Abstract
The pharmacological modulation of disease-relevant carbohydrate–protein interactions represents an underexplored area of medicinal chemistry. One particular challenge in the design of glycomimetic compounds is the inherent instability of the glycosidic bond toward enzymatic cleavage. This problem has traditionally been approached by employing S-, N-, or C-glycosides with reduced susceptibility toward glycosidases. The application of ring-extended glycomimetics is an innovative approach to circumvent this issue. On the example of the bacterial adhesin FimH, it was explored how design principles from pyranose glycomimetics transfer to analogous septanose structures. A series of ring-extended FimH antagonists exhibiting the well-proven pharmacophore necessary for targeting the tyrosine-gate of FimH was synthesized. The resulting septanoses were evaluated for their affinity to the conformationally rigid isolated lectin domain of FimH (FimHLD), as well as a structurally flexible full-length FimH (FimHFL) construct. Some elements of potent mannoside-based FimH antagonists could be successfully transferred to septanose-based ligands, ultimately resulting in a 32-fold increase in binding affinity. Interestingly, the canonical ca. 100-fold loss of binding affinity between FimHLD and FimHFL is partly mitigated by the more flexible septanose antagonists, hinting at potentially differing interaction features of the flexible glycomimetics with intermediately populated states during the conformational transition of FimHFL.
Keywords: Glycomimetics, septanose, urinary tract infections, bacterial lectin, antiadhesive therapy
Graphical Abstract

Introduction
Many biological surfaces are densely covered with a layer of carbohydrates, the so-called glycocalyx.[1] Recognition of these cell surface carbohydrate structures is accomplished by specialized lectins, constituting an important factor in cell–cell adhesion, pathogen recognition, and immune signaling processes. As a consequence, the pharmacological modulation of disease-relevant carbohydrate recognition events by means of glycomimetic ligands represents a promising drug design approach.[2–3]
In terms of physicochemical and pharmacokinetic properties, the development of glycomimetic drugs is associated with a number of particular challenges. Specifically, low bioavailability and short plasma half-lives as well as rapid metabolic turnover of glycosidic bonds by glycosidases hamper glycomimetic drug design.[2–3] The design of drug molecules mimicking other classes of biomolecules has similarly been challenged. Thus, degradation of peptidic drugs or nucleotide-based therapeutics by proteases or nucleases, respectively, has historically been a major concern.[4–7] One strategy to prevent metabolic lability focused on the incorporation of chemically disparate peptide or nucleotide analogs imitating the features of the natural building blocks but do not show their metabolic instability. Thus, modern strategies towards peptidic compounds include the use of peptide bond mimics such as β-amino acids or D-amino acid residues to prevent or delay proteolytic digestion.[4–5, 8–9] Similarly, modification of oligonucleotides with phosphothioate backbones or incorporation of non-natural nucleobases such as N-methyl pseudouridine can efficiently prolong the half-life of RNA therapeutics.[6–7, 10] For carbohydrates, similar efforts have mainly focused on modifying the glycosidic bond. Thus, researchers have attempted to replace O-glycosidic linkages with S-, N-, or C-glycosides in order to prevent glycosidase cleavage.[3, 11–14] The synthesis and application of such glycomimetics have been reviewed recently.[15–16] Inspired by the use of β-amino acids in peptide analogs, we sought to explore the compatibility of septanoses, ring-expanded homologs of pyranoses that are typically not recognized by glycosidases[17] with common design principles in glycomimetic drug discovery. For this, the bacterial adhesin FimH was selected as a well-characterized lectin target with abundant structure–activity relationship data.[18–21] In a previous study, we demonstrated that 1, a septanose analog of the FimH ligand n-heptyl α-d-mannopyranoside (2) (Figure 1), is able to bind to the mannose binding site.[22] The respective crystal structure revealed that the septanose ring can assume a conformation superimposable with the mannose hydrogen bond network in the FimH binding site (Figure 2A). However, the conformational flexibility of the septanose ring in solution ultimately imposed an energetic burden and resulted in an entropic penalty reducing the overall affinity by a factor of ten.
Figure 1.
Ligands synthesized and evaluated for binding to FimH; n-heptyl septanose (1) and n-heptyl mannoside (2) have been studied earlier.[22] Sepantoside 3 and septanoses 4 – 9 were synthesized and evaluated in the current communication.
Figure 2.
A) Crystal structure of n-heptyl mannosides (2) in complex with FimHLD (PDB 4XO8). B) Crystal structure of septanose 1 in complex with FimHLD (PDB 5CGB). Best scored poses of 7a (C) and 6 (D) from shape-based ligand-alignment docking with POSIT. The biphenyl pseudoaglycones of 7a and 6 interact with the tyrosine gate residues of FimH, while polar interactions in the mannose binding site are preserved.
With this insight into the consequence of exchanging the carbohydrate scaffold itself, the present study aims to leverage the increased flexibility of septanoses to access additional exit vectors for aromatic moieties targeting the so-called tyrosine gate of FimH.[23–24] In contrast to the glycosidic bond in mannosides, which has a strong preference for the gauche-trans or gauche-gauche conformation attributed to the exo-anomeric effect,[25] the torsional energy barriers are typically much lower in alkyl ether bonds. Initial modeling studies (see below) indicated that both biphenyl, as well as elongated biphenylmethyl pseudoaglycones were able to adopt a conformation that orients the aromatic moieties towards the tyrosine gate. Thus, both pseudoaglycone series can be seen as functional analogs of biphenyl mannosides, despite their differing chemical structure. FimH is known to undergo a conformational transition from an open state, in which the mannose binding site is solvent accessible, to a closed state with a solvent-shielded mannose hydrogen bond network.[19, 26–28] Experimentally, binding to both states can be approximated by employing either the full length protein (FimHFL), in which this mechanism is left intact, or the isolated lectin domain (FimHLD) construct, which is locked in the solvent-shielded conformation unable of undergoing a major conformational reorganization of its binding site. In general, binding to FimHFL is associated with an approximately 100-fold loss in binding affinity due to an entropic penalty associated with the loss of protein flexibility. We hypothesized that more flexible carbohydrate analogs bearing biphenyl modifications, which generated highly potent pyranose ligands for FimH,[18–19, 21, 29–31] might be able to address intermediate conformations along the conformational transition trajectory, altering the associated potential energy landscape, and, thus, enable a more potent interaction with FimHFL.
Results and Discussion
Ligand design and molecular docking.
Interestingly, a flexible n-heptyl substituent in the anomeric position of the septanose (→3) was also able to adjust to the tyrosine gate conformation as observed in the crystal structure (Figure 2C). As this modification introduced a glycosidic linkage, a potential weak spot regarding glycosidase metabolism, further studies mainly focused on substitutions in position C2 of the septanose. For mannoside ligands of FimH, it has been repeatedly shown that pronounced hydrophobic interactions with residues in the tyrosine gate (Tyr48, Tyr137, Ile52) can significantly boost binding affinity.[18, 20–21, 29] In particular, rigid and electron poor biphenyl aglycones lead to highly potent antagonists displaying sub-nanomolar affinity.[21] Based on this information, docking studies with similar modifications at position C2 of the septanose core were performed to evaluate which linker would enable the best fit of the aromatic moieties within the tyrosine gate. As shown in Figure 2, compounds with methylene-linked biphenyl substituents (→ i.e., 7a) displayed favorable interactions with tyrosine gate residues. In particular, a close contact of 3.8 Å to Tyr48 indicated favorable π-stacking interactions (Figure 2C), which had been similarly observed for the respective biphenyl mannosides ligands.[21] A direct arylation of the C2 hydroxyl group of the 1-deoxyseptanose with a biphenyl substituent (→ 6, 9a–c) seemed similarly effective in reaching Tyr48 (Figure 2D).
Synthesis.
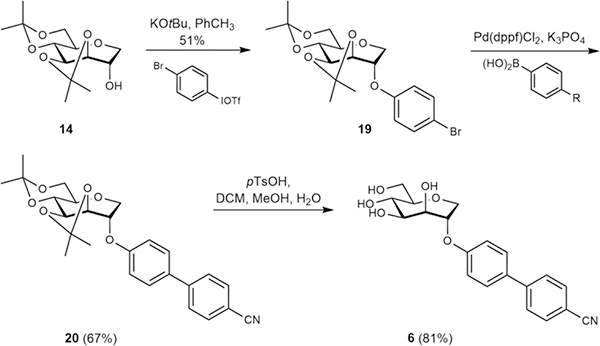
The new septanoses were obtained by the following three different strategies. Firstly, n-heptyl 2-deoxy septanoside (3), was prepared by a de novo route that leveraged our recent experience with synthesizing 2-deoxy sugars by allyl Grignard addition to protected furanoses followed by functionalization (Scheme 1).[32] Secondly, the majority of the ligands were obtained from protected 1-deoxy septanose 14 (Scheme 2), which we have previously used in the original synthesis of compound 1.[22, 33] Thirdly, functionalization of the free hydroxyl at C2 – to one degree or another – followed by deprotection yielded compounds 1 and 4–9 (Scheme 3). Details of the syntheses for all the ligands are given in the following paragraphs, the Experimental Section.
Scheme 1.
Synthesis of n-heptyl 2-deoxy septanoside 3.
Scheme 2.
Synthesis of protected C2-O-methyl biphenyl septanoses 7a-b and 9a-c.
Scheme 3.
Synthesis of C2-O- biphenyl septanose 6.
2-Deoxy septanoside 3 was obtained by addition of allyl Grignard reagent onto tri-O-benzyl arabinose 10 (Scheme 1) to give adduct 11 in 72% chemical yield (62:38 dr). The diastereoselectivity, though modest, favored the C4 R configuration (shown) as confirmed through the structural characterization of septanoside 3 (vide infra). This preference was expected based on our recent preparation of 3-amino septanosides that involved allyl Grignard addition to protected furanoses.[32] Ozonolysis of homo-allylic alcohol 11 delivered septanose 12 (80%) that was directly converted to the protected n-heptyl α-septanoside 13 (57%, 90:10 α:β) under modified Fischer glycosidation conditions. Hydrogenolysis of the benzyl groups of 13 then provided 2-deoxy septanoside 3 in 95% yield. The configuration at the anomeric center as well as the stereogenic center at C3, the stereocenter set in the Grignard addition, was determined by NMR spectroscopy. Accordingly, the 13C chemical shift of C1 (97.7 ppm) was consistent with values of other α-septanosides[34] and intramolecular NOEs between H2a, H4, and H6 defined the “bottom” face of the septanose ring. The strong crosspeak between H2e and H3 and the lack of a crosspeak between H5 and any other hydrogens such as H3 supported the assigned configuration at C3.
The route to C2-O-methylene biphenyl compounds 7 and 9, via the bis-acetonide protected precursors of 17 and 18, is depicted in Scheme 2. These syntheses leveraged the facile alkylation of the C2-hydroxy septanose 14 with either 4-bromo benzyl bromide or 2-chloro-4-bromo benzyl bromide to give 15 (82%) and 16 (93%), respectively. The alkylating agent at this step of the synthesis set the proximal aromatic ring (i.e., either phenyl, as for 7, or o-chlorophenyl as for 9) of the pseudoaglycones. These compounds were poised for Pd(0) mediated Suzuki couplings with commercially available boronic acids that converted compound 15 into biphenyl analogs 17a and 17b (37–58%), containing p-cyano and p-carboxamido groups, respectively.[21] Similarly, o-chloro-p-bromo derivative 16 was converted to compounds 18a-c (42–75%) containing m- or p-cyano and p-carboxamido groups. Removal of the two acetonide groups by acid-mediated transacetalization provided compounds 7a, 7b and 9a-c in 24–89% yields. The synthesis of septanose 6 (Scheme 3) followed the same strategy used for the preparation of 7 and 9 but replaced the initial alkylation with a novel arylation reaction recently introduced by Olofsson and co-workers.[35–36] Arylation of 14 with bis-(p-bromophenyl) iodonium triflate under the reported conditions gave C2-O-aryl septanose 19 in 51% yield. Suzuki coupling of 19 with p-cyano phenyl boronic under the established conditions delivered compound 20 (67%). Finally, conversion to 6 was accomplished via acid-mediated removal of the acetonides on 20 (81%). For compounds 8a–c (Scheme 4), arylation of intermediate 14 was achieved in a nucleophilic substitution reaction with 4-bromo-2-chloro-1-fluorobenzene. Coupling of aryl bromide 21 then yielded protected precursors 22a–c, which were then converted to compounds 8a–c by treatment with pTsOH.
Scheme 4.
Synthesis of C2-O-biphenyl septanoses 8a–c.
Alkylation reactions, followed by removal of the acetonide protecting groups, characterized the syntheses of compounds 1, 4, 5, and 7c. The alkylation reactions that afforded protected analogs 23-26 are shown in Scheme 5. Based on the alkylating agent used, acetonide-protected n-heptyl 23 (60%), benzyl 24 (76%), phenyl propyl 25 (64%) and biphenylmethyl 26 (77%) analogs were prepared. Following the transacetalization protocol, these compounds were deprotected to give compounds 1, 4, 5, and 7c, in 26–52% yield. The ability to prepare this collection of analogs from a common starting material, namely compound 14, underscores its value to the investigation.
Scheme 5.
Synthesis of C2-O- alkylated septanoses 1, 4, 5, and 7c.
Binding affinity to the lectin domain of FimH (FimHLD).
To determine the potency of the ligands under study, a competitive fluorescence polarization assay (FPA) was employed.[18] Resulting affinity data (Table 1) were analyzed to extract the impact of three relevant design parameters by comparison with related mannosides or septanoses.
Table 1.
Affinities of FimH antagonists determined in the competitive fluorescence polarization assay.a

|
KD (μM) |

|
KD (nM)b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| FimHLD | FimHFL | ||||||||
| FimHLD | FimHFL | ||||||||
|
| |||||||||
|
|
1 | 1.94 | 31.76 |
|
2 | 28.3 | 3600 | ||
| See Fig. 1 | 3 | NB | NB | ||||||
|
|
n=1 | 4 | 80.12 | 417.00 | |||||
| n=3 | 5 | 7.05 | 125.20 | ||||||
|
|
6 | 0.26 | 42.25 |
|
R=CN | 27 | 2.0 | 208 | |
| R=CONH2 | 28 | 2.8 | 240 | ||||||
| R=H | 29 | 15.1 | 1100 | ||||||
|
|
R=CN | 7a | 1.44 | 63.13 | |||||
| R=CONH2 | 7b | 2.72 | 116.22 | ||||||
| R=H | 7c | 5.67 | 109.60 | ||||||

|
R=CN | 8a | 0.10 | 6.99 |

|
R=CN | 30 | 0.5 | 84 |
| R=CONH2 | 8b | 0.12 | 7.34 | R=CONH2 | 31 | 0.3 | 86 | ||
| R=H | 8c | 0.06 | 6.11 | R=H | 32 | 2.8 | 458 | ||
|
|
R=p-CN | 9a | 1.30 | 37.84 | |||||
| R=m-CN | 9b | 1.44 | 35.51 | ||||||
| R=p-CONH2 | 9c | 1.58 | 59.37 | ||||||
Aglycone flexibility.
On the basis of ligands 4, 5, 6, and 7c, the influence of size and flexibility of the hydrophobic substitution can be derived. The benzyl moiety of 4 is too short to reach the tyrosine gate, which results in a loss in binding affinity by a factor of 10 compared to the phenylpropyl analog 5. By further restricting the conformational flexibility of the hydrophobic moiety (→ 7c), only a slight increase in binding affinity could be observed. Even though all three ligands present an aromatic substituent, no benefit is observed compared to the purely aliphatic substituent in 1. This could be attributed to the flexibility of the n-heptyl pseudoaglycone enabling interactions with both tyrosines of the tyrosine gate. A shift of the attachment position from C2 (→ 1) to C1 (→ 3) resulted in full abrogation of FimH affinity. To examine if further constraining the conformation of the hydrophobic pseudoaglycone substituent could reduce entropy penalties upon binding, compound 6, which lacked the methylene linker unit, was synthesized. This modification increased the affinity by a factor of 6 compared to 7a. and yielded a potent ligand with a KD of 260 nM determined by FPA. The markedly increased affinity of 6 enabled the use of isothermal titration calorimetry (ITC) to corroborate the affinity data and shed light on the thermodynamic driving forces behind the interaction with FimH. Unfortunately, the low solubility of 6 imposed significant challenges on this experiment (see experimental section below). The binding affinity of 6 as determined by ITC (KD = 838 nM) was approx. 3-fold lower than recorded in the FPA assay (Figure 3, Table S3). Compared with previously reported data for 1, the interaction was characterized by a more pronounced enthalpic signature. Simultaneously, an increased entropy penalty was observed. This data is in line with the interpretation that the biphenyl pseudoaglycone of 6 engages in stronger interactions with the tyrosine gate compared to the aliphatic n-heptyl chain in 1, whereas the resulting loss in protein flexibility, potentially in combination with a lack of residual pseudoaglycone flexibility in the complex, causes an entropic penalty. Unfortunately, the thermodynamic fingerprint of the parent compound 7a could not be determined due to its weaker affinity and insufficient solubility.
Figure 3.
Thermodynamic fingerprints of 6 and 8a–c interacting with FimHLD. Numeric data is given in Table S3.
Electronic modulation of aglycone biphenyl.
The addition of electron withdrawing substituents on the aromatic ring interacting with Tyr48 has previously proved useful in strengthening π-stacking contacts.[21] Thus, cyano (→7a) and carboxamido (→7b) groups were introduced in the para-position of the terminal aromatic ring, yielding a 4-fold and 2-fold increase in binding affinity to FimHLD. Most likely, the gain of affinity is a result of improved aromatic interactions to Tyr48, as observed in the docking pose (Figure 2C). Intriguingly, a counterintuitive trend was observed in the C2-arylated series (8a–c). Here, the electron withdrawing substituents in 8a-b slightly reduce affinity compared with the unmodified biphenyl derivative 8c. This observation suggests that the interaction with the tyrosine gate is not governed by π-stacking contacts, as reported for analogous biphenyl mannosides 30–32, where electron withdrawing groups significantly increase affinity. This hypothesis is corroborated by microcalorimetric data (Figure 3), as none of the modifications effect a measurable difference in the thermodynamic fingerprint of 8a–c.
Biphenyl ortho-modification.
The introduction of an ortho-chloro substituent in the proximal biphenyl ring was previously beneficial in case of biphenyl mannoside FimH ligands. Substituents in the ortho position address a shallow hydrophobic pocket lining the tyrosine gate and typically add a favorable contribution to affinity by a factor of 5–10.[31] By adding this modification to septanose ligands with electron withdrawing groups in the distal aromatic ring (→ 8a-c/9a-c), we sought to capitalize on this effect. For biphenyl septanose 8a, a slight affinity improvement by a factor of 2.6 compared to 6 was indeed achieved. This trend is also reflected in the ITC data (Figure 3, Table S3). In the biphenylmethyl series, neither ligand with an ortho-chloro substituent displayed any significant affinity increase compared with 7a. A docking analysis of 9a revealed that the ortho-chloro group was rotated out of the tyrosine gate in the best scored pose and points towards the solvent (Figure S44). Apparently, the tyrosine gate conformation used in the docking study is not able to favorably accommodate this modification. This observation might explain why barely any contribution to affinity was observed.
A clear conclusion can be drawn from a comparison of the C2-biphenyl compound (6, 8a–c) with C2-biphenylmethyl derivatives (7a–c, 9a–c). Here, a preference for the shorter, less flexible pseudoaglycones is revealed. It is likely that the improved affinity of biphenyl septanoses 6 and 8a–c is a result of the reduced number of rotatable bonds and, consequently, a reduced entropy penalty upon binding compared with the analogous biphenylmethyl derivatives. Whereas aglycone optimization in the latter series only results in a minor improvement (e.g., factor 1.5 from 1 to 9a), analogous modifications proved to be more successful in biphenyl septanoses (e.g., factor 32 from 1 to 8c). This result still lags behind the successes reported for mannosides ligands (e.g., a factor 56.6 when comparing 2 to 30). It is conceivable that the biphenyl pseudoaglycone exit vectors provided by the septanose scaffold in a binding-competent conformation are not optimally suited to enable a beneficial stacking interaction with the tyrosine gate, highlighting an opportunity for further optimization campaigns.
Binding affinity to full length FimH (FimHFL).
The FPA with the full-length FimH construct mostly reflect the trends observed for the isolated lectin domain FimHLD and can be concisely summarized.
Aglycone flexibility.
Affinity loss from a short benzyl linker (→ 4) could be partly recouped upon linker elongation (→ 5) or extension of the pseudoaglycone (→ 7c). The determination of FimHFL affinity for the more potent compound 6 was severely complicated by its low solubility. The reported best fit value of 42 μM is associated with large errors (see experimental section) due to incomplete saturation of the protein even at the highest experimentally achievable compound concentration. Therefore, this result should be interpreted with caution.
Electronic modulation of aglycone biphenyl.
Whereas no benefit in binding affinity was observed for the carboxamido modification in para-position of the outer aromatic ring (→ 7b), a cyano group (→ 7a) gave an approximately 2-fold increase. In the biphenyl series, the introduction of electron withdrawing substituents was not met with a significant affinity improvement. In fact, the unmodified biphenyl derivative 8c is slightly more potent, although overlapping confidence intervals (Table S1) prevent the extraction of a clear trend.
Biphenyl ortho-modification.
As observed for the FimHLD construct, the ortho-chloro modification resulted in an affinity gain by factor 6 for biphenyl septanose 8a. Interestingly, the introduction of an ortho-chloro group in the biphenylmethyl series (→9a-c) was met with a sizeable affinity improvement to FimHFL, despite the fact that no apparent benefit was observed for FimHLD. This might indicate that certain conformational states allowing a favorable interaction in the hydrophobic pocket are more abundantly populated in the flexible full-length construct.
As mentioned above, the conformational transition in FimHFL is typically associated with an approximately 100-fold loss in ligand binding affinity, which was consistently observed across multiple SAR studies on biphenyl mannosides ligands.[19, 21, 27–28] The septanose ligands presented here show a similar trend of reduced binding affinity for the full-length protein construct. However, for some compounds, the reduction of affinity is much smaller than the expected two orders of magnitude (Table 2). As evident from the ratio between FimHFL and FimHLD affinity, the expected 100-fold affinity loss is attenuated to a factor of ca. 5- to 40-fold, in particular for more flexible septanose ligands. We hypothesize that compounds based on the more flexible septanose scaffold, in combination with a flexible aglycone structure, might be able to interact favorably with intermediate conformations along the induced-fit trajectory, which might reduce the energetic penalty associated with the conformational transition.
Table 2.
FimHFL/FimHLD affinity ratios of selected ligands.
| Ligand | KD FimHFL / KD FimHLD |
|---|---|
|
| |
| 1 | 16.4 |
| 4 | 5.2 |
| 5 | 17.8 |
| 7a | 43.8 |
| 7b | 42.7 |
| 7c | 19.3 |
| 9a | 29.1 |
| 9b | 24.7 |
| 9c | 37.6 |
Conclusions
The present study was designed to assess if previous knowledge from pyranose ligands can be transferred to ring-extended septanose analogs in order to generate a new class of glycomimetics evading glycosidase recognition. In addition, we wanted to explore, if the residual flexibility of septanoses can be used as a design feature when binding to a flexible receptor target is required. It was concluded that some features from mannose-based glycomimetics can be successfully applied to septanoses. In particular, the choice of a biphenyl aglycone with an ortho-chloro substitution proved to successfully increase binding affinity. However, the introduction of electron withdrawing groups to the para-position of the outer aromatic ring did not yield the expected affinity gain (Table 1). Similar to mannoside ligands, the septanoses reported here display a weaker binding affinity for the flexible FimHFL construct compared with the rigid receptor FimHLD. However, a relative comparison reveals that flexible septanose ligands are less affected by a shift in the conformational equilibrium of the mannose binding site. As a result, the ratio of FimHFL/FimHLD affinity is less than the 100-fold loss in binding affinity commonly observed for pyranose ligands, in particular when flexible aglycone moieties are employed (Table 2). This indicates that the inherent flexibility of the seven membered ring core could prove beneficial when a ligation of less structured carbohydrate binding sites is required.
Experimental Section
Molecular docking.
Marvin 17.21.0 (Chemaxon, https://www.chemaxon.com) and the Open Eye docking toolkit was used for ligand preparation, receptor generation, and docking.[37] The crystal structure of FimHLD in complex with 1 was retrieved from the protein databank (PDB 5CGB) and prepared using SPRUCE and MakeReceptor.[38] A box with side lengths of 15.3 Å × 15.7 Å × 21.7 Å centered around the crystallographic ligand was generated. All crystallographic water molecules outside of the mannose binding pocket were removed. A library of 400 three dimensional conformers for each ligand was prepared using OMEGA classic.[39–40] The ligands were then docked into the receptor binding site using the shape-based ligand-alignment algorithm POSIT with an additional post pose prediction relaxation step.[41]
Protein production and purification.
FimHLD and FimHFL were produced and purified as reported earlier.[26, 42]
Fluorescence polarization assay.
The binding affinity to FimHLD was determined by measuring the change in fluorescence polarization of reported tracer 3′-chloro-N-(2-(3-(3′,6′-dihydroxy-3-oxo-3H-spiro-[isobenzofuran-1,9′-xanthen]-5-yl)thioureido)ethyl)-4′-(α-d-mannopyranosyloxy)biphenyl-4-carboxamide in the presence of 3–9.[18] For this, a serial ligand dilution starting with a final concentration of 500 μM was prepared in buffer containing 20 mM HEPES (pH 7.4), 150 mM NaCl, 50 μg/mL BSA, and a final concentration of 5% DMSO, and mixed with FimHLD and tracer to final concentrations of 50 nM, respectively. After an equilibration time of 24 h, fluorescence polarization was measured using a Tecan Spark or Tecan Infinite 1000 Pro (Tecan Trading AG, Männedorf, Switzerland) microplate reader (Ex.: 485 nm, Em.: 535 nm) using black, flat bottom, NBS-treated 96-well microtiter plates (Corning Inc., New York, USA). KD values were determined by fitting the resulting data to an analytical competitive binding model using Prism version 8.4.3 (Graphpad Software Inc.,California, USA).[43] For FimHFL, ligand serial dilutions starting from a final concentration of 5 mM, as well as FimHFL and tracer concentrations of 300 nM and 500 nM were used, respectively. For mannosides 27 and 28, a starting concentration of 50 μM was chosen. The low solubility of 6, 8a, and 8c necessitated an increase in the final DMSO concentration to 10%. Nevertheless, it was not possible to achieve a ligand concentration higher than 500 μM, which partly resulted in incomplete protein saturation in experiments with FimHFL.
Isothermal titration calorimetry.
Microcalorimetric data was recorded using a MicroCal ITC200 instrument (MicroCal, Northampton, USA). Thermograms were recorded at 25 °C using a reference power of 6 μcal s−1, a stirring speed of 750 rpm, feedback mode high, and a filter period of 2 s. All protein samples were extensively dialyzed against assay buffer (20 mM Hepes, 150 mM NaCl, pH 7.4) before use and ligand samples were dissolved in the dialysate buffer to minimize dilution effects. A DMSO concentration of 5% or 10% was employed to achieve sufficient ligand solubility. Baseline correction and integration was performed with NITPIC.[44–45] Sedphat was used for global nonlinear regression analysis of experimental data and determination of confidence intervals.[45–46]. The relatively low heat content in the experiment with 6 resulted in lower than optimal signal to noise ratios and large relative errors in peak integration. Low ligand solubility precluded an experiment setup with increased ligand and protein concentrations to enhance the measured heat. Thus, the resulting confidence intervals in the fitting parameters are relatively large.
Synthesis
General Experimental.
Reactions were performed under an atmosphere of dry nitrogen unless otherwise noted. Reagents and solvents used in reactions were used as supplied by the manufacturer. Thin-layer chromatography (TLC) plates (F254, 250 nm) were used to monitor reaction progress and 200–300 mesh silica, with the solvent mixtures specified, was used for preparative column chromatography. 1H and 13C NMR spectra were obtained using either a Bruker AVANCE III 400 MHz or a Bruker AVANCE 500 MHz NMR spectrometer. 1H NMR chemical shifts are reported relative to tetramethylsilane (TMS at 0.00 ppm) or solvent residual peak (CHCl3 in CDCl3 at 7.26 ppm, CHD2OX in CD3OD at 3.31 ppm). 13C NMR chemical shifts are reported relative to tetramethylsilane (TMS at 0.00 ppm) or solvent residual peak (CDCl3 at 77.2 ppm, CD3OD at 49.0 ppm). HRMS were collected on an Applied Biosystems QSTAR Elite instrument using electrospray ionization (ESI) and time of flight (TOF) detection. The purity of tested compounds was determined by HPLC using an Agilent 1200 instrument equipped with a Waters Atlantis T3 C18 3 μM 2.1×100 mm column. The mobile phase was a gradient of 0–95% MeCN + 0.1% TFA in water + 0.1% TFA over 20 min. The analytes were detected by UV at 254 nm or by evaporative light scattering.
(2R,3R,4R,5R)-1,3,4-benzyloxy-octen-7-ene-1,5-diol (11).
2,3,5-Tri-O-benzyl arabinose 10 (0.779 g, 1.85 mmol) was dissolved in dry THF (15 mL) and the solution was cooled on an ice bath to 0 °C under N2. To this solution was added allylmagnesium bromide (11 mL of a 1.0 M solution in diethyl ether, 11.1 mmol, 6.0 eq) dropwise via syringe (5 mL min−1). The solution was allowed to warm to rt and then stirred for an additional 24h. The mixture was then cooled to 0°C on an ice bath and quenched by the addition of 10 mL sat’d NH4Cl (aq). The mixture was extracted with EtOAc (3 × 25 mL), the combined organic layers were dried over Na2SO4, and the solvent was removed under reduced pressure. The residue was purified via column chromatography using 2:8 EtOAc:DCM as eluent, yielding two diastereomeric products. Compound 11, the C4-R diastereomer, was isolated as a light-yellow oil (0.385 g, 45% yield) Rf 0.53 (2:8 EtOAc:DCM); 1H NMR (CDCl3) 400 MHz δ 7.33 (m, 15H), 5.76 (dddd, J = 14.0, 10.2, 5.8, 5.8 Hz, 1H), 5.07 (dd, J = 16.6, 9.5 Hz, 1H), 4.74 (d, J = 11.4 Hz, 1H), 4.55 (m, 5H), 4.07 (m, 1H), 3.91 (m, 1H), 3.80 (dd, J = 6.2, 4.8, 0.9 Hz, 1H), 3.70 (m, 2H), 3.58 (ddd, J = 4.1, 3.0, 0.7 Hz, 1H), 2.98 (dd, J = 5.5, 5.5 Hz, 1H), 2.65 (dd, J = 5.5, 5.5 Hz, 1H), 2.29 (m, 2H); 13C{1H} NMR (CDCl3) 125 MHz δ 138.0, 137.9, 134.9, 128.5, 128.3, 128.1, 128.0, 127.9 (2), 127.8, 117.5, 80.4, 78.1, 74.3, 73.7, 73.5, 71.1, 70.1, 39.0; HRMS (ESI-TOF) m/z cald for C29H35O5 [M+H]+ 463.2485, found 463.2498.
n-Heptyl α-2-deoxy-4,5,7-tri-O-benzyl-D-glycero-D-galactoseptanoside (13).
Homoallylic alcohol 11 (0.220 g, 0.476 mmol) was dissolved in dry DCM (30 mL) and transferred to a long neck round bottom flask that had both an inlet and outlet and no ground glass joints. The solution was cooled to −78°C and O3 gas was bubbled through the mixture using the WELSBACH ozone generator. Once a blue color was observed in the solution, the formation of O3 was stopped and the flask was purged with O2 gas until the color dissipated and the mixture was clear. With the reaction still at −78°C, a solution of triphenylphosphine (0.440 g) in 4 mL DCM was added dropwise, and the mixture was allowed warm to rt over 14 h. The solvent was removed under vacuum and the residue was purified via column chromatography using hexanes:EtOAc as eluent to give 2-deoxy-D-glycero-D-galactoseptanose 12 (0.145 g, 67%) as a clear, colorless oil with Rf 0.67 (3:7 EtOAc:Hexanes). The material was not extensively characterized but rather carried on to the subsequent reaction.1H and 13C NMR spectra of 11, though, showed signals consistent with three species presumed to be the acyclic aldehydo-diol and the associated a- and b- lactols. Septanose 13 (0.145 g, 0.312 mmol) was dissolved in 10 mL of a 3:1 solution of n-heptanol and DCM (10 mL total volume) to which p-toluenesulfonic acid monohydrate (0.064 g, 0.374 mmol) was added. The mixture was stirred at rt for 8h, then triethylamine (52 μL, 0.374 mmol) was added. Solvents were removed under vacuum and by azeotroping with water and the residue was purified via column chromatography using 3:7 EtOAc:Hexanes as eluent. The α glycoside 13 (0.090 g, 51% yield) was isolated as a clear oil. Rf 0.64 (3:7 EtOAc:Hexanes); 1H NMR (CDCl3) 500 MHz δ 7.34 (m, 15H), 5.07 (d, J = 11.5 Hz, 1H), 4.94 (dd, J = 7.5, 7.5 Hz, 1H), 4.89 (d, J = 11.1 Hz, 1H), 4.82 (d, J = 11.0 Hz, 1H), 4.77 (d, J = 11.1 Hz, 1H), 4.67 (s, 2H), 3.96 (d, J = 9.7 Hz, 1H), 3.89 (d, J = 10.0 Hz, 1H), 3.78 (m, 3H), 3.66 (d, J = 10.0 Hz, 1H), 3.45 (m, 2H), 2.72 (s, 1H), 2.27 (dd, J = 15.0, 6.1 Hz, 1H), 1.93 (m, 1H), 1.56 (t, J = 5.8 Hz, 2H), 1.32 (m, 8H), 0.88 (t, J = 6.5 Hz, 3H); 13C{1H} NMR (CDCl3) 125 MHz δ 138.4, 138.3, 138.0, 128.7, 128.5, 128.4, 127.9, 97.8, 91.5, 80.4, 76.0, 74.7, 73.6, 70.1, 67.9, 67.8, 66.7, 37.0, 31.9, 29.6, 29.2, 26.1, 22.7, 14.2; HRMS (ESI-TOF) m/z calcd for C35H50NO6 [M+ NH4]+ 580.3638, found 580.3688.
n-Heptyl α-2-deoxy-D-glycero-D-galactoseptanoside (3).
In a vial the benzyl protected n-heptyl 2-deoxyseptanoside 13 (0.010 g, 0.018 mmol) was dissolved in dry THF (3 mL). 10% Pd/C (2 mg, 25% wt) was added and then the flask was purged with N2 and then purged with hydrogen. After purging, the hydrogen atmosphere was maintained with a balloon and the reaction was allowed to stir at rt for 14h. The mixture was then filtered through a short pad of celite and the solvent was removed under vacuum to deliver 3 (0.005 g, 95%). 1H NMR (CH3OD) 500 MHz δ 4.82 (dd, J = 9.3, 5.6 Hz, 1H), 3.84 (ddd, J = 9.3, 6.8, 6.8 Hz, 1H), 3.75 (dd, J = 11.6, 3.2 Hz, 1H), 3.70 (dd, J = 11.7, 4.7 Hz, 1H), 3.64 (ddd, J = 10.0, 3.5, 0.9 Hz, 1H), 3.47 (ddd, J = 11.5, 9.6, 0.9 Hz, 1H), 3.38 (dd, J = 9.4, 6.7, 6.7 Hz, 1H), 3.33 (dd, J = 10.1, 8.2 Hz, 1H), 3.16 (t, J = 8.7 Hz, 1H), 2.05 (ddd, J = 14.8, 5.6, 1.0 Hz, 1H), 1.86 (ddd, J = 14.8, 11.2, 9.4 Hz, 1H), 1.54 (m, 2H), 1.31 (m, 8H), 0.90 (t, J = 7.0 Hz, 3H); 13C NMR (CH3OD) 125 MHz δ 99.1, 83.9, 74.0, 70.2, 68.7, 68.3, 64.0, 39.2, 33.0, 30.7, 30.3, 27.3, 23.7, 14.4; HRMS (ESI-TOF) m/z calcd for C14H29O6 [M+H]+ 293.1964, found 293.1966.
2-O-(4-bromobenzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (15).
Into a 10 mL round bottom flask was added 3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 14 (0.0416 g, 0.152 mmol, 1.0 eq.) in 1.5 mL dry DMF. Tetrabutylammonium iodide (TBAI) (0.005 g, 0.015 mmol, 0.1 eq.) and sodium hydride (0.015 g, .61 mmol, 4.0 eq.) were added at 0 °C, and the flask was stirred for 30 minutes under N2. 4-bromo-1-bromomethylbenzene (0.152 g, 0.61 mmol, 4.0 eq.) was added and the reaction was stirred overnight at rt under N2. The mixture was quenched by the addition of 1.0 mL methanol at 0 °C and the solvent was removed under reduced pressure. The residue was redissolved in EtOAc (10 mL), washed with water (2 × 10 mL) and brine (1 × 10 mL). The organic layer was dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was purified via column chromatography using 3:7 EtOAc:hexane as eluent to give 15 (0.0548 g, 82%) as a clear colorless oil. Rf 0.56 (3:7 EtOAc:Hexanes); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.48 (d, J = 8.2 Hz, 2H), 7.26 (d, J = 8.2 Hz, 2H), 4.73 (d, J = 12.2 Hz, 1H), 4.63 (d, J = 12.2 Hz, 1H), 4.35 (dd, J = 7.8, 7.8 Hz, 1H), 4.29 (dd, J = 7.8, 7.8 Hz, 1H), 3.92 (m, 2H), 3.75 (dd, J = 8.6, 8.6 Hz, 1H), 3.60 (m, 2H), 3.29 (dd, J = 12.9, 10.0 Hz, 1H), 3.22 (ddd, J = 8.9, 6.9, 6.1 Hz, 1H), 1.52 (s, 3H), 1.47 (s, 3H), 1.42 (s, 6H); 13C NMR (100 MHz, CDCl3): δ (ppm) 137.4, 131.7, 129.7, 121.8, 109.6, 99.3, 80.9, 79.2, 79.1, 74.3, 73.7, 72.1, 70.2, 62.6, 27.8, 27.4, 24.8, 20.6: HRMS (ESI-TOF) m/z [M+Na]+ calcd for C20H2779BrO6Na+ 465.0883, found 465.0882.
2-O-(2-chloro-4-bromobenzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (16).
Into a 10 mL round bottom flask was added 3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 14 (0.040 g, 0.146 mmol, 1.0 eq.) in 1.5 mL dry DMF. Tetrabutylammonium iodide (TBAI) (0.005 g, 0.015 mmol, 0.1 eq.) and sodium hydride (0.014 g, .58 mmol, 4.0 eq.) were added at 0 °C, and the flask was stirred for 30 minutes under N2. 4-bromo-2-chloro-1-bromomethylbenzene (0.166 g, 0.58 mmol, 4.0 eq.) was added, and the reaction was stirred overnight at rt under N2. The mixture was quenched by the addition of 1.0 mL methanol at 0 °C and then the solvent was removed under reduced pressure. The residue was redissolved in EtOAc (10 mL), washed with water (2 × 10 mL) and brine (1 × 10 mL). The organic layer was dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was isolated via column chromatography using 1:4 EtOAc:hexane as eluent to give 15 (0.065 g, 93%) as a clear colorless oil. Rf = 0.38 (1:4 EtOAc:Hexane); 1H NMR (500 MHz, CDCl3): δ (ppm) 7.55 (m, 1H), 7.49 (m, 1H), 7.43 (m, 1H), 4.84 (d, J = 13.2 Hz, 1H), 4.73 (d, J = 13.7 Hz, 1H), 4.39 (dd, J = 8.3, 8.3 Hz, 1H), 4.33 (dd, J = 8.3, 8.3 Hz, 1H), 4.00 (dd, J = 13.0, 2.5 Hz, 1H), 3.95 (dd, J = 11.9, 5.9 Hz, 1H), 3.80 (dd, J = 8.9, 8.9 Hz, 1H), 3.67 (ddd, J = 9.3, 9.3, 2.2 Hz, 1H), 3.64 (dd, J = 12.1, 6.8 Hz, 1H), 3.34 (dd, J = 12.9, 9.8 Hz, 1H), 3.27 (ddd, J = 9.0, 6.5, 6.5, Hz, 1H), 1.56 (s, 3H), 1.50 (s, 3H), 1.45 ( s, 3H), 1.447 (s, 3H); 13C NMR (125 MHz, CDCl3): δ (ppm) 135.1, 133.6, 131.8, 130.4, 130.0, 121.5, 109.5, 99.1, 80.6, 79.7, 79.0, 74.1, 73.5, 74.1, 73.5, 70.0, 69.3, 62.4, 27.6, 27.2, 24.6, 20.3; HRMS (ESI-TOF) m/z calcd for C20H26BrClO6Na+ [M+Na]+ 499.0493, found 499.0469.
2-O-(4-[4-cyanophenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (17a).
Into a 20 mL vial containing 2-O-(4-bromophenyl)-3,4;5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 15 (0.0303 g, 0.068 mmol, 1.0 eq.) was added 4-cyanophenylboronic acid (0.011 g, 0.075 mmol, 1.1 eq.), [1,1’-Bis(diphenylphosphino)-ferrocene]palladium(II) dichloride:DCM adduct (0.0028 g, 0.0034 mmol, 0.05 eq.), and potassium phosphate tribasic (0.022 g, 0.103 mmol, 1.5 eq). The vial was vacuum evacuated and refilled with N2 gas three times. Anhydrous DMF (1.0 mL) was added and the solution was degassed by applying a vacuum to the vial and swirling, followed by N2 flush. After two degassing cycles the vial was stirred under N2 at 80 °C for 12 h. The reaction was allowed to cool to rt and diluted with ethyl acetate (15 mL), washed with water (1 × 15 mL) and brine (1 × 15 mL), dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was isolated via chromatography using 3:7 EtOAc:hexane as eluent to yield 17a as a clear, colorless oil (0.0186 g, 58%). Rf 0.38 (3:7 EtOAc:Hexane); 1H NMR (500 MHz, CDCl3): δ (ppm) 7.72 (d, J = 8.3 Hz, 2H), 7.68 (d, J = 8.2 Hz, 2H), 7.57 (d, J = 8.2 Hz, 2H), 7.49 (d, J = 8.1 Hz, 2H) 4.84 (d, J = 12.2 Hz, 1H), 4.73 (d, J = 12.2 Hz, 1H), 4.37 (dd, J = 8.2, 8.2 Hz, 1H), 4.30 (d, J = 8.7, 8.7 Hz, 1H), 3.96 (dd, J = 12.8, 2.1 Hz, 1H), 3.91 (dd, J = 12.0, 5.8 Hz, 1H), 3.75 (dd, J = 9.0, 9.0 Hz, 1H), 3.64 (ddd, J = 10.8, 9.2, 2.1 Hz, 1H), 3.59 (dd, J = 12.0, 6.8 Hz, 1H), 3.31 (dd, J = 12.7, 9.9 Hz, 1H), 3.23 (ddd, J = 8.8, 6.5, 6.5 Hz, 1H), 1.53 (s, 3H), 1.46 (s, 3H), 1.42 (s, 3H), 1.41 (s, 3H); 13C NMR (125 MHz, CDCl3): δ (ppm) 145.4, 138.9, 138.6, 132.6, 128.5, 127.7, 127.3, 119.0, 111.0, 109.4, 99.2, 80.8, 79.1, 79.0, 74.1, 73.5, 72.2, 70.1, 62.4, 27.6, 27.1, 24.6, 20.4; HRMS (ESI-TOF) m/z [M+H]+ calcd for C27H32NO6+ 466.2224, found 466.2201.
2-O-(4-[4-aminocarbonylphenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (17b).
Into a 20 mL vial containing 2-O-(4-bromophenyl)-3,4;5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 15 (0.0255 g, 0.058 mmol, 1.0 eq.) was added 4-aminocarbonylphenylboronic acid (0.0104 g, 0.063 mmol, 1.1 eq.), [1,1’-Bis(diphenylphosphino)-ferrocene]palladium(II) dichloride:DCM adduct (0.0024 g, 0.0029 mmol, 0.05 eq.), and potassium phosphate tribasic (0.018 g, 0.086 mmol, 1.5 eq). The vial was vacuum evacuated and refilled with N2 gas three times. Anhydrous DMF (1.0 mL) was added, and the solution was degassed by applying a vacuum to the vial and swirling, followed by N2 flush. After two degassing cycles the vial was stirred under N2 at 80 °C for 12 h. After, the reaction was allowed to cool to rt and diluted with EtOAc (15 mL), washed with water (1 × 15 mL) and brine (1 × 15 mL), dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was isolated via chromatography using 7:3 EtOAc:hexane as eluent to yield 17b as a clear, colorless oil (0.0104 g, 37%). Rf 0.30 (7:3 EtOAc:Hexane); 1H NMR (500 MHz, CDCl3) δ (ppm) 7.89 (d, J = 8.1 Hz, 2H), 7.67 (d, J = 8.2 Hz, 2H), 7.60 (d, J = 8.1 Hz, 2H), 7.47 (d, J = 8.0 Hz, 2H) 4.83 (d, J = 12.2 Hz, 1H), 4.73 (d, J = 12.2 Hz, 1H), 4.37 (dd, J = 8.2, 8.2 Hz, 1H), 4.30 (dd, J = 8.2, 8.2 Hz, 1H), 3.96 (dd, J = 10.7, 2.1 Hz, 1H), 3.91 (dd, J = 12.0, 5.9 Hz, 1H), 3.75 (dd, J = 9.0, 9.0 Hz, 1H), 3.65 (ddd, J = 10.8, 9.2, 2.1 Hz, 1H), 3.59 (dd, J = 12.0, 6.9 Hz, 1H), 3.31 (dd, J = 12.6, 9.9 Hz, 1H), 3.23 (ddd, J = 8.8, 6.5, 6.5 Hz, 1H), 1.53 (s, 3H), 1.46 (s, 3H), 1.42 (s, 3H), 1.41 (s, 3H); 13C NMR (125 MHz, CDCl3) δ (ppm) 169.0, 144.6, 139.4, 138.2, 132.0, 130.4, 128.0, 127.3, 109.4, 99.1, 80.8, 79.0, 78.9, 74.1, 73.5, 72.3, 70.1, 62.4, 27.6, 27.1, 24.6, 20.4; HRMS (ESI-TOF) m/z [M+Na]+ calcd for C27H33NO7Na+ 506.2149, found 506.2139.
2-O-(2-chloro-4-[4-cyanophenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (18a).
Into a 20 mL vial containing 2-O-(2-chloro-4-bromophenyl)-3,4;5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 16 (0.0251 g, 0.053 mmol, 1.0 eq.) was added 4-cyanophenylboronic acid (0.0085 g, 0.058 mmol, 1.1 eq.), [1,1’-Bis(diphenylphosphino)-ferrocene]palladium(II) dichloride:DCM adduct (0.0013 g, 0.0016 mmol, 0.03 eq.), and potassium phosphate tribasic (0.017 g, 0.079 mmol, 1.5 eq). The vial was evacuated under vacuum and refilled with N2 gas three times. Anhydrous DMF (1.0 mL) was added and was degassed by applying a vacuum to the vial and swirling to allow gasses to bubble off, followed by N2 flush. After two degassing cycles the vial was stirred under N2 at 80 °C for 12 h. Then, the mixture was allowed to cool to rt and diluted with EtOAc (15 mL). The mixture was washed with water (1 × 15 mL) and brine (1 × 15 mL), dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was isolated via chromatography using 3:7 EtOAc:hexane as eluent to yield 18a as a clear oil (0.0120 g, 46%, 53% BRSM). Rf 0.33 (3:7 EtOAc:Hexane); 1H NMR (400 MHz, CDCl3) δ (ppm) 7.76–7.74 (m, 2H), 7.70–7.66 (m, 3H), 7.60 (d, J = 1.8 Hz, 1H), 7.49 (dd, J = 7.9, 1.8 Hz, 1H) 4.94 (d, J = 13.3 Hz, 1H), 4.83 (d, J = 13.3 Hz, 1H), 4.41 (dd, J = 8.4, 7.7 Hz, 1H), 4.33 (dd, J = 8.4, 7.7 Hz, 1H), 4.02 (dd, J = 12.8, 2.3 Hz, 1H), 3.93 (dd, J = 12.0, 5.9 Hz, 1H), 3.79 (dd, J = 9.0, 9.0 Hz, 1H), 3.71 (ddd, J = 10.9, 9.5, 2.3 Hz, 1H), 3.63 (dd, J = 12.0, 7.0 Hz, 1H), 3.35 (dd, J = 12.8, 9.8 Hz, 1H) 3.27 (ddd, J = 9.0, 6.8, 6.1 Hz, 1H), 1.56 (s, 3H), 1.49 (s, 3H), 1.44 (s, 3H), 1.43 (s, 3H); 13C NMR (125 MHz, CDCl3) δ (ppm) 144.0, 139.9, 136.4, 134.2, 133.6, 132.8, 129.9, 127.9, 127.7, 125.6, 118.7, 116.3, 111.6, 109.5, 99.2, 80.7, 79.8, 79.0, 74.1, 73.5, 70.0, 69.6, 62.4, 27.6, 27.2, 24.6, 20.4. HRMS (ESI-TOF) m/z calcd for [M+Na]+ C27H3035ClNO6Na+ 522.1654, found 522.1682.
2-O-(2-chloro-4-[3-cyanophenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (18b).
Into a 20 mL vial containing 2-O-(2-chloro-4-bromophenyl)-3,4;5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 16 (0.0213 g, 0.045 mmol, 1.0 eq.) was added 3-cyanophenylboronic acid (0.0070 g, 0.049 mmol, 1.1 eq.), [1,1’-Bis(diphenylphosphino)-ferrocene]palladium(II) dichloride:DCM adduct (0.0011 g, 0.0013 mmol, 0.03 eq.), and potassium phosphate tribasic (0.0142 g, 0.067 mmol, 1.5 eq). The vial was evacuated under vacuum and refilled with N2 gas three times. Anhydrous DMF (1.0 mL) was added and was degassed by applying a vacuum to the vial and swirling to allow gasses to bubble off, followed by N2 flush. After two degassing cycles the vial was stirred under N2 at 80 °C for 12 h. The mixture was then allowed to cool to rt and diluted with EtOAc (15 mL), washed with water (1 × 15 mL) and brine (1 × 15 mL), dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was purified via chromatography using 3:7 EtOAc:hexane as eluent to yield 18b as a clear oil (0.0168 g, 75%). Rf 0.40 (3:7 EtOAc:Hexane); 1H NMR (500 MHz, CDCl3) δ (ppm) 7.88 (s, 1H), 7.83–7.82 (m, 1H), 7.74–7.69 (m, 2H), 7.62–7.59 (m, 2H), 7.50 (dd, J = 7.9, 1.9 Hz, 1H), 4.96 (d, J = 13.4 Hz, 1H), 4.86 (d, J = 13.4 Hz, 1H), 4.44 (dd, J = 8.0, 8.0 Hz, 1H), 4.36 (dd, J = 8.0, 8.0 Hz, 1H), 4.05 (dd, J = 12.8, 2.3 Hz, 1H), 3.96 (dd, J = 12.1, 6.1 Hz, 1H), 3.82 (dd, J = 8.9, 8.9 Hz, 1H), 3.73 (ddd, J = 10.9, 8.9, 2.2 Hz, 1H), 3.66 (dd, J = 12.1, 7.1 Hz, 1H), 3.38 (dd, J = 12.8, 9.8 Hz, 1H), 3.29 (ddd, J = 8.8, 6.8, 6.2 Hz, 1H), 1.59 (s, 3H), 1.52 (s, 3H), 1.47 ( s, 3H), 1.46 (s, 3H); 13C NMR (125 MHz, CDCl3) δ (ppm) 140.8, 139.6, 136.2, 133.6, 131.4, 131.3, 130.6, 130.0, 129.8, 127.8, 125.4, 118.6, 113.2, 109.5, 99.1, 80.7, 79.8, 79.0, 74.1, 73.5, 70.0, 69.5, 62.4, 27.6, 27.2, 24.6, 20.4; HRMS (ESI-TOF) m/z [M+Na]+ calcd for C27H3035ClNO6Na+ 522.1654, found 522.1659.
2-O-(2-chloro-4-[4-aminocarbonylphenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (18c).
Into a 20 mL vial containing 2-O-(2-chloro-4-bromophenyl)-3,4;5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 16 (0.0182 g, 0.038 mmol, 1.0 eq.) was added 4-aminocarbonylphenylboronic acid (0.0070 g, 0.042 mmol, 1.1 eq.), [1,1’-Bis(diphenylphosphino)-ferrocene]palladium(II) dichloride:DCM adduct (0.0010 g, 0.001 mmol, 0.03 eq.), and potassium phosphate tribasic (0.0121 g, 0.057 mmol, 1.5 eq). The vial was vacuum evacuated and refilled with N2 gas three times. Anhydrous DMF (1.0 mL) was added, and the solution was degassed by applying a vacuum to the vial and swirling, followed by N2 flush. After two degassing cycles the vial was stirred under N2 at 80 °C for 12 h. After, the reaction was allowed to cool to rt and diluted with EtOAc (15 mL), washed with water (1 × 15 mL) and brine (1 × 15 mL), dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was purified via chromatography using 7:3 EtOAc:hexane as eluent to yield 18c as a clear, colorless oil (0.0083 g, 42%). Rf 0.21 (7:3 EtOAc:Hexane); 1H NMR (500 MHz, CDCl3) δ (ppm) 7.93–7.92 (m, 2H), 7.70–7.63 (m, 4H), 7.53–7.52 (m, 1H), 4.95 (d, J = 13.4 Hz, 1H), 4.85 (d, J = 13.4 Hz, 1H), 4.43 (dd, J = 8.1, 8.1 Hz, 1H), 4.35 (dd, J = 8.1, 8.1 Hz, 1H), 4.05 (dd, J = 12.7, 2.1 Hz, 1H), 3.96 (dd, J = 12.1, 6.1 Hz, 1H), 3.82 (dd, J = 9.0, 9.0 Hz, 1H), 3.73 (ddd, J = 10.4, 8.8, 2.3 Hz, 1H), 3.65 (dd, J = 11.9, 6.7 Hz, 1H), 3.37 (dd, J = 12.6, 9.6 Hz, 1H), 3.29 (ddd, J = 8.9, 6.7, 6.2 Hz, 1H), 1.58 (s, 3H), 1.51 (s, 3H), 1.46 ( s, 3H), 1.45 (s, 3H); 13C NMR (125 MHz, CDCl3) δ (ppm) 169.0, 143.1, 140.7, 135.7, 133.5, 132.6, 129.9, 128.1, 127.9, 127.2, 125.6, 109.5, 99.1, 80.7, 79.7, 79.0, 74.1, 73.5, 70.0, 69.6, 62.4, 27.6, 27.2, 24.6, 20.4. HRMS (ESI-TOF) m/z [M+Na]+ calcd for C27H3235ClNO7Na+ 540.1760, found 540.1758.
2-O-(4-[4-cyanophenyl]benzyl)-1,6-anhydro-D-glycero-D-galactitol (7a)
Into a 10 mL round bottom flask was added 2-O-(4-[4-cyanophenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 17a (0.0186 g, 0.040 mmol, 1.0 eq.) in EtOH/water (3.0 mL, 1:3). Amberlite IR-120 resin was added until pH ≤ 3, and the mixture was refluxed at 110 °C for 4 hr, or until TLC showed complete consumption of starting material. The mixture was cooled to rt and the resin was filtered off, washing with methanol (3 × 7 mL). Solvent was removed from the filtrate under reduced pressure and the residue was purified via column chromatography using 3:7 iPrOH:DCM as eluent to give 7a (0.006 g, 39%) as a clear, colorless oil. Rf 0.5 (3:7 iPrOH:DCM); 1H NMR (500 MHz, CD3OD) δ (ppm) 7.84 (d, J = 8.4 Hz, 2H), 7.81 (d, J = 8.4 Hz, 2H), 7.70 (d, J = 7.8 Hz, 2H), 7.52 (d, J = 7.8 Hz, 2H), 4.74 (d, J = 12.2 Hz, 1H), 4.69 (d, J = 12.2 Hz, 1H), 4.15 (dd, J = 12.8, 5.0 Hz, 1H), 4.12 (d, J = 5.9 Hz, 1H), 3.94 (d, J = 7.4 Hz, 1H), 3.80–3.76 (m, 2H), 3.66 (dd, J = 8.1, 8.1 Hz, 1H), 3.60 (dd, J = 11.7, 7.4 2H), 3.55 (dd, J = 12.9, 6.7 Hz, 1H), 3.42 (ddd, J = 7.9, 7.9, 2.5 Hz, 1H); 13C NMR (125 MHz, CD3OD): δ (ppm) 146.7, 140.6, 139.8, 133.8, 129.7, 128.8, 128.2, 119.8, 111.9, 85.5, 80.0, 76.0, 74.5, 72.8, 71.5, 70.3, 64.5; HRMS (ESI-TOF) m/z [M+Na]+ calcd for C21H23NO6Na+ 408.1418, found 408.1427.
2-O-(4-[4-aminocarbonylphenyl]benzyl)-1,6-anhydro-D-glycero-D-galactitol (7b)
Into a 10 mL round bottom flask was added 2-O-(4-[4-aminocarbonylphenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (17b) (0.0104 g, 0.022 mmol, 1.0 eq.) in EtOH/water (3.0 mL, 1:3). Amberlite IR-120 resin was added until pH ≤ 3, and the mixture was refluxed at 80 °C for 4 h, or until TLC showed complete consumption of starting material. The mixture was cooled to rt and the resin was filtered off, washing with methanol (3 × 7 mL). Solvent was removed from the filtrate under reduced pressure and the residue was purified via column chromatography using 1:1 iPrOH:DCM as eluent to give 7b (0.008 g, 89%) as a clear, colorless oil. Rf 0.5 (3:7 iPrOH:DCM); 1H NMR (500 MHz, CD3OD): δ (ppm) 7.96 (d, J = 8.3 Hz, 2H), 7.72 (d, J = 8.5 Hz, 2H), 7.66 (d, J = 8.3 Hz, 2H), 7.48 (d, J = 8.3 Hz, 2H), 4.72 (d, J = 12.0 Hz, 1H), 4.66 (d, J = 12.0 Hz, 1H), 4.15 (dd, J = 13.0, 5.2 Hz, 1H), 4.12 (dd, J = 5.8, 1.8 Hz, 1H), 3.94 (dd, J = 7.5, 1.6 Hz, 1H), 3.80–3.75 (m, 2H), 3.68 (dd, J = 8.5, 7.8 Hz, 1H), 3.61 (dd, J = 11.7, 6.9 Hz, 1H), 3.53 (dd, J = 12.9, 6.7 Hz, 1H), 3.41 (ddd, J = 8.7, 7.1, 2.9 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ (ppm) 170.0, 145.5, 140.6, 139.8, 133.7, 129.6, 129.3, 128.1, 127.9, 85.4, 79.9, 75.9, 74.5, 72.9, 71.4, 70.5, 64.3; HRMS (ESI-TOF) m/z [M+Na]+ calcd for C21H25NO7Na+ 426.1523, found 426.1525.
2-O-(2-chloro-4-[4-cyanophenyl]benzyl)-1,6-anhydro-D-glycero- D-galactitol (9a)
Into a 10 mL round bottom flask was added 2-O-(2-chloro-4-[4-cyanophenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 18a (0.012 g, 0.024 mmol, 1.0 eq.) in EtOH/water (3.0 mL, 1:3). Amberlite IR-120 resin was added until pH ≤ 3, and the mixture was refluxed at 110 °C for 4 hr, or until TLC showed complete consumption of starting material. The mixture was cooled to rt and the resin was filtered off, washing with methanol (3 × 7 mL). Solvent was removed from the filtrate under reduced pressure and the residue was purified via column chromatography using 1:9 MeOH:DCM as eluent to give 9a (0.0043 g, 43%) as a clear, colorless oil. Rf 0.57 (1:9 MeOH:DCM); 1H NMR (500 MHz, CD3OD) δ (ppm) 7.84 (s, 4H), 7.75 (s, 1H), 7.69–7.67 (m, 2H), 4.83 (d, J = 12.7 Hz, 1H), 4.78 (d, J = 12.7 Hz, 1H), 4.21 (dd, J = 13.0, 4.8 Hz, 1H), 4.16 (d, J = 5.9 Hz, 1H), 3.95 (d, J = 7.4 Hz, 1H), 3.86–3.83 (m, 1H), 3.78 (dd, J = 11.5, 1.9 Hz, 1H), 3.67 (dd, J = 7.9, 7.9 Hz, 1H), 3.63–3.58 (m, 2H), 3.44 (ddd, J = 8.0, 8.0, 1.8 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ (ppm) 145.2, 141.4, 137.8, 134.9, 133.9, 131.4, 128.9, 128.8, 126.8, 119.6, 112.6, 85.5, 80.7, 76.0, 74.4, 71.5, 70.3, 70.2, 64.5; HRMS (ESI-TOF) m/z [M+H]+ calcd for C21H2335ClNO7+ 420.1208, found 420.1204.
2-O-(2-chloro-4-[3-cyanophenyl]benzyl)-1,6-anhydro-D-glycero-D-galactitol (9b)
Into a 10 mL round bottom flask was added 2-O-(2-chloro-4-[3-cyanophenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 18b (0.0168 g, 0.0336 mmol, 1.0 eq.) in EtOH/water (4.0 mL, 1:3). Amberlite IR-120 resin was added until pH ≤ 3, and the mixture was refluxed at 110 °C for 4 hr, or until TLC showed complete consumption of starting material. The mixture was cooled to rt and the resin was filtered off, washing with methanol (3 × 7 mL). Solvent was removed from the filtrate under reduced pressure and the residue purified via column chromatography using 1:9 MeOH:DCM as eluent to give 9b (0.0034 g, 24%) as a clear, colorless oil. Rf 0.59 (1:9 MeOH:DCM); 1H NMR (500 MHz, CD3OD) δ (ppm) 8.04 (s, 1H), 7.97 (d, J = 8.0 Hz, 1H) 7.77–7.74 (m, 2H), 7.70–7.64 (m, 3H), 4.83 (d, J = 12.4 Hz, 1H), 4.78 (d, J = 12.4 Hz, 1H), 4.21 (dd, J = 12.9, 5.1 Hz, 1H), 4.16 (dd, J = 5.8, 1.3 Hz, 1H), 3.95 (dd, J = 7.6, 1.5 Hz, 1H), 3.87–3.83 (m, 1H), 3.79 (dd, J = 11.5, 2.4 Hz, 1H), 3.67 (dd, J = 7.7, 7.7 Hz, 1H), 3.64–3.58 (m, 2H), 3.44 (ddd, J = 8.3, 8.3, 2.3 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ (ppm) 142.0, 141.2, 137.5, 134.9, 132.7, 132.5, 131.6, 131.4, 131.2, 128.7, 126.7, 119.5, 114.2, 85.5, 80.7, 76.0, 74.5, 71.5, 70.3, 70.2, 64.5; HRMS (ESI-TOF) m/z [M+H]+ calcd for C21H2335ClNO7+ 420.1208, found 420.1187.
2-O-(2-chloro-4-[4-aminocarbonylphenyl]benzyl)-1,6-anhydro-D-glycero-D-galactitol (9c)
Into a 10 mL round bottom flask was added 2-O-(2-chloro-4-[4-aminocarbonylphenyl]benzyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 18c (0.0083 g, 0.016 mmol, 1.0 eq.) in EtOH/water (3.0 mL, 1:3). Amberlite IR-120 resin was added until pH ≤ 3, and the mixture was refluxed at 110 °C for 4 hr, or until TLC showed complete consumption of starting material. The mixture was cooled to rt and the resin was filtered, washing with methanol (3 × 7 mL). Solvent was removed from the filtrate under reduced pressure and the residue was purified via column chromatography using alumina as the stationary phase and 2:8 water:acetonitrile as eluent to give 9c (0.0022 g, 31%) as a clear, colorless oil. Rf 0.70 (2:8 water:ACN); 1H NMR (500 MHz, CD3OD) δ (ppm) 8.01–7.99 (m, 2H), 7.77–7.74 (m, 3H), 7.67 (s, 2H), 4.83 (d, J = 12.2 Hz, 1H), 4.78 (d, J = 12.2 Hz, 1H), 4.23 (dd, J = 13.0, 5.1 Hz, 1H), 4.18 (d, J = 5.9 Hz, 1H), 3.96 (d, J = 7.5 Hz, 1H), 3.86 (ddd, J = 11.7, 5.8, 5.8 Hz, 1H), 3.79 (dd, J = 11.7, 2.4 Hz, 1H), 3.70 (dd, J = 8.0, 8.0 Hz, 1H), 3.65–3.58 (m, 2H), 3.45 (ddd, J = 7.7, 7.7, 2.5 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ (ppm) 171.9, 144.0, 142.4, 137.0, 134.8, 134.3, 131.4, 129.4, 128.8, 128.0, 126.7, 85.5, 80.7, 76.0, 74.5, 71.6, 70.3, 70.2, 64.5; HRMS (ESI-TOF) m/z [M+Na]+ calcd for C21H2435ClNO7Na+ 460.1134, found 460.1127.
2-O-(4-bromophenyl)-3,4;5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (19).
3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 14 (0.028 g, 0.10 mmol, 1.0 eq.) was dissolved in toluene (1.0 mL), added into a 10 mL round bottom flask, and stirred at room temp for 5 minutes. Potassium tert-butoxide (0.0344 g, 0.31 mmol, 3.0 eq.) and bis-(4-bromophenyl) iodonium triflate (0.134 g, 0.31 mmol, 3.0 eq.) were added to the flask and the solution was stirred for 1 h as a yellow color formed. Upon consumption of starting material and the appearance of a new spot by TLC - presumed to be the product - the reaction was diluted with 20 mL EtOAc, silica gel (2.0 g) was added, and the solvent was removed under reduced pressure. The silica-adsorbed reaction mixture was purified via column chromatography using 4:6 EtOAc:hexanes to yield 19 as a clear colorless oil (0.0192 g, 44%, 51% BRSM). Rf 0.75 (4:6 EtOAc:Hexane); 1H NMR (400 MHz, CDCl3): δ (ppm) 7.38 (d, J = 9.2 Hz, 2H), 6.87 (d, J = 9.2 Hz, 2H), 4.50 (dd, J = 8.7, 8.0 Hz, 1H), 4.37 (dd, J = 8.8, 8.0 Hz, 1H), 4.33 (dd, J = 9.5, 2.2 Hz, 1H), 3.96–3.91 (m, 2H), 3.85 (dd, J = 9.0, 9.0 Hz, 1H), 3.63 (dd, J = 12.0, 7.0 Hz, 1H), 3.34 (dd, J = 13.0, 9.7 Hz, 1H), 3.28 (ddd, J = 9.0, 6.8, 6.1 Hz, 1H), 1.50 (s, 6H), 1.43 (s, 3H), 1.42 (s, 3H); 13C NMR (100 MHz, CDCl3): δ (ppm) 156.8, 132.7, 118.4, 114.3, 110.1, 99.5, 79.6, 79.0, 78.7, 74.6, 73.8, 69.0, 62.6, 27.7, 27.3, 24.7, 20.6; HRMS (ESI-TOF) m/z [M+H]+ calcd for C19H2679BrO6+ 429.0907, found 429.0899.
2-O-(4-[4-cyanophenyl]phenyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (20).
Into a 20 mL vial containing 2-O-(4-bromophenyl)-3,4;5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 19 (0.0756 g, 0.18 mmol, 1.0 eq.) was added 4-cyanophenylboronic acid (0.0388 g, 0.26 mmol, 1.5 eq.), and Cs2CO3 (0.086 g, 0.26 mmol, 1.5 eq). The vial was vacuum evacuated and refilled with Ar. Degassed dioxane /H2O (2.0/0.4 mL) was added under Ar, following addition of Pd(Ph3P)4 (0.0203 g, 0.0176 mmol, 0.1 eq.) and the reaction mixture was stirred under Ar at 80 °C for 1 hr. The mixture was allowed to cool to rt and the solvent was removed under reduced pressure. The residue was purified by flash chromatography on silica gel (PE/EE, 6:1–4:1) to give 20 (0.053 g, 67%) as white solid; 1H NMR (400 MHz, CDCl3) δ (ppm) 7.70 (d, J = 8.3 Hz, 2H), 7.63 (d, J = 8.3 Hz, 2H), 7.53 (d, J = 8.8 Hz, 2H), 7.09 (d, J = 8.8 Hz, 2H) 4.55 (dd, J = 8.8, 7.8 Hz, 1H), 4.47 (ddd, J = 9.3, 9.3 2.0 Hz, 1H), 4.40 (dd, J = 8.8, 7.6 Hz, 1H), 4.00 (dd, J = 13.0, 2.2 Hz, 1H), 3.95 (dd, J = 12.0, 5.9 Hz, 1H), 3.89 (dd, J = 9.0, 9.0 Hz, 1H), 3.65 (dd, J = 12.1, 6.8 Hz, 1H), 3.38 (dd, J = 13.0, 9.6 Hz, 1H), 3.31 (ddd, J = 8.9, 6.5, 6.3 Hz, 1H), 1.51 (s, 6H), 1.44 (s, 6H); 13C NMR (100 MHz, CDCl3) δ (ppm) 158.2, 145.3, 132.83, 132.80, 128.7, 127.4, 119.3, 117.0, 110.6, 110.1, 99.5, 79.6, 79.1, 78.4, 74.7, 73.8, 69.1, 62.6, 27.7, 27.3, 24.7, 20.7; HRMS (ESI-TOF) m/z [M+Na]+ calcd for C26H29NO6Na+ 474.1887, found 474.1888.
2-O-(4-[4-cyanophenyl]phenyl)-1,6-anhydro-D-glycero-D-galactitol (6).
Into a 10 mL round bottom flask was added 2-O-(4-[4-cyanophenyl]phenyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 20 (0.020 g, 0.044 mmol, 1.0 eq.) in DCM/MeOH/water (1.0 mL/2.0 mL/30 μL). TsOH monohydrate (0.012 g, 0.06 mmol) was added and the mixture was stirred at 50 °C overnight. The mixture was cooled to rt and solvent was removed under reduced pressure and the residue was purified by flash chromatography on silica gel (DCM/MeOH, 9:1–6:1); the product was redissolved in CH3CN/MeOH/H2O, filtered through a 0.45 μM filter and the filtrate was lyophilized to give 6 (0.013 g, 81%) as white solid; 1H NMR (500 MHz, CD3OD) δ (ppm) 7.80–7.76 (m, 4H), 7.64 (d, J = 8.7 Hz, 2H), 7.12 (d, J = 8.7 Hz, 2H), 4.69 (q, J = 5.9 Hz, 1H), 4.30 (dd, J = 13.0, 5.1 Hz, 1H), 4.19 (dd, J = 5.9, 1.8 Hz, 1H), 3.99 (dd, J = 7.2, 1.8 Hz, 1H), 3.78 (dd, J = 11.8, 2.9 Hz, 1H), 3.73 (t, J = 7.5 Hz, 1H), 3.65–3.59 (m, 2H), 3.46 (td, J = 7.8, 2.8 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ (ppm) 160.0, 133.8, 133.7, 129.6, 129.6, 128.3, 119.9, 117.5, 117.5, 111.2, 86.3, 78.4, 76.1, 73.9, 71.4, 70.0, 64.5,; HRMS (ESI-TOF) m/z [M+Na]+ calcd for C20H21NO6Na+ 394.1261, found 394.1245.
2-O-(4-bromo-2-chlorophenyl)-3,4;5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (21).
To a solution of 14 (0.273 g, 0.995 mmol) in DMF (7.5 mL) was added NaH (60%, 0.16 g, 4.0 mmol) at 0°C. The reaction mixture was stirred at 0°C for 10 minutes then at rt for another 0.5 h. 4-Bromo-2-chloro-1-fluorobenzene (0.485 mL, 4.0 mmol) was added to the reaction mixture in one portion at rt. The reaction mixture was stirred at 65°C for 24 h and then diluted with toluene and quenched carefully with MeOH. Solvent was removed under reduced pressure. The residue was redissolved into DCM, washed with water, brine and dried over Na2SO4, filtered and the solvent was removed under reduced pressure. The product was purified via column chromatography using (1:6–1:4) EtOAc:petroleum ether as eluent to give 21 (0.410 g, 88% yield) as a honeylike solid. 1H NMR (500 MHz, CDCl3) δ 7.49 (d, J = 2.4 Hz, 1H), 7.32 (dd, J = 8.8, 2.4 Hz, 1H), 7.04 (d, J = 8.8 Hz, 1H), 4.59 (dd, J = 8.7, 7.7 Hz, 1H), 4.47 – 4.30 (m, 2H), 4.01 – 3.88 (m, 2H), 3.81 (t, J = 9.1 Hz, 1H), 3.61 (dd, J = 12.1, 7.0 Hz, 1H), 3.45 (dd, J = 12.9, 9.9 Hz, 1H), 3.28 (ddd, J = 9.1, 7.0, 5.9 Hz, 1H), 1.50 (s, 3H), 1.49 (s, 3H), 1.43 (s, 3H), 1.42 (s, 3H); 13C NMR (126 MHz, CDCl3) δ 153.0, 133.0, 130.8, 126.0, 118.9, 114.5, 110.0, 99.4, 81.3, 79.5, 78.9, 74.5, 73.7, 69.2, 62.5, 27.6, 27.3, 24.5, 20.5; ESI-MS m/z calcd for C19H24BrClNaO6 [M+Na]+: 485.03; found: 487.15.
2-O-[4-(4’-cyanophenyl)-2-chlorophenyl]-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (22a).
To a solution of 21 (0.0458 g, 98.7μmmol) in dioxane/water (2.0 mL/0.4 mL) was added 4-cyanophenylboronic acid (0.0368 g. 0.25 mmol) and Cs2CO3 (0.0545 g, 0.167 mmol) at rt. The reaction mixture was degassed with Argon, then Pd(PPh3)4 (0.0192 g, 16.69 μmol) was added under Argon. The mixture was stirred for 1.5 h at 80°C, then cooled to rt, co-evaporated with toluene. The residue was purified via column chromatography using (1:4–1:3) EtOAc:petroleum ether as eluent to give 22a (0.033 g, 68% yield) as a white foam. 1H NMR (500 MHz, CDCl3) δ 7.75 – 7.69 (m, 2H), 7.65 – 7.58 (m, 3H), 7.45 (dd, J = 8.6, 2.3 Hz, 1H), 7.28 – 7.22 (m, 1H), 4.64 (dd, J = 8.8, 7.7 Hz, 1H), 4.49 (td, J = 9.2, 2.3 Hz, 1H), 4.41 (dd, J = 9.1, 7.6 Hz, 1H), 4.00 (dd, J = 13.0, 2.4 Hz, 1H), 3.95 (dd, J = 12.1, 5.9 Hz, 1H), 3.85 (t, J = 9.1 Hz, 1H), 3.64 (dd, J = 12.1, 6.9 Hz, 1H), 3.51 (dd, J = 13.0, 9.8 Hz, 1H), 3.32 (ddd, J = 9.1, 6.9, 5.9 Hz, 1H), 1.53 (s, 3H), 1.51 (s, 3H), 1.44 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 154.0, 143.9, 134.0, 132.8, 129.3, 127.5, 126.6, 118.9, 117.5, 111.2, 110.0, 99.4, 80.9, 79.5, 78.9, 74.6, 73.7, 69.2, 62.5, 27.6, 27.2, 24.6, 20.5; ESI-MS m/z calcd for C26H28ClNaO6 [M+Na]+: 508.15; found: 508.36.
2-O-[4-(4’-carboxamidophenyl)-2-chlorophenyl]-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (22b).
Following the procedure described for 22a, starting with 21 (0.075 g, 162 μmol), 4-aminocarbonylphenylboronic acid (0.040 g, 240 μmol), Cs2CO3 (0.0528 g, 162 μmol), Pd(PPh3)4 (0.0187 g, 16.2 μmol) in dioxane/water (2.0 mL/0.4 mL), stirred at 80°C for 1 h, purified via column chromatography using (3:7–4:6) acetone:petroleum ether as eluent to give 22b (0.065 g, 80% yield) as a honeylike solid. 1H NMR (500 MHz, CDCl3) δ 7.88 (d, J = 8.5 Hz, 2H), 7.69 – 7.51 (m, 3H), 7.49 – 7.40 (m, 1H), 7.23 (d, J = 8.6 Hz, 1H), 6.47 (brs, 1H, NH2), 6.14 (brs, 1H, NH2), 4.63 (dd, J = 8.7, 7.7 Hz, 1H), 4.47 (td, J = 9.3, 2.4 Hz, 1H), 4.39 (dd, J = 9.1, 7.6 Hz, 1H), 3.99 (dd, J = 12.9, 2.3 Hz, 1H), 3.93 (dd, J = 12.1, 5.9 Hz, 1H), 3.84 (t, J = 9.1 Hz, 1H), 3.62 (dd, J = 12.1, 6.9 Hz, 1H), 3.48 (dd, J = 13.0, 9.8 Hz, 1H), 3.34 – 3.27 (m, 1H), 1.52 (s, 2H), 1.49 (s, 3H), 1.42 (s, 5H); 13C NMR (126 MHz, CDCl3) δ 169.4, 153.5, 143.2, 134.8, 132.2, 132.1, 132.1, 131.7, 129.2, 128.8, 128.7, 128.3, 127.0, 126.6, 125.2, 117.5, 110.0, 99.3, 80.9, 79.5, 78.8, 74.5, 73.6, 69.2, 62.4, 27.6, 27.2, 24.5, 20.5; ESI-MS m/z calcd for C26H30ClNO7 [M+Na]+: 526.16; found: 526.23.
2-O-[4-phenyl-2-chlorophenyl]-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (22c).
Following the procedure described for 22a, starting with 21 (0.0624 g, 134 μmol), 4-phenylboronic acid (0.0246 g, 202 μmol), Cs2CO3 (0.044 g, 134 μmol), Pd(PPh3)4 (0.015 g, 13.4 μmol) in dioxane/water (2.0 mL/0.4 mL), stirred at 80°C for 1 h, purified via column chromatography using (3:7–4:6) ethyl acetate:petroleum ether as eluent to give 22c (0.046 g, 74%) as white solid.
1H NMR (500 MHz, CDCl3) δ 7.60 (d, J = 2.3 Hz, 1H), 7.54 – 7.50 (m, 2H), 7.47 – 7.40 (m, 3H), 7.37 – 7.31 (m, 1H), 7.28 – 7.21 (m, 2H), 4.64 (dd, J = 8.7, 7.7 Hz, 1H), 4.47 (td, J = 9.3, 2.3 Hz, 1H), 4.40 (dd, J = 9.1, 7.7 Hz, 1H), 4.02 (dd, J = 13.0, 2.4 Hz, 1H), 3.94 (dd, J = 12.1, 6.0 Hz, 1H), 3.86 (t, J = 9.1 Hz, 1H), 3.64 (dd, J = 12.1, 7.0 Hz, 1H), 3.49 (dd, J = 13.0, 9.8 Hz, 1H), 3.31 (ddd, J = 9.1, 7.0, 5.9 Hz, 1H), 1.54 (s, 3H), 1.51 (s, 3H), 1.45 (s, 6H); 13C NMR (126 MHz, CDCl3) δ 152.8, 139.5, 136.4, 129.7, 129.1, 129.0, 127.5, 126.9, 126.4, 125.1, 117.7, 109.9, 99.3, 81.0, 79.5, 78.9, 74.4, 73.7, 69.2, 62.4, 27.6, 27.3, 24.5, 20.4; ESI-MS m/z calcd for C26H29ClNO6 [M+Na]+: 483.15; found: 483.25.
2-O-[4-(4’-cyanophenyl)-2-chlorophenyl]-1,6-anhydro-D-glycero-D-galactitol (8a).
Into a 10 mL round bottom flask was added 2-O-(4-[4’-cyanophenyl]-2-chlorophenyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 22a (0.033 g, 67.9 μmol, 1.0 eq.) in DCM/MeOH/water (1.0 mL/2.0 mL/30 μL). TsOH monohydrate (0.018 g, 101 μmol, 1.5 eq.) was added and the mixture was stirred at 50 °C for 7.5h. The mixture was cooled to rt and solvent was removed under reduced pressure and the residue was purified by flash chromatography on silica gel (DCM/MeOH, 9:1); the product was redissolved in CH3CN/MeOH/H2O, filtered through a 0.45 μM filter and the filtrate was lyophilized to give 8a (0.0228 g, 84%) as white solid. 1H NMR (500 MHz, MeOD) δ 7.76 (m, 4H), 7.74 (d, J = 2.3 Hz, 1H), 7.60 (dd, J = 8.6, 2.4 Hz, 1H), 7.31 (d, J = 8.6 Hz, 1H), 4.74 (q, J = 5.9 Hz, 1H), 4.30 (dd, J = 13.1, 5.2 Hz, 1H), 4.24 (dd, J = 6.0, 1.8 Hz, 1H), 4.06 (dd, J = 7.4, 1.8 Hz, 1H), 3.80 (dd, J = 11.7, 2.8 Hz, 1H), 3.76 – 3.68 (m, 2H); 13C NMR (126 MHz, MeOD) δ 155.3, 145.2, 134.6, 133.9, 130.0, 128.5, 127.9, 125.7, 119.7, 117.4, 111.9, 85.9, 80.1, 76.1, 73.9, 71.6, 69.9, 64.5; HR-MS m/z calcd for C20H20ClNNaO6 [M+Na]+: 428.0871; found: 428.0871.
2-O-[4-(4’-carboxamidophenyl)-2-chlorophenyl]-1,6-anhydro-D-glycero-D-galactitol (8b).
Following the procedure described for 8a, starting with 22b (0.060 g, 119 μmol), DCM/MeOH/water (1.8 mL/3.5 mL/50 μL), TsOH monohydrate (0.0308 g, 178 μmol, 1.5 eq.), 50°C overnight. Solvent was removed under reduced pressure and the residue was purified by flash chromatography on silica gel (DCM/MeOH, 6:1); the product was redissolved in CH3CN/MeOH/H2O, filtered through a 0.45 μM filter and the filtrate was lyophilized to give 8b (0.0114 g, 22%) as white solid. 1H NMR (500 MHz, MeOD) δ 7.94 (d, J = 8.5 Hz, 2H), 7.76 – 7.66 (m, 2H), 7.60 (dd, J = 8.6, 2.3 Hz, 1H), 7.30 (d, J = 8.7 Hz, 1H), 4.73 (q, J = 5.9 Hz, 1H), 4.30 (dd, J = 13.1, 5.2 Hz, 1H), 4.25 (dd, J = 5.9, 1.8 Hz, 1H), 4.06 (dd, J = 7.5, 1.8 Hz, 1H), 3.80 (dd, J = 11.7, 2.8 Hz, 1H), 3.76 – 3.68 (m, 2H), 3.63 (dd, J = 11.8, 7.4 Hz, 1H), 3.51 (ddd, J = 10.2, 7.5, 2.9 Hz, 1H); 13C NMR (126 MHz, MeOD) δ 154.8, 144.0, 135.6, 133.7, 129.4, 127.64, 127.61, 125.6, 117.5, 85.9, 80.1, 76.0, 73.9, 71.6, 70.0, 64.5; HR-MS m/z calcd for C20H22ClNNaO7 [M+Na]+: 446.0977; found:446.0977.
2-O-[4-phenyl-2-chlorophenyl]-1,6-anhydro-D-glycero-D-galactitol (8c).
Following the procedure described for 8a, starting with 22c (0.046 g, 99 μmol), DCM/MeOH/water (1.0 mL/2.0 mL/30 μL), TsOH monohydrate (0.025 g, 149 μmol, 1.5 eq.), 50°C overnight. purified via was purified by flash chromatography on silica gel (DCM/MeOH, 9:1), the product was redissolved in CH3CN/H2O, filtered through a 0.45 μM filter and the filtrate was lyophilized to give 22c (0.031 g, 82%) as white solid. 1H NMR (500 MHz, MeOD) δ 7.64 (d, J = 2.3 Hz, 1H), 7.58 – 7.54 (m, 2H), 7.52 (dd, J = 8.6, 2.3 Hz, 1H), 7.42 (t, J = 7.7 Hz, 2H), 7.36 – 7.30 (m, 1H), 7.26 (d, J = 8.6 Hz, 1H), 4.70 (q, J = 5.9 Hz, 1H), 4.30 (dd, J = 13.1, 5.2 Hz, 1H), 4.25 (dd, J = 5.8, 1.8 Hz, 1H), 4.07 (dd, J = 7.5, 1.8 Hz, 1H), 3.81 (dd, J = 11.7, 2.8 Hz, 1H), 3.76 – 3.68 (m, 2H), 3.63 (dd, J = 11.7, 7.4 Hz, 1H), 3.51 (ddd, J = 8.4, 7.4, 2.8 Hz, 1H); 13C NMR (126 MHz, MeOD) δ 154.2, 140.7, 137.1, 130.0, 129.7, 128.4, 127.6, 127.5, 125.5, 117.6, 85.8, 80.2, 75.9, 73.9, 71.6, 70.0, 64.5; HR-MS m/z calcd for C19H21ClNaO6 [M+Na]+: 403.0919.; found: 403.0918.
2-O-benzyl-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (24).
Into a 10 mL round bottom flask was added 3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 14 (0.018 g, 0.066 mmol, 1.0 eq.) in dry DMF (1.0 mL). Tetrabutylammonium iodide (TBAI) (0.002 g, 0.007 mmol, 0.1 eq.) and sodium hydride (0.007 g, 0.264 mmol, 4.0 eq.) were added at 0 °C and the flask was stirred for 30 min under N2. Benzyl bromide (0.031 mL, 0.264 mmol, 4.0 eq.) was added, and the reaction was stirred overnight at rt under N2. The mixture was quenched by the addition of methanol (1.0 mL) at 0 °C and then the solvent was removed under reduced pressure. The residue was redissolved in EtOAc (10 mL), washed with water (2 × 10 mL) and brine (1 × 10 mL). The organic layer was dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was isolated via column chromatography using 1:3 EtOAc:hexane as eluent to give 24 (0.018 g, 76%) as a colorless oil. Rf 0.33 (2:8 EtOAc:Hexane); 1H NMR (400 MHz, CDCl3) δ (ppm) 7.40–7.31 (m, 5H), 4.80 (d, J = 12.1 Hz, 1H), 4.69 (d, J = 12.4 Hz, 1H), 4.37 (dd, J = 8.2, 8.2 Hz, 1H), 4.30 (dd, J = 8.6, 8.6 Hz, 1H), 3.96 (dd, J = 12.7, 2.2 Hz, 1H), 3.92 (dd, J = 12.1, 6.0 Hz, 1H), 3.77 (dd, J = 8.9, 8.9 Hz, 1H), 3.64 (ddd, J = 9.9, 9.9, 2.4 Hz, 1H), 3.61 (dd, J = 12.1, 7.0 Hz, 1H), 3.30 (dd, J = 12.7, 9.9 Hz, 1H), 3.24 (ddd, J = 12.8, 9.2, 6.5 Hz, 1H), 1.53 (s, 3H), 1.48 (s, 3H), 1.43 (s, 6H); 13C NMR (100 MHz, CDCl3) δ (ppm) 138.4, 128.6, 128.1, 128.0, 109.6, 99.3, 81.0, 79.2, 79.1, 74.2, 73.8, 72.9, 70.3, 62.7, 27.8, 27.4, 24.9, 20.6; HRMS (ESI-TOF) m/z [M+Na]+ calcd for C20H28O6Na+ 387.1778, found 387.1773.
2-O-(3-phenylpropyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (25).
Into a 10 mL round bottom flask was added 3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 14 (0.017 g, 0.062 mmol, 1.0 eq.) in dry DMF (1.0 mL). Tetrabutylammonium iodide (TBAI) (0.002 g, 0.0062 mmol, 0.1 eq.) and sodium hydride (0.006 g, .25 mmol, 4.0 eq.) were added at 0 °C and the flask was stirred for 30 min under N2. 1-bromo-3-phenylpropane (0.038 mL, 0.25 mmol, 4.0 eq.) was added, and the reaction was stirred overnight at rt under N2. The mixture was quenched by the addition of methanol (1.0 mL) at 0 °C and then the solvent was removed under reduced pressure. The residue was redissolved in EtOAc (10 mL), washed with water (2 × 10 mL) and brine (1 × 10 mL). The organic layer was dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was isolate via column chromatography using 1:3 EtOAc:hexane as eluent to give 25 (0.016 g, 64% yield) as a colorless oil. Rf 0.45 (1:3 EtOAc:Hexane); 1H NMR (500 MHz, CDCl3) δ (ppm) 7.33–7.31 (m, 2H), 7.25–7.21 (m, 3H), 4.31–4.30 (m, 2H), 3.96 (dd, J = 12.0, 5.9 Hz 1H), 3.92 (dd, J = 12.6, 2.2 Hz, 1H), 3.79 (dd, J = 8.7, 8.7 Hz, 1H), 3.72 (dt, J = 9.5, 6.2 Hz, 1H), 3.64 (dd, J = 12.1, 7.1 Hz, 1H), 3.60 (dd, J = 9.5, 6.6 Hz, 1H) 3.50 (dd, J = 7.4, 7.4 Hz 1H), 3.29 (dd, J = 12.6, 9.9 Hz 1H), 3.26 (dd, J = 9.1, 6.6 Hz, 1H), 2.79–2.68 (m, 2H), 1.95 (qu, J = 6.9 Hz, 2H), 1.55 (s, 3H), 1.52 (s, 3H), 1.46 (s, 3H), 1.45 (s, 3H); 13C NMR (125 MHz, CDCl3) δ (ppm) 141.9, 128.5, 128.3, 125.8, 109.3, 99.1, 80.6, 79.6, 78.9, 74.1, 73.5, 70.0, 62.5, 32.1, 31.4, 27.7, 27.2, 24.6, 20.3; HRMS (ESI-TOF) m/z [M+K]+ calcd for C22H32O6K+ 431.1830, found 431.1817.
2-O-(4-methylbiphenyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol (26).
Into a 10 mL round bottom flask was added 3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 14 (0.029 g, 0.106 mmol, 1.0 eq.) in dry DMF (1.0 mL). Tetrabutylammonium iodide (TBAI) (0.004 g, 0.011 mmol, 0.1 eq.) and sodium hydride (0.010 g, 0.424 mmol, 4.0 eq.) were added at 0 °C, and the flask was stirred for 30 min under N2. 4-bromomethylbiphenyl (0.105 mL, 0.424 mmol, 4.0 eq.) was added, and the reaction was stirred overnight at rt under N2. The mixture was quenched by the addition of methanol (1.0 mL) at 0 °C and then the solvent was removed under reduced pressure. The residue was redissolved in EtOAc (10 mL), washed with water (2 × 10 mL) and brine (1 × 10 mL). The organic layer was dried with Na2SO4, filtered, and the solvent was removed under reduced pressure. The product was isolate via column chromatography using 1:4 EtOAc:hexane as eluent to give 26 (0.036 g, 77% yield) as a clear colorless oil. Rf 0.44 (1:4 EtOAc:Hexane); 1H NMR (500 MHz, CDCl3) δ (ppm) 7.65–7.62 (m, 4H), 7.51–7.48 (m, 4H), 7.40 (t, J = 7.6 Hz, 1H) 4.87 (d, J = 12.0, 1H), 4.77 (d, J = 12.0 Hz, 1H), 4.42 (dd, J = 8.3, 8.3 Hz, 1H), 4.35 (dd, J = 8.3, 8.3 Hz, 1H), 4.02 (dd, J = 12.8, 2.3 Hz, 1H), 3.96 (dd, J = 12.0, 5.9 Hz, 1H), 3.81 (dd, J = 9.0, 9.0 Hz, 1H), 3.70 (ddd, J = 9.6, 9.6, 2.4 Hz, 1H), 3.65 (dd, J = 12.0, 7.1 Hz, 1H), 3.36 (dd, J = 12.7, 9.9 Hz, 1H), 3.28 (ddd, J = 8.8, 6.6, 6.4 Hz, 1H), 1.58 (s, 3H), 1.52 (s, 3H) 1.48 (s, 3H), 1.47 (s, 3H); 13C NMR (125 MHz, CDCl3): δ (ppm) 140.9, 140.8, 137.2, 128.8, 128.3, 127.3, 127.2, 127.1, 109.4, 99.1, 80.8, 79.0, 78.9, 74.0, 73.6, 72.5, 70.1, 62.4, 27.6, 27.2, 24.7, 20.4; HRMS (ESI-TOF) m/z calcd for C26H32O6Na+ 463.2091, found 463.2079.
2-O-benzyl-1,6-anhydro-D-glycero-D-galactitol (4).
Into a 10 mL round bottom flask was added 2-O-benzyl-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 24 (0.0183 g, 0.0502 mmol, 1.0 eq.) in EtOH/water (3.0 mL, 1:3). Amberlite IR-120 resin was added until the pH ≤ 3, and the flask was refluxed at 110 °C for 4 hr, or until TLC analysis showed complete consumption of starting material. The mixture was cooled to rt and the resin was filtered, washing with methanol (3 × 7 mL). Solvent was removed from the filtrate under reduced pressure and the residue was purified via column chromatography using 1:9 MeOH:DCM as eluent to give 4 (0.0075 g, 52%) as a clear, colorless oil. Rf 0.32 (1:9 MeOH:DCM); 1H NMR (500 MHz, CD3OD) δ (ppm) 7.39–7.34 (m, 4H), 7.29 (t, J = 7.0, Hz, 1H), 4.67 (d, J = 11.9 Hz, 1H), 4.62 (d, J = 11.9 Hz, 1H), 4.12 (dd, J = 7.9, 5.1 Hz, 1H), 4.11–4.10 (m, 1H), 3.93 (d, J = 7.6 Hz, 1H), 3.79–3.73 (m, 2H), 3.65 (dd, J = 7.7, 7.7 Hz, 1H), 3.56 (dd, J = 11.6, 3.4 Hz, 1H), 3.52 (dd, J = 12.9, 6.5 Hz, 1H), 3.41 (ddd, J = 8.4, 8.4, 2.5 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ (ppm) 139.8, 129.4, 129.0, 128.7, 85.4, 79.8, 75.9, 74.5, 73.3, 71.5, 70.3, 64.5; HRMS (ESI-TOF) m/z [M+H]+ calcd for C14H21O6+ 285.1333, found 285.1307.
2-O-(3-phenylpropyl)-1,6-anhydro-D-glycero-D-galactitol (5).
Into a 10 mL round bottom flask was added 2-O-(3-phenylpropyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 25 (0.0155 g, 0.0395 mmol, 1.0 eq.) in EtOH/water (3.0 mL, 1:3). Amberlite IR-120 resin was added until the pH ≤ 3, and the mixture was refluxed at 110 °C for 4 hr, or until TLC showed complete consumption of starting material. The mixture was cooled to rt and the resin was filtered off, washing with methanol (3 × 7 mL). Solvent was removed from the filtrate under reduced pressure and the residue was purified via column chromatography using 1:9 MeOH:DCM as eluent to give 5 (0.0032 g, 26%) as a clear, colorless oil. Rf 0.33 (1:9 MeOH:DCM); 1H NMR (500 MHz, CD3OD) δ (ppm) 7.27 (t, J = 7.4 Hz, 2H), 7.21 (d, J = 7.4 Hz, 2H), 7.17 (t, J = 7.1 Hz, 1H), 4.15 (dd, J = 13.0, 5.0 Hz, 1H), 4.05 (d, J = 5.8 Hz, 1H), 3.90 (d, J = 7.3 Hz, 1H), 3.78 (d, J = 11.4 Hz, 1H), 3.64 (dd, J = 8.0, 8.0 Hz, 1H), 3.61–3.54 (m, 4H), 3.48 (dd, J = 12.7, 6.8 Hz, 1H), 3.40 (dd, J = 7.9, 7.9 Hz, 1H), 2.71 (t, J = 7.7 Hz, 2H), 1.90 (qu, J = 7.3 Hz, 2H); 13C NMR (125 MHz, CD3OD) δ (ppm) 143.2, 129.5, 129.4, 126.8, 85.4, 80.6, 75.9, 74.5, 71.5, 70.7, 70.4, 64.5, 33.3, 32.9; HRMS (ESI-TOF) m/z [M+H]+ calcd for C16H25O6+ 313.1646, found 313.1612.
2-O-(4-methylbiphenyl)-1,6-anhydro-D-glycero-D-galactitol (7c).
Into a 10 mL round bottom flask was added 2-O-(4-methylbiphenyl)-3,4:5,7-di-O-isopropylidene-1,6-anhydro-D-glycero-D-galactitol 26 (0.036 g, 0.082 mmol, 1.0 eq.) in EtOH/water (4.0 mL, 1:3). Amberlite IR-120 resin was added until pH ≤ 3, and the mixture was refluxed at 110 °C for 4 hr, or until TLC showed complete consumption of starting material. The mixture was cooled to rt and the resin was filtered off, washing with methanol (3 × 7 mL). Solvent was removed from the filtrate under reduced pressure and the residue was purified via column chromatography using 1:9 MeOH:DCM as eluent to give 7c (0.0125 g, 43%) as a clear, colorless oil. Rf 0.43 (1:9 MeOH:DCM); 1H NMR (500 MHz, CD3OD) δ (ppm) 7.63–7.61 (m, 4H), 7.47–7.43 (m, 4H), 7.36–7.33 (m, 1H), 4.72 (d, J = 11.9 Hz, 1H), 4.67 (d, J = 11.9 Hz, 1H), 4.15 (dd, J = 13.0, 5.0 Hz, 1H), 4.12 (d, J = 5.5 Hz, 1H), 3.95 (d, J = 7.7 Hz, 1H), 3.80–3.77 (m, 2H), 3.66 (dd, J = 8.1, 8.1 Hz, 1H), 3.60 (dd, J = 11.7, 7.4 Hz, 1H), 3.54 (dd, J = 12.9, 6.6 Hz, 1H), 3.43 (ddd, J = 8.1, 8.1, 2.5 Hz, 1H); 13C NMR (125 MHz, CD3OD) δ (ppm) 142.1, 142.0, 138.9, 129.9, 129.5, 128.4, 128.0, 127.9, 85.4, 79.8, 76.0, 74.5, 73.0, 71.5, 70.4, 64.5; HRMS (ESI-TOF) m/z [M+Na]+ calcd for C20H24O6Na+ 383.1465, found 383.1487.
Supplementary Material
Highlights.
Septanoses are ring-expanded glycomimetics that are not susceptible to glycosidase cleavage
Structure–activity relationships from pyranose glycomimetics binding to FimH cannot be easily transferred to related septanoses
Septanoses diverge from the canonical 100-fold loss in binding affinity for the more flexible FimHFL construct
Acknowledgements
The authors are thankful to the Chemaxon Academic Program and the OpenEye Academic Licensing Program for providing licenses for the molecular modeling tools used in this study. Support for this work was provided by NIH grant R21 AI142363.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Gagneux P, Hennet T, Varki A, in Essentials of Glycobiology, 10.1101/glycobiology.4e.7 (Eds.: th, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Mohnen D, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH), Cold Spring Harbor (NY), 2022, pp. 79–92. [DOI] [PubMed] [Google Scholar]
- [2].Ernst B, Magnani JL, Nat Rev Drug Discov 2009, 8, 661–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hevey R, Pharmaceuticals 2019, 12, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee AC, Harris JL, Khanna KK, et al. , Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Werle M, Bernkop-Schnurch A, Amino Acids 2006, 30, 351–367. [DOI] [PubMed] [Google Scholar]
- [6].Sahin U, Kariko K, Tureci O, Nat Rev Drug Discov 2014, 13, 759–780. [DOI] [PubMed] [Google Scholar]
- [7].Sasso JM, Ambrose BJB, Tenchov R, et al. , J Med Chem 2022, 65, 6975–7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seebach D, Beck AK, Bierbaum DJ, Chem Biodivers 2004, 1, 1111–1239. [DOI] [PubMed] [Google Scholar]
- [9].Cabrele C, Martinek TA, Reiser O, et al. , J Med Chem 2014, 57, 9718–9739. [DOI] [PubMed] [Google Scholar]
- [10].Nance KD, Meier JL, ACS Cent Sci 2021, 7, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mydock-McGrane L, Cusumano Z, Han Z, et al. , J Med Chem 2016, 59, 9390–9408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chalopin T, Alvarez Dorta D, Sivignon A, et al. , Org Biomol Chem 2016, 14, 3913–3925. [DOI] [PubMed] [Google Scholar]
- [13].Schwardt O, Rabbani S, Hartmann M, et al. , Bioorganic & Medicinal Chemistry 2011, 19, 6454--6473. [DOI] [PubMed] [Google Scholar]
- [14].Feng GJ, Guo YF, Tang Y, et al. , J Med Chem 2023, 66, 12536–12543. [DOI] [PubMed] [Google Scholar]
- [15].Kelemen V, Borbás A, in Recent Trends in Carbohydrate Chemistry, 10.1016/b978-0-12-817467-8.00005-0, 2020, pp. 161–215. [DOI] [Google Scholar]
- [16].Leusmann S, Menova P, Shanin E, et al. , Chem Soc Rev 2023, 52, 3663–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pote AR, Pascual S, Planas A, et al. , Int J Mol Sci 2021, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kleeb S, Pang L, Mayer K, et al. , J Med Chem 2015, 58, 2221–2239. [DOI] [PubMed] [Google Scholar]
- [19].Mayer K, Eris D, Schwardt O, et al. , J Med Chem 2017, 60, 5646–5662. [DOI] [PubMed] [Google Scholar]
- [20].Pang L, Kleeb S, Lemme K, et al. , ChemMedChem 2012, 7, 1404–1422. [DOI] [PubMed] [Google Scholar]
- [21].Schönemann W, Cramer J, Mühlethaler T, et al. , ChemMedChem 2019, 14, 749–757. [DOI] [PubMed] [Google Scholar]
- [22].Sager CP, Fiege B, Zihlmann P, et al. , Chem Sci 2018, 9, 646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wellens A, Lahmann M, Touaibia M, et al. , Biochemistry 2012, 51, 4790–4799. [DOI] [PubMed] [Google Scholar]
- [24].Fiege B, Rabbani S, Preston RC, et al. , ChemBioChem 2015, 16, 1235–1246. [DOI] [PubMed] [Google Scholar]
- [25].Ruda A, Aytenfisu AH, Angles d'Ortoli T, et al. , Phys Chem Chem Phys 2023, 25, 3042–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sauer MM, Jakob RP, Eras J, et al. , Nature Communications 2016, 7, 10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Cramer J, Jiang X, Schönemann W, et al. , RSC Chem Biol 2020, 1, 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Eris D, Preston RC, Scharenberg M, et al. , ChemBioChem 2016, 17, 1012–1020. [DOI] [PubMed] [Google Scholar]
- [29].Han Z, Pinkner JS, Ford B, et al. , J Med Chem 2012, 55, 3945–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Han Z, Pinkner JS, Ford B, et al. , J Med Chem 2010, 53, 4779–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pang L, Kleeb S, Lemme K, et al. , ChemMedChem 2012, 7, 1404–1422. [DOI] [PubMed] [Google Scholar]
- [32].Bosko C, Vannam R, Peczuh MW, Tetrahedron Lett 2022, 93. [Google Scholar]
- [33].Pero B, Peczuh MW, J Org Chem 2022, 87, 7474–7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Saha J, Peczuh MW, Adv Carbohydr Chem Biochem 2011, 66, 121–186. [DOI] [PubMed] [Google Scholar]
- [35].Bielawski M, Zhu M, Olofsson B, Adv Synth Catal 2007, 349, 2610–2618. [Google Scholar]
- [36].Tolnai GL, Nilsson UJ, Olofsson B, Angew Chem Int Ed Engl 2016, 55, 11226–11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].OEDOCKING 4.1.1.0, OpenEye Scientific Software, Inc., Santa Fe, NM, http://www.eyesopen.com/. [Google Scholar]
- [38].Spruce 1.4.0.0, OpenEye Scientific Software, Santa Fe, NM, http://www.eyesopen.com/. [Google Scholar]
- [39].OMEGA 4.1.2.0, OpenEye Scientific Software, Santa Fe, NM, http://www.eyesopen.com/. [Google Scholar]
- [40].Hawkins PC, Skillman AG, Warren GL, et al. , J Chem Inf Model 2010, 50, 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kelley BP, Brown SP, Warren GL, et al. , J Chem Inf Model 2015, 55, 1771–1780. [DOI] [PubMed] [Google Scholar]
- [42].Rabbani S, Jiang X, Schwardt O, et al. , Anal Biochem 2010, 407, 188–195. [DOI] [PubMed] [Google Scholar]
- [43].Wang ZX, FEBS letters 1995, 360, 111–114. [DOI] [PubMed] [Google Scholar]
- [44].Scheuermann TH, Brautigam CA, Methods 2015, 76, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Brautigam CA, Zhao H, Vargas C, et al. , Nat Protoc 2016, 11, 882–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Piszczek G, Methods 2015, 76, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.