Abstract
Key Clinical Message
Subacute thyroiditis which is typically characterized by cervical pain and fever is caused by viral infection and is seen after SARS‐CoV‐2 vaccination. Here we report a post‐vaccination subacute thyroiditis after SARS‐CoV‐2 vaccination.
Abstract
Subacute thyroiditis (SAT) is possibly caused by a viral infection and is typically characterized by cervical pain and fever. SAT associated with SARS‐CoV‐2 infection or SARS‐CoV‐2 vaccination has been reported, albeit in limited numbers. A 34‐year‐old woman was referred to our clinic with typical SAT symptoms. The diagnosis was confirmed through thyroid scintigraphy after receiving the SARS‐CoV‐2 vaccination, despite testing negative for COVID‐19 via RT‐PCR. There is a theoretical correlation between SARS‐CoV‐2 vaccination and SAT. Vaccination may have a direct or indirect impact on the thyroid, but further studies are required to confirm this relationship. A systematic review of the literature of similar cases was performed for comparison. Ultimately, the overall benefits of SARS‐CoV‐2 vaccination outweigh the potential adverse effects. Therefore, these types of reports should not divert attention from the actual reality.
Keywords: case report, COVID‐19; SARS‐CoV‐2, subacute thyroiditis, vaccination
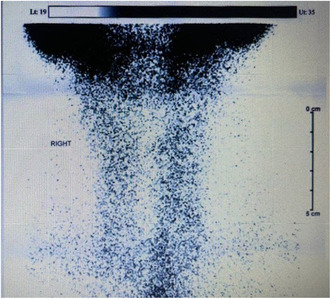
Thyroid scintigraphy of thyroid in the patient suspecting to SAT.
1. INTRODUCTION
Subacute thyroiditis (SAT) is a self‐limited inflammatory disorder that occurs in the thyroid gland. 1 SAT is possibly caused by a viral infection and is typically characterized by anterior cervical pain radiating to the pharynx and jaw, along with fever. 2 Laboratory studies reveal suppressed levels of TSH, elevated T3 and T4 levels, as well as increased WBC, CRP, and ESR levels. While anti‐thyroid peroxidase antibodies (Anti‐TPO) are typically found in painless SAT, they are usually absent or present in low titers in painful SAT. 3 Thyroid scintigraphy evaluates the iodine‐123 iodide or 99mTc‐pertechnetate uptake and distribution, which is expected to be markedly reduced in SAT. 4 , 5
SAT could play a role as a component of the COVID‐19 presentation with the signs mentioned above. 6 In addition, SAT has been rarely reported after SARS‐CoV‐2 vaccination in some case reports. 1 , 7 It is suggested that SARS‐CoV‐2 vaccination may trigger autoimmune reactions of which SAT is the most prevalent example of it. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA syndrome) is the other suggested reason behind SAT after vaccination due to the exposure to adjuvants. ASIA syndrome of Shoenfold's syndrome has been discussed first in 2011, as the reason behind several autoimmune conditions, which are induced following exposure to substances with adjuvant activity and should not be disregarded as a potential cause. 8 , 9 , 10
We report a case of SAT following SARS‐CoV‐2 vaccination along with a literature review.
2. CASE HISTORY
A 34‐year‐old woman presented to our clinic with fatigue, sweating, anterior cervical pain, and fever lasting for 8 days. The patient mentioned receiving a SARS‐CoV‐2 vaccination 4 weeks ago, specifically the first dose of the BBIBP‐CorV (Sinopharm, Beijing CNBG (inactivated virus vaccine)) vaccine. She has a medical history of gastroesophageal reflux disease (GERD) and had a previous episode of COVID‐19 about 7 months ago, for which she did not require antiviral or corticosteroid therapy or hospitalization. She did not report any personal or familial history of thyroid diseases before, and she had no symptoms related to upper respiratory tract infections during these days. A physical examination was conducted, revealing an oral temperature of 38.5°C and tenderness in the anterior cervical‐thyroid anatomical area without any erythema.
3. METHODS
The nasopharyngeal swab polymerase chain reaction (PCR) test was negative for SARS‐CoV‐2. abratoryL studies, including CBC, inflammatory markers, thyroid function tests, and thyroid scintigraphy, shown in the table and figure below, were consistent with SAT probably associated with the preceding vaccine. Ultrasonography reported an upper limit of normal thyroid size with regular margins, decreased echogenicity, heterogeneous echotexture with hypoechoic areas, and normal vascularity in both lobes without nodules or cervical lymphadenopathy.
4. OUTCOME AND RESULTS
The treatment with 12.5 mg/day of prednisolone was initiated for the patient. During the follow‐up visit after 2 weeks, the primary symptoms had resolved, so the corticosteroid dose was gradually tapered.
5. DISCUSSION
5.1. SAT after vaccination
Several cases have been reported as subacute thyroiditis after COVID‐19 infection and SARS‐CoV‐2 vaccination which some of them are enlisted in Table 1 to facilitate any comparison. Now, subacute thyroiditis is a known complication or clinical manifestation of COVID‐19 with several reports from all over the world. SARS‐CoV‐2 could affect thyroid cells directly through ACE2 receptors (angiotensin‐converting enzyme) and the following inflammation. 11
TABLE 1.
Previous reported cases of post‐SARS‐CoV‐2 and COVID‐19‐associated SAT.
| Author | Age/gender | Type of vaccine | Para clinical Test for COVID‐19 | Time passed from vaccination or COVID‐19 diagnosis | ignsS and symptoms | Thyroid tests | Inflammatory markers | Thyroid function in reassessment | Ultrasonography (US) | Thyroid scintigraphy | Treatment initiative and follow‐up | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Post‐vaccination Case 1 |
Das et al. 24 | 47/F | First dose of the ChAdOx1 nCoV‐19 (Astra Zeneca) vaccine | N/A | 3 weeks | Fever/neck pain and tenderness/restlessness/difficult swallowing/weight loss |
TSH ↓ T4 ↑ T3 Anti‐TPO (−) Anti‐TG (−) Anti‐TRAb (−) |
– | N/A |
ulky thyroid with hypoechoic nodulesB Without any cystic changes, calcification or increased vascularity |
No uptake, consistent with thyroiditis | 40 mg of propranolol every day/Symptoms resolved in 8 weeks |
| Post‐vaccination case 2 | Chatzi et al. 1 | 35/F |
First dose of SARS‐CoV‐2 mRNA (Pfizer/BioNTech) |
N/A | 12 days | eck pain/fatigue/palpitationN |
TSH ↓ FT4 ↑ Anti‐TPO (−) Anti‐TG (−) Anti‐TRAb (−) |
WBC → ESR = 75 CRP = 498 (↑↑) |
N/A | Increased gland dimensions with heterogeneous appearance and with hypoechogenic regions | Low uptake from the thyroid parenchyma with irregular gland margins | Prednisolone |
| Post‐vaccination case 3 | Chatzi et al. | 32/F |
Second dose of SARS‐CoV‐2 mRNA (Pfizer/BioNTech) |
N/A | 4 days | Neck pain/fatigue |
TSH ↓↓ FT4 → Anti‐TPO (−) Anti‐TG (−) Anti‐TRAb (−) |
WBC → ESR = 40 CRP = 10 (↑) |
N/A | Increased gland dimensions with heterogeneous appearance and hypoechogenic regions | Low uptake from the thyroid parenchyma with irregular gland margins | Prednisolone |
| Post‐vaccination case 4 | Iremli et al. 25 | Prednisolone |
Low Uptake from the thyroid parenchyma with irregular gland Margins |
Increased Gland dimensions with heterogeneous appearance and Hypoechogenic regions |
N/A | Neck pain/fatigue |
TSH ↓↓ FT4 → Anti‐TPO (−) Anti‐TG (−) Anti‐TRAb (−) |
WBC → ESR = 40 CRP = 10 (↑) |
4 days | N/A |
Second dose of SARS‐CoV‐2 mRNA (Pfizer/BioNTech) |
Methylprednisolone started with 16 mg/day And propranolol 25 mg every 12 h |
| Post‐vaccination case 5 | Iremli et al. | 34/F |
First dose of SARS‐CoV‐2 Vaccine (Vero Cell), Inactivated (CoronaVacR, Sinovac Life Sciences, Beijing) |
Negative PCR | 4 days | Anterior neck pain and tenderness/fatigue/and weight loss |
TSH ↓ FT4 FT3 ↑↑ Anti‐TPO (−) Anti‐TG (−) Anti‐TRAb (−) |
WBC → ESR = 19 CRP = 6(↑) |
Thyrotoxicosis after 4 weeks | Bilateral focal hypoechoic areas with decreased blood flow |
– |
Methylprednisolone was started with 16 mg/day And propranolol 25 mg every 12 h |
| Post‐vaccination case 6 | Iremli et al. | 37/F |
First dose of SARS‐CoV‐2 Vaccine (Vero Cell), Inactivated (CoronaVacR, Sinovac Life Sciences, Beijing) |
Negative PCR | 7 days |
Neck pain and mild Tenderness on palpation over the right lobe of the thyroid Gland |
TSH → FT4 → FT3 ↑ Anti‐TPO (−) Anti‐TG (−) Anti‐TRAb (−) |
WBC → ESR = 25 CRP = 2.4 (→) |
Thyrotoxicosis after 4 weeks | Bilateral hypoechoic areas with irregular borders and reduced blood flow in Doppler US |
– |
Followed up with no specific treatment and she was asymptomatic in Week 8 |
| Post‐vaccination case 7 | Saygılı et al. 26 | 38/F |
Second dose of SARS‐CoV‐2 Vaccine (Vero Cell), Inactivated (CoronaVacR, Sinovac Life Sciences, Beijing) |
N/A | 2 weeks | Swelling in the neck, pain, fatigue, loss of appetite and sweating |
TSH ↓↓ FT4 ↑↑ FT3 Anti‐TPO (−) Anti‐TG (−) |
WBC → ESR = 78 CRP = 8.76 (↑↑) |
Hypothyroidism after about 1 month | Increased size of the right thyroid lobe, an irregularly demarcated hypoechoic area |
– |
Treatment started with 275 mg naproxen sodium and 20 mf propranolol, both of them twice a day After 14 days most of the complaints were resolved |
| Post‐vaccination case 8 | Soltanpoor et al. 27 | 34/F | First dose of COVAXIN (The Bharat Biotech COVID‐19 Vaccine) | Negative chest CT | 5–7 days | Intermittent mild fever/Palpitation/anterior neck pain/mild thyroid enlargement and tenderness |
TSH ↓ T4 ↑↑ T3 ↑ |
WBC → ESR = 60 CRP = 9.8 (→) |
N/A |
– |
Moderate to severe decreased thyroid radiotracer uptake accompanied by high background activity, compatible with subacute thyroiditis |
15 mg/day prednisolone, 20 mg propranolol, twice per day; The patient had a significant resolution of symptoms after 2 weeks |
| Post‐vaccination case 9 | Siolos et al. 28 | 51/F | First dose of the BNT162B2 SARS‐CoV‐2 (Pfizer‐BioNTech) | Negative PCR | 4 days | Mild anterior neck pain/fever/nausea |
TSH ↓ FT4 ↑ T3 → Anti‐TPO (−) Anti‐TG (−) Anti‐TRAb (−) |
ESR = 103 CRP = 135 (↑↑) |
Euthyroid after 8 weeks |
– |
Decreased uptake of 99mTc‐pertechnetate by the thyroid gland |
16 mg/day of Methylprednisolone After 2 days fever and neck pain resolved |
| Post‐vaccination case 10 | Siolos et al. | 39/F | Did not mention the first or second dose/ChAdOx1‐S [recombinant] (AstraZeneca) | Negative PCR |
– |
No symptoms But abnormal thyroid function test |
TSH ↓ FT4 ↑ T3 → Anti‐TPO (+) Anti‐TG (+) Anti‐TRAb (−) |
ESR = 17 CRP = 1 (→) |
Euthyroidism after 8 weeks | Profoundly hypoechoic left lobe with decreased blood flow | Decreased uptake and thyroid | Followed up with no specific treatment and laboratory tests were back to normal after 2 months |
| urrent Cstudy | – | 34/F | With the first dose of BBIBP‐CorV (Sinopharm, Beijing CNBG) | Negative PCR | 4 weeks | Fever/fatigue/sweating/anterior neck pain and tenderness |
TSH ↓ T4 ↑ T3 ↑ Anti‐TPO N/A Anti‐TG N/A Anti‐TRAb N/A |
ESR = 73 CRP = +3 D‐dimer ↑ |
N/A | Upper limit of normal size with regular margins, decreased echogenicity, heterogeneous echotexture with hypoechoic areas, normal vascularity in both lobes | Decreased uptake of 99mTc‐pertechnetate by the thyroid gland |
12.5 mg/day of prednisolone Symptoms were resolved after 2 weeks |
|
COVID Case 1 |
Davoodi et al. 6 | 33/M | – | Positive PCR during SAT diagnosis | 10 | Fever/chills/sore throat/body ache/lethargy |
TSH ↓ TT4 ↑ TT3 ↑ Anti‐TPO (−) Anti‐TRAb (−) |
WBC ↑ ESR = 84 CRP = 37.9 (↑↑) |
Euthyroidism after 7 weeks |
A heterogeneous Thyroid gland with bilateral ill‐defined hypoechoic |
N/A |
Dexamethasone 4 mg every 8 h for 5 days and oral prednisone 25 mg daily with taper 7 weeks after he became Euthyroid |
|
COVID Case 2 |
Chakraborty U et al. 29 | 58/M | – | Positive PCR during SAT diagnosis | 4 | Fever/sore throat/neck tenderness/neck swelling/tachycardia |
TSH ↓ TT4 ↑ TT3 ↑ Anti‐TPO (−) Anti‐TRAb (−) |
WBC → ESR = 110 CRP = 16.6 (↑) |
Hypothyroidism after 4 weeks | Increased vascularity of the thyroid gland and diffuse enlargement of the thyroid gland with hypoechogenicity and a solitary nodule in each lobe | Poor and patchy uptake of radiotracer in the thyroid gland with high circulating and background radioactivity | Initiate with 30 mg/day prednisolone then taper and 40 mg/day propranolol |
|
COVID Case 3 |
Mari Des J. et al. 30 | 47/F | – | Positive PCR during SAT diagnosis | 14 | Neck pain |
TSH ↓↓ TT4 → TT3 Anti‐TPO (−) Anti‐TRAb (−) |
WBC → ESR = N/A CRP = 5 (↑) |
Hypothyroidism after 8 weeks | A slightly enlarged right thyroid lobe, with ill‐defined hypoechogenicity and normal vascularity in both lobes | N/A |
Celecoxib (dosage N/A) After 4 weeks resolve symptoms |
| COVID case 4 | Khatri et al. 31 | 41/F | – | Negative PCR during SAT diagnosis | 14 |
Neck pain/neck swelling/fever/odynophagia/headache/chills/palpitations/fatigue/weight loss/hand Tremors |
TSH ↓ T4 ↑ T3 ↑ Anti‐TPO (+) Anti‐TRAb (−) |
WBC → ESR = 107 CRP = 36 (↑↑) |
Euthyroid after about 6 weeks | A heterogeneous thyroid gland with bilateral patchy ill‐defined hypoechoic areas | N/A |
Oral ibuprofen 600 mg every 6 h and prednisone 40 mg daily Complete symptoms resolution after 6 weeks from discharge |
| COVID case 5 | Mattar SAM et al. 32 | 34/M | – | Positive PCR during SAT diagnosis | 9 | Neck pain/tachycardia/diffuse asymmetric tender goiter/cervical lymphadenopathy |
TSH ↓ FT4 ↑ FT3 ↑ Anti‐TPO (−) and Anti‐TRAb (−) |
WBC ↑ ESR = N/A CRP = 122 (↑↑) |
Euthyroid after 10 weeks |
An enlarged thyroid gland with heterogeneous echotexture. Both lobes had hypoechoic areas with ill‐defined margins corresponding to the hard regions palpable. Color flow Doppler showed reduced blood flow in both lobes. A few cervical lymph nodes with normal morphology were seen |
N/A | Initiate prednisolone at a dose of 20 mg. Atenolol at a dosage of 25 mg every morning |
| COVID case 6 | Chong et al. 33 | 37/M | – | Negative PCR during SAT diagnosis | 30 | Anterior neck pain with tenderness/fatigue/chills/palpitation/weight loss |
TSH ↓ FT4 ↑ FT3 ↑ Anti‐TPO (−) and Anti‐TRAb (−) |
WBC → ESR = 31 CRP = 14 (↑) |
Hypothyroidism after 3 weeks | Thyroid gland echotexture is diffusely heterogeneous | N/A | Aspirin and propranolol |
| COVID case 7 | Brancatella et al. 34 | 18/F | – | Negative PCR during SAT diagnosis | 19 | Fever/fatigue/palpitations/anterior neck pain with tenderness |
TSH ↓ FT4 ↑ FT3 ↑ Anti‐TPO (−) Anti‐TRAb (−) TgAb (+) |
WBC ↑ ESR = 90 CRP = 6.9 (↑) |
Euthyroid after 9 weeks | Multiple hypoechoic areas | N/A |
Initiate with prednisone (25 mg/d) then tapered Resolve symptom within 1 week |
| COVID case 8 | Mehmood MA et al. 35 | 29/F | – | Negative PCR during SAT diagnosis | 49 | Fever/back pain/odynophagia/palpitation/weight loss/tachycardia/hand tremor |
TSH ↓ FT4 ↑ FT3 ↑ Anti‐TPO (−) and Anti‐TRAb (−) |
WBC → ESR = 84 CRP = 44 (↑) |
Euthyroid after 10 weeks | Heterogeneously enlarged thyroid gland | N/A |
initiate prednisone (20 mg) and atenolol (25 mg) daily then increase to 40 mg and 50 mg respectively Then tapered. Recovery during 10 weeks |
| COVID case 9 | Asfuroglu Kalkan et al. 36 | 41/F | – | Positive PCR during SAT diagnosis | 0 | Fever/neck pain and tender to palpitation |
TSH ↓ FT4 ↑ FT3 ↑ Anti‐TPO (−) and Anti‐TRAb (−) |
WBC ↑ ESR = 134 CRP = 101 (↑↑) |
N/A | A relative diffuse decrease in vascularity and parenchyma was heterogeneous. | N/A | Prednisolone 16 mg daily and (drug for COVID‐19 such as hydroxychloroquine) |
| COVID case 10 | Ruggeri RM et al. 4 | 43/F | – | Negative PCR during SAT diagnosis | 45 | Pain and tenderness of neck/fatigue/tremors/palpitations/cervical and submandibular lymphadenopathy |
TSH ↓ FT4 ↑ FT3 ↑ Anti‐TPO (−) Anti‐TRAb (−) |
WBC → ESR = 60 CRP = 8.8 () |
Euthyroid after 4 weeks |
A diffusely Enlarged and hypoechogenic thyroid gland |
Markedly reduced 99mTc uptake in the gland | Oral prednisone (25 mg/day as the starting dose, gradually tapered) resolution symptoms during 4 weeks |
Abbreviations: TSH: ↓ 0.01–0.2 μIU/mL, ↓↓ <0.01 μIU/mL. T3, FT3, T4, FT4, WBC, CRP and etc.: → within normal; ↑, increased; ↓, decreased; ↑↑, 1.5 times more than normal upper limit; ↓↓, 1.5 times lower than normal lower limit; TSH, thyroid‐stimulating hormone; (F)T3, (free) triiodothyronine; (F)T4, thyroxine; anti‐TPO, thyroid peroxidase Antibody; WBC, white blood cell; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; Anti‐Tg, anti‐thyroglobulin antibody; TRAB, thyroid receptor antibodies; N/A, not assessed.
Thyroid disorders following viral infections or vaccinations have been discussed in the past decades. After the COVID‐19 pandemic and the production of vaccines with different structures and their injection, thyroid‐related disorders were seen. 12 Studies have shown that these disorders have occurred with the injection of almost all types of COVID‐19 vaccines, although the highest rate has occurred with mRNA‐based vaccines, followed by viral vectors, and less in inactive vaccines. These disorders include subacute thyroiditis, Graves' disease, focal painful thyroiditis, and finally silent thyroiditis. 13 Of course, in some cases, concurrent Graves' disease and SAT have been reported, too. 14
Subacute thyroiditis, which is also referred to as granulomatous or de Quervain's thyroiditis, is a self‐limiting autoimmune condition that occurs in response to a viral infection or the resulting inflammatory state. It usually presents with fever, neck pain, and palpitations; however, in some cases, patients do not develop any significant symptoms. 12 Typical SAT has three sequential phases, which include thyrotoxicosis, hypothyroidism, and euthyroidism. It is believed that this condition occurs after a recent viral infection in genetically predisposed patients, and the relationship between numerous HLAs (such as HLA‐Bw35, HLA‐B67, HLA‐B35, HLA‐DRB108, HLA‐DRB101, HLA‐B18:01, HLA‐DRB101, and HLA‐C*04:01) and SAT has been established. 15 , 16
SAT mostly affects middle‐aged women, and as we mentioned earlier, it is common after receiving viral vaccinations. Jafarzadeh et al. 13 gathered data on 50 SAT cases, of which 62% occurred following mRNA‐based vaccines, 24% with inactivated vaccines, and 12% with viral vector vaccines. In one patient, there was no data about the type of vaccine. They also stated that most patients were female, with a mean age of 39.5 years, and 58% of patients experienced SAT after the first vaccine injection. Similar to our study, the patient was a 34‐year‐old woman who had a history of receiving the first dose of an inactivated viral vaccine before developing SAT.
The diagnosis of SAT is based on laboratory findings, ultrasonography (US), and scintigraphy features. Elevated erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP), as well as abnormal thyroid function tests, are seen in patients. In the US, hypoechoic and heterogeneous pieces with obscure borders and feeble vascularization are characteristic features. In line with our study, as shown in Table 2, our patient had high levels of ESR, CRP, T3, T4, and low TSH. On US evaluation, there was an upper limit‐sized thyroid with regular margins and decreased echogenicity, heterogeneous echotexture with hypoechoic areas. 17 The thyroid scintigraphy usually shows a low or absent uptake, similar to our case, Figure 1, Scintigraphy (99mTc scan) demonstrates poor radiotracer uptake of both lobes with increased background and salivary gland uptake consistent with SAT. 18
TABLE 2.
Laboratory results of the patient on admission date and follow‐up.
| Laboratory tests | Admission | After 8 weeks | Reference range |
|---|---|---|---|
| ESR | 73 | 5 | 0–20 |
| CRP | +3 | Neg | Neg |
| D‐dimer | 727 | Neg | Neg: <500 |
| TSH | 0.1 | 4.64 | 0.4–5.5 |
| T4 | 16.8 | 8.5 | 4.5–12.5 |
| T3 | 489 | – | 84–172 |
| WBC | 9100 | 9300 | 4400–11,300 |
| Lymphocytosis | 1700 | 3350 | 800–4000 |
| Hgb | 9.7 | 12.7 | 12.5–15.3 |
| MCV | 74.9 | 76.4 | 80–96.1 |
| MCH | 23.8 | 25.4 | 27.7–35 |
| PLT | 302,000 | 250,000 | 150,000–450,000 |
Abbreviations: CRP, C‐reactive protein; ESR, elevated erythrocyte sedimentation rate; Hgb, hemoglobin; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; PLT, platelet; TSH, thyroid‐stimulating hormone; WBC, white blood cell.
FIGURE 1.
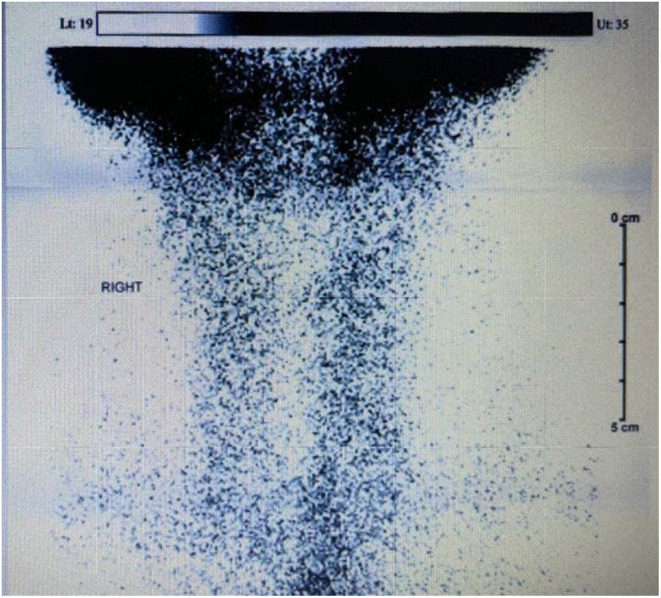
Scintigraphy (99mTc scan) demonstrates poor radiotracer uptake of both lobes with increased background and salivary gland uptake consistent with SAT.
Steroids and non‐steroidal anti‐inflammatory drugs (NSAIDs) are mostly effective in treating the symptoms and normalizing the laboratory markers of SAT. 19 In our study, after diagnosing SAT, we prescribed prednisolone (12.5 mg daily) for the patient, and in a 2‐week follow‐up, her condition improved.
5.2. A relation behind the vaccine injection and thyroid diseases
As stated in the studies, thyroid cells produce the SARS‐CoV‐2 receptor called angiotensin‐converting enzyme 2 (ACE2) and the transmembrane protease serine 2 (TMPRSS2). Therefore, SARS‐CoV‐2 can assault thyroid tissue, leading to thyroid dysfunction during and after COVID‐19 infection. Antibodies against SARS‐CoV‐2S protein have been reported to react with thyroid peroxidase (TPO) and can directly bind to ACE2‐expressing thyroid cells. These antibodies may play a role in initiating autoimmunity through molecular mimicry in susceptible individuals. Additionally, studies have reported a positive relationship between clinical severity associated with COVID‐19 and thyroid dysfunction. 13 , 17 , 20
Vaccines, like infections, may play a role in the development of autoimmune conditions through different mechanisms, such as molecular mimicry, epitope spreading, polyclonal activation, and the presentation of enigmatic antigenic determinants. Molecular mimicry between vaccine antigens and thyroid proteins can trigger an autoimmune response. Several factors, such as tissue damage, prolonged inflammatory response, and genetic background, can also cause autoimmune diseases. 13
Based on the above explanations, patients infected with SARS‐CoV‐2 or vaccinated may be at risk for thyroid dysfunction, especially those with a prolonged inflammatory response and a genetic background.
However, it was observed by Clarke et al. 21 that thyroid and adrenal function were found to be preserved ≥3 months after the onset of COVID‐19. Although a considerable number of individuals had chronic fatigue, changes in thyroid or adrenal function could not explain their symptoms. In addition, Goyal et al. 22 conducted longitudinal cohort research in which subjects examined at a short time (<1 year) after a primarily mild and asymptomatic SARS‐CoV‐2 infection did not show signs of thyroid autoimmune or dysfunction progressing. In our opinion, the risk of subacute thyroiditis due to SARS‐CoV‐2 infection or vaccination is different for each person and occurs rarely. However, according to the history of autoimmune disorders and the specific conditions of each person, therapeutic and preventive protocols should be undertaken in susceptible people.
The cases presented, including those outlined in Table 1, occurred following SARS‐CoV‐2 vaccination. This occurrence is considered coincidental, and the theories discussed represent potential pathways rather than established causation. Further investigations are essential to provide a more comprehensive understanding of this potential correlation.
It is important to emphasize that SARS‐CoV‐2 vaccination has resulted in a substantial reduction in mortality and disease severity among the general population. The occasionally reported adverse events should not undermine the significant benefits associated with vaccination. 23
6. CONCLUSION
Despite numerous studies, it is still impossible to definitively determine the effect of being infected with COVID‐19 or receiving a SARS‐CoV‐2 vaccine on the occurrence of autoimmune thyroid disease. This issue has become more complicated, especially considering several factors, including the different types of vaccines administered to different individuals. We hereby present a documented instance of SAT following SARS‐CoV‐2 vaccination, accompanied by a thorough a brief review concerning COVID‐associated and post‐vaccination cases of SAT. We have organized the relevant data in Table 1 to facilitate comparisons.
AUTHOR CONTRIBUTIONS
Ozra Akha: Conceptualization; investigation; validation; writing – original draft. Mahdi Mazandarani: Investigation; writing – original draft; writing – review and editing. Soroush Azari: Data curation; writing – original draft. Niloofar Daneshfar: Conceptualization; project administration; writing – original draft; writing – review and editing. Kimia Rasouli: Investigation; writing – review and editing. Keyvan Heydari: Conceptualization; investigation; writing – original draft; writing – review and editing. Golvash Tavakolian: Writing – original draft. Aref Hoseini: Conceptualization; data curation; investigation; project administration; resources; supervision; validation; writing – original draft; writing – review and editing.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interest relevant to the contents of this article.
ETHICS STATEMENT
The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal.
ACKNOWLEDGMENTS
We acknowledge Ahmad Pakdel (ORCID: 0000‐0003‐2597‐4138) and Hamidreza Zalpoor (ORCID: 0000‐0002‐8057‐2804) for contributing to this study.
Akha O, Mazandarani M, Azari S, et al. Subacute thyroiditis after SARS‐CoV‐2 vaccination: A case report. Clin Case Rep. 2024;12:e8678. doi: 10.1002/ccr3.8678
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article (and its additional information files).
REFERENCES
- 1. Chatzi S, Karampela A, Spiliopoulou C, Boutzios G. Subacute thyroiditis after SARS‐CoV‐2 vaccination: a report of two sisters and summary of the literature. Hormones (Athens). 2021;21:177‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao N, Wang S, Cui XJ, et al. Two‐years prospective follow‐up study of subacute thyroiditis. Front Endocrinol (Lausanne). 2020;11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hennessey JV. Subacute thyroiditis. In: Feingold KR, Anawalt B, Boyce A, et al., eds. Endotext. MDText.com, Inc; 2000.Copyright © 2000–2021. [Google Scholar]
- 4. Ruggeri RM, Campennì A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS‐COV‐2: an endocrine complication linked to the COVID‐19 pandemic. Hormones (Athens). 2021;20(1):219‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giovanella L, Avram AM, Iakovou I, et al. EANM practice guideline/SNMMI procedure standard for RAIU and thyroid scintigraphy. Eur J Nucl Med Mol Imaging. 2019;46(12):2514‐2525. [DOI] [PubMed] [Google Scholar]
- 6. Davoodi L, Oladi Z, Jafarpour H, Zakariaei Z, Soleymani E, Razavi A. A 33‐year‐old man with COVID‐19 presented with subacute thyroiditis: a rare case report and literature review. New Microbes New Infect. 2021;41:100871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bornemann C, Woyk K, Bouter C. Case report: two cases of subacute thyroiditis following SARS‐CoV‐2 vaccination. Front Med (Lausanne). 2021;8:737142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watad A, Sharif K, Shoenfeld Y. The ASIA syndrome: basic concepts. Mediterr J Rheumatol. 2017;28(2):64‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bragazzi NL, Hejly A, Watad A, Adawi M, Amital H, Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101412. [DOI] [PubMed] [Google Scholar]
- 10. Ruggeri RM, Giovanellla L, Campennì A. SARS‐CoV‐2 vaccine may trigger thyroid autoimmunity: real‐life experience and review of the literature. J Endocrinol Invest. 2022;45(12):2283‐2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rotondi M, Coperchini F, Ricci G, et al. Detection of SARS‐COV‐2 receptor ACE‐2 mRNA in thyroid cells: a clue for COVID‐19‐related subacute thyroiditis. J Endocrinol Invest. 2021;44:1085‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Soltanpoor P, Norouzi G. Subacute thyroiditis following COVID‐19 vaccination. Clin Case Rep. 2021;9(10):e04812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jafarzadeh A, Nemati M, Jafarzadeh S, Nozari P, Mortazavi S. Thyroid dysfunction following vaccination with COVID‐19 vaccines: a basic review of the preliminary evidence. J Endocrinol Invest. 2022;45(10):1835‐1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Altay FP, Nar A, Tütüncü NB. A case report of concurrent graves' disease and subacute thyroiditis following SARS‐CoV‐2 vaccination: an autoimmune/inflammatory syndrome (ASIA). Endocr Metab Immune Disord Drug Targets. 2023;23(2):242‐246. [DOI] [PubMed] [Google Scholar]
- 15. Ohsako N, Tamai H, Sudo T, et al. Clinical characteristics of subacute thyroiditis classified according to human leukocyte antigen typing. J Clin Endocrinol Metab. 1995;80(12):3653‐3656. [DOI] [PubMed] [Google Scholar]
- 16. Stasiak M, Tymoniuk B, Michalak R, Stasiak B, Kowalski ML, Lewiński A. Subacute thyroiditis is associated with HLA‐B* 18: 01,‐DRB1* 01 and‐C* 04: 01—the significance of the new molecular background. J Clin Med. 2020;9(2):534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trimboli P, Cappelli C, Croce L, Scappaticcio L, Chiovato L, Rotondi M. COVID‐19‐associated subacute thyroiditis: evidence‐based data from a systematic review. Front Endocrinol. 2021;12:707726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Viola N, Brancatella A, Sgrò D, Santini F, Latrofa F. Clinical, biochemical features and functional outcome of patients with SARS‐CoV‐2‐related subacute thyroiditis: a review. Endocrine. 2023;79(3):448‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mattar SAM, Koh SJQ, Chandran SR, Cherng BPZ. Subacute thyroiditis associated with COVID‐19. BMJ Case Rep. 2020;13(8):e237336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Christensen J, O'Callaghan K, Sinclair H, et al. Risk factors, treatment and outcomes of subacute thyroiditis secondary to COVID‐19: a systematic review. Intern Med J. 2022;52(4):522‐529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clarke SA, Phylactou M, Patel B, et al. Normal adrenal and thyroid function in patients who survive COVID‐19 infection. J Clin Endocrinol Metab. 2021;106(8):2208‐2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goyal A, Gupta Y, Kalaivani M, Tandon N. Mild and asymptomatic SARS‐CoV‐2 infection is not associated with progression of thyroid dysfunction or thyroid autoimmunity. Clin Endocrinol (Oxf). 2022;98:277‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Esmaeilzadeh A, Maleki AJ, Moradi A, et al. Major severe acute respiratory coronavirus‐2 (SARS‐CoV‐2) vaccine‐associated adverse effects; benefits outweigh the risks. Expert Rev Vaccines. 2022;21(10):1377‐1394. [DOI] [PubMed] [Google Scholar]
- 24. Das L, Bhadada SK, Sood A. Post‐COVID‐vaccine autoimmune/inflammatory syndrome in response to adjuvants (ASIA syndrome) manifesting as subacute thyroiditis. J Endocrinol Invest. 2021;45:465‐467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. İremli BG, Şendur SN, Ünlütürk U. Three cases of subacute thyroiditis following SARS‐CoV‐2 vaccine: postvaccination ASIA syndrome. J Clin Endocrinol Metab. 2021;106(9):2600‐2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saygılı ES, Karakilic E. Subacute thyroiditis after inactive SARS‐CoV‐2 vaccine. BMJ Case Rep. 2021;14(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soltanpoor P, Norouzi G. Subacute thyroiditis following COVID‐19 vaccination. Clin Case Rep. 2021;9(10):e04812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siolos A, Gartzonika K, Tigas S. Thyroiditis following vaccination against COVID‐19: report of two cases and review of the literature. Metabol Open. 2021;12:100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chakraborty U, Ghosh S, Chandra A, Ray AK. Subacute thyroiditis as a presenting manifestation of COVID‐19: a report of an exceedingly rare clinical entity. BMJ Case Rep. 2020;13(12):e239953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. San Juan MDJ, Florencio MQV, Joven MH. Subacute thyroiditis in a patient with coronavirus disease 2019. AACE Clin Case Rep. 2020;6(6):e361‐e364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khatri A, Charlap E, Kim A. Subacute thyroiditis from COVID‐19 infection: a case report and review of literature. Eur Thyroid J. 2021;9(6):324‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mattar SAM, Koh SJQ, Rama Chandran S, Cherng BPZ. Subacute thyroiditis associated with COVID‐19. BMJ Case Rep. 2020;13(8):e237336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chong WH, Shkolnik B, Saha B, Beegle S. Subacute thyroiditis in the setting of coronavirus disease 2019. Am J Med Sci. 2021;361(3):400‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brancatella A, Ricci D, Viola N, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis after Sars‐COV‐2 infection. J Clin Endocrinol Metab. 2020;105(7):2367‐2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mehmood MA, Bapna M, Arshad M. A case of post‐COVID‐19 subacute thyroiditis. Cureus. 2020;12(12):e12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with Covid‐19 infection. J Endocrinol Invest. 2020;43(8):1173‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its additional information files).


