Abstract
Attempts to explain the origin and diversification of vertebrates have commonly invoked the evolution of feeding ecology, contrasting the passive suspension feeding of invertebrate chordates and larval lampreys with active predation in living jawed vertebrates. Of the extinct jawless vertebrates that phylogenetically intercalate these living groups, the feeding apparatus is well-preserved only in the early diverging stem-gnathostome heterostracans. However, its anatomy remains poorly understood. Here, we use X-ray microtomography to characterize the feeding apparatus of the pteraspid heterostracan Rhinopteraspis dunensis (Roemer, 1855). The apparatus is composed of 13 plates arranged approximately bilaterally, most of which articulate from the postoral plate. Our reconstruction shows that the oral plates were capable of rotating around the transverse axis, but likely with limited movement. It also suggests the nasohypophyseal organs opened internally, into the pharynx. The functional morphology of the apparatus in Rhinopteraspis precludes all proposed interpretations of feeding except for suspension/deposit feeding and we interpret the apparatus as having served primarily to moderate the oral gape. This is consistent with evidence that at least some early jawless gnathostomes were suspension feeders and runs contrary to macroecological scenarios that envisage early vertebrate evolution as characterized by a directional trend towards increasingly active food acquisition.
Keywords: heterostracan, pteraspid, feeding, Devonian, Palaeozoic, ostracoderm
1. Introduction
Feeding figures prominently in attempts to understand the evolutionary origins of vertebrates [1,2]. In contrast to invertebrate chordates, which exclusively suspension feed with either a ciliated pharynx or a mucus net [3], the dorso-ventrally closing jaws of living jawed vertebrates (crown-gnathostomes) and ‘placoderms’ [4–6] or anteroposteriorly moving system of cartilages in cyclostomes (hagfishes and lampreys) [7–10] allow for a far broader range of feeding strategies. The evolution of these unique vertebrate feeding modes plays a major role in attempts to explain the evolution of vertebrate anatomy and the origins of its modern diversity [11,12]. Prominently, the New Head Hypothesis [13–17] argues that the shift from suspension feeding to predation accompanied the emergence of neural crest, neurogenic placodes and the accompanying evolution of a prechordal head. The fossil record of the earliest vertebrates with a prechordal head (i.e. parts formed from trabecular elements of the neurocranium anterior to the notochord [18]) provides a test of this scenario [19], but the required data are currently lacking.
In particular, heterostracans, an extinct group of jawless stem-gnathostomes, have been a focus of the debate over feeding in early vertebrates. This is because their oral region is more commonly and completely preserved than in any other such group, and they are often interpreted as one of the earliest diverging lineages of stem-gnathostomes [1,20–23]. As such, heterostracans have the potential to inform on the feeding ecology of the earliest members of the gnathostome lineage [24]. The heterostracan feeding apparatus is best known in pteraspids, where the oral region is characterized by distinctive macromeric dermal plates [25–29]. The function of these plates has been much debated, variously interpreted as biting [26] or slicing [30] ‘jaws’, a cyclostome-like feeding apparatus [31–34], a sediment scoop [29] or a filtering structure [35–39]. Equally varied are the inferred ecologies, with heterostracans interpreted as active predators [26], macrophagous selective predators [14,40], hagfish-like scavengers [31–34], herbivores [30], detritivores [29,41] or suspension feeders (including filter feeding) [12,27,42–45]. The most recent investigation suggests that heterostracans were suspension feeders because the analysed oral plates exhibited no evidence of the wear anticipated of a ‘tooth-like’ function [27].
The difficulty in studying the articulated heterostracan oral apparatus in situ contributes to this lack of consensus. In the rare cases where they are preserved, articulated heterostracan oral apparatuses consist of small plates suspended in encasing sediment. As a result, previous reconstructions of the oral apparatus have focused either on the gross arrangement of the apparatus, in which the morphology and detailed arrangement of the individual plates are not characterized [29,31,33,34], or describe isolated elements with little or no reference to articulated apparatuses [29,46,47]. Evidently, the feeding ecology of heterostracans remains in its infancy and so we sought to advance understanding through a detailed characterization and reconstruction of the heterostracan oral apparatus. We used X-ray microtomography to characterize the three-dimensionally articulated oral apparatus of an exceptionally well-preserved specimen of the pteraspid heterostracan Rhinopteraspis dunensis (Roemer, 1855). Using computed tomography, we generated volumetric models of the components of the oral apparatus and used these models to reconstruct their three-dimensional arrangement in vivo. We use this reconstruction to assess competing hypotheses of heterostracan feeding.
2. Material and methods
(a) . Specimens and locality
Rhinopteraspis dunensis (Roemer, 1855) NHMUK PV P 73217 is housed in the collections of the Natural History Museum, London (NHMUK). In the museum catalogue, the specimen is listed as being collected from ‘Odenspiel Quarry’, likely corresponding to Jaeger Steinbruch, a quarry near the village of Odenspiel, Reichshof, North-Rhine Westphalia, Germany or, possibly, outcrops in the local area [48,49]. The Jaeger Quarry and surrounding outcrops expose sandstones and mudstones belonging to the Siegenian (?upper Pragian or lower Emsian, Lower Devonian) Odenspiel Formation [50,51], deposited on the northern margin of the Rhenohercynian Basin, which was a marginal transgressive and regressive delta-dominated setting [52]. The Odenspiel Formation falls within the ‘Pararhenotypics subfacies' of Jansen [53], representing a marginal marine, intertidal lagoonal setting preserving a restricted fauna of fish, bivalve molluscs, lingulid and terebratulid brachiopods, and eurypterids [48,49,54–56].
(b) . Terminology
Various terminologies have been applied to the pteraspid oral region. Here, we follow the terminology of Randle & Sansom [57] and, where that is not possible, that of Blieck [58]; a comparison to terminologies used in especially relevant studies is given in electronic supplementary material, table S1. We extend existing terminology to describe the nature and arrangement of the oral plates (figure 1g). For the anatomical axes of the animal as a whole, we use dorsal/ventral in the dorsoventral axis, rostral/caudal for the sagittal axis, and dextral/sinistral for the transverse axis. When describing the oral apparatus itself we also use lateral/medial to describe lateral positions relative to the sagittal axis, ad/aboral to describe the surfaces of plates relative to the mouth (i.e. adoral is the surface of a plate facing the mouth, aboral the surface facing away) and proximal/distal to refer to positions on the oral plates relative to their articulation with the postoral plate, with oral plate tips being distal.
Figure 1.
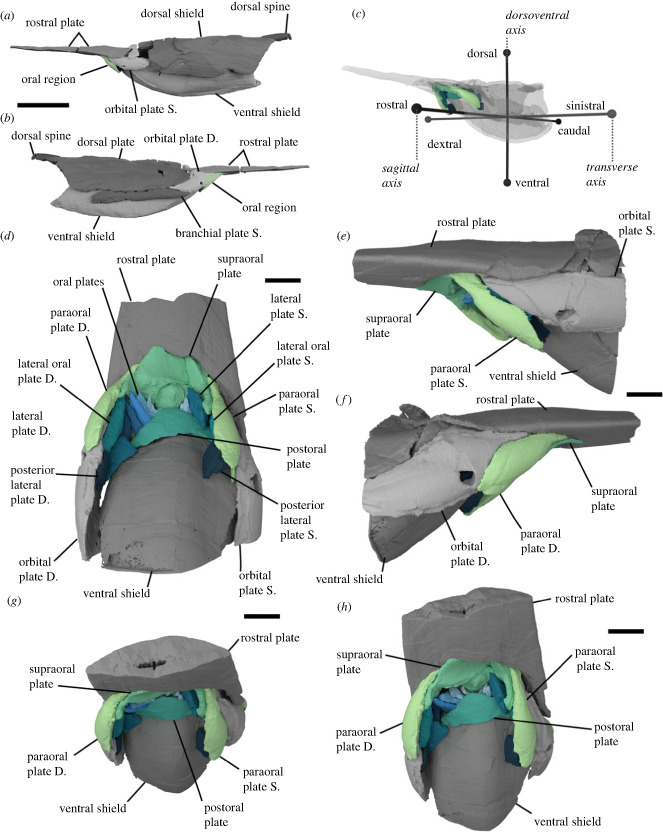
Rhinopteraspis dunensis NHMUK PV P 73217. (a–c) Rendering of head shield based on computed tomographic data in (a) sinistral, (b) dextral view and (c) transparent with scheme of anatomical axes. (d–h) Renders based on higher resolution data showing the oral apparatus in more detail in (d), ventral view, (e) sinistral view, (f) dextral view, (g) rostral view, (h) rostro-ventral view. Green and blue parts of three-dimensional renders represent oral region. Abbreviations: S, sinistral (left), D, dextral (right). Scale bars represent 5 cm in panels (a,b), 1 cm in (d–h).
(c) . Computed tomography
Rhinopteraspis dunensis NHMUK PV P 73217 (figure 1; electronic supplementary material, figure S1) was scanned using a Nikon Metrology XTH 225ST X-ray tomography instrument based in Bristol Palaeobiology, University of Bristol. Two scans were undertaken, each composed of two stacked scans. The first scan included the whole headshield at a voltage of 223 kV and a current of 193 µA, with 3141 projections, and with a 1 mm Sn filter, obtaining a dataset with 63.41 µm voxel resolution (figure 1a,b). The second scan targeted the oral region specifically, including the posterior third of the rostrum, the orbital, oral and pineal parts of the headshield, and the anterior quarter of the dorsal and ventral discs, at a voltage of 180 kV and a current of 178 µA, with 3141 projections of eight frames and 708 ms each, and with a 0.5 mm Cu filter, achieving a voxel resolution of 22.91 µm (figure 1d–h, 2).The resulting tomographic datasets were segmented in Mimics v.25 (materialize) to create three-dimensional models. All three-dimensional models were visualized in Blender 3.5 (blender.org).
Figure 2.
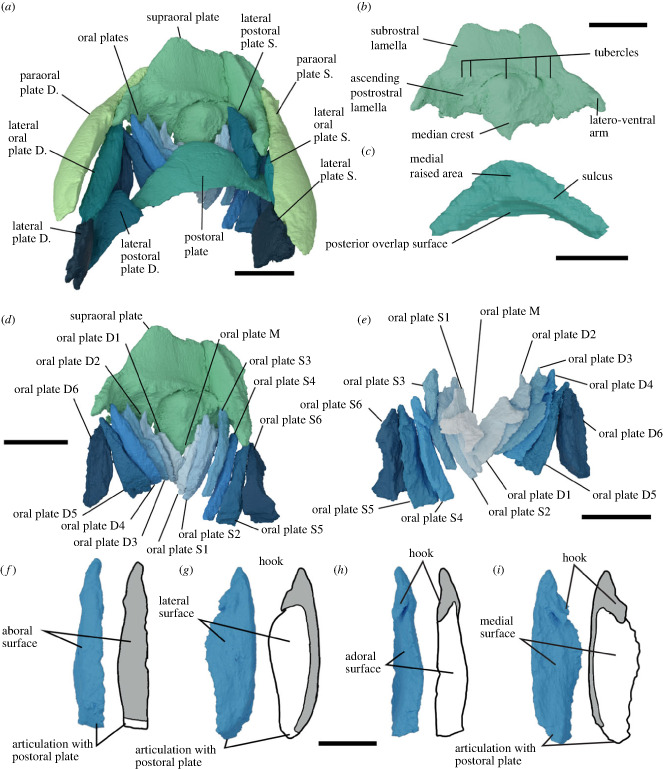
Rhinopteraspis dunensis NHMUK PV P 73217 oral region. (a) Oral apparatus and surrounding plates as preserved, in ventral view, (b) ventral view of supraoral plate with three fragments rearticulated, (c) postoral plate in dorsal view, (d) oral apparatus as preserved with ventral plates removed, (e) dorsal view of oral plates as preserved, (f–i) oral plate R4 in aboral, (f) lateral, (g) adoral (h) and medial (i) views, alongside drawings depicting the inferred extent of dermal ornament in grey, based on comparison with isolated plates of Loricopteraspis dairydinglensis [25,45,59]. S, sinistral (left), D, dextral (right). Scale bars represent 1 cm in (a–e), 0.5 cm in (f–i).
(d) . Reconstruction and animation
Three-dimensional models of the higher resolution scan set of Rhinopteraspis were imported into Blender for retrodeformation (figure 3). Use of Blender tools for retrodeformation followed the recommendations and techniques set out by Herbst et al. [60]. Initial retrodeformation focused on the best-preserved plates and those with clearly delineated articulations [61], including the rostral, orbital, supraoral and paraoral plates. After rearticulating and repairing deformation (see electronic supplementary material, information), these elements provided a framework to delimit the dorsal and lateral extent of the oral plate array. The oral plates were aligned, maintaining their preserved order, within this delimited area by placing their dorsal tips close to the margin of the mouth and rotating the plates caudoventrally to match the angle of the surrounding plates. The postoral plates were then fitted to the proximal ends of the oral plates. The proximal ends of the oral plates were then readjusted into articulation with the sulcus preserved in the postoral plates (figure 2c), while maintaining alignment with the closely articulated lateral oral- and paraoral plates and each other. Finally, the ventral disc was retrodeformed and articulated with the postoral plate. The reconstructed specimen was animated in Blender to simulate the movement of the oral plates (electronic supplementary material, figure S3). Empties (single geometry-less points that act as handles for object transformation without interfering in the render process) were placed between each oral plate and the postoral plate below, so that the local x-axis of each was aligned with the approximate outer boundary of the postoral plate. Each oral plate was then parented (=linked) to the empty below it. These empties were then animated to rotate around their local x-axes, causing the parented oral plate to also rotate around that axis.
Figure 3.
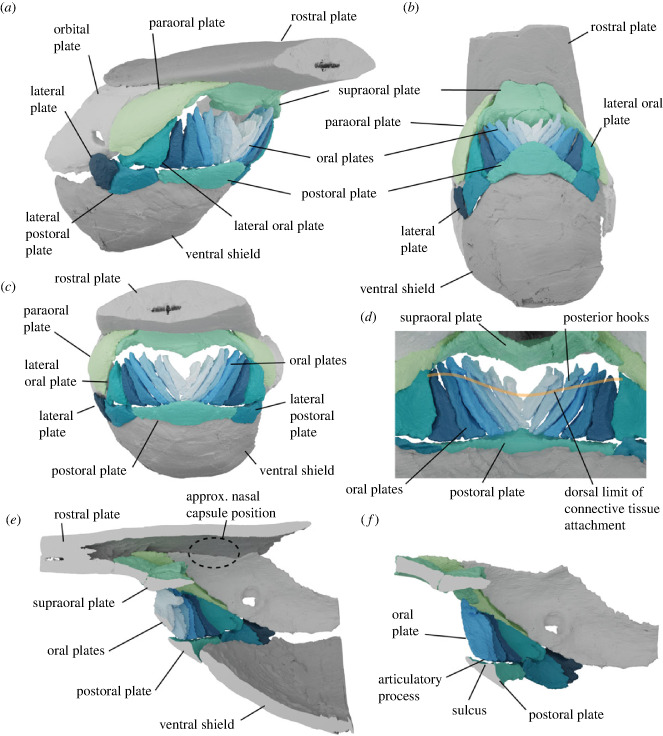
Reconstruction of Rhinopteraspis dunensis based on NHMUK PV P 73217; anterior section of rostrum not shown. (a) Rostrolateral view, (b) ventral view, (c) rostral view, (d) caudal view of articulated oral plates, (e) sagittal cross-section through centre of the head, (f) close up cross-section lateral to (e) showing junction between base of oral plate and the postoral plate.
3. Description
NHMUK PV P 73217 is an almost-complete three-dimensional specimen of Rhinopteraspis dunensis, preserving the entire headshield and articulated body scales (figure 1a,b; electronic supplementary material, figure S1). The specimen has been crushed laterally, with the oral region and ventral shield displaced rostro-dorsally (figure 1). Otherwise, the specimen is complete and individual oral elements appear to have maintained their original shape and relative location, as evidenced by their approximately symmetrical arrangement. The dermal skeletal anatomy of pteraspid heterostracans is well characterized in numerous taxa [25,62] to which that of Rhinopteraspis dunensis NHMUK PV P 73217 conforms [39,63,64]. The headshield is composed of large dorsal and ventral shields separated by paired cornual and branchial plates, with a dorsal spine set into the posterior margin of the dorsal shield (figure 1a,b; electronic supplementary material, figure S1). Anterior to the dorsal shield is an elongate rostrum that is separated from the dorsal shield by paired orbital plates (figure 1a,b; electronic supplementary material, figure S1). The anterior length of the rostrum is broken off from the rest of the specimen (figure 1a,b; electronic supplementary material, figure S1). The unpaired pineal plate is indistinguishable from the top of the orbital plates in the scan data.
The oral region is bordered dorsally by the supraoral plate, laterally by paired paraoral plates, and caudally by the postoral plate (figure 1). In previous descriptions of pteraspids, including Rhinopteraspis, the subrostral lamella and ascending postrostral lamella have been characterized as part of the rostral plate [58,59], although Friman & Bardenheuer described paired plates in this position, they termed them ‘subrostral plates’ [39]. In NHMUK PV P 73217 they comprise a separate structure, broken into three parts (postmortem), which we term the supraoral plate (figures 1d,g,h and 2a,b). The supraoral plate is trapezoidal in shape, narrowing rostrally. A pronounced furrow runs around its lateral and rostral margins, which is overlapped by the paraoral, orbital and rostral plates; the margins of the plate sweep ventro-caudally to form two latero-ventral arms (figure 2b). The rostral half of the ventral surface of the supraoral plate (the subrostral lamella) is surmounted by a superficial layer of ornament and made convex by a prominent median crest, aligned rostro-caudally (figure 2b). The caudalmost half (the ascending postrostral lamella) curves upwards into the mouth, lacks dentine ornament (as in Rhinopteraspis cornubica [59, p. 373]) and is effectively divided into two furrows by the median crest. The rostral border of this area presumably represents the position of the oral opening; this border is marked by a row of tubercles, comprising one large medial tubercle and two pairs of smaller tubercles on either side (figure 2b). The paired paraoral plates are elongate, taper rostrally, and overlie large rostro-lateral overlap surfaces on the orbital plates, rostral to the orbits (figures 1d–f and 2a). We interpret the ‘olfactory grooves’ identified in Rhinopteraspis cornubica by Tarlo [59, fig. 1] as the overlap surfaces between the paraoral plates and supraoral plate (figure 2a) [37].
The postoral plate is bow-shaped and originally symmetrical, although the right process is damaged (figure 2a,c). The ventral surface is smoothly convex. The inner surface has a caudal overlap surface that is concave to curve around the anterior rim of the ventral shield, and a dorsal surface that forms the ventral margin of the oral opening, bearing paired sulci for the oral plates that are interrupted medially by a raised area (figure 2c). Paired lateral plates and lateral postoral plates lie between the postoral plate and the orbital plates (figures 1 and 2). The extensive overlap between the plates surrounding the mouth and the larger head shield plates strongly suggests that they comprised an integrated structural unit with little or no movement relative to each other.
The oral apparatus itself is composed of 13 imbricated oral plates and one pair of lateral oral plates (figures 1 and 2; electronic supplementary material, figure S2). The aboral, mediolateral surfaces of each oral plate, as well as the lateral surfaces of the hook, are all faced with tuberculated ornament (figure 2f–i). However, the lateral and adoral surfaces of the oral plates, as well as the ventral side of the hook and the proximal end of the plates, are all unornamented, instead exhibiting a porous surface texture reflecting open vascular canals. The plates are arranged bilaterally about the midline into sinistral and dextral series. Although plate pairs that occur in equivalent positions on either side of the midline are similar, they do not exhibit mirror-image symmetry. In particular, the unpaired medial plate is not symmetrical but, rather, is continuous with the sinistral series (figure 2e; electronic supplementary material, figure S2). Each oral plate has the same general morphology of a main limb with a rhomboidal cross-section, a distal hook (except for the most laterally placed plates), and a proximal articulation surface for the postoral plate (figure 2; electronic supplementary material, figure S2). There are six oral plates in the sinistral series (S1–6) and six in the dextral series (D1–6), each preserved inclined at varying degrees (maximum about 45°) to each other along their coronal axis. The more medially placed the plate, the more inclined it is along its long axis to provide a fit with the adjacent oral plate; the lateral and medial faces of the plates overlap and imbricate, inclined at increasing angles relative to the sagittal plane, from medial to lateral. The distal hooks of the oral plates curve adorally, while the proximal ends of the plates are notched, reflecting the ventral limit of the external dermal ornament, serving as articulation with the postoral plate (except for the medial plate M).
Although the oral plates have similar morphologies, individually they vary in relative proportion depending on their position within the apparatus (figure 2; electronic supplementary material, figure S2). The distal hook of the unpaired medial oral plate M is as long as the main proximal limb. This plate is preserved overlying the lateral two oral plates D1 and S1. The lateral side of its proximal end is notched to fit with the left medial face of D1. Plate M curves dextrally such that it fits the curvature of the dextral face of the adjacent oral plate S1. The main proximal limb of oral plates D1 and S1 are twice as long that of M, with a narrow base; similarly, it may not have articulated with the postoral plate, but the hooks in D1 and S1 are as large and very similar in shape. These plates fit around oral plates S2 and D2, which fit around S3 and D3, etc.; these pairs have narrow bases and slightly smaller hooks than S1 and D1. Plates S4 and D4 have a slightly broader base and proportionally shorter hook. This trend continues laterally, with increasingly shorter hooks and wider bases in S5 and D5. Finally, S6 and D6 have no perceptible hook and a very broad base. The lateral edges of plates S6 and D6 is concave, fitting the medial edge of the lateral oral plates.
The lateral oral plates are morphologically distinct from the oral plates (figure 2; electronic supplementary material , figure S2), approximately triangular, tapering rostrally with a curved medial margin that matches the lateral profile of the lateral-most oral plates (figure 2a; electronic supplementary material , figure S2). The better-preserved sinistral lateral oral plate appears to have a distinct notch in its posterior side (electronic supplementary material, figure S2A,C), although this is difficult to corroborate from the dextral lateral oral plate.
4. Reconstruction
The combined width of the bases of the oral plates, when aligned perpendicular to a sagittal plane, matches the length of the sulcus on the postoral plate (figure 3) and the complementary symmetry exhibited by adjacent plates indicates that they were capable of almost completely filling the width of the oral opening. In this position, the lateral-facing surface of each oral plate overlaps the adoral surface of its outer neighbour (figure 3). The lateral surfaces of the outermost oral plates fit closely with the lateral oral plates (figure 3c,d). M, S1 and D1 appear to lie on top of the neighbouring oral plates rather than contacting the postoral plate. When viewed caudally, this brings the hooks of the plates into alignment, forming a plane above the posterior unornamented surface of the plates (figure 3d). This reconstruction also suggests that the oral apparatus fits together such that the tops of the oral plates extend to the top of the mouth to almost meet the supraoral plate. The medial crest of the supraoral plate creates a convex dorsal margin of the mouth, and the increasing length of the oral plates in lateral positions means that their dorsal tips also follow this convex line. Thus, when fully closed, there would have remained a short but wide opening (cf. [65]) into which projected the dorsal tips of the oral plates and the medial crest and associated tubercles of the dorsal oral plate. There is no evidence that the plates intercalated with the tubercles demarcating the dorsal margin of the mouth at the rostral margin of the ascending postrostral lamella, figure 2b). When modelled to open around the axis of the sulcus on the postoral plate synchronously, the oral plates do not overlap as they rotate (figure 4; electronic supplementary material, figure S3). Instead, their placement along the curved axis of the postoral plate sulcus causes them to splay outwards (figure 4; electronic supplementary material, figure S3).
Figure 4.
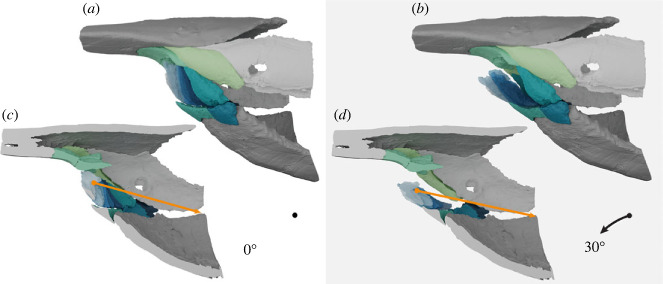
Reconstruction of Rhinopteraspis dunensis based on NHMUK PV P 73217 in lateral view with oral plates animated to open to 30°. (a,b) Lateral view at estimated resting position (a) and rotated aborally to 30° relative to resting (b). (c,d) Lateral view bisected, at estimated resting position (c) and rotated aborally to 30° relative to resting (d). Orange line is a hypothetical adductor muscle illustrating that the angle of this places a constraint on angle of rotation.
5. Discussion
Based upon our three-dimensional reconstruction of the oral region in Rhinopteraspis, we are able to consider the oral plates as an integrated apparatus and test hypotheses of its form and function. The endoskeleton of heterostracans is completely unknown beyond what can be inferred from the dermal skeleton [1]. Hence, we attempt to consider the constraints imposed by the dermal skeleton, without speculating as to endoskeletal structure.
(a) . Interpretation of the oral apparatus of Rhinopteraspis
Our reconstruction indicates that, even when the mouth was fully closed by occlusion of the oral plates, there would have remained a short but wide gape that was effectively divided in two by the median crest of the supraoral plate (figure 2c). The associated tubercles of the supraoral plate and tips of the oral plate would have projected into this space, further occluding it. There is no evidence for separate upper oral plates as inferred by Stensio [33,34]. The morphologies of individual oral plates are closely comparable to those observed in isolated oral plates of Loricopteraspis dairydinglensis recovered through acid digestion of limestone [27,47,66]. The open vasculature on the lateral surfaces and adoral surfaces, as well as the ventral side of the hook and the proximal end of each plate, is significant. Hence, these parts of the plates must have been embedded in soft tissue, the upper limit of which would have been the proximal surface of the large projecting hooks on the adoral side of the oral plates. The medial plates would have been supported entirely by this soft tissue which must have provided the basis of any movement of the plates and so may have included unmineralized cartilage, muscles or tendons.
The large, curved overlap surface at a 45° angle between the postoral plate and the ventral plate in Rhinopteraspis (figure 2c) suggests that movement of the postoral plate would not have been possible and that the postoral plate was static during feeding. By contrast, the imbricated nature of the oral plates strongly suggests that they were mobile relative to the postoral plate. Any movement requires rotation around the point of their attachment to the postoral plate. The rhomboid cross-section and the complementary symmetry of the oral plates would have prevented them either moving independently, or moving in an entirely sagittal plane (electronic supplementary material, figure S3). Rather, they must have moved as an integrated unit, splaying ventro-laterally as the plates rotated aborally on the sulcus of the postoral plate. The medial plates (M, S1, D1) that do not articulate with the postoral plate are the exceptions: their cross-sectional shape and ab/adoral overlap would have precluded their movement relative to the other plates. If forward pointing denticles were present on the lateral surfaces of the oral plate hooks, as in Loricopteraspis and Pteraspis [27,66], these structures would not have bordered the oral opening but, rather, the junction between each plate and its neighbour, linked by soft tissue. Rather than being involved in food capture or processing, this ornamentation may have helped to prevent particles from becoming lodged in the spaces between the plates [66] and their associated soft tissue, preventing fouling of the oral apparatus (cf. Hamann & Blanke [3]).
Where it has been made explicit in a reconstruction, previous hypotheses of heterostracan feeding function in taxa with separate oral plates have assumed significant movement and a degree of rotation of the oral apparatus around the transverse axis [26,29,31]. Although these reconstructions are not intended as precise models of function, they do indicate the degree of rotation that is envisaged: the following values are approximations taken from reconstructions, to the nearest 5°. In Errivaspis White [29, fig. 49] reconstructed the oral apparatus acting as a sediment scoop, with the postoral plate rotating aborally by 30° and the oral plates a further 25°. Janvier [31, fig. 2] reconstructed the oral plates as a hagfish-like apparatus moving both into and out of the mouth, with the oral plates and postoral plate rotating together aborally by approximately 80° from resting position and then adorally by approximately 85° from resting position. Janvier [65, fig. 12] reconstructed a generalized pteraspid opening the oral plates, with the postoral plate rotating 40° aborally and the oral plates an additional 50°. Bendix-Almgreen reconstructed two cyathaspids with pteraspid-like oral plates in which only the oral plates themselves moved: Allocryptaspis [30, fig. 4A], where the oral plates rotate adorally by 55° and Anglaspis [30, fig. 4E] where the oral plates rotate adorally by 55°.
However, our reconstruction suggests that movement was far more limited than previous reconstructions infer. In most of these reconstructions the postoral plate(s) is assumed to move significantly in addition to the oral plates, either inwards [31] or outwards [29,65]. An immobile postoral plate reduces the potential for rotation of the apparatus considerably. An absolute maximum for movement is probably given by an angle of 180° relative to the angle of the adductor musculature, otherwise it would be impossible to return the elements to a resting position (figure 4d). Even with the least conservative assumption, as this musculature runs parallel to the rostrocaudal axis of the animal this limits the oral plates to a rotation of about 55° relative to the hypothetical resting angle flush with the other dermal plates in our reconstruction. Rotation seems likely to be even more limited than this based on the poorly developed joint between the oral plates and the postoral plate, as well as the suspension of the small median oral plates in soft tissue. A structural analogue in a living vertebrate for heterostracan oral plates might be the branchiostegal plates in osteichthyans, which support the branchiostegal membrane, and make limited, coordinated movements to aid the suction pump [67].
Because the aboral surfaces of the oral plates lack any kind of attachment surfaces the oral apparatus of Rhinopteraspis could only have been moved from the adoral side with rostro-caudal movements. This is inconsistent with a gnathostome-like organization of paired mandibular adductor muscles. However, living cyclostomes operate the oral apparatus by moving cartilages rostrally and caudally along the floor of the pharynx. In hagfishes, keratinous toothlets are mounted on a cartilaginous dental plate that is pulled anteriorly along a basal plate to evert the lingual apparatus, and posteriorly to return it to resting position [7,8,10]. In lampreys, a medial piston cartilage is protracted rostrally, moving a medial apical cartilage, the action of which brings keratinous toothlets in front of the apical cartilage into contact with other toothlets in a rasping action [9,68]. A medial groove along the visceral surface of the ventral shield in some pteraspids has been cited as evidence for a cyclostome-like medial structure of the oral mechanism [1].
(b) . Nasohypophyseal anatomy in heterostracans
Our reconstruction provides indirect evidence on the nasohypophyseal anatomy of Rhinopteraspis. Several authors have suggested a hagfish-like morphology in heterostracans where an external nasohypophyseal duct opens into a prenasal sinus supported by a hypothetical palatino-subnasal lamina [31–34,69]. However, there is insufficient space in our reconstruction above the oral plates for such an opening, no direct evidence for any kind of palatino-subnasal lamina, and the dorsal border of the oral plates matches the bilobate shape of the supraoral plate [69]. An alternative scenario sees heterostracans as having gnathostome-like paired nostrils [31,45,59]. We find no evidence for a gnathostome-like paired nostrils, and specifically the paired ‘olfactory grooves’ identified in Rhinopteraspis [59] appear to be overlap surfaces. The remaining possibility, which we consider most likely, is that the nasal cavities and hypophyseal organ opened into the roof of the mouth [70].
Interpretations of the nasohypophyseal region in heterostracans are closely tied to efforts to ally them to extant vertebrate groups [33,34,59,69,70]. Rather than an explicitly cyclostome or gnathostome model, our reconstruction is instead consistent with the nasal and hypophyseal organs in heterostracans opening into the oralobranchial cavity [70]. This would be most clearly comparable to the anatomy of galeaspids, where these organs open into the spaces confluent with the oralobranchial cavity [71]. Based on the relative positions of the large, paired nasal organs in heterostracans, the position of which can be inferred from the ventral surface of the dorsal headshield [72] any nasohypophyseal opening(s) would most likely be positioned dorsalocaudally relative to the supraoral plate. The division of the ascending lamella of the supraoral plate into two furrows, a feature seen in both pteraspid [41, plate 16, fig. 5] and cyathaspid heterostracans [73, figs. 123C, 135B] could be explained as a way of channelling inflowing water to the paired olfactory organs.
(c) . Comparison of oral anatomy with other heterostracans
The shape and arrangement of the oral plates in Rhinopteraspis is closely comparable to that in other Pteraspidiformes, which are thought to comprise a clade within the heterostracans [28]. The shape of individual oral plates conforms closely to the morphologies of isolated plates acid prepared from an articulated specimen of Loricopteraspis dairydinglensis [47, p. 37, 66]. The arrangement of these plates is similar to articulated specimens of Protopteraspis [26] and Errivaspis [29] (figure 5). Importantly, the posterior alignment of the hooks in Rhinopteraspis (figure 3) can also be seen in other pteraspids where the adoral side of an articulated oral apparatus is visible: Mylopteraspidella [34, fig. 44, p. 197] and Protopteraspis [26], as well as in Errivaspis [29, figs. 41–44]. Together, these indicate that our interpretation of the feeding apparatus of Rhinopteraspis is more broadly applicable within pteraspids. However, varied body shapes and positions of the oral opening indicate some diversity in feeding ecology (e.g. Doryaspis [77], Drepanaspis [78]).
Figure 5.
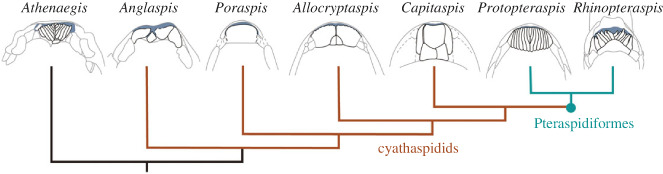
The diversity of the anatomy of the oral region in heterostracans, showing Athenaegis [74], Anglaspis [44], Poraspis [75], Allocryptaspis [36], Capitaspis [76], Protopteraspis [26] and Rhinopteraspis (this study). Oral plates shown in black, other parts of the dermal headshield in grey. Blue indicates the oral opening. Phylogeny taken from [28].
Outside the pteraspidiform clade [28], heterostracan oral anatomies are considerably more varied. Athenaegis, the oldest articulated heterostracan, is often assumed to be an outgroup to all other macromeric heterostracans [28,57,79,80], and its oral apparatus is interpreted as being pteraspid-like in the sense of comprising a fan of finger-like plates [74] (figure 5). By contrast, cyathaspids, a likely paraphyletic grade of macromeric heterostracans [28], have varied systems of one or several plates that cover the same region of the headshield as the oral apparatuses of Athenaegis and pteraspids (e.g. Anglaspis [44], Poraspis [75], Capitaspis [76] and Allocryptaspis [36], figure 5). However, the homologies of individual plates in these cyathaspid oral plate systems are difficult to reconcile, either within cyathaspids or by comparison with pteraspids. In amphiaspids, the oral aperture occurs at the end of a tube in a single, fused headshield [81]. Meanwhile, the oral apparatuses of the ‘tessellate heterostracan’ taxa are completely unknown [41]. Thus, while the oral apparatus of Rhinopteraspis may be representative of pteraspids, Athenaegis and, by implication, macromeric heterostracans primitively, it may not be representative of heterostracans more generally, which may have exhibited greater diversity in terms of feeding ecology. Further investigation is required to assess the ubiquity of suspension-feeding within the group, and the position in the water column at which they fed.
(d) . Implications for feeding in pteraspids and ancestral heterostracans
Our model of the articulated oral apparatus can be used to consider the various feeding strategies that have been proposed for pteraspid heterostracans. These strategies can be roughly divided into macrophagy, predation, deposit/detritus feeding, and microphagy or suspension feeding [82].
Hypotheses of macrophagous and predatory heterostracan feeding are based on analogy between heterostracan oral plates and the gnathostome mandible (e.g. [26]), or more rarely the hagfish oral apparatus (e.g. [31]). However, the oral apparatus in Rhinopteraspis is poorly constructed for biting or grasping. Although the ascending lamella bears tubercles that have been interpreted as the upper ‘jaw’ analogue [59], they do not occlude with the tips of the oral plates. Moreover, the angle of approach of the plates to the preoral plate during closing is oblique, with a low mechanical advantage, and would have been poorly suited to generating force. Finally, the oral plates themselves would not have formed a firm biting surface, with a poorly developed joint with the postoral plate even in the broad-based lateral oral plates (e.g. plates S/D4–6), and the medial oral plates (e.g. plates M, S1, D1) suspended in soft tissue or with very narrow-based articulations with the postoral plate. Reconstructions that interpret the oral plates as elements analogous to hagfish-like toothlets [31] can be similarly rejected because of the junction between the proximal tips of the oral plates and the sulcus in the postoral plate. All hypotheses of predatory ecology that require considerable aboral rotation of the oral plates and postoral plate (see §5a) are physically impossible and, as such, can be rejected.
Deposit or detritus feeding interpretations typically envisage the oral apparatus as a ‘scoop’ that would have been used to acquire sediment from the bottom of the water body [29]. As with predation, this requires the plates to evert substantially from their resting position, aided by movement of the postoral plate [29, figs. 49,50]. We can reject this based on our reconstruction of Rhinopteraspis, where significant movement of the oral plates is limited. However, we cannot rule out burial of the snout/mouth in the substrate as a means of deposit feeding. The presence or absence of wear, which is often used as a line of evidence in discussion of deposit feeding [27,83], is not visible in Rhinopteraspis at the resolution of our scan data. We note, however, that the elongated snout in pteraspids such as Rhinopteraspis, along with the strongly convex ventral abdomen, would have restricted the ability of the animal to place its mouth in contact with the substrate. Constraints imposed by the snout would also place the latero-ventral orbits in contact with the substrate if the animal was deposit feeding; this is in marked contrast with the dorsally placed eyes of contemporaneous osteostracans and galeaspids that are assumed to be deposit feeders [1,71,84].
Under interpretations of suspension feeding or deposit feeding, rostral rotation of the oral plates would have resulted in a greater area of gape, increasing intake. The occluded oral apparatus leaves a restricted opening with digitate margins defined by the tips of the oral plates and the tubercles of the dorsal oral plate that would have served to prevent fouling (cf. [3,66]) while facilitating inflow in a manner analogous to straining functions seen in animals as diverse as flamingos, brachiopods, oysters and gastropods [85]. It is possible that the exposed tips of the oral plates in Rhinopteraspis acted as an analogous structure along with the tubercles on the supraoral plate. The limited movement acted to control the entry of larger particles into the mouth and may also have served to provide a means to expel larger particles trapped between the plates [3]. It has been suggested that some cyathaspid heterostracans had an endostyle like that which aids suspension feeding in larval lampreys and invertebrate chordates [45,86]. However, the evidence for this is limited to the groove on the visceral surface of the ventral plate the position of which is likely incompatible with this identity [87].
(e) . Implications for early vertebrate evolution
The New Head and New Mouth hypotheses [11,14] have argued for a long-term evolutionary shift towards increasingly active food acquisition, from the filter-feeding of invertebrate chordates to macrophagous predation in living jawed vertebrates. The feeding ecology of heterostracans is key to supporting or refuting these hypotheses, as this group represents one of the earliest diverging members of the gnathostome stem-lineage. A diversity of feeding ecologies have been suggested for heterostracans, from macrophagous predation, through scavenging and herbivory, to deposit and filter feeding. Our three-dimensional reconstruction of the feeding apparatus of Rhinopteraspis dunensis precludes all proposed feeding strategies bar suspension feeding or deposit feeding by burial of the mouth in the substrate.
Comparison to other heterostracans suggests that while this feeding ecology may not be shared by all members of the group, the anatomy that underlies it is present in pteraspids and the oldest and earliest well-known heterostracan [74]; as such it may be plesiomorphic for the clade. In other armoured stem-gnathostomes (osteostracans, galeaspids, thelodonts, pituriaspids, anaspids, arandaspids and astraspids) there is little information on the anatomy of their feeding apparatus [1,71,84,88–91], but marked variation in their anatomy suggests a range of ecological roles. Meanwhile, evidence that filter feeding in ammocoete larval lampreys represents an independent evolutionary innovation [23] (although see [92]) suggests that suspension feeding has evolved separately amongst jawless vertebrates at least once. Taken together with evidence for macrophagy in earlier diverging lampreys, hagfish and conodonts [82,93,94], it is clear that early vertebrates and stem-gnathostomes established a diversity of feeding ecologies long before the origin of jaws. This finding is consistent with recent demonstrations that vertebrate innovations and elaborations cannot be characterized by a directional trend towards increasingly active food acquisition [95–97], but, rather, increasing ecological diversity.
6. Conclusion
The lack of knowledge of the three-dimensional anatomy of early vertebrate feeding apparatuses has obscured their feeding ecology, hindering the testing of macroecological scenarios that seek to explain early vertebrate evolution. Using X-ray microtomography, we have reconstructed the oral apparatus of the pteraspid heterostracan Rhinopteraspis dunensis. The oral apparatus in Rhinopteraspis is composed of one medial and six pairs of bilaterally arranged oral plates, plus a pair of lateral oral plates. Inferred articulation of these plates indicates that range of motion was limited; the oral plates could only move in concert and could not rotate far. When occluded, the oral plates left a short wide gape to the mouth, closed partially by a convex crest extending from the supraoral plate and the distal tips of the oral plates. The reconstructed anatomy precludes all proposed feeding modes bar suspension or deposit feeding in Rhinopteraspis. Heterostracans more generally show a far wider range of oral anatomies and body shapes than in pteraspids [36], a trend reflected in jawless vertebrates more broadly: a diversity of feeding ecologies was established early in vertebrate evolution, long before the origin of jaws. Given the existence of contemporary macrophagous vertebrate predators and scavengers, the presence of suspension feeding heterostracans is incompatible with a directional trend in vertebrates towards increasingly active food acquisition.
Acknowledgements
CT data were obtained at the XTM Facility, Palaeobiology Research Group, University of Bristol; many thanks to Liz Martin-Silverstone (University of Bristol) for help with CT scanning. Thanks also to Emma Bernard (NHMUK) for specimen access.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The CT data and the three-dimensional models deriving from that data which underlie this work are made freely downloadable. CT data are available at Morphosource, both the higher resolution scan set [98] and the lower resolution scan set [99]. The three-dimensional models are available via Dryad [100].
Supplementary material is available online [101].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
R.P.D.: conceptualization, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; A.S.J.: conceptualization, formal analysis, funding acquisition, investigation, methodology, visualization, writing—original draft, writing—review and editing; S.G.: conceptualization, formal analysis, funding acquisition, investigation, visualization, writing—review and editing; A.L.: investigation, writing—review and editing; M.G.: investigation, writing—review and editing; Z.J.: conceptualization, funding acquisition, investigation, writing—review and editing; S.L.: conceptualization, funding acquisition, investigation, methodology, writing—review and editing; E.R.: conceptualization, funding acquisition, writing—review and editing; P.C.J.D.: writing—review and editing; I.J.S.: conceptualization, funding acquisition, investigation, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by the Leverhulme Trust (RPG-2021-271 ‘Feeding without jaws: innovations in early vertebrates'). R.P.D. is currently supported by the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement no. 101062426 (DEADSharks). S.G. was also supported by a Royal Society Dorothy Hodgkin Research Fellowship (DH160098). M.G. is funded by the Natural Environment Research Council (GW4+ DTP studentship NE/S007504/1) while P.C.J.D. is funded by the Leverhulme Trust (RF-2022-167) and the John Templeton Foundation (JTF 62220; JTF 62574).
References
- 1.Janvier P. 1996. Early vertebrates. Oxford monographs on geology and geophysics, vol. 33. Oxford, UK: Clarendon Press. [Google Scholar]
- 2.Anderson PSL, Friedman M, Brazeau MD, Rayfield EJ. 2011. Initial radiation of jaws demonstrated stability despite faunal and environmental change. Nature 476, 206-209. ( 10.1038/nature10207) [DOI] [PubMed] [Google Scholar]
- 3.Hamann L, Blanke A. 2022. Suspension feeders: diversity, principles of particle separation and biomimetic potential. J. R. Soc. Interface 19, 20210741. ( 10.1098/rsif.2021.0741) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson PSL, Westneat MW. 2007. Feeding mechanics and bite force modelling of the skull of Dunkleosteus terrelli, an ancient apex predator. Biol. Lett. 3, 77-79. ( 10.1098/rsbl.2006.0569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeLaurier A. 2019. Evolution and development of the fish jaw skeleton. Wiley Interdiscip. Rev. Dev. Biol. 8, 1-36. ( 10.1002/wdev.337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smithwick FM. 2015. Feeding ecology of the deep-bodied fish Dapedium (Actinopterygii, Neopterygii) from the Sinemurian of Dorset, England. Palaeontology 58, 293-311. ( 10.1111/pala.12145) [DOI] [Google Scholar]
- 7.Clark AJ, Summers AP. 2007. Morphology and kinematics of feeding in hagfish: possible functional advantages of jaws. J. Exp. Biol. 210, 3897-3909. ( 10.1242/jeb.006940) [DOI] [PubMed] [Google Scholar]
- 8.Clubb BL, Clark AJ, Uyeno TA. 2019. Powering the hagfish ‘bite’: the functional morphology of the retractor complex of two hagfish feeding apparatuses. J. Morphol. 280, 827-840. ( 10.1002/jmor.20986) [DOI] [PubMed] [Google Scholar]
- 9.Hilliard RW, Potter IC, Macey DJ. 1985. The dentition and feeding mechanism in adults of the Southern Hemisphere lamprey Geotria australis Gray. Acta Zool. 66, 159-170. ( 10.1111/j.1463-6395.1985.tb00834.x) [DOI] [Google Scholar]
- 10.Dawson JA. 1963. The oral cavity, the ‘jaws’ and the horny teeth of Myxine glutinosa. In The biology of Myxine (eds Brodal AB, Fänge R), pp. 231-255. Oslo, Norway: Universitetsforlaget. [Google Scholar]
- 11.Mallatt J. 1996. Ventilation and the origin of jawed vertebrates: a new mouth. Zool. J. Linn. Soc. 117, 329-404. ( 10.1111/j.1096-3642.1996.tb01658.x) [DOI] [Google Scholar]
- 12.Mallatt J. 1984. Feeding ecology of the earliest vertebrates. Zool. J. Linn. Soc. 82, 261-272. ( 10.1111/j.1096-3642.1984.tb00643.x) [DOI] [Google Scholar]
- 13.Northcutt RG. 2005. The new head hypothesis revisited. J. Exp. Zool. B Mol. Dev. Evol. 304, 274-297. ( 10.1002/jez.b.21063) [DOI] [PubMed] [Google Scholar]
- 14.Gans C, Northcutt RG. 1983. Neural crest and the origin of vertebrates: a new head. Science 220, 268-273. ( 10.1126/science.220.4594.268) [DOI] [PubMed] [Google Scholar]
- 15.Northcutt RG. 1996. The origin of craniates: neural crest, neurogenic placodes, and homeobox genes. Israeli J. Zool. 42, 273-313. [Google Scholar]
- 16.Gans C. 1989. Stages in the origin of vertebrates: analysis by means of scenarios. Biol. Rev. 64, 221-268. ( 10.1111/j.1469-185X.1989.tb00471.x) [DOI] [PubMed] [Google Scholar]
- 17.Gans C. 1993. Evolutionary origin of the vertebrate skull. In The skull: patterns of structural and systematic diversity, vol. 2, pp. 1-35. Chicago, IL: University of Chicago Press. [Google Scholar]
- 18.Kuratani S, Ahlberg PE. 2018. Evolution of the vertebrate neurocranium: problems of the premandibular domain and the origin of the trabecula. Zool. Lett. 4, 1-10. ( 10.1186/s40851-017-0083-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Northcutt GR, Gans C. 1983. The genesis of neural crest and epidermal placodes: a reinterpretation of vertebrate origins. Q Rev. Biol. 58, 1-28. ( 10.1086/413055) [DOI] [PubMed] [Google Scholar]
- 20.Donoghue PCJ, Forey PL, Aldridge RJ. 2000. Conodont affinity and chordate phylogeny. Biol. Rev. 75, 191-251. ( 10.1111/j.1469-185X.1999.tb00045.x) [DOI] [PubMed] [Google Scholar]
- 21.Keating JN, Donoghue PCJ. 2016. Histology and affinity of anaspids, and the early evolution of the vertebrate dermal skeleton. Proc. R. Soc. B 283, 20152917. ( 10.1098/rspb.2015.2917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyashita T, et al. 2019. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological–molecular conflict in early vertebrate phylogeny. Proc. Natl Acad. Sci. USA 116, 2146-2151. ( 10.1073/pnas.1814794116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyashita T, Gess RW, Tietjen K, Coates MI. 2021. Non-ammocoete larvae of Palaeozoic stem lampreys. Nature 591, 408-412. ( 10.1038/s41586-021-03305-9) [DOI] [PubMed] [Google Scholar]
- 24.Keating JN, Marquart CL, Marone F, Donoghue PCJ. 2018. The nature of aspidin and the evolutionary origin of bone. Nat. Ecol. Evol. 2, 1501-1506. ( 10.1038/s41559-018-0624-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blieck ARM. 1984. Hétérostracés, pteraspidiformes: systemastique, phylogenie, biostratigraphie et biogeographie, 1-25. Cahiers du Palaéontologie (section Vertébrés). Paris, France: Editions du CNRS. [Google Scholar]
- 26.Kiaer J. 1928. The structure of the mouth of the oldest known vertebrates, pteraspids and cephalaspids. Palaeobiologica 1, 117-134. [Google Scholar]
- 27.Purnell MA. 2002. Feeding in extinct jawless heterostracan fishes and testing scenarios of early vertebrate evolution. Proc. R. Soc. Lond. B 269, 83-88. ( 10.1098/rspb.2001.1826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randle E, Sansom RS. 2017. Phylogenetic relationships of the 'higher heterostracans' (Heterostraci: Pteraspidiformes and Cyathaspididae), extinct jawless vertebrates. Zool. J. Linn. Soc. 181, 910-926. ( 10.1093/zoolinnean/zlx025) [DOI] [Google Scholar]
- 29.White EI. 1935. The ostracoderm Pteraspis Kner and the relationships of the agnathous vertebrates. Phil. Trans. R. Soc. Lond. B 225, 381-457. ( 10.1098/rstb.1935.0015) [DOI] [Google Scholar]
- 30.Bendix-Almgreen SE. 1986. Silurian ostracoderms from Washington Land (North Greenland), with comments on cyathaspid structure, systematics and phyletic position. Rapport Grønlands Geologiske Undersøgelse 132, 89-123. ( 10.34194/rapggu.v132.7970) [DOI] [Google Scholar]
- 31.Janvier P. 1974. The structure of the naso-hypophysial complex and the mouth in fossil and extant cyclostomes, with remarks on amphiaspiforms. Zool. Scr. 3, 193-200. ( 10.1111/j.1463-6409.1974.tb00816.x) [DOI] [Google Scholar]
- 32.Jarvik E. 1980. Basic structure and evolution of vertebrates. London, UK: Academic Press. [Google Scholar]
- 33.Stensiö E. 1958. Les Cyclostomes fossiles ou Ostracodermes. In Traité de zoologie (ed. Grassé PP), pp. 173-425. Paris, France: Masson. [Google Scholar]
- 34.Stensiö E. 1964. Les Cyclostomes fossiles ou Ostracodermes. In Traité de paleontologie (ed. Piveteau J), pp. 96-383. Paris, France: Masson. [Google Scholar]
- 35.Janvier P. 2015. Facts and fancies about early fossil chordates and vertebrates. Nature 520, 483-489. ( 10.1038/nature14437) [DOI] [PubMed] [Google Scholar]
- 36.Elliott DK, Reed RC, Loeffler EJ. 2004. A new species of Allocryptaspis (Heterostraci) from the Early Devonian, with comments on the structure of the oral area in cyathaspids. In Recent advances in the origin and early radiation of vertebrates, pp. 455-472. Munich, Germany: Verlag Dr. Friedrich Pfeil. [Google Scholar]
- 37.Lingham-Soliar T. 2014. The vertebrate integument volume 1: origin and evolution. In Vertebrate integument volume 1: origin and evolution, vol. 1, pp. 1-268. Heidelburg, Germany: Springer Berlin. [Google Scholar]
- 38.Stiefel KM. 2021. Evolutionary trends in large pelagic filter-feeders. Hist. Biol. 33, 1477-1488. ( 10.1080/08912963.2019.1711072) [DOI] [Google Scholar]
- 39.Friman L, Bardenheuer P. 1984. A new reconstruction of Rhinopteraspis dunensis (Agnatha, Heterostraci) with some remarks on pteraspid ontogeny. Munster Forsch Geol Paläont. 61, 147-173. [Google Scholar]
- 40.Denison RH. 1961. Feeding mechanisms of Agnatha and early gnathostomes. Am. Zool. 1, 177-181. ( 10.1093/icb/1.2.177) [DOI] [Google Scholar]
- 41.Dineley DL, Loeffler EJ. 1976. Ostracoderm faunas of the Delorme and associated Siluro-Devonian Formations, North West Territories, Canada. Palaeontol. Assoc. Special Papers Palaeontol. 18, 1-214. [Google Scholar]
- 42.Romer AS. 1959. The vertebrate story. Chicago, IL: University of Chicago Press. [Google Scholar]
- 43.Mallatt J. 1985. Reconstructing the life cycle and the feeding of ancestral vertebrates. In Evolutionary biology of primitive fishes (eds Foreman RE, Gorbman A, Dodd JM, Olson R). New York, NY: Plenum Press. [Google Scholar]
- 44.Blieck A, Heintz N. 1983. The cyathaspids of the Red Bay Group (Lower Devonian) of Spitsbergen. Polar Res. 1, 49-74. ( 10.1111/j.1751-8369.1983.tb00731.x) [DOI] [Google Scholar]
- 45.Halstead LB. 1973. The heterostracan fishes. Biol. Rev. 48, 279-332. ( 10.1111/j.1469-185X.1973.tb01005.x) [DOI] [Google Scholar]
- 46.Ball HW, Dineley DL. 1961. The old red sandstone of Brown Clee Hill and the adjacent area. I. Stratigraphy. Bulletin of the British Museum (Natural History), Geology 5, 175-242. [Google Scholar]
- 47.White EI. 1961. The Old Red Sandstone of Brown Clee Hill and the adjacent area. II. Palaeontology. Bulletin of the British Museum (Natural History), Geology 5, 243-310. [Google Scholar]
- 48.Conzen T, Gerlach R. 1993. Fische aus dem Unterdevon. Archäologie im Rheinland 1993, 22-23. [Google Scholar]
- 49.Hartkopf-Fröder C, Knaak M, Weber HM, Wrede V. 2015. Der Steinruch Jaeger bei Odenspiel - ein Unterdevon-Fossilfundpunkt mit großem Potential. Archäologie im Rheinland 2015, 39-41. [Google Scholar]
- 50.Becker RT, Marshall JEA, Da Silva A, Agterberg FP, Gradstein FM, Ogg JG. 2020. Chapter 22 – The Devonian Period. In Geologic time scale 2020, pp. 733-810. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 51.Poschmann M, Schoenemann B, Mccoy VE. 2016. Telltale eyes: the lateral visual systems of Rhenish Lower Devonian eurypterids (Arthropoda, Chelicerata) and their palaeobiological implications. Palaeontology 59, 295-304. ( 10.1111/pala.12228) [DOI] [Google Scholar]
- 52.Stets J, Schäfer A. 2009. The Siegenian delta: land–sea transitions at the northern margin of the Rhenohercynian Basin. Geol. Soc. Spec. Publ. 314, 37-72. ( 10.1144/SP314.3) [DOI] [Google Scholar]
- 53.Jansen U. 2016. Brachiopod faunas, facies and biostratigraphy of the Pridolian to lower Eifelian succession in the Rhenish Massif (Rheinisches Schiefergebirge, Germany). In Devonian climate, sea level and evolutionary events (eds Becker RT, Königshof P, Brett CE), pp. 45-122. London, UK: Geological Society, Special Publications. [Google Scholar]
- 54.Weber HM, Hartkopf-Fröder C. 2016. Reste eines seltenen Seeskorpions aus dem Unterdevon von Odenspiel. Archäologie im Rheinland 2016, 52-53. [Google Scholar]
- 55.Hartkopf-Fröder C, Weber HM. 2016. From Emsian coastal to Famennian marine environments: palaeogeographic evolution and biofacies in the Bergisch Gladbach-Paffrath Syncline area (Rhenish Massif, Germany). Munster Forsch Geol Paläont. 108, 46-75. [Google Scholar]
- 56.Schmidt W. 1959. Grundlagen einer Pteraspiden-Stratigraphie im Unterdevon der Rheinische Geosynklinale. Fortschritte in der Geologie von Rheinland und Westfalen 5, 1-82. [Google Scholar]
- 57.Randle E, Sansom RS. 2016. Exploring phylogenetic relationships of Pteraspidiformes heterostracans (stem-gnathostomes) using continuous and discrete characters. J. Syst. Palaeontol. 2019, 1-17. ( 10.1080/14772019.2016.1208293) [DOI] [Google Scholar]
- 58.Blieck A. 1980. Le genre Rhinopteraspis Jaekel (Vertébrés, Hétérostracés) du Dévonien Inférieur. Bull. Mus. Natl Hist. nat, Paris, 4è sér. 2, 25-47. [Google Scholar]
- 59.Tarlo B. 1961. Rhinopteraspis cornubica (McCoy), with notes on the classification and evolution of the pteraspids. Acta Palaeontol. Pol. 6, 367-400. [Google Scholar]
- 60.Herbst EC, Meade LE, Lautenschlager S, Fioritti N, Scheyer TM. 2022. A toolbox for the retrodeformation and muscle reconstruction of fossil specimens in Blender. R. Soc. Open Sci. 9, 220519. ( 10.1098/rsos.220519) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lautenschlager S. 2016. Reconstructing the past: methods and techniques for the digital restoration of fossils. R. Soc. Open Sci. 3, 160342. ( 10.1098/rsos.160342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keating JN, Marquart CL, Donoghue PCJ. 2015. Histology of the heterostracan dermal skeleton: insight into the origin of the vertebrate mineralised skeleton. J. Morphol. 276, 657-680. ( 10.1002/jmor.20370) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fahlbusch K. 1957. Pteraspis dunensis Roemer: eine neuarbeitung der pteraspidenfunde (agnathen) von Overath. Palaeontographica Abteilung A 108, 1-56. [Google Scholar]
- 64.Fahlbusch K. 1966. Eine Pteraspiden-Fauna aus dem Unterdevon von Alken an der Mosel. Senckenbergiana lethaea. 47, 165-191. [Google Scholar]
- 65.Janvier P. 1981. The phylogeny of the Craniata, with particular reference to the significance of fossil ‘agnathans’. J. Vertebr. Paleontol. 1, 121-159. ( 10.1080/02724634.1981.10011886) [DOI] [Google Scholar]
- 66.Grohganz M, Ferron HG, Johanson Z, Donoghue PCJ. 2023. Testing hypotheses of pteraspid heterostracan feeding using computational fluid dynamics. J. Vertebr. Paleontol. 43, e2272974. ( 10.1080/02724634.2023.2272974) [DOI] [Google Scholar]
- 67.Farina SC, Near TJ, Bemis WE. 2015. Evolution of the branchiostegal membrane and restricted gill openings in actinopterygian fishes. J. Morphol. 276, 681-694. ( 10.1002/jmor.20371) [DOI] [PubMed] [Google Scholar]
- 68.Dawson J. 1905. The breathing and feeding mechanism of the lampreys. Biol. Bull. 9, 1-21. ( 10.2307/1535799) [DOI] [Google Scholar]
- 69.Janvier P, Blieck ALB. 1993. Halstead and the heterostracan controversy. Modern Geol. 18, 89-105. [Google Scholar]
- 70.Novitskaya LI. 1983. Morfologia drevnikh vestcheliostnik [Morphology of ancient agnathans]. Moscow, Russia: Nauka. [Google Scholar]
- 71.Gai Z, Donoghue PCJ, Zhu M, Janvier P, Stampanoni M. 2011. Fossil jawless fish from China foreshadows early jawed vertebrate anatomy. Nature 476, 324-327. ( 10.1038/nature10276) [DOI] [PubMed] [Google Scholar]
- 72.Whiting HP, Tarlo LB. 1965. The brain of the heterostraci (Agnatha). Nature 207, 829-831. ( 10.1038/207829a0) [DOI] [Google Scholar]
- 73.Denison R. 1964. The Cyathaspididae a family of Silurian and Devonian jawless vertebrates. Fieldiana Geol. 13, 309-473. [Google Scholar]
- 74.Soehn KL, Wilson MVH. 1990. A complete, articulated heterostracan from Wenlockian (Silurian) beds of the Delorme Group, Mackenzie Mountains, Northwest Territories, Canada. J. Vertebr. Paleontol. 10, 405-419. ( 10.1080/02724634.1990.10011825) [DOI] [Google Scholar]
- 75.Elliott DK, Petriello MA. 2011. New poraspids (Agnatha, Heterostraci) from the Early Devonian of the western United States. J. Vertebr. Paleontol. 31, 518-530. ( 10.1080/02724634.2011.557113) [DOI] [Google Scholar]
- 76.Elliott DK, Petriello MA. 2013. A new cyathaspid (Agnatha, Heterostraci) with an articulated oral cover from the Late Silurian of the Canadian Arctic. J. Vertebr. Paleontol. 33, 29-34. ( 10.1080/02724634.2012.717568) [DOI] [Google Scholar]
- 77.Pernègre VN. 2003. The genus Doryaspis White (Heterostraci) from the Lower Devonian of Vestspitsbergen, Svalbard. J. Vertebr. Paleontol. 22, 735-746. ( 10.1671/0272-4634(2002)022[0735:TGDWHF]2.0.CO;2) [DOI] [Google Scholar]
- 78.Gross W. 1963. Drepanaspis gemuendenensis Schlüter Neuuntersuchung. Palaeontographica Abteilung A 121, 133-155. [Google Scholar]
- 79.Lundgren M, Blom H. 2013. Phylogenetic relationships of the cyathaspidids (Heterostraci). GFF 135, 74-84. ( 10.1080/11035897.2013.770792) [DOI] [Google Scholar]
- 80.Elliott DK, Lassiter L, Blieck A. 2021. A phylogenetic analysis of the Cyathaspididae (Vertebrata: Pteraspidomorphi: Heterostraci). Acta Palaeontol. Pol. 66, 631-640. ( 10.4202/app.00811.2020) [DOI] [Google Scholar]
- 81.Novitskaya LI. 1971. Les Amphiaspides (Heterostraci) du Devonien de la Sibérie, vol. 12, Cahiers de Paléontologie, Section Vertebres, pp. 1-130. Paris, France: Centre National de la Recherche Scientifique. [Google Scholar]
- 82.Purnell MA. 2001. Scenarios, selection, and the ecology of early vertebrates. In Major events in early vertebrate evolution (ed. Ahlberg PE), pp. 187-208. Systematics Association special volume series. London, UK: Taylor and Francis. [Google Scholar]
- 83.Denison RH. 1973. Growth and wear of the shield in Pteraspididae (Agnatha). Palaeontographica Abteilung A 143, 1-10. [Google Scholar]
- 84.Janvier P. 1985. Les Céphalaspides du Spitsberg. Anatomie, phylogénie et systématique des Ostéostracés siluro-dévoniens. Révision des Ostéostracés de la Formation de Wood Bay (Dévonien inférieur du Spitsberg). Cahiers de Paléontologie, Section Vertebres. Paris, France: Centre National de la Recherche Scientifique.
- 85.Rudwick MJS. 1964. The function of zigzag deflexions in the commissures of fossil brachiopods. Palaeontology 7, 135-171. [Google Scholar]
- 86.Halstead Tarlo L, Whiting H. 1965. A new interpretation of the internal anatomy of the Heterostraci (Agnatha). Nature 206, 148-150. ( 10.1038/206148a0) [DOI] [Google Scholar]
- 87.Miyashita T. 2016. Fishing for jaws in early vertebrate evolution: a new hypothesis of mandibular confinement. Biol. Rev. Camb. Phil. Soc. 91, 611-657. ( 10.1111/brv.12187) [DOI] [PubMed] [Google Scholar]
- 88.Gagnier PY. 1993. Sacabambaspis janvieri, Vertébré ordovicien de Bolivie. 1, Analyse morphologique. Ann. Paléontol. 79, 19-69. [Google Scholar]
- 89.Dearden RP, Lanzetti A, Giles S, Johanson Z, Jones AS, Lautenschlager S, Randle E, Sansom IJ. 2023. The oldest three-dimensionally preserved vertebrate neurocranium. Nature 621, 782-787. ( 10.1038/s41586-023-06538-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blom H, Märss T, Miller CG. 2002. Silurian and earliest Devonian birkeniid anaspids from the Northern Hemisphere. Trans. R. Soc. Edinb. Earth Sci. 92, 263-323. ( 10.1017/S0263593300000250) [DOI] [Google Scholar]
- 91.Donoghue PCJ, Smith MP. 2002. The anatomy of Turinia pagei (Powrie), and the phylogenetic status of the Thelodonti. Trans. R. Soc. Edinb. Earth Sci. 92, 15-37. ( 10.1017/S026359330000002X) [DOI] [Google Scholar]
- 92.Mallatt J. 2023. Vertebrate origins are informed by larval lampreys (ammocoetes): a response to Miyashita et al., 2021. Zool. J. Linn. Soc. 197, 287-321. ( 10.1093/zoolinnean/zlac086) [DOI] [Google Scholar]
- 93.Purnell M. 1995. Microwear on conodont elements and macrophagy in the first vertebrates. Nature 374, 798-800. 10.1038/374798a0 [DOI] [Google Scholar]
- 94.Purnell M, Donoghue PCJ. 1997. Architecture and functional morphology of the skeletal apparatus of ozarkodinid conodonts. Phil. Trans. R. Soc. B 352, 1545-1564. ( 10.1098/rstb.1997.0141) [DOI] [Google Scholar]
- 95.Ferrón HG, Donoghue PCJ. 2022. Evolutionary analysis of swimming speed in early vertebrates challenges the ‘New Head Hypothesis’. Commun. Biol. 5, 863. ( 10.1038/s42003-022-03730-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gai Z, Li Q, Ferrón HG, Keating JN, Wang J, Donoghue PCJ, Zhu M. 2022. Galeaspid anatomy and the origin of vertebrate paired appendages. Nature 609, 959-963. ( 10.1038/s41586-022-04897-6) [DOI] [PubMed] [Google Scholar]
- 97.Gai Z, Lin X, Shan X, Ferrón HG, Donoghue PCJ. 2023. Postcranial disparity of galeaspids and the evolution of swimming speeds in stem-gnathostomes. Natl Sci. Rev. 10, nwad050. ( 10.1093/nsr/nwad050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dearden RP, et al. 2024 Media 000546818: A close up scan of the oral region of Nhmuk Pv P 73217. MorphoSource 000546818. ( 10.17602/M2/M546818) [DOI]
- 99.Dearden RP, et al. 2024. Media 000545620: The entire specimen of Nhmuk Pv P 73217. MorphSource 000545620. ( 10.17602/M2/M545620) [DOI]
- 100.Dearden RP, et al. 2024. The three-dimensionally articulated oral apparatus of a Devonian heterostracan sheds light on feeding in Palaeozoic jawless fishes [Dataset]. Dryad Digital Repository. ( 10.5061/dryad.612jm64b4) [DOI] [PubMed]
- 101.Dearden RP, et al. 2024. The three-dimensionally articulated oral apparatus of a Devonian heterostracan sheds light on feeding in Palaeozoic jawless fishes. Figshare. ( 10.6084/m9.figshare.c.7109022) [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dearden RP, et al. 2024 Media 000546818: A close up scan of the oral region of Nhmuk Pv P 73217. MorphoSource 000546818. ( 10.17602/M2/M546818) [DOI]
- Dearden RP, et al. 2024. Media 000545620: The entire specimen of Nhmuk Pv P 73217. MorphSource 000545620. ( 10.17602/M2/M545620) [DOI]
- Dearden RP, et al. 2024. The three-dimensionally articulated oral apparatus of a Devonian heterostracan sheds light on feeding in Palaeozoic jawless fishes [Dataset]. Dryad Digital Repository. ( 10.5061/dryad.612jm64b4) [DOI] [PubMed]
- Dearden RP, et al. 2024. The three-dimensionally articulated oral apparatus of a Devonian heterostracan sheds light on feeding in Palaeozoic jawless fishes. Figshare. ( 10.6084/m9.figshare.c.7109022) [DOI] [PubMed]
Data Availability Statement
The CT data and the three-dimensional models deriving from that data which underlie this work are made freely downloadable. CT data are available at Morphosource, both the higher resolution scan set [98] and the lower resolution scan set [99]. The three-dimensional models are available via Dryad [100].
Supplementary material is available online [101].


