawford AH. Intraoperative experience with neuromotor evoked potentials. A review of 60 consecutive cases. Spine. 1999;24(19):2033–2034. [DOI] [PubMed] [Google Scholar]
- 125.Luk KD, Hu Y, Wong YW, Cheung KM. Evaluation of various evoked potential techniques for spinal cord monitoring during scoliosis surgery. Spine. 2001;26(16):1772-1777. [DOI] [PubMed] [Google Scholar]
- 126.MacDonald DB, Al Zayed Z, Al Saddigi A. Four-limb muscle motor evoked potential and optimized somatosensory evoked potential monitoring with decussation assessment: Results in 206 thoracolumbar spine surgeries. Eur Spine J. 2007;16(S2):171-187. doi: 10.1007/s00586-007-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Makarov MR, Samchukov ML, Birch JG, Cherkashin AM, Sparagana SP, Delgado MR. Somatosensory evoked potential monitoring of peripheral nerves during external fixation for limb lengthening and correction of deformity in children. J Bone Joint Surg Br. 2012;94(10):1421-1426. [DOI] [PubMed] [Google Scholar]
- 128.Manninen PH. Monitoring evoked potentials during spinal surgery in one institution. Can J Anaesth. 1998;45(5 Pt 1):460-465. [DOI] [PubMed] [Google Scholar]
- 129.Melachuri SR, Kaur J, Melachuri MK, et al. The diagnostic accuracy of somatosensory evoked potentials in evaluating neurological deficits during 1057 lumbar interbody fusions. J Clin Neurosci. 2019;61:78-83. [DOI] [PubMed] [Google Scholar]
- 130.Melachuri SR, Melachuri MK, Anetakis K, Crammond DJ, Balzer JR, Thirumala PD. Diagnostic accuracy of thresholds less than or equal to 8 mA in pedicle screw testing during lumbar spine procedures to predict new postoperative lower extremity neurological deficits. Spine. 2021;46(2):E139. [DOI] [PubMed] [Google Scholar]
- 131.Melachuri SR, Kaur J, Melachuri MK, Crammond DJ, Balzer JR, Thirumala PD. The diagnostic accuracy of somatosensory evoked potentials in evaluating neurological deficits during 1036 posterior spinal fusions. Neurol Res. 2017;39(12):1073-1079. [DOI] [PubMed] [Google Scholar]
- 132.Melachuri SR, Stopera C, Melachuri MK, et al. The efficacy of somatosensory evoked potentials in evaluating new neurological deficits after spinal thoracic fusion and decompression. J Neurosurg Spine. 2020:1-6. [DOI] [PubMed] [Google Scholar]
- 133.May DM, Jones SJ, Alan Crockard H. Somatosensory evoked potential monitoring in cervical surgery: Identification of pre- and intraoperative risk factors associated with neurological deterioration. J Neurosurg. 1996;85(4):566-573. doi: 10.3171/jns.1996.85.4.0566. [DOI] [PubMed] [Google Scholar]
- 134.Miller SM, Donegan SW, Voigt N, et al. Transcranial motor-evoked potentials for prediction of postoperative neurologic and motor deficit following surgery for thoracolumbar scoliosis. Orthop Rev. 2019;1:11. doi: 10.4081/or.2019.7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meyer PR, Jr, Cotler HB, Gireesan GT. Operative neurological complications resulting from thoracic and lumbar spine internal fixation. Clin Orthop Relat Res. 1988;237:125-131. [PubMed] [Google Scholar]
- 136.Mochida K, Komori H, Okawa A, Shinomiya K. Evaluation of motor function during thoracic and thoracolumbar spinal surgery based on motor-evoked potentials using train spinal stimulation. Spine. 1997;22(12):1385-1393. doi: 10.1097/00007632-199706150-00018. [DOI] [PubMed] [Google Scholar]
- 137.Muramoto A, Imagama S, Ito Z, et al. The cutoff amplitude of transcranial motor evoked potentials for transient postoperative motor deficits in intramedullary spinal cord tumor surgery. Spine. 2014;39(18):E1086-E1094. [DOI] [PubMed] [Google Scholar]
- 138.Muramoto A, Imagama S, Ito Z, et al. The cutoff amplitude of transcranial motor-evoked potentials for predicting postoperative motor deficits in thoracic spine surgery. Spine. 2013;38(1):E21-E27. doi: 10.1097/brs.0b013e3182796b15. [DOI] [PubMed] [Google Scholar]
- 139.Noonan KJ, Walker T, Feinberg JR, Nagel M, Didelot W, Lindseth R. Factors related to false- versus true-positive neuromonitoring changes in adolescent idiopathic scoliosis surgery. Spine. 2002;27(8):825-830. [DOI] [PubMed] [Google Scholar]
- 140.Neira VM, Ghaffari K, Bulusu S, et al. Diagnostic accuracy of neuromonitoring for identification of new neurologic deficits in pediatric spinal fusion surgery. Anesth Analg. 2016;123(6):1556-1566. doi: 10.1213/ane.0000000000001503. [DOI] [PubMed] [Google Scholar]
- 141.Noordeen MH, Lee J, Gibbons CE, Taylor BA, Bentley G. Spinal cord monitoring in operations for neuromuscular scoliosis. J Bone Joint Surg Br. 1997;79(1):53-57. [DOI] [PubMed] [Google Scholar]
- 142.Oya J, Burke JF, Vogel T, Tay B, Chou D, Mummaneni P. The accuracy of multimodality intraoperative neuromonitoring to predict postoperative neurologic deficits following cervical laminoplasty. World Neurosurgery. 2017;106:17-25. doi: 10.1016/j.wneu.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 143.Padberg AM, Wilson-Holden TJ, Lenke LG, Bridwell KH. Somatosensory- and motor-evoked potential monitoring without a wake-up test during idiopathic scoliosis surgery. An accepted standard of care. Spine. 1998;23(12):1392-1400. [DOI] [PubMed] [Google Scholar]
- 144.Padberg AM, Russo MH, Lenke LG, Bridwell KH, Komanetsky RM. Validity and reliability of spinal cord monitoring in neuromuscular spinal deformity surgery. J Spinal Disord. 1996;9(2):150. doi: 10.1097/00002517-199604000-00012. [DOI] [PubMed] [Google Scholar]
- 145.Papastefanou SL, Henderson LM, Smith NJ, Hamilton A, Webb JK. Surface electrode somatosensory-evoked potentials in spinal surgery: Implications for indications and practice. Spine. 2000;25(19):2467-2472. [DOI] [PubMed] [Google Scholar]
- 146.Paradiso G, Lee GYF, Sarjeant R, Hoang L, Massicotte EM, Fehlings MG. Multimodality intraoperative neurophysiologic monitoring findings during surgery for adult tethered cord syndrome: Analysis of a series of 44 patients with long-term follow-up. Spine. 2006;31(18):2095-2102. [DOI] [PubMed] [Google Scholar]
- 147.Park P, Wang AC, Sangala JR, et al. Impact of multimodal intraoperative monitoring during correction of symptomatic cervical or cervicothoracic kyphosis. J Neurosurg Spine. 2011;14(1):99-105. doi: 10.3171/2010.9.spine1085. [DOI] [PubMed] [Google Scholar]
- 148.Park JH, Lee SH, Kim ES, Eoh W. Analysis of multimodal intraoperative monitoring during intramedullary spinal ependymoma surgery. World Neurosurgery. 2018;120:e169-e180. doi: 10.1016/j.wneu.2018.07.267. [DOI] [PubMed] [Google Scholar]
- 149.Pastorelli F, Di Silvestre M, Plasmati R, et al. The prevention of neural complications in the surgical treatment of scoliosis: The role of the neurophysiological intraoperative monitoring. Eur Spine J. 2011;1:S105–S114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pastorelli F, Di Silvestre M, Vommaro F, et al. Intraoperative monitoring of somatosensory (SSEPs) and transcranial electric motor-evoked potentials (tce-MEPs) during surgical correction of neuromuscular scoliosis in patients with central or peripheral nervous system diseases. Eur Spine J. 2015;24(S7):931-936. doi: 10.1007/s00586-015-4282-6. [DOI] [PubMed] [Google Scholar]
- 151.Pelosi L, Lamb J, Grevitt M, Mehdian SMH, Webb JK, Blumhardt LD. Combined monitoring of motor and somatosensory evoked potentials in orthopaedic spinal surgery. Clin Neurophysiol. 2002;113(7):1082-1091. [DOI] [PubMed] [Google Scholar]
- 152.Bello JP, Pérez-Lorensu PJ, Roldán-Delgado H, et al. Role of multimodal intraoperative neurophysiological monitoring during positioning of patient prior to cervical spine surgery. Clin Neurophysiol. 2015;126(6):1264-1270. doi: 10.1016/j.clinph.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 153.Qiu J, Liu W, Shi B, et al. Intra-Operative neurophysiological monitoring in patients with intraspinal abnormalities undergoing posterior spinal fusion. Orthop Surg. 2022;14(8):1615-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Quiñones-Hinojosa A, Lyon R, Zada G, et al. Changes in transcranial motor evoked potentials during intramedullary spinal cord tumor resection correlate with postoperative motor function. Neurosurgery. 2005;56(5):982–993. [PubMed] [Google Scholar]
- 155.Quraishi NA, Lewis SJ, Kelleher MO, Sarjeant R, Rampersaud YR, Fehlings MG. Intraoperative multimodality monitoring in adult spinal deformity: Analysis of a prospective series of one hundred two cases with independent evaluation. Spine. 2009;34(14):1504-1512. [DOI] [PubMed] [Google Scholar]
- 156.Ruschel L, Aragão A, Oliveira M, Milano J, Neto M, Ramina R. Correlation of intraoperative neurophysiological parameters and outcomes in patients with intramedullary tumors. Asian Journal of Neurosurgery. 2021;16(02):243-248. doi: 10.4103/ajns.ajns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sakaki K, Kawabata S, Ukegawa D, et al. Warning thresholds on the basis of origin of amplitude changes in transcranial electrical motor-evoked potential monitoring for cervical compression myelopathy. Spine. 2012;37(15):E913-E921. doi: 10.1097/brs.0b013e31824caab6. [DOI] [PubMed] [Google Scholar]
- 158.Schär RT, Sutter M, Mannion AF, et al. Outcome of L5 radiculopathy after reduction and instrumented transforaminal lumbar interbody fusion of high-grade L5-S1 isthmic spondylolisthesis and the role of intraoperative neurophysiological monitoring. Eur Spine J. 2017;26(3):679-690. [DOI] [PubMed] [Google Scholar]
- 159.Schwartz DM, Auerbach JD, Dormans JP, et al. Neurophysiological detection of impending spinal cord injury during scoliosis surgery. J Bone Joint Surg Am. 2007;89(11):2440-2449. [DOI] [PubMed] [Google Scholar]
- 160.Skaggs DL, Choi PD, Rice C, et al. Efficacy of intraoperative neurologic monitoring in surgery involving a vertical expandable prosthetic titanium rib for early-onset spinal deformity. J Bone Jt Surg Am Vol. 2009;91(7):1657-1663. doi: 10.2106/jbjs.g.00202. [DOI] [PubMed] [Google Scholar]
- 161.Skinner SA, Nagib M, Bergman TA, Maxwell RE, Msangi G. The initial use of free-running electromyography to detect early motor tract injury during resection of intramedullary spinal cord lesions. Neurosurgery. 2005;56(2 Suppl):299–314. [DOI] [PubMed] [Google Scholar]
- 162.Sutter M, Eggspuehler A, Grob D, et al. The diagnostic value of multimodal intraoperative monitoring (MIOM) during spine surgery: A prospective study of 1,017 patients. Eur Spine J. 2007;16(S2):162-170. doi: 10.1007/s00586-007-0418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sutter M, Eggspuehler A, Jeszenszky D, et al. The impact and value of uni- and multimodal intraoperative neurophysiological monitoring (IONM) on neurological complications during spine surgery: A prospective study of 2728 patients. Eur Spine J. 2019;28(3):599-610. doi: 10.1007/s00586-018-5861-0. [DOI] [PubMed] [Google Scholar]
- 164.Sutter MA, Eggspuehler A, Grob D, Porchet F, Jeszenszky D, Dvorak J. Multimodal intraoperative monitoring (MIOM) during 409 lumbosacral surgical procedures in 409 patients. Eur Spine J. 2007;2(2):S221-S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Smith PN, Balzer JR, Khan MH, et al. Intraoperative somatosensory evoked potential monitoring during anterior cervical discectomy and fusion in nonmyelopathic patients–a review of 1,039 cases. Spine J. 2007;7(1):83-87. [DOI] [PubMed] [Google Scholar]
- 166.Stechison MT, Panagis SG. Reinhart SS. Somatosensory evoked potential. Acta Neurochir. 1995;135(1):56-61. [DOI] [PubMed] [Google Scholar]
- 167.Takahashi M, Imagama S, Kobayashi K, et al. Validity of the alarm point in intraoperative neurophysiological monitoring of the spinal cord by the monitoring working group of the Japanese society for spine surgery and related research. Spine. 2021;46(20):E1069-E1076. doi: 10.1097/brs.0000000000004065. [DOI] [PubMed] [Google Scholar]
- 168.Tanaka S, Hirao J, Oka H, Akimoto J, Takanashi J, Yamada J. Intraoperative monitoring during decompression of the spinal cord and spinal nerves using transcranial motor-evoked potentials: The law of twenty percent. J Clin Neurosci. 2015;22(9):1403-1407. doi: 10.1016/j.jocn.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 169.Taylor AJ, Combs K, Kay RD. et al. Neuromonitoring for cervical spondylotic myelopathy surgery causes confusion. Spine. 2021;46(22):E1185-E1191. doi: 10.1097/brs.0000000000004070. [DOI] [PubMed] [Google Scholar]
- 170.Thirumala PD, Bodily L, Tint D, et al. Somatosensory-evoked potential monitoring during instrumented scoliosis corrective procedures: Validity revisited. Spine J. 2014;14(8):1572-1580. doi: 10.1016/j.spinee.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 171.Tiruchelvarayan R, Tang MH, Perera S, Lo YL. Outcomes following aggressive surgical resection of intra-medullary spinal cord tumours with intra-operative neuro-monitoring. Proc Singapore Healthc. 2013;22(3):183-190. doi: 10.1177/201010581302200305. [DOI] [Google Scholar]
- 172.Tohmeh A, Somers C, Howell K. Saphenous somatosensory-evoked potentials monitoring of femoral nerve health during prone transpsoas lateral lumbar interbody fusion. Eur Spine J. 2022;31(7):1658-1666. doi: 10.1007/s00586-022-07224-9. [DOI] [PubMed] [Google Scholar]
- 173.Traba A, Romero JP, Arranz B, Vilela C. A new criterion for detection of radiculopathy based on motor evoked potentials and intraoperative nerve root monitoring. Clin Neurophysiol. 2018;129(10):2075-2082. doi: 10.1016/j.clinph.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 174.Yu T, Wang Y, Zhang XW, et al. Multimodal intraoperative monitoring during reduction of spine burst fracture and dislocation prevents neurologic injury. Medicine. 2018;10:97. doi: 10.1097/md.0000000000010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tsirikos AI, Aderinto J, Tucker SK, Noordeen HH. Spinal cord monitoring using intraoperative somatosensory evoked potentials for spinal trauma. J Spinal Disord Tech. 2004;17(5):385-394. doi: 10.1097/01.bsd.0000095825.98982.1a. [DOI] [PubMed] [Google Scholar]
- 176.Tsirikos AI, Duckworth AD, Henderson LE. et al. Med Princ Pract. 2020;29(1):6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ushirozako H, Hasegawa T, Ebata S, et al. Weekly teriparatide administration and preoperative anterior slippage of the cranial vertebra next to fusion segment < 2 mm promote osseous union after posterior lumbar interbody fusion. Spine. 2019;44(5):E288-E297. [DOI] [PubMed] [Google Scholar]
- 178.Ushirozako H, Yoshida G, Kobayashi S, et al. Transcranial motor evoked potential monitoring for the detection of nerve root injury during adult spinal deformity surgery. Asian Spine J. 2018;12(4):639-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ushirozako H, Yoshida G, Imagama S, et al. Efficacy of transcranial motor evoked potential monitoring during intra- and extramedullary spinal cord tumor surgery: A prospective multicenter study of the monitoring committee of the Japanese society for spine surgery and related research. Global Spine Journal. 2021:219256822110114. doi: 10.1177/21925682211011443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ushirozako H, Yoshida G, Kobayashi S, et al. Impact of total propofol dose during spinal surgery: Anesthetic fade on transcranial motor evoked potentials. J Neurosurg Spine. 2019;30(5):705-713. doi: 10.3171/2018.10.spine18322. [DOI] [PubMed] [Google Scholar]
- 181.van der Wal EC, Klimek M, Rijs K. Scheltens-de Boer M, biesheuvel K, harhangi BS. Intraoperative neuromonitoring in patients with intradural extramedullary spinal cord tumor: A single-center case series. World Neurosurg. 2021;147:e516-e523. [DOI] [PubMed] [Google Scholar]
- 182.Vitale MG, Moore DW, Matsumoto H, et al. Risk factors for spinal cord injury during surgery for spinal deformity. J Bone Joint Surg Am. 2010;92(1):64-71. [DOI] [PubMed] [Google Scholar]
- 183.Wang S, Zhuang Q, Zhang J, et al. Intra-operative MEP monitoring can work well in the patients with neural axis abnormality. Eur Spine J. 2016;25(10):3194-3200. doi: 10.1007/s00586-015-4205-6. [DOI] [PubMed] [Google Scholar]
- 184.Wang S, Zhang J, Tian Y, et al. Intraoperative motor evoked potential monitoring to patients with preoperative spinal deficits: Judging its feasibility and analyzing the significance of rapid signal loss. Spine J. 2017;17(6):777-783. doi: 10.1016/j.spinee.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 185.Wilent WB, Rhee JM, Harrop JS, et al. Therapeutic impact of traction release after C5 nerve root motor evoked potential (MEP) alerts in cervical spine surgery. Clinical Spine Surgery: A Spine Publication. 2020;33(10):E442-E447. doi: 10.1097/bsd.0000000000000969. [DOI] [PubMed] [Google Scholar]
- 186.Wilent WB, Bryan Wilent W, Tesdahl EA, et al. Utility of motor evoked potentials to diagnose and reduce lower extremity motor nerve root injuries during 4,386 extradural posterior lumbosacral spine procedures. Spine J. 2020;20(2):191-198. doi: 10.1016/j.spinee.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 187.Yoshida G, Imagama S, Kawabata S, et al. Adverse events related to transcranial electric stimulation for motor-evoked potential monitoring in high-risk spinal surgery. Spine. 2019;44(20):1435-1440. [DOI] [PubMed] [Google Scholar]
- 188.Yoshida G, Ando M, Imagama S, et al. Alert timing and corresponding intervention with intraoperative spinal cord monitoring for high-risk spinal surgery. Spine. 2019;44(8):E470-E479. [DOI] [PubMed] [Google Scholar]
- 189.Yoshida G, Ushirozako H, Kobayashi S, et al. Intraoperative neuromonitoring during adult spinal deformity surgery: Alert-positive cases for various surgical procedures. Spine Deform. 2019;7(1):132-140. [DOI] [PubMed] [Google Scholar]
- 190.Yoshida G, Ushirozako H, Machino M, et al. Transcranial motor-evoked potentials for intraoperative nerve root monitoring during adult spinal deformity surgery: A prospective multicenter study. Spine. 2022;47(22):1590-1598. doi: 10.1097/brs.0000000000004440. [DOI] [PubMed] [Google Scholar]
- 191.Yu T, Li QJ, Zhang XW, et al. Multimodal intraoperative monitoring during surgical correction of scoliosis to avoid neurologic damage. Medicine. 2019;98(15):e15067. doi: 10.1097/md.0000000000015067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhuang Q, Wang S, Zhang J, et al. How to make the best use of intraoperative motor evoked potential monitoring? Experience in 1162 consecutive spinal deformity surgical procedures. Spine. 2014;39(24):E1425-E1432. [DOI] [PubMed] [Google Scholar]
- 193.Zuccaro M, Zuccaro J, Samdani AF, Pahys JM, Hwang SW. Intraoperative neuromonitoring alerts in a pediatric deformity center. Neurosurg Focus. 2017;43(4):E8. doi: 10.3171/2017.7.focus17364. [DOI] [PubMed] [Google Scholar]
- 194.Welch WC, Rose RD, Balzer JR, Jacobs GB. Evaluation with evoked and spontaneous electromyography during lumbar instrumentation: A prospective study. J Neurosurg. 1997;87(3):397-402. doi: 10.3171/jns.1997.87.3.0397. [DOI] [PubMed] [Google Scholar]
- 195.Velayutham P, Cherian VT, Rajshekhar V, Babu KS. The effects of propofol and isoflurane on intraoperative motor evoked potentials during spinal cord tumour removal surgery - a prospective randomised trial. Indian J Anaesth. 2019;63(2):92-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Koffie RM, Morgan CD, Giraldo JP, et al. Should somatosensory and motor evoked potential monitoring Be used routinely in all posterior cervical operations for degenerative conditions of the cervical spine? World Neurosurgery. 2022;162:e86-e90. doi: 10.1016/j.wneu.2022.02.080. [DOI] [PubMed] [Google Scholar]
- 197.Wada K, Imagama S, Matsuyama Y, et al. Comparison of intraoperative neuromonitoring accuracies and procedures associated with alarms in anterior versus posterior fusion for cervical spinal disorders: A prospective multi-institutional cohort study. Medicine. 2022;101(49):e31846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kim JH, Lenina S, Mosley G, et al. The efficacy of intraoperative neurophysiological monitoring to detect postoperative neurological deficits in transforaminal lumbar interbody fusion surgery. Operative Neurosurgery. 2019;16(1):71-78. doi: 10.1093/ons/opy061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Huang SL, Qi HG, Liu JJ, Li JL, Huang YJ, Xiang L. Alarm value of somatosensory evoked potential in idiopathic scoliosis surgery. World Neurosurg. 2016;92:397-401. [DOI] [PubMed] [Google Scholar]
- 200.Gundanna M, Eskenazi M, Bendo J, Spivak J, Moskovich R. Somatosensory evoked potential monitoring of lumbar pedicle screw placement for in situ posterior spinal fusion. Spine J. 2003;3(5):370-376. doi: 10.1016/s1529-9430(03)00144-x. [DOI] [PubMed] [Google Scholar]
- 201.DiCindio S, Theroux M, Shah S, et al. Multimodality monitoring of transcranial electric motor and somatosensory-evoked potentials during surgical correction of spinal deformity in patients with cerebral palsy and other neuromuscular disorders. Spine. 2003;28(16):1851-1855. doi: 10.1097/01.brs.0000083202.62956.a8. [DOI] [PubMed] [Google Scholar]
- 202.Ishida W, Casaos J, Chandra A, et al. Diagnostic and therapeutic values of intraoperative electrophysiological neuromonitoring during resection of intradural extramedullary spinal tumors: A single-center retrospective cohort and meta-analysis. J Neurosurg Spine. Published online. 2019:1-11. [DOI] [PubMed] [Google Scholar]
- 203.Ille S, Wagner A, Joerger AK, Wostrack M, Meyer B, Shiban E. Predictive value of transcranial evoked potential monitoring for intramedullary spinal cord tumors. J Neurol Surg Cent Eur Neurosurg. 2021;82(4):325-332. [DOI] [PubMed] [Google Scholar]
- 204.James WS, Rughani AI, Dumont TM. A socioeconomic analysis of intraoperative neurophysiological monitoring during spine surgery: National use, regional variation, and patient outcomes. Neurosurg Focus. 2014;37(5):E10. [DOI] [PubMed] [Google Scholar]
- 205.Magit DP, Hilibrand AS, Kirk J, et al. Questionnaire study of neuromonitoring availability and usage for spine surgery. J Spinal Disord Tech. 2007;20(4):282-289. [DOI] [PubMed] [Google Scholar]
- 206.Traynelis VC, Abode-Iyamah KO, Leick KM, Bender SM, Greenlee JDW. Cervical decompression and reconstruction without intraoperative neurophysiological monitoring. J Neurosurg Spine. 2012;16(2):107-113. [DOI] [PubMed] [Google Scholar]
- 207.Ajiboye RM, D’Oro A, Ashana AO, et al. Routine use of intraoperative neuromonitoring during ACDFs for the treatment of spondylotic myelopathy and radiculopathy is questionable. Spine. 2017;42(1):14-19. doi: 10.1097/brs.0000000000001662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: Results of a large multicenter survey. Electroencephalogr Clin Neurophysiol. 1995;96(1):6-11. [DOI] [PubMed] [Google Scholar]
- 209.Alemo S, Sayadipour A. Role of intraoperative neurophysiologic monitoring in lumbosacral spine fusion and instrumentation: A retrospective study. World Neurosurg. 2010;73(1):72-76. [DOI] [PubMed] [Google Scholar]; discussion e7.
- 210.Hamilton DK, Smith JS, Sansur CA, et al. Rates of new neurological deficit associated with spine surgery based on 108,419 procedures: A report of the scoliosis research society morbidity and mortality committee. Spine. 2011;36(15):1218-1228. [DOI] [PubMed] [Google Scholar]
- 211.Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 15: Electrophysiological Monitoring and Lumbar Fusion. Journal of Neurosurgery: Spine. 2005;2(6):725-732. doi: 10.3171/spi.2005.2.6.0725. [DOI] [PubMed] [Google Scholar]
- 212.MacDonald DB, Stigsby B. Al homoud I, abalkhail T, mokeem A. Utility of motor evoked potentials for intraoperative nerve root monitoring. J Clin Neurophysiol. 2012;29(2):118. [DOI] [PubMed] [Google Scholar]
- 213.Weiss DS. Spinal cord and nerve root monitoring during surgical treatment of lumbar stenosis. Clin Orthop Relat Res. 2001;384(384):82-100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Accuracy of Intraoperative Neuromonitoring in the Diagnosis of Intraoperative Neurological Decline in the Setting of Spinal Surgery—A Systematic Review and Meta-Analysis by Mohammed Ali Alvi, Brian K. Kwon, Nader Hejrati, Lindsay A. Tetreault, Nathan Evaniew, Andrea C. Skelly, and Michael G. Fehlings in Global Spine Journal