Abstract
(R)-9-(2-Phosphonylmethoxypropyl)adenine (PMPA), an acyclic nucleoside phosphonate analog, is one of a new class of potent antiretroviral agents. Previously, we showed that PMPA treatment for 28 days prevented establishment of persistent simian immunodeficiency virus (SIV) infection in macaques even when therapy was initiated 24 h after intravenous virus inoculation. In the present study, we tested regimens involving different intervals between intravenous inoculation with SIV and initiation of PMPA treatment, as well as different durations of treatment, for the ability to prevent establishment of persistent infection. Twenty-four cynomolgus macaques (Macaca fascicularis) were studied for 46 weeks after inoculation with SIV. All mock-treated control macaques showed evidence of productive infection within 2 weeks postinoculation (p.i.). All macaques that were treated with PMPA for 28 days beginning 24 h p.i. showed no evidence of viral replication following discontinuation of PMPA treatment. However, extending the time to initiation of treatment from 24 to 48 or 72 h p.i. or decreasing the duration of treatment reduced effectiveness in preventing establishment of persistent infection. Only half of the macaques treated for 10 days, and none of those treated for 3 days, were completely protected when treatment was initiated at 24 h. Despite the reduced efficacy of delayed and shortened treatment, all PMPA-treated macaques that were not protected showed delays in the onset of cell-associated and plasma viremia and antibody responses compared with mock controls. These results clearly show that both the time between virus exposure and initiation of PMPA treatment as well as the duration of treatment are crucial factors for prevention of acute SIV infection in the macaque model.
We recently used the simian immunodeficiency virus (SIV)-infected macaque model to evaluate the efficacy of (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA), which is an acyclic nucleoside phosphonate analog and a potent antiretroviral compound (1, 2) in the setting of acute retroviral infection (23). In that study, PMPA prevented SIV infection even when treatment was started 24 h after intravenous virus inoculation (23). The increased antiretroviral efficacy of PMPA in SIV-challenged macaques (23), compared with that of other nucleoside analogues such as zidovudine (AZT) (15, 24, 29), may be related to ease of phosphorylation and the longer intracellular half-life for active phosphorylated metabolites of acyclic nucleoside phosphonates than for other nucleoside analogs (1). Although PMPA is highly potent when administered during de novo or early in SIV infection, the optimal treatment regimen of PMPA for preventing establishment of persistent SIV infection has not yet been determined. Therefore, we undertook the present study to determine the impact of increasing intervals between virus inoculation and initiation of PMPA treatment and varying the duration of treatment on the effectiveness of treatment in preventing the establishment of persistent infection.
MATERIALS AND METHODS
Macaques.
The subjects were 24 cynomolgus macaques (Macaca fascicularis), 12 males and 12 females, 2.5 to 3.5 years old. All animals were determined to be clinically healthy and free of type D retrovirus and SIV before virus inoculation. The macaques were assigned to study groups as summarized in Table 1 and Fig. 1 to 3. Treatment regimens are described in detail below. Care and husbandry were in strict conformance with federal guidelines. All procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington.
TABLE 1.
Summary of virological and immunological status following various treatment regimens
| Group | Determination | No. positive at indicated wk p.i./no. tested
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 6 | 8 | 12 | 16 | 20 | 24 | 32 | 46 | ||
| A (mock-treated control; macaques 95012, 95023, 95032, 95041) | PBMC viremiaa | 0/4 | 4/4 | 3/4 | 3/4 | 3/4 | 3/4 | 4/4 | 4/4 | 3/4 | 3/4 | 4/4 | 3/4 |
| Plasma SIV RNA | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 3/4 | 4/4 | 3/3 | 3/3 | 3/4 | |
| PCR-viral DNAb | 3/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 3/4 | NDc | ND | 4/4 | |
| SIV antibodies | 0/4 | 0/4 | 0/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | |
| B (24-h postexposure, 28-day treatment; macaques 95025, 95044, 95054, M94312) | PBMC viremia | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 |
| Plasma SIV RNA | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | |
| PCR-viral DNA | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | |
| SIV antibodies | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 1/4 | |
| C (48-h postexposure, 28-day treatment; macaques 95026, 95035, 95043, 95059) | PBMC viremia | 0/4 | 0/4 | 0/4 | 0/4 | 2/4 | 3/4 | 3/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 |
| Plasma SIV RNA | 0/4 | 0/4 | 0/4 | 0/4 | 2/4 | 4/4 | 3/4 | 3/4 | 3/4 | 3/4 | 2/4 | 2/4 | |
| PCR-viral DNA | 0/4 | 0/4 | 0/4 | 0/4 | 2/4 | 3/4 | 2/4 | 2/4 | 2/4 | ND | ND | 4/4 | |
| SIV antibodies | 0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 2/4 | 3/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | |
| D (72-h postexposure, 28-day treatment; macaques 95017, 95038, 95061, M95033) | PBMC viremia | 0/4 | 0/4 | 0/4 | 0/4 | 1/4 | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 |
| Plasma SIV RNA | 0/4 | 0/4 | 0/4 | 0/4 | 3/4 | 3/4 | 3/4 | 4/4 | 2/4 | 2/4 | 2/4 | 2/4 | |
| PCR-viral DNA | 0/4 | 0/4 | 0/4 | 0/4 | 2/4 | 3/4 | 3/4 | 3/4 | 1/4 | ND | ND | 2/4 | |
| SIV antibodies | 0/4 | 0/4 | 0/4 | 0/4 | 2/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | |
| E (24-h postexposure, 10-day treatment; macaques 95016, 95020, 95033, 95053) | PBMC viremia | 0/4 | 0/4 | 0/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 |
| Plasma SIV RNA | 0/4 | 0/4 | 1/4 | 1/4 | 2/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | 1/4 | |
| PCR-viral DNA | 0/4 | 0/4 | 0/4 | 1/4 | 2/4 | 0/4 | 2/4 | 2/4 | 1/4 | ND | ND | 1/4 | |
| SIV antibodies | 0/4 | 0/4 | 0/4 | 0/4 | 0/4 | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 | 3/4 | |
| F (24-h postexposure, 3-day treatment; macaques 95015, 95030, M95018, 95052) | PBMC viremia | 0/4 | 0/4 | 1/4 | 3/4 | 1/4 | 1/4 | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 | 2/4 |
| Plasma SIV RNA | 1/4 | 3/4 | 3/4 | 4/4 | 3/4 | 2/4 | 2/4 | 3/4 | 2/4 | 3/4 | 2/4 | 2/4 | |
| PCR-viral DNA | 0/4 | 0/4 | 1/4 | 4/4 | 3/4 | 4/4 | 4/4 | 3/4 | 2/4 | ND | ND | 2/4 | |
| SIV antibodies | 0/4 | 0/4 | 0/4 | 3/4 | 3/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | 4/4 | |
PBMC were titrated from 106 to 101 cells and then cocultured with C8166 cells to isolate infectious virus in this in vitro assay.
A nested PCR was used to detect SIV long terminal repeat DNA in 106 PBMCs.
ND, not determined.
FIG. 1.
Plasma viral load levels in mock-treated and PMPA-treated macaques after intravenous inoculation with uncloned SIVmne. SIV RNA levels in plasma were measured by a sensitive quantitative competitive RT-PCR assay as described in the text. Threshold sensitivity for the assay was 300 copy eq/ml of plasma.
FIG. 3.
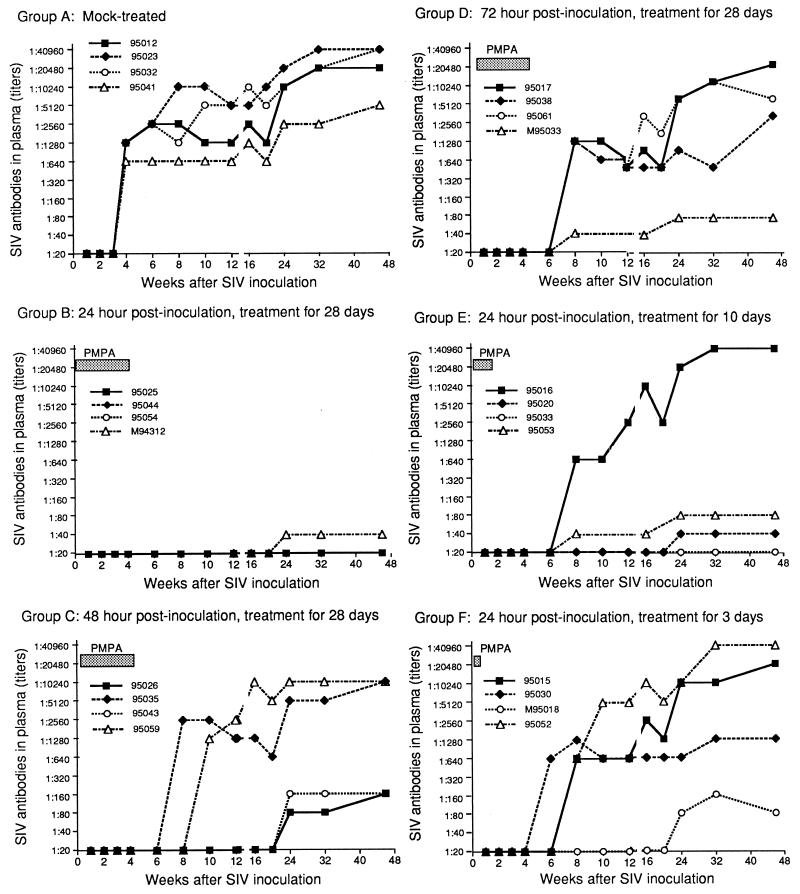
Anti-SIV IgG antibody response in mock-treated and PMPA-treated macaques after intravenous inoculation with uncloned SIVmne. Titers were expressed as the reciprocal of the highest dilution that yielded positive immunofluorescent staining. The lowest titer of SIV-positive antibody in this assay was 1:40.
Virus inoculum.
Virus used for inoculation was derived from the cell culture supernatant of uncloned SIVmne propagated in HuT 78 cells (3). The cell supernatant was filtered (0.45-μm-pore-size filter; Nalgene, Rochester, N.Y.) and frozen in aliquots in liquid nitrogen. The titer of this virus stock was 105 tissue culture infectious doses per milliliter as determined in human T-cell lines. The stock was diluted immediately before inoculation. All macaques were inoculated intravenously with 1.0 ml of 103 tissue culture infectious doses, which is equivalent to 10 times the 50% animal infectious dose (25). Such a dose results in 100% infectivity in macaques. Intravenous inoculation was used to minimize potential differences between animals in transmission across mucosal barriers.
PMPA preparation and treatment regimen.
PMPA was dissolved in water, and the pH was adjusted to 7.0 with 0.1 N NaOH. The volume of the solution was adjusted with distilled water to a PMPA concentration of 30 mg/ml and filter sterilized (0.2-μm-pore-size filter; Nalgene). The macaques were divided into six groups of four animals each (groups A, B, C, D, E, and F) and inoculated intravenously with 10 times the 50% animal infectious dose of SIVmne. The four macaques in group A served as an infection control group: after inoculation they were mock treated with sterile, physiological saline for 28 days. The macaques in groups B to F were treated with PMPA beginning 24, 48, or 72 h postinoculation (p.i.). PMPA (30 mg/kg) was administered once daily via the subcutaneous route for 3, 10, or 28 days. The treatment regimens for macaque groups A to F are summarized in Table 1.
Clinical observations.
The macaques were observed daily for general physical condition including appetite, stool consistency, activity level, and appearance. At specific intervals they were anesthetized with ketamine for thorough physical examination. At these times, weight and body temperature were recorded and blood was drawn for complete blood counts, serum biochemistry, virology, and lymphocyte subset analyses. Blood draws were performed weekly during PMPA treatment (i.e., the first 4 weeks p.i.), every 2 weeks for the next 6 weeks, and then once a month until the end of the study (46 weeks p.i.). The data obtained from the physical examinations and blood analyses were used to monitor the course of SIV infection, SIV-induced disease, and potential drug toxicity.
Processing of blood samples.
EDTA-anticoagulated blood was collected as described above, and the course of SIV infection was followed for 46 weeks. The EDTA-anticoagulated blood obtained from the femoral vein was centrifuged to separate plasma and buffy coats. Plasma was aliquoted and used for assays of SIV RNA, anti-SIV immunoglobulin G (IgG) antibodies, and immunoblotting. Peripheral blood mononuclear cells (PBMC) were separated from the buffy coat by centrifugation through Ficoll-Hypaque density gradients (Pharmacia, Piscataway, N.J.).
SIV RNA in plasma (plasma-associated virus).
Virus-associated SIV RNA in plasma was quantified via a real-time reverse transcriptase (RT)-mediated PCR (RT-PCR) assay as described in detail elsewhere (22). Briefly, plasma virion-associated RNA was extracted with commercial reagents (Purescript; Gentra Systems, Minneapolis, Minn.). After a random-primed reverse transcription, real-time quantitative PCR analysis was performed with primers to a highly conserved region of SIV gag sequence and a fluorochrome-labeled internally hybridizing oligonucleotide probe (22). Duplicate RT-PCRs were performed for each sample, along with a reaction in which no RT was included as a control to detect any DNA contamination of the test samples. The nominal threshold sensitivity for the assay was 300 copy eq/ml of plasma.
Assessment of virus infectivity.
The frequency of infected cells was measured by cocultivation of serial 10-fold dilutions (106 to 101) of PBMC or lymph node mononuclear cells (LNMC) prepared from biopsied lymph nodes with target cells (C8166) in 24-well tissue culture plates or cell culture flasks for virus isolation (23, 26). The cells were maintained in RPMI 1640 medium to which were added 2 mM l-glutamine, 50 μg of gentamicin per ml, and 10% heat-inactivated fetal calf serum. Cultures were examined twice weekly for syncytial cytopathic effects, and culture supernatants were sampled weekly for detection of SIV p27 antigens by antigen capture assay (Coulter, Hialeah, Fla.). All cultures were maintained for 4 weeks by weekly passage of culture on to fresh target cells. The results of virus isolation and detection were used for estimating the frequency of infectious cells or the level of cell-associated virus. For example, 106 PBMC or LNMC needed for detection of SIV were determined as one infectious cell frequency; 105 and 104 PBMC that yielded a positive SIV were expressed as 10 and 100 infectious cells per 106 PBMC, or 1- to 2-log-higher levels of cell-associated virus.
Virus isolation from PBMC or LNMC.
Approximately 106 PBMC were cultured for 2 days in RPMI 1640 containing 5 μg of phytohemagglutinin (Sigma) per ml for activation of T lymphocytes. The supernatant of culture was removed, and the cell pellets were resuspended in RPMI 1640 medium supplemented with 8 U of human interleukin-2 (Boehringer Mannheim) per ml and cocultivated with C8166 cells. The basic methods for cell culture and virus isolation were the same as those described for infectivity assays described above. Culture supernatants were sampled for measuring the levels of SIV p27 antigen by the use of a capture enzyme-linked immunosorbent assay (Coulter).
PCR for SIV DNA in PBMC.
PCR detection of SIV nucleic acid sequences was performed on DNA extracted from PBMC, using a nested set of oligonucleotide primers specific for SIV long terminal repeat regions as described previously (23, 24). Briefly, 1 μg of PBMC DNA was amplified in each reaction mixture containing 0.2 mM deoxynucleoside triphosphates, 2.0 mM MgCl2, Amplitaq buffer, 2.5 U of Taq polymerase (Amplitaq; Perkin-Elmer Cetus, Norwalk, Conn.), and 10 nM primers (National Bioscience, Plymouth, Mass.). Samples were amplified with external primers, the products were diluted 1:100, and the internal nested primers were used to amplify a fragment of 850 bp. Specific DNA bands were detected on ethidium bromide-stained agarose gels. Analysis was done for PBMC collected at multiple time points from 1 to 46 weeks p.i.
Antibody determination.
Anti-SIV IgG antibody titers in plasma were detected by an immunofluorescence antibody (IFA) assay (25, 27) and expressed as the reciprocal of the highest twofold dilution (duplicate per dilution) that gave positive immunofluorescence staining. Briefly, plasma from experimental macaques was diluted 1:20 to 1:40,960 in phosphate-buffered saline. SIV-infected C8166 cells attached to Teflon-coated slides (Cel-Line Associates, Newfield, N.J.) were used as target cells for binding SIV antibodies from the diluted plasma. After incubation and washing, fluorescein-conjugated goat anti-monkey IgG (Organon Teknika Cappel, Malvern, Pa.) was added. Cells showing fluorescence were considered to be positive for the presence of SIV antibody. The lower limit of the IFA assay for anti-SIV IgG antibody titer was 1:20. SIV-specific antibodies to viral proteins were detected by Western blotting (3, 4) using a 0.45-μm-pore-size Immobilon membrane (Millipore, Bedford, Mass.). Briefly, 1,000-fold-concentrated SIV was separated on sodium dodecyl sulfate–10 to 20% polyacrylamide electrophoresis gradient gels and transferred by electrophoresis as described previously (3) except that a 0.45-μm-pore-size Immobilon membrane (Millipore) was used instead of nitrocellulose. On Western immunoblots, each strip contained approximately 10 μg of viral proteins.
Lymphocyte subset analysis.
CD4+ and CD8+ lymphocyte data were obtained from all macaques before, during, and after PMPA treatment. Specific lymphocyte subsets were determined by incubating EDTA-anticoagulated blood samples with a panel of mouse anti-human monoclonal antibodies that react with macaque lymphocytes (23, 26). Specific CD4+ cells and other lymphocyte subsets were analyzed by flow cytometry using a FACScan (Becton Dickinson, San Jose, Calif.). Absolute cell numbers were calculated from total and differential leukocyte counts and the percentage of lymphocytes with T-cell markers.
Statistical analysis.
Data obtained from virologic, immunologic, and hematologic studies were analyzed by χ2 and analysis of variance.
RESULTS
Virologic and serologic studies.
All macaques were monitored at predetermined intervals for levels of plasma virion-associated SIV RNA, PBMC-associated virus, PCR SIV DNA in PBMC, and SIV antibody responses. The summary and outcome of the various PMPA treatments are shown in Table 1. To facilitate comparison of data for individual macaques described in the text, the study groups and the macaque numbers in each group are shown in the same order in Fig. 1 to 3 and in Table 1. Comparisons of virologic data between group A (the mock-treated control) and groups B (treated starting 24 h p.i.), C (treated starting 48 h p.i.) and D (treated starting 72 h p.i.) demonstrate the effects of the time interval between intravenous virus inoculation and the initiation of PMPA treatment. Similarly, the differences between group A and groups B, E, and F demonstrate the effects of various durations of PMPA treatment (28, 10, and 10 days, respectively). In addition to the virologic status shown in Table 1, detailed data for PBMC-associated SIV (Fig. 2), plasma-associated virus (Fig. 1), and SIV antibody responses (Table 1 and Fig. 3) were used as criteria for determining SIV infection.
FIG. 2.
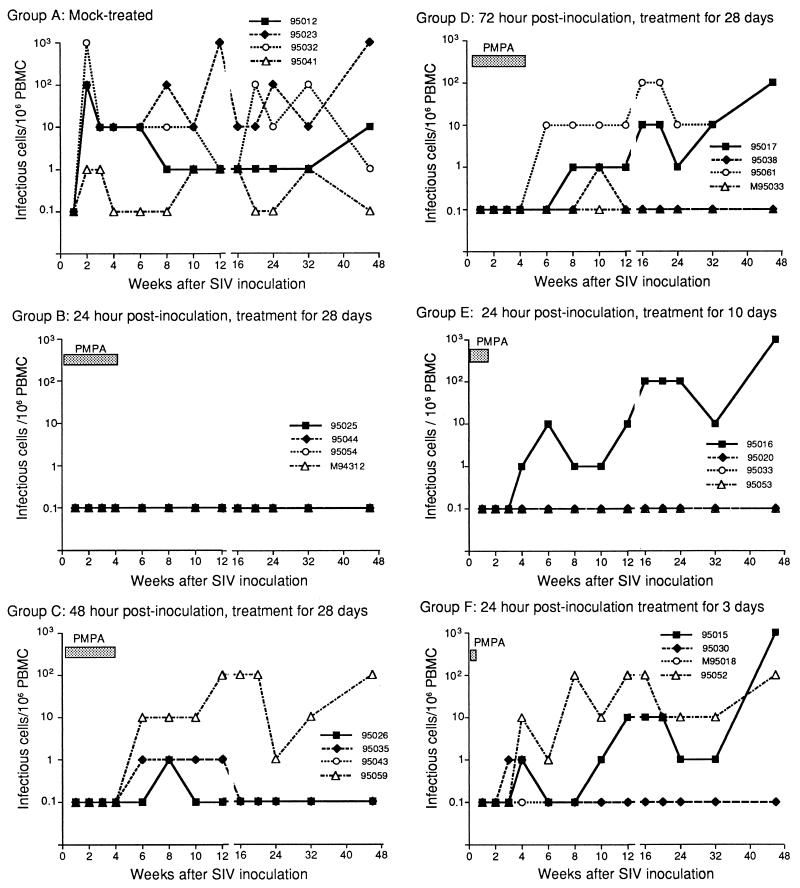
Frequencies of infectious cells in PBMC from macaques. The frequency of infectious cells was measured by cocultivation of serial dilutions of PBMC with target cells for detection of viral replication as described in the text.
All four of the mock-treated macaques in group A developed persistent infection as determined by plasma viremia (17,000 to 130,000 copy eq of SIV RNA/ml of plasma) by week 1 p.i. (Fig. 1), by PBMC-associated virus (100 to 1,000 infectious cells per 106 PBMC, except in macaque 95041, which had 1 infectious cell per 106 PBMC) starting at week 2 p.i. (Table 1 and Fig. 2), and by the presence of anti-SIV IgG antibodies (antibody titers ranging from 1:640 to 1:1,280) beginning at week 4 p.i. The SIV infection and antibody response in the control macaques persisted throughout the 46-week study. However, in macaque 95041 these levels were 1 to 2 logs lower than in other control macaques.
When PMPA treatment was initiated 24 h p.i. and continued for 28 days (group B) (Table 1 and Fig. 1 to 3), three of the four macaques showed no evidence of SIV infection by virologic and serologic detection throughout the 46-week observation period. The fourth macaque (M94312) had a very low titer (1:40) of antibody at week 24 p.i. with no increase in the scope of antigen specificity or titer of antibody at week 46, despite negative virus isolation from PBMC and lymph node biopsies, negative PCR SIV DNA, and undetectable plasma SIV RNA.
When PMPA treatment was initiated 48 h p.i. and continued for 28 days (group C) (Table 1 and Fig. 1 to 3), all four macaques showed no evidence of infection over the first 4 weeks p.i. during PMPA treatment. Upon withdrawal of PMPA, however, two macaques (95026 and 95043) showed transient viremia as determined by PBMC-associated virus (1 infectious cell per 106 PBMC) at 6 to 12 weeks p.i. (Fig. 2) and by measurable plasma viral RNA levels (1,000 to 22,000 copy eq/ml, respectively) at 8 to 12 weeks p.i. (Fig. 1); thereafter, their PBMC and plasma no longer showed any evidence of virus. These two macaques also had low levels of SIV-specific antibodies to viral proteins detectable by immunoblotting beginning at week 16 p.i. The other two macaques in this group (95059 and 95035) developed persistent infection. Macaque 95059 had continuously high levels of both PBMC-associated virus (10 to 100 infectious cells per 106 PBMC) and plasma viral RNA (mean of 1,630,000 copy eq/ml of plasma) and high titers (1:10,240) of anti-SIV IgG antibody. Likewise, macaque 95035 had moderately high titers (1:2,560 to 1:5,120) of anti-SIV IgG antibody beginning 8 weeks p.i. and lasting until the end of the observation period. In the first 2 weeks after discontinuation of PMPA, plasma SIV RNA went from undetectable to 90,000,000 copy eq/ml and then decreased in the post-acute phase of infection, equilibrating at approximately 200,000 copy eq/ml during weeks 24 to 46 (Fig. 1).
When PMPA treatment was initiated 72 h p.i. and continued for 28 days (group D) (Table 1 and Fig. 1 to 3), the four macaques did not show any evidence of viral replication during the treatment period. Macaque M95033 did not show evidence of infection in any of the virologic assays performed throughout the 46-week study but had very low levels of antibody (1:40) detectable by IFA and immunoblotting beginning 8 weeks p.i.; antibody persisted at a titer of 1:80 from weeks 24 through 46 p.i. Macaques 95017 and 95061 became persistently infected, showing high levels of PBMC-associated virus (10 to 100 infectious cells per 106 PBMC) and plasma viral RNA and increasing antibody titers from 6 weeks p.i. until the end of the study. Interestingly, macaque 95038 had only transiently detectable viremia as determined by plasma SIV RNA (37,000 copy eq/ml) only at week 6 p.i. and by PBMC-associated virus (1 infectious cell per 106 PBMC) only at week 10 p.i.; thereafter the PBMC and plasma no longer showed any detectable virus. However, this macaque had a measurable anti-SIV antibody (1:640 to 1:1,280) beginning 8 weeks p.i. and persisting to the end of the observation period.
When PMPA treatment was initiated 24 h p.i. and continued for only 10 days (group E) (Table 1 and Fig. 1 to 3), three of the four macaques showed no signs of persistent viremia by virologic detection. Of these three animals, macaque 95053 had very low levels of anti-SIV IgG antibody, with titers of 1:40 beginning 8 weeks p.i. and 1:80 from weeks 24 through 46. This macaque had a very low level of plasma SIV RNA (600 copy eq/ml) only at week 6 p.i. Macaque 95020 had an even lower antibody titer (1:40) beginning 24 weeks p.i., detectable by a very weak band for SIV gp120 on immunoblotting. Macaque 95033 had no detectable antibody throughout the 46-week study. However, one macaque (95016) had a persistent infection as determined by increasing plasma SIV RNA beginning at week 3 p.i. (1,700 copy eq/ml of SIV RNA), by increasing PBMC-associated virus beginning at week 4 p.i. (1 infectious cell per 106 PBMC), and by increasing antibody response beginning at week 8 p.i. (anti-SIV IgG titer of 1:640). By the end of the study, this macaque had 31,000,000 copy eq of SIV RNA/ml of plasma, 10 to 100 infectious cells per 106 PBMC of PBMC-associated virus, and 1:40,960 titer of anti-SIV antibody.
When PMPA treatment was initiated 24 h p.i. and continued for only 3 days (group F) (Table 1 and Fig. 1 to 3), two macaques (95030 and M95018) apparently had transient infection with detectable plasma SIV RNA levels (6,000 to 730,000 copy eq/ml in macaque 95030 and 2,400 to 25,000 copy eq/ml in macaque M95018) beginning 1 to 2 weeks p.i. By 8 weeks p.i., plasma SIV RNA was undetectable in these two animals. It remained undetectable in macaque M95018 through the end of the study, but low levels (1,400 to 2,000 copy eq/ml) were detectable in macaque 95030 at 16 and 24 weeks p.i. Interestingly, macaque M95018 had no evidence of viral infection by PBMC-associated virus isolation and showed only low titers (1:80) of antibody throughout the study. Macaque 95030 showed a persistent antibody response (antibody titer of 1:640 to 1:1,280) beginning 6 weeks p.i. and lasting to the end of the study. The two remaining macaques (95015 and 95052) had a persistent infection as determined by plasma SIV RNA beginning 2 to 3 weeks p.i. (10,000 to 22,000 copy eq/ml) and by PBMC-associated virus (1 to 10 infectious cells per 106 PBMC) beginning 4 weeks p.i.; the infection lasted through the end of the study. At 46 weeks p.i., the mean level of infection in these two macaques was 17,000,000 copy eq of SIV RNA/ml of plasma and 10 to 100 infectious cells per 106 PBMC. Both macaques also had persistent antibody responses beginning 4 to 8 weeks p.i. (antibody titer of 1:640) and lasting through the end of the study (antibody titer of 1:20,480 to 1:40,960 at 46 weeks p.i.).
Clinical status and drug toxicity.
None of the PMPA-treated macaques showed signs of toxic side effects throughout the maximum duration of treatment (28 days); there were no abnormalities in complete blood counts, serum chemistry and biochemistry profiles, general physical condition, or neurobehavioral activities. However, 8 of the 16 PMPA-treated macaques (groups B to E) showed transient decreases of serum phosphorus during treatment (the first 4 weeks p.i.). Of these eight macaques, one (95035) had a severe decrease (1.1 to 2.5 mg/dl) and seven had a mild decrease (2.9 to 3.5 mg/dl) of serum phosphorus. In contrast, untreated controls (group A) and macaques treated with PMPA for only 3 days (group F) showed normal values of serum phosphorus ranging from 4.0 to 6.4 mg/dl (mean, 4.99 ± 0.49) and from 4.1 to 6.1 mg/dl (mean, 5.24 ± 0.23), respectively. All four mock-treated macaques showed a transient mild decrease in leukocyte counts at 2 weeks p.i., and three showed a moderate lymphadenopathy at 4 weeks p.i. Beginning 10 weeks p.i., mock-treated macaques with high viral load (i.e., based on levels of plasma SIV RNA and infectious cells per 106 PBMC) developed persistent lymphadenopathy and recurrent skin rashes.
Of the 20 PMPA-treated macaques, seven showed no clinical signs of SIV infection and six showed only transient clinical evidence of infection. The remaining seven PMPA-treated macaques, which had persistently high viral loads, exhibited recurrent rashes and/or lymphadenopathy similar to those observed in the mock-treated macaques.
Two mock-treated macaques with high viral load had severe thrombocytopenia beginning 46 weeks p.i. Similarly, four PMPA-treated macaques with persistent viremia and high viral loads also developed moderate to severe thrombocytopenia beginning 46 weeks p.i.
Lymphocyte subsets.
To determine whether the antiviral effect of PMPA treatment also improves responses of CD4+ and CD8+ lymphocytes in PBMC, the PMPA-treated macaques were grouped according to the level of viremia and then compared with mock-treated macaques. The mock-treated macaques (n = 4) showed a decrease in the mean CD4+ cells from 2,300 ± 446 cells/mm3 at virus inoculation to 1,585 ± 461 cells/mm3 over the course of 20 weeks p.i. The PMPA-treated macaques that were virus negative (n = 7) and only transiently iremic (n = 6) showed slight increases in the mean CD4+ cells, from 2,015 ± 693 cells/mm3 at virus inoculation to 2,436 ± 310 cells/mm3 by week 20 p.i. The PMPA-treated, persistently viremic macaques (n = 7) showed a slight decrease in the mean CD4+ cells from 2,073 ± 497 cells/mm3 before inoculation to 1,515 ± 186 cells/mm3 by week 20 p.i.; this was similar to the level of the mock-treated macaques. There was no significant difference in the CD4+/CD8+ ratios between PMPA-treated macaques and mock-treated macaques before week 32 p.i. (data not shown). However, beginning at week 46 p.i., two of the four mock-treated macaques showed further decreases in CD4+ cells (range, 572 to 583 cells/mm3; mean, 578 ± 8 cells/mm3) as well as CD4+/CD8+ ratios (range, 0.31 to 0.63; mean, 0.47 ± 0.23). Similarly, six of seven PMPA-treated, persistently viremic macaques showed further decreases in CD4+ cells (range, 665 to 1,288 cells/mm3; mean, 985 ± 266 cells/mm3) as well as CD4+/CD8+ ratios (range, 0.33 to 0.76; mean, 0.45 ± 0.18). The remaining two mock-treated macaques, seven PMPA-protected macaques, and six PMPA-treated, transiently viremic macaques had normal or slightly increased levels of CD4+ cells and CD4/CD8 ratios. Approximately 50% of both mock-treated controls and PMPA-treated macaques showed an intermediate to persistent increase in CD20 cell counts (or B cells).
Evaluation of efficacy.
On the basis of virologic, immunologic, and clinical results, we confirmed our previous finding that PMPA at a dose of 30 mg/kg once daily by subcutaneous injection for 28 days is safe and well tolerated. PMPA treatment that was begun 24 h after viral inoculation and continued for 28 days completely prevented the establishment of persistent SIVmne infection throughout the 46-week study. PMPA treatment for the same duration (28 days) but not begun until 48 or 72 h p.i. was less efficacious. Furthermore, reducing the duration of treatment from 28 days to 10 days diminished protection in one of the four macaques on this regimen, and limiting the duration to 3 days further reduced the efficacy of PMPA against acute SIVmne infection. However, all the macaques that did become infected in the PMPA-treated groups showed delays in PBMC-associated virus, plasma viremia, and antibody responses compared with mock controls.
DISCUSSION
Previously, we showed that PMPA treatment at 30 mg/kg daily for 28 days completely prevented detectable acute SIV infection and establishment of persistent infection in macaques, even when therapy was started 24 h after intravenous virus inoculation (23). In the present study, we explored the temporal parameters of postinoculation PMPA treatment, with three major aims: (i) to understand the impact of increasing delays between inoculation and onset of treatment, as well as the impact of various durations of treatment on antiviral effectiveness; (ii) to identify the most effective regimen for postinoculation PMPA treatment in M. fascicularis inoculated intravenously with SIVmne; and (iii) to gain insight into basic aspects of early SIV viral replication following intravenous inoculation.
Our results show that a 4-week regimen of PMPA completely protects macaques from acute SIV infection and establishment of persistent infection if treatment is initiated within 24 h p.i. but provides less protection if treatment is begun 48 or 72 h p.i. The highest efficacy achieved when treatment was begun 24 h p.i. indicated that the level of virus infection established within 24 h after intravenous inoculation was still low enough to be preventable by an effective (28-day) regimen of antiretroviral treatment, plus perhaps a contribution from immune responses. The antiretroviral effect of PMPA treatment most probably involved blocking the spread of virus from those CD4+ cells already infected by the time treatment was initiated and then maintaining the blockade until this population of cells had decreased through death and clearance, thereby precluding reemergence of extensive viral replication after drug treatment was withdrawn. The failure of similar 28-day treatment regimens beginning 48 or 72 h p.i. implies that within 2 to 3 days of systemic exposure, the virus can establish a level or type of infection that does not decay sufficiently over a 28-day treatment period to preclude reemergence upon withdrawal of treatment. Overall, there seems to be a short temporal window during which postinoculation PMPA treatment can block establishment of persistent infection. After this time, PMPA treatment may dramatically decrease viral replication but cannot prevent or eradicate persistent infection (16, 28, 30).
The duration of postinoculation PMPA treatment also was critical in blocking the establishment of persistent SIV infection. Even when started within 24 h p.i., a 3-day course of PMPA treatment was largely ineffective and a 10-day course protected only half of the macaques tested, while a 28-day course was 100% effective. These results clearly establish that the mechanism of postinoculation PMPA treatment effects is not through the blockade of initial infection by the inoculating virus and provide insight into the size and life span of the infected cell population established in the period between inoculation and the initiation of treatment. The average clearance half life of the productively infected cells contributing the majority of virions to the plasma virus pool in SIV-infected macaques is estimated at less than 2 days (16), similar to values for human immunodeficiency virus (HIV)-infected humans (11, 17, 18, 32). Assuming that complete blockade by PMPA of new infections occurs, the pool of such cells will decay at this rate during the treatment period. In this model, the effectiveness of a given treatment regimen will thus depend on the size of the infected cell pool at the initiation of treatment and on whether the duration of treatment provides a sufficient number of clearance half-lives for the pool of infected cells to decay below the threshold required for reemergence of virus upon discontinuation of treatment. In this model, back-calculation for the number of treatment half-lives comprised by different treatment durations allows provisional estimation of the pool size of productively infected cells present at the initiation of treatment. This depends on assumptions about the pool size of residual productively infected cells required for reemergence of measurable plasma virus following discontinuation of treatment.
One additional factor contributing to differences in the reemergence of virus upon discontinuation of treatment in identically inoculated animals receiving identical PMPA treatment regimens likely involves differences in the size or nature of the infected cell pool present at the initiation of treatment (10, 13, 31). Marked differences in viral replication patterns were observed between identically inoculated mock-treated control animals, similar to other reports (10, 13, 31), with comparable or greater variability in the heterogeneity of viral replication patterns in identically inoculated, identically PMPA-treated animals, except for the uniform protection observed in those that received 28-day treatment beginning 24 h p.i. (group B).
Infection of longer-lived cells may also play a role in the results obtained. Thus, although the majority of virus in the plasma compartment is derived from productively infected cells with a clearance half-life of less than 2 days (16), decay characteristics of the plasma virus compartment with sustained antiretroviral treatment indicate the presence of additional compartments with decay half-lives on the order of approximately 14 to 40 days (14, 17, 18). This compartment may reflect contributions both from virus produced by longer-lived cells such as macrophage lineage cells and from the activation of viral replication from latently infected cells. Recent studies indicate that this latently infected cell compartment can persist for prolonged periods, even in the face of continuous effective antiretroviral treatment that suppresses viral replication to below detectable levels (8, 9, 33). However, the time when this compartment is first established has not been determined. This factor, which is critical for understanding retroviral pathogenesis and designing effective treatment for HIV infection, may also help determine the effectiveness of different postinoculation treatment regimens. Given the prolonged lifetime of these cells, short-term antiretroviral treatment is unlikely to prevent establishment of persistent infection if initiated after the establishment of such a latently infected cell compartment. Identification of the time interval during which this compartment is first established, and evaluation of its contribution to the type of results that we observed, will be important objectives for future studies.
In this study, we also investigated the time kinetics of viral infection, including antibody responses, in macaques that became infected in the face of incompletely protective PMPA treatment. In saline-treated macaques, evidence of productive viral infection developed within 1 to 2 weeks p.i. No such evidence was observed in macaques that received 4 weeks of PMPA treatment starting 24 h p.i. For macaques that achieved incomplete protection, the main effect of PMPA treatment was to delay the onset of viral infection and antibody response until 1 to 3 weeks after PMPA treatment ended. PMPA likely delayed the spread of infection by preventing de novo infection from cells already infected at the initiation of treatment to new uninfected targets. Stopping PMPA treatment before the end of 4 weeks of treatment presumably resulted in the resumption of virus spread from infected cells whose life span exceeded our treatment period, such as latently infected cells with proviral DNA or perhaps infected cells sequestered in sites that are functionally inaccessible to PMPA treatment. However, these PMPA-treated, SIV-infected macaques had markedly different patterns of viral replication, as reflected by plasma SIV RNA measurements, than did mock-treated controls, including 50% of macaques in which plasma SIV RNA was detectable only transiently following the withdrawal of PMPA. These macaques also showed lower antibody responses, consistent with lower levels of viral replication, than did macaques with persistent infection. In these cases, PMPA treatment may have suppressed early virus replication sufficiently to allow development of immune responses capable of holding viral replication comparatively in check upon withdrawal of treatment. The present study did not include a rechallenge with infectious virus of those macaques manifesting only apparently transient infection, to determine whether such transient viral replication was associated with induction of protective immune responses. However, it is intriguing to speculate that such protection, if it occurs, may be similar to the apparent increased resistance to HIV infection and demonstrable immune responses to HIV, seen in some seronegative subjects with repeated exposures, consistent with prior abortive or transient infection (19–21).
There is a need for a regimen of antiretroviral treatment that completely inhibits de novo viral infection and eliminates the long-lived infected cells in HIV type 1 (HIV-1)-infected patients (6, 8, 9, 18). Such a regimen would be clinically beneficial and could prevent the development of drug-resistant viruses. Current regimens can prevent viral replication, but the long-lived infected cells remain a formidable challenge. In this study we found that a 4-week regimen of PMPA treatment beginning 24 h after virus exposure seems to be effective against acute SIV in macaques presumably because it suppresses viral spread during the period usually associated with the initial burst of acute viral replication, allowing decay of the infected cell pool already established by the time treatment is initiated. Unlike PMPA, AZT given postexposure is incompletely effective against acute SIV infection in macaques (15, 24, 29). The greater efficacy of PMPA compared with AZT may be related to the rapid intracellular formation and long half-life of PMPA’s active metabolites in macaques (9a).
The course of acute SIV infection in macaques as well as primary HIV-1 infection in humans suggests that the first week after virus exposure is a critical time during which antiviral therapy can be most effective. Unfortunately, it is not always possible to know when exposure has occurred among the general population. Among the infant population, however, it can be assumed that neonates born to HIV-infected mothers have been exposed to the virus. Epidemiological studies suggest that about 65 to 70% of infants with congenital HIV-1 infection became infected shortly before or during delivery (5, 7, 12). Beginning PMPA treatment regimen within 24 h after birth could have a significant role in reducing the risk of maternal transmission of HIV-1 infection to these infants.
ACKNOWLEDGMENTS
This study was supported in part by USPHS NIH NIAID contracts N01-AI-15120 and N01-AI-65311 and by NIH grant RR00166.
We thank Roberta Black for invaluable consultation on this study and for review of the manuscript, T. A. Wiltrout for technical assistance with RT-PCR SIV RNA, and Marj Domenowske for illustration service.
REFERENCES
- 1.Balzarini J, Zhang H, Herdewijn P, Johns D G, De Clercq E. Intracellular metabolism and mechanism of anti-retrovirus action of 9-(2-phosphonylmethoxyethyl)adenine, a potent anti-human immunodeficiency virus compound. Proc Natl Acad Sci USA. 1991;88:1499–1503. doi: 10.1073/pnas.88.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D, DeClerq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(-2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–338. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benveniste R E, Arthur L O, Tsai C-C, Sowder R, Copeland T D, Henderson L E, Oroszlan S. Isolation of a lentivirus from a macaque with lymphoma: comparison to HTLV-III/LAV and other lentiviruses. J Virol. 1986;60:483–490. doi: 10.1128/jvi.60.2.483-490.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benveniste R E, Morton W R, Clark E A, Tsai C-C, Ochs H D, Ward J M, Kuller L, Knott W B, Hill R W, Gale M J, Thouless M E. Inoculation of baboons and macaques with simian immunodeficiency virus/mne, a primate lentivirus closely related to human immunodeficiency virus type 2. J Virol. 1988;62:2091–2101. doi: 10.1128/jvi.62.6.2091-2101.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertolli J, St. Louis M E, Simonds R J, Nieburg P, Kamenga M, Brown C, Tarande M, Quinn T, Ou C Y. Estimating the timing of mother-to-child transmission of human immunodeficiency virus in a breast-feeding population in Kinshasa, Zaire. J Infect Dis. 1996;174:722–726. doi: 10.1093/infdis/174.4.722. [DOI] [PubMed] [Google Scholar]
- 6.Cavert W, Notermans D W, Staskus K, Wietgrefe S W, Zupancic M, Gebhard K, Henry K, Zhang Z Q, Mills R, McDade H, Gouldsmit J, Danner S A, Haase A T. Kinetics of response in lymphoid to antiretroviral therapy of HIV-1 infection. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 7.Chouquet C, Burgard M, Richardson S, Rouzioux C, Costagliola D. Timing of mother-to-child HIV-1 transmission and diagnosis of infection based on polymerase chain reaction in the neonatal period by a non-parametric method. AIDS. 1997;11:1183–1199. doi: 10.1097/00002030-199709000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho D D, Richman D D, Siliciano R F. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 9a.Fridland, A. Personal communication.
- 10.Hirsch V M, Fuerst T R, Sutter G, Carroll M W, Yang L C, Goldstein S, Piatak M, Elkins W R, Montefiori D C, Moss B, Lifson J D. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J Virol. 1996;70:3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho D D, Neumann A U, Perelson A S, Chen W, Leonard J M, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 12.Kalish L A, Pitt J, Lew J, Landesman S, Diaz C, Hershow R, Hollinger F B, Pagano M, Smeriglio V, Moye J for the Women and Infants Transmission Study (WITS) Defining the time of fetal or perinatal acquisition of human immunodeficiency virus type 1 infection on the basis of age at first positive culture. J Infect Dis. 1997;175:712–715. doi: 10.1093/infdis/175.3.712. [DOI] [PubMed] [Google Scholar]
- 13.Lifson J D, Nowak M, Goldstein S, Rossio J L, Kinter A, Vasquez G, Wiltrout T A, Bromn C, Schneider D, Wahl L, Lloyd A, Elkins W R, Fauci A S, Hirsch V M. The extent of early viral replication is a critical determinant of the natural history of AIDS virus infection. J Virol. 1997;75:9508–9514. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lifson, J. D., M. Nowak, A. Lloyd, and V. M. Hirsch. Clues to primate lentiviral pathogenesis from the study of SIV dynamics. In M. Girrard and B. Dodet (ed.), Retroviruses of human AIDS and related animal diseases, in press. Pasteur Vaccins, Paris, France.
- 15.Martin L N, Murphey-Corb M, Soike K F, Davison-Fairburn B, Baskin G B. Effects of initiation of 3′-azido-3′-deoxythymidine (zidovudine) treatment at different times after infection of rhesus monkeys with simian immunodeficiency virus. J Infect Dis. 1993;168:825–835. doi: 10.1093/infdis/168.4.825. [DOI] [PubMed] [Google Scholar]
- 16.Nowak M A, Lloyd A L, Vasquez G M, Wiltrout T A, Wahl L M, Bischofberger N, Williams J, Kinter A, Fauci A S, Hirsch V M, Lifson J D. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J Virol. 1997;71:7518–7525. doi: 10.1128/jvi.71.10.7518-7525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 18.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D. Decay characteristics of HIV-infected compartments during combination therapy. Nature. 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 19.Rowland-Jones S L, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, Whitby D, Sabally S, Gallimore A, Corrah T. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 20.Rowland-Jones S L, McMichael A. Immune responses in HIV-exposed seronegatives: have they repelled the virus? Opin Immunol. 1995;7:448–455. doi: 10.1016/0952-7915(95)80087-5. [DOI] [PubMed] [Google Scholar]
- 21.Shearer G M, Clerici M. Protective immunity against HIV infection: has nature done the experiment for us? Immunol Today. 1996;17:21–24. doi: 10.1016/0167-5699(96)80564-0. [DOI] [PubMed] [Google Scholar]
- 22.Suryanarayana K, Wiltrout T A, Vasquez G M, Hirsch V M, Lifson J D. Plasma SIV RNA viral load by real time quantification of product generation in RT PCR. AIDS Res Hum Retroviruses. 1998;14:183–189. doi: 10.1089/aid.1998.14.183. [DOI] [PubMed] [Google Scholar]
- 23.Tsai C-C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 24.Tsai C-C, Follis K E, Grant R F, Nolte R E, Bartz C R, Benveniste R E, Sager P R. Effect of dosing frequency on ZDV prophylaxis in macaques infected with simian immunodeficiency virus. J Acquired Immune Defic Syndr. 1993;6:1086–1092. [PubMed] [Google Scholar]
- 25.Tsai C-C, Follis K E, Grant R F, Nolte R E, Wu H, Benveniste R E. Infectivity and pathogenesis of titered dosages of simian immunodeficiency virus (SIV) experimentally inoculated into longtailed macaques (Macaca fascicularis) Lab Anim Sci. 1993;43:411–416. [PubMed] [Google Scholar]
- 26.Tsai C-C, Follis K E, Sabo A, Grant R F, Bartz C, Nolte R E, Benveniste R E, Bischofberger N. Preexposure prophylaxis with 9-(2-phosphonylmethoxyethyl)adenine against simian immunodeficiency virus infection in macaques. J Infect Dis. 1994;169:260–266. doi: 10.1093/infdis/169.2.260. [DOI] [PubMed] [Google Scholar]
- 27.Tsai C-C, Follis K E, Yarnall M, Deaver L E, Benveniste R E, Sager P R. In vitro screening for antiretroviral agents against simian immunodeficiency virus (SIV) Antiviral Res. 1990;14:87–98. doi: 10.1016/0166-3542(90)90046-a. [DOI] [PubMed] [Google Scholar]
- 28.Tsai C-C, Follis K E, Beck T W, Sabo A, Bischofberger N, Dailey P J. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res Hum Retroviruses. 1997;13:707–712. doi: 10.1089/aid.1997.13.707. [DOI] [PubMed] [Google Scholar]
- 29.Van Rompay K K A, Marthas M L, Ramos R A, Mandel C P, McGowan E K, Joye S M, Pederson N C. Simian immunodeficiency virus (SIV) infection of infant rhesus macaques as a model to test antiretroviral drug prophylaxis and therapy; oral 3′-azido-3′-deoxythymidine prevents SIV infection. Antimicrob Agents Chemother. 1992;36:2381–2386. doi: 10.1128/aac.36.11.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Rompay K K A, Cherrington J M, Marthas M L, Berardi C J, Mulato A S, Spinner A, Tarara R P, Canfield D R, Telm S, Bischofberger N, Pederson N C. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob Agents Chemother. 1996;40:2586–2591. doi: 10.1128/aac.40.11.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P R, Hu S-L, Haigwood N L. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load in early infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei H, Ghosh S K, Taylor M E, Johnson V A, Emini E R, Deutsch P, Lifson J D, Bonhoeffer S, Nowak M A, Hahn B H, Saag M S, Shaw G M. Viral dynamics in human immunodeficiency virus type 1 infection. Nature. 1995;373:117–122. doi: 10.1038/373117a0. [DOI] [PubMed] [Google Scholar]
- 33.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]