Abstract
Cell-free human immunodeficiency virus type 1 (HIV-1) can be taken up and released by a monolayer of primary human gingival cells and remain infectious for CD4+ cells. Virus-sized latex particles covalently coated with purified native HIV-1 envelope glycoprotein gp120 are also transported through the primary epithelial cells. This process is significantly stimulated by increasing the intracellular cyclic AMP (cAMP) concentration. Inhibition experiments with mannan and α-methyl-mannopyranoside indicated that mannosyl groups are involved in the interaction between gp120 and gingival cells. An increase of cellular oligomannosyl receptors by incubation with the mannosidase inhibitor deoxymannojirimycin augmented transcellular transport of the gp120-coated particles. The results suggest that infectious HIV can penetrate gingival epithelia by a cAMP-dependent transport mechanism involving interaction of the lectin-like domain of gp120 and mannosyl residues on glycoproteins on the mucosal surface. Penetration of HIV could be inhibited by soluble glycoconjugates present in oral mucins.
Little is known about the mechanisms of virus entrance into the organism through the cellular mucosal barrier. Human immunodeficiency virus (HIV) must pass epithelial cells which are part of the mucosal barrier to infect CD4+ cells (9, 20). Virus entry may occur if the integrity of the mucosa is compromised. Alternatively, entry via receptor-mediated uptake that involves receptors distinct from CD4, which is not expressed on epithelial cells, may be feasible. Virus transport through the epithelial cell monolayers is suggested by several experiments. During incubation of HIV type 1 (HIV-1)-infected mononuclear blood cells, with no cell-free virus present, on the apical site of monolayers of immortalized cells a basolateral release of infectious virus was shown (4). Furthermore, infection of neonate and adult macaques with cell-free simian immunodeficiency virus via the upper alimentary tract has been demonstrated, suggesting virus transport through the mucosal barrier (1, 2). However, no data on the penetration of HIV through primary human epithelial cells are available. Therefore, we studied the transport of HIV-1 through gingival epithelial cells grown as a monolayer.
MATERIALS AND METHODS
Primary culture of epithelial cells.
Epithelial cells were obtained from biopsies of the gingiva of a healthy male donor. The biopsies were washed several times with phosphate-buffered saline and cultured after trypsinization in Dulbecco modified Eagle medium medium containing 10% fetal calf serum (FCS). Fibroblast growth was suppressed by the addition of recombinant epidermal growth factor (10 μg/liter; Sigma, Deisenhofen, Germany) to the culture medium. The epithelial character of the primary cells and the formation of tight junctions were confirmed morphologically by electron microscopy.
The expression of cytokeratin and CD4 receptor was investigated by immunocytology. Cells cultivated on coverslips for 5 days were washed with cold phosphate-buffered saline and fixed with acetone for 10 min at room temperature. The nonspecific binding sites were blocked with 50 mM Tris–50 mM NaCl–10 mM CaCl2–0.1% normal goat serum, pH 7.5. After being washed, the cells were incubated with anticytokeratin receptor (CK5; ICN ImmunoBiological) or anti-CD4 receptor (OKT4; Dianova), and the primary antibodies were visualized by alkaline phosphatase and monoclonal antialkaline phosphatase staining (7).
Two-compartment culture system.
For transepithelial transport experiments, primary epithelial cells (104/ml) were grown on a polycarbonate filter membrane (9-mm diameter; 3.0-μm pore diameter; Becton Dickinson) separating a basal and an apical chamber and cultivated for 10 days until confluence was observed. The development of an epithelial monolayer was examined by confocal laser microscopy. To further test for confluence, a fluorescein solution (0.2 mg/ml) or fluorescent particles (106 particles/ml, each particle 0.1 μm in diameter) were added to the apical chamber and fluorescence activity in the basal chambers was measured after 45 min of incubation at 4°C to inhibit cell membrane diffusion. For calibration of paracellular diffusion, membranes were covered with a layer of 15% polyacrylamide gel, leaving free circular areas by placing small cylinders of defined size on the filter before gel casting. Fluorescence activity which diffused from the apical to the basal chamber through different areas of uncovered epithelial cells was measured after the 45-min incubation.
Viral transepithelial transport.
HIV-1 strain IIIb (1.8 × 105 50% tissue culture infective doses [TCID50]/ml) harvested from HIV-1-infected H9 cells was cleared from all debris by centrifugation (10 min, 200 × g) and filtered through a 0.2-μm-pore-size filter membrane. The cell-free virus was diluted 1:10 with Hanks buffer and was placed in the apical compartment (for details see Results). After a 45-min incubation, medium from the lower compartment was harvested. The amount of infectious HIV-1 in the basal chamber of the culture unit was determined by a standard titration assay. Titration of HIV was performed in triplicate in 24-well tissue culture plates on MT4 cells seeded at a concentration of 2 × 104/ml. Samples were diluted serially (1:10) in culture medium (RPMI 1640 supplemented with 10% FCS and 5% glutamine). The titration was evaluated between 10 and 14 days postinfection when a prominent cytopathic effect (CPE) was visible. Medium was replaced twice a week, with cells being diluted as required. Values of TCID50 per milliliter were determined as described in reference 22. All experiments were performed in triplicate.
Inhibition studies.
For inhibition studies on MT4 and epithelial cells mannan (5 mg/ml final concentration), α-methyl-mannopyranoside (αMMP; 100 mM final concentration), or mucin (30 mg/ml) was added to the dilution buffer. Monosaccharide analysis after hydrolysis of the mucin showed that the mannose content was about 1% of the total mass. The HIV-1 specificity of the CPE in MT4 cells was confirmed by determination of p24 core protein content by a p24 antigen capture assay (Coulter). Control experiments indicated that relevant concentrations of the glycoconjugate inhibitors neither reduced the titer of HIV-1 nor inhibited the CPE in MT4 cells, a result which is in agreement with reference 16. Experiments were done in triplicate.
Viral intake and release.
HIV-1 strain IIIB (1.8 × 105 TCID50/ml) was placed on epithelial cells (106/petri dish). For inhibition studies mannan (5 mg/ml final concentration), αMMP (100 mM), or mucin (30 mg/ml) was added to the culture medium. After a 1-h incubation, the cells were washed three times with trypsin (0.25%) and incubated for 10 min at 37°C with trypsin solution to inactivate all virus particles adsorbed at the cell surface. The cells were harvested and subcultured for different incubation times (see Table 1). Subsequently, the cell-free supernatant of each subculture was titrated on MT4 cells as described above.
TABLE 1.
Detection of cell-free HIV-1 taken up by epithelial monolayera
| Time after trypsin treatment | HIV concn (TCID50/ml) in the
supernatant after treatment with:
|
|||
|---|---|---|---|---|
| No inhibitor | Mannan (5 mM) | αMMP (100 mM) | Mucin (1 mM) | |
| 60 min | 103 | 100 | 101 | 101 |
| 120 min | 103 | 101 | 101 | 101 |
| 160 min | 103 | 101 | 101 | 101 |
| 24 h | 103 | 101 | 101 | 101 |
HIV-1 strain IIIb (1.8 × 104 TCID50/ml) was placed on epithelial cells (106/petri dish) with or without inhibitors. After a 1-h incubation, the cells were treated with trypsin to inactivate all extracellular HIV particles. Cells were incubated with fresh medium, and the supernatants were harvested at different time points. Infectious virus in the supernatant was titrated on MT4 cells (see Materials and Methods). All experiments were performed in triplicate. The differences between the infectious titers with and without inhibitors are highly significant for all incubation times (P < 0.001 by the Mann-Whitney U test).
Preparation of biotinyl-mannan.
To avoid non-mannosyl-mediated binding of mannan, the oligopeptide tail of mannan was digested by proteinase K treatment (20 μg of protease K/100 mg of mannan) for 2 h at 37°C, resulting in a protein content reduction of from 5% to below 0.1% of the total mass. Mannan was separated from free amino acids as well as from the enzyme molecules by affinity chromatography with Galanthus nivalis agglutinin (GNA). Bound mannan was specifically eluted with 100 mM αMMP. The residual peptide core of mannan (80 mg of mannan/ml of Na2CO3; 50 mM; pH 8.5) was biotinylated by overnight incubation with HN-hydroxy-succinimide-capronyl-biotin (0.2 mg/ml). Biotinylated and nonbiotinylated mannan molecules were separated from hydrolyzed capronyl-biotinyl by GNA affinity chromatography. In order to separate the biotinylated mannan conjugates from nonbiotinylated carbohydrates, i.e., mannan and αMMP, the lipophilic biotinyl conjugates were retained on a reversed-phase cartridge (SEP-PAC-Cartridge, C18; Waters, Eschborn, Germany) and eluted by a stepwise gradient of methanol-water (0 to 10% [vol/vol]). The eluate was lyophilized and stored at −20°C until use.
gp120 preparation, characterization of lectin-like activity, and coupling to microbeads.
Cell-free supernatant of HIV-1 strain IIIB-infected human H9 cells was treated with 0.5% Nonidet P-40 and protease inhibitor (phenylmethylsulfonyl fluoride; 5 mM). Debris was eliminated by ultracentrifugation at 100,000 × g for 2 h at 4°C. The viral envelope glycoprotein was purified by GNA affinity chromatography as described by Gilljam (13) followed by immunoaffinity chromatography using human serum immunoglobulins with high anti-HIV-1 gp120 titers (demonstrated by Western blot analysis). The purity and specificity of the gp120 was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in conjunction with silver staining and by immunoblotting with an HIV-1 gp120-specific monoclonal antibody (clone RL16.76.1; Immunotech, Hamburg, Germany). The sensitivity of the staining was enhanced by a luminol-containing substrate as described in reference 25.
To test the lectin properties of the native HIV-1 gp120, the envelope glycoprotein was electrophoresed on a polyacrylamide-SDS gel, blotted onto a polystyrol surface, and stained with the biotinyl-mannan conjugate (Tris buffer, 1% glycine, 0.2% Tween 20, 5 mM CaCl2; pH 7.3), a mouse monoclonal antibiotin antibody (Boehringer GmbH, Mannheim, Germany), and a secondary peroxidase-labelled antimouse antibody (Dako, Hamburg, Germany), followed by incubation with a chemiluminescence substrate as described above. To reduce nonspecific binding of the monoclonal antibiotin antibody to the immobilized gp120, carbohydrates of the antibody were oxidized with periodate (3). The periodate oxidation eliminated the mannosyl-specific lectin binding of the monoclonal antibody after dot blotting. The purified HIV-1 gp120 was adsorbed onto a polystyrol surface. The lectin-like binding properties of the immobilized gp120 were determined by incubating with biotin-labelled mannan overnight at 37°C, and the amount of gp120-bound biotinyl-mannan was quantified with a biotin-specific monoclonal antibody. Different concentrations of various soluble carbohydrates (high-mannose-type glycans [5 to 9 mannose molecules per glycan] derived from RNAse B), hybrid-type glycans (derived from ovalbumin), complex-type glycans (derived from fetuin; Oxford Glycosystems, Oxford, United Kingdom), αMMP, and glucose were coincubated with the biotinylated mannan complex (for details, see the legend for Fig. 1). The 50% inhibitory concentrations (IC50) were estimated by four-parameter logistic spline interpolation after equilibrium incubation (18).
FIG. 1.
Binding of biotinyl-mannan to immobilized gp120. Isolated gp120 was immobilized to polystyrol, and the binding of a mannan-biotinyl conjugate was quantified in the presence of different inhibitory carbohydrates. , mannan; ▾, high-mannose-type glycans (derived from RNAse B); ⧫, hybrid-type glycans (derived from ovalbumin); , complex-type glycans (derived from fetuin); ▪, αMMP; ▴, glucose. Data are mean values of double determinations and given as the ratio of absorption in the presence of an inhibitor (B) and absorption with no inhibitor (B0), expressed as percentages. Curves were extrapolated by a four-parameter spline curve.
For transepithelial transport studies, gp120 was covalently coupled to monodispersed carboxylated fluorescent microparticles (0.1 μm in diameter; Polysciences, Warlington, Pa.) as described previously (21). The active groups of the control beads and the remaining gp120-coated beads were blocked with glycine. The attachment of gp120 to the fluorescent particles was quantified by measuring the binding of a monoclonal anti-HIV gp120 antibody to the beads.
Transepithelial transport of particles.
The gp120-coated particles were diluted (105 particles/ml) in Hanks buffer and placed in the apical chamber after the epithelial cells were washed three times with Hanks buffer. The apical chamber was transferred into a new basal chamber, and Hanks buffer was changed after 10, 40, and 90 min of incubation. The buffer harvested at the indicated time points was centrifuged (14,000 × g for 15 min), the pellet was resuspended, and the fluorescence activity was measured. A combination of forskolin (FSK; 10 μM) and 3-isobutyl-1-methyl-xanthin (IBMX; 10 μM) (17) was used to study the effect of cyclic AMP (cAMP) and epithelial transport. Preincubation of the epithelial cells for 2 h increased the intracellular cAMP level eightfold (cAMP immunoassay; Biomol, Plymouth Meeting, Pa.). Glycine-coated microparticles were used as the control for nonspecific paracellular flow under the experimental conditions; the fluorescence of the controls was subtracted from the fluorescence activities found in the respective experiments with gp120-coated particles.
Inhibition experiments.
Before addition to the apical chamber, the gp120-coated particles (105 particles/ml) were preincubated for 10 min with mannan (5 mg/ml final concentration) or αMMP (100 mM final concentration). After incubation for 10, 40, and 90 min at 37°C, the solution in the basal chamber was changed and the fluorescence activity was measured as described above.
To increase the amount of high-mannose-type glycans on the epithelial cells, cells were preincubated for 2 h with 10 mM deoxymannojirimycin (10). To control the increase of high-mannose-type glycan expression, the filter membrane was cut and placed in 300 μl of lysis buffer (0.2 M Tris-HCl, 2% SDS, 0.1% dithiothreitol). After centrifugation at 3,000 × g for 5 min, the glycoproteins of the supernatant were separated by SDS-PAGE and the glycoproteins blotted onto nitrocellulose were incubated with a GNA-digoxigenin conjugate (1 μg/ml; Boehringer GmbH) for 1 h and stained with an anti-digoxigenin-peroxidase conjugate (0.1 μg/ml; Boehringer GmbH). Bound peroxidase was detected after incubation of the nitrocellulose with a chemiluminescent substrate and exposure to photon-sensitive film (Kodak X-AR) as described previously (25).
RESULTS
The degree of paracellular leakage of the epithelial cell monolayer was tested by incubation with fluorescein- or glycine-coated fluorescent microbeads. With an uncovered membrane (maximal flow rate) about 2% of the upper-compartment fluorescence activity was detected in the lower compartment. In all experiments the paracellular flow was always less than 4% (mean, 1.8%) of the maximal flow rate, i.e., less than 0.05% of the input particles.
After cell-free infectious HIV-1 was placed on the epithelial monolayer, the quantity of infectious virus on the basal side of the epithelial monolayer was determined by titration of infectious virus. Approximately 5% (103 TCID50/ml) of the virus placed in the upper compartment was found in the basal chamber after a 45-min incubation. Preincubation with mannan (5 mM) or αMMP (100 mM) reduced the amount of infectious HIV-1 in the basal compartment by 1 order of magnitude (i.e., to 102 TCID50/ml). Mucin inhibited the transepithelial transport of cell-free HIV-1 to a similar extent (102 TCID50/ml). The differences between the results in the absence and presence of inhibitors are highly significant (P < 0.001 by the Mann-Whitney U test). Supernatant of uninfected epithelial cells did not induce a CPE. Epithelial cells were incubated with cell-free HIV-1 for 30 min, the cell supernatant was removed, and the cell surfaces were treated with trypsin. Infectious virus particles were released for several hours into the basal chamber (Table 1). Cellular uptake and release were inhibited by 2 orders of magnitude after coincubation of the virus and the epithelial cells with mannosyl derivatives (Table 1). Mucin could also significantly inhibit the viral uptake and release of cell-free HIV-1 (Table 1). Incubation of mannosyl derivatives with the CD4+ indicator cells and cell-free HIV did not show any inhibition of CPE. Supernatants of epithelial cells without treatment with HIV did not induce CPE in MT4 cells.
After the dot blotting of native HIV-1, gp120 was shown to bind to biotinylated mannan. This binding was effectively inhibited by glycans with a terminal oligomannosyl structure. The IC50 of high-mannose-type glycans (IC50 = 0.20 μM), glycans of the hybrid type (IC50 = 0.37 μM), and mannan (IC50 = 0.24 μM) were comparable (Fig. 1). The IC50 was 40 μM for complex-type glycans which contain a trimannosyl core. Monosaccharides such as αMMP and glucose also inhibited binding, but only at much higher concentrations (IC50 = 275 μM for αMMP, IC50 = 1,460 μM for glucose).
To demonstrate that transepithelial transport was mediated by HIV-1 gp120 and not by receptors from the H9 cell line used to grow the virus, fluorescent polystyrrol microspheres coupled to purified gp120 were placed in the apical chamber. The amount of gp120-coupled particles was quantified in the medium of the basal chamber, and the number of glycine-coated particles (control) was subtracted (Fig. 2). Compared to transport through unstimulated cells, the transport of gp120-coated particles through the epithelial monolayer was increased by 50% upon preincubation with a combination of FSK and IBMX compounds (Fig. 2). To increase the number of glycan receptors on the epithelial cells, the cells were preincubated with deoxymannojirimycin, which inhibits the mannosidase I in the Golgi apparatus (10). This pretreatment resulted in a further augmentation of the cAMP-stimulated increase of particles in the basal compartment (Fig. 2). The competitive inhibitors mannan and αMMP reduced the transport of gp120-coated particles in FSK- and IBMX-treated cells to about 50% of the level for unstimulated cells (Fig. 2).
FIG. 2.
Particles in the basal chamber after 10, 40 and 90 min of incubation at 37°C. The figure shows the transport of gp120-coated particles (106/ml) without preincubation (◘) and after preincubation of the cells with 10 μM FSK and 10 μM IBMX (▴) or with FSK, IBMX, and 10 mM deoxymannojirimycin (⧫). The effect of preincubating the HIV-gp120 particles with 5 mg of mannan per ml (░⃞) or 100 mM αMMP (▾) and using FSK- and IBMX-treated cells is also shown. The transcytosis rate of the glycine-coated control particles was subtracted separately for each individual experiment to correct for the nonspecific transcytosis referred to above. Additionally, the absolute transcytosis rates of the glycine-coated particles are shown (○). All data are given as means ± standard errors of the means of quadruplicate measurements.
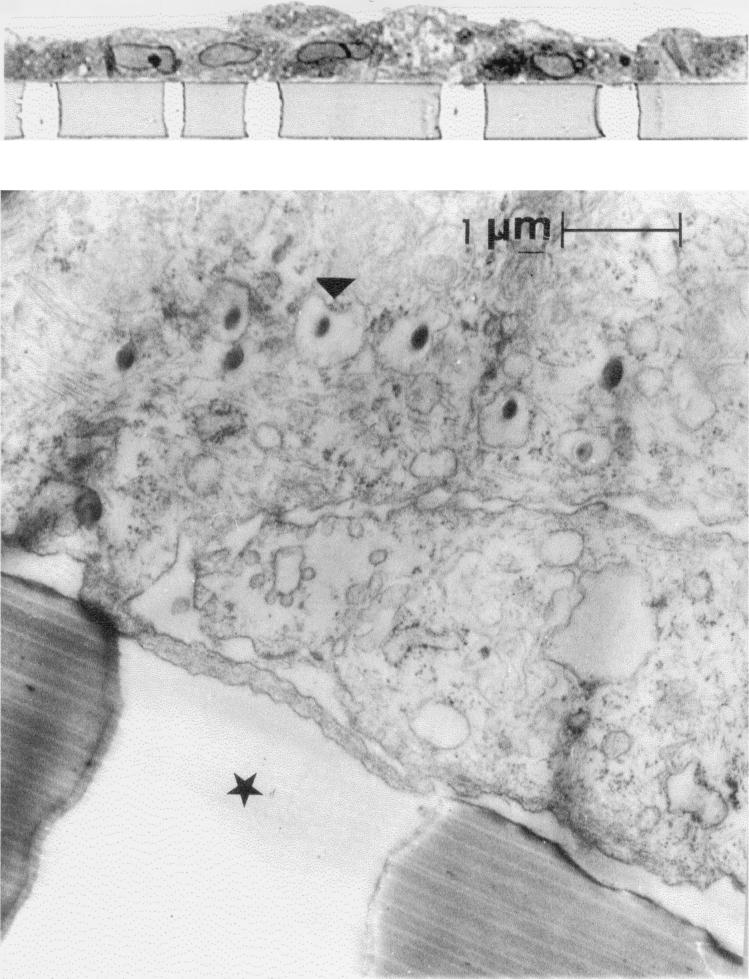
At the beginning of the incubation of gp120-coated particles with epithelial cells a rapid increase of particles in the lower compartment was observed (Fig. 2). At later times (40 min of incubation) we did not detect any significant increase of gp120-coated particles in the basal chamber despite the fact that a high concentration of such particles in the apical chamber was present. Further evidence of transepithelial transport is provided by electron microscopy studies showing gp120-coated particles in the endosomes of epithelial cells (Fig. 3).
FIG. 3.
Light microscopy results (upper panel) and ultrastructure (lower panel) of the epithelial monolayer. The epithelial cells were grown on a filter membrane with a pore diameter size of 3.0 μm for 10 days until confluence and were incubated for 10 min at 37°C with fluorescent particles covalently coupled to gp120. The gp120 particles were taken up into cellular vesicles. The arrowhead shows the vesicular uptake of a gp120-coated particle. The star indicates the membrane pore.
Lectin staining after SDS-PAGE and subsequent Western blotting of epithelial cell lysates showed several mannosylated glycoproteins which might be involved in gp120 binding (data not shown).
DISCUSSION
To elucidate cellular events during HIV entrance into the body, we cultivated primary human epithelial cells from gingival biopsies until confluence on a porous filter membrane separating the chambers of a two-compartment cultivation system. Cell-free infectious virus was present in the basal compartment after 45 min of incubation. The virus particles retained their infectivity for CD4+ cells.
To confirm the uptake and release of viral particles, epithelial cells grown in tissue culture dishes were incubated with cell-free HIV-1. After the removal or inactivation by trypsin treatment of extracellular virus and the feeding of cells with fresh medium, the supernatant of the epithelial cells was collected at different time points. Infectious virus was released from these cells for several hours (Table 1), indicating that during trypsin treatment infectious HIV particles were presumably taken up by and protected inside the epithelial cells and released later. These experiments imply that HIV-1 can be taken up by the epithelial monolayer without losing infectivity.
The rapid basolateral release of infectious virus is in accordance with a recent report showing that HIV is transported through a monolayer of immortalized cells derived from a colon carcinoma by cell-cell contact (4). Investigations of the infection of subepithelial cells of macaques via incubation of epithelia of the vagina with cell-free simian immunodeficiency virus (24) also support our results.
There are several reports that gp120 has lectin-like properties (14, 15, 27). Biotinylated mannan was shown to specifically bind to immobilized native HIV-1 gp120. The binding was effectively inhibited by glycans with a terminal-oligomannosyl structure. The IC50 of a high-mannose-type glycan, a hybrid-type glycan, and the linear nonpolar oligomannosyl-glycan mannan were comparable (Fig. 1). A possible interaction with the peptide core of mannan was unlikely due to the extensive protease digestion during preparation of the biotinyl-mannan. These data are in accordance with reports which demonstrated a lectin-like activity of recombinant HIV-1 gp120 by using mannosyl-containing receptor analogs (14, 15).
To test the biological relevance of these findings for the epithelium-virus interaction, HIV-1 was preincubated with the linear nonpolar mannosyl oligomer mannan or αMMP in a concentration 1,000 times higher than the IC50. The mixture was transferred to the apical side of the epithelial monolayer grown on filter membranes. Preincubation with inhibitors reduced the amount of infectious HIV-1 on the basal side by a factor of approximately 10 (Table 1). Cellular uptake and release were also inhibited by 2 orders of magnitude by coincubation of the virus and the epithelial cells with mannosyl derivatives (Table 1). These data indicate that the receptors for HIV-1 gp120 on primary human epithelial cells are the oligomannosyl residues of cellular surface glycoproteins interacting with the lectin-like domain on the HIV-1 gp120 molecules. Although in our experiments we cannot exclude infection of epithelial cells, the short time course of the appearance of virus on the basolateral side of the monolayer renders a productive infection of epithelial cells unlikely. Coincubation of cell-free infectious virus with the epithelial cells in the presence of FCS inhibited viral transepithelial transport in epithelial cells without diminishing the infectivity for CD4+ MT4 cells (data not shown). This can be explained by the excess of oligomannosyl residues present in glycoconjugates of FCS and might explain the negative results concerning transport of cell-free HIV-1 reported by others (4). The high level of nonspecific binding of the untreated monoclonal antibiotin antibody which we found with immobilized gp120 can also be explained by attached glycan residues.
HIV infection is frequently transmitted via the genital route, whereas transmission via the oral route is less common (6). The differences in susceptibility to HIV infection may be explained by the amount and structure of the mucins found on the surfaces of oral and genital mucosa. Oligosaccharides in human oral mucins contain approximately 2% mannose molecules (19, 26), while during midcycle oligomannosyl residues are not found in mucins of vaginal secretions (28). To test the biological effect of mannosyl residues containing mucins, mucin with a mannose content of about 1% was used to study the inhibition of transepithelial transport of HIV-1. The data showed that about 1 μmol of mucin could inhibit viral uptake by epithelial cells of cell-free HIV-1 (Table 1 and Results) to an extent similar to that of mannan.
It was reported that membrane molecules of the host cells are integrated into the viral envelope during budding (4, 12). These receptors possibly could mediate the interaction between HIV and epithelial cells. To demonstrate that HIV-1 gp120 is strongly involved in transepithelial transport, purified gp120 was coupled to fluorescent polystyrol microspheres. After the particles were placed in the apical chamber, a greater number of gp120-coupled particles than glycine-coated particles (control) was detected in the basal chamber (Fig. 2), indicating an accelerated passage of gp120-coated particles through the monolayer of human epithelial cells. Further evidence for transepithelial transport comes from electron microscopy studies showing gp120-coated particles in endosomes of epithelial cells (Fig. 3).
Dependence of transepithelial transport on the adenylate cyclase system (17) was demonstrated for immunoglobulins (5, 23). The intracellular cAMP concentration can be maximally increased by a combination of FSK, a terpene activating adenylate cyclase, and IBMX, an inhibitor of phosphodiesterase. Compared to transport through unstimulated cells, the transport of gp120-coated particles through the epithelial monolayer was increased by 50% upon preincubation with a combination of these compounds (Fig. 2). The results indicate that the active transcellular transport process of gp120-coated particles can be accelerated by activation of the adenylate cyclase system.
Despite a high concentration of gp120-coated particles in the apical chamber, we did not detect any further significant transcellular transport of those particles to the basal chamber after 40 to 90 min of incubation, indicating a saturation of transepithelial transport. The transcellular transport of gp120-coated particles might be limited by the availability of cellular receptors for gp120, which must recycle through the epithelial cells, or of other factors required for active transport. Lectin staining after SDS-PAGE and subsequent Western blotting of epithelial-cell lysates showed several mannosylated glycoproteins which might be involved in the gp120 binding (data not shown).
To increase the number of glycan receptors on the epithelial cells, we preincubated the cells with deoxymannojirimycin, which inhibits the mannosidase I in the Golgi apparatus (10). We were able to show a further increase in the FSK- and IBMX-stimulated transmembrane transportation of particles into the basal compartment (Fig. 2).
The small soluble-receptor analogs mannan and αMMP were used as competitive inhibitors to demonstrate that lectin-oligosaccharide interactions are involved in the transport of gp120-coated particles. As expected, the preincubation of the gp120-coated particles with the inhibitors reduced the transport rate to about the rate for unstimulated cells (Fig. 2). Epidemiological evidence points to an association of low concentrations of mannose-binding lectin (MBL) in serum, caused by variant alleles in the MBL gene, with an increased risk of HIV infection (11). MBL binds to oligosaccharides with a high mannose content which are present on HIV-1 gp120 and which can inhibit HIV infection of CD4+ T-cell lines. MBL could compete with the lectin-like domain of gp120 for mannosyl group-containing binding sites on the surface of CD4− epithelial cells. This could inhibit the transepithelial transport of infectious HIV and could reduce the number of infectious particles which are available for infection of CD4+ cells located below the epithelial layer.
The presented data show that cell-free HIV-1 can penetrate a gingival epithelial monolayer, an in vitro model of the cellular mucosal barrier, by active transport which can be stimulated by FSK and IBMX. The virus transport is mediated by a gp120-oligomannosyl interaction leading to a transepithelial transport of HIV-1 and can be competitively inhibited by oligomannosyl glycoconjugates. In vivo, this protective mechanism might be incomplete, particularly when the number of virus particles is high (1, 2, 24) or when the amount of soluble oligomannosyl residues is reduced, as shown for the midcycle vaginal secretion (28). The results might also give an explanation for an innate, nonspecific protection for heterosexual women previously reported (8). Inhibition of transepithelial transport by oligomannosyl derivatives might be useful to protect the organism from viral entrance and prevent subsequent infection. Further studies are in progress to identify the oligomannosyl-specific domain of gp120.
ACKNOWLEDGMENTS
The gingival biopsies were kindly provided by S. Hägewald. We thank B. Baum, A. van Nieuw Amerongen, B. Guggenheim, and K. Koschel for helpful discussions.
This research was supported in part by the Deutsche Forschungsgemeinschaft (DFG).
REFERENCES
- 1.Baba T W, Jeong Y S, Pennick D, Bronson R, Greene M F, Ruprecht R M. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 2.Baba T W, Trichel A M, An L, Liska V, Martin L N, Murphey-Corb M, Ruprecht R M. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science. 1996;268:2395–2398. doi: 10.1126/science.272.5267.1486. [DOI] [PubMed] [Google Scholar]
- 3.Bobbit L A. Periodate oxidation of carbohydrates. Adv Carbohydr Chem Biochem. 1956;11:1–41. doi: 10.1016/s0096-5332(08)60115-0. [DOI] [PubMed] [Google Scholar]
- 4.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithlieal cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 5.Bomsel M, Prydz K, Parton R G, Gruenberg J, Simons K. Endocytosis in filter-grown Madin-Darby canine kidney cells. J Cell Biol. 1989;109:3243–3258. doi: 10.1083/jcb.109.6.3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Transmission of HIV possibly associated with exposure of mucous membrane to contaminated blood. Morbid Mortal Weekly Rep. 1997;46:620–623. [PubMed] [Google Scholar]
- 7.Cordell J L, Falini B, Erber W N, Ghosh A K, Abdulaziz Z, MacDonald S, Pulford K A, Stein H, Mason D Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes) J Histochem Cytochem. 1984;32:219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- 8.Fowke K R, Nagelkerke N J D, Kimani J, Simonsen J N, Anzala A O, Bwayo J J, MacDonald K S, Ngugi E N, Plummer F A. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet. 1996;348:1347–1351. doi: 10.1016/S0140-6736(95)12269-2. [DOI] [PubMed] [Google Scholar]
- 9.Frankel S S, Wenig B M. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science. 1996;272:115–117. doi: 10.1126/science.272.5258.115. [DOI] [PubMed] [Google Scholar]
- 10.Fuhrmann U, Bause E, Legler G, Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984;307:755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- 11.Garred P, Madsen H O, Balslev U, Hofmann B, Pedersen C, Gerstorf J, Svejgaard A. Susceptibility to HIV infection and progression of AIDS in relation to variant alleles of mannose-binding lectin. Lancet. 1997;349:236–240. doi: 10.1016/S0140-6736(96)08440-1. [DOI] [PubMed] [Google Scholar]
- 12.Gelderblom H, Reupke H, Winkel T, Kunze R, Pauli G. MHC-antigens: constituents of the envelopes of human and simian immunodeficiency viruses. Z Naturforsch Sect C. 1987;42:1328–1334. doi: 10.1515/znc-1987-11-1230. [DOI] [PubMed] [Google Scholar]
- 13.Gilljam G. Envelope glycoproteins of HIV-1, HIV-2, and SIV purified with Galanthus nivalis agglutinin induce strong immune responses. AIDS Res Hum Retroviruses. 1993;9:431–438. doi: 10.1089/aid.1993.9.431. [DOI] [PubMed] [Google Scholar]
- 14.Haidar M, Seddiki N, Gluckman J C, Gattegno L. Carbohydrate binding properties of the envelope glycoproteins of human immunodeficiency virus type 1. Glycoconj J. 1992;9:315–323. doi: 10.1007/BF00731092. [DOI] [PubMed] [Google Scholar]
- 15.Haidar M, Seddiki N, Gluckman J C, Gattegno L. The role of calcium and N-linked glycans in the oligomerization and carbohydrate binding properties of human immunodeficiency virus external envelope glycoprotein. Glycoconj J. 1994;11:73–79. doi: 10.1007/BF00731146. . (Erratum, 11:500.) [DOI] [PubMed] [Google Scholar]
- 16.Hammar L, Hirsch I, Machado A A, De Mareuil J, Baillon J G, Bolmont C, Chermann J C. Lectin-mediated effects on HIV type 1 infection in vitro. AIDS Res Hum Retroviruses. 1995;11:87–95. doi: 10.1089/aid.1995.11.87. [DOI] [PubMed] [Google Scholar]
- 17.Hansen S H, Casanova J E. Gs alpha stimulates transcytosis and apical secretion in MDCK cells through cAMP and protein kinase A. J Cell Biol. 1994;126:677–687. doi: 10.1083/jcb.126.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooton J, Gibbs C, Paetkau V. Interaction of interleukin-2 with cells: quantitative analysis of effects. J Immunol. 1985;135:2464–2473. [PubMed] [Google Scholar]
- 19.Loomis R E, Prakobphol A, Levine M J, Reddy M S, Jones P C. Biochemical and biophysical comparison of two mucins from human submandibular-sublingual saliva. Arch Biochem Biophys. 1987;258:452–464. doi: 10.1016/0003-9861(87)90366-3. [DOI] [PubMed] [Google Scholar]
- 20.Ludewig B, Holzmeister J, Gentile M, Gelderblom H R, Rokos K, Becker Y, Pauli G. Replication pattern of human immunodeficiency virus type 1 in mature Langerhans cells. J Gen Virol. 1995;76:1317–1325. doi: 10.1099/0022-1317-76-6-1317. [DOI] [PubMed] [Google Scholar]
- 21.Molday R S, Dreyer W J, Rembaum A, Yen S P. New immunolatex spheres: visual markers of antigens on lymphocytes for scanning electron microscopy. J Cell Biol. 1975;64:75–88. doi: 10.1083/jcb.64.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed C J, Münch H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 23.Rodewald R. Distribution of immunoglobulin G receptors in the small intestine of the young rat. J Cell Biol. 1980;85:18–32. doi: 10.1083/jcb.85.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spira A I, Marx P A, Patterson B K, Mahoney M, Koup R K, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorpe S J, Abel P, Henderson D, Jones N, Feizi T. Expression of blood group antigens in urinary tract tumours: prospective fluorescence study using cryostat sections of fresh frozen tissues. J Clin Pathol. 1986;39:1165–1176. doi: 10.1136/jcp.39.11.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veerman E C, Valentijn Benz M, Bank R A, Nieuw Amerongen A V. Isolation of high molecular weight mucins from human whole saliva by ultracentrifugation. J Biol Buccale. 1989;17:307–312. [PubMed] [Google Scholar]
- 27.Yahi N, Baghdiguian S, Moreau H, Fantini J. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J Virol. 1992;66:4848–4854. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yurewicz E C, Moghissi K S. Purification of human midcycle cervical mucin and characterization of its oligosaccharides with respect to size, composition, and microheterogeneity. J Biol Chem. 1981;256:11895–11904. [PubMed] [Google Scholar]