Abstract
The role of human immunodeficiency virus (HIV) strain variability remains a key unanswered question in HIV dementia, a condition affecting around 20% of infected individuals. Several groups have shown that viruses within the central nervous system (CNS) of infected patients constitute an independently evolving subset of HIV strains. A potential explanation for the replication and sequestration of viruses within the CNS is the preferential use of certain chemokine receptors present in microglia. To determine the role of specific chemokine coreceptors in infection of adult microglial cells, we obtained a small panel of HIV type 1 brain isolates, as well as other HIV strains that replicate well in cultured microglial cells. These viruses and molecular clones of their envelopes were used in infections, in cell-to-cell fusion assays, and in the construction of pseudotypes. The results demonstrate the predominant use of CCR5, at least among the major coreceptors, with minor use of CCR3 and CXCR4 by some of the isolates or their envelope clones.
Human immunodeficiency virus (HIV) dementia (HIVD), also called the AIDS dementia complex, is a primary disorder of the central nervous system (CNS) that affects about 20% of HIV-infected individuals (32). Although the pathogenesis of HIVD has been the subject of many studies, there is no consensus on the cause of brain dysfunction. Neuropathological and virological studies have implicated brain microglial cells and microglia-derived giant cells as the principal CNS cells infected with HIV (3, 35, 39), leading most investigators to believe that microglial cells are the central element in the development of this complication. Microglial cells may produce viral proteins that have neurotoxic properties, or perhaps HIV-infected cells are induced to secrete soluble neurotoxins like platelet-activating factor, tumor necrosis factor alpha, or nitric oxide (15, 22, 23, 30).
A key unanswered question in the development of HIVD is the role of viral strain variability. While only a proportion of infected individuals demonstrate CNS dysfunction, the relative contributions of viral and host factors are unknown. Data from a number of groups have indicated that HIV penetrates the CNS early during the course of systemic HIV infection (5, 8). For example, HIV can be isolated from the cerebrospinal fluid of 50% of individuals early in the course of infection, and HIV sequences obtained from the CNS segregate independently of sequences prevalent in the systemic circulation (11, 17, 26, 40). One potential explanation for the isolated evolution of HIV strains within the CNS is that replication within microglia results in adaptation of the virus for this cell type (36).
Chemokine receptors, which were recently found to have an essential role in mediating HIV entry in conjunction with CD4, are responsible for some of the key differences in HIV cellular tropism (6, 9, 13, 14, 19). Isolates using CXCR4 as a coreceptor replicate in peripheral blood lymphocytes and in some immortalized T-cell lines, whereas HIV type 1 (HIV-1) isolates that replicate in monocyte-derived macrophages (MDM) utilize CCR5, the second major HIV coreceptor discovered. Several other chemokine receptors, like CCR3, and orphan receptors such as STRL33 and GPR15 have been shown to have coreceptor activity with different assays (10, 18, 29). The potential role of these and other chemokine receptors in HIV pathogenesis is yet to be explored.
Microglial cells express several chemokine receptors, including CCR3, CCR5, and CXCR4 (25, 27). Therefore, viruses could theoretically use any of these coreceptors to enter microglial cells. In fact, clearly T-cell line-tropic viruses like HIV-1HXB will replicate in some microglial preparations (36). However, for the most part, viruses that replicate in microglial cells also replicate in MDM (36, 37), and they would be expected to at the very least use CCR5 as a coreceptor. However, He and collaborators (25) have recently suggested that utilization of CCR3 as a coreceptor is an important correlate of replication in fetal microglial cells, since some viruses obtained from the brain (JRFL and YU-2) (28) were capable of utilizing CCR3 in a fusion assay, and antibodies against CCR3 inhibited infection of fetal microglial cells.
To determine the role of chemokine coreceptors in infection of adult microglial cells, we have obtained a small panel of brain isolates (20, 21), as well as other HIV strains that replicate well in cultured microglial cells. These viruses, as well as molecular clones of their envelope (env) genes, have been used in infection and cell-to-cell fusion assays to determine their chemokine coreceptor utilization. The results demonstrate the predominant use of CCR5, at least among the major coreceptors so far identified, but with some use of CCR3 and CXCR4 by some envelope clones.
MATERIALS AND METHODS
Cells.
Microglial cultures were prepared as previously described (1, 36, 41) from fresh adult human brain tissue obtained from donors undergoing temporal lobectomy for medication-resistant epilepsy. CD4-expressing human osteosarcoma (HOS-CD4) cells engineered to express chemokine receptors were obtained from the AIDS Reference Reagent Program. 293T and U87 cells were obtained from M. Malim (Department of Microbiology, University of Pennsylvania) and B. Doranz (Department of Pathology and Laboratory Medicine, University of Pennsylvania), respectively. The microglial cells were maintained in M/M medium (Dulbecco’s modified Eagle’s medium [DMEM] supplemented with 5% fetal bovine serum, 5% giant cell tumor supernatant [IGEN Inc., Rockville, Md.]) as described previously (36); 293T and U87 cells were maintained in DMEM supplemented with 10% fetal bovine serum with penicillin and streptomycin (each at 100 U/ml).
HIV isolates.
The isolate HIV-1BORI, which was obtained from an individual with a primary HIV-1 infection, was provided by G. Shaw (University of Alabama, Birmingham). HIV-1BORI-15 was selected by 15 sequential passages of the parental BORI isolate in microglial cells (36). The primary brain HIV-1 strains DS-br, RC-br, and KJ-br were isolated by S. Gartner (20, 21) in MDM. Virus stocks of these isolates were prepared in macrophages. Molecular HIV clone YU-2(RF-1), originally cloned by Li et al. (28), was obtained from R. Fouchier (University of Pennsylvania). HIV-1Ada-M was obtained from the AIDS repository. HIV-189.6 was obtained from R. Collman (University of Pennsylvania) (7).
Microglial infection.
Microglial cells were plated in 24-well plates at a density of 2 × 105 cells/well and infected with cell-free virus stocks standardized by p24gag antigen content (34 ng/ml). The cultures were exposed to the virus inocula for 16 h at 37°C; the inocula were then removed and replaced by fresh medium. The culture supernatants were removed at regular intervals, and fresh medium was added. The supernatants were stored at −80°C until the p24gag antigen concentration was measured with an antigen capture assay (NEN DuPont, Boston, Mass.).
Infection of cells expressing chemokine coreceptors.
293T cells expressing chemokine receptors were prepared by transiently transfecting 3 × 105 cells/well in a six-well plate with 2 μg each of pT4 (31), which expresses the CD4 molecule, and plasmids expressing CCR3 (34), CCR5 (9), or CXCR4 (19), using calcium phosphate. The following day, transfected cells were replated in 96-well plates and infected with cell-free virus stocks (73 ng of p24gag viral antigen per ml). Cultures were exposed to inocula for 5 h at 37°C. Culture supernatants were removed on days 3, 6, and 8 after infection, and p24gag antigen concentrations were measured (NEN DuPont).
Approximately 2 × 104 HOS-CD4 cells expressing chemokine coreceptors were plated in each well of a 24-well plate, incubated overnight with 10 ng of p24gag of the appropriate HIV-1 isolate, washed extensively with DMEM, and maintained in DMEM supplemented with 10% fetal calf serum and 1 μg of puromycin per ml. Supernatants from the infected cells were removed every 2 to 3 days to monitor p24gag antigen concentration.
PCR amplification of HIV env genes.
The env genes from isolates HIV-1BORI-15, HIV-1RC-br, HIV-1DS-br, and HIV-1KJ-br were molecularly cloned from the genomic DNA extracted from cultured human microglia 3 days after infection. HIV-1BORI was cloned from DNA extracted from infected peripheral blood mononuclear cells. Plasmid YU-2(RF-1) was used to clone the YU-2 envelope. The pCR3.1-JRFL env expression vector was provided by B. Doranz (12), and the BaL env expression plasmid was obtained from J. Moore (Aaron Diamond AIDS Research Center, New York, N.Y.).
The env genes were amplified by PCR using AmpliWax beads (Perkin-Elmer, Norwalk, Conn.) in a DNA Thermal Cycler (Perkin-Elmer). The reaction mixtures contained a total volume of 50 μl and were composed of 1× PCR buffer II (Perkin-Elmer), 2.5 mM MgCl2, 0.4 mM deoxynucleoside triphosphates, 1 μM each env 1 primer (5′ AGAAAGAGCAGAAGACAGTGGCAATGA 3′) and env 2 primer (5′ TTTTGACCACTTGCCACCCAT 3′), and 2.5 U of Amplitaq DNA polymerase (Perkin-Elmer). The reaction conditions were a denaturing step at 93°C for 1 min, 30 cycles with a denaturing step of 93°C for 30 s, an annealing step of 62°C for 30 s, an extension step of 72°C for 4 min, and a final extension step of 72°C for 10 min.
The PCR products were visualized by agarose gel electrophoresis, and bands of the appropriate size were purified by using Geneclean (Bio 101, La Jolla, Calif.). env PCR products from HIV-1BORI and HIV-1BORI-15 were ligated by T/A cloning into pCR2.1 (Invitrogen, San Diego, Calif.), and those clones in the proper orientation were directionally subcloned into pcDNA3.1(−) (Invitrogen) for use in the fusion assays and for the preparation of pseudotypes. env PCR products from the brain isolates were ligated by unidirectional T/A cloning directly into pCR3.1-Uni (Invitrogen). Individual recombinants with full-length inserts were then selected for use in fusion and pseudotype assays, based on restriction mapping.
The molecular clones were sequenced in the C2-V4 regions by using a single primer, 5′-ACAGTACAATGTACACATGG-3′. The sequences were analyzed by using CLUSTAL to ensure that each set of clones were derived from a distinct parent virus.
Western analysis.
293T cells (106) were infected with vaccinia virus encoding T7 RNA polymerase (vTF1.1; a gift from B. Moss, National Institute of Allergy and Infectious Diseases [NIAID]) to drive env expression and then transfected with envelope clones and pNL4-3-LucR+E− (a gift from N. Landau, Aaron Diamond AIDS Research Center) as described below. Twenty-four hours later, the cells were lysed in 1 ml of lysis buffer (10 mM Tris [pH 7.5], 0.15 M NaCl, 2 mM EDTA, 0.5% Nonidet P-40, aprotinin [2 μg/ml], phenylmethylsulfonyl fluoride [50 μg/ml]), and 30 μl was chromatographed in a sodium dodecyl sulfate–7.5% polyacrylamide gel. The proteins were transferred to a polyvinylidene difluoride membrane by using a semidry apparatus, then probed with a rabbit polyclonal anti-gp120 serum (R2143; a gift of P. Earl, NIAID) followed by horseradish peroxidase-conjugated goat anti-rabbit serum (Boehringer Mannheim), and visualized by chemiluminescence (Amersham).
Fusion assays.
env clones were tested in a cell-cell fusion assay (13, 33, 34) for the ability to mediate fusion with various coreceptors. env-expressing effector cells were mixed with target cells expressing CD4 and coreceptor. Effector cells also contained vaccinia virus encoding T7 RNA polymerase (vTF1.1) which drove luciferase production upon fusion with the target cells. Effector cells were prepared by infecting 293T cells with vTF1.1 at a multiplicity of infection of 10 for 45 min and then transfecting these cells with constructs expressing env, using calcium phosphate for 4 h. Effector cells were incubated overnight in rifampin to inhibit vaccinia virus replication. Target cells were prepared by transfecting QT6 cells with equal amounts of CD4 and coreceptor plasmids (9, 19, 31, 34) for 4 h at 37°C and incubated overnight. To assay for fusion, effector cells were washed and mixed with target cells in the presence of rifampin and cytosine arabinoside. Fusion proceeded for 9 h at 37°C, and cells were lysed with 0.5% Nonidet P-40. Luciferase activity was measured by adding 50 μl of lysate to 50 μl of luciferase substrate (Promega) and determining chemiluminescence in a luminometer (13).
Production of viral pseudotypes.
Pseudotypes were prepared essentially as described by Deng et al. (9). In brief, 293T cells were cotransfected by using calcium phosphate (5 Prime-3 Prime, Boulder, Colo.) with equivalent amounts of pNL4-3-LucR−E− or pNL4-3-LucR+E− (gifts from N. Landau) together with plasmids expressing the appropriate env gene. Cells (3 × 105/well) were transfected in six-well plates with 3 μg of each plasmid; alternatively, 106 cells were transfected in 10-cm dishes with 15 μg of each plasmid. Supernatants containing the pseudotyped viruses were collected 2 to 3 days later, either centrifuged (10 min at 1,750 × g on a Beckman centrifuge) or filtered through a 0.45-μm-pore-size filter to remove cellular debris, and then stored at −80°C.
Infection of U87 cells with HIV pseudotypes.
U87 cells (105/well) were plated and grown overnight in a 24-well plate. The following day they were transfected with 2 μg each of pT4, which expresses the CD4 molecule (31), and plasmids expressing the desired chemokine receptor. After 24 h, the transfected cells were infected with 200 μl of pseudotyped virus in the presence of Polybrene (8 μg/ml), and 500 μl of medium was added 16 to 24 h later. On the third day after the pseudotype infection, the cells were lysed with 150 μl of buffer as instructed for the Promega luciferase assay system. Luciferase activity was measured by adding 50 μl of luciferase substrate to 20 μl of lysate in 75- by 12-mm tubes (Sarstedt) and reading light activity in a Lumat LB 9501 luminometer (Berthold). In all experiments, light activity is reported as relative light units per 10 s.
Infection of microglia with pseudotyped virus.
Pseudotypes expressing the HIV-1BaL envelope were prepared as indicated above. Microglial cells were cultured in 96-well plates for 18 days and then pretreated with anti-CCR5 antibody 45531.111 (R&D Systems, Minneapolis, Minn.), anti-CCR3 antibody 7B11 (AIDS repository), or anti-CXCR-4 antibody 12G5 (17) (obtained from J. Hoxie) at 20 μg/ml for 45 min at 4°C. The cells were then infected with 200 μl of pseudotyped virus for 16 h at 37°C, the medium was replaced, and the cells were lysed 3 days later with 100 μl of lysis buffer; then 40 μl was combined with 100 μl of luciferase substrate, and chemiluminescence read as indicated above.
RESULTS
Infections of microglial cultures with brain isolates.
Several publications have documented the infectability of cultured adult microglia with either primary or laboratory HIV-1 strains (36, 37). Since microglia are the principal cell type infected in the brains of individuals with HIVD, we expected that most or all isolates obtained from the brain would infect microglial cultures. Three HIV isolates cultured from the brain tissue of two adults (HIV-1RC-br and HIV-1DS-br) and one infant (HIV-1KJ-br), all of whom had died with HIVD or HIV encephalopathy, and a well-documented isolate from the frontal lobe (HIV-1JRFL) were used to infect microglial tissue as described in Materials and Methods. The p24gag concentrations in the supernatants on day 17 after infection (which is approximately the time of peak replication) are shown in Table 1. In addition, we used a control dualtropic virus, HIV-189.6. All of the viruses replicated in the microglial cultures, but infection with HIV-1KJ-br resulted in consistently lower p24gag concentrations in eight similar experiments using microglia obtained from different donors. In subsequent experiments, we also used two related isolates, HIV-1BORI, a virus isolated during an acute infection, and a derivative obtained by sequential passage in microglia (HIV-1BORI-15) that replicates to high titer and induces giant syncytia in microglia (36).
TABLE 1.
Replication of CNS HIV isolates in microglia
| Isolate | p24gag concn (ng/ml) day 17 postinfection |
|---|---|
| DS-br | 280 |
| JRFL | 114 |
| KJ-br | 26 |
| RC-br | 138 |
| 89.6 | 23 |
Cytopathicity in microglial cultures.
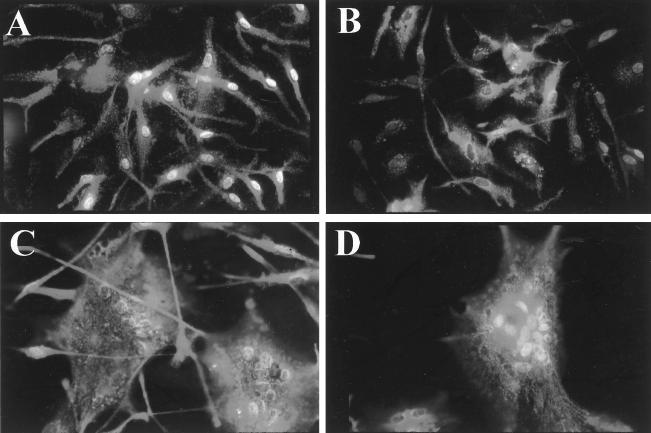
The most characteristic finding in HIV infection of the nervous system is the multinucleated giant cell, which is thought to represent the fusion of infected microglial cells. Multinucleated giant cell formation was also seen following in vitro infection of microglial cultures (36, 37). Since the chemokine coreceptors are involved in cell-to-cell fusion, we wanted to assess syncytium formation by the viruses used in these experiments (Fig. 1). Cultures infected with the brain isolates HIV-1DS-br and HIV-1RC-br (Fig. 1C and D) demonstrated marked cytopathology, while HIV-1KJ-br-infected (Fig. 1B) and mock-infected (Fig. 1A) microglial cultures had little or no syncytium formation. These findings were repeated in assays of microglial cultures from seven other donors. Similarly, infection with HIV-1BORI induced little or no cytopathology, whereas infection with the sequentially passaged isolate induced extensive fusion (36).
FIG. 1.
Multinucleated giant cell formation in uninfected microglial cells (A) and in microglial cultures infected with HIV-1KJ-br (B), HIV-1DS-br (C), or HIV-1RC-br (D). Microglial cultures were infected as described in Materials and Methods, and syncytium formation was monitored 12 days after infection. Syncytium formation was also quantified by counting the number of nuclei and the number of cells and was consistent with these photomicrographs, which are representative of seven experiments with each of the illustrated isolates.
Three types of experiments were then performed to determine the chemokine coreceptor utilization of those HIV isolates that replicated in the microglial cultures: (i) replication in 293T cells transfected with chemokine coreceptor molecules and CD4, (ii) cloning and expression of env genes, which were then tested in a cell-to-cell fusion assay, and (iii) preparation and assay of pseudotyped viruses using the same cloned envelopes.
Replication in 293T cells transfected with chemokine receptors.
We transfected 293T cells with CD4 and one of the major chemokine coreceptors (CCR3, CCR5, or CXCR4), and used these cells in infections as indicated in Materials and Methods. We first performed fusion experiments with CCR3 and did not see any signal above background with our env clones (see below). We then switched to CCR3P, which expresses CCR3 at up to 20-fold-higher levels than the original construct (34). CCR3P was then used for all subsequent experiments, and all results reported here are for that construct.
Infectivity was determined by assay for p24gag 6 days after infection, and the values are reported in Table 2. All of the viruses replicated in the 293T-CD4-CCR5 cells, but there was some variability in the use of the other coreceptors. For example, isolates HIV-1DS-br and HIV-1YU-2 replicated in cells expressing CCR3 (high-expressing construct CCR3P), whereas HIV-1DS-br, HIV-1KJ-br, and HIV-1BORI had p24 values above background in the cells expressing CXCR4.
TABLE 2.
Infection of 293T cells transfected with chemokine coreceptors
| Coreceptor | p24 concn (pg/ml) 6 days after infectiona
|
||||
|---|---|---|---|---|---|
| DS-br | KJ-br | BORI | BORI-16 | YU-2 | |
| None (CD4) | 420 | — | — | 150 | — |
| CCR3 | 1,300 | — | — | 140 | 2,400 |
| CCR5 | 5,900 | 330 | 1,400 | 420 | 19,400 |
| CXCR4 | >800 | 120 | 160 | 140 | — |
See text for details. —, ≤50 pg/ml.
In related experiments, we also used a panel of HOS engineered to express the HIV coreceptors (9). This allowed us to determine whether CCR1, CCR2b, or CCR4 could be used by any of these HIV isolates. None of the isolates infected HOS cells expressing CD4 in conjunction with these other coreceptors (data not shown). The isolates replicated in HOS-CCR5 cells but not in HOS-CCR3 cells. However, the level of expression of CCR3 in those cells is probably lower than in the 293T cells transfected with the CCR3P construct (34).
These results indicated that the HIV-1 strains that replicated well in the microglial cells could use CCR5 as a coreceptor. However, the use of other chemokine receptors remained in some question, since we did not determine whether all of the chemokine receptors were expressed at similar levels on the surface of the 293T cells. We therefore used other assays to address the same question.
Envelope-mediated cell-to-cell fusion.
The envelope genes from the panel of HIV strains that replicated in microglia were then molecularly cloned as described in Materials and Methods. Two to five functional clones were obtained for each brain isolate.
For the cell-to-cell fusion assay, the env gene was expressed in 293T cells (effectors); these were fused with QT6 cells (targets) expressing the CD4 molecule together with one of several coreceptor molecules. The QT6 cells were also transfected with a plasmid expressing the luciferase gene under the control of the T7 promoter, which was activated when cell fusion allowed the translocation of the T7 polymerase protein expressed in the effector cells. The extent of fusion was therefore quantified by chemoluminescence.
Data from representative experiments are shown in Fig. 2. Three envelope clones obtained from HIV-1BORI-15 and one env clone obtained from HIV-1BORI clone all showed strong fusion with CCR5-expressing targets, as well as with CCR3-expressing targets (Fig. 2A). Control envelopes from HIV-1JRFL and HIV-1YU-2 fused cells expressing either CCR5 or CCR3, as previously reported (6, 25). Expression of other coreceptors, including CCR1, CCR2, CCR4, and CXCR4, did not lead to fusion of HIV-1BORI or HIV-1BORI-15 env-expressing cells (data not shown). These results indicated that the pattern of coreceptor utilization had not changed after extensive passage in microglia, in spite of the accelerated replication and fusogenicity of the passaged virus.
FIG. 2.
Use of chemokine coreceptors in a cell-to-cell fusion assay. (A) The env genes from isolates HIV-1BORI and HIV-1BORI-15 were PCR amplified and cloned in an expression vector as described in the text. Fusion assays were then performed with QT6 cells transiently expressing several chemokine coreceptors as previously described (13). Note that the CCR3 construct (CCR3P) was designed to increase surface expression. In this assay, cell-to-cell fusion results in expression of the luciferase gene, which is monitored by chemiluminescence (relative light units). Three HIV-1BORI-15 envelope clones [BORI-15 (4C), BORI-15 (5C), and BORI-15 (7A)] and one HIV-1BORI envelope clone [BORI (11A)] were used. Envelopes from HIV-1JRFL and HIV-1YU-2, two viruses obtained directly from the brain, were used as controls for expression of CCR3. The envelope genes from brain isolates DS-br and RC-br were amplified and cloned as described in the text, and a fusion assay was performed as described previously (13). The envelope from the dualtropic isolate HIV-189.6 was used as a control for expression of CCR3. These experiments are representative of two to three assays performed with these envelopes.
Fusion assay with one env clone of each brain-derived virus, HIV-1DS-br and HIV-1RC-br, demonstrated that these env clones in particular utilized CCR5 in fusion but not CCR3 (Fig. 2B), whereas the control dualtropic virus HIV-189.6 could mediate fusion with both CCR3-expressing target cells and CCR5-expressing cells. These fusion data suggested that the envelopes from our brain-derived viruses and microglial cell-passaged virus could all utilize CCR5 but that the utilization of CCR3 was not universally present.
env-pseudotyped virus infection of U87-MG cells.
To test the coreceptor utilization by envelope proteins in the context of virions, we used a pseudotyped virus system described by Deng and collaborators (9). env-pseudotyped luciferase-expressing viruses were used to infect with U87 cells transiently transfected with CD4 and various chemokine coreceptors. Since in this system the nef gene has been replaced by the luciferase gene, viral entry was quantified by measuring chemiluminescence.
env clones from each of the viruses were individually tested in multiple experiments, and the geometric mean was obtained (Table 3).
TABLE 3.
Coreceptor use of env clones pseudotyped with luciferase-expressing virus
| Virus (clone) | Relative light units/10 sa
|
|||
|---|---|---|---|---|
| No coreceptor | CCR3 | CCR5 | CXCR4 | |
| DS (12b) | 215 | 249 | 2,333 | 565 |
| DS (C13) | 503 | 530 | 7,507 | 898 |
| DS (C17) | 401 | 1,706 | 45,524 | 1,186 |
| RC (7) | 282 | 377 | 12,336 | 510 |
| RC (48) | 251 | 424 | 4,469 | 610 |
| RC (52) | 222 | 157 | 765 | 124 |
| RC (56) | 138 | 312 | 18,353 | 117 |
| RC (59) | 149 | 240 | 1,370 | 178 |
| KJ (A1) | 124 | 564 | 2,164 | 2,270 |
| KJ (A7) | 135 | 215 | 3,065 | 146 |
| BORI (11A) | 320 | 2,235 | 14,473 | 590 |
| BORI-15 (4C) | 340 | 1,989 | 59,399 | 603 |
| BORI-15 (5C) | 259 | 269 | 2,663 | 399 |
| BORI-15 (7A) | 779 | 478 | 9,820 | 1,166 |
| BaL | 543 | 99,308 | 453,053 | 269 |
| JRFL | 378 | 2,328 | 50,838 | 424 |
| YU-2 | 205 | 483 | 9,072 | 189 |
Geometric mean of multiple experiments (3 to 15) except for clones KJ (A1) and KJ (A7), which were assayed twice. Values in boldface were significant (0.0007 ≤ P ≤0.068); values underlined represent reproducible use of a coreceptor that did not reach significance, using Wilcoxon’s signed ranks test.
Pseudotyped viruses containing the envelopes of HIV-1BORI or HIV-1BORI-15 registered positive with target cells expressing CCR5 (as did the control pseudotypes using envelope from HIV-1BaL, HIV-1JRFL, and HIV-1YU-2). One clone obtained from HIV-1BORI-15 (4C) also used CCR3. This result is somewhat different than that obtained with the cell fusion assay (Fig. 2A), perhaps because of differences in expression and sensitivity. Pseudotypes expressing these envelopes did not register any activity with target cells expressing CXCR4. These results indicated that adaptation to growth in microglia, and induction of syncytia in that cell type, did not change the coreceptor utilization, which was predominantly CCR5.
The envelopes from the brain isolates HIV-1DS-br, HIV-1RC-br, and HIV-1KJ-br had a more complex pattern. All of these env clones had in common the ability to mediate fusion with CCR5 when used in the pseudotyped virus assay, and this receptor gave the highest level of chemiluminescence for all of the clones. Nevertheless, some of the env clones had the ability to utilize CCR3 or CXCR4 in addition to CCR5, although always with a much lower signal. We noted that the HIV-1JRFL envelope used both CCR3 and CCR5, as previously described (25, 34), but in this assay the HIV-1YU-2 envelope did not use CCR3, unlike its behavior in the fusion assay. This discrepancy may reflect differences in the sensitivity of these assays.
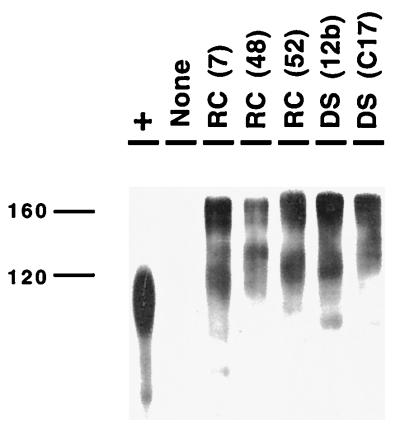
To ensure that differences in luciferase activity noted with several of the HIV-1RC-br env clones in the pseudotype assay did not represent aberrant processing of the envelope, we used a Western analysis for selected clones (see Materials and Methods). As shown in Fig. 3, clones that resulted in low levels of luciferase activity [e.g., RC(52) and DS(12b)] generated precursor and surface (gp120) proteins of the appropriate mobility.
FIG. 3.
Western blot of selected env clones. Selected env clones from the HIV-1RC-br and HIV-1DS-br isolates were resolved by sodium dodecyl sulfate–7.5% polyacrylamide gel electrophoresis transferred to a polyvinylidene difluoride membrane, and probed with a polyclonal rabbit anti-gp120 serum followed by peroxidase-conjugated goat anti-rabbit serum, and the bound antibody was detected by enhanced chemiluminescence (Amersham). Recombinant gp120 prepared in a baculovirus system was used as a positive control (+ lane); the slight difference in mobility may be due to differences in glycosylation. “None” represents a lysate from cells transfected with pNL4-3-LucR+E− only. All of the env clones analyzed resulted in proteins that were processed into gp120.
Antibody blocking of microglial infection.
To determine whether antibodies against CCR3, CCR5, and CXCR4 could block infection of microglia, we used pseudotyped virus containing env from HIV-1BaL. These pseudotypes registered positively with cells expressing either CCR3 or CCR5 (Table 3) and were high titered, making these pseudotypes particularly suitable for infection of a primary cell. As shown in Fig. 4, exposure of microglial cells with anti-CCR5 (45531) for 45 min prior to infection resulted in a marked reduction in the luciferase activity. Neither the anti-CCR3 antibody (7B11) nor the anti-CXCR4 antibody (12G5) had a marked effect on infection. However, the anti-CCR3 antibody inhibited infection of HIV-1BaL pseudotypes in U87 cells transiently transfected with CD4 and CCR3 (data not shown).
FIG. 4.
Blocking microglial infection with antibodies against coreceptors. Monoclonal antibodies against CCR3, CCR5, and CXCR4 and a control anti-human herpesvirus 6 p41 antibody (see Materials and Methods for details) were used to block infection of cultured microglial cells with pseudotyped virus expressing the HIV-1BaL env. Three days after infection, the cells were lysed, and levels of chemiluminescence were determined and expressed as relative light units/10 s. The anti-CCR5 antibody was the only one that had an effect on infection.
DISCUSSION
HIV infection of microglia appears to be an essential component of HIVD, since this is the predominant cell type harboring HIV sequences and expressing HIV proteins in the CNS of HIV-infected individuals (3, 24, 38, 39). Several lines of evidence suggest that strain variability could play a role in infection of microglial cells. First, HIV infection of the CNS occurs only in a proportion of HIV-infected individuals, in spite of the nearly universal presence of macrophage-tropic strains, most of which can replicate at least to some extent in microglia (36). Furthermore, studies from several groups have documented that HIV sequences obtained from the CNS cluster together, suggesting an independent evolution, perhaps even adaptation for CNS replication (17, 26, 40). Finally, in vitro, some HIV strains replicate preferentially in microglial cells rather than MDM (36).
Chemokine receptor utilization is a key determinant of cellular tropism (reviewed in reference 4), and since microglial cells express CCR3, CCR5, CXCR4, and perhaps other coreceptors, it is reasonable to propose that differential utilization of chemokine coreceptors could be partly responsible for specific tropism for this cell type. To determine whether in fact viruses that replicate well in microglia have a unique pattern of utilization of chemokine coreceptors, we obtained three viruses cultured from the CNS of individuals with dementia or encephalopathy (the pediatric counterpart of HIVD) and one strain that had been adapted for microglial growth by sequential passage. Infection of microglia with this adapted isolate results in particularly large syncytia. These viruses were used to infect cells engineered to express CD4 in conjunction with one of the major coreceptors. Envelope clones from each isolate were then used in a cell-to-cell fusion assay, and pseudotyped viruses were prepared.
All of the brain isolates as well as the isolate passaged in microglia used CCR5 as the principal coreceptor in all of the assays, although there was some replication in cells expressing CXCR4 or CCR3. This was more noticeable in the isolate adapted to microglia (HIV-1BORI-15), which used CCR3 in the fusion assay performed with a construct that expresses CCR3 well (CCR3P). One of the env clones obtained from this virus also used CCR3 in the pseudotype assay. However, this pattern of chemokine receptor use was similar to that of its parent (HIV-1BORI), indicating that adaptation into a syncytium-forming phenotype for microglia was not associated with alteration in the use of these major coreceptors. Nor was this pattern particularly unique for this virus, since many other isolates use multiple coreceptors, including CCR3 and STRL33 (2, 4, 10, 18, 29). Our data reinforce the importance of CCR5 as a coreceptor for macrophage-like cells but do not exclude the possibility that other coreceptors among the many recently described could influence entry into microglial cells.
He et al. (25) recently reported that CCR3 and CCR5 function as coreceptors for HIV-1 infection of fetal microglia and showed that an antibody directed against CCR3 could inhibit infection of those cells. While we found no compelling evidence for CCR3 predominance among CNS viruses, our experiments were performed with several of the same brain isolates in adult microglia. Perhaps the developmental stage of the microglia or even the influence of other cell types present in the experiments performed by He et al. may account for the discrepancies between these two studies.
We were initially concerned that the isolation procedures for four of the brain isolates or clones could have altered the chemokine coreceptor use, and thus we obtained env clones from DNA extracted from microglia, ensuring that they represent viruses that can enter these cells. Although the results were reasonably uniform, there was some variability among the clones (Table 3), suggesting that direct cloning of envelopes from infected CNS might offer an alternative approach for studies of the utilization of coreceptors in the brain. This is an important area for future studies as the number of potential coreceptors expands, since many of them are expressed in CNS tissues. These experiments also stress the importance of studying coreceptor utilization on primary cells and with multiple clones.
ACKNOWLEDGMENTS
A.V.A. and J.T.C.S. contributed equally to this work.
This work was supported by PHS grants NS-27405, NS-35743, training grant AI-07325, and the Medical Scientist Training Program.
We thank E. Berger (NIAID) for the CXCR4 molecular clone, S. Peiper (University of Louisville) for the CCR3 clone, M. Parmentier (Université libre de Bruxelles, Brussels, Belgium) for the CCR5 clone, N. Landau (Aaron Diamond AIDS Research Center) for the pNL4-3-Luc vectors, J. Moore (Aaron Diamond AIDS Research Center) for the BaL env expression vector, P. Earl (NIAID) for the R2143 serum, and J. Hoxie (University of Pennsylvania) for the 12G5 antibody. Other reagents were obtained from the AIDS Research Reference and Reagent Program. We thank B. Doranz and other members of the Doms laboratory for many helpful comments and advice, and we thank H. Sheng for technical help.
REFERENCES
- 1.Albright A V, Strizki J, Harouse J M, Lavi E, O’Connor M, González-Scarano F. HIV-1 infection of cultured human adult oligodendrocytes. Virology. 1996;217:211–219. doi: 10.1006/viro.1996.0108. [DOI] [PubMed] [Google Scholar]
- 2.Alkahatib G, Liao F, Berger E A, Farber J M, Peden K W C. A new SIV co-receptor STRL33. Nature. 1997;388:238. doi: 10.1038/40789. [DOI] [PubMed] [Google Scholar]
- 3.Bagasara O, Lavi E, Bobroski L, Tawadros R, Pestaner J P, Pomerantz R J. Cellular reservoirs of HIV-1 in the central nervous system of infected-individuals: identification by the combination of in situ PCR and immunohistochemistry. AIDS. 1996;10:573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Berger, E. A. 1997. HIV entry and tropism: the chemokine receptor connection. AIDS 11(Suppl. A):S3–S16. [PubMed]
- 5.Carne C A, Teddr R S, Smith A. Acute encephalopathy coincident with seroconversion for anti-HTLV-III. Lancet. 1985;ii:1206–1208. doi: 10.1016/s0140-6736(85)90740-8. [DOI] [PubMed] [Google Scholar]
- 6.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu J, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The b-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 7.Collman R, Balliet J W, Gregory S A, Friedman H, Kolson D L, Nathanson N, Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992;66:7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis L E, Hjelle B L, Miller V E, Palmer D L, Llewellyn A L, Merlin T L, Young S A, Mills R G, Wachsman W, Wiley C A. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 9.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 10.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 11.Di Stefano M, Wilt S, Gray F, Dubois-Dalcq M, Chiodi F. HIV type 1 V3 sequences and the development of dementia during AIDS. AIDS Res Hum Retroviruses. 1996;12:471–476. doi: 10.1089/aid.1996.12.471. [DOI] [PubMed] [Google Scholar]
- 12.Doranz B J, Lu Z-H, Rucker J, Zhang T-Y, Sharron M, Cen Y-H, Wang Z-X, Guo H-H, Du J-G, Accavitti M A, Doms R W, Peiper S C. Two distinct CCR5 domains can mediate coreceptor usage by human immunodeficiency virus type 1. J Virol. 1997;71:6305–6314. doi: 10.1128/jvi.71.9.6305-6314.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the b-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 14.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 15.Dreyer E B, Kaiser P K, Offerman J T, Lipton S A. HIV coat protein neurotoxicity is prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- 16.Endres M J, Clapham P R, Marsh M, Ahuja M, Turner J D, McKnight A, Thomas J F, Stoebenau-Haggerty B, Choe S, Vance P J, Wells T N C, Power C A, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 17.Epstein L G, Kuiken C, Blumberg B M, Hartman S, Sharer L R, Clement M, Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissue-specific evolution within host-determined quasispecies. Virology. 1991;180:583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- 18.Farzan M, Choe H, Martin K, Marcon L, Hoffman W, Karlsson G, Sun Y, Barrett P, Marchand N, Sullivan N, Gerard N, Gerard C, Sodroski J. Two orphan seven-transmembrane segment receptors which are expressed in CD4-positive cells support simian immunodeficiency virus infection. J Exp Med. 1997;186:405–411. doi: 10.1084/jem.186.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G-protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 20.Gartner S, Popovic M. Macrophage tropism of HIV-1. AIDS Res Hum Retroviruses. 1990;6:1017–1021. doi: 10.1089/aid.1990.6.1017. [DOI] [PubMed] [Google Scholar]
- 21.Gartner S, McDonald R A, Hunter E A, Bouwman F, Liu Y, Popovic M. Gp120 sequence variation in brain and in T-lymphocyte human immunodeficiency virus type 1 primary isolates. J Hum Virol. 1997;1:3–18. [PubMed] [Google Scholar]
- 22.Gelbard H A, Nottet H S L M, Swindells S, Jett M, Dzenko K A, Genis P, White R, Wang L, Choi Y-B, Zhang D, Lipton S A, Tourtellotte W W, Epstein L G, Gendelman H E. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol. 1994;68:4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giulian D, Yu J, Li X, Tom D, Li J, Wendt E, Lin S-N, Schwarcz R, Noonan C. Study of receptor-mediated neurotoxins released by HIV-1 infected mononuclear phagocytes found in human brain. J Neurosci. 1996;16:3139–3153. doi: 10.1523/JNEUROSCI.16-10-03139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass J D, Wesselingh S L, Selnes O A, McArthur J C. Clinical-neuropathological correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- 25.He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay C R, Sodroski J, Gabuzda D. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 26.Korber B T, Kunstman K J, Patterson B K, Furtado M, McEvilly M M, Levy R, Wolinsky S M. Genetic differences between blood and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994;68:7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavi E, Strizki J M, Ulrich A M, Zhang W, Fu L, Wang Q, O’Connor M, Hoxie J A, Gonzalez-Scarano F. CXCR-4 (fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Kappes J C, Conway J A, Price R W, Shaw G M, Hahn B H. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: Identification of replication-competent and -defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao F, Alkahatib G, Peden K W C, Sharma G, Berger E A, Farber J M. STRL33, a novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–2023. doi: 10.1084/jem.185.11.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipton S A, Sucher N J, Kaiser P K, Dreyer E B. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- 31.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 32.McArthur J C, Hoover D R, Bacellar H, Miller E N, Cohen B A, Becker J T, Graham N M H, McArthur J H, Selnes O A, Jacobson L P, Visscher B R, Concha M, Saah A. Dementia in AIDS patients: incidence and risk factors. Neurology. 1993;43:2245–2251. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 33.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994;68:5411–5418. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rucker J, Edinger A L, Sharron M, Samson M, Lee B, Berson J F, Yi Y, Margulies B, Collman R G, Doranz B J, Parmentier M, Doms R W. Utilization of chemokine receptors, orphan receptors, and herpesvirus-encoded receptors by diverse human and simian immunodeficiency viruses. J Virol. 1997;71:8999–9007. doi: 10.1128/jvi.71.12.8999-9007.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharer L R. Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol. 1992;51:3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Strizki J M, Albright A V, Sheng H, O’Connor M, Perrin L, Gonzalez-Scarano F. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J Virol. 1996;70:7564–7662. doi: 10.1128/jvi.70.11.7654-7662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watkins B A, Dorn H H, Kelly W B, Armstrong R C, Potts B J, Michaels F, Kufta C V, Dubois-Dalq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science. 1990;249:549–552. doi: 10.1126/science.2200125. [DOI] [PubMed] [Google Scholar]
- 38.Wiley C A. Quantitative neuropathologic assessment of HIV-1 encephalitis. Curr Top Microbiol Immunol. 1995;202:55–61. doi: 10.1007/978-3-642-79657-9_4. [DOI] [PubMed] [Google Scholar]
- 39.Wiley C A, Schrier R D, Nelson J, Lampert P W, Oldstone M B A. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong J K, Ignacio C C, Torriani F, Havlir D, Fitch N J S, Richman D D. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. J Virol. 1997;71:2059–2071. doi: 10.1128/jvi.71.3.2059-2071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yong V W, Antel J P. Culture of glial cells from human brain biopsies. In: Fedoroff S, Richardson A, editors. Protocols for neural cell culture. Totowa, N.J: Humana Press; 1992. pp. 81–96. [Google Scholar]