Abstract
Background and Objectives
Efgartigimod, which has been well tolerated and efficacious in individuals with generalized myasthenia gravis (MG), is available in Japan not only for the treatment of anti–acetylcholine receptor–positive (AChR+) but also anti–muscle-specific receptor tyrosine kinase (MuSK+) and seronegative generalized MG. We report details of the use of efgartigimod for generalized MG in clinical practice in Japan.
Methods
We included patients with generalized MG in the 2021 survey of Japan Myasthenia Gravis Registry (JAMG-R) study group who received an initial cycle of efgartigimod between May and September 2022. We defined “responders” as patients who achieved a score ≥2 points for MG activities of daily living (MG-ADL) in the first treatment cycle. The MG composite and the Revised scale of the 15-item Myasthenia Gravis–Quality of Life scale (MG-QOL15-r) were also evaluated.
Results
Of 1,343 JAMG-R patients, 36 (2.7%) started efgartigimod (female 68%, age 53 years). Their serologic profiles were as follows: AChR+, n = 19 (53%); MuSK+, n = 6 (17%); and seronegative, n = 11 (31%). Twenty-six patients (72%) had refractory MG. There were 81 cycles of efgartigimod during the 26-week observation in 34 patients (average, 2.4 cycles). The mean interval between cycles was 5.9 weeks. A continuous 4-weekly infusion of efgartigimod was performed in 65 (80%) of 81 cycles. In the first cycle, the MG-ADL score of the 34 patients decreased significantly from 10.5 ± 4.3 to 6.9 ± 5.1 (p = 0.003). Similarly, the mean MG composite and MG-QOL15-r decreased from 18.4 ± 13.6 to 11.8 ± 9.6 (p = 0.004) and from 19.2 ± 6.3 to 14.2 ± 8.3 (p = 0.007), respectively. Twenty-one (62%) patients were responders. Therapeutic responses were observed in the subsequent cycles. The duration of effectiveness of efgartigimod was varied among the responders; 4 responders had only a single effective cycle. Significant improvement was observed in the MuSK+ patients. Prednisolone dose of 7 patients was reduced. Our examination of the patients' postintervention status revealed that 6 patients achieved minimal manifestations. COVID-19 occurred in 5 patients. We failed to detect clinical or laboratory findings associated with responders.
Discussion
Efgartigimod can be considered for the treatment of patients with generalized MG who do not achieve minimal manifestations, with a broad flexibility of patient selection and treatment schedules.
Introduction
Generalized myasthenia gravis (MG) is a neuromuscular disorder caused by pathogenic immunoglobulin G (IgG) autoantibodies against the nicotinic acetylcholine receptor (AChR) or muscle-specific receptor tyrosine kinase (MuSK), both of which functionally interfere with normal synaptic transmission.1 Recent advances in immunotherapy have been proven to be effective for the management of MG.2,3 Approximately 15%–25% of individuals with generalized MG remain refractory to the current therapy, although the definition of refractory MG can differ among investigators.4,5 The Japan Myasthenia Gravis Registry (JAMG-R) study group is made up of motivated neurologists who specialize in MG care who have provided a large amount of detailed and reliable patient information for over 10 years.6 The goal of the JAMG-R study group is to promote high-quality medical care for patients with MG, based on clinical data from multiple institutions.
The neonatal Fc receptor (FcRn) is a major histocompatibility complex class I–like molecule that recycles IgG, extending its half-life by approximately four-fold compared with that of other immunoglobulins that are not recycled by FcRn.7,8 Efgartigimod is a human IgG1 antibody Fc-fragment, a natural ligand of FcRn, which has been engineered for increased affinity to FcRn. Targeting the FcRn is expected to provide novel therapies for various autoimmune disorders. The randomized double-blind and placebo-controlled ADAPT phase III trial recruited patients with generalized MG in 15 countries in Europe, North America, and Japan,9 and the results of the trial demonstrated that efgartigimod was well tolerated and efficacious in patients with generalized MG. Efgartigimod is now used in the United States and European counties for the treatment of anti–AChR-positive (AChR+), generalized MG. By contrast, efgartigimod is available in Japan not only for patients with AChR+ generalized MG but also for those with anti–MuSK-positive (MuSK+) and seronegative generalized MG.10 We conducted this study to determine the current aspects of efgartigimod treatment for generalized MG in clinical practices in Japan.
Methods
Patients
The diagnosis of MG was made based on the patient exhibiting the typical history and signs of fluctuating weakness in the voluntary muscles, the presence of serum anti-AChR or anti-MuSK antibody, and abnormal findings of neuromuscular junction transmission.1 In addition, patients were diagnosed with seronegative MG if they had the following attributes: (1) a clinical history of fatigable weakness; (2) objective fatigable weakness on physical examination; and (3) electrophysiologic abnormalities, either ≥10% decrease in the compound muscle action potential amplitudes at 3-Hz repetitive stimulation or motor unit potential variation or abnormal single-fiber electromyography in at least 1 cranial or limb muscle. We found that 1,343 patients with generalized MG visited one of the JAMG-R sites between April and October 2021 (Figure 1A), and we evaluated the patients' currents status including severity, subtype, and treatment. We collected the clinical data of onset age, disease duration, sex, past treatment regimen(s), and disease status at its worst (eTable 1). The severity of MG and postintervention status of each patient was determined according to the Myasthenia Gravis Foundation of America (MGFA) standards.11 We also investigated the patients' scores on scales for MG activities of daily living (MG-ADL), quantitative MG (QMG), and an MG composite.12,13 The patients' self-perceived health-related quality of life (QOL) was evaluated using the revised 15-item MG-QOL scale (MG-QOL15-r).14 We collected clinical data during the COVID-19 pandemic; the patients' respiratory testing was thus often restricted and could not be evaluated for all the patients. Thus, instead of a quantitative MG score, we used the MG composite to evaluate the patients' current disease severity. Minimal symptom expression was defined by the MG-ADL 0 or 1 point. Refractory MG is defined as occurring in patients whose symptoms cannot be well controlled, even if with ≥2 immunosuppressive oral therapy or with early fast-acting therapy, or who cannot tolerate therapy for adequate control due to side effects and/or burdens.6
Figure 1. Study Flow.
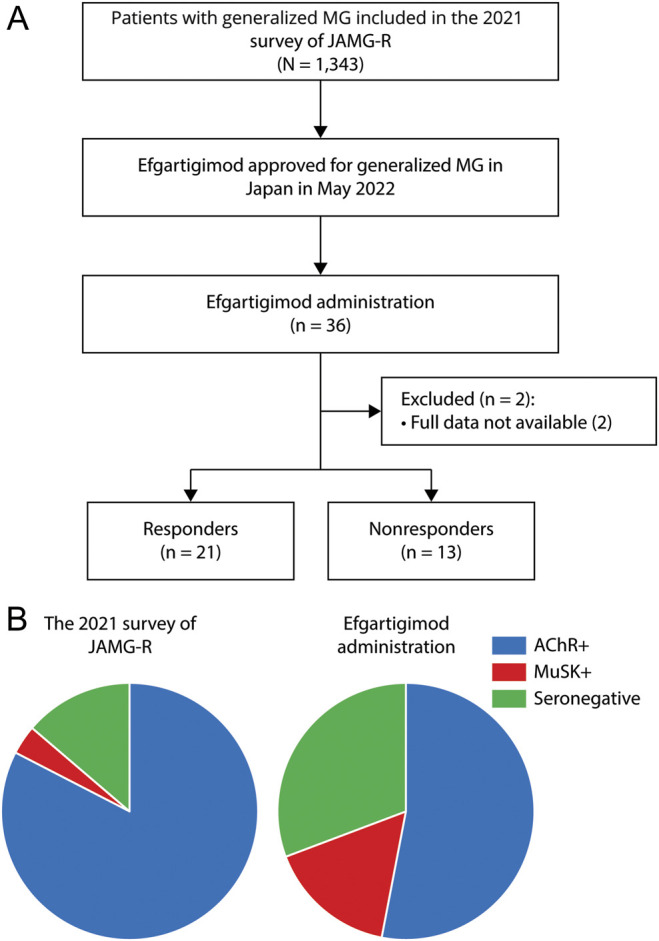
Study flow (A) and autoantibody profiles (B). AChR+ = anti–acetylcholine receptor–positive; JAMG-G = Japan Myasthenia Gravis Registry; MuSK+ = anti–muscle-specific receptor tyrosine kinase-positive.
Efgartigimod was approved by the Japanese Government for the treatment of generalized MG in May 2022. We included patients in the 2021 JAMG-R study with generalized MG who received an initial cycle of efgartigimod between May and September 2022. Efgartigimod (10 mg/kg) was administered as 4 infusions per cycle (1infusion per week). Assessments were performed weekly during consecutive infusions of efgartigimod and at 1 week after the final infusion of each cycle up to the end of the 26-week observational period. If the final cycle was started within the 26-week period, we extended the observational period until the end of the final cycle. We defined “responders” as patients who achieved a respective score of ≥2 points for MG-ADL in the first treatment cycle. Definition of responders was also supported by an improvement or unchanged of the MG composite and the MG-QOL15-r.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was part of the JAMG-R study approved by the ethics committee of each neurologic center (Institutional Review Board No. R3-1 at Hanamaki General Hospital, the primary investigating institute). Written informed consent was obtained from all study participants.
Statistical Analyses
The statistical evaluation of the patients' clinical information was performed using JMP ver. 16.0.0 software (SAS, Cary, NC). Categorical variables were compared by the χ2 test. Continuous variables were compared by an analysis of variance (ANOVA) and the Mann-Whitney U test.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
Among the 1,343 patients with generalized MG included in the 2021 JAMG-R survey, 36 (2.7%) started efgartigimod treatment. Their demographic and clinical features are summarized in the Table 1. The mean age was 53.0 years, with female predominancy. The average disease duration before the stating efgartigimod was 12.9 years.
Table.
Summary of the 36 Patients With Generalized Myasthenia Gravis (MG) Being Treated With Efgartigimod
| Age, y | 53.0 ± 14.7 |
| Female, n (%) | 25 (68) |
| Disease duration, y | 12.9 ± 14.7 |
| MG subtype, n (%) | |
| AChR+ early onset | 4 (11) |
| AChR+ late onset | 5 (14) |
| AChR+ thymoma associated | 10 (28) |
| MuSK+ | 6 (17) |
| Seronegative | 11 (31) |
| Worst MGFA classification, n (%) | |
| II, mild | 4 (11) |
| III, moderate | 19 (53) |
| IV, severe | 8 (22) |
| V, crisis | 5 (14) |
| Worst quantitative MG score | 18.9 ± 6.3 |
| Oral prednisolone | 33 (92) |
| Calcineurin inhibitors | 35 (97) |
| Thymectomy | |
| Thymectomy for thymoma | 10/10 (100%) |
| Thymectomy for non-thymoma | 6/26 (23%) |
| Fast-acting treatment, n (%) | |
| Plasmapheresis | 26 (72) |
| Intravenous immunoglobulin | 26 (72) |
| Intravenous high-dose methylprednisolone | 28 (78) |
| Molecular targeting drugs, n (%) | |
| Rituximab | 2 (6) |
| Eculizumab | 5 (14) |
| MG status, n (%) | |
| Minimal manifestations or better | 0 (0) |
| Refractory | 26 (72) |
Abbreviations: AChR+ = anti–acetylcholine receptor–positive; MG = myasthenia gravis; MGFA = MG Foundation of America; MuSK+ = anti–muscle-specific receptor tyrosine kinase–positive.
Disease subsets based on the 36 patients' serologic profiles revealed 19 (53%) patients with AChR+ MG, 6 (17%) patients with MuSK+ MG, and 11 (31%) patients with seronegative MG. The autoantibody profiles of patients receiving efgartigimod differed between the 36 patients receiving efgartigimod and the 2021 JAMG-R survey (Figure 1B). The worst MGFA classification showed that 32 patients had class III or overdisease, including 5 patients who experienced a myasthenic crisis. The worst quantitative MG score was 18.9, higher than the 2021 JAMG-R survey score at 14.5.
The therapeutic history of the 36 patients showed that oral prednisolone was used in 33 patients. All but 1 patient also used calcineurin inhibitors. During their clinical courses, 33 patients received fast-acting treatments such as plasmapheresis, intravenous immunoglobulin (IVIg), and intravenous high-dose methylprednisolone (IVMP). Regardless of the treatment, the postintervention statuses showed that none of the patients had achieved minimal manifestations. There were 26 (72%) patients with refractory MG.
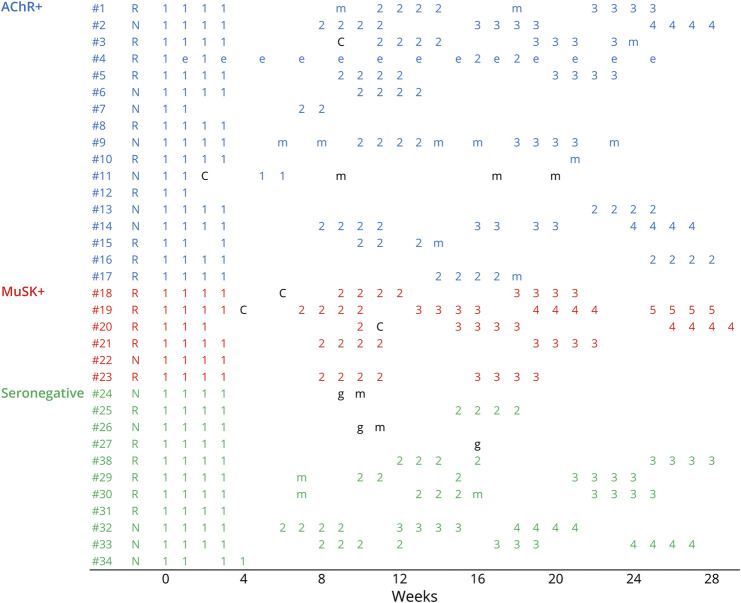
After we excluded the cases of 2 AChR+ patients with insufficient data, we evaluated the clinical features of 34 patients with generalized MG. The disease subsets were divided into 4 AChR+ early-onset patients (patients #1 to #4), 4 AChR+ late-onset patients (#5 to #8), 9 AChR+ thymoma–associated patients (#9 to #17), 6 MuSK+ patients (#18 to #23), and 11 seronegative patients (#24 to #34) (Figure 2). There were 81 cycles of efgartigimod during the observation period, and the average number of cycles was 2.4. In the clinical practice, the criteria of entering additional cycles were based on the individual investigators. Ten patients including 5 responders and 5 nonresponders received only a single cycle. We evaluated the 47 intervals between cycles in the remaining 24 patients. The average interval between cycles was 5.9 weeks (range 2–21 weeks).
Figure 2. Cycles of Efgartigimod.
Time courses of efgartigimod in 34 patients with generalized MG. Numbers = the numbers of cycles of efgartigimod treatment. AChR+ = anti–acetylcholine receptor–positive; MuSK+ = anti–muscle-specific receptor tyrosine kinase-positive; C = COVID-19; e = eculizumab; g = intravenous immunoglobulin; m = intravenous high-dose methylprednisolone; N = nonresponder; R = responder.
A continuous 4-weekly infusion of efgartigimod (the complete cycle) was basically defined. However, the complete cycles were performed in 65 (80%) of the 81 cycles. In fact, the skipping or discontinuation of the consecutive 4 infusions of efgartigimod (i.e., an incomplete cycle) was allowable. The incomplete cycles were observed in 16 cycles in 12 patients. The reasons for the incomplete cycles were IgG decrease in 9 cycles, COVID-19 in 2 cycles, the patients' business concerns in 4 cycles, and the year-end closing of the hospital in 1 cycle.
To evaluate the effectiveness of efgartigimod, we used primarily the MG-ADL score, as was done in the ADAPT trial.9 The present patients’ MG-ADL scores at baseline ranged from 1 to 17 points; the MG-ADL scores of 4 patients were <5 points. The MG condition of these 4 patients was improved by the fast-acting treatment performed just before the administration of efgartigimod. One AChR+ patient with the MG-ADL score 1 point (#11) experienced severe neck weakness, a symptom that was not taken into account by the MG-ADL scale.
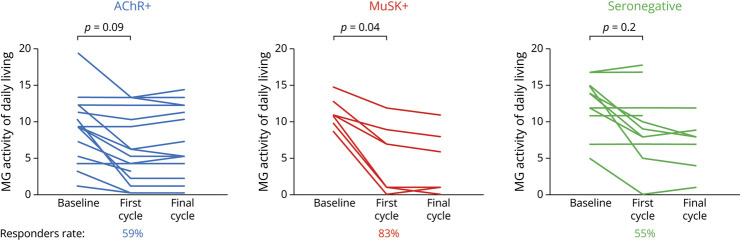
In the first cycle, the MG-ADL scores of the 34 patients decreased from 10.5 ± 4.3 to 6.9 ± 5.1 points (p = 0.003). Figure 3 illustrates the changes in the patients' MG-ADL scores after the first cycle of efgartigimod stratified by autoantibody status. The significant improvement of MG-ADL scores was observed in the MuSK+ patients but not in the AChR+ or seronegative patients. This tendency was observed at the final cycle during the 26-week observational period.
Figure 3. Therapeutic Responses to Efgartigimod.
Changes in the patients' myasthenia gravis activities of daily living. AChR+ = anti–acetylcholine receptor–positive; MuSK+ = anti–muscle-specific receptor tyrosine kinase-positive.
Of the 34 patients, 21 (62%) achieved a ≥2-point improvement on the MG-ADL and on the MG composite and MG-QOL15-r scales. Thus, there were 21 responders and 13 nonresponders. The responder rate was higher in the MuSK+ patients at 83% compared with 59% in the AChR+ patients and 55% in the seronegative patients. Notably, 4 patients (1AChR+ patient [#17] and 3 MuSK+ patients [#18, #20, and #23]) achieved a ≥9-point improvement on the MG-ADL scale. Minimal symptom expression defined by an MG-ADL score 0 or 1 was observed in 7 patients. The mean MG composite and mean MG-QOL15-r score of the 34 patients decreased from 18.4 ± 13.6 to 11.8 ± 9.6 (p = 0.004) and from 19.2 ± 6.3 to 14.2 ± 8.3 (p = 0.007), respectively. These findings supported the discrimination between responders and nonresponders evaluated by the MG-ADL.
Clinical improvement was usually observed soon after the first or second infusion in the first cycle. Among the 21 responders, 16 underwent additional cycles of efgartigimod. The effectiveness was usually repeatedly observed in the subsequent cycles. However, 2 patients (1AChR+ patient [#3] and 1 MuSK+ patient [#19]) had not achieved any improvement at the third cycle. The duration of effectiveness of efgartigimod varied among the responders. The efficacy of efgartigimod was long-lasting in 4 responders (3 AChR+ patients [#8, #10, and #12] and 1 seronegative patient [#31]), resulting in the use of only a single cycle during the 26-week observation. By contrast, 1 seronegative responder (#27) hoped to change to the previous IVIg therapy after she underwent a first cycle because she complained of a sudden cessation of efficacy of efgartigimod.
Among the 13 nonresponders, 8 patients began an additional cycle of efgartigimod. During the subsequent cycles, these patients again failed to achieve an MG-ADL score of ≥2 points; however, they noted an improvement of limited muscle weakness and a reduction of fatigue, which were not evaluated by the MG-ADL scale. Among 5 of the nonresponders who underwent a single cycle, 3 patients (1with AChR+ [#11] and 2 seronegative patients [#24 and #26]) required fast-acting treatment during the observation period.
The maximal reduction of IgG (65%) was observed 1 week after the final infusion in the first cycle. The IgG reduction rate was similar among the AChR+, MuSK+, and seronegative groups. Again, we failed to detect the differences in IgG reduction rate between responders and nonresponders (64% vs 65%, p = 0.8). The maximal reduction of pathogenetic autoantibodies was 45%. These rates were also similar between the anti-AChR and anti-MuSK antibodies.
Regarding concomitant treatment, oral prednisolone was used by 30 patients at the baseline, at the daily dose of 12.6 ± 1.5 mg. At the end of the observation period, a reduction of the prednisolone dose was documented in 7 patients (from 13.4 to 9.5 mg/d in average). Calcineurin inhibitors were administered in all but 2 patients (due to drug-induced liver dysfunction). These drugs (tacrolimus in 27 patients and cyclosporine in 5 patients) were not changed.
During the 6 months before the introduction of efgartigimod, 27 patients had received fast-acting treatments. Although 15 patients were free from additional fast-acting treatments, 12 other patients underwent a fast-acting treatment (including 4 patients who did so after the discontinuation of efgartigimod). The indication of additional IVMP depended on individual investigators. IVMP was performed for 8 patients (7 responders and 1 nonresponder).
Shifts from other molecular targeting drugs were performed: eculizumab (n = 4) and rituximab (n = 2) to efgartigimod. Among the patients who changed from eculizumab to efgartigimod, 1 AChR+ patient (#12) showed a remarkable effect of efgartigimod, although the patient had received only 2 infusions. The other 2 AChR+ patients (#9 and #14) were nonresponders. The combination of efgartigimod and eculizumab was effective in 1 AChR+ patient (#4), and she had had the most intractable MG. Regarding the shift from rituximab to efgartigimod, 1 MuSK+ patient (#23) was a responder, but the other seronegative patient (#34) was not a responder.
We compared the patients' postintervention status between the baseline and 1 week after the final cycle (Figure 4). No patients achieved minimal manifestations at the baseline. The efgartigimod treatment resulted in favorable outcomes; it should be emphasized that 6 patients achieved minimal manifestations (2 AChR+ patients, 3 MuSK+ patients, and 1 seronegative patient). Of these 6 patients, the daily dose of prednisone was ≤5 mg in 3 patients.
Figure 4. Outcome of Patients.
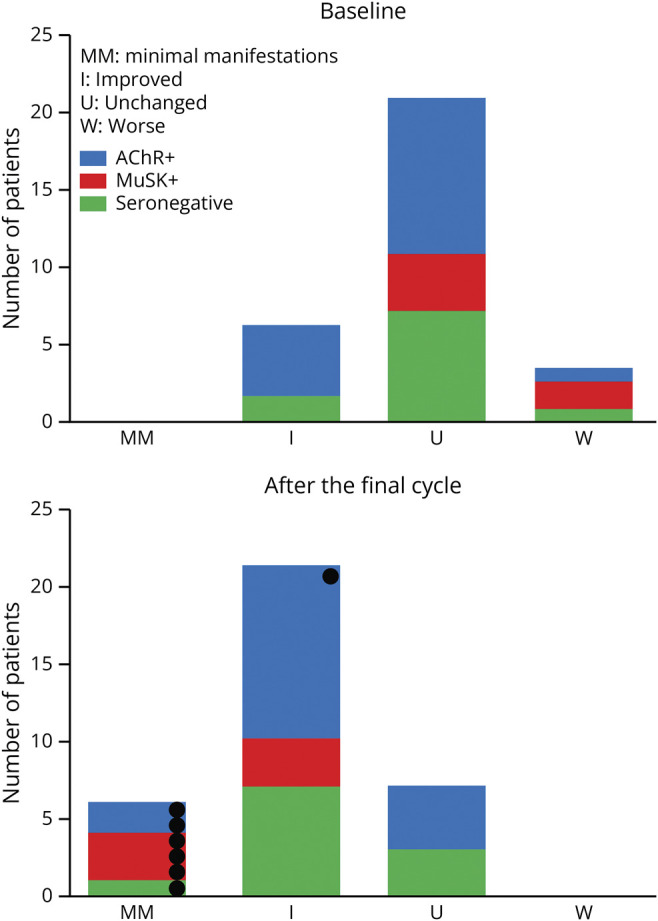
Changes in the patients' postintervention status. Black circles indicate patients with minimal symptom expression. AChR+ = anti–acetylcholine receptor–positive; MuSK+ = anti–muscle-specific receptor tyrosine kinase–positive.
There were 13 adverse events in 11 patients. COVID-19 occurred in 5 patients both during an efgartigimod cycle (n = 2) or interval (n = 3). The exacerbation of MG due to COVID-19 was not so serious that additional treatment for MG was necessary. Other adverse events included headache (n = 3), urinary tract infection (n = 1), diarrhea (n = 2), nausea (n = 1), and fatigue (n = 1). Headaches appeared at the first cycle and were generally mild. Patients who experienced these adverse events were able to continue the efgartigimod treatment. Of interest, 1 MuSK+ patient (#21) noticed fatigue that resembled the fatigue experienced due to the patient's IVMP treatment and differed from myasthenic fatigue.
We investigated the potential factors involved in the efgartigimod-responder status (eTable 2), and we found no associations with various clinical factors including age, gender, disease duration, MG severity, autoantibody status, complete cycle of efgartigimod, IgG deduction, and adverse events.
Discussion
The present patients with generalized MG had the average MG-ADL score of 10.5 ± 4.3 points, which is comparable with the ADAPT efgartigimod group's score at 9.2 ± 2.6 points. Our patients' MG composite and MG-QOL15-r scores were also similar to those in the ADAPT trial. However, we suspect that the present patients had more severe MG and more frequently refractory MG compared with the ADAPT patients. Most of our patients received fast-acting treatment or molecular targeted drugs, which did not meet the inclusion criteria of the ADAPT trial.
Recent attention has focused on the management of patients with refractory MG experiencing an unsatisfactory QOL and social disadvantages.4,5 The unfavorable condition with side effects that can be induced by treatment with corticosteroids and other immunosuppressive agents requires a new therapeutic strategy. The ratio of refractory MG in this study was 72% (n = 26/36), much higher than that 24% (n = 328/1,343) in the 2021 survey from the JAMG-R study group (n = 328/1,343).6
Treatment cycles in the ADAPT trial were based on the duration of the individual patient's clinical effect.9 In clinical settings, therapeutic schedules have been flexibly adjusted by both physicians and patients. We observed that 6 of the patients in this study received 4 or 5 cycles of efgartigimod, whereas in the ADAPT trial, the number of cycles was limited to 3. The interval period between cycles was 5.9 weeks in this study that was equal to 5.8 weeks in the ADAPT extension study.15 The continuous 4-weekly infusion of efgartigimod was not performed in 20% of the present patients' cycles, for several reasons; our early vigilance regarding an IgG decrease caused incomplete cycles or delayed the initiation of the subsequent cycle. In clinical settings, we speculate that an incomplete performance of the planned infusion may be allowable. Subcutaneous intravenous formulations of efgartigimod will provide convenience and be useful for maintaining a regular schedule.
Although patients with refractory MG were preferentially enrolled in this study, the responder rate in the first cycle was 62%. The ADAPT group reported a 68% responder rate, with a broad range of improvement. Among the 21 responders in these analyses, 6 patients had a narrow response, presenting a 2-point or 3-point reduction in the MG-ADL score, and 4 patients showed a marked response presenting a ≥9-point reduction in the MG-ADL score. Some patients complained that the duration of the efficacy of efgartigimod was shorter than expected because it was reported that clinical improvement lasting ≥6 weeks was usually achieved.8 The ADAPT report indicated that 17 of 84 patients in the efgartigimod group received only 1 0treatment cycle. It was reported that the clinical benefit of efgartigimod was correlated with the patients' IgG reduction initially but persisted even after the IgG levels returned to baseline values.8,9 Taken together, the past and present findings indicate that a sustainable efficacy of efgartigimod can be expected in some patients.
Reproducible efficacy was recognized after the subsequent cycles in both the ADAPT trial and this study. By contrast, 8 of the present nonresponders entering subsequent cycles failed to gain a ≥2-point improvement in their MG-ADL score. The efficacy of efgartigimod may be predictable after the first cycle. Among 21 patients in the ADAPT trial who were nonresponders during the first cycle, 19 were retreated and 7 of them were responders in the second cycle.9 We think that it is worth repeating at least 1 or 2 additional cycles of efgartigimod in first-cycle nonresponders.
Patients with AChR+ MG accounted for 77% of the ADAPT participants, indicating similar rates of responders between AChR+ and non-AChR patients. Our cohort's autoantibody profiles showed higher prevalences of MuSK+ and seronegative patients compared with the ADAPT patients. Our results suggest that favorable responses to efgartigimod can be expected, especially in MuSK+ patients. Seronegative MG tends to present refractory courses in younger patients, reflecting unmet medical needs.16,17 We speculate that efgartigimod is also potentially effective for patients with refractory seronegative MG.
Based on our observations, it is likely that the IgG decrease among this cohort was temporary and produced no serious problems. We recommend measuring patients' IgG at baseline and at 1 week after the first cycle because IgG monitoring is useful for both drug safety and the prediction of efgartigimod efficacy. There is no reported evidence regarding the threshold of IgG levels that is associated with infections. We estimate that close attention may be necessary only when IgG levels decrease to <100 mg/dL (excluding the specific condition of immunodeficiency).18
In the ADAPT trial, 19% of the patients in the efgartigimod group had never been treated with steroid or nonsteroidal immunosuppressant therapy. In Japan, efgartigimod should be administered in patients with generalized MG who have received the combination of low-dose prednisolone and calcineurin inhibitors. Although the steroid dose was fixed in the ADAPT trial,9 we observed a reduction of the prednisolone dose in 7 of the present patients. By contrast, additional IVMP may be beneficial for generalized MG in combination with efgartigimod.17
Our analyses revealed that efgartigimod was generally tolerable, but 5 patients contracted COVID-19 during their efgartigimod treatment. We speculate that the association between the IgG decrease and COVID-19 is not strong. Frequent visits to a hospital and/or increased ADLs may be related to COVID-19. The observation period of the study also included the seventh and eighth peaks of the COVID-19 pandemic in Japan.
In this study, we report real-world data of efgartigimod treatment for myasthenia gravis including postintervention status. A current goal of MG treatment is to achieve minimal manifestations, which is associated with maintaining a favorable QOL. We emphasize that 6 of the present patients achieved minimal manifestations, although they had frequently required a fast-acting treatment. More patients may achieve minimal manifestations during a longer follow-up.19
There are several study limitations to consider. First, we included 36 patients with MG among 1,343 patients who were registered in the 2021 survey of JAMG-R. Recruiting patients with MG in the JAMG-R was not performed only for this study.6 There was 1-year interval between the initial registration and the start of efgartigimod treatment. Second, we did not evaluate the costs of efgartigimod compared with treatments such as plasmapheresis or IVIG. An incremental cost-effectiveness ratio and the suitable choice of molecular targeting drugs for generalized MG should be examined in further studies. Third, we also failed to detect clinical or laboratory factors associated with responders. Clinical studies with more patients to find biomarkers that can predict treatment responses including the duration of effectiveness are necessary. In conclusion, efgartigimod can be considered as a treatment choice for patients with generalized MG who do not achieve minimal manifestations, providing a broad flexibility of patient selection and treatment schedules.
Appendix. Authors
| Name | Location | Contribution |
| Shigeaki Suzuki, MD, PhD | Department of Neurology, Keio University School of Medicine, Tokyo, Japan | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Akiyuki Uzawa, MD, PhD | Department of Neurology, Graduate School of Medicine, Chiba University, Japan | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Yuriko Nagane, MD, PhD | Department of Neurology, Hanamaki General Hospital, Japan | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Masayuki Masuda, MD, PhD | Department of Neurology, Tokyo Medical University, Japan | Major role in the acquisition of data |
| Shingo Konno, MD, PhD | Department of Neurology, Toho University Ohashi Medical Center, Tokyo, Japan | Major role in the acquisition of data |
| Tomoya Kubota, MD, PhD | Division of Health Sciences, Department of Clinical Laboratory and Biomedical Sciences, Osaka University Graduate School of Medicine, Japan | Major role in the acquisition of data |
| Makoto Samukawa, MD, PhD | Department of Neurology, Kindai University Faculty of Medicine, Sayama, Japan | Major role in the acquisition of data |
| Kei Ishizuchi, MD, PhD | Department of Neurology, Keio University School of Medicine, Tokyo, Japan | Major role in the acquisition of data |
| Daiki Tokuyasu, MD | Department of Neurology, Keio University School of Medicine, Tokyo, Japan | Major role in the acquisition of data |
| Hideo Handa, MD | Department of Neurology, Graduate School of Medicine, Chiba University, Japan | Major role in the acquisition of data |
| Manato Yasuda, MD, PhD | Department of Neurology, Graduate School of Medicine, Chiba University, Japan | Major role in the acquisition of data |
| Naoki Kawaguchi, MD, PhD | Department of Neurology, Neurology Chiba Clinic, Japan; | Major role in the acquisition of data |
| Takashi Kimura, MD, PhD | Department of Neurology, Hyogo Medical University, Nishinomiya, Japan | Major role in the acquisition of data |
| Yasushi Suzuki, MD, PhD | Department of Neurology, National Hospital Organization Sendai Medical Center, Sendai, Japan | Major role in the acquisition of data |
| Takamichi Sugimoto, MD, PhD | Department of Clinical Neuroscience and Therapeutics, Hiroshima University, Japan | Major role in the acquisition of data |
| Naoya Minami, MD, PhD | Department of Neurology, National Hospital Organization Hokkaido Medical Center, Sapporo, Japan | Major role in the acquisition of data |
| Masanori P. Takahashi, MD, PhD | Division of Health Sciences, Department of Clinical Laboratory and Biomedical Sciences, Osaka University Graduate School of Medicine, Japan | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Hiroyuki Murai, MD, PhD | Department of Neurology, International University of Health and Welfare, Narita, Japan | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Kimiaki Utsugisawa, MD, PhD | Department of Neurology, Hanamaki General Hospital, Japan | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
Study Funding
This work was supported by a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI, no. JP20H03592.
Disclosure
S. Suzuki has received personal fees from Alexion Pharmaceuticals, Argenx, UCB Pharma, the Japan Blood Products Organization, and Asahi Kasei Medical. A. Uzawa has received honoraria from Alexion Pharmaceuticals and Argenx. Y. Nagane has received speaker honoraria from Argenx, Alexion Pharmaceuticals, and the Japan Blood Products Organization. M. Masuda and S. Konno declare no potential conflicts of interest related to this article. T. Kubota has received honoraria for lectures from Alexon Pharmaceuticals, Argenx, and UCB Pharma. M. Samukawa, K. Ishizuchi, D. Tokuyasu, H. Handa, M. Yasuda, N. Kawaguchi, T. Kimura, Y. Suzuki, T. Sugimoto, and N. Minami declare no potential conflicts of interest related to this article. M.P. Takahashi reports unrestricted research grants from Japan Blood Products Organization, Astellas Pharma, Mitsubishi Tanabe Pharma, and Pfizer outside the submitted work and honoraria for lectures from Argenx, Alexion Pharmaceuticals, and UCB Pharma. H. Murai has served as a paid consultant for Alexion, AstraZeneca Rare Disease, Argenx, and UCB and has received speaker honoraria from the Japan Blood Products Organization and Chugai Pharmaceutical and research support from the Ministry of Health, Labour and Welfare, Japan. K. Utsugisawa has served as a paid consultant for UCB Pharma, Argenx, Janssen Pharma, Viela Bio, Chugai Pharma, Hanall BioPharma, and Mitsubishi Tanabe Pharma and has received speaker honoraria from Argenx, Alexion Pharmaceuticals, and the Japan Blood Products Organization. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Punga AR, Maddison P, Heckmann JM, Guptill JT, Evoli A. Epidemiology, diagnostics, and biomarkers of autoimmune neuromuscular junction disorders. Lancet Neurol. 2022;21(2):176-188. doi: 10.1016/S1474-4422(21)00297-0 [DOI] [PubMed] [Google Scholar]
- 2.Narayanaswami P, Sanders DB, Wolfe G, et al. International consensus guidance for management of myasthenia gravis: 2020 update. Neurology. 2021;96(3):114-122. doi: 10.1212/WNL.0000000000011124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vanoli F, Mantegazza R. Antibody therapies in autoimmune neuromuscular junction disorders: approach to myasthenic crisis and chronic management. Neurotherapeutics. 2022;19(3):897-910. doi: 10.1007/s13311-022-01181-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider-Gold C, Hagenacker T, Melzer N, Ruck T. Understanding the burden of refractory myasthenia gravis. Ther Adv Neurol Disord. 2019;12:1756286419832242. doi: 10.1177/1756286419832242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran C, Biswas A, Mendoza M, Katzberg H, Bril V, Barnett C. Performance of different criteria for refractory myasthenia gravis. Eur J Neurol. 2021;28(4):1375-1384. doi: 10.1111/ene.14675 [DOI] [PubMed] [Google Scholar]
- 6.Suzuki S, Masuda M, Uzawa A, et al. Japan MG Registry: chronological surveys over 10 years. Clin Exp Neuroimmunol. 2023;14(1):5-12. doi: 10.1111/cen3.12731 [DOI] [Google Scholar]
- 7.Ulrichts P, Guglietta A, Dreier T, et al. Neonatal Fc receptor antagonist efgartigimod safely and sustainably reduces IgGs in humans. J Clin Invest. 2018;128(10):4372-4386. doi: 10.1172/JCI97911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard JF Jr., Bril V, Burns TM, et al. Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92(23):e2661-e2673. doi: 10.1212/WNL.0000000000007600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard JF Jr., Bril V, Vu T, et al. Safety, efficacy, and tolerability of efgartigimod in patients with generalised myasthenia gravis (ADAPT): a multicentre, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2021;20(7):526-536. doi: 10.1016/S1474-4422(21)00159-9 [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Uzawa A, Murai H. Efgartigimod for generalized myasthenia gravis with or without anti-acetylcholine receptor antibodies: a worldwide and Japanese perspective. Expert Rev Clin Immunol. 2022;18(12):1207-1215. doi: 10.1080/1744666X.2022.2136167 [DOI] [PubMed] [Google Scholar]
- 11.Jaretzki A 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task Force of the Medical Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55(1):16-23. doi: 10.1212/wnl.55.1.16 [DOI] [PubMed] [Google Scholar]
- 12.Wolfe GI, Herbelin L, Nations SP, Foster B, Bryan WW, Barohn RJ. Myasthenia gravis activities of daily living profile. Neurology. 1999;52(7):1487-1489. doi: 10.1212/wnl.52.7.1487 [DOI] [PubMed] [Google Scholar]
- 13.Burns TM, Conaway M, Sanders DB, MG Composite and MG-QOL15 Study Group. The MG composite: a valid and reliable outcome measure for myasthenia gravis. Neurology. 2010;74(18):1434-1440. doi: 10.1212/WNL.0b013e3181dc1b1e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns TM, Sadjadi R, Utsugisawa K, et al. International clinimetric evaluation of the MG-QOL15, resulting in slight revision and subsequent validation of the MG-QOL15r. Muscle Nerve. 2016;54(6):1015-1022. doi: 10.1002/mus.25198 [DOI] [PubMed] [Google Scholar]
- 15.Howard JF Jr, Bril V, Vu T, et al. Long-term safety and efficacy of efgartigimod in patients with generalized myasthenia gravis: Interim results of the ADAPT+ study. Neurology. 2022;99(23_supplment_2):S37-S38. doi: 10.1212/01.wnl.0000903308.81107.e2 [DOI] [Google Scholar]
- 16.Tomschik M, Hilger E, Rath J, et al. Subgroup stratification and outcome in recently diagnosed generalized myasthenia gravis. Neurology. 2020;95(10):e1426-e1436. doi: 10.1212/WNL.0000000000010209 [DOI] [PubMed] [Google Scholar]
- 17.Uzawa A, Suzuki S, Kuwabara S, et al. Effectiveness of early cycles of fast-acting treatment in generalised myasthenia gravis. J Neurol Neurosurg Psychiatry. 2023;94(6):467-473. doi: 10.1136/jnnp-2022-330519 [DOI] [PubMed] [Google Scholar]
- 18.Ishizuchi K, Takizawa T, Ohnuki Y, et al. Immunodeficiency in patients with thymoma-associated myasthenia gravis. J Neuroimmunol. 2022;371:577950. doi: 10.1016/j.jneuroim.2022.577950 [DOI] [PubMed] [Google Scholar]
- 19.Mantegazza R, Wolfe GI, Muppidi S, et al. Post-intervention status in patients with refractory myasthenia gravis treated with eculizumab during REGAIN and its open-label extension. Neurology. 2021;96(4):e610-e618. doi: 10.1212/WNL.0000000000011207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.