Abstract
Background
Patients with predominantly antibody deficiency (PAD) have lower anti–severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike antibody levels after initial 2-dose SARS-CoV-2 vaccination than healthy controls do; however, the anti-spike antibody responses and neutralization function in patients with PAD following subsequent immunizations remain understudied.
Objective
We sought to characterize anti-spike antibody responses in adults with PAD over the course of 5 SARS-CoV-2 vaccine doses and identify diagnostic and immunophenotypic risk factors for low antibody response.
Methods
We evaluated anti-spike antibody levels in 117 adult patients with PAD and 192 adult healthy controls following a maximum of 5 SARS-CoV-2 immunizations. We assessed neutralization of the SARS-CoV-2 wild-type strain and the Omicron BA.5 variant and analyzed infection outcomes.
Results
The patients with PAD had significantly lower mean anti-spike antibody levels after 3 SARS-CoV-2 vaccine doses than the healthy controls did (1,439.1 vs 21,890.4 U/mL [P < .0001]). Adults with secondary PAD, severe primary PAD, and high-risk immunophenotypes had lower mean anti-spike antibody levels following vaccine doses 2, 3, and/or 4 but not following vaccine dose 5. Compared with patients with mild and moderate PAD, patients with severe PAD had a higher rate of increase in anti-spike antibody levels over 5 immunizations. A strong positive correlation was observed between anti-spike antibody levels and neutralization of both the SARS-CoV-2 wild-type strain and the Omicron BA.5 variant. Most infections were managed on an outpatient basis.
Conclusions
In all of the patients with PAD, anti-spike antibody levels increased with successive SARS-CoV-2 immunizations and were correlated with neutralization of both the SARS-CoV-2 wild-type strain and the Omicron BA.5 variant. Secondary PAD, severe primary PAD, and high-risk immunophenotypes were correlated with lower mean anti-spike antibody levels following vaccine doses 2 through 4. Patients with severe PAD had the highest rate of increase in anti-spike antibody levels over 5 immunizations. These data suggest a clinical benefit to sequential SARS-CoV-2 immunizations, particularly among high-risk patients with PAD.
Key words: Predominantly antibody deficiency, SARS-CoV-2, common variable immunodeficiency, anti-spike antibody, Omicron BA.5 variant, neutralization, CD19+ B cells, CD4+ T cells, class-switched memory B cells, rituximab
Predominantly antibody deficiency (PAD) is the most frequently diagnosed inborn error of immunity and the most widespread primary immunodeficiency disorder globally. PAD is defined clinically by increased susceptibility to infection, low antibody levels, and/or impaired vaccine responses.1,2 The spectrum of PAD disease ranges from infectious manifestations only to complications including autoimmunity and lymphoproliferative disease, with the potential for end-organ damage and reduced life expectancy.3
Current research indicates that immunodeficient individuals who contract severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) often have more severe illness and a higher mortality rate than the overall population.4 The data specific to individuals with PAD show increased morbidity due to SARS-CoV-2 infection than among the general population, with approximately 9% to 10% of patients with PAD progressing to severe infection requiring hospitalization.5,6 Severe SARS-CoV-2 cases have been further linked to specific primary and/or secondary defects in immune defense pathways.7,8 Together, these findings suggest that patient immunophenotypes may affect both risk for and protection against SARS-CoV-2.
Anti-spike antibody levels are a known correlate of protection against severe SARS-CoV-2 infection.9, 10, 11 Data from our group and others have shown that patients with an underlying immune deficiency diagnosis, including PAD, demonstrate lower anti-spike antibody responses to the initial 2-dose SARS-CoV-2 vaccination series than healthy controls do.12, 13, 14, 15 Prior studies have revealed that the underlying severity of PAD and immunophenotypic markers of disease severity correlate with vaccination response, including lower anti-spike antibody levels in patients with secondary PAD (such as following a B-cell depletion therapy) and severe primary PAD (such as in those with low CD4+ T-cell counts, low CD19+ B-cell counts, and low class-switched memory B-cell counts).12
As the SARS-CoV-2 pandemic has evolved, additional vaccine doses have been recommended by the US Centers for Disease Control and Prevention.16 However, data on the SARS-CoV-2 vaccination response, neutralization response, and infectious outcomes in patients with PAD are limited. We sought to characterize these responses and outcomes over 5 SARS-CoV-2 vaccine doses in patients with PAD, with a focus on diagnostic and immunophenotypic risk factors, as well as to investigate the neutralization of both wild-type SARS-CoV-2 and the Omicron BA.5 variant.
Methods
Informed consent was obtained from patients with PAD at Mass General Brigham under an institutional review board–approved protocol (protocol no. 2021P002414), as previously described.12 Adult patients with PAD (aged >18 years) who had received the initial 2-dose SARS-CoV-2 vaccination series between December 18, 2020, and June 14, 2022, were included and longitudinally followed to assess anti-spike antibody levels over sequential SARS-CoV-2 immunizations. Informed consent was also obtained from healthy controls at Mass General Brigham under institutional review board protocols (protocol nos. 2020P001081 and 2020P002274), as described in previously published cohorts.17,18 Adult healthy controls (aged >18 years) had anti-spike antibodies to SARS-CoV-2 evaluated following up to a total of 3 SARS-CoV-2 vaccines.
PAD diagnoses were confirmed by manual chart review by a clinical immunologist and met the consensus definitions.1,19,20 In all, 7 patients did not have samples available for a serial dilution measure of an exact titer and were excluded from further analysis. A total of 117 patients with PAD were included. Patients with any confounding variables at the time of immunodeficiency diagnosis (eg, clonal lymphocyte population or ongoing immunosuppression) were classified as having secondary PAD. Patients with primary PAD were subclassified as having mild antibody deficiency (IgG subclass deficiency, specific antibody deficiency, and primary hypogammaglobulinemia), moderate antibody deficiency (uncomplicated common variable immunodeficiency [CVID]), and severe antibody deficiency (complicated PAD that encompassed the diagnoses of activated phosphoinositide 3-kinase delta syndrome, transmembrane activator and CAML interactor (TACI) deficiency, nuclear factor kappa B subunit 1 [NFKB1] deficiency, and complicated CVID or specific antibody deficiency, defined as a presence of co-occurring autoinflammatory clinical features20 but without a known genetic etiology).
We evaluated subjects’ demographic and clinical characteristics, including type of PAD and vaccine type(s) received. Immune testing available in the electronic medical record was reviewed at time points both before first immunoglobulin replacement therapy (IgRT) (for immunoglobulin levels, specifically) and in closest proximity to SARS-CoV-2 vaccination (for peripheral blood flow cytometry, specifically). Recent treatment regimens were examined, with a focus on B-cell depletion therapy (eg, rituximab) received in close proximity to vaccination (defined as 6 months before or 1 month after the initial 2-dose series or the last vaccine dose received). We evaluated for a history of SARS-CoV-2 infection, infection outcomes, use of preexposure prophylaxis with tixagevimab and cilgavimab (Evusheld, AstraZeneca, Cambridge, United Kingdom), use of any antiviral rescue therapy, and level of medical care needed.
Serologic assays were performed through the Massachusetts General pathology laboratory using the Roche Elecsys Anti–SARS-CoV-2 S-antibody test (evaluating antibodies to the SARS-CoV-2 spike (S) protein receptor binding domain [“anti-spike antibody”]) and the Roche Elecsys Anti–SARS-CoV-2 N-antibody test (evaluating antibodies to the SARS-CoV-2 nucleocapsid domain [“antinucleocapsid antibody”]). These tests are semiquantitative and correlate with neutralizing immunity.21,22 The Roche S-antibody assay reports results in absorbance units per mL (U/mL), with values of 0.8 U/mL or higher considered reactive, and the Roche N-antibody assay reports that cutoff index values of 1.0 or higher are considered reactive.23
Neutralization was measured using a SARS-CoV-2 pseudovirus neutralization assay as previously described,12,18 evaluating wild-type SARS-CoV-2 and the Omicron BA.5 variant. Briefly, lentiviral particles encoding luciferase and ZsGreen reporter genes were pseudotyped with SARS-CoV-2 spike protein and produced in 293T cells, titered using ZsGreen expression by flow cytometry, and used in an automated neutralization assay with 50 to 250 infectious units of pseudovirus coincubated with 3-fold serial dilutions of serum for 1 hour. Neutralization was determined on 293T-ACE2 cells. Percentage of neutralization was determined by subtracting background luminescence measured in cell control wells (cells only) from sample wells and dividing by virus control wells (virus and cells only). The values of the half-maximum effective concentration of each sample against the pseudoviruses (pNT50) were calculated by taking the inverse of the 50% inhibitory concentration.
All anti-spike antibody levels were log-transformed and are reported as geometric means (±95% CI). To account for differences in time from last vaccination to anti-spike antibody measurement, 1-way analysis of covariance was used to compare anti-spike antibody levels after each SARS-CoV-2 vaccine dose, and the adjusted geometric means are reported. The Spearman rank correlation coefficient (ρ) was used to examine anti-spike antibody levels in relation to neutralization function and immunophenotype. A mixed linear model with random intercept and slope was used to analyze the extent to which anti-spike antibody levels changed over SARS-CoV-2 immunizations while adjusting for time since vaccination, rituximab use, IgRT, tixagevimab and cilgavimab prophylaxis, and acquired SARS-CoV-2 infections. The linear mixed model was implemented using the lme4 package24 in R, version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria). Statistical analyses were completed with SAS 9.4 (SAS Institute, Cary, NC) and Prism, version 7.01 (Reston, Va); 2-tailed P values less than .05 were considered significant.
Results
Demographics of patients with PAD
This study included 192 adult healthy controls and 117 adult patients with PAD (88.9% with primary PAD and 11.1% with secondary PAD). In contrast to the healthy controls, the patients with PAD were significantly older (average age 52.5 vs 47.5 years), predominantly non-Hispanic White (98.3%), and mainly female (68.4%) (Table I). Patients with primary PAD were stratified by their disease severity; 23.1% had mild PAD, 31.7% had moderate PAD, and 45.2% had severe PAD. All of the patients with PAD received at least 2 doses of a SARS-CoV-2 vaccine, with 93.2%, 67.5%, and 38.5% of them additionally receiving vaccine doses 3, 4, and 5, respectively. For their initial immunization, 41.9% of the patients with PAD received the mRNA-1273 (Moderna, Cambridge, Mass) vaccine, 49.6% received the BNT162b2 (Pfizer, New York, NY) vaccine, and 8.6% received the Ad26.COV2.S (Janssen, Titusville, NJ) vaccine. All subsequent vaccinations beyond the primary series in the PAD cohort were mRNA-based vaccine platforms (mRNA-1273 [Moderna] or BNT162b2 [Pfizer]). The earliest introduction of the bivalent vaccine, directed against both the wild-type strain and Omicron BA.5 variant, occurred at vaccine dose 4 in the PAD cohort, with 37 patients with PAD (31.6%) receiving a bivalent vaccine at any time. The average time between a vaccine dose and subsequent anti-spike antibody measurement was between 51.9 and 83.3 days for patients with PAD, which was statistically different between the healthy controls and patients with PAD for vaccine doses 2 (P < .0001) and 3 (P < .0001) (Table I). Therefore, analysis of covariance was used to compare anti-spike antibody levels after each SARS-CoV-2 vaccine dose, adjusting for time since last vaccination.
Table I.
Patient demographics
| Characteristic | PAD (n = 117) | Healthy controls (n = 192) | P value |
|---|---|---|---|
| Age (y), average (SD) | 52.5 (15.9) | 47.5 (13.5) (n missing = 60) |
.008 |
| Age (y), min-max | 19-81 | 23-76 | |
| Female sex, % (no.) | 68.4 (80) | 55.3 (104) | .02 |
| Race and ethnicity, % (no.) | |||
| Non-Hispanic White | 98.3 (115) | 25.3 (48) | <.0001 |
| Unknown | 0 | 1 (2) | — |
| Vaccine, initial dose, % (no.) | |||
| mRNA-1273 (Moderna) | 41.9 (49) | 19.5 (37) | — |
| BNT162b2 (Pfizer) | 49.6 (58) | 54.7 (104) | — |
| Ad26.COV2.S (Janssen) | 8.6 (10) | 25.3 (48) | — |
| Unknown | 0 | 1.6 (3) | — |
| SARS-CoV-2 vaccines received, % (no.) | |||
| Dose 1 | 100 (117) | 100 (192) | — |
| Dose 2 | 100 (117) | 76.0 (146) | — |
| Dose 3 | 93.2 (109) | 39.1 (75) | — |
| Dose 4 | 67.5 (79) | 0 | — |
| Dose 5 | 38.5 (45) | 0 | — |
| Dose >5 | 10.3 (12) | 0 | — |
| Bivalent SARS-CoV-2 vaccines received, % (no.) | |||
| At any time | 31.6 (37) | — | — |
| At dose 1 | 0 | — | — |
| At dose 2 | 0 | — | — |
| At dose 3 | 0 | — | — |
| At dose 4 | 4.3 (5) | — | — |
| At dose 5 | 23.1 (27) | — | — |
| After dose 5 | 5.1 (6) | — | — |
| Average time, vaccine to blood draw (d), % (no.) | |||
| Dose 1 | 70.6 (11) | 71.8 (78) | 1.0 |
| Dose 2 | 61.8 (67) | 154.8 (118) | <.0001 |
| Dose 3 | 83.3 (76) | 25.8 (59) | <.0001 |
| Dose 4 | 58.1 (53) | — | |
| Dose 5 | 51.9 (31) | — | |
| Average time between vaccines (d), % (no.) | |||
| Between doses 1 and 2 | 38.8 (117) | 61.7 (146) | — |
| Between doses 2 and 3 | 182.1 (107) | 224.9 (75) | — |
| Between doses 3 and 4 | 210.7 (79) | — | — |
| Between doses 4 and 5 | 189.4 (45) | — | — |
| After dose 5 | 127.1 (12) | — | — |
| PAD type, % (no.) | |||
| CVID | 28.2 (33) | — | — |
| Complicated PAD | 40.2 (47) | — | — |
| Secondary hypogammaglobulinemia | 11.1 (13) | — | — |
| Primary hypogammaglobulinemia | 9.4 (11) | — | — |
| Specific antibody deficiency | 9.4 (11) | — | — |
| IgG subclass deficiency | 1.7 (2) | — | — |
| Immunosuppression (around SARS-CoV-2 vaccine), % (no.) | |||
| Yes: any, 1 mo after | 23.1 (27) | — | — |
| Yes: any, 1 mo before | 22.2 (26) | — | — |
| Yes: B-cell depletion, 6 mo before to 1 mo after | 8.6 (10) | — | — |
| No. of SARS-CoV-2 infections, % (no.) | |||
| 0 | 58.1 (68) | — | — |
| 1 | 33.3 (39) | — | — |
| 2 | 7.7 (9) | — | — |
| 3 | 0.9 (1) | — | — |
| Timing of SARS-CoV-2 infections, % (no.) | |||
| Before dose 1 | 5.1 (6) | — | — |
| Between dose 1 and 2 | 0.9 (1) | — | — |
| Between dose 2 and 3 | 7.7 (9) | — | — |
| Between dose 3 and 4 | 18.0 (21) | — | — |
| Between dose 4 and 5 | 11.1 (13) | — | — |
| After dose 5 | 3.4 (4) | — | — |
| Positive for nucleocapsid antibody, % (no.) | |||
| All patients with PAD | 82.2 (60) (n missing = 44) |
— | — |
| Receiving prior IgRT | 89.5 (17) | — | |
| Not receiving prior IgRT | 79.6 (43) | — | .49∗ |
| With prior SARS-CoV-2 infection | 91.4 (53) | — | |
| Without prior SARS-CoV-2 infection | 46.7 (7) | — | .0004∗ |
Min-max, Minimum-maximum.
P value according to the Fisher exact test.
Antibody response to SARS-CoV-2 vaccine is lower in patients with PAD than in healthy controls
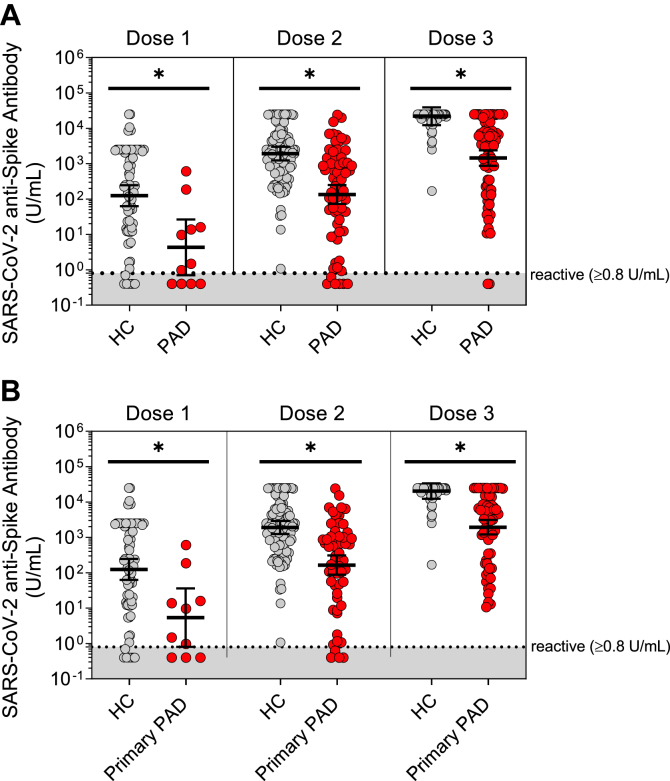
We observed significantly lower anti-spike antibody levels in the patients with PAD than in the healthy controls following up to 3 SARS-CoV-2 vaccine doses (Fig 1, A). Following vaccine dose 3, the mean anti-spike antibody level in the patients with PAD was 1,439.1 U/mL, compared with 21,890.4 U/mL in the healthy controls (P < .0001).
Fig 1.
Mean anti-spike antibody levels following 3 SARS-CoV-2 vaccine doses in adults with PAD versus in healthy controls. SARS-CoV-2 anti-spike antibody levels (in U/mL), shown in log scale as geometric means, adjusted for time from last vaccination. A, Levels compared between healthy adult controls (HCs) (gray circles [n = 192]) and all adult patients with PAD (red circles [n = 117]) after vaccination doses 1 to 3, as indicated. B, Levels compared between adult HCs (gray circles [n = 192]) and adult patients with primary PAD (red circles [n = 104]) after vaccination doses 1 to 3, as indicated. Symbols represent unique individuals, bars represent adjusted geometric means (95% CIs) of total indicated patients (n), and shading represents the assay’s lower limit of reactivity. ∗P < .05 by analysis of covariance analysis.
Given that secondary PAD is a known risk factor for lower anti-spike antibody responses,12 we performed additional analyses excluding patients with secondary PAD and still found that the patients with primary PAD produced significantly lower mean anti-spike antibody levels than the healthy controls following up to 3 SARS-CoV-2 vaccine doses (1,942.5 vs 20,422.3 U/mL [P < .0001] [Fig 1, B]).
Anti-spike antibody levels positively correlate with neutralization of the wild-type and Omicron BA.5 variant of SARS-CoV-2 in patients with PAD
Among the patients with primary PAD, there was a strong positive correlation (ρ = 1.0; P < .0001) between anti-spike antibody levels and neutralization of wild-type SARS-CoV-2 after 2 doses of the SARS-CoV-2 vaccine, with the correlation maintaining significance through vaccine dose 4 (Table II). In contrast, no significant correlation was detected for the Omicron BA.5 variant after 2 doses of the SARS-CoV-2 vaccine, potentially because of patient exposure to only the monovalent vaccine and wild-type SARS-CoV-2 strain at that time. We observed a significant positive correlation following vaccine doses 3 (ρ = 0.8; P < .0001) and 4 (ρ = 0.6; P = .003) for the Omicron BA.5 variant, which is consistent with the peak prevalence of this variant and the first introduction of the SARS-CoV-2 bivalent vaccine at vaccine dose 4 in the PAD cohort. Stratifying by all patients with PAD who had (n = 6) or had not (n = 58) received a bivalent SARS-CoV-2 vaccine at any time, we observed a trend toward higher neutralization of the Omicron BA.5 variant in patients with PAD who had received a bivalent vaccine, although this difference did not reach statistical significance (half-maximum effective concentration of each sample against the pseudoviruses [pNT50] = 366.5 vs 139.8 [P = .5] [see Table E1 in the Online Repository at www.jaci-global.org]).
Table II.
Correlation between anti-spike antibody level and neutralization function among patients with primary PAD
| Strain | Spearman rank correlation coefficient |
|||
|---|---|---|---|---|
| Post–dose 2 coefficient (P value) n = 5 | Post–dose 3 coefficient (P value) n = 27 | Post–dose 4 coefficient (P value) n = 24 | Post–dose 5 coefficient (P value) n = 6 | |
| Wild-type SARS-CoV-2 |
1.00 (<.0001) | 0.82 (<.0001) | 0.62 (.002) | 0.94 (.06) |
| Omicron BA. 5 variant |
0.90 (.3) | 0.77 (<.0001) | 0.60 (.003) | 0.66 (.3) |
Patients with PAD with secondary and severe primary disease have lower anti-spike antibody levels following up to 4 SARS-CoV-2 vaccines
Secondary PAD is a known risk factor for low anti-spike antibody levels following an initial 2-dose SARS-CoV-2 vaccination.12 Therefore, we chose to compare vaccine responses between adults with primary and secondary PAD following subsequent SARS-CoV-2 vaccines. Patients with secondary PAD, as compared with primary PAD, trended toward lower anti-spike antibody levels following vaccine dose 3 (393.0 vs 2,102.4 U/mL [P = .05]), with the difference reaching statistical significance following vaccine dose 4 (459.0 vs 5,018.0 U/mL [P = .005]). In contrast, anti-spike antibody levels were not statistically different between patients with secondary and primary PAD following vaccine dose 5 (4,387.0 vs 8,903.9 U/mL [P = .2] [Fig 2, A]).
Fig 2.
Mean anti-spike antibody levels following 5 SARS-CoV-2 vaccine doses, compared between patients with PAD by clinical disease type. SARS-CoV-2 anti-spike antibody levels (in U/mL), shown in log scale as geometric means, adjusted for time from last vaccination. A, Levels compared between adult patients with secondary PAD (purple circles [n = 13]), and adult patients with primary PAD (red circles [n = 104]), after vaccination doses 2 to 5, as indicated. B, Symbols with bars represent adjusted geometric means (±95% CIs) of the patients with mild (green circles), moderate (orange circles), and severe (red circles) primary PAD, and the dotted line indicates mean SARS-CoV-2 anti-spike antibody levels in adult healthy controls (HCs) following vaccine dose 3 as a reference. C, Patients segregated by disease severity after vaccine dose 2 (n = 57), dose 3 (n = 64), dose 4 (n = 47), and dose 5 (n = 26) in adult patients with mild (green circles [n = 24]), moderate (orange circles [n = 33]), and severe (red circles [n = 47]) primary PAD. Symbols represent unique individuals, bars represent adjusted geometric means (±95% CIs) of the total indicated patients (n), respectively; shading represents the assay’s lower limit of reactivity, and the dotted line indicates mean SARS-CoV-2 anti-spike antibody levels in adult healthy controls (HCs) following vaccine dose 3 as a reference. ∗P < .05 by analysis of covariance analysis. In post hoc analysis, statistical significance was driven by differences between the groups with severe versus mild and moderate PAD.
Among the patients with primary PAD, our group previously reported lower anti-spike antibody levels following initial 2-dose SARS-CoV-2 immunization in patients with severe disease than in those with moderate and mild disease.12 Here, we further compared SARS-CoV-2 vaccine responses between the primary PAD disease severity groups following up to 5 vaccine doses. We observed lower anti-spike antibody levels in patients with severe PAD than in patients with moderate and mild PAD following vaccine doses 2 (64.2 vs 707.0 vs 305.4 U/mL [P = .02]), 3 (755.2 vs 3356.1 vs 8454.3 U/mL [P = .001]), and 4 (2729.2 vs 9857.0 vs 6951.4 U/mL [P = .02] [Fig 2, B and C]). In post hoc analysis, statistical significance was driven by differences between the groups with severe versus mild and moderate PAD. This finding may be explained by the more severe immunophenotypes seen in patients with severe PAD.12 In contrast, no significant differences in anti-spike antibody levels were observed in patients with mild PAD versus in patients with moderate PAD versus in patients with severe PAD after vaccine dose 5 (7,563.4 vs 12,280.8 vs 6,674.8 U/mL [P = .2]).
Patients with primary PAD with high-risk immunophenotypes have lower anti-spike antibody levels following up to 4 SARS-CoV-2 vaccines
We further investigated anti-spike antibody responses in patients with PAD with high-risk immunophenotypes, such as low absolute CD19+ B-cell count, low absolute CD4+ T-cell count, and impaired B-cell maturation, as they have previously been identified as correlating with low anti–SARS-CoV-2 spike antibody levels following initial series immunization.12 We collected the results of peripheral blood flow cytometry testing in closest proximity to vaccination, which occurred at a median (quartile 1–quartile 3) of 265 days (range 42.5-444 days) after vaccine dose 1.
Among the patients with PAD, we again observed that low absolute CD19+ B-cell counts correlated with lower anti-spike antibody levels. Following vaccine doses 2 through 4, patients with PAD with B-cell counts of 90 cells/μL or less (the lower limit of the normal adult laboratory reference range) had significantly lower mean anti-spike antibody levels than did those with B-cell counts higher than 90 cells/μL (eg, after dose 4, 1,557.4 vs 7,702.5 U/mL [P = .001] [Fig 3, A and see Fig E1, A in the Online Repository at www.jaci-global.org]). Furthermore, among all immunophenotypes analyzed, total absolute counts of CD19+ B cells emerged as a predominant immunophenotypic factor positively associated with anti-spike antibody levels, as measured by the Spearman rank correlation coefficient following vaccine doses 1 through 4 (Table III). In contrast, following vaccine dose 5, anti-spike antibody levels were no longer significantly lower in patients with low CD19+ B-cell counts than in those with CD19+ B-cell counts in the normal range (5,080.2 vs 10,888.6 U/mL; [P = .1]).
Fig 3.
Mean anti-spike antibody levels following 5 SARS-CoV-2 vaccine doses, compared between patients with PAD by immunophenotype. SARS-CoV-2 anti-spike antibody levels (in U/mL) in adult patients with PAD, shown in log scale as geometric means, adjusted for time from last vaccination, at postvaccine time points, as indicated. Segregated by patients with PAD with absolute B-cell counts higher than 90 cells/μL (blue circles and line) and absolute B-cell counts of 90 cells/μL or lower (orange circles and line) (A), absolute T-cell counts higher than 419.0 cells/μL (blue circles and line) and absolute T-cell counts of 419.0 cells/μL or lower (orange circles and line) (B), and class-switched memory B-cell frequency of at least 2.0% of CD19+ cells (blue circles and line) and class-switched memory B-cell frequency less than 2.0% of CD19+ cells (orange circles and line) (C). Symbols with bars represent adjusted geometric means (±95% CIs) of the total set of patients, and the dotted line indicates mean SARS-CoV-2 anti-spike antibody levels in adult healthy controls (HCs) following vaccine dose 3 as a reference. ∗P < .05 by analysis of covariance analysis.
Table III.
Correlation between anti-spike antibody level and immunophenotype among patients with primary PAD
| Immune parameter | Spearman rank correlation coefficient |
||||
|---|---|---|---|---|---|
| Post–dose 1 coefficient (P value) | Post–dose 2 coefficient (P value) | Post–dose 3 coefficient (P value) | Post–dose 4 coefficient (P value) | Post–dose 5 coefficient (P value) | |
| IgG (mg/dL) | 0.8 (.02) | 0.1 (.4) | 0.2 (.1) | 0.1 (.4) | 0.2 (.3) |
| IgA (mg/dL) | 0.5 (.2) | 0.2 (.1) | 0.4 (.002) | 0.4 (.009) | 0.3 (.2) |
| IgM (mg/dL) | –0.8 (.02) | 0.3 (.05) | 0.3 (.01) | 0.2 (.1) | 0.06 (.8) |
| CD3+ T cells (cells/uL) | –0.05 (.9) | 0.4 (.01) | 0.2 (.2) | 0.2 (.1) | 0.2 (.4) |
| CD4+ T cells (cells/μL) | 0.3 (.5) | 0.4 (.006) | 0.2 (.1) | 0.3 (.08) | 0.2 (.5) |
| CD8+ T cells (cells/μL) | –0.7 (.04) | 0.5 (.1) | 0.1 (.5) | 0.1 (.4) | 0.4 (.06) |
| CD19+ B cells (cells/μL) | 0.8 (.01) | 0.5 (.001) | 0.5 (<.001) | 0.3 (.04) | –0.09 (.7) |
| CD16/56+CD3– natural killer cells (cells/μL) | 0.4 (.4) | 0.2 (.3) | 0.08 (.6) | –0.05 (.7) | –0.1 (.6) |
Similar to our previous findings,12 we found that low absolute CD4+ T-cell counts were significantly associated with lower anti-spike antibody levels. After the initial 2-dose SARS-CoV-2 vaccine series, patients with PAD with CD4+ T-cell counts of 419.0 cells/μL or less (the lower limit of the adult laboratory reference range) had significantly lower anti-spike antibody levels than did patients with PAD with CD4+ T-cell counts higher than 419.0 cells/μL (33.1 vs 289.5 U/mL [P = .04] [Fig 3, B and see Fig E1, B). However, with subsequent SARS-CoV-2 vaccine doses, no statistical differences in anti-spike antibody levels were observed between the 2 cohorts.
Finally, we observed a significant association between low class-switched memory B-cell counts and low anti-spike antibody levels. We found a significant difference in anti-spike antibody levels between patients with less than 2.0% versus at least 2.0% (of total CD19+ cells) class-switched memory B cells after vaccine dose 4 (2,512.1 vs 8,197.8 U/mL [P = .01] [Fig 3, C and see Fig E1, C). Here again, however, there was no statistical difference in mean anti-spike antibody levels between patients with PAD with less than 2.0% versus at least 2.0% class-switched memory B cells following vaccine dose 5 (7,621.3 vs 11,665.0 U/mL [P = .3]).
Confounder analysis results
Rituximab is a B-cell–depleting agent known to inhibit the humoral response to SARS-CoV-2 vaccination in autoimmune and immunodeficient patients.12,25, 26, 27, 28 In this study, we observed significantly lower anti-spike antibody levels in patients with primary PAD with a history of rituximab use than in those without a history of rituximab use after vaccine doses 3 (670.1 vs 2,863.3 U/mL [P = .04]) and 4 (1,432.9 vs 7,105.6 U/mL [P = .001]), but not after vaccine dose 5 (6,641.2 vs 10,024.2 U/mL [P = .3] [Fig 4, A and see Fig E2, A in the Online Repository at www.jaci-global.org]). A similar trend was observed in all patients with PAD, regardless of whether they had the primary or secondary subtype of PAD (see Fig E3, in the Online Repository at www.jaci-global.org).
Fig 4.
Mean anti-spike antibody levels following 5 SARS-CoV-2 vaccine doses, compared between patients with PAD by clinical confounder. SARS-CoV-2 anti-spike antibody levels (in U/mL) in adult patients with primary PAD, shown in log scale as geometric means, adjusted for time from last vaccination, at postvaccine time points, as indicated. Segregated by patients with PAD who received prior rituximab therapy (orange circles and line) and did not receive prior rituximab therapy (blue circles and line) (A), received prior IgRT (orange circles and line) and did not receive prior IgRT (blue circles and line) (B), received prior tixagevimab and cilgavimab (Evusheld [orange circles and line]) and did not receive prior Evusheld (blue circles and line) (C), and had prior SARS-CoV-2 infection (orange circles and line) and did not have prior SARS-CoV-2 infection (blue circles and line) (D). Symbols with bars represent adjusted geometric means (±95% CIs) of the total set of patients, and the dotted line indicates mean SARS-CoV-2 anti-spike antibody levels in adult healthy controls (HCs) following vaccine dose 3 as reference. ∗P < .05 by analysis of covariance analysis.
To test the theory that passive antibody transfer may produce higher anti-spike antibody levels in patients receiving IgRT, we compared anti-spike antibody levels between patients who did (n = 85) and did not (n = 19) receive IgRT (Fig 4, B and see Fig E2, B). We found that patients who received IgRT had significantly lower anti-spike antibody levels than those patients who did not receive IgRT, specifically after vaccine doses 3 and 4. This lower response may be explained by the more severe immunophenotypes seen in the patients who received IgRT. After vaccine dose 5, there was no significant difference in anti-spike antibody levels between those who did and did not receive IgRT (9,194.6 vs 7,437.9 U/mL [P = .7]). In addition, the rate of seropositivity for antinucleocapsid antibody trended to be higher in patients with PAD who received IgRT than in those who did not receive IgRT. However, this difference did not reach statistical significance (Table I).
We evaluated anti-spike antibody responses between those patients who received tixagevimab and cilgavimab prophylaxis (n=15) and those who did not (n=89). Notably, no significant differences in anti-spike antibody levels were observed between these 2 cohorts, even up to vaccine dose 5 (Fig 4, C and see Fig E2, C).
Similarly, no significant differences in anti-spike antibody levels were observed between patients who had a natural history of SARS-CoV-2 infection at any time during the study (n = 28) and those with no such history (n = 76) (Fig 4, D and see Fig E2, D). As expected, a natural history of SARS-CoV-2 infection was strongly correlated with seropositivity for antinucleocapsid antibody (91.4 vs 46.7% [P = .0004] [Table I]).
Lastly, there were no significant differences in anti-spike antibody levels between patients younger than 50 years and patients aged 50 years or older, although anti-spike antibody levels did increase with cumulative SARS-CoV-2 vaccine doses regardless of age (see Fig E4 in the Online Repository at www.jaci-global.org).
Patients with severe PAD have a higher rate of increase in anti-spike antibody levels over 5 sequential SARS-CoV-2 vaccines
Anti-spike antibodies in all of the patients with PAD increased over 5 SARS-CoV-2 vaccine doses (P < .0001) (see Table E2 in the Online Repository at www.jaci-global.org). A mixed linear model was created to analyze the rate of increase in anti-spike antibody levels over time in patients with PAD stratified by disease severity. The unadjusted model demonstrated significantly lower anti-spike antibody levels in patients with severe PAD than in patients mild or moderate PAD at baseline, following vaccine dose 1. Additionally, the unadjusted model demonstrated a higher rate of increase in anti-spike antibody levels over 5 sequential SARS-CoV-2 vaccine doses in patients with severe PAD than in patients with mild or moderate PAD (Fig 5). These findings were significant even after adjustment for a history of rituximab use, IgRT therapy, tixagevimab and cilgavimab prophylaxis, or prior SARS-CoV-2 infection (see Table E3 in the Online Repository at www.jaci-global.org). Additionally, we did not identify a significant difference in the uptake of sequential SARS-CoV-2 vaccine doses, up to vaccine dose 5, between patients with severe PAD and patients with mild and moderate PAD (see Table E4 in the Online Repository at www.jaci-global.org).
Fig 5.
Linear mixed model of SARS-CoV-2 vaccine response among patients with PAD by clinical disease severity. Linear mixed model of mean anti–SARS-CoV-2 spike antibody levels following vaccine doses, as indicated, in patients with primary PAD, stratified by clinical disease severity (mild in green, moderate in orange, and severe in red). Shaded areas represent ±95% CIs. Further adjustment for time since vaccination, use of tixagevimab and cilgavimab, IgRT (mg/kg per month), and prior SARS-CoV-2 infection did not significantly change these findings (see Table E3).
Infection outcomes in patients with PAD
Over the course of this study, there were 36 patients with PAD (30.8%) who contracted SARS-CoV-2 infection once and 9 patients with PAD (7.7%) who contracted SARS-CoV-2 infection more than once. Overall, SARS-CoV-2 infections were well tolerated, with no patient deaths. Of those patients who were infected once, 2 (5.7%) remained asymptomatic and 24 (68.6%) required treatment; of those patients, 15 (60.0%) received nirmatrelvir copackaged with ritonavir (Paxlovid, Pfizer). One patient (2.9%) required hospitalization and another patient (2.9%) required admission to the intensive care unit. Among those patients with PAD who acquired multiple SARS-CoV-2 infections, 1 underwent emergency department evaluation and none were hospitalized. Three patients (33.3%) required treatment with their first infection, and 6 (66.7%) required treatment with their second infection (as detailed in Table E5 in the Online Repository at www.jaci-global.org).
Discussion
Elucidation of the humoral immune response to SARS-CoV-2 vaccination in patients with PAD provides critical information needed by public health officials and clinicians to develop immunization guidelines for patients at high risk for SARS-CoV-2 morbidity.
In this study, we evaluated anti-spike antibody responses in adults with PAD over 5 SARS-CoV-2 vaccine doses and analyzed diagnostic and immunophenotypic risk factors for low antibody response. Consistent with prior research, we observed significantly lower anti-spike antibody levels in adults with PAD than in adult healthy controls. We identified secondary PAD and severe primary PAD as diagnostic risk factors for lower anti–SARS-CoV-2 spike antibody levels, which improved with subsequent vaccine doses (particularly following vaccine dose 5). Of note, patients with severe primary PAD demonstrated a higher rate of increase in anti-spike antibody levels over 5 sequential SARS-CoV-2 vaccine doses than did the cohorts with mild or moderate PAD. These data suggest a cumulative benefit to sequential SARS-CoV-2 immunization in the cohort of patients with severe PAD immunodeficiency, consistent with US Centers for Disease Control and Prevention guidelines outlining additional immunization doses in patients with moderate-to-severe immunocompromise.16
In terms of immunophenotype, we identified low absolute CD19+ B-cell count as a significant risk factor for low anti-spike antibody levels following SARS-CoV-2 immunization. Consistent with prior studies, we also identified low CD4+ T-cell count and low class-switched memory B-cell count as being risk factors for low SARS-CoV-2 vaccine responses in adults with primary PAD. Similar to our findings regarding high-risk clinical phenotypes, we observed that anti-spike antibody levels between low and high-risk immunophenotypes were no longer significantly different after the fifth vaccine dose, indicating that additional SARS-CoV-2 immunization may be particularly beneficial in patients with low CD19+ B-cell counts, low CD4+ T-cell counts, and/or low class-switched memory B-cell counts.
B-cell–depleting agents, such as rituximab, are commonly used in patients with PAD to treat autoimmune and lymphoproliferative complications, but they are associated with lower SARS-CoV-2 vaccine responses.12,25, 26, 27, 28 Similarly, our study showed that rituximab use in patients with PAD was associated with significantly lower anti-spike antibody levels, specifically following vaccine doses 3 and 4. These data may be helpful in guiding clinicians who have patients with PAD who are receiving or about to start receiving a B-cell–depleting agent. Consideration of vaccination with recovery of B-cell counts or spaced 2 to 4 weeks before subsequent rituximab infusions can be considered.29 Continued careful surveillance of this patient population with regard to SARS-CoV-2 is warranted, especially in the dual setting of an immunodeficiency disorder and pharmacologic immunosuppression.
Anti–SARS-CoV-2 spike antibody levels have been correlated with antiviral neutralization function.12 In this study, we found that anti-spike antibody levels did in fact positively correlate with neutralization of both wild-type SARS-CoV-2 and the Omicron BA.5 variant. There was a very strong positive correlation for wild-type SARS-CoV-2 following vaccine doses 2 through 4, and a modestly strong positive correlation for the Omicron BA.5 variant following vaccine doses 3 and 4. Correlation with the Omicron BA.5 variant increased following subsequent vaccinations, likely driven by the development and uptake of the bivalent vaccine and natural exposure to Omicron BA.5 at the later time points.
There are several limitations to our study. Although it was a large study of patients with PAD, the patients with PAD were predominantly female and non-Hispanic White, which demographically, was statistically different from the healthy control cohort. Prior studies have shown differences in anti-spike antibody response based on sex and race, such as longer anti-spike antibody half-lives after SARS-CoV-2 infection in females and higher anti-spike antibody levels after SARS-CoV-2 vaccination among participants who self-identify as White.30,31 Thus, the external validity of this study may be limited, and as a result, there is a need for future studies including patients with PAD who are from more diverse backgrounds and are matched with healthy controls. In addition, the timing between each vaccine dose and its corresponding anti-spike antibody level measurement was not fixed. To account for this, we performed adjusted analyses accounting for the time since vaccination. In the confounder analysis, patients with either continuous or sporadic rituximab therapy use were included, which could have affected the response to vaccination and thus requires future investigation. Our data set was also underpowered to look at sequential B-cell counts during the course of the SARS-CoV-2 vaccination series, and therefore, our data are limited to correlations with single–data point immunophenotypes. It is also important to note that the healthy controls received up to 3 SARS-CoV-2 vaccine doses, whereas the patients with PAD received up to 5. Therefore, we were unable to directly compare data between the 2 cohorts after vaccine dose 3. Although a strength of this study is the assay used to measure anti-spike antibody levels, which allowed for dilutions up to 25,000 U/mL, we acknowledge that testing approaching the upper limit of the assay may be nearing a nonlinear detection range. Finally, we observed strong correlations between anti-spike antibody levels and neutralization function for wild-type SARS-CoV-2 and the Omicron BA.5 variant, but additional research is needed to elucidate the neutralization response to evolving variants and among healthy controls.
In conclusion, this study provides novel insights into anti-spike antibody responses in adults with PAD over 5 sequential SARS-CoV-2 vaccine doses. Risk factors for a lower anti-spike antibody response included secondary PAD, severe primary PAD, rituximab use, low CD19+ B-cell count, low CD4+ T-cell count, and low class-switched memory B-cell count. For most of these risk factors, we identified a significant increase in the anti-spike antibody response by the fifth SARS-CoV-2 vaccine dose, highlighting the importance of additional immunizations in these patients. This study contributes important data regarding response to SARS-CoV-2 vaccination and clinical and immunophenotypic risk factors, which underscore the importance of additional SARS-CoV-2 vaccine doses in patients with PAD.
Disclosure statement
Supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award K23AI163350 [to S.B.]), the American Academy of Allergy Asthma & Immunology (faculty development awards [to S.B. and J.R.F.]), and the National Institute on Minority Health and Health Disparities of the National Institute of Health (award R01MD017816 [to J.R.F.]). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure of potential conflict of interest: J. R. Farmer is an ongoing consultant for Pharming and has received investigator-initiated research grants from Pfizer, Bristol-Myers Squibb, and Pharming with no direct relation to the work presented. The rest of the authors declare that they have no relevant conflicts of interest.
Key messages.
-
•
Diagnostic and immunophenotypic risk factors for lower anti-spike antibody levels following SARS-CoV-2 vaccination (particularly following vaccine doses 2 through 4) in adults with PAD included secondary PAD, severe primary PAD, low CD19+ B-cell count, low CD4+ T-cell count, and low class-switched memory B-cell count.
-
•
In all patients with PAD, anti-spike antibody levels increased over 5 sequential SARS-CoV-2 immunizations and were positively correlated with both wild-type SARS-CoV-2 and Omicron BA.5 variant neutralizations.
-
•
Patients with PAD who are at risk of low anti-spike antibody responses to SARS-CoV-2 immunization were identified, and the need for additional SARS-CoV-2 vaccine doses to optimize anti-spike antibody responses, particularly among high-risk patients with PAD, was highlighted.
Supplementary data
References
- 1.Boni1lla F.A., Khan D.A., Ballas Z.K., Chinen J., Frank M.M., Hsu J.T., et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136:1186. doi: 10.1016/j.jaci.2015.04.049. 205.e1-78. [DOI] [PubMed] [Google Scholar]
- 2.Cheraghi T., Kalantari A., Shabestari M.S., Abolhassani H., Eibel H., Hammarström L. 1st ed. Academic Press; Cambridge, MA: 2021. Inborn errors of immunity. [Google Scholar]
- 3.Durandy A., Kracker S., Fischer A. Primary antibody deficiencies. Nat Rev Immunol. 2013;13:519–533. doi: 10.1038/nri3466. [DOI] [PubMed] [Google Scholar]
- 4.Delavari S., Abolhassani H., Abolnezhadian F., Babaha F., Iranparast S., Ahanchian H., et al. Impact of SARS-CoV-2 pandemic on patients with primary immunodeficiency. J Clin Immunol. 2021;41:345–355. doi: 10.1007/s10875-020-00928-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farmer J.R., Galbraith A., Ong M.S. Association of inborn errors of immunity with severe COVID-19 and post-acute sequelae of COVID-19. J Allergy Clin Immunol Pract. 2023;11:2616. doi: 10.1016/j.jaip.2023.05.029. 7.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weifenbach N., Jung A., Lotters S. COVID-19 infection in CVID patients: what we know so far. Immun Inflamm Dis. 2021;9:632–634. doi: 10.1002/iid3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W., Wang W. Auto-antibodies against type I IFNs are associated with severe COVID-19 pneumonia. Signal Transduct Target Ther. 2021;6:96. doi: 10.1038/s41392-021-00514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheetham N.J., Kibble M., Wong A., Silverwood R.J., Knuppel A., Williams D.M., et al. Antibody levels following vaccination against SARS-CoV-2: associations with post-vaccination infection and risk factors in two UK longitudinal studies. Elife. 2023;12 doi: 10.7554/eLife.80428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldblatt D., Alter G., Crotty S., Plotkin S.A. Correlates of protection against SARS-CoV-2 infection and COVID-19 disease. Immunol Rev. 2022;310:6–26. doi: 10.1111/imr.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perry J., Osman S., Wright J., Richard-Greenblatt M., Buchan S.A., Sadarangani M., et al. Does a humoral correlate of protection exist for SARS-CoV-2? A systematic review. PLoS One. 2022;17 doi: 10.1371/journal.pone.0266852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barmettler S., DiGiacomo D.V., Yang N.J., Lam T., Naranbhai V., Dighe A.S., et al. Response to severe acute respiratory syndrome coronavirus 2 initial series and additional dose vaccine in patients with predominant antibody deficiency. J Allergy Clin Immunol Pract. 2022;10:1622. doi: 10.1016/j.jaip.2022.03.017. 34.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delmonte O.M., Bergerson J.R.E., Burbelo P.D., Durkee-Shock J.R., Dobbs K., Bosticardo M., et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J Allergy Clin Immunol. 2021;148:1192–1197. doi: 10.1016/j.jaci.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Leeuwen L.P.M., GeurtsvanKessel C.H., Ellerbroek P.M., de Bree G.J., Potjewijd J., Rutgers A., et al. Immunogenicity of the mRNA-1273 COVID-19 vaccine in adult patients with inborn errors of immunity. J Allergy Clin Immunol. 2022;149:1949–1957. doi: 10.1016/j.jaci.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tandon M., DiGiacomo D.V., Zhou B., Hesterberg P., Rosenberg C.E., Barmettler S., et al. Response to SARS-CoV-2 initial series and additional dose vaccine in pediatric patients with predominantly antibody deficiency. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1217718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention, COVID-19 vaccines for people who are moderately or severely immunocompromised, Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/immuno.html. Accessed August 20, 2023.
- 17.Naranbhai V., Garcia-Beltran W.F., Chang C.C., Berrios Mairena C., Thierauf J.C., Kirkpatrick G., et al. Comparative immunogenicity and effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 vbaccines. J Infect Dis. 2022;225:1141–1150. doi: 10.1093/infdis/jiab593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Beltran W.F., Lam E.C., St Denis K., Nitido A.D., Garcia Z.H., Hauser B.M., et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell. 2021;184:2523. doi: 10.1016/j.cell.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonilla F.A., Barlan I., Chapel H., Costa-Carvalho B.T., Cunningham-Rundles C., de la Morena M.T., et al. International consensus document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4:38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bousfiha A., Jeddane L., Picard C., Al-Herz W., Ailal F., Chatila T., et al. Human inborn errors of immunity: 2019 update of the IUIS phenotypical classification. J Clin Immunol. 2020;40:66–81. doi: 10.1007/s10875-020-00758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel E.U., Bloch E.M., Clarke W., Hsieh Y.H., Boon D., Eby Y., et al. Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol. 2021;59:e02257. doi: 10.1128/JCM.02257-20. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio-Acero R., Castelletti N., Fingerle V., Olbrich L., Bakuli A., Wolfel R., et al. In search of the SARS-CoV-2 protection correlate: head-to-head comparison of two quantitative S1 Assays in pre-characterized oligo-/asymptomatic patients. Infect Dis Ther. 2021:1–14. doi: 10.1007/s40121-021-00475-x. [DOI] [PubMed] [Google Scholar]
- 23.Suhandynata R.T., Bevins N.J., Tran J.T., Huang D., Hoffman M.A., Lund K., et al. SARS-CoV-2 serology status detected by commercialized platforms distinguishes previous infection and vaccination adaptive immune responses. J Appl Lab Med. 2021;6:1109–1122. doi: 10.1093/jalm/jfab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bates DM M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67:1–48. [Google Scholar]
- 25.Bonelli M.M., Mrak D., Perkmann T., Haslacher H., Aletaha D. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis. 2021;80:1355–1356. doi: 10.1136/annrheumdis-2021-220408. [DOI] [PubMed] [Google Scholar]
- 26.Mrak D., Tobudic S., Koblischke M., Graninger M., Radner H., Sieghart D., et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis. 2021;80:1345–1350. doi: 10.1136/annrheumdis-2021-220781. [DOI] [PubMed] [Google Scholar]
- 27.Troldborg A., Thomsen M.K., Bartels L.E., Andersen J.B., Vils S.R., Mistegaard C.E., et al. Time since rituximab treatment is essential for developing a humoral response to COVID-19 mRNA vaccines in patients with rheumatic diseases. J Rheumatol. 2022;49:644–649. doi: 10.3899/jrheum.211152. [DOI] [PubMed] [Google Scholar]
- 28.Egri N., Calderon H., Martinez R., Vazquez M., Gomez-Caverzaschi V., Pascal M., et al. Cellular and humoral responses after second and third SARS-CoV-2 vaccinations in patients with autoimmune diseases treated with rituximab: specific T cell immunity remains longer and plays a protective role against SARS-CoV-2 reinfections. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1146841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtis J.R., Johnson S.R., Anthony D.D., Arasaratnam R.J., Baden L.R., Bass A.R., et al. American College of Rheumatology Guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: Version 5. Arthritis Rheumatol. 2023;75:E1–E16. doi: 10.1002/art.42372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei J., Matthews P.C., Stoesser N., Maddox T., Lorenzi L., Studley R., et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nature Communications. 2021;12:6250. doi: 10.1038/s41467-021-26479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin C., Nazareth J., Jarkhi A., Pan D., Das M., Logan N., et al. Ethnic differences in cellular and humoral immune responses to SARS-CoV-2 vaccination in UK healthcare workers: a cross-sectional analysis. EClinicalMedicine. 2023;58:101926. doi: 10.1016/j.eclinm.2023.101926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.