To the editor,
Drug induced ILD accounts for 16-48% of Nitrofurantoin induced related adverse events reported in registry studies (1). However, in the UK, prophylactic use of Nitrofurantoin is recommended in women who have 3 or more UTI per year (2) and National trend of prescribing Nitrofurantoin in England has increased by 7% in the last 10 years (3). We reviewed our ILD database from the year 2012 to 2020 and identified 10 patients diagnosed with Nitrofurantoin induced Interstitial Lung disease. In this case series, we documented age, sex, initial renal function, eosinophil count, spirometry and radiological findings. We also focused on various CT findings and reversibility of radiological abnormality following cessation of Nitrofurantoin.
We retrospectively reviewed electronic notes of the selected patients. All of them were female, median age 80 years, range of 23 years, mean 82 +/-8SD. 8 out of 10 patients have documented history of lower urinary tract infection. Nitrofurantoin induced chronic lung changes are thought to be due to disturbance of equilibrium between oxidative and anti-oxidative stress, resulting in production of toxic metabolites causing lung injury and long term fibrosis (4). This may explain prevalence of chronic pulmonary changes in the elderly with decreased creatinine clearance which results in accumulation of nitrofurantoin and its metabolites. There is paucity of data to indicate gender as a risk factor for drug induced interstitial lung disease, however, all the patients from this review were female with history of recurrent UTI.
The exact dose of prophylactic nitrofurantoin is not specified in all case notes. In our series, the average duration of Nitrofurantoin treatment is 17 months prior to presentation. This matches with a case series from Grampian which reported 13 cases of Nitrofurantoin induced lung disease between 2009 and 2012, mean duration of treatment was 14 months (5).
The mean predicted FVC was 80% at presentation and TLCo was 60%. Pre-treatment mean eGFR was 76ml/min/m2 and mean eosinophil count at presentation was 0.19mm3.
4 out of 10 patients were prescribed oral steroid. We also note, all but 2 patients had normal peripheral blood eosinophil count and those 2 patients improved almost immediately after cessation of Nitrofurantoin treatment. There is no reported death until the time of this data collection. Table 1 summarises the demographic characteristics of the patient, length of treatment with Nitrofurantoin, CT scan pattern, need of Prednisolone treatment and FVC at presentation (if available).
Table 1.
Summary of 10 cases.
| Age | Gender | Time to presentation (months) |
History of recurrent UTI | CT Pattern | Treatment with Prednisolone | Initial FVC (% predicted) | |
|---|---|---|---|---|---|---|---|
| Case 1 | 74 | F | 12 | Yes | Ground glass, traction bronchiectasis | N | 83 |
| Case 2 | 78 | F | 11 | Yes | Traction bronchiectasis | Y | 57 |
| Case 3 | 67 | F | 12 | Yes | Peripheral subpleural opacification | Y | 71 |
| Case 4 | 86 | F | Unknown | Yes | Bilateral bronchiectasis | N | 66 |
| Case 5 | 84 | F | Unknown | Ground glass, traction bronchiectasis | N | Unknown | |
| Case 6 | 79 | F | Unknown | Yes | Ground glass, traction bronchiectasis | N | Unknown |
| Case 7 | 81 | F | 36 | Ground glass | Y | Unknown | |
| Case 8 | 89 | F | 24 | Yes | Inflammatory changes, hilar lymphadenopathy | N | 98 |
| Case 9 | 90 | F | 12 | Yes | Pulmonary fibrosis, atypical distribution | N | 136 |
| Case 10 | 80 | F | 12 | Yes | Ground glass, traction bronchiectasis | Y | 64 |
Although, symptoms resolved after cessation of Nitrofurantoin, there were residual changes at chest imaging. Several case reports (8-11) were reviewed and CT changes varied from bilateral reticular septal thickening, architectural distortion and honeycombing to diffuse ground glass opacification. Interestingly, 6 out of 10 cases in this series, showed peribronchial ground glass infiltration, not uniform, resulting in varying degrees of patchy bronchial dilatation. Many showed arcades outlining secondary pulmonary lobules. When combined with peribronchial consolidation, this pattern fits most with organising pneumonia. Figure 1 shows 4 representative CT sections, demonstrating a variety of features including peribronchial and perilobular distribution of opacification, traction bronchiectasis, honeycombing and ground glass opacification.
Figure 1.
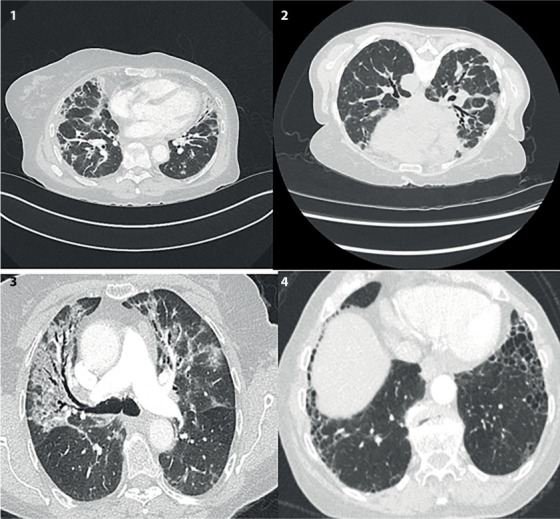
Coronal section of 4 representative CTs. Clockwise 1) Peribronchial vascular thickening and peripheral consolidation, some volume loss of middle and right lower lobe. 2) Traction bronchiectasis and interstitial lines outlining secondary pulmonary lobules. 3) Anterior, basal and subpleural honeycombing (post treatment cessation). 4) Bilateral upper lobe ground glass changes and traction bronchiectasis.
In summary, the patients in this series, improved symptomatically with or without treatment with steroid following cessation of nitrofurantoin therapy, but irreversible changes in the chest imaging may contribute to long term morbidity. Cautious counselling should be undertaken prior to Nitrofurantoin prescription regardless of renal function. MHRA recommended close monitoring for signs of pulmonary, hepatic, neurological and gastro intestinal side effects during treatment and is contra-indicated if eGFR<45 ml/min/1.73m (6,7).
References
- Skeoch S, Weatherley N, Swift A, et al. Drug-Induced Interstitial Lung Disease: A Systematic Review. J Clin Med. 2018 Oct 15;7(10):356. doi: 10.3390/jcm7100356. doi: 10.3390/jcm7100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JR, Guran LA, Gregory WT, McDonagh MS. Nitrofurantoin vs other prophylactic agents in reducing recurrent urinary tract infections in adult women: a systematic review and meta- analysis. Am J Obstet Gynecol. 2016;215(5):548–60. doi: 10.1016/j.ajog.2016.07.040. [DOI] [PubMed] [Google Scholar]
- Hammond A, Stuijfzand B, Avison MB, Hay AD. Antimicrobial resistance associations with national primary care antibiotic stewardship policy: Primary care-based, multilevel analytic study. PLoS One. 2020 May 14;15(5):e0232903. doi: 10.1371/journal.pone.0232903. doi: 10.1371/journal.pone.0232903. PMID: 32407346; PMCID: PMC7224529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd MR. Metabolic activation and lung toxicity: a basis for cell-selective pulmonary damage by foreign chemicals. Environ Health Perspect. 1984;55:47–51. doi: 10.1289/ehp.845547. doi:10.1289/ehp.845547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall ADL, Dempsey OJ. Is nitrofurantoin lung on the increase? BMJ. 2013;346:f3897. doi: 10.1136/bmj.f3897. doi: 10.1136/bmj.f3897. [DOI] [PubMed] [Google Scholar]
- Urinary Tract Infection (Recurrent): Antimicrobial prescribing. NICE guidelines, May 2018. https://www.nice.org.uk/guidance/ng112/documents/draft-guideline. [Google Scholar]
- Nitrofurantoin now contraindicated in most patients with an estimated glomerular filtration rate (eGFR) of less than 45 ml/min/1.73m2. GOV.UK, 12 February 2015. https://www.gov.uk/drug-safety-update/nitrofurantoin-now-contraindicated-in-most-patients-with-an-estimated-glomerular-filtration-rate-egfr-of-less-than-45-ml-min-1-73m2. [Google Scholar]
- Tatley M. Pulmonary Reactions with Nitrofurantoin. MEDSAFE- New Zealand Medicines and Medical Devices Safely Authority Prescriber Update. 2002;23(2):24–25. https://medsafe.govt.nz/profs/puarticles/nitrofurant. [Google Scholar]
- Boyd MR. Metabolic activation and lung toxicity: a basis for cell-selective pulmonary damage by foreign chemicals. Environ Health Perspect. 1984;55:47–51. doi: 10.1289/ehp.845547. doi:10.1289/ehp.845547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahid B, Wilderore B. Nitrofurantoin pulmonary toxicity: a brief review. The Internet Journal of Pulmonary Medicine. 2005;6(2) [Google Scholar]
- Chin AJ, Rashid S, Gharibeh TR, Kibbe PS, Wynbrandt JH. Interstitial lung disease secondary to long-term nitrofurantoin use. Am J Case Rep. 2020;21:e920386. doi: 10.12659/AJCR.920386. Published 2020 Apr 2. doi:10.12659/AJCR.920386. [DOI] [PMC free article] [PubMed] [Google Scholar]


