Abstract
Background
The need for accessible cellular biomarkers of neurodegeneration in carriers of the fragile X mental retardation 1 (FMR1) premutation (PM) alleles.
Objective
To assess the mitochondrial status and respiration in blood lymphoblasts from PM carriers manifesting the fragile X-associated tremor/ataxia syndrome (FXTAS) and non-FXTAS carriers, and their relationship with the brain white matter lesions.
Methods
Oxygen consumption rates (OCR) and ATP synthesis using a Seahorse XFe24 Extracellular Flux Analyser, and steady-state parameters of mitochondrial function were assessed in cultured lymphoblasts from 16 PM males (including 11 FXTAS patients) and 9 matched controls. The regional white matter hyperintensity (WMH) scores were obtained from MRI.
Results
Mitochondrial respiratory activity was significantly elevated in lymphoblasts from PM carriers compared with controls, with a 2- to 3-fold increase in basal and maximum OCR attributable to complex I activity, and ATP synthesis, accompanied by unaltered mitochondrial mass and membrane potential. The changes, which were more advanced in FXTAS patients, were significantly associated with the WMH scores in the supratentorial regions.
Conclusion
The dramatic increase in mitochondrial activity in lymphoblasts from PM carriers may represent either the early stages of disease (specific alterations in short-lived blood cells) or an activation of the lymphocytes under pathological situations. These changes may provide early, convenient blood biomarkers of clinical involvements.
Key Words: Fragile X-associated tremor/ataxia syndrome, Fragile X mental retardation 1 premutation, Cellular biomarkers, Mitochondrial respiration, Blood lymphoblasts, White matter hyperintensities
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS), first described in 2001 [1], is a late-onset progressive neurodegenerative disorder affecting carriers of common premutation (PM) alleles of the fragile X mental retardation 1 (FMR1) gene. These alleles contain non-coding region CGG expansions of 56-200 repeats [2]. In females these alleles may expand further into the full mutation range (>200) over one generation [3], leading to developmental abnormality - the fragile X syndrome [4]. The population frequency of PM is estimated to be 1 in 250-468 in males and 1 in 113-209 in females [5], with approximately 40% of male carriers and 8-16% of female carriers being affected with FXTAS as they get older [1, 5, 6].
In addition to the core features of prominent tremor and ataxia, a proportion of FXTAS patients also exhibit parkinsonism, psychiatric problems, cognitive decline, and peripheral neuropathy. White matter lesions of the middle cerebellar peduncles (MCP) occur in 50% of patients, while MRI changes may also include less specific cerebellar and cerebral white matter T2 hyperintensities, as well as cortical atrophy. Neuropathology reveals ubiquitin-positive intranuclear inclusions in both the brain and non-central nervous system organs [5, 6]. The broad range of manifestations of FXTAS may reflect different stages of disease and/or differences in genetic penetrance, so that the term ‘FXTAS spectrum’ appears more appropriate [6]. Moreover, some carriers may only manifest non-syndromic psychiatric, neurological or MRI changes, or cognitive decline.
An important breakthrough in understanding the link between the PM and brain changes was the finding in the blood of carriers of elevated levels of FMR1 transcripts that correlated with the size of the CGG repeat expansion [7]. Toxicity of the expanded FMR1 mRNA is implicated in neurodegeneration; this has been supported by Drosophila and mouse models, which showed that an increase in FMR1 mRNA resulted in neural cell death [8, 9]. This toxicity has been linked to the dysregulation of cellular mechanisms in the form of mitochondrial dysfunction found in cultured fibroblasts (carriers with and without FXTAS), and post-mortem brains (FXTAS patients) [10, 11, 12], but the specific roles of the expanded FMR1 mRNA in neurodegenerative processes are not fully understood. Difficult access to human brain tissue has hampered research into the pathomechanisms involved in different stages of this process.
In this study, we investigated aspects of mitochondrial and non-mitochondrial respiratory activity in the most accessible human tissue-blood using cultured lymphoblasts from adult male PM carriers affected with FXTAS spectrum, against the background of baseline data from age-matched healthy non-carriers. We demonstrate unexpected changes in the lymphoblasts from carriers of the PM alleles, especially the affected ones, compared with controls, in the form of highly increased measures of mitochondrial and non-mitochondrial respiratory activity, which correlated with the degree of white matter lesions in specified areas of the brain.
Subjects and Methods
Sample
The study was approved by the La Trobe University Human Ethic Committee (No. 01/85). All participants gave informed consent for their involvement in the study. The sample included 16 Caucasian male PM carriers aged between 48 and 81 years, with the exception of 1 additional 18-year-old male. The PM carriers were recruited from fragile X families which were identified through clinical admissions of children with the fragile X syndrome to the Victorian Clinical Genetic Services of the Royal Children's Hospital in Melbourne and who were cascade tested.
Eleven carriers were placed in the FXTAS spectrum category based on the presence of the MCP sign on MRI associated with varying degrees of action tremor/ataxia (n = 9) or with dementia in the absence of neurological changes (n = 2). Five carriers were outside the FXTAS spectrum (3 asymptomatic, 1 with fibromyalgia, and 1 young adult with high functioning autism - none showing the MCP sign). CGG repeat sizes ranged from 56 to 160, and FMR1 mRNA levels ranged from 2.32 to 5.38. The age-matched 9 healthy controls recruited from the general population provided baseline data for mitochondrial respiratory activity in cultured lymphoblasts; their age ranged between 45 and 83 years, and their CGG repeat numbers were <40.
Clinical and Neuroimaging Assessments
Apart from clinical history and standard neurological examination conducted by two neurologists (E.S. and D.Z.L.) with relevant experience, the Addenbrooke Cognitive Examination-Revised (ACE-R) [13] was used as a screening measure for dementia.
MRI scans were performed on 1.5-tesla Siemens or General Electric scanners and included 2-dimensional dual echo proton density with T2 weighting and/or fluid-attenuated inversion recovery (FLAIR) turbo spin echo axial images with a 5-mm slice thickness and a 1.5-mm gap.
Visual White Matter Hyperintensity Rating
The extent and severity of supratentorial and infratentorial deep white matter hyperintensity (DWMH) and periventricular WMH (PVWMH) were evaluated from the proton density/T2 and/or the FLAIR digital images by an experienced neuroradiologist (N.T.) using a visual semi-quantitative method. The evaluation was performed blinded to clinical data and was repeated 1 week apart. The DWMH rating was based on the method described by Wahlund et al. [14]. Since DWMH and PVWMH may result from different pathological processes and vary in extent and severity between different clinical scenarios [5, 6], the latter was separately rated as described by van Straaten et al. [15]. DWMHs were defined as areas >3 mm of increased signal on T2 and proton density or FLAIR images, and were rated in 6 regions for each side of the brain: supratentorial frontal, parieto-occipital, temporal, subcortical white matter, infratentorial, and basal ganglia regions. The basal ganglia included the deep nuclei and capsules. Infratentorial changes included WMH in the MCP and adjacent deep white matter of the cerebellar hemispheres, which is one of the major criteria for the diagnosis of FXTAS [1, 5, 6]. PVWMHs were defined as confluent hyperintensities adjacent to the frontal or occipital horns (caps) or the bodies (bands) of the lateral ventricles. When PVWMHs were >10 mm in transverse diameter they were given a score of 2, and any excess was included in the DWMH score. Respective scores were totalled to give total supratentorial DWMH (here we excluded the basal ganglia), total infratentorial DWMH, and total PVWMH scores. A more detailed description of this method has been given in [16]. Chronic lacunes were perceived as well-defined areas >3 mm with signal characteristics similar to cerebrospinal fluid. There were 2 lacunes in 1 subject and 1 lacune in 2 subjects, but they were not included in the WMH score rating.
Laboratory Protocols
A detailed description of the laboratory methods, including isolation of PBMC and transformation with EBV 30, cell culture, assessments of mitochondrial membrane potential and mitochondrial mass, Seahorse respirometry, ATP, reactive oxygen species (ROS) assays, CGG repeat sizing, and FMR1 mRNA expression, is given in the online supplementary material (for all online suppl. material, see www.karger.com/doi/10.1159/000446803).
Statistical Analyses
Testing for normality of the distribution of molecular variables was conducted using Shapiro-Wilk test statistics at 5% significance level, and the variables that did not fit the normal distribution model were transformed. We used generalized estimating equations to compare the molecular scores between PM carriers (FXTAS and non-FXTAS groups separately and combined) and non-carrier age-matched controls. This method takes into account correlations within each experimental unit. WMH scores were considered as count data [as in [16]], and Poisson regression was applied to examine relationships between individual WMH scores and each molecular measure. False discovery rates were used to compute p values after adjusting for multiple testing, with p < 0.05 considered significant. All analyses were carried out using STATA statistical software (version 13.1, StataCorp, College Station, Tex., USA).
Results
Mitochondrial Respiratory Activity
We first explored the possibility that there may be alterations in mitochondrial respiratory activity (involving the flux of electrons to molecular oxygen from complexes I or II via complexes III and IV, as well as ATP synthesis and consumption) in blood lymphoblasts of PM carriers compared with the age-matched non-carriers, using a Seahorse XFe24 Extracellular Flux Analyser. The results show a significant increase in both basal and maximum oxygen consumption rates (MaxOCR), as well as ATP levels well above normal control values in the total sample of PM carriers (fig. 1; table 1); oxygen consumption associated with mitochondrial complex I was also significantly increased in the total PM sample. Predictably, there were highly significant intercorrelations between all elevated Seahorse respiratory measures in the total PM group, ranging from 0.503 to 0.950 (data not shown).
Fig. 1.
Box plots based on data for major components of mitochondrial respiratory function in lymphoblasts from the total sample of male PM carriers compared with non-carrier age-matched controls. C = Controls; PM = PM carriers; CI = complex I; NR = non-respiratory oxygen consumption; PL = proton leak. In each experiment, data were collected and averaged from 4 separate wells for each individual cell line. Each PM (n = 16) and control (n = 9) cell line was assayed in at least 3 independent experiments and the means were calculated. The y-axis represents z-scores for standardized molecular variables listed along the x-axis.
Table 1.
Summary statistics and comparison between male PM carriers (FXTAS, non-FXTAS, and all PM) and age-matched non-carriers (controls) using the generalized estimating equations method
| Variable | Controls (n = 9) | PM |
p value |
||||
|---|---|---|---|---|---|---|---|
| FXTAS (n = 11) | non-FXTAS (n = 5) | all PM (n =16) | P1 | P2 | P3 | ||
| Basal | 135.1± 75.4 | 284.7 ± 91.4 | 304.5 ± 175 | 290.9 ± 117 | 0.0003 | 0.0028 | 0.0002 |
| ATP | 77.12± 56.3 | 175.2 ± 65.7 | 188.6 ± 121 | 179.4 ± 82.7 | 0.0014 | 0.0390* | 0.0008 |
| ATP% | 53.15± 7.71 | 60.47 ± 8.52 | 59.37 ± 6.71 | 60.13 ± 7.79 | 0.0574 | 0.1440 | 0.0404* |
| MaxOCR | 139.5± 78.0 | 361.5 ± 144 | 347.7 ± 208 | 357.2 ± 159 | 0.00001 | 0.0200 | 0.00003 |
| MaxOCR% | 102.5± 33.4 | 130.2 ± 30.2 | 115.4 ± 36.9 | 125.6 ± 31.9 | 0.0271* | 0.4300 | 0.0524 |
| CI | 91.11± 66.7 | 277.9 ± 121 | 270.0 ± 181 | 275.4 ± 1356 | 0.0045 | 0.0220 | 0.00004 |
| CI% | 57.02± 12.7 | 74.48 ± 6.68 | 72.53 ± 10.1 | 73.87 ± 7.59 | 0.0001 | 0.0615 | 0.0005 |
| CII | 10.56± 9.00 | 18.23 ± 9.95 | 15.24 ± 10.8 | 17.30 ± 9.94 | 0.1802 | 0.6171 | 0.2713 |
| CII%a | 6.820± 3.08 | 6.440 ± 4.29 | 4.330 ± 1.08 | 5.780 ± 3.69 | 0.6965 | 0.0549 | 0.3681 |
| NR | 44.13± 13.5 | 66.33 ± 26.7 | 70.11 ± 26.6 | 67.51 ± 25.9 | 0.0349* | 0.0147 | 0.0056 |
| NR% | 35.02± 15.5 | 19.25 ± 8.26 | 23.86 ± 10.6 | 20.69 ± 8.97 | 0.0054 | 0.0989 | 0.0111 |
| PLb | 4.120± 1.95 | 6.78 ± 1.61 | 7.750 ± 3.39 | 7.080 ± 2.24 | 0.0017 | 0.0008 | 0.0002 |
| PL% | 11.88± 4.79 | 16.20 ± 6.57 | 17.76 ± 7.22 | 16.69 ± 6.58 | 0.0949 | 0.0703 | 0.0394* |
| mtMass | 1.090± 0.17 | 0.930 ± 0.14 | 1.010 ± 0.12 | 0.950 ± 0.14 | 0.0251* | 0.3788 | 0.0477* |
| MMP | 1.250± 0.15 | 1.290 ± 0.32 | 1.000 ± 0.15 | 1.210 ± 0.31 | 0.5222 | 0.0080 | 0.9150 |
| ATPS | 172.0± 69.1 | 194.2 ± 65.5 | 174.6 ± 73.1 | 188.1 ± 66.1 | 0.3524 | 0.4413 | 0.2937 |
| ROS | 0.950± 0.31 | 0.630 ± 0.42 | 0.520 ± 0.17 | 0.590 ± 0.35 | <0.0001 | <0.0001 | <0.0001 |
Data are expressed as means ± SD. p values: for comparisons between controls and FXTAS (p1), controls and non-FXTAS (p2), and controls and all PM (p3). Seahorse respirometry measures: these were derived from OCR before and after the sequential addition of pharmacological agents. Basal = Basal OCR in pmol/min; ATP = ATP synthesis after the addition of 2 uM oligomycin (ATP synthase inhibitor); MaxOCR = uncoupled (maximum) respiration after adding 1 uM of CCCP (uncoupling protonophore); CI = mitochondrial complex I activity (after addition of 1 uM of complex I inhibitor rotenone; CII = mitochondrial complex II activity (after addition of 5 uM antimycin complex II inhibitor; NR = non-respiratory oxygen consumption processes not driven by electron transport. In each measurement period the OCR was measured and averaged over 3 measurement cycles (time points), each including a mixing step of 3 min, a wait of 2 min, and a measurement time of 3 min. Other cellular/mitochondrial measures: PL = proton leak representing mito-chondrial activities other than ATP synthesis; mtMass = mitochondrial mass (normalized to control cell line); MMP = mitochondrial membrane potential (normalized to control cell line); ATPS = steady-state ATP (nM/106 cells normalized to control cell line); ROS = fluorescence relative to control cell line. MaxOCR%, CI%, CII%, NR%, and PL% are the respective measures taken as the percentage of the respective maximum pmol/min values. * p > 0.05, after adjusting for multiple testing using false discovery rate.
Comparisons adjusted for age.
Variables transformed using square root function.
Other components of mitochondrial respiratory function such as proton leak were also significantly elevated. The increase in non-respiratory oxygen consumption compared with controls was significant in the total PM group and in the non-FXTAS subsample. The absence of a significant change in ATPS (steady-state ATP) levels shows that the elevated rates of ATP synthesis and electron transport are matched by increases in the rate of consumption of ATP by cellular activities. Although ATP synthesis was increased to similar levels in both the FXTAS and non-FXTAS subsamples, this was not statistically significant in the latter after adjustment for multiple comparisons. However, ATP synthesis is the main contributor to the basal OCR, for which the increase was significant in both these groups.
Dramatic elevations of mitochondrial respiration and ATP synthesis rates were not associated with any increase in the mitochondrial mass. This data combined with unchanged mitochondrial membrane potential shows no indication of an impairment or blockade of mitochondrial electron transport at any stage in the electron transport chain. Consistent with the above, there was no significant change in MaxOCR, ATP, mitochondrial complex II, or proton leak taken as the percentage of the respective maximum values (picomole/min) (table 1). This proportional increase in all the major components of mitochondrial respiration suggests normally functioning, though hyperactive, mitochondrial systems. However, a modest but significant (in the total PM and FXTAS group) increase in mitochondrial complex I as a percentage of MaxOCR matched by a corresponding decrease in non-respiratory oxygen consumption as a percentage of MaxOCR would suggest that the cellular capacity for energy generation by oxidative phosphorylation may be increased more than the cellular consumption of oxygen by other means.
In an attempt to identify possible cellular triggers of the OCR elevation, we also assessed the levels of intracellular ROS - a potential source of oxidative stress. Contrary to expectations, the ROS levels were significantly decreased in both FXTAS and non-FXTAS carrier groups compared with non-carriers (table 1). This suggests that increased cellular antioxidant defences (e.g. mitochondrial superoxide dismutase and glutathione peroxidase) might be able to more than negate any increases in ROS production that are expected to accompany the elevated mitochondrial activity in the lymphoblasts.
Relationships between Cellular Respiration Measures and WMH Scores
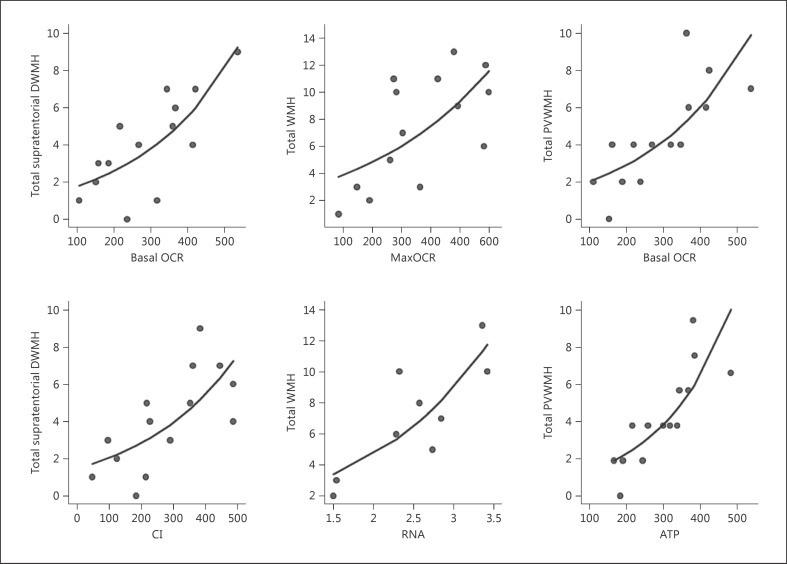
The data in table 1 showing that the FXTAS group manifested more obvious changes in cellular bioenergetics status than the non-FXTAS group of PM carriers already suggest that these changes may indeed be relevant to the clinical neurological status. However, in order to obtain more direct evidence for such an association in a small sample, we selected semi-quantitative measures of white matter lesions in specific brain areas as the covariate of our newly identified cellular respiratory biomarkers, as well as of the already established genotypic changes including CGG repeats and the levels of FMR1 mRNA. The results shown in table 2 provide evidence for strong associations between the elevated mitochondrial respiratory activity (levels of basal OCR, oxygen consumption by respiratory ATP synthesis, MaxOCR, and oxygen consumption by complex I in uncoupled mitochondria), non-respiratory OCR, and the total WMH, total supratentorial DWMH, parietal-occipital DWMH, and total PVWMH. That the statistical significance of these relationships has not merely been determined by individual outlying values in small samples is illustrated in several examples of scatter plots (fig. 2). Importantly, those hyperintensities also showed significant positive correlations with both genetic characteristics of PM alleles: the size of the CCG expansion and the levels of FMR1 mRNA (total WMH, total PVWMH, and total infratentorial DWMH) or with RNA only (total supratentorial DWMH, parieto-occipital DWMH, and frontal DWMH). All these relationships remained significant after adjustment for age (wherever appropriate) and multiple relationships.
Table 2.
Relationship between cellular biomarkers, CGG expansion size and FMR1 transcript levels (predictors) and the local and total WMH scores (outcome) in the total sample of PM carriers using Poisson regression
| Outcome | p-o DWMH |
Total supratentorial DWMH |
Total infratentorial DWMH |
Total WMH |
Total PVWMH |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| coef. | p value | coef. | p value | coef. | p value | coef. | p value | coef. | p value | |
| Basal OCR | 0.599 | 0.0010 | 0.380 | 0.0006 | 0.191 | 0.105 | 0.452 | 0.00004 | 0.364 | 0.0005 |
| ATP | 0.803 | 0.0022 | 0.538 | 0.0008 | 0.307 | 0.069 | 0.455 | 0.0001 | 0.570 | 0.0002 |
| MaxOCR | 0.404 | 0.0065 | 0.281 | 0.0012 | 0.133 | 0.136 | 0.219 | 0.0005 | 0.248 | 0.0022 |
| CI | 0.424 | 0.0126 | 0.328 | 0.0013 | 0.147 | 0.157 | 0.253 | 0.0006 | 0.299 | 0.0017 |
| NR | 1.980 | 0.0377* | 1.277 | 0.0203 | −0.041 | 0.940 | 0.637 | 0.1017 | 1.026 | 0.0452* |
| PL | 0.575 | 0.2740 | 0.800 | 0.0105 | 0.019 | 0.963 | 1.451 | 0.0004 | 1.095 | 0.0389* |
| ROS | 82.92 | 0.0935 | 24.04 | 0.4981 | 20.34 | 0.610 | 22.39 | 0.3980 | 39.26 | 0.2260 |
| CGG | −0.450 | 0.7158 | −0.460 | 0.5490 | 3.325 | 0.00003 | 1.360 | 0.0139 | 1.055 | 0.1377 |
| mRNA | 11.79 | 0.6887 | 18.03 | 0.2178 | 27.55 | 0.020 | 64.76 | 0.0022 | 28.38 | 0.0152 |
Only the variables which showed significant changes compared with non-carriers and defined here as ‘biomarkers’ were included in regression analysis. coef. = Regression coefficients (multiplied by 100); p-o DWMH = parieto-occipital region of supratentorial deep white matter; CI = complex I; NR = non-respiratory oxygen consumption; PL = proton leak. Sample sizes ranged from 9 to 14 for individual measures.
p > 0.05, after adjusting for multiple testing using false discovery rate.
Fig. 2.
Scatter plots and fitted lines of the total supratentorial DWMH (outcome) versus molecular (predictor) measures based on data from the total PM sample. CI = Complex I. The lines were fitted using the Poisson regression model.
Important anecdotal information has been provided by a case of the young adult male with high-functioning autism, whom we included in this study amongst older carriers because his large CGG repeat size broadened the range of PM alleles. Despite his age and the absence of any neurological changes (MRI data were not available), his parameters representing mitochondrial activity were usually above, and never below, the average for the total PM (or FXTAS only) sample. This suggests that the size of the CGG repeat expansion within the PM range may have an effect on the observed alterations in respiratory measures in these types of cells, but it requires confirmation in a much larger sample covering a broader range of expanded PM alleles.
Discussion
The preceding data on cellular function in blood lymphoblasts derived from a sample of adult (mainly affected) male carriers of FMR1 PM alleles showed dramatic elevation (over healthy non-carriers) of all the measures representing mitochondrial respiration, including basal OCR and ATP synthesis, OCR associated with mitochondrial complex I, non-respiratory OCR, and proton leak. There was no evidence for mitochondrial damage in these cells. These changes were not paralleled by an increase in mitochondrial mass, which suggested elevated mitochondrial activity rather than mitochondrial biogenesis; however, a possible role of an excessive autophagic degradation rate may be considered. Although there are fundamental differences between cells grown in culture and in vivo, our evidence for the involvement of blood lymphocytes in neurodegenerative processes in PM carriers is based on the presence of obvious differences in cellular functions between PM carriers and healthy non-carriers, where the timing and conditions of the cultures were the same in both groups. This evidence is further supported by the positive regression relationship between the elevation of lymphoblast respiratory functions and the severity of white matter pathology in the brains of the affected PM carriers. More specific interpretation of these findings is difficult at present because of the paucity of existing data on the involvement of blood lymphocytes in neurodegenerative processes linked to unstable mutations or otherwise, but this report may pave the way for further investigation into this phenomenon.
Considering the earlier rare finding of the presence of mitochondrial dysfunction in animal brain tissue or human fibroblasts carrying PM alleles [10, 11, 12], the observed increase in mitochondrial activity in blood lymphoblasts may represent early stages of disease-specific processes in response to cellular stress, preceding a subsequent decline of mitochondrial function as the result of adverse consequences of hyperactivity. These long-term consequences, however, may not pertain to blood cells because of their rapid turnover, in contrast with fibroblasts and especially brain cells. On the other hand, it is likely that the observed mitochondrial hyperactivity represents an activation of blood lymphocytes in their reported neuroprotective and sometimes neuroantagonistic roles. Indirect evidence for the postulated role of blood lymphocytes in mediating increased oxidative stress to neurones leading to cell death, or in alleviating inflammatory aspects of neurodegenerative processes, has been presented in rare experimental or human studies of Alzheimer's disease [17, 18, 19]. Moreover, important and relevant findings on lymphocyte recruitment, activation, and infiltration in the CNS under pathological situations, including Parkinson's disease and ALS, have recently been reviewed [20].
Possible sources of activation of cellular stress signalling pathways in carriers of the PM alleles have been linked to FMR1 mRNA. It has been claimed that the ‘toxic’ effect of elevated FMR1 transcripts leads to sequestration of some important RNA-binding proteins, such as purine-rich element binding protein (Pur α) [21], nuclear ribonucleoprotein A2/B1 (hnRNP A2/B1) [22], Sam68 [23], and DGCR8-DROSHA complex [24], which are deposited in the intranuclear inclusions (reviewed in Garcia-Arocena et al. [25]). Alternatively, the elevated RNA may act as a trigger of neuronal stress responses leading to the overexpression of candidate proteins involved in neuroprotection [6, 21, 22, 26].
Some other sources of activation of cellular stress signalling pathways in PM cells may be linked to the accumulation of ‘toxic’ peptides generated by aberrant (RAN) translation [27, 28], including the recently reported polyglycine-containing protein (FMRpolyG) [28]. On the other hand, the involvement of co-transcriptional processes of R-loop formation as a potential source of primary cellular stress has recently been suggested. This formation is promoted by the highly GC-rich FMR1 5′ UTR region [29], consequently leading to an R-loop-dependent activation of response to the DNA damage at the FMR1 locus. The hypothesis of DNA damage and activation of the DNA damage repair processes may be supported by the finding of decreased cell viability and upregulation of the phosphorylated DNA repair-associated histone variant γH2AX in human neuroblastoma-derived cell lines in conjunction with a large increase in expanded (95 CGGs) FMR1 mRNA [30]. In view of our present findings, it will be important in future studies to explore these possibilities and their potential links with mitochondrial hyperactivation in the blood cells of PM-affected carriers.
Notably, our analysis showed highly significant associations between the degree of total white matter lesion load, especially of periventricular and deep white matter, and the elevated rate of oxygen consumption, ATP, or complex I activity, in spite of the small sample size. We chose the WMH scores as potential correlates of those cellular biomarkers not only because the loss of brain white matter is a major feature of brain pathology in older/affected PM carriers [5, 6] and has been linked to the elevation of ‘toxic’ FMR1 mRNA [present data and [6]] but also because they are a more accurate and specific representation of brain damage than clinical assessments. Moreover, brain changes visible on functional MRI, fibre track changes [31, 32, 33, 34] and brain atrophy, particularly in the cerebellum [35, 36], have been shown to occur well before the onset of clinically diagnosed FXTAS. Thus, our finding of an association between the alterations in cultured blood lymphocytes and the extent of white matter lesions show that, although these alterations may reflect only an initial stage of pathological processes in these types of cells, they are obviously relevant to the progression of (or potential for) neurodegenerative changes leading to FXTAS. Therefore, we suggest that measures of mitochondrial activity in lymphoblasts may be considered early and convenient cellular biomarkers of neurodegenerative processes in PM carriers.
It is of particular interest that, although almost all the changes (except for mitochondrial membrane potential and non-respiratory oxygen consumption) were more evident in the FXTAS group, we also observed a significant elevation of oxygen consumption in a subsample of non-FXTAS PM allele carriers, including basal OCR, MaxOCR, mitochondrial complex I activity, and proton leak, as well as non-respiratory consumption. If this is confirmed in a larger independent sample, it will suggest continuity of the underlying cellular/neurological processes in carriers of PM alleles from subclinical forms to the fully manifested FXTAS as the individuals get older. It also raises the possibility that similar cellular and molecular processes may underlie atypical manifestations such as non-syndromic features in the low penetrance forms.
In conclusion, this study identified new cellular biomarkers in the form of highly elevated mitochondrial respiratory activity measures in cultured lymphoblasts from a small sample of FXTAS and non-FXTAS adult male PM carriers with a broad spectrum of clinical manifestations compared with matched healthy non-carriers. These biomarkers were found to correlate with the extent of white matter lesions, which also showed significant relationships with the level of increase of ‘toxic’ FMR1 mRNA. Our findings warrant confirmation in much larger and more diversified samples of PM carriers, using a broader range of mitochondrial and other cellular metabolism and survival tests, and also including fresh non-transformed leucocytes. This potential discovery of new aspects of cellular pathomechanisms in carriers of the PM alleles linked to neurodegenerative processes may facilitate the identification of suitable targets for future treatment techniques.
Acknowledgements
This study was supported by the National Institutes of Child Health and Human Development (grant R01 HD 36071 to D.Z.L. and F.T.). The work was also supported by funds from the La Trobe University Department of Microbiology (P.R.F.), School of Life Sciences (P.R.F.) and ‘Understanding Disease’ Research Focus Area (S.J.A., D.Z.L., and P.R.F.). The authors thank the many families who participated in this study.
Disclosure Statement
The authors have no conflicts of interest to declare.
Supplementary Material
Supplementary data
References
- 1.Hagerman RJ, Hagerman PJ. Fragile X-associated tremor/ataxia syndrome. Ann NY Acad Sci. 2015;1338:58–70. doi: 10.1111/nyas.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maddalena A, Richards CS, McGinniss MJ, et al. Technical standards and guidelines for fragile X: the first of series of disease specific supplements to the Standard Guidelines for Clinical Genetics Laboratories of the ACMG. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001;3:200–205. doi: 10.1097/00125817-200105000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat X site results in genetic instability. Resolution of Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 4.Hagerman RJ, The physical and behavioural phenotype . Fragile X Syndrome. In: Hagerman RJ, Hagerman PJ, editors. Diagnosis, Treatment and Research. ed 3. Baltimore: John Hopkins University Press; 2002. pp. pp 3–109. [Google Scholar]
- 5.Hagerman RJ, Hagerman PJ. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013;12:786–798. doi: 10.1016/S1474-4422(13)70125-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loesch DZ, Hagerman R, Unstable mutations in the FMR1 gene and the phenotypes . Tandem Repeat Polymorphisms: Genetic Plasticity, Neural Diversity and Disease) In: Hannah AJ, editor. Texas: Landes Bioscience; 2011. [Google Scholar]
- 7.Tassone F, Hagerman RJ, Taylor AK, et al. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin P, Zarnescu DC, Zhang F, et al. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39:739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 9.Willemsen R, Hoogeveen-Westerveld M, Reis S, et al. The FMR1 CGG repeat mouse displays ubiquitin-positive intranuclear neuronal inclusions; implications for the cerebellar tremor/ataxia syndrome. Hum Mol Genet. 2003;12:949–959. doi: 10.1093/hmg/ddg114. [DOI] [PubMed] [Google Scholar]
- 10.Ross-Inta C, Omanska-Klusek A, Wong S, et al. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010;429:545–552. doi: 10.1042/BJ20091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napoli E, Ross-Inta C, Wong S, et al. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2011;20:3079. doi: 10.1093/hmg/ddr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan ES, Cao Z, Hulsizer S, et al. Early mitochondrial abnormalities in hippocampal neurons cultured from FMR1 premutation mouse model. J Neurochem. 2012;123:613–621. doi: 10.1111/j.1471-4159.2012.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21:1078–1085. doi: 10.1002/gps.1610. [DOI] [PubMed] [Google Scholar]
- 14.Wahlund LO, Barkhof F, Scheltens P, et al;, European Task Force on Age-Related White Matter Changes A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. 2001;32:1318–1322. doi: 10.1161/01.str.32.6.1318. [DOI] [PubMed] [Google Scholar]
- 15.van Straaten ECI, Harvey D, Scheltens P, et al;, Alzheimer's Disease Cooperative Study group Periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J Neurol. 2008;255:1302–1308. doi: 10.1007/s00415-008-0874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trost N, Cook M, Hammersley E, et al. White matter changes in patients with Parkinson's disease carrying small CGG expansion FMR1 alleles: a pilot study. Neurodegener Dis. 2014;14:67–76. doi: 10.1159/000356190. [DOI] [PubMed] [Google Scholar]
- 17.Shrestha R, Millington O, Brewer J, Bushell T. Lymphocytes protect cortical neurones against excitotoxicity mediated by kainic acid, an in vitro model for neurodegeneration. Kathmandu Univ Med J (KUMJ) 2013;11:132–138. doi: 10.3126/kumj.v11i2.12488. [DOI] [PubMed] [Google Scholar]
- 18.Cornelius C, Salinaro AT, Scuto M, et al. Cellular stress response, sirtuins and UCP proteins in Alzheimer's disease: role of vitagenes. Immun Ageing. 2013;10:4. doi: 10.1186/1742-4933-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesarwani P, Murali AK, Al-Khami AA, Mehrotra S. Redox regulation of T-cell function: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2013;18:1497–1534. doi: 10.1089/ars.2011.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almolda B, Gonzales B, Castellano B. Microglial cells the regulators of lymphocyte responses in the CNS? Front Cell Neurosci. 2015;9:440. doi: 10.3389/fncel.2015.00440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin P, Duan R, Qurashi A, et al. Pur α binds to rCGG repeats and modulates repeat-mediated neurodegeneration in a Drosophila model of fragile X tremor/ataxia syndrome. Neuron. 2007;55:556–564. doi: 10.1016/j.neuron.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sofola OA, Jin P, Qin Y, et al. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat- induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007;55:565–571. doi: 10.1016/j.neuron.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sellier C, Rau F, Liu Y, et al. Sam68 sequestration and partial loss of function are associated with splicing alterations in FXTAS patients. EMBO J. 2010;29:1248–1261. doi: 10.1038/emboj.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellier C, Freyermuth F, Tabet R, et al. Sequestration of DROSHA and DGCR8 by expanded CGG RNA repeats alters microRNA processing in fragile X-associated tremor/ataxia syndrome. Cell Rep. 2013;3:869–880. doi: 10.1016/j.celrep.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qurashi A, Li W, Zhou JY, Peng J, Jin P. Nuclear accumulation of stress response mRNAs contributes to the neurodegeneration caused by Fragile X premutation rCGG repeats. PLoS Genet. 2011;7:e1002102. doi: 10.1371/journal.pgen.1002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buijsen RAM, Sellier C, Severijnen L-A WFM, et al. FMRpolyG-positive inclusions in CNS and non-CNS organs of a fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome. Acta Neuropathol Commun. 2014;2:162–166. doi: 10.1186/s40478-014-0162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Todd PK, Ackall FY, Hur J, et al. Transcriptional changes and developmental abnormalities in a zebra fish model of myotonic dystrophy type 1. Dis Model Mech. 2014;7:143–155. doi: 10.1242/dmm.012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loomis EW, Sanz LA, Chedin F, et al. Transcription-associated R-loop formation across the human FMR1 CGG-repeat region. PLoS Genet. 2014;10:e1004294. doi: 10.1371/journal.pgen.1004294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoem G, Raske CR, Garcia-Arocena D, et al. CGG repeat length threshold for FMR1 RNA pathogenesis in a cellular model for FXTAS. Hum Mol Genet. 2011;20:2161–2170. doi: 10.1093/hmg/ddr101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang JY, Hagerman RJ, Rivera SM. A multimodal imaging analysis of subcortical gray matter in fragile X premutation carriers. Mov Disord. 2013;28:1278–1284. doi: 10.1002/mds.25473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang JY, Hessl D, Iwahashi C, et al. Influence of the fragile X mental retardation (FMR1) gene on the brain and working memory in men with normal FMR1 alleles. Neuroimage. 2013;65:288–298. doi: 10.1016/j.neuroimage.2012.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JY, Hessl D, Schneider A, et al. Fragile X-associated tremor/ataxia syndrome: influence of the FMR1 gene on motor fiber tracts in males with normal and premutation alleles. JAMA Neurol. 2013;70:1022–1029. doi: 10.1001/jamaneurol.2013.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang JY, Hessl DH, Hagerman RJ, et al. Age-dependent structural connectivity effects in fragile X premutation. Arch Neurol. 2012;9:482–489. doi: 10.1001/archneurol.2011.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battistella G, Niederhauser J, Fornari E, et al. Brain structure in asymptomatic FMR1 premutation carriers at risk for fragile X-associated tremor/ataxia syndrome. Neurobiol Aging. 2013;34:1700–1707. doi: 10.1016/j.neurobiolaging.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto R, Javan AK, Tassone F, et al. A voxel-based morphometry study of grey matter loss in fragile X-associated tremor/ataxia syndrome. Brain. 2011;134:863–878. doi: 10.1093/brain/awq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data