Abstract
Patients with type 1 diabetes (T1D) have a greater risk of cardiovascular disease. Proconvertase subtilisin-kexin 9 (PCSK9) is involved in the atherosclerosis process. This study aimed to determine the relationship between PCSK9 levels and epicardial adipose tissue (EAT) volume and cardiometabolic variables in patients with T1D. This was an observational cross-sectional study including 73 patients with T1D. Clinical, biochemical and imaging data were collected. We divided the patients into two groups according to their glycemic control and the EAT index (iEAT) percentile. We performed a correlation analysis between the collected variables and PCSK9 levels; subsequently, we performed a multiple regression analysis with the significant parameters. The mean age was 47.6 ± 8.5 years, 58.9% were men, and the BMI was 26.9 ± 4.6 kg/m2. A total of 31.5%, 49.3% and 34.2% of patients had hypertension, dyslipidemia and smoking habit, respectively. The PCSK9 concentration was 0.37 ± 0.12 mg/L, which was greater in patients with worse glycemic control (HbA1c > 7.5%), dyslipidemia and high EAT volume (iEAT > 75th percentile). The PCSK9 concentration was positively correlated with age (r = 0.259; p = 0.027), HbA1c (r = 0.300; p = 0.011), insulin dose (r = 0.275; p = 0.020), VLDL-C level (r = 0.331; p = 0.004), TG level (r = 0.328; p = 0.005), and iEAT (r = 0.438; p < 0.001). Multiple regression analysis revealed that 25% of the PCSK9 variability was explained by iEAT and HbA1c (p < 0.05). The PCSK9 concentration is associated with metabolic syndrome parameters, poor glycemic control and increased EAT volume in patients with T1D.
Keywords: Type 1 diabetes, Biomarkers, Epicardial adipose tissue, Cardiometabolic risk factors, Cardiometabolic traits, Cardiovascular disease risk
Subject terms: Endocrine system and metabolic diseases, Endocrinology, Medical research, Biomarkers, Predictive markers
Introduction
Patients with type 1 diabetes (T1D) have a greater risk of cardiovascular disease (CVD)1–3 than does the general population. The molecular processes underlying this risk are unclear, and multiple mechanisms have been proposed. Hyperglycemia and hypoglycemia contribute to mechanisms related to atherosclerosis, such as oxidative stress, nonenzymatic glycosylation, endothelial dysfunction and inflammatory pathways3–5. The presence of diabetic kidney disease (DKD), metabolic syndrome, insulin resistance and obesity in patients with T1D dramatically increases the risk of CVD3,4,6. Although plasma cholesterol and triglyceride levels are usually normal in patients with T1D, lipoprotein composition may be altered, with dysfunctional and proatherogenic HDL lipoproteins6, which could partly account for the increased cardiovascular risk. However, these risk factors and comorbidities do not fully explain the heightened risk observed, suggesting that other factors could be involved.
Epicardial adipose tissue (EAT) is a visceral fat depot surrounding the myocardium and coronary arteries under the visceral pericardium. It is an important source of nutrients for cardiomyocytes and favors homeostasis of the myocardium, but in some conditions, it can also produce a wide range of inflammatory mediators7,8. Several studies have demonstrated that increased EAT volume is a cardiovascular risk factor9–11, and Shmilovich et al.13 postulated that the threshold value of the EAT index (iEAT) that predicts the development of major cardiovascular events in a cohort of healthy individuals was the 95th percentile, which corresponded to 68.1 cm3/m2 of body surface area. In type 2 diabetes or obesity, there is an increase in the volume of epicardial adipose tissue, which implies an increased risk of developing CVD8,12.
On the other hand, increased levels of the circulating proconvertase subtilisin-kexin 9 (PCSK9) are found in patients with type 2 diabetes, obesity and CVD and are associated with CVD severity14–18. The main action of PCSK9 is the regulation of low-density lipoprotein receptor (LDLR) recycling, forming a complex that is internalized and promotes the degradation of LDLR by hepatocytes. LDLR function is to bind plasmatic low-density lipoprotein (LDL) and remove it from circulation to supply cholesterol to peripheral cells. However, in addition to its role in lipid metabolism, PCSK9 is involved in various processes related to atherosclerosis, affecting both endothelial cell and cardiomyocyte function and proinflammatory pathways19–21.
As T1D is increasingly associated with insulin resistance and obesity the investigation of these two parameters, EAT volume and PCSK9 concentration, in this setting, could add new information in this population. This study aimed to determine the relationship between circulating PCSK9 levels, metabolic status and EAT volume in patients with T1D.
Methods
This was an observational transversal study including 73 Caucasian patients with T1D who were followed since their diagnosis between 1985 and 1994 at a tertiary university hospital in Barcelona, Spain22. The diagnosis of T1D was established according to international guidelines23. All the subjects provided written informed consent to participate. The protocol was approved by the clinical research ethics committee of the Hospital de la Santa Creu i Sant Pau (IIBSP-DIA-2011-114). All procedures performed involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The following baseline characteristics were recorded: sex, age, body mass index (BMI), waist circumference (WC), time since diagnosis, type of therapy (basal-bolus regimen or use of continuous subcutaneous insulin infusion), dose of insulin expressed as units per kg per day (UI/kg/day), smoking status and the presence of comorbidities/chronic diabetic complications. Dyslipidemia was defined as the presence of any of the following: triglyceride (TG) ≥ 1.7 mmol/L, LDL cholesterol (LDL-C) > 4.2 mmol/L or hypolipidemic treatment. Hypertension was defined as the presence of three or more systolic blood pressure measurements ≥ 140 mmHg and/or diastolic blood pressure measurements ≥ 90 mmHg or antihypertensive treatment. Overweight was defined as a BMI ranging from 25 to 29.9 kg/m2, and obesity was considered to be present if the BMI was greater than or equal to 30 kg/m2. High WC was defined as a WC > 102 cm in men and > 88 cm in women. A hypertriglyceridemic waist was defined as a high WC and TG ≥ 1.7 mmol/L.
The biochemical parameters analyzed included total cholesterol (TC), TG, high-density lipoprotein cholesterol (HDL-C), LDL-C, very low-density lipoprotein cholesterol (VLDL-C), apolipoprotein B (ApoB), HbA1c levels and the urine albumin-to-creatinine ratio (ACR), as previously described22. The PCSK9 concentration was determined via ELISA according to the manufacturer’s instructions (Bio Vendor, Ref # RD191473200R).
A cardiac computed tomography angiography (CCTA) exam using a 256-slice CT scanner (Brilliance iCT 256; Philips Healthcare, Amsterdam, the Netherlands) was performed on all participants. The data is expressed as the EAT volume (cm3) indexed to the body surface area (m2), EAT index cm3/m2. The details of the procedure are described in detail in22,24.
The statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL, USA) version 21.0 for Windows. Variables are expressed as the mean ± standard deviation (SD) for continuous variables and as absolute numbers with percentages for categorical variables. The Kolmogorov‒Smirnov test was used to assess the distribution of continuous variables. We used the parametric chi-square test to compare categorical variables. Student’s t test was used to compare categorical and continuous variables with a normal distribution, and the Mann–Whitney U test was performed for nonparametric variables. The Pearson correlation test was used to analyze the correlation between continuous variables with a normal distribution, and Spearman’s rho test was performed for the nonparametric variables. Statistically significant correlations between the studied variables and the PCSK9 concentration were further analyzed by a multiple regression model and the backward method, in which nonparametric variables were previously transformed into logarithms. p < 0.05 was considered to indicate statistical significance.
Ethics approval and consent to participate
The protocol was approved by the clinical research ethics committee of the Hospital de la Santa Creu i Sant Pau (IIBSP-DIA-2011-114). All the subjects provided written informed consent to participate.
Results
The baseline demographic, clinical and biochemical characteristics of the 73 patients included are shown in Table 1. There were 15 (20.5%) patients with obesity, 43 (58.9%) with overweight, 20 (27.4%) with high WC, and 5 (6.8%) with hypertriglyceridemic waist. The mean iEAT was 39.8 ± 22.4 cm3/m2 and 16 subjects (21.9%) had an iEAT > 75th percentile.
Table 1.
Baseline clinical, metabolic and biochemical characteristics.
| Sex M/F | 43 (58.9)/30 (41.1) |
| Age, years | 47.2 ± 8.5 |
| Time since diagnose, years | 22.4 ± 2.2 |
| Insulin dose, Ui/kg/day | 0.6 ± 0.2 |
| Weight, kg | 77.8 ± 17.7 |
| BMI, kg/m2 | 26.9 ± 4.6 |
| WC, cm | 93.9 ± 13.1 |
| Type of treatment, BBT/CSII | 62 (84.9)/11 (15.1) |
| Smoking status | |
| Smoker | 25 (34.2) |
| Former smoker | 22 (30.1) |
| Non smoker | 26 (35.6) |
| Dyslipidemia | 40 (54.8) |
| Hypolipidemic treatment | 34 (46.6) |
| Statin treatment | 33 (45.2) |
| Statins and ezetimibe treatment | 1 (1.4) |
| Hypertension | 23 (31.5) |
| Diabetic retinopathy | 13 (18.1) |
| Diabetic nephropathy | 8 (11) |
| Diabetic neuropathy | 12 (16.4) |
| CAD | 1 (1.4) |
| Stroke | 1 (1.4) |
| Peripheral arterial disease | 2 (2.8) |
| HbA1c, % (mmol/mol) | 7.6 (60 mmol/mol) ± 1.1 |
| ACR, mg/mmol | 2.2 ± 0.7 |
| Total cholesterol, mmol/L | 4.77 ± 0.72 |
| HDL cholesterol, mmol/L | 1.48 ± 0.30 |
| LDL cholesterol, mmol/L | 2.83 ± 0.56 |
| VLDL cholesterol, mmol/L | 0.45 ± 0.32 |
| Triglycerides, mmol/L | 1.02 ± 0.9 |
| Apolipoprotein B, g/L | 0.78 ± 0.16 |
| PCSK9, mg/L, | 0.37 ± 0.12 |
Male (M); Female (F); Waist circumference (WC); Basal-bolus therapy (BBT); Continuous Subcutaneous Insulin Infusion (CSII); Coronary artery disease (CAD); Urine Albumin to Creatinine Ratio (ACR); Proconvertase subtilisin-kexin 9 (PCSK9).
The data are expressed as the mean ± SD or n (%).
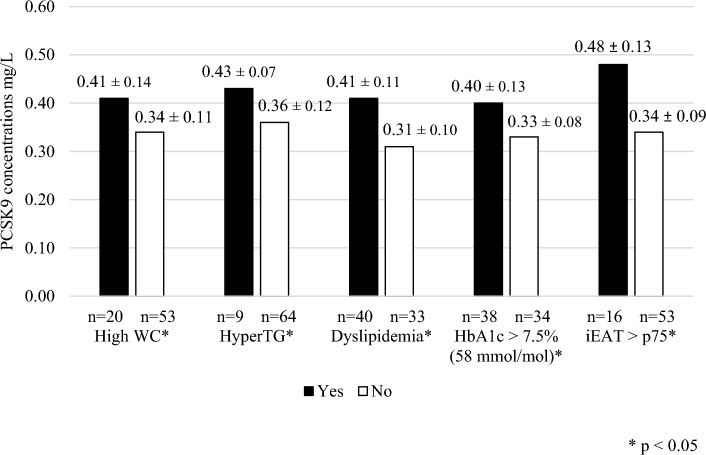
The mean PCSK9 concentration was 0.37 ± 0.12 mg/L. The PCSK9 concentration was significantly greater in T1D patients with a high WC, TG ≥ 1.7 mmol/L, dyslipidemia, HbA1c > 7.5% (58 mmol/mol), or an iEAT above the 75th percentile (Fig. 1). We did not find differences in the PCSK9 plasma concentration according to sex or with hypertension, obesity, overweight, chronic complications, or smoking (data not shown).
Figure 1.
Comparative PCSK9 plasma concentration according to clinical and biochemical variables. Figure shows the difference in the PCSK9 concentration according to clinical, metabolic and biochemical parameters. Proconvertase subtilisin-kexin 9 (PCSK9); Waist circumference (WC); Hypertriglyceridemia (HyperTG); Epicardial adipose tissue index (iEAT).
Correlation analysis revealed positive correlations between PCSK9 concentration and age (r = 0.259; p = 0.027), HbA1c (r = 0.300; p = 0.011), insulin dose (r = 0.275; p = 0.020), VLDL-C level (r = 0.331; p = 0.004), TG level (r = 0.328; p = 0.005), and iEAT (r = 0.438; p < 0.001). The correlation between PCSK9 and iEAT is shown in Fig. 2.
Figure 2.
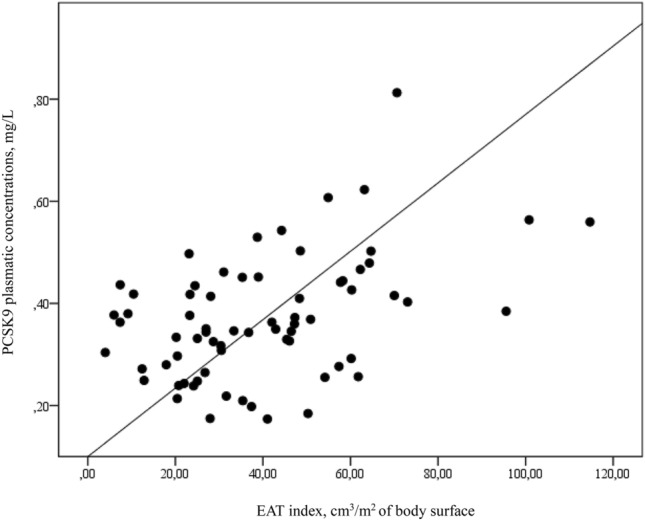
Correlation between EAT index and PCSK9 plasmatic concentration. Figure shows the correlation between the PCSK9 plasma concentration and the EAT index. r = 0.438, p < 0.001. Proconvertase subtilisin-kexin 9 (PCSK9); Epicardial adipose tissue (EAT).
Among the quantitative variables significantly correlated with the PCSK9 plasma concentration, multiple regression analysis was performed to analyze the predictors of the PCSK9 concentration; these included age, HbA1c, insulin dose, TG level and iEAT. The results showed that 25% of the PCSK9 variability was explained by iEAT and HbA1c (p < 0.05) (Table 2).
Table 2.
Multiple linear regression function for PCSK9.
| Nonstandardized coefficients | Typified coefficients | t | P value | IC (95%) for B | ||
|---|---|---|---|---|---|---|
| B | Typical error | |||||
| iEAT | 0.002 | 0.002 | 0.374 | 3.389 | 0.001 | 0.001 to 0.003 |
| HbA1c | 0.029 | 0.012 | 0.266 | 2.411 | 0.019 | 0.005 to 0.052 |
| Constant | 0.070 | 0.091 | 0.769 | 0.445 | − 0.112 to 0.252 | |
To evaluate the adjustment of the regression model to the observed data, different statistics were used, such as the multiple correlation coefficient (R = 0.498) and the coefficient of determination (R2 = 0.248). The R2 value indicates that 25% of the variation in PCSK9 is explained by the model. The corrected R2 is 0.225. The significance value of F (Fisher distribution) = 10.56 with a P value < 0.001 confirms that the model is appropriate.
Proconvertase subtilisin-kexin 9 (PCSK9); Epicardial adipose tissue index (iEAT).
Discussion
In the present study, we confirm the relationship between the PCSK9 concentration and the degree of metabolic control in patients with T1D and also with components of metabolic syndrome. The main novelty of our study is the positive association between the iEAT and the PCSK9 plasma concentration, which remained significant after adjustment for other confounding variables, such as age, insulin dose and TG level (Fig. 3).
Figure 3.
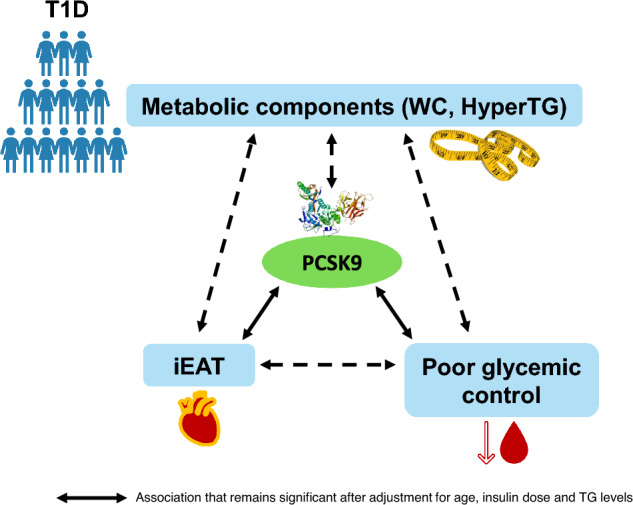
A graphical summary of results is presented in this figure. Elevated plasma concentration of PCSK9 is associated with components of metabolic syndrome, poor glycemic control and high EAT volume in patients with T1D. Type 1 diabetes (T1D); Waist circumference (WC); Hypertriglyceridemia (HyperTG); Proconvertase subtilisin-kexin 9 (PCSK9); Epicardial adipose tissue index (iEAT).
PCSK9 and glycemic control
A higher concentration of PCSK9 has been described in T1D patients with poor glycemic control25–27. Some studies have shown that insulin induces PCSK9 expression, principally through SREBP-1c and HNF-1α transcription factors28–31, suggesting a potential link between poor glycemic control, insulin dose and PCSK9 levels in patients with T1D. However, Laugier-Robiolle et al.25 reported that the association between PCSK9 concentration and poor glycemic control remained significant after adjusting for insulin dose, as occurred in our cohort. In vitro studies have demonstrated that the increase in cholesterol concentration, may lead to β-cell dysfunction and worsening/progression of diabetes32,33. In this context, we could hypothesize that the association between PCSK9 levels and glycemic control is mediated by increased cholesterol concentration. However, in subjects with familial hypercholesterolemia, including those with a gain of function mutation in PCSK9, epidemiological studies have shown a lower DM prevalence34.Thus, the underlying mechanism explaining the relationship between PCSK9 concentration and poor glycemic control remains to be clarified.
PCSK9 and components of metabolic syndrome
The relationship between PCSK9 levels and insulin resistance parameters has been reported in previous studies in patients with and without type 2 diabetes35–37, but information on T1D is scarce. In general population and in patients with type 2 diabetes several studies have reported an association of PCSK9 levels with obesity38–40, WC and different metabolic parameters35such as TG and HOMA score35. In our study we also found a relationship between increased levels of PCSK9 in patients with high WC, but not with overweight and obesity. Concerning atherogenic dyslipidemia, the association between PCSK9 concentration and elevated TG levels or the prevalence of small dense LDL (sdLDL) has already been described in previous studies25,27,41,42 in patients with T1D and type 2 diabetes25,27,41,42. In our cohort, we also found an association between increased levels of PCSK9 and the presence of dyslipidemia and hyperTG. Notably, treatment with statins and fibrates increases plasma PCSK9 levels19,41,43, likely due to counterregulation of LDLR overexpression caused by statins43. Although this could partially explain the association between the PCSK9 concentration and dyslipidemia found in our study, this fact does not justify the association with the presence of hyperTG because none of the patients were receiving treatment with fibrates.
PCSK9 and iEAT
Studies concerning the relationship between PCSK9 and EAT are scarce, and no study has analyzed such association in patients with T1D. Dozio et al. studied the relationship between PCSK9 and EAT in subjects with CVD. They found a positive correlation between the local expression of PCSK9 by EAT and its thickness but they did not find a positive association between plasma PCSK9 levels and EAT volume in these patients44. The authors argue that the EAT-induced inflammation may upregulate PCSK9 expression, thus explaining the association between the local expression of PCSK9 by EAT and its thickness44. In contrast, Baragetti et al.45 reported that nondiabetic individuals with the loss-of-function PCSK9 R46L variant, which implies decreased PCSK9 concentration, had greater epicardial fat thickness. These discrepancies point to a complex relationship between PCSK9 alterations and the accumulation of EAT, which deserves future research. As EAT thickness is a marker for visceral adiposity and the related metabolic and cardiovascular risk, PCSK9 might be one of the factors responsible for the development of atherogenic dyslipidemia and increased CVD risk in patients with T1D.The main limitations of our study are its cross-sectional nature and the small sample size of our cohort, making larger studies necessary to confirm our data. Another limitation of the study is the presence of statin treatment, which could partially explain the association between the PCK9 concentration and dyslipidemia, although would not justify the association with hyperTG because none of the patients were receiving treatment with fibrates. However, the main strength of this study is that, to the best of our knowledge, this is the first study to evaluate parameters related to metabolic syndrome and EAT measured by CCTA in patients with T1D. Furthermore, this procedure has been performed in well-characterized patients with T1D, treated with intensive insulin treatment and followed in our hospital since their diagnosis.
Conclusion
In patients with T1D, an elevated plasma concentration of PCSK9 is associated with components of metabolic syndrome, poor glycemic control and high EAT volume. EAT thickness is a marker for visceral adiposity and related metabolic alterations, and PCSK9 might be one of the factors responsible for the development of atherogenic dyslipidemia. In this context, further studies, including those with newly drugs such as monoclonal antibody against PCSK9 and synthetic small RNA that interferes PCSK9 transcription46,47, could contribute to acquire a more detailed understanding of the link between PCSK9 and EAT volume and to determine its contribution to dyslipidemia and increased CVD risk associated with T1D.
Author contributions
H.S., A.P. and J.L.S.Q. designed the study. H.S., C.C., G.C., J.A., and I.M. carried out the patient assessments. S.B. and J.L.S.Q. undertook the laboratory work. All the authors analyzed and interpreted the study data. H.S. wrote the initial draft of the manuscript. I.M., J.L.S.Q., F.B.V. and A.P. reviewed and edited the manuscript. All the authors read and approved the final version of the manuscript.
Funding
This work was supported by FIS PI16/00471 and PI20/00334 from the Instituto de Salud Carlos III/Ministry of Health, co-financed by the European Regional Development Fund (FEDER “A way to make Europe”/“Investing in your future”) and a grant from the Sociedad Española de Diabetes (SED). CIBERDEM (CB07/08/0016) is an Instituto de Salud Carlos III Project.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jose Luís Sanchez-Quesada, Email: jsanchezq@santpau.cat.
Antonio Pérez, Email: aperez@santpau.cat.
References
- 1.Livingstone SJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9:e1001321. doi: 10.1371/journal.pmed.1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley RR, Peters SAE, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:198–206. doi: 10.1016/S2213-8587(14)70248-7. [DOI] [PubMed] [Google Scholar]
- 3.Colom C, Rull A, Sanchez-Quesada JL, Pérez A. Cardiovascular disease in type 1 diabetes mellitus: Epidemiology and management of cardiovascular risk. J. Clin. Med. 2021;10:1798. doi: 10.3390/jcm10081798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergès B. Cardiovascular disease in type 1 diabetes: A review of epidemiological data and underlying mechanisms. Diabetes Metab. 2020;46:442–449. doi: 10.1016/j.diabet.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 5.Schofield, J., Ho, J., Soran, H., J. Cardiovascular Risk in Type 1 Diabetes Mellitus. Diabetes Therapy10, 773–789 (2019). [DOI] [PMC free article] [PubMed]
- 6.Tell S, Nadeau KJ, Eckel RH. Lipid management for cardiovascular risk reduction in type 1 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2020;27:207–214. doi: 10.1097/MED.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ansaldo AM, Montecucco F, Sahebkar A, Dallegri F, Carbone F. Epicardial adipose tissue and cardiovascular diseases. Int. J. Cardiol. 2019;278:254–260. doi: 10.1016/j.ijcard.2018.09.089. [DOI] [PubMed] [Google Scholar]
- 8.Berg, G., Miksztowicz, V., Morales, C. & Barchuk, M. Epicardial adipose tissue in cardiovascular disease. in Advances in Experimental Medicine and Biology vol. 1127 131–143 (Springer, 2019). [DOI] [PubMed]
- 9.Konwerski M, Asecka AG, Opolski G, Grabowski M, Mazurek T. Role of epicardial adipose tissue in cardiovascular diseases: A review. Biology. 2022 doi: 10.3390/biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goeller M, et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J. Cardiovasc. Comput. Tomogr. 2018;12:67–73. doi: 10.1016/j.jcct.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahabadi AA, et al. Association of epicardial fat with cardiovascular risk factors and incident myocardial infarction in the general population: The Heinz Nixdorf recall study. J. Am. Coll. Cardiol. 2013;61:1388–1395. doi: 10.1016/j.jacc.2012.11.062. [DOI] [PubMed] [Google Scholar]
- 12.Lee YJ, et al. Increased epicardial adipose tissue (EAT) volume in type 2 diabetes mellitus and association with metabolic syndrome and severity of coronary atherosclerosis. Clin. Endocrinol. (Oxf.) 2009;70:876–882. doi: 10.1111/j.1365-2265.2008.03411.x. [DOI] [PubMed] [Google Scholar]
- 13.Shmilovich H, et al. Threshold for the upper normal limit of indexed epicardial fat volume: Derivation in a healthy population and validation in an outcome-based study. Am. J. Cardiol. 2011;108:1680–1685. doi: 10.1016/j.amjcard.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, et al. Proprotein convertase subtilisin-kexin type 9 as a biomarker for the severity of coronary artery disease. Ann. Med. 2015;47:386–393. doi: 10.3109/07853890.2015.1042908. [DOI] [PubMed] [Google Scholar]
- 15.Peng J, et al. Association of circulating proprotein convertase subtilisin/kexin type 9 concentration, prothrombin time and cardiovascular outcomes: A prospective cohort study. Thromb. J. 2021;19:1–13. doi: 10.1186/s12959-021-00344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luquero A, Badimon L, Borrell-Pages M. PCSK9 functions in atherosclerosis are not limited to plasmatic LDL-cholesterol regulation. Front. Cardiovasc.Med. 2021 doi: 10.3389/fcvm.2021.639727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cammisotto V, et al. Proprotein convertase subtilisin kexin type 9 (PCSK9) beyond lipids: The role in oxidative stress and thrombosis. Antioxidants. 2022 doi: 10.3390/antiox11030569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunimura A, et al. Relationship between serum proprotein convertase subtilisin/kexin type 9 concentration and prevalence of coronary artery calcium in a community-based sample of Japanese men. J. Atheroscler. Thromb. 2023;30:767–777. doi: 10.5551/jat.63549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glerup S, Schulz R, Laufs U, Schlüter KD. Physiological and therapeutic regulation of PCSK9 activity in cardiovascular disease. Basic Res. Cardiol. 2017;112:32. doi: 10.1007/s00395-017-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dounousi E, et al. Association between PCSK9 levels and markers of inflammation, oxidative stress, and endothelial dysfunction in a population of nondialysis chronic kidney disease patients. Hindawi Oxid. Med. Cell. Longev. 2021;667:7012. doi: 10.1155/2021/6677012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Z, Pothineni NVK, Goel A, Lüscher TF, Mehta JL. PCSK9 and inflammation: Role of shear stress, pro-inflammatory cytokines, and LOX-1. Cardiovasc. Res. 2020;116:908–915. doi: 10.1093/cvr/cvz313. [DOI] [PubMed] [Google Scholar]
- 22.Colom C, et al. Associations between epicardial adipose tissue, subclinical atherosclerosis and high-density lipoprotein composition in type 1 diabetes. Cardiovasc. Diabetol. 2018;17:1–10. doi: 10.1186/s12933-018-0794-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Associatio AD. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes—2021. Diabetes Care. 2020;44:S15–S33. doi: 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalo-Calvo DD, et al. Soluble LRP1 is an independent biomarker of epicardial fat volume in patients with type 1 diabetes mellitus. Sci. Rep. 2018;8:1054. doi: 10.1038/s41598-018-19230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laugier-Robiolle S, et al. Glycaemic control influences the relationship between plasma PCSK9 and LDL cholesterol in type 1 diabetes. Diabetes Obes. Metab. 2017;19:448–451. doi: 10.1111/dom.12819. [DOI] [PubMed] [Google Scholar]
- 26.Levenson AE, et al. PCSK9 is increased in youth with type 1 diabetes. Diabetes Care. 2017;40:e85–e87. doi: 10.2337/dc16-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bojanin D, et al. Association between proprotein convertase subtilisin/kexin 9 (PCSK9) and lipoprotein subclasses in children with type 1 diabetes mellitus: Effects of glycemic control. Atherosclerosis. 2019;280:14–20. doi: 10.1016/j.atherosclerosis.2018.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J. Clin. Endocrinol. Metab. 2009;94:2537–2543. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costet P, et al. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J. Biol. Chem. 2006;281:6211–6218. doi: 10.1074/jbc.M508582200. [DOI] [PubMed] [Google Scholar]
- 30.Miao J, et al. Role of insulin in the regulation of proprotein convertase subtilisin/kexin type 9. Arterioscler. Thromb. Vasc. Biol. 2015;35:1589–1596. doi: 10.1161/ATVBAHA.115.305688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morelli MB, Wang X, Santulli G. Functional role of gut microbiota and PCSK9 in the pathogenesis of diabetes mellitus and cardiovascular disease. Atherosclerosis. 2019;289:176–178. doi: 10.1016/j.atherosclerosis.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion. Diabetes. 2007;56:2328–2338. doi: 10.2337/db07-0056. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Shi J, Su Q, Yang Z, Qin L. Correlation between circulating PCSK9 levels and gestational diabetes mellitus in a Chinese population. Front. Endocrinol. 2022;13:826757. doi: 10.3389/fendo.2022.826757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.González-Lleó AM, Sánchez-Hernández RM, Boronat M, Wägner AM. Diabetes and familial hypercholesterolemia: Interplay between lipid and glucose metabolism. Nutrients. 2022;14:1503. doi: 10.3390/nu14071503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan DC, et al. Plasma proprotein convertase subtilisin Kexin type 9 as a predictor of carotid atherosclerosis in asymptomatic adults. Heart Lung Circ. 2016;25:520–525. doi: 10.1016/j.hlc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Baass A, et al. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin. Chem. 2009;55:1637–1645. doi: 10.1373/clinchem.2009.126987. [DOI] [PubMed] [Google Scholar]
- 37.Hamamura H, et al. Serum proprotein convertase subtilisin/kexin type 9 (PCSK9) is independently associated with insulin resistance, triglycerides, lipoprotein(a) levels but not low-density lipoprotein cholesterol levels in a general population. J. Atheroscler. Thromb. 2021;28:329–337. doi: 10.5551/jat.56390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levenson AE, et al. Obesity and type 2 diabetes are associated with elevated PCSK9 levels in young women. Pediatr. Diabetes. 2017;18:755–760. doi: 10.1111/pedi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tóth Š, et al. Elevated circulating PCSK9 concentrations predict subclinical atherosclerotic changes in low risk obese and non-obese patients. Cardiol. Ther. 2017;6:281–289. doi: 10.1007/s40119-017-0092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mba CM, Mbacham W, Sobngwi E, Mbanya JC. Is PCSK9 associated with plasma lipid levels in a sub-saharan african population of patients with obesity and type 2 diabetes? Diabetes Metab. Syndr. Obes. 2019;12:2791–2797. doi: 10.2147/DMSO.S234243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arsenault BJ, et al. Effect of atorvastatin, cholesterol ester transfer protein inhibition, and diabetes mellitus on circulating proprotein subtilisin kexin type 9 and lipoprotein(a) levels in patients at high cardiovascular risk. J. Clin. Lipidol. 2018;12:130–136. doi: 10.1016/j.jacl.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Waluś-Miarka, M., Kapusta, M., Miarka, P., Kawalec, E. & Idzior-Waluś, B. Serum PCSK9 correlates with PTX3 and apolipoproteins B, A1, and C3 concentrations in patients with type 2 diabetes. Cardiovasc. Ther.2021, 956161 (2021). [DOI] [PMC free article] [PubMed]
- 43.Silbernagel G, Scharnagl H, Kleber ME, Stojakovic T, März W. Circulating proprotein convertase subtilisin-kexin type 9, all-cause mortality, and cardiovascular mortality: The Ludwigshafen Risk and Cardiovascular Health study. Eur. J. Prev. Cardiol. 2017;24:1095–1101. doi: 10.1177/2047487317693938. [DOI] [PubMed] [Google Scholar]
- 44.Dozio, E. et al. PCSK9 expression in epicardial adipose tissue: Molecular Association with local tissue inflammation. Mediators Inflamm.2020, 1348913 (2020). [DOI] [PMC free article] [PubMed]
- 45.Baragetti A, et al. PCSK9 deficiency results in increased ectopic fat accumulation in experimental models and in humans. Eur. J. Prev. Cardiol. 2017;24:1870–1877. doi: 10.1177/2047487317724342. [DOI] [PubMed] [Google Scholar]
- 46.Santulli G, Jankauskas SS, Gambardella J. Inclisiran: A new milestone on the PCSK9 road to tackle cardiovascular risk. Eur. Heart J. Cardiovasc. Pharmacother. 2021;7:e11–e12. doi: 10.1093/ehjcvp/pvab014. [DOI] [PubMed] [Google Scholar]
- 47.Marfella R, et al. Evidence of an anti-inflammatory effect of PCSK9 inhibitors within the human atherosclerotic plaque. Atherosclerosis. 2023;378:117180. doi: 10.1016/j.atherosclerosis.2023.06.971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.